Abstract
Autophagy is the process whereby cytoplasmic cargo (e.g., protein and organelles) are sequestered within a double membrane–enclosed transport vesicle and degraded after vesicle fusion with the vacuole/lysosome. Current evidence suggests that the Vps34 phosphatidylinositol 3-kinase is essential for macroautophagy, a starvation-induced autophagy pathway (Kihara et al., 2001). Here, we characterize a requirement for Vps34 in constitutive autophagy by the cytoplasm-to-vacuole targeting (Cvt) pathway. First, we show that transient disruption of phosphatidylinositol (PtdIns) 3-phosphate (PtdIns[3]P) synthesis through inactivation of temperature-sensitive Vps34 or its upstream activator, Vps15, blocks the Cvt and macroautophagy pathways. Yet, PtdIns(3)P-binding FYVE domain-containing proteins, which mediate carboxypeptidase Y (CPY) transport to the vacuole by the CPY pathway, do not account for the requirement of Vps34 in autophagy. Using a genetic selection designed to isolate PtdIns(3)P-binding effectors of Vps34, we identify Etf1, an uncharacterized type II transmembrane protein. Although Etf1 does not contain a known 3-phosphoinositide–binding domain (i.e., FYVE or Phox), we find that Etf1 interacts with PtdIns(3)P and that this interaction requires a basic amino acid motif (KKPAKK) within the cytosolic region of the protein. Moreover, deletion of ETF1 or mutation of the KKPAKK motif results in strong sorting defects in the Cvt pathway but not in macroautophagy or in CPY sorting. We propose that Vps34 regulates the CPY, Cvt, and macroautophagy pathways through distinct sets of PtdIns(3)P-binding effectors and that Vps34 promotes protein trafficking in the Cvt pathway through activation/localization of the effector protein Etf1.
Keywords: Cvt vesicle; Vps15; phosphoinositide; vacuole; Apg
Introduction
In both yeast and mammalian cells, autophagy has been characterized as a nonclassical membrane-trafficking pathway, which functions to transport proteins and organelles directly from the cytoplasm of the cell to the lumen of the vacuole/lysosome (Dunn, 1990a,b; Baba et al., 1994). In yeast, several distinct autophagy pathways have been identified, including the cytoplasm-to-vacuole targeting (Cvt)* pathway and macroautophagy (Harding et al., 1996; Scott et al., 1996; Baba et al., 1997). The Cvt pathway functions constitutively under vegetative growth conditions to deliver specific cytosolic components, such as the hydrolase precursor, aminopeptidase (AP)I, to the vacuole lumen where the API zymogen is proteolytically clipped to its active form (Klionsky et al., 1992). In contrast, macroautophagy is an inducible pathway, stimulated in response to nitrogen/carbon starvation to carry out the bulk transport of cytoplasmic organelles and proteins, including newly synthesized API, to the vacuole (Takeshige et al., 1992; Baba et al., 1994, 1997). The Cvt and macroautophagy pathways are mechanistically similar, each entailing the sequestration of cytoplasmic cargo into a double membrane transport intermediate known as the Cvt vesicle (150 nm) or the autophagosome (400–900 nm), respectively, which then dock and fuse with the vacuole/lysosome (Takeshige et al., 1992; Baba et al., 1997; Scott et al., 1997).
Genetic and biochemical studies in yeast have resolved autophagy into multiple essential stages, which include cargo recruitment, Cvt vesicle/autophagosome formation, and the docking/fusion of these transport vesicles with the vacuole/lysosome (Kim and Klionsky, 2000; Klionsky and Emr, 2000). Most autophagy mutants in yeast block a specific stage of both the Cvt and macroautophagy pathways, indicating that there is significant genetic overlap between constitutive and inducible autophagic pathways (Tsukada and Ohsumi, 1993; Harding et al., 1995, 1996). For instance, two ubiquitin-like conjugation systems in which the multispecificity Apg7 E1-activating enzyme covalently links Apg12, a ubiquitin-like molecule, with Apg5 and also links Aut7/Apg8 with phosphatidylethanolamine are required for the formation/sequestration of both the Cvt vesicle and autophagosome (Kim and Klionsky, 2000; Ohsumi, 2001). Components of the autophagic transport machinery appear to be conserved in higher eukaryotes, since human orthologues of Apg12, Apg5, and Aut7/Apg8 have been identified (Mizushima et al., 1998; Kabeya et al., 2000). Several proteins which regulate the fusion of Cvt and macroautophagy pathway transport intermediates with the vacuole have also been defined to include the vacuolar t-SNARE Vam3, the SNAP-25 homologue Vam7, and the rab GTPase Ypt7 (Darsow et al., 1997; Sato et al., 1998; Kim et al., 1999; Wurmser et al., 2000).
A relatively small group of specialized factors are believed to control interconversion between the Cvt and macroautophagy pathways. A pivotal player underlying the shift from Cvt trafficking to macroautophagy is the Tor protein kinase, which regulates the activity of ribosomal protein S6 through phosphorylation of p70S6 kinase and the expression of starvation-specific genes (Brown et al., 1994; Beck and Hall, 1999). Hence, exposure of cells to rapamycin, a Tor kinase inhibitor, reproduces the effect of nutrient starvation and induces macroautophagy (Noda and Ohsumi, 1998). In addition, stimulation of macroautophagy results in the transcriptional upregulation of AUT7/APG8, an event critical to the formation of the macroautophagic transport intermediate, the autophagosome (Kirisako et al., 1999; Huang et al., 2000). Proteins which function in the Cvt pathway but not macroautophagy, such as Tlg2 (a t-SNARE), Cvt9, and Cvt20, have also been identified and are believed to function as key determinants which promote the Cvt pathway over macroautophagy under nutrient-rich conditions (Abeliovich et al., 1999; Kim et al., 2001b; Leber et al., 2001).
The lipid, phosphatidylinositol (PtdIns) 3-phosphate (PtdIns[3]P), may also play a regulatory role in autophagy. PtdIns(3)P is a member of a family of phosphorylated derivatives of phosphatidylinositol, which serve as integral membrane second messengers, functioning to localize and/or modify the activity of specific downstream effector proteins (Rameh and Cantley, 1999; Wurmser et al., 1999; Sato et al., 2001). Phosphoinositides are required for cell growth, apoptosis, and membrane trafficking, necessitating the acute maintenance of phosphoinositide levels through the antagonistic action of lipid kinases and phosphatases/lipases, which mediate phosphoinositide synthesis and turnover, respectively (Wurmser et al., 1999). It has been found that treatment of a human cell line with wortmannin, a phosphoinositide (PI) 3-kinase inhibitor, impaired macroautophagy and, conversely, that exogenous addition of PtdIns(3)P to these cells promoted macroautophagy, strongly implicating a role for PtdIns(3)P in this process (Petiot et al., 2000). Consistent with these observations, Vps34, the only known Saccharomyces cerevisiae PtdIns 3-kinase, coimmunoprecipitates with two proteins which regulate autophagy, Apg6/Vps30, and Apg14 (Kihara et al., 2001). Additionally, deletion of VPS34 disrupts nitrogen starvation–induced formation of the autophagosome (Kihara et al., 2001). In Hansenula polymorpha, it has been shown that a homologue of Vps34 is required in the autophagy and subsequent degradation of peroxisomes (Kiel et al., 1999). This data links yeast Vps34 and its human counterpart, hVps34 (Volinia et al., 1995), to autophagy, although the relevant PtdIns(3)P-binding effector(s) of Vps34 has not been defined.
The Vps34 PtdIns 3-kinase is best characterized as a key regulator in the carboxypeptidase Y (CPY) pathway, which delivers newly synthesized proteins, such as the hydrolase CPY, from the trans-Golgi network to an endosomal compartment and, subsequently, to the vacuole (Schu et al., 1993). Recruitment of Vps34 to the Golgi/endosome and activation of its PtdIns 3-kinase activity is performed by Vps15, a membrane-associated serine/threonine protein kinase (Stack et al., 1995). Multiple proteins essential for CPY transport, including Vac1/Vps19 and Vps27, contain either a FYVE domain or, in the case of Vam7, a Phox homology (PX) domain, two motifs which bind PtdIns(3)P (Burd and Emr, 1998; Wurmser et al., 1999; Sato et al., 2001; Wishart et al., 2001). Mutations within the FYVE domain of Vac1 or the PX domain of Vam7 that disrupt PtdIns(3)P binding also impair the vacuolar transport of CPY (Burd et al., 1997; Burd and Emr, 1998; Sato et al., 1998; Cheever et al., 2001), confirming a direct role for PtdIns(3)P in Golgi-to-vacuole sorting.
In this paper, we present evidence indicating that FYVE domain–containing proteins (e.g., Vac1 and Vps27) do not account for the requirement of Vps34 in the formation of Cvt vesicles or autophagosomes, raising the possibility that additional as yet undefined effectors of Vps34 exist. We identify Etf1 as a novel PtdIns(3)P-binding protein and provide data supporting a role for Etf1 as a downstream effector of Vps34 specific to autophagy.
Results
A genetic screen for potential effectors of the Vps34 PtdIns 3-kinase
Vps34 PtdIns 3-kinase activity is required for CPY pathway trafficking between the Golgi apparatus and vacuole (Stack et al., 1995). It has also been suggested that Vps34 functions in macroautophagy (Kiel et al., 1999; Kihara et al., 2001). Although FYVE and PX domain–containing proteins, which bind PtdIns(3)P and function as effectors of Vps34 in the CPY pathway, have been characterized (Burd et al., 1997; Burd and Emr, 1998; Cheever et al., 2001), the relevant effector(s) of Vps34 in autophagy is unknown.
