Abstract
Actin participates in several intracellular trafficking pathways. We now find that actin, bound to the surface of purified yeast vacuoles in the absence of cytosol or cytoskeleton, regulates the last compartment mixing stage of homotypic vacuole fusion. The Cdc42p GTPase is known to be required for vacuole fusion. We now show that proteins of the Cdc42p-regulated actin remodeling cascade (Cdc42p → Cla4p → Las17p/Vrp1p → Arp2/3 complex → actin) are enriched on isolated vacuoles. Vacuole fusion is dramatically altered by perturbation of the vacuole-bound actin, either by mutation of the ACT1 gene, addition of specific actin ligands such as latrunculin B or jasplakinolide, antibody to the actin regulatory proteins Las17p (yeast Wiskott-Aldrich syndrome protein) or Arp2/3, or deletion of actin regulatory genes. On docked vacuoles, actin is enriched at the “vertex ring” membrane microdomain where fusion occurs and is required for the terminal steps leading to membrane fusion. This role for actin may extend to other trafficking systems.
Keywords: yeast vacuoles; membrane fusion; actin; latrunculin B; jasplakinolide
Introduction
Proteins are sorted by selective vesicular traffic (Jahn and Sudhof, 1999). The membrane fusion stage of trafficking is unexpectedly complex. It requires the coordinated action of chaperones (such as NSF and α-SNAP), SNAREs, GTPases (Rab and Rho families), lipids (steroid and phosphoinositides), and regulated calcium fluxes.
Actin has a central role in several trafficking events (Qualmann et al., 2000; Foti et al., 2001; Goode and Rodal, 2001; Lechler et al., 2001). Actin filaments are required for the transport and spatial targeting of secretory proteins (Pruyne et al., 1998; Guo et al., 2001), organelle inheritance during cell division (Catlett and Weisman, 2000), and the maintenance of Golgi structure (Mullholland et al., 1997; Valderrama et al., 1998). In different systems, membrane fusion has been shown to be promoted by stabilization of F-actin (Koffer et al., 1990; Jahraus et al., 2001), F-actin disassembly (Vitale et al., 1991; Muallem et al., 1995), or actin remodeling, i.e., disassembly plus reassembly (Bernstein et al., 1998; Lang et al., 2000). Actin remodeling also accompanies synaptic stimulation (Colicos et al., 2001). Actin ligands can modulate phagosome–endosome fusion in vitro (Jahraus et al., 2001), and actin binds to purified endosomal and lysosomal vesicles, an association which can nucleate actin polymerization (Mehrabian et al., 1984; Taunton, 2001). Actin and myosin V are involved directly in vacuole movement into the bud during cell division (Hill et al., 1996; Catlett et al., 2000). Rho-GTPases regulate actin rearrangement (Hall, 1998) by signaling to multiple downstream effector complexes such as the Wiskott-Aldrich syndrome protein (WASp)* and the Arp2/3 complex (Higgs and Pollard, 1999). In each of these studies, it was assumed that the relevant actin molecules are cytosolic or cytoskeletal, though none of the data precludes a role for organelle-bound actin.
We study membrane fusion with vacuoles from Saccharomyces cerevisiae (Wickner and Haas, 2000). Purified yeast vacuoles undergo homotypic fusion in simple buffers containing ATP. All of the proteins and lipids needed for fusion are bound to the vacuole membrane. The reaction occurs in three stages termed priming, docking, and fusion. Priming, initiated by the ATPase Sec18p, releases Sec17p (Mayer et al., 1996) and disassembles a cis complex of SNAREs (Ungermann et al., 1998a). Priming liberates the “HOPS” complex (for homotypic fusion and vacuole protein sorting)/VPS class C complex (Sato et al., 2000; Seals et al., 2000), which then associates with GTP-bound Ypt7p to initiate docking (Price et al., 2000). Completion of docking requires SNAREs (Ungermann et al., 1998b), the vacuole membrane potential (Ungermann et al., 1999), phosphoinositides (Mayer et al., 2000), and the Rho-GTPases Cdc42p and Rho1p (Eitzen et al., 2001; Müller et al., 2001). Docking culminates in a transient release of vacuole lumenal calcium (Peters and Mayer, 1998). Calcium activates calmodulin, which binds to the V0 domain of the vacuolar ATPase, triggering the formation of trans-pairs of V0 plus the t-SNARE Vam3p, leading to organelle fusion (Peters et al., 2001).
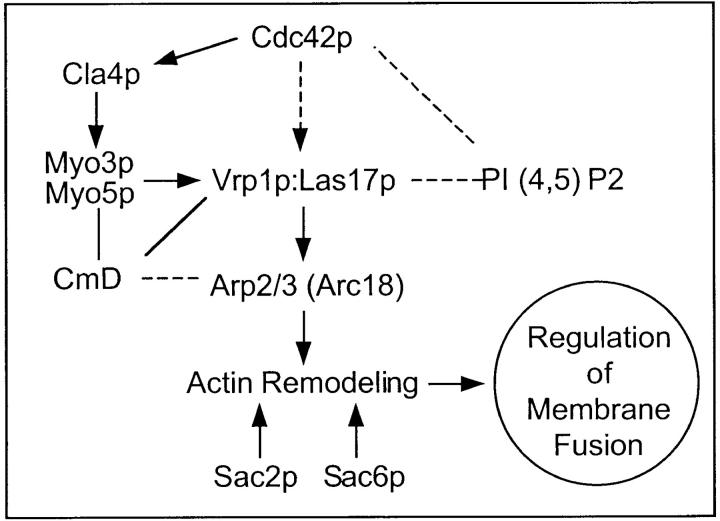
Two Rho-GTPases which are required for vacuole fusion, Cdc42p and Rho1p (Eitzen et al., 2001; Müller et al., 2001), can regulate actin structure (Pringle et al., 1995; Helliwell et al., 1998) through a well-studied cascade which includes Las17p/Bee1p (yeast WASp) and the Arp2/3 complex (Fig. 1) . A recent screen of a library of yeast strains with defined gene deletions (Seeley et al., 2002) suggested that this cascade of actin regulatory genes is needed to maintain normal vacuole structure. We now report that the proteins of this regulatory cascade, from Cdc42p to Las17p and Arp2/3p, and actin itself, are found on purified yeast vacuoles, are essential for fusion, and allow actin action at the final stage of the fusion pathway. This role of actin in vacuole fusion may extend to other membrane fusion events.
Figure 1.
A signaling pathway which regulates actin remodeling. Arrows depict known protein interactions. Dashed lines and arrows depict pathways seen in mammalian cells. Lines show other interacting factors.
Results
Cdc42p, a Rho-GTPase which regulates actin structure, is required for vacuole fusion and normal vacuole copy number in vivo (Eitzen et al., 2001; Müller et al., 2001). These studies showed that the fusion of purified vacuoles was blocked by antibodies to Cdc42p and that vacuoles which were isolated from strains with temperature-sensitive Cdc42p were thermolabile for fusion. Fig. 1 depicts a schematic pathway in which Cdc42p and phosphatidylinositol 4,5-bisphosphate (PI[4,5]P2) govern a regulatory cascade which controls actin remodeling. Genetic (Seeley et al., 2002) and biochemical (Mayer et al., 2000) data show that PI(4,5)P2 is required for vacuole fusion, possibly as a guanine nucleotide exchange factor for Cdc42p (Zheng et al., 1996) or an activating ligand for Las17p. Vacuoles have abnormal structure in strains with gene deletions for Cla4p, Vrp1p, Myo3p, Myo5p, Arp2p, Arc18p, Sac2p, or Sac6p or when point mutations are introduced into actin (Fig. 2 A). Vacuoles are also fragmented in the las17–16 strain, which contains a COOH-terminal 21 amino acid truncation that removes the Arp2/3 activation domain of Las17p (Fig. 2 A) (Duncan et al., 2001). Each of these proteins directly modulates actin structure or its assembly (Adams et al., 1989; Higgs and Pollard, 1999, 2000; Vaduva et al., 1999; Evangelista et al., 2000; Prehoda et al., 2000; Rozelle et al., 2000). Cla4p, a p20-activated kinase (PAK), is a downstream effector of Cdc42p (Gladfetter et al., 2001; Mosch et al., 2001). Cla4p modifies Myo3p and Myo5p (Lechler et al., 2000), which interact with Las17p/Bee1p, the yeast homologue of WASp (Madania et al., 1999; Lechler et al., 2000). Yeast verprolin, Vrp1p, also binds directly to Las17p (Madania et al., 1999; Lechler et al., 2001; Martinez-Quiles et al., 2001). Las17p and Vrp1p regulate actin remodeling through activation of the Arp2/3 complex (Winter et al., 1999; Evangelista et al., 2000). Because actin and its regulatory proteins might affect vacuole structure either directly or indirectly through altered endocytic or biosynthetic protein and lipid delivery to the vacuole, we have characterized their presence and functions on the purified organelle. In accord with the finding that isolated vacuoles bear Cdc42p (Müller et al., 2001), we find that Cla4p, Vrp1p, Las17p, Arp3p, and actin itself are present on purified vacuoles (Fig. 2 B) at levels which meet or exceed their concentration in the whole cell. However, only a small portion of the total cellular complement of these proteins is vacuolar, since this organelle comprises ∼1/30th of the cell protein. Actin on the vacuole is thus accompanied by at least a proportionate amount of its normal regulatory proteins, even though both actin and its regulatory proteins, such as Las17p and Arp2/3 complex, are largely found in cytoplasmic cables and plasma membrane patches (Winter et al., 1999).
