Abstract
Integrin α6β4 signaling proceeds through Src family kinase (SFK)–mediated phosphorylation of the cytoplasmic tail of β4, recruitment of Shc, and activation of Ras and phosphoinositide-3 kinase. Upon cessation of signaling, α6β4 mediates assembly of hemidesmosomes. Here, we report that part of α6β4 is incorporated in lipid rafts. Metabolic labeling in combination with mutagenesis indicates that one or more cysteine in the membrane-proximal segment of β4 tail is palmitoylated. Mutation of these cysteines suppresses incorporation of α6β4 in lipid rafts, but does not affect α6β4-mediated adhesion or assembly of hemidesmosomes. The fraction of α6β4 localized to rafts associates with a palmitoylated SFK, whereas the remainder does not. Ligation of palmitoylation-defective α6β4 does not activate SFK signaling to extracellular signal–regulated kinase and fails to promote keratinocyte proliferation in response to EGF. Thus, compartmentalization in lipid rafts is necessary to couple the α6β4 integrin to a palmitoylated SFK and promote EGF-dependent mitogenesis.
Keywords: keratinocyte; proliferation; palmitoylation; hemidesmosome; cysteine
Introduction
The α6β4 integrin is a laminin-5 receptor with unique functions in epithelial growth and carcinoma invasion (Mainiero et al., 1997; Shaw et al., 1997). The cytoplasmic tail of β4 is large and bears no homology to the short tails of other integrin β subunits. Upon matrix binding, the β4 tail is phosphorylated on tyrosine and interacts with the adaptor Shc, inducing Ras/extracellular signal–regulated kinase (ERK) signaling (Mainiero et al., 1995, 1997). In addition, β4 activates phosphatidylinositol-3 kinase (PI-3K) and Rac (Shaw et al., 1997). Upon dephosphorylation, the β4 tail associates with the keratin cytoskeleton, leading to assembly of hemidesmosomes (Spinardi et al., 1993; Murgia et al., 1998; Dans et al., 2001). α6β4 cooperates with multiple receptor protein tyrosine kinases. Activation of the EGF receptor (EGF-R) and Met enhances phosphorylation of β4 and Shc signaling, causing disruption of hemidesmosomes and increased cell motility and proliferation (Mariotti et al., 2001; Trusolino et al., 2001). Conversely, matrix binding to α6β4 increases activation of ErbB2/Neu (Falcioni et al., 1997). Regulated joint α6β4/receptor protein tyrosine kinase signaling promotes epithelial cell survival, proliferation, and migration (Mainiero et al., 1997; Mariotti et al., 2001; Weaver et al., 2002). Deregulation of this system plays a crucial role in carcinoma invasion and growth (Shaw et al., 1997; Mariotti et al., 2001; Trusolino et al., 2001). Mice with a targeted deletion of the β4 tail display proliferation defects in the epidermis and intestinal epithelium, highlighting the physiological significance of α6β4 signaling (Murgia et al., 1998).
The lipid rafts—subdomains of the plasma membrane enriched in cholesterol and glycosphingolipids—promote membrane compartmentalization of signaling components (Simons and Toomre, 2000). Because of the biophysical properties of their lipid anchor, GPI-linked receptors and palmitoylated signaling proteins, such as certain G proteins, H-Ras, many Src family kinases (SFKs), and eNOS, are concentrated in rafts (Resh, 1999). Here, we report that the α6β4 integrin is incorporated in lipid rafts in a palmitoylation-dependent manner, and this is necessary to couple the integrin to a palmitoylated SFK (pSFK) and to promote mitogenic signaling.
Results and discussion
Lipid rafts are resistant to extraction in Triton X-100, and because of their low density, float on sucrose or OptiPrep™ gradients. To examine if α6β4 localizes to lipid rafts, HaCat keratinocytes were solubilized in Triton X-100 and subjected to sucrose gradient fractionation. The rafts were recovered from fractions 2 and 3, as indicated by the relative enrichment of caveolin-1 and the pSFK Yes in these fractions. The Triton X-100–soluble cellular fraction was distributed over fractions 7–10, as shown by blotting with anti-transferrin receptor and anti-tubulin, whereas the insoluble material remained in the pellet fraction, P (Fig. 1 A). Immunoblotting with anti-β4 showed that a significant part of α6β4 (∼15% of the total) cofractionates with the rafts, whereas the remainder is in the Triton X-100–soluble fraction and, to a minor extent, in the pellet fraction (Fig. 1 A). As shown in Fig. 1 B, treatment of HaCat cells with the cholesterol-chelating agents methyl-β-cyclodextrin and saponin disrupted the association of α6β4 with lipid rafts. Thus, a fraction of α6β4 partitions in the low density fractions of sucrose gradients in a cholesterol-dependent manner, as expected of a lipid raft component.
Figure 1.
