Abstract
We have studied the formation of different types of cell matrix adhesions in cells that bind to fibronectin via either α5β1 or αvβ3. In both cases, cell adhesion to fibronectin leads to a rapid decrease in RhoA activity. However, α5β1 but not αvβ3 supports high levels of RhoA activity at later stages of cell spreading, which are associated with a translocation of focal contacts to peripheral cell protrusions, recruitment of tensin into fibrillar adhesions, and fibronectin fibrillogenesis. Expression of an activated mutant of RhoA stimulates αvβ3-mediated fibrillogenesis. Despite the fact that α5β1-mediated adhesion to the central cell-binding domain of fibronectin supports activation of RhoA, other regions of fibronectin are required for the development of α5β1-mediated but not αvβ3-mediated focal contacts. Using chimeras of β1 and β3 subunits, we find that the extracellular domain of β1 controls RhoA activity. By expressing both β1 and β3 at high levels, we show that β1-mediated control of the levels of β3 is important for the distribution of focal contacts. Our findings demonstrate that the pattern of fibronectin receptors expressed on a cell dictates the ability of fibronectin to stimulate RhoA-mediated organization of cell matrix adhesions.
Keywords: integrin; Rho–GTPase; fibronectin; cell matrix adhesion; matrix assembly
Introduction
Cell adhesion is indispensable for embryonic development and tissue function and wound healing in the adult (Hynes and Zhao, 2000). Adhesive connections between cells are essential for the maintenance of tissue integrity and polarity and occur in three distinct structures termed adherens junctions, desmosomes, and tight junctions (Gumbiner, 1996). In adherens junctions, the homophilic interaction of clustered cadherins connects cells with each other, and probably via their connection with the cytoskeleton, cadherins can act as signaling molecules (Vleminckx and Kemler, 1999). An analogous process but with different players takes place in the adhesive contacts between cells and the ECM. In cell matrix adhesions, clustered integrins (each made up of a noncovalently linked α and β subunit) bind to ECM components via their globular head domains and connect to the actin cytoskeleton via adaptor proteins that bind their short cytoplasmic tails (van der Flier and Sonnenberg, 2001; Hynes, 2002). Cell matrix adhesions also act as signaling units by their capacity to organize the actin cytoskeleton and to accumulate various signaling intermediates (Geiger et al., 2001).
It has been reported that integrin-mediated cell adhesion can inhibit the formation of adherens junctions (Monier-Gavelle and Duband, 1997; Weaver et al., 1997; Gimond et al., 1999; von Schlippe et al., 2000). However, the mechanism by which they do so remains poorly understood. One potential explanation may be the fact that the formation and maintenance of adherens junctions are regulated by small GTPases of the Rho family (Fukata and Kaibuchi, 2001) and that integrins modulate the activity and localization of Rho–GTPases and their effector proteins (Schwartz and Shattil, 2000). Rho–GTPases cycle between an inactive, GDP-bound state and an active, GTP-bound state. In the latter, they are able to bind and activate a variety of effector proteins which modulate the organization of the actin cytoskeleton (Hall and Nobes, 2000). Although integrin-mediated adhesion regulates the actions of Rho–GTPases, which in turn affect cell–cell adhesion, the converse process can also take place: the activity of Rho–GTPases can be modulated by cadherins in adherens junctions (Fukata and Kaibuchi, 2001), and in turn Rho–GTPases regulate the actin cytoskeletal dynamics which govern the morphological changes during cell adhesion and spreading on ECM. Cdc42 and Rac1 stimulate the formation of small cell matrix adhesions termed “focal complexes” associated with filopodia (in the case of Cdc42) or lamellipodia (in the case of Rac1) (Nobes and Hall, 1995). On the other hand, RhoA is implicated in the formation of actin stress fibers and the maturation of cell matrix adhesions to large junctional complexes termed “focal contacts” (Ridley and Hall, 1992; Rottner et al., 1999).
Besides mediating adhesion to ECM components, integrins participate in the assembly of an ECM. For instance, the assembly of a fibronectin matrix, an essential structure for cell migration during embryogenesis and wound healing (Hynes, 1990; George et al., 1993), requires integrins (Mosher, 1995; Schwarzbauer and Sechler, 1999), although other nonintegrin receptors are probably equally important (Woods, 2001). Of the integrins, α5β1 is the typical fibronectin receptor involved in matrix assembly (Wu et al., 1993; Zhang et al., 1993), but other integrins can compensate to some extent for its absence (Wennerberg et al., 1996; Yang and Hynes, 1996). Integrin affinity can be regulated, and it has turned out that the ability of β3 integrins to mediate fibronectin matrix assembly depends on their affinity state (Wu et al., 1995, 1996). Regardless of the integrin involved, its connection to the actin cytoskeleton is crucial for fibronectin fibrillogenesis. Fibronectin fibrils coalign with actin stress fibers (Heggeness et al., 1978; Hynes and Destree, 1978), and F-actin–disrupting agents or inhibition of RhoA prevent matrix assembly (Christopher et al., 1997; Zhang et al., 1997; Zhong et al., 1998). RhoA-mediated contractility may generate the tension required for the exposure of self-assembly sites within the first two type III repeats of fibronectin (Hocking et al., 1994; Zhong et al., 1998; Sechler et al., 2001). Indeed, studies on fibronectin fibrillogenesis in live cells demonstrate considerable stretching of fibronectin molecules and unfolding of their globular modules upon association with the cell surface (Ohashi et al., 1999; Baneyx et al., 2001).
It is still unclear why α5β1 is such an efficient mediator of fibronectin fibrillogenesis compared with other integrins. Part of the explanation may lie in the fact that it binds fibronectin with high affinity. On the other hand, one might speculate that different fibronectin-binding integrins generate different degrees of tension/contractility by their effect on the activity of RhoA or their association with the actin cytoskeleton. Fibronectin fibrillogenesis is accompanied by a specific translocation of α5β1 together with the cytoskeletal protein tensin from focal contacts into yet another type of cell matrix adhesions termed “fibrillar adhesions” (Pankov et al., 2000; Zamir et al., 2000). This process may be of critical importance for the assembly of a fibronectin matrix, since a fragment of tensin was found to inhibit both the translocation of α5β1 and fibronectin fibrillogenesis without an effect on focal contacts (Pankov et al., 2000). Thus, the specific association of α5β1 with tensin may partly explain the efficiency with which this integrin supports fibrillogenesis.
In the current study, we have used two independently generated β1 integrin–deficient cell lines in which we ectopically expressed β1 or increased the expression of β3. We established that these cell lines bind to fibronectin via α5β1 or via αvβ3, respectively. We then used these cell lines to investigate the role of β1 and β3 integrins in the regulation of (a) cell–cell adhesion, (b) the activity of Rho–GTPases, (c) the development of the various types of cell matrix adhesions, and (d) fibronectin matrix assembly.
Results
Increased levels of αvβ3 induce spreading and scattering but do not stimulate GTP loading on RhoA in β1-deficient cells
Expression of β1 causes the disruption of cell–cell junctions in β1-deficient GD25 fibroblastoid cells and induces a morphological epithelial-mesenchymal–like transition in β1-deficient GE11 epithelioid cells (Gimond et al., 1999). To investigate if this effect is specific for β1 integrins, we generated GD25 and GE11 cells with increased surface expression levels of the integrin αvβ3 (GDβ3 and GEβ3, respectively). Attempts to generate such a cell line by ectopic expression of the αv subunit were unsuccessful (unpublished data), suggesting that the amount of the β3 subunit was limiting. Indeed, ectopic expression of β3 resulted in increased surface expression of αvβ3 in these cells (see Fig. 2). The morphology of the GDβ3 and GEβ3 cells resembled that of GDβ1 and GEβ1 cells; cell–cell contacts were disrupted and spreading was enhanced compared with the parental cells (Fig. 1 A). Notably, GEβ3 cells (and to a lesser extent GDβ3 cells) were considerably more spread than the β1-expressing cells (cell height at a distance of 4 μm from the nucleus was 6.7 ± 0.72 for GE11, 4.03 ± 0.45 for GEβ1, and 2.36 ± 0.23 for GEβ3 cells).
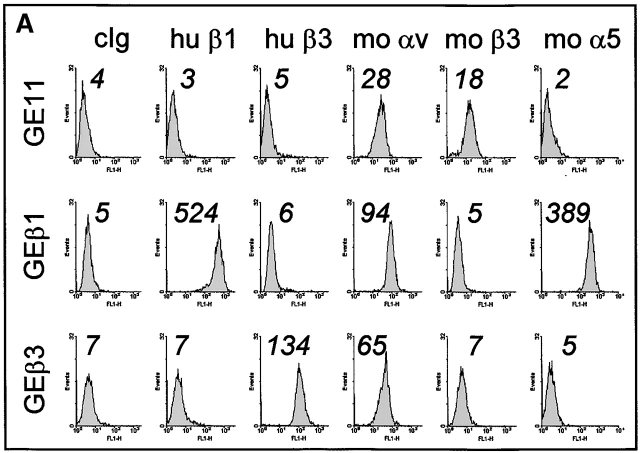
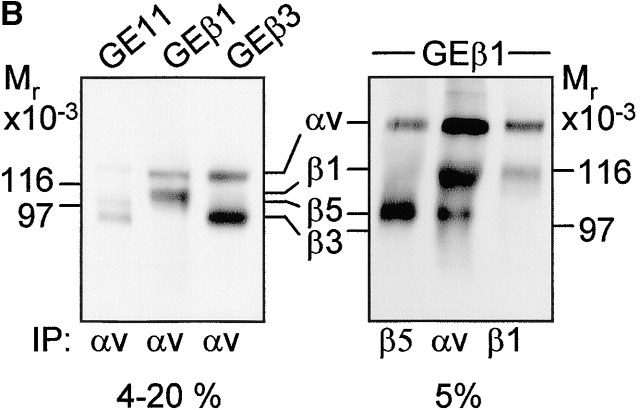
Figure 2.
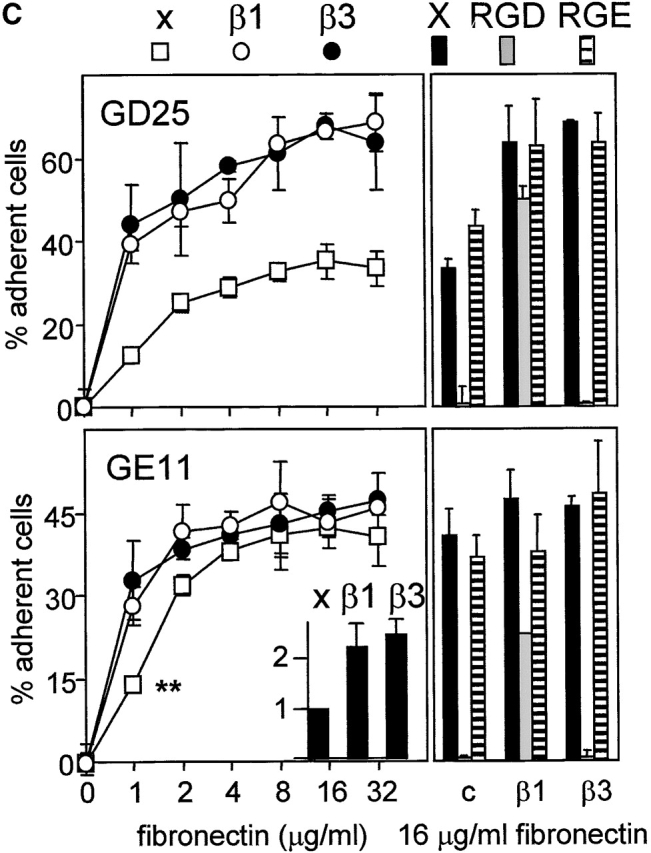
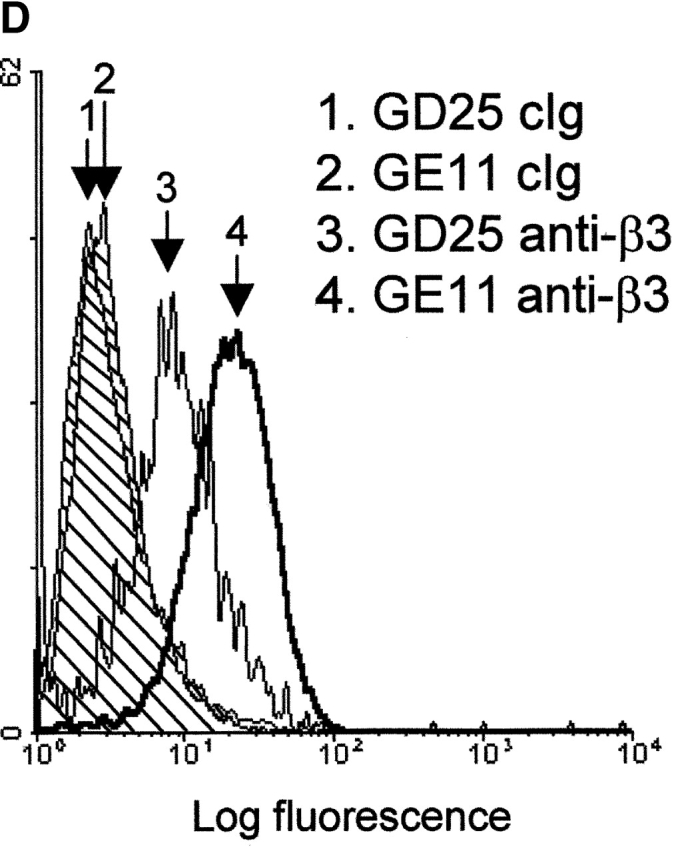
Integrin expression profiles and adhesion to fibronectin. (A) FACS® analysis showing surface expression of the indicated human (hu) and mouse (mo) integrin subunits on GE11, GEβ1, and GEβ3 cells (cIg, control Ig). Numbers indicate mean fluorescence units. (B) Immunoprecipitations of the indicated biotinylated integrin subunits from cell lysates of GE11, GEβ1, and GEβ3 cells were separated on 4–20% (left) or 5% SDS PAGE (right) and subjected to Western blotting using HRPO-labeled streptavidin. (C) The line graphs show adhesion of control (□) or β1- (○) or β3-transduced (•) GD25 or GE11 cells to wells coated with the indicated concentrations of fibronectin. Mean ± SD of one out of three experiments performed in triplicate is shown. **For GE11, GEβ1, and GEβ3 cells, the relative adhesion to wells coated with 1 μg/ml of fibronectin calculated from three individual experiments is shown in the inset. The column graphs show the adhesion to wells coated with 16 μg/ml of fibronectin in the absence (black bars) or presence of 0.5 mg/ml GRGDSP peptide (white bars) or control GRGESP peptide (hatched bars). (D) FACS® analysis showing expression of endogenous mouse β3 on GD25 (profile 3) and GE11 cells (profile 4).
