Abstract
OBJECTIVES: The aim of this study was to estimate the prevalence of diabetes, impaired glucose tolerance (IGT), and impaired fasting glucose (IFG) in first-degree relatives (FDR) of people with type 2 diabetes mellitus. METHODS: A cross-sectional study of FDR of type 2 diabetes patients was conducted between 2003 and 2005. A total of 2,368 FDR of type 2 diabetes outpatients aged 30-60 years (614 men and 1754 women) from Isfahan Endocrine and Metabolism Research Center (Iran) were examined. All subjects underwent a standard 75 g 2-h oral glucose tolerance test (OGTT). IGT, IFG and type 2 diabetes were diagnosed according to the criteria of the American Diabetes Association (ADA). The mean (SD) age of participants was 43.1 (6.9) years. RESULTS: The prevalence of type 2 diabetes, IGT and IFG were 10.3% (95% CI: 9.1-11.5), 19.5% (17.9-21.1) and 17.3% (15.8-18.8) respectively. The prevalence rates were significantly higher than those reported for a control population of the same age (type 2 diabetes, 6.0% (95% CI: 5.7-6.2) and IGT 9.6 (95% CI: 9.3-9.9)). IGT was more frequent among women (OR: 0.66; 95% CI: 0.51-0.87), whereas diabetes (OR: 1.31; 95% CI: 0.96-1.78) and IFG (OR: 1.41; 95% CI: 1.10-1.80) were higher in men. Multivariate analysis revealed that age and obesity or abdominal obesity were significantly associated with diabetes, IGT and IFG. CONCLUSIONS: FDR of people with type 2 diabetes in Iran are at higher risk of IGT and type 2 diabetes than the population at large. Risk increases with age and obesity. These findings may be useful for the identification of persons at risk of developing type 2 diabetes and strongly support the regular screening of FDR of type 2 diabetes patients.
Keywords: type 2 diabetes, impaired glucose tolerance, first-degree relatives, risk factors, prevalence
Introduction
Type 2 diabetes mellitus is an important public health problem worldwide, and its prevalence is increasing in both developed and developing nations [1]. Family members of people with diabetes are at higher risk of developing diabetes [2-5]. The inheritance pattern is, however, unclear. Though a series of candidate genes has been investigated, none have been identified that contribute significantly to the development of the disease. None of the susceptible genes that have been identified to date causes diabetes in the absence of other genetic or environmental contributing factors, which is consistent with a multifactorial or polygenic origin for this disorder. Although genetic factors play a key role in the development of type 2 diabetes, in the vast majority of patients, diabetes is brought about by a combination of genetic and environmental factors. Familial clustering of diabetes may support a genetic predisposition to diabetes. With the increasing prevalence of diabetes mellitus worldwide [1] the number of first-degree relatives (FDR) of people with type 2 diabetes, and thus an increased risk of developing diabetes, will also increase, which means that identifying risk factors associated with susceptibility to diabetes becomes increasingly important. While much is known about the impact of diabetes on FDR in developed nations, few studies have been undertaken in developing nations and none in Iran. Genetic and environmental exposures, as well as the availability of medical care, are different in Iran and the study of these factors is worthwhile. Accurate information regarding the prevalence of diabetes, IGT, IFG and associated risk factors in FDR of people with diabetes is important to gain a better understanding of the etiology of the disease and, if possible, to prevent or delay its progression and complications in developing countries.
The objective of this study was to estimate the prevalence of diabetes, impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) in FDR of people with type 2 diabetes mellitus and to conduct a preliminary investigation of the determinants of diabetes, IGT and IFG in FDR of patients with type 2 diabetes.
Subjects and methods
Our sample contained 2,368 FDR (614 men and 1754 women) from a consecutive sample of patients with type 2 diabetes attending outpatient clinics at the Isfahan Endocrine and Metabolism Research Center, which is part of the Isfahan University of Medical Sciences, Iran. The sample of FDR was recruited between 2003 and 2005. The tenets of the Declaration of Helsinki were followed, institutional ethical committee approval was granted, and a declaration of informed consent was signed by each participant.
