Abstract
The mammalian nuclear lamina protein lamin B1 is posttranslationally modified by farnesylation, endoproteolysis, and carboxymethylation at a carboxyl-terminal CAAX motif. In this work, we demonstrate that the CAAX endoprotease Rce1 is required for lamin B1 endoproteolysis, demonstrate an independent pool of proteolyzed but nonmethylated lamin B1, as well as fully processed lamin B1, in interphase nuclei, and show a role for methylation in the organization of lamin B1 into domains of the nuclear lamina. Deficiency in the endoproteolysis or methylation of lamin B1 results in loss of integrity and deformity of the nuclear lamina. These data show that the organization of the nuclear envelope and lamina is dependent on a mechanism involving the methylation of lamin B1, and they identify a potential mechanism of laminopathy involving a B-type lamin.
Keywords: confocal microscopy; cancer chemotherapy; Zmpste24; isoprenylcysteine carboxyl methyltransferase; farnesyltransferase inhibitor
Introduction
The nuclear lamina is a dense intermediate filament polymer underlying the nuclear envelope of higher eukaryotes (Goldman et al., 2002; Hutchison, 2002). It interacts closely with both the inner membrane of the nuclear envelope and the underlying chromatin, and these associations are thought to be important in maintaining the stability of the interphase nucleus. Interactions between peripheral heterochromatin domains and the nuclear lamina may be important in the control of gene expression and gene silencing (Labrador and Corces, 2002).
In somatic cells, the nuclear lamina is focally concentrated in regions of peripheral chromatin attachment (Paddy et al., 1990; Belmont et al., 1993). The nonhomogeneous distribution of the lamina at the nuclear periphery is likely to arise from a combination of factors, including interactions with chromatin, binding of inner nuclear membrane proteins, expression patterns of the lamin proteins, and posttranslational modifications of the lamin proteins. The lamina contains both A-type and B-type lamins, which are type V intermediate filament proteins that form the building blocks of the polymer (Stuurman et al., 1998). The B-type lamins, including lamins B1 and B2, are ubiquitously expressed and are essential developmental proteins in Drosophila melanogaster and Caenorhabditis elegans models (Lenz-Bohme et al., 1997; Liu et al., 2000). The A-type lamins, which include lamin A and its truncated splice variant lamin C, are expressed in most, but not all, terminally differentiated cells (Lehner et al., 1987).
All lamin proteins except lamin C terminate in a CAAX motif (cysteine, aliphatic, aliphatic, any of several amino acids) that triggers a series of sequential posttranslational modifications at the carboxyl terminus (Stuurman et al., 1998). First, a farnesyl moiety is attached to the thiol group of the cysteine (the “C” of the CAAX motif) by protein farnesyltransferase. Next, the terminal three amino acids (i.e., the “AAX”) are removed by one of two endoproteases, discussed later in this paragraph. Finally, the carboxylate anion of the then carboxyl-terminal farnesylcysteine is methylated by isoprenylcysteine carboxyl methyltransferase (Icmt). Both endoproteolysis and carboxymethylation are dependent on farnesylation of the cysteine residue and are membrane-associated processing events (Dai et al., 1998; Otto et al., 1999). Farnesylation is important for the peripheral localization and membrane association of the lamins. Endoproteolysis and subsequent methylation increase the hydrophobicity of the carboxyl terminus and may stabilize the membrane association. CAAX endoproteases include Rce1 (Otto et al., 1999), which processes farnesylated Ras proteins as well as other CAAX proteins, and Zmpste24, which almost certainly is involved in the processing of the carboxyl terminus of pre–lamin A (Bergo et al., 2002b). In this paper, we demonstrate that the Rce1 CAAX endoprotease is required for lamin B1 endoproteolysis.
Deficiencies of the mammalian CAAX processing enzymes cause severe phenotypic changes in mouse models. Rce1 deficiency causes death late in embryonic development despite apparently normal organogenesis (Kim et al., 1999). A deficiency in Zmpste24 causes defective processing of pre–lamin A, spontaneous bone fractures, and muscle weakness (Bergo et al., 2002b; Pendas et al., 2002). Icmt deficiency is lethal at mid-gestation (Bergo et al., 2001), perhaps because of agenesis of the liver (Lin et al., 2002). A deficiency in protein farnesyltransferase, caused by a knockout of the gene for the β-subunit of the enzyme, is lethal at embryonic day 6 to 7 (unpublished data). All of the enzymes involved in the processing of CAAX proteins are potential antineoplastic targets, and various farnesyltransferase inhibitors (FTIs) have already been tested in human clinical trials (Bergo et al., 2000, 2002a; Sebti and Hamilton, 2001). Interestingly, defective Zmpste24-mediated processing of pre–lamin A results in a muscle weakness phenotype similar to that observed in lamin A/C deficiency in mice (Sullivan et al., 1999; Bergo et al., 2002b; Pendas et al., 2002). In humans, specific mutations in lamins A and C cause Emery-Dreifuss muscular dystrophy and an axonal neuropathy, as well as cardiomyopathy, partial lipodystrophy, and mandibuloacral dysplasia (Burke and Stewart, 2002). More recently, mutations in lamin A have been linked to Hutchinson-Gilford progeria syndrome in humans (De Sandre-Giovannoli et al., 2003; Eriksson et al., 2003) and an analogous progeria-like disease in a mouse model (Mounkes et al., 2003).
To extend our knowledge of the effects of CAAX processing defects and to clarify the role of CAAX processing in the organization of the nuclear lamina, we developed a monoclonal antibody that serves as a marker of CAAX endoproteolysis of lamin B1. We identify the lamin B1 CAAX endoprotease, show that lamin B1 is differentially “CAAX processed” in interphase and mitotic cells, and demonstrate the presence of a carboxymethylation-dependent lamin receptor in the nuclear envelope. Furthermore, we provide evidence for the methylation–dependent organization of lamin B1 into subdomains of the nuclear lamina, and describe pathological changes in the nuclear envelope that result from defective proteolytic processing of lamin B1.
Results
A monoclonal antibody specific for CAAX-processed lamin B1
A monoclonal antibody, 8D1, that reacted with the nuclear envelope of HeLa cells (Fig. 1 A) recognized lamin B1, as determined by two-dimensional electrophoresis experiments (Fig. 1 B). This antibody reacted differently with different isoforms of lamin B1 and failed to react with a bacterially expressed His-tagged lamin B1 (Fig. 1 D), suggesting that eukaryotic posttranslational modification is required for the formation of the epitope. To determine whether the region of lamin B1 recognized by the antibody was near to the carboxyl-terminal CAAX motif, the last 13 or 25 residues of lamin B1 were fused to the carboxyl terminus of lamin C. Antibody 8D1 reacted with the construct containing the last 13 residues of lamin B1 (Fig. 1 C). Expression of His-tagged lamin B1 in HeLa cells in the presence of an FTI (Fig. 1 D) prevented the acquisition of the epitope in newly synthesized lamin B1 (although preexisting endogenous protein in the nuclear lamina remained immunoreactive, which is consistent with the slow turnover of the protein once inserted into the lamina; Moir et al., 2000). Sequence alignment of the carboxyl-terminal 40 residues of lamin B1, lamin B2, lamin C, and construct lamin C-C13 suggests that the specificity of the antibody is derived from sequence differences immediately upstream of the CAAX motif in lamin B1 and lamin B2, in addition to farnesylation of the cysteine residue (Fig. 1 E). To test whether further CAAX modification in the form of carboxymethylation of the cysteine (after endoproteolysis) is required for antibody reactivity, mouse embryonic fibroblasts from wild-type and Icmt−/− mice (Bergo et al., 2000) were subjected to Western blot with 8D1 (Fig. 1 F). Antibody 8D1 reacted with lamin B1 from wild-type and knockout mouse cell lines. The loss of the carboxymethylated isoform of lamin B1 in the Icmt-deficient cells was confirmed by two-dimensional electrophoresis and Western blot of total lamin B1 (Fig. 1 G). The loss of the most neutral isoform of lamin B1 is consistent with a lack of neutralization of the carboxylate anion by a methyl group.
Figure 1.