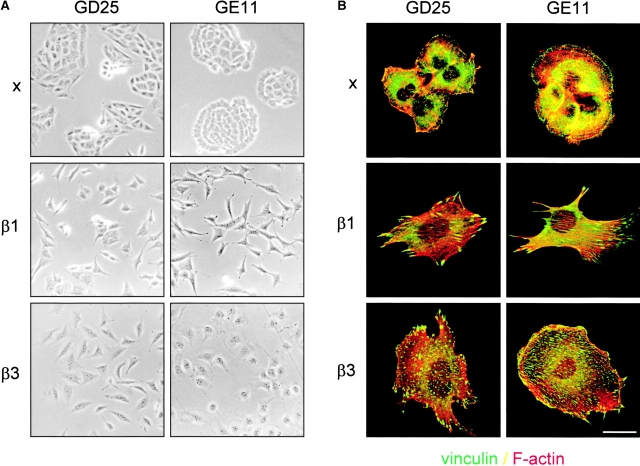
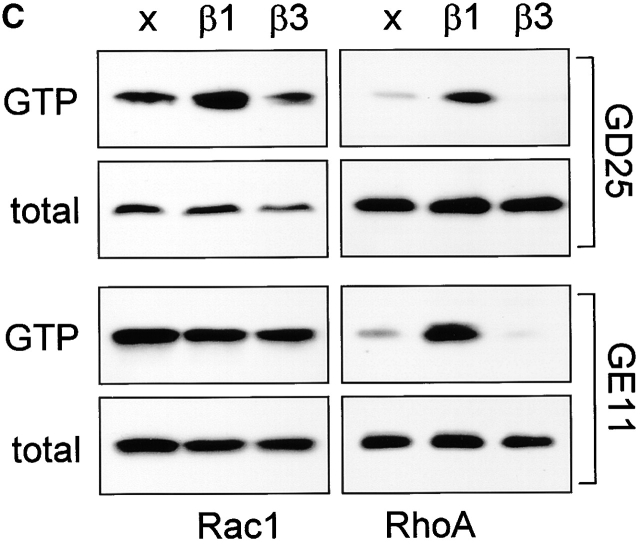
Figure 1.
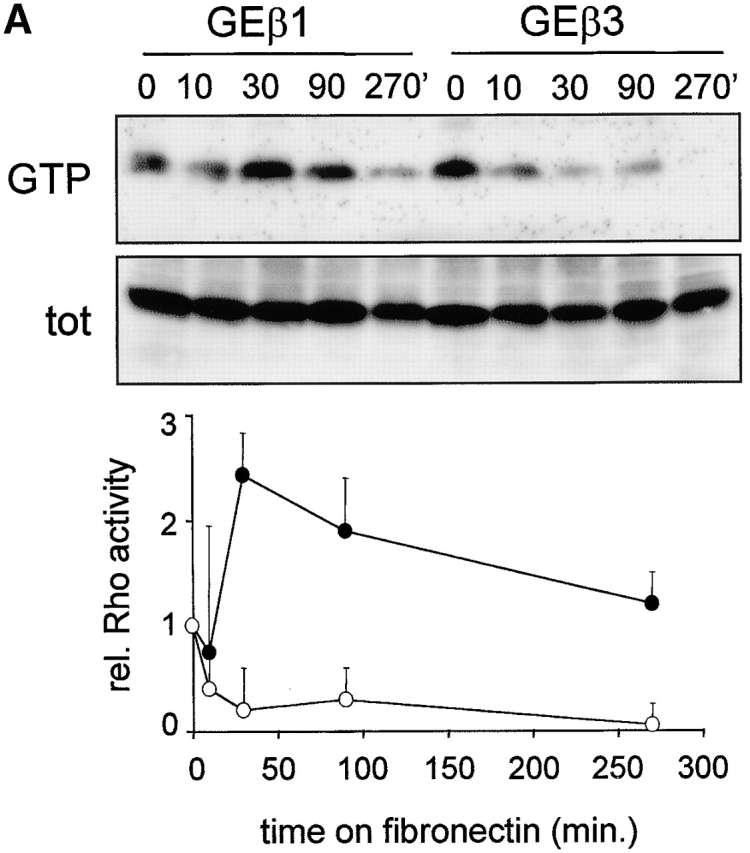
Ectopic expression of β3 in β1 integrin-deficient cells stimulates cell scattering and formation of cell matrix adhesions but not RhoA–GTP loading. (A) Microphotographs of GD25 fibroblastoid and GE11 epithelioid β1-deficient cells ectopically expressing the integrin β1 or β3 subunit. (B) GD25 and GE11 cells expressing the indicated integrins were grown in complete medium for 1 d on glass coverslips, fixed, permeabilized, stained for vinculin (FITC) and F-actin (phalloidin:TR), and analyzed by fluorescence microscopy. Bar, 5 μm. (C) GD25 or GE11 cells transduced with integrin β subunits as indicated were grown in standard culture medium, lysed, and processed for RhoA and Rac1 activity assays as described in the Materials and methods. One representative experiment of three is shown.
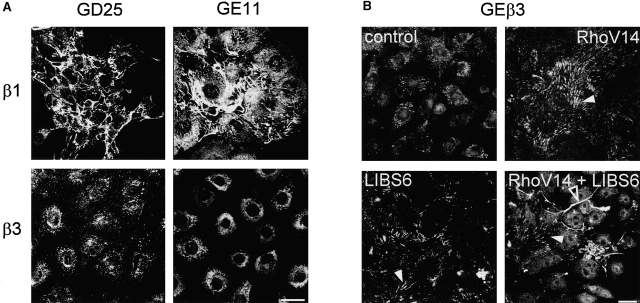
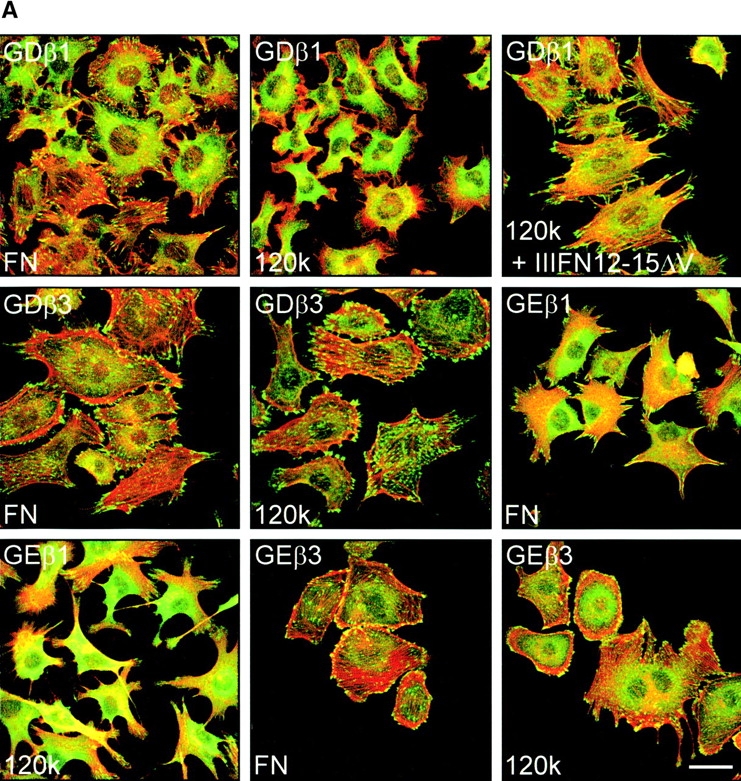
GD25 cells contain very small vinculin-containing cell matrix adhesions, and vinculin and F-actin are localized at the borders of epithelial islands in GE11 cells. Expression of β1 integrins leads to increased formation of F-actin stress fibers and cell matrix adhesions in both cell types (Fig. 1 B) (Wennerberg et al., 1996; Gimond et al., 1999). Similar to the effect of β1 expression, increased size and numbers of vinculin-positive cell matrix adhesions were observed in GDβ3 and GEβ3 cells (Fig. 1 B). However, in contrast to the localization of cell matrix adhesions in cell protrusions of β1-expressing cells they were more randomly distributed over the entire basal surface of the GDβ3 and GEβ3 cells.
We have reported previously that RhoA activity is increased after β1 expression in GD25 or GE11 cells (Gimond et al., 1999). However, it remained unclear if RhoA activity is induced by β1 integrins and required for the disruption of cell–cell junctions or if RhoA is activated as a consequence of the loss of cell–cell adhesion. Therefore, we tested if the scattered phenotype caused by increased levels of αvβ3 is also accompanied by increased RhoA activity. A strong increase in RhoA–GTP levels was indeed observed upon expression of β1 but in marked contrast, GTP-bound RhoA was barely detectable in GDβ3 and GEβ3 cells (Fig. 1 C). GTP loading on Rac1 was only weakly affected by the expression of either β1 or the increased expression of β3; a small increase in Rac1 activity was consistently detected in GDβ1 cells, whereas the levels of GTP-bound Rac1 in GE11, GEβ1, and GEβ3 cells were similar. Thus, similar to the expression of β1, increased levels of β3 integrins induce scattering of β1-deficient cells, but only β1 integrins stimulate high levels of RhoA activity and the organization of focal contacts in cell protrusions.
Expression of fibronectin receptors and adhesion to fibronectin by β1- versus β3-transduced cells
To be able to use these cell lines for a comparative study of β1 and β3 integrin–mediated responses to fibronectin, we analyzed their expression profiles of fibronectin-binding integrins and their adhesiveness to fibronectin. GDβ3 (unpublished data) and GEβ3 cells strongly expressed αvβ3 (Fig. 2, A, hu β3, and B, left panel). High surface expression of the α5 subunit of the α5β1 fibronectin-binding integrin was induced in GDβ1 (unpublished data) and GEβ1 cells (Fig. 2 A, mo α5), but αv expression levels were also increased (Fig. 2 A, mo αv). As reported for GDβ1 cells (Retta et al., 2001), we observed that αvβ3 levels were suppressed in GEβ1 cells (Fig. 2, A, mo β3, and B, left panel). Immunoprecipitations with anti-αv, -β1, and -β5 antibodies demonstrated that the αvβ1 vitronectin/fibronectin receptor and the αvβ5 vitronectin receptor are expressed on GEβ1 cells (Fig. 2 B, right panel). No αvβ6 was detected (unpublished data). Thus, β1-transduced cells may use α5β1 and αvβ1, whereas β3-transduced cells may use αvβ3 for binding to fibronectin.
