Abstract
Bloom syndrome (BS) is a hereditary disorder characterized by pre- and postnatal growth retardation, genomic instability, and cancer. BLM, the gene defective in BS, encodes a DNA helicase thought to participate in genomic maintenance. We show that BS human fibroblasts undergo extensive apoptosis after DNA damage specifically when DNA replication forks are stalled. Damage during S, but not G1, caused BLM to rapidly form foci with γH2AX at replication forks that develop DNA breaks. These BLM foci recruited BRCA1 and NBS1. Damaged BS cells formed BRCA1/NBS1 foci with markedly delayed kinetics. Helicase-defective BLM showed dominant-negative activity with respect to apoptosis, but not BRCA1/NBS1 recruitment, suggesting catalytic and structural roles for BLM. Strikingly, inactivation of p53 prevented the death of damaged BS cells and delayed recruitment of BRCA1/NBS1. These findings suggest that BLM is an early responder to damaged replication forks. Moreover, p53 eliminates cells that rapidly assemble BRCA1/NBS1 without BLM, suggesting that BLM is essential for timely BRCA1/NBS1 function.
Keywords: DNA damage; DNA repair; DNA replication; γH2AX; S phase checkpoint
Introduction
Bloom syndrome (BS) is a rare genetic disorder caused by inactivating mutations in BLM, a member of the RECQ helicase family (Hickson et al., 2001). There are five human RECQ-like proteins (RECQL1, BLM, WRN, RECQL4, and RECQ5), each having 3′ to 5′ DNA helicase activity but little sequence similarity outside the helicase motifs. Three of these helicases (BLM, WRN, and RECQL4) cause a clinical syndrome when deficient (van Brabant et al., 2000). These syndromes (Bloom, Werner, and Rothmund-Thomson, respectively) all show genomic instability and cancer susceptibility, but each also has distinctive features. The unique features of BS are severe pre- and postnatal growth retardation and a wide spectrum of cancers that develop at a young age. Other BS phenotypes include facial sun sensitivity, immunodeficiency, and male sterility/female subfertility (German, 1969, 1997). Werner syndrome, by contrast, is an adult progeria characterized by multiple signs of aging that develop two to three decades prematurely. These aging phenotypes include cataracts, thin gray hair, atherosclerosis, osteoporosis, and type II diabetes. Werner syndrome individuals also develop cancer, but >50% mesenchymal tumors, which are rare in the general aged population (Martin, 1997). Rothmund-Thomson syndrome presents with skin and skeletal abnormalities and a high incidence of skin and bone neoplasms (Vennos and James, 1995). Thus, BS is the only RECQ-related disorder that results in the range and types of cancer observed in the general aged population (German, 1993). BS patients die predominantly of cancer in the second or third decade of life, much earlier than individuals with Werner or Rothmund-Thomson. Thus, BLM must participate in a process that is vital from early development and that, when defective, predisposes individuals to very early onset of cancer.
Many genes that are mutated in human disorders are structurally or functionally conserved in lower eukaryotes, including yeast (Frei and Gasser, 2000b). Nonetheless, human disease genes rarely correct phenotypes resulting from mutations in the corresponding yeast gene. A notable exception is BLM and WRN, which complement defects in SGS1, the single RECQ homologue in the budding yeast Saccharomyces cerevisiae. BLM or WRN suppresses the hyper-recombination phenotype of sgs1 mutant yeast, although only BLM restores the slow growth and hydroxyurea (HU) resistance of top1:sgs1 double mutants (Yamagata et al., 1998). The HU sensitivity of sgs1 mutants is noteworthy because HU inhibits ribonucleotide reductase, thereby hindering DNA replication fork progression (Koc et al., 2003). Like BS cells, sgs1 mutants are genomically unstable, accumulating chromosome translocations, chromosome deletions, and sister chromatid exchanges, the most common chromosomal abnormality in BS (Onoda et al., 2000). Fission yeast (Schizosaccharomyces pombe) show similar phenotypes upon inactivation of its single RECQ homologue, RQH1/RAD12. Rqh1/rad12 mutants are hypersensitive to HU (Stewart et al., 1997), showing chromosomal abnormalities and hyper-recombination after exposure. Thus, yeast BLM orthologues may be cell cycle–dependent sensors and/or early responders to DNA damage. Indeed, recent findings suggest that Sgs1p signals to the S phase checkpoint machinery, intriguingly through protein–protein interactions outside the helicase domain (Frei and Gasser, 2000b).
Indirect evidence suggests that BLM may likewise play a role in sensing and repairing DNA damage in mammalian cells (van Brabant et al., 2000); for example, the recent observation that Epstein Barr virus–transformed BS lymphoblasts fail to form NBS1/MRE11 foci after exposure to HU (Franchitto and Pichierri, 2002). Here, we examine the role of BLM in the DNA damage response using human fibroblasts with intact damage checkpoints. We show that BS cells, or normal cells that express a helicase-defective BLM, die by apoptosis in response to diverse genotoxins. This sensitivity was limited to S phase, during which time damage rapidly mobilized BLM from its location in promyelocytic leukemia protein (PML) bodies to DNA breaks. We show that BLM is required for rapid recruitment of BRCA1 and NBS1 to the damage, but interestingly, this recruitment did not require BLM helicase activity. Of particular importance, BS cells deficient in p53 function did not die when damaged in S phase, nor did they delay BRCA1/NBS1 recruitment into foci. We suggest that our findings help explain the growth retardation and cancer predisposition of BS.
Results
hTERT immortalized BS and complemented fibroblasts
To study BLM function in untransformed cells, we developed isogenic human fibroblasts that lack or express BLM. First, we used a retrovirus to express the catalytic subunit of telomerase (hTERT) into normal (normal human fibroblast [NHF]) and BS (Bloom syndrome fibroblast [BSF]) strains. hTERT immortalizes cells (Bodnar et al., 1998; Vaziri et al., 1999) without disrupting cell cycle checkpoints (Jiang et al., 1999; Toouli et al., 2002). hTERT rescued three NHFs (82-6, HCA2, and WI-38) and two BSFs (HG2654 and HG1013) from replicative senescence (Kim et al., 1999; unpublished data), as expected.
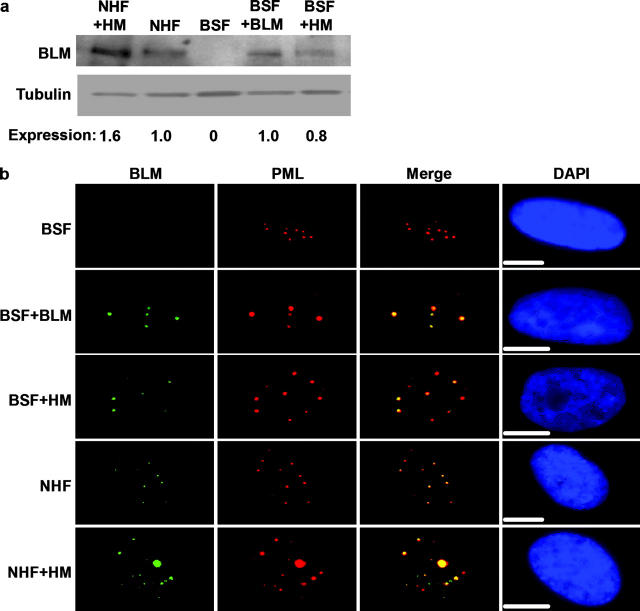
We next used retroviruses to introduce a control (insertless) vector or one that expresses either wild-type BLM or a mutant protein (helicase mutant [HM]) harboring a single amino acid substitution (K695T) that abolishes helicase activity (Neff et al., 1999). Wild-type or HM BLM is toxic when overexpressed in untransformed cells (Yankiwski et al., 2000; unpublished data). We therefore selected an uncloned population of low expressers from mass infected cultures (see Materials and methods). Western analysis confirmed that BLM is undetectable in control BS cells and that the retrovirus expressed wild-type and mutant BLM at levels similar to that of endogenous BLM in normal cells (Fig. 1 a). Immunostaining showed that >90% of BS cells expressed the retroviral proteins, and that they localized primarily to PML nuclear bodies (Fig. 1 b), the sites to which endogenous BLM localizes when it is expressed in S and G2 (Bischof et al., 2001b).
Figure 1.
Human cells expressing wild-type or mutant BLM proteins. (a) Protein expression. NHFs and BSFs were immortalized by hTERT and then infected with retroviruses carrying no insert (NHF and BSF) or cDNAs encoding wild-type (+BLM) or mutant (+HM) BLM proteins as described in the Materials and methods. Proteins from asynchronous cultures were analyzed for BLM and tubulin (control) by Western blotting and quantified by densitometry. Relative BLM levels (Expression) were determined by dividing the BLM signal by the tubulin signal, setting the NHF value at 1.0. (b) BLM localization. Cells described in part a were stained for BLM (green) and PML (red), and the images were merged using Adobe Photoshop®. Nuclei were counterstained with DAPI (blue). Bars, 10 μm.
