Abstract
Leukocytes roll on selectins at nearly constant velocities over a wide range of wall shear stresses. Ligand-coupled microspheres roll faster on selectins and detach quickly as wall shear stress is increased. To examine whether the superior performance of leukocytes reflects molecular features of native ligands or cellular properties that favor selectin-mediated rolling, we coupled structurally defined selectin ligands to microspheres or K562 cells and compared their rolling on P-selectin. Microspheres bearing soluble P-selectin glycoprotein ligand (sPSGL)-1 or 2-glycosulfopeptide (GSP)-6, a GSP modeled after the NH2-terminal P-selectin–binding region of PSGL-1, rolled equivalently but unstably on P-selectin. K562 cells displaying randomly coupled 2-GSP-6 also rolled unstably. In contrast, K562 cells bearing randomly coupled sPSGL-1 or 2-GSP-6 targeted to a membrane-distal region of the presumed glycocalyx rolled more like leukocytes: rolling steps were more uniform and shear resistant, and rolling velocities tended to plateau as wall shear stress was increased. K562 cells treated with paraformaldehyde or methyl-β-cyclodextrin before ligand coupling were less deformable and rolled unstably like microspheres. Cells treated with cytochalasin D were more deformable, further resisted detachment, and rolled slowly despite increases in wall shear stress. Thus, stable, shear-resistant rolling requires cellular properties that optimize selectin–ligand interactions.
Keywords: selectin; PSGL-1; rolling; adhesion; leukocyte
Introduction
Lymphocyte recirculation or leukocyte recruitment into inflamed tissues requires that cells tether to and roll on vascular surfaces under flow. Reversible binding of selectins to cell-surface glycoconjugates mediates this first adhesive interaction (Vestweber and Blanks, 1999; McEver, 2001). L-selectin, expressed on leukocytes, binds to ligands on endothelial cells and other leukocytes. P- and E-selectin, expressed on activated platelets and/or endothelial cells, bind to ligands on leukocytes, platelets, and some endothelial cells. Each selectin has a membrane-distal C-type lectin domain that binds to oligosaccharides with terminal sialylated and fucosylated structures such as sialyl Lewis x (sLex).* The major leukocyte ligand for P- and L-selectin is P-selectin glycoprotein ligand (PSGL)-1, a homodimeric mucin (McEver and Cummings, 1997; McEver, 2001). P-selectin binds in a stereospecific manner to an NH2-terminal region of PSGL-1 through recognition of tyrosine sulfate residues, adjacent peptide determinants, and fucose and sialic residues on a properly positioned core-2 O-glycan (Leppänen et al., 1999, 2000; Somers et al., 2000).
Leukocyte rolling requires rapid formation and breakage of adhesive bonds that are subjected to applied force (Lawrence and Springer, 1991). Biochemical measurements reveal that selectin-ligand interactions have rapid dissociation rates and, in some instances, rapid association rates (Mehta et al., 1998; Nicholson et al., 1998; Wild et al., 2001). These kinetics may be necessary but not sufficient to initiate or support rolling. For example, an EGF domain substitution in L-selectin enhances tethering of cells to L-selectin ligands under flow, but does not alter the apparent kon in the absence of flow (Dwir et al., 2000). This suggests that subtle differences in the orientations of selectins or their ligands may regulate bond association or dissociation rates under flow. Selectin–ligand bonds have been suggested to have high tensile strength, based on the ability of transient cell tethers on low selectin densities to resist premature dissociation by increasing shear forces (Alon et al., 1995, 1997). However, these tethers may have more than one bond so that single bonds need not have high strength (Ramachandran et al., 2001).
In vivo, leukocytes roll stably in a narrow range of velocities despite wide variations in wall shear stress (Atherton and Born, 1973; Firrell and Lipowsky, 1989). These shear-resistant interactions allow leukocytes to roll for longer intervals, which enhances conversion from rolling to integrin-mediated firm adhesion and then transendothelial migration (Jung et al., 1998). Leukocytes rolling on selectins in vitro exhibit similar shear-resistant rolling, which is associated with an increase in selectin bond number as wall shear stress increases (Chen and Springer, 1999). This automatic braking system has been ascribed to intrinsic molecular features of selectins and their ligands; higher wall shear stresses are postulated to overcome repulsive forces, favor molecular contacts, and increase bond formation (Chen and Springer, 1999). However, cellular features may also contribute to rolling behavior. Rolling cells deform as wall shear stress rises (Firrell and Lipowsky, 1989; Lei et al., 1999), and they stretch microvilli and extrude membrane tethers (Shao et al., 1998; Schmidtke and Diamond, 2000; Park et al., 2002). These properties may increase contact area and the probability of bond formation, and reduce force on individual bonds. The abilities of these cellular features to stabilize rolling have not been addressed in detail.
Perfusing ligand-coupled microspheres over immobilized selectins has enabled study of selectin-mediated tethering and rolling in a cell-free environment. Microspheres coated with sLex tether to and roll on E-, P-, or L-selectin (Brunk et al., 1996; Brunk and Hammer, 1997; Greenberg et al., 2000; Rodgers et al., 2000). These data establish that a purified selectin and a minimal carbohydrate ligand are sufficient to support rolling of a rigid particle. The extent to which specific molecular or cellular features augment rolling dynamics is less well understood. Unlike those of leukocytes, the rolling velocities of sLex-coated microspheres increase rapidly with wall shear stress until detachment. It is not known whether this reflects deficient components of the sLex glycoconjugates that are present in natural ligands or deficient features of the microspheres that are present in cells. Microspheres coated with native PSGL-1 or NH2-terminal fragments of recombinant soluble PSGL-1 roll on immobilized P-selectin (Goetz et al., 1997; Rodgers et al., 2001; Park et al., 2002). Where studied, the rolling velocities of these microspheres also increase rapidly with wall shear stress until detachment. It is not known whether this shear sensitivity reflects components missing in the PSGL-1 fragments, suboptimal presentation of PSGL-1 due to random coupling, or missing features normally contributed by cells. Tyrosine sulfation of the PSGL-1 fragments enhances microsphere rolling on P-selectin, but there is controversy as to the importance of this modification for rolling (Ramachandran et al., 1999; Rodgers et al., 2001). The densities of sLex or PSGL-1 derivatives on microspheres have been measured by different antibodies, precluding direct comparisons of rolling behavior (Rodgers et al., 2000). Selectin ligands expressed on cells may be heterogeneous because of variations in posttranslational modifications. Thus, it is difficult to know whether differences in rolling between cells and microspheres are due to differences in the ligands or to other differences between cells and microspheres.
To distinguish the contributions of molecular and cellular features to rolling on a selectin, we used well-characterized ligands: a minimal sLex glycoconjugate, small GSPs modeled after the NH2-terminal region of PSGL-1, and a recombinant soluble form of PSGL-1 comprising the entire extracellular sequence. These ligands were directionally coupled through a COOH-terminal biotin to streptavidin-coated microspheres or to streptavidin-coated K562 cells, which do not express functional selectin ligands. Microspheres or cells decorated with defined ligands at matched densities were perfused over immobilized P-selectin at different wall shear stresses.
Results
Preparation of ligand-coupled microspheres
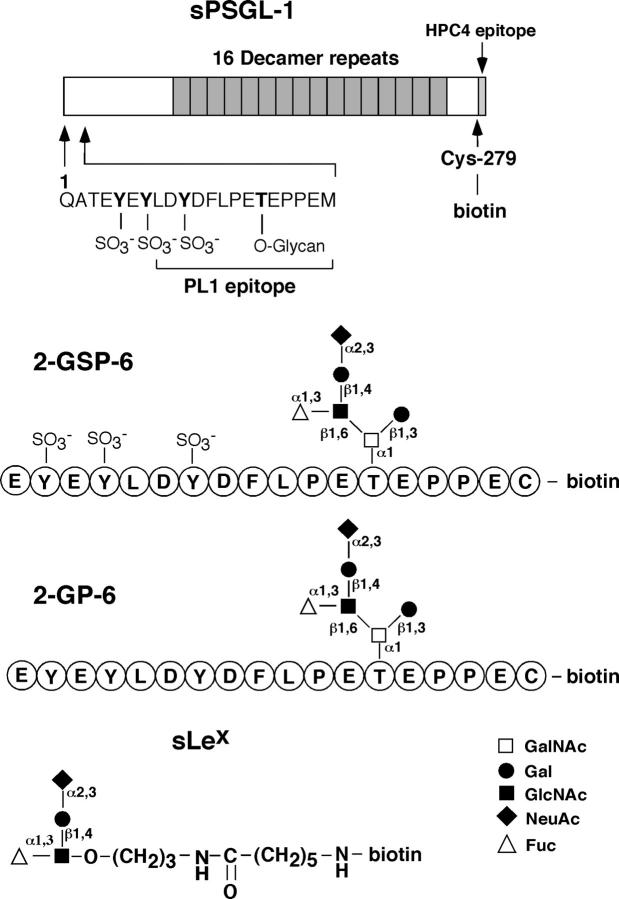
For flow chamber studies, we prepared four well-defined ligands with widely varying affinities for P-selectin (Fig. 1) . Recombinant sPSGL-1 was coexpressed in CHO cells with an α1-3 fucosyltransferase and a core-2 β1-6-N-acetylglucosaminyltransferase to confer the glycosylation required for optimal binding to selectins (Li et al., 1996b). Like native PSGL-1 (Li et al., 1996a), sPSGL-1 is a Ser/Thr-rich mucin that is predicted to extend ∼40 nm. P-selectin binds to a small NH2-terminal region that includes three sulfated tyrosines and a core-2 O-glycan capped with sLex at a nearby threonine. This region contains the epitope for PL1, a monoclonal antibody (mAb) that blocks binding of PSGL-1 to P-selectin. 2-glycosulfopeptide (GSP)-6, a glycosulfopeptide modeled after this region, binds with equivalent affinity to P-selectin (Leppänen et al., 1999). 2-glycopeptide (GP)-6, which lacks sulfate on the tyrosines, binds with much lower affinity to P-selectin. sLex, a simple tetrasaccharide that lacks all peptide determinants and the core-2 O-glycan presentation, binds with very low affinity to P-selectin (Leppänen et al., 2000).