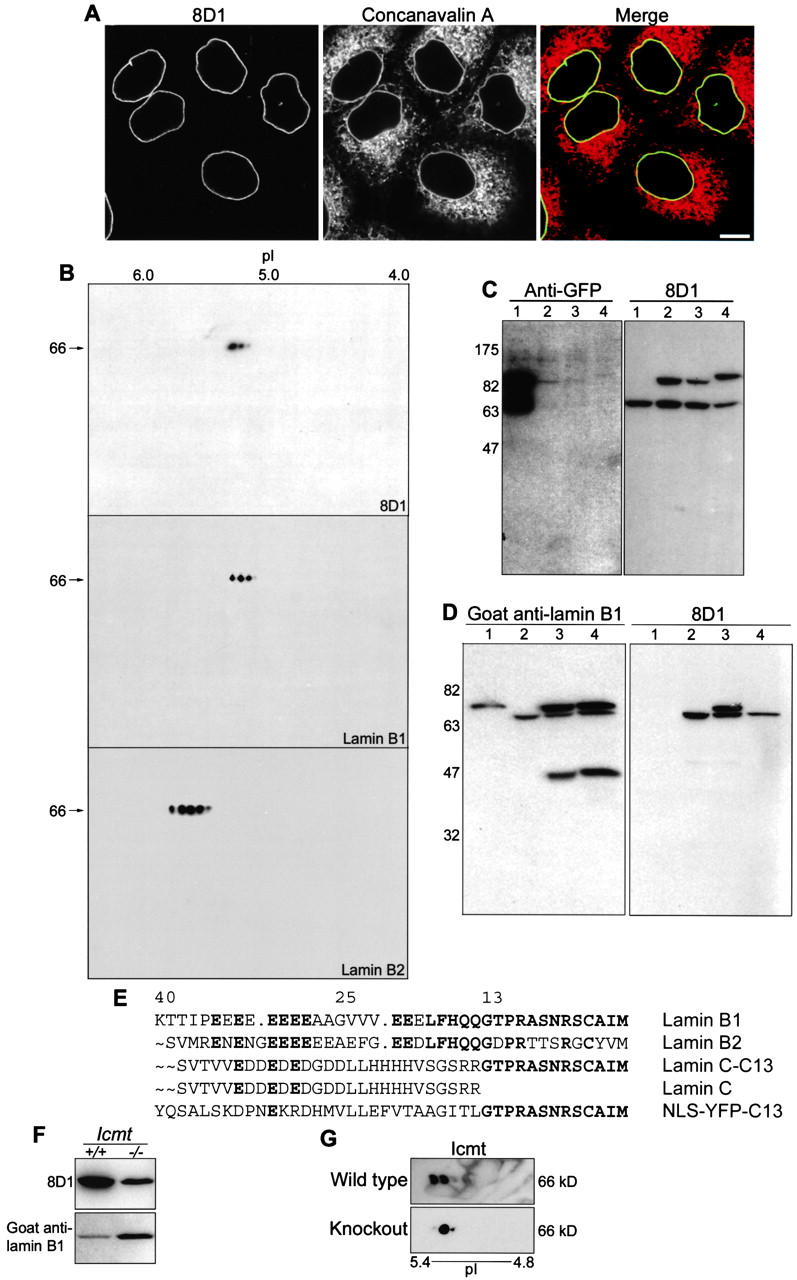
Monoclonal antibody 8D1 reacts with farnesylated lamin B1. (A) Immunofluorescence with 8D1 (green in merge) and the endoplasmic reticulum marker rhodamine– concanavalin A (red in merge) show partial colocalization at the nuclear envelope of HeLa cells. (B) HeLa nuclear envelope proteins were separated by two-dimensional electrophoresis on 13-cm, pH 4–7, gradients and immunoblotted with 8D1 and commercial antilamin antibodies. 8D1 reactivity co-migrated with the reactivity of a commercial anti–lamin B1 antibody, but shows differential reactivity with isoforms of lamin B1, suggesting a posttranslational modification is required for formation of the epitope. No cross reactivity with lamin B2 is present. (C) Replicate Western blots with polyclonal anti-GFP and 8D1. YFP–lamin C (lane 1) was fused upstream of 13 (lane 2) or 25 (lane 3) residues of the carboxyl terminus of lamin B1 and expressed in HeLa cells. GFP–lamin B1 is expressed as a control (lane 4). 8D1 reacted with lamin C fusion proteins containing the last 13 residues of lamin B1 and, therefore, binds near the CAAX motif. (D) 8D1 reacts with a eukaryotic posttranslational modification of lamin B1. Western blot with polyclonal anti–lamin B1 and 8D1; (lane 1) His6-Xpress-lamin B1 expressed in bacteria; (lane 2) HeLa lysate; and (lane 3) His6-Xpress-lamin B1 expressed in HeLa and (lane 4) in the presence of 100 μM FTI III. Lower molecular mass band represents a carboxyl- terminal apoptotic fragment of lamin B1. (E) Sequence alignment of the carboxyl terminal 40 residues of human lamin B1, lamin B2, construct lamin C-C13, human lamin C, and construct NLS-YFP-C13 (last 13 residues of lamin B1 fused directly to YFP). (F) Western blot of whole cell lysates from Icmt+/+ and Icmt−/− mouse embryonic fibroblasts with 8D1 and polyclonal anti–lamin B1 antibodies. 8D1 reacts with lamin B1 that is not methylated. (G) Two-dimensional electrophoresis on 13-cm, pH 4–7, gradients of total protein from Icmt+/+ (wild type) and Icmt−/− (knockout) mouse embryonic fibroblasts followed by immunolabeling for total lamin B1 shows loss of the most neutral isoform of lamin B1 in Icmt-deficient cells. Bars, 10 μm.
Rce1 is the lamin B1 CAAX endoprotease
To determine whether endoproteolysis of lamin B1 after farnesylation was required to create the epitope for antibody 8D1, mouse embryonic fibroblasts from wild-type embryos and embryos deficient in the known CAAX endoproteases, Rce1 and Zmpste24, were labeled with 8D1 and examined by immunofluorescence microscopy and Western blot (Fig. 2, A and B). Rce1+/+ and Zmpste24+/+ cells possessed endoproteolyzed, 8D1-positive lamin B1 at the nuclear periphery. Zmpste24−/− cells were also positive, but Rce1−/− cells failed to react with the antibody (also confirmed by Western blotting; Fig. 2 C), suggesting that CAAX processing of lamin B1 may be compromised in these cells. Optical sectioning by confocal microscopy could not achieve the required resolution in the Z-axis to obtain a mid-nuclear section in these fibroblast nuclei. Therefore, intranuclear signals of total lamin B1 and 8D1-positive lamin B1 may be, in part, the result of signals contributed from either basal or apical sections of the nuclear lamina. However, large intranuclear foci that readily label with the polyclonal antibody are not present in the 8D1 label; such foci have previously been reported in mouse fibroblasts (Moir et al., 1994). In addition, intranuclear invaginations of the nuclear envelope (Fricker et al., 1997), which are extensions of the peripheral nuclear membrane and lamina, label with both the polyclonal antibody and 8D1.
Figure 2.
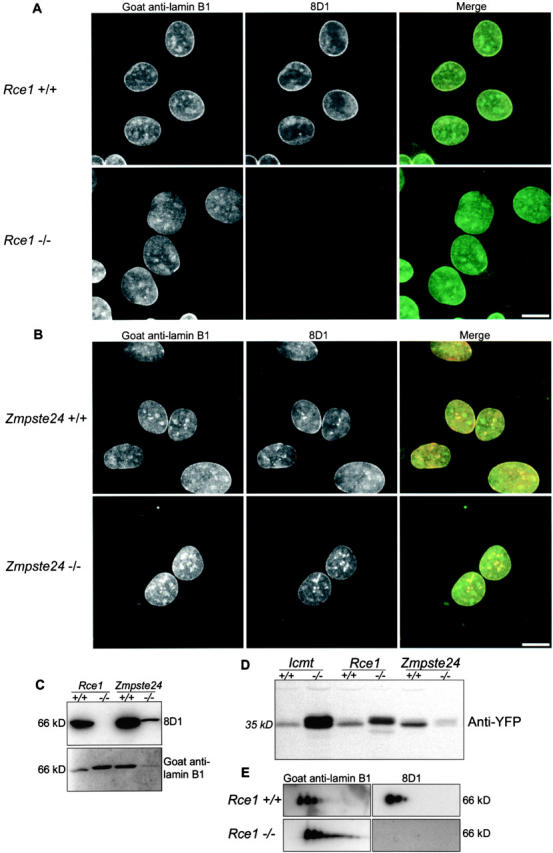
Rce1 is the lamin B1 CAAX endoprotease. Mouse embryonic fibroblasts from control mice and animals with homozygous deficiency of either Rce1 (A) or Zmpste24 (B) were double labeled with goat anti–lamin B1 (green in merge) and 8D1 (red in merge). Images represent single confocal sections. Rce1-deficient cells do not label with 8D1. (C) Western blot of whole cell lysates from wild-type and Rce1- or Zmpste24-deficient mouse embryonic fibroblasts with 8D1 and polyclonal anti–lamin B1 antibodies confirms the lack of an 8D1 reactivity in Rce1−/− cells. (D) A heterologous reporter containing the last 40 residues of lamin B1 including the CAAX motif, NLS-YFP-C40, was expressed in wild-type and CAAX processing–deficient cells and analyzed by SDS-PAGE and Western blot with an anti-GFP antibody. Primary antibody was detected with an alkaline phosphatase–conjugated secondary antibody and chromogenic substrate on the membrane. The protein migrates slower when expressed in Rce1−/− cells compared with wild- type cells or cells deficient in Icmt or Zmpste24, indicating a defect in proteolysis. The doublet in Icmt−/− cells is due to partial endoproteolysis. (E) Two-dimensional electrophoresis of total protein from wild-type and Rce1 deficient fibroblasts immunolabeled with polyclonal anti–lamin B1 and 8D1. Rce1 deficiency results in the loss of the methylated isoform of lamin B1. No isoforms of lamin B1 are reactive with 8D1 in Rce1−/− cells. Bars, 10 μm.
