Abstract
Here we describe the phenotypic characterization of the cta4 + gene, encoding a novel member of the P4 family of P-type ATPases of fission yeast. The cta4Δ mutant is temperature sensitive and cold sensitive lethal and displays several morphological defects in cell polarity and cytokinesis. Microtubules are generally destabilized in cells lacking Cta4p. The microtubule length is decreased, and the number of microtubules per cell is increased. This is concomitant with an increase in the number of microtubule catastrophe events in the midzone of the cell. These defects are likely due to a general imbalance in cation homeostasis. Immunofluorescence microscopy and membrane fractionation experiments revealed that green fluorescent protein–tagged Cta4 localizes to the ER. Fluorescence resonance energy transfer experiments in living cells using the yellow cameleon indicator for Ca2+ indicated that Cta4p regulates the cellular Ca2+ concentration. Thus, our results reveal a link between cation homeostasis and the control of cell shape, microtubule dynamics, and cytokinesis, and appoint Ca2+ as a key ion in controlling these processes.
Keywords: Schizosaccharomyces pombe; P-type ATPase; endoplasmic reticulum; calcium; microtubule
Introduction
The P-type ATPases are present in all living cells where they mediate ion transport across membranes on the expense of ATP hydrolysis. Among the ions transported by these pumps are protons, calcium, sodium, potassium, and heavy metals such as manganese, iron, copper, and zinc. The formation of a phosphorylated intermediate during the catalytic cycle is a characteristic of P-type ATPases that distinguishes them from V ATPases and F ATPases (Pedersen and Carafoli, 1987a,b).
Within the protein family of P-type ATPases, the Ca2+-ATPases are of special interest, since calcium plays a key role in signal transduction in eukaryotic cells (Clapham, 1995). The transient elevation in cytosolic-free calcium stimulates several Ca2+-binding proteins and their targets that act to elicit downstream signaling pathways. Ubiquitous effectors of calcium signaling are Ca2+/calmodulin (CaM)*-dependent protein kinases and Ca2+/CaM-dependent phosphatase, calcineurin, which act by modulating the phosphorylation state of diverse proteins including transcription factors (Stull, 2001). There is a growing body of evidence that the cellular response to a rise in calcium depends on the amplitude, frequency, duration, and location of the Ca2+ signal (Sanders et al., 1999). The calcium signal is terminated when cytosolic-free Ca2+ concentration is reduced to basal levels by Ca2+-ATPases and Ca2+/H+ exchangers that transport calcium from the cell or sequester it in organelles. In the last case, refilling of intracellular compartments safeguards the Ca2+ release during subsequent signaling events and provides a lumenal space with specific Ca2+ concentration required for diverse biochemical reactions taking place in those compartments (Corbett and Michalak, 2000).
Two main classes of Ca2+-ATPases have been described: sarco/ER Ca2+-ATPases (SERCA) and plasma membrane Ca2+-ATPases, which differ from one another in their subcellular distribution, biochemical characteristics, and mode of regulation (Guerini and Carafoli, 1999; Carafoli and Brini, 2000). In addition, the secretory pathway Ca2+-ATPases initially characterized in budding yeast (Rudolph et al., 1989) has emerged as a separate class (Gunteski-Hamblin et al., 1992; Sorin et al., 1997).
The fission yeast Schizosaccharomyces pombe is an excellent model system for eukaryotic cell biology. Several components of Ca2+-mediated signaling of animal cells have been identified and characterized in fission yeast. A temperature-sensitive Ca2+-binding site CaM mutant exhibits broken spindles and defects in chromosome segregation (Moser et al., 1997). CaM is localized to the spindle pole bodies and sites of polarized cell growth in S. pombe (Moser et al., 1997). In cells undergoing cytokinesis, CaM was found on both sides of septum. Similar CaM redistribution at the cell equator was observed in dividing animal cells where CaM activation by elevation of free Ca2+ was proposed to trigger the formation of the cleavage furrow (Li et al., 1999). Thus, there are several indications that Ca2+ and CaM have an important role in regulating aspects of the cytokinesis both in animal cells and fission yeast, but more direct evidence for Ca2+ affecting this process is lacking.
Recently, the gene ppb1 + encoding for the catalytic subunit of calcineurin was isolated from S. pombe (Yoshida et al., 1994; Plochocka-Zulinska et al., 1995). Mating, microtubule distribution, chromosome segregation, spindle pole body, and nuclear positioning were impaired in calcineurin-deficient cells (Yoshida et al., 1994). The lack of ppb1 + resulted in branched cells with multiple septa that fail to cleave. These observations further indicate that Ca2+ homeostasis can have profound effects on cytoskeleton functions in fission yeast.
In spite of these findings, little is known about how calcium signals are generated and controlled in S. pombe, and so far little is known about the molecular identity of transporters, which deplete cytosolic calcium. The cta3 + gene product was identified previously as a Ca2+-ATPase (Ghislain et al., 1990) and is related to the ENA1 Na+-ATPase of Saccharomyces cerevisiae. Cta3p is required for Na+ tolerance in S. pombe (Nishikawa et al., 1999). The Ca2+/H+ exchangers were shown to be responsible for Ca2+ transport in membranes of the secretory pathway organelles, but Ca2+-ATPase activity has so far not been detected in membrane preparations (Okorokov et al., 2001). Although the genes encoding for several putative calcium ATPases were identified by the S. pombe genome-sequencing project, no genetic analysis has been performed on the corresponding null mutants. Thus, the involvement of each individual pump in calcium homeostasis and the role of the pumps in signal transduction and diverse cellular functions have not been established. Toward this end, we have here determined the subcellular localization of the putative calcium ATPase SPAC2E11.07C and analyzed the physiological consequences of its gene deletion. To follow the preexisting nomenclature of P-type ATPases in fission yeast, SPAC2E11.07C was named cta4 + (calcium/cation transporting ATPase). The cta4 + gene encodes a novel member of the P4 family of P-type ATPases that is localized in the ER. cta4 + is not an essential gene, but cta4Δ mutants display several morphological defects, an imbalance in cation homeostasis and are temperature sensitive and cold sensitive lethal. Microtubules are generally destabilized in cells lacking Cta4p. The microtubule length is decreased, and the number of microtubules per cell is increased concomitant with an increase in the number of microtubule catastrophe events in the midzone of the cell. Fluorescence resonance energy transfer (FRET) experiments in living cells using the fluorescent yellow cameleon indicator for Ca2+ indicated that a deletion of cta4 + causes an elevation of cellular calcium levels. Our results reveal a link between control of cell shape, microtubule dynamics, cytokinesis, and cation homeostasis, and appoint Ca2+ as a key regulatory ion. Hence, our data points to a new level of control over these important processes.
Results
Analysis of the cta4 + gene
The full-length SPAC2E11.07C gene (sequence data available from GenBank/EMBL/DDBJ under accession no. O14072) referred to here as cta4 + encodes a 1211–amino acid–long protein. Twelve transmembrane spanning domains (TMDs) and three defined hydrophilic cytosolic domains linking TMDs 2 and 3, 4 and 5, and 6 and 7 were predicted by topological analysis. Sequence analysis indicates that Cta4p has all five highly conserved domains characteristic of ion transporting P-type ATPases (Tables I and II). These include the potential phosphorylation site represented by Asp485 and the domains involved in ATP binding, all located in a large hydrophilic loop between TMDs 6 and 7. Search for homologues in the GenBank database revealed that Cta4p is related to the S. cerevisiae ORF Yel031p, which encodes the Spf1 ATPase that belongs to the family of P4-ATPases with unknown substrate specificity (Catty et al., 1997). The Cta4p sequence shares specific amino acid sequence motifs intrinsic for P4 ATPases with the Spf1 amino acid sequence. These include one Cys residue preceding the consensus motif GDG×ND and the ××S4×FTS14×GR××LV×× sequence (Tables I and II). Whereas the highest sequence identity was obtained with the SPF1 gene product in amino acid sequence comparisons (49% overall identity), other S. cerevisiae cation ATPases, such as Na+-ATPase ENA1 and Ca2+-ATPases PMR1 and PMC1, showed a relatively low sequence similarity with Cta4p (14.2, 15.1, and 12.6% overall amino acid identities, respectively). The Cta4p sequence showed low overall identity (13.7%) with Cta3p of S. pombe (Ghislain et al., 1990). Besides SPF1 of S. cerevisiae, human KIAA1825 protein expressed in brain (sequence data available from GenBank/EMBL/DDBJ under accession no. AB058728) (Nagase et al., 2001) had a high sequence similarity to Cta4p (37.7% overall amino acid identity).
Table I. Cta4 ATPase belongs to the P4 family of P-type ATPases.
| P-type consensusa | Cta4 sequence | Putative function in Cta4 |
|---|---|---|
| LTGES | 307LSGES311 | Unknown |
| F/SDKTGTLT | 484FDKTGTLT491 | Phosphorylation site |
| KGA-- | 614KGAPE618 | ATP binding |
| M•TGD | 706MITGD710 | ATP binding |
| GDG•ND | 823CGDTND828 | Coupling ATP-binding domain to domain involved in ion transport |
Underline denotes conservative replacement, indicated by • in the consensus.