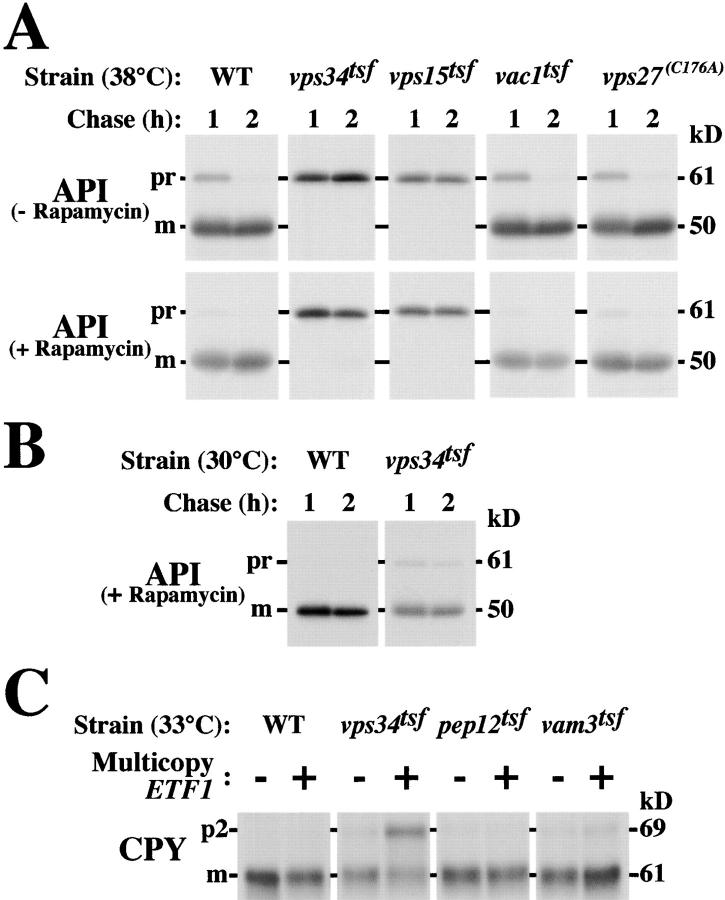
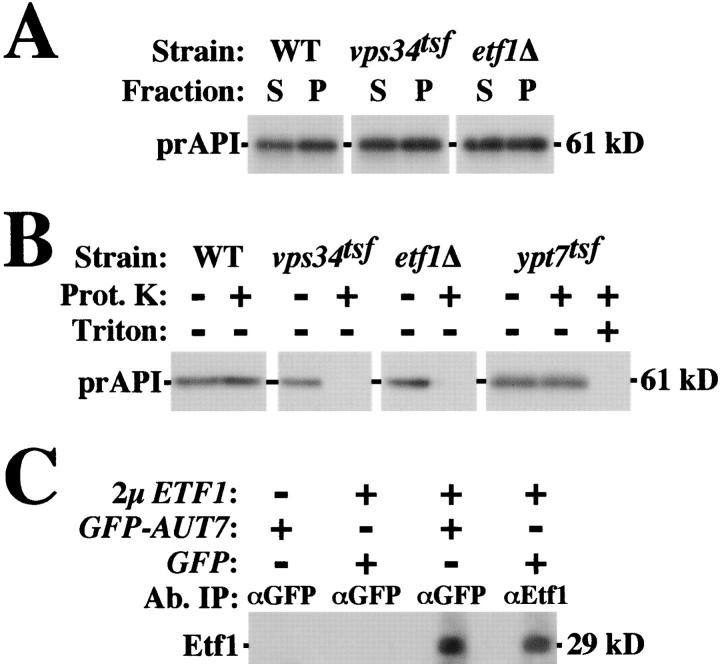
To address whether the activity of identified PtdIns(3)P-binding FYVE domain proteins (i.e., Vac1/Vps19, Vps27, Pib1, and Pib2) (Burd and Emr, 1998) are required for autophagy, cells were pulse labeled with [35S]methionine, chased for 1 or 2 h, and immunoprecipitated for API, a cargo of the Cvt and macroautophagy pathways which undergoes proteolytic processing to its mature form upon successful transport to the vacuole lumen (Klionsky et al., 1992). At the nonpermissive temperature of 38°C, a transient block in the synthesis of PtdIns(3)P through inactivation of the Vps34tsf (Stack et al., 1995) or a new temperature-sensitive allele of VPS15 (vps15 tsf; see Materials and methods), resulted in significant transport defects by both the Cvt and rapamycin-induced macroautophagy pathways as evidenced by the 61-kD precursor form of API at the 2-h chase point (Fig. 1 A). Under permissive conditions of 30°C, both vps34 tsf and vps15 tsf strains predominantly exhibited mature API after chasing for 2 h (Fig. 1 B; data not depicted). Previous experiments implicating a functional role for Vps15 and Vps34 in autophagy were limited by the use of vps15Δ and vps34Δ strains in which multiple pleiotropic phenotypes result (e.g., severe growth defects, loss of vacuolar hydrolase activity, and aberrant vacuolar morphology) (Herman and Emr, 1990; Herman et al., 1991). Thus, interpretation of these previous experiments is complicated by the possibility that deletion of VPS34 has secondary effects on autophagy. Our experiments, in which inactivation of Vps34 PtdIns 3-kinase activity is transient, suggest a direct role for PtdIns(3)P in the Cvt and macroautophagy pathways. In contrast, high temperature–induced inactivation of the Vac1tsf or mutation of the Vps27 FYVE domain (vps27 [C176A]), which resulted in severe CPY missorting defects (Burd et al., 1997; unpublished data), did not disrupt Cvt or macroautophagy transport, since API is delivered to the vacuole and converted to the 50-kD mature form with similar kinetics to wild-type cells (Fig. 1 A). Similarly, inactivation of other FYVE domain proteins Fab1, Pib1, and Pib2 failed to cause defects in the autophagy of API (Gary et al., 1998; Shin et al., 2001) (unpublished data). This data suggests that FYVE domain–containing effectors of Vps34 do not account for the requirement of Vps34 PtdIns 3-kinase activity in this transport pathway.
Figure 1.
Multicopy ETF1 specifically enhances CPY missorting in the vps34tsf strain. (A) Wild-type (WT), vps34 tsf, vps15 tsf, vac1 tsf, or vps27 (C176A) strains were grown overnight at 26°C and pulse labeled with [35S]methionine for 10 min at 38°C. After a 1- or 2-h chase with or without 0.2 μg/ml rapamycin, labeled cells were lysed and immunoprecipitated for API. Immunoprecipitates were resolved by SDS-PAGE and fluorography, revealing API maturation defects in vps34 tsf and vps15 tsf but not vac1 tsf or vps27 (C176A) strains at 38°C. (B) Wild-type and vps34 tsf cells were grown, pulse labeled with [35S]methionine for 10 min, and chased for 1 or 2 h under permissive conditions for the vps34 tsf (30°C). API was immunoprecipitated and assayed as described above. (C) Wild-type, vps34 tsf, pep12 tsf (CBY9), and vam3 tsf cells transformed with a 2 μm vector or multicopy ETF1 (as indicated) were grown overnight at 27°C, shifted to 33°C for ∼4 h. Each strain was pulse labeled with [35S]methionine for 10 min and chased for 30 min at 33°C. CPY immunoprecipitates derived each strain were analyzed by SDS-PAGE and fluorography, revealing that multicopy ETF1 synthetically enhances CPY missorting in the vps34 tsf but not wild-type, pep12 tsf, or vam3 tsf cells.
Recently, a family of 15 yeast proteins, which contain PX domains, a motif implicated in the binding of 3-PIs have been identified (Wishart et al., 2001). Evidence suggests that one such PX protein, Vam7 regulates the fusion of autophagosomes with the vacuole, leaving the relevant effector of Vps34 that participates in the formation of macroautophagy transport vesicles undefined (Sato et al., 1998; Kihara et al., 2001). Therefore, one approach we are attempting to identify a PtdIns(3)P-binding protein(s) that regulates the formation of autophagosomes is to analyze the remaining 14 yeast PX proteins for potential roles in autophagy.
In this paper, the results of a second approach that we have also undertaken are presented. We observed that overexpression of a GFP–EEA1–FYVE domain fusion or VAM7 resulted in scorable dominant negative CPY–invertase secretion defects (∼5%) in the vps34 tsf at 33°C, perhaps through titration of the available pool of PtdIns(3)P (unpublished data). Based on this, we reasoned that the overexpression of an autophagy pathway effector of Vps34 might cause a CPY missorting phenotype by competing with endogenous PtdIns(3)P-binding proteins, which function in the CPY pathway for PtdIns(3)P. Since this could provide a means of identifying a new effector protein(s) of the autophagy (or CPY) pathway, we performed a gene dosage–dependent screen for multicopy genomic library plasmids, which at the semipermissive temperature of 33°C, enhanced CPY missorting in the vps34 tsf strain (DDY3407). Using a colorimetric plate assay sensitive to the secretion of a CPY–invertase fusion protein, 57 library plasmids that induced CPY secretion were isolated after screening 60,000 transformants. The majority of these plasmids (39), encoding factors such as CPY, Vps4, and Vps3, caused CPY missorting in both wild-type and vps34 tsf cells (Table I). However, 18 isolates resulted in CPY missorting in the vps34 tsf strain but not in wild-type cells. One such plasmid, isolated five times, encoded a mutant version of Vps34 that is truncated within its COOH-terminal kinase domain. Overproduction of this kinase-deficient form of Vps34 caused 75% CPY missorting phenotypes in the vps34 tsf strain at semipermissive temperature (Table I), presumably by titrating Vps15, the upstream regulator of Vps34 (Stack et al., 1995). The most prevalent isolate of the screen (obtained 13 times) contained a 12.8-kb genomic insert. The dominant negative phenotype was ultimately traced to a 1.5-kb fragment, which contains a single uncharacterized ORF, YJL178C (Table I).
Table I.
Multicopy enhancers of vps34 tsf missorting
| Relevant gene
|
Number of times isolated |
CPY missorting
|
||
|---|---|---|---|---|
| WT | vps34tsf | |||
| % | ||||
| YJL178C | 13 | 0 | 50 | |
| vps34 (kinase deficient) | 5 | 0 | 75 | |
| PRC1 (CPY) | 6 | 50 | 70 | |
| VPS4 | 1 | 5 | 15 | |
| VPS3 | 1 | 5 | 50 | |
| NHX1/VPS44 | 6 | 5 | 50 | |
| APYI | 1 | 5 | 50 | |
| SUC2 | 5 | 0 | 0 | |
VPS4 encodes an AAA-type ATPase required for endosomal-to-vacuole sorting. VPS3 is required for Golgi-to-endosome trafficking. NHX1/VPS44 is predicted to encode an Na+/H+ exchanger. APY1 encodes a vacuolar aminopeptidase. SUC2 encodes the secreted protein invertase (which scores as a false positive in our selection of colonies which secrete the CPY–invertase fusion). WT, wild type. The Saccharomyces Genome Database is available at http://genome-www.stanford.edu/Saccharomyces. 18 isolates of this selection have not been fully characterized.
After a 4-h shift to 33°C, overexpression of YJL178C resulted in ∼50% CPY missorting defects in the vps34 tsf strain but failed to cause significant CPY missorting when overexpressed in wild-type cells or mutant strains harboring temperature-conditional alleles of the endosomal t-SNARE (pep12 tsf) or the vacuolar t-SNARE, (vam3 tsf) (Fig. 1 C) (Burd et al., 1997; Darsow et al., 1997). Since overexpression of YJL178C specifically enhanced the CPY missorting phenotype of the vps34 tsf, we designated this ORF ETF1 (enhancer of vps thirty-four missorting 1).
