Abstract
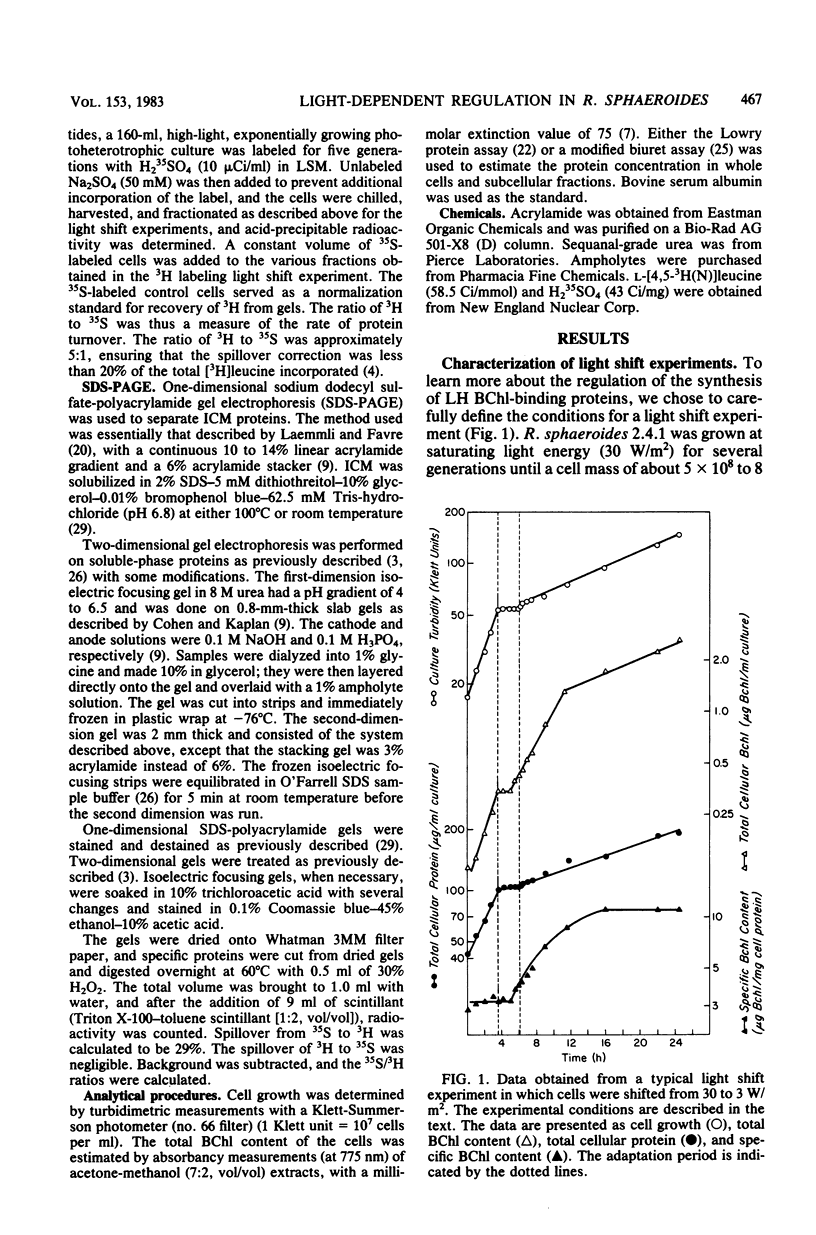
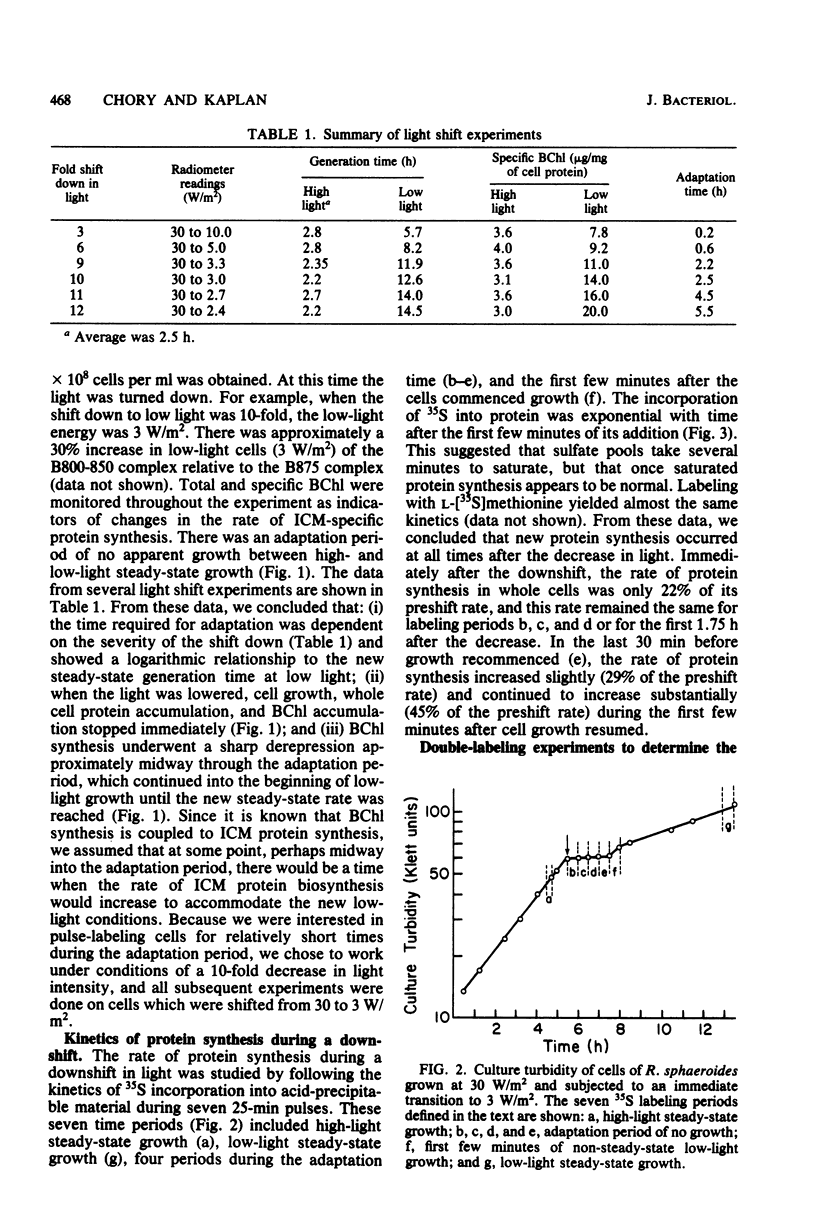
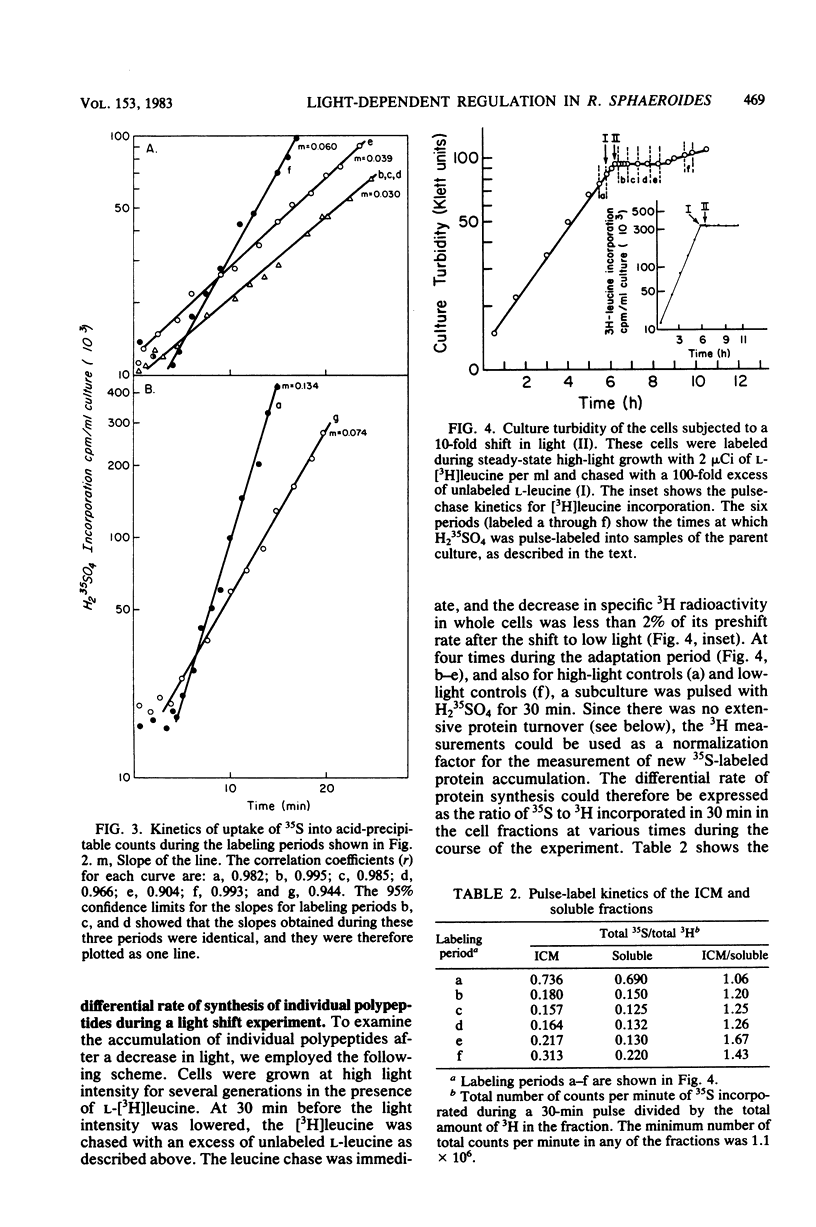
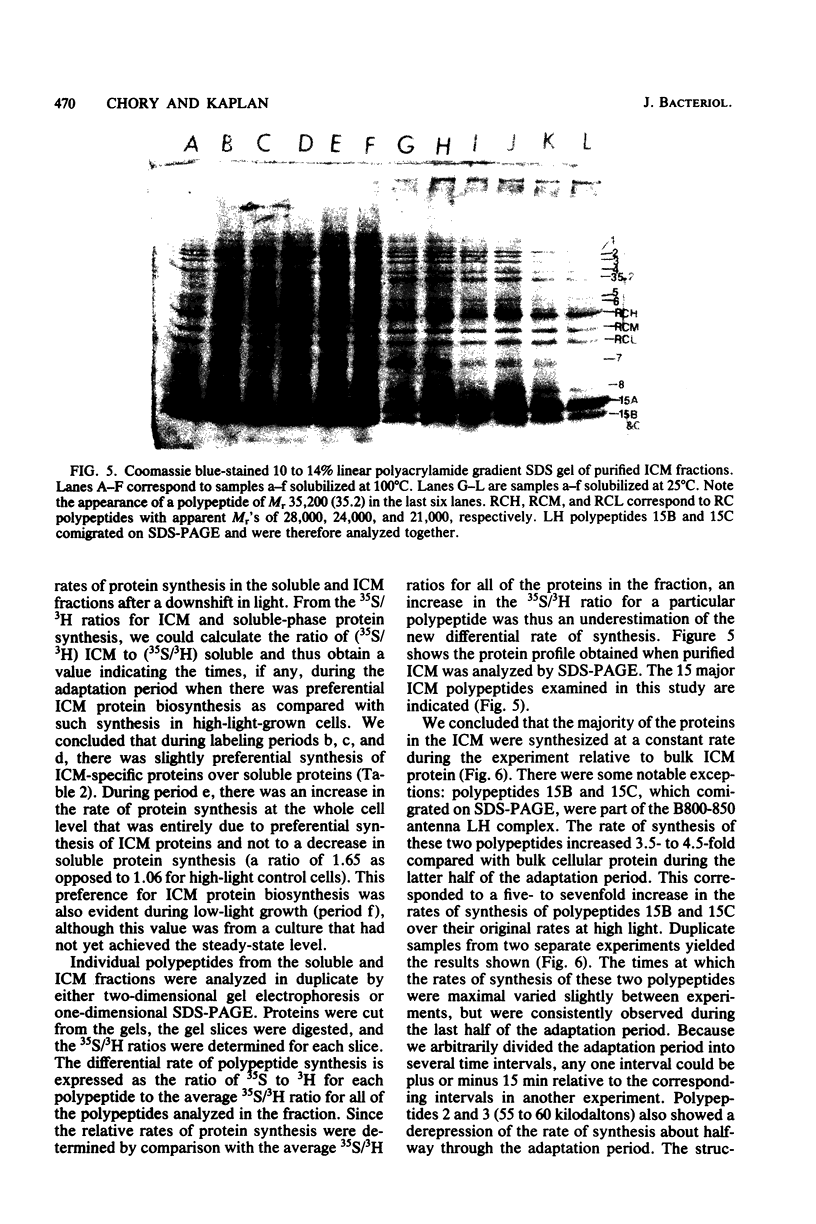
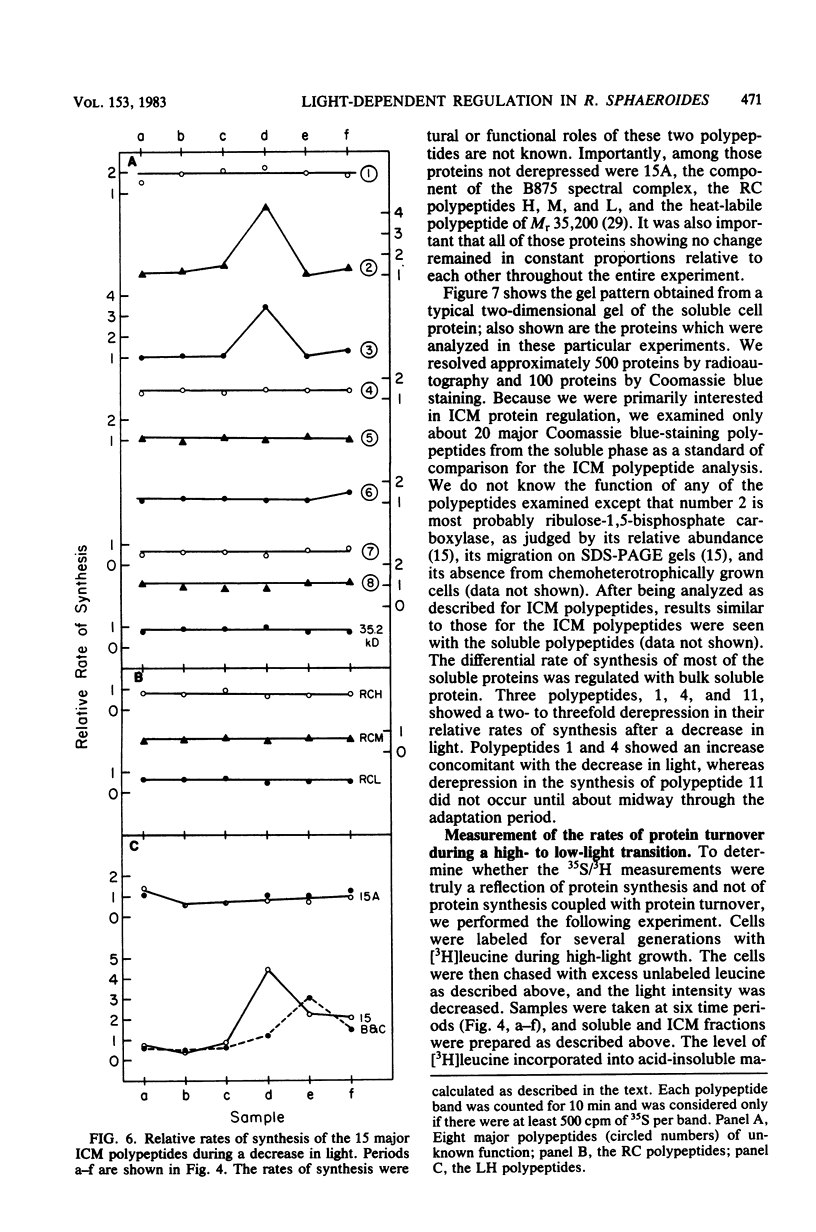
Cells of Rhodopseudomonas sphaeroides grown under saturating light conditions (30 W/m2) and then shifted to low light intensity (3 W/m2) required 2.5 h to adapt to the new lower light conditions. After the shift, cell growth, whole cell protein accumulation, and bacteriochlorophyll accumulation ceased immediately. Approximately midway into the adaptation period, bacteriochlorophyll synthesis commenced at a new, higher rate, which continued through the beginning of the low-light growth period until new steady-state levels were reached. Immediately after the downshift, the rate of cellular protein synthesis declined to 22% of its preshift rate. Pulse-labeling of protein throughout the adaptation period and comparison with a steady-state prelabel culture revealed that synthesis of two of the three light-harvesting proteins, as well as two additional high-molecular-weight photosynthetic membrane proteins, was derepressed three- to fivefold compared