Figure 1.
Selectin ligands coupled to microspheres or K562 cells. Biotin was covalently attached to the COOH-terminal cysteine of sPSGL-1, 2-GSP-6, or 2-GP-6, or to a spacer group on sLex. The biotinylated ligands were bound to streptavidin-coated microspheres or K562 cells.
sPSGL-1, 2-GSP-6, and 2-GP-6 each contained a COOH- terminal cysteine, which allowed specific attachment of biotin to the sulfhydryl group. sLex was attached to biotin through a synthetic linker. Each biotinylated ligand was coupled to streptavidin coated on 6-μm microspheres. Microspheres were prepared with equivalent densities of sPSGL-1, 2-GSP-6, or 2-GP-6, as measured by flow cytometry with PL1 (Fig. 2 A). Similar levels of sP-selectin bound to microspheres decorated with each ligand, establishing their functional equivalence by this assay (Fig. 2 B). The relative levels of sLex were measured by flow cytometry with the anti-sLex mAb HECA-452 (Fig. 2 C). HECA-452 bound equivalently to microspheres coated with 2-GSP-6 or 2-GP-6, as expected because each peptide contains a single core-2 O-glycan capped with sLex. HECA-452 bound to approximately fivefold more sites on sPSGL-1-microspheres, presumably because each sPSGL-1 molecule has several fucosylated O-glycans. HECA-452 bound to approximately threefold more sites on sLex-microspheres than on 2-GSP-6- or 2-GP-6-microspheres. Binding studies with 125I-labeled PL1 established that each microsphere displayed ∼23,000 molecules of sPSGL-1, 2-GSP-6, or 2-GP-6. By using these data to calibrate the HECA-452 binding measured by flow cytometry, each sLex-microsphere was estimated to display ∼70,000 sLex molecules.
Figure 2.
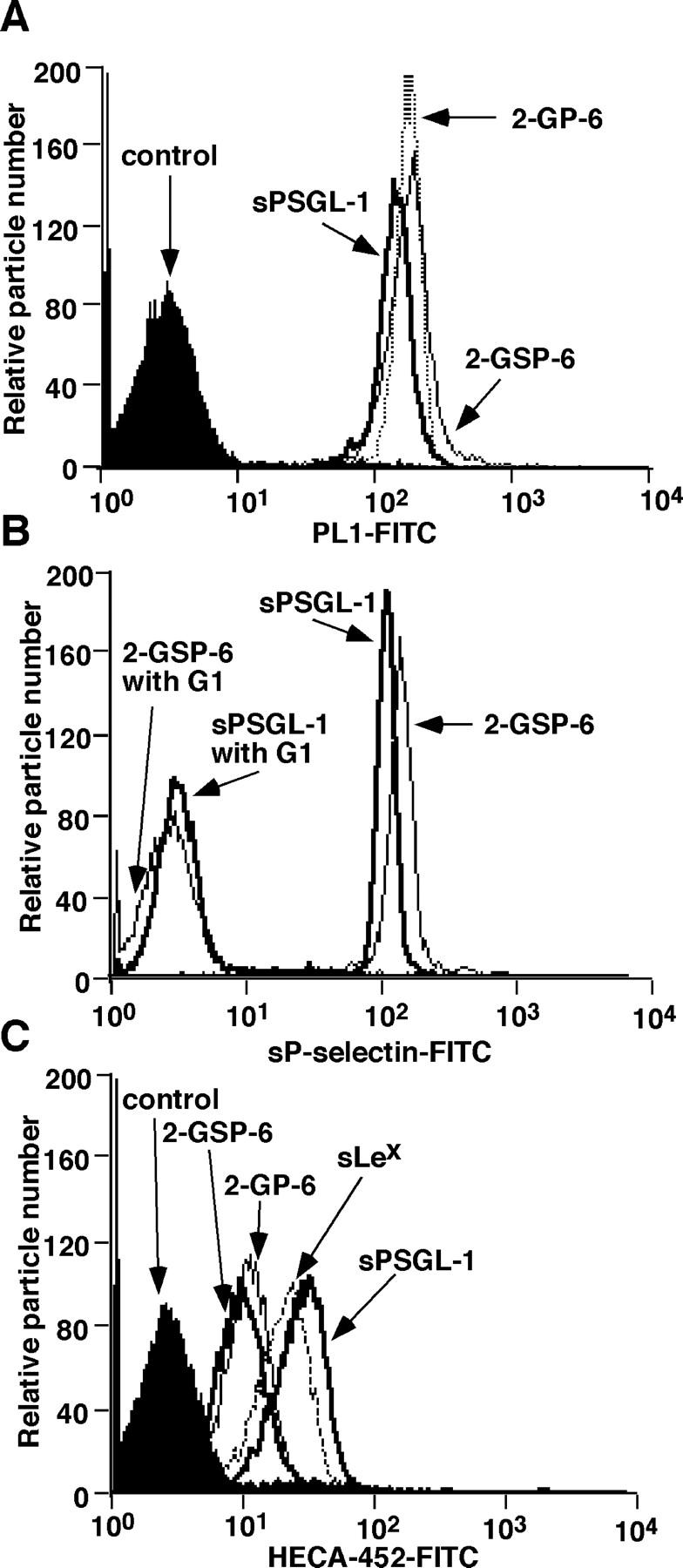
Surface densities of selectin ligands on microspheres measured by flow cytometry. (A) Microspheres were incubated with anti–PSGL-1 mAb PL1 or with an isotype-matched control mAb. Bound antibody was detected with FITC-conjugated goat anti–mouse IgG. (B) Microspheres were incubated with sP-selectin complexed with the nonblocking anti–P-selectin mAb S12 in the presence or absence of the blocking anti–P-selectin mAb G1. Bound sP-selectin was detected with FITC-conjugated goat anti–mouse IgG. (C) Microspheres were incubated with the anti-sLex mAb HECA-452 or with an isotype-matched control mAb. Bound mAb was detected with FITC-conjugated goat anti–rat IgM.
Microspheres bearing 2-GSP-6 or sPSGL-1 tether to and roll on P-selectin equivalently under flow
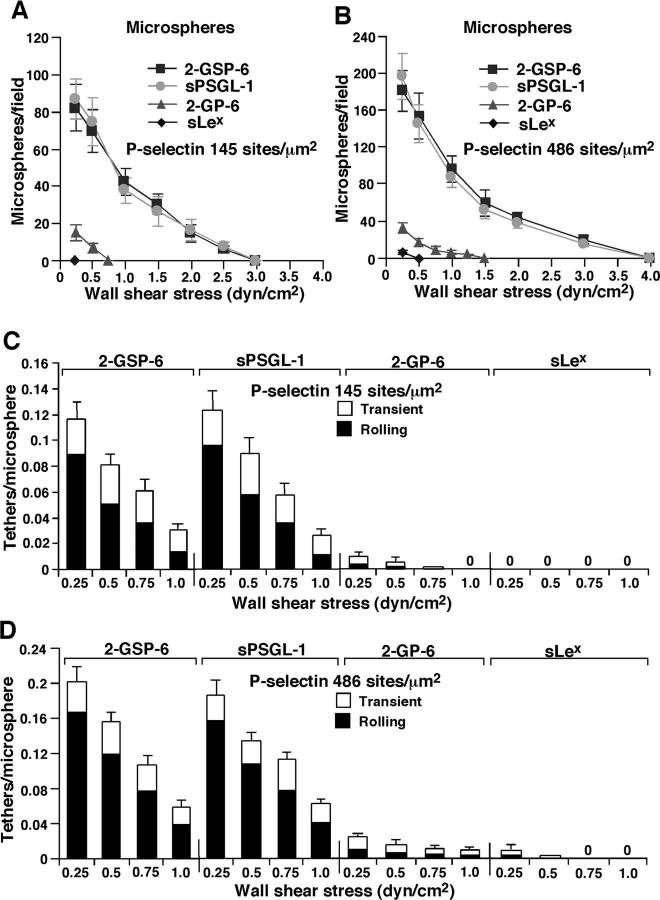
We compared the number of rolling ligand-coupled microspheres that accumulated on intermediate or high densities of P-selectin as a function of wall shear stress (Fig. 3, A and B) . Rolling was specific, because it was Ca2+ dependent and was blocked by anti–PSGL-1 mAb PL1 or anti–P-selectin mAb G1 (unpublished data). Similar numbers of microspheres displaying 2-GSP-6 or sPSGL-1 rolled on P-selectin. Only small numbers of microspheres bearing 2-GP-6 rolled on P-selectin, and rolling was much faster and more irregular than for microspheres displaying 2-GSP-6 or sPSGL-1. No microspheres bearing the isolated sLex glycan rolled, except on high density P-selectin at the lowest wall shear stress tested, even though these microspheres displayed approximately threefold more sLex than microspheres decorated with 2-GP-6. We also measured the initial tethering of microspheres to intermediate or high densities of P-selectin as a function of wall shear stress (Fig. 3, C and D). Microspheres coupled with 2-GSP-6 or sPSGL-1 tethered at identical rates and most tethers converted into rolling interactions. Fewer microspheres bearing 2-GP-6 tethered to P-selectin, and most were transient tethers that did not lead to rolling. Microspheres bearing sLex tethered only on high density P-selectin at the lowest wall shear stresses tested, and virtually all of these tethers were transient. These data demonstrate that the peptide, sulfate, and glycan components of the NH2-terminal re-gion of PSGL-1 all contribute significantly to binding to P-selectin under applied force. In other experiments, we covalently coupled the COOH-terminal cysteines of 2-GSP-6 or sPSGL-1 to the amino groups on microspheres. At matched densities, these microspheres rolled equivalently to streptavidin-coated microspheres coupled with biotinylated ligands (unpublished data). Thus, rolling behavior was due to association and dissociation of bonds between selectins and their ligands rather than of bonds between streptavidin and biotin.
