Abstract
Functions of bone morphogenetic proteins (BMPs) are initiated by signaling through specific type I and type II serine/threonine kinase receptors. In previous studies, we have demonstrated that the type IB BMP receptor (BMPR-IB) plays an essential and specific role in osteoblast commitment and differentiation. To determine the role of BMP receptor signaling in bone formation in vivo, we generated transgenic mice, which express a truncated dominant-negative BMPR-IB targeted to osteoblasts using the type I collagen promoter. The mice are viable and fertile. Tissue-specific expression of the truncated BMPR-IB was demonstrated. Characterization of the phenotype of these transgenic mice showed impairment of postnatal bone formation in 1-mo-old homozygous transgenic mice. Bone mineral density, bone volume, and bone formation rates were severely reduced, but osteoblast and osteoclast numbers were not significantly changed in the transgenic mice. To determine whether osteoblast differentiation is impaired, we used primary osteoblasts isolated from the transgenic mice and showed that BMP signaling is blocked and BMP2-induced mineralized bone matrix formation was inhibited. These studies show the effects of alterations in BMP receptor function targeted to the osteoblast lineage and demonstrate a necessary role of BMP receptor signaling in postnatal bone growth and bone formation in vivo.
Keywords: BMP; receptor; transgenic mice; osteoblast differentiation; bone formation
Introduction
Bone morphogenetic proteins (BMPs)* are multifunctional growth factors that mediate a variety of biological functions, including osteoblast differentiation and bone formation (Urist, 1965; Wozney et al., 1988; Wozney 1992). BMPs signal through serine/threonine kinase receptors composed of type I and type II components. Three subtypes of type I receptors, which mediate BMP signaling, have been identified, including the types IA and IB BMP receptors and the type IA activin receptor (Koenig et al., 1994; ten Dijke et al., 1994; Yamaji et al., 1994; Macias-Silva et al., 1998). These receptors are expressed differentially in various tissues. For example, the type IA BMP receptor is highly expressed throughout mouse development and in adult tissues (Dewulf et al., 1995), whereas the type IB BMP receptor is expressed in mesenchymal precartilage condensations during mouse development and in chondrocytes and osteoblasts in adult mice (Yamaji et al., 1994; Dewulf et al., 1995; Ishidou et al., 1995). Relative levels of expression of these receptors and their timing of expression may be critical for determination of the appropriate biological response.
To examine the role of type I BMP receptor in osteoblast differentiation, we have previously stably expressed truncated dominant-negative (kinase domain deletion) and constitutively active types IA and IB BMP receptors in a clonal osteoblast precursor cell line, 2T3. Overexpression of the truncated type IB BMP receptor completely blocked BMP2-induced mineralized bone matrix formation and inhibited expression of genes that are associated with osteoblast differentiation, such as alkaline phosphatase, osteocalcin, and the bone specific transcription factor, Runx2/Cbfa1 (Chen et al., 1998). 2T3 cells expressing the truncated BMPR-IB were respecified to differentiate into mature adipocytes. Consistent with these results, overexpression of the constitutively active BMPR-IB in 2T3 cells induced BMP-independent mineralized bone matrix formation (Chen et al., 1998). These results demonstrate that BMPR-IB plays an important and specific role in osteoblast commitment and differentiation in vitro.
To determine the role of BMP receptors in bone formation in vivo, we have now generated transgenic mice, which overexpress the truncated BMPR-IB transgene. Expression of truncated BMPR-IB was targeted to osteoblasts by using the type I collagen promoter, which is specific for the osteoblast lineage. Characterization of the phenotypes of these transgenic mice revealed impairment in BMP receptor signaling as well as bone growth and bone formation in homozygous transgenic mice. Our results show that BMPs and BMP receptor signaling are necessary components for osteoblast differentiation and postnatal bone formation in vivo.
Results
Generation of the truncated BMPR-IB transgenic mouse lines
We first constructed two truncated, HA-tagged BMPR-IB transgenes, one driven by the type I collagen enhancer/basal promoter (0.7 kb) and the other by the 2.3-kb type I collagen promoter. These two promoters have been shown to direct bone-specific expression of reporter genes in vivo (Rossert et al., 1995, 1996). Using these type I collagen promoters to direct expression of the truncated BMPR-IB transgene, we generated two types of transgenic mice. Three founder transgenic mice for type I collagen enhancer/basal promoter (0.7 kb) (tg[Col-0.7]) and one founder mouse for 2.3-kb type I collagen promoter (tg[Col-2.3]) were generated and analyzed in these studies. One female founder mouse that contains high copy numbers of Col1a1(0.7)-trBMPR-IB transgene was very small and did not breed. This may be due to the defects in the female reproductive organs (Yi et al., 2001). The other three founder mice were bred to wild-type nontransgenic CB6F1 mice to establish the lines of mice. Heterozygous mice were then interbred to produce homozygous mice. Transgenic mice were genotyped by Southern blot analysis and by PCR and the genotype of homozygous mice was further confirmed by back breeding. The PCR primers for detection of the truncated BMPR-IB transgene have been described previously (Chen et al., 1998). Both heterozygous and homozygous mice for two types of transgenic mice were viable and fertile and survived into adulthood. The phenotypes of all these transgenic mouse lines are similar.
Tissue-specific expression of the truncated BMPR-IB transgene
We have examined expression of truncated BMPR-IB in homozygous transgenic mice by RT-PCR as described (Chen et al., 1998). Total RNA was extracted from different tissues including bones of 1-mo-old transgenic mice and from bones of 2-mo-old transgenic mice. Three different lines of mice were analyzed and expression levels of the truncated BMPR-IB transgene were determined and compared with those of endogenous BMPR-IB. Truncated BMPR-IB mRNA was strongly expressed in bone tissues, and in contrast, only a weak expression was detectable in skin and muscle of 1-mo-old transgenic mice (Fig. 1 A, top, tg[Col-0.7] and bottom, tg[Col-2.3]). In all other tissues, including brain, liver, heart, lung, and kidney, expression of truncated BMPR-IB transgene was undetectable (Fig. 1 A). Expression of the truncated BMPR-IB transgene was stable, as strong expression of the transgene was observed in bones in both 1- and 2-mo-old transgenic mice (Fig. 1 B). We also determined expression of the truncated BMPR-IB in bones by Western blot analysis. Cell lysates were extracted from calvariae of wild-type and tg(Col-2.3) transgenic mice. Immunoblotting using an anti-HA antibody demonstrated expression of the truncated BMPR-IB protein in bones of transgenic mice, but not in those of wild-type mice (Fig. 1 C).
Figure 1.