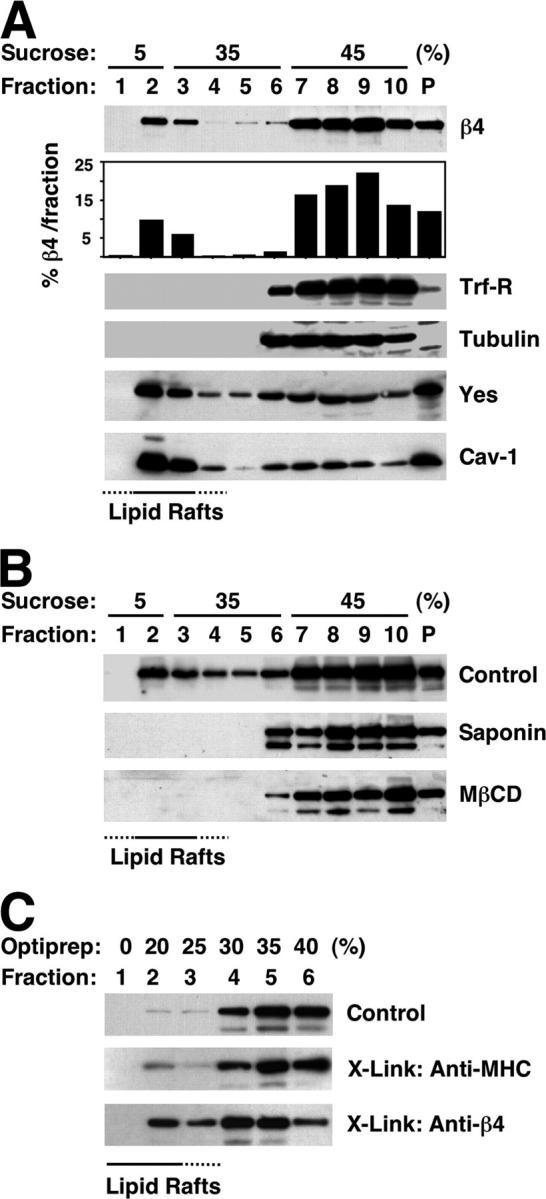
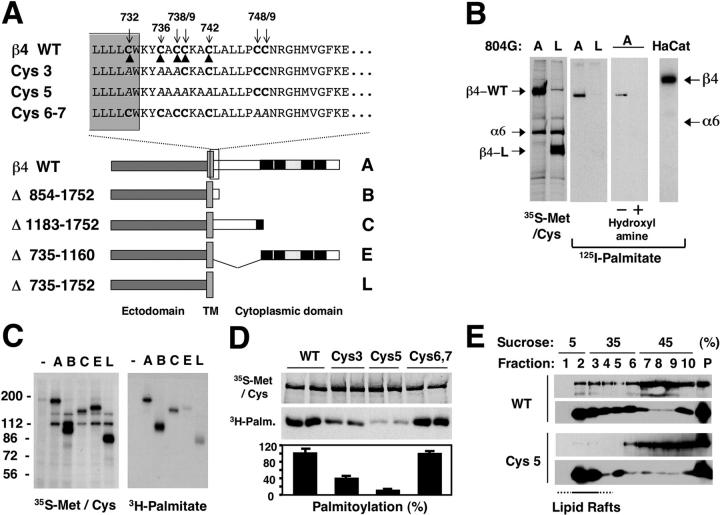
Incorporation of α6β4 in lipid rafts. (A) After lysis in Triton X-100, HaCat cells were fractionated by sucrose gradient ultracentrifugation. Equal aliquots were blotted with antibodies to β4, transferrin receptor (Trf-R), tubulin, Yes, and caveolin-1 (Cav-1). The percentage of β4 recovered from each fraction was estimated by densitometry. (B) HaCat cells were left untreated or treated with 0.2% saponin or 10 mM MβCD before lysis and ultracentrifugation. Fractions were probed with anti-β4. (C) HaCat cells were incubated in suspension with mAb W6.32 (anti-MHC) or 3E1 (anti-β4) followed by anti–mouse IgGs, or were treated with anti–mouse IgGs only. Cells were lysed in Triton X-100 and subjected to OptiPrep™ gradient ultracentrifugation. Blotting was with anti-β4.
Antibody-mediated cross-linking was used to mimic the effect of ligand-induced aggregation of α6β4 and to study its effect on the incorporation of the integrin in rafts. Fractionation was on OptiPrep™ gradients. Fig. 1 C shows that antibody-mediated ligation of α6β4 greatly increased the amount of integrin recovered from rafts (4–30%), whereas cross-linking of type I MHC did not exert this effect. In addition, the amount of α6β4 recovered from the raft fraction of untreated suspended cells was much lower than that usually obtained from the same fraction of stably adherent cells (compare Fig. 1 C with Fig. 1 A; 4 vs. 15%). These results suggest that ligand-induced aggregation promotes incorporation of α6β4 in lipid rafts.
The juxtamembrane segment of the β4 tail contains a cluster of cysteines, which may be palmitoylated (Fig. 2 A). To examine this possibility, rat bladder 804G cells expressing a wild-type (A) or a tail-less (L) human β4 were metabolically labeled with 16-[125I]iodohexadecanoic acid ([125I]IC16) palmitate analogue or [35S]methionine/cysteine and were immunoprecipitated with the anti–human β4 mAb 3E1. Fig. 2 B shows that β4 incorporated [125I]IC16, but α6 did not. Deletion of the β4 tail prevented palmitoylation of β4. In addition, treatment with alkali released the [125I] radioactive signal from β4, implying that the radioactive palmitate analogue was attached to β4 through a thio–esther bond. Notably, β4 was found to be palmitoylated to a higher apparent stoichiometry in HaCat keratinocytes (Fig. 2 B), primary human keratinocytes, and squamous carcinoma A431 cells (unpublished data). Although other integrin β subunits do not contain potential palmitoylation sites, the cytoplasmic segments of α3, α6, α8, and αE contain one membrane-proximal cysteine. We did not detect any palmitoylation of α3β1 and α6β1 (unpublished data). Thus, β4 may be the only integrin subunit modified by palmitoylation.