Figure 2.
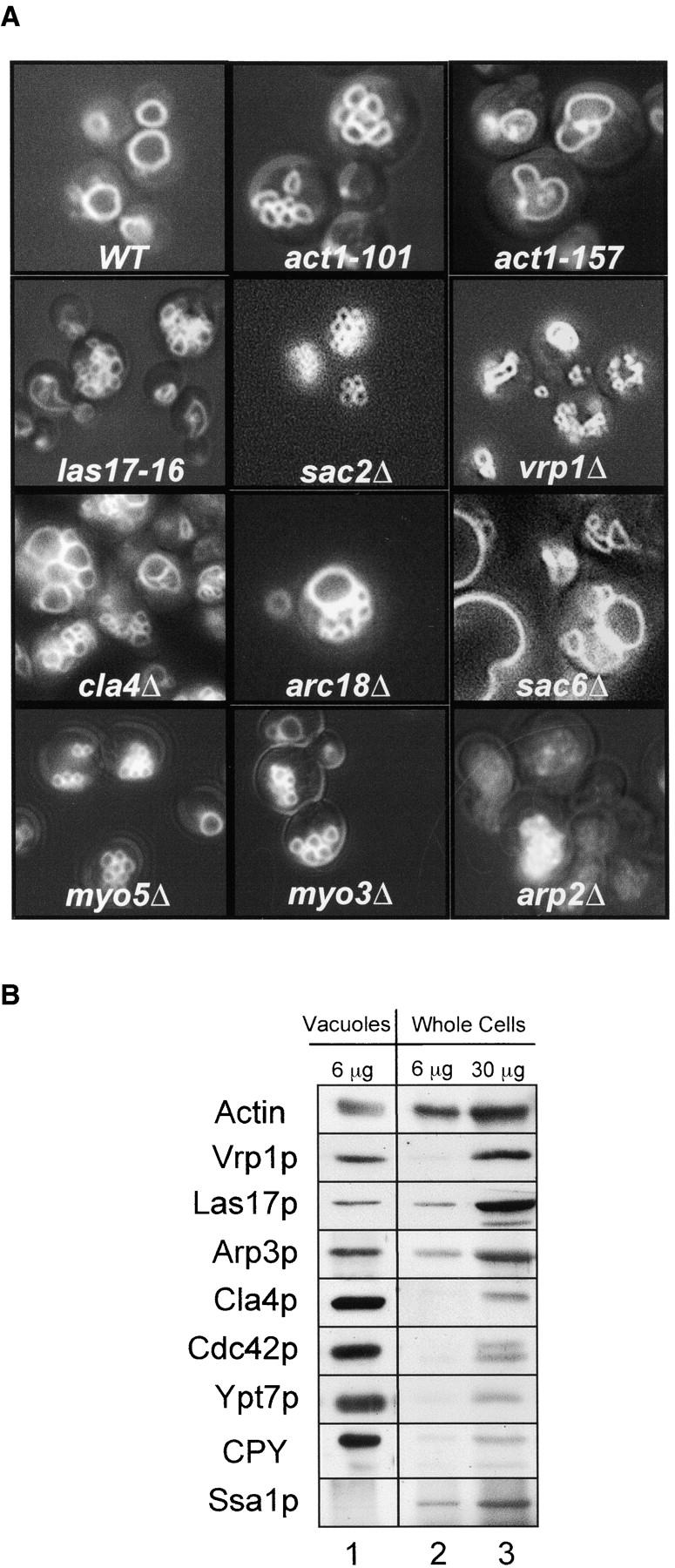
Actin affects vacuole morphology. (A) Vacuole morphology of gene deletion or actin or LAS17 mutant strains. The strains RLY 574 (arp2Δ), DDY2266 (las17–16), GEY1290 (myo3Δ), GEY1090 (myo5Δ), GEY4840 (sac2Δ), GEY1290 (sac6Δ), GEY3370 (vrp1Δ), GEY2980 (cla4Δ), GEY3700 (arc18Δ), GEY0394–101 (act1–101), GEY0394–157 (act1–157), and the wild-type strain BY4742 (WT) were grown overnight in YPD, stained with FM4–46 and examined for vacuole morphology. The width of the field shown in each of the nine panels is 25 μm. (B) Immunoblot analysis of vacuoles and whole cell lysate prepared from strain GEY6024 for actin, actin signaling proteins (Vrp1p, Las17p, Arp3p, Cla4p, and Cdc42p), vacuolar proteins (Ypt7p and CPY), and a cytosolic chaperone (Ssa1p).
To test the role of these proteins in vacuole fusion, we prepared an antibody to a peptide from the NH2-terminal region of Las17p. We assay homotypic fusion of vacuoles by mixing vacuoles from two strains (Wickner and Haas, 2000). One strain has normal vacuolar lumenal proteases but is deleted for the gene encoding the Pho8p phosphatase. The other strain is deleted for the vacuolar lumenal protease Pep4p and therefore accumulates catalytically inactive pro-Pho8p. During incubation with ATP, fusion allows the Pep4p protease to gain access to pro-Pho8p, yielding mature, active phosphatase which is assayed colorimetrically. Vacuole fusion is inhibited by antibodies to Las17p (Fig. 3 A, left, No depletion). The inhibitory antibodies can be specifically depleted by beads bearing the immobilized peptide to which the antibodies were raised (Fig. 3 A, left, compare depletion by immobilized LAS17 peptide with YPT7 peptide).
Figure 3.
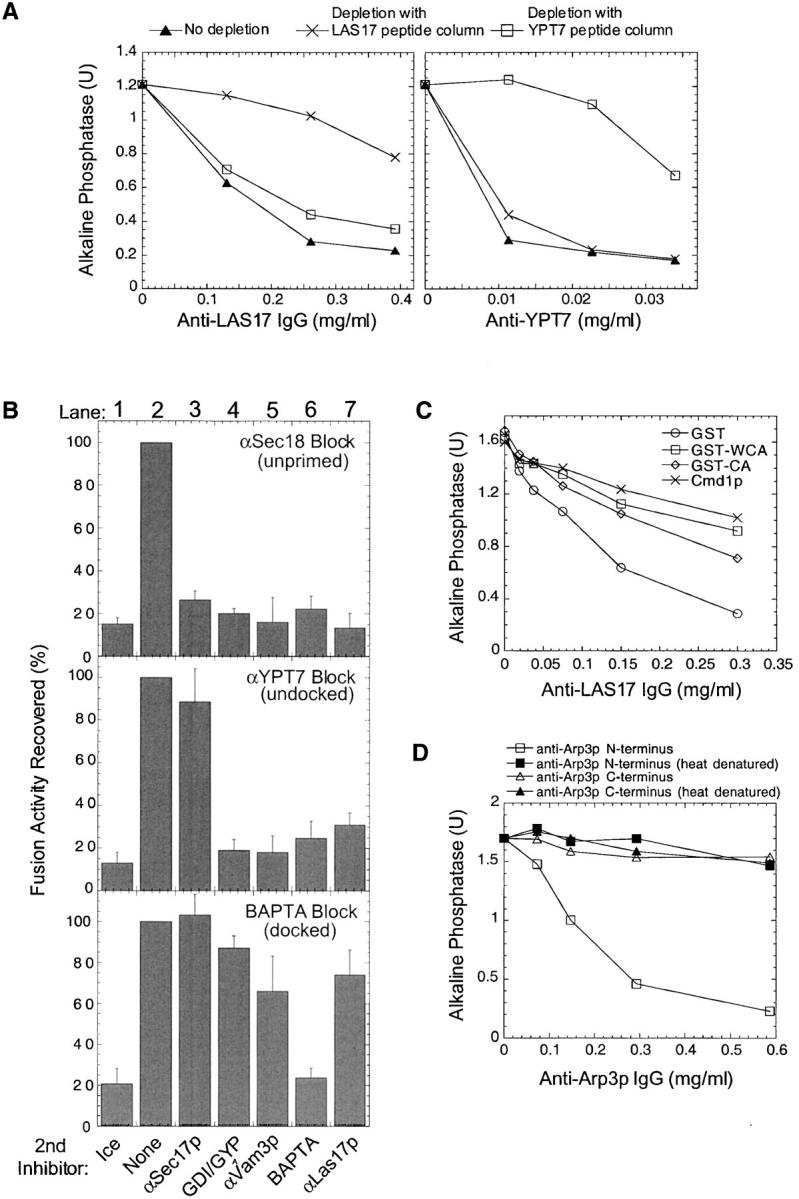
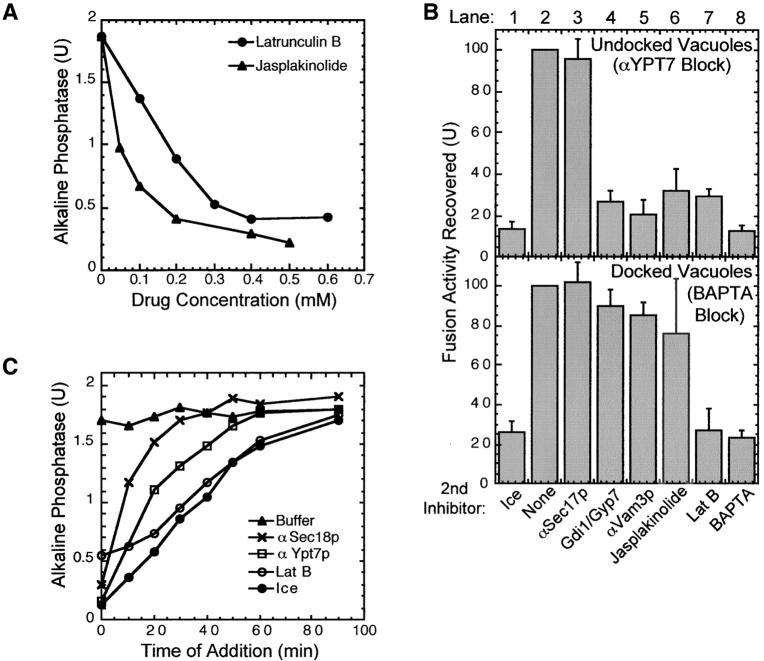
Actin regulatory proteins are required for vacuole fusion. (A) Las17p antibodies inhibit vacuole fusion. Standard fusion reactions were incubated at 27°C in the presence of increasing concentrations of anti-LAS17 peptide antibodies (left) or anti-YPT7 peptide antibodies (right) that were either untreated (▴) or were immunodepleted by incubation with immobilized LAS17 peptide (×) or immobilized YPT7 peptide (□) (as described in Materials and methods). Titration curves for immunodepleted samples were normalized by volume to the nonimmunodepleted samples. (B) Las17p antibody no longer inhibits after docking. Standard fusion reactions (7×) were first incubated with either 50 μg/ml anti-Sec18p, 64 μg/ml anti-Ypt7 antibodies, or 3.5 mM BAPTA for 30, 35, or 45 min, respectively. Sec18p (10 μg/ml), Ypt7p peptide (30 μg/ml), or 4 mM calcium was then added to reverse these blocks, and aliquots (30 μl) were immediately distributed into tubes containing the indicated second inhibitors and incubated at 27°C (lanes 2–7) or on ice (lane 1) for 90 min total. (C) Bypass of Las17p function. Fusion reactions with 8 μg/ml IB2 and 1 μg/ml Sec18p were incubated with increasing concentrations of anti-Las17 antibodies in the presence or absence of 1 mg/ml calmodulin, 0.5 mg/ml GST, 0.5 mg/ml GST–WCA, or 0.5 mg/ml GST–CA. (D) Arp3p antibodies inhibit vacuole fusion. Standard fusion reactions were incubated with an increasing amount of NH2- and COOH-terminal Arp3p antibodies which had been dialyzed into PS buffer and used directly or heat denatured by incubation at 95°C for 5 min.