Catty et al., 1997.
Table II.
Identification of P4-ATPase signatures in Cta4 *
| P4-ATPases signaturesa | Cta4 sequence |
|---|---|
| D LI•T--•PPA ELP | 435MIITSVV PSELP 446 |
| M FC***•**C V | 821MCGDGTNDV 829 |
| ••S-A C•P SFTSK N---•--•--••-E QGRC A•LVT N• | 959 DA SAA A PFTSKLAVVSSITNIVRQGRC T LVAL 990 |
Underline denotes conservative replacement, indicated by • in the consensus. Bold denotes conserved amino acids in the P4-ATPase specific sequences. Highly conserved amino acids in P-type ATPases are indicated by
in the consensus.
Catty et al., 1997.
Cta4-GFP is localized to the ER
To investigate the intracellular localization of the Cta4 ATPase in fission yeast, a fusion protein between Cta4p at its COOH terminus and the green fluorescent protein (GFP) was generated (see Materials and methods). The Cta4-GFP gave a weak signal in live cells, but the fusion protein was readily detected by indirect immunofluorescence (IF) microscopy using antibodies against GFP (Fig. 1 A). The Cta4-GFP fusion protein is replacing the endogenous cta4 + allele and is thus expressed from the natural cta4 + promoter and is fully functional (see below). The localization pattern of the Cta4-GFP was found to be similar to that of Sec61, a known ER marker (Broughton et al., 1997) as revealed by IF. To test for colocalization of these two proteins, Sec61 fused to the c-myc epitope tag was expressed from a plasmid in the strain carrying Cta4-GFP (Fig. 1 A). Digital confocal microscopy revealed a partial colocalization of both proteins, suggesting that the Cta4 ATPase at least in part resides in the ER.
Figure 1.
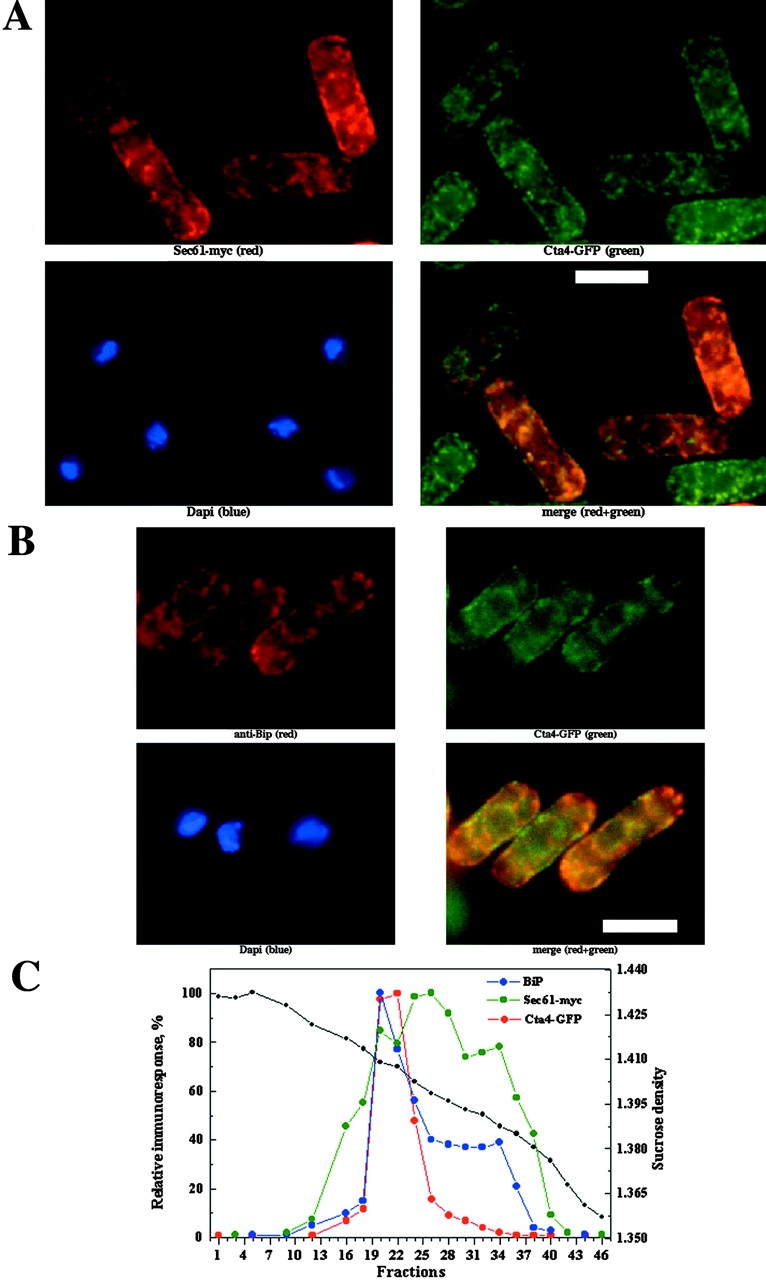
Cellular localization of Cta4p. (A and B) Yeast cells were grown to early log phase, fixed and stained with antibodies, and examined by IF microscopy (as described in Materials and methods). (A) GFP-tagged Cta4p cells transformed with plasmid-encoding Sec61-myc fusion protein (Broughton et al., 1997) were subjected to IF and stained with anti-GFP (green), anti-myc (red), and DAPI (blue). The cell to cell variation in expression of Sec61-myc is due to unequal partitioning of the multicopy plasmid. Bar, 10 μm. (B) Wild-type cells expressing GFP-tagged Cta4p stained with anti-GFP (green), anti-BiP (red), and DAPI (blue). The merged images show colocalization of Cta4p with Sec61 and BiP (yellow) in the deconvolved image. (C) Cta4p is comigrating with ER membranes. Total membranes were isolated from the cells expressing both Cta4-GFP and Sec61-myc fusion proteins, fractionated on a sucrose density gradient, and submitted to immunoblot analysis. Dot blots of individual fractions were used for quantification of the relative levels of Cta4, Sec61, and BiP in membrane fractions. A fraction with higher immunoresponse was considered as 100% in each case.
These data were further confirmed by subcellular fractionation of total membranes isolated from cells expressing both Cta4-GFP and Sec61-myc fusion proteins. Immunoblots of membrane fractions showed that Cta4-GFP comigrated with Sec61-myc at positions within the gradient corresponding to 41–47% sucrose where membrane vesicles enriched with ER were shown previously to migrate (Fig. 1 C) (Okorokov et al., 2001). However the Sec61-myc fusion protein showed a wider distribution than Cta4-GFP and was also detected in membrane fractions migrating at 32–41% sucrose corresponding to the Golgi and/or Golgi-like membranes. Thus, it is likely that this broader distribution of Sec61-myc corresponds to Golgi and that Cta4-GFP is more restricted to the ER. In animal cells, a Sec61 homologue was found in the ER-to-Golgi intermediate compartment in addition to the ER, thus supporting this idea (Greenfield and High, 1999; Kamhi-Nesher et al., 2001). Another possible explanation for the broader distribution of Sec61-myc might be its overexpression from a plasmid.
To verify the ER localization of Cta4 ATPase, the antibody against another ER organelle marker, chaperone BiP (Pidoux and Armstrong, 1993), was used. IF microscopy demonstrated colocalization of Cta4-GFP and BiP (Fig. 1 B). Distribution of BiP in membrane fractions was more restricted than that of Sec61-myc and was similar to that of Cta4. Both BiP and Cta4 proteins were concentrated in fractions 20–22 (Fig. 1 C). Therefore, it could be concluded from these results that Cta4 ATPase is localized to the ER compartment.
cta4 + regulates cation homeostasis
To further investigate the function of Cta4p in fission yeast, the cta4 + gene was disrupted with the ura4 + marker gene, and the resulting cta4Δ phenotype was compared with that of wild type. Tetrad analysis revealed that cta4 + was not an essential gene; however, cta4Δ cells exhibited poor growth at 25°C compared with that at 30°C and died at 36°C, indicating that some aspect of cell cycle progression was impaired in these conditions (Fig. 2 A). Growth of Cta4-GFP cells was not impaired at 25 and 36°C, pointing out that this allele is fully functional.
Figure 2.