Figure 3.
Rolling and tethering of ligand-coupled microspheres on P-selectin. (A and B) Microspheres coupled with the indicated ligand were perfused over P-selectin at the indicated density. After 5 min, the number of rolling microspheres was quantified. (C and D) The measured number of microspheres that tethered during the first 60 s was normalized by dividing by the number of cells delivered across the field of view in the focal plane of the substrate. The percentage of tethers that were transient or that were converted to rolling adhesion is also indicated. The data represent the mean ± SD of five experiments.
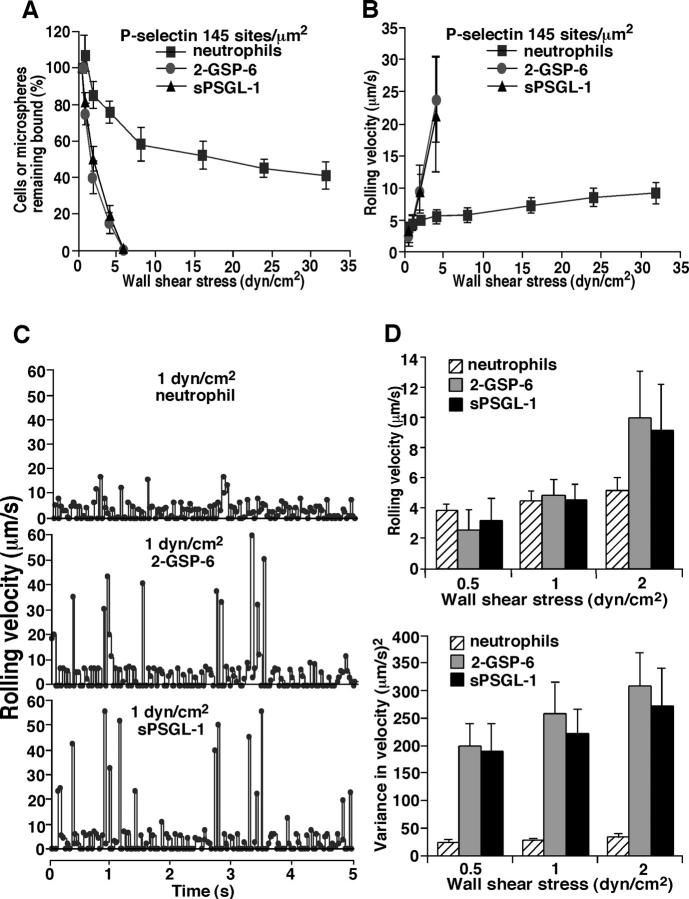
We next compared the rolling behavior of microspheres displaying 2-GSP-6 or sPSGL-1. As wall shear stress was increased, both types of microspheres detached at the same rates (Fig. 4 A). Like microspheres bearing other selectin ligands, their rolling velocities increased linearly at identical increments until they detached (Fig. 4 B). This behavior differed markedly from that of neutrophils, which resisted detachment and developed a plateau in mean rolling velocity despite large increases in wall shear stress. Frame-by-frame analysis revealed that neutrophils rolled comparatively smoothly, with little fluctuation in the velocities of rolling steps. By contrast, both types of microspheres rolled more irregularly, with periodic large increases in rolling velocities (Fig. 4 C). At lower wall shear stresses where most microspheres remained attached, mean rolling velocities were similar to those of neutrophils. However, the variances of the velocity rates were much higher for microspheres, confirming the irregular rolling behavior in a larger population (Fig. 4 D). These results demonstrate that microspheres coupled with 2-GSP-6 or sPSGL-1 tether to and roll on P-selectin with very similar features. Thus, the mucin stalk of PSGL-1 does not contribute to microsphere interactions with P-selectin under the flow conditions tested.
Figure 4.
Detachment resistance and rolling properties of neutrophils or ligand-coupled microspheres on P-selectin. Neutrophils or microspheres were allowed to accumulate on P-selectin at 0.5 dyn/cm2. Wall shear stress was then increased every 30 s, and the percentage of remaining adherent cells (A) and their rolling velocities (B) was determined. (C) Frame-by-frame velocities of representative neutrophils or microspheres rolling on P-selectin at 145 sites/μm2. (D) Mean velocities and variances of velocities for neutrophil or microsphere populations rolling on P-selectin at 145 sites/μm2. The data represent the mean ± SD of five experiments.
The more stable rolling of neutrophils than of ligand-coupled microspheres might be due to greater strength of bonds between native PSGL-1 on neutrophils and P-selectin or to less force acting on the tethers of neutrophils than of microspheres. The force applied to an adhesive tether depends on the tether angle, which is based on the length of the lever arm and the radius of the cell or microsphere. The lever arm is a function of the surface topology of the cell or microsphere on which ligands are presented. We used flow reversal experiments on very low densities of sP-selectin to measure the lever arms of neutrophils and ligand-coupled microspheres (Table I). The lever arm for neutrophils was similar to a previously determined value (Alon et al., 1997), whereas the lever arm for microspheres was much shorter, consistent with their lack of microvilli. The shorter lever arm translated into a larger tether angle, but when corrected for the radius (4.25 μm for neutrophils, 3 μm for microspheres), the estimated tether forces under flow were only slightly greater for microspheres than for neutrophils. Analysis of transient tether lifetimes as a function of tether force yielded similar estimates for the unstressed dissociation rates and mechanical strengths of tethers of neutrophils and microspheres (Table I). These data suggest that the more stable rolling of neutrophils than of microspheres is not due to superior dissociation kinetic or mechanical properties of bonds between native PSGL-1 and P-selectin. Therefore, we examined whether cellular rather than molecular features might explain the more stable rolling of leukocytes.
Table I. Dissociation rates and reactive compliance values for transient tethers of neutrophils or 2-GSP-6– or sPSGL-1–coupled microspheres on sP-selectin.
| Lever arm | Tether angle | k 0 off | a | |
|---|---|---|---|---|
| μm | ° | s − 1 | Å | |
| Neutrophils | 3.6 ± 0.5 | 58.6 ± 3.7 | 1.0 ± 0.1 | 0.8 ± 0.1 |
| 2-GSP-6 microspheres | 0.8 ± 0.3 | 80.0 ± 0.3 | 0.9 ± 0.2 | 0.8 ± 0.1 |
| sPSGL-1 microspheres | 1.0 ± 0.3 | 76.3 ± 4.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
The tether angle was derived from the radius of the neutrophil (4.25 μm) or microsphere (3 μm) and from the lever arm (Goldman et al., 1967), which was measured by flow reversal of transient tethers as described in Materials and methods. The lever arm and tether angle data represent the mean ± SD from measurements of 25 tethers subjected to flow reversal. The koff 0 and a data represent the mean ± SD of 500–1,000 transient tethers from each of five independent experiments.
