Abstract
Force-initiated signal transduction can occur either via membrane-based ionic mechanisms or through changes in cytoskeletal–matrix linkages. We report here the stretch-dependent binding of cytoplasmic proteins to Triton X-100 cytoskeletons of L-929 cells grown on collagen-coated silicone. Triton X-100–insoluble cytoskeletons were stretched by 10% and incubated with biotinylated cytoplasmic proteins. Analysis with two-dimensional gel electrophoresis showed stretch-dependent binding of more than 10 cytoplasmic protein spots. Bound cytoplasmic proteins were purified by a photocleavable biotin tag and stretch-dependent binding of paxillin, focal adhesion kinase, and p130Cas was found, whereas the binding of vinculin was unchanged and actin binding decreased with stretch. Paxillin binding upon stretch was morphologically and biochemically similar in vitro and in vivo, that is, enhanced in the periphery and inhibited by the tyrosine phosphatase inhibitor, phenylarsine oxide. Thus, we suggest that transduction of matrix forces occurs through force-dependent conformation changes in the integrated cytoskeleton.
Keywords: mechanotransduction; focal contact; cytoskeleton; force; tyrosine phosphatase
Introduction
There is a critical role for force in controlling cell signal pathways (Chen et al., 1997), development (Grill et al., 2001), wound healing (Timmenga et al., 1991), and in the control of cell growth (Damien et al., 2000) that is lost in many cancers (Saiga et al., 1989). At a molecular level, there is evidence that calcium movements (Wirtz and Dobbs, 1990; Pommerenke et al., 1996; Glogauer et al., 1998; Okuda et al., 1999) or alterations in ion gating (Drummond et al., 2000) trigger cell responses. To sense sustained forces, cells may use mechanisms other than ion channels such as force-dependent changes in the cell cytoskeleton. Recent studies using matrix-coated beads and patterned substrata indicate that force is transduced at the site of matrix–cell contact into changes in the strength of integrin–cytoskeleton bonds and the assembly of focal contact proteins (Wang et al., 1993; Choquet et al., 1997; Balaban et al., 2001) that is affected by the tyrosine kinase, c-Src (Felsenfeld et al., 1999). Two major hypotheses for the assembly of proteins at focal contact sites involve either an (a) local change in the ionic environment or (b) cytoskeletal alteration in response to force. To differentiate between these possibilities, we have prepared detergent-insoluble cytoskeletons of spread cells before stretching, which lack a surrounding membrane and therefore could not be affected by ion movements. Previous reports have shown that purified cytoskeletal proteins can bind to isolated focal adhesion models (Avnur et al., 1983; Ball et al., 1986; Cattelino et al., 1999), suggesting that interactions can be observed in vitro. We show here that stretching the cytoskeletons alone causes the binding of focal contact proteins.
Results and discussion
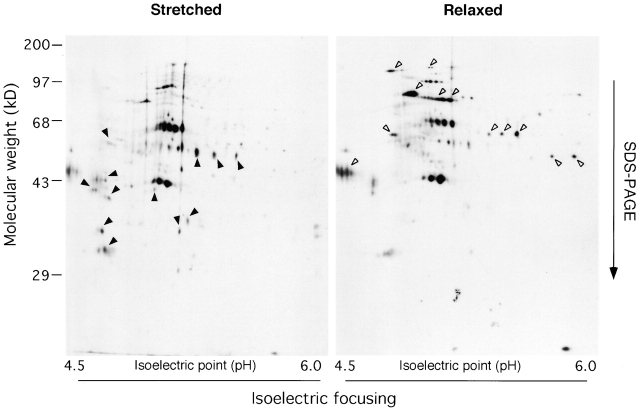
To test whether or not the stretching of cytoskeletons could lead to cytoplasmic protein binding, we designed a protocol to screen for cytoplasmic proteins that bound in a stretch-dependent manner to Triton X-100–insoluble cytoskeletons (see Materials and methods and Fig. 1). Treatment with 0.25% Triton X-100 released most cytoplasmic and membrane proteins including the majority of focal contact proteins, paxillin, focal adhesion kinase (FAK),* p130Cas, and vinculin (unpublished data); but the cytoskeleton stayed attached to the collagen-coated silicone substrate during biaxial stretch of 10% as measured by changes in the dimensions of rhodamine-phalloidin–labeled cytoskeletons. Cytoplasmic proteins were biotinylated (see Materials and methods) to differentiate them from the Triton X-100–insoluble cytoskeleton proteins. To control for general effects of mechanical disturbance and the traction forces normally generated under routine culture conditions (Galbraith and Sheetz, 1999; Horwitz and Parsons, 1999), we compared binding of cytoplasmic proteins to cytoskeletons after stretch with binding after relaxation (cells were incubated overnight on a prestretched substratum [Sawada et al., 2001], treated with Triton X-100, and then cytoskeletons were relaxed). After incubation of Triton X-100–insoluble cytoskeletons with biotinylated cytoplasmic proteins, the bound complex was washed and solubilized for two-dimensional (2-D) gel electrophoresis and blot analysis with HRP-conjugated Streptavidin (Amersham Pharmacia Biotech) in more than 10 separate experiments. We reproducibly found more than 10 major and several minor stretch-specific protein spots (Fig. 2, left). After relaxation of the cytoskeletons, we found more than 10 major and several minor spots that were not found in the stretched samples (Fig. 2, right). In a separate experiment, we found no difference in the binding of cytoplasmic proteins to cytoskeletons after incubation of cells for 16 h on an unstretched or prestretched (but unrelaxed) substrate (unpublished data), which indicated that prestretching the substrate had no effect. Thus, stretching or relaxing of a collagen substrate causes the reproducible binding of cytoplasmic proteins to Triton X-100–insoluble cytoskeletons.