ETF1 encodes a type II transmembrane protein
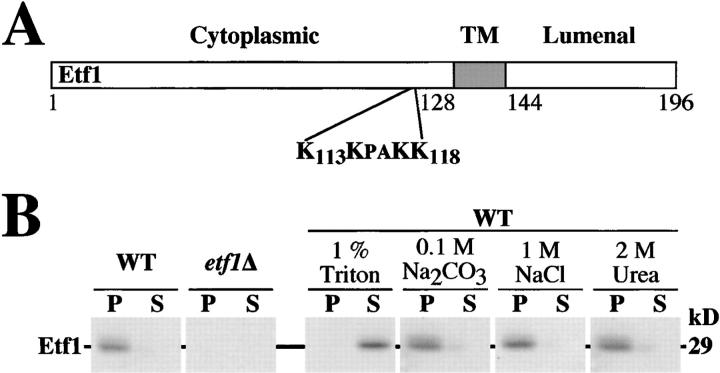
The Saccharomyces Genome Database (http://genome-www.stanford.edu/Saccharomyces/), reports that YJL178C (ETF1) is an uncharacterized ORF, which encodes a 196 amino acid protein. A hydropathy analysis revealed a single hydrophobic region (aa 128–144) and MTOP (Prediction of Membrane Topology; http://psort.nibb.ac.jp/) predicted that the protein integrates into the membrane in a type II orientation, with the NH2-terminal 127 amino acids exposed to the cytoplasm and a 52 amino acid lumenal COOH terminus (Fig. 2 A). When [35S]methionine-labeled spheroplasts were osmotically lysed and separated into soluble/cytosolic (S100) and membrane/pelletable (P100) fractions by a 100,000 g spin, polyclonal antiserum raised against the cytoplasmic 123 amino acids of the ORF recognized a single protein, which migrates at ∼29 kD (occasionally as a doublet) in wild-type cells. No protein was detected in cells deleted for ETF1 (Fig. 2 B). Association of Etf1 with the membrane fraction was sensitive to 1% triton but not to 1 M Na2CO3, 1 M NaCl, or 1 M urea, confirming that Etf1 is a membrane protein. Unexpectedly, BLAST searches of the nonredundant protein database failed to identify proteins with significant homology to Etf1. Perhaps this indicates that the function of Etf1 is specific to S. cerevisiae or, more likely, is performed by a nonhomologous protein in other organisms. Further examination revealed a basic patch of amino acids (K113KPAKK118) within the cytoplasmic region of Etf1 (Fig. 2 A). This is particularly intriguing, since in the context of profilin, gelsolin, and FYVE domain proteins, basic amino acid clusters have been implicated in the binding of acidic PIs (Xian et al., 1995; Burd and Emr, 1998; Chaudhary et al., 1998).
Figure 2.
ETF1 encodes a type II transmembrane protein. (A) Analysis of the primary amino acid sequence using MTOP indicates that Etf1 is a type II membrane-spanning protein with a single transmembrane (TM) region (aa 128–144). A basic cluster (aa 113–118: KKPAKK) is present within the cytoplasmic region of Etf1. (B) Wild-type or etf1Δ-7 (etf1Δ) cells were converted to spheroplasts, labeled with [35S]methionine for 20 min, and osmotically lysed. The lysates were either subjected to 1% Triton, 0.1 M Na2CO3, 1 M NaCl, 2 M urea, or left mock treated on ice for 10 min. Lysates were then centrifuged at 100,000 g to yield a high speed membrane pellet (P) and supernatant (S). Etf1 was immunoprecipitated from each fraction and analyzed by SDS-PAGE and fluorography.
Etf1 interacts with PtdIns(3)P
Our data suggests that overproduced Etf1 enhances CPY missorting specifically in the context of the vps34 tsf allele by titrating a component of the Vps34 signaling cascade. We could not detect interactions between Etf1 and Vps15/Vps34 by native immunoprecipitation or changes in the endogenous levels of PtdIns(3)P synthesis in cells deleted for or overexpressing ETF1 (unpublished data). This indicates that Etf1 is unlikely to sequester Vps15 or Vps34, which led us to test the possibility that Etf1 binds and titrates PtdIns(3)P.
A maltose-binding protein (MBP) fusion of ETF1 lacking the transmembrane domain (aa 124–144) was expressed in bacteria and affinity purified. Approximately 100 ng of the fusion protein was incubated with nitrocellulose strips spotted with 60 pmol of the most prevalent phospholipids and PIs in yeast. Probing these blots with polyclonal antiserum that recognizes Etf1 did not reveal Etf1 binding to phosphatidylcholine, phosphatidylserine, or PtdIns. In contrast, Etf1 bound PtdIns(3)P with 3–6-fold greater affinity than PtdIns(4)P, PtdIns (3,5)P2, and PtdIns(4,5)P2 (Fig. 3 A). Although this method is not highly quantitative, we also attempted to estimate binding affinities of Etf1 or the EEA1–FYVE domain for PIs spotted on nitrocellulose. The relative affinity of Etf1 for PtdIns(3)P was comparable to the FYVE domain of EEA1, but the EEA1–FYVE domain exhibited higher specificity for PtdIns(3)P (Fig. 3 B). Similar results were obtained when purified MBP–Etf1 was found to associate with liposomes containing 2% PtdIns(3)P approximately threefold better than PtdIns- or PtdIns(4,5)P2-containing liposomes (Fig. 3 C). When gradients of PtdIns(3)P or PtdIns(4,5)P2 (60–15 pmol) were spotted onto nitrocellulose, Etf1 bound PtdIns(3)P with approximately threefold greater affinity than PtdIns(4,5)P2, whereas 100 ng of an MBP fusion of etf1 in which each lysine within the K113KPAKK118 motif was mutated to alanine (Etf1[K113–118A]) failed to exhibit significant interactions with PtdIns(3)P (Fig. 3 D). Thus, the K113KPAKK118 motif within Etf1 plays a critical role in promoting Etf1 interactions with PtdIns(3)P in vitro. Other amino acids within Etf1 may also be important in mediating the association of Etf1 with PtdIns(3)P.
Figure 3.
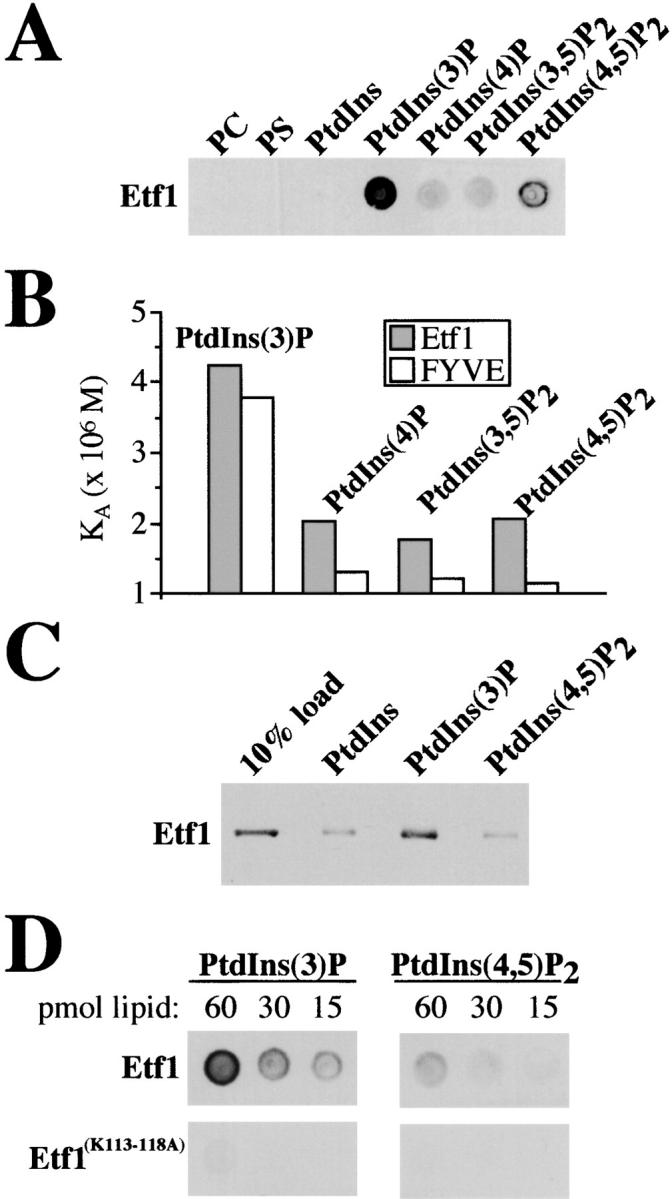
Etf1 associates with PtdIns(3)P. (A) MBP-Etf1 (∼ 100 ng) purified from E. coli was incubated with nitrocellulose strips spotted with 60 pmol of the indicated lipid. After extensive washing of the nitrocellulose, immunoblot analysis revealed that Etf1 interacts with PtdIns(3)P. (B) Affinities of Etf1 and the EEA1–FYVE domain for PtdIns(3)P were compared by incubating ∼100 ng of GST–Etf1 or GST–EEA1–FYVE with 60, 30, or 15 pmol of the indicated PI spotted on nitrocellulose. Monoclonal antibody specific to GST was used to detect protein–lipid interactions. Quantification of protein bound to each PI was estimated by the Scatchard method and expressed as an association constant (K A). (C) 25 μg of liposomes comprised equally of phosphatidylcholine (PC), phosphatidylserine (PS), and PtdIns were supplemented with either PtdIns(3)P or PtdIns(4,5)P2 (2% final molar concentration) and incubated with 1 μg of purified MBP–Etf1 or MBP–Etf1(K113–118A). The relative amounts pelletable/liposome-bound Etf1 was compared with 10% of the total quantity Etf1 added to the binding reaction by SDS-PAGE and Western blot. (D) PtdIns(3)P or PtdIns(4,5)P2 (60 to 15 pmol) were spotted onto nitrocellulose and incubated with 100 ng of purified MBP-Etf1 or MBP-Etf1(K113–118A). The relative affinity of Etf1 for each PI was determined by immunoblot analysis. The above experiments were repeated with independent PI stocks; representative examples are shown.
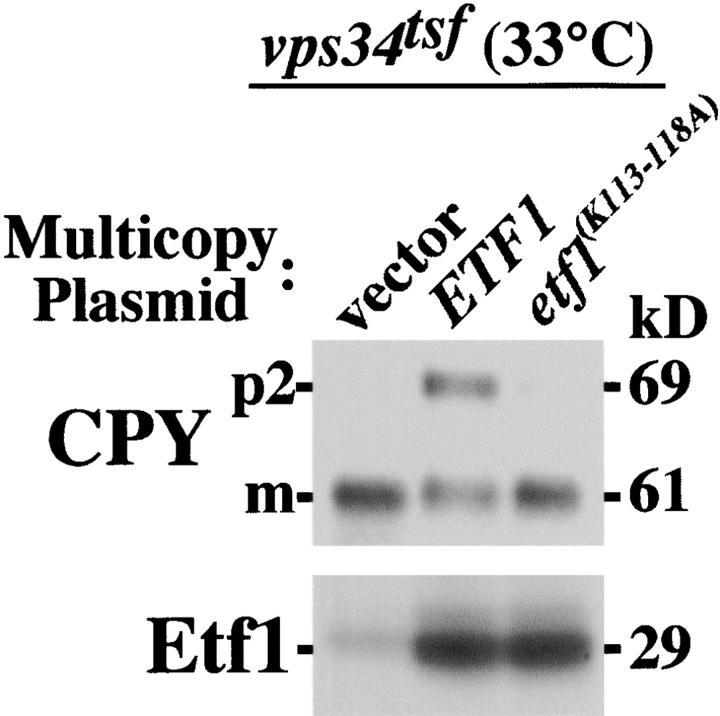
To determine if the K113KPAKK118 motif and hence the PtdIns(3)P-binding activity of Etf1 is required for dominant interference of multicopy ETF1 on the vacuolar transport of CPY, we assayed CPY sorting in vps34 tsf cells, which overexpressed wild-type ETF1 or etf1 (K113–118A). Although overproduction of wild-type Etf1 resulted in 50% CPY missorting, full-length Etf1(K113–118A) failed to exert dominant negative defects on CPY trafficking, even though the mutant version of the protein could be detected (Fig. 4) . These results indicate that Etf1 is capable of binding PtdIns(3)P and that amino acid substitutions within the protein, which disrupt PtdIns(3)P-binding activity, also curtail the dominant-negative effects of multicopy ETF1 on CPY sorting in the vps34 tsf. We propose that overexpression of ETF1 is likely to titrate PtdIns(3)P from a Vps34 effector(s) (i.e., Vac1, Vps27, and Vam7), which mediate vacuolar protein transport of CPY. Moreover, the data is suggestive of a high affinity interaction between Etf1 and PtdIns(3)P, raising the possibility that PtdIns(3)P regulates the endogenous function of Etf1.