GD25 cells poorly adhered to fibronectin, but the expression of β1 integrins or the increased expression of αvβ3 induced a similar increase in adhesion to fibronectin (Fig. 2 C). Likewise, GEβ1 and GEβ3 cells adhered better to fibronectin than the parental GE11 cells. At a concentration of 1 μg/ml fibronectin, ∼30% of the GEβ1 and GEβ3 cells but only 15% of the GE11 cells had adhered (Fig. 2 C, asterisks, and insert). Cell adhesion to fibronectin of nontransduced and β3-transduced GD25 and GE11 cells was blocked by a GRGDSP peptide (but not by a GRGESP control), whereas adhesion of β1-transduced cells was only poorly sensitive to inhibition by RGD (Fig. 2 C) but could be completely blocked by the anti–mouse α5 antibody, BMA5 (unpublished data). This is in line with the fact that binding of αvβ3 occurs exclusively via the RGD site in the central cell-binding domain (CCBD)* of fibronectin, whereas α5β1 binds the RGD and the synergy region (Aota et al., 1994; Bowditch et al., 1994; Danen et al., 1995). The surprisingly strong adhesion of parental GE11 but not GD25 cells to fibronectin was completely blocked by the RGD peptide, suggesting that endogenous αvβ3 was responsible. In line with this, we observed that the expression of αvβ3 on GE11 cells was stronger than that on GD25 cells (Fig. 2 D).
In conclusion, β1-transduced cells express the αvβ1 and α5β1 fibronectin receptors and predominantly use α5β1 for adhesion to fibronectin, whereas β3-transduced cells use αvβ3, but the efficiency of adhesion to fibronectin of both cell types is similar.
Integrin α5β1- but not αvβ3-mediated adhesion to fibronectin stimulates RhoA–GTP loading and localization of focal contacts in cellular protrusions
Having established that β1- and β3-transduced cells adhere with similar efficiency to fibronectin, whereas only β1 integrins stimulate RhoA activity, we next examined the regulation of RhoA–GTP loading during spreading of these cells on fibronectin. In line with the findings in cells grown in standard medium (Fig. 1 C), Rac1 GTP levels were similar in β1- or β3-transduced cells spreading on fibronectin under serum-free conditions (unpublished data). In GEβ1 cells, RhoA–GTP levels were high in suspension, low at initial adhesion (t = 10 min), but strongly increased at later time points after adhesion (t = 30 min) followed by a gradual decrease during further cell spreading (Fig. 3 A). A similar pattern, albeit with somewhat slower kinetics, was seen with GDβ1 cells (see Fig. 6 B). High levels of GTP-bound RhoA that disappeared during the early stages of cell adhesion were also observed in suspended GEβ3 (Fig. 3 A) and GDβ3 cells (unpublished data), but the increase in RhoA–GTP loading during later stages of cell spreading (t = 30 and 90 min) was not observed in these cells.
Figure 3.
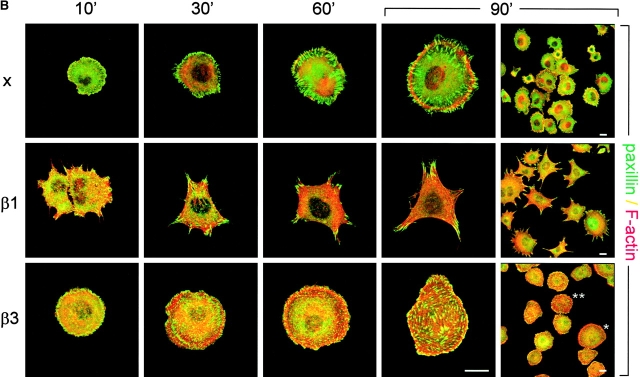
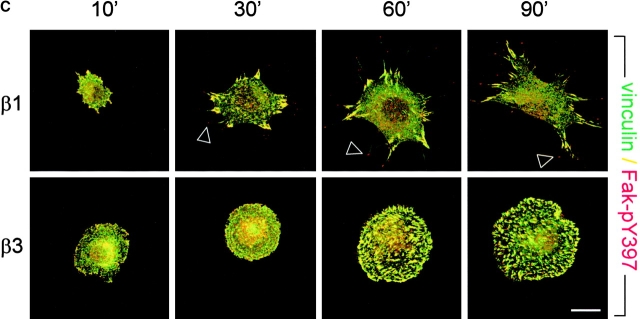
Regulation of GTP–RhoA levels and organization of cell matrix adhesions during cell spreading on fibronectin. (A) Cells were serum starved overnight, maintained in suspension for 2 h, and subsequently plated in the absence of serum on dishes coated with 10 μg/ml fibronectin and processed for RhoA activity assays. The Western blot of one representative experiment is shown, and the graph indicates the mean ± SD of three experiments in which the amount of GTP-bound RhoA is shown relative to that in suspended cells (•, GEβ1; ○, GEβ3). (B and C) GE11 cells expressing the indicated integrins were plated in the absence of serum on fibronectin-coated coverslips for the indicated times, fixed, permeabilized, stained for paxillin (FITC) and F-actin (phalloidin:TR) (B) or vinculin (FITC) and phospho-specific pY397-FAK (TR) (C), and analyzed by fluorescence microscopy. Bars, 5 μm.
Figure 6.
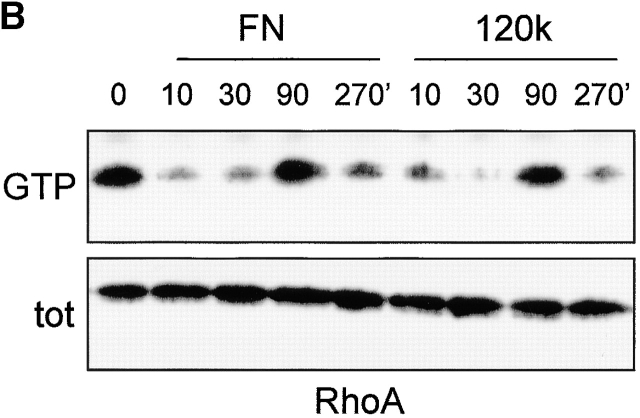
Roles of fibronectin regions other than the CCBD in β1- and β3-mediated regulation of cell matrix adhesions and RhoA–GTP loading. (A) GD25 and GE11 cells transduced with the indicated integrin subunits were serum starved overnight and plated for 120 min in the absence or presence of 0.5 μg/ml GST–IIIFN12-15ΔV on glass coverslips coated with 10 μg/ml fibronectin or 5 μg/ml fibronectin 120-kD chymotryptic fragment as indicated. Cells were fixed, permeabilized, stained for paxillin (FITC) and F-actin (phalloidin:TR), and analyzed by fluorescence microscopy. Bar, 10 μm. (B) GDβ1 cells were serum starved overnight, maintained in suspension for 2 h in the presence of cyclohexamide, plated for the indicated times on dishes coated with 10 μg/ml fibronectin or 5 μg/ml 120-kD chymotryptic fragment, and processed for RhoA activity assays (note that GTP–RhoA levels are high in suspended cells and in cells plated 90 min on FN or the 120-kD fragment).
To study the development and distribution of cell matrix adhesions in response to adhesion to fibronectin via α5β1 or αvβ3, spreading assays were performed on fibronectin-coated coverslips in the absence of serum in order to rule out any effect of vitronectin, a serum component that binds αv integrins. Since the exaggerated cell spreading and random distribution of cell matrix adhesions was most prominent in GEβ3 cells (Fig. 1, A and B), we compared GEβ1 and GEβ3 cells in these assays. In spreading GE11 cells, a cortical ring of F-actin was observed and cell matrix adhesions stained for paxillin were distributed along this ring (Fig. 3 B). In GEβ1 cells, focal contacts assembled between 10 and 30 min after α5β1-mediated adhesion to fibronectin, the vast majority of which localized to peripheral cell protrusions that were connected by long F-actin stress fibers. In contrast, F-actin and cell matrix adhesions initially became organized in a cortical ring in spreading GEβ3 cells, but at later time points some GEβ3 cells did develop large focal contacts that were distributed randomly over the basal cell surface and were connected by short F-actin cables (Fig. 3 B, compare 60 min with 90 min and also * with ** in bottom right; 27 ± 11% of GEβ3 cells showed random distribution of short F-actin cables and focal contacts rather than circular distribution at 90 min). Similarly, vinculin was clustered in focal contacts that localized in cell protrusions of GEβ1 cells, whereas it was detected in randomly distributed focal contacts in GEβ3 cells (Fig. 3 C). Intriguingly, staining for the autophosphorylated (active) form of FAK revealed a partial colocalization with vinculin in both cell lines, but it was also detected at the tips of cell protrusions of GEβ1 cells. Finally, talin was found together with vinculin in the adhesions present in β1- and β3-transduced cells (unpublished data). From these findings we conclude that α5β1- but not αvβ3-mediated adhesion to fibronectin stimulates RhoA–GTP loading and the distribution of focal contacts in peripheral cell protrusions.
Altered localization of tensin and impaired fibronectin fibrillogenesis in GDβ3 and GEβ3 cells that can be partially rescued by activated RhoA
In analogy with the complete rescue of cell adhesion to fibronectin by the increased expression of αvβ3 in β1-deficient cells, low levels of fibronectin-binding integrins in general might also explain the inefficient fibronectin matrix assembly in β1-deficient cells. Therefore, we assessed fibronectin matrix assembly in GDβ3 and GEβ3 cells. Our findings confirmed that expression of β1 induced the formation of a dense meshwork of fibronectin fibrils in GD25 cells (Wennerberg et al., 1996) and showed a similar induction in GEβ1 cells (Fig. 4 A). However, despite similar adhesiveness of β3-transduced cells to immobilized fibronectin increased levels of αvβ3 did not stimulate fibronectin fibrillogenesis. In confluent cultures of GD25, GE11, GDβ3, and GEβ3 cells, some short fibronectin fibrils could be seen, but fibrillogenesis was minimal compared with that in GDβ1 and GEβ1 cells (unpublished data). Similarly, β1-expressing cells effectively incorporated exogenous biotinylated fibronectin into a matrix, whereas cells expressing β3 at high levels did not (unpublished data; see Fig. 8, B and C).
Figure 4.
Absence of fibronectin matrix assembly in β1-deficient cells ectopically expressing β3 and partial rescue by V14RhoA. (A) GD25 or GE11 cells expressing the indicated integrins were grown on glass coverslips for 36 h in standard culture medium, fixed, stained with antifibronectin antibodies, and analyzed by immunofluorescence microscopy. Bar, 20 μm. (B) GEβ3 cells, either untransfected or transiently expressing V14RhoA, were seeded for 4 h on fibronectin-coated glass coverslips, washed, and subsequently incubated for 20 h in medium containing fibronectin-depleted serum supplemented with 10 μg/ml biotinylated fibronectin in the absence or presence of 5 μM LIBS6 antibody as indicated. Cells were fixed, stained with TR-conjugated streptavidin, and analyzed by fluorescence microscopy. Filled arrowheads indicate short (< 5 μm) and open arrowheads indicate longer fibrils (10–20 μm). Bar, 20 μm.
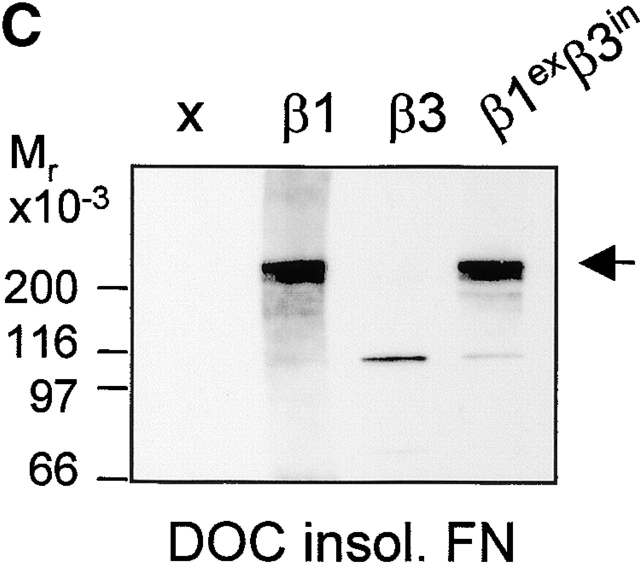
Figure 8.