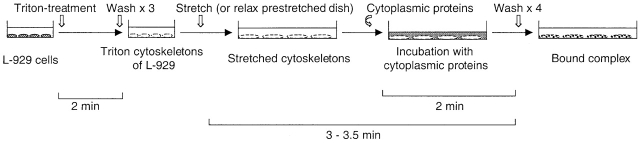
Figure 1.
Diagram of protocol for stretch-dependent binding of cytoplasmic proteins to Triton X-100–insoluble cytoskeletons. L-929 cells were cultured on a collagen-coated silicone substrate, and cytoskeletons were prepared by treating with 0.25% Triton X-100/ISO (+) buffer for 2 min (as described in Materials and methods). Triton X-100–insoluble cytoskeletons were either left unstretched or stretched (or relaxed from prestretch) with ISO (+) after washing three times. Then, the ISO (+) buffer was replaced with the cytoplasmic lysate solution (as described in Materials and methods), incubated for 2 min at room temperature, and washed four times with ISO (+) buffer.
Figure 2.
2-D gels of biotinylated proteins that bound to stretched or relaxed cytoskeletons. The complex of the cytoskeleton with the biotinylated cytoplasmic proteins (Fig. 1) was solubilized with 1 ml of a rehydration buffer (8 M urea, 2% CHAPS, 20 mM DTT, 0.5% IPG buffer [Amersham Pharmacia Biotech]) for isoelectrical focusing (the first dimension of 2-D gel electrophoresis). Immobiline dry strip (pH 4–7; Amersham Pharmacia Biotech) was rehydrated with 350 μl of each sample and subjected to isoelectrical focusing followed by SDS-PAGE. Biotinylated cytoplasmic proteins in 2-D gels were visualized with affinity blotting using HRP-conjugated Streptavidin. Arrowheads mark the spots that were found specifically in Stretched or Relaxed.
Numerous controls were performed to test for binding of biotinylated proteins to the substratum or collagen, but the pattern of stretch-specific spots was not repeated. Further, adhesion of the cells to the collagen substrate was necessary, since stretching of substrata when cells were not spread (for example, when cells were cultured on a noncoated silicone substrate) did not cause stretch-dependent binding. Interestingly, if the cells had reached confluence then stretching of a confluent monolayer of Triton X-100–insoluble cytoskeletons did not result in stretch-dependent binding (unpublished data). These results indicate that cell cytoskeleton–matrix linkages must be stretched to cause stretch-dependent binding.
Since focal contacts grow in response to stretch (Balaban et al., 2001), we tested if focal contact proteins would bind to the cytoskeletons in response to a 10% stretch of the cytoskeletons (Fig. 3 A). To enable us to purify bound cytoplasmic proteins from Triton X-100–insoluble cytoskeletons, we labeled the cytoplasmic proteins with a photocleavable form of biotin (Olejnik et al., 1995). Biotinylated proteins eluted from the cytoskeletons by high salt were bound to avidin beads, released by UV irradiation, and subjected to SDS-PAGE followed by immunoblot analysis. Using monospecific antibodies to paxillin, FAK, p130Cas, and vinculin, we reproducibly found increased binding of the first three to stretched cytoskeletons, but vinculin was not changed with cells on a collagen matrix (Fig. 3 B). In addition, we also found stretch-dependent binding of PKB/Akt (King et al., 1997). Interestingly, actin binding decreased by ∼50% (n = 5) in the samples from the stretched cytoskeletons (Fig. 3 B). Since the binding of vinculin and actin remained constant or decreased, respectively, it is clear that stretch does not cause all focal contact proteins to bind to the cytoskeletons. Thus, we identified four focal contact or membrane-bound proteins, paxillin, FAK, p130Cas, and PKB/Akt, that bound to the cytoskeletons in a stretch-dependent manner. Since paxillin, FAK, and p130Cas are known to form complexes (Harte et al., 1996; Bang et al., 2000; Yano et al., 2000), we tested if these proteins formed a complex in our lysate. Although we observed coimmunoprecipitation of FAK and p130Cas, paxillin was not precipitated with either an antibody against FAK or p130Cas (unpublished data). Furthermore, neither FAK nor p130Cas was precipitated with an antipaxillin antibody (unpublished data). Therefore, it seems likely that three of the proteins (paxillin, Akt/PKB, and FAK-p130Cas complex) bind independently to the cytoskeletons.
Figure 3.