Asynchronous BS cells are mildly sensitive to genotoxic agents
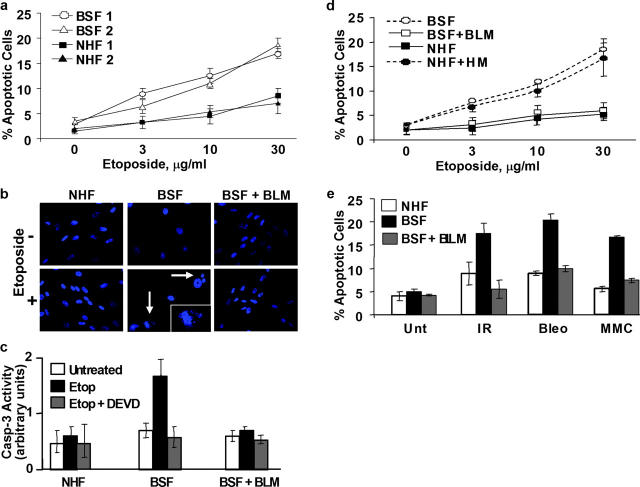
To assess sensitivity to DNA damage, we treated asynchronous cultures for 1 h with etoposide, a topoisomerase II inhibitor that causes double strand DNA breaks (Sinha, 1995). 6 h later, we scored the cells for signs of apoptosis: loss of mitochondrial membrane potential (Fig. 2 a), nuclear blebbing (Fig. 2 b), and activated caspase-3 (Fig. 2 c) (Green and Reed, 1998). At all concentrations tested, the two BSFs were one- to twofold more sensitive than two NHFs (Fig. 2 a). More importantly, wild-type BLM rescued this sensitivity (Fig. 2, b–d). Conversely, the HM mutant rendered NHFs as sensitive as BSF (Fig. 2 d), suggesting that it has dominant-negative activity. Comparable results were obtained when we similarly treated with X-rays, bleomycin (a radiomimetic), or mitomycin C (an alkylating agent); in all cases, BSFs were one- to twofold more sensitive than NHFs (Fig. 2 e).
Figure 2.
BS cells are sensitive to DNA damaging agents. (a) Etoposide sensitivity. Asynchronous NHF1 and 2 (82-6 and HCA2) and BSF1 and 2 (HG1013 and HG2654) cells were treated with the indicated doses of etoposide for 1 h and scored 6 h later for loss of mitochondrial membrane potential (% Apoptotic Cells), as described in the Materials and methods. (b and c) BS cells die by apoptosis. NHFs (82-6), BSFs (HG2654), and complemented BSFs (BSF + BLM) were synchronized in S phase as described in the text and the Materials and methods and then left untreated (−) or treated (+) for 1 h with etoposide (20 μg/ml) or HU (30 mM; not depicted). 6 h later, cells were fixed and stained with DAPI to observe nuclear morphology (b). Nuclear blebbing or fragmentation (arrows and inset) was observed in ∼50% treated BSFs and in <5% treated NHF and BSF + BLM cells. Alternatively, untreated or etoposide-treated (Etop) cells were analyzed for activated caspase-3 (Casp-3 Activity) in the absence or presence of DEVD, a caspase-3 inhibitor (c), as described in the Materials and methods. (d) Effect of wild-type and mutant BLM. Asynchronous BSF (HG2654), complemented BSF (BSF + BLM), NHFs (82-6), and mutant BLM expressing NHF (NHF + HM) were treated with the indicated doses of etoposide for 1 h and scored for apoptosis as described in part a. (e) Sensitivity to other agents. Asynchronous NHF, BSF, and BSF + BLM cells were untreated (Unt), X irradiated (5 Gy; IR), or treated for 2 h with 20 μg/ml bleomycin (Bleo) or 2 μg/ml mitomycin C (MMC), and scored for apoptosis as described in part a. Error bars show the standard error from at least two independent experiments.
BSFs are sensitive in S phase, but not G1
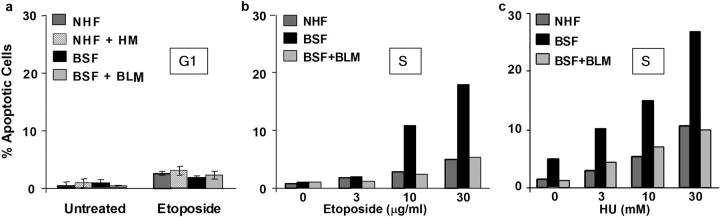
Because BLM expression peaks in S phase and G2 (Bischof et al., 2001b), and mutant RECQ proteins render yeast hypersensitive to replication inhibitors such as HU (Yamagata et al., 1998), we asked whether BSFs were selectively sensitive to DNA damage during S phase. We cultured NHF, NHF + HM, BSF, and BSF + BLM cells in serum-deficient medium for 72 h. All cell types became quiescent (>95% G1 DNA content; <2% cells in S phase), similar to unmodified cells (unpublished data). Thus, the hTERT immortalization and other infections did not perturb normal growth control. We then stimulated quiescent cells to enter G1 by providing serum for 4–6 h and challenged with etoposide or bleomycin for 1 h. 6 h later, we assessed the cells for apoptosis. All four cell types showed low sensitivity to etoposide (Fig. 3 a) and bleomycin (not depicted). Thus, BS cells were not hypersensitive to DNA damage in G1.
Figure 3.
BS cells are sensitive to damage in S phase but not G1. (a) Lack of sensitivity during G1. NHFs (82-6), mutant BLM-expressing NHFs (NHF + HM), BSFs (HG2654), and complemented BSF (BSF + BLM) were synchronized in G1 as described in the text and Materials and methods, treated with etoposide (20 μg/ml) for 1 h, and scored for apoptosis 6 h later as described for Fig. 2 a. (b and c) Hypersensitivity during S phase. NHF, BSF, and BSF + BLM cells were synchronized in S phase as described in the text and the Materials and methods, treated with indicated doses of etoposide (b) or HU (c) for 1 h, and scored for apoptosis 6 h later. Shown is a representative experiment, which was repeated at least twice.
To examine sensitivity in S phase, we serum stimulated quiescent NHF, BSF, and BSF + BLM cells for 8 h and then added aphidicolin for 12–14 h to arrest them at the G1/S boundary. We then cultured in drug-free medium to allow progression into S phase. After 1 h (early S phase), we added etoposide for 1 h and scored for apoptosis 6 h later. In contrast to their relative insensitivity in G1, BSFs were hypersensitive to etoposide in early S (Fig. 3 b). Moreover, BLM complementation attenuated this sensitivity (Fig. 3 b). Cell death was somewhat greater when BSFs were damaged during S phase rather than asynchronous growth (Fig. 2, a and d, versus Fig. 3 b), and greater yet when the synchrony was improved (see next section). Thus, BSFs were hypersensitive to DNA damage selectively during S phase.
Of significance, both asynchronous (not depicted) and S phase (Fig. 3 c) BSFs underwent apoptosis when treated with HU, which stalls replication forks (Koc et al., 2003). At 10–30 mM, a 1-h exposure to HU caused as much cell death in 6 h as a 1-h exposure to etoposide (Fig. 3, a and b). A lower dose (1 mM), which still retards replication forks, also caused apoptosis, albeit after a longer interval (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200304016/DC1), consistent with the report that BS lymphoblasts die 36 h after exposure to 2 mM HU (Franchitto and Pichierri, 2002). This result suggests that BSFs are hypersensitive to replication stress.
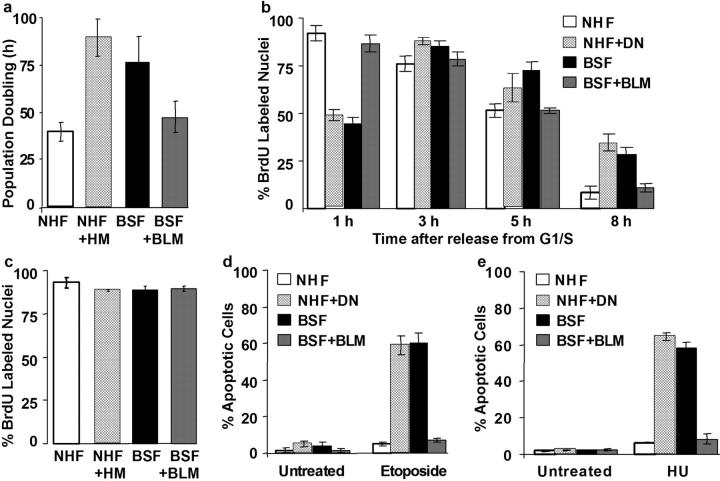
Slow growth of BLM-deficient cells
hTERT-immortalized BSFs proliferated slowly relative to hTERT-expressing NHFs (doubling time 80–100 h versus ∼40 h; Fig. 4 a), as did unmodified BSFs (not depicted). BLM complementation accelerated the growth of BSFs, whereas the HM mutant retarded the growth of NHFs (Fig. 4 a). These growth rate differences affected the kinetics of S phase entry after synchronization. We followed S phase entry after release from a G1/S block using 1-h pulses with BrdU. Greater than 80% of NHF and BSF + BLM cells incorporated BrdU 1 h after release, but <50% of BSF and NHF + HM cells did so. However, 3 h after release, >80% of BSF and NHF + HM cells incorporated BrdU (Fig. 4, b and c). These findings indicate that the slow growth of BSF and NHF + HM cells is not due to a smaller growth fraction. Moreover, their slower entry into and exit from S phase (Fig. 4 b) suggest that the slow growth is not due solely to an increase in G1, consistent with the cell cycle distributions of asynchronous cells determined by flow cytometry (unpublished data). We therefore adjusted the synchronization protocol in subsequent experiments (see Materials and methods) and assessed sensitivity to etoposide and HU when >80% of cells were in early S (Fig. 4 c). Under these conditions, BSF and NHF + HM cells showed ∼10-fold more apoptosis than NHF or BSF + BLM cells (Fig. 4, d and e). Unmodified BSF cells also underwent extensive apoptosis when given HU during S phase (Fig. S2 a, available at http://www.jcb.org/cgi/content/full/jcb.200304016/DC1), indicating that the cell death we observed was not due to hTERT expression. We conclude that BLM is crucial for cell survival during replication stress.
