Abstract
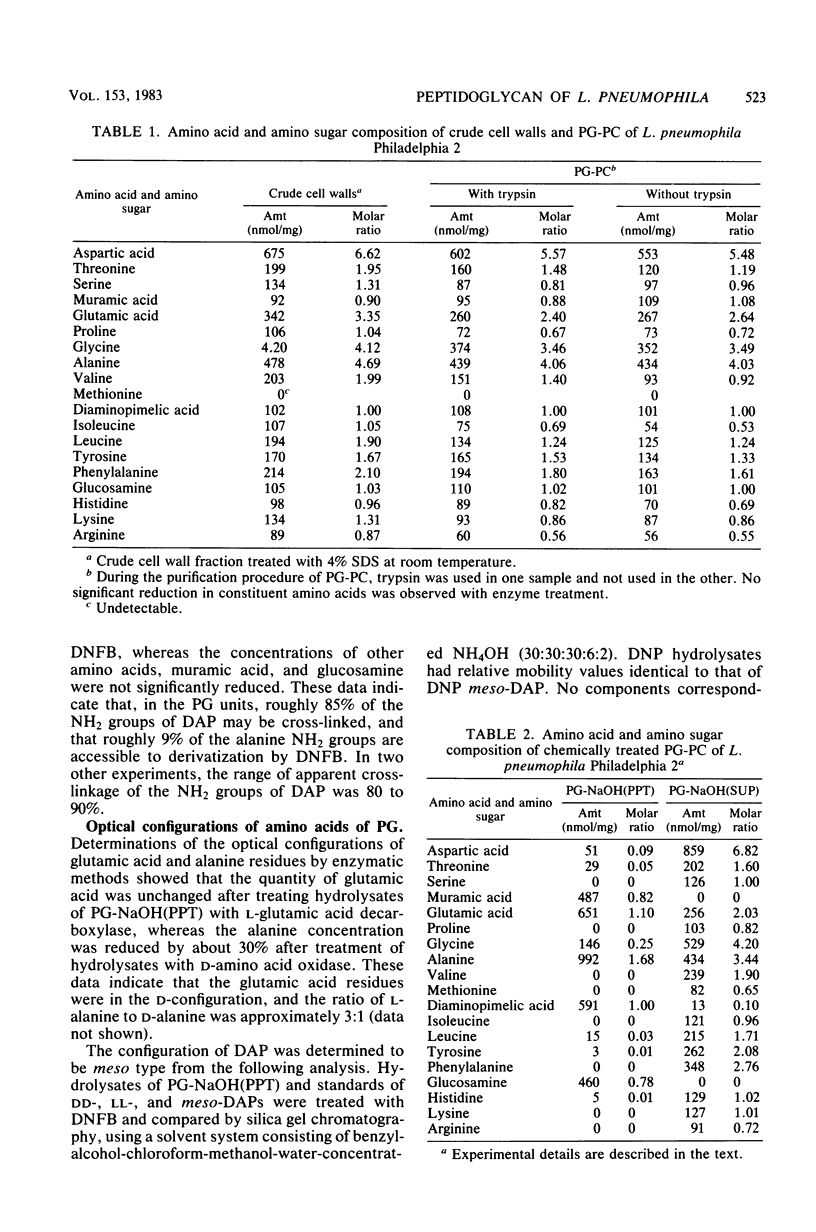
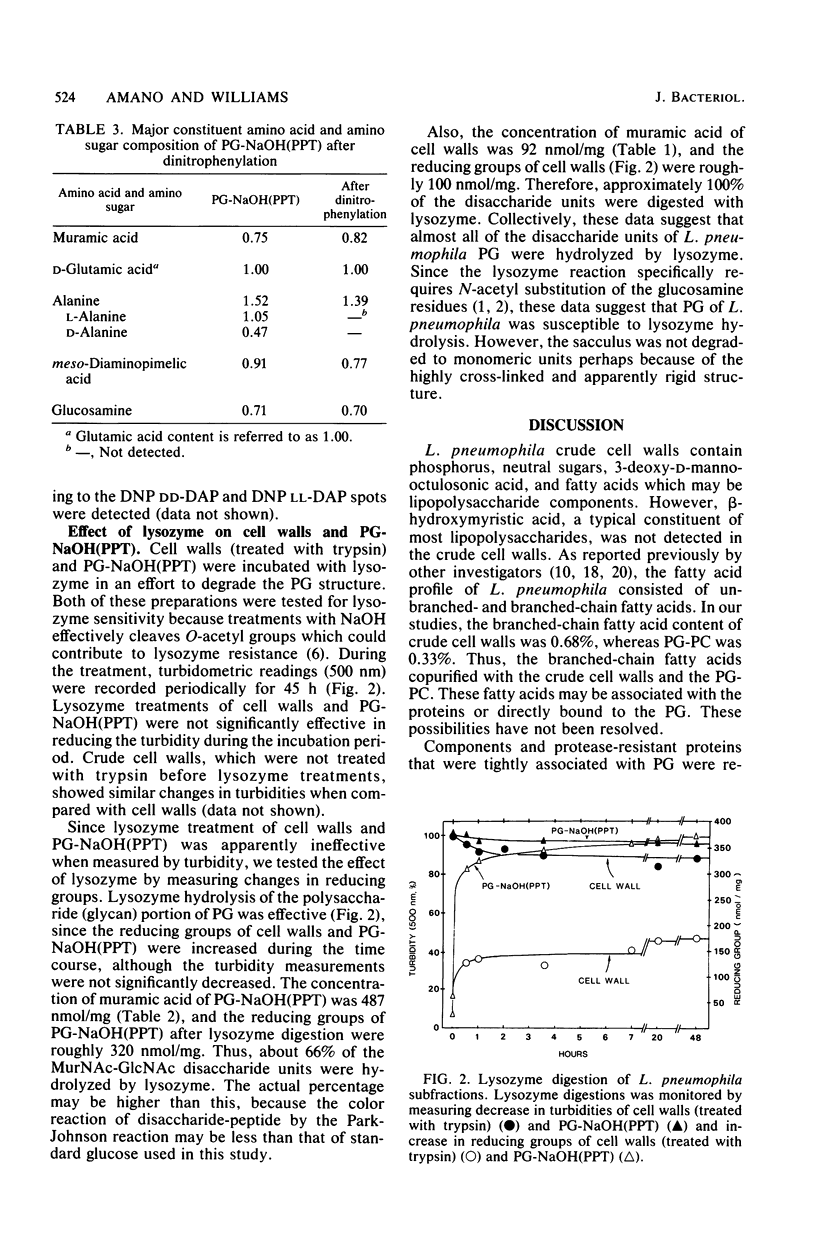
Peptidoglycan (PG) from Legionella pneumophila was composed of muramic acid, glucosamine, glutamic acid, alanine, and meso-diaminopimelic acid in a molar ratio of 0.8:0.8:1.1:1.7:1. Partially purified PG contained trypsin-insensitive proteins which were extracted by 1 N NaOH hydrolysis without apparent dissolution of the PG. Lysozyme hydrolysis of purified PG or cell walls caused an increase in reducing groups which correlated with roughly 70 to 100% digestion of disaccharides. However, there was no significant decrease in turbidity during lysozyme hydrolysis of purified PG or cell wall. Additionally, 80 to 90% of the meso-diaminopimelic acid epsilon-amino groups were not susceptible to dinitrophenylation. Collectively, the PG of L. pneumophila was sensitive to lysozyme hydrolysis and insensitive to alkali dissolution, and 80 to 90% of the NH2 groups of meso-diaminopimelic acid were apparently involved in cross-linkages between peptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Hayashi H., Araki Y., Ito E. The action of lysozyme on peptidoglycan with N-unsubstituted glucosamine residues. Isolation of glycan fragments and their susceptibility to lysozyme. Eur J Biochem. 1977 Jun 1;76(1):299–307. doi: 10.1111/j.1432-1033.1977.tb11596.x. [DOI] [PubMed] [Google Scholar]
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Goundry J. The action of dilute alkali on the glycyl residues of staphylococcal peptidoglycan. Biochem J. 1970 Jan;116(2):313–315. doi: 10.1042/bj1160313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Bricas E., Ghuysen J. M., Dezélée P. The cell wall peptidoglycan of Bacillus megaterium KM. I. Studies on the stereochemistry of alpha, alpha'-diaminopimelic acid. Biochemistry. 1967 Aug;6(8):2598–2607. doi: 10.1021/bi00860a043. [DOI] [PubMed] [Google Scholar]