K562 cells bearing randomly coupled sPSGL-6 tether to and roll on P-selectin more efficiently than K562 cells bearing randomly coupled 2-GSP-6
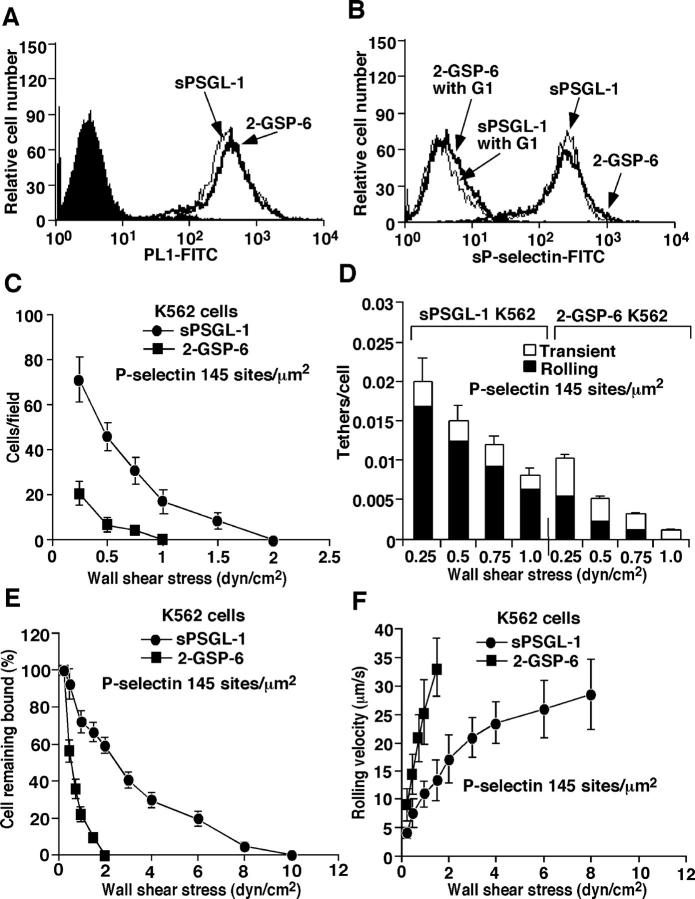
To determine how our defined selectin ligands functioned when coupled to the surface of a cell, we used hematopoietic K562 cells, which do not express functional selectin ligands. Strep-tavidin was randomly bound to the surfaces of biotinylated cells, and then biotinylated 2-GSP-6 or sPSGL-1 was coupled to strep-tavidin on the cells at matched densities (Fig. 5 A). Similar levels of sP-selectin bound to K562 cells decorated with each ligand, establishing their functional equivalence by this assay (Fig. 5 B). However, under flow, more K562 cells bearing sPSGL-1 rolled on P-selectin than did K562 cells bearing 2-GSP-6 (Fig. 5 C). Compared with K562 cells bearing 2-GSP-6, more K562 cells bearing sPSGL-1 tethered to P-selectin, and more of these tethers converted into rolling adhesions (Fig. 5 D). K562 cells displaying sPSGL-1 also resisted detachment and reached a near plateau in rolling velocities in response to increases in wall shear stress (Fig. 5, E and F). Thus, unlike microspheres, K562 cells randomly coupled with sPSGL-1 tether to and roll on P-selectin much better than K562 cells randomly coupled with 2-GSP-6. Ligand-coupled K562 cells tethered to and rolled on P-selectin less well than neutrophils, most likely due to their larger size (14 μm diameter), an ∼50% lower ligand density (measured by flow cytometry and adjusted for differences in surface area), the lack of ligand dimerization, the random ligand distribution on the cell surface, and other factors that distinguish K562 cells from neutrophils.
Figure 5.
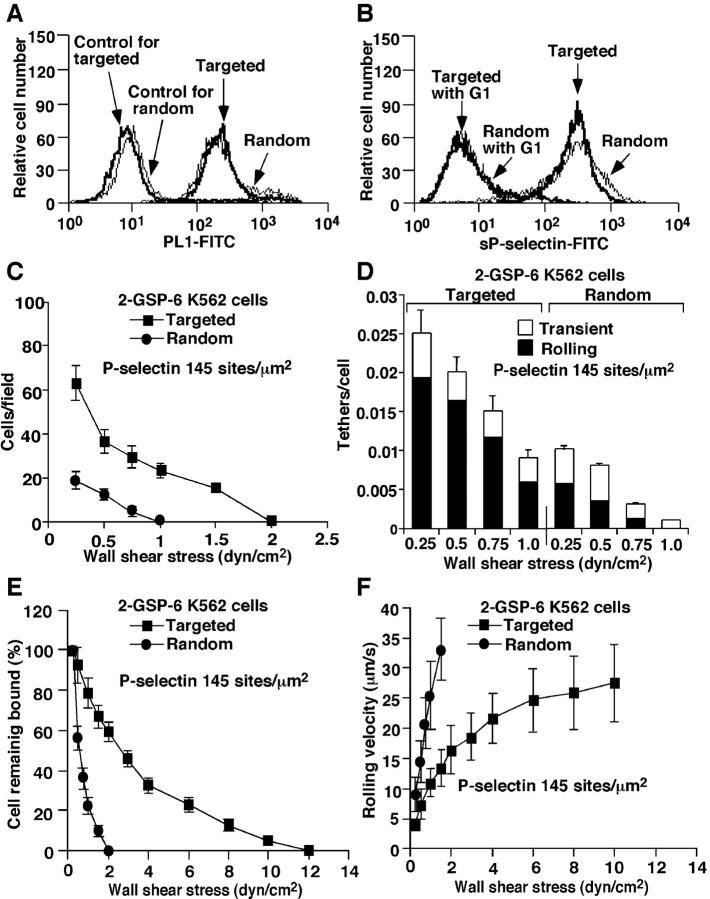
Tethering and rolling of ligand-coupled K562 cells on P-selectin. Binding of anti-PSGL-1 mAb PL1 (A) or sP-selectin (B) was measured as in Fig. 2. The accumulated number of rolling cells (C), the tethering rates (D), the detachment resistance (E), and the mean rolling velocities (F) were measured as in Fig. 3. The data represent the mean ± SD of five experiments.
Targeting coupling of 2-GSP-6 to the membrane-distal region of a mucin enhances K562 cell interactions with P-selectin under flow
The enhanced tethering and rolling of K562 cells bearing sPSGL-1 could reflect the ability of the mucin stalk to project the P-selectin–binding domain above most of the cellular glycocalyx. To test this hypothesis, we expressed the full transmembrane form of PSGL-1 in transfected K562 cells. PSGL-1 on these cells does not bind P-selectin, because the cells lack the α1-3-fucosyltransferase required to confer binding (Snapp et al., 1997). We bound biotinylated mAb PL1 to the NH2-terminal region of PSGL-1 and coupled streptavidin to the mAb. Biotinylated 2-GSP-6 was then bound to streptavidin, which targeted the glycosulfopeptide to the membrane-distal region of the extended but nonfunctional PSGL-1. Cells were prepared with matched densities of this targeted coupled 2-GSP-6 or of 2-GSP-6 that was coupled to randomly distributed streptavidin (Fig. 6 A). These cells bound equivalent amounts of sP-selectin (Fig. 6 B). However, significantly more cells bearing targeted coupled 2-GSP-6 than randomly coupled 2-GSP-6 tethered to and rolled on P-selectin (Fig. 6, C and D). Cells displaying targeted 2-GSP-6 converted more tethers to rolling events (Fig. 6 D), and they rolled much more stably and resisted detachment by increasing wall shear stress (Fig. 6, E and F). Cells bearing targeted 2-GSP-6 or randomly coupled sPSGL-1 rolled similarly (Figs. 5 and 6), indicating that appropriate cell-surface presentation allowed 2-GSP-6 to function like sPSGL-1. These results demonstrate that the mucin stalk of PSGL-1 enhances the ability of cells, but not microspheres, to tether to and roll on P-selectin under flow.
Figure 6.
Rolling and tethering of K562 cells with 2-GSP-6 coupled to randomly distributed streptavidin or with 2-GSP-6 targeted to a membrane-distal region of a glycoform of PSGL-1 that does not bind selectins. (A) Surface densities of random or targeted 2-GSP-6 were measured by binding of anti–PSGL-1 mAb PL1 as described in Materials and methods. The binding of sP-selectin (B), the accumulated number of rolling cells (C), the tethering rates (D), the detachment resistance (E), and the mean rolling velocities (F) were measured as in Figs. 2 and 3. The data represent the mean ± SD of five experiments.
Cellular perturbations alter the stability of rolling of ligand-coupled K562 cells on P-selectin
The ligand density on microspheres, adjusted for surface area, was at least threefold greater than the density on K562 cells. Yet K562 cells bearing sPSGL-1 or 2-GSP-6 targeted to the membrane-distal regions of PSGL-1 rolled much more stably than microspheres displaying either ligand. Ligand-coupled K562 cells resisted detachment and only gradually increased their rolling velocities as wall shear stress was increased. Because the same ligands were coupled to both microspheres and K562 cells, the superior rolling of K562 cells implied that cellular features such as deformation might contribute to stable rolling. As an initial test of this hypothesis, we fixed K562 cells with paraformaldehyde and then coupled sPSGL-1 or 2-GSP-6 to the fixed cells; this excluded possible effects of fixation on the ligands themselves. Fixation abrogated global deformation, as measured by micropipette aspiration, in the force ranges experienced by cells in the flow chamber (Table II). Fixation also prevented micropipette-controlled, PL1-coated beads from extruding thin membrane tethers from ligand-coupled cells, even at forces 20-fold higher than those required to pull tethers from unfixed cells (unpublished data).