Figure 4.
Multicopy etf1(K113–118A) fails to elicit dominant-negative CPY missorting defects in the vps34tsf. vps34 tsf (DDY3407) cells transformed with multicopy ETF1 or etf1 (K113–118A) were grown at 27°C and shifted to 33°C for ∼4 h. After a 10-min pulse label with [35S]methionine and a 30-min chase at 33°C, cells were subjected to CPY and Etf1 immunoprecipitation and analyzed by SDS-PAGE/fluorography.
Etf1 is essential for autophagic trafficking by the Cvt pathway
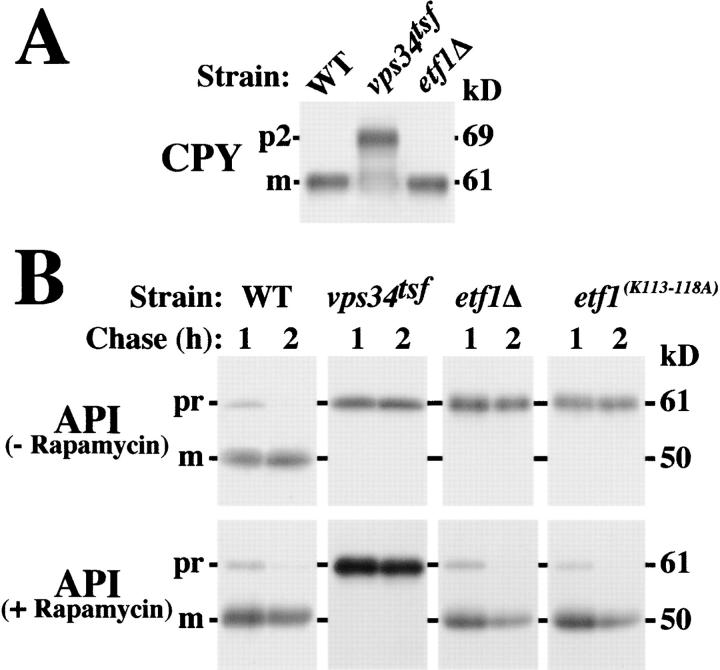
Vps34 PtdIns 3-kinase activity plays an essential signaling role in promoting the transport of a wide array of cargoes to the vacuole by the CPY, macroautophagy, and Cvt sorting pathways (Fig. 1 A) (Stack et al., 1995; Kihara et al., 2001). Our data suggests that Etf1 may carry out an effector function downstream of Vps34. Whereas Vps34 is required for CPY transport, pulse-labeling cells deleted for ETF1 with [35S]methionine did not reveal a requirement for Etf1 in the trafficking of CPY to the vacuole (Fig. 5 A). Therefore, we assayed whether Etf1, like Vps34, is required for autophagy.
Figure 5.
ETF1 is required for Cvt pathway trafficking. Cells were grown overnight at 26°C and pulse labeled with [35S]methionine for 10 min at 38°C. (A) After a 30 min chase, labeled wild-type, vps34 tsf, and etf1Δ cells were lysed and immunoprecipitated for CPY. Samples were resolved by SDS-PAGE and fluorography. (B) After 1- or 2-h chases in the presence or absence of 0.2 μg/ml rapamycin, API immunoprecipitates derived from labeled wild-type, vps34 tsf, etf1Δ, and etf1 (K113–118A) strains were analyzed by SDS-PAGE and fluorography, revealing defects in API maturation in the etf1Δ cells by the Cvt pathway but not macroautophagy.
Pulse-labeled cells either maintained under vegetative growth conditions to assay the Cvt pathway or treated with rapamycin to assay macroautophagy were immunoprecipitated for API at 1- and 2-h chase points. At 38°C, vegetatively growing wild-type cells mediated vacuolar transport of API, whereas the vps34 tsf strain and etf1Δ cells each exhibited >95% blocks in the conversion of API to its 50-kD mature form (Fig. 5 B). Because the vacuolar maturation of CPY proceeded with normal kinetics in the absence of Etf1 function (Fig. 5 A) and like API maturation requires the activity of proteinase B (Van Den Hazel et al., 1996), these data indicate that the API processing block did not result from pleiotropic defects in the transport of hydrolases to the vacuole in etf1Δ cells. Moreover, expression of etf1 (K113–118A) failed to complement the API transport defects of the etf1Δ background, even though the mutant protein was produced (Fig. 5 B; data not depicted). This suggests that amino acids within Etf1 required for PtdIns(3)P binding are also necessary for Cvt trafficking of API. After induction of macroautophagy by rapamycin, API maturation was blocked in the vps34 tsf strain. In contrast, API was transported to the vacuole and matured in etf1Δ and etf1 (K113–118A) strains with kinetics similar to wild-type cells (Fig. 5 B).
By EM, we confirmed that macroautophagy was not blocked in cells deleted for ETF1. At the permissive temperature of 26°C, rapamycin-treated vps34 tsf cells that lack the gene-encoding proteinase A (PEP4), a hydrolase critical to vacuolar degradation of autophagic bodies (Tsukada and Ohsumi, 1993), exhibited ∼6–10 autophagic bodies within the lumen of the majority of vacuoles (Fig. 6 A, arrows), suggesting that the macroautophagy pathway is intact. Similar results were obtained in wild-type or ypt7 tsf cells (at permissive temperature), which have been deleted for PEP4 (Fig. 6 C; data not depicted). At the nonpermissive temperature of 38°C, the vps34 tsf/pep4Δ strain failed to generate observable autophagosomes/autophagic bodies (Fig. 6 B) consistent with the proposed role of Vps34 in the formation of autophagosomes (Kihara et al., 2001). In contrast, at 38°C the ypt7 tsf/pep4Δ strain accumulated cytoplasmic autophagosomes (Fig. 6 D, arrows), verifying a requirement for Ypt7 in the fusion of autophagosomal intermediates with the vacuole (Kim et al., 1999; Wurmser et al., 2000). Much like pep4Δ cells, etf1Δ/pep4Δ cells exhibited ∼3–10 autophagic bodies within the lumen of the vacuole, indicating that the macroautophagy pathway is predominantly intact even in the absence of Etf1 function (Fig. 6 E). Because Etf1 directly interacts with PtdIns(3)P and, like Vps34, is essential for the autophagy of API by the Cvt pathway, we postulate that Etf1 functions as a downstream effector of Vps34 specific to the Cvt pathway. Additionally, based on our observation that Etf1 is not a key mediator of macroautophagy it is intriguing to speculate that as yet undefined autophagy effectors of Vps34 exist (see Discussion).
Figure 6.
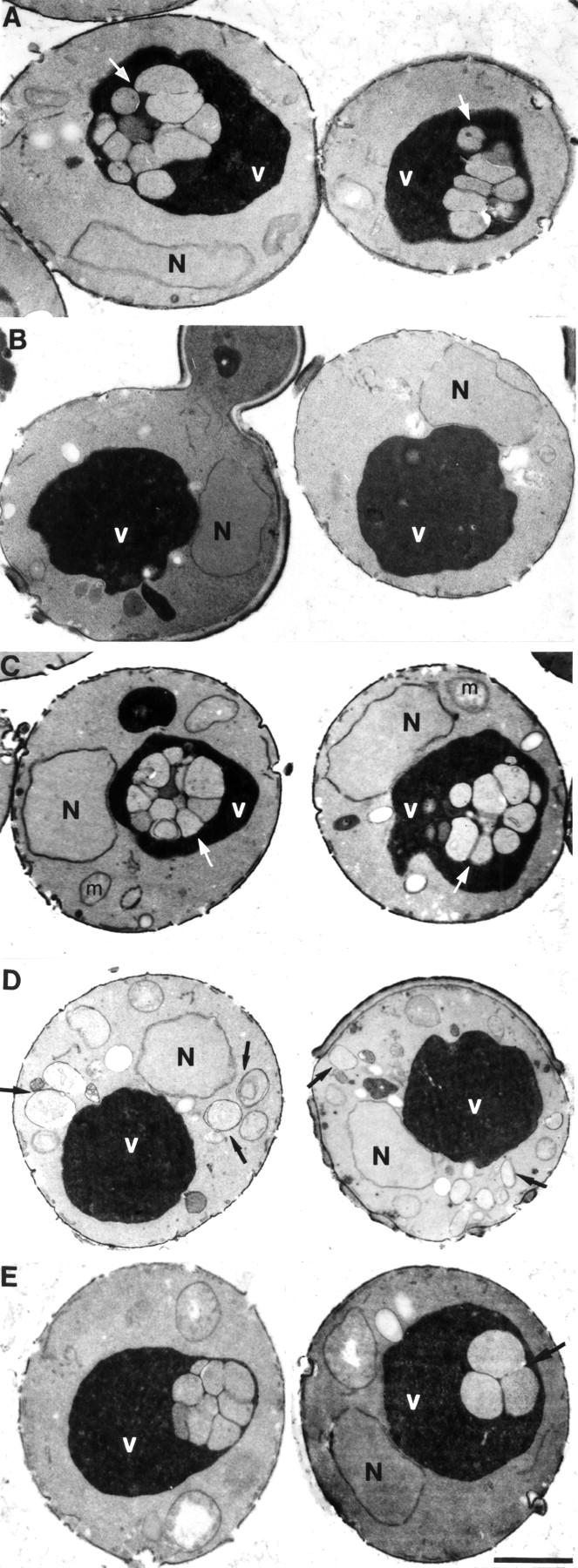
etf1 Δ cells exhibit autophagic bodies within the lumen of the vacuole by EM. Cells were grown to mid-log phase at 26°C and treated with 0.2 μg/ml rapamycin at either 26 or 38°C for 2 h. Strains were then fixed with 3% glutaraldehyde, converted to spheroplasts, and visualized by EM. (A) vps34 tsf at 26°C. (B) vps34 tsf at 38°C. (C) ypt7 tsf at 26°C. (D) ypt7 tsf at 38°C. (E) etf1Δ. Arrows indicate autophagosomes and autophagic bodies. N, nucleus; V, vacuole; m, mitochondria. Bar, 0.5 μm.