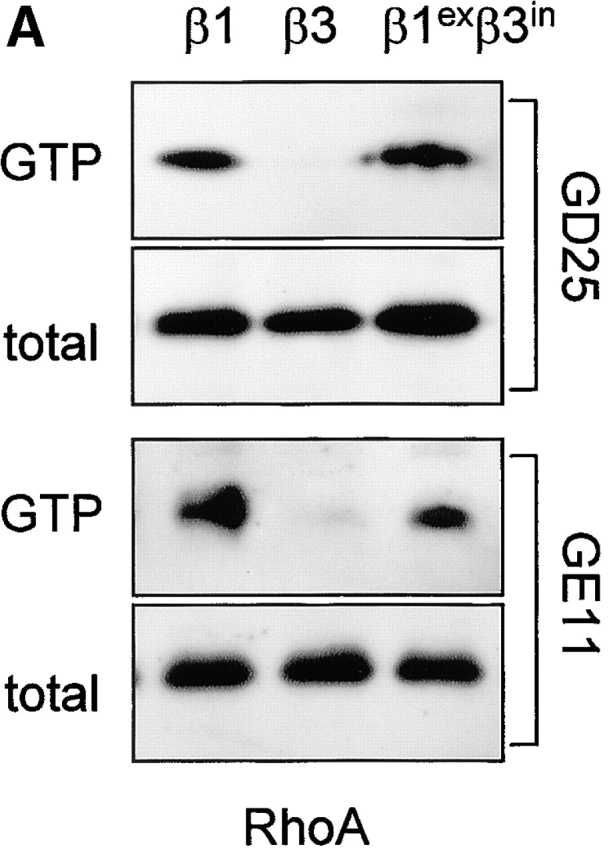
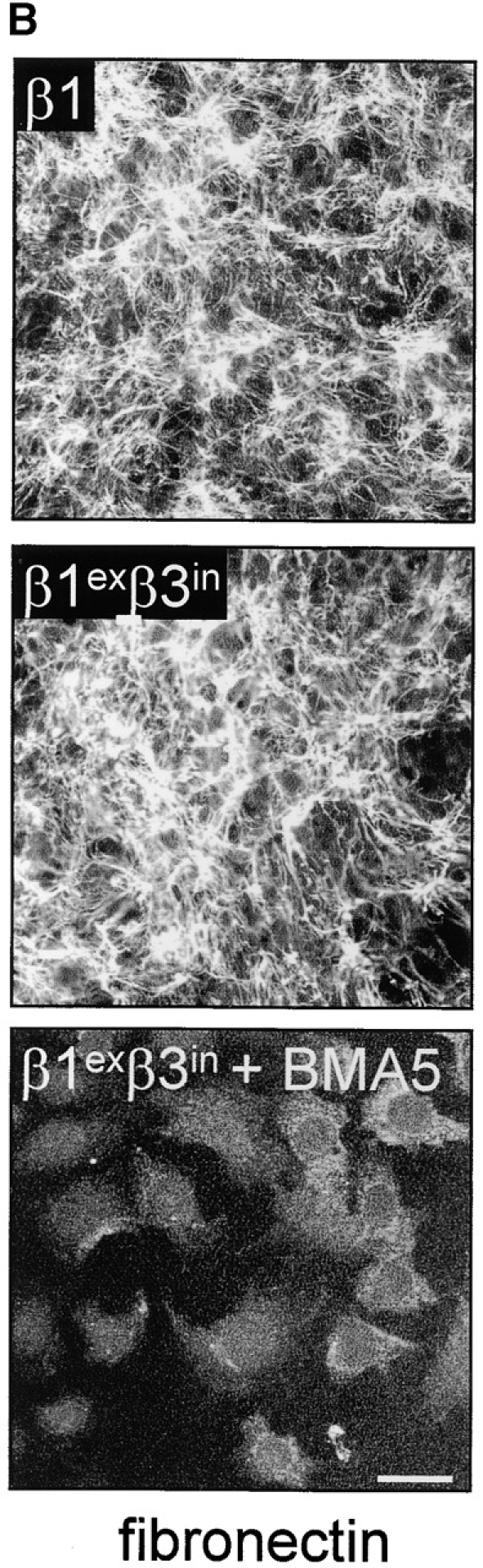
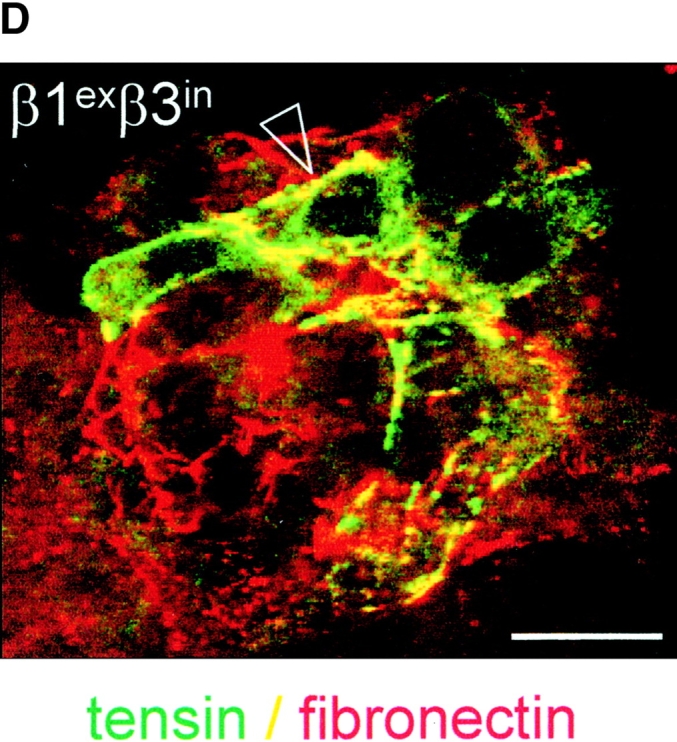
The role of the cytoplasmic domain in RhoA activation, fibronectin matrix assembly, and tensin recruitment. (A) GD25 and GE11 cells expressing the indicated wild-type or chimeric integrin subunits were grown in standard culture medium, lysed, and processed for RhoA activity assays. (B) GEβ1 and GEβ1exβ3in cells were serum starved overnight, seeded for 4 h on fibronectin-coated glass coverslips in serum-free medium, washed, and subsequently incubated in the absence or presence of 0.5 μg/ml BMA5 anti-α5 antibody for 20 h in medium containing fibronectin-depleted serum supplemented with 10 μg/ml biotinylated fibronectin. Cells were fixed, stained with TR-conjugated streptavidin, and analyzed by fluorescence microscopy. Bars, 20 μm. (C) GE11 cells expressing the indicated wild-type or chimeric integrin subunits were serum starved, seeded for 4 h on fibronectin-coated dishes in serum-free medium, washed, and subsequently incubated for 20 h in medium containing fibronectin-depleted serum supplemented with 10 μg/ml biotinylated fibronectin. Cells were lysed in DOC buffer, and insoluble material was separated by SDS-PAGE and subjected to Western blotting using HRPO-conjugated streptavidin. (D) GDβ1exβ3in cells were transiently transfected with GFP-tensin, serum starved, seeded for 4 h on fibronectin-coated glass coverslips, washed, and subsequently incubated for 20 h in medium containing fibronectin-depleted serum supplemented with 10 μg/ml biotinylated fibronectin. Cells were fixed, stained with TR-conjugated streptavidin, and analyzed by fluorescence microscopy. A cluster of ∼10 cells is shown; the arrowhead points to localization of tensin along fibronectin fibrils. Bar, 20 μm.
Since the inability of β3-transduced GD25 and GE11 cells to assemble a fibronectin matrix correlated with their inability to activate RhoA, we wondered if expression of a dominant active mutant of RhoA could induce fibronectin fibrillogenesis in GEβ3 cells. Therefore, we transiently expressed V14RhoA in GEβ3 cells and observed that the formation of many short fibronectin fibrils (< 5 μm) was stimulated, but it did not result in the assembly of a matrix comparable to that produced by GEβ1 cells (Fig. 4 B, top right). We next tested if the inability of high levels of αvβ3 to induce efficient fibronectin matrix assembly could be rescued by the addition of an activating anti-β3 antibody as has been reported for αvβ3 in CHO cells (Wu et al., 1996). Treatment of GEβ3 cells with LIBS6 weakly stimulated the assembly of exogenously added fibronectin into thin short fibrils along the periphery of the cells (Fig. 4 B, bottom left). When V14RhoA-expressing GEβ3 cells were treated with LIBS6, the formation of long fibrils (10–20 μm) was observed, similar to those observed in GEβ1 cells although their number was small (Fig. 4 B, bottom right).
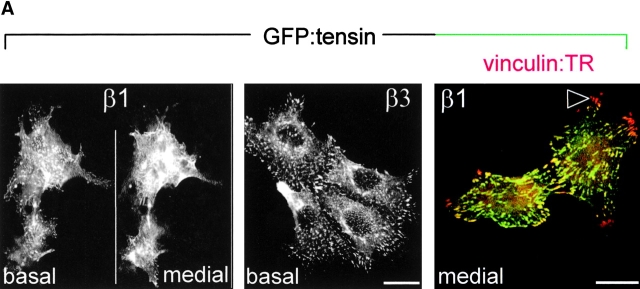
The cytoskeletal protein tensin colocalizes with α5β1 but not αvβ3 in the fibrillar adhesions associated with fibronectin fibrillogenesis (Pankov et al., 2000; Zamir et al., 2000). To investigate where tensin resides in cells lacking α5β1, we investigated tensin localization in β1- and β3-transduced GD25 and GE11 cells. Since we were unable to detect endogenous mouse tensin, we transiently expressed GFP-tensin in these cells. GFP-tensin was localized in fibrillar adhesions, (where vinculin was not concentrated) at the basal and apical surface of GDβ1 cells but not of GDβ3 cells (Fig. 5 A). In GDβ3 cells, tensin colocalized with vinculin in focal contacts. As a control, GFP-vinculin was localized in focal contacts in both GDβ1 and GDβ3 cells (unpublished data). Identical findings were obtained for tensin and vinculin localization in GE11 cells transduced with β1 or β3 (unpublished data). Furthermore, we observed colocalization of tensin with exogenously added biotinylated fibronectin in fibrillar adhesions in GEβ1 cells in confluent and subconfluent cultures, whereas tensin was localized in focal contacts of GEβ3 cells where biotinylated fibronectin was not detected (Fig. 5 B).
Figure 5.
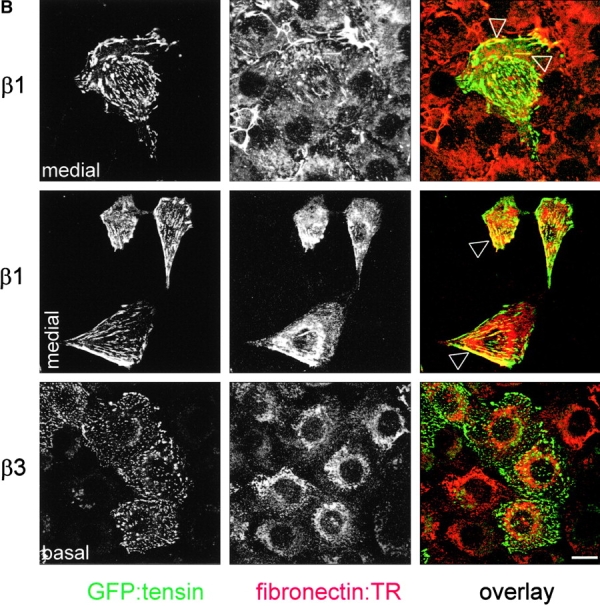
Absence of tensin-containing fibrillar adhesions in β1-deficient cells ectopically expressing β3. (A) GD25 cells expressing the indicated integrins were transiently transfected with GFP-tensin, seeded in standard culture medium on glass coverslips for 24 h, fixed, and permeabilized for analysis by confocal fluorescence microscopy. Shown is the localization of GFP-tensin alone (black and white pictures) or in combination with vinculin (TR; color picture). Note the absence of tensin in many of the peripheral focal contacts that stain for vinculin in GDβ1 cells (arrowhead). (B) GE11 cells expressing the indicated integrins and transiently expressing GFP-tensin were seeded for 4 h on fibronectin-coated glass coverslips, washed, and subsequently incubated for 20 h in medium containing fibronectin-depleted serum supplemented with 10 μg/ml biotinylated fibronectin. Cells were fixed, stained with TR-conjugated streptavidin, and analyzed by fluorescence microscopy. Note that coalignment of fibronectin and tensin can be observed in confluent (top) and subconfluent cultures (middle) of GEβ1 (arrowheads) but not GEβ3 cells. Focal planes are as indicated. Bars, 10 μm.
Thus, in line with the requirement of RhoA for fibronectin matrix assembly increased expression of αvβ3, which does not stimulate RhoA activity, does not support this process. Expression of dominant active RhoA or activating anti-β3 antibodies can both stimulate the initial steps of αvβ3-mediated fibronectin fibril formation. However, in combination they induce long fibronectin fibrils, indistinguishable from those induced by α5β1, though the process is inefficient. Moreover, under conditions in which RhoA activity is undetectable (GDβ3 and GEβ3 cells) tensin-containing fibrillar adhesions fail to form. Instead, tensin is colocalized with vinculin in focal contacts that are randomly distributed over the basal surface.
