Abstract
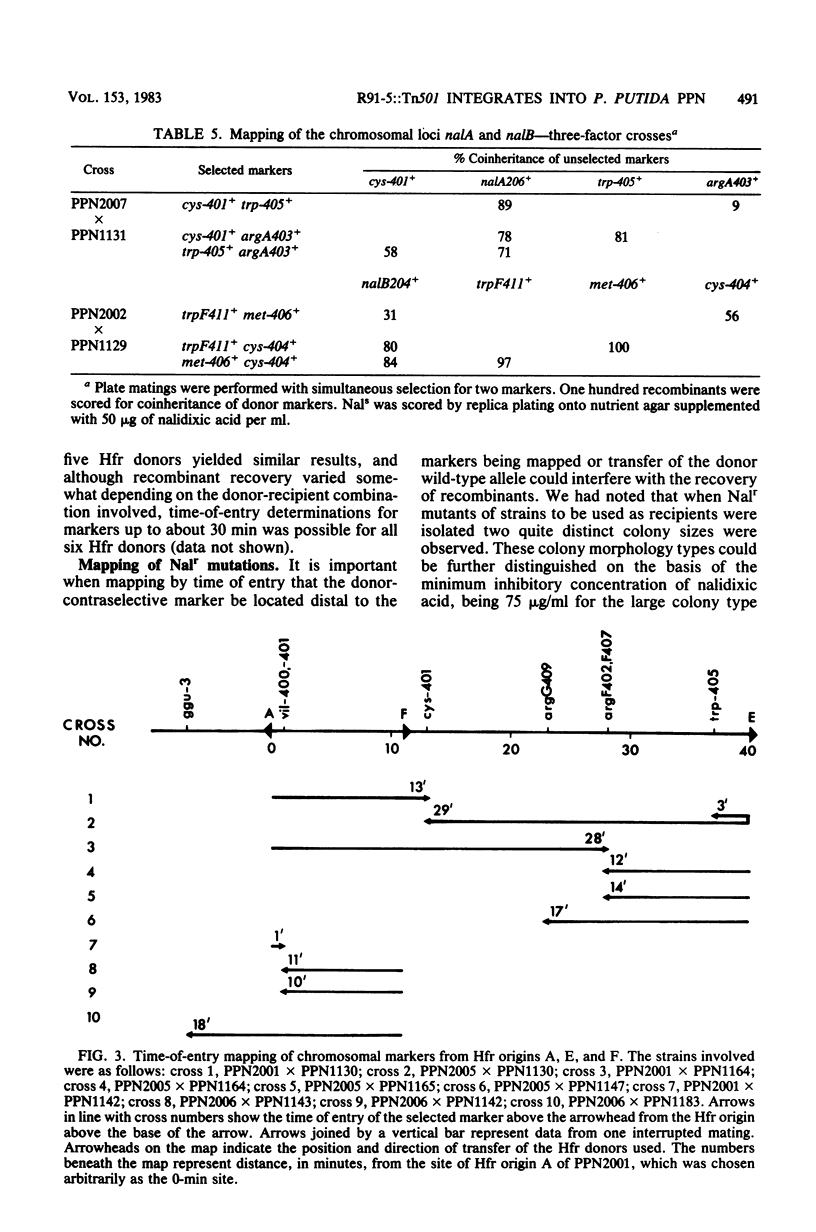
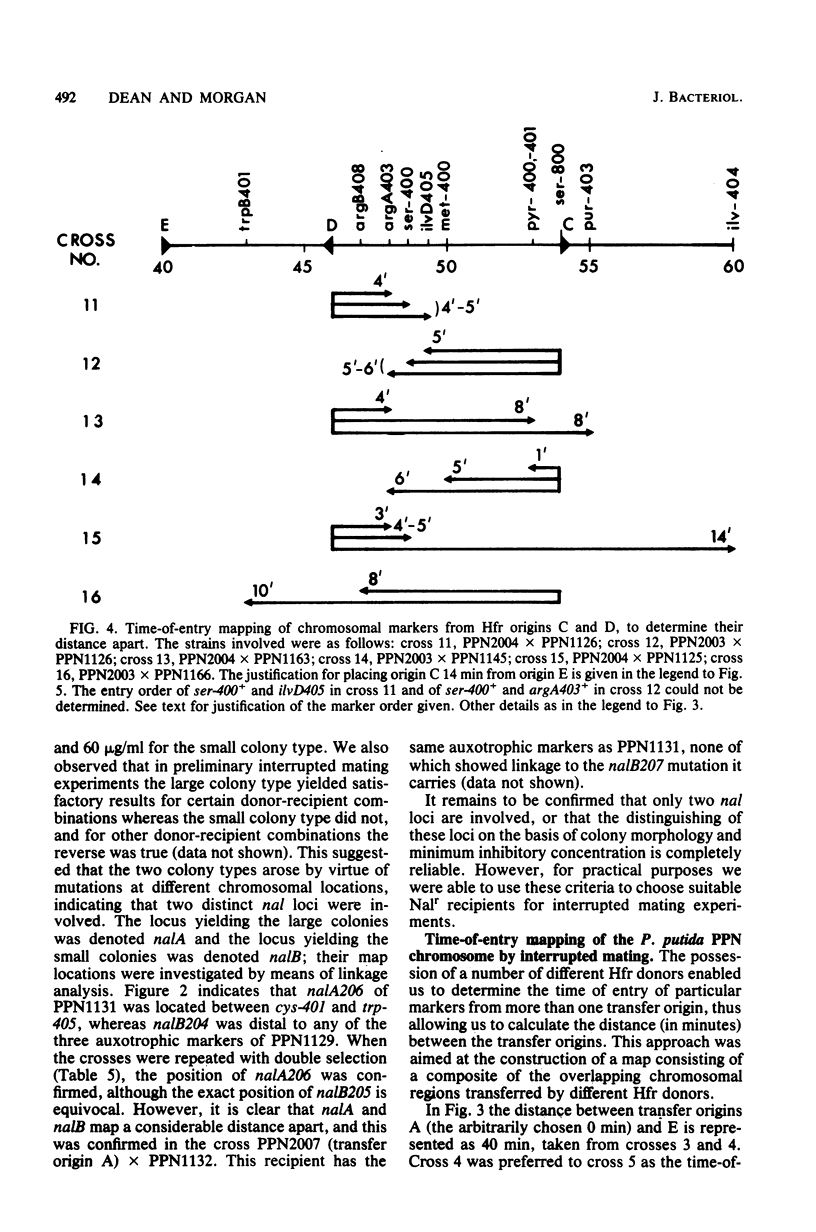
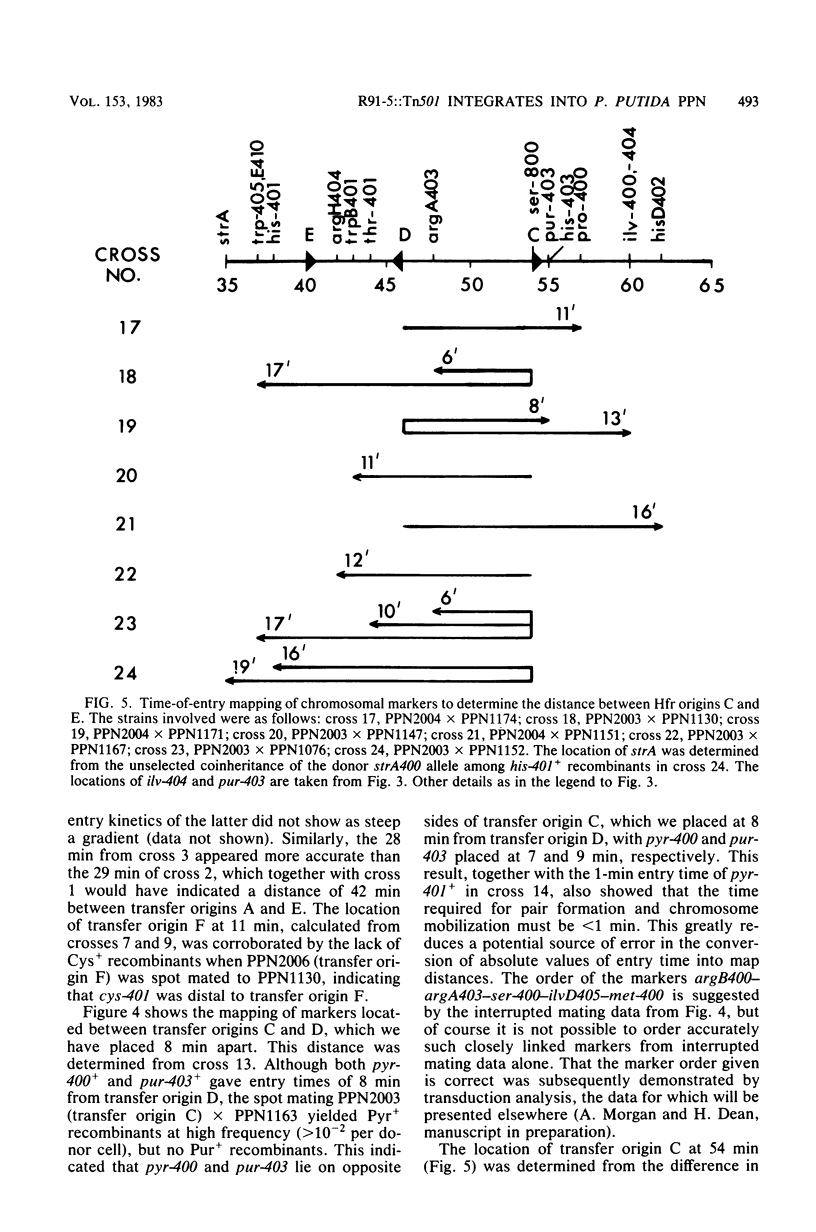
Derivatives of the Pseudomonas aeruginosa plasmid R91-5, loaded with the transposon Tn501, were transferred to P. putida PPN. Over 90% of exconjugants, which arose at a frequency of ca. 10(-6) per donor cell, exhibited high-frequency (greater than 10(-2) per donor cell) polarized transfer of chromosomal markers. In one instance it was demonstrated by transduction that the plasmid had been inserted into a gene required for serine biosynthesis. The integrated nature of the plasmid in this and other P. putida (R91-5::Tn501) derivatives was supported by the failure to detect covalently closed circular DNA in these strains. The transfer origins of six different Hfr donors have been characterized genetically, and time-of-entry kinetics obtained from interrupted matings have enabled the construction of a circular genetic map 103 min in length and containing 35 markers. The genetic map of P. putida PPN shows significant differences in marker order to that of P. aeruginosa PAO.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedik M., Fennewald M., Shapiro J. Transposition of a beta-lactamase locus from RP1 into Pseudomonas putida degradative plasmids. J Bacteriol. 1977 Feb;129(2):809–814. doi: 10.1128/jb.129.2.809-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan J. M., Helman Z. M., Krishnapillai V. Transfer-deficient mutants of the narrow-host-range plasmid R91-5 of Pseudomonas aeruginosa. J Bacteriol. 