Table II. Deformability of K562 cells measured by micropipette aspiration.
| Cell treatment | Pressure required to deform cell to a fixed position within the pipette (pN/μm2) |
|---|---|
| None | 19 ± 8 |
| Paraformaldehyde | >50a |
| MβCD | 38 ± 9 |
| αCD | 19 ±1 |
| Cytochalasin D | 6 ± 4 |
The data represent the mean ± SD of pressures measured for four to five cells.
aNo deformation measured at pressures >50 pN/μm2.
Fixed and nonfixed cells displaying matched densities of sPSGL-1 or targeted 2-GSP-6 were perfused over P-selectin. Strikingly, fixed cells bearing sPSGL-1 (Fig. 7) or 2-GSP-6 (unpublished data) rolled much less stably than nonfixed cells. Fixed cells behaved much like ligand-coupled microspheres. They detached rapidly in response to increasing wall shear stress (Fig. 7 A), and their rolling velocities increased linearly until they detached (Fig. 7 B). Even at lower shear stresses, frame-by-frame analysis of representative fixed cells revealed periodic large increases in rolling velocity (Fig. 7 C), which was confirmed by an increase in the variance of rolling velocity in a population of fixed cells (Fig. 7 D). Coupling sPSGL-1 to an approximately sevenfold higher density on unfixed K562 cells increased detachment resistance and elicited a more obvious plateau in rolling velocities as wall shear stress was increased. Despite the high ligand density, fixation of cells eliminated these favorable rolling properties (Fig. 7, E and F). Fixed neutrophils also rapidly increased their rolling velocities and detached readily as wall shear stress was increased (unpublished data). These data demonstrate that fixation-sensitive features allow ligand-coupled cells to roll stably on P-selectin over a broad range of wall shear stresses.
Figure 7.
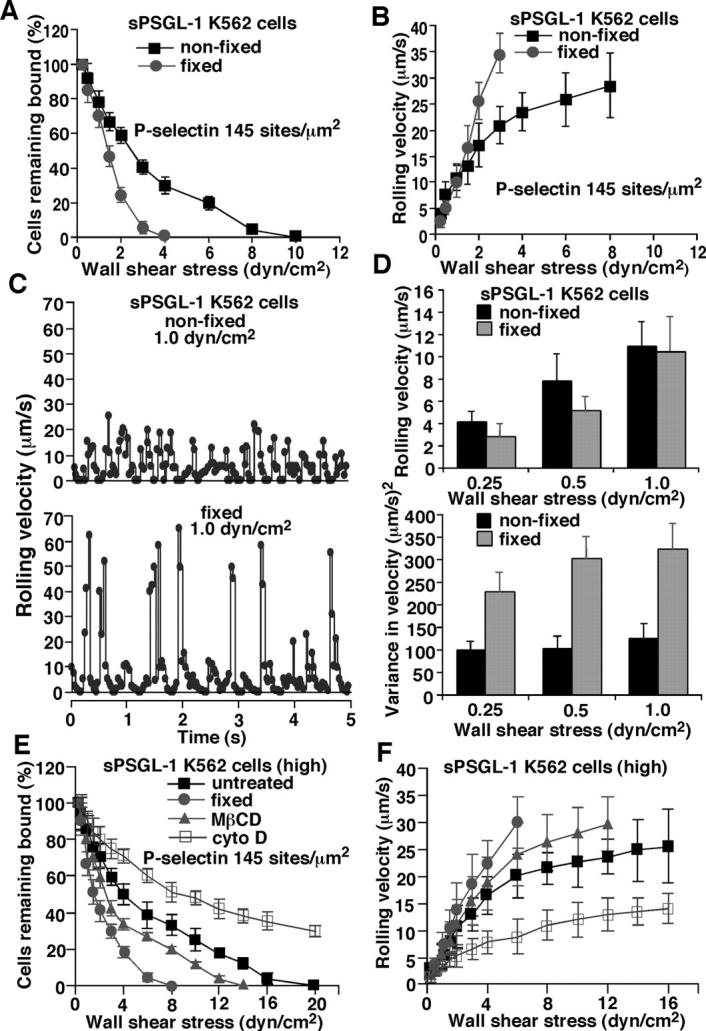
Effects of cellular perturbations on detachment resistance and rolling properties of sPSGL-1–coupled K562 cells on P-selectin. Cells were fixed with paraformaldehyde or treated with MβCD or cytochalasin D before coupling of sPSGL-1. Untreated and treated cells displayed equivalent surface densities of sPSGL-1 (unpublished data). (A and B) Detachment resistance and mean rolling velocities of nonfixed or fixed cells were measured as in Fig. 3. (C) Frame-by-frame velocities of representative nonfixed and fixed cells rolling on P-selectin at 145 sites/μm2. (D) Mean velocities and variances of velocities for populations of nonfixed or fixed cells rolling on P-selectin at 145 sites/μm2. (E and F) sPSGL-1 was coupled to approximately sevenfold higher density on untreated cells, fixed cells, or cells treated with MβCD or cytochalasin D. Detachment resistance and mean rolling velocities were measured as in Fig. 3. Cells treated with αCD, an inactive analogue of MβCD, or with DMSO, the diluent for cytochalasin D, rolled identically to untreated cells (unpublished data). The data represent the mean ± SD of five experiments.
As an alternative to fixation, we treated cells with the cholesterol-chelating agent methyl-β-cyclodextrin (MβCD) or the inactive structural analog α-cyclodextrin (αCD). MβCD, but not αCD, decreased deformability of the cells, although to a lesser extent than fixation did (Table II). MβCD-treated cells rolled less stably than untreated or α-CD–treated cells, but more stably than fixed cells (Fig. 7, E and F). Treatment with cytochalasin D, which facilitates depolymerization of actin filaments, markedly increased cell deformability (Table II) and enhanced rolling on P-selectin (Fig. 7, E and F). Indeed, cytochalasin D–treated K562 cells resembled neutrophils in their detachment resistance and nearly uniform rolling velocities over a wide range of wall shear stresses.
Discussion
Ligand-coupled microspheres tether to and roll on selectins under flow, indicating that the kinetic and mechanical features of selectin–ligand interactions are sufficient to confer rolling adhesion. However, the automatic braking system that stabilizes rolling over a broad range of wall shear stresses has been observed only on cells. It has not been clear whether the superior rolling behavior of cells is due to expression of molecularly distinct selectin ligands or to physical features of cells that optimize rolling. By coupling structurally defined ligands to both microspheres and cells, we demonstrate that cellular features are essential to enable stable rolling on a selectin over a spectrum of wall shear stresses. We show that the ligand molecular components that enhance binding affinity to P-selectin also enhance microsphere rolling on P-selectin. In addition, a cell must present its ligands in accessible positions on the surface to confer stable rolling.
Studies with glycosulfopeptides have demonstrated that sulfated tyrosines, peptide components, and an O-glycan capped with sLex must be presented in a stereochemically precise array for high affinity binding of PSGL-1 to P-selectin (Leppänen et al., 1999, 2000; Somers et al., 2000). These combined features allow the glycosulfopeptide to bind to a much broader surface of the lectin domain of P-selectin than that to which sLex alone binds (Somers et al., 2000). We observed that microspheres bearing matched densities of 2-GSP-6 or sPSGL-1 rolled equivalently on P-selectin. Although sPSGL-1 is much longer than 2-GSP-6, the nano-meter-scale dimensions of these molecules are small compared to the radius of the microsphere, and the lever arms of adhesive tethers measured by flow reversal were indistinguishable. Therefore, the forces applied to the bonds between P-selectin and 2-GSP-6 or sPSGL-1 are expected to be the same, unless the numbers of bonds are different. That microspheres bearing 2-GSP-6 or sPSGL-1 rolled similarly on P-selectin suggests no detectable molecular differences in the ligands that affect rolling. In contrast, microspheres bearing 2-GP-6, which lacks sulfate on the tyrosine residues, tethered to and rolled on P-selectin much less efficiently than microspheres displaying sPGSL-1 or 2-GSP-6. In turn, microspheres displaying only sLex tethered and rolled less efficiently than microspheres bearing 2-GP-6. Indeed, tethering or rolling of sLex-coupled microspheres could be detected only at very high densities of P-selectin and at very low wall shear stresses. Since the same force should be applied to all P-selectin–ligand bonds, the different microsphere rolling behaviors indicate distinct kinetic and mechanical properties of bonds for each ligand, in agreement with the biochemical studies. These results are consistent with the virtual inability of cells to roll on P-selectin if they express sLex determinants without PSGL-1 or express recombinant PSGL-1 where phenylalanines have replaced the tyrosines (Snapp et al., 1997; Liu et al., 1998; Ramachandran et al., 1999). Some studies emphasize that microspheres bearing sLex or nonsulfated PSGL-1–derived glycopeptides could roll on P-selectin, but analysis of these data reveals that rolling was far less stable than for microspheres bearing fully modified glycosulfopeptides. Only rolling fluxes were comparable, presumably because small numbers of microspheres rapidly rolling across the field of view yielded the same flux value as large numbers of slowly rolling microspheres (Rodgers et al., 2000, 2001).