The FDR of patients with type 2 diabetes included siblings or children. They reported to the clinics in the morning after an overnight fast. Subjects were asked to abstain from vigorous exercise in the evening and morning before examination. Smokers were encouraged to abstain from smoking in the morning of the investigations. On arrival in the clinic, the information on family history in the questionnaire completed by FDR was verified first. Then height and weight were measured using standard apparatus with subjects in light clothes and without shoes. Weight was measured to the nearest 0.1 kg on a calibrated beam scale. Height, waist and hip circumference were measured to the nearest 0.5 cm with a measuring tape. Waist was measured midway between the lower rib margin and the iliac-crest at the end of a gentle expiration. Hip circumference was measured over the greater trochanters directly over the underwear. Body mass index (BMI, kg/m2) is recognized as the measure of overall obesity. Normal BMI was defined as BMI < 25, overweight as BMI 25-29.99, and obesity as BMI ≥ 30. A waist-to-hip ratio (WHR) of <0.8 in women and <0.95 in men was considered normal. Resting blood pressure (BP) was measured after subjects had been seated for 10 minutes by using a mercury sphygmomanometer and appropriately sized cuffs, using standard techniques. Those FDR with fasting plasma glucose (FPG) ≥ 200 mg/dl were considered to be diabetic. If FPG was ≥126 and <200 mg/dl, a second FPG was measured on another day. If the second FPG was also ≥126 mg/dl, participants were considered to be diabetic. Subjects with FPG < 126 mg/dl underwent a standard oral glucose tolerance test (OGTT) according to ADA criteria (75 g glucose 2-h) [6]. Venous blood was sampled 0, 30, 60, and 120 min. after oral glucose administration. Plasma samples obtained after centrifugation were analyzed on the same day. FPG ≥ 126 mg/dl or 2-h plasma glucose of ≥200 mg/dl defined diabetes mellitus. IGT was defined as FPG < 126 mg/dl, and 2-h plasma glucose concentration ≥140 mg/dl and <200 mg/dl. A FPG within the range of 100 to 126 mg/dl and 2-h plasma glucose <140 mg/dl were considered to indicate IFG, whereas a FPG below 100 mg/dl and 2-h plasma glucose <140 mg/dl were considered to be signs of normal glucose tolerance [6, 7].
Glycosylated hemoglobin (HbA1c) (measured by ion-exchange chromatography and used as an indicator of diabetic control), total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol (measured using standardized procedures), and low-density lipoprotein (LDL) cholesterol (calculated by the Friedewald equation [8] provided total triglycerides did not exceed 400 mg/dl) were assessed. All the blood sampling procedures were performed in the central laboratory of the Isfahan Endocrine and Metabolism Research Center using an enzyme-linked method.
Statistical analysis
Statistical methods used included Student's t-test, chi squared test and stepwise binary logistic regression. Age-adjusted means were calculated and compared using general linear models. Multiple logistic regressions were carried out with the SPSS for Windows (SPSS Inc., Chicago, IL, USA) to obtain the odds ratio (OR), accompanied by 95% confidence intervals (CI). We considered the following covariates in the multivariate-adjusted analyses: age, gender, BMI, waist circumference (WC), triglyceride, LDL, HDL, total cholesterol and systolic and diastolic BP. Adjustments for age were examined in separate models. Prevalence rates of diabetes, IGT and IFG were age-adjusted, using the direct method of adjustment, within the WHO world standard population [9]. All tests for statistical significance were two-tailed, with the level of significance at p < 0.05.
Results
Subject characteristics
Differences in the distribution of risk factors among 614 male and 1754 female FDR of people with type 2 diabetes are shown in Table 1. Women had lower waist circumference, height and weight, WHR, FPG, 30 and 60 minutes plasma glucose, triglyceride and BP and were younger than men. Men had lower BMI, hip circumference, 2-hour plasma glucose, HDL and LDL than women.
Table 1. Age-adjusted means (SE) of selected characteristics among 614 men and 1754 women.