1978 Sep;135(3):911–919. doi: 10.1128/jb.135.3.911-919.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus C. F., Gunsalus I. C. Transduction and the clustering of genes in fluorescent Pseudomonads. Proc Natl Acad Sci U S A. 1968 May;60(1):168–175. doi: 10.1073/pnas.60.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Plasmids in Pseudomonas. Annu Rev Genet. 1976;10:7–30. doi: 10.1146/annurev.ge.10.120176.000255. [DOI] [PubMed] [Google Scholar]
- Chandler P. M., Krishnapillai V. Characterization of Pseudomonas aeruginosa derepressed R-plasmids. J Bacteriol. 1977 May;130(2):596–603. doi: 10.1128/jb.130.2.596-603.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P. M., Krishnapillai V. Phenotypic properties of R factors of Pseudomonas aeruginosa: R factors transferable only in Pseudomonas aeruginosa. Genet Res. 1974 Jun;23(3):251–257. doi: 10.1017/s0016672300014907. [DOI] [PubMed] [Google Scholar]
- Haas D., Watson J., Krieg R., Leisinger T. Isolation of an Hfr donor of Pseudomonas aeruginosa PAO by insertion of the plasmid RP1 into the tryptophan synthase gene. Mol Gen Genet. 1981;182(2):240–244. doi: 10.1007/BF00269664. [DOI] [PubMed] [Google Scholar]