Unlike neutrophils, ligand-coupled microspheres rolled irregularly and detached rapidly as wall shear stress was increased. Previous studies noted this rolling behavior but did not resolve why it differed from that of cells (Brunk et al., 1996; Brunk and Hammer, 1997; Greenberg et al., 2000; Rodgers et al., 2000, 2001; Park et al., 2002). It has been proposed that contact forces from elevated wall shear stress increase selectin-ligand bond number by overcoming the flexibility and electrostatic repulsion of mucin ligands, which hinder bond formation (Chen and Springer, 1999). If this were true, 2-GSP-6 on microspheres would bind more readily to P-selectin, because it lacks the postulated anti-adhesive features in the mucin stalk of sPSGL-1. However, extended mucins such as PSGL-1 may be rigid rather than flexible (Li et al., 1996a). Microspheres bearing either ligand exhibited identical rolling behavior on P-selectin. Thus, a selectin ligand, even if it lacks a mucin component, cannot stabilize microsphere rolling velocities as wall shear stress is increased.
In marked contrast to ligand-coupled microspheres, K562 cells coupled with sPSGL-1 tethered to and rolled on P-selectin much better than K562 cells coupled with 2-GSP-6. Directional coupling of 2-GSP-6 to the membrane-distal region of a PSGL-1 glycoform that could not bind to selectins also enhanced K562 cell rolling on P-selectin. These results demonstrate that a selectin ligand must be properly positioned on a cell surface, which has much more complex and dynamic topography than a microsphere surface. PSGL-1 is longer than most glycoproteins and some proteoglycans that comprise the cell-surface glycocalyx. The mucin stalk may extend the P-selectin–binding site of PSGL-1 or of a directionally coupled glycosulfopeptide to the outer limits of the bulk glycocalyx, enhancing the probability of contact with P-selectin. Similarly, the consensus repeats of P-selectin extend the lectin domain well above the cell surface, favoring interactions with PSGL-1 on rolling leukocytes (Patel et al., 1995).
Notably, K562 cells bearing sPSGL-1 or 2-GSP-6 targeted to the outer limits of the glycocalyx rolled more like leukocytes than ligand-coupled microspheres. They rolled more uniformly, resisted detachment, and tended to plateau their rolling velocities as wall shear stress was increased. Fixation of the cells before ligand coupling eliminated these features, such that the fixed cells rolled unstably, as did microspheres. The fixation-induced rolling defects were particularly evident at wall shear stresses above 1.5 dyn/cm2, where deformation of unfixed cells should be greater. However, even at lower shear stresses, the rolling velocities of fixed cells were more irregular than the velocities of unfixed cells. Thus, fixation-sensitive cellular features are required to stabilize selectin-dependent rolling over a wide range of wall shear stresses. These features likely include deformation of the cell contact area, microvillus extension, and membrane tether extrusions. Micropipette experiments confirmed that fixation eliminated these features at the force ranges applied to adherent cells in the flow chamber. MβCD treatment also reduced deformability and impaired rolling. Conversely, cytochalasin D increased deformability and further stabilized rolling, as previously demonstrated for neutrophils (Finger et al., 1996a; Sheikh and Nash, 1998). At low to intermediate shear stresses, cell deformation may only be observed by side-view microscopes that provide a clearer image of the contact area (Lei et al., 1999). Even small increases in contact area, coupled to closer apposition of potentially interacting molecules, may increase the probability of bond formation under flow. It has been argued that deformation occurs too slowly to contribute to stabilization of rolling (Chen and Springer, 1999). However, leukocytes visibly deform while rolling on postcapillary venules at higher wall shear stresses (Firrell and Lipowsky, 1989). Cells that initially roll suboptimally may stabilize rolling as wall shear stress promotes deformation at the adhesive substrate. Membrane tether extrusions may stabilize rolling by reducing the force on tethers and by allowing the cell to slip or roll downstream from the tether (Shao et al., 1998; Schmidtke and Diamond, 2000; Park et al., 2002). More tethers may develop as the cell rolls. Multiple tethers should increase tether lifetime, increasing the opportunity for new bonds, and thus new tethers, to form.
The automatic braking system that stabilizes leukocyte rolling velocities has been suggested to explain the threshold wall shear stress below which rolling on selectins does not occur (Chen and Springer, 1999). This shear threshold requirement is particularly evident for L-selectin–dependent leukocyte rolling (Finger et al., 1996b). Ligand-coupled microspheres rolling on L–selectin exhibit a shear threshold requirement, indicating that molecular features are sufficient for this phenomenon (Greenberg et al., 2000). In contrast, our results demonstrate that cellular properties are indispensable to stabilize rolling over a broad range of wall shear stresses. Thus, the shear threshold effect and the automatic braking system can be distinguished by their respective dependence on molecular and cellular properties. In addition to cellular deformation and extrusion of membrane tethers, cells modulate tethering and rolling by molecular features of selectins or their ligands that affect their presentation on the cell surface (Patel et al., 1995; Von Andrian et al., 1995; Li et al., 1998; Setiadi et al., 1998; Dwir et al., 2000, 2001; Ramachandran et al., 2001). Signaling may further regulate selectin function (Spertini et al., 1991). Therefore, selectin-dependent rolling is sensitive to intrinsic kinetic and mechanical features of selectin–ligand bonds, which are coupled to cellular features that modulate bond formation and dissociation.
Materials and methods
Proteins, glycoconjugates, and cells
P-selectin purified from platelet membranes (mP-selectin) and recombinant soluble P-selectin (sP-selectin) were prepared as described (Ushiyama et al., 1993). The following mAbs were used as purified proteins: anti–P-selectin mAbs G1 and S12 (murine IgG1; Geng et al., 1990); anti–PSGL-1 mAbs PL1 and PL2 (murine IgG1; Moore et al., 1995); and anti-sLex mAb HECA-452 (rat IgM; American Type Culture Collection; Picker et al., 1991). Monovalent sLex coupled to biotin by a linker was from Glycotech. The GSPs 2-GP-6 and 2-GSP-6 were synthesized as described (Leppänen et al., 1999, 2000).
PCR was used to construct a cDNA encoding the entire extracellular domain of human PSGL-1, including a single cysteine at residue 279 of the mature protein, followed by an eight-residue epitope for the Ca2+-dependent mAb HPC4 (Stearns et al., 1988). The construct was ligated into the expression vector pEE14.1 and stably transfected into CHO cells expressing α1-3-fucosyltransferase VII and core-2 β1-6-N-acetylglucosaminyltransferase I (Ramachandran et al., 1999). sPSGL-1 was purified from conditioned medium by HPC4 chromatography (Mehta et al., 1997).
Human neutrophils were isolated from healthy donors (Zimmerman et al., 1985). Transfected K562 cells expressing full-length PSGL-1 were prepared as described (Ramachandran et al., 2001).
Biotinylation of glycoconjugates and proteins
2-GP-6, 2-GSP-6, and sPSGL-1 (20 μg) were biotinylated at COOH-terminal cysteine residues by incubation with 4 mM biotin-HPDP (Pierce Chemical Company) for 90 min. Biotinylated glycopeptides were resolved from unreacted glycopeptides and biotin by HPLC. Biotinylated sPSGL-1 was dialyzed against PBS to remove free biotin. PL1 (500 μg) in 500 μl 0.1 M sodium bicarbonate, pH 7.5, was incubated with 50 μl NHS-biotin (1 mg/ml in DMSO; Pierce Chemical Co.) for 2 h. Biotinylated PL1 was recovered by gel filtration and dialyzed against PBS.
Coupling glycoconjugates or sPSGL-1 to microspheres or K562 cells
Streptavidin-coated, polystyrene microspheres (6-μm diameter; 2 × 107; Polysciences) were incubated with 5 ng biotinylated 2-GP-6 or 2-GSP-6, 50 ng biotinylated sLex, or 500 ng biotinylated sPSGL-1 in 100 μl PBS for 60 min and then washed. In some experiments, the COOH-terminal cysteine of 2-GSP-6 was directly coupled to amino groups on 6-μm Polybead amino microspheres (Polysciences) as follows: Microspheres (107) in 1 ml PBS were incubated with 20 mM SPDP (Pierce Chemical Co.) in 50 μl DMSO for 30 min, washed, incubated with 5 ng 2-GSP-6 in 500 μl PBS overnight, and washed. Microspheres were stored at 4°C in PBS with 0.1% sodium azide.