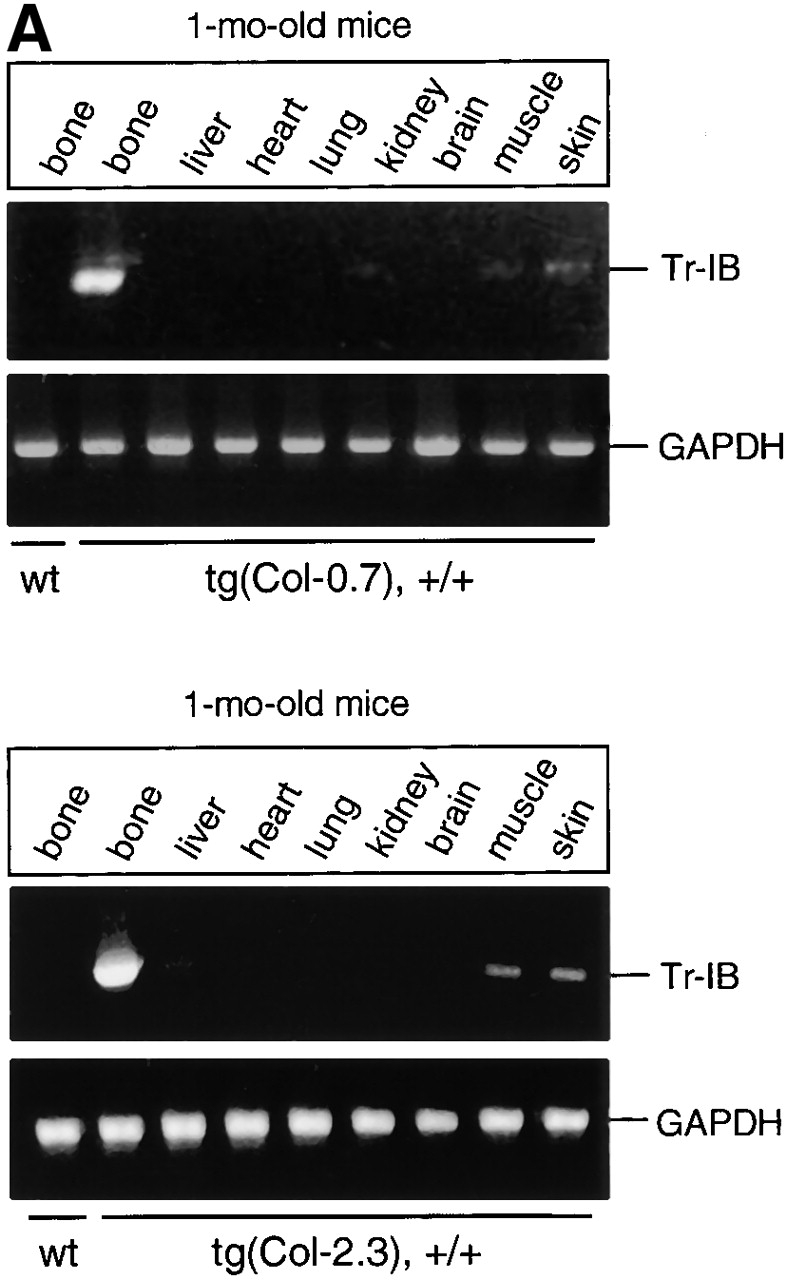


Tissue-specific expression of the truncated BMPR-IB transgene. (A) mRNA expression of the truncated BMPR-IB (Tr-IB) transgene in 1-mo-old transgenic mice. Bone and other tissues were collected from 1-mo-old tg(Col-0.7) and tg(Col-2.3) homozygous transgenic mice. The truncated BMPR-IB transgene was amplified using the primers described previously (Chen et al., 1998). (B) mRNA expression of truncated (Tr-IB) and endogenous (En-IB) BMPR-IB in bone tissues in 1- and 2-mo-old tg(Col-0.7) and tg(Col-2.3) transgenic mice. (C) Protein expression of the truncated BMPR-IB (Tr-IB) transgene in bones. Calvarial bones were collected from 1-mo-old wild-type and tg(Col-2.3) transgenic mice. The cell lysates were extracted using lysis buffer and then sonicated on ice. Western blot was performed using anti-HA antibody.
Changes in bone development in truncated BMPR-IB transgenic mice
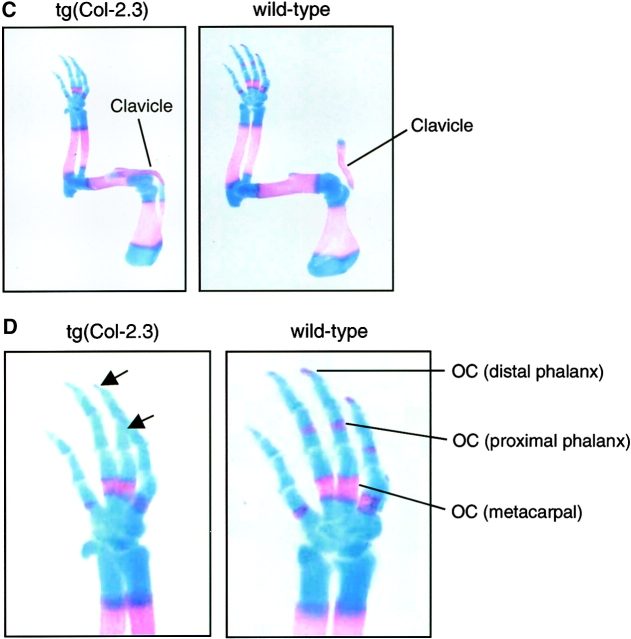
To examine changes in bone development, we analyzed three litters of 18.5-dpc embryos of tg(Col-2.3) transgenic mice by whole-embryo Alizarin red and Alcian blue staining. Bone development was normal in both wild-type and heterozygous embryos. In homozygous embryos, the sizes of embryos were smaller than those of wild-type littermates. Mineralization of calvarial bones, including frontal, parietal and interparietal bones, was delayed in these homozygous embryos (Fig. 2, A and B). In contrast to the phenotypes observed in Runx2/Cbfa1 heterozygous knockout mice, clavicle bones developed normally in tg(Col-2.3) homozygous embryos (Fig. 2, A and C). In 18.5–d postcoitum (dpc) wild-type embryos, the ossification centers were formed in distal and proximal phalangeal bones. In contrast, no ossification center was formed in these bones in the transgenic littermates (Fig. 2, C and D). This is consistent with the overall delay in mineralization in transgenic mice.
Figure 2.
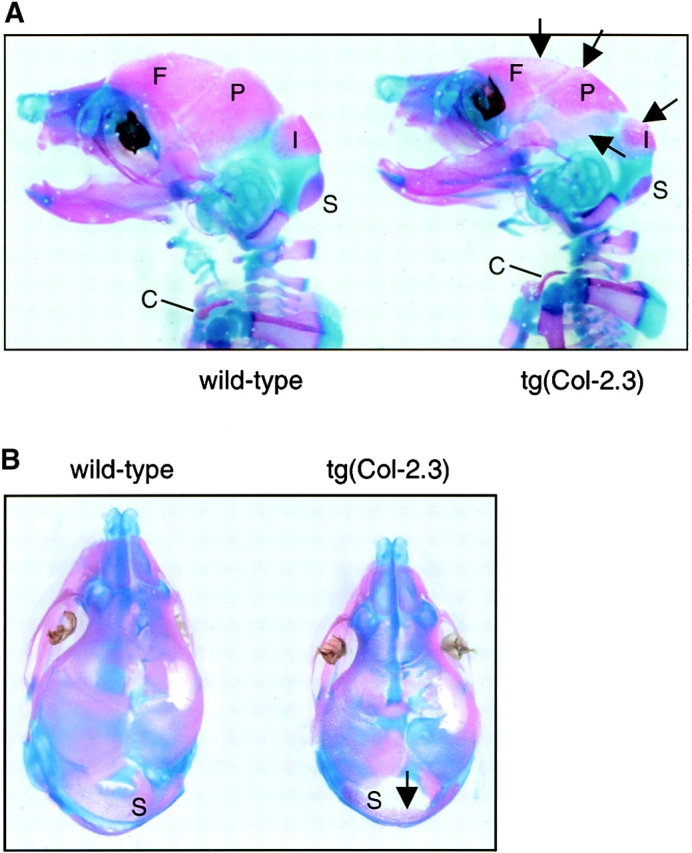
Alizarin red and Alcian blue staining of transgenic embryos. 18.5-dpc mouse embryos were killed and processed for Alizarin red and Alcian blue staining. The sizes of tg(Col-2.3) homozygous transgenic embryos were smaller than those of wild-type embryos. (A and B) Delayed ossification of frontal (F), parietal (P), interparietal (I), and supraoccipital (S) bones were noted in homozygous transgenic embryos and indicated by arrows (A and B). The wild-type embryos showed normal mineralization in calvarial bones. The clavicle (C) developed normally in both wild-type and homozygous transgenic embryos (A and C). (C and D) The formation of ossification centers (OCs) of distal and proximal phalangeal bones were observed in wild-type embryos but not in homozygous transgenic embryos (D, arrows).
Impairment of bone growth and reduction of bone mineral density in truncated BMPR-IB transgenic mice
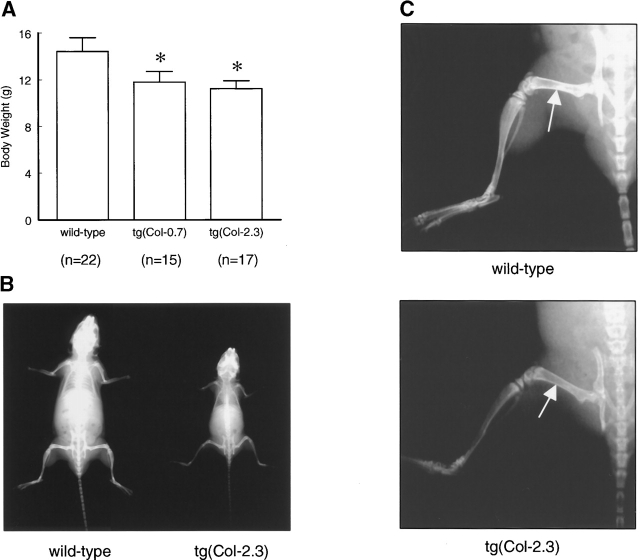
To determine whether bone growth is impaired in the transgenic mice, we measured body weight and analyzed changes in bone growth and bone mineral density (BMD) in 1-mo-old wild-type and three lines of homozygous mice by radiographic analysis and by bone densitometry. The average body weights of tg(Col-0.7) and tg(Col-2.3) mice were significantly and consistently less (12 and 22%, respectively) than those of the wild-type littermates throughout their life (Fig. 3 A). Radiographic analysis confirmed the reduction in size and also showed that bone densities in the hind limbs in tg(Col-2.3) homozygous mice were less than those of wild-type mice (Fig. 3, B and C).