Reversible inhibitors of the vacuole fusion reaction allow us to determine the last reaction stage that is sensitive to anti-Las17p antibodies (Fig. 3 B). Staging experiments were performed with three reversible inhibitors: antibodies to the priming catalyst Sec18p, antibodies to the docking catalyst Ypt7p, or the fusion inhibitor BAPTA. Incubation of vacuoles with anti-Sec18p antibodies prevents priming. The fusion reaction pathway resumes when excess Sec18p is added (Fig. 3 B, top, lane 2). This reaction remains sensitive to anti-Las17p antibodies (Fig. 3 B, top, lane 7), showing that Las17p is required for the normal fusion reaction pathway. Vacuoles undergo priming in fusion reactions incubated with anti-YPT7 peptide antibodies, since upon reversal of this block with excess YPT7 peptide, fusion reactions are resistant to anti-Sec17p antibodies (Fig. 3 B, middle, compare lane 2 with 3). At this stage, docking is incomplete, since after reversal of the anti-YPT7 block, reactions remain sensitive to either Gdi1p/Gyp7p (Fig. 3 B, middle, lane 4), which extracts Ypt7p (Eitzen et al., 2000), antibody to the t-SNARE Vam3p (Fig. 3 B, middle, lane 5), or the calcium chelator BAPTA (Fig. 3 B, middle, lane 6). The reaction also remains sensitive to anti-Las17p antibodies after reversal of the anti-YPT7 block (Fig. 3 B, middle, lane 7). In the presence of BAPTA, vacuoles complete docking but do not fuse (Peters and Mayer; 1998; Ungermann et al., 1999). Upon reversal of the BAPTA block with calcium, vacuoles complete fusion (Fig. 3 B, bottom, lane 2). At this stage, reactions are no longer sensitive to inhibitors of priming and docking (Fig. 3 B, bottom, lanes 3–5) or to anti-Las17p antibodies (Fig. 3 B, bottom, lane 7). Readdition of BAPTA (Fig. 3 B, bottom, lane 6) or incubation on ice (Fig. 3 B, bottom, lane 1) inhibits fusion. These data suggest that Las17p, which helps initiate actin polymerization, may only be needed until docking is complete, but do not specify the reaction stage where actin functions to promote fusion.
The sensitivity of vacuole fusion to antibody to an NH2-terminal region of Las17p is partially suppressed by the addition of pure recombinant WCA domain of Las17p (Fig. 3 C). The COOH-terminal WCA domain of Las17p stimulates the Arp2/3 complex in yeast and higher eukaryotes (Machesky et al., 1999; Winter et al., 1999). We also found that addition of the CA domain partially suppresses anti-Las17p inhibition, although less efficiently than the WCA domain (Fig. 3 C). Whereas the CA domain of WASp acts as a dominant-negative inhibitor in mammalian extracts (Machesky et al., 1999), the CA domain of yeast stimulates Arp2/3 function, though less efficiently than WCA (Winter et al., 1999). The reaction sensitivity to anti-Las17p is also suppressed by the addition of calmodulin (Fig. 3 C), in accord with the finding that calmodulin activates the Arp2/3 complex, which is a downstream effector of Las17p/WASp (Schaerer-Brodbeck and Riezman, 2000a). Thus, actin remodeling may be at least one of the functions of calmodulin in vacuole fusion. Calmodulin also regulates many other cellular processes and specifically associates with the vacuolar Vtc complex (Müller et al., 2002); further studies will be needed to establish its full range of functions in vacuole fusion. We also used commercially available Arp3p antibodies to directly test the requirement of the Arp2/3 complex in our reaction. An antibody to an NH2-terminal domain of Arp3p antibody inhibits vacuole fusion, whereas antibody to the COOH-terminal domain of Arp3 or heat-denatured antibodies do not inhibit (Fig. 3 D). These biochemical studies complement the genetic results (Fig. 2 A) and indicate that the pathway from Cdc42p through Las17p to the Arp2/3 complex regulates vacuole fusion. Therefore, we sought direct tests of the role of actin itself in vacuole fusion.
Actin mutations affect vacuole fusion
Several actin mutants were examined for their vacuole morphology in vivo. Strains bearing the act1–101 allele have fragmented vacuoles, whereas strains bearing the act1–157 allele have abnormally large, lobed vacuoles (Fig. 2 A). Purified vacuoles from these strains, when incubated in vitro without added cytosolic proteins, are strikingly defective for in vitro fusion when compared with their isogenic ACT1 counterparts (Fig. 4 A, lanes 1–3). Though we have not found conditions which allow fusion of vacuoles from the act1–101 strain, fusion is restored to act1–157 vacuoles when given cytosol (lane 17) or a mixture of the purified chaperone IB2 (Slusarewicz et al., 1997), calmodulin, and Sec18p (lane 14), a mixture which we term “pure components” (P.C.). This agrees well with the failure of in vivo fusion of act1–101 vacuoles but a fused, albeit abnormal, structure of act1–157 vacuoles (Fig. 2 A). These P.C. which stimulate salt-washed vacuoles (Ungermann et al., 1999), also show some stimulation of wild-type (ACT1) vacuole fusion (Fig. 4 A, compare lane 1 with 13), but only act1–157 vacuoles exhibit complete P.C. dependence (Fig. 4 A, compare lane 2 with 14). This fusion is sensitive to normal fusion inhibitors (Fig. 4 B). Immunoblot analysis was performed on purified vacuoles from ACT1, act1–157, or act1–101 strains (Fig. 4 C). Vacuoles from actin mutant strains have normal levels of bound proteins such as actin, calmodulin, Sec18p, Nyv1p (v-SNARE), Ypt7p (the vacuolar Rab), and Vps39p (a subunit of HOPS), and lumenal carboxypeptidase Y, showing that they are of substantially normal composition. However, the act1–101 mutation causes a striking deficiency in vacuolar Cla4p, Vrp1p, Las17p, and Arp3p, though these proteins are present at normal levels in total cell extracts (Fig. 4 D). The inability of even cytosol to rescue the fusion of vacuoles from act1–101 strains (Fig. 4 A, lane 18) may reflect this striking loss of actin regulatory proteins. There was also a modest diminution of vacuolar Vam3p levels (Fig. 4, C and D), which may contribute to the fusion defect of these vacuoles.
Figure 4.