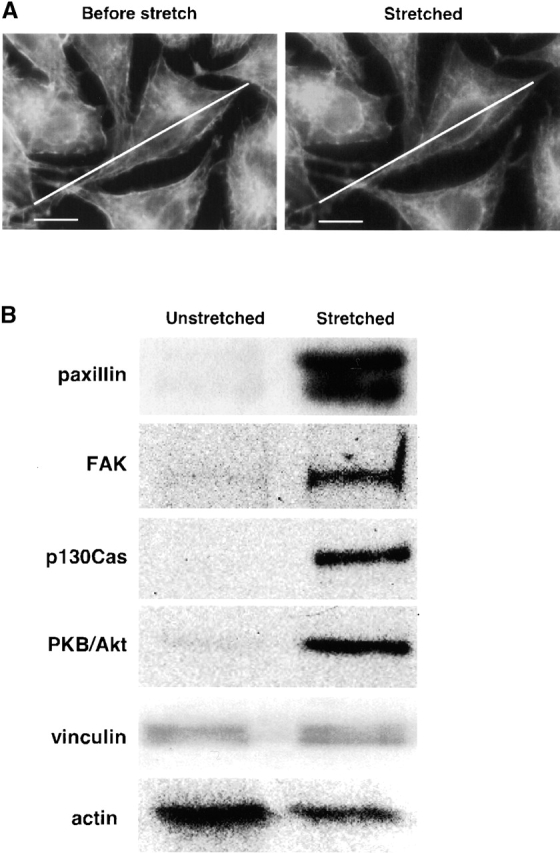
Focal contact proteins bind preferentially to stretched cytoskeletons. (A) Micrographs showing that Triton X-100–insoluble cytoskeletons are stretched 10%. Triton X-100–insoluble cytoskeletons on a collagen-coated silicone membrane (StageFlexer®; Flexcell International) were incubated with rhodamine-phalloidin (Molecular Probes) for 2 min, and washed three times with ISO (+) buffer. Images were obtained with an Olympus BX50 microscope with a 60×, 0.9 NA water immersion objective. Focus was adjusted to identify the peripheral margins (lower surface of cells), and images were obtained before stretch (Before stretch) and 5 min after stretch (Stretched). The diagonal lines show the length of the cell before stretch. (B) Western blots of focal contact proteins bound to unstretched and stretched cytoskeletons. L-929 cytoplasmic proteins tagged with a photocleavable biotin (NHS-PC-LC-biotin) were added to Triton X-100–insoluble cytoskeletons of L-929 cells on a stretchable silicone dish (Sawada et al., 2001), and cytoskeletons were stretched or left unstretched (Fig. 1). After washing, bound cytoplasmic proteins were eluted with 1 ml of 1 M NaCl in HYPO buffer (as described in Materials and methods), precipitated with avidin beads (immobilized neutravidin; Pierce Chemical Co.) after sevenfold dilution with HYPO buffer, and released from the bead complex by irradiation with 302 nm UV light (10 min). After photocleavage, proteins were eluted with 120 μl of HYPO buffer, and 40 μl of the sample was subjected to 10% SDS-PAGE followed by immunoblotting with antibodies to paxillin, FAK, p130Cas, PKB/Akt (Transduction Laboratories), vinculin (Upstate Biotechnology), or actin (Santa Cruz Biotechnology). Bar, 10 μm.
Because green fluorescent protein (GFP) paxillin was reported to assemble at focal contact sites in response to force in some cell systems (Riveline et al., 2001), we tested if paxillin would respond similarly in our system. After L-929 cells were transfected with GFP paxillin on a collagen-coated silicone substrate, there were relatively few focal contacts labeled with transfected GFP paxillin (Fig. 4 A, left). However, when the cells were stretched biaxially (10% in each dimension) the GFP fluorescence at the focal contacts increased dramatically, particularly at the periphery of the cells (Fig. 4 A, middle). Relaxation of the stretched cells resulted in the rapid (<2 min) loss of the peripheral GFP paxillin assembly (Fig. 4 A, right). Immunocytochemical analysis showed that endogenous paxillin behaved similarly (Fig. 4 B). This stretch-dependent paxillin accumulation was inhibited by addition of a tyrosine phosphatase inhibitor, phenylarsine oxide (PAO; 20 μM) (Stover et al., 1991), whereas it was not inhibited by a tyrosine kinase inhibitor, genistein (100 μM) (Akiyama et al., 1987) (Fig. 4 B), implying that tyrosine dephosphorylation of some molecule(s) was required for assembly as seen in other systems (Choquet et al., 1997).
Figure 4.