Other regions besides the CCBD in fibronectin regulate cell matrix adhesions in β1- but not β3-transduced cells
Despite their low levels of RhoA activity, GDβ3 and GEβ3 cells did generate focal contacts (even though their distribution was different from that in β1-expressing cells). Besides the interactions with the CCBD in fibronectin, other regions in the fibronectin molecule can stimulate the formation of focal contacts and F-actin stress fibers (Woods, 2001). We analyzed the formation of cell matrix adhesions in cells adhering via α5β1 or αvβ3 to a 120-kD chymotryptic fragment of fibronectin that contains the CCBD. Under these conditions, GDβ1 and to a lesser extent GEβ1 cells displayed few small cell matrix adhesions with most of the paxillin being localized diffusely in the cytoplasm (Fig. 6 A). Formation of focal contacts in GDβ1 cells could be stimulated by the addition of a fibronectin type 3 repeat (IIIFN)12-15ΔV GST fusion protein, which contains the HepII domain that has been reported to stimulate RhoA-dependent processes (Woods, 2001). In complete contrast, the formation of focal contacts in cells binding to the 120-kD fragment through αvβ3 was indistinguishable from that seen on fibronectin (Fig. 6 A).
The inability of β1-transduced cells to assemble focal contacts on the 120-kD fibronectin fragment may be due to inefficient stimulation of RhoA activity under those conditions. Therefore, we compared the regulation of RhoA–GTP levels in β1-expressing cells spreading on fibronectin and on the CCBD only. RhoA–GTP levels were high in suspension and were suppressed during the early stages of spreading (10 and 30 min) on fibronectin or the 120-kD chymotryptic fragment (Fig. 6 B). However, RhoA–GTP levels were transiently stimulated at later time points, e.g., 60 min (unpublished data) and 90 min (Fig. 6 B), on both substrates. Similar results but with somewhat faster kinetics were obtained with GEβ1 cells (unpublished data). Treatment of the cells with cyclohexamide ruled out that synthesis and deposition of cellular fibronectin was responsible for the activation of RhoA on 120-kD coated surfaces.
These findings demonstrate that, although RhoA–GTP loading can be supported by the CCBD, fibronectin-stimulated focal contact formation in cells binding through α5β1 requires interactions with domains of fibronectin other than the CCBD, such as the HepII domain, whereas the focal contacts in cells binding through αvβ3 can be stimulated by the CCBD exclusively.
Low levels of GTP-bound RhoA in β3-transduced cells cannot be explained by increased activity of FAK or p190RhoGAP
Although the results above clearly showed that focal contacts in the β1-deficient cells are formed in the absence of high levels of RhoA activity, the reason for the impaired RhoA activation in these cells remained unclear. Therefore, we examined the two pathways that have been reported to play an important role in integrin-mediated suppression of RhoA–GTP levels, namely FAK (Ren et al., 2000) and p190Rho GTPase-activating protein (GAP) (Arthur et al., 2000). FAK became rapidly phosphorylated on its major autophosphorylation site, Tyr397, after adhesion to fibronectin in GEβ1 cells, whereas the response was somewhat delayed in GEβ3 cells (Fig. 7). This delay appeared to be specific, since adhesion-induced phosphorylation of p130Cas occurred with similar kinetics in GEβ1 and GEβ3 cells. Tyrosine phosphorylation of p190RhoGAP was similar in GEβ1 and GEβ3 cells spreading on fibronectin and gradually increased with time. Thus, tyrosine phosphorylation of FAK and p190RhoGAP, which correlates with the activity of these proteins, is not increased or more sustained in β3-transduced cells, demonstrating that a failure to inactivate these pathways does not explain the low level of GTP-bound RhoA in the β3-transduced cells.
Figure 7.

Fibronectin-stimulated tyrosine phosphorylation of FAK, p130Cas, and p190RhoGAP. GEβ1 and GEβ3 cells were serum starved overnight and replated on dishes coated with 10 μg/ml fibronectin in serum-free medium. Cells were lysed in modified RIPA buffer at the indicated time points for immunoprecipitation with antibodies indicated on the left. Immunoprecipitates or total lysates were separated on 8% SDS-PAGE followed by Western blotting with antibodies indicated on the right.
The extracellular region of the β subunit determines RhoA–GTP loading, fibronectin matrix assembly, and tensin recruitment
One possible explanation for the specific stimulation of RhoA activity and RhoA-dependent processes, such as fibronectin matrix assembly by α5β1, is that specific amino acids in the β1 cytoplasmic domain can recruit specific signaling intermediates, e.g., tensin, which ultimately leads to RhoA activation. To test this idea, we expressed a chimeric β subunit consisting of the β1 extracellular and transmembrane domain and the β3 cytoplasmic domain (β1exβ3in) in GD25 and GE11 cells. The level of expression of this chimera was comparable to that of control wild-type β1, it was accompanied by a similar strong expression of α5, and the cells adopted a scattered morphology that resembled that of wild-type β1-expressing cells (unpublished data). When we measured RhoA activity in these cells, similar amounts of GTP-bound RhoA were observed in β1exβ3in- and β1-expressing cells (Fig. 8 A).
We next examined the ability of this chimera to stimulate the assembly of a fibronectin matrix. In line with the levels of GTP-bound RhoA in these cells, the extent of fibril formation observed in GEβ1exβ3in was similar to that in GDβ1 (Fig. 8, B and C). In addition, matrix assembly of β1- (unpublished data) and that of β1exβ3in-expressing cells was completely blocked by BMA5 blocking anti–mouse α5 antibody (Fig. 8 B), indicating that the chimeric β subunit bound fibronectin as a functional α5β1exβ3in heterodimer. We also tested if the formation of tensin-containing fibrillar adhesions was affected by the replacement of the β1 cytoplasmic tail, and again we observed no effect; tensin efficiently localized to fibrillar adhesions at the apical surface of GDβ1exβ3in cells where biotinylated fibronectin was incorporated into fibrils (Fig. 8 D). Finally, we expressed an inverse chimeric integrin β subunit, β3exβ1in, in GD25 and GE11 cells and observed that it behaved similar to β3 with respect to RhoA activation and fibronectin matrix assembly (unpublished data).
Together, these findings demonstrate that the stimulation of RhoA activity, organization of focal contacts in cell protrusions, fibronectin matrix assembly, and formation of tensin-containing fibrillar adhesions is dictated by the extracellular region of the β subunit.
High levels of expression of both β1 and β3 leads to an intermediate phenotype
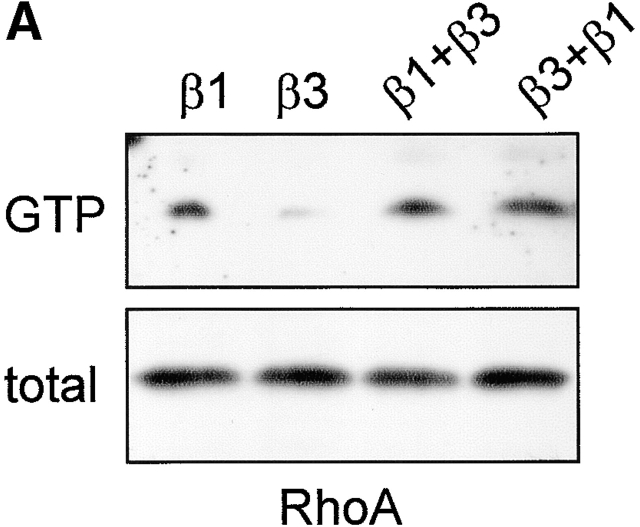
Given the suppression of αvβ3 levels in β1-transduced GD25 (Retta et al., 2001) and GE11 cells (Fig. 2, A and B), the enhanced RhoA activation that we observed in these cells may also be due to a loss of inhibitory functions of β3 integrins. To test this, we ectopically expressed β3 in GEβ1 cells (GE[β1+β3]), and, vice versa, β1 was expressed in GEβ3 cells (GE[β3+β1]). The surface levels of the α5, β1, and β3 subunits were similar to those in cells transduced with a single subunit (unpublished data). Importantly, RhoA–GTP loading in β1-expressing cells was not inhibited by the increased expression of αvβ3 (Fig. 9 A).
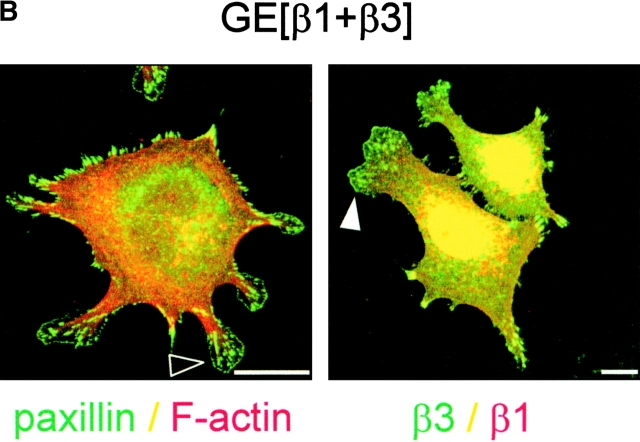
Figure 9.
Regulation of αvβ3 levels by β1 affects focal contact maturation not RhoA–GTP loading. (A) GE11 cells expressing the indicated integrin subunits were grown in standard culture medium, lysed, and processed for RhoA activity assays. (B) GE[β1+β3] cells were plated in the absence of serum on fibronectin-coated coverslips for 90 min, fixed, permeabilized, and stained for paxillin (FITC) and F-actin (phalloidin:TR) or with polyclonal anti-β1 (TR) and FITC-conjugated monoclonal anti-β3 as indicated. Arrowheads indicate membrane blebs containing many small matrix adhesions that stain for paxillin (open arrowhead) and β3 integrin (filled arrowhead). Bars, 5 μm.
Despite the inability of αvβ3 to suppress RhoA–GTP levels in β1-expressing cells, the organization of focal contacts was altered. Compared with GEβ1 cells, GE[β1+β3] cells growing in standard culture medium were considerably more spread, a characteristic feature of GEβ3 cells (unpublished data). Moreover, spreading of GE[β1+β3] cells on fibronectin under serum-free conditions led to an intermediate phenotype. Some protrusions with focal contacts at their tips were still formed, but many cells showed a cortical organization of F-actin, and most protrusions ended in membrane blebs (Fig. 9 B). Moreover, despite the stimulation of RhoA–GTP loading in GE[β3+β1] cells, they retained the typical pancake-like spread phenotype of GEβ3 cells with part of the cells showing the protrusions ending in membrane blebs as described above (unpublished data). Finally, since the ectopic expression of β3 in GEβ1 cells only minimally affected overall RhoA–GTP levels and morphology but did affect the organization of cell matrix adhesions locally at the ends of the protrusions, we wondered if the membrane blebs were formed due to high concentrations of αvβ3 integrins, which we found is associated with Rac1 but not RhoA activity. Indeed, many small paxillin-containing cell matrix adhesions were localized at those sites, and these adhesions contained large amounts of αvβ3 (Fig. 9 B, right panel).
These findings demonstrate that the ability of β1 integrins to regulate αvβ3 levels is an important aspect of their role in the distribution/maturation of cell matrix adhesions, but it is not involved in the stimulation of overall RhoA–GTP loading by β1.
Discussion
The ECM regulates many cellular functions mainly via signaling by integrins (Giancotti, 2000; Danen and Yamada, 2001; Schwartz, 2001). To study the role of the β1 integrins in ECM signaling, cell lines have been generated that lack the β1 subunit common to this subfamily (Fässler and Meyer, 1995; Gimond et al., 1999). These cells show impaired adhesion, migration, and matrix assembly on fibronectin (Wennerberg et al., 1996; Gimond et al., 1999). Notably, the β1-deficient cells do express low levels of αvβ3, an integrin that can bind fibronectin and that might be expected to compensate for the lack of β1 as far as interactions with fibronectin are concerned. In the current study, we increased the level of αvβ3 surface expression in the independently generated GD25 fibroblastoid and GE11 epithelioid β1-deficient cell lines and tested if this results in similar changes as observed after the induction of β1 expression. We find that increased expression of β3- in β1-deficient cells causes the disruption of cell–cell contacts, but unlike the expression of β1 in these cells this process is not accompanied by an increased activity of the small GTPase, RhoA.
We propose the following model for integrin-mediated control of the development and organization of cell matrix adhesions: during the initial stages of cell spreading on fibronectin when RhoA activity is low, focal complexes are formed that gradually increase in size and convert to focal contacts (Ridley and Hall, 1992; Rottner et al., 1999). This early phase is efficiently stimulated by αvβ3-mediated binding to the CCBD, but it requires additional interactions in cells which bind via α5β1. Subsequently, at later stages of cell spreading when the number of focal contacts containing αvβ3 continues to increase randomly in the cell, focal contacts containing α5β1 disappear from the center while the new ones are formed at the tips of cell protrusions. This lattter process is accompanied by an increase in RhoA-mediated tension, which is neccessary for the formation of tension-containing fibrillar adhesions, and assembly of a fibronectin matrix.