K562 cells (5 × 106 in 200 μl PBS) were incubated with 50 μl NHS-biotin (1 mg/ml in DMSO) for 10 min at 4°C, washed, incubated with 50 μg/ml streptavidin (Pierce Chemical Co.) in 250 μl PBS for 60 min at 4°C, and washed. Some streptavidin-coated K562 cells were fixed with 2% paraformaldehyde for 20 min and then washed with PBS with 0.5% FBS. Alternatively, streptavidin-coated cells were incubated with 10 mM MβCD or αCD (Sigma-Aldrich) in 100 μl HBSS for 15 min at 37°C and then diluted into 1 ml HBSS with 0.5% human serum albumin. In other experiments, streptavidin-coated cells were incubated with 10 μM cytochalasin D (Sigma-Aldrich) or control DMSO diluent for 20 min at 37°C. Biotinylated 2-GSP-6 or sPSGL-1 was then coupled to streptavidin-coated K562 cells. Cell deformability was measured by the pressure required to aspirate a portion of a cell a fixed distance into a 8.3-μm diameter micropipette (Schmid-Schonbein et al., 1981; Evans and Yeung, 1989). To examine the rigidity of specific subcellular structures, a 5-μm PL1-coated bead coated fitted into a micropipette was allowed to contact a K562 cell expressing PSGL-1 held by another micropipette. A controlled aspiration pressure drove the bead into the micropipette with a known velocity in the absence of adhesion forces. A slower velocity signified binding of PL1 to PSGL-1 and extrusion of a membrane tether after apparent separation of the bead from the cell body (Shao and Hochmuth, 1996, 1999). A force just over 20 pN, based on the difference in bead velocities with and without tethers, repeatedly extruded tethers from unfixed cells.
mAb-targeted coupling of 2-GSP-6 or sPGSL-1 was achieved by incubating transfected K562 cells expressing full-length PSGL-1 with 10 μg/ml biotinylated PL1 for 30 min at 4°C. The cells were washed, incubated with streptavidin, washed, incubated with biotinylated 2-GSP-6 or sPSGL-1, and washed again.
Flow cytometry and site density measurements
All incubations were at 4°C. Microspheres or cells (2 × 106) were incubated with 10 μg/ml PL1, HECA-452, or isotype-matched control murine IgG1 or rat IgM for 30 min, washed, and incubated with FITC-conjugated goat anti–mouse IgG (H + L; Caltag) or FITC-conjugated goat anti–rat IgM (Pharmingen) for 30 min. To measure the density of mAb-targeted 2-GSP-6, cells were incubated with 10 μg/ml biotinylated PL1 for 30 min, washed, and then incubated with FITC-conjugated streptavidin for 30 min. Control incubations omitted biotinylated 2-GSP-6 or biotinylated PL1. sP-selectin dimerized by preincubation with nonblocking anti-P-selectin mAb S12 (22 μg/ml sP-selectin and 5 μg/ml S12 for 1 h) in the presence or absence of 50 μg/ml blocking anti–P-selectin mAb G1 was incubated with microspheres or cells for 1 h, followed by washing and incubation with FITC-conjugated goat anti–mouse IgG. Binding was analyzed on a Becton-Dickinson FACscan using CellQuest software. Site densities of GSP or sPSGL-1 on neutrophils or microspheres were independently measured by binding of 125I-labeled PL1 (Ushiyama et al., 1993).
Tethering and rolling of microspheres and cells under flow
Microspheres or cells (106/ml in HBSS containing 0.5% human serum albumin) were perfused over adsorbed mP-selectin in a parallel-plate flow chamber. Site densities of P-selectin were determined by binding of 125I- labeled mAb G1 (Moore et al., 1995). After 5 min, the accumulated number of rolling cells was measured with a videomicroscopy system coupled to a digitized image analysis system (Inovision; Ramachandran et al., 1999). To measure resistance to detachment, microspheres (2 × 106/ml) or neutrophils (106/ml) were allowed to accumulate at 0.25 or 0.5 dyn/cm2. Wall shear stress was increased every 30 s, and the percentage of remaining adherent microspheres or cells was determined. Tethering rates were measured as described (Ramachandran et al., 1999). Rolling velocities were measured by tracking individual microspheres or cells frame by frame. The pooled data from all microspheres or cells were used to calculate the mean velocity and variance of velocity for the population (Ramachandran et al., 1999).
Determination of dissociation rate constants and strengths of transient tethers
Transient tether dissociation kinetics as a function of wall shear stress were measured on very low densities of sP-selectin (Ramachandran et al., 1999, 2001). The dependence of the measured apparent tether koff on applied force was assumed to follow the Bell (1978) equation: koff = koff 0 exp (aFb/kT), where koff 0 is the dissociation rate in the absence of applied force, a is the reactive compliance, Ft is the force on the tether, k is Boltzmann's constant, and T is the absolute temperature. The force on Ft was calculated based on the tether angle (Goldman et al., 1967), which was derived after measuring the lever arm of the tether by modification of a previously described method (Alon et al., 1997). Briefly, the lever arm was defined as half the distance moved by a microsphere or cell tethered to very low density sP-selectin during flow reversal. Flow reversal was achieved by two electropneumatically actuated ball values (Whitey Co.), whose directions were simultaneous changed by air pressure from a PicoTM electronic controller (Rockwell Automation). Forward and reverse flow were controlled by separate Hamilton syringe pumps, only one of which controlled flow through the chamber at any given time. Fully developed reverse flow was attained within two video frames (0.067 s), as determined by measuring the velocity of freely suspended cells.
Acknowledgments
We thank Dr. Lynda Pierini for suggesting MβCD as an agent to alter cellular deformability.
This work was supported by National Institutes of Health grants HL 65631, AI 44902, and AI 48075. W.D. Marcus was supported by a United Negro College Fund–Merck postdoctoral fellowship.
Footnotes
Abbreviations used in this paper: αCD, α-cyclodextrin; GP, glycopeptide; GSP, glycosulfopeptide; mAb, monoclonal antibody; MβCD, methyl-β-cyclodextrin; mP-selectin, membrane-derived P-selectin; PSGL, P-selectin glycoprotein ligand; sLex, sialyl Lewis x; sP-selectin, soluble P-selectin; sPSGL-1, soluble PSGL-1.
References
- Alon, R., D.A. Hammer, and T.A. Springer. 1995. Lifetime of the P-selectin: carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 374:539–542. [DOI] [PubMed] [Google Scholar]
- Alon, R., S.Q. Chen, K.D. Puri, E.B. Finger, and T.A. Springer. 1997. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J. Cell Biol. 138:1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton, A., and G.V.R. Born. 1973. Relationship between the velocity of rolling granulocytes and that of the blood flow in venules. J. Physiol. 233:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, G.I. 1978. Models for the specific adhesion of cells to cells: A theoretical framework for adhesion mediated by reversible bonds between cell surface molecules. Science. 200:618–627. [DOI] [PubMed] [Google Scholar]
- Brunk, D.K., and D.A. Hammer. 1997. Quantifying rolling adhesion with a cell-free assay: E-selectin and its carbohydrate ligands. Biophys. J. 72:2820–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk, D.K., D.J. Goetz, and D.A. Hammer. 1996. Sialyl Lewisx/E-selectin-mediated rolling in a cell-free system. Biophys. J. 71:2902–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S.Q., and T.A. Springer. 1999. An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J. Cell Biol. 144:185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwir, O., G.S. Kansas, and R. Alon. 2000. An activated L-selectin mutant with conserved equilibrium binding properties but enhanced ligand recognition under shear flow. J. Biol. Chem. 275:18682–18691. [DOI] [PubMed] [Google Scholar]
- Dwir, O., G.S. Kansas, and R. Alon. 2001. Cytoplasmic anchorage of L-selectin controls leukocyte capture and rolling by increasing the mechanical stability of the selectin tether. J. Cell Biol. 155:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, E., and A. Yeung. 1989. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys. J. 56:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger, E.B., R.E. Bruehl, D.F. Bainton, and T.A. Springer. 1996. a. A differential role for cell shape in neutrophil tethering and rolling on endothelial selectins under flow. J. Immunol. 157:5085–5096. [PubMed] [Google Scholar]
- Finger, E.B., K.D. Puri, R. Alon, M.B. Lawrence, U.H. Von Andrian, and T.A. Springer. 1996. b. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 379:266–269. [DOI] [PubMed] [Google Scholar]
- Firrell, J.C., and H.H. Lipowsky. 1989. Leukocyte margination and deformation in mesenteric venules of rat. Am. J. Physiol. 256:H1667–H1674. [DOI] [PubMed] [Google Scholar]
- Geng, J.-G., M.P. Bevilacqua, K.L. Moore, T.M. McIntyre, S.M. Prescott, J.M. Kim, G.A. Bliss, G.A. Zimmerman, and R.P. McEver. 1990. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 343:757–760. [DOI] [PubMed] [Google Scholar]