Figure 3.
Impairment of bone growth in truncated BMPR-IB transgenic mice. (A) The comparison of the body weight between 1-mo-old wild-type and homozygous transgenic mice. Three litters of wild-type and transgenic mice were pooled. The body weights of tg(Col-0.7) and tg(Col-2.3) transgenic mice are 12 and 22% lower compared with age-matched wild-type mice. (B and C) x-ray analysis of tg(Col-2.3) transgenic mouse. Radiographs of 1-mo-old wild-type (left) and homozygous transgenic mouse (right) is shown in (B). Note the significantly smaller size of the homozygous transgenic mouse was compared with its wild-type littermate. The density of the long bones was also decreased in the transgenic mouse as indicated by arrows (C).
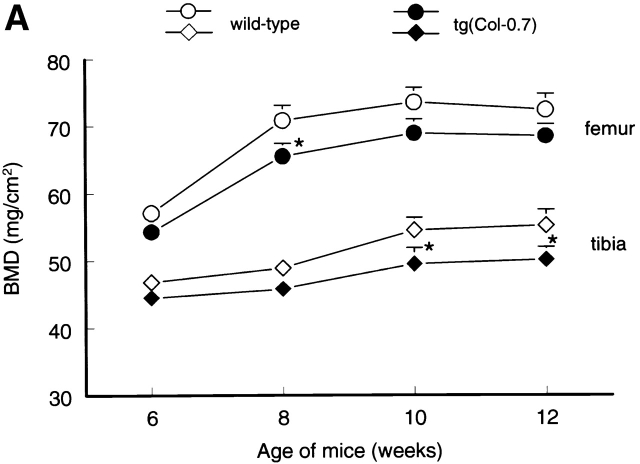
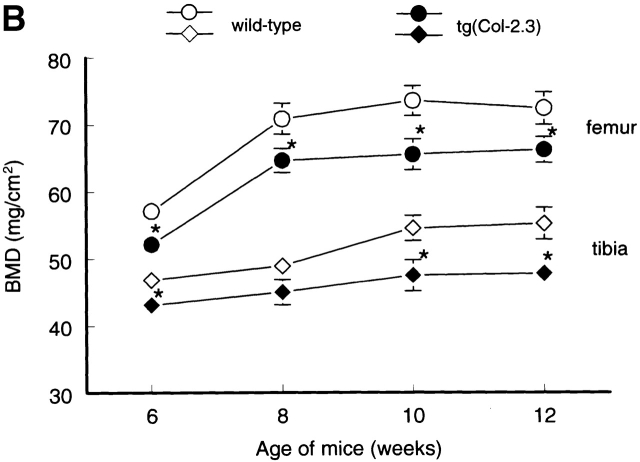
To determine changes in bone formation during bone modeling and remodeling phases in the transgenic mice, we first analyzed temporal changes in BMD in wild-type CB6F1 mice using PIXImus bone densitometer. In wild-type CB6F1 mice, BMD increased with age and plateaued after 10 wk (Fig. 4), suggesting that bone modeling continues until peak bone mass is achieved at 10 wk of age in CB6F1 mice. Bone remodeling starts thereafter. BMDs in three litters of transgenic mice and age-matched wild-type mice were then compared. BMD in tg(Col-0.7) and tg(Col-2.3) transgenic mice was reduced 5–9% and 8–13%, respectively relative to sex and age-matched wild-type mice after adjusting for body weight (Fig. 4, A and B). These results demonstrate that BMP signaling through BMP IB receptor and its interacting components play an important role in bone formation and maintaining BMD in adult animals.
Figure 4.
Temporal changes in BMD in wild-type and transgenic mice. The BMDs in distal femora and proximal tibiae were monitored using PIXImus bone densitometer in 6-, 8-, 10-, and 12-wk-old wild-type and transgenic mice. Significant reduction in BMD in tg(Col-0.7) (A) and tg(Col-2.3) (B) homozygous transgenic mice was observed compared with the sex- and age-matched wild-type mice. The body weight was adjusted after measurements. *P < 0.05, two-way analysis of variance followed by Dunnett's test.
Osteopenia in truncated BMPR-IB transgenic mice
Differences in bone formation between transgenic and wild-type mice were confirmed by bone histomorphometric analyses. Comparison of trabecular bone volumes in wild-type and transgenic mice revealed a 26% reduction in bone volume in 1-mo-old tg(Col-0.7) mice and 34% reduction in bone volume in 1-mo-old tg(Col-2.3) mice (Fig. 5, A and B). Because trabecular bone volume represents the percentage of bone in a well-defined area of bone marrow cavity, these results suggest that homozygous transgenic mice develop osteopenia postnatally due to disruption in BMP receptor signaling. To investigate whether dynamic changes in bone formation in transgenic mice, we measured bone formation rates (BFRs) and found that these were reduced by 58% in tg(Col-2.3) mice (Fig. 5, C and D).
Figure 5.



Histological and histomorphometric analyses of proximal tibia of 1-mo-old wild-type and transgenic mice. (A and B) von Kossa–stained tibiae were used to measure the trabecular bone volume (BV) in the entire area of the bone marrow cavity 1–3 mm from the growth plates and expressed as a percentage of total tissue volume (TV) (n = 10). The bone volumes in tg(Col-0.7) and tg(Col-2.3) transgenic mice were significantly reduced compared with those of wild-type mice. *P < 0.05, t test. (C and D) The BFRs/BS were measured in the same area and expressed as μm3/μm2/d (n = 10). The BFRs were significantly reduced in tg(Col-2.3) transgenic mice compared with the wild-type mice. *P < 0.05, t test. (E and F) Bone sections were stained with Hematoxylin and Eosin (E) (C: cortical bone; T: trabecular bone) and the numbers of osteoblasts in wild-type and tg(Col-2.3) transgenic mice were counted in hematoxylin and eosin–stained sections (F). Osteoblasts in the same area were also stained for ALP activity (G and H). (I and J) Tibiae were stained with Goldner method and osteoid thickness in the same area was measured. In tg(Col-2.3) transgenic mice, the osteoid thickness was significantly reduced compared with that of wild-type mice (I and J). *P < 0.05, t test.
BMP-2 has been shown to stimulate osteoblast proliferation and differentiation in vitro. It has been reported that the proliferation of the prechondrogenic cells was reduced in BMPR-IB knockout mice (Yi et al., 2000). To analyze histological changes in bone and changes in osteoblast numbers in transgenic mice, we stained bone sections with hematoxylin and eosin. Trabecular bones were reduced in transgenic mice but cortical bones were similar to the wild-type mice (Fig. 5 E). Osteoblast numbers were enumerated in wild-type and tg(Col-2.3) mice and a slight reduction in osteoblast numbers were found in transgenic mice compared with their wild-type littermates (Fig. 5 F). To examine osteoblast function, we stained osteoblasts cytochemically for alkaline phosphatase (ALP) activity. Osteoblasts on the surfaces of trabecular bone, endosteum, and periosteum were stained with ALP in both wild-type and transgenic mice (Fig. 5, G and H)
Because BMP-2 has also recently been shown to stimulate osteoclast differentiation and survival in vitro (Itoh et al., 2001), in the present studies, we also stained sections for tartrate-resistant acid phosphatase activity, which delineates osteoclasts, and compared osteoclast numbers in wild-type and tg(Col-2.3) mice. We found that there was no significant difference in osteoclast numbers between wild-type and transgenic mice (unpublished data).
To examine changes in matrix mineralization in transgenic mice, we also measured osteoid thickness in transgenic mice. Using plastic-embedded sections of proximal tibiae from 1-mo-old wild-type and tg(Col-2.3) mice stained by Goldner method, we found that there was a significant decrease (31%) in osteoid thickness in the transgenic mice compared with wild-type littermates (Fig. 5, I and J).