To confirm a defect of CAAX endoproteolysis of lamin B1 in Rce1−/− cells, a heterologous reporter with the last 40 residues of lamin B1 containing the CAAX motif (construct NLS-YFP-C40, which was small enough to discern molecular mass differences due to endoproteolysis) was expressed in wild-type cells and cells deficient in Rce1 or Zmpste24 (Fig. 2 D). The construct expressed in Rce1−/− cells migrated slower in SDS-PAGE compared with protein expressed in wild-type cells or cells deficient in either Icmt or Zmpste24. The construct expressed in Icmt−/− cells runs as a doublet, suggesting partial endoproteolysis in these cells. Two-dimensional electrophoresis of lamin B1 from Rce1−/− cells demonstrates a loss of the methylated isoform of lamin B1, which is consistent with an absence of carboxymethylation after CAAX endoproteolysis (Fig. 2 E). Rce1−/− cells still contain multiple isoforms of lamin B1, three of which are common to the wild-type control. Therefore, these charge differences are likely to arise from modifications upstream of the CAAX motif of lamin B1 and are consistent with the sequential addition of negative charge, possibly in the form of phosphorylation events. Importantly, the same isoforms that are labeled with 8D1 in the wild-type cells do not label in the knockout line, suggesting that upstream modifications present in these isoforms do not affect the ability of 8D1 to recognize lamin B1. The posttranslational modifications recognized by antibody 8D1 are summarized in Table I.
Table I. Summary of reactivity of antibody 8D1 on lamin B1.
| CAAX modification | Carboxyl terminus of lamin B1 |
8D1 reactive |
|---|---|---|
| Unmodified | NRSCAIM | No |
| Farnesylated | NRSC(f)AIM | No |
| Farnesylated and endoproteolyzed |
NRSC(f) | Yes |
| Farnesylated, endoproteolyzed and carboxymethylated |
NRSC(f)-ME | Yes |
A 15-carbon farnesyl moiety is covalently attached to the cysteine residue to create a farnesyl cysteine (C(f)), followed by endoproteolysis (release of AIM) and carboxymethylation (-ME).
Proteolysis of lamin B1 requires 40 residues of upstream sequence
Various lengths of sequence upstream from the CAAX motif of lamin B1 were fused to the carboxyl terminus of a reporter protein, NLS-YFP, that included a nuclear localization sequence. Constructs included 13, 25, 40, 172, 200, and 371 residues from the carboxyl terminus of lamin B1, and were designed based upon the domain structure of the protein (Fig. 3 A). These constructs were used in combination with antibody 8D1, a marker of CAAX endoproteolysis of lamin B1, to study this processing event in HeLa cells. Antibody 8D1 failed to recognize the fusion protein with 13 or 25 carboxyl-terminal residues (Fig. 3 B). The NLS-YFP-C40 protein ran as a doublet on SDS-PAGE, indicating partial carboxyl-terminal proteolysis in untreated cells but not in FTI-treated cells, in which CAAX modification was prevented. Moreover, antibody 8D1 reacted only with the lower molecular mass, proteolyzed isoform (Fig. 3, C and D). These results show that at least 40 residues of the carboxyl-terminal sequence of lamin B1 are required for endoproteolysis. Constructs with larger carboxyl-terminal regions of lamin B1 all underwent endoproteolysis and all were 8D1 immunoreactive. A polyglutamate tract is situated ∼30 residues upstream of the carboxyl terminus of lamin B1 and lamin B2 and may constitute a secondary recognition motif for the endoprotease, in addition to the farnesyl-CAAX motif.
Figure 3.
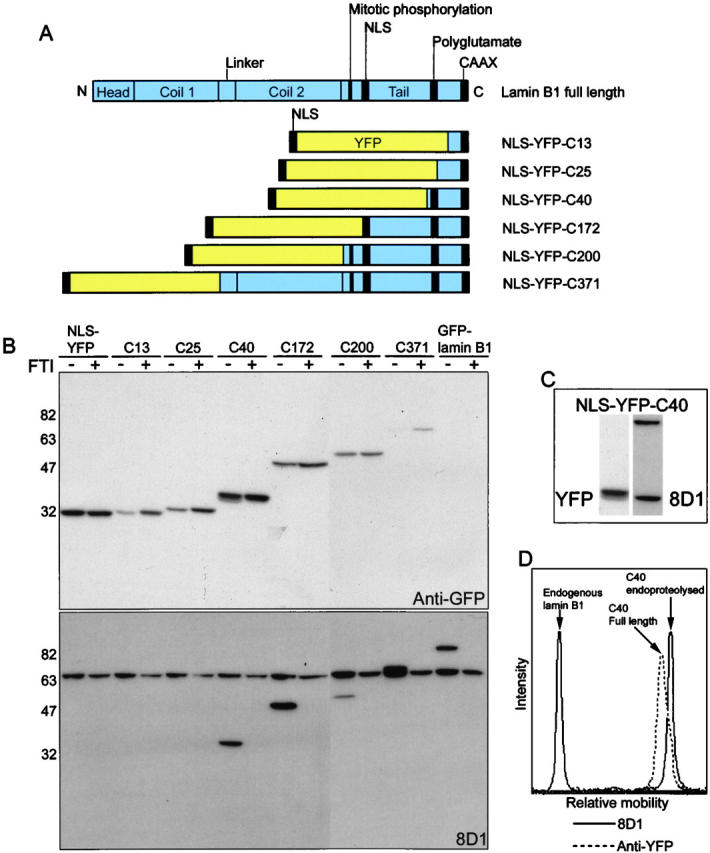
The lamin B1 CAAX endoprotease requires 40 residues of upstream sequence. (A) Schematic representation of full-length lamin B1 and carboxyl-terminal tail constructs. Increasing lengths of the lamin B1 carboxyl terminus were fused to the carboxyl terminus of NLS-YFP. (B) Constructs were expressed in HeLa cells in the absence and presence of 50 μM FTI III and analyzed by Western blot with anti-GFP and 8D1. Constructs are indicated above lanes; the numbers represent the number of residues from the carboxyl terminus of full-length lamin B1. 8D1 reactivity is acquired when 40 residues of lamin B1 sequence are present and is dependent on farnesylation. (C) Construct NLS-YFP-C40 is shown as an inset with 8D1 and anti-YFP signals aligned, indicating that 8D1 recognizes a lower molecular weight band corresponding to proteolyzed lamin B1 carboxyl terminus. The data are derived from a single nitrocellulose membrane that was reprobed to ensure perfect alignment of bands from the two antibodies. (D) The relative mobility of bands recognized by the antibodies is represented. 8D1 (solid line) recognizes endogenous lamin B1 and the reporter, which co-migrates with the shoulder present in the anti-YFP label (dotted line), corresponding to the lower molecular weight, endoproteolyzed species.
Differential CAAX processing of lamin B1 in interphase nuclei
Characterization of antibody 8D1 by Western blotting after two-dimensional gel electrophoresis demonstrated differentially processed pools of lamin B1 in free-cycling interphase cells. Furthermore, we were able to differentiate the mature, methylated isoform and the farnesylated, endoproteolyzed but nonmethylated isoforms from unprocessed lamin B1 using a combination of two-dimensional electrophoresis and immunolabeling with 8D1. The relationship between these pools was examined by an analysis of nuclear protein fractions from cells treated with cycloheximide or an FTI (Fig. 4 A). Western blotting with goat anti–lamin B1 polyclonal antibody showed that the three major isoforms of lamin B1 are reduced to two after inhibition of protein synthesis with cycloheximide for 4 h. The intensity of the immunolabeling of acidic isoforms was increased by treatment with an FTI, suggesting that these isoforms represent a pool of nonfarnesylated lamin B1. Labeling of the same blot membranes with 8D1 allows discrimination of the proteolyzed and methylated pools of lamin B1 from the nonproteolyzed forms. The 8D1-positive isoforms of lamin B1 in the immunoblots represent methylated (fully processed, most neutral) and endoproteolyzed, nonmethylated (more acidic) isoforms. Thus, the depletion of the precursor pool by inhibiting either protein synthesis or farnesyltransferase activity does not result in the relentless progression toward the mature methylated pool (even over a 24-h period or one cell cycle in the case of the FTI treatment). This suggests that interphase nuclei contain an independent pool of proteolyzed, but nonmethylated, lamin B1 and that progression to the mature methylated form of lamin B1 may be regulated by other posttranslational modifications (e.g., phosphorylation).