Figure 4.
Slow growth and S phase kinetics of BLM-deficient cells. (a) Proliferation rate. We plated NHF, NHF + HM, BSF, and BSF + BLM cells at subconfluent densities, counted cells every 2–4 d, and calculated population doubling times while the cells proliferated exponentially. (b) S phase kinetics. We synchronized cells at the G1/S boundary, released for the indicated times, added BrdU for 1 h, and then fixed and stained for BrdU as described in the text and the Materials and methods. (c) Optimized synchronization. We adjusted the synchronization of BSF, NHF + HM, NHF, and BSF + BLM cells as described in the Materials and methods and verified that ∼90% initiated S phase 1 h after release from a G1/S block by pulsing with BrdU for 1 h and scoring the % BrdU-labeled nuclei. (d and e) Sensitivity during S phase. NHF, NHF + HM, BSF, and BSF + BLM cells were synchronized at the G1/S boundary, released for 1 h, and given 20 μg/ml etoposide (d) or 30 mM HU (e) for 1 h. We scored apoptosis 6 h later as described for Fig. 2 a.
Death of BSFs is p53 dependent
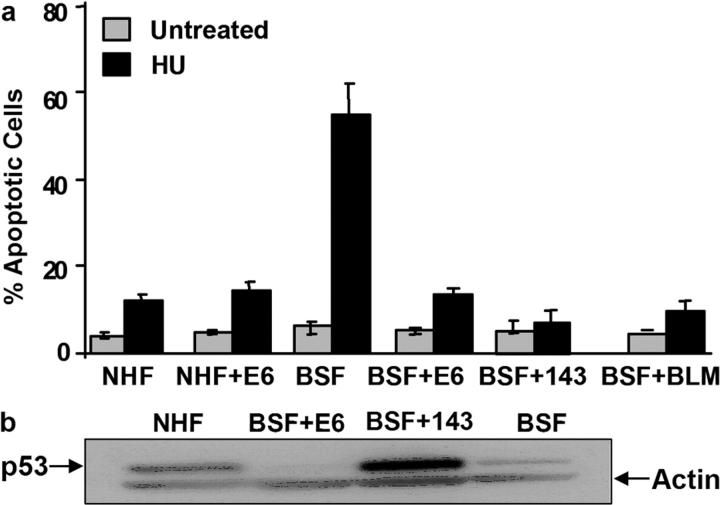
Because the p53 tumor suppressor is a critical regulator of apoptosis, we asked whether the death of BSFs is p53 dependent. First, we reduced p53 in NHFs and BSFs by expressing E6, a viral protein that stimulates p53 degradation (Dalal et al., 1996). We then synchronized the cells in early S phase and stalled replication forks with HU. E6 did not alter the modest apoptotic response of NHFs; however, it completely abolished the robust apoptotic response of BSFs (Fig. 5 a). Second, we expressed a dominant-negative p53 protein (V143A) (Matas et al., 2001) in BSFs. p53-V143 also abolished the apoptotic response to HU (Fig. 5a). Western analysis confirmed that p53 levels were similar in NHFs and BSFs, and that p53 was reduced by E6 in NHFs (unpublished data) and elevated by expression of p53-V143A in BSFs (Fig. 5 b). These results indicate that BLM deficiency during replication stress engages the apoptotic function of p53.
Figure 5.
BS cells undergo p53-dependent apoptosis. (a) Apoptosis. NHFs (WI-38) and BSFs (HG2654) were infected with retroviruses expressing either E6 or p53-V143A. The cells were then synchronized and treated in early S phase for 1 h with HU (30 mM). Apoptosis was scored 6 h later as described for Fig. 2 a. Error bars, standard error from two experiments. (b) p53 levels. Lysates from proliferating NHFs and BSFs infected with control, E6-expressing, or p53-V143A–expressing retroviruses were analyzed for p53 or actin (control) by Western blotting.
BLM rapidly localizes to double strand breaks during S phase
Stalled replication forks can develop double strand breaks (Ward and Chen, 2001; Xu and Stern, 2003), which are rapidly marked by phosphorylated histone H2AX (γH2AX), foci of which are a very early marker of broken DNA (Paull et al., 2000; Celeste et al., 2002). We therefore asked whether BLM localized to DNA breaks marked with γH2AX when replication forks stall.
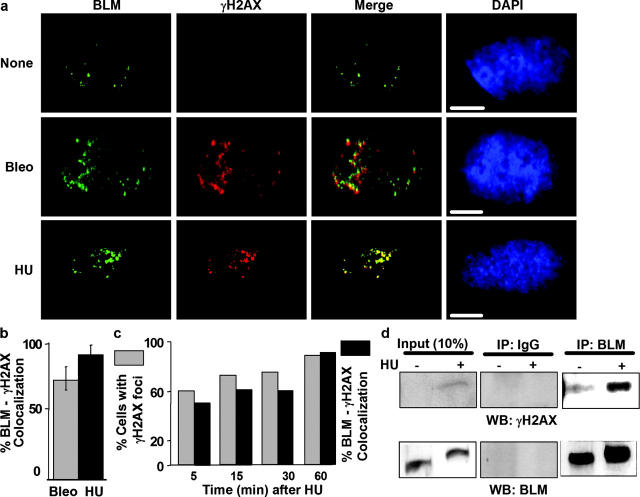
As expected (Paull et al., 2000; Bischof et al., 2001b; Furuta et al., 2003), undamaged NHFs in early S showed diffuse γH2AX and punctate BLM immunostaining (Fig. 6 a). When these cells were given bleomycin, which causes random double strand breaks (Kaufmann and Kies, 1998), multiple γH2AX foci formed in most cells within 1 h (Fig. 6, a–c). Many (∼70%) of these foci costained for BLM (Fig. 6, a and b). When the cells were given HU, multiple γH2AX foci also formed, indicating that some stalled forks acquired double strand breaks (Ward and Chen, 2001; Xu and Stern, 2003). BLM localized to nearly 90% of these foci (Fig. 6, a and b). BLM localized to γH2AX foci rapidly, lagging behind γH2AX focus formation by only a few minutes (Fig. 6 c). Unmodified NHFs (lacking hTERT) (Fig. S2 b) and complemented BS cells (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200304016/DC1) also showed rapid γH2AX/BLM colocalization in response to HU. Because bleomycin breaks DNA randomly, whereas HU causes breaks at replication forks, these results suggest that BLM localizes to replication forks that develop double strand breaks. It is possible, however, that BLM localizes to any double strand break during S phase. Whatever the case, immunoprecipitation and Western analysis showed that ∼70% of γH2AX coprecipitated with BLM when Jurkat cells were given HU (Fig. 6 d), suggesting that BLM and γH2AX reside in a common complex after replication forks stall. Moreover, >70% of the BLM/γH2AX foci that formed after HU treatment did not contain PML (unpublished data), consistent with our finding that BLM dissociates from PML bodies in a fraction of asynchronous cells treated with agents that break DNA (Bischof et al., 2001b). Together, these findings suggest that BLM is recruited from PML bodies to associate with double strand breaks during S phase.
Figure 6.
BLM localizes with γH2AX. (a) BLM/γH2AX foci. NHF (82-6) cells were synchronized in early S phase and not treated (None) or treated with 20 μg/ml bleomycin (Bleo) or 30 mM HU for 1 h. Cells were then immunostained for BLM (green) and γH2AX (red), and the images were merged. DAPI staining (blue) marks cell nuclei. Bars, 10 μm. (b) Quantitation of BLM/γH2AX coincidence. Merged images from part a were analyzed for the percentage of γH2AX foci that costained for BLM, counting ∼100 nuclei per experiment. Error bars, standard error from two experiments. (c) Kinetics of γH2AX/BLM colocalization. NHFs in early S phase were given 30 mM HU and stained for γH2AX and BLM at the indicated intervals thereafter. Gray bars, % cells with >10 γH2AX foci. Black bars, % cells in which >80% of γH2AX foci costained for BLM. (d) BLM–γH2AX complex. Asynchronous Jurkat cells were untreated (−) or treated (+) with 10 mM HU for 6 h. Lysates were prepared, 90% was immunoprecipitated (IP) by control (IgG) or anti-BLM antibody, and immunoprecipitates were analyzed by Western blotting (WB) using anti-γH2AX or anti-BLM antibodies. 10% of each lysate was analyzed directly by Western blotting (Input).