Figure 2.
Palmitoylation of β4 is required for incorporation of α6β4 in rafts. (A) Constructs encoding wild-type and mutant forms of β4. The β4 tail contains two pairs of type III Fn-like modules (black boxes) interrupted by a connecting segment (gray dotted box). The predicted end of transmembrane domain (TM) and membrane-proximal segment of the tail of β4 and various alanine mutants is shown above. (B) 804G cells expressing wild-type (A) or tail-less (L) β4 and HaCat cells were metabolically labeled with [35S]Met/Cys or [125I]IC16 and immunoprecipitated with mAb 3E1. The indicated lane was soaked overnight in 1 M hydroxylamine before fluorography. (C) 804G cells and their derivatives expressing wild-type β4 (A) or the indicated mutant versions of β4 (B, C, E, and L) were metabolically labeled with [35S]Met/Cys or [3H]palmitate and immunoprecipitated with mAb 3E1. (D) 293-T cells expressing wild-type β4 (A) or the indicated mutant forms of β4 (Cys 3, Cys 5, and Cys 6,7) were metabolically labeled with [35S]Met/Cys or [3H]palmitate and immunoprecipitated with mAb 3E1. The ratio of 3H to 35S radioactivity incorporated by mutant forms of β4 was measured by PhosphorImager analysis. (E) 804G cells expressing human wild-type β4 (WT) or palmitoylation-defective β4 (Cys 5) were lysed with Triton X-100 and subjected to sucrose gradient ultracentrifugation. Proteins from each fraction were probed with anti–human β4 N20 or with anti-caveolin N20.
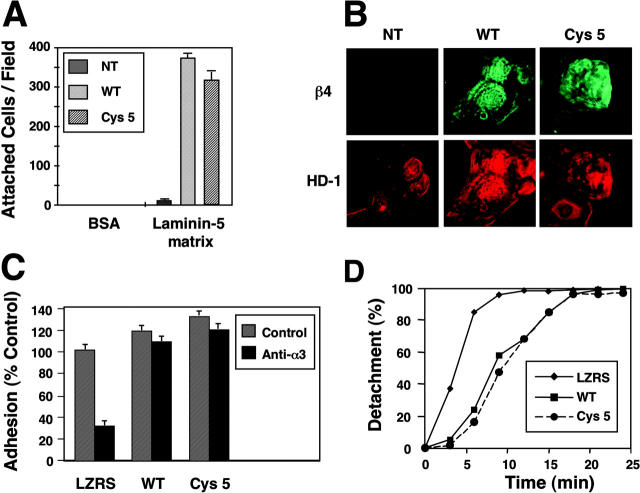
To identify the region of β4 tail modified by palmitoylation, we examined various cytoplasmic deletion mutant forms of human β4 (Fig. 2 A). Metabolic labeling with [3H]palmitate revealed that mutants B and C were palmitoylated as efficiently as wild-type β4. By contrast, mutants L and E incorporated very little [3H]palmitate (Fig. 2 C), possibly because of nonphysiological, partially compensatory palmitoylation of Cys 732, which resides at the boundary between the transmembrane and cytoplasmic domain of β4. These results suggest that the membrane-proximal segment of the tail of β4 comprises the major site(s) of palmitoylation. Next, we introduced alanine permutations at each one of the seven membrane-proximal cysteines. None of these individual mutations reduced palmitoylation of β4 by a significant degree (unpublished data). However, simultaneous replacement of the first three cysteines (β4 Cys 3) reduced the incorporation of [3H]palmitate by ∼50%, and permutation of the first five (β4 Cys 5) abolished it almost completely. In contrast, replacement of the last two cysteines (β4 Cys 6–7) did not affect palmitoylation of β4 (Fig. 2 D). These results imply that the first five cysteines in the membrane-proximal segment of β4 tail comprise the major site(s) of palmitoylation. We suspect that mutagenesis did not allow us to identify a single palmitoylation site in β4 because mutation of a specific cysteine in a cluster of potential sites may result in palmitoylation of an adjacent, not necessarily physiological site. Finally, we examined if α6β4 localizes to lipid rafts in a palmitoylation-dependent manner. 804G cells expressing equivalent levels of human β4 or the palmitoylation-defective mutant β4 Cys 5 were subjected to Triton X-100 extraction and sucrose gradient centrifugation. Notably, the β4 Cys 5 mutant was excluded from the lipid raft fraction (Fig. 2 E), indicating that palmitoylation of β4 is required for incorporation of α6β4 in lipid rafts. To examine if palmitoylation of β4 and incorporation of α6β4 in lipid rafts play a role in ligand binding, 293-T cells were transfected with vectors encoding α6 in combination with either β4 or β4 Cys 5. Immunoblotting and FACS® analysis showed that the expression levels of wild-type and mutant α6β4 were comparable. The cells were plated on laminin-5 at 4°C because at this temperature the function of α3β1, which also mediates adhesion to laminin-5, is suppressed (Xia et al., 1996). Untransfected 293-T cells did not attach to laminin-5 at 4°C, whereas cells expressing wild-type α6β4 attached to a significant extent, indicating that adhesion to laminin-5 at 4°C requires expression of α6β4. The palmitoylation-defective α6β4 mutant promoted attachment to laminin-5 as effectively as wild-type α6β4 (Fig. 3 A), suggesting that palmitoylation of β4 is not required for α6β4-mediated binding to laminin-5. To assess the ability of the palmitoylation-deficient