Impairment of periosteal bone formation in truncated BMPR-IB transgenic mice
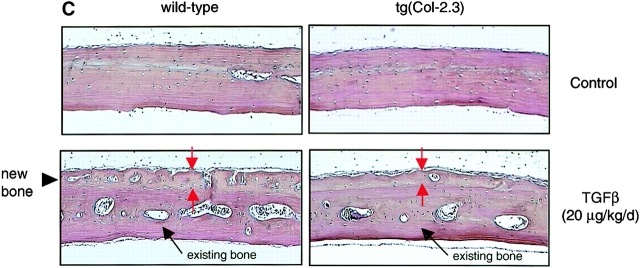
We analyzed periosteal bone formation of calvariae in 1-mo-old transgenic mice. BMP-2 was injected subcutaneously (20 μg/kg/d; daily for 5 d) over calvariae (n = 5). Administration of BMP-2 induced new bone formation over calvariae of wild-type mice but not those of homozygous tg(Col-2.3) mice (Fig. 6 A). In heterozygous mice, BMP-2 induced new bone formation on calvariae but the effects were 30–40% less in comparison to their wild-type littermates (unpublished data). These results strongly imply that responses of the mice are related to the levels of the transgene. To further confirm the de novo bone formation over the surface of calvariae, we labeled calvarial bones by injecting Calcein and Alizarin red after BMP-2 treatment. A significant new bone formation indicated by Calcein and Alizarin red labeling was observed in wild-type but not in homozygous tg(Col-2.3) mice (Fig. 6 B).
Figure 6.
Impairment of periosteal bone formation in truncated BMPR-IB transgenic mice. (A and B) The responsiveness of tg(Col-2.3) homozygous transgenic mice to BMP-2. BMP-2 (20 μg/kg/d, daily for 5 d) in 20 μl PBS containing 0.1% BSA were injected into mice subcutaneously, adjacent to the calvarial bones. Significant amounts of new woven bone, indicated by red arrows and a black arrow head, were formed on the periosteal surface of calvariae in wild-type mice receiving BMP-2, but not in homozygous transgenic mice receiving BMP-2. The existing bones were indicated by a black arrow (A). Similarly, BMP-2 (20 μg/kg/d, ×5d) was administered subcutaneously and Calcein and Alizarin red were injected 7 and 2 d before the animals were killed. The new bone formation during the two fluorochrome injection (5-d period) was observed in wild-type mice (indicated by white arrows and a black arrowhead) but not in tg(Col-2.3) mice (B). (C) Effects of TGFβ on periosteal bone formation. TGFβ (20 μg/kg/d, daily for 5 d) was injected subcutaneously over calvariae. Significant amounts of new woven bone, indicated by red arrows and a black arrowhead, were formed on the periosteal surface of calvariae in wild-type and homozygous transgenic mice. (D and E) In vitro bone formation assay. Calvariae were isolated from 4-d-old wild-type and tg(Col-2.3) mice and cultured for 7 d in BGJ medium. At the end of the incubation, calvariae were fixed and processed for histomorphometric analyses. Osteoblasts on the BS were indicated by red arrows and existing bones were indicated by yellow arrows (D). The thickness of calvariae were measured and presented in (E).
To further examine the specificity of the blockage of BMP receptor signaling in transgenic mice, TGFβ (20 μg/kg/d; daily for 5 d), a known inducer of bone formation in this assay (Marcelli et al., 1990), was injected subcutaneously over calvariae of 1-mo-old wild-type and transgenic mice. TGFβ induced extensive new bone formation in both wild-type and tg(Col-2.3) homozygous transgenic mice (Fig. 6 C). These results indicate that BMP signaling is specifically blocked in truncated BMPR-IB–expressing transgenic mice.
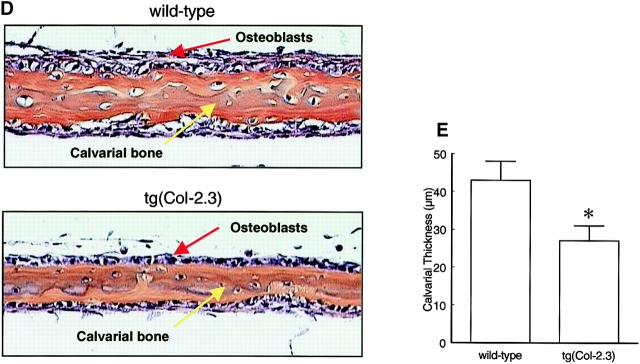
Although BMP-2 failed to induce new bone formation in homozygous transgenic mice in vivo, it was still unclear whether normal bone formation was impaired in these transgenic mice since de novo bone formation is not observed on calvariae of both wild-type and homozygous transgenic mice without exogenous BMP-2 treatment. To assess the role of endogenous BMP signaling in normal bone formation, calvariae were isolated from wild-type and tg(Col-2.3) mice and cultured for 7 d (Traianedes et al., 1998), after which they were fixed and processed for histomorphometric analyses. Thickness of calvariae from transgenic mice was found to be 37% less than those of wild-type mice (Fig. 6, D and E). Because newly formed bones can be identified on calvariae of transgenic mice, these results suggest that BMP signaling is a necessary growth signal for normal bone formation but may not be obligatory. Other bone growth regulatory factors may compensate the loss of BMP signaling or complete blockage of BMP receptor signaling may not have been achieved in these viable mouse lines.
Blockage of BMP signaling in truncated BMPR-IB transgenic mice
To examine BMP signaling in osteoblasts, primary osteoblasts were isolated from calvariae of 3-d-old wild-type and transgenic mice by trypsin/collagenase sequential digestion, transfected with Flag-Smad1 cDNA construct, and the nuclear translocation of Smad1 was determined by the immunofluorescence staining. Smad1 nuclear translocation was clearly inhibited in cells from transgenic mice (Fig. 7 A). These results were also supported by a reporter assay using a 9 × SBE-OC-pGL3 reporter gene, containing nine copies of a Smad1 response element (Kusanagi et al., 2000) linked to the osteocalcin basal promoter. Addition of BMP-2 stimulated luciferase activity of this reporter gene in a dose-dependent manner in osteoblasts from wild-type mice. In contrast, low concentration of BMP-2 (25 and 50 ng/ml) failed to activate the reporter gene in cells isolated from tg(Col-2.3) mice, although high concentrations of BMP-2 (100 and 200 ng/ml) retained some activity in this regard (Fig. 7 B). These results confirm disruption of BMP signaling in osteoblasts of transgenic mice.
Figure 7.
Blockage of BMP signaling in truncated BMPR-IB transgenic mice. (A) Flag-Smad1 expression plasmid was transfected in primary osteoblasts isolated from calvariae of wild-type and tg(Col-2.3) mice in the absence or presence of BMP-2 (50 ng/ml). The experiment of nuclear localization of Smad1 was performed by immunofluorescence staining. Nuclear translocation of Flag-Smad1 was observed in osteoblasts from wild-type mice after BMP-2 treatment but not in osteoblasts from transgenic mice with BMP-2 treatment (50 ng/ml). The DAPI nuclear staining was performed as control in this experiment. (B) 9 × SBE-OC-pGL3 reporter and β-gal control plasmids were cotransfected in primary osteoblasts isolated from calvariae of wild-type and transgenic mice. The luciferase activity was measured by luciferase assay kit using Luminometer. BMP-2 induced luciferase activity in a dose-dependent manner in osteoblasts from wild-type mice. In transgenic mice, BMP-2–induced luciferase activity was significantly inhibited. *P < 0.05, two-way analysis of variance followed by Dunnett's test.
Impairment of osteoblast differentiation in truncated BMPR-IB transgenic mice
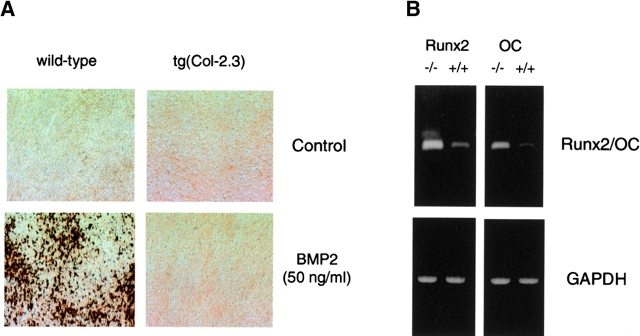
To further analyze whether osteoblast differentiation capacity is impaired in the transgenic mice, primary osteoblasts isolated from calvariae of wild-type and tg(Col-2.3) homozygous transgenic mice were cultured in an osteoblast differentiation medium containing glycerophosphate and ascorbic acid. As we had previously reported, in these prolonged cultures, bone matrix deposition and mineralization was induced by BMP-2 after 10-d incubation in cultures of osteoblasts isolated from wild-type mice (Chen et al., 1998). In contrast, there was no obvious mineralized bone matrix formation in cultures of osteoblasts isolated from homozygous transgenic mice under the same conditions even in the presence of BMP-2 (50 ng/ml) (Fig. 8 A).