BSFs do not form NBS1 and BRCA1 foci immediately after replication stress
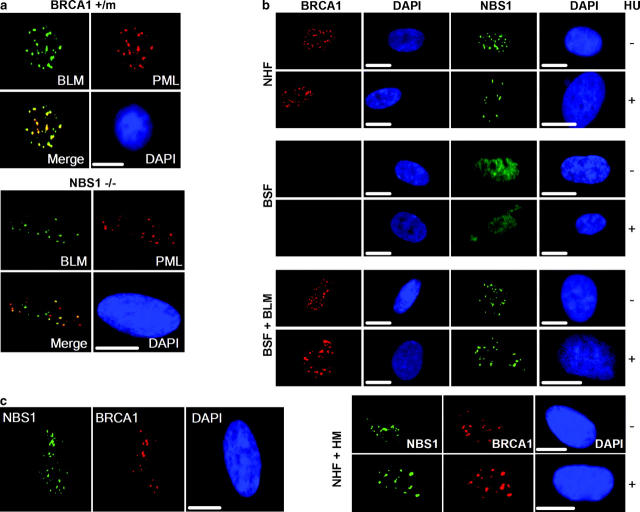
γH2AX foci are thought to recruit proteins such as NBS1 (Nijmegen breakage syndrome protein 1) and BRCA1 (breast cancer–associated protein 1) (Celeste et al., 2002; Fernandez-Capetillo et al., 2003; Kobayashi et al., 2002), which are important for optimal double strand break repair (D'Amours and Jackson, 2002). BRCA1 colocalized with BLM in a fraction of HU-treated carcinoma cells (Wang et al., 2000), and BS lymphoblasts were reported deficient in NBS1 foci (Franchitto and Pichierri, 2002). To determine the relationship between BLM, BRCA1, and NBS1, we localized BLM in human carcinoma cells carrying a dominant-negative BRCA1 allele (Scully et al., 1999) and hTERT-immortalized, NBS1-deficient fibroblasts (see Materials and methods). In undamaged cells, BLM localized primarily to PML bodies (Fig. 7 a). Further, BLM localized to many of the γH2AX foci that formed after exposure to HU (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200304016/DC1). These findings suggest that proper BLM localization does not depend on BRCA1 or NBS1. However, the rapid response of BRCA1 and NBS1 to replication stress depended on BLM.
Figure 7.
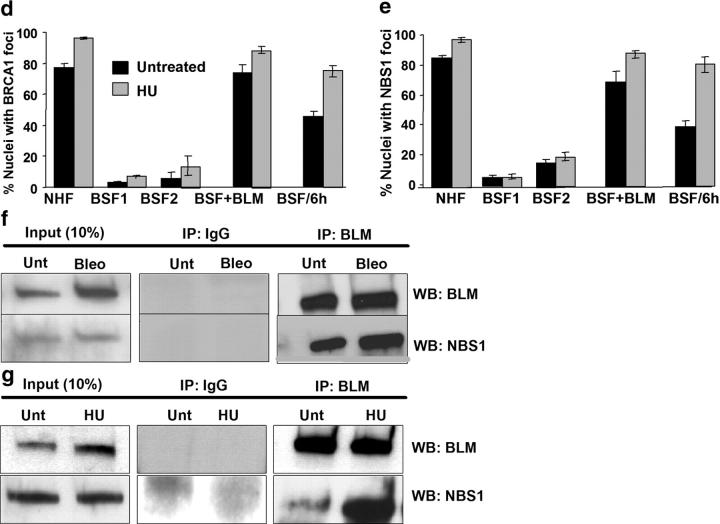
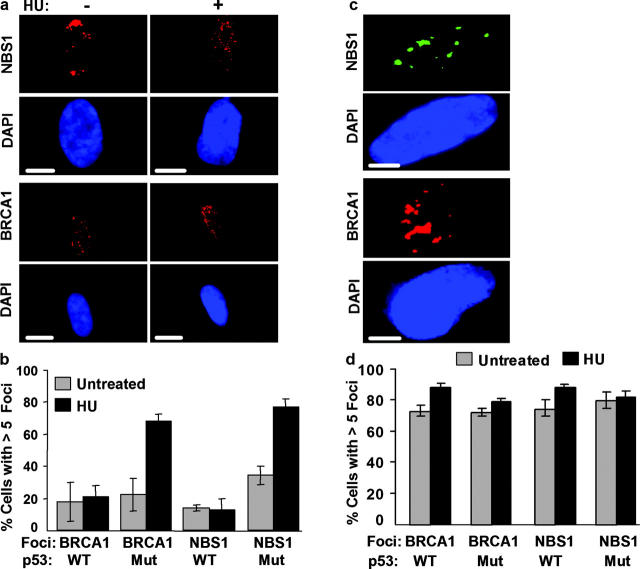
Effects of BLM deficiency on BRCA1 and NBS1 foci. (a) BLM localization in BRCA1- or NBS1-deficient cells. Asynchronous BRCA1 mutant cells (HCC1937, BRCA1+/m) and NBS1-deficient cells (GM07166A, NBS1−/−) were immunostained for BLM and PML, and the images were merged. Bars, 10 μm. (b) NHF (82-6), BSF (HG2654), complemented BSF (BSF + BLM), and NHF expressing mutant BLM (NHF + HM) were synchronized in early S phase, left untreated (−) or treated (+) with 30 mm HU for 1 h, and immunostained for BRCA1 and NBS1. Nuclei were counterstained with DAPI (blue). Bars, 10 μm. (c) Delayed BRCA1 and NBS1 focus formation in BSF. BSFs were synchronized and treated with HU as described in part b. 6 h later, they were immunostained for BRCA1 and NBS1. (d and e) Quantification of nuclei with BRCA1 or NBS1 foci. NHF (82-6), BSF (HG1013, BSF1; HG2654, BSF2), and complemented HG2654 (BSF + BLM) cells were synchronized in early S phase, left untreated or treated with HU (30 mM) for 1 h, and then immunostained for BRCA1 (d) or NBS1 (e) foci. BSFs (HG2654) were also stained 6 h after treatment with HU (BSF/6h). Nuclei with greater than five BRCA1 or NBS1 foci were scored as positive. Error bars, standard error from two experiments. (f and g) NBS1–BLM complexes. Asynchronous Jurkat cells were untreated (Unt) or treated with 10 μg/ml bleomycin (Bleo) for 6 h (f), and NHFs (82-6) were synchronized in early S and untreated (Unt) or treated with 30 mM HU for 1 h. Lysates were prepared, 90% was immunoprecipitated (IP) by control (IgG) or anti-BLM antibody, and immunoprecipitates were analyzed by Western blotting (WB) using anti-BLM or anti-NBS1 antibodies. 10% of each lysate was analyzed directly by Western blotting (Input).
In undamaged NHFs, BRCA1 was barely detectable in G0/G1 (not depicted) but formed multiple (greater than five) foci in early S (Fig. 7, b and d), consistent with BRCA1, like BLM, being expressed during S phase (Scully and Livingston, 2000). A fraction (25–30%) of BRCA1 foci colocalized with BLM and PML in undamaged cells, but the majority (∼80%) colocalized with BLM and γH2AX 1 h after replication was stalled by HU (not depicted). BSFs formed many fewer BRCA1 foci. Cells with greater than five BRCA1 foci comprised <20% of undamaged BSFs in S phase, and this percentage did not increase 1 h after exposure to 30 mM (Fig. 7, b and d) or 1 mM HU (Fig. S5 a, available at http://www.jcb.org/cgi/content/full/jcb.200304016/DC1). Western analysis showed that BLM did not alter BRCA1 protein levels (not depicted), indicating that BLM affects BRCA1 localization, not expression. NBS1 focus formation was also impaired in BSFs. Undamaged NHFs formed NBS1 foci during S phase, as reported (Lombard and Guarente, 2000; Miura et al., 2001). However, NBS1 foci were largely absent from BSFs; <20% of undamaged cells contained greater than five foci and this did not change 1 h after HU treatment (Fig. 7, b and e; Fig. S5 a). Failure to form BRCA1 and NBS1 foci was due to BLM deficiency because these foci formed normally in BLM-complemented BSFs (Fig. 7, b, d, and e; Fig. S5 a). Further, this failure was not due to the synchronization method, because BSFs synchronized by mimosine and then treated for 1 h with HU in early S also formed very few BRCA1 or NBS1 foci (Fig. S5, b and c).
BRCA1 was shown to reside in a complex with BLM in a fraction of damaged, asynchronous HeLa cells (Wang et al., 2000). Immunoprecipitation showed that NBS1 also resides in a complex with BLM in asynchronous Jurkat cells treated with bleomycin and NHFs treated with HU in S phase (Fig. 7, f and g). Taken together, these findings suggest that BLM acts upstream of NBS1 and BRCA1 to assemble repair complexes at damaged replication forks.