mutant form of β4 to promote assembly of hemidesmosomes, we introduced β4 and β4 Cys 5 in β4-deficient keratinocytes from a patient affected by junctional epidermolysis bullosa with pyloric atresia (PA-JEB). As reported previously (Gagnoux-Palacios et al., 1997), transient transfection of PA-JEB keratinocytes with wild-type β4 caused assembly of hemidesmosome-like adhesions containing HD-1/plectin (Fig. 3 B) and BPAG-2 (unpublished data). Introduction of β4 Cys 5 resulted in formation of hemidesmosome-like structures similar to those nucleated by wild-type β4 (Fig. 3 B), indicating that palmitoylation of β4 is not required for assembly of hemidesmosomes. To examine the role of β4 palmitoylation in keratinocyte adhesion, we used retroviral transduction to generate PA-JEB keratinocytes expressing similar levels of β4 and β4 Cys 5. PA-JEB keratinocytes expressing β4 Cys 5 adhered to laminin-5 in the presence of anti-α3β1 antibodies as well as PA-JEB keratinocytes expressing wild-type β4 (Fig. 3 C), confirming that palmitoylation of β4 is not required for α6β4-mediated adhesion. Upon assembly of hemidesmosomes, keratinocytes become more resistant to detachment with trypsin/EDTA (Gagnoux-Palacios et al., 2001). Analysis of the kinetics of cell detachment revealed that β4 Cys 5 delays trypsin/EDTA-induced cell detachment as effectively as the wild-type β4 (Fig. 3 D). Thus, α6β4-mediated adhesion and assembly of hemidesmosomes does not require palmitoylation of β4 and incorporation of the integrin in lipid rafts.
Figure 3.
Palmitoylation of β4 is not necessary for α6β4-mediated adhesion and assembly of hemidesmosomes. (A) 293-T cells were left untransfected (NT) or transfected with α6 in combination with wild-type β4 or β4 Cys 5, and were then plated on laminin-5 matrix at 4°C. Three experiments yielded essentially identical results. (B) Immortalized β4-deficient PA-JEB keratinocytes were left untransfected (NT) or transiently transfected with α6 in combination with wild-type β4 or β4 Cys 5. Double-immunofluorescent staining with anti-β4 and anti–HD-1/plectin indicates that wild-type β4 and β4 Cys 5 are both recruited to the basal cell surface and colocalize with plectin in hemidesmosome-like adhesions. In untransfected cells, plectin codistributes instead with the actin cytoskeleton. (C) PA-JEB keratinocytes transduced with empty vector (LZRS), wild-type β4, or β4 Cys 5 were plated in the presence of anti-α3 mAb PIB5 on laminin-5 matrix for 30 min at 37°C. Results are expressed as percentages of the adhesion of control transfectants in the absence of the inhibitory mAb. (D) PA-JEB keratinocytes stably transduced with empty vector (LZRS), wild-type β4, or β4 Cys 5 were plated on laminin-5 matrix for 48 h and then treated with trypsin/EDTA. The number of cells that had detached by the indicated times was determined by direct counting. Four experiments yielded essentially the same result.
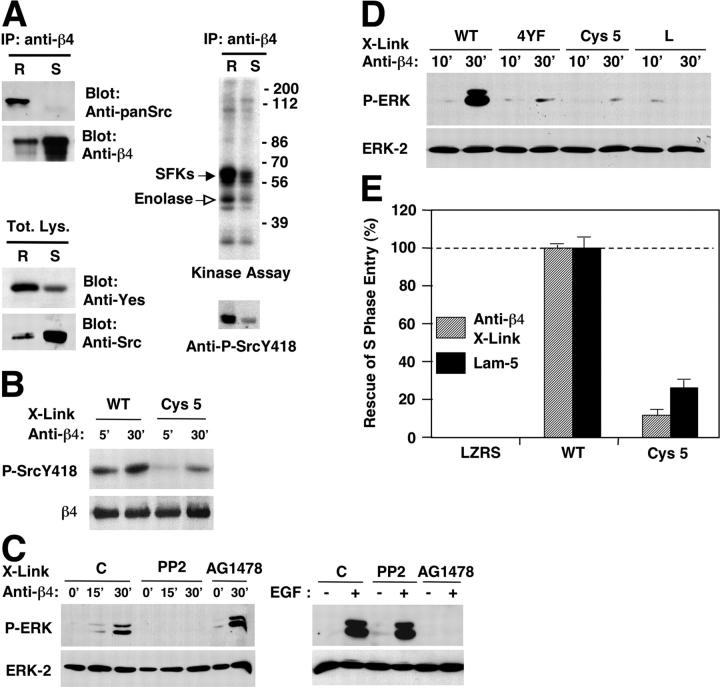
Prior reports have indicated that α6β4 signaling is mediated by a pSFK (Mariotti et al., 2001). We asked whether α6β4 associates with a pSFKs in lipid rafts. After antibody-mediated ligation of α6β4, the lipid raft and the Triton X-100–soluble fractions of HaCat keratinocytes were immunoprecipitated with anti-β4 and probed by blotting with anti-panSrc, which recognizes Src, Fyn, and Yes, and as a control, with anti-β4. We found that the lipid raft fraction of α6β4 is associated with SFKs, but the Triton X-100–soluble fraction, which is much larger, is not (Fig. 4 A). Upon introduction in HaCat cells, a GPI-linked form of GFP localized to lipid rafts, but did not coimmunoprecipitate with SFKs, providing a control for specificity (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200305006/DC1). We were unable to identify Yes in association with α6β4 immunoprecipitated from the lipid raft fraction (unpublished data), possibly due to the low stoichiometry or relative instability of the association of α6β4 with Yes and the low affinity of currently available antibodies reacting specifically with this kinase. However, immunoblotting experiments indicated that Yes is highly enriched in the lipid raft fraction of HaCat keratinocytes, whereas Src is not (Fig. 4 A). HaCat keratinocytes express very low levels of the other pSFK, Fyn (unpublished data). These results suggest that the lipid raft fraction of α6β4 is preferentially associated with a pSFK.