Regulation of Rho–GTPase activity in cells expressing β1 or β3 integrins
Besides regulation by soluble factors, the activity and the localization of Rho–GTPases and their effector proteins is influenced by cell–cell and cell–ECM adhesion (Schwartz and Shattil, 2000; Fukata and Kaibuchi, 2001). We found previously that RhoA activity is barely detectable in β1-deficient cell lines and is increased upon expression of β1 (Gimond et al., 1999). However, since RhoA can be suppressed by cadherin signaling (Noren et al., 2001) it was difficult to determine if RhoA is implicated in β1-induced scattering or if its activation is caused by the loss of cell–cell contacts. Since we now observe that RhoA activity is associated specifically with β1 expression, whereas both β1- and β3-transduced cells are scattered, we conclude that β1 integrins specifically support high levels of RhoA activity.
Cell spreading upon attachment to fibronectin is accompanied by activation of Cdc42, which in turn activates Rac1 (Price et al., 1998). Around the same time (within 5 min after attachment) RhoA activity is down-regulated, a Src-dependent process that is probably important for relieving the tension that would otherwise interfere with the initial phase of cell spreading (Ren et al., 1999; Arthur et al., 2000). Subsequently, RhoA activity is increased and stimulates further cytoskeletal rearrangement after which GTP–RhoA levels gradually decrease again to reach a steady-state level of intermediate activity (Barry et al., 1997; Ren et al., 1999). We observed previously that among different ECM proteins fibronectin is particularly efficient in the stimulation of these RhoA-dependent processes (Danen et al., 2000). Our current findings demonstrate that the pattern of fibronectin receptors expressed by a cell dramatically affects the activation of RhoA and as a consequence the ability of cells to organize their cell matrix adhesions.
Both p190RhoGAP and FAK have been implicated in the suppression of RhoA activity in the early phase of integrin-mediated spreading (Arthur et al., 2000; Ren et al., 2000). Our observation that tyrosine phosphorylation of p190RhoGAP and FAK is not stronger or more sustained in cells adhering to fibronectin via αvβ3 compared with cells adhering via α5β1 suggests that suppression by these pathways cannot explain the low levels of GTP–RhoA in the β3-transduced cells. Parenthetically, there is evidence for the converse process, control of phosphorylation of FAK by the RhoA/Rho kinase pathway (Flinn and Ridley, 1996; Sinnett-Smith et al., 2001), and this may explain our observation that tyrosine phosphorylation of FAK is somewhat delayed in GEβ3 cells spreading on fibronectin. This in turn might cause the turnover rate of focal contacts to be low, which explains why so many focal contacts are randomly distributed in the cell. This exlpanation is supported by the fact that also in FAK knockout fibroblasts, the turnover of focal contacts is impaired (Ilic et al., 1995). Alternative mechanisms that could explain the specific activation of RhoA by β1 integrins may involve the FAK-associated GAP for RhoA, Graf (Taylor et al., 1998, 1999), or the activation of specific guanine exchange factors, although at this moment to our knowledge no guanine exchange factor for RhoA has been shown to be affected by integrin-mediated adhesion.
The finding that swapping of the β1 and β3 cytoplasmic tails affects neither RhoA activity nor the downstream functional effects argues against a model in which specific combinations of amino acids that bind specific signaling intermediates (e.g., tensin) are present in the cytoplasmic domain of the β1 but not of the β3 subunit. An alternative explanation is that specific α subunits are involved, i.e., α5 but not αv may stimulate RhoA activity. Cross-talk between integrins and other receptors has been demonstrated, and differential modulation by α5β1 and αvβ3 of other receptors that regulate RhoA activity could also be involved. In this respect, the RhoA activity assays that were performed with cells growing in complete medium indicate that lysophosphatidic acid is unable to support high levels of GTP-bound RhoA in cells lacking β1 integrins, suggesting that α5β1 may support Edg receptor signaling. On the other hand, the results of RhoA activity assays with cells spreading on the CCBD in the absence of serum demonstrate that the α5β1-dependent RhoA activation on fibronectin does not depend on collaboration with cellular receptors that bind regions of fibronectin other than the CCBD, such as the HepII domain (e.g., syndecans) (Woods, 2001). Nevertheless, in line with findings with mouse fibroblasts (Saoncella et al., 1999) we observe that such collaboration does regulate the formation of cell matrix adhesions in cells adhering via α5β1.
A recent study shows that overexpression of β3 in CHO cells is associated with increased RhoA activity, whereas overexpression of β1 is associated with Rac1 activity (Miao et al., 2002). It is not mentioned in this report how the overexpression of one subunit affects the levels of the other integrins, and it is possible that cross-talk between β1 and β3 integrins occurs in the CHO cells that express both integrins. Nevertheless, these findings suggest major differences in the regulation of Rho–GTPases between CHO cells and the two cell lines used in our study. On the other hand, in complete agreement with our finding that Rac1 activity is similar in β1- and β3-expressing cells, others have reported recently that αIIbβ3-mediated adhesion of CHO cells and clustering of IL2R chimeras of either β1 or β3 cytoplasmic tails in fibroblasts stimulates Rac1 activity (Berrier et al., 2002). A major complicating factor in comparing the results of various studies is the difference in possibilities for cross-talk between the integrins and other receptors, such as Edg receptors, syndecans, or cadherins, in the different cell types.
What is the nature of the cell matrix adhesions in GDβ3 and GEβ3 cells?
Even though αvβ3- and α5β1-mediated cell matrix adhesions are formed when RhoA activity is low (RhoA activity is stimulated ∼30 min in GEβ1 and somewhat later in GDβ1 cells, whereas cell matrix adhesions begin to form as early as 10 min after cell adhesion), they both are sensitive to inhibition with the Y-27632 Rho kinase inhibitor (unpublished data). However, the fact that αvβ3-mediated focal contacts are stimulated by the CCBD, whereas α5β1-mediated focal contacts require additional stimuli suggests that they develop by different mechanisms. Probably due to the absence of increased RhoA activity during later stages of cell spreading in β1-deficient cells, the αvβ3-mediated focal contacts remain randomly distributed instead of translocating to the tips of cell protrusions and giving rise to fibrillar adhesions as is the case with α5β1-mediated focal contacts.
Integrins are indirectly connected with the actin cytoskeleton via scaffolding proteins such as vinculin, paxillin, and tensin that cluster with integrins in cell matrix adhesions (Geiger et al., 2001). In two-dimensional in vitro culture systems, segregation of components of cell matrix adhesions gives rise to focal contacts and fibrillar adhesions of which the latter are specifically enriched in the integrin α5β1 and tensin (Pankov et al., 2000; Zamir et al., 2000). It has been shown recently that cells in vivo or in three-dimensional matrices in vitro contain cell matrix adhesions that combine aspects of focal contacts and fibrillar adhesions (Cukierman et al., 2001). Interestingly, the adhesions formed in GDβ3 and GEβ3 cells appear to mimic those three-dimensional adhesions to some extent: they contain vinculin, paxillin, and talin, and in addition they also retain tensin. The low level of GTP-bound RhoA observed in β1-deficient cells plated on a two-dimensional surface and the weaker mechanical stretching of cells grown in a three-dimensional environment may each result in a similar low level of tension, which apparently is insufficient for this translocation process.
Fibronectin matrix assembly
Besides mediating cell adhesion to ECM, integrins are also actively involved in the formation of the ECM, for example, in fibronectin matrix assembly (Schwarzbauer and Sechler, 1999). α5β1 is the typical integrin involved in fibronectin fibrillogenesis, but in mouse embryonic fibroblasts lacking both α5β1 and another β1 integrin fibronectin receptor, α4β1, matrix assembly can occur and was shown to depend on αv integrins (Yang and Hynes, 1996). By contrast, others have shown that a CHO cell line lacking α5β1 fails to produce a fibronectin matrix despite the presence of αv integrins (Wu et al., 1993; Zhang et al., 1993). Furthermore, in immortalized fibroblasts derived from β1-null embryonic stem cells the fibronectin matrix is severely impaired with residual assembly in highly confluent cultures that is mediated by the fibronectin/vitronectin receptor αvβ3 and can be suppressed by vitronectin (Wennerberg et al., 1996; Sakai et al., 1998a,b; Zhang et al., 1999). Thus, the ability of other integrins, besides α5β1, to mediate fibronectin matrix assembly depends on the cell type studied, which may be explained by expression levels or activation state of the integrin involved. Indeed, αIIbβ3 and αvβ3 can promote fibronectin matrix assembly in CHO cells provided that they are stimulated by activating mutations or by certain anti-β3 antibodies (LIBS) (Wu et al., 1995, 1996).
We observe in GDβ3 and GEβ3 cells that high levels of αvβ3 cannot support fibronectin matrix assembly, which is associated with the inability of these cells to support high levels of RhoA activity. Moreover, we report for the first time that expression of a dominant active mutant of RhoA, V14RhoA, can partially restore fibronectin fibrillogenesis in the absence of α5β1. This strongly suggests that part of the explanation for the efficiency with which α5β1 supports matrix assembly lies in its ability to support high levels of RhoA activity.
Inhibition of adherens junctions by integrin-mediated adhesion
Rho–GTPases are critically involved in the regulation of adherens junctions (Fukata and Kaibuchi, 2001). In GD25 fibroblastoid cells and GE11 epithelioid cells, desmosomes are absent and cell–cell contacts occur through adherens junctions (Gimond et al., 1999). RhoA and Rac1 are both required for the formation of adherens junctions (Braga et al., 1997). However, we showed previously that Rac1 activity is also required for the β1-induced disruption of adherens junctions (Gimond et al., 1999). We now find that in cells in which RhoA activity is barely detectable (β3) and in cells with high levels of GTP-bound RhoA (β1) adherens junctions are disrupted. This demonstrates that in our experimental system integrin-mediated stimulation of high RhoA activity is not required for the induction of a scattered phenotype. However, the morphological epithelial-mesenchymal–like transition observed in GEβ3 cells is much less complete than that in GEβ1 cells. Thus, RhoA activity may be required for the more complete switch in morphology from epithelioid to fibroblastoid.
Control of β3 levels by β1 integrins
We find that the ability of β1 integrins to regulate levels of αvβ3 is crucial for the development of a complete fibroblastoid phenotype. Thus, constitutive high levels of expression of αvβ3 in GEβ1 cells leads to a phenotype that appears to be intermediate between that of GEβ1 and GEβ3 cells with cellular protrusions ending in membrane blebs containing small cell matrix adhesions rather than pointed membrane protrusions with focal contacts. Since the level of αvβ3 does not affect the overall RhoA–GTP levels in β1-expressing cells, the inefficient formation of focal contacts in doubly transduced cells must be due to inhibition of RhoA effector pathways or to very local alterations in RhoA activity. Decreased activity of the RhoA–Rho kinase pathway and loss of stress fibers, focal contacts, and fibronectin matrix assembly is observed in many different types of transformed cells. Intriguingly, in these cells the expression of α5β1 is almost invariably switched to the expression of αvβ3 (Plantefaber and Hynes, 1989; Giancotti and Ruoslahti, 1990). Our finding, that these integrins differently regulate the activity of Rho–GTPases and their downstream functions may explain the dramatic morphological changes in tumor cells that undergo such a switch in integrin expression profile.
Materials and methods
Antibodies and other materials
The following mAbs against integrin subunits were used: human β1 (clone TS2/16), human β3 (clone LM609) (Cheresh and Harper, 1987), provided by Dr. David Cheresh (Scripps Research Institute, La Jolla, CA), mouse α5 (clone BMA5) (Fehlner-Gardiner et al., 1996) provided by Dr. Bosco Chan (University of Western Ontario, London, Canada), mouse αv (clone RMV-7; PharMingen), and mouse β3 (clone 2C9.G2; PharMingen). The activating anti-β3 antibody (clone LIBS6) (Frelinger et al., 1991) was a gift from Dr. Mark Ginsberg (Scripps Research Institute, La Jolla, CA). Polyclonal antisera directed against integrin β1, β5, and β6 cytoplasmic domains were provided by Dr. Ulrike Mayer (University of Manchester, Manchester, UK) and Dr. Ed Roos (The Netherlands Cancer Institute, Amsterdam, Netherlands). Other mAbs were directed against fibronectin, FAK, p130Cas, paxillin, Rac1, p190RhoGAP, and phosphotyrosine (clones 10, 77, 21, 165, 102, 30, and RC20, respectively, obtained from Transduction Laboratories), RhoA (clone 26C4; Santa Cruz Biotechnology, Inc.), and vinculin (clone VIIF9; Glukhova et al., 1990) provided by Dr. Marina Glukhova (Institut Curie, Paris, France). Polyclonal antiserum against talin was provided by Dr. Kenneth Yamada (National Institutes of Health, Bethesda, MD) and polyclonal anti-phosphoY397-FAK was obtained from Biosource. Texas red (TR)-conjugated Phalloidin was obtained from Molecular Probes and TR- and HRP-conjugated Streptavidin were purchased from Pierce Chemical Co. Cyclohexamide was obtained from Sigma-Aldrich and used at a concentration of 25 μg/ml. Human plasma fibronectin was purified as described (Danen et al., 2000), and biotinylated fibronectin was prepared using EZ-link Sulfo-NHS-Biotin (Pierce Chemical Co.) according to the manufacturer's protocol. Fibronectin-depleted serum was prepared by passing FBS over a gelatin sepharose column (Amersham Biosciences) twice. GRGDSP and GRGESP peptides were obtained from Life Technologies. The 120-kD chymotryptic fragment of fibronectin was purchased from Chemicon. GST fusion proteins were isolated from bacterial lysates using glutathione-conjugated agarose beads (Amersham Biosciences), washed, eluted using 10 mM reduced glutathione, dialyzed, and analyzed by SDS-PAGE.