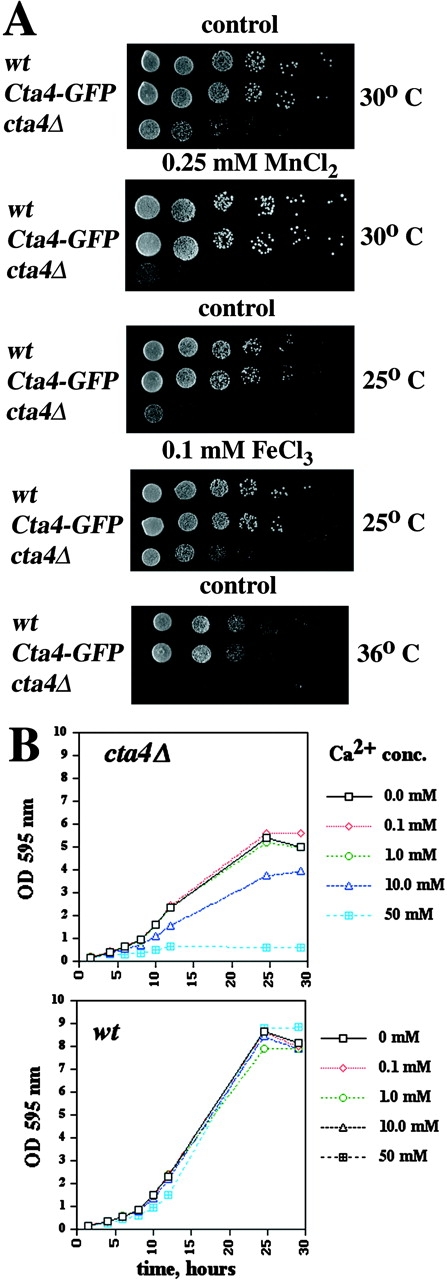
Phenotypes of cta4 Δ . (A) Disruption of cta4 + confers a temperature-sensitive phenotype and causes an imbalance in cation homeostasis. Cells were serially diluted in fivefold steps, spotted onto YES plates containing cations as indicated, and incubated for 3 d at 25, 30, or 36°C. Strains used were Fy1180, Hu185, and Hu285. (B) The effect of Ca2+ on the growth of the cta4Δ mutant. Growth curves showing the growth inhibition by different concentrations of CaCl2 at 30°C.
Yeast P-type ATPase mutants commonly display phenotypic defects in response to variations in the concentration of cations in the growth media (Rudolph et al., 1989; Cunningham and Fink, 1996). Consistent with this idea, we found that growth of cta4Δ cells was completely inhibited by a high concentration, that is, 100 mM of CaCl2. Next, growth inhibition of lower concentrations of CaCl2 was investigated using liquid cultures (Fig. 2 B). It was clear that the growth of wild-type cells was unaffected by addition of up to 50 mM CaCl2, whereas the growth of cta4Δ cells was partially inhibited already by the addition of 10 mM CaCl2 and completely inhibited by the addition of 50 mM CaCl2. The cta4 + deletion mutant was also sensitive to 0.25 mM MnCl2 (Fig. 2 A). Furthermore, the cta4Δ growth at 25°C was improved by high concentrations of Fe3+ (0.1 mM FeCl3) (Fig. 2 A), and the lethality of cta4Δ at 36°C could be overcome by K+ (300 mM KCl), ruling out that Cl− could be toxic to cta4Δ cells (unpublished data). Collectively, these results showed that the homeostasis of different ions is severely compromised in cta4Δ cells. Although the data did not elucidate which cation is transported by the cta4 + gene product, they strongly suggest that Cta4 ATPase might have a crucial function in maintenance of cation homeostasis in fission yeast. This conclusion was further reinforced by the observation that overexpression of cta4 + from the regulatable nmt1 + promoter was deleterious to wild-type cells, but growth was restored by the addition of 100–200 mM CaCl2 (unpublished data).
The Ca2+/CaM-dependent protein phosphatase 2B, calcineurin, was shown to be responsible for a maintenance of cation homeostasis in S. cerevisiae by regulating the expression of Ca2+ and Na+ ATPases (Nakamura et al., 1993; Cunningham and Fink, 1996; Mendoza et al., 1996). In S. pombe, the calcineurin A subunit-like protein encoded by the ppb1 + gene is the target of cyclosporin A (CsA) (Yoshida et al., 1994). cta4Δ cells were found to be susceptible to 10 μg/ml CsA (Fig. 3), whereas wild-type strain remained insensitive to threefold higher drug concentration. This result indicates that cta4 + acts in the same or parallel pathways as calcineurin, since ppb1 + seems necessary for the viability of the cta4Δ mutant.
Figure 3.
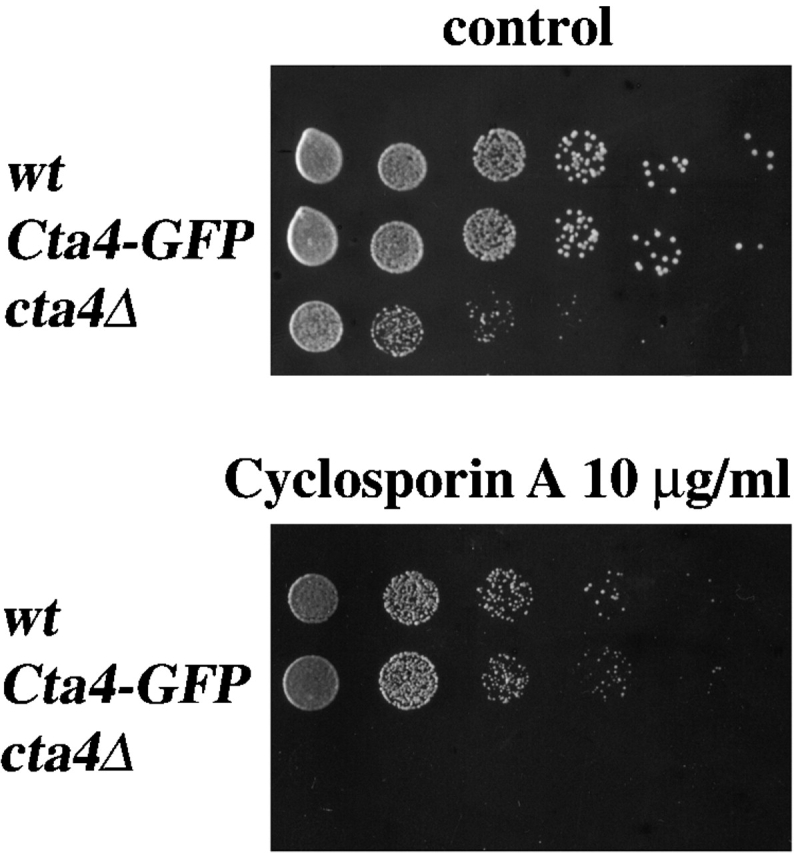
Viability of cta4 Δ depends on calcineurin. Cells were serially diluted in fivefold steps, spotted onto YES plates containing 10 μg/ml CoA, and incubated for 3 d at 30°C. Strains used were Fy1180, Hu185, and Hu285.
Functional similarity with the S. cerevisiae Spf1 ATPase
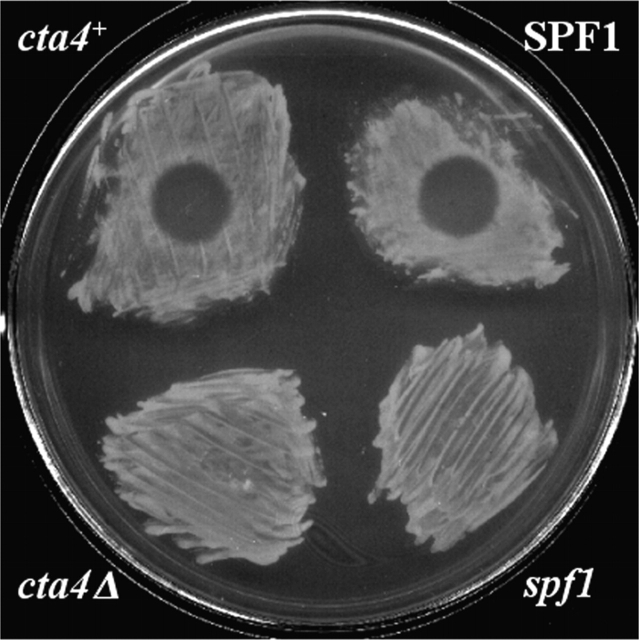
Because Cta4 ATPase shares 49% homology with S. cerevisiae Spf1p whose deletion confers resistance to Pichia farinosa killer toxin, SMKT (Suzuki and Shimma, 1999), we investigated the effect of SMKT on fission yeast wild-type and cta4Δ cells. In the halo assay, the wild-type strain displayed a clear sensitivity to SMKT, whereas cta4Δ was resistant to the toxin (Fig. 4). It was proposed that the resistance to killer toxin in spf1 null might be due to an alteration in glycosylation of the cell wall components (Suzuki and Shimma, 1999). The enzymes involved in the glycosylation process require Mn2+ for their activity (Kaufman et al., 1994). Therefore, the resistance to SMKT displayed by cta4Δ cells is not surprising, since the cta4Δ mutant also exhibits defects in Mn2+ homeostasis (Fig. 2 A). Thus, both Spf1 and Cta4 could have similar functions both with respect to Mn2+ homeostasis and the response to SMKT. It has been shown recently that SMKT interacts with the plasma membrane of wild-type but not mutant spf1 cells (Suzuki et al., 2001), raising a possibility that a structure and/or targeting of some membrane component, which binds the toxin, is similarly affected in S. cerevisiae spf1 and S. pombe cta4 mutant cells.
