Abstract
Two chlorobenzoate-degradative plasmids were studied by the hybridization of the restriction endonuclease-generated fragments of one plasmid after transfer to a nitrocellulose filter with nick-translated radioactive DNA of the other plasmid as a probe. Two strains harboring the 3-chlorobenzoic acid-degradative plasmids were isolated in two different parts of the world at two different times. The plasmids are now found to be closely related to each other by hybridization studies. The chlorobenzoate-degradative plasmid from Pseudomonas sp. strain B13 (termed pB13) has a 6-kilobase deletion but otherwise is homologous with previously described plasmid pAC25.
Full text
PDF
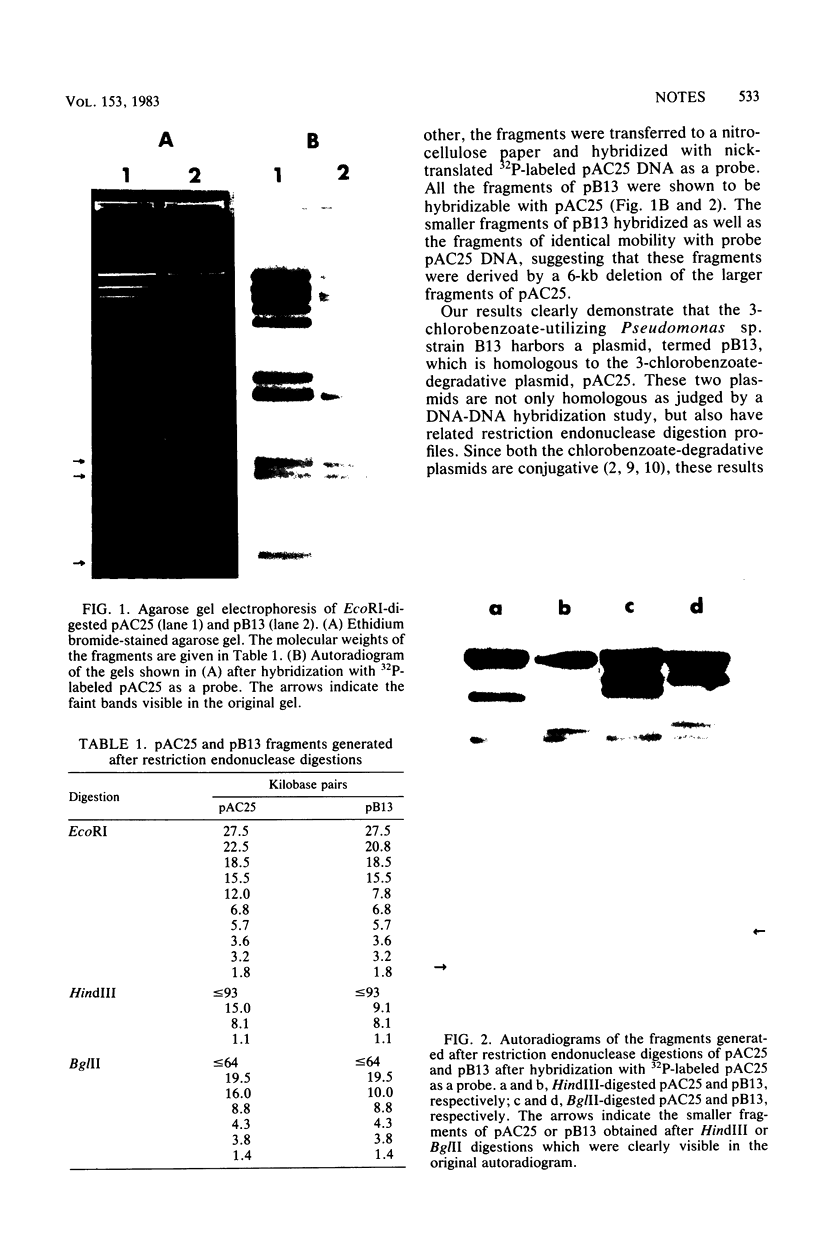

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatterjee D. K., Kellogg S. T., Hamada S., Chakrabarty A. M. Plasmid specifying total degradation of 3-chlorobenzoate by a modified ortho pathway. J Bacteriol. 1981 May;146(2):639–646. doi: 10.1128/jb.146.2.639-646.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Hellwig M., Reineke W., Knackmuss H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeenes D. J., Williams P. A. Excision and integration of degradative pathway genes from TOL plasmid pWW0. J Bacteriol. 1982 Apr;150(1):188–194. doi: 10.1128/jb.150.1.188-194.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Feary T. W., Blevins W. T. Pyruvate carboxylase deficiency in pleiotropic carbohydrate-negative mutant strains of Pseudomonas aeruginosa. J Bacteriol. 1974 Jun;118(3):999–1009. doi: 10.1128/jb.118.3.999-1009.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Construction of haloaromatics utilising bacteria. Nature. 1979 Feb 1;277(5695):385–386. doi: 10.1038/277385a0. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Charbit A., Benson S., Caruso M., Laux R., Meyer R., Banuett F. Perspectives for genetic engineering of hydrocarbon oxidizing bacteria. Basic Life Sci. 1981;18:243–272. doi: 10.1007/978-1-4684-3980-9_15. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wheatcroft R., Williams P. A. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J Gen Microbiol. 1981 Jun;124(2):433–437. doi: 10.1099/00221287-124-2-433. [DOI] [PubMed] [Google Scholar]