Figure 4.
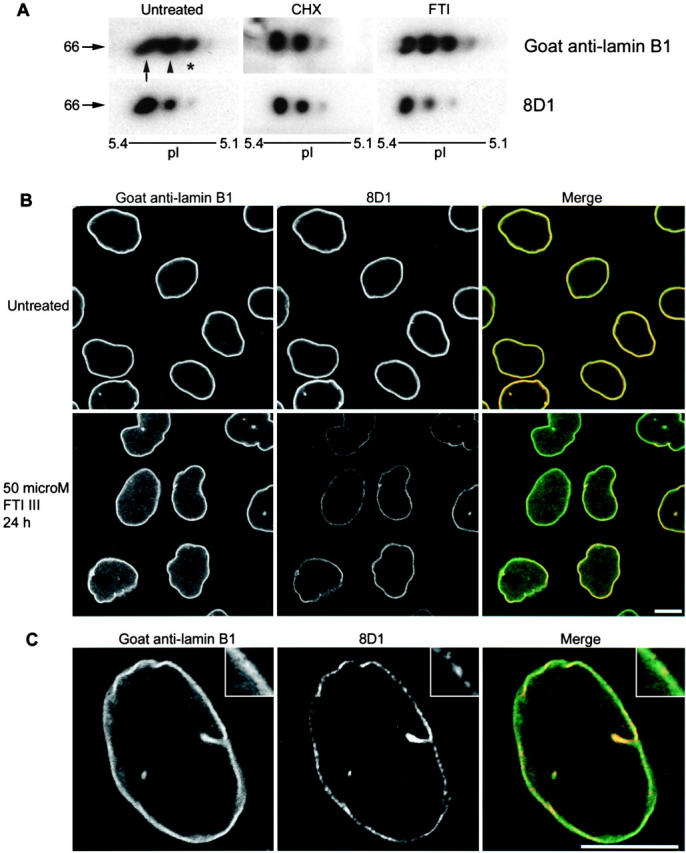
Differential CAAX processing of lamin B1 in interphase cells. (A) Two-dimensional electrophoresis of proteins from purified HeLa nuclei and immunolabeling with goat anti–lamin B1 and 8D1 antibodies demonstrates differential CAAX processing of lamin B1. Relative molecular mass and pI are shown. Nuclei purified from cells treated with cycloheximide (CHX) or farnesyltransferase inhibitor (FTI) demonstrate stability of two isoforms of lamin B1, representing methylated (arrow) and proteolyzed (arrowhead) isoforms. An acidic isoform of lamin B1 (asterisk), depleted by CHX treatment and increased by FTI treatment, may represent a nonfarnesylated pool of lamin B1. (B) Single confocal sections of untreated and FTI-treated HeLa cells labeled with goat anti–lamin B1 (green in merge) and 8D1 (red in merge) antibodies. FTI treatment increased the intranuclear pool of lamin B1 and decreased the mature pool of lamin B1 at the nuclear periphery even though total lamin B1 was maintained. The 8D1 signal was quantitatively lower in the nuclear lamina of FTI-treated cells and showed a discontinuous pattern. (C) FTI-treated cells were labeled with dilutions of polyclonal antibody to achieve similar signal intensities for both antibodies. Subdomains of CAAX-processed lamin B1 identified by 8D1 are present in the lamina (shown at a higher magnification in the inset), compared with a more continuous pattern seen with the polyclonal antibody. Bars, 10 μm.
CAAX processing organizes domains within the nuclear envelope
The extent to which differentially “CAAX-modified” pools of lamin B1 may reflect an organization within the nuclear lamina and nucleoplasm was examined by confocal microscopy of HeLa cells that were labeled with both 8D1 and goat anti–lamin B1 (Fig. 4 B). In untreated HeLa cells, both 8D1 and the polyclonal antibody show a characteristic distribution of lamin B1 in the peripheral nuclear lamina. A weak punctate intranuclear distribution of lamin B1 (Moir et al., 2000) was observed only with the polyclonal antibody and is likely the nonfarnesylated precursor pool. After 24 h of FTI treatment, the intranuclear nonfarnesylated pool was easily visible; it diffusely filled the nucleoplasm and was excluded from nucleoli. The peripheral lamin B1 signal detected by the polyclonal antibody was unchanged compared with controls, suggesting that lamin B1 may be directed to the nuclear periphery by alternative mechanisms, albeit less efficient than farnesylation. Analysis of the 8D1 signal in FTI-treated cells revealed a significant decrease in the amount of mature lamin B1 despite the normal levels of total lamin B1. Furthermore, the remaining 8D1-positive lamin B1 was not uniformly distributed throughout the lamina but was concentrated in small domains not readily visible in untreated cells. To ensure that this apparent organization of CAAX-processed lamin B1 was not due to different intensities of the label, FTI-treated HeLa cells were labeled with dilutions of the polyclonal antibody to achieve a similar intensity to the 8D1 label (Fig. 4 C). The same subdomains of the nuclear lamina could still be distinguished by 8D1.
Evidence for a carboxymethyl-lamin receptor in the nuclear envelope
Examination of the localization of NLS-YFP-C13 (Fig. 5 A) and NLS-YFP-C25 (not depicted) transiently expressed in HeLa cells, showed that those proteins were localized throughout the endomembrane system and plasma membrane. In contrast, NLS-YFP-C40 localized to the nuclear envelope of HeLa cells and was proteolyzed (Fig. 5 B). To determine whether retention in the nuclear envelope resulted from interactions with chromatin (e.g., interactions between the polyglutamate tract and basic histone residues) or was dependent on endoproteolysis and methylation, NLS-YFP-C40 was expressed in wild-type and CAAX processing–deficient cells (Fig. 5 C). NLS-YFP-C40 localized to the nuclear envelope in each wild-type cell line and in Zmpste24−/− cells, but not in Rce1−/− or Icmt−/− cells. Farnesylation was not affected in these cells as a reticular pattern of NLS-YFP-C40, which colocalized with concanavalin A, was seen in the cytoplasm, indicating association with endomembranes (Fig. 5 D). In addition, expression of the construct in the presence of an FTI resulted in a diffuse nucleoplasmic accumulation (unpublished data). The intranuclear levels of the reporter were greater in Icmt-deficient cells compared with Rce1-deficient cells and may indicate differences in the stability of membrane association resulting from incomplete processing. These results indicate that carboxymethylation is a minimum requirement for the peripheral nuclear localization of the carboxyl-terminal tail of lamin B1 fused to a heterologous reporter. Although full-length lamin B1 localizes and is retained at the nuclear envelope by integration in the nuclear lamina polymer through homo- and possibly heterotypic interactions of the rod domain, in addition to binding to known inner nuclear membrane proteins, our data suggest the presence of a carboxymethyl-lamin receptor at the nuclear envelope involved in binding of the carboxyl-terminal end of the tail domain dependent on the degree of posttranslational modification.
Figure 5.