Figure 4.
Loss of cta4 + confers a resistance to P. farinosa killer toxin SMKT. The wild-type (Fy1180) and cta4 mutant cells (Hu285) were spread on the MB plates on which 5 μl of 100 μM SMKT solution was spotted (arrowhead). The S. cerevisiae strains used were CS202A and CS202B.
cta4 + is required for cytokinesis and microtubule integrity
Next, the effect of cta4 + gene disruption on cell morphology was investigated. cta4Δ cells were frequently multiseptated and formed hyphae-like structures, in which cells were not separated even when grown at the permissive temperature of 30°C. These multicellular structures were often branched and aggregated (Fig. 5 A). Lowering or increasing the temperature from 30°C or adding CaCl2 to the cells aggravated the phenotype. The multiseptated phenotype suggested that Cta4p is required for the final stages of cytokinesis. The aberrant cell shapes exhibited by cta4Δ also suggested that the cells had defects in the cytoskeleton. To test if microtubule function was perturbed in cta4Δ cells, growth in the presence of the microtubule destabilizing drug thiabendazole (TBZ) was assayed. cta4Δ cells were found to be sensitive to TBZ, indicating that Cta4p is normally required to stabilize microtubules (Fig. 5 B). To examine whether loss of cta4 + leads to changes in microtubule assembly, microtubules in cta4Δ cells were visualized using monoclonal antitubulin antibodies by IF microscopy of cells grown at 25°C. Cytoplasmic microtubules in the mutant cells appeared abnormally short, and the number of microtubules per cell was increased in cta4Δ cells compared with wild-type cells (Fig. 6, A and B). Measurements of the microtubule length indicated that the microtubules in interphase cta4Δ cells were significantly shorter (t = −3.573; P = 0.001) than those of wild-type cells. The microtubules in cta4Δ cells rarely reached lengths greater than 4 μm, whereas those of wild-type cells extended up to 8 μm (Fig. 6 C). Furthermore, the number of microtubules per interphase cell was markedly increased in cta4Δ cells to three to six microtubules per cell, whereas wild-type cells had only one to three (Fig. 6 D). Thus, Cta4p generally stabilizes microtubules.
Figure 5.
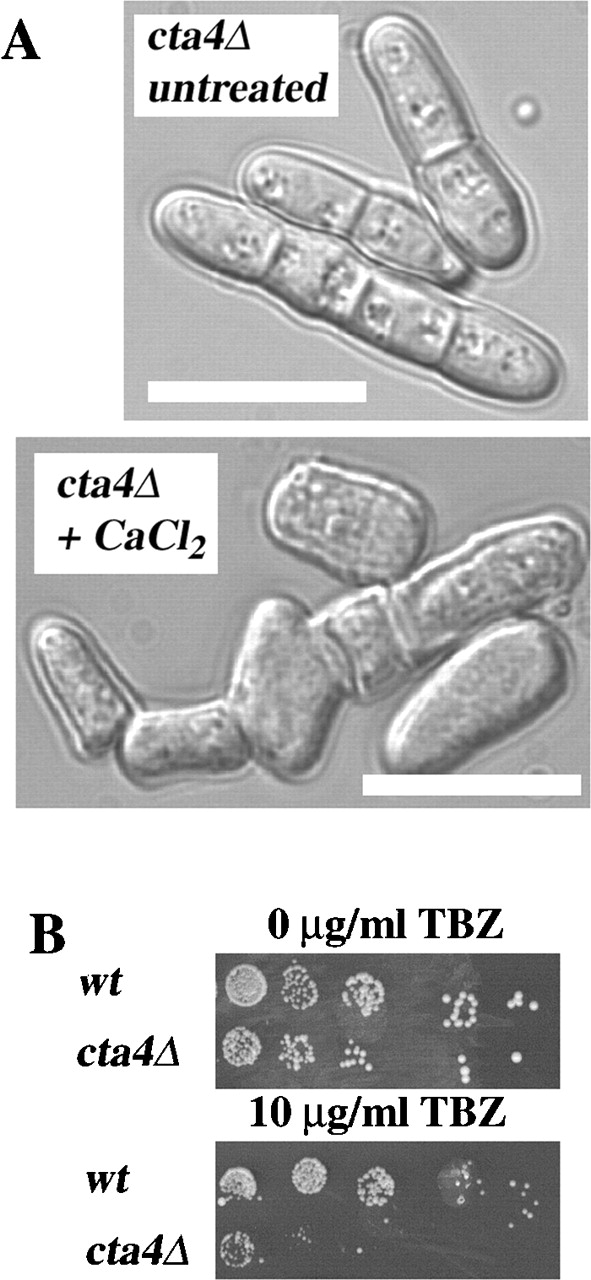
Cell shape defects, cytokinesis defects, and TBZ sensitivity of the cta4 Δ mutant. (A) Aberrant cell morphology of cta4Δ cells. Phase–contrast micrographs of S. pombe wild-type and cta4Δ cells grown in YES medium at 30°C. Bars, 10 μm. (B) TBZ sensitivity of cta4Δ. Strains grown at 30°C were 972 and Hu285.
Figure 6.
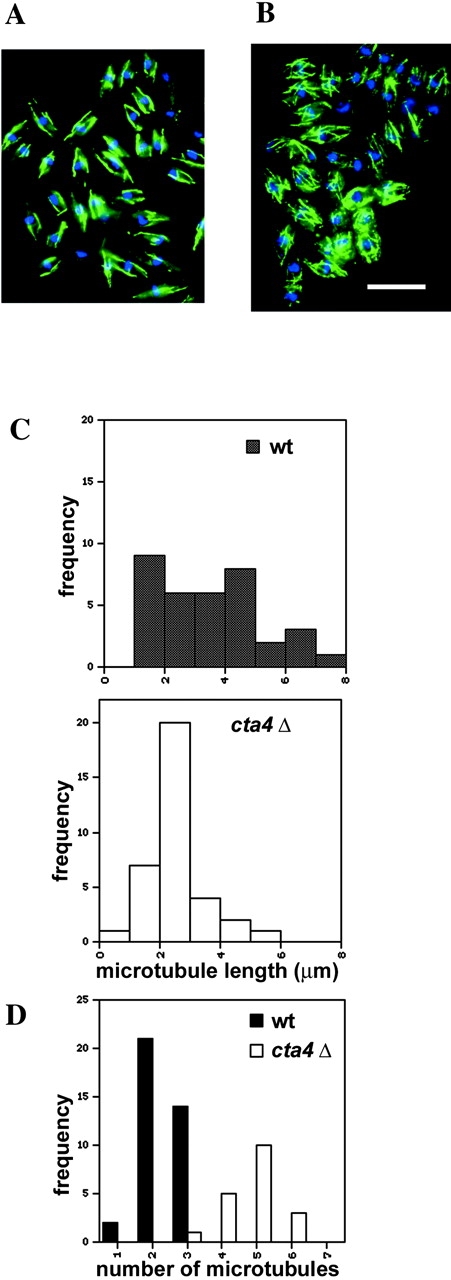
Microtubule distribution in cta4 Δ cells. (A and B) IF microscopy images of wild-type (A) and cta4Δ cells (B) grown at 25°C and fixed and stained with anti-TAT1 (green) and DAPI (blue). Bar, 10 μm. (C) Analysis of microtubule length (cta4Δ, n = 47; wt, n = 35) (D) Analysis of the number of microtubules per interphase cell (cta4Δ, n = 47; wt, n = 35).
To test if the shortening and increase in microtubule number per cell were due to altered dynamic properties of the microtubules, we investigated the microtubule dynamics in living wild-type and cta4Δ cells using strains expressing α-tubulin fused to GFP (Ding et al., 1998; Pidoux et al., 2000). The analysis of time-lapse movies of α-tubulin–GFP fusion confirmed the alterations observed in fixed cells, namely that the number of microtubules per cell was markedly increased in cells lacking Cta4p (Fig. 7, A and B). In addition, cells lacking cta4 + exhibited changes in the microtubule growth rate and in the occurrence of microtubule catastrophe events. Length measurements of individual microtubules revealed that, although the microtubules of wild-type cells commonly reached a length of >5 μm before they suddenly started to shrink, those of cta4Δ cells underwent catastrophe events already at lengths of ∼3 μm (Fig. 7 C). Moreover, the microtubule catastrophe events in cta4Δ cells were not limited to a cortical region near the cell tip, whereas those in wild-type cells in 89% of the cases occurred in the cell tip region (Fig. 7 D). Thus, the time-lapse analysis revealed that the dynamic features of microtubules were strongly altered in cta4Δ cells, leading to a failure in guiding the microtubules toward the cell tip so that the cell growth axis could not be maintained.
Figure 7.