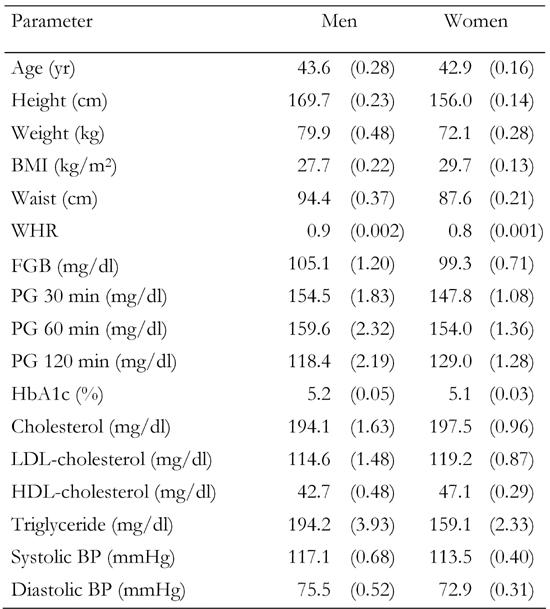
Data are age-adjusted means (standard error in parentheses). Means were calculated using general linear models. BMI: body mass index. WHR: waist-to-hip ratio. FGB: fasting glucose baseline. PG: plasma glucose. BP: blood pressure.
Prevalence
Of the 2,368 FDR of people with type 2 diabetes (614 men and 1754 women), 1261 had normal OGTT (317 men and 944 women), 243 had diabetes (78 men and 165 women), 458 had IGT (86 men and 372 women) and 406 had IFG (132 men and 274 women). 51.6% of the men and 53.8% of women had normal OGTT. Nearly half of FDR aged 30-60 years were diabetic or had impaired glucose regulation (48.4%). The overall prevalence of diabetes was 10.3% (95% CI: 9.0-11.5). The prevalence of IGT and IFG were 19.3% (95% CI: 17.8-20.9) and 17.2% (95% CI: 15.6, 18.7) respectively. The prevalence of diabetes was higher in men (12.9%; 95% CI: 10.1-15.4) than women (9.4%; 95% CI: 8.1-10.9). The prevalence of IFG was also higher in men (21.7%; 95% CI: 18.3-24.8) than women (15.8%; 95% CI: 13.9-17.3), whereas prevalence of IGT was higher in women (21.4%; 95% CI: 19.3-23.1) than men (14.0%; 95% CI: 11.3-16.8). The prevalence of type 2 diabetes and IGT were significantly higher than those reported for the general population (type 2 diabetes, 6.0% (95% CI: 5.7-6.2) and IGT 9.6 (95% CI: 9.3-9.9) (Table 2). As expected, there was a statistically increasing prevalence of diabetes and IGT with increasing age. When age was adjusted to the WHO world standard population, the age-adjusted prevalence rates of diabetes, IGT and IFG were 10.6%, 19.6% and 17.2% respectively.
Table 2. Prevalence of type 2 diabetes, impaired glucose tolerance and impaired fasting glucose in the study group and in the general population (Iran).
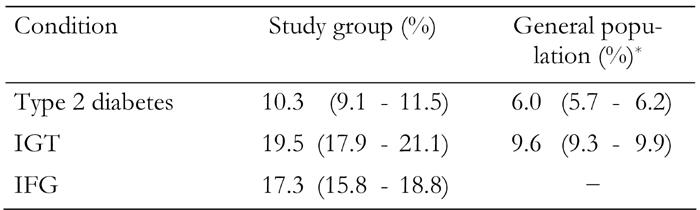
Data are prevalence ratios (95% CI in parentheses). IGT: impaired glucose tolerance. IFG: impaired fasting glucose. * According to International Diabetes Federation, 2006.
Risk factors
To determine the influence of potential factors on diabetes, IGT and IFG, univariate analysis was first performed (Table 3). Age-adjusted OR showed that subjects who had diabetes were more likely to be men, to be older and to have higher systolic and diastolic BP, total cholesterol, LDL and HDL cholesterol, BP, BP, total cholesterol, LDL and HDL cholesterol, triglyceride, BMI, WC and WHR. Those who had IGT were more likely to be women, to be older and to have higher total and HDL cholesterol, BMI and WC. For all variables, there was a fairly consistent 'dose response' across the range of values. For example, the prevalence of diabetes and IGT was higher in older age groups, amongst those with higher cholesterol, BMI and abdominal obesity.
Table 3. Prevalence rates (%) of diabetes mellitus, impaired glucose tolerance and impaired fasting glucose in first-degree relatives of patients with type 2 diabetes by selected characteristics.