Figure 8.
Impairment of osteoblast differentiation in truncated BMPR-IB transgenic mice. (A) von Kossa staining of cultures of primary osteoblasts isolated from calvariae of wild-type and tg(Col-2.3) homozygous mice. The cells were cultured for 10 d in the presence and absence of 50 ng/ml BMP-2. After incubation, the cells were fixed with 10% formalin and stained by von Kossa method. (B) mRNA expression of Runx2/Cbfa1 and osteocalcin. mRNA was extracted from long bones of 1-mo-old wild-type and tg(Col-2.3) mice. Significant reduction of mRNA expression of Runx2/Cbfa1 and OC in homozygous mice was observed by RT-PCR in comparison to their wild-type littermates.
Expression of osteoblast marker genes were also analyzed in tg(Col-2.3) mice. Runx2/Cbfa1 and osteocalcin were detected in the long bones of 1-mo-old wild-type mice by RT-PCR. In homozygous transgenic mice, expression of Runx2/Cbfa1 and osteocalcin were significantly reduced compared with that in wild-type mice (Fig. 8 B), suggesting that inhibition of bone formation in transgenic mice in vivo is partly due to impairment of osteoblast differentiation.
Discussion
BMPs were originally identified from bone matrix using an ectopic bone formation assay (Urist, 1965; Wozney et al., 1988). Careful examination of the ectopic bone formation model indicates that it closely mimics the normal processes of endochondral bone formation (Wozney, 1992). The function of BMPs including BMP-2, BMP-6, and BMP-7 in ectopic bone formation and fracture repair (Wozney et al., 1988; Sampath et al., 1992; Gitelman et al., 1994) and the roles of BMPs and BMP receptors in embryonic development (Dudley et al., 1995; Luo et al., 1995; Mishina et al., 1995; Winnier et al., 1995; Zhang and Bradley, 1996) have been well characterized, but the physiological roles of BMPs and BMP receptor signaling in normal bone formation in postnatal life are still poorly understood.
Previously, we demonstrated that type IB BMP receptors play an essential role in osteoblast commitment and differentiation (Chen et al., 1998). In the present studies, we established a transgenic mouse model and analyzed the function of BMP receptors in normal bone formation in vivo. We found that BMP receptor signaling is necessary for normal bone growth and bone formation in postnatal and adult mice. The osteoblast differentiation capacity are clearly impaired in these transgenic mice but a role for BMP receptor signaling in osteoblast proliferation and apoptosis cannot be ruled out from the present studies.
BMPs may stimulate osteoblast differentiation in part by inducing expression and activation of a transcription factor, Runx2/Cbfa1. Targeted disruption of Runx2/Cbfa1 reveals that Runx2/Cbfa1 is an osteoblast-specific transcription factor. Homozygous Runx2/Cbfa1-deficient mice die soon after delivery due to inability to breathe, with the most pronounced effect in these homozygous knockout mice being a complete lack of both endochondral and intramembranous ossification (Komori et al., 1997; Otto et al., 1997). In transgenic mice overexpressing mutant Runx2/Cbfa1 (DNA binding domain) driven by the osteocalcin promoter, Runx2/Cbfa1 function in osteoblasts is blocked. The rates of bone formation are reduced 70%, and expression of osteoblast marker genes is inhibited in the postnatal animals (Ducy et al., 1999). This reduction in bone formation is similar to what we observed in homozygous truncated BMPR-IB transgenic animals. BMP-7 and BMP-2 induce Runx2/Cbfa1 expression in vitro (Ducy et al., 1997; Chen et al., 1998) and inhibition of BMP signaling reduces Runx2/Cbfa1 expression in vitro and in vivo (Chen et al., 1998; this study).
Null mutations of BMP receptors have been created to understand the roles of BMP receptor signaling in embryonic development and postnatal life. Null mutation of the BMPR-IA gene is embryonic-lethal. Animals die at embryonic day 9.5 dpc, with defects in mesoderm formation, suggesting that BMPR-IA is essential for the mesoderm formation during gastrulation (Mishina et al., 1995). In contrast, mice lacking BMPR-IB are viable but exhibit defects in the interphalangeal joints (Yi et al., 2000). In these BMPR-IB–deficient mice, proliferation of prechondrogenic cells and chondrocyte differentiation in the phalangeal region are markedly reduced. In adult mutant mice, the proximal interphalangeal joint is absent, and the phalanges are replaced by a single rudimentary element, whereas the distal phalanges are unaffected. The lengths of the radius, ulna and tibia are normal, but the metacarpals/metatarsals are reduced. The appendicular defects in BMPR-IB–null mutant mice resemble those seen in mice homozygous for the GDF5bp-j null allele of the GDF5 locus (Storm et al., 1994). Because GDF5 has been shown to play a critical role in cartilage formation (Chang et al., 1994; Thomas et al., 1996, 1997; Storm and Kingsley, 1999) and bind to BMPR-IB with high affinity (Nishitoh et al., 1996), these results suggest that BMPR-IB plays a nonredundant role in cartilage formation in vivo.
BMP ligands may utilize multiple type I receptors to mediate signaling during osteoblast differentiation and bone formation. This hypothesis is supported by the observations from BMPR-IB and BMP-7 double mutant mice, in which severe appendicular skeletal defects were observed in forelimbs and hindlimbs with almost completely absent ulnae and shortened radii (Yi et al., 2000). Because BMP-7 binds efficiently to both BMPR-IB and ActR-IA, and because ActR-IA also mediates specifically BMP signaling (Macias-Silva et al., 1998), it is conceivable that BMPR-IB and ActR-IA play important synergistic or overlapping roles in bone formation in vivo.
As mentioned earlier, BMPR-IB–null mutant mice show no dramatic skeletal changes (Yi et al., 2000), although the absence of BMPR-IB and BMP-7 ligands leads to synergistic defects in cartilage and bone during development. It is possible that in the null mutant mice, BMPR-IB is required during early development and the surviving animals compensate through one of the other type I BMP receptors. In the present studies, we targeted the mutant dominant-negative BMPR-IB to the osteoblast lineage using the type I collagen promoter. In this way, we may have been avoided this possible complication. The dominant negative approach, we and others have taken to explore the role of BMP receptors, may alter several of the BMP pathways, and thus is more complex than simple knockout of the BMPR-IB gene.
The dominant-negative approach of overexpression of inactive mutant receptors to block receptor signaling is well established in studies of BMP signaling during embryonic development and specific functions of BMP signaling in vitro (Maeno et al., 1994; Suzuki et al., 1994; Kawakami et al., 1996; Zou and Niswander, 1996; Glinka et al., 1997; Thomas et al., 1997; Zou et al., 1997; Chen et al., 1998; Frisch and Wright, 1998; Volk et al., 2000). It has been shown that the mutant BMP type I receptors bind type II BMP or activin receptors but fail to mediate signaling due to a lack of or mutation in the kinase domain of the receptors. The mutant type I receptors partially or completely block the interacting type II receptor signaling and the degree of inhibition depends on the expression levels of the mutant type I receptors similar to what we have observed in our transgenic model. Because BMPR-IB interacts with BMPR-II, ActR-II and ActR-IIB (Liu et al., 1995; Yamashita et al., 1995; Onishi et al., 1998), which also interacts with other type I BMP receptors, it is likely that signaling via other type I BMP receptors is also affected in our transgenic mice. Nevertheless, our studies clearly show a role for BMP receptor signaling in bone formation in postnatal animals. TGF-β induces new bone formation over calvariae in both wild-type and truncated BMPR-IB transgenic mice, strongly suggesting that the blockage of receptor signaling by truncated BMPR-IB is restricted to BMP signaling pathway.
An issue in using the type I collagen promoter to drive the truncated BMPR-IB transgene is that overexpression of the truncated BMPR-IB reduces Runx2/Cbfa1 expression, which in turn may inactivate the type I collagen promoter. This is likely not the case in our studies because: (a) Runx2/Cbfa1 response elements are not located in the type I collagen enhancer/basal promoter and similar phenotypes were observed in transgenic mice irrespective of whether the type I collagen enhancer/basal promoter or the 2.3-kb type I collagen promoter was used to generate the mice; (b) There was no decrease in the expression of the truncated BMPR-IB transgene in transgenic mice as the animals aged; and (c) A persistent decrease in BMD in the transgenic mice at different ages was observed up to 12 wk of age. These observations suggest that transcription of the type I collagen gene is only partly dependent on Runx2/Cbfa1 activation. In fact, the osteoblast-specific enhancer region of the type I collagen promoter has been identified in vitro and in vivo by Rossert et al. (1996), and no Runx2/Cbfa1 response element was found in this region (Kern et al., 2001).