Figure 4.
Compartmentalization of α6β4 signaling in lipid rafts. (A) After antibody-mediated ligation of α6β4, HaCat cells were lysed with Triton X-100 and subjected to OptiPrep™ gradient ultracentrifugation. The lipid raft (R) and Triton X-100–soluble (S) fractions were either immunoprecipitated with mAb 3E1 and probed with anti-β4 or anti-pan-Src, or directly probed with antibodies reacting with Yes or Src. The lipid raft and Triton X-100–soluble fractions were also immunoprecipitated with mAb 3E1 and subjected to kinase assay or immunoblotting with anti-phospho Src Y418. (B) PA-JEB keratinocytes expressing wild-type β4 or β4 Cys 5 were plated on dishes coated with mAb 3E1 for the indicated times. Total lysates were probed with anti-phospho Src Y418 or anti-β4 N20. (C) HUVECs transiently transfected with vectors encoding α6β4 were left untreated (C) or treated with the SFK inhibitor PP2 or the EGF-R inhibitor AG1478. Cells were plated on dishes coated with mAb 3E1 for the indicated times or stimulated with EGF. Total proteins were probed with anti-phospho-ERK and anti-ERK-2. (D) HUVECs were transiently transfected with α6 in combination with wild-type β4, phosphorylation-defective β4 (4YF), β4 Cys 5, or tail-less β4 (L). Cells were plated for the indicated times on dishes coated with mAb 3E1. Blotting was as in B. (E) PA-JEB keratinocytes stably transduced with empty vector (LZRS), wild-type β4, and β4 Cys 5 were deprived of mitogens, detached, and plated on coverslips coated with purified laminin-5 or incubated in suspension with mAb 3E1, and were then plated on coverslips coated with anti–mouse IgGs. After 24 h of incubation with EGF, the cells were subjected to anti-BrdU staining. The data represent the average and SDs of values obtained from three experiments and are expressed as percentage of rescue.
To examine if α6β4 activates SFK signaling in lipid rafts, HaCat cells were subjected to anti-β4 cross-linking and sucrose gradient fractionation. The lipid raft and the Triton X-100–soluble fractions were immunoprecipitated with anti-β4 and subjected to kinase assay or blotting with anti-phospho Src Y418, which monitors phosphorylation of tyrosine in the activation loop of SFKs. The α6β4-associated pSFK from the lipid raft fraction underwent autophosphorylation, as shown by increased incorporation of 32P and reactivity with the anti-phospho Src Y418 antibody, and it also phosphorylated the exogenous substrate enolase. By contrast, the Triton X-100–soluble fraction displayed low kinase activity (Fig. 4 A). Furthermore, we observed that the β4 Cys 5 mutant promoted activation of SFKs less efficiently than wild-type β4, implying that palmitoylation of β4 is required for activation of SFKs (Fig. 4 B). These findings indicate that α6β4 is coupled to SFK signaling in lipid rafts.
To examine the role of lipid rafts in α6β4 signaling to ERK, we transiently transfected vectors encoding various mutant forms of α6β4 in β4-negative human umbilical venous endothelial cells (HUVECs; Dans et al., 2001). To confirm that α6β4 signaling to ERK is mediated by an SFK, cells transfected with wild-type α6β4 were treated with the SFK inhibitor PP2. As expected, α6β4-mediated activation of ERK was suppressed by PP2, but not by the EGF-R inhibitor AG1478. By contrast, EGF-R–mediated activation of ERK was inhibited by AG1478, but not by PP2 (Fig. 4 C). Then, we asked if localization to lipid rafts is necessary for α6β4-mediated SFK signaling to ERK. Cells were transfected with constructs encoding α6 in combination with wild-type β4, phosphorylation-defective β4 (4F), palmitoylation-defective β4 (Cys 5), or tail-less β4 (L). Immunoblotting showed that the cells expressed comparable amounts of wild-type, palmitoylation-defective, and 4YF β4, and somewhat higher levels of tail-less β4 (unpublished data). Ligation of wild-type α6β4 caused activation of ERK, whereas ligation of the palmitoylation-defective, 4YF, or tail-less mutant did not induce this event (Fig. 4 D). These results indicate that palmitoylation of β4 and, by inference, localization of α6β4 to lipid rafts are necessary for efficient signaling to ERK.