- Goetz, D.J., D.M. Greif, H. Ding, R.T. Camphausen, S. Howes, K.M. Comess, K.R. Snapp, G.S. Kansas, and F.W. Luscinskas. 1997. Isolated P-selectin glycoprotein ligand-1 dynamic adhesion to P- and E-selectin. J. Cell Biol. 137:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, A.J., R.G. Cox, and H. Brenner. 1967. Slow viscous motion of a sphere parallel to a plane wall–Couette flow. Chem. Eng. Sci. 22:653–660. [Google Scholar]
- Greenberg, A.W., D.K. Brunk, and D.A. Hammer. 2000. Cell-free rolling mediated by L-selectin and sialyl Lewisx reveals the shear threshold effect. Biophys. J. 79:2391–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, U., K.E. Norman, K. Scharffetter-Kochanek, A.L. Beaudet, and K. Ley. 1998. Transit time of leukocytes rolling through venules controls cytokine- induced inflammatory cell recruitment in vivo. J. Clin. Invest. 102:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, M.B., and T.A. Springer. 1991. Leukocytes roll on a selectin at physiologic flow rates: Distinction from and prerequisite for adhesion through integrins. Cell. 65:859–873. [DOI] [PubMed] [Google Scholar]
- Lei, X., M.B. Lawrence, and C. Dong. 1999. Influence of cell deformation on leukocyte rolling adhesion in shear flow. J. Biomech. Eng. 121:636–643. [DOI] [PubMed] [Google Scholar]
- Leppänen, A., P. Mehta, Y.-B. Ouyang, T. Ju, J. Helin, K.L. Moore, I. van Die, W.M. Canfield, R.P. McEver, and R.D. Cummings. 1999. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. J. Biol. Chem. 274:24838–24848. [DOI] [PubMed] [Google Scholar]
- Leppänen, A., S.P. White, J. Helin, R.P. McEver, and R.D. Cummings. 2000. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J. Biol. Chem. 275:39569–39578. [DOI] [PubMed] [Google Scholar]
- Li, F., H.P. Erickson, J.A. James, K.L. Moore, R.D. Cummings, and R.P. McEver. 1996. a. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J. Biol. Chem. 271:6342–6348. [DOI] [PubMed] [Google Scholar]
- Li, F., P.P. Wilkins, S. Crawley, J. Weinstein, R.D. Cummings, and R.P. McEver. 1996. b. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J. Biol. Chem. 271:3255–3264. [PubMed] [Google Scholar]
- Li, X., D.A. Steeber, M.L.K. Tang, M.A. Farrar, R.M. Perlmutter, and T.F. Tedder. 1998. Regulation of L-selectin-mediated rolling through receptor dimerization. J. Exp. Med. 188:1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W.-J., V. Ramachandran, J. Kang, T.K. Kishimoto, R.D. Cummings, and R.P. McEver. 1998. Identification of N-terminal residues on P-selectin glycoprotein ligand-1 required for binding to P-selectin. J. Biol. Chem. 273:7078–7087. [DOI] [PubMed] [Google Scholar]
- McEver, R.P. 2001. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb. Haemost. 86:746–756. [PubMed] [Google Scholar]
- McEver, R.P., and R.D. Cummings. 1997. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Invest. 100:485–492. [PubMed] [Google Scholar]
- Mehta, P., K.D. Patel, T.M. Laue, H.P. Erickson, and R.P. McEver. 1997. Soluble monomeric P-selectin containing only the lectin and epidermal growth factor domains binds to P-selectin glycoprotein ligand-1 on leukocytes. Blood. 90:2381–2389. [PubMed] [Google Scholar]
- Mehta, P., R.D. Cummings, and R.P. McEver. 1998. Affinity and kinetic analysis of P-selectin binding to P-selectin glycoprotein ligand-1. J. Biol. Chem. 273:32506–32513. [DOI] [PubMed] [Google Scholar]
- Moore, K.L., K.D. Patel, R.E. Bruehl, L. Fugang, D.A. Johnson, H.S. Lichenstein, R.D. Cummings, D.F. Bainton, and R.P. McEver. 1995. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J. Cell Biol. 128:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, M.W., A.N. Barclay, M.S. Singer, S.D. Rosen, and P.A. Van der Merwe. 1998. Affinity and kinetic analysis of L-selectin (CD62L) binding to glycosylation-dependent cell-adhesion molecule-1. J. Biol. Chem. 273:763–770. [DOI] [PubMed] [Google Scholar]
- Park, E.Y., M.J. Smith, E.S. Stropp, K.R. Snapp, J.A. DiVietro, W.F. Walker, D.W. Schmidtke, S.L. Diamond, and M.B. Lawrence. 2002. Comparison of PSGL-1 microbead and neutrophil rolling: microvillus elongation stabilizes p-selectin bond clusters. Biophys. J. 82:1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, K.D., M.U. Nollert, and R.P. McEver. 1995. P-selectin must extend a sufficient length from the plasma membrane to mediate rolling of neutrophils. J. Cell Biol. 131:1893–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker, L.J., T.K. Kishimoto, C.W. Smith, R.A. Warnock, and E.C. Butcher. 1991. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 349:796–799. [DOI] [PubMed] [Google Scholar]
- Ramachandran, V., M.U. Nollert, H. Qiu, W. Liu, R.D. Cummings, C. Zhu, and R.P. McEver. 1999. Tyrosine replacement in P-selectin glycoprotein ligand-1 affects distinct kinetic and mechanical properties of bonds with P- and L-selectin. Proc. Natl. Acad. Sci. USA. 96:13771–13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran, V., T. Yago, T.K. Epperson, M.M.A. Kobzdej, M.U. Nollert, R.D. Cummings, C. Zhu, and R.P. McEver. 2001. Dimerization of a selectin and its ligand stabilizes cell rolling and enhances tether strength in shear flow. Proc. Natl. Acad. Sci. USA. 98:10166–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers, S.D., R.T. Camphausen, and D.A. Hammer. 2000. Sialyl Lewisx-mediated, PSGL-1-independent rolling adhesion on P-selectin. Biophys. J. 79:694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers, S.D., R.T. Camphausen, and D.A. Hammer. 2001. Tyrosine sulfation enhances but is not required for PSGL-1 rolling adhesion on P-selectin. Biophys. J. 81:2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schonbein, G.W., K.L. Sung, H. Tozeren, R. Skalak, and S. Chien. 1981. Passive mechanical properties of human leukocytes. Biophys. J. 36:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke, D.W., and S.L. Diamond. 2000. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J. Cell Biol. 149:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiadi, H., G. Sedgewick, S.L. Erlandsen, and R.P. McEver. 1998. Interactions of the cytoplasmic domain of P-selectin with clathrin-coated pits enhance leukocyte adhesion under flow. J. Cell Biol. 142:859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, J.Y., and R.M. Hochmuth. 1996. Micropipette suction for measuring piconewton forces of adhesion and tether formation from neutrophil membranes. Biophys. J. 71:2892–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, J.Y., and R.M. Hochmuth. 1999. Mechanical anchoring strength of L-selectin, β2 integrins, and CD45 to neutrophil cytoskeleton and membrane. Biophys. J. 77:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, J.Y., H.P. Ting-Beall, and R.M. Hochmuth. 1998. Static and dynamic lengths of neutrophil microvilli. Proc. Natl. Acad. Sci. USA. 95:6797–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh, S., and G.B. Nash. 1998. Treatment of neutrophils with cytochalasins converts rolling to stationary adhesion on P-selectin. J. Cell. Physiol. 174:206–216. [DOI] [PubMed] [Google Scholar]
- Snapp, K.R., A.J. Wagers, R. Craig, L.M. Stoolman, and G.S. Kansas. 1997. P-selectin glycoprotein ligand-1 is essential for adhesion to P-selectin but not E-selectin in stably transfected hematopoietic cell lines. Blood. 89:896–901. [PubMed] [Google Scholar]
- Somers, W.S., J. Tang, G.D. Shaw, and R.T. Camphausen. 2000. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 103:467–479. [DOI] [PubMed] [Google Scholar]
- Spertini, O., G.S. Kansas, J.M. Munro, J.D. Griffin, and T.F. Tedder. 1991. Regulation of leukocyte migration by activation of the leukocyte adhesion molecule-1 (LAM-1) selectin. Nature. 349:691–694. [DOI] [PubMed] [Google Scholar]
- Stearns, D.J., S. Kurosawa, P.J. Sims, N.L. Esmon, and C.T. Esmon. 1988. The interaction of a Ca2+-dependent monoclonal antibody with the protein C activation peptide region. Evidence for obligatory Ca2+ binding to both antigen and antibody. J. Biol. Chem. 263:826–832. [PubMed] [Google Scholar]
- Ushiyama, S., T.M. Laue, K.L. Moore, H.P. Erickson, and R.P. McEver. 1993. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J. Biol. Chem. 268:15229–15237. [PubMed] [Google Scholar]
- Vestweber, D., and J.E. Blanks. 1999. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 79:181–213. [DOI] [PubMed] [Google Scholar]
- Von Andrian, U.H., S.R. Hasslen, R.D. Nelson, S.L. Erlandsen, and E.C. Butcher. 1995. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 82:989–999. [DOI] [PubMed] [Google Scholar]
- Wild, M.K., M.C. Huang, U. Schulze-Horsel, P.A. van Der Merwe, and D. Vestweber. 2001. Affinity, kinetics, and thermodynamics of E-selectin binding to E-selectin ligand-1. J. Biol. Chem. 276:31602–31612. [DOI] [PubMed] [Google Scholar]
- Zimmerman, G.A., T.M. McIntyre, and S.M. Prescott. 1985. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J. Clin. Invest. 76:2235–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]