- Elliott J. A., Johnson W., Helms C. M. Ultrastructural localization and protective activity of a high-molecular-weight antigen isolated from Legionella pneumophila. Infect Immun. 1981 Feb;31(2):822–824. doi: 10.1128/iai.31.2.822-824.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty W. R., Makula R. A., Feeley J. C. Cellular lipids of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):631–634. doi: 10.7326/0003-4819-90-4-631. [DOI] [PubMed] [Google Scholar]
- Flesher A. R., Ito S., Mansheim B. J., Kasper D. L. The cell envelope of the Legionnaires' disease bacterium. Morphologic and biochemical characteristics. Ann Intern Med. 1979 Apr;90(4):628–630. doi: 10.7326/0003-4819-90-4-628. [DOI] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Guerrant G. O., Lambert M. S., Moss C. W. Identification of diaminopimelic acid in the Legionnaires disease bacterium. J Clin Microbiol. 1979 Dec;10(6):815–818. doi: 10.1128/jcm.10.6.815-818.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel J. A., Finnerty W. R., Feeley J. C. Fine structure of the Legionnaires' disease bacterium. In-vitro and in-vivo studies of four isolates. Ann Intern Med. 1979 Apr;90(4):652–655. doi: 10.7326/0003-4819-90-4-652. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mayberry W. R. Dihydroxy and monohydroxy fatty acids in Legionella pneumophila. J Bacteriol. 1981 Aug;147(2):373–381. doi: 10.1128/jb.147.2.373-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade J. E., Shepard C. C., Fraser D. W., Tsai T. R., Redus M. A., Dowdle W. R. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977 Dec 1;297(22):1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Weaver R. E., Dees S. B., Cherry W. B. Cellular fatty acid composition of isolates from Legionnaires disease. J Clin Microbiol. 