To address the physiological significance of α6β4 incorporation in lipid rafts, we examined the ability of β4 Cys 5 to promote EGF-dependent mitogenesis. After synchronization in G0, PA-JEB keratinocytes stably transduced with retroviral vectors encoding β4, β4 Cys 5, or empty virus were plated on laminin-5, or they were incubated with mAb 3E1 and plated on dishes coated with anti–mouse IgGs. BrdU incorporation and anti-BrdU staining indicated that wild-type β4 significantly enhanced the ability of PA-JEB keratinocytes to progress through the cell cycle on laminin-5. Similar results were obtained after antibody-mediated ligation of α6β4. By contrast, the palmitoylation-defective β4 was not able to rescue EGF-mediated proliferation of PA-JEB keratinocytes (Fig. 4 E), providing evidence that α6β4-dependent mitogenic signaling requires palmitoylation of β4 and incorporation of α6β4 in lipid rafts.
Although α6β4, like other integrins, does not contain a kinase domain, ligation of α6β4 causes phosphorylation of the cytoplasmic tail of β4, and hence, recruitment of Shc and other signal transducers. In order for this to occur, the integrin must associate with a tyrosine kinase. Here, we have shown that compartmentalization in lipid rafts is necessary to couple α6β4 to a pSFK and thus reconstitute its ability to activate signaling and promote epithelial mitogenesis. These results provide direct evidence that compartmentalization in lipid rafts is required for α6β4 signaling. Because it is known that part of the EGF-R localizes to lipid rafts (Waugh et al., 1999), and our prior analyses have indicated that the EGF-R activates β4 signaling through the integrin-associated pSFK (Mariotti et al., 2001), it is possible that incorporation in lipid rafts is also necessary for EGF-R–dependent activation of β4 signaling.
How does matrix binding activate α6β4 signaling? At steady state, only a fraction of α6β4 is palmitoylated, and hence localized to lipid rafts. However, antibody- or ligand-induced oligomerization of α6β4 increases the amount of integrin recovered in the raft fraction, suggesting that matrix binding to α6β4 increases the integrin's affinity for lipid rafts. In addition, palmitoylation is a reversible process (Resh, 1999), allowing for regulated incorporation of α6β4 in lipid rafts. We envision that matrix-induced aggregation of α6β4-containing rafts promotes signaling by bringing the integrin in close proximity to the pSFK, and possibly by excluding a negative regulatory tyrosine phosphatase, as implied by the observation that vanadate greatly enhances phosphorylation of β4 (Dans et al., 2001). In addition, because H-Ras, which is palmitoylated and localizes to lipid rafts, activates PI-3K more efficiently than other Ras isoforms (Yan et al., 1998; Roy et al., 1999), the association with lipid rafts may explain the ability of α6β4 to activate PI-3K, and hence, Rac, more effectively than other integrins (Shaw et al., 1997). Thus, compartmentalization in lipid rafts potentially explains several specific aspects of α6β4 signaling.
Although other integrins do not appear to be palmitoylated, prior reports suggest that membrane compartmentalization plays a role in signaling by many integrins. Certain β1 and αv integrins associate, through caveolin-1, with pSFKs, thereby activating Shc signaling to ERK (Wary et al., 1998). Although these integrins are soluble in Triton X-100, it is possible that they associate with lipid rafts through a Triton X-100–sensitive interaction with uPAR, which is GPI linked and localized to rafts (Wei et al., 1999). The α3β1, α6β1, and certain other integrins associate with tetraspanins (Hemler, 2001). Because many tetraspanins are palmitoylated and also tend to form oligomers, they could promote integrin incorporation in lipid raft-like domains. Accordingly, α6β1 associates with detergent-resistant microdomains to promote survival signaling in oligodendrocytes (Baron et al., 2003). Finally, αvβ3, αIIbβ3, and α2β1 combine with the integrin-associated protein in cholesterol-dependent microdomains distinct from classical rafts (Green et al., 1999), and α4β1 and αLβ2 have been shown to colocalize with the lipid raft marker GM-1 in T cells (Leitinger and Hogg, 2002). We anticipate that future experiments will reveal that the mechanism of membrane compartmentalization illustrated here also operates, with some variations, in other integrin-signaling systems.
Materials and methods
Antibodies and chemicals
The antibodies to β4, BPAG-2, and HD-1/plectin were characterized previously (Hieda et al., 1992; Murgia et al., 1998). Other antibodies and chemicals were from New England Biolabs, Inc. (anti-phospho-ERK), Santa Cruz Biotechnology, Inc. (anti-ERK-2, anti-Src N-16, anti-pan Src SRC2, anti-caveolin-1 N-20, and anti-β4 C-20 and N-20), Transduction Laboratories (anti-Yes mAb), Zymed Laboratories (anti-Transferrin mAb), BD Biosciences (anti-tubulin mAb), CHEMICON International (anti-α3 mAb P1B5 and anti-GFP mAb 2510), Biosource International (anti-phospho-Src Y418 pAb), Calbiochem (PP2 and AG1478), Molecular Probes, Inc. (anti-GFP A6455), Sigma-Aldrich (saponin and methyl-β-cyclodextrin), and Boehringer (BrdU and anti-BrdU mAb).
