Abstract
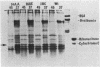
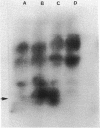
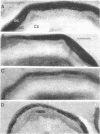
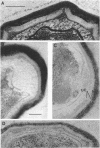
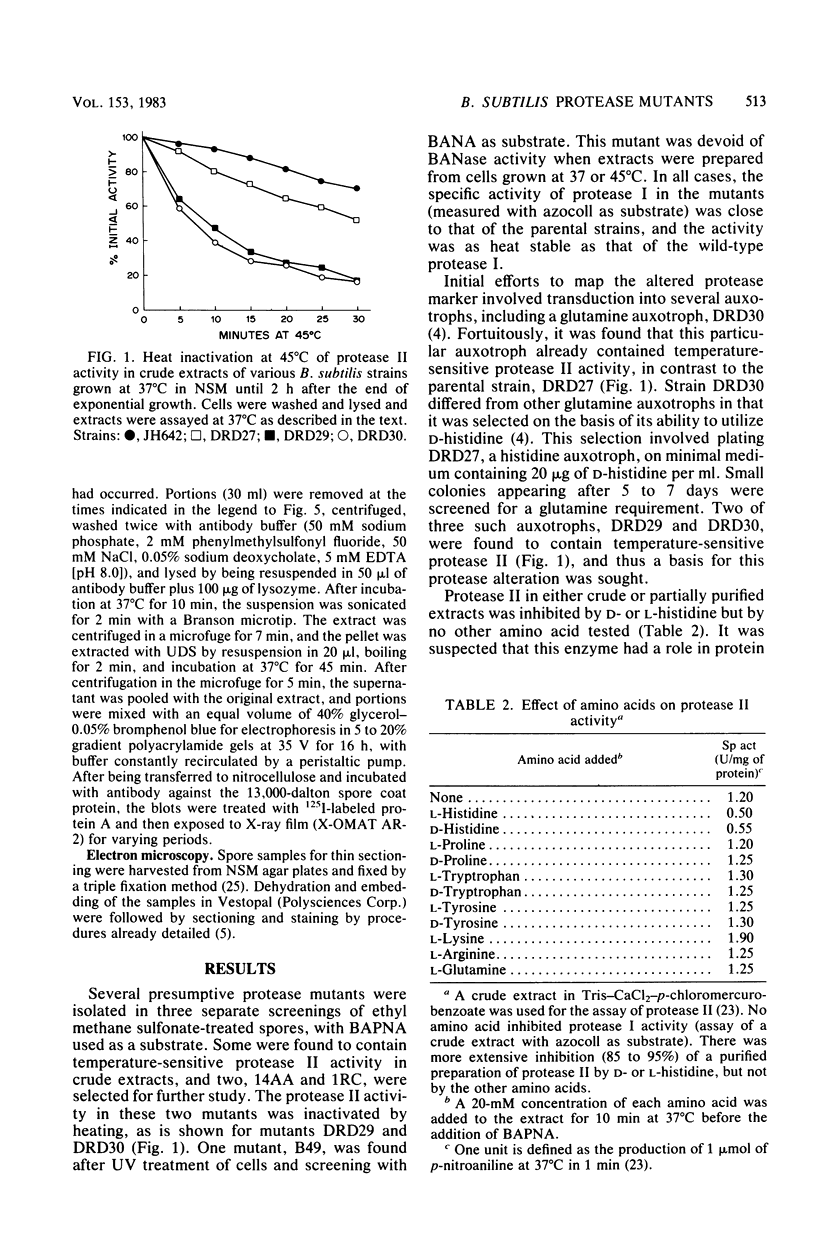
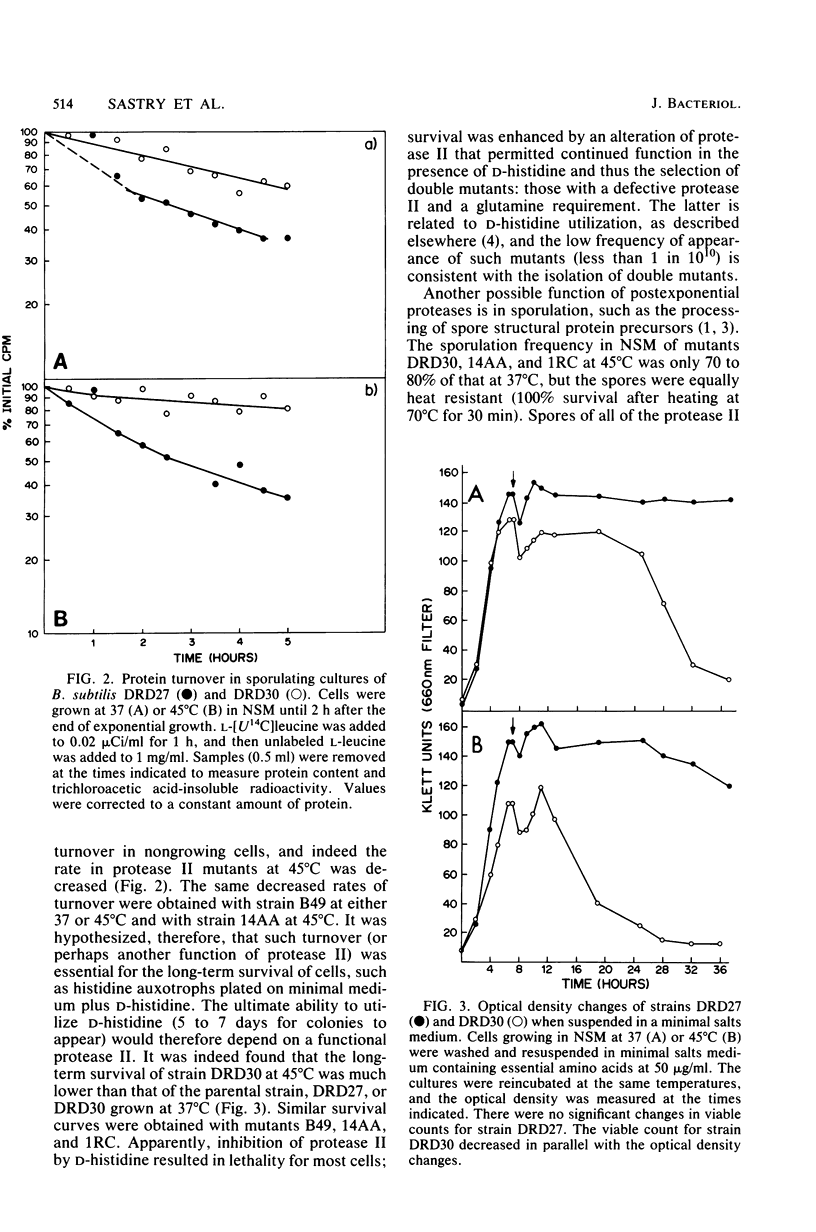
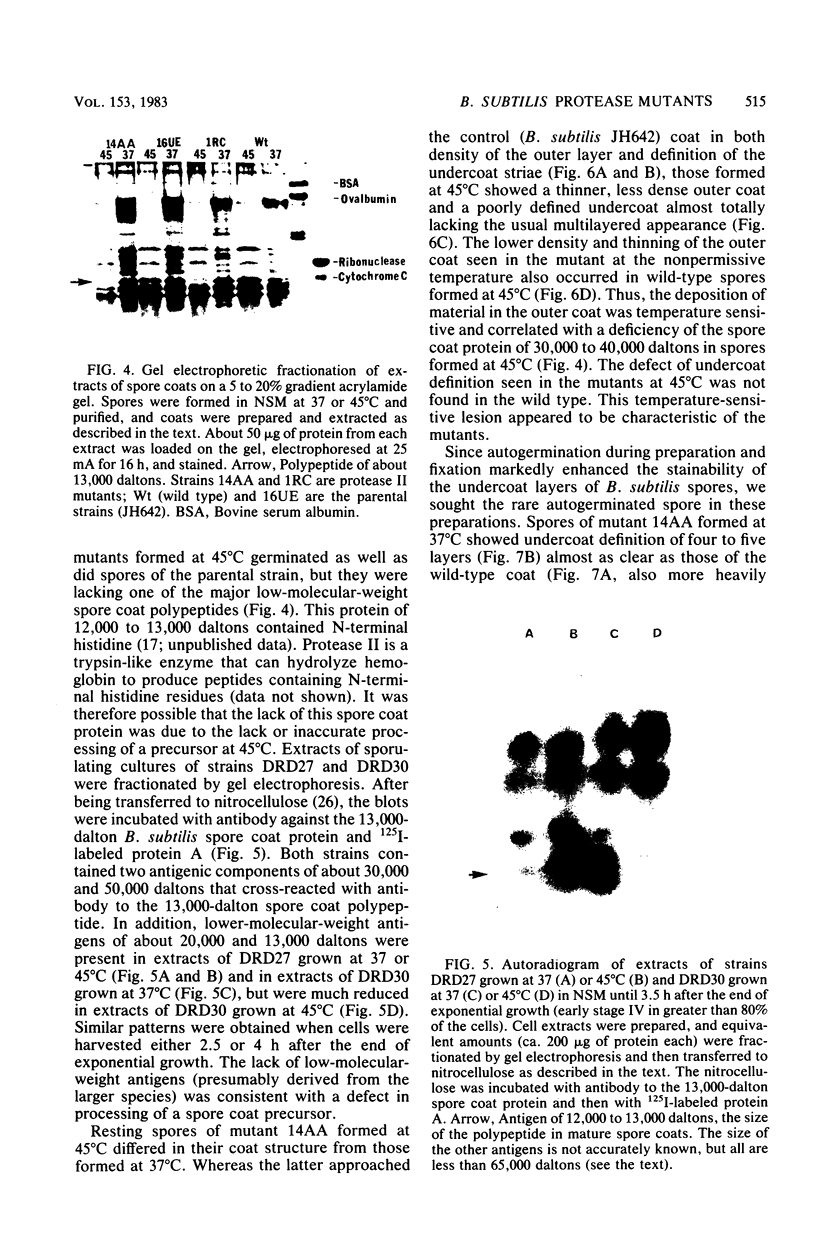
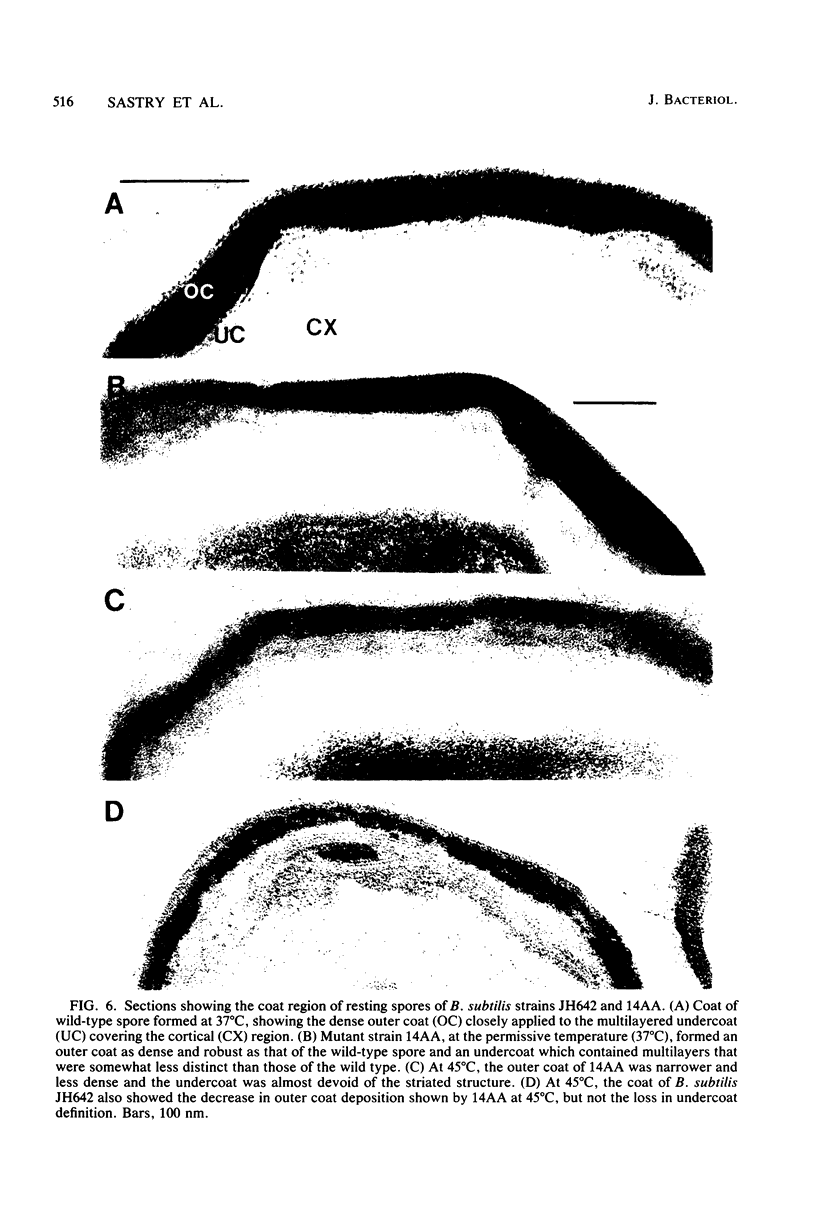
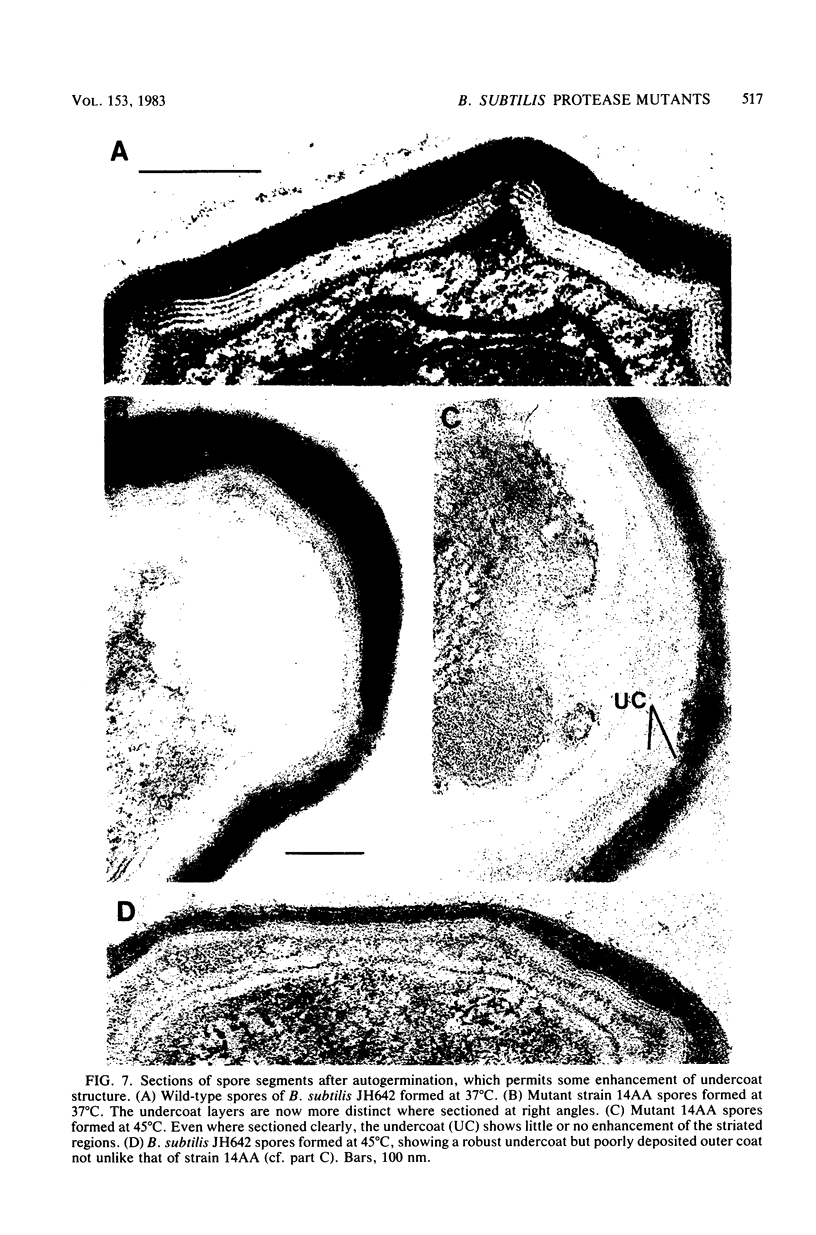
A colony screening procedure was devised to detect Bacillus subtilis mutants containing temperature-sensitive trypsin-like intracellular protease activity. The enzyme was characterized as a non-sulfhydryl serine protease on the basis of inhibitor studies. It was also inhibited by D- or L-histidine but not by any other amino acid tested. The long-term survival at 45 degrees C of these mutants in a minimal salts medium was decreased, with rapid lysis occurring within 24 h. A D-histidine function in long-term survival and inhibition accounted for the presence of additional protease mutants among survivors of histidine auxotrophs selected for their ability to utilize D-histidine. In addition to being lysed when incubated