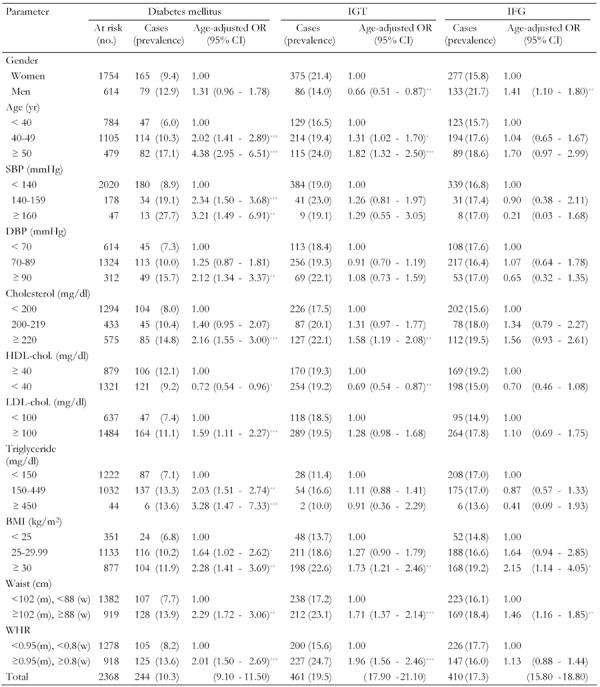
Odds ratio (with 95% CI in parentheses) calculated by binary logistic regression analysis. Total number of at risk is not the same for each variable because of missing values. IGT: impaired glucose tolerance. IFG: impaired fasting glucose. SBP: systolic blood pressure. DBP: diastolic blood pressure. HDL: high-density lipoprotein. LDL: low-density lipoprotein. BMI: body mass index. WHR: waist-to-hip ratio. w: women. m: men. OR: odds ratio. CI: confidence interval. * p < 0.5, ** p < 0.01, *** p < 0.001.
To determine the independent predictors of the prevalence of diabetes, IGT and IFG a forward stepwise binary logistic regression was performed to test 10 predictor variables: age, systolic and diastolic BP, total cholesterol, HDL and LDL cholesterol, triglyceride, BMI, WC, all included as continuous variables, and gender. 267 subjects were excluded from these analyses because of missing information on risk factors, leaving 2101 subjects to be analyzed. Three separate models were computed for diabetes, IGT and IFG (Table 4). Older age, higher WC, higher cholesterol, higher triglyceride and systolic BP significantly increased the risk of diabetes. For the IGT group, age, cholesterol and BMI significantly increased the risk of IGT. Age, WC and HDL significantly increased the risk of IFG. No other variables were significant.
Table 4. Risk factors related to prevalence of diabetes, impaired glucose tolerance and impaired fasting glucose for first-degree relatives of patients with type 2 diabetes (multiple logistic regression analysis).
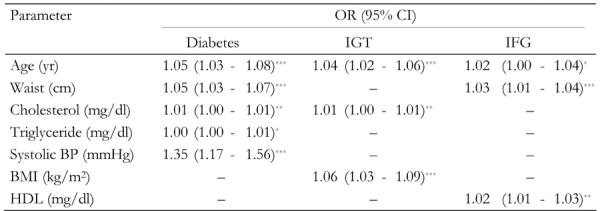
BMI: body mass index. HDL: high-density lipoprotein. IGT: impaired glucose tolerance. IFG: impaired fasting glucose. * p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
In this cross-sectional study, FDR of patients with type 2 diabetes show increased prevalence of type 2 diabetes, IGT and IFG. In most cases, this is accompanied by unfavorable BMI, WC and lipid profile. Prevalence rates of type 2 diabetes in general populations in various studies from around the world show considerable variations. A comparison between these studies is difficult because estimates of prevalence of abnormal glucose tolerance depend on methodological factors and the applied definition of diabetes, the IGT and IFG used, as well as the composition of the community examined by age and gender.
The prevalence of diabetes in the general population of Tehran over 30 years old was 7.2% (7.6% women and 7.1% men) [10]. Primary results of the national program for the prevention and control of type 2 diabetes showed that 3.6% (4.3% women and 2.6% men) of the general population of Iran aged 30 years and over had diabetes [11]. The prevalence of diabetes in the general population over 20 years old in central Iran was 5.4% and 7.1% in men and women and 6.7% and 5.3% in urban and rural areas respectively [12]. The prevalence of IGT was 3.7% and 6.2% in men and women and that of IFG was 0.4% and 0.5% in men and women respectively in the general population [12]. The International Diabetes Federation estimated that 6.0% of the general population of Iran aged 20-79 years had diabetes and 7.7% had IGT [13]. Recently, the National Survey of Risk Factors for Non-Communicable Diseases of Iran estimated that 7.7% of adults aged 24-64 had diabetes and 16.8% of Iranian adults had IFG [14]. Diabetes prevalence varied from 1.3% in rural areas to 14.5% in large cities.