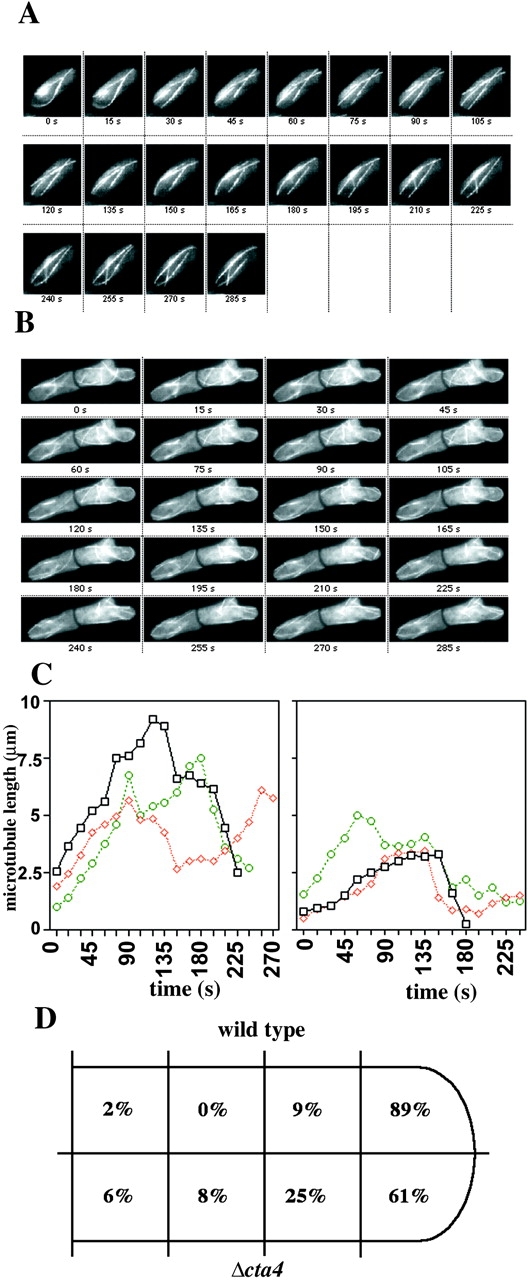
Microtubule dynamics are altered in cta4 Δ cells. (A) A time-lapse series of images of wild-type cells expressing GFP-tagged α-tubulin. (B) A time-lapse series of cta4Δ cells expressing GFP-tagged α-tubulin. (C) Growth of individual microtubules in wild-type (left) and cta4Δ (right) cells. (D) The schematic drawing is representing the frequencies of catastrophe event in different parts of the cell. Strains used were Fy2773 and Hu326.
cta4 + controls the nuclear Ca2+ levels
Considering that the cta4 + deletion mutant displayed a Ca2+-sensitive phenotype, it was tempting to speculate that Cta4 ATPase would play a role in controlling cytosolic-free calcium levels. To test this possibility, we explored the FRET-based technique using “cameleon” reporter constructs expressed in living cells to measure Ca2+ levels by time-lapse yellow fluorescent protein (YFP)/cyan fluorescent protein (CFP) ratio imaging (Miyawaki et al., 1997). The cameleon reporter consists of a CFP-M13-CaM-YFP protein fusion, which gives a relative increase in YFP emission (at 535 nm) when Ca2+ levels are high. This is due to FRET from CFP to YFP caused by proximity of CFP and YFP when M13 interacts with Ca2+-bound CaM. The yellow cameleon YC2 was cloned into pREP3x plasmid (see Materials and methods), and the resulting plasmid was introduced into wild-type S. pombe cells and cells lacking cta4 + by transformation. To increase the signal for accurate determination of the FRET signal, a nuclear localization signal was added to the YC2 protein fusion and strong expression (thiamine) was used from the pREP3x nmt1 + promoter. The ratio imaging measurements indicated that the ratio of 535:480 fluorescence increased significantly (t = 4.125, P < 0.001) in untreated cta4Δ cells compared with wild-type cells and in cta4Δ cells treated with Ca2+ compared with wild-type cells treated with Ca2+ (t = 3.154, P = 0.003). The 535:480 ratio in Ca2+-treated cta4Δ cells was on average 1.59 (n = 21), whereas Ca2+-treated wild-type cells gave 535:480 ratios on average of 1.39 (n = 18). The 535:480 ratios were generally stable over time. It was noticed that all aberrantly shaped or round cta4Δ cells examined gave particularly high FRET 535:480 ratios on average of 1.78 (n = 15): for example, the cell nucleus indicated in red in Fig. 8 C. Thus, the cell shape defects caused by loss of cta4 + are associated with elevated nuclear Ca2+ level.
Figure 8.
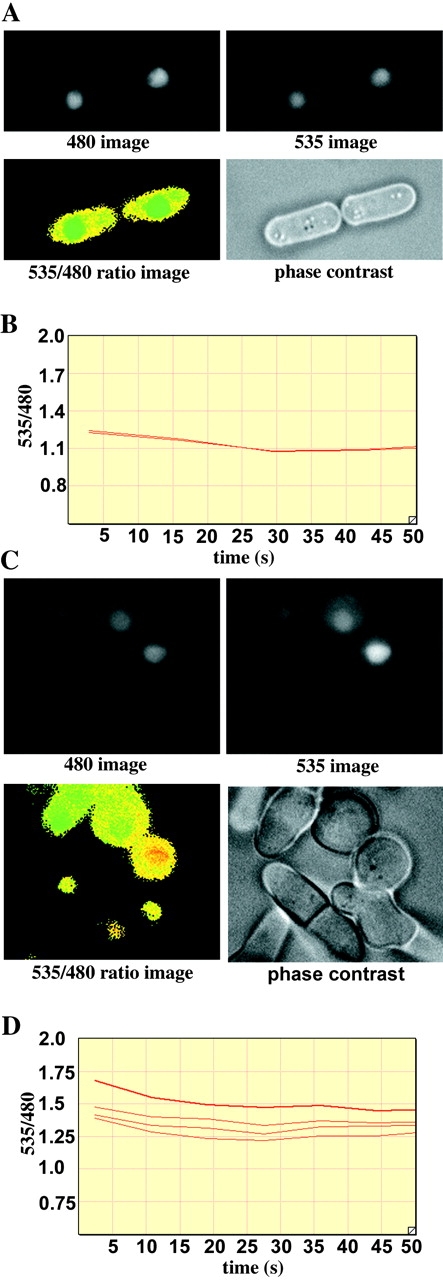
Measurement of [Ca2 + ]nuc in live fission yeast cells using yellow cameleon YC.2. (A and C) Images of wild-type (A) and cta4Δ (C) cells. Yeast cells expressing the yellow cameleon pREP3x-YC2 plus NLS were grown at 30°C and treated with 100 mM CaCl2 for 1 h and then subjected to ratio imaging. The raw data image of CFP (480 nm) and YFP (535 nm) emissions are shown. The 535:480 ratio image (bottom left) indicates the [Ca2+]nuc using a “rainbow” scale in which green indicates low, yellow indicates intermediate, and red indicates high YFP to CFP ratios. (B and D) Time course analysis of the 535:480 emission ratios on the same yeast cells as in A and C. Wild-type (B) and cta4Δ (D) cells were subjected to time-lapse ratio imaging analysis at 30°C. The y axis shows the ratio of the YFP to CFP signal in the nuclei of the cells, which indicates [Ca2+]nuc. The x axis shows the real time in seconds.
Discussion
In the present work, we undertook a genetic approach to gain insight into the role of a putative P-type ATPase in fission yeast. Cta4 ATPase belongs to a P4 subfamily of the P-type ATPases with an unknown substrate specificity and subcellular localization (Catty et al., 1997). This recently identified subfamily comprises, in addition to cta4 +, SPF1 and Yor291 of S. cerevisiae and several homologous genes found in the genomes of Plasmodium, Caenorhabditis, Tetrahymena, and Arabidopsis (Catty et al., 1997; Suzuki and Shimma, 1999; Axelsen and Palmgren, 2001). Although amino acid homology between P4 ATPases could be as little as 20%, these pumps contain specific conserved amino acid signatures, suggesting that they might have common substrate and/or common mechanism of regulation and related cellular functions. The cta4 + gene product is most closely related to the budding yeast ATPase Spf1. The function of Spf1p is little understood. It has been shown that spf1 disruption leads to a resistance to P. farinosa killer toxin and impaired glycosylation (Suzuki and Shimma, 1999) and that Spf1/Cod1 regulates a degradation of integral ER membrane protein hydroxymethylglutaryl–coenzyme A (CoA) reductase (Cronin et al., 2000). Gene disruption experiments indicated that the cta4 + gene was nonessential for viability. Yet the cta4-null mutant displayed pleiotropic cellular phenotypes. The cell growth was reduced at 25°C and arrested at 36°C. Loss of cta4 + resulted in multiseptated hyphae-like structures in which cells did not separated and were often branched and aggregated. The microtubule structure was destabilized. Adaptation to cation stress was also impaired. Similarly to spf1 null, cta4Δ cells were resistant to SMKT killer toxin. However, whether the spf1 and cta4 genes are functionally interchangeable remains to be determined. It is of note that contrary to cta4Δ cells, lack of Spf1 ATPase did not cause visible changes in ion homeostasis, since spf1 growth was independent of calcium and manganese (Suzuki and Shimma, 1999). On the other hand, the addition of high CaCl2 (200 mM) to extracellular medium could restore a defect in hydroxymethylglutaryl-CoA reductase degradation displayed by spf1 cells (Cronin et al., 2000).