Despite failure to form BRCA1 or NBS1 foci in early S, BSFs formed these foci later in S phase (6–8 h after release from G1/S) (not depicted). Moreover, although many BSFs did not form BRCA1/NBS1 foci 1 h after replication forks stalled, they formed these foci 6 h later (Fig. 7, c–e). Thus, BLM deficiency delayed, but did not abrogate, BRCA1 and NBS1 recruitment into foci. Of interest, NHFs expressing the HM mutant were completely normal in assembling BRCA1/NBS1 foci during early S and 1 h after replication forks stalled (Fig. 7 b). Thus, despite dominant-negative activity with respect to growth and apoptosis, the HM mutant did not have dominant-negative activity with respect to BRCA1/NBS1 focus formation. This finding suggests that BLM has a structural role, which does not depend on its helicase activity, in assembling BRCA1/NBS1 complexes.
p53-deficient BSFs rapidly assemble NBS1 and BRCA1 foci after replication stress
In response to DNA damage, p53 halts cell cycle progression, presumably to allow repair, or induces apoptosis or senescence if the damage is severe (Ryan et al., 2001). Recent findings suggest that p53 may directly modulate repair or interact with repair proteins (Lu et al., 2003; Maser and DePinho, 2003; Rubbi and Milner, 2003). Because p53 mediated the apoptotic response of BSFs to damaged replication forks (Fig. 5), we asked whether p53 prevented the rapid assembly of BRCA1/NBS1 foci in BSFs. In contrast to BSFs with wild-type p53, 70–80% of BSFs with mutant p53 (p53-V143A) assembled BRCA1 and NBS1 foci 1 h after HU stalled replication forks (Fig. 8, a and b). Loss of p53 function also partially restored the ability of undamaged BSF to form NBS1, but not BRCA1, foci in S phase (Fig. 8 b). NHFs with mutant p53, in contrast, showed no change in their relatively low incidence of apoptosis (Fig. 5 a), or ability to form BRCA1/NBS1 foci (Fig. 8, c and d) 1 h after HU stalled replication forks. These findings suggest that p53 acts downstream of BLM, delaying BRCA1/NBS1 focus formation in the absence of BLM.
Figure 8.
Localization of BRCA1 and NBS1 in BS cells with mutant p53. (a) Effect of mutant p53 in BSFs. BSFs (HG2654) expressing p53-V143A were synchronized in early S phase and left untreated (−) or treated (+) with 30 mM HU for 1 h. They were then immunostained for NBS1 or BRCA1 as indicated, and the nuclei were counterstained with DAPI (blue). Bars, 10 μm. (b) Quantitation. BSFs with wild-type (WT) or mutant (p53-V143A; Mut) p53 were synchronized in early S phase, challenged with 30 mM HU for 1 h, and immunostained for BRCA1 and NBS1. Cells were scored for nuclei containing greater than five BRCA1 or NBS1 foci. (c) Effect of mutant p53 in NHFs. NHFs (82-6) were synchronized in early S phase, treated with 30 mM HU for 1 h, and immunostained for NBS1 and BRCA1. Nuclei were counterstained with DAPI (blue). Bars, 10 μm. (d) Quantitation. BSFs with wild-type (WT) or mutant (p53-V143A; Mut) p53 were synchronized, challenged with HU, and immunostained as described in part b. Cells were scored for nuclei containing greater than five BRCA1 or NBS1 foci.
Discussion
BLM associates with double strand breaks during S phase
Studies in yeast and human cells suggest a pivotal role for RECQ-like helicases in maintaining genomic integrity during S phase (Bernstein et al., 2002; D'Amours and Jackson, 2002). Among the five human RECQ helicases, BLM appears to be particularly important in this regard (Ababou et al., 2000; Dutertre et al., 2000; Sengupta et al., 2003). Although in vitro data suggest that Xenopus RECQ proteins play a direct role in DNA replication (Liao et al., 2000), yeast and mammalian cell studies are more consistent with a role for BLM in responding to DNA damage during replication (Hickson, 2003), and particularly in repairing stalled replication forks (Imamura et al., 2001; Ababou et al., 2002; Sengupta et al., 2003). In contrast to many studies, we used isogenic, hTERT-immortalized, but untransformed, BS fibroblasts in which we expressed normal or helicase-deficient BLM proteins. These proteins were expressed at levels comparable to endogenous BLM in NHFs, and localized to PML bodies. Although BLM can localize to the nucleolus in some transformed cells, we and others find BLM predominantly in PML bodies in normal cells (Yankiwski et al., 2000; Bischof et al., 2001b). Our results show unambiguously that BSFs, both unmodified and hTERT immortalized, are hypersensitive to genotoxic stress only during S phase. Virtually all the hypersensitivity was eliminated by expressing wild-type BLM, and the HM mutant conferred hypersensitivity to normal cells, indicating that the hypersensitivity is a consequence of BLM deficiency. We did not observe such striking cell cycle–dependent sensitivity in hTERT-immortalized Werner syndrome fibroblasts (unpublished data).
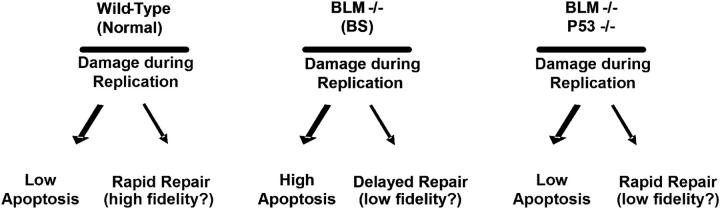
In response to damage during S phase, BLM promptly left the PML bodies and associated with DNA double strand breaks, as assessed by loss of costaining with PML and gain of costaining with γH2AX. The kinetics of costaining with γH2AX, and ability of HU to cause BLM relocalization, suggests that BLM rapidly localizes to double strand breaks at replication forks. These results, and the finding that BSFs fail to rapidly assemble BRCA1 or NBS1 foci after damage, suggest that BLM may be an important initial coordinator of processes that ensure the faithful repair of damage during DNA replication (Fig. 9). In the absence of BLM function, the majority of cells damaged during S phase die by apoptosis. We hypothesize that the survivors may repair the damage by suboptimal, low fidelity processes and thus may harbor a high incidence of the chromosomal abnormalities characteristic of BS cells.
Figure 9.
Model for how BLM and p53 status regulate apoptosis and repair proficiency. Shown are experimentally verified and predicted outcomes in normal (wild-type) or BS cells (BLM −/−), with wild-type or deficient p53 function (p53+/+ or p53−/−).
Regarding the cells we generated for this study, we consistently observed a gradual increase in growth rate and loss of sensitivity to genotoxic stress when BSF and NHF + HM cells were continually subcultured (unpublished data). For this reason, we prepared fresh cultures from frozen stocks every few weeks. These findings suggest that the genomic instability caused by BLM deficiency can produce mutational variants that eventually overtake the culture. Transformed cells, particularly those in which the p53 pathway is defective, may be unsuitable for studying normal cellular consequences of BLM deficiency. Lesions in the p53 pathway and/or the use of asynchronous cultures may also explain apparent discrepancies in the literature, such as the insensitivity of BLM-null chicken cells to HU (Imamura et al., 2001) or the resistance of BS lymphoblastoid cells to some DNA damaging agents (Wang et al., 2001).
BS cells are prone to apoptosis
Stalled forks can develop double strand breaks, which typically cause senescence in NHFs (Robles and Adami, 1998). However, we observed no evidence of senescence when BSFs experienced damage during replication; rather, the cells died by apoptosis. Thus, BLM deficiency renders cells highly susceptible to apoptosis, suggesting a possible explanation for the pre- and postnatal growth retardation seen in BS. Endogenous oxidative metabolism continually generates DNA lesions (Ames, 1998), which can stall replication forks and generate double strand breaks. Under these conditions, BLM and other proteins must act quickly to repair the damage. In the absence of BLM, many cells may fail to repair the damage rapidly enough, whereupon p53 signals those cells to die. Individuals with BS may continually lose cells, owing to excessive apoptosis, particularly during pre- and postnatal development, when cell proliferation is extensive. Excessive apoptosis would leave many tissues with chronic cellular insufficiency, and hence small size, thereby explaining the pre- and postnatal growth retardation. BS is rare among humans, and it is hypothesized that many BS embryos die in utero (German, 1997). Consistent with this idea, most blm−/− mice die before birth due to massive apoptosis (Chester et al., 1998).
Role of BLM in BRCA1 and NBS1 focus formation
BLM expands the list of proteins that associate with γH2AX at DNA breaks (Tauchi et al., 2002; Shang et al., 2003; Xu and Stern, 2003). BLM localized to γH2AX foci within minutes of their formation. As γH2AX interacts directly with NBS1 (Kobayashi et al., 2002), BLM may facilitate the NBS1–γH2AX interaction. Consistent with this view, the HM mutant was proficient at recruiting BRCA1 and NBS1, despite its inability to prevent apoptosis after DNA damage during S phase. Thus, although BLM helicase activity is essential for preventing cell death, it is dispensable for rapid BRCA1/NBS1 recruitment. These findings suggest that BLM has both an enzymatic role and a structural role in the rapid response to damage during S phase. Interestingly, WRN may also have both catalytic and structural roles in DNA repair (Chen et al., 2003).