In summary, our studies provide in vivo evidence that BMP receptor signaling is necessary for normal murine postnatal bone growth and bone formation.
Materials and methods
Construction of type I collagen promoter–truncated BMPR-IB transgenes
The type I collagen enhancer/basal promoter (Col-0.7) and 2.3-kb type I collagen promoter (Col-2.3) were used to drive truncated BMPR-IB expression in transgenic mice. The type I collagen enhancer/basal promoter contains four copies of the osteoblast-specific enhancer and a basal promoter of the type I collagen gene. To construct Col1a1(0.7)-trBMPR-IB (tg[Col-0.7]) transgene, the CMV promoter in pcDNA3 vector was removed by MfeI/HindIII digestion and replaced by a polylinker containing multiple cloning sites. The type I collagen minimal promoter and the upstream matrix attachment region was released from pJA-ClacZM vector, obtained from Dr. Benoit de Crombrugghe (Anderson Cancer Center, Houston, TX) and then cloned into the ClaI/KpnI sites of the polylinker of the modified pcDNA3 vector. Truncated BMPR-IB cDNA (kinase domain deletion) (Chen et al., 1998) was then cloned into the multiple cloning sites of the modified pcDNA3 vector. To monitor expression of the mutant BMPR-IB, the HA-epitope was tagged at the C-terminal end of the mutant BMPR-IB. The transgene was released by AscI/NaeI digestion. To generate the Col1a1(2.3)-trBMPR-IB (tg[Col-2.3]) transgene, the lacZ reporter gene was removed from the pJ251 vector, obtained from Dr. Benoit de Crombrugghe, which contains the 2.3-kb mouse type I collagen promoter, by BamHI digestion. The truncated BMPR-IB cDNA was cloned into the BamHI site of the pJ251 vector. The transgene was released by KpnI/NarI digestion.
Generation of truncated BMPR-IB transgenic mice
The tg(Col-0.7) and the tg(Col-2.3) transgenes were excised from vector sequences and purified by agarose gel electrophoresis and binding to Genclean glass beads (BIO101, Inc.). Approximately 1 to 2 μl of a solution of DNA at a concentration of 2 μg/ml was microinjected into the pronuclei of fertilized one-cell mouse embryos (Hogan et al., 1994). The one-cell embryos were derived from mating of CB6F1 (C57BL/6 × BALB/c) males and females obtained from Harlan Sprague Dawley, Inc. The injected embryos were reimplanted into B6D2F1 (C57BL/6 × DBA/2) pseudopregnant females. The presence of the transgene in the resulting pups was determined by preparing genomic DNA from a small piece of tail tissue and assaying by Southern blot analysis (Meinkoth and Wahl, 1984). Homozygous transgenic mice were derived from heterozygous transgenic mice and confirmed by mating to CB6F1 nontransgenic mice.
RNA analysis
Total RNA was isolated from different organs of 1-mo-old homozygous transgenic mice and from bone tissues (calvariae and long bones) of 1-mo-old wild-type and transgenic mice using RNAzol B solution (Biotex Lab). RNA expression was analyzed by RT-PCR as described (Chen et al., 1998). DNAse I-treated total RNA was reverse transcribed using oligo(dT). cDNA were amplified using the primers specific for truncated BMPR-IB, osteocalcin and Runx2/Cbfa1.
Western blot analysis
To determine protein expression of the truncated BMPR-IB transgene, the cell lysates were extracted from calvariae of wild-type and transgenic mice, mixed with sample buffer, and run on SDS-PAGE gels (Mini-PROTEIN II Ready gels; Bio-Rad Laboratories). The proteins were transblotted onto a PVDF membrane (Bio-Rad Laboratories) in transblotting buffer (20 mM Tris, 150 mM glycine, 20% methanol, pH 8.0) at 4°C for 1 h. The membrane was blocked with 5% BSA (Sigma-Aldrich) in TBS for 2 h at room temperature, and incubated with anti-HA polyclonal antibody (200 mg/ml, 1:200 dilution) in TBS for 2 h at room temperature. Incubation with horse-radish peroxidase conjugated protein A (Kirkegaard & Perry Lab) was performed at room temperature for 2 h. The membrane was then washed five times with TBS containing 0.1% Triton X-100 for 5 min and twice with TBS. Immunostaining was detected using enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech).
Alizarin red and Alcian blue staining of embryonic skeleton
18.5-dpc embryos from transgenic mice and wild-type littermates were dissected to remove the skin, muscle, and fat and then preserved in acetone for 1 wk to further remove fat, with daily change of the acetone. Embryos were stained with Alizarin red (0.09%) and Alcian blue (0.05%) in a solution containing ethanol, glacial acetic acid, and water (67:5:28) for 48 h. After staining, embryos were transferred to 1% potassium hydroxide for 3 d to dissolve the soft tissue and then rinsed with water. Embryos were preserved in a solution containing glycerin: 1% potassium hydroxide (8:2) to remove excess stains (Kaufman, 1992).
X-Ray analyses of bone structure and BMD measurements
To evaluate changes in bone structure in the transgenic mice, animals were x-rayed using a Faxitron radiographic inspection unit (Model 8050-020; Field Emission Corporation, Inc.).
A PIXImus bone densitometer (Lunar Corporation), which utilizes Dual Energy x-ray Absorptiometry (DEXA) technology, was also used to monitor changes in BMD in the transgenic mice. The advantage of this type of analysis is that it is non-invasive and therefore the temporal changes in BMD were monitored in transgenic and wild-type mice. BMD was monitored in 3 litters of animals from three different lines of transgenic mice at 6, 8, 10, and 12 wk of age.
Histological and histomorphometric analyses
Histomorphometric analyses were performed on tibial metaphysis in 1-mo-old wild-type and transgenic mice. Half of bone samples were decalcified in 14% EDTA and embedded in paraffin and the sections of bone samples were stained with H&E and tartrate-resistant acid phosphatase. Histological changes in trabecular and cortical bones and growth plates were analyzed and changes in osteoblast and osteoclast numbers were quantitated.
The other half of bone samples were embedded in methylmethacrylate. The sections of bone samples were used for the measurements of BFR or stained with von Kossa and Goldner methods to determine the bone volume and osteoid thickness. The trabecular bone volume was quantitated in the entire area of the bone marrow cavity 1–3 mm from the growth plates and expressed as a percentage of tissue volume. Values were expressed as the mean ± standard error calculated from at least three nonconsecutive sections per mouse from 10 transgenic mice and 10 age-matched wild-type mice.
To analyze the changes in BFRs, double fluorescence labeling was performed and analyzed in tibial metaphysis. Tetracycline (15 mg/kg i.p.; Sigma-Aldrich) was administered to 1-mo-old wild-type and transgenic mice, and this was followed by injection of a calcein label (20 mg/kg i.p.; Sigma-Aldrich) 4 d later, mice were sacrificed 48 h after the second label was injected, and bone tissues were removed and fixed in 70% ethanol for 48 h. The specimens were dehydrated through a graded series of ethanol (70–100%) and embedded in methylmethacrylate without prior decalcification. 7-μm sections were cut using a Leica 2165 rotary microtome, and the unstained sections were viewed using fluorescence microscopy, and the following dynamic indices of bone formation were measured (Uy et al., 1997): (a) Labeled bone surface (BS) or mineralizing surface; (b) Mineral appositional rate (μm/day) = mean distance between two fluorescent labels divided by the number of days between labels; and (c) BFR/BS, μm3/μm2/day = mineralizing surface × mineral appositional rate/BS.
In vivo periosteal bone formation assay
Recombinant BMP-2 and TGFβ were injected subcutaneously over the parietal bone of the calvariae of transgenic mice and wild-type littermates that were 1-mo-old (Chen et al., 1997). Mice received either vehicle (20 μl PBS containing 0.1% BSA) alone or BMP-2 or TGFβ (20 μg/kg/d, daily for 5 d). Mice were sacrificed 14 d after commencing injections and calvarial bones were removed. The bones were decalcified in 14% EDTA and bissected coronally midway between the coronal and lambdoid sutures. The samples were dehydrated through graded alcohols and embedded in paraffin. Transverse sections were cut at 3-μm thickness and stained with an hematoxylin and eosin stain. Newly formed bone were identified by its woven structure in contrast to preexisting bone with lamellar structure. The hematoxylin and eosin–stained slides were viewed by the Nikon E400 microscope that was linked to a color video monitor (Sony model PVM-14M2MDU Trinitron Color Video Monitor; Sony Corp.) and the images were captured.