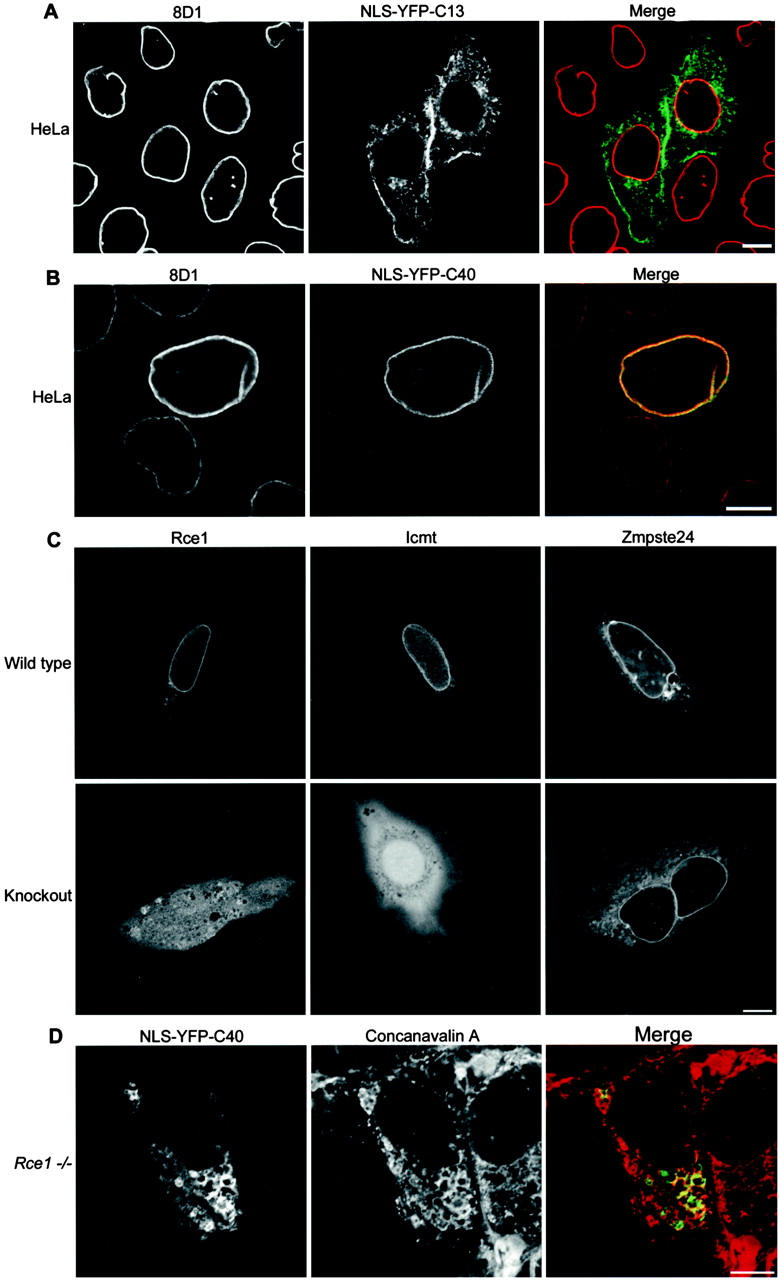
A carboxymethyl-dependent lamin receptor in the nuclear envelope. (A) NLS-YFP-C13 (green in merge), representing the terminal 13 residues of lamin B1, was expressed in HeLa cells; the cells were fixed and labeled with 8D1 (red in merge). A single confocal section is shown. NLS-YFP-C13 localized throughout the endomembrane system and plasma membrane. The absence of 8D1 reactivity confirms that the construct was not proteolyzed despite membrane association. (B) NLS-YFP-C40 (green in merge) expressed in HeLa cells localized to the nuclear envelope and was 8D1 positive (red in merge), confirming that CAAX processing took place. A single confocal section is shown. (C) Single confocal sections through the mid-nucleus of wild-type and knockout cell lines, deficient in individual CAAX-processing enzymes, expressing NLS-YFP-C40, show failure to localize to the nuclear envelope in Rce1−/− and Icmt−/− cells. The nuclear envelope localization of NLS-YFP-C40 was not affected in Zmpste24−/− cells. Intranuclear levels of the expressed protein were more pronounced in Icmt−/−cells compared with Rce1−/− cells. (D) Farnesylation and membrane association was not impaired in Rce1- or Icmt-deficient cells. NLS-YFP-C40 is partially colocalized (merge) with rhodamine–concanavalin A in Icmt−/− cells (not depicted) and Rce1−/− cells. Bars, 10 μm.
Defective CAAX processing results in morphological changes in the nuclear lamina
Because methylation of lamin B1 may be required for an interaction with a component of the nuclear envelope, the effects of deficiencies in the CAAX processing of lamin B1 on the nuclear lamina were examined by immunofluorescent labeling of mouse embryonic fibroblasts (Fig. 6 A). Defects in proteolysis of lamin B1 (in Rce1−/− cells) and carboxymethylation of all lamins (in Icmt−/− cells) were associated with an increase in morphological changes in the nuclear lamina reminiscent of those caused by mutations in lamin A/C (Vigouroux et al., 2001). Herniations of chromatin, evident by phase-contrast microscopy (Fig. 6 B), were less frequent in control cells than in Rce1−/− cells (1.3 ± 0.6% vs. 4.1 ± 1.9%, P = 0.07) or in Icmt−/− cells (1.9 ± 0.4% vs. 11.9 ± 3.2%, P = 0.01). Defects in the nuclear lamina were also less frequent in control cells than in Rce1−/− cells (0.6 ± 1.0% vs. 12.4 ± 4.2%, P = 0.01) or Icmt−/− cells (0.7 ± 0.9% vs. 9.1 ± 3.9% for control and knockout, respectively, P = 0.02). Morphological changes were frequently localized to one pole of the nucleus, which was consistent with previous studies (Sullivan et al., 1999; Vigouroux et al., 2001). Wild-type cells and Zmpste24−/− cells were not significantly different in herniations (5.9 ± 2.0% vs. 5.0 ± 2.5%, P = 0.58) or in lamina defects (5.5 ± 3.0% vs. 5.5 ± 1.9%, P = 0.99).
Figure 6.
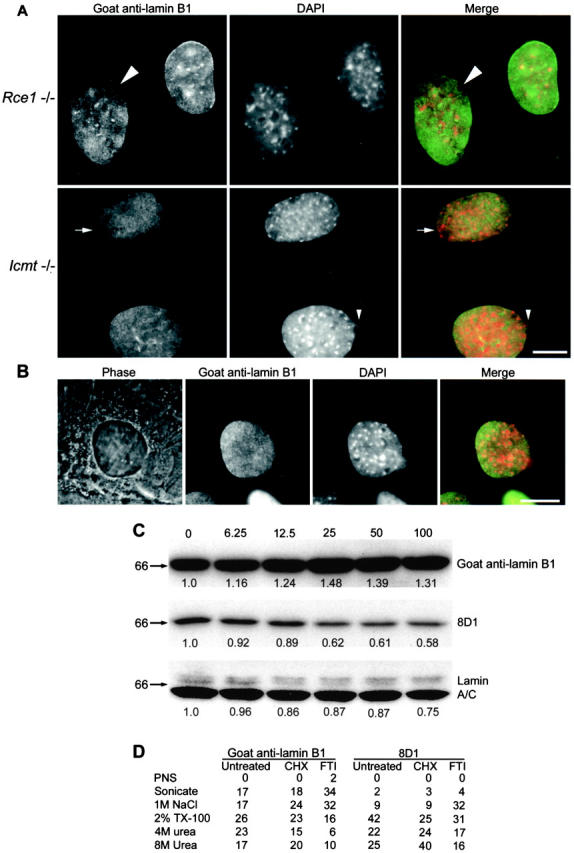
Defective CAAX processing of lamin B1 leads to morphological changes in the nuclear lamina. (A) Epifluorescence images of Rce1−/− and Icmt−/− cells labeled with goat anti–lamin B1 (green in merge) and DAPI (red in merge) for DNA. Defects in the lamina included abnormal distribution of lamin B1 in the periphery (arrows), often localized to one pole of the nucleus; peripheral lamina defects with no lamin B1 present (large arrowheads); and herniation of chromatin through peripheral defects (small arrowheads). (B) An epifluorescence image of an Icmt−/− cell labeled with a polyclonal anti–lamin B1 antibody (green in merge) and DAPI (red in merge) shows a large herniation of chromatin (DAPI) and relative paucity of lamin staining overlying the herniation. The herniation is visible by phase-contrast microscopy of the same cell as an irregularity and protrusion of the nuclear envelope. (C) HeLa cells treated with doubling doses of an FTI were analyzed by Western blot with goat anti–lamin B1 and 8D1 antibodies. Over the period of a cell cycle, mature, 8D1-positive lamin B1 decreased to ∼60% of control levels. Total lamin B1 expression was up-regulated. Pre–lamin A accumulated in a band of higher molecular weight. Lanes were loaded with equal amounts of total protein. (D) HeLa cells were treated with 50 μM farnesyltransferase inhibitor (FTI) III for 24 h or with 20 μg/ml cycloheximide (CHX) for 4 h, or were untreated. Nuclei were purified and subjected to differential extraction. Samples were analyzed by Western blot with goat anti–lamin B1 and 8D1 antibodies and quantitated by densitometry. The amounts of total lamin B1 and mature (8D1 positive) lamin B1 in each extracted fraction are shown as a percentage of the total amount released. PNS, postnuclear supernatant. Bars, 10 μm.
The effects of farnesyltransferase inhibition on the lamina were further demonstrated by quantitation of total and mature lamin B1 protein with 8D1 and goat anti–lamin B1 antibodies on Western blots of cells treated with increasing doses of an FTI (Fig. 6 C). Total lamin B1 was slightly up-regulated, which accounts for the maintenance of lamin B1 in the peripheral lamina despite the increase in the intranuclear lamin B1 levels. The mature pool of lamin B1 decreased to 60% of control levels after treatment with 25 μM FTI III (25% of the recommended dose). Thus, 8D1 could be useful for monitoring the effects of FTI therapy (Adjei et al., 2000).
The effect of the decrease in mature lamin B1 on the nuclear lamina was measured by differential extraction of proteins under increasingly stringent conditions (Fig. 6 D). FTI treatment shifted the extraction profile of the total lamin B1 in the direction of the less stringent conditions compared with control cells. This is due in part to the increased nucleoplasmic pool and in part to a compromise in nuclear lamina integrity because the 8D1-positive mature lamin B1 in the lamina also exhibits the shift toward release under less stringent extraction conditions. The extraction data reveal the effect of FTI treatment and deficient CAAX modification on the nuclear lamina; our data demonstrate the functional relationship between differential CAAX processing of lamin B1 and the organization of lamin B1 within the nucleoplasm and nuclear lamina.