Vps34 and Etf1 regulate the formation of Cvt vesicles
Like macroautophagy, the Cvt pathway has been resolved into multiple mechanistically distinct stages including the recruitment of cytoplasmic cargo to the membrane, formation/sequestration of membrane-associated cargo into Cvt vesicles, and fusion of these transport intermediates with the vacuole (Kim and Klionsky, 2000). It follows that if Etf1 functions as a downstream effector of Vps34, then both proteins are likely to act at a common stage of the Cvt pathway. It has been shown that Vps34 is required for the formation of autophagosomes, the transport intermediate of the macroautophagy pathway (Kihara et al., 2001). To determine if Vps34 and Etf1 function at a common step of the Cvt pathway, we assayed for vps34- and etf1-dependent defects in recruitment of API to the membrane and in the formation/sequestration of Cvt intermediates.
Under vegetative conditions, cells were pulse labeled with [35S]methionine, chased for 20 min, and osmotically lysed. Lysates were separated into a low speed pellet (P5), which is enriched for Cvt intermediates (Scott et al., 1997), and soluble (S5) fractions. In wild-type cells, precursor API distributes equally between the soluble and pelletable fractions, and no change in this distribution was observed in the vps34 tsf strain at nonpermissive temperature or in cells deleted for ETF1, indicating that neither Vps34 nor Etf1 function in recruiting pAPI to the membrane (Fig. 7 A). Subsequent resuspension and exposure of the labeled P5 pool of pAPI to proteinase K revealed that pAPI was protease sensitive in the vps34 tsf at nonpermissive temperature and in etf1Δ cells but not in wild-type cells or a mutant strain lacking Ypt7 function (Fig. 7 B). Thus, Vps34 and Etf1 are each required for Cvt vesicle formation, consistent with our proposed functional role for Etf1 as a PtdIns(3)P-responsive effector of Vps34.
Figure 7.
Vps34 and Etf1 each regulate the formation/sequestration of the Cvt vesicle. Wild-type, vps34 tsf, and etf1Δ spheroplasts were pulse labeled with [35S]methionine for 10 min at 38°C and chased 20 min. Spheroplasts were osmotically lysed and separated by a 5,000 g spin into a low speed pellet (P) and soluble (S) fractions. (A) Analysis of API immunoprecipitates of the P and S fractions by SDS-PAGE and fluorography indicate that Vps34 and Etf1 are not required for recruitment of prAPI to Cvt vesicles. (B) P fractions derived from wild-type, vps34 tsf, etf1Δ, and ypt7 tsf cells were either proteinase K or mock treated and then immunoprecipitated for API. SDS-PAGE/fluorography revealed that the P fraction pool of prAPI is protease accessible in both the vps34 tsf and etf1Δ cells. (C) Detergent extracts of strains overexpressing GFP, GFP-AUT7, and/or ETF1 were subjected to native immunoprecipitation using polyclonal antiserum specific to GFP or Etf1 as indicated. Immunoprecipitates were resolved by SDS-PAGE and analyzed for the presence of Etf1 by Western blot. For direct immunoprecipitation of Etf1 using Etf1 antiserum, an ∼15% load is shown.
To gain mechanistic insights into the potential function of Etf1, we assayed if Etf1 forms complexes in vivo with other proteins known to function during the vesicle formation stage of autophagy. By Western blot, Etf1 did not coimmunoprecipitate with Apg1 or Apg12 (unpublished data). In contrast, immunoprecipitates obtained from detergent extracts of yeast cooverexpressing both ETF1 and GFP-AUT7/APG8, revealed that ∼15% of the total pool Etf1 coprecipitated with GFP-Aut7 but not with GFP alone (Fig. 7 C). We did not test for interactions between Etf1 and Aut7 when each protein was produced at single copy levels. It has been documented that Aut7 is covalently linked to phosphatidylethanolamine in a multistep reaction analogous to the ubiquitin conjugation system and that this modification plays a role in recruiting Aut7 to membranes (Kim and Klionsky, 2000; Ohsumi, 2001). Deletion of ETF1 did not influence the subcellular fractionation pattern of GFP-Aut7 (unpublished data), suggesting that Etf1 may modulate the activity of an Aut7-containing protein complex rather than by influencing its localization or the coupling of Aut7 to phosphatidylethanolamine.
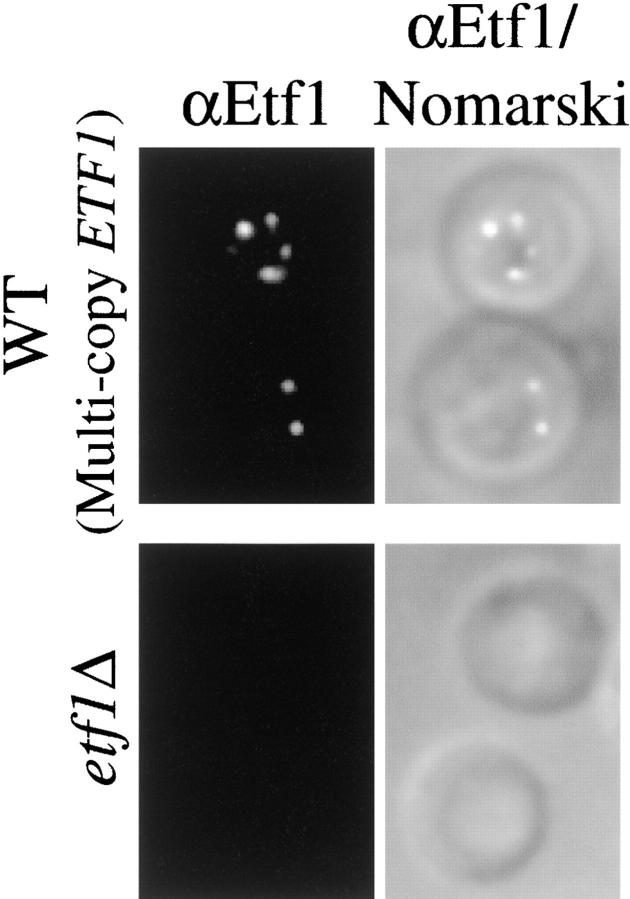
Etf1 is enriched in prevacuolar membrane compartments
By indirect immunofluorescence (IF), affinity-purified antiserum raised against Etf1 localizes the protein to multiple cytoplasmic compartments that are often clustered adjacent to the vacuole in wild-type cells expressing multicopy ETF1 (Fig. 8) . Little or no background staining was observed in cells deleted for ETF1 (Fig. 8), and it could Etf1 be detected at single copy levels (unpublished data). Therefore, like PtdIns(3)P (Burd and Emr, 1998), Etf1 is enriched in prevacuolar compartments. Whereas characterized cargoes of the Cvt vesicle (i.e., API and Aut7/Apg8) are delivered to the vacuole and subsequently clipped by vacuolar proteases (Klionsky et al., 1992; Kirisako et al., 1999; Huang et al., 2000), [35S]methionine pulse-labeling experiments demonstrated that Etf1 stability is not proteinase A–/proteinase B–sensitive (unpublished data), indicating that Etf1 is not consumed during autophagy, since it is not transported into the vacuole lumen. Association of Etf1 and Aut7 must therefore be disrupted before the complete formation/sequestration of the Cvt vesicle.
Figure 8.
Etf1 localization by immunofluorescence. Wild-type and etf1Δ cells were grown at 26°C and converted to spheroplasts. After fixation with 4% formaldehyde, spheroplasts were immobilized on multiwell slides and probed with affinity-purified Etf1 polyclonal antibody as described in Materials and methods. Etf1 immunofluorescence and an overlay of fluorescence and Nomarski images are presented.
PIs function as integral membrane second messengers characterized to modify the activity and/or localization of target effector proteins (Rameh and Cantley, 1999; Wurmser et al., 1999; Sato et al., 2001). Hence, we tested whether PtdIns(3)P synthesis is required for the proper subcellular localization of Etf1. By IF, significant changes in Etf1 distribution were not observed as a result of Vps34tsf inactivation, perhaps because detection of Etf1 using IF necessitates ETF1 overexpression (unpublished data). Therefore, we localized Etf1 by subcellular fractionation, since by this method endogenous levels of Etf1 can be detected. [35S]methionine-labeled spheroplasts were osmotically lysed and separated by differential centrifugation into low and high speed membrane pellets (P13 and P100, respectively) and the soluble/cytosolic (S100) fraction. In wild-type cells, single copy levels of the Etf1 protein distributed to each membrane fraction (60% P13, 40% P100, and 0% S100). Strikingly, inactivation of Vps34tsf PtdIns 3-kinase activity for 10 min at 38°C mislocalized the P100 pool of Etf1 to the P13 fraction (>95% P13, <5% P100, and 0% S100) (Table II). We could not detect a change in the cellular distribution of Etf1 in the fab tsf strain at 38°C (Table II) in which PtdIns(3,5)P2 synthesis was disrupted (Gary et al., 1998). In addition, interrupting Cvt vesicle formation through inactivation of Aut7 or CPY pathway trafficking by shifting vac1 tsf and vps4 tsf cells to 38°C for 10 min did not significantly alter Etf1 fractionation relative to wild-type cells (Table II). Thus, Etf1 localization requires the synthesis of PtdIns(3)P by Vps34 but not other PIs (i.e., PtdIns[3,5]P2) or the activity of proteins that mediate the autophagy and CPY pathways. Collectively, therefore, these results indicate that PtdIns(3)P plays a role in the localization of Etf1 through its direct interactions with Etf1 (see Discussion).
Table II. Vps34 PtdIns 3-kinase activity regulates Etf1 localization: Etf1 distribution by subcellular fractionation.
| Strain | P13 | P100 | S100 | ||
|---|---|---|---|---|---|
|
|
%
|
||||
| WT | 60 | 40 | 0 | ||
| vps34tsf | >95 | <5 | 0 | ||
| fab1tsf | 60 | 40 | 0 | ||
| aut7Δ | 60 | 40 | 0 | ||
| vac1tsf | 60 | 40 | 0 | ||
| vps4tsf | 50 | 50 | 0 | ||
WT, wild type.