In European societies, the prevalence of age-adjusted type 2 diabetes in people over 25 years of age is 3% to 10% [15]. The prevalence of diabetes in the US is approximately 7% [16]. The prevalence of diabetes and IGT in FDR of people with type 2 diabetes, 10.3% and 19.5%, as reported in this study, are considerably higher than they are in the general population and require serious consideration since diabetes is a debilitating chronic disease.
Type 2 diabetes appears to have strong genetic associations. Studies in twins have demonstrated that concordance rates for type 2 diabetes in monozygotic twins range between 34% and 83% [17]. The broad range of observed correlations suggests both a complex genetic predisposition and an interaction between genetic and environmental factors in the pathogenesis of type 2 diabetes. People who have one FDR suffering from diabetes have a 40% risk of having this disease. If diabetes is present in both parents, this risk is doubled [18].
The present study found a higher prevalence of type 2 diabetes and IFG in men and a higher prevalence of IGT in women in a way that is consistent with previous studies [19-25]. The potential reason or reasons for this gender difference in glucose metabolism has not been explored, but some studies suggest that female sex hormones may contribute [26-30], while others failed to see any effect on glucose metabolism [31-33].
The excess risk of diabetes and IGT associated with FDR of patients with type 2 diabetes was amplified in the presence of overweight and obesity. Obesity is associated with type 2 diabetes in the general population. However, our findings confirm this association in FDR of people with type 2 diabetes. The FDR of people with type 2 diabetes who were overweight or obese were at much higher risk of diabetes and IGT than non-obese relatives. Several studies have shown that measures of obesity show strong heritability [34]. This suggests that genetic factors besides lifestyle, obesity and dyslipidemia may be among the risk factors for diabetes and IGT.
Another finding that requires further elaboration is the high prevalence of diabetes and IGT in the high cholesterol group. This is most likely because diabetes and IGT are associated with a higher prevalence of additional cardiovascular risk factors, which collectively result in a high risk profile. Aggregation of multiple risk factors, including obesity, high BP and hyperlipidemia, has been shown to increase the development of coronary heart disease [35]. It seems that diabetes and IGT tend to coexist with other cardiovascular risk factors. The lipid and lipoprotein disturbances are most likely related to the impaired glucose metabolism. Indeed, insulin resistance seems to play a major role in dyslipidemia in subjects with both normal and abnormal glucose tolerance [36], and appears to be the common element accounting for the cluster of atherogenic metabolic abnormalities found in the metabolic syndrome (IGT, hypertension and dyslipidemia), which confers a high risk for cardiovascular disease [36].
Our study has several strengths and limitations. The strengths include the large sample consisting of both men and women, the sound representativeness of the FDR of people with type 2 diabetes, and information on potential determinants of impaired glucose regulation. As a cross-sectional study, the present analysis is limited in its ability to elucidate causal relationships between risk factors and diabetes, IGT and IFG. Another limitation was that study participants were aged 30-60 years, and results may not apply to the broader age groups. Despite the above limitations, the findings here add to our understanding of the prevalence and risk factors of diabetes, IGT and IFG in FDR of people with type 2 diabetes in Iran. Furthermore, this study provides new data from Iran, a developing country that has been underrepresented in past studies.
In summary, the findings of this study illustrate for the first time the prevalence of diabetes, IGT and IFG in FDR of patients with type 2 diabetes in Iran. Our results emphasize the importance of controlling for all known diabetes risk factors, especially overweight and obesity, in FDR of people with type 2 diabetes. These findings may prove useful in identifying a specific subset of the population at particular risk of developing metabolic disturbances known to predispose to cardiovascular disease and strongly support the regular screening of FDR of patients with type 2 diabetes.