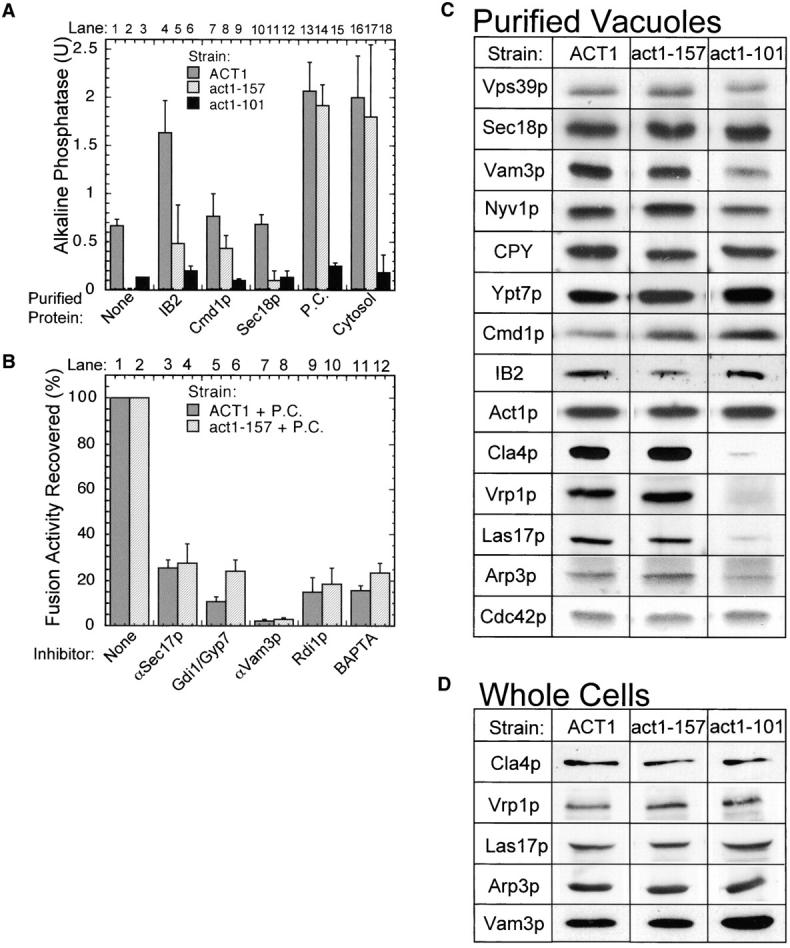
Actin mutations impair vacuole fusion. (A) Vacuoles isolated from strains bearing wild-type actin (GEY0394/0398), act1–157 (GEY0394–157/0398–157), or act1–101 (GEY0394–101/0398–101) were tested for fusion in the absence of additional soluble components (None) or in the presence of IB2, calmodulin, Sec18p, P.C. (IB2, Sec18p, and Cmd1p), or cytosol (0.5 mg/ml). (B) Standard fusion reactions of ACT1 or act1–157 vacuoles are blocked by inhibitors of the normal fusion pathway. (C) Immunoblot analysis of purified vacuoles (5 μg/lane) isolated from strain GEY0394 (ACT1) or the actin mutant strains GEY0394–157 (act1–157), and GEY0394–101 (act1–101). (D) Immunoblot analysis of whole cell lysates (30 μg/lane) from the strains in C.
Actin ligands block vacuole fusion
Latrunculin B and jasplakinolide bind to yeast actin monomers or filaments, respectively (Ayscough 2000; Morton et al., 2000). These drugs inhibit vacuole fusion (Fig. 5 A). As for anti-Las17p (Fig. 3 B), reversible reaction inhibitors were used to determine the stage at which the reaction becomes resistant to latrunculin B or jasplakinolide (Fig. 5 B). Vacuoles were allowed to prime in the presence of an antibody to a peptide from the Ypt7p sequence (Eitzen et al., 2001). Upon addition of excess YPT7 peptide to neutralize the antibody, the subsequent docking and fusion (Fig. 5 B, top, lane 2) remained sensitive to added jasplakinolide or latrunculin B (Fig. 5 B, top, lanes 6 and 7). In a parallel incubation, vacuoles were allowed to complete docking in the presence of the fusion inhibitor BAPTA. Upon reversal of the BAPTA block, the vacuoles went on to fuse when incubated at 27°C (Fig. 5 B, bottom, lane 2). Fusion was no longer sensitive to Gdi1p/Gyp7p or anti-Vam3p antibodies, though these blocked the reaction completely if added from the start (unpublished data), indicating that the vacuoles had completed docking in the presence of BAPTA (Peters and Mayer, 1998; Ungermann et al., 1999). Although latrunculin B still inhibited the fusion of these docked vacuoles (Fig. 5 B, bottom, lane 7), jasplakinolide did not (Fig. 5 B, bottom, lane 6). To verify that latrunculin acts as a late stage inhibitor of vacuole fusion, its kinetics of inhibition were examined. The drug was added to aliquots of a standard fusion reaction at various times, and each aliquot was further incubated for a total time of 90 min at 27°C. Latrunculin addition yielded a kinetic curve of inhibition that was similar to samples placed on ice, which represents the membrane fusion curve (Fig. 5 C). The curve of acquired resistance to latrunculin B was well resolved from the kinetics of acquiring resistance to priming (anti-Sec18p) and docking (anti-Ypt7p) inhibitors. Together, these data suggest that actin remodeling, revealed by sensitivity of the vacuole fusion reaction to anti-Las17p or jasplakinolide, occurs during docking, although the formation of F-actin from monomer, shown by latrunculin B sensitivity, is needed during membrane fusion.
Figure 5.
Actin ligands inhibit vacuole fusion. (A) Inhibition of vacuole fusion by latrunculin B and jasplakinolide. Drugs were added to standard fusion reactions. (B) Standard vacuole fusion reactions were arrested while undocked by incubation with 64 μg/ml Ypt7 antibodies or when docked (but not fused) by incubation with 3.5 mM BAPTA for 35 or 45 min, respectively. The indicated second inhibitors were added after reversal of the initial blocks by the addition of either 30 μg/ml YPT7 peptide or 4 mM calcium, respectively. (C) Kinetic analysis of inhibition by latrunculin B. A large standard fusion reaction was incubated at 27°C. At various times, 30-μl aliquots were placed on ice (defining the fusion curve) or added to tubes containing anti-Sec18p antibodies (priming curve), anti-Ypt7p antibodies (docking curve), or latrunculin B (300 μM final concentration), and further incubated at 27°C for 90 min total.
To determine whether actin remodeling is needed for docking itself or whether it is only needed during docking to allow subsequent fusion, we performed a quantitative microscopic assay of docking (Mayer and Wickner, 1997; Wang et al., 2002). During our standard in vitro incubations, docking creates large clusters of five or more vacuoles (Fig. 6 B). Docking requires ATP-dependent priming (Fig. 6 A) and is blocked by the extraction of Ypt7p by Gdi1p (Fig. 6 C). Neither jasplakinolide nor latrunculin B, at concentrations which block the fusion reaction, inhibit the formation of docked vacuole clusters (Fig. 6, D and F), and docking remains sensitive to Gdi1p (i.e., Ypt7p dependent) in the presence of either drug (Fig. 6, E and G). Thus actin is needed for vacuole fusion rather than for docking, though the jasplakinolide-sensitive aspects of actin remodeling occur during the docking reaction.
Figure 6.
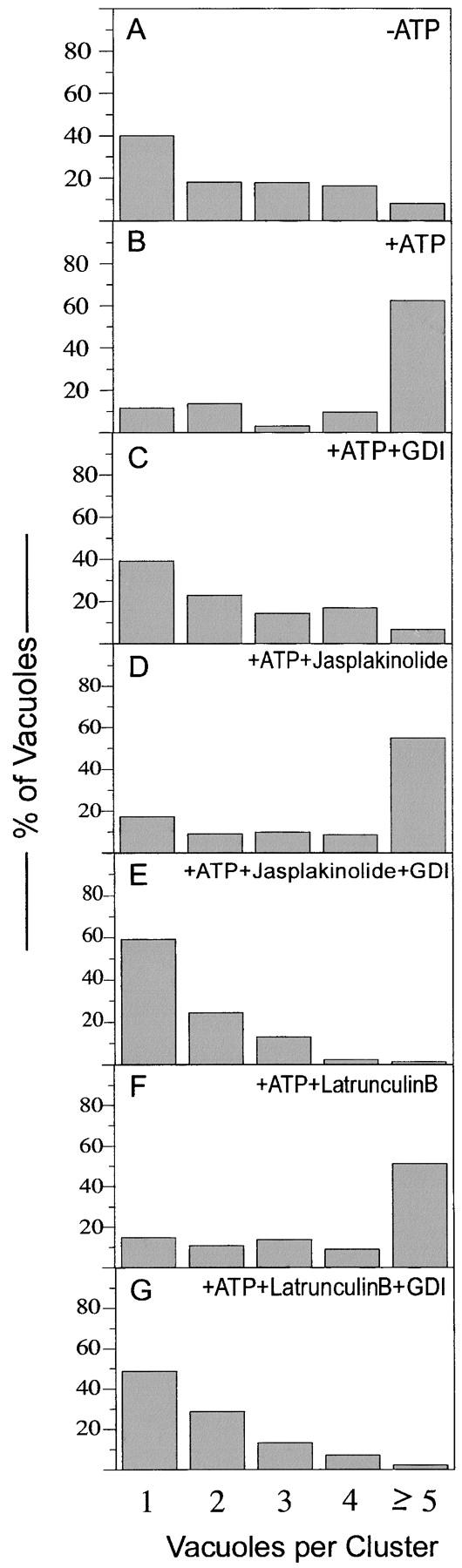
Actin remodeling is not needed for Ypt7-dependent docking. The quantitative microscopic assay of vacuole docking was performed as described (Wang et al., 2002). Approximately 700 vacuoles were counted for each reaction, and the percentage of vacuoles in each cluster size was scored. Gdi1p (100 μg/ml), jasplakinolide (250 μM), and latruculin B (400 μM) were added as indicated.