Two-step recruitment of lamin B1 to postmitotic lamina
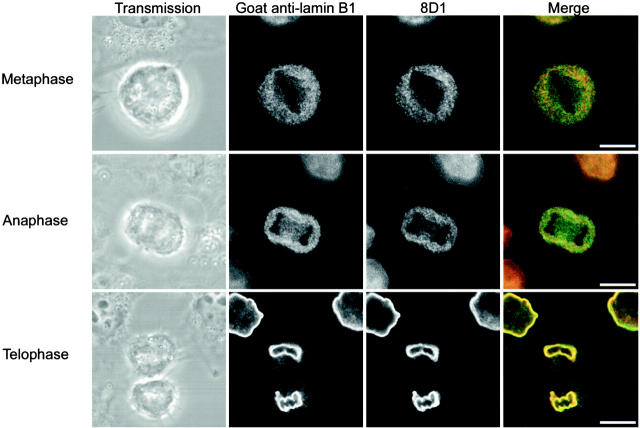
Examination of untreated mitotic HeLa cells by double label immunofluorescence reveals a persistence of an 8D1-negative, nonproteolyzed pool of lamin B1 during mitosis and in the subsequent recruitment of lamin B1 to the reforming nuclear envelope (Fig. 7). The polyclonal antibody labels the spindle region in the metaphase and anaphase cell, whereas 8D1 does not. In the telophase/early G1 cell shown, nuclear envelope–associated lamin B1 was 8D1 positive, whereas cytoplasmic lamin B1 (which has yet to associate with the reforming envelope) was nonproteolyzed and 8D1 negative. These data confirm that the differentially processed isoforms of lamin B1, identified biochemically, correlate with the organization of lamin B1 in the nucleoplasm and nuclear lamina in both interphase and mitosis.
Figure 7.
Two-step recruitment of lamin B1 at the end of mitosis. Single confocal sections of fluorescent labels and transmission images of mitotic HeLa cells double labeled with goat anti–lamin B1 (green in merge) and 8D1 (red in merge) antibodies demonstrate an unprocessed pool of lamin B1 throughout mitosis that localizes to the spindle region in metaphase and anaphase. In a telophase/early G1 cell, 8D1-positive mature lamin B1 is localized in the reforming envelope; a pool of 8D1-negative, nonproteolyzed lamin B1 was seen in the cytoplasm. Lamin B1 was recruited to the reforming nuclear envelope in two steps, dependent on the degree of carboxyl-terminal modification. Bars, 10 μm.
Discussion
Our data support the identification of the lamin B1 endoprotease as Rce1, an enzyme with broad specificity for farnesylated CAAX substrates. Rce1 substrates include Ras, Gγ subunits, and a-factor in yeast (Kim et al., 1999; Otto et al., 1999; Dolence et al., 2000; Trueblood et al., 2000). Despite this relatively broad specificity, the processing of lamin B1 requires at least 40 residues of upstream sequence in addition to the CAAX motif. The inability of Rce1 to process shorter carboxyl-terminal peptides of lamin B1 (13 or 25 residues) stands in contrast with previous demonstrations of Rce1 activity on synthetic farnesylated tetrapeptides (Dolence et al., 2000), on CAAX motifs fused to heterologous reporters, and on short (9 residue) Ras sequences fused to reporters (Choy et al., 1999). The previously held view that Rce1 requires a farnesylated CAAX motif as its only recognition motif does not hold true for lamin B1 processing. Alternatively, the carboxyl terminus of lamin B1 may include additional posttranslational modifications that prevent endoproteolysis (e.g., phosphorylation) upstream of the CAAX motif.
Although Ras requires full CAAX processing (i.e., endoproteolysis and methylation) and a second upstream signal (in the form of palmitoylation or a polybasic domain) to localize to the plasma membrane (Hancock et al., 1991; Choy et al., 1999), the carboxyl terminus of lamin B1 can direct a reporter protein to the plasma membrane in the absence of endoproteolysis and methylation or any secondary targeting signals. Association with endomembrane structures suggests that trafficking through the Golgi complex is a likely route to the plasma membrane, rather than direct association with the plasma membrane itself (Choy et al., 1999).
The retention of the carboxyl terminus of lamin B1 in the nuclear envelope was dependent on carboxymethylation, confirming that protein–protein interactions in the extreme carboxyl terminus are present and extending the notion of an isoprenyl-lamin receptor in the nuclear envelope to include carboxymethylation. Because it has eight transmembrane domains, the lamin B receptor has previously been proposed as an isoprene receptor (Hennekes and Nigg, 1994). The degree to which the farnesyl moiety interacts with the putative carboxymethyl-lamin receptor is not known, but will certainly influence identification of candidate inner membrane proteins for this role. Because lamin A undergoes further proteolytic processing upstream of the CAAX modifications (Kilic et al., 1999), stable carboxymethyl-dependent interactions in the nuclear envelope are probably limited to B-type lamins. The activation of a cryptic splice site through a single base change in the lamin A gene has recently been described in patients with Hutchinson-Gilford progeria syndrome (Eriksson et al., 2003) and results in a 50–amino acid deletion in the tail domain, including the pre–lamin A protease site upstream of the CAAX motif. Lamin A in these patients is expected to be constitutively farnesylated and carboxymethylated; it is tempting to speculate that this isoform of lamin A may compete with B-type lamin interactions for binding to a carboxymethyl-lamin receptor.
The presence of a carboxymethyl-lamin receptor in the inner nuclear membrane is made more significant by the finding that lamin B1 exists in differentially carboxymethylated states. Furthermore, the stability of these pools suggests additional regulation of the progression toward the fully processed methylated state. Coupled with the fact that the mature protein is shown to occupy subdomains of the nuclear lamina, we propose that methylation of lamin B1 may be a novel mechanism for higher-order organization of the nuclear lamina. Interactions with chromatin have been mapped to the tail domain of lamin B1 immediately downstream of the rod domain (Taniura et al., 1995; Stierle et al., 2003). Although carboxymethylation may not influence lamin–chromatin interactions themselves, methylation-dependent binding of an inner membrane protein may organize peripheral chromatin interactions into domains along the lamina polymer. Interphase phosphorylation of the lamina has been proposed as a mechanism for generating heterogeneity in the nuclear lamina independent of expression patterns of the lamin proteins (Ottaviano and Gerace, 1985; Worman et al., 1988). Carboxymethylation provides an additional, and possibly dynamic (Chelsky et al., 1987), mechanism for regulation of domains within the lamina.
The nuclear lamina has been proposed as a structural component in the binding of insulator elements responsible for compartmentalization of chromatin domains (Labrador and Corces, 2002). The boundary activity exhibited by components of the yeast nuclear pore complex support the relationship between peripheral nuclear structure and function (Ishii et al., 2002). Yeast cells do not contain lamin proteins. It is tempting to speculate that the creation of subdomains in the nuclear lamina by carboxymethylation of B-type lamins may provide a similar mechanism for boundary activity or binding of insulator elements in higher eukaryotes that express lamin proteins.
The nuclear lamina defects in cells deficient in Rce1 or Icmt establish a potential mechanism for a novel laminopathy involving a B-type lamin. The phenotypic changes seen in these animal models (Kim et al., 1999; Bergo et al., 2001) may be accounted for, at least in part, by lamina defects introduced by aberrant processing of lamin B1. The pathogenetic mechanism of such a B-type laminopathy may depend on the loss of interaction with a putative carboxymethyl-lamin receptor in the nuclear envelope. Furthermore, FTIs (Sebti and Hamilton, 2001) cause previously unidentified changes in the expression and localization of lamin B1 (Dalton et al., 1995), and potentially important consequences for the integrity and organization of the nuclear lamina. More recently, the longstanding cancer chemotherapeutic agent, methotrexate, has been shown to exert its antiproliferative effect, at least in part through the inhibition of Icmt (by increasing a metabolite intermediate that directly inhibits the enzyme) and, therefore, carboxylmethylation of prenylated proteins (Winter-Vann et al., 2003). Because all of the CAAX processing enzymes are potential chemotherapeutic targets (Bergo et al., 2000, 2002a), an understanding of the effects of inhibition on nuclear structure, gene expression, and peripheral chromatin silencing are essential in the development of agents acting on these targets.
Materials and methods
Reagents
Goat anti–lamin B1 antibody (model C20; Santa Cruz Biotechnology, Inc.) was characterized by two-dimensional electrophoresis and confirmed to be lamin B1 specific. Primary antibodies used included anti–lamin B1 (Chemicon), anti–lamin A/C (Novocastra), anti–lamin B2 (Novocastra), and anti-GFP (CLONTECH Laboratories, Inc.). Anti–mouse, anti–goat, and anti–rabbit secondary antibodies were obtained from Jackson ImmunoResearch Laboratories, conjugated to HRP or AP (for Western blot), or conjugated to Alexa 488 or Alexa 568 (Molecular Probes) for immunofluorescence microscopy. FTI III, a cell-permeable farnesyltransferase inhibitor, was obtained from Calbiochem.