Several lines of evidence suggest that cta4 + is involved in Ca2+ homeostasis. First, cta4Δ was unable to grow when calcium was supplied to medium and when it was chelated by EGTA (unpublished data). Second, calcium measurements in cta4Δ cells using fluorescent indicator yellow cameleon showed that nuclear calcium levels were increased in cta4-null cells in comparison with wild-type cells. It is likely that nuclear Ca2+ levels are indicative of the cytoplasmic Ca2+ levels, since Ca2+ ions can diffuse through the nuclear pores (Lipp et al., 1997). Thus, loss of cta4 + function reduces the ability to sequester Ca2+ to internal stores, presumably the ER (see below). The consequence of this is an increase in intracellular Ca2+.
The cta4 mutant cells were sensitive to inhibition of calcineurin, Ca2+/CaM-dependent protein phosphatase type 2B, by CsA. The ppb1 + gene of S. pombe encodes a catalytic subunit of calcineurin. In this respect, it is noteworthy that pleiotropic phenotypes of cta4Δ were reminiscent of those reported previously for ppb1Δ mutants regarding growth characteristics, septation, cell shape defects, and microtubule integrity (Yoshida et al., 1994). Moreover, S. pombe mutants lacking cta4 + were, like S. cerevisiae calcineurin mutants, sensitive to Ca2+ and Mn2+ cation stress. Thus, it is possible that calcineurin function is defective in cta4-null cells. It remains to be determined if the cta4 + and ppb1 + genes interact and if these two gene products share an essential overlapping function. Finally, cta4 + overexpression was toxic to wild-type cells, and this could be overcome by addition of Ca2+ (unpublished data). Therefore, both elevated and lowered Ca2+ cytosolic levels may be deleterious to S. pombe. Since cta4 + deletion is not lethal to S. pombe cells, it could be concluded that other Ca2+ transporters deplete cytosolic Ca2+ in this genetic background, although to a lesser extent than cta4 + normally does. Therefore, the precise regulation of Ca2+ homeostasis fails and, consequently, Ca2+ signals.
From our results it could be presumed that Cta4p might transport Ca2+. However, direct biochemical evidence is needed to establish the exact substrate specificity of Cta4p. Our attempts to measure ATP-dependent Ca2+ transport in isolated membranes of S. pombe 972 have shown that all Ca2+ uptake was abolished by protonophore FCCP, indicating that Ca2+ transport was due to Ca2+/H+ exchange (Okorokov et al., 2001). The similar result was obtained with S. pombe strain Hu237 used for determination of subcellular localization of Cta4p (unpublished data). Further experiments will be necessary to find the conditions favoring a detection of biochemical activity of Cta4 ATPase. The biochemical characterization of Cta4p will contribute to our comprehension of the physiological function of P4 ATPases.
Cta4p localizes to the ER in S. pombe. In animal cells, ER is equipped with SERCA-type Ca2+-ATPase and is a main Ca2+ store compartment (Mendolesi and Pozzan, 1998; Carafoli and Brini, 2000). The ATPases belonging to SERCA-type were also found in plant, protozoa, and insect (Liang et al., 1997; Lockyer et al., 1998; Talla et al., 1998). Interestingly, the gene encoding for SERCA-type Ca2+ pump has not been identified in yeast, although Ca2+-ATPase activity could be detected in the S. cerevisiae membranes derived from the ER (Okorokov and Lehle, 1998; unpublished data). These observations raise a possibility that Cta4p could represent a primary ancient pump serving the ER. It is likely that the evolution of the ER as an organelle was driven by a wide range of functions supporting the development of complex signaling networks within the eukaryotic cell. This may have been the driving force leading to the appearance of additional specialized ATPases, such as SERCA, which may sequester calcium ions into the ER.
The pleiotropic defects exhibited by cta4Δ could be interpreted by either a direct or an indirect involvement of cta4 + in microtubule integrity, cell shape, and cytokinesis through regulated changes in Ca2+ concentrations. Since Ca2+ is a well-known secondary messenger, any transient elevations in the intracellular Ca2+ concentration would result in Ca2+ binding to multiple classes of Ca2+-binding proteins, each of which can, in its turn, regulate multiple downstream signaling pathways. On the other hand, Ca2+ is emerging as the regulatory ion for many ER/Golgi functions. Oscillations in free Ca2+ concentrations in the ER of animal cells were shown to control diverse processes, including protein synthesis, chaperone function, and glycoprotein processing (Corbett and Michalak, 2000). The budding yeast secretory pathway requires Ca2+ for proper glycosylation, sorting, and ER-associated protein degradation (Antebi and Fink, 1992; Durr et al., 1998; Okorokov and Lehle, 1998). Further studies are required to identify components of the Ca2+ signaling machinery, which depend on Cta4p. However, some speculations about possible downstream targets can be made already.
We showed that loss of cta4 + enables yeast cells to complete cytokinesis. Previously, this process was shown to be dependent, in part, on the cps1 + gene encoding β-(1,3)-d-glucan synthase (Ishiguro et al., 1997; Liu et al., 2000). Expression of a homologue of cps1 + in budding yeast, FKS2, is induced by PKC together with calcineurin in a Ca2+-dependent manner (Zhao et al., 1998). cps1 + also appears to be dependent on calcineurin, since the cps1 ts mutant is hypersensitive to CsA (Ishiguro et al., 1997). In addition, cps1 ts mutants are, like cta4Δ, multiseptated and branched. Considering our supposition that calcineurin function might be compromised in cta4Δ, then defects in cytokinesis could be explained through changes in Cps1 activity, and this raises the possibility that cta4 + and cps1 + act in the same pathway.
There is a strong link between cell shape/polarity and microtubules in fission yeast (Sawin and Nurse, 1998). Therefore, our data provide evidence that microtubule integrity relies on cta4 + function. From this study, we cannot distinguish direct from indirect effects of Ca2+ on microtubules. Thus, additional studies will be necessary to gain a deeper comprehension of the involvement of cta4 + in this process. One of the possibilities is that cta4 + would regulate microtubule integrity, controlling the stability and/or deposition of microtubule-associated proteins. A recent study in animal cells has shown that Ca2+-binding proteins, such as S100A1 and S100B, might have a role in the in vivo regulation of the state of assembly of microtubules in a Ca2+-regulated manner (Sorci et al., 2000). Also, in S. pombe, microtubule-associated factors, such as the CLIP170-like protein Tip1, are involved in microtubule dynamics (Brunner and Nurse, 2000). The phenotypes of tip1Δ null and cta4Δ are related, since the mutants cells are not rod-shaped like wild-type S. pombe cells. Furthermore, both mutants show shorter microtubules and an increase in the frequency of microtubule catastrophe events throughout the cell rather than exclusively in the cell tips, which are the regions of polarized growth (Fig. 7) (Brunner and Nurse, 2000). In both cases, the changes in cell shape are associated with a defective guidance mechanism for microtubules. In this respect, since Tip1p is a microtubule-binding protein it would be interesting to investigate if Tip1p is directly or indirectly dependent on Ca2+ and/or Cta4p.
We provide evidence that the Ca2+ concentration is crucial for establishing the correct cell polarity by regulating microtubule dynamics. This finding is not without precedence, since studies in plant root tips demonstrate that root hair polarity is dependent on a Ca2+ gradient that increases in Ca2+ concentration toward the tip of the root hair (Bibikova et al., 1997; Wymer et al., 1997; Gadella et al., 1999). Furthermore, the polarity marked by this gradient is dependent on microtubules (Bibikova et al., 1999). Thus, the Ca2+-dependent mechanisms operating with respect to cell polarity may likely be of general significance in eukaryotes.
Further measurements of cytosolic Ca2+ are needed to clarify whether a Ca2+ gradient exists within the yeast cell and if there is a correlation between localized high Ca2+ and the sites of polarized growth. At the moment, it is tempting to speculate that increased Ca2+ at the ends of the cells would be a guiding signal for microtubule growth and a factor that induces the occurrence of microtubule catastrophe events.
Materials and methods
Strains and media
Media used were prepared according to standard methods (Moreno et al., 1991). Strains used in this study are listed in Table III.