Immunofluorescence staining
Primary osteoblasts were isolated from the calvariae of 3-d-old wild-type and transgenic mice and transfected with Flag-Smad1. Cells were serum starved with αMEM containing 0.2% FCS for 16 h, and treated with BMP-2 (50 ng/ml) for 15 min. Cells were washed three times with PBS and fixed with 3.8% paraformaldehyde-PBS. After 15 min of incubation with 0.1% Triton-PBS, cells were blocked with 1% BSA-PBS, incubated with anti-Flag antibody (1:500) for 2 h, and washed six times with 0.1% Triton-PBS, followed by incubation with fluorescein isothiocyanate-conjugated anti–mouse IgG antibody (Jackson Immunoresearch Laboratories, Inc.). Cells were extensively washed with PBS and visualized by fluorescence microscope.
DNA transfection and luciferase assay
Oligonucleotides containing nine copies of GCCG Smad1 binding element (9 × SBE) was synthesized and cloned in front of osteocalcin basal promoter (−155/+1) in pGL3 basic vector (9 × SBE-OC-pGL3). The 9 × SBE-OC-pGL3 reporter construct and β-gal control plasmid was cotransfected in osteoblasts isolated from the calvariae of 3-d-old wild-type and transgenic mice using lipofactamine plus reagents (GIBCO BRL). After transfection, the medium was replaced with fresh medium containing 1% FCS in the presence and absence of BMP-2 (25–200 ng/ml) (R&D Systems). After a 48-h incubation, the cells were washed with PBS and lysed with lysis buffer and luciferase activity was measured with luciferase assay reagents (Promega) using a Luminometer. The luciferase activity was then normalized by the β-gal activity.
Mineralized bone matrix formation assay
Primary osteoblasts were isolated from the calvariae of transgenic and wild-type mice and osteoblast differentiation was monitored using a mineralized matrix formation assay as described by Bharagava et al. (1986). The cells were plated in 24-well culture plates at density of 2 × 104 cells/well and cultured with α minimal essential medium (αMEM) supplemented with 10% FCS. When the cells reached confluency (day 0), the medium was changed to αMEM containing 5% FCS, 100 μg/ml ascorbic acid and 5 mM β-glycerol phosphate with or without 50 ng/ml of BMP-2. The cells were incubated for 10 d. The medium was changed every other day and fresh reagents were added. von Kossa stain of mineralized bone matrix was performed as follows. The cell cultures were washed with PBS twice, fixed in phosphate buffered formalin for 10 min, and then washed with water, and serially dehydrated in 70%, 95%, and 100% ethanol, twice each and air dried. The plates were rehydrated from 100% to 95% to 80% ethanol to water before staining. The water was removed, a 2% silver nitrate solution was added, and the plates were exposed to sunlight for 20 min, after which the plates were rinsed with water. 5% sodium thiosulfate was added for 3 min and the plates were then rinsed with water. The modified van Gieson stain was then used as a counterstain after the von Kossa stain. The unmineralized collagen matrix can be recognized by the yellow/red van Gieson stain. The acid fuchsin solution (5 part of 1% acid fuchsin, 95 part of picric acid, and 0.25 part of 12 M HCl) was added for 5 min. The plates were washed with water, and then 2 × 95% ethanol and 2 × 100% ethanol, and dried for image analysis.
Acknowledgments
We would like to thank Dr. Benoit de Crombrugghe for providing us pJA-ClacZM and pJ251 vectors. We would also like to thank Nancy Garrett for her help in preparing this manuscript.
This work was supported in part by a grant from National Osteoporosis Foundation to D. Chen, and grant R01-AR44728 from the National Institutes of Health to S.E. Harris.
S.E. Harris's present address is Department of Oral Biology, School of Dentistry, University of Missouri-Kansas City, Kansas City, MO 64108.
Footnotes
Abbreviations used in this paper: ALP, alkaline phosphatase; BFR, bone formation rate; BMD, bone mineral density; BMP, bone morphogenetic protein; BS, bone surface; dpc, day(s) postcoitum.
References
- Bharagava, U., M. Bar-lev, C.G. Bellows, and J.E. Aubin. 1986. Ultrastructural analysis of bone nodules formed in vitro by isolated fetal rat calvaria cells. Bone. 9:155–163. [DOI] [PubMed] [Google Scholar]
- Chang, S.C., B. Hoang, J.T. Thomas, S. Vukicevic, F.P. Luyten, N.J.P. Ryba, C.A. Kozak, A.H. Reddi, and M. Moos, Jr. 1994. Cartilage-derived morphogenetic proteins. J. Biol. Chem. 269:28227–28234. [PubMed] [Google Scholar]
- Chen, D., M.A. Harris, G. Rossini, C.R. Dunstan, S.L. Dallas, J.Q. Feng, G.R. Mundy, and S.E. Harris. 1997. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4 and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif. Tissue Int. 60:283–290. [DOI] [PubMed] [Google Scholar]
- Chen, D., X. Ji, M.A. Harris, J.Q. Feng, G. Karsenty, A.J. Celeste, V. Rosen, G.R. Mundy, and S.E. Harris. 1998. Differential roles for BMP receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J. Cell Biol. 142:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewulf, N., K. Verschueren, O. Lonnoy, A. Moren, S. Grimsby, K.V. Spiegle, K. Miyazono, and D. Huylebroeck. 1995. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 136:2652–2663. [DOI] [PubMed] [Google Scholar]
- Ducy, P., R. Zhang, V. Geoffroy, A.L. Ridall, and G. Karsenty. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 89:747–754. [DOI] [PubMed] [Google Scholar]
- Ducy, P., M. Starbuck, M. Priemel, J. Shen, G. Pinero, V. Geoffroy, M. Amling, and G. Karsenty. 1999. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 13:1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, A.T., K.M. Lyons, and E.J. Roberson. 1995. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 9:2795–2807. [DOI] [PubMed] [Google Scholar]
- Frisch, A., and C.V. Wright. 1998. XBMPRII, a novel Xenopus type II receptor mediating BMP signaling in embryonic tissues. Development. 125:431–442. [DOI] [PubMed] [Google Scholar]
- Gitelman, S.E., M.S. Kobrin, J.Q. Ye, A.R. Lopez, A. Lee, and R. Derynck. 1994. Recombinant Vgr-1/BMP-6–expressing tumors induce fibrosis and endochondral bone formation in vitro. J. Cell Biol. 126:1595–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka, A., W. Wu, D. Onichtchouk, C. Blumenstock, and C. Niehrs. 1997. Head induction by simultaneous repression of Bmp and Wnt signaling in Xenopus. Nature. 389:517–519. [DOI] [PubMed] [Google Scholar]
- Hogan, B., R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the Mouse Embryos: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY. 226–230.
- Hoodless, P.A., T. Haerry, S. Abdollah, M. Stapleton, M.B. O'Connor, L. Attisano, and J.L. Wrana. 1996. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 85:489–500. [DOI] [PubMed] [Google Scholar]
- Ishidou, Y., I. Kitajima, H. Obama, I. Maruyama, F. Murata, T. Imamura, N. Yamada, P. ten Dijke, K. Miyazono, and T. Sakou. 1995. Enhanced expression of type I receptors for bone morphogenetic proteins during bone formation. J. Bone Miner. Res. 10:1651–1659. [DOI] [PubMed] [Google Scholar]
- Itoh, K., N. Udagawa, T. Katagiri, S. Iemura, N. Ueno, H. Yasuda, K. Higashio, J.M.W. Quinn M.T. Gillespie, J. Martin, T. Suda, and N. Takahashi. 2001. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-κ B ligand. Endocrinology. 142:3656–3662. [DOI] [PubMed] [Google Scholar]
- Kaufman, M.H. 1992. The atlas of mouse development. Academic Press, London. 495–507.