Vertex enrichment of actin
We have defined the membrane microdomains of docked vacuoles as outside (not in contact with another vacuole), boundary (apposed to another vacuole in a docked cluster), or vertex (where boundary membranes, or outside and boundary membranes, meet). The vertices form a ring around the boundary membrane of adjacent vacuoles, and vacuole homotypic fusion occurs at this ring in vivo and in vitro (Wang et al., 2002). Proteins which catalyze docking and fusion are enriched at these vertex rings, providing a mechanistic rationale for the observed vertex ring fusion pathway. Since actin is required for vacuole fusion, we tested whether it accumulates at the vertex rings of docked vacuoles. Rhodamine-labeled rabbit muscle G-actin was added to vacuoles in the presence of the lipophilic dye MDY-64. When these vacuoles were clumped by cosedimentation (Fig. 7 A, top), there was no apparent clustering of bound actin at any microdomain. However, when these vacuoles were incubated under authentic docking conditions (Fig. 7 A, middle), actin bound selectively to the vertices as shown by ratiometric analysis of the two fluors. This vertex enrichment, like those seen for other docking and fusion factors (Wang et al., 2002), is prevented when SNARE disassembly is blocked (Wang et al., 2000) by an added excess of Sec17p (Fig. 7 A, bottom).
Figure 7.
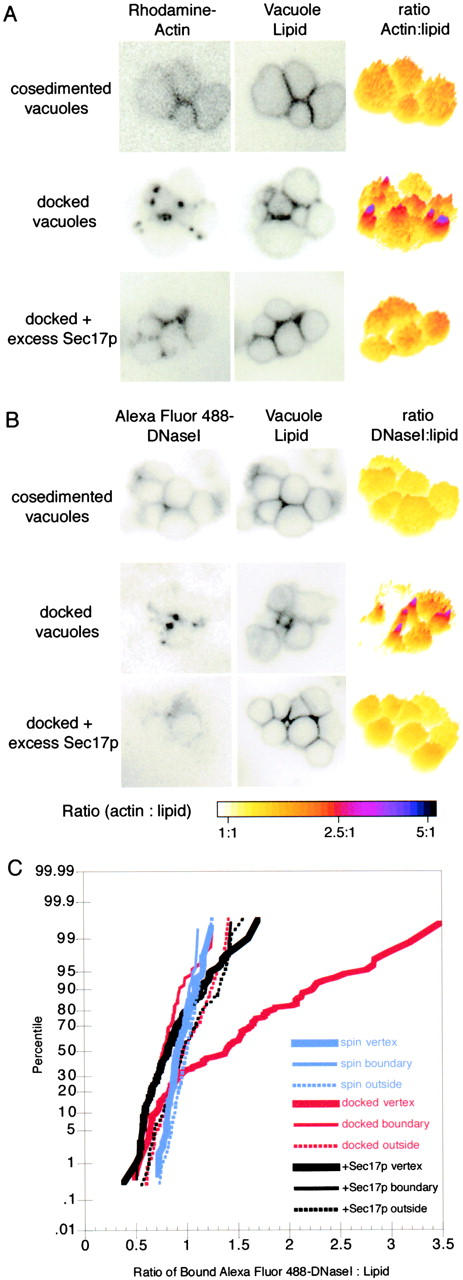
Actin is enriched at the vertices of docked vacuoles. Purified vacuoles were either docked by the physiological Ypt7p-dependent pathway or clustered by cosedimentation as described in Wang et al. (2002). (A) Rhodamine-actin (4 μM, total rabbit muscle actin) was added at the beginning of the reaction, or Alexa Fluor 488–DNaseI (0.2 μM) was added to docked or cosedimented vacuoles (B). After a 30-min incubation at 27°C, vacuoles were fixed with 1% formaldehyde for 20 min at room temperature, sedimented at 3000 g for 2 min, resuspended in PS buffer, and subjected to microscopic analysis (as described in Materials and methods). Ratiometric images of representative clusters are shown. (C) Cumulative distribution plots of the ratios of pixel values (rhodamine:MDY-64 lipid label) obtained for each morphometric domain (vertex, boundary, and outside edge midpoints) and each treatment. Each curve represents an average of 410 individual ratio measurements on 110–115 vacuole clusters from three independent experiments.
A similar pattern of vertex-specific labeling was seen with vacuoles which formed docked clusters in the presence of Alexa Fluor 488–labeled DNaseI (Fig. 7 B). DNaseI binds selectively to actin and thus serves as a measure of the spatial distribution of vacuole-bound actin. Quantitation of the ratios of Alexa Fluor 488–DNaseI to lipidic fluor was performed for the outside edge, boundary midpoints, and vertices of many vacuole clusters. Each ratio was plotted according to its percentile value in its cohort (Fig. 7 C), showing that there is a clear enrichment of Alexa Fluor 488–DNaseI–decorated actin at the vertices of vacuoles under authentic docking conditions.
Discussion
Our studies show that vacuole-bound actin is needed for homotypic fusion of this organelle in the absence of cytoskeleton or cytosol. Proteins of the well-established pathways of actin cytoskeleton regulation are needed for normal vacuole structure in vivo (Fig. 2 A) and are found on purified vacuoles at levels which cannot be due to cytosolic contamination (Fig. 2 B). Antibody to the Las17p/Bee1p, the yeast WASp homologue, inhibits vacuole fusion (Fig. 3), and this inhibition can be modulated (Fig. 3 C) by high levels of either the WCA domain of Las17p or by calmodulin, which are known to interact directly with Arp2/3 complex. Antibody to Arp3p itself also blocks vacuole fusion (Fig. 3 D). Mutations in actin have striking effects on vacuole structure in vivo (Fig. 2 A) and fusion in vitro (Fig. 4), and well-studied actin ligands (Morton et al., 2000) show fusion stage-specific inhibition of the vacuole fusion reaction (Figs. 5 and 6). We found that blocking F-actin depolymerization (jasplakinolide) no longer inhibits vacuoles that have undergone docking (BAPTA block), whereas blocking F-actin assembly with latrunculin B still prevents the final stage of membrane fusion (Fig. 5 B). These results suggest that disassembly of actin, though not needed for docking, is required to produce fusion-competent docked vacuoles and that actin reassembly is needed for fusion. Thus Cdc42p may be essential for vacuole fusion (Eitzen et al., 2001; Müller et al., 2001) because it governs the state of vacuole-bound actin. Rho1p may even regulate vacuole fusion by controlling actin via a MAPK cascade (Helliwell et al., 1998).
Actin is required for other yeast trafficking reactions as well. However, it is unclear in these studies whether this reflects a need for actin cytoskeleton or for actin on the surface of organelles. Actin is needed for mitochondrial motility (Simon et al., 1995) and inheritance (Hermann et al., 1997; Boldogh et al., 2001), vacuole inheritance (Hill et al., 1996), and the polarized delivery of secretory vesicles to the bud tip (Novick and Botstein, 1985; Mullholland et al., 1997; Pruyne et al., 1998). Cdc42p regulates polarized exocytosis in yeast (Pringle et al., 1995; Zhang et al., 2001) and controls actin polymerization through activation of Las17p and the Arp2/3 complex (Lechler et al., 2001). Cdc42p also interacts with proteins that mediate secretory vesicle docking and fusion in a manner that is independent of actin cytoskeleton (Adamo et al., 2001; Zhang et al., 2001). It is unclear though whether Cdc42p also controls actin-related organelle inheritance.
Actin and its regulatory proteins are required for endocytosis in yeast (Kubler and Riezman, 1993; Munn et al., 1995; Geli and Riezman, 1996) and mammals (Lamaze et al., 1997). These actin regulatory proteins include Myo5p (Geli et al., 1998), calmodulin (Kubler et al., 1994), which is also needed for vacuole homotypic fusion (Peters and Mayer, 1998), Arc35p (Schaerer-Brodbeck and Riezman, 2000b), a subunit of the Arp2/3 complex, and Pan1p, which interacts with the endocytic machinery and activates the Arp2/3 complex (Duncan et al., 2001).
Immunoblot analysis of purified vacuoles and whole cell lysate revealed an enrichment of actin regulatory proteins from Cdc42p to the Arp2/3 complex on the vacuole membrane (Fig. 2). Although the Arp2/3 complex or its subcomponents have not previously been localized to the vacuole membrane, they have been found on other subcellular organelles such as the nucleus (Weber et al., 1995; Yan et al., 1997) and the mitochondria (Boldogh et al., 2001). We now show that these proteins, which are part of a well-studied actin remodeling cascade (Higgs and Pollard, 1999), also regulate membrane fusion. Clearly the major site of Arp2/3 localization is cortical actin patches at the plasma membrane where it is involved in cell growth via regulation of vesicle delivery (Moreau et al., 1996; Lechler et al., 2001), though these studies did not preclude a continuing role in vesicle docking and membrane fusion. In addition to Arp3p and Las17p antibody inhibition, we also show that calmodulin and Las17p WCA domain, which are known to interact with and stimulate Arp2/3 function (Winter et al., 1999; Schaerer-Brodbeck and Riezman, 2000b), can stimulate membrane fusion of vacuoles which have been blocked by the addition of an NH2-terminal Las17p peptide antibody (Fig. 3 C). Additionally, specific actin mutations cause the striking loss of vacuolar localization of several actin regulatory proteins, coincident with the inability of these vacuoles to fuse in vitro (Fig. 4 C). These data thus establish a requirement for actin remodeling for vacuole fusion.