with bulk cellular protein. Finally, the synthesis of at least three soluble proteins showed light-dependent regulation after the light downshift. These results are discussed in terms of the light-dependent regulation of synthesis of the photosynthetic membrane macromolecular components and the division of protein synthesis between the photosynthetic membranes and the soluble cell phase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard J., Sistrom W. R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972 Feb;15(2):209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- BULL M. J., LASCELLES J. The association of protein synthesis with formation of pigments in some photosynthetic bacteria. Biochem J. 1963 Apr;87:15–28. doi: 10.1042/bj0870015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Holland I. B. Regulation of the synthesis of surface protein in the cell cycle of E. coli B/r. Cell. 1979 Oct;18(2):287–296. doi: 10.1016/0092-8674(79)90048-5. [DOI] [PubMed] [Google Scholar]
- Broglie R. M., Hunter C. N., Delepelaire P., Niederman R. A., Chua N. H., Clayton R. K. Isolation and characterization of the pigment-protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):87–91. doi: 10.1073/pnas.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J., Crofts A. R. Analysis of the pigment content of an antenna pigment-protein complex from three strains of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1978 Jun 8;502(3):409–416. doi: 10.1016/0005-2728(78)90074-9. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R. SOME OBSERVATIONS ON THE SYNTHESIS AND FUNCTION OF THE PHOTOSYNTHETIC APPARATUS IN RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1543–1553. doi: 10.1073/pnas.46.12.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. K., Kaplan S. Characterization of the three major intracytoplasmic membrane polypeptides isolated from Rhodopseudomonas sphaeroides. J Biol Chem. 1981 Jun 10;256(11):5909–5915. [PubMed] [Google Scholar]
- Cohen L. K., Kaplan S. The non-detergent solubilization and isolation of intracytoplasmic membrane polypeptides from Rhodopseudomonas sphaeroides. J Biol Chem. 1981 Jun 10;256(11):5901–5908. [PubMed] [Google Scholar]