Acknowledgments
We are grateful to Mr. Majid Abyar for computer technical assistance. This study could not have been concluded without the contribution of the first-degree relatives of the diabetics who consented to participate.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Grill V, Persson G, Carlsson S, Norman A, Alvarsson M, Ostensson CG, Svanstrom L, Efendic S. Family history of diabetes in middle-aged Swedish men is a gender unrelated factor which associates with insulinopenia in newly diagnosed diabetic subjects. Diabetologia. 1999;42:15–23. doi: 10.1007/s001250051106. [DOI] [PubMed] [Google Scholar]
- 3.Park HS, Yim KS, Cho SI. Gender differences in familial aggregation of obesity-related phenotypes and dietary intake pattern in Korean families. Ann Epidemiol. 2004;14:486–491. doi: 10.1016/j.annepidem.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Li JK, Ng MC, So WY, Chiu CK, Ozaki R, Tong PC, Cockram CS, Chan JC. Phenotypic and genetic clustering of diabetes and metabolic syndrome in Chinese families with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2006;22:46–52. doi: 10.1002/dmrr.577. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Cosegregation of obesity with familial aggregation of type 2 diabetes mellitus. Diabetes Obes Metab. 2000;2:149–154. doi: 10.1046/j.1463-1326.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 6.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 7.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.Waterhouse JA, Muir CS, Correa P, Powell J, editors. Cancer incidence in five continents. Vol. 3. IARC Scientific Publications; Lyon, France: 1976. p. 456. [Google Scholar]
- 10.Azizi F. Diabetes mellitus in the Islamic republic of Iran. IDF Bull. 1996;41:38–93. [Google Scholar]
- 11.Azizi F, Guoya MM, Vazirian P, Dolatshati P, Habbibian S. Screening for type 2 diabetes in the Iranian national programme: a preliminary report. East Mediterr Health J. 2003;9(5-6):1122–1127. [PubMed] [Google Scholar]
- 12.Sadeghi M, Roohafza H, Shirani S, Poormoghadas M, Kelishadi R, Baghaii A, Sarraf-Zadegan N. Diabetes and associated cardiovascular risk factors in Iran: the Isfahan Healthy Heart Programme. Ann Acad Med Singapore. 2007;36(3):175–180. [PubMed] [Google Scholar]
- 13.Diabetes Atlas. 3rd ed International Diabetes Federation; 20076. [Google Scholar]
- 14.Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, Safaie A, Forouzanfar M, Gregg EW. Prevalence of diabetes mellitus and impaired fasting glucose in the adult population of Iran: the national survey of risk factors for non-communicable diseases of Iran. Diabetes Care. 2007 doi: 10.2337/dc07-0959. In press. [DOI] [PubMed] [Google Scholar]
- 15.Stewart J, Kendrick D, Nottingham Diabetes Blood Pressure Study Group. Setting and negotiating targets in people with type 2 diabetes in primary care. Diabet Med. 2005;22:683–687. doi: 10.1111/j.1464-5491.2005.01496.x. [DOI] [PubMed] [Google Scholar]
- 16.Skyler JS, Oddo C. Diabetes trends in the USA. Diabetes Metab Res Rev. 2002;18(Supl 3):S21–S26. doi: 10.1002/dmrr.289. [DOI] [PubMed] [Google Scholar]
- 17.Bener A, Zirie M, Al-Rikabi A. Genetic, obesity, and environmental risk factors associated with type 2 diabetes. Croat Med J. 2005;46:302–307. [PubMed] [Google Scholar]
- 18.Yuturu S, Bridges JF, Dhanireddy RR. Preliminary evidence of genetic anticipation in type 2 diabetes mellitus. Med Sci Monit. 2005;11:262–265. [PubMed] [Google Scholar]
- 19.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care. 2003;26(3):868–874. doi: 10.2337/diacare.26.3.868. [DOI] [PubMed] [Google Scholar]
- 20.Glumer C, Jorgensen T, Borch-Johnsen K. Prevalence of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care. 2003;26:2335–2340. doi: 10.2337/diacare.26.8.2335. [DOI] [PubMed] [Google Scholar]
- 21.Qiao Q, Hu G, Tuomilehto J, Nakagami T, Balkau B, Borch-Johnsen K, Ramachandran A, Mohan V, Iyer SR, Tominaga M et al. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care. 2003;26:1770–1780. doi: 10.2337/diacare.26.6.1770. [DOI] [PubMed] [Google Scholar]