Part of the functions of calmodulin and PI(4,5)P2 in vacuole fusion may be the regulation of actin. Calmodulin mediates the signal of docking-induced calcium release (Peters and Mayer, 1998). It interacts directly with the Vo and Vtc complexes (Peters et al., 2001; Müller et al., 2002), Myo2p (Cheney et al., 1993; Brockerhoff et al., 1994), and the Arp2/3 complex (Schaerer-Brodbeck and Riezman, 2000b). Our current studies (Fig. 3 C) show that calmodulin can at least partially bypass the inhibition caused by antibody to Las17p. Genetic (Seeley et al., 2002) and biochemical (Mayer et al., 2000) data show that PI(4,5)P2 is required for vacuole fusion, perhaps in part to function as a guanine nucleotide exchange factor for Cdc42p (Zheng et al., 1996) or as an activating ligand for Las17p. PI(4)P 5-kinase has been shown to govern actin polymerization in yeast (Desrivieres et al., 1998) and mammals (Shibasaki et al., 1997).
Although our combined genetic and biochemical data clearly show that actin has a central role in vacuole fusion, the regulation of this process and the mode of actin action are unknown. Actin structure is altered by overexpression of Nrf1p (Murray and Johnson, 2000), a protein which is directly involved in vacuole fusion (Müller et al., 2002) and interacts genetically with Cdc42p (Murray and Johnson, 2000). Actin remodeling on the vacuole is regulated by the Cdc42p–Las17p–Arp2/3 cascade. Though Cdc42p action requires the prior activation of Ypt7p (Eitzen et al., 2001), the mechanisms which connect these GTPases are unclear. Recently, we reported (Wang et al., 2002) that docked vacuoles show selective accumulation of fusion proteins at a ring around the edge of their apposed membranes and that this ring is the site of membrane fusion. Vacuolar-bound actin is also enriched at these vertices during docking (Fig. 7). Vertex enrichment is a critical subreaction of membrane fusion (Wang et al., 2002). Actin may contribute to this protein localization and might distort or destabilize the bilayer membrane on each vacuole as a prerequisite to fusion itself.
Materials and methods
Yeast strains and genetic modifications
Yeast strains are listed in Table I. The endogenous ACT1 gene was replaced with the act1–101 and act1–157 mutant alleles or wild-type ACT1 (including the downstream HIS3 gene) by PCR amplification from strains DDY 338, DDY1544, and DDY354, respectively (Holtzman et al., 1994; Belmont et al., 1999) using primers TCTACTACATCAGCTTTTAG and GAAGGGCAATTGCATAAAC. Transformants were selected on SD-his (Bio101). Proper integration was checked by genomic DNA PCR using primers AATCTTTGTTAAAGAATAGG and CTAATGGCGGTTCTACAAG. In vivo vacuole morphology was examined by fluorescence microscopy after FM4–64 staining (Seeley et al., 2002).
Table I. Yeast strains.
| Strain | Genotype | Source |
|---|---|---|
| K91-1A | Mata, ura3, lys1, Δpho8::AL134, Δpho13::pPH13 | Y. Kanekoa |
| DKY6281 | Matα, his3, leu2, ura3, lys2, suc2, Δpho8::TRP1 | D. Klionskyb |
| RLY 574 | Matα, his3, leu2, trp1, Δarp2::TRP1 | R. Lic |
| DDY2266 | Mata, his3, leu2, ura3 lys2,, las17-16::LEU2 | D. Drubind |
| BY4742 | Matα, his3Δ1, leu2Δ0, ura3Δ0, lys2Δ0 | Research Genetic |
| GEY1290 | BY4742, Δmyo3::kanMx | Research Genetic |
| GEY1090 | BY4742, Δmyo5::kanMx | Research Genetic |
| GEY4840 | BY4742, Δsac2::kanMx | Research Genetic |
| GEY1290 | BY4742, Δsac6::kanMx | Research Genetic |
| GEY3370 | BY4742, Δvrp1::kanMx | Research Genetic |
| GEY2980 | BY4742, Δcla4::kanMx | Research Genetic |
| GEY3700 | BY4742, Δarc18::kanMx | Research Genetic |
| GEY6024 | BY4742, Δpep4::kanMx | Research Genetic |
| GEY6028 | BY4742, Δpho8::kanMx | Research Genetic |
| GEY0394 | BY4742, Δpep4::kanMx, Δact1::ACT1(HIS3) | This study |
| GEY0398 | BY4742, Δpho8::kanMx, Δact1::ACT1(HIS3) | This study |
| GEY0394-101 | BY4742, vpep4::kanMx, Δact1::act1-101(HIS3) | This study |
| GEY0398-101 | BY4742, Δpho8::kanMx, Δact1::act1-101(HIS3) | This study |
| GEY0394-157 | BY4742, Δpep4::kanMx, Δact1::act1-157(HIS3) | This study |
| GEY0398-157 | BY4742, Δpho8::kanMx, Δact1::act1-157(HIS3) | This study |
Osaka University, Osaka, Japan.
University of Michigan, Ann Arbor, MI.
Harvard Medical School, Boston, MA.
University of California, Berkeley, CA.
Biochemical reagents
Reagents were dissolved or dialyzed in PS buffer (20 mM PIPES-KOH, pH 6.8, 200 mM sorbitol) unless otherwise noted. Alexa Fluor 488–DNaseI (Molecular Probes) was dissolved in PBS/50% glycerol at 166 μM, and rhodamine-labeled rabbit actin (a gift from Dr. H. Higgs, Dartmouth Medical School) was a mixture of 30 μM unlabeled actin and 10 μM labeled actin. Jasplakinolide (Molecular Probes) and latrunculin B (Biomol) were dissolved in DMSO at 10 mM. Amino (yG18) and carboxy (yK17) Arp3p antibodies (Santa Cruz Biotechnology, Inc.) were dialyzed into PS buffer. The following inhibitors were used at the final concentrations listed: affinity-purified anti-YPT7 peptide antibodies (50 μg/ml), IgG fraction of anti-Las17 peptide antibodies (350 μg/ml), affinity-purified anti-Sec18p antibodies (50 μg/ml), anti-Vam3p IgG (150 μg/ml), anti-Sec17p IgG (250 μg/ml), Arp3p IgGs (300 μg/ml), Gdi1p (100 μg/ml), Gyp7p (30 μg/ml), Rdi1p (500 μg/ml), latrunculin B (400 μM), jasplakinolide (250 μM), and BAPTA (5 mM).
Protein preparation
Sec18p, Gyp7p, Gdi1p, Rdi1p, calmodulin (Cmd)1p, and IB2 were prepared as described (Brockerhoff et al., 1992; Slusarewicz et al., 1997; Eitzen et al., 2001). GST, GST–WCA, and GST–CA were expressed in Escherichia coli DH5α transformed with the vector pGEX-4T1 (Amersham Biosciences) only or with vector containing in frame BamH1–EcoR1PCR products encoding the COOH-terminal 125 (WCA) or 66 (CA) amino acids of Las17p. These proteins were prepared as described for Rdi1p (Eitzen et al., 2001). Cytosol was prepared from strain K91–1A grown to an OD600 ∼4, lysed by vortexing 5 min with glass beads in lysis buffer (125 mM KCl, 5 mM MgCl2, 20 mM PIPES-KOH, pH 6.8, 200 mM sorbitol, 1 mM ATP, 6.6 ng/ml leupeptin, 16.6 ng/ml pepstatin, 16.6 μM o-phenanthroline, 3.3 μM Pefabloc SC), and cleared by centrifugation (12,000 g for 10 min and then 150,000 g for 60 min at 4°C). Whole cell lysates were similarly prepared in lysis buffer from strains grown to an OD600 ∼1 except that 1% Triton X-100 was added after glass bead vortexing and the lysate was cleared by centrifugation at 3,000 g for 4 min at 4°C. YPT7 and LAS17 peptide antibodies were raised against TEAFEDDYNDAINIRC and FEMEECFAGLLFVDINEASHC conjugated to KLH, respectively. These peptides (3 mg/ml resin) and Sec18p (10 mg/ml resin) were conjugated to Sulfolink resin (Pierce Chemical Co.) according to the manufacturer's protocol. IgG fractions were prepared from sera by protein A sepharose adsorption (Harlow and Lane, 1988). Immobilized peptides and protein were used for affinity purification and immunodepletion of antibodies. For immunodepletion experiments, IgGs (0.5 ml) were incubated with 0.1 ml packed resin in PBS for 1 h at 4°C. Flow-through samples were saved as immunodepleted fractions. The resin was washed twice with 1 ml of PBS, and bound antibodies were eluted with 0.25 ml of 100 mM glycine-HCl, pH 2.5. All samples were dialyzed into PS buffer.
Standard vacuole fusion reactions
Vacuoles were isolated from strains grown in YPD to an OD600 ∼0.9. Fusion reactions (Haas, 1995) contain 3 μg of vacuoles lacking proteinase A and 3 μg of vacuoles lacking alkaline phosphatase in 30 μl fusion reaction buffer (FRB: 125 mM KCl, 5 mM MgCl2, 20 mM PIPES-KOH, pH 6.8, 200 mM sorbitol, 1 mM ATP, 40 mM creatine phosphate, 0.5 mg/ml creatine kinase, 10 μM CoA, 6.6 ng/ml leupeptin, 16.6 ng/ml pepstatin, 16.6 μM o-phenanthroline, 3.3 μM Pefabloc SC). Standard vacuole fusion reactions also contained P.C. (200 μg/ml calmodulin, 8 μg/ml IB2, and 1 μg/ml Sec18p). Reactions were incubated for 90 min at 27°C before assaying for alkaline phosphatase. Staging experiments with reversible blocking reagents were as described previously (Eitzen et al., 2001).