Cells, constructs, and expression methods
Vectors encoding human α6, β4, and the β4 mutants B, C, E, and L were described previously (Spinardi et al., 1993; Mainiero et al., 1997). The β4 Cys mutants were generated with QuikChange® (Stratagene). HaCat, HUVECs, rat bladder 804G, and 293-T HEK cells were transfected as described previously (Dans et al., 2001; Mariotti et al., 2001). Immortalized PA-JEB keratinocytes (Gagnoux-Palacios et al., 1997) were cultured in serum-free keratinocyte growth medium (GIBCO BRL). Retroviral particles were recovered from Phoenix packaging cells transfected with pLZRS-IRES-zeo encoding β4 or the β4 Cys 5 mutant, and were used to transduce PA-JEB keratinocytes.
Biochemical methods
For fractionation, cells were lysed on ice for 30 min with 0.5% Triton X-100 in TNE (25 mM Tris, pH 7.5, 150 mM NaCl, and 2 mM EDTA) with inhibitors. After Dounce homogenization, the lysates were subjected to either sucrose or OptiPrep™ gradient ultracentrifugation. When indicated, cells were pretreated with 0.2% saponin for 10 min at 4°C or with 10 mM MβCD for 1 h at 37°C before detergent extraction. To mimic ligand-induced aggregation of α6β4, cells were detached with EDTA, incubated in suspension with 10 μg/ml anti-β4 mAb 3E1 for 20 min on ice, washed, and then incubated with 10 μg/ml anti–mouse IgGs for 5 min at 37°C. For immunoblotting, proteins from each fraction were precipitated with TCA. For immunoprecipitation, the lipid raft and soluble fractions were diluted with an equal volume of TNE, 1% Triton X-100, and 10% sucrose. After labeling with Tran 35S-label (ICN Biomedicals), IC16 (Peseckis et al., 1993), or [3H]palmitate (ICN Biomedicals; Wolven et al., 1997), cells were lysed in 50 mM Tris, pH 8.0, 150 mM NaCl, 1% Nonidet® P-40, 0.5% sodium deoxycholate, and 2 mM EDTA with phosphatase and protease inhibitors, and were immunoprecipitated with mAb 3E1. A PhosphorImager was used to quantify the results. Kinase assays were as described previously (Mariotti et al., 2001).
Adhesion and immunofluorescence
To test adhesion, cells were plated for 1 h at 4°C or for 30 min in the presence of anti-α3 on wells coated with laminin-5 matrix (Mariotti et al., 2001). The relative resistance of PA-JEB keratinocytes to trypsin/EDTA-induced detachment was measured as described previously (Gagnoux-Palacios et al., 2001). Cells were fixed with 3.7% PFA and permeabilized with 0.1% Triton X-100 before immunofluorescent staining.
Cell cycle progression
PA-JEB keratinocytes stably transduced with pLZRS, pLZRS-β4, or pLZRS-β4 Cys5 were deprived of growth factors, detached, and either plated on coverslips coated with 5 μg/ml human laminin-5 (CHEMICON International) or incubated in suspension with the mAb 3E1 and then plated on coverslips coated with 10 μg/ml anti–mouse IgGs. Cells were cultured for 16 h in serum-free keratinocyte growth medium supplemented with 10 ng/ml EGF and 50 μg/ml bovine pituitary extract, labeled with BrdU, and then stained with anti-BrdU mAbs. The results were expressed as percentage of rescue (R), according to the formula R =([X−L]/[F−L]) × 100 (where X is the percentage of BrdU-positive cells expressing β4 Cys 5; F is the percentage of BrdU-positive cells expressing wild-type β4; and L is the percentage of BrdU-positive cells transduced with pLZRS, on either laminin-5 or the anti-β4 substrate).
Online supplemental materials
Fig. S1 shows a control for the coimmunoprecipitation of α6β4 with pSFKs from the lipid raft fraction. HaCat keratinocytes were transiently transfected with a vector encoding a GPI-linked form of GFP, obtained by fusing GFP to the COOH terminus of CD55. After Triton X-100 extraction and sucrose density fractionation, the lipid raft and the soluble fractions were immunoprecipitated with anti-GFP mAbs and subjected to blotting with either anti panSrc or anti-GFP pAbs. Online supplemental material available at http://www.jcb.org/cgi/content/full/jcb.200305006/DC1.
Supplemental Material
Acknowledgments
We thank A. Galmiche, G.P. Nolan, and K. Owaribe for reagents, and members of the Giancotti laboratory for discussions.
This work was supported by National Institutes of Health awards R37 CA58976 (to F.G. Giancotti), R01 GM57966 (to M.D. Resh), and P30 CA08748 (to the Memorial Sloan-Kettering Cancer Center).
The online version of this article includes supplemental material.
L. Gagnoux-Palacios' present address is INSERM U385, School of Medicine, University of Nice, 06107 Nice, France.
M. Dans' present address is Department of Dermatology, University of Pennsylvania, Philadelphia, PA 19104.
W. van't Hof's present address is Athersys Inc., Cleveland, OH 44115.
A. Mariotti's present address is CePO/ISREC, 1066 Epalinges, Switzerland.
Abbreviations used in this paper: [125I]IC16, 16-[125I]iodohexadecanoic acid; EGF-R, EGF receptor; ERK, extracellular signal–regulated kinase; HUVEC, human umbilical venous endothelial cell; PA-JEB, junctional epidermal bullosa with pyloric atresia; PI-3K, phosphatidylinositol-3 kinase; pSFK, palmitoylated Src family kinase; SFK, Src family kinase.