Discussion
Etf1 functions as an effector of Vps34 PtdIns 3-kinase signaling in autophagy
Although transient disruption of Vps34 or Vps15 activity resulted in autophagy defects by both macroautophagy and the Cvt pathway, inactivation of Vac1 or Vps27, previously characterized effectors of Vps34, failed to cause significant autophagy defects (Fig. 1 A). Our genetic screen for proteins, which function as potential downstream effectors of Vps34, resulted in the identification of Etf1, which encodes a type II transmembrane protein that strongly bound PtdIns(3)P in vitro (Fig. 3). Deletion of ETF1 or expression of a mutant version of ETF1, which failed to bind PtdIns(3)P, impaired autophagic transport of API to the vacuole by the Cvt pathway, indicating that both Vps34-mediated synthesis of PtdIns(3)P and PtdIns(3)P-binding by Etf1 are required for the Cvt pathway. This data functionally and biochemically links Vps34 to Etf1. Consistent with these findings, Vps34 and Etf1 were each essential for the formation of Cvt vesicles, a common stage of the autophagic transport reaction. Based on these results, we propose that Vps34 and Etf1 coordinately regulate formation of Cvt vesicles through PtdIns(3)P-triggered activation/localization of Etf1.
PIs have been characterized as signaling molecules, which function to recruit effector proteins from the cytosol to specific target membranes (Rameh and Cantley, 1999; Wurmser et al., 1999; Sato et al., 2001; Wishart et al., 2001). Etf1 is fundamentally different from these proteins in that it contains a transmembrane domain, obviating a role for PtdIns(3)P in redistributing Etf1 from the cytosol to the membrane fraction (Fig. 2 and Table II). It is possible that PtdIns(3)P could modulate an intrinsic activity of Etf1, since PIs are also known to influence the activity of membrane proteins (e.g., gating of membrane-spanning ion channels) (Baukrowitz and Fakler, 2000). Given that the P100 pool of Etf1 was almost completely lost upon inactivation of Vps34 (Table II), we believe that PtdIns(3)P functions as a localization determinant for Etf1, which in combination with other determinants present within the primary sequence of the protein, concentrates Etf1 into a specific subset of PtdIns(3)P-containing membrane compartments. Restriction of Etf1 localization by PtdIns(3)P may serve to bring the protein into proximity of an Etf1-interacting protein(s). One candidate binding partner of Etf1 is Aut7, which coprecipitated with Etf1 from detergent extracts (Fig. 7 C). The finding that Etf1 formed a complex with Aut7 but did not appear to influence the localization of Aut7 by subcellular fractionation could indicate that Etf1 modulates the activity of this or another protein in an Aut7 complex (e.g., Cvt19) (Kim et al., 2002). Although critical for autophagy by the Cvt pathway, integration of Etf1 into an Aut7-containing complex is not essential for macroautophagy (Fig. 5 B). Etf1 may thus function to redirect Aut7 and/or proteins associated with Aut7 from macroautophagy into the Cvt pathway.
Etf1 basic patch (KKPAKK) is required for PI binding
Etf1 does not contain a previously characterized PI-binding motif. By analogy with profilin, gelsolin and the FYVE domain, which each contain a cluster of basic amino acids that participate in the binding of specific PIs (Xian et al., 1995; Burd and Emr, 1998; Chaudhary et al., 1998; Kutateladze et al., 1999), we predicted that the K113KPAKK118 sequence present within the cytosolic region of Etf1 promotes contact between Etf1 and the acidic PI, PtdIns(3)P. Multiple uncharacterized ORFs have been found to contain KKPAKK motifs by the genome sequencing projects of C. elegans (e.g., yk307b2.3, W07E6.1, and AAC78177.2) and Drosophila melanogaster (e.g., AAF48891.1, AAF47091.1, AAF57753.1, and AAA73062.1). Future experiments will be required to determine whether PIs regulate the function of these putative PI-binding proteins.
The Vps34 PtdIns 3-kinase is a multifunctional regulator of autophagy
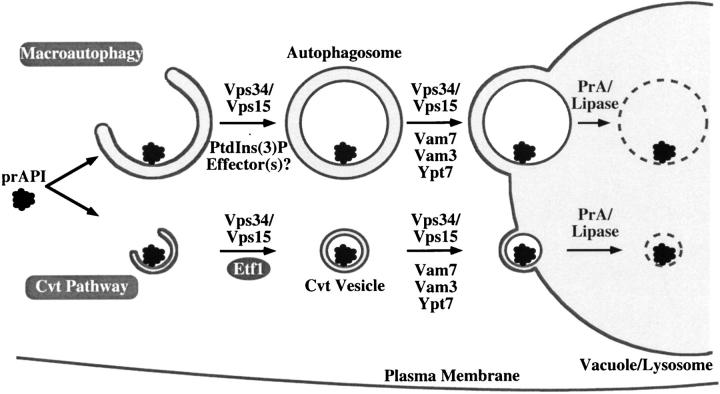
Etf1 is required for the Cvt branch of autophagy but not macroautophagy or CPY transport (Fig. 5). In contrast, Vps34 and its upstream regulator, the Vps15 protein kinase, function in each of these transport pathways (Fig. 1 A) (Stack et al., 1995; Kihara et al., 2001). PtdIns(3)P promotes trafficking of CPY from the Golgi to the vacuole by modulating the activity/localization of Vac1, Vps27, and Vam7, which bind PtdIns(3)P by virtue of FYVE or PX domains (Burd and Emr, 1998; Cheever et al., 2001). These proteins do not appear to play a critical role in autophagosome or Cvt vesicle formation (Fig. 1 A) (Darsow et al., 1997; Sato et al., 1998). One interpretation of these findings is that an additional effector(s) of Vps34 that functions in macroautophagy exists but as yet remains undefined (Fig. 9 and Table III). Alternatively, since the Vps34 kinase signals more than a dozen effectors including Etf1, FYVE domain– and PX domain–containing proteins (Burd and Emr, 1998; Cheever et al., 2001; Yu and Lemmon, 2001; Nice et al., 2002), it is also possible that the simultaneous disruption of the activity/localization of many PtdIns(3)P-binding effectors in the vps34 mutant reduces the overall cellular transport capacity and indirectly blocks macroautophagy.
Figure 9.
Distinct sets of PtdIns(3)P-binding effectors mediate the signaling effects of the Vps34 PtdIns 3-kinase. Etf1 serves as a transmembrane effector of Vps34 in the formation of Cvt vesicles through its basic amino acid cluster (KKPAKK). This suggests that an unidentified PtdIns(3)P-binding protein carries out the formation of autophagosomes in the macroautophagy pathway. The PX protein Vam7, vacuolar t-SNARE Vam3, and rab GTPase Ypt7 are key mediators in the vacuolar fusion of autophagic transport intermediates of both the Cvt and macroautophagy pathways.
Table III. 3-phosphoinositide regulated pathways in yeast.
| PtdIns(3)P regulated pathways | PtdIns(3)P effector | Stage | Motif |
|---|---|---|---|
| CPY pathway | Vac1 | Golgi-to-endosome | FYVE domain |
| CPY pathway | Vps27 | Endosome maturation | FYVE domain |
| CPY pathway | Vam7 | Endosome/vacuole fusion | PX domain |
| Vacuole membrane recycling/turnover | Fab1 | Unknown | FYVE domain |
| Cvt pathway | Etf1 | Cvt vesicle formation | KKPAKK |
| Cvt pathway | Vam7 | Cvt vesicle/vacuole fusion | PX domain |
| Macroautophagy | Unknown | Autophagosome formation | Unknown |
| Macroautophagy | Vam7 | Autophagosome/vacuole fusion | PX domain |
Proteins which contain either FYVE domains or PX motifs execute Vps34 functions in the CPY sorting pathway (Vac1, Vps27, and Vam7) and in vacuole membrane recycling/turnover (Fab1).
Our selection of multicopy vps34 tsf enhancers has not identified additional Vps34 effector candidates. Perhaps, Etf1 was prone to isolation by this approach, since the capacity of Etf1 to titrate PtdIns(3)P in the CPY pathway is potentiated by its transmembrane motif, which may tether the protein to PtdIns(3)P-enriched compartments. An examination of the sequence of known Apg proteins for basic amino acid clusters revealed several promising candidates that may ultimately be shown to also mediate interactions with PtdIns(3)P (unpublished data). In addition, each of the 15 yeast PX domain–containing proteins have been demonstrated to bind 3-PIs (Cheever et al., 2001; Yu and Lemmon, 2001) and two of these, Cvt13 and Cvt20, participate in the Cvt pathway but not macroautophagy (Nice et al., 2002).
The work of Kihara et al. (2001) and our lab strongly implicates a requirement for Vps34 in the formation of autophagosomes and Cvt vesicles (Figs. 6 and 7). It is possible that Vps34 also regulates fusion of Cvt vesicles and autophagosomes with the vacuole through the SNAP-25 homologue, Vam7, and its partner t-SNARE, Vam3. Mutations within the PX domain of Vam7 that disrupt PtdIns(3)P-binding impair the fusion of CPY-containing endosomes with the vacuole (Sato et al., 1998; Cheever et al., 2001). Vam7 activity is also required for autophagy (Sato et al., 1998), raising the possibility that the PtdIns(3)P-binding capacity of the Vam7 PX domain plays an additional modulatory role in autophagosome and Cvt vesicle fusion with the vacuole. A model summarizing the multiple proposed roles of Vps34 in autophagy and other membrane trafficking pathways is presented (Fig. 9 and Table III).
A growing number of proteins have been found to promote autophagy by the Cvt pathway, including two ubiquitin-like protein conjugation systems, a t-SNARE, and several factors which couple cargo recruitment to vesicle formation/sequestration (Abeliovich et al., 1999; Kim and Klionsky, 2000; Kim et al., 2001b; Leber et al., 2001; Ohsumi, 2001). Further experiments will be necessary to define each of the effectors functioning downstream of Vps34 in autophagy and which components of the autophagic machinery are regulated by PtdIns(3)P.
Materials and methods
Strains
For a list of S. cerevisiae strains used in this study, please see Table IV. Yeast were grown in standard yeast extract, peptone, dextrose/fructose, or, following transformation by the lithium acetate method (Ito et al., 1983), synthetic medium containing essential amino acids (Burd et al., 1997). The entire ETF1 and AUT7 genes were disrupted by transforming SEY6210 with a 1.1-kb HIS3 PCR fragment flanked by ∼50 bp of sequence present upstream and downstream of the respective ORFs. Histidine prototrophs were analyzed by PCR to confirm gene disruption.