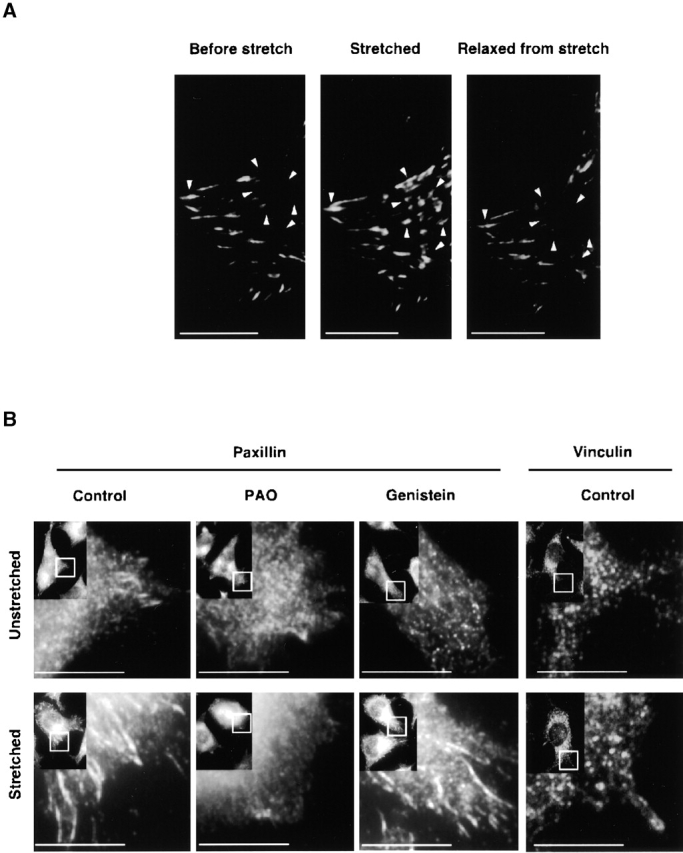
Fluorescence micrographs of the stretch-dependent distribution of GFP paxillin, endogenous paxillin, and vinculin in intact L-929 cells. (A) L-929 cells transiently transfected with GFP paxillin were cultured on collagen-coated silicone membranes in a StageFlexer system. Cells were stretched by 10%, held for 5 min, and subsequently relaxed by allowing the stretched silicone membrane to return to its original size. GFP fluorescence was observed with an Olympus BX50 microscope using a 60×, 0.9 NA water immersion objective before stretch (left), 2 min after stretch (middle), and 2 min after relaxation of stretch (right). Arrowheads indicate sites of GFP paxillin assembly dynamics: Before stretch, Stretched, and Relaxed from stretch. (B) Antipaxillin or antivinculin antibody distribution in L-929 cells cultured on silicone membranes was measured either for unstretched (top), stretched (for 2 min; bottom), stretched PAO treated (20 μM, for 10 min), or stretched genistein treated (100 μM, for 10 min) cells. Cells were fixed with 3.7% formaldehyde/PBS, permeabilized with 0.1% Triton X-100/PBS, and subjected to immunostaining using an antipaxillin or an antivinculin antibody. Stretched samples were relaxed after fixation. The rectangle in the inset (low magnification) indicates the area of each micrograph. Bars, 10 μm.
Since GFP vinculin was also reported to assemble at the focal contact sites in a force-dependent manner (Balaban et al., 2001; Riveline et al., 2001), we also examined the response of vinculin to the stretch. Consistent with the binding to the stretched cytoskeletons (Fig. 3 B), we did not observe stretch-dependent accumulation at focal contact sites either of endogenous vinculin (Fig. 4 B) or of transfected GFP vinculin (unpublished data).
To test whether or not paxillin would bind to the stretched cytoskeletons in a location and manner similar to intact cells, we added cytoplasmic proteins from HEK 293 cells stably transfected with GFP paxillin (293-GFP-pax) (see Materials and methods). The GFP moiety enabled us to discriminate the added paxillin from the small amount of endogenous paxillin that remained in Triton X-100–insoluble cytoskeletons of L-929. After biaxial stretch, we found that GFP paxillin bound with a punctate distribution concentrated at the lower surface of the cells (Fig. 5 A, top), which is similar to the distribution of endogenous paxillin binding in stretched intact cells (Fig. 4 B). Using cytoplasmic proteins from HEK 293 cells stably transfected with GFP (no paxillin), we found that GFP alone did not bind to Triton X-100–insoluble cytoskeletons whether stretched or not (unpublished data). Therefore, the in vitro GFP paxillin binding to Triton X-100–insoluble cytoskeletons is analogous to in vivo stretch-dependent paxillin binding.
Figure 5.