BSFs that survive damage during S phase eventually formed BRCA1 and NBS1 foci, albeit with delayed kinetics. Thus, BLM is not unconditionally necessary for BRCA1 and NBS1 focus formation, but rather is necessary only for the rapid response. In the absence of BLM, another RECQ helicase may substitute for BLM, but its recruitment may be less efficient or require events that take several hours to complete. BLM also enhanced the ability of BRCA1 and NBS1 to form foci during S phase in undamaged cells. However, of the BRCA1 and NBS1 foci that formed during S phase, only 20–30% colocalized with BLM in PML bodies. Why then are BRCA1 and NBS1 foci so rare in BS cells? One possibility is that BRCA1 and NBS1 transiently shuttle into the PML body, where BLM facilitates modifications that allow these proteins to form foci outside the PML body. Consistent with this possibility, BLM may be necessary for NBS1 phosphorylation (Franchitto and Pichierri, 2002).
Yeast RECQ homologues have been proposed to act as sensors of damage encountered during DNA synthesis (Frei and Gasser, 2000a,b), a role also ascribed to mammalian BRCA1 and the MRE11/RAD50/NBS1 complex. Loss of any of these proteins sensitizes cells to DNA damage and gives rise to a mutator phenotype. BLM and MRE11/RAD50/NBS1 were recently identified as part of a large complex, which BRCA1 was proposed to anchor to the nuclear matrix (Wang et al., 2000). Our previous finding that BLM associates tightly with the nuclear matrix (Bischof et al., 2001b), and our finding that BLM is required for rapid recruitment of BRCA1 to damage-induced foci, raises the possibility that BLM provides the scaffold for assembly of the BRCA1 repair complex. Assembly of this complex may prevent the death of cells with damaged replication forks. This possibility is consistent with the finding that BLM is rapidly cleaved during apoptosis induced by severe genotoxic stress (Bischof et al., 2001a).
Role of p53 in BS phenotypes
p53 was crucial for the apoptotic death of BS cells. This apoptosis was not accompanied by an increase in BAX or p21 expression (not depicted), genes for which p53 is a known transactivator. Thus, p53 may induce apoptosis independent of its transactivation activity, consistent with the finding that p53 is transcriptionally inactive during S phase (Gottifredi et al., 2001; Takimoto and El-Deiry, 2001). p53 may mediate the death of damaged BSFs by directly inducing mitochondria-mediated apoptosis (Mihara et al., 2003), or by virtue of it transrepression activity (Ryan et al., 2001).
Loss of p53 function allowed BS cells to survive replication stress, but the survivors may acquire chromosomal aberrations (unpublished data). This scenario provides a plausible explanation for the extraordinarily high cancer incidence seen in BS. The excessive apoptosis that likely occurs in the proliferative tissues of BS individuals would also create a chronic need for cell replacement and hence cell division, which increases the risk of oncogenic mutations. Because mutations in the p53 pathway prevent the death of BS cells, there is intense selection for BS cells that lose p53 function. Loss of p53 function alone is a strong risk factor for genomic instability and the development of cancer (Donehower, 1996). However, BLM deficiency may exacerbate the loss of genomic integrity due to faulty repair by BLM-deficient BRCA1/NBS1 complexes. In BS cells with wild-type p53 function, these complexes assemble after a delay, perhaps owing to time required to recruit a substitute helicase. In cells with defective p53 function, however, these complexes assemble rapidly, but may lack the substitute helicase and repair may be faulty. This model (Fig. 9) is consistent with recent findings showing that BLM and p53 deficiencies synergize to up-regulate homologous recombination (Sengupta et al., 2003).
Materials and methods
Cells
NHFs WI-38, HCA2, and 82-6 have been described (Kim et al., 1999; Itahana et al., 2002). BS fibroblasts HG2654 and HG1013 (provided by J. German, Weill Medical College of Cornell University, New York, NY) were cultured as previously described (Bischof et al. 2001b). HG2654 (blmash) is homozygous for a 6-bp deletion and 7-bp insertion. HG1013 has A inserted at nt 1610 and C→T at nt 2328. NBS1-deficient fibroblasts GM07166A (from the Coriell Institute for Medical Research, Camden, NJ) were immortalized with hTERT as previously described (Kim et al., 1999). BRCA1-mutated cells HCC1937 (from the American Type Culture Collection) were cultured as previously described (Scully et al., 1999). Jurkat cells (provided by T. Kohwi-Shigematsu, Lawrence Berkeley National Laboratory) were cultured in RPMI plus 10% fetal calf serum.
Retroviruses
We produced amphotropic retroviruses and infected cells as previously described (Kim et al., 1999; Itahana et al., 2002). We cloned the wild-type BLM cDNA into pMSCVhyg (CLONTECH Laboratories, Inc.) and HM mutant cDNA (Neff et al., 1999) into pBabe-puro (Morgenstern and Land, 1990).
BLM complemented BSFs and HM-expressing NHFs
hTERT-expressing cells were infected on three consecutive days with control (insertless), BLM-expressing, or BLM-HM–expressing viruses. To select for low expressers, we cultured cells in antibiotics (0.75 μg/ml puromycin or 100 μg/ml hygromycin) for 1–2 d, allowed recovery for 14–21 d, and then selected again for 7 d.
Synchronization
For G1 synchronization, we seeded cells in serum-containing medium and, 24 h later, shifted them to medium containing 0.2% serum for 72–96 h. We then added medium containing 10% serum for 4–6 h. To synchronize cells at the G1/S boundary and accommodate the different doubling times of NHFs and BSFs, we serum deprived (0.2%) the cells for 72–96 h, cultured for 8 (NHF and BSF + BLM) or 12 h (BSF and NHF + HM) in 10% serum, and then cultured in medium containing 10% serum and either 2 μg/ml aphidicolin or 2 mM HU for 12 (NHF and BSF + BLM) or 16 h (BSF and NHF + HM). To release from the G1/S block, cells were washed extensively and cultured in drug-free, serum-containing medium for 1 (NHF and BSF + BLM) or 3 h (BSF and NHF + HM) before addition of genotoxic agents. To synchronize cells with mimosine, we cultured cells in low serum (0.2%) for 72 h, added 10% serum for 6 h, and then added 0.25 mM mimosine for 16 h. The cells were washed extensively and then cultured in drug-free serum containing medium for 3 h before addition of genotoxic agents.
Antibodies
We used affinity-purified rabbit (Neff et al., 1999) or goat anti-BLM (C-18 and K-20; Santa Cruz Biotechnology, Inc.), rabbit (Neomarkers) or mouse (ab-1; Oncogene Research Products) anti-BRCA1, rabbit anti-NBS1 (Novus Biologicals; Oncogene Research Products), mouse (PG-M3; Santa Cruz Biotechnology, Inc.) or rabbit (Chemicon) anti-PML, mouse anti-actin (Chemicon), mouse anti-p53, and mouse anti-tubulin (ab-1; Oncogene Research Products) antibodies. Rabbit anti-γH2AX was previously described (Burma et al., 2001). We detected BrdU incorporation by immunofluorescence, using a commercially available kit (Roche).
Immunofluorescence
We seeded 2 × 104 cells in four-well chamber slides, fixed with ice-cold methanol for 20 min at −20°C or 4% paraformaldehyde for 5 min at room temperature, permeabilized with 0.5% Triton X-100 for 5 min, and blocked with 10% serum from the secondary antibody species. We incubated cells in primary antibodies for 1 h at room temperature or overnight at 4°C and in fluorochrome-conjugated secondary antibodies for 0.5–1 h. To visualize DNA, the last wash contained 0.4 μg/ml DAPI. We mounted slides in Vectashield and viewed by epifluorescence. Images were captured with a CCD camera and merged using Adobe Photoshop®.
Drug treatments and apoptosis assay
We treated cells with etoposide, bleomycin, or HU (Sigma-Aldrich) for 1 h at the indicated concentrations, washed, and cultured in drug-free medium for 6 h. We detected apoptosis by collapse of mitochondrial membrane potential using the MitoCapture reagent (Biovision) (Kaminker et al., 2001), morphology of nuclei after DAPI staining, and caspase-3 activity using a commercially available kit (Oncogene Research Products). In brief, cell lysates were incubated with a synthetic caspase-3 substrate conjugated to a colorimetric tag in the presence or absence of the caspase-3 inhibitor DEVD-CHO for 5 h. Caspase-3 activity is proportional to optical density at 405 nm. The graph shows activity in arbitrary units.
Western analysis
We prepared protein lysates in 5% SDS, separated 30 μg protein using 4–15% SDS-PAGE, transferred the proteins to a nylon membrane, blocked, and incubated with primary and secondary antibodies, as previously described (Bischof et al., 2001b). We detected secondary antibodies by ECL (Amersham Biosciences).
Immunoprecipitation
We employed a protocol previously described (Yannone et al., 2001). We precleared 500–1,000 μg cell lysate with ultra-link beads (Pierce Chemical Co.), incubated the supernatant with either goat anti-IgG or anti-BLM antibodies overnight at 4°C, and clarified the supernatants by centrifugation. We incubated the supernatants with fresh ultra-link beads, washed the beads, and released the bound proteins using Laemmli sample buffer. We separated the released proteins by 4–15% SDS-PAGE, as previously described (Bischof et al., 2001b).