- 22.The DECODE Study Group. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care. 2003;26:61–69. doi: 10.2337/diacare.26.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Williams JW, Zimmet PZ, Shaw JE, de Courten MP, Cameron AJ, Chitson P, Tuomilehto J, Alberti KG. Gender differences in the prevalence of impaired fasting glycaemia and impaired glucose tolerance in Mauritius: does sex matter? Diabet Med. 2003;20:915–920. doi: 10.1046/j.1464-5491.2003.01059.x. [DOI] [PubMed] [Google Scholar]
- 24.Blake DR, Meigs JB, Muller DC, Najjar SS, Andres R, Nathan DM. Impaired glucose tolerance, but not impaired fasting glucose, is associated with increased levels of coronary heart disease risk factors: results from the Baltimore Longitudinal Study on Aging. Diabetes. 2004;53:2095–2100. doi: 10.2337/diabetes.53.8.2095. [DOI] [PubMed] [Google Scholar]
- 25.Novoa FJ, Boronat M, Saavedra P, Diaz-Cremades JM, Varillas VF, La Roche F, Alberiche MP, Carrillo A. Differences in cardiovascular risk factors, insulin resistance, and insulin secretion in individuals with normal glucose tolerance and in subjects with impaired glucose regulation: the Telde Study. Diabetes Care. 2005;28:2388–2393. doi: 10.2337/diacare.28.10.2388. [DOI] [PubMed] [Google Scholar]
- 26.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 27.van Genugten RE, Utzschneider KM, Tong J, Gerchman F, Zraika S, Udayasankar J, Boyko EJ, Fujimoto WY, Kahn SE American Diabetes Association GENNID Study Group. Effects of sex and hormone replacement therapy use on the prevalence of isolated impaired fasting glucose and isolated impaired glucose tolerance in subjects with a family history of type 2 diabetes. Diabetes. 2006;55:3529–3535. doi: 10.2337/db06-0577. [DOI] [PubMed] [Google Scholar]
- 28.Bonds DE, Lasser N, Qi L, Brzyski R, Caan B, Heiss G, Limacher MC, Liu JH, Mason E, Oberman A et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women's Health Initiative randomized trial. Diabetologia. 2006;49:459–468. doi: 10.1007/s00125-005-0096-0. [DOI] [PubMed] [Google Scholar]
- 29.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 30.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;38:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 31.Duncan AC, Lyall H, Roberts RN, Petrie JR, Perera MJ, Monaghan S, Hart DM, Connell JM, Lumsden MA. The effect of estradiol and a combined estradiol/progestagen preparation on insulin sensitivity in healthy postmenopausal women. J Clin Endocrinol Metab. 1999;84:2402–2407. doi: 10.1210/jcem.84.7.5836. [DOI] [PubMed] [Google Scholar]
- 32.Davidson MH, Maki KC, Marx P, Maki AC, Cyrowski MS, Nanavati N, Arce JC. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch Intern Med. 2000;160:3315–3325. doi: 10.1001/archinte.160.21.3315. [DOI] [PubMed] [Google Scholar]
- 33.Vehkavaara S, Westerbacka J, Hakala-Ala-Pietila T, Virkamaki A, Hovatta O, Yki-Jarvinen H. Effect of estrogen replacement therapy on insulin sensitivity of glucose metabolism and preresistance and resistance vessel function in healthy postmenopausal women. J Clin Endocrinol Metab. 2000;85:4663–4670. doi: 10.1210/jcem.85.12.7034. [DOI] [PubMed] [Google Scholar]
- 34.Selby JV, Newman B, Quesenberry CP Jr, Fabsitz RR, Carmelli D, Meaney FJ, Slemenda C. Genetic and behavior on body fat distribution. Int J Obes. 1990;14:593–602. [PubMed] [Google Scholar]
- 35.Laakso M, Sarlund H, Mykkanen L. Insulin resistance is associated with lipid and lipoprotein abnormalities in subjects with varying degrees of glucose tolerance. Arteriosclerosis. 1990;10:223–231. doi: 10.1161/01.atv.10.2.223. [DOI] [PubMed] [Google Scholar]
- 36.Folsom AR, Szklo M, Stevens J, Liao F, Smith R, Eckfeldt JH. A prospective study of coronary heart disease in relation to fasting insulin, glucose and diabetes. Diabetes Care. 1997;20:915–942. doi: 10.2337/diacare.20.6.935. [DOI] [PubMed] [Google Scholar]