- Donohue T. J., Cain B. D., Kaplan S. Alterations in the phospholipid composition of Rhodopseudomonas sphaeroides and other bacteria induced by Tris. J Bacteriol. 1982 Nov;152(2):595–606. doi: 10.1128/jb.152.2.595-606.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley R. T., Lueking D. R., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Polypeptide insertion into growing membrane. J Biol Chem. 1978 Jan 25;253(2):458–464. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Different molecular forms of D-ribulose-1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Biol Chem. 1977 Feb 10;252(3):943–949. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lueking D. R., Campbell T. B., Burghardt R. C. Light-induced division and genomic synchrony in phototrophically growing cultures of Rhodopseudomonas sphaeroides. J Bacteriol. 1981 May;146(2):790–797. doi: 10.1128/jb.146.2.790-797.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okamura M. Y., Steiner L. A., Feher G. Characterization of reaction centers from photosynthetic bacteria. I. Subunit structure of the protein mediating the primary photochemistry in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1394–1403. doi: 10.1021/bi00704a013. [DOI] [PubMed] [Google Scholar]
- Sauer K., Austin L. A. Bacteriochlorophyll-protein complexes from the light-harvesting antenna of photosynthetic bacteria. Biochemistry. 1978 May 16;17(10):2011–2019. doi: 10.1021/bi00603a033. [DOI] [PubMed] [Google Scholar]
- Shepherd W. D., Kaplan S. Effect of heat and 2-mercaptoethanol on intracytoplasmic membrane polypeptides of Rhodopseudomonas sphaeroides. J Bacteriol. 1978 Aug;135(2):656–667. doi: 10.1128/jb.135.2.656-667.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd W. D., Kaplan S., Park J. T. Penicillin-binding proteins of Rhodopseudomonas sphaeroides and their membrane localization. J Bacteriol. 1981 Aug;147(2):354–361. doi: 10.1128/jb.147.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto J., Huang Kao M. Y. Effects of incident light levels on photosynthetic membrane polypeptide composition and assembly in Rhodopseudomonas sphaeroides. J Bacteriol. 1977 Feb;129(2):1102–1109. doi: 10.1128/jb.129.2.1102-1109.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto J., Lascelles J. Coupling between bacteriochlorophyll and membrane protein synthesis in Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):799–803. doi: 10.1073/pnas.70.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]