Table III. Yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| S. pombe | ||
| 972 | h− | |
| Fy1180 | h+ otr1R(SphI)::ade6+ ura4-D18 leu1-32 ade6-M210 | R. Allshirea |
| Hu185 | h+ cta4-GFP::kanMX6 otr1R(SphI)::ade6 + ura4-D18 leu1-32 ade6-M210 | This study |
| Hu285 | h− cta4:: ura4+ ura4-D18 leu1-32 ade6-M216 | This study |
| Fy2796 | h− cta4:: ura4+ ura4-D18 leu1-32 ade6-M210 | This study |
| Fy2773 | h− leu1-32 ura4-D18 ade6-M210 ars1::nmt1-a-Tub-GFP-LEU2+ | A. Pidouxa |
| Hu326 | h− cta4:: ura4+ ura4-D18 leu1-32 ade6-M210 ars1::nmt1-α-Tub-GFP-LEU2+ | This study |
| Hu237 | cta4-GFP::kanMX6 otr1R(SphI)::ade6+ ura4-D18 leu1-32 ade6-M210 pREP- nmt1-Sec61-myc-LEU2+ | This study |
| S. cerevisiae | ||
| CS202A | MATa his3Δ1 leu2-3 ura3-52 trp1-289 spf1::LEU2 | Suzuki and Shimma, 1999 |
| CS202B | MATa his3Δ1 leu2-3 ura3-52 trp1-289 | Suzuki and Shimma, 1999 |
University of Edinburgh, Edinburgh, UK.
IF microscopy
S. pombe cells were prepared for IF microscopy according to the formaldehyde fixation procedure with some modifications (Hagan and Ayscough, 2000). Log phase cultures were incubated for 5–30 min in YES plus 1.2 M sorbitol before harvest. In most cases PEMAL (PEM plus 5 or 0.03% milk, 0.1 M l-lysine HCl, cleared by centrifugation during 30 min at 20,000 g) was used instead of PEMBAL. As primary antibodies, rabbit anti-GFP (Molecular Probes), mouse anti-myc (Sigma-Aldrich), and rabbit anti-BiP (Pidoux and Armstrong 1993) were used. FITC- or Texas red–conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories or Sigma-Aldrich. Cells were visualized using a ZEISS Axioskop II imaging microscope equipped with a Hamamatsu C4742–95 CCD camera. Z-series digital confocal deconvolution analysis using Openlab software (version 2:25; Improvision), was performed using 0.2–0.3-μm sample z spacing and nearest neighbor deconvolution method. An object magnification of 100× and a lens aperture of 1.4 were used.
Live analysis of S. pombe cells
We performed GFP and CFP/YFP time-lapse analysis using the ratio imaging module of Openlab software version 2.25 and a ZEISS Axioskop II imaging microscope equipped with a Hamamatsu C4742–95 CCD camera. The yeast cells were embedded in 10 μl 1% soft agar in PMG medium under a 22 × 22-mm no. 1 coverslip (Propper) and subjected to time-lapse video capture using a 10% neutral density filter to reduce photobleaching of the GFP.
Membrane fractionation
Yeast cells were grown to late log phase. After incubation in 1.2 M sorbitol and 30 mM mercaptoethanol, pH 8.5, for 10 min at 25°C, they were washed with 1.2 M sorbitol and 50 mM NaH2PO4 adjusted with citric acid to pH 5.8. Spheroplasts were then isolated by incubation of the cells with lytic enzymes from Tritrichoderma at 30°C in the same buffer. Spheroplasts lysis and isolation of membranes followed published procedures (Okorokov and Lehle, 1998). The resuspended total membranes were loaded onto a step gradient formed of 56, 52, 48, 45, 42, 39, 36, 33, 30, and 25% sucrose (wt/wt). After centrifugation at 140,000 g for 2 h 45 min, the membrane fractions were collected from the bottom and stored at −70°C.
Immunoblotting
Yeast membranes from the sucrose gradient fractions (10 μl) were spotted on nitrocellulose membrane and probed with antibodies. Anti-GFP and anti-myc antibodies were purchased from Molecular Probes and Sigma-Aldrich, respectively. Anti-BiP antibodies were provided by Prof. J. Armstrong (University of Sussex, Brighton, UK). The blots were developed with peroxidase-conjugated secondary antibody.
Recombinant DNA
All procedures with recombinant DNA were performed according to standard techniques (Maniatis et al., 1982). The YC2 construct was PCR amplified from the original DNA clone (Miyawaki et al., 1997) using oligonucleotides that add a PKKKRKV (SV40) nuclear localization signal fused to the YC2 NH2 terminus and cloned into pREP3× multicopy plasmid digested with SalI. The expression of cta4 + was induced in PMG medium lacking thiamine. The cta4 + gene was tagged at its endogenous site with GFP using the method from Bahler et al. (1998).
Acknowledgments
We thank A. Mutvei for comments on the article, C. Retamal for help with immunomeasurements, K. Cunningham and S. Suzuki for S. cerevisiae strains, C. Stirling for Sec61-myc, S. Suzuki for SMKT toxin, K. Gull for the Tat1 antibody, J. Armstrong for BiP antibody, A. Pidoux for the Fy2773 strain, and R. Tsien for cameleon pYC2.
This work was supported by the Swedish Medical Research Council VR-M grant no. 12562 to K. Ekwall, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro grant E-26/171.374/99 to L. Okorokova, a Natural Science Council VR-N open postdoc grant to H. Appelgran, and a Wennergren stipend to M. Tabish.
Footnotes
Abbreviations used in this paper: CaM, calmodulin; CoA, coenzyme A; CsA, cyclosporin A; CYP, cyan fluorescent protein; FRET, fluorescence resonance energy transfer; GFP, green fluorescent protein; IF, immunofluorescence; SERCA, sarco/ER Ca2+-ATPases; TBZ, thiabendazole; TMD, transmembrane spanning domain; YFP, yellow fluorescent protein.
References
- Antebi, A., and G.R. Fink. 1992. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel-like distribution. Mol. Biol. Cell. 3:633–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen, K.B., and M.G. Palmgren. 2001. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 126:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler, J., J.Q. Wu, M.S. Longtine, N.G. Shah, A. McKenzie III, A.B. Steever, A. Wach, P. Philippsen, and J.R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 14:943–951. [DOI] [PubMed] [Google Scholar]
- Bibikova, T.N., A. Zhigilei, and S. Gilroy. 1997. Root hair growth is directed by calcium and endogeneous polarity. Planta. 203:495–505. [DOI] [PubMed] [Google Scholar]
- Bibikova, T.N., E.B. Blancaflor, and S. Gilroy. 1999. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17:657–665. [DOI] [PubMed] [Google Scholar]
- Broughton, J., D. Swennen, B.M. Wilkinson, P. Joye, C. Gaillardin, and C.J. Stirling. 1997. Cloning of SEC61 homologues from Schizosaccharomyces pombe and Yarrowia lipolytica reveals the extent of functional conservation within this core component of the ER translocation machinery. J. Cell Sci. 110:2715–2727. [DOI] [PubMed] [Google Scholar]
- Brunner, D., and P. Nurse. 2000. CLIP170-like tip1p spatially organizes microtubular dynamics in fission yeast. Cell. 102:695–704. [DOI] [PubMed] [Google Scholar]
- Carafoli, E., and M. Brini. 2000. Calcium pumps: structural basis for and mechanism of calcium transmembrane transport. Curr. Opin. Chem. Biol. 4:152–161. [DOI] [PubMed] [Google Scholar]
- Catty, P., A. de Kerchove d'Exaerde, and A. Goffeau. 1997. The complete inventory of the yeast Saccharomyces cerevisiae P-type transport ATPases. FEBS Lett. 409:325–332. [DOI] [PubMed] [Google Scholar]
- Clapham, D.E. 1995. Calcium signaling. Cell. 80:259–268. [DOI] [PubMed] [Google Scholar]
- Corbett, E.F., and M. Michalak. 2000. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem. Sci. 25:307–311. [DOI] [PubMed] [Google Scholar]
- Cronin, S.R., A. Khoury, D.K. Ferry, and R.Y. Hampton. 2000. Regulation of HMG-CoA reductase degradation requires the P-Type ATPase Cod1p/Spf1p. J. Cell Biol. 148:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, K.W., and G.R. Fink. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, D.-Q., Y. Chikashige, T. Haraguchi, and Y. Hiraoka. 1998. Oscillatory nuclear movement in fission yeast meiotic prophase is driven by astral microtubules, as revealed by continuous observation of chromosomes and microtubules in living cells. J. Cell Sci. 111:701–712. [DOI] [PubMed] [Google Scholar]
- Durr, G., J. Strayle, R. Plemper, S. Elbs, S.K. Klee, P. Catty, D.H. Wolf, and H.K. Rudolph. 1998. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell. 9:1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadella, T.W.J., G.N.M. van der Krogt, Jr., and T. Bisseling. 1999. GFP-based FRET microscopy in living plant cells. Trends Plant Sci. 4:287–291. [DOI] [PubMed] [Google Scholar]
- Ghislain, M., A. Goffeau, D. Halachmi, and Y. Eilam. 1990. Calcium homeostasis and transport are affected by disruption of cta3, a novel gene encoding Ca2+-ATPase in Schizosaccharomyces pombe. J. Biol. Chem. 265:18400–18407. [PubMed] [Google Scholar]
- Greenfield, J.J., and S. High. 1999. The Sec61 complex is located in both the ER and the ER-Golgi intermediate compartment. J. Cell Sci. 112:1477–1486. [DOI] [PubMed] [Google Scholar]
- Guerini, D., and E. Carafoli. 1999. The calcium pumps. Calcium as a Cellular Regulator. E. Carafoli and C.B. Klee, editors. Oxford University Press, Oxford, UK. 249–278.