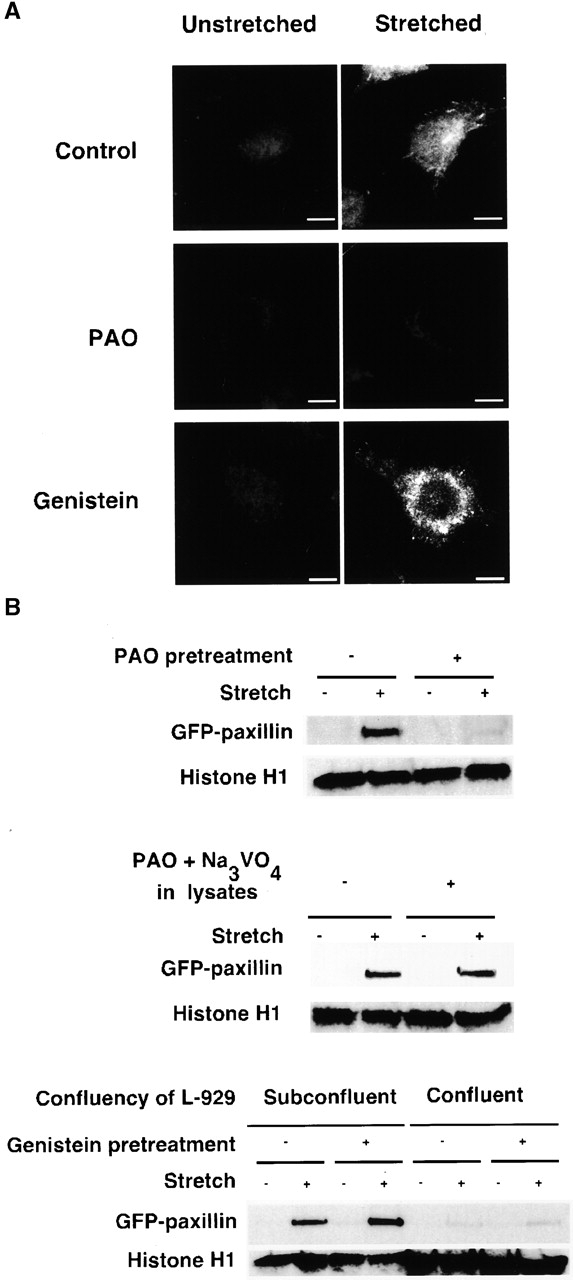
In vitro GFP paxillin binding to Triton X-100–insoluble cytoskeletons. (A) Triton X-100–insoluble cytoskeletons of L-929 cells in a StageFlexer system either unstretched (left) or stretched (10%; right) were incubated with 293-GFP-pax lysates (supplemented with 0.5 mM ATP, 2% BSA) for 2 min. After four washes with ISO (+) buffer, the bound complex of cytoplasmic proteins and cytoskeletons (Fig. 1) was fixed with 3.7% formaldehyde/ISO buffer. Stretched samples were relaxed after fixation, and the distribution of bound GFP paxillin was visualized with fluorescence microscopy (Olympus BX50 microscope with a 60×, 0.9 NA water immersion objective). PAO (20 μM; center) or genistein (100 μM; bottom) was added 10 min before permeabilization and stretching. (B) Triton X-100–insoluble cytoskeletons of L-929 cells in silicone dishes either unstretched or stretched by 10% were incubated with 293-GFP-pax lysates for 2 min. After four washes with ISO (+) buffer, the bound complex of cytoplasmic proteins and cytoskeletons (Fig. 1) were solubilized with 500 μl of SDS sample buffer (100 mM Tris-HCl, pH 6.8, 36% glycerol, 4% SDS, 10 mM DTT, 0.01% bromophenol blue). 50 μl of each sample was subjected to SDS-PAGE followed by immunoblotting with antibodies to GFP (CLONTECH Laboratories, Inc.) and histone H1 (Santa Cruz Biotechnology, Inc.). PAO (20 μM; top), genistein (100 μM; bottom), or solvent (0.1% DMSO) was added 10 min before permeabilization and stretching. Triton X-100–insoluble cytoskeletons were prepared from 4 × 105 cells per dish with the exception of the confluent culture (four right lanes on bottom) where 1.2 × 106 cells per dish were used. Bar, 10 μm.
We tested if cell confluence, tyrosine phosphatase, or kinase inhibitors might influence the level of GFP paxillin binding to stretched cytoskeletons (GFP alone did not bind to stretched cytoskeletons). With subconfluent L-929 monolayers (4 × 105 cells per dish), we observed stretch-dependent GFP paxillin binding (Fig. 5 B) (cytoskeleton number as measured by histone H1 levels was constant), which was consistent with our microscopic observation (Fig. 5 A). However, in confluent L-929 monolayers (1.2 × 106 cells per dish) GFP paxillin did not bind to stretched Triton X-100–insoluble cytoskeletons (Fig. 5 B, bottom; note the increased amount of histone H1 in the four right lanes). Treatment of the cells with PAO before Triton extraction decreased the amount of GFP paxillin bound to the stretched cytoskeletons (Fig. 5, A, center, and B, top), whereas genistein caused an increase in the amount bound (Fig. 5, A and B, bottom). Addition of PAO to lysates did not affect the stretch-dependent GFP paxillin binding even when another tyrosine phosphatase inhibitor, sodium orthovanadate (Na3VO4; 1 mM), was added and DTT was excluded to ensure the inhibition of tyrosine phosphatase activity by PAO (Stover et al., 1991) (Fig. 5 B, center). Furthermore, the lysates obtained from 293-GFP-pax cells pretreated with PAO showed equivalent stretch-dependent GFP paxillin binding to controls (unpublished data). Because PAO inhibition was only observed when L-929 cells were pretreated and GFP paxillin binding to cytoskeletons was ATP independent (unpublished data), the binding is likely to be independent of either protein kinase or phosphatase activity.
Cytoskeletons from PAO-treated cells could be stretched by at least twofold more than controls (unpublished data), indicating that they had decreased integrity. Early reinforcement of extracellular matrix linkages with the cytoskeleton is inhibited by PAO (Choquet et al., 1997). An expected result of PAO treatment of dynamic linkages (i.e., linkages that break and reform) would be a decrease in the strength of cytoskeleton–matrix linkages, since they could not reform. Treatment of the cytoskeletons with PAO did not cause any change in the Triton cytoskeleton integrity perhaps because of the lack of dynamics. Thus, cytoskeleton integrity appears to be important for stretch-dependent binding.