Lamin B1 constructs
Full-length lamin B1 cDNA was obtained from the IMAGE consortium (clone 2969826; HGMP Resource Centre), amplified by PCR to introduce restriction sites, and ligated into pTRC-His A (Invitrogen) with the BamHI and XhoI sites to introduce a polyhistidine tag and an Xpress epitope tag at the amino terminus. The coding region of the construct was amplified by PCR with Pfu DNA polymerase (Promega) and introduced into pcDNA 3.1 (Invitrogen) for mammalian cell expression with the KpnI and XhoI sites. GFP–lamin B1 was constructed by amplifying the EGFP sequence from pEGFP-tubulin (CLONTECH Laboratories, Inc.) and introducing it upstream of the lamin B1 sequence in pcDNA 3.1 with KpnI and BamHI restriction sites. Tail domain constructs of lamin B1 were constructed by PCR amplification and ligation downstream of NLS-YFP. Lamin C coding sequence (IMAGE clone 3355388; HGMP Resource Centre) was amplified by PCR and introduced between NLS-YFP and lamin B1 carboxyl termini C13 and C25. Lengths of lamin B1 carboxyl terminus used in these constructs included extreme carboxyl termini (C13 and C25); polyglutamate tract (C40); carboxyl-terminal domain including the NLS (C172); entire carboxyl-terminal domain including juxta-rod sequence (C200); and linker 2, coil 2, and rod domain together in C371. All constructs were sequenced and confirmed to be free of mutations.
Cell culture and cell fractionation
HeLa cells were grown in DME supplemented with 10% FCS and l-glutamine (Invitrogen). Mouse embryonic fibroblasts were cultured as described by Bergo et al. (2001). Cells were transfected with Lipofectamine 2000 (Invitrogen). Cells were treated with 20 μg/ml cycloheximide for 4 h where indicated to inhibit protein synthesis. FTI treatment was performed for 24 h in all cases at a dose of 50 μM FTI III, unless otherwise stated. Nuclei were prepared at 4°C by Dounce homogenization in the absence of detergent as described previously (Collas, 1998). A protease inhibitor cocktail containing 1 μg/ml aprotinin, 1 mM phenylmethanesulphonyl fluoride, and 1 μg/ml leupeptin was included during all steps of the isolation procedure.
Monoclonal antibody production
Purified nuclei were digested with 20 μg/ml DNase I and purified through a sucrose cushion, and hydrophobic proteins were enriched by phase separation in Triton X-114 (Bordier, 1981). Balb/c mice were immunized, and fusion of splenocytes to SP2 mouse myeloma cells and selection was performed as described previously (Vaux and Gordon, 1985). Hybridomas were screened by indirect immunofluorescence of HeLa cells as described in Immunocytochemistry.
Extraction of purified nuclei and two-dimensional electrophoresis
Aliquots of purified nuclei for two-dimensional electrophoresis were solubilized immediately in isoelectric focusing sample buffer (9 M urea, 2% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, 20 mM DTT, and 0.5% ampholyte). Isoelectric focusing was performed on an IPGphor flatbed electrophoresis unit with 13-cm linear immobilized pH gradient strips (pH 4–7; Amersham Pharmacia Biotech) for a total of 41,500 V-h (500 V for 1 h, 1,000 V for 1 h, and 8,000 V for 5 h). Equilibration for second dimension was performed in 6 M urea, 30% glycerol, 2% SDS, 50 mM Tris, pH 6.8, with 60 mM DTT, followed by 135 mM iodoacetamide for 20 min each. A 10% reducing SDS gel was used for the second dimension. For differential extraction, purified nuclei from 5 × 107 cells were pelleted and resuspended in 300 μl of nuclear isolation buffer (10 mM Hepes, pH 7.4, 2 mM MgCl2, 25 mM KCl, 250 mM sucrose, 1 mM DTT, and protease inhibitors) and sonicated for two 5-s bursts at 10-μm amplitude (Soniprep 150; Sanyo). Insoluble material was pelleted in a microcentrifuge at 20,000 g for 5 min, and the supernatant was kept for analysis. The pellet was resuspended in 300 μl of nuclear extraction buffer (1 M NaCl, 20 mM Hepes, pH 7.4, and protease inhibitor cocktail) and incubated at RT with agitation for 20 min (Otto et al., 2001). The procedure was repeated in nuclear extraction buffer with 2% Triton X-100, 4 M urea, and, finally, 8 M urea in sequential extractions. Samples were dialyzed against 1% SDS and 50 mM Tris, pH 6.8, before gel electrophoresis. Bands were quantitated with IMAGEQuant software (Molecular Dynamics). Denaturing protein electrophoresis was performed with standard methods as described in Laemmli (1970). Protein concentration was determined with a protein assay kit (Bio-Rad Laboratories). Western blotting was performed in 5% milk in PBS at optimal antibody concentrations. Where applicable, membranes were stripped in 2% SDS and reprobed with a second primary antibody.
Immunocytochemistry
Cells were grown on glass coverslips, washed in cold PBS, and fixed in 4% PFA in PBS for 20 min at RT, permeabilized in 0.5% Triton X-100 in PBS for 5 min, and blocked in 0.2% bovine gelatin in PBS. Antibody labeling at optimum dilutions was performed for 45 min in a humidified chamber. Labeled cells were mounted in Mowiol and counterstained with DAPI. Confocal microscopy was performed on a confocal laser-scanning microscope (model MRC-1024 [Bio-Rad Laboratories]; model Lasersharp 2000 [Bio-Rad Laboratories]). Epifluorescence microscopy was performed on an Axioplan II microscope (Carl Zeiss, Inc.) fitted with a Spot CCD camera (Diagnostic Instruments). Confocal images were viewed in Confocal Assistant, and all images and figures were arranged in Adobe Photoshop.
Acknowledgments
We thank P. Cook, S. Ordway and G. Howard for comments on the manuscript.
This work was supported in part by Medical Research Council grant G9719283, Wellcome Trust Joint Infrastructure Fund programme 057227/Z/99, National Institutes of Health grants HL4163 and AG15451, and grant 11KT-0087 from the University of California Tobacco-related Disease Research Program.
M.S. Hollinshead's present address is Wright-Fleming Institute, Imperial College Faculty of Medicine, St. Mary's Campus, London W2 1PG, UK.
Abbreviations used in this paper: FTI, farnesyltranferase inhibitor; Icmt, isoprenylcysteine carboxyl methyltransferase.