Online supplemental material
The supplemental material (Figs. S1–S5) is available at http://www.jcb.org/cgi/content/full/jcb.200304016/DC1. The supplemental figures show that low dose HU causes apoptosis in BSFs (Fig. S1), unmodified BSFs behave similarly to hTERT-immortalized BSFs (Fig. S2), BLM localizes to g-H2AX foci in BLM-complemented cells (Fig. S3), BLM localizes to g-H2AX foci after replication stress in BRCA1 mutant or NBS1-deficient cells (Fig. S4), and BRCA1 and NBS1 foci form in BS cells after low dose HU or mimosine synchronization (Fig. S5).
Supplemental Material
Acknowledgments
We thank Drs. James German for the BS cells, Robert Weinberg (Whitehead Institute, Cambridge, MA) for the hTERT cDNA, Nathan Ellis (Memorial Sloan-Kettering Cancer Center, New York, NY) for the wild-type and mutant BLM cDNAs, and Junko Oshima (University of Washington, Seattle, WA) and James Smith (University of Texas, San Antonio, TX) for cells.
This work was supported by National Institutes of Health (NIH) research grant AG11658 (J. Campisi), NIH training grant AG00226, and Californian Breast Cancer Research Program award 8FB-0148 (A.R. Davalos).
The online version of this article includes supplemental material.
Abbreviations used in this paper: BS, Bloom syndrome; BSF, Bloom syndrome fibroblast; HM, helicase mutant; HU, hydroxyurea; NHF, normal human fibroblast; PML, promyelocytic leukemia protein.
References
- Ababou, M., S. Dutertre, Y. Lecluse, R. Onclercq, B. Chatton, and M. Amor-Gueret. 2000. ATM-dependent phosphorylation and accumulation of endogenous BLM protein in response to ionizing radiation. Oncogene. 19:5955–5963. [DOI] [PubMed] [Google Scholar]
- Ababou, M., V. Dumaire, Y. Lecluse, and M. Amor-Gueret. 2002. Bloom's syndrome protein response to ultraviolet-C radiation and hydroxyurea-mediated DNA synthesis inhibition. Oncogene. 21:2079–2088. [DOI] [PubMed] [Google Scholar]
- Ames, B.N. 1998. Micronutrients prevent cancer and delay aging. Toxicol. Lett. 102–103:5–18. [DOI] [PubMed]
- Bernstein, C., H. Bernstein, C.M. Payne, and H. Garewal. 2002. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat. Res. 511:145–178. [DOI] [PubMed] [Google Scholar]
- Bischof, O., S. Galande, F. Farzaneh, T. Kohwi-Shigematsu, and J. Campisi. 2001. a. Selective cleavage of BLM, the Bloom syndrome protein, during apoptotic cell death. J. Biol. Chem. 276:12068–12075. [DOI] [PubMed] [Google Scholar]
- Bischof, O., S.H. Kim, J. Irving, S. Beresten, N.A. Ellis, and J. Campisi. 2001. b. Regulation and localization of the Bloom syndrome protein in response to DNA damage. J. Cell Biol. 153:367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar, A.G., M. Ouellette, M. Frolkis, S.E. Holt, C.P. Chiu, G.B. Morin, C.B. Harley, J.W. Shay, S. Lichtsteiner, and W.E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science. 279:349–352. [DOI] [PubMed] [Google Scholar]
- Burma, S., B.P. Chen, M. Murphy, A. Kurimasa, and D.J. Chen. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276:42462–42467. [DOI] [PubMed] [Google Scholar]
- Celeste, A., S. Petersen, P.J. Romanienko, O. Fernandez-Capetillo, H.T. Chen, O.A. Sedelnikova, B. Reina-San-Martin, V. Coppola, E. Meffre, M.J. Difilippantonio, et al. 2002. Genomic instability in mice lacking histone H2AX. Science. 296:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., S. Huang, A. Davalos, R. Schiestl, J. Campisi, and J. Oshima. 2003. WRN, the protein deficient in Werner syndrome, plays a critical structural role in optimizing DNA repair. Aging Cell. 2:191–199. [DOI] [PubMed] [Google Scholar]
- Chester, N., F. Kuo, C. Kozak, C.D. O'Hara, and P. Leder. 1998. Stage-specific apoptosis, developmental delay, and embryonic lethality in mice homozygous for a targeted disruption in the murine Bloom's syndrome gene. Genes Dev. 12:3382–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal, S., Q. Gao, E.J. Androphy, and V. Band. 1996. Mutational analysis of human papillomavirus type 16 E6 demonstrates that p53 degradation is necessary for immortalization of mammary epithelial cells. J. Virol. 70:683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amours, D., and S.P. Jackson. 2002. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat. Rev. Mol. Cell Biol. 3:317–327. [DOI] [PubMed] [Google Scholar]
- Donehower, L.A. 1996. The p53-deficient mouse: a model for basic and applied cancer studies. Semin. Cancer Biol. 7:269–278. [DOI] [PubMed] [Google Scholar]
- Dutertre, S., M. Ababou, R. Onclercq, J. Delic, B. Chatton, C. Jaulin, and M. Amor-Gueret. 2000. Cell cycle regulation of the endogenous wild type Bloom's syndrome DNA helicase. Oncogene. 19:2731–2738. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo, O., A. Celeste, and A. Nussenzweig. 2003. Focusing on foci: H2AX and the recruitment of DNA-damage response factors. Cell Cycle. 2:426–427. [PubMed] [Google Scholar]
- Franchitto, A., and P. Pichierri. 2002. Bloom's syndrome protein is required for correct relocalization of RAD50/MRE11/NBS1 complex after replication fork arrest. J. Cell Biol. 157:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei, C., and S.M. Gasser. 2000. a. RecQ-like helicases: the DNA replication checkpoint connection. J. Cell Sci. 113:2641–2646. [DOI] [PubMed] [Google Scholar]
- Frei, C., and S.M. Gasser. 2000. b. The yeast Sgs1p helicase acts upstream of Rad53p in the DNA replication checkpoint and colocalizes with Rad53p in S-phase-specific foci. Genes Dev. 14:81–96. [PMC free article] [PubMed] [Google Scholar]
- Furuta, T., H. Takemura, Z.Y. Liao, G.J. Aune, C. Redon, O.A. Sedelnikova, D.R. Pilch, E.P. Rogakou, A. Celeste, H.T. Chen, et al. 2003. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA-double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J. Biol. Chem. 278:20303–20312. [DOI] [PubMed] [Google Scholar]
- German, J. 1969. Bloom's syndrome. I. Genetical and clinical observations in the first twenty-seven patients. Am. J. Hum. Genet. 21:196–227. [PMC free article] [PubMed] [Google Scholar]
- German, J. 1993. Bloom syndrome: a Mendelian prototype of somatic mutational disease. Medicine (Baltimore). 72:393–406. [PubMed] [Google Scholar]
- German, J. 1997. Bloom's syndrome. XX. The first 100 cancers. Cancer Genet. Cytogenet. 93:100–106. [DOI] [PubMed] [Google Scholar]
- Gottifredi, V., S. Shieh, Y. Taya, and C. Prives. 2001. p53 accumulates but is functionally impaired when DNA synthesis is blocked. Proc. Natl. Acad. Sci. USA. 98:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D.R., and J.C. Reed. 1998. Mitochondria and apoptosis. Science. 281:1309–1312. [DOI] [PubMed] [Google Scholar]
- Hickson, I.D. 2003. RecQ helicases: caretakers of the genome. Nat. Rev. Cancer. 3:169–178. [DOI] [PubMed] [Google Scholar]
- Hickson, I.D., S.L. Davies, J.L. Li, N.C. Levitt, P. Mohaghegh, P.S. North, and L. Wu. 2001. Role of the Bloom's syndrome helicase in maintenance of genome stability. Biochem. Soc. Trans. 29:201–204. [DOI] [PubMed] [Google Scholar]
- Imamura, O., K. Fujita, A. Shimamoto, H. Tanabe, S. Takeda, Y. Furuichi, and T. Matsumoto. 2001. Bloom helicase is involved in DNA surveillance in early S phase in vertebrate cells. Oncogene. 20:1143–1151. [DOI] [PubMed] [Google Scholar]
- Itahana, K., G.P. Dimri, E. Hara, Y. Itahana, Y. Zou, P.Y. Desprez, and J. Campisi. 2002. A role for p53 in maintaining and establishing the quiescence growth arrest in human cells. J. Biol. Chem. 277:18206–18214. [DOI] [PubMed] [Google Scholar]
- Jiang, X.R., G. Jimenez, E. Chang, M. Frolkis, B. Kusler, M. Sage, M. Beeche, A.G. Bodnar, G.M. Wahl, T.D. Tlsty, and C.P. Chiu. 1999. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 21:111–114. [DOI] [PubMed] [Google Scholar]
- Kaminker, P.G., S.H. Kim, R.D. Taylor, Y. Zebarjadian, W.D. Funk, G.B. Morin, P. Yaswen, and J. Campisi. 2001. TANK2, a new TRF1-associated poly(ADP-ribose) polymerase, causes rapid induction of cell death upon overexpression. J. Biol. Chem. 276:35891–35899. [DOI] [PubMed] [Google Scholar]