Table IV. Strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| SEY6210 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | Robinson et al., 1988 |
| BHY10 | SEY6210; CPY-INV::LEU2 | Burd et al., 1997 |
| DDY3407 | BHY10; vps34 tsf | Burd et al., 1997 |
| vps34 tsf | SEY6210; vps34Δ::TRP1; pRS416.vps34 tsf | Stack et al., 1995 |
| vps15 tsf | BHY10; vps15Δ-1::HIS3; pRS414.vps15-155 | This study |
| vac1 tsf | CBY57; pRS413.vac1-30 | Burd et al., 1997 |
| vps27(C176A) | SEY6210; vps27Δ::HIS3; pRS416.vps27 (C176A) | M. Babsta |
| vam3 tsf | TDY2; pVAM3-6.416 | Darsow et al., 1997 |
| etf1Δ-7 | SEY6210; etf1Δ::HIS3 | This study |
| ypt7 tsf f | SEY6210; ypt7Δ::HIS3 pRS414.ypt7-38 | Wurmser et al., 2000 |
| fab1 tsf | SEY6210; fab1Δ::LEU2; pRS416.fab1-2 | Gary et al., 1998 |
| apg8Δ | SEY6210; apg8Δ-2::HIS3 | This study |
| vps4 tsf | MBY2; pMB59 | Babst et al., 1997 |
| pep12 tsf | CBY9 | Burd et al., 1997 |
| etf1 (K113-118A) | etf1Δ-7; pRS415.etf1 (K113-118A) | This study |
| TVY1 | SEY6210; pep4Δ::LEU2 | Lab strain |
| vps34 tsf /pep4Δ | TVY1; vps34Δ-2::TRP1; pRS416.vps34 tsf | This study |
| ypt7 tsf /pep4Δ | TVY1; ypt7Δ-6::HIS3; pRS414.ypt7-38 | This study |
| etf1Δ/pep4Δ | TVY1; etf1Δ-1::HIS3 | This study |
University of California, San Diego, CA.
DNA methods
Standard procedures were used to carry out DNA modifications, ligations (using enzymes from Roche and New England Biolabs), bacterial transformations, and minipreps (Maniatis et al., 1982). QIAquick Gel Extraction (QIAGEN) was used to purify DNA fragments.
pRS414.VPS15 was made by ligating a 4.8-kb XhoI/BamHI VPS15 fragment into pRS414 cut with XhoI/BamHI (Sikorski and Hieter, 1989).
Selection of the vps15 tsf was conducted by randomly mutagenizing the region of VPS15 that codes for aa 9–781 using Taq polymerase (PerkinElmer) under error-prone PCR conditions (Stack et al., 1995). This PCR product was cotransformed with pRS414.VPS15 linearized by AatII/StuI into BHY10; vps15Δ-1, where these DNA fragments were allowed to recombine. Temperature-conditional vps15 mutants were isolated using a colorimetric CPY–invertase secretion assay (Burd et al., 1997), yielding vps15–155.
pRS415 and pRS426 versions of ETF1 and etf1 (K113–118A) were constructed by PCR amplifying a 1.9-kb EagI/SalI fragment containing ETF1 or, for etf1 (K113–118A), by amplifying a 1.4-kb EagI/PstI and 477-bp PstI/SalI fragment (the PstI site was introduced into ETF1 as a result of mutagenizing each lysine between aa 113–118). These PCR products were then ligated into each vector cut with EagI/SalI.
For generating polyclonal serum that recognizes Etf1, a 375-bp BglII/AatII PCR fragment of ETF1 coding for aa 1–123 was ligated into pGexKG cut with BamHI/AatII. Standard procedures were used to raise polyclonal anti-Etf1 serum using GST–Etf1 as the antigen (Darsow et al., 1997).
MBP fusions of ETF1 were constructed by PCR amplifying a 375-bp BglII/AatII fragment, coding for aa 1–123, and a 79-bp AatII/EcoRI fragment, coding for aa 145–175 (AatII was introduced into etf1 as a result of deleting the transmembrane domain, aa 124–144), and simultaneously ligating these inserts into pMalC2. The same approach was followed in constructing MBP-etf1 (K113–118A) using the pRS415.etf1 (K113–118A) as a PCR template.
pRS416.vps34 tsf (Stack et al., 1995), pVAM3–6.416 (Darsow et al., 1997), pMB59 (Babst et al., 1997), pRS416.fab1–2 (Gary et al., 1998), pRS413.vac1–30 (Burd et al., 1997), and pRS414.ypt7–38 (Wurmser et al., 2000) plasmids have each been described.
Cloning of ETF1
DDY3407 was transformed with a multicopy Sau3A-digested genomic library cloned into the BamHI site of pYEP24 (a gift from Mark Rose, Princeton University, Princeton, NJ) and grown at 27°C for several days. Colonies were transferred to yeast extract/peptone/fructose plates, grown for 18 h at 27°C, and then incubated at 33°C for 6 h. CPY–invertase secreting colonies were selected, and the plasmids overexpressed by each colony were isolated as described (Burd et al., 1997). Each genomic insert was restriction mapped to determine the size of the fragment and sequenced (100–200 bp for each insert). CPY missorting phenotypes were also determined by secretion of the CPY–invertase fusion in DDY3407 cells overexpressing the GFP–EEA1–FYVE fusion or VAM7.
YJL178C (ETF1) plasmids, initially contained an ∼12.8-kb genomic fragment predicted to code for nine complete ORFs (chromosome X; approximate coordinates 87,000–99,500), necessitating deletion analysis to identify the ORF that conferred CPY missorting in DDY3407 at 33°C. The plasmids were sequentially digested with PvuII and NheI and self-ligated after each digest. The resulting 5.6-kb genomic fragment-containing plasmid tested positive for CPY–invertase secretion in DDY3407 at 33°C and was treated with Bsu36I/PvuII/klenow to remove a 1.5-kb fragment containing only the intact YJL178C. This fragment ligated into pRS426 cut with SmaI was sufficient to impair CPY sorting in DDY3407 at 33°C.
Pulse labeling and protein immunoprecipitation
Protein transport assays and immunoprecipitations were performed as described (Gary et al., 1998). Where indicated, 0.2 μg/ml rapamycin (Sigma-Aldrich) was added to cells during the chase period to induce macroautophagy (Noda and Ohsumi, 1998; Abeliovich et al., 1999). Antiserum specific to API was a gift from Daniel Klionsky (University of Michigan, Ann Arbor, MI).
API subcellular fractionation and protease protection assays were performed as described (Scott et al., 1997) except that 5 OD600 U of spheroplasts were subjected to a 10-min pulse label with [35S]methionine and chased for 20 min before osmotic lysis and immunoprecipitation of prAPI from S5 and P5 fractions. The Pipes 6.8 lysis buffer contained 2 mM MgCl2.
Immunoprecipitation and subsequent detection of full-length Etf1(K113–118A) was performed by briefly glass bead lysing etf1 (K113–118A) cells (15 s) followed by immediate denaturation of the sample. Efficient lysis was achieved through multiple lysis/denaturation cycles. Etf1 subcellular fractionations were performed as described (Wurmser et al., 2000).
For coimmunoprecipitation studies, multicopy plasmids expressing ETF1 and GFP or a single copy plasmid encoding GFP–AUT7 coupled to the CUP1 promoter (Kim et al., 2001a) were transformed into etf1Δ cells as indicated (Fig. 7 C). GFP–AUT7 expression was induced with 10−4 M CuSO4 for 12 h. 10 OD600 U of each strain were homogenized in 2 mls of PBS containing protease inhibitors and 4 mM EDTA. Lysates were cleared of unbroken cells by a low speed spin and then centrifuged at 100,000 g for 15 min at 4°C. The resulting pellet was resuspended in 1.5 mls of PBS containing protease inhibitors, 4 mM EDTA, and 0.5% IGEPAL CA-630 (NP-40; Sigma-Aldrich). After centrifugation at 13,000 g for 5 min, resuspensions were incubated with protein A–sepharose (Amersham Biosciences) prebound to GFP- (a gift from Charles Zuker, University of California, San Diego, CA) or Etf1-specific antibody for 30 min. Beads were washed twice with PBS + 0.5% IGEPAL CA-630 and subjected to SDS-PAGE and anti-Etf1 Western blot.
Lipid binding assays
Immobilization of lipids on nitrocellulose and incubation of recombinant protein with the blot were performed as described previously (Dowler et al., 1999) except that 100 ng of purified Etf1 protein was used per binding reaction and 60–15 pmol of lipids were spotted (Fig. 3, A, C, and D). In Fig. 3 B, bacterial extracts containing ∼100 ng of GST–Etf1 (aa 1–123) and GST–EEA1-FYVE domain (aa 1257–1411 of EEA1) fusions were incubated with nitrocellulose strips spotted with 60–15 pmol of the indicated PI. Blots were probed with monoclonal anti-GST antibody (Calbiochem) and binding quantified by scanning blot exposures in Scion Image 1.62a (National Institutes of Health). Scatchard plots were used to determine binding affinities.
Liposome-binding assays were performed as described (Burd and Emr, 1998), using ∼1 μg of purified Etf1 protein. Multiple independent PI stocks (Cell Signals, Inc.) were used in ascertaining PI-binding specificity of Etf1.
Microscopy
For EM, etf1Δ, ypt7 tsf, and vps34 tsf cells were grown at 26°C and maintained at mid-log phase. Cells were treated with 0.2 μg/ml rapamycin to induce macroautophagy for 2 h. During this 2 h period, etf1Δ, vps34 tsf, and ypt7 tsf cells were either incubated at 26°C or (for vps34 tsf and ypt7 tsf) shifted to 38°C. 50 OD600 U of each strain were fixed and processed for EM as described previously (Babst et al., 1997; Darsow et al., 1997).
Localization of Etf1 by indirect immunofluorescence was performed by converting 10 OD600 U of etf1Δ or wild-type cells expressing pRS426.ETF1 to spheroplasts and fixing for 1 h in 4% formaldehyde, otherwise following previously described methods (Babst et al., 1997). 1:5,000 diluted affinity-purified antiserum raised against GST-Etf1 was incubated with cells overnight and after washing, 1:300 goat anti–rabbit A568 secondary antibody (Alexa). Cells were observed as described (Babst et al., 1997).
Acknowledgments
We are grateful to Daniel Klionsky for helpful discussions, API antiserum, and the GFP–AUT7 construct; Tammie McQuistan for carrying out whole cell electron microscopic analysis (Immunoelectron microscopy Core B of Program Project grant CA58689 headed by M. Farquhar), Peter Olson for his help in constructing the vps15 tsf allele, Chris Burd for determining that Pib1/Pib2 are not essential for API transport, Patricie Burda for constructing HA-tagged VPS15 and VPS34 fusions, Mark Rose for a multicopy genomic library, Markus Babst and David Katzmann for construction of the vps27 FYVE domain (C176A) point mutant, Charles Zuker for GFP antibody, and Trey Sato for critically reading the article. A.E. Wurmser is a member of the Biomedical Sciences Graduate Program. S.D. Emr is an investigator of the Howard Hughes Medical Institute.
A.E. Wurmser is supported by the Program Project Grant (National Institutes of Health, CA 58689).
Footnotes
Abbreviations used in this paper: AP, aminopeptidase; CPY, carboxypeptidase Y; Cvt, cytoplasm-to-vacuole targeting; IF, immunofluorescence; MBP, maltose-binding protein; PI, phosphoinositide; PtdIns, phosphatidylinositol; PX, phox; PtdIns(3)P, PtdIns 3-phosphate.