- Kawakami, Y., T. Ishikawa, M. Shimabara, N. Tanda, M. Enomoto-Iwamoto, M. Iwamoto, T. Kuwana, A. Ueki, S. Noji, and T. Nohno. 1996. BMP signaling during bone pattern determination in the developing limb. Development. 122:3557–3566. [DOI] [PubMed] [Google Scholar]
- Kern, B., J. Shen, M. Starbuck, and G. Karsenty. 2001. Cbfa1 contributes to the osteoblast-specific expression of type I collagen genes. J. Biol. Chem. 276:7101–7107. [DOI] [PubMed] [Google Scholar]
- Koenig, B.B., J.S. Cook, D.H. Wolsing, J. Ting, J.P. Tiesman, P.E. Correa, C.A. Olson, A.L. Pecquet, F. Ventura, R.A. Grant, et al. 1994. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol. Cell. Biol. 14:5961–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori, T., H. Yagi, S. Nomura, A. Yamaguchi, K. Sasaki, K. Deguchi, Y. Shimizu, R.T. Bronson, Y. Gao, M. Inada, et al. 1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 89:755–764. [DOI] [PubMed] [Google Scholar]
- Kusanagi, K., H. Inoue, Y. Ishidou, H.K. Mishima, M. Kawabata, and K. Miyazono. 2000. Characterization of a bone morphogenetic protein-responsive Smad-binding element. Mol. Biol. Cell. 11:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F., F. Ventura, J. Doody, and J. Massague. 1995. Human type II receptor for bone morphogenetic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol. Cell Biol. 15:3479–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, G., C. Hofmann, A.L.J.J. Bronchers, M. Sohocki, A. Bradley, and G. Karsenty. 1995. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Gene Dev. 9:2808–2820. [DOI] [PubMed] [Google Scholar]
- Macias-Silva, M., P.A. Hoodless, S.J. Tang, M. Buchwald, and J.L. Wrana. 1998. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 273:25628–25636. [DOI] [PubMed] [Google Scholar]
- Maeno, M., R.C. Ong, A. Suzuki, N. Ueno, and H. Kung. 1994. A truncated bone morphogenetic protein 4 receptor alters the fate of ventral mesoderm to dorsal mesoderm: roles of animal pole tissue in the development of ventral mesoderm. Proc. Natl. Acad. Sci. USA. 91:10260–10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli, C., A.J.P. Yates, and G.R. Mundy. 1990. In vivo effects of human recombinant transforming growth factor beta on bone turnover in normal mice. J. Bone Miner. Res. 5:1087–1096. [DOI] [PubMed] [Google Scholar]
- Meinkoth, J., and G. Wahl. 1984. Hybridization of nucleic acids immobilized on solid supports. Anal. Biochem. 138:267–284. [DOI] [PubMed] [Google Scholar]
- Mishina, Y., A. Suzuki, N. Ueno, and R.B. Behringer. 1995. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Gene Dev. 9: 3027–3037. [DOI] [PubMed] [Google Scholar]
- Nishitoh, H., H. Ichijo, M. Kimura, T. Matsumoto, F. Makishima, A. Yamaguchi, H. Yamashita, S. Enomoto, and K. Miyazono. 1996. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J. Biol. Chem. 271:21345–21352. [DOI] [PubMed] [Google Scholar]
- Onishi, T., Y. Ishidou, T. Nagamine, K. Yone, T. Imamura, M. Kato, T.K. Sampath, P. ten Dijke, and T. Sakou. 1998. Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone. 22:605–612. [DOI] [PubMed] [Google Scholar]
- Otto, F., A.P. Thornell, T. Crompton, A. Denze, K.C. Gilmour, I.R. Rosewell, G.W.H. Stamp, R.S.P. Beddington, S. Mundlos, B.R. Olsen, et al. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 89:765–771. [DOI] [PubMed] [Google Scholar]
- Rossert, J.A., H. Eberspaecher, and B. de Crombrugghe. 1995. Separate cis-acting DNA elements of the mouse pro-1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J. Cell Biol. 129:1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossert, J.A., S.S. Chen, H. Eberspaecher, C.N. Smith, and B. de Crombrugghe. 1996. Identification of a minimal sequence of the mouse pro-1(I) collagen promoter that confers high-level osteoblast expression in transgenic mice and that binds a protein selectively present in osteoblasts. Proc. Natl. Acad. Sci. USA. 93:1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath, T.K., J.C. Maliakal, P.V. Hauschka, W.K. Jones, H. Sasak, R.F. Tucker, K.H. White, J.E. Coughlin, M.M. Tucker, and R.H. Pang. 1992. Recombinant human osteogenic protein-1 (hOP-1) induces bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J. Biol. Chem. 267:20352–20362. [PubMed] [Google Scholar]
- Storm, E.E., and D.M. Kingsley. 1999. GDF5 coordinates bone and joint formation during digit development. Dev. Biol. 209:11–27. [DOI] [PubMed] [Google Scholar]
- Storm, E.E., T.V. Huynh, N.G. Copeland, N.A. Jenkins, D.M. Kingsley, and S.J. Lee. 1994. Limb alterations in brachypodism mice due to mutations in a new member of the TGF-superfamily. Nature. 368:639–643. [DOI] [PubMed] [Google Scholar]
- Suzuki, A., R.S. Thies, N. Yamaji, J.J. Song, J.M. Wozney, K. Murakami, and N. Ueno. 1994. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc. Natl. Acad. Sci. USA. 91:10255–10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke, P., H. Yamashita, T.K. Sampath, A.H. Reddi, M. Estevez, D.L. Riddle, H. Ichijo, C.H. Heldin, and K. Miyazono. 1994. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 269:16985–16988. [PubMed] [Google Scholar]
- Thomas, J.T., K. Lin, M. Nandedkar, M. Camargo, J. Cervenka, and F.P. Luyten. 1996. A human chondrodysplasia due to a mutation in a TGF superfamily member. Nat. Genet. 12:315–317. [DOI] [PubMed] [Google Scholar]
- Thomas, J.T., M.W. Kilpatrick, K. Lin, L. Erlacher, P. Lembessis, T. Costa, P. Tsipouras, and F.P. Luyten. 1997. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat. Genet. 17:58–64. [DOI] [PubMed] [Google Scholar]
- Traianedes, K., M.R. Dallas, I.R. Garrett, G.R. Mundy, and L.F. Bonewald. 1998. 5-Lipoxygenase metabolites inhibit bone formation in vitro. Endocrinology. 139: 3178–3184. [DOI] [PubMed] [Google Scholar]
- Urist, M.R. 1965. Bone formation by autoinduction. Science. 150:893–899. [DOI] [PubMed] [Google Scholar]
- Uy, H.L., G.R. Mundy, B.F. Boyce, B.M. Story, C.R. Dunstan, J.J. Yin, G.D. Roodman, and T.A. Guise. 1997. Tumor necrosis factor enhances parathyroid hormone-related protein-induced hypercalcemia and bone resorption without inhibiting bone formation in vivo. Cancer Res. 57:3194–3199. [PubMed] [Google Scholar]
- Volk, S.W., M. D'Angelo, D. Diefenderfer, and P.S. Leboy. 2000. Utilization of bone morphogenetic protein receptors during chondrocyte maturation. J. Bone Miner. Res. 15:1630–1639. [DOI] [PubMed] [Google Scholar]
- Winnier, G., M. Blessing, P.A. Labosky, and B.L.M. Hogan. 1995. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9:2105–2116. [DOI] [PubMed] [Google Scholar]
- Wozney, J.M. 1992. The bone morphogenetic protein family and osteogenesis. Mol. Reprod. Dev. 32:160–167. [DOI] [PubMed] [Google Scholar]
- Wozney, J.M., V. Rosen, A.J. Celeste, L.M. Mitsock, M.J. Whitters, R. Kriz, R. Hewick, and E.A. Wang. 1988. Novel regulators of bone formation: Molecular clones and activities. Science. 242:1528–1534. [DOI] [PubMed] [Google Scholar]
- Yamaji, N., A.J. Celeste, R.S. Thies, J.J. Song, S.M. Bernier, D. Goltzman, K.M. Lyons, J. Nove, V. Rosen, and J.M. Wozney. 1994. A mammalian serine/threonine kinase receptor specifically binds BMP-2 and BMP-4. Biochem. Bioph. Res. Comm. 205:1944–1951. [DOI] [PubMed] [Google Scholar]
- Yamashita, H., P. ten Dijke, D. Huylebroeck, T.K. Sampath, M. Andries, J.C. Smith, C. Heldin, and K. Miyazono. 1995. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J. Cell Biol. 130:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, S.E., A. Daluiski, R. Pederson, V. Rosen, and K.M. Lyons. 2000. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 127:621–630. [DOI] [PubMed] [Google Scholar]
- Yi, S.E., P.S. LaPolt, B.S. Yoon, J.Y. Chen, J.K.H. Lu, and K.M. Lyons. 2001. The type I BMP receptor BmprIB is essential for female reproductive function. Proc. Natl. Acad. Sci. USA. 98:7994–7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., and A. Bradley. 1996. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 122:2977–2986. [DOI] [PubMed] [Google Scholar]
- Zou, H., and L. Niswander. 1996. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 272:738–741. [DOI] [PubMed] [Google Scholar]
- Zou, H., R. Wieser, J. Massagué, and L. Niswander. 1997. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 11:2191–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]