A decrease in cytoskeleton integrity would result in lower forces in the Triton cytoskeleton after stretch. Hence, it seems that force on the Triton cytoskeleton is critical for binding. Further, force on cytoskeleton–integrin–matrix linkages appears to be critical. When cells become confluent, the area of adherence to the matrix substrate is decreased and cell–cell contacts are increased. Thus, the loss of stretch-dependent paxillin binding in confluent cells (Fig. 5 B) implicates the matrix–cytoskeleton linkage. We suggest that force on the collagen–integrin–cytoskeleton linkage causes protein or domain unfolding, which results in binding.
From the analysis of the biotinylated proteins that bind to the cytoskeletons, it is clear that a distinct subset of proteins binds to stretched cytoskeletons that is different from the proteins that bind to relaxed cytoskeletons. The limited number of proteins that bind in a specific manner is consistent with a selective binding process. Because we used an extracted cytoskeleton and a cytoplasm that had been manipulated (diluted, biotinylated, and column filtered), there may be additional components that bind in vivo to cytoskeletons. However, in these studies there is a close correspondence of the binding in vivo and in vitro. Stretch causes in vivo and in vitro binding of GFP paxillin to sites of maximum stress, and PAO treatment blocks that binding. Stretch-dependent binding is lost in cytoskeletons from confluent cells, which correlates with the in vivo loss of stretch-dependent activation of mitogen-activated protein kinases (Sawada et al., 2001) when the cells become confluent (unpublished data). Vinculin shows no change in stretch-dependent binding in vivo or in vitro under these conditions. Further, there must be collagen attachment for stretch-dependent binding. Thus, we suggest that stretch-dependent binding to the cytoskeleton represents the cellular mechanism of response to the stretch of collagen–cytoskeleton linkages.
Force could be transduced into a biochemical signal through the localization of soluble proteins to focal sites on the cytoskeleton similar to the activation of many proteins by membrane binding, for example, PKC. Mechanical stresses on membranes caused by osmotic swelling (Viana et al., 2001) or fluid shear (Satlin et al., 2001) can activate membrane channels (stress channels), and substrate stretch could also affect ion movements. However, the stretch-dependent in vitro binding of focal contact proteins to the Triton cytoskeletons we observed is independent of ion currents. Thus, we show here that traction stresses on cytoskeleton–integrin–extracellular matrix linkages cause the binding of focal contact proteins. We currently hypothesize that a major component of the in vivo mechanism for sensing sustained matrix forces involves the direct effects of force on the cytoskeleton, although ion currents could influence the response.
Force-dependent changes in cytoskeletal protein conformation through protein domain unfolding or conformation change could open new binding sites. For example, relatively low forces are needed to unfold spectrin, and the unfolding of fibronectin opens new binding sites (Ingham et al., 1997). It is now critical to determine which proteins are altered in conformation by force on the cytoskeleton.
In the context of tissues, cells have to exert regulated levels of force on the extracellular matrix and adjacent cells. In cells in vitro, the level of applied force is critical for the survival of cells (Chen et al., 1997) and is linked to the activation of mitogen-activated protein kinase signaling pathways (MacKenna et al., 1998; Kippenberger et al., 2000; Sawada et al., 2001). After transformation by tyrosine kinases such as v-Src, cells no longer require force generated on matrices to grow since they can grow on soft agar, which further indicates that tyrosine kinase/phosphatase pathways are involved in transducing matrix traction forces to biochemical changes. We suggest based upon these studies that one mechanism of sensing force is through force-dependent changes in the cytoskeleton network, which causes binding of cytoplasmic focal contact proteins and initiates intracellular signaling cascade(s).
Materials and methods
Cells, transfection, and plasmids
Mouse fibroblastic L-929 cells and HEK 293 cells were cultured in DME supplemented with 10% FBS at 37°C, 5% CO2, and 100% humidity. Transfection was performed with FuGene 6 (Roche) according to the manufacturer's protocol. GFP paxillin expression vector, GFP vinculin expression vector, and those parent GFP expression vector (pRK-GFP) were provided by K. Yamada (National Institutes of Health, Bethesda, MD). To isolate cell lines stably expressing transfected GFP paxillin or GFP (without paxillin), pcDNA3 that carried a neomycin-resistant gene (Invitrogen) was cotransfected and clones were selected using G-418 (GIBCO BRL).
Triton X-100–insoluble cytoskeletons
L-929 cultures (subconfluent except in the experiments for Fig. 5 B) were plated on collagen-coated (type I; Sigma-Aldrich) silicone, either the StageFlexer system (Flexcell International) or the stretchable silicone dishes (Sawada et al., 2001). Cells were washed once with ISO (isotonic) buffer (20 mM Hepes, pH 7.5, 150 mM NaCl, 0.5 mM EDTA, 1 mM DTT, 4 mM MgCl2, 1 mM PMSF, 15 μg/ml aprotinin), treated with 0.25% Triton X-100/ISO (+) buffer (ISO buffer supplemented with 0.5 mM ATP, 2% BSA) for 2 min and washed three times with ISO (+) buffer (Fig. 1).