References
- Abeliovich, H., T. Darsow, and S.D. Emr. 1999. Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE-Sec1p complex composed of Tlg2p and Vps45p. EMBO J. 18:6005–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba, M., K. Takeshige, N. Baba, and Y. Ohsumi. 1994. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J. Cell Biol. 124:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba, M., M. Osumi, S.V. Scott, D.J. Klionsky, and Y. Ohsumi. 1997. Two distinct pathways for targeting proteins from the cytoplasm to the vacuole/lysosome. J. Cell Biol. 139:1687–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst, M., T.K. Sato, L.M. Banta, and S.D. Emr. 1997. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 16:1820–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz, T., and B. Fakler. 2000. KATP channels gated by intracellular nucleotides and phospholipids. Eur. J. Biochem. 267:5842–5848. [DOI] [PubMed] [Google Scholar]
- Beck, T., and M.N. Hall. 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 402:689–692. [DOI] [PubMed] [Google Scholar]
- Brown, E.J., M.W. Albers, T.B. Shin, K. Ichikawa, C.T. Keith, W.S. Lane, and S.L. Schreiber. 1994. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 369:756–758. [DOI] [PubMed] [Google Scholar]
- Burd, C.G., and S.D. Emr. 1998. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to ring FYVE domains. Mol. Cell. 2:1–20. [DOI] [PubMed] [Google Scholar]
- Burd, C.G., M. Peterson, C.R. Cowles, and S.D. Emr. 1997. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol. Biol. Cell. 8:1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary, A., J. Chen, Q.M. Gu, W. Witke, D.J. Kwiatkowski, and G.D. Prestwich. 1998. Probing the phosphoinositide 4,5-bisphosphate binding site of human profilin I. Chem. Biol. 5:273–281. [DOI] [PubMed] [Google Scholar]
- Cheever, M.L., T.K. Sato, T. de Beer, T.G. Kutateladze, S.D. Emr, and M. Overduin. 2001. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat. Cell Biol. 3:613–618. [DOI] [PubMed] [Google Scholar]
- Darsow, T., S.E. Rieder, and S.D. Emr. 1997. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell Biol. 138:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler, S., R.A. Currie, C.P. Downes, and D.R. Alessi. 1999. DAPP1: a dual adaptor for phosphotyrosine and 3-phosphoinositides. Biochem. J. 342:7–12. [PMC free article] [PubMed] [Google Scholar]
- Dunn, W.A., Jr. 1990. a. Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J. Cell Biol. 110:1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, W.A., Jr. 1990. b. Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J. Cell Biol. 110:1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary, J.D., A.E. Wurmser, C.J. Bonangelino, L.S. Weisman, and S.D. Emr. 1998. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J. Cell Biol. 143:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, T.M., K.A. Morano, S.V. Scott, and D.J. Klionsky. 1995. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J. Cell Biol. 131:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, T.M., A. Hefner-Gravink, M. Thumm, and D.J. Klionsky. 1996. Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole protein targeting pathway. J. Biol. Chem. 271:17621–17624. [DOI] [PubMed] [Google Scholar]
- Herman, P.K., and S.D. Emr. 1990. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:6742–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, P.K., J.H. Stack, J.A. DeModena, and S.D. Emr. 1991. A novel protein kinase homolog essential for protein sorting to the yeast lysosome-like vacuole. Cell. 64:425–437. [DOI] [PubMed] [Google Scholar]
- Huang, W.P., S.V. Scott, J. Kim, and D.J. Klionsky. 2000. The itinerary of a vesicle component, Aut7p/Cvt5p, terminates in the yeast vacuole via the autophagy/Cvt pathways. J. Biol. Chem. 275:5845–5851. [DOI] [PubMed] [Google Scholar]
- Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya, Y., N. Mizushima, T. Ueno, A. Yamamoto, T. Kirisako, T. Noda, E. Kominami, Y. Ohsumi, and T. Yoshimori. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19:5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel, J.A., K.B. Rechinger, I.J. van der Klei, F.A. Salomons, V.I. Titorenko, and M. Veenhuis. 1999. The Hansenula polymorpha PDD1 gene product, essential for the selective degradation of peroxisomes, is a homologue of Saccharomyces cerevisiae Vps34p. Yeast. 15:741–754. [DOI] [PubMed] [Google Scholar]
- Kihara, A., T. Noda, N. Ishihara, and Y. Ohsumi. 2001. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 152:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., and D.J. Klionsky. 2000. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu. Rev. Biochem. 69:303–342. [DOI] [PubMed] [Google Scholar]
- Kim, J., V.M. Dalton, K.P. Eggerton, S.V. Scott, and D.J. Klionsky. 1999. Apg7p/Cvt2p is required for the cytoplasm-to-vacuole targeting, macroautophagy, and peroxisome degradation pathways. Mol. Biol. Cell. 10:1337–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., W.P. Huang, and D.J. Klionsky. 2001. a. Membrane recruitment of Aut7p in the autophagy and cytoplasm to vacuole targeting pathways requires Aut1p, Aut2p, and the autophagy conjugation complex. J. Cell Biol. 152:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., Y. Kamada, P.E. Stromhaug, J. Guan, A. Hefner-Gravink, M. Baba, S.V. Scott, Y. Ohsumi, W.A. Dunn, Jr., and D.J. Klionsky. 2001. b. Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole. J. Cell Biol. 153:381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., W.P. Huang, P.E. Stromhaug, and D.J. Klionsky. 2002. Convergence of multiple autophagy and cytoplasm to vacuole targeting components to a perivacuolar membrane compartment prior to de novo vesicle formation. J. Biol. Chem. 277:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako, T., M. Baba, N. Ishihara, K. Miyazawa, M. Ohsumi, T. Yoshimori, T. Noda, and Y. Ohsumi. 1999. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J. Cell Biol. 147:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky, D.J., and S.D. Emr. 2000. Autophagy as a regulated pathway of cellular degradation. Science. 290:1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky, D.J., R. Cueva, and D.S. Yaver. 1992. Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J. Cell Biol. 119:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutateladze, T.G., K.D. Ogburn, W.T. Watson, T. de Beer, S.D. Emr, C.G. Burd, and M. Overduin. 1999. Phosphatidylinositol 3-phosphate recognition by the FYVE domain. Mol. Cell. 3:805–811. [DOI] [PubMed] [Google Scholar]
- Leber, R., E. Silles, I.V. Sandoval, and M.J. Mazon. 2001. Yol082p, a novel CVT protein involved in the selective targeting of aminopeptidase I to the yeast vacuole. J. Biol. Chem. 276:29210–29217. [DOI] [PubMed] [Google Scholar]
- Maniatis, T., E.F. Fritsch, and J. Sambrook. 1982. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Mizushima, N., H. Sugita, T. Yoshimori, and Y. Ohsumi. 1998. A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J. Biol. Chem. 273:33889–33892. [DOI] [PubMed] [Google Scholar]
- Nice, D.C., T.K. Sato, P.E. Stromhaug, S.D. Emr, and D.J. Klionsky. 2002. Cooperative binding of the cytoplasm to vacuole targeting pathway proteins, Cvt13 and Cvt20, to PtdIns(3)P at the pre-autophagosomal structure is required for selective autophagy. J. Biol. Chem. 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda, T., and Y. Ohsumi. 1998. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 273:3963–3966. [DOI] [PubMed] [Google Scholar]
- Ohsumi, Y. 2001. Molecular dissection of autophagy: two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2:211–216. [DOI] [PubMed] [Google Scholar]
- Petiot, A., E. Ogier-Denis, E.F. Blommaart, A.J. Meijer, and P. Codogno. 2000. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275:992–998. [DOI] [PubMed] [Google Scholar]
- Rameh, L.E., and L.C. Cantley. 1999. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274:8347–8350. [DOI] [PubMed] [Google Scholar]
- Robinson, J.S., D.J. Klionsky, L.M. Banta, and S.D. Emr. 1988. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 8:4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T.K., T. Darsow, and S.D. Emr. 1998. Vam7p, a SNAP-25-like molecule, and Vam3p, a syntaxin homolog, function together in yeast vacuolar protein trafficking. Mol. Cell. Biol. 18:5308–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T.K., M. Overduin, and S.D. Emr. 2001. Location, location, location: membrane targeting directed by PX domains. Science. 294:1881–1885. [DOI] [PubMed] [Google Scholar]
- Schu, P.V., K. Takegawa, M.J. Fry, J.H. Stack, M.D. Waterfield, and S.D. Emr. 1993. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 260:88–91. [DOI] [PubMed] [Google Scholar]
- Scott, S.V., A. Hefner-Gravink, K.A. Morano, T. Noda, Y. Ohsumi, and D.J. Klionsky. 1996. Cytoplasm-to-vacuole targeting and autophagy employ the same machinery to deliver proteins to the yeast vacuole. Proc. Natl. Acad. Sci. USA. 93:12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, S.V., M. Baba, Y. Ohsumi, and D.J. Klionsky. 1997. Aminopeptidase I is targeted to the vacuole by a nonclassical vesicular mechanism. J. Cell Biol. 138:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, M.E., K.D. Ogburn, O.A. Varban, P.M. Gilbert, and C.G. Burd. 2001. FYVE Domain targets Pib1p ubiquitin ligase to endosome and vacuolar membranes. J. Biol. Chem. 276:41388–41393. [DOI] [PubMed] [Google Scholar]
- Sikorski, R.S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack, J.H., D.B. DeWald, K. Takegawa, and S.D. Emr. 1995. Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. J. Cell Biol. 129:321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige, K., M. Baba, S. Tsuboi, T. Noda, and Y. Ohsumi. 1992. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 119:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada, M., and Y. Ohsumi. 1993. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 333:169–174. [DOI] [PubMed] [Google Scholar]
- Van Den Hazel, H.B., M.C. Kielland-Brandt, and J.R. Winther. 1996. Review: biosynthesis and function of yeast vacuolar proteases. Yeast. 12:1–16. [DOI] [PubMed] [Google Scholar]
- Volinia, S., R. Dhand, B. Vanhaesebroeck, L.K. MacDougall, R. Stein, M.J. Zvelebil, J. Domin, C. Panaretou, and M.D. Waterfield. 1995. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J. 14:3339–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart, M.J., G.S. Taylor, and J.E. Dixon. 2001. Phoxy lipids: revealing PX domains as phosphoinositide binding modules. Cell. 105:817–820. [DOI] [PubMed] [Google Scholar]
- Wurmser, A.E., J.D. Gary, and S.D. Emr. 1999. Phosphoinositide 3-kinases and their FYVE domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. J. Biol. Chem. 274:9129–9132. [DOI] [PubMed] [Google Scholar]
- Wurmser, A.E., T.K. Sato, and S.D. Emr. 2000. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J. Cell Biol. 151:551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian, W., R. Vegners, P.A. Janmey, and W.H. Braunlin. 1995. Spectroscopic studies of a phosphoinositide-binding peptide from gelsolin: behavior in solutions of mixed solvent and anionic micelles. Biophys. J. 69:2695–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J.W., and M.A. Lemmon. 2001. All phox homology (PX) domains from Saccharomyces cerevisiae specifically recognize phosphatidylinositol 3-phosphate. J. Biol. Chem. 276:44179–44184. [DOI] [PubMed] [Google Scholar]