1977 Aug;6(2):140–143. doi: 10.1128/jcm.6.2.140-143.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neblett T. R., Riddle J. M., Dumoff M. Surface topography and fine structure of the Legionnaires' disease bacterium. A study of six isolates from hospitalized patients. Ann Intern Med. 1979 Apr;90(4):648–651. doi: 10.7326/0003-4819-90-4-648. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- PERKINS H. R. THE ACTION OF HOT FORMAMIDE ON BACTERIAL CELL WALLS. Biochem J. 1965 Jun;95:876–882. doi: 10.1042/bj0950876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner M. R., Tang J., Barzilay I., Khorana H. G. Structure of the lipopolysaccharide from an Escherichia coli heptose-less mutant. I. Chemical degradations and identification of products. J Biol Chem. 1979 Jul 10;254(13):5906–5917. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., DiGiorgio S., Darner J., Wilhelm A. Detection of Legionella pneumophila capsular-like envelope antigens by counterimmunoelectrophoresis. J Clin Microbiol. 1981 Apr;13(4):637–642. doi: 10.1128/jcm.13.4.637-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Umemoto T., Ota T., Sagawa H., Kato K., Takada H., Tsujimoto M., Kawasaki A., Ogawa T., Harada K., Kotani S. Chemical and biological properties of a peptidoglycan isolated from Treponema pallidum kazan. Infect Immun. 1981 Feb;31(2):767–774. doi: 10.1128/iai.31.2.767-774.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., LEUTGEB W. Autolytic enzymes as a source of error in the preparation and study of gram-negative cell walls. J Gen Microbiol. 1963 Jan;30:127–130. doi: 10.1099/00221287-30-1-127. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]