- Kaufmann, W.K., and P.E. Kies. 1998. DNA signals for G2 checkpoint response in diploid human fibroblasts. Mutat. Res. 400:153–167. [DOI] [PubMed] [Google Scholar]
- Kim, S.H., P. Kaminker, and J. Campisi. 1999. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 23:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, J., H. Tauchi, S. Sakamoto, A. Nakamura, K. Morishima, S. Matsuura, T. Kobayashi, K. Tamai, K. Tanimoto, and K. Komatsu. 2002. NBS1 localizes to gamma-H2AX foci through interaction with the FHA/BRCT domain. Curr. Biol. 12:1846–1851. [DOI] [PubMed] [Google Scholar]
- Koc, A., L.J. Wheeler, C.K. Mathews, and G.F. Merrill. 2003. Replication-independent MCB gene induction and deoxyribonucleotide accumulation at G1/S in Saccharomyces cerevisiae. J. Biol. Chem. 278:9345–9352. [DOI] [PubMed] [Google Scholar]
- Liao, S., J. Graham, and H. Yan. 2000. The function of Xenopus Bloom's syndrome protein homolog (xBLM) in DNA replication. Genes Dev. 14:2570–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard, D.B., and L. Guarente. 2000. Nijmegen breakage syndrome disease protein and MRE11 at PML nuclear bodies and meiotic telomeres. Cancer Res. 60:2331–2334. [PubMed] [Google Scholar]
- Lu, X., G. Lozano, and L.A. Donehower. 2003. Activities of wildtype and mutant p53 in suppression of homologous recombination as measured by a retroviral vector system. Mutat. Res. 522:69–83. [DOI] [PubMed] [Google Scholar]
- Martin, G.M. 1997. The Werner mutation: does it lead to a “public” or “private” mechanism of aging? Mol. Med. 3:356–358. [PMC free article] [PubMed] [Google Scholar]
- Maser, R.S., and R.A. DePinho. 2003. Take care of your chromosomes lest cancer take care of you. Cancer Cell. 3:4–6. [DOI] [PubMed] [Google Scholar]
- Matas, D., A. Sigal, P. Stambolsky, M. Milyavsky, L. Weisz, D. Schwartz, N. Goldfinger, and V. Rotter. 2001. Integrity of the N-terminal transcription domain of p53 is required for mutant p53 interference with drug-induced apoptosis. EMBO J. 20:4163–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara, M., S. Erster, A. Zaika, O. Petrenko, T. Chittenden, P. Pancoska, and U.M. Moll. 2003. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell. 11:577–590. [DOI] [PubMed] [Google Scholar]
- Miura, M., H. Watanabe, T. Sasaki, K. Tatsumi, and M. Muto. 2001. Dynamic changes in subnuclear NP95 location during the cell cycle and its spatial relationship with DNA replication foci. Exp. Cell Res. 263:202–208. [DOI] [PubMed] [Google Scholar]
- Morgenstern, J.P., and H. Land. 1990. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 18:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, N.F., N.A. Ellis, T.Z. Ye, J. Noonan, K. Huang, M. Sanz, and M. Proytcheva. 1999. The DNA helicase activity of BLM is necessary for the correction of the genomic instability of Bloom syndrome cells. Mol. Biol. Cell. 10:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda, F., M. Seki, A. Miyajima, and T. Enomoto. 2000. Elevation of sister chromatid exchange in Saccharomyces cerevisiae sgs1 disruptants and the relevance of the disruptants as a system to evaluate mutations in Bloom's syndrome gene. Mutat. Res. 459:203–209. [DOI] [PubMed] [Google Scholar]
- Paull, T.T., E.P. Rogakou, V. Yamazaki, C.U. Kirchgessner, M. Gellert, and W.M. Bonner. 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10:886–895. [DOI] [PubMed] [Google Scholar]
- Robles, S.J., and G.R. Adami. 1998. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 16:1113–1123. [DOI] [PubMed] [Google Scholar]
- Rubbi, C.P., and J. Milner. 2003. p53 is a chromatin accessibility factor for nucleotide excision repair of DNA damage. EMBO J. 22:975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, K.M., A.C. Phillips, and K.H. Voudsen. 2001. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 13:332–337. [DOI] [PubMed] [Google Scholar]
- Scully, R., and D.M. Livingston. 2000. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature. 408:429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully, R., S. Ganesan, K. Vlasakova, J. Chen, M. Socolovsky, and D.M. Livingston. 1999. Genetic analysis of BRCA1 function in a defined tumor cell line. Mol. Cell. 4:1093–1099. [DOI] [PubMed] [Google Scholar]
- Sengupta, S., S.P. Linke, R. Pedeux, Q. Yang, J. Farnsworth, S.H. Garfield, K. Valerie, J.W. Shay, N.A. Ellis, B. Wasylyk, and C.C. Harris. 2003. BLM helicase-dependent transport of p53 to sites of stalled DNA replication forks modulates homologous recombination. EMBO J. 22:1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, Y.L., A.J. Bodero, and P.L. Chen. 2003. NFBD1, a novel nuclear protein with signature motifs of FHA and BRCT, and an internal 41-amino acid repeat sequence, is an early participant in DNA damage response. J. Biol. Chem. 278:6323–6329. [DOI] [PubMed] [Google Scholar]
- Sinha, B.K. 1995. Topoisomerase inhibitors. A review of their therapeutic potential in cancer. Drugs. 49:11–19. [DOI] [PubMed] [Google Scholar]
- Stewart, E., C.R. Chapman, F. Al-Khodairy, A.M. Carr, and T. Enoch. 1997. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 16:2682–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto, R., and W.S. El-Deiry. 2001. DNA replication blockade impairs p53-transactivation. Proc. Natl. Acad. Sci. USA. 98:781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi, H., S. Matsuura, J. Kobayashi, S. Sakamoto, and K. Komatsu. 2002. Nijmegen breakage syndrome gene, NBS1, and molecular links to factors for genome stability. Oncogene. 21:8967–8980. [DOI] [PubMed] [Google Scholar]
- Toouli, C.D., L.I. Huschtscha, A.A. Neumann, J.R. Noble, L.M. Colgin, B. Hukku, and R.R. Reddel. 2002. Comparison of human mammary epithelial cells immortalized by simian virus 40 T-Antigen or by the telomerase catalytic subunit. Oncogene. 21:128–139. [DOI] [PubMed] [Google Scholar]
- van Brabant, A.J., R. Stan, and N.A. Ellis. 2000. DNA helicases, genomic instability, and human genetic disease. Annu. Rev. Genomics Hum. Genet. 1:409–459. [DOI] [PubMed] [Google Scholar]
- Vaziri, H., J.A. Squire, T.K. Pandita, G. Bradley, R.M. Kuba, H. Zhang, S. Gulyas, R.P. Hill, G.P. Nolan, and S. Benchimol. 1999. Analysis of genomic integrity and p53-dependent G1 checkpoint in telomerase-induced extended-life-span human fibroblasts. Mol. Cell. Biol. 19:2373–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennos, E.M., and W.D. James. 1995. Rothmund-Thomson syndrome. Dermatol. Clin. 13:143–150. [PubMed] [Google Scholar]
- Wang, X.W., A. Tseng, N.A. Ellis, E.A. Spillare, S.P. Linke, A.I. Robles, H. Seker, Q. Yang, P. Hu, S. Beresten, et al. 2001. Functional interaction of p53 and BLM DNA helicase in apoptosis. J. Biol. Chem. 276:32948–32955. [DOI] [PubMed] [Google Scholar]
- Wang, Y., D. Cortez, P. Yazdi, N. Neff, S.J. Elledge, and J. Qin. 2000. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 14:927–939. [PMC free article] [PubMed] [Google Scholar]
- Ward, I.M., and J. Chen. 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276:47759–47762. [DOI] [PubMed] [Google Scholar]
- Xu, X., and D.F. Stern. 2003. NFBD1/KIAA0170 is a chromatin-associated protein involved in DNA damage signaling pathways. J. Biol. Chem. 278:8795–8803. [DOI] [PubMed] [Google Scholar]
- Yamagata, K., J. Kato, A. Shimamoto, M. Goto, Y. Furuichi, and H. Ikeda. 1998. Bloom's and Werner's syndrome genes suppress hyperrecombination in yeast sgs1 mutant: implication for genomic instability in human diseases. Proc. Natl. Acad. Sci. USA. 95:8733–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankiwski, V., R.A. Marciniak, L. Guarente, and N.F. Neff. 2000. Nuclear structure in normal and Bloom syndrome cells. Proc. Natl. Acad. Sci. USA. 97:5214–5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannone, S.M., S. Roy, D.W. Chan, M.B. Murphy, S. Huang, J. Campisi, and D.J. Chen. 2001. Werner syndrome protein is regulated and phosphorylated by DNA-dependent protein kinase. J. Biol. Chem. 276:38242–38248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.