- Harayama S., Tsuda M., Iino T. High frequency mobilization of the chromosome of Escherichia coli by a mutant of plasmid RP4 temperature-sensitive for maintenance. Mol Gen Genet. 1980;180(1):47–56. doi: 10.1007/BF00267351. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Isolation and characterization of an R' plasmid in Pseudomonas aeruginosa. J Bacteriol. 1978 Mar;133(3):1078–1082. doi: 10.1128/jb.133.3.1078-1082.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapillai V. A novel transducing phage. Its role in recognition of a possible new host-controlled modification system in Pseudomonas aeruginosa. Mol Gen Genet. 1972;114(2):134–143. doi: 10.1007/BF00332784. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V. DNA insertion mutagenesis in a Pseudomonas aeruginosa R plasmid. Plasmid. 1979 Apr;2(2):237–246. doi: 10.1016/0147-619x(79)90042-8. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V., Royle P., Lehrer J. Insertions of the transposon Tn1 into the Pseudomonas aeruginosa chromosome. Genetics. 1981 Mar-Apr;97(3-4):495–511. doi: 10.1093/genetics/97.3-4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesinger T., Haas D., Hegarty M. P. Indospicine as an arginine antagonist in Escherichia coli and Pseudomonas aeruginosa. Biochim Biophys Acta. 1972 Mar 14;262(2):214–219. doi: 10.1016/0005-2787(72)90235-3. [DOI] [PubMed] [Google Scholar]
- Morgan A. F. Isolation and characterization of Pseudomonas aeruginosa R' plasmids constructed by interspecific mating. J Bacteriol. 1982 Feb;149(2):654–661. doi: 10.1128/jb.149.2.654-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D., Broda P. R plasmids R91 and R91a from Pseudomonas aeruginosa share only the gene for carbenicillin resistance. J Bacteriol. 