at 45 degrees C under nongrowth conditions, all of the protease mutants had a decreased rate of protein turnover and produced spores deficient in a major low-molecular-weight spore coat polypeptide. The morphology of the undercoat layers was altered, but there was no effect on spore heat resistance or on germination. The missing spore coat polypeptide appeared to be processed from a larger precursor by cleavage to produce N-terminal histidine. A defect in this protease could account for the lack of processing and thus the absence of this polypeptide in spore coats.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I. Synthesis of Bacillus cereus spore coat protein. J Bacteriol. 1981 Jan;145(1):541–547. doi: 10.1128/jb.145.1.541-547.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. A new assay for cathepsin B1 and other thiol proteinases. Anal Biochem. 1972 May;47(1):280–293. doi: 10.1016/0003-2697(72)90302-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Alterations of spore coat processing and protein turnover in a Bacillus cereus mutant with a defective postexponential intracellular protease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1254–1258. doi: 10.1073/pnas.74.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. R., Aronson A. I. Selection of Bacillus subtilis mutants impaired in ammonia assimilation. J Bacteriol. 1980 Feb;141(2):985–988. doi: 10.1128/jb.141.2.985-988.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Tipper D. J. Bacillus subtilis spore coats: complexity and purification of a unique polypeptide component. J Bacteriol. 1978 Sep;135(3):1091–1106. doi: 10.1128/jb.135.3.1091-1106.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. Effects of mutational loss of specific intracellular proteases on the sporulation of Bacillus subtilis. J Bacteriol. 1973 May;114(2):612–617. doi: 10.1128/jb.114.2.612-617.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Spizizen J. Increased rate of asporogenous mutations following treatment of Bacillus subtilis spores with ethyl methanesulfonate. Mutat Res. 1971 Sep;13(1):93–96. doi: 10.1016/0027-5107(71)90130-8. [DOI] [PubMed] [Google Scholar]