In vitro microscopic docking assay and image processing
Docking assays were performed as described previously (Wang et al., 2002) with minor modifications. Reactions (30 μl) contain 5 μg of vacuoles (from strain DKY6281) labeled with the lipophilic dye MDY-64 (3 μM; Molecular Probes). At the end of the reaction, vacuoles were mixed with 40 μl of 0.6% agarose in PS buffer. Aliquots (15 μl) were then observed by fluorescence microscopy. Images were acquired with an Olympus BX51 microscope equipped with a 60×/1.4 NA plan Apochromat objective, 100 W Mercury arc lamp, and Cooke Sensicam QE CCD camera. The microscope and camera were controlled by IP lab software (Scanalytics). An Endow GFP filter cube (Chroma Technology Corp.) was used for rhodamine-actin and Alexa Fluor 488–DNaseI. The camera was operated at normal gain setting without binning. An automation script was written to facilitate reproducible image acquisition. Image processing and quantitative analysis were according to Wang et al. (2002).
Acknowledgments
We thank Dr. David Drubin (University of California, Berkeley, CA) and Dr. Rong Li (Harvard Medical School, Boston, MA) for discussions and mutant strains, Dr. Harry Higgs (Dartmouth Medical School) for reagents and helpful suggestions, Dr. Alex Merz for discussions, and Dr. Nathan Margolis for technical assistance.
This work was supported by grants from the National Institute of General Medical Sciences and the Human Frontier Science Program.
G. Eitzen's current address is Dept. of Cell Biology, University of Alberta, Edmonton, Alberta, Canada T6G 2H7.
Footnotes
Abbreviations used in this paper: Cmd, calmodulin; PAK, p20-activated kinase; P.C., pure components; PI(4,5)P2, phosphatidylinositol 4,5-bisphosphate; WASp, Wiskott-Aldrich syndrome protein.
References
- Adamo, J.E., J.J. Moskow, A.S. Gladfelter, D. Viterbo, D.J. Lew, and P.J. Brennwald. 2001. Yeast Cdc42p functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell Biol. 155:581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, A.E.M., D. Botstein, and D.G. Drubin. 1989. A yeast actin-binding protein is encoded by SAC6, a gene found by suppression of an actin mutation. Science. 243:231–233. [DOI] [PubMed] [Google Scholar]
- Ayscough, K.R. 2000. Endocytosis and the development of cell polarity in yeast require a dynamic F-actin cytoskeleton. Curr. Biol. 10:1587–1590. [DOI] [PubMed] [Google Scholar]
- Belmont, L.D., G.M.L. Patterson, and D.G. Drubin. 1999. New actin mutants allow further characterization of the nucleotide binding cleft and drug binding sites. J. Cell Sci. 112:1325–1336. [DOI] [PubMed] [Google Scholar]
- Bernstein, B.W., M. DeWitt, and J.R. Bamburg. 1998. Actin disassembles reversibly during electrically induced recycling of synaptic vesicles in cultured neurons. Brain Res. Mol. Brain Res. 53:236–251. [DOI] [PubMed] [Google Scholar]
- Boldogh, I.R., H.C. Yang, W.D. Nowakowski, S.L. Karmon, L.G. Hays, J.R. Yates, and L.A. Pon. 2001. Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc. Natl. Acad. Sci. USA. 98:3162–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff, S.E., C.G. Edmonds, and T.N. Davis. 1992. Structural analysis of wild-type and mutant yeast calmodulins by limited proteolysis and electrospray ionization mass spectrometry. Protein Sci. 1:504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff, S.E., R.C. Stevens, and T.N. Davis. 1994. The unconventional myosin, Myo2p, is a calmodulin target at sites of cell growth in Saccharomyces cerevisiae. J. Cell Biol. 124:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett, N.L., and L.S. Weisman. 2000. Divide and multiple: organelle partitioning in yeast. Curr. Opin. Cell Biol. 12:509–516. [DOI] [PubMed] [Google Scholar]
- Catlett, N.L., J.E. Deux, F. Tang, and L.S. Weisman. 2000. Two distinct regions in a yeast myosin-V tail domain are required for the movement of different cargoes. J. Cell Biol. 150:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney, R.E., M.K. O'Shea, J.E. Heuser, M.V. Coelho, J.S. Wolenski, E.M. Espreafico, P. Forscher, R.E. Larson, and M.S. Mooseker. 1993. Brain mysoin V is a two-headed unconventional myosin with motor activity. Cell. 75:13–23. [DOI] [PubMed] [Google Scholar]
- Colicos, M.A., B.E. Collins, M.J. Sailor, and Y. Goda. 2001. Remodeling of synaptic actin induced by photoconductive stimulation. Cell. 107:605–616. [DOI] [PubMed] [Google Scholar]
- Desrivieres, S., F.T. Cooke, P.J. Parker, and M.N. Hall. 1998. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 273:15787–15793. [DOI] [PubMed] [Google Scholar]
- Duncan, M.C., M.J.T.V. Cope, B.L. Goode, B. Wendland, and D.G. Drubin. 2001. Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nat. Cell Biol. 3:687–690. [DOI] [PubMed] [Google Scholar]
- Eitzen, G., E. Will, D. Gallwitz, A. Haas, and W. Wickner. 2000. Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J. 19:6713–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen, G., N. Thorngren, and W. Wickner. 2001. Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking and fusion. EMBO J. 20:5650–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista, M., B.M. Klebl, A.H.Y. Tong, B.A. Webb, T. Leeuw, E. Leberer, M. Whiteway, D.Y. Thomas, and C. Boone. 2000. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 148:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti, M., A. Audhya, and S.D. Emr. 2001. Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol-4-phosphate that functions in the control of the actin cytoskeleton and actin morphology. Mol. Biol. Cell. 12:2396–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli, M.I., and H. Riezman. 1996. Role of the type I myosins in receptor-mediated endocytosis in yeast. Science. 272:533–535. [DOI] [PubMed] [Google Scholar]
- Geli, M.I., A. Wesp, and H. Riezman. 1998. Distinct functions of calmodulin are required for the uptake step of receptor-mediated endocytosis in yeast: the type I myosin Myo5p is one of the calmodulin targets. EMBO J. 17:635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfetter, A.S., J.J. Moskow, T.R. Zyla, and D.J. Lew. 2001. Isolation and characterization of effector-loop mutants of CDC42 in yeast. Mol. Biol. Cell. 12:1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode, B.L., and A.A. Rodal. 2001. Modular complexes that regulate actin assembly in budding yeast. Curr. Opin. Microbiol. 4:703–712. [DOI] [PubMed] [Google Scholar]
- Guo, W., F. Tamanoi, and P. Novick. 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3:353–360. [DOI] [PubMed] [Google Scholar]
- Haas, A. 1995. A quantitative assay to measure homotypic fusion in vitro. Methods Cell Sci. 17:283–294. [Google Scholar]
- Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science. 279:509–514. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies, a Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 309–311.