1979 Jun;138(3):1036–1037. doi: 10.1128/jb.138.3.1036-1037.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Royle P. L., Matsumoto H., Holloway B. W. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1981 Jan;145(1):145–155. doi: 10.1128/jb.145.1.145-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M., Richmond M. H. Characterization of a translocation unit encoding resistance to mercuric ions that occurs on a nonconjugative plasmid in Pseudomonas aeruginosa. J Bacteriol. 1977 Mar;129(3):1227–1233. doi: 10.1128/jb.129.3.1227-1233.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Holloway B. W. A mutant sex factor of Pseudomonas aeruginosa. Genet Res. 1972 Feb;19(1):91–108. doi: 10.1017/s0016672300014294. [DOI] [PubMed] [Google Scholar]
- Vicente M., Pedro M. A., Torrontegui G., Cánovas J. L. The uptake of glucose and gluconate by Pseudomonas putida. Mol Cell Biochem. 1975 Apr 30;7(1):59–64. doi: 10.1007/BF01732164. [DOI] [PubMed] [Google Scholar]
- Watson J. M., Holloway B. W. Chromosome mapping in Pseudomonas aeruginosa PAT. J Bacteriol. 1978 Mar;133(3):1113–1125. doi: 10.1128/jb.133.3.1113-1125.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis L. The genetics of dissimilarity pathways in Pseudomonas. Annu Rev Microbiol. 1975;29:505–524. doi: 10.1146/annurev.mi.29.100175.002445. [DOI] [PubMed] [Google Scholar]