- Gunteski-Hamblin, A.-M., D.M. Clarke, and G.E. Shull. 1992. Molecular cloning and tissue distribution of alternatively spliced mRNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry. 31:7600–7608. [DOI] [PubMed] [Google Scholar]
- Hagan, I.M., and K.R. Ayscough. 2000. Fluorescence microscopy in yeast. Protein Localization by Fluorescence Microscopy. A Practical Approach. V.J. Allan, editor. Oxford University Press, Oxford. 179–206.
- Ishiguro, J., A. Saitou, A. Durán, and J.C. Ribas. 1997. cps1 +, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to Cyclosporin A and Papulacandin B. J. Bacteriol. 179:7653–7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhi-Nesher, S., M. Shenkman, S. Tolchinsky, S.V. Fromm, R. Ehrlich, and G.Z. Lederkremer. 2001. A novel quality control compartment derived from the endoplasmic reticulum. Mol. Biol. Cell. 12:1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, R.J., M. Swaroop, and P. Murtha-Riel. 1994. Depletion of manganese within the secretory pathway inhibits O-linked glycosylation in mammalian cells. Biochemistry. 33:9813–9819. [DOI] [PubMed] [Google Scholar]
- Li, C.-J., R. Heim, P. Lu, Y. Pu, R.Y. Tsien, and D.C. Chang. 1999. Dynamic redistribution of calmodulin in HeLa cells during cell division as revealed by a GFP-calmodulin fusion protein technique. J. Cell Sci. 112:1567–1577. [DOI] [PubMed] [Google Scholar]
- Liang, F., K.W. Cunningham J.F. Harper, and H. Sze. 1997. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 94:8579–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp, P., D. Thomas, M.J. Berridge, and M.D. Bootman. 1997. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J. 16:7166–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., H. Wang, and M.K. Balasubramanian. 2000. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J. Cell Sci. 113:1223–1230. [DOI] [PubMed] [Google Scholar]
- Lockyer, P.J., E. Puente, J. Windass, F. Earley, J.M. East, and A.G. Lee. 1998. Cloning and expression of an insect Ca2+-ATPase from Heliothis virescens. Biochim. Biophys. Acta. 1369:14–18. [DOI] [PubMed] [Google Scholar]
- Maniatis, T., E.F. Fritsch, and J. Sambrook. 1982. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 545 pp. [Google Scholar]
- Mendolesi, J., and T. Pozzan. 1998. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem. Sci. 23:10–14. [DOI] [PubMed] [Google Scholar]
- Mendoza, I., F.J. Quintero, R.A. Bressan, P.M. Hasegawa, and J.M. Pardo. 1996. Activated calcineurin confers high tolerance to ion stress and alters the budding pattern and cell morphology of yeast cells. J. Biol. Chem. 271:23061–23067. [DOI] [PubMed] [Google Scholar]
- Miyawaki, A., J. Llopis, R. Heim, J.M. McCaffery, J.A. Adams, M. Ikura, and R.Y. Tsien. 1997. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 388:882–887. [DOI] [PubMed] [Google Scholar]
- Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795–823. [DOI] [PubMed] [Google Scholar]
- Moser, M.J., M.R. Flory, and T.N. Davis. 1997. Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J. Cell Sci. 110:1805–1812. [DOI] [PubMed] [Google Scholar]
- Nagase, T., M. Nakayama, D. Nakajima, R. Kikuno, and O. Ohara. 2001. Prediction of the coding sequences of unidentified human genes. XIX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 8:85–95. [DOI] [PubMed] [Google Scholar]
- Nakamura, T., Y. Liu, D. Hirata, H. Namba, S. Harada, T. Hirokawa, and T. Miyakawa. 1993. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast as an important determinant to high salt stress conditions. EMBO J. 12:4063–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, T., H. Aiba, and T. Mizuno. 1999. The cta3 + gene that encodes a cation-transporting P-type ATPase is induced by salt stress under control of the Wis1-Sty1 MAPKK-MAPK cascade in fission yeast. FEBS Lett. 455:183–187. [DOI] [PubMed] [Google Scholar]
- Okorokov, A.L., and L. Lehle. 1998. Ca2+-ATPases of Saccharomyces cerevisiae: diversity and possible role in protein sorting. FEMS Microbiol. Lett. 162:83–91. [DOI] [PubMed] [Google Scholar]
- Okorokov, L.A., F.E. Silva, and A.L. Okorokova Facanha. 2001. Ca2+ and H+ homeostasis in fission yeast: a role of Ca2+/H+ exchange and distinct V-H+-ATPases of the secretory pathway organelles. FEBS Lett. 505:321–324. [DOI] [PubMed] [Google Scholar]
- Pedersen, P.L., and E. Carafoli. 1987. a. Ion motive ATPases. I. Ubiquity, properties, and significance for cell function. Trends Biochem. Sci. 12:146–150. [Google Scholar]
- Pedersen, P.L., and E. Carafoli. 1987. b. Ion motive ATPases. II. Energy coupling and work output. Trends Biochem. Sci. 12:186–189. [Google Scholar]
- Pidoux, A.L., and J. Armstrong. 1993. The BiP protein and the endoplasmic reticulum of Schizosaccharomyces pombe: fate of the nuclear envelope during cell division. J. Cell Sci. 105:1115–1120. [DOI] [PubMed] [Google Scholar]
- Pidoux, A.L., S. Uzawa, P.E. Perry, W.Z. Cande, and R.C. Allshire. 2000. Live analysis of lagging chromosomes during anaphase and their effect on spindle elongation rate in fission yeast. J. Cell Sci. 113:4177–4191. [DOI] [PubMed] [Google Scholar]
- Plochocka-Zulinska, D., G. Rasmussen, and C. Rasmussen. 1995. Regulation of calcineurin gene expression in Schizosaccharomyces pombe. J. Biol. Chem. 270:24794–24799. [DOI] [PubMed] [Google Scholar]
- Rudolph, H.K., A. Antebi, G.R. Fink, C.M. Buckley, T.E. Dorman, J. LeVitre, L.S. Davidow, J.I. Mao, and D.T. Moir. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 58:133–145. [DOI] [PubMed] [Google Scholar]
- Sanders, D., C. Brownlee, and J.F. Harper. 1999. Communication with calcium. Plant Cell. 11:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin, K.E., and P. Nurse. 1998. Regulation of cell polarity by microtubules in fission yeast. J. Cell Biol. 142:457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci, G., A.L. Agnelett, and R. Donato. 2000. Effects of S100A1 and S100B on microtubule stability. An in vitro study using triton-cytoskeletons from astrocyte and myoblast cell lines. Neuroscience. 99:773–783. [DOI] [PubMed] [Google Scholar]
- Sorin, A., G. Rosas, and R. Rao. 1997. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 272:9895–9901. [DOI] [PubMed] [Google Scholar]
- Stull, J.T. 2001. Ca2+-dependent cell signaling through calmodulin-activated protein phosphatase and protein kinase minireview series. J. Biol. Chem. 276:2311–2312. [DOI] [PubMed] [Google Scholar]
- Suzuki, C., and Y. Shimma. 1999. P-type ATPase spf1 mutants show a novel resistance mechanism for the killer toxin SMKT. Mol. Microbiol. 32:813–823. [DOI] [PubMed] [Google Scholar]
- Suzuki, C., Y. Ando, and S. Machida. 2001. Interaction of SMKT, a killer toxin produced by Pichia farinosa, with the yeast cell membranes. Yeast. 18:1471–1478. [DOI] [PubMed] [Google Scholar]
- Talla, E., R.L. de Mendonça, I. Degand, A. Goffeau, and M. Ghislain. 1998. Schistosoma mansoni Ca2+-ATPase SMA2 restores viability to yeast Ca2+-ATPase-deficient strains and functions in calcineurin-mediated Ca2+ tolerance. J. Biol. Chem. 273:27831–27840. [DOI] [PubMed] [Google Scholar]
- Wymer, C.L., T.N. Bibikova, and S. Gilroy. 1997. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 12:427–439. [DOI] [PubMed] [Google Scholar]
- Yoshida, T., T. Toda, and M. Yanagida. 1994. A calcineurin-like gene ppb1 + in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 107:1725–1735. [DOI] [PubMed] [Google Scholar]
- Zhao, C., U.S. Jung, P. Garrett-Engele, T. Roe, M.S. Cyert, and D.E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell Biol. 18:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]