Plasmids
The cDNA encoding the human integrin αv subunit was digested from the pcDNA/αv vector (provided by Dr. David Cheresh) as a BamHI-XbaI fragment and cloned into a biscistronic retroviral vector to create LZRS-αv-IRES-zeo. LZRS-β1-IRES-neo encoding the human integrin β1 subunit was described previously (Gimond et al., 1999). The cDNA encoding the human integrin β3 subunit was amplified from pcDNA/β3 (Danen et al., 1996) by PCR with Pwo DNA polymerase using a 5′ primer containing a ClaI site followed by a Kozak sequence and a 3′ primer containing a SnabI site and cloned into a bicistronic retroviral vector to create the LZRS-β3-IRES-neo vector. For the generation of LZRS-β1exβ3in-IRES-neo encoding a β1exβ3in chimeric integrin subunit, the β3 cytoplasmic tail was amplified from pcDNA/β3 by PCR with Pwo DNA polymerase using a 5′ primer in which the codons encoding Lysine716 and Leucine717 were mutated from aaa·ctc to aag·ctt, creating a HindIII site and a 3′ primer containing a SnabI site. This fragment was then fused to the internal HindIII site at that same location 3′ of the transmembrane domain of β1 in LZRS-β1-IRES-neo. For the generation of the expression vector encoding the converse chimera, LZRS-β3exβ1in-IRES-neo, the β3 extracellular and transmembrane region was amplified from pcDNA/β3 with Pwo DNA polymerase using a 5′ primer containing a ClaI site followed by a Kozak sequence and a 3′ primer in which the codons encoding Lysine716 and Leucine717 were mutated from aaa·ctc to aag·ctt, creating a HindIII site, and this fragment was then fused to the internal HindIII site at that same location 5′ of the β1 cytoplasmic tail in LZRS-β1-IRES-neo. Plasmids encoding GFP-tagged tensin and vinculin (Kioka et al., 1999; Zamir et al., 2000) were gifts from Dr. Kenneth Yamada. The retroviral V14RhoA construct was a gift from Dr. John Collard (The Netherlands Cancer Institute, Amsterdam, Netherlands). The cDNA encoding GST–IIIFN12-15ΔV (Gaultier et al., 2002) was a gift from Dr. Dominique Alfandari (University of Virginia, Charlottesville, VA). All constructs were verified by DNA sequence analysis.
Cell culture, cDNA transfections, and retroviral transductions
The GD25 and GE11 cell lines have been described previously: GD25 β1-deficient fibroblastic cells were developed through in vitro differentiation of β1 knockout embryonic stem cells (Fässler and Meyer, 1995) and GE11 β1-deficient epithelioid cells were isolated from β1 chimeric embryos (Gimond et al., 1999). Both cell lines were immortalized with SV-40 large T transducing retrovirus and cultured in DME supplemented with 10% FBS, penicillin, and streptomycin.
cDNA transfections in GD25 and GE11 cells were done using the Effectene kit from QIAGEN according to the manufacturer's protocol. Ecotrophic Phoenix packaging cells (Kinsella and Nolan, 1996) were transfected with amplification-deficient retroviral vectors using the calcium phosphate method to produce virus-containing culture supernatants. Subsequently, retroviral transductions were performed by culturing 105 cells for 8 h with 1 ml cell-free Phoenix supernatant in the presence of 10 μg/ml DOTAP (Boehringer). Cells were then maintained overnight in fresh medium. Transduced cells were sequentially bulk sorted three times by FACS® for integrin surface expression.
Immunofluorescence and flow cytometry
For immunofluorescence, cells were fixed in 2% PFA for 15 min and permeabilized in 0.2% Triton X-100 for 5 min with the exception of fibronectin matrix staining, which was performed on fixed but nonpermeabilized cells unless costaining of cytoskeletal components was performed. The coverslips were then blocked with 2% BSA for 1 h and incubated with primary antibodies for 1 h at RT. After washing with PBS, cells were incubated with FITC or TR-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) or with TR-conjugated phalloidin (Molecular Probes) for 1 h at RT. Preparations were then washed in PBS, mounted in MOWIOL 4-88 solution supplemented with DABCO (Calbiochem), and analyzed with a confocal Leica TCS NT microscope.
For flow cytometry and cell sorting, cells were trypsinized, collected in culture medium, washed once with PBS, and incubated with primary antibodies in PBS containing 2% serum for 1 h at 4°C. Cells were then washed in PBS, incubated with FITC-conjugated secondary antibodies for 1 h at 4°C, washed again, and analyzed on a FACScan® or sorted on a FACStar plus® (Becton Dickinson).
Adhesion assays
Wells of 96-well tissue culture plates were coated with various concentrations of fibronectin in PBS overnight at 4°C, blocked with 2% heat-denatured BSA for 2 h at 37°C, and washed once with PBS. Asynchronously growing cells were trypsinized, collected in culture medium, washed once with PBS, resuspended in DME/0.5% BSA, and added to the wells at 2 × 104 cells per well. After 20 min of incubation at 37°C, unattached cells were removed by rinsing of the plates with PBS, and the remaining attached cells were lysed and stained at 37°C overnight in 3.75 mM p-nitrophenyl N-acetyl-β-d-glucosamide/0.05 M sodium citrate/0.25% Triton X-100. The OD405 was determined in triplicate wells and related to the OD405 measured in wells in which all 2 × 104 cells were stained to calculate the percentage of adhered cells.
Biotinylation, immunoprecipitation, and Western blot analysis
For biotinylation of integrins at the cell surface, adherent cultures were serum starved for 30 min, washed with PBS, incubated with 0.5 mg/ml sulfo-NHS-biotin (Pierce Chemical Co.) in PBS for 30 min, and washed three times with PBS. For immunoprecipitation of biotinylated integrins, cells were lysed in Nonidet P-40 lysis buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1% Nonidet P-40, 10% glycerol, 10 mM MgCl2, supplemented with a protease inhibitor mix [Sigma-Aldrich]) and for immunoprecipitation of FAK, p130Cas, and p190RhoGAP, cells were lysed in modified RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1% sodium deoxycholate, 0.1% SDS, 1 mM sodium orthovanadate, and a protease inhibitor mix [Sigma-Aldrich]). Lysates were clarified by centrifugation at 14,000 rpm for 20 min at 4°C. To remove nonspecific binding, clarified lysates were then incubated overnight at 4°C with control IgG, and complexes were collected with 10 μl GammaBind (Amersham Biosciences). For immunoprecipitations, cleared lysates were incubated with 2 μg/ml of the specific antibodies for 2 h at 4°C, and immune complexes were collected with 10 μl GammaBind (Amersham Biosciences), washed three times with lysis buffer, and solubilized in Laemmli sample buffer. Total cell lysates or immunoprecipitates were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore), and analyzed by Western blotting followed by ECL using the SuperSignal system (Pierce Chemical Co.).
DOC insolubility assays
Cells were plated on fibronectin-coated dishes in serum-free medium for 4 h and subsequently incubated for an additional 20 h in medium containing 10% fibronectin-depleted serum and 10 μg/ml biotinylated fibronectin. After washing with PBS, cells were lysed in DOC buffer (20 mM Tris-HCl, pH 8.5, 1% sodium deoxycholate [DOC], 2 mM N-ethylmaleimide, 2 mM iodoacetic acid, 2 mM EDTA, 2 mM PMSF) and passed five times through a 23 GA needle. DOC-insoluble material was collected by centrifugation at 14,000 rpm for 20 min at 4°C, washed once with DOC buffer, resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore), and analyzed by Western blotting using HRPO-conjugated streptavidin.
Biochemical assays for activity of Rho–GTPases
Cells growing subconfluently in standard medium or cells that had been serum starved overnight and replated on fibronectin-coated dishes were lysed in 1 ml Nonidet P-40 lysis buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1% Nonidet P-40, 10% glycerol, 10 mM MgCl2, supplemented with a protease inhibitor mix [Sigma-Aldrich]), and lysates were clarified by centrifugation at 14,000 rpm for 20 min at 4°C. A 1% aliquot was removed for determination of total quantities of the GTPase being analyzed. Clarified lysates were then incubated for 45 min at 4°C with a GST fusion protein of the Rho-binding domain of the Rho effector protein Rhotekin (Ren et al., 1999) or with a biotinylated peptide corresponding to the Cdc42/Rac interactive binding motif in PAK1B (provided by J. Collard; some Rac assays were repeated with a GST fusion protein of the Rac-binding domain of PAK1B and gave identical results). Complexes were bound to glutathione- or streptavidin-conjugated beads, respectively, and washed three times in Nonidet P-40 lysis buffer. The samples were analyzed by 14% SDS-PAGE and Western blotting using RhoA and Rac1 antibodies to detect bound activated GTPases.
Acknowledgments
We thank Dominique Alfandari, Bosco Chan, David Cheresh, John Collard, Mark Ginsberg, Marina Glukhova, Ed Roos, Ulrike Mayer, and Kenneth Yamada for their generous gifts of antibodies and plasmids. We thank Lauran Oomen and Lenny Brocks for their excellent assistance with the confocal microscopy and Roumen Pankov for his advice on the DOC insolubility assays.
This work was supported by grants from the Dutch Cancer Society (NKI 1999-2117 and NKI 2001-2488) and the German Research Foundation (SFB-413).
C. Brakebusch's and R. Fässler's present address is Department of Molecular Medicine, Max Planck Institute of Biochemistry, 82152 Martinsried, Germany.
Footnotes
Abbreviations used in this paper: CCBD, central cell-binding domain; IIIFN, fibronectin type 3 repeat; GAP, GTPase-activating protein; TR, Texas red.