Observation of stretch-dependent (GFP) paxillin assembly in intact cells
Subconfluent L-929 cells transiently transfected with GFP paxillin on the collagen-coated (type I) silicone membrane (Flexcell International) were stretched biaxially (10% in each dimension), sustained for 2 min, and the GFP fluorescence was observed by fluorescence microscopy. 5 min after stretch, the silicone substrate was relaxed to its original size, and the fluorescence was recorded. For endogenous paxillin measurements, L-929 cells cultured on silicone membranes were stretched likewise, sustained for 2 min, and fixed with 3.7% formaldehyde/PBS. After fixation, the cells on the silicone substrate were permeabilized with 0.1% Triton X-100/PBS, subjected to immunostaining using antipaxillin antibody (Transduction Laboratories) or antivinculin antibody (Upstate Biotechnology) and compared with cells that had been fixed before stretch.
Lysates from 293-GFP-pax cells or L-929 cells
HEK 293 cells stably transfected with GFP paxillin expression vector (293-GFP-pax cells) or L-929 cells were scraped off tissue culture plastic, washed twice with ISO buffer, resuspended 1:1.5 (packed cells:buffer) in HYPO (hypotonic) buffer (20 mM Hepes, pH 7.5, 0.5 mM EDTA, 1 mM DTT, 4 mM MgCl2, 1 mM PMSF, 15 μg/ml aprotinin), homogenized, and cleared of cell debris and nuclei by centrifugation (2 × 105 g, 30 min). The supernatant was filtrated using PD-10 column (Amersham Pharmacia Biotech) preequilibrated with ISO buffer. We usually prepared ∼7 ml of lysates containing ∼5 mg/ml cytoplasmic proteins from 4 × 103 cm2 of confluent culture.
Biotinylation of lysates from L-929 cells
After passage over the PD-10 column preequilibrated with ISO buffer, lysates from L-929 cells were incubated with NHS-biotin or NHS-PC-LC-biotin (100 μg/ml) (Pierce Chemical Co.) for 2 h at 4°C and then passed over a second PD-10 column preequilibrated with ISO buffer to remove unbound biotin.
Acknowledgments
We thank K. Yamada for providing plasmids for GFP paxillin, GFP vinculin, and GFP. K. De Vos was particularly helpful in the 2-D gel analysis and microscopy. We also thank K. Nakamura, H. Ichijo, and K. Doi for consistent support, and K. Miller, G. Giannone, G. von Weichert, and M. Tamada for helpful discussions.
This work was supported by National Institutes of Health grant GM36277.
Footnotes
Abbreviations used in this paper: 2-D, two-dimensional; FAK, focal adhesion kinase; GFP, green fluorescent protein; PAO, phenylarsine oxide.
References
- Akiyama, T., J. Ishida, S. Nakagawa, H. Ogawara, S. Watanabe, N. Itoh, M. Shibuya, and Y. Fukami. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262:5592–5595. [PubMed] [Google Scholar]
- Avnur, Z., J.V. Small, and B. Geiger. 1983. Actin-independent association of vinculin with the cytoplasmic aspect of the plasma membrane in cell-contact areas. J. Cell Biol. 96:1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban, N.Q., U.S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472. [DOI] [PubMed] [Google Scholar]
- Ball, E.H., C. Freitag, and S. Gurofsky. 1986. Vinculin interaction with permeabilized cells: disruption and reconstitution of a binding site. J. Cell Biol. 103:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang, O.S., E.J. Kim, J.G. Chung, S.R. Lee, T.K. Park, and S.S. Kang. 2000. Association of focal adhesion kinase with fibronectin and paxillin is required for precartilage condensation of chick mesenchymal cells. Biochem. Biophys. Res. Commun. 278:522–529. [DOI] [PubMed] [Google Scholar]
- Cattelino, A., C. Albertinazzi, M. Bossi, D.R. Critchley, and I. de Curtis. 1999. A cell-free system to study regulation of focal adhesions and of the connected actin cytoskeleton. Mol. Biol. Cell. 10:373–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.S., M. Mrksich, S. Huang, G.M. Whitesides, and D.E. Ingber. 1997. Geometric control of cell life and death. Science. 276:1425–1428. [DOI] [PubMed] [Google Scholar]
- Choquet, D., D.P. Felsenfeld, and M.P. Sheetz. 1997. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 88:39–48. [DOI] [PubMed] [Google Scholar]
- Damien, E., J.S. Price, and L.E. Lanyon. 2000. Mechanical strain stimulates osteoblast proliferation through the estrogen receptor in males as well as females. J. Bone Miner. Res. 15:2169–2177. [DOI] [PubMed] [Google Scholar]
- Drummond, H.A., F.M. Abboud, and M.J. Welsh. 2000. Localization of beta and gamma subunits of ENaC in sensory nerve endings in the rat foot pad. Brain Res. 884:1–12. [DOI] [PubMed] [Google Scholar]
- Felsenfeld, D.P., P.L. Schwartzberg, A. Venegas, R. Tse, and M.P. Sheetz. 1999. Selective regulation of integrin–cytoskeleton interactions by the tyrosine kinase Src. Nat. Cell Biol. 1:200–206. [DOI] [PubMed] [Google Scholar]