- Helliwell, S.B., A. Schmidt, Y. Ohya, and M.N. Hall. 1998. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol. 8:1211–1214. [DOI] [PubMed] [Google Scholar]
- Hermann, G.J., E.J. King, and J.M. Shaw. 1997. The yeast gene MDM20 is necessary for mitochondrial inheritance and organization of the actin cytoskeleton. J. Cell Biol. 137:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs, H.N., and T.D. Pollard. 1999. Regulation of actin polymerization by Arp 2/3 complex and WASp/Scar proteins. J. Biol. Chem. 274:32531–32534. [DOI] [PubMed] [Google Scholar]
- Higgs, H.N., and T.D. Pollard. 2000. Activation by Cdc42 and PIP2 of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp 2/3 complex. J. Cell Biol. 150:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, K.L., N.L. Catlett, and L.S. Weisman. 1996. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J. Cell Biol. 135:1535–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman, D.A., K.F. Wertmann, and D.G. Drubin. 1994. Mapping actin surfaces required for functional interactions in vivo. J. Cell Biol. 126:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn, R., and T.C. Sudhof. 1999. Membrane fusion and exocytosis. Annu. Rev. Biochem. 68:863–911. [DOI] [PubMed] [Google Scholar]
- Jahraus, A., M. Egeberg, B. Hinner, A. Habermann, E. Sackman, A. Pralle, H. Faulstich, V. Rybin, H. Defacque, and G. Griffiths. 2001. ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol. Biol. Cell. 12:155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffer, A., P.E.R. Tatham, and B.D. Gomperts. 1990. Changes in the state of actin during the exocytotic reaction of permeabilized rat mast cells. J. Cell Biol. 111:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler, E., and H. Riezman. 1993. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 12:2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler, E.F., E.F. Schimmoller, and H. Riezman. 1994. Calcium-independent calmodulin requirement for endocytosis in yeast. EMBO J. 13:5539–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze, C., L.M. Fujimoto, H.L. Yin, and S.L. Schmid. 1997. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J. Biol. Chem. 272:20332–20335. [DOI] [PubMed] [Google Scholar]
- Lang, T., I. Wacker, I. Wunderlich, A. Rohrbach, G. Giese, T. Soldati, and W. Almers. 2000. Role of actin cortex in the subplamalemal transport of secretory granules in PC-12 cells. Biophys. J. 78:2863–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler, T., A. Shevchenko, A. Shevchenko, and R. Li. 2000. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 148:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler, T., G.A. Jonsdottir, S.K. Klee, D. Pellman, and R. Li. 2001. A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp 2/3-activating motor complex in yeast. J. Cell Biol. 155:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky, L.M., R.D. Mullins, H.N. Higgs, D.A. Kaiser, L. Blanchoin, R.C. May, M.E. Hall, and T.D. Pollard. 1999. Scar, a WASp related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. USA. 96:3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madania, A., P. Dumoulin, S. Grava, H. Kitamoto, C. Scharer-Brodbeck, A. Soulard, V. Moreau, and B. Winsor. 1999. The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol Biol Cell. 10:3521–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Quiles, N., R. Rohatgi, I.M. Anton, M. Medina, S.P. Saville, H. Miki, H. Yamaguchi, T. Takenawa, J.H. Hartwig, R.S. Geha, and N. Ramesh. 2001. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat. Cell Biol. 3:484–491. [DOI] [PubMed] [Google Scholar]
- Mayer, A., and W. Wickner. 1997. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF). J. Cell Biol. 136:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, A., W. Wickner, and A. Haas. 1996. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 85:83–94. [DOI] [PubMed] [Google Scholar]
- Mayer, A., D. Scheglmann, S. Dove, A. Glatz, W. Wickner, and A. Haas. 2000. Phosphatidylinositol-(4,5)-bisphosphate regulates two steps of homotypic vacuole fusion. Mol. Biol. Cell. 11:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian, M., K.J. Bame, and L.H. Rome. 1984. Interaction of rat liver lysosomal membranes with actin. J. Cell Biol. 99:680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau, V., A. Madania, R.P. Martin, and B. Winsor. 1996. The Saccharomyces cerevisiae actin-related protein Arp2 is involved in the actin cytoskeleton. J. Cell Biol. 134:117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton, W.M., K.R. Ayscough, and P.J. McLaughlin. 2000. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2:376–378. [DOI] [PubMed] [Google Scholar]
- Mosch, H.U., T. Kohler, and G.H. Braus. 2001. Different domains of the essential GTPase Cdc42p required for growth and development of Saccharomyces cerevisiae. Mol. Cell. Biol. 21:235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem, S., K. Kwiatkowska, X. Xu, and H.L. Yin. 1995. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J. Cell Biol. 128:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, O., D.I. Johnson, and A. Mayer. 2001. Cdc42p functions at the docking stage of yeast vacuole membrane fusion. EMBO J. 20:5657–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, O., M.J. Bayer, C. Peters, J.S. Andersen, M. Mann, and A. Mayer. 2002. The Vtc proteins in vacuole fusion: coupling NSF activity to Vo trans-complex formation. EMBO J. 21:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullholland, J., A. Wesp, H. Riezman, and D. Botstein. 1997. Yeast actin cytoskeletal mutants accumulate a new class of Golgi-derived secretory vesicle. Mol. Biol. Cell. 8:1481–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn, A.L., B.J. Stevenson, M.I. Geli, and H. Riezman. 1995. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell. 6:1721–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, J.M., and D.I. Johnson. 2000. Isolation and characterization of Nrf1p, a novel negative regulator of the Cdc42p GTPase in Schizosaccharomyces pombe. Genetics. 154:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick, P., and D. Botstein. 1985. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 40:405–416. [DOI] [PubMed] [Google Scholar]
- Peters, C., and A. Mayer. 1998. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 396:575–580. [DOI] [PubMed] [Google Scholar]
- Peters, C., M.J. Bayer, S. Buhler, J.S. Andersen, M. Mann, and A. Mayer. 2001. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 409:581–587. [DOI] [PubMed] [Google Scholar]
- Prehoda, K.E., J.A. Scott, R.D. Mullins, and W.A. Lim. 2000. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 290:801–806. [DOI] [PubMed] [Google Scholar]
- Price, A., D. Seals, W. Wickner, and C. Ungermann. 2000. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol. 148:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle, J.R., E. Bi, H.A. Harkins, J.E. Zahner, C. DeVirgilio, J. Chant, K. Corado, and H. Fares. 1995. Establishment of cell polarity in yeast. Cold Spring Harb. Symp. Quant. Biol. 60:729–744. [DOI] [PubMed] [Google Scholar]
- Pruyne, D.W., D.H. Schott, and A. Bretscher. 1998. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143:1931–1945. [DOI] [PubMed] [Google Scholar]
- Qualmann, B., M.M. Kessels, and R.B. Kelley. 2000. Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 150:F111–F116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozelle, A.L., L.M. Machesky, M. Yamamoto, M.H.E. Driessens, R.H. Insall, M.G. Roth, K. Luby-Phelps, G. Marriott, A. Hall, and H.L. Yin. 2000. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr. Biol. 10:311–320. [DOI] [PubMed] [Google Scholar]
- Sato, T.K., P. Rehling, and S.D. Emr. 2000. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell. 6:661–671. [DOI] [PubMed] [Google Scholar]
- Schaerer-Brodbeck, C., and H. Riezman. 2000. a. Saccharomyces cerevisiae Arc35p works through two genetically separable calmodulin functions to regulate the actin and tubulin cytoskeletons. J. Cell Sci. 113:521–532. [DOI] [PubMed] [Google Scholar]
- Schaerer-Brodbeck, C., and H. Riezman. 2000. b. Functional interactions between the p35 subunit of the Arp2/3 complex and calmodulin in yeast. Mol. Biol. Cell. 11:1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals, D., G. Eitzen, N. Margolis, W. Wickner, and A. Price. 2000. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. USA. 97:9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, E.S., M. Kato, N. Margolis, W. Wickner, and G. Eitzen. 2002. The genomics of homotypic vacuole fusion. Mol. Biol. Cell. 13:782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki, Y., H. Ishihara, N. Kizuki, T. Asano, Y. Oka, and Y. Yazaki. 1997. Massive actin polymerization induced by phosphatidylinositol-4-phosphate 5-kinase in vivo. J. Biol. Chem. 272:7578–7581. [DOI] [PubMed] [Google Scholar]
- Simon, V.R., T.C. Swayne, and L.A. Pon. 1995. Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J. Cell Biol. 130:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarewicz, P., Z. Xu, K. Seefeld, A. Haas, and W.T. Wickner. 1997. IB2 is a small cytosolic protein that participates in vacuole fusion. Proc. Natl. Acad. Sci. USA. 94:5582–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton, J. 2001. Actin filament nucleation by endosomes, lysosomes and secretory vesicles. Curr. Opin. Cell Biol. 13:85–91. [DOI] [PubMed] [Google Scholar]
- Ungermann, C., B.J. Nichols, H.R.B. Pelham, and W. Wickner. 1998. a. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J. Cell Biol. 140:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann, C., K. Sato, and W. Wickner. 1998. b. Defining the functions of trans-SNARE pairs. Nature. 396:543–548. [DOI] [PubMed] [Google Scholar]
- Ungermann, C., W. Wickner, and Z. Xu. 1999. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc. Natl. Acad. Sci. USA. 96:11194–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaduva, G., N. Martinez-Quiles, I.M. Anton, N.C. Martin, R.S. Geha, A.K. Hopper, and N. Ramesh. 1999. The human WASP-interacting protein, WIP, activates the cell polarity pathway in yeast. J. Biol. Chem. 274:17103–17108. [DOI] [PubMed] [Google Scholar]
- Valderrama, F., T. Babia, I. Ayala, J.W. Kok, J. Renau-Piqueras, and G. Egea. 1998. Actin microfilaments are essential for the cytological positioning and morphology of the Golgi complex. Eur. J. Cell Biol. 76:9–17. [DOI] [PubMed] [Google Scholar]
- Vitale, M.L., A. Rodrigues del Castillo, L. Tchakarov, and J.-M. Trifaro. 1991. Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J. Cell Biol. 113:1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., C. Ungermann, and W. Wickner. 2000. The docking of primed vacuoles can be reversibly arrested by excess Sec17p (αSNAP). J. Biol. Chem. 275:22862–22867. [DOI] [PubMed] [Google Scholar]
- Wang, L., E.S. Seeley, W. Wickner, and A.J. Merz. 2002. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 108:357–369. [DOI] [PubMed] [Google Scholar]
- Weber, V., M. Harata, H. Hauser, and U. Wintersberger. 1995. The actin-related protein Act3p of Saccharomyces cerevisiae is located in the nucleus. Mol. Biol. Cell. 6:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner, W., and A. Haas. 2000. Yeast vacuole fusion: a window on organelle trafficking mechanisms. Annu. Rev. Biochem. 69:247–275. [DOI] [PubMed] [Google Scholar]
- Winter, D., T. Lechler, and R. Li. 1999. Activation of the yeast Arp 2/3 complex by Bee1p, a WASP-family protein. Curr. Biol. 9:501–504. [DOI] [PubMed] [Google Scholar]
- Yan, C., N. Leibowitz, and T. Melese. 1997. A role for the divergent actin gene, ACT2, in nuclear pore structure and function. EMBO J. 16:3572–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., E. Bi, P. Novick, L. Du, K.G. Kozminski, J.H. Lipschutz, and W. Guo. 2001. Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 276:46745–46750. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., J.A. Glaven, W.J. Wu, and R.A. Cerione. 1996. Phosphatidylinositol 4,5-bisphosphate provides an alternative to guanine nucleotide exchange factors by stimulating the dissociation of GDP from Cdc42Hs. J. Biol. Chem. 271:23815–23819. [DOI] [PubMed] [Google Scholar]