References
- Adjei, A.A., J.N. Davis, C. Erlichman, P.A. Svingen, and S.H. Kaufmann. 2000. Comparison of potential markers of farnesyltransferase inhibition. Clin. Cancer Res. 6:2318–2325. [PubMed] [Google Scholar]
- Belmont, A.S., Y. Zhai, and A. Thilenius. 1993. Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopy tomography. J. Cell Biol. 123:1671–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo, M.O., G.K. Leung, P. Ambroziak, J.C. Otto, P.J. Casey, and S.G. Young. 2000. Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J. Biol. Chem. 275:17605–17610. [DOI] [PubMed] [Google Scholar]
- Bergo, M.O., G.K. Leung, P. Ambroziak, J.C. Otto, P.J. Casey, A.Q. Gomes, M.C. Seabra, and S.G. Young. 2001. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J. Biol. Chem. 276:5841–5845. [DOI] [PubMed] [Google Scholar]
- Bergo, M.O., P. Ambroziak, C. Gregory, A. George, J.C. Otto, E. Kim, H. Nagase, P.J. Casey, A. Balmain, and S.G. Young. 2002. a. Absence of the CAAX endoprotease Rce1: effects on cell growth and transformation. Mol. Cell. Biol. 22:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo, M.O., B. Gavino, J. Ross, W.K. Schmidt, C. Hong, L.V. Kendall, A. Mohr, M. Meta, H. Genant, Y. Jiang, et al. 2002. b. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl. Acad. Sci. USA. 99:13049–13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604–1607. [PubMed] [Google Scholar]
- Burke, B., and C.L. Stewart. 2002. Life at the edge: the nuclear envelope and human disease. Nat. Rev. Mol. Cell Biol. 3:575–585. [DOI] [PubMed] [Google Scholar]
- Chelsky, D., J.F. Olson, and D.E. Koshland, Jr. 1987. Cell cycle-dependent methyl esterification of lamin B. J. Biol. Chem. 262:4303–4309. [PubMed] [Google Scholar]
- Choy, E., V.K. Chiu, J. Silletti, M. Feoktistov, T. Morimoto, D. Michaelson, I.E. Ivanov, and M.R. Philips. 1999. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 98:69–80. [DOI] [PubMed] [Google Scholar]
- Collas, P. 1998. Nuclear envelope disassembly in mitotic extract requires functional nuclear pores and a nuclear lamina. J. Cell Sci. 111:1293–1303. [DOI] [PubMed] [Google Scholar]
- Dai, Q., E. Choy, V. Chiu, J. Romano, S.R. Slivka, S.A. Steitz, S. Michaelis, and M.R. Philips. 1998. Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J. Biol. Chem. 273:15030–15034. [DOI] [PubMed] [Google Scholar]
- Dalton, M.B., K.S. Fantle, H.A. Bechtold, L. DeMaio, R.M. Evans, A. Krystosek, and M. Sinensky. 1995. The farnesyl protein transferase inhibitor BZA-5B blocks farnesylation of nuclear lamins and p21ras but does not affect their function or localization. Cancer Res. 55:3295–3304. [PubMed] [Google Scholar]
- De Sandre-Giovannoli, A., R. Bernard, P. Cau, C. Navarro, J. Amiel, I. Boccaccio, S. Lyonnet, C.L. Stewart, A. Munnich, M. Le Merrer, and N. Levy. 2003. Lamin A truncation in Hutchinson-Gilford progeria. Science. 300:2055. [DOI] [PubMed] [Google Scholar]
- Dolence, J.M., L.E. Steward, E.K. Dolence, D.H. Wong, and C.D. Poulter. 2000. Studies with recombinant Saccharomyces cerevisiae CaaX prenyl protease Rce1p. Biochemistry. 39:4096–4104. [DOI] [PubMed] [Google Scholar]
- Eriksson, M., W.T. Brown, L.B. Gordon, M.W. Glynn, J. Singer, L. Scott, M.R. Erdos, C.M. Robbins, T.Y. Moses, P. Berglund, et al. 2003. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 423:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker, M., M. Hollinshead, N. White, and D. Vaux. 1997. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J. Cell Biol. 136:531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, R.D., Y. Gruenbaum, R.D. Moir, D.K. Shumaker, and T.P. Spann. 2002. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 16:533–547. [DOI] [PubMed] [Google Scholar]
- Hancock, J.F., K. Cadwallader, H. Paterson, and C.J. Marshall. 1991. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 10:4033–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekes, H., and E.A. Nigg. 1994. The role of isoprenylation in membrane attachment of nuclear lamins. A single point mutation prevents proteolytic cleavage of the lamin A precursor and confers membrane binding properties. J. Cell Sci. 107:1019–1029. [DOI] [PubMed] [Google Scholar]
- Hutchison, C. 2002. Lamins: building blocks or regulators of gene expression? Nat. Rev. Mol. Cell Biol. 3:848–858. [DOI] [PubMed] [Google Scholar]
- Ishii, K., G. Arib, C. Lin, G. Van Houwe, and U.K. Laemmli. 2002. Chromatin boundaries in budding yeast: the nuclear pore connection. Cell. 109:551–562. [DOI] [PubMed] [Google Scholar]
- Kilic, F., D.A. Johnson, and M. Sinensky. 1999. Subcellular localization and partial purification of prelamin A endoprotease: an enzyme which catalyzes the conversion of farnesylated prelamin A to mature lamin A. FEBS Lett. 450:61–65. [DOI] [PubMed] [Google Scholar]
- Kim, E., P. Ambroziak, J.C. Otto, B. Taylor, M. Ashby, K. Shannon, P.J. Casey, and S.G. Young. 1999. Disruption of the mouse Rce1 gene results in defective Ras processing and mislocalization of Ras within cells. J. Biol. Chem. 274:8383–8390. [DOI] [PubMed] [Google Scholar]
- Labrador, M., and V.G. Corces. 2002. Setting the boundaries of chromatin domains and nuclear organisation. Cell. 111:151–154. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed] [Google Scholar]
- Lehner, C.F., R. Stick, H.M. Eppenberger, and E.A. Nigg. 1987. Differential expression of nuclear lamin proteins during chicken development. J. Cell Biol. 105:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz-Bohme, B., J. Wismar, S. Fuchs, R. Reifegerste, E. Buchner, H. Betz, and B. Schmitt. 1997. Insertional mutation of the Drosophila nuclear lamin Dm0 gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae. J. Cell Biol. 137:1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X., J. Jung, D. Kang, B. Xu, K.S. Zaret, and H. Zoghbi. 2002. Prenylcysteine carboxylmethyltransferase is essential for the earliest stages of liver development in mice. Gastroenterology. 123:345–351. [DOI] [PubMed] [Google Scholar]
- Liu, J., T.R. Ben-Shahar, D. Riemer, M. Treinin, P. Spann, K. Weber, A. Fire, and Y. Gruenbaum. 2000. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol. Biol. Cell. 11:3937–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir, R.D., M. Montag-Lowy, and R.D. Goldman. 1994. Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J. Cell Biol. 125:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir, R.D., M. Yoon, S. Khuon, and R.D. Goldman. 2000. Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 151:1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounkes, L.C., S. Kozlov, L. Hernandez, T. Sullivan, and C.L. Stewart. 2003. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature. 423:298–301. [DOI] [PubMed] [Google Scholar]
- Ottaviano, Y., and L. Gerace. 1985. Phosphorylation of the nuclear lamins during interphase and mitosis. J. Biol. Chem. 260:624–632. [PubMed] [Google Scholar]
- Otto, J.C., E. Kim, S.G. Young, and P.J. Casey. 1999. Cloning and characterization of a mammalian prenyl protein-specific protease. J. Biol. Chem. 274:8379–8382. [DOI] [PubMed] [Google Scholar]
- Otto, H., M. Dreger, L. Bengtsson, and F. Hucho. 2001. Identification of tyrosine-phosphorylated proteins associated with the nuclear envelope. Eur. J. Biochem. 268:420–428. [DOI] [PubMed] [Google Scholar]
- Paddy, M.R., A.S. Belmont, H. Saumweber, D.A. Agard, and J.W. Sedat. 1990. Interphase nuclear envelope lamins form a discontinuous network that interacts with only a fraction of the chromatin in the nuclear periphery. Cell. 62:89–106. [DOI] [PubMed] [Google Scholar]
- Pendas, A.M., Z. Zhou, J. Cadinanos, J.M. Freije, J. Wang, K. Hultenby, A. Astudillo, A. Wernerson, F. Rodriguez, K. Tryggvason, and C. Lopez-Otin. 2002. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31:94–99. [DOI] [PubMed] [Google Scholar]
- Sebti, S., and A.D. Hamilton. 2001. Farnesyltransferase Inhibitors in Cancer Therapy. Humana Press, Totowa, New Jersey. 280 pp.
- Stierle, V., J. Couprie, C. Ostlund, I. Krimm, S. Zinn-Justin, P. Hossenlopp, H.J. Worman, J.C. Courvalin, and I. Duband-Goulet. 2003. The carboxyl-terminal region common to lamins A and C contains a DNA binding domain. Biochemistry. 42:4819–4828. [DOI] [PubMed] [Google Scholar]
- Stuurman, N., S. Heins, and U. Aebi. 1998. Nuclear lamins: their structure, assembly, and interactions. J. Struct. Biol. 122:42–66. [DOI] [PubMed] [Google Scholar]
- Sullivan, T., D. Escalante-Alcalde, H. Bhatt, M. Anver, N. Bhat, K. Nagashima, C.L. Stewart, and B. Burke. 1999. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniura, H., C. Glass, and L. Gerace. 1995. A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J. Cell Biol. 131:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueblood, C.E., V.L. Boyartchuk, E.A. Picologlou, D. Rozema, C.D. Poulter, and J. Rine. 2000. The CaaX proteases, Afc1p and Rce1p, have overlapping but distinct substrate specificities. Mol. Cell. Biol. 20:4381–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux, D.J., and S. Gordon. 1985. Intracellular antigens associated with the cytoplasmic surface of phagolysosomes. J. Cell Sci. 77:109–127. [DOI] [PubMed] [Google Scholar]
- Vigouroux, C., M. Auclair, E. Dubosclard, M. Pouchelet, J. Capeau, J.C. Courvalin, and B. Buendia. 2001. Nuclear envelope disorganization in fibroblasts from lipodystrophic patients with heterozygous R482Q/W mutations in the lamin A/C gene. J. Cell Sci. 114:4459–4468. [DOI] [PubMed] [Google Scholar]
- Winter-Vann, A.M., B.A. Kamen, M.O. Bergo, S.G. Young, S. Melnyk, S.J. James, and P.J. Casey. 2003. Targeting Ras signaling through inhibition of carboxyl methylation: an unexpected property of methotrexate. Proc. Natl. Acad. Sci. USA. 100:6529–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman, H.J., I. Lazaridis, and S.D. Georgatos. 1988. Nuclear lamina heterogeneity in mammalian cells. Differential expression of the major lamins and variations in lamin B phosphorylation. J. Biol. Chem. 263:12135–12141. [PubMed] [Google Scholar]