References
- Baron, W., L. Decker, H. Colognato, and C. ffrench-Constant. 2003. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr. Biol. 13:151–155. [DOI] [PubMed] [Google Scholar]
- Dans, M., L. Gagnoux-Palacios, P. Blaikie, S. Klein, A. Mariotti, and F.G. Giancotti. 2001. Tyrosine phosphorylation of the β4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J. Biol. Chem. 276:1494–1502. [DOI] [PubMed] [Google Scholar]
- Falcioni, R., A. Antonini, P. Nistico, S. Di Stefano, M. Crescenzi, P.G. Natali, and A. Sacchi. 1997. α6β4 and α 6β1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp. Cell Res. 236:76–85. [DOI] [PubMed] [Google Scholar]
- Gagnoux-Palacios, L., Y. Gache, J.P. Ortonne, and G. Meneguzzi. 1997. Hemidesmosome assembly assessed by expression of a wild-type integrin β4 cDNA in junctional epidermolysis bullosa keratinocytes. Lab. Invest. 77:459–468. [PubMed] [Google Scholar]
- Gagnoux-Palacios, L., M. Allegra, F. Spirito, O. Pommeret, C. Romero, J.P. Ortonne, and G. Meneguzzi. 2001. The short arm of the laminin γ2 chain plays a pivotal role in the incorporation of laminin-5 into the extracellular matrix and in cell adhesion. J. Cell Biol. 153:835–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, J.M., A. Zhelesnyak, J. Chung, F.P. Lindberg, M. Sarfati, W.A. Frazier, and E.J. Brown. 1999. Role of cholesterol in formation and function of a signaling complex involving αvβ3, integrin-associated protein (CD47), and heterotrimeric G proteins. J. Cell Biol. 146:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler, M.E. 2001. Specific tetraspanin functions. J. Cell Biol. 155:1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda, Y., Y. Nishizawa, J. Uematsu, and K. Owaribe. 1992. Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J. Cell Biol. 116:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger, B., and N. Hogg. 2002. The involvement of lipid rafts in the regulation of integrin function. J. Cell Sci. 115:963–972. [DOI] [PubMed] [Google Scholar]
- Mainiero, F., A. Pepe, K.K. Wary, L. Spinardi, M. Mohammadi, J. Schlessinger, and F.G. Giancotti. 1995. Signal transduction by the α6β4 integrin: distinct β4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 14:4470–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero, F., C. Murgia, K.K. Wary, A.M. Curatola, A. Pepe, M. Blumemberg, J.K. Westwick, C.J. Der, and F.G. Giancotti. 1997. The coupling of α6β4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 16:2365–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti, A., P.A. Kedeshian, M. Dans, A.M. Curatola, L. Gagnoux-Palacios, and F.G. Giancotti. 2001. EGF-R signaling through Fyn kinase disrupts the function of integrin α6β4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J. Cell Biol. 155:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia, C., P. Blaikie, N. Kim, M. Dans, H.T. Petrie, and F.G. Giancotti. 1998. Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin β4 cytoplasmic domain. EMBO J. 17:3940–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peseckis, S.M., I. Deichaite, and M.D. Resh. 1993. Iodinated fatty acids as probes for myristate processing and function. Incorporation into pp60v-src. J. Biol. Chem. 268:5107–5114. [PubMed] [Google Scholar]
- Resh, M.D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta. 1451:1–16. [DOI] [PubMed] [Google Scholar]
- Roy, S., R. Luetterforst, A. Harding, A. Apolloni, M. Etheridge, E. Stang, B. Rolls, J.F. Hancock, and R.G. Parton. 1999. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1:98–105. [DOI] [PubMed] [Google Scholar]
- Shaw, L.M., I. Rabinovitz, H.H. Wang, A. Toker, and A.M. Mercurio. 1997. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 91:949–960. [DOI] [PubMed] [Google Scholar]
- Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39. [DOI] [PubMed] [Google Scholar]
- Spinardi, L., Y.L. Ren, R. Sanders, and F.G. Giancotti. 1993. The β4 subunit cytoplasmic domain mediates the interaction of α6β4 integrin with the cytoskeleton of hemidesmosomes. Mol. Biol. Cell. 4:871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusolino, L., A. Bertotti, and P.M. Comoglio. 2001. A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell. 107:643–654. [DOI] [PubMed] [Google Scholar]
- Wary, K.K., A. Mariotti, C. Zurzolo, and F.G. Giancotti. 1998. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 94:625–634. [DOI] [PubMed] [Google Scholar]
- Waugh, M.G., D. Lawson, and J.J. Hsuan. 1999. Epidermal growth factor receptor activation is localized within low-buoyant density, non-caveolar membrane domains. Biochem. J. 337:591–597. [PMC free article] [PubMed] [Google Scholar]
- Weaver, V.M., S. Lelievre, J.N. Lakins, M.A. Chrenek, J.C. Jones, F. Giancotti, Z. Werb, and M.J. Bissell. 2002. β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Y., X. Yang, Q. Liu, J.A. Wilkins, and H.A. Chapman. 1999. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolven, A., H. Okamura, Y. Rosenblatt, and M.D. Resh. 1997. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol. Biol. Cell. 8:1159–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, Y., S.G. Gil, and W.G. Carter. 1996. Anchorage mediated by integrin α6β4 to laminin 5 (epiligrin) regulates tyrosine phosphorylation of a membrane-associated 80-kD protein. J. Cell Biol. 132:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, J., S. Roy, A. Apolloni, A. Lane, and J.F. Hancock. 1998. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 273:24052–24056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.