- Kerjan P., Keryer E., Szulmajster J. Characterization of a thermosensitive sporulation mutant of Bacillus subtilis affected in the structural gene of an intracellular protease. Eur J Biochem. 1979 Aug 1;98(2):353–362. doi: 10.1111/j.1432-1033.1979.tb13194.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J. Characterization of a protein inhibitor of intracellular protease from Bacillus subtilis. FEBS Lett. 1977 Feb 15;74(1):59–61. doi: 10.1016/0014-5793(77)80752-7. [DOI] [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Millet J., Larribe M., Aubert J. P. Mutant thermosensible de B. subtilis affecté dans la sporulation et la sérylprotéase extracellulaire. Biochimie. 1976;58(1-2):109–117. doi: 10.1016/s0300-9084(76)80361-6. [DOI] [PubMed] [Google Scholar]
- Munoz L., Sadaie Y., Doi R. H. Spore coat protein of Bacillus subtilis. Structure and precursor synthesis. J Biol Chem. 1978 Oct 10;253(19):6694–6701. [PubMed] [Google Scholar]
- Pandey N. K., Aronson A. I. Properties of the Bacillus subtilis spore coat. J Bacteriol. 1979 Mar;137(3):1208–1218. doi: 10.1128/jb.137.3.1208-1218.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava O. P., Aronson A. I. Isolation and characterization of a unique protease from sporulating cells of Bacillus subtilis. Arch Microbiol. 1981 May;129(3):227–232. doi: 10.1007/BF00425256. [DOI] [PubMed] [Google Scholar]
- Stepanov V. M., Strongin A. Y., Izotova L. S., Abramov Z. T., Lyublinskaya L. A., Ermakova L. M., Baratova L. A., Belyanova L. P. Intracellular serine protease from Bacillus subtilis. Structural comparison with extracellular serine proteases-subtilisins. Biochem Biophys Res Commun. 1977 Jul 11;77(1):298–305. doi: 10.1016/s0006-291x(77)80196-4. [DOI] [PubMed] [Google Scholar]
- Stevenson K. E., Vaughn R. H., Crisan E. V. Fixation of mature spores of Clostridium botulinum. J Bacteriol. 1972 Mar;109(3):1295–1297. doi: 10.1128/jb.109.3.1295-1297.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Yuan K., Johnson W. C., Tipper D. J., Setlow P. Comparison of various properties of low-molecular-weight proteins from dormant spores of several Bacillus species. J Bacteriol. 1981 Jun;146(3):965–971. doi: 10.1128/jb.146.3.965-971.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]