References
- Aota, S., M. Nomizu, and K. Yamada. 1994. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 269:24756–24761. [PubMed] [Google Scholar]
- Arthur, W., L. Petch, and K. Burridge. 2000. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10:719–722. [DOI] [PubMed] [Google Scholar]
- Baneyx, G., L. Baugh, and V. Vogel. 2001. Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. USA. 98:14464–14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, S., H. Flinn, M. Humphries, D. Critchley, and A. Ridley. 1997. Requirement for Rho in integrin signalling. Cell Adhes. Commun. 4:387–398. [DOI] [PubMed] [Google Scholar]
- Berrier, A.L., R. Martinez, G.M. Bokoch, and S.L. LaFlamme. 2002. Integrin β tails activate Rac1. J. Cell Sci. 115:4285–4291 [DOI] [PubMed] [Google Scholar]
- Bowditch, R., M. Hariharan, E. Tominna, J. Smith, K. Yamada, E. Getzoff, and M. Ginsberg. 1994. Identification of a novel integrin binding site in fibronectin. Differential utilization by beta 3 integrins. J. Biol. Chem. 269:10856–10863. [PubMed] [Google Scholar]
- Braga, V., L. Machesky, A. Hall, and N. Hotchin. 1997. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J. Cell Biol. 137:1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh, D., and J. Harper. 1987. Arg-Gly-Asp recognition by a cell adhesion receptor requires its 130-kDa alpha subunit. J. Biol. Chem. 262:1434–1437. [PubMed] [Google Scholar]
- Christopher, R., A. Kowalczyk, and P. McKeown-Longo. 1997. Localization of fibronectin matrix assembly sites on fibroblasts and endothelial cells. J. Cell Sci. 110:569–581. [DOI] [PubMed] [Google Scholar]
- Cukierman, E., R. Pankov, D. Stevens, and K. Yamada. 2001. Taking cell-matrix adhesions to the third dimension. Science. 294:1708–1712. [DOI] [PubMed] [Google Scholar]
- Danen, E., and K. Yamada. 2001. Fibronectin, integrins, and growth control. J. Cell. Physiol. 189:1–13. [DOI] [PubMed] [Google Scholar]
- Danen, E., S. Aota, A. van Kraats, K. Yamada, D. Ruiter, and G. van Muijen. 1995. Requirement for the synergy site for cell adhesion to fibronectin depends on the activation state of integrin alpha 5 beta 1. J. Biol. Chem. 270:21612–21618. [DOI] [PubMed] [Google Scholar]
- Danen, E., A. van Kraats, I. Cornelissen, D. Ruiter, and G. van Muijen. 1996. Integrin beta 3 cDNA transfection into a highly metastatic alpha v beta 3-negative human melanoma cell line inhibits invasion and experimental metastasis. Biochem. Biophys. Res. Commun. 226:75–81. [DOI] [PubMed] [Google Scholar]
- Danen, E., P. Sonneveld, A. Sonnenberg, and K. Yamada. 2000. Dual stimulation of Ras/mitogen-activated protein kinase and RhoA by cell adhesion to fibronectin supports growth factor–stimulated cell cycle progression. J. Cell Biol. 151:1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fässler, R., and M. Meyer. 1995. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 9:1896–1908. [DOI] [PubMed] [Google Scholar]
- Fehlner-Gardiner, C., S. Uniyal, C. von Ballestrem, and B. Chan. 1996. Differential utilization of VLA-4 (alpha 4 beta 1) and -5 (alpha 5 beta 1) integrins during the development of mouse bone marrow-derived mast cells. Differentiation. 60:317–325. [DOI] [PubMed] [Google Scholar]
- Flinn, H., and A. Ridley. 1996. Rho stimulates tyrosine phosphorylation of focal adhesion kinase, p130 and paxillin. J. Cell Sci. 109:1133–1141. [DOI] [PubMed] [Google Scholar]
- Frelinger, A., III, X. Du, E. Plow, and M. Ginsberg. 1991. Monoclonal antibodies to ligand-occupied conformers of integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J. Biol. Chem. 266:17106–17111. [PubMed] [Google Scholar]
- Fukata, M., and K. Kaibuchi. 2001. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat. Rev. Mol. Cell Biol. 2:887–897. [DOI] [PubMed] [Google Scholar]
- Gaultier, A., H. Cousin, T. Darribere, and D. Alfandari. 2002. ADAM13 disintegrin and cysteine-rich domains bind to the second heparin-binding domain of fibronectin. J. Biol. Chem. 277:23336–23344. [DOI] [PubMed] [Google Scholar]
- Geiger, B., A. Bershadsky, R. Pankov, and K. Yamada. 2001. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2:793–805. [DOI] [PubMed] [Google Scholar]
- George, E., E. Georges-Labouesse, R. Patel-King, H. Rayburn, and R. Hynes. 1993. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 119:1079–1091. [DOI] [PubMed] [Google Scholar]
- Giancotti, F., and E. Ruoslahti. 1990. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 60:849–859. [DOI] [PubMed] [Google Scholar]
- Giancotti, F. 2000. Complexity and specificity of integrin signalling. Nat. Cell Biol. 2:E13–E14. [DOI] [PubMed] [Google Scholar]
- Gimond, C., A. van der Flier, S. van Delft, C. Brakebusch, I. Kuikman, J. Collard, R. Fassler, and A. Sonnenberg. 1999. Induction of cell scattering by expression of beta1 integrins in beta1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function. J. Cell Biol. 147:1325–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova, M., M. Frid, and V. Koteliansky. 1990. Developmental changes in expression of contractile and cytoskeletal proteins in human aortic smooth muscle. J. Biol. Chem. 265:13042–13046. [PubMed] [Google Scholar]
- Gumbiner, B. 1996. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 84:345–357. [DOI] [PubMed] [Google Scholar]
- Hall, A., and C. Nobes. 2000. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness, M., J. Ash, and S. Singer. 1978. Transmembrane linkage of fibronectin to intracellular actin-containing filaments in cultured human fibroblasts. Ann. NY Acad. Sci. 312:414–417. [DOI] [PubMed] [Google Scholar]
- Hocking, D., J. Sottile, and P. McKeown-Longo. 1994. Fibronectin's III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J. Biol. Chem. 269:19183–19187. [PubMed] [Google Scholar]
- Hynes, R.O. 1990. Fibronectins. Springer-Verlag New York, Inc., New York. 546 pp.
- Hynes, R. 2002. Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687. [DOI] [PubMed] [Google Scholar]
- Hynes, R., and A. Destree. 1978. Relationships between fibronectin (LETS protein) and actin. Cell. 15:875–886. [DOI] [PubMed] [Google Scholar]
- Hynes, R., and Q. Zhao. 2000. The evolution of cell adhesion. J. Cell Biol. 150:F89–F96. [DOI] [PubMed] [Google Scholar]
- Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 12:539–544 [DOI] [PubMed] [Google Scholar]
- Kinsella, T., and G. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405–1413. [DOI] [PubMed] [Google Scholar]
- Kioka, N., S. Sakata, T. Kawauchi, T. Amachi, S. Akiyama, K. Okazaki, C. Yaen, K. Yamada, and S. Aota. 1999. Vinexin: a novel vinculin-binding protein with multiple SH3 domains enhances actin cytoskeletal organization. J. Cell Biol. 144:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, H., S. Li, Y. Hu, S. Yuan, Y. Zhao, B. Chen, W. Puzon-McLaughlin, T. Tarui, J. Shyy, Y. Takada, and S. Usami. 2002. Differential regulation of Rho GTPases by β1 and β3 integrins: the role of the extracellular domain of integrin in intracellular signaling. J. Cell Sci. 115:2199–2206. [DOI] [PubMed] [Google Scholar]
- Monier-Gavelle, F., and J. Duband. 1997. Cross talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by beta1 and beta3 integrins in migrating neural crest cells. J. Cell Biol. 137:1663–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher, D. 1995. Organization of the provisional fibronectin matrix: control by products of blood coagulation. Thromb. Haemost. 74:529–533. [PubMed] [Google Scholar]
- Nobes, C., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 81:53–62. [DOI] [PubMed] [Google Scholar]
- Noren, N., C. Niessen, B. Gumbiner, and K. Burridge. 2001. Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276:33305–33308. [DOI] [PubMed] [Google Scholar]
- Ohashi, T., D. Kiehart, and H. Erickson. 1999. Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc. Natl. Acad. Sci. USA. 96:2153–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov, R., E. Cukierman, B. Katz, K. Matsumoto, D. Lin, S. Lin, C. Hahn, and K. Yamada. 2000. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J. Cell Biol. 148:1075–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantefaber, L., and R. Hynes. 1989. Changes in integrin receptors on oncogenically transformed cells. Cell. 56:281–290. [DOI] [PubMed] [Google Scholar]
- Price, L., J. Leng, M. Schwartz, and G. Bokoch. 1998. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell. 9:1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X., W. Kiosses, and M. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X., W. Kiosses, D. Sieg, C. Otey, D. Schlaepfer, and M. Schwartz. 2000. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 113:3673–3678. [DOI] [PubMed] [Google Scholar]
- Retta, S., G. Cassara, M. D'Amato, R. Alessandro, M. Pellegrino, S. Degani, G. De Leo, L. Silengo, and G. Tarone. 2001. Cross talk between beta(1) and alpha(V) integrins: beta(1) affects beta(3) mRNA stability. Mol. Biol. Cell. 12:3126–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A., and A. Hall. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 70:389–399. [DOI] [PubMed] [Google Scholar]
- Rottner, K., A. Hall, and J. Small. 1999. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9:640–648. [DOI] [PubMed] [Google Scholar]
- Sakai, T., O. Peyruchaud, R. Fassler, and D. Mosher. 1998. a. Restoration of beta1A integrins is required for lysophosphatidic acid-induced migration of beta1-null mouse fibroblastic cells. J. Biol. Chem. 273:19378–19382. [DOI] [PubMed] [Google Scholar]
- Sakai, T., Q. Zhang, R. Fassler, and D. Mosher. 1998. b. Modulation of beta1A integrin functions by tyrosine residues in the beta1 cytoplasmic domain. J. Cell Biol. 141:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoncella, S., F. Echtermeyer, F. Denhez, J. Nowlen, D. Mosher, S. Robinson, R. Hynes, and P. Goetinck. 1999. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and stress fibers. Proc. Natl. Acad. Sci. USA. 96:2805–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M. 2001. Integrin signaling revisited. Trends Cell Biol. 11:466–470. [DOI] [PubMed] [Google Scholar]
- Schwartz, M., and S. Shattil. 2000. Signaling networks linking integrins and rho family GTPases. Trends Biochem. Sci. 25:388–391. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer, J., and J. Sechler. 1999. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr. Opin. Cell Biol. 11:622–627. [DOI] [PubMed] [Google Scholar]
- Sechler, J., H. Rao, A. Cumiskey, I. Vega-Colon, M. Smith, T. Murata, and J. Schwarzbauer. 2001. A novel fibronectin binding site required for fibronectin fibril growth during matrix assembly. J. Cell Biol. 154:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith, J., J. Lunn, D. Leopoldt, and E. Rozengurt. 2001. Y-27632, an inhibitor of Rho-associated kinases, prevents tyrosine phosphorylation of focal adhesion kinase and paxillin induced by bombesin: dissociation from tyrosine phosphorylation of p130(CAS). Exp. Cell Res. 266:292–302. [DOI] [PubMed] [Google Scholar]
- Taylor, J., J. Hildebrand, C. Mack, M. Cox, and J. Parsons. 1998. Characterization of graf, the GTPase-activating protein for rho associated with focal adhesion kinase. Phosphorylation and possible regulation by mitogen-activated protein kinase. J. Biol. Chem. 273:8063–8070. [DOI] [PubMed] [Google Scholar]
- Taylor, J., M. Macklem, and J. Parsons. 1999. Cytoskeletal changes induced by GRAF, the GTPase regulator associated with focal adhesion kinase, are mediated by Rho. J. Cell Sci. 112:231–242. [DOI] [PubMed] [Google Scholar]
- van der Flier, A., and A. Sonnenberg. 2001. Function and interactions of integrins. Cell Tissue Res. 305:285–298. [DOI] [PubMed] [Google Scholar]
- Vleminckx, K., and R. Kemler. 1999. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays. 21:211–220. [DOI] [PubMed] [Google Scholar]
- von Schlippe, M., J. Marshall, P. Perry, M. Stone, A. Zhu, and I. Hart. 2000. Functional interaction between E-cadherin and alphav-containing integrins in carcinoma cells. J. Cell Sci. 113:425–437. [DOI] [PubMed] [Google Scholar]
- Weaver, V., O. Petersen, F. Wang, C. Larabell, P. Briand, C. Damsky, and M. Bissell. 1997. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137:231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg, K., L. Lohikangas, D. Gullberg, M. Pfaff, S. Johansson, and R. Fassler. 1996. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J. Cell Biol. 132:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, A. 2001. Syndecans: transmembrane modulators of adhesion and matrix assembly. J. Clin. Invest. 107:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C., J. Bauer, R. Juliano, and J. McDonald. 1993. The alpha 5 beta 1 integrin fibronectin receptor, but not the alpha 5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J. Biol. Chem. 268:21883–21888. [PubMed] [Google Scholar]
- Wu, C., V. Keivens, T. O'Toole, J. McDonald, and M. Ginsberg. 1995. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 83:715–724. [DOI] [PubMed] [Google Scholar]
- Wu, C., P. Hughes, M. Ginsberg, and J. McDonald. 1996. Identification of a new biological function for the integrin alpha v beta 3: initiation of fibronectin matrix assembly. Cell Adhes. Commun. 4:149–158. [DOI] [PubMed] [Google Scholar]
- Yang, J., and R. Hynes. 1996. Fibronectin receptor functions in embryonic cells deficient in alpha 5 beta 1 integrin can be replaced by alpha V integrins. Mol. Biol. Cell. 7:1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir, E., M. Katz, Y. Posen, N. Erez, K. Yamada, B. Katz, S. Lin, D. Lin, A. Bershadsky, Z. Kam, and B. Geiger. 2000. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2:191–196. [DOI] [PubMed] [Google Scholar]
- Zhang, Q., M. Magnusson, and D. Mosher. 1997. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol. Biol. Cell. 8:1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., T. Sakai, J. Nowlen, I. Hayashi, R. Fassler, and D. Mosher. 1999. Functional beta1-integrins release the suppression of fibronectin matrix assembly by vitronectin. J. Biol. Chem. 274:368–375. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., A. Morla, K. Vuori, J. Bauer, R. Juliano, and E. Ruoslahti. 1993. The alpha v beta 1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J. Cell Biol. 122:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, C., M. Chrzanowska-Wodnicka, J. Brown, A. Shaub, A. Belkin, and K. Burridge. 1998. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]