- Galbraith, C.G., and M.P. Sheetz. 1999. Keratocytes pull with similar forces on their dorsal and ventral surfaces. J. Cell Biol. 147:1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glogauer, M., P. Arora, D. Chou, P.A. Janmey, G.P. Downey, and C.A. McCulloch. 1998. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J. Biol. Chem. 273:1689–1698. [DOI] [PubMed] [Google Scholar]
- Grill, S.W., P. Gonczy, E.H. Stelzer, and A.A. Hyman. 2001. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature. 409:630–633. [DOI] [PubMed] [Google Scholar]
- Harte, M.T., J.D. Hildebrand, M.R. Burnham, A.H. Bouton, and J.T. Parsons. 1996. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J. Biol. Chem. 271:13649–13655. [DOI] [PubMed] [Google Scholar]
- Horwitz, A.R., and J.T. Parsons. 1999. Cell migration—movin' on. Science. 286:1102–1103. [DOI] [PubMed] [Google Scholar]
- Ingham, K.C., S.A. Brew, S. Huff, and S.V. Litvinovich. 1997. Cryptic self-association sites in type III modules of fibronectin. J. Biol. Chem. 272:1718–1724. [DOI] [PubMed] [Google Scholar]
- King, W.G., M.D. Mattaliano, T.O. Chan, P.N. Tsichlis, and J.S. Brugge. 1997. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol. Cell. Biol. 17:4406–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippenberger, S., A. Bernd, S. Loitsch, M. Guschel, J. Muller, J. Bereiter-Hahn, and R. Kaufmann. 2000. Signaling of mechanical stretch in human keratinocytes via MAP kinases. J. Invest. Dermatol. 114:408–412. [DOI] [PubMed] [Google Scholar]
- MacKenna, D.A., F. Dolfi, K. Vuori, and E. Ruoslahti. 1998. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J. Clin. Invest. 101:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda, M., M. Takahashi, J. Suero, C.E. Murry, O. Traub, H. Kawakatsu, and B.C. Berk. 1999. Shear stress stimulation of p130(cas) tyrosine phosphorylation requires calcium-dependent c-Src activation. J. Biol. Chem. 274:26803–26809. [DOI] [PubMed] [Google Scholar]
- Olejnik, J., S. Sonar, E. Krzymanska-Olejnik, and K.J. Rothschild. 1995. Photocleavable biotin derivatives: a versatile approach for the isolation of biomolecules. Proc. Natl. Acad. Sci. USA. 92:7590–7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommerenke, H., E. Schreiber, F. Durr, B. Nebe, C. Hahnel, W. Moller, and J. Rychly. 1996. Stimulation of integrin receptors using a magnetic drag force device induces an intracellular free calcium response. Eur. J. Cell Biol. 70:157–164. [PubMed] [Google Scholar]
- Riveline, D., E. Zamir, N.Q. Balaban, U.S. Schwarz, T. Ishizaki, S. Narumiya, Z. Kam, B. Geiger, and A.D. Bershadsky. 2001. Focal contacts as mechanosensors. Externally applied local mechanical force induces growth of focal contacts by an mdia1-dependent and rock-independent mechanism. J. Cell Biol. 153:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiga, T., I. Kashu, K. Tabuchi, and O. Midorikawa. 1989. Increased contraenvironmental-pressure cell division capability: a decisive force in metastasis and invasion of mouse lung adenocarcinoma cell lines. Invasion Metastasis. 9:254–268. [PubMed] [Google Scholar]
- Satlin, L.M., S. Sheng, C.B. Woda, and T.R. Kleyman. 2001. Epithelial Na(+) channels are regulated by flow. Am. J. Physiol. Renal Physiol. 280:F1010–F1018. [DOI] [PubMed] [Google Scholar]
- Sawada, Y., K. Nakamura, K. Doi, K. Takeda, K. Tobiume, M. Saitoh, K. Morita, I.I. Komuro, K. De Vos, M. Sheetz, and H. Ichijo. 2001. Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MAP kinase. J. Cell Sci. 114:1221–1227. [DOI] [PubMed] [Google Scholar]
- Stover, D.R., H. Charbonneau, N.K. Tonks, and K.A. Walsh. 1991. Protein-tyrosine-phosphatase CD45 is phosphorylated transiently on tyrosine upon activation of Jurkat T cells. Proc. Natl. Acad. Sci. USA. 88:7704–7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmenga, E.J., T.T. Andreassen, H.J. Houthoff, and P.J. Klopper. 1991. The effect of mechanical stress on healing skin wounds: an experimental study in rabbits using tissue expansion. Br. J. Plast. Surg. 44:514–519. [DOI] [PubMed] [Google Scholar]
- Viana, F., E. de la Pena, B. Pecson, R.F. Schmidt, and C. Belmonte. 2001. Swelling-activated calcium signalling in cultured mouse primary sensory neurons. Eur. J. Neurosci. 13:722–734. [DOI] [PubMed] [Google Scholar]
- Wang, N., J.P. Butler, and D.E. Ingber. 1993. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 260:1124–1127. [DOI] [PubMed] [Google Scholar]
- Wirtz, H.R., and L.G. Dobbs. 1990. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science. 250:1266–1269. [DOI] [PubMed] [Google Scholar]
- Yano, H., H. Uchida, T. Iwasaki, M. Mukai, H. Akedo, K. Nakamura, S. Hashimoto, and H. Sabe. 2000. Paxillin alpha and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA. 97:9076–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]