Abstract
Glutamate and its synthetic analogues N-methyl-D-aspartate (NMDA), kainate, and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) are potent dilator agents in the cerebral circulation. The close linkage between neural activity-based release and actions of glutamate on neurons and the related decrease in cerebral vascular resistance is a classic example in support of the concept of tight coupling between increased neural activity and cerebral blood flow. However, mechanisms involved in promoting cerebral vasodilator responses to glutamatergic agents are controversial. Here we review the development and current status of this important field of research especially in respect to cerebrovascular responses to NMDA receptor activation.
Keywords: Glutamate, cerebral circulation, cerebral arteries, nitric oxide, neurons, ischemia, NMDA receptors, rats, rabbits, piglets
1. Introduction
Glutamate is one of the most prevalent neurotransmitters in the brain (Bonvento et al., 2002; Kang et al., 2005; Fellin et al., 2006) and it can activate a number of ionotropic receptors on neurons and astroglia (Garthwaite, 1991; Aoki et al., 1997; Guerguerian et al., 2002). The three types of ionotropic glutamatergic receptors are characterized by the names of their synthetic analogues: N-methyl-D-aspartate (NMDA), kainate, and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) (McDonald et al., 1990; Garthwaite et al., 1991; Lerma et al., 1997). Activation of ionotropic glutamatergic receptors on neurons leads to calcium entry, membrane depolarization, activation of intracellular signaling pathways, and the subsequent production and release of vasoactive agents which can diffuse to vascular smooth muscle and dilate cerebral arteries (Garthwaite, 1991; Domoki et al., 2002). Under some circumstances, additional neurons or astroglia may be involved in transmitting or modifying the initial signal from the target neurons to the resistance blood vessels in brain (Filosa et al., 2004; Fellin et al., 2006; Filosa et al., 2006). Recent evidence suggests that activation of the metabotropic glutamate receptors situated on the astrocytes also produce rapid vasodilation of nearby arterioles (Zonta et al., 2003).
The linkage between neural activity-based release and actions of glutamate on parenchymal cells, and the associated decrease in cerebral vascular resistance, provide for the tight coupling between cerebral metabolism and blood flow. However, the chemical nature of the dilator agents and the relative roles of neurons, astroglia, and endothelium in promoting dilator responses have become controversial (Domoki et al., 2002; Fiumana et al., 2003; Zonta et al., 2003; Simandle et al., 2005; Ayata & Moskowitz, 2006; Leffler et al., 2006 a,b). The purpose of this review is to critically examine the literature concerning the factors mediating glutamatergic-mediated dilation in the circulation of the cerebral cortex.
2. Defining the “Playing Field” for responses to glutamatergic agents
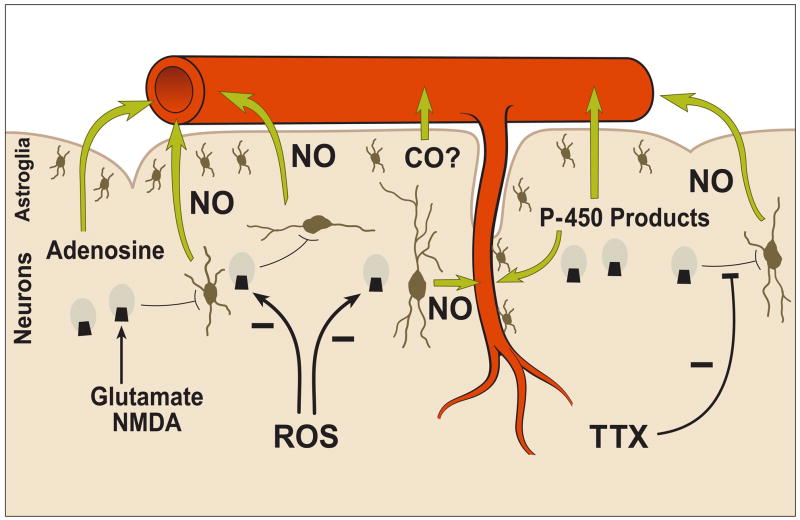
The most comprehensive approach for examining the mechanisms involved in glutamatergic effects on the cerebral circulation is to consider the cerebral blood vessels, astroglia, and neurons as functionally inter-related components of the “neuronal-vascular axis,” as originally introduced in 1998 (Veltkamp et al., 1998; Domoki et al., 1999a), or as the more recently designated “neurovascular unit” (Hawkins and Davis, 2005) (Figure 1). The tight coupling of brain metabolism and blood flow is a well established concept (Roy and Sherrington, 1890) and provides the functional basis for the neurovascular unit (Busija and Leffler, 1987a; Busija, et al., 1988). However, the precise inter-relationships among the components of the neurovascular unit, which account for metabolism/blood flow coupling, is only now being defined for various stimuli. In particular, the potential for a prominent role of astroglia (Filosa et al., 2004; 2006; Mulligan and MacVicar, 2004; Zonta, et al., 2003; Koehler et al., 2006; Lalo et al., 2006) and interneurons (Cauli et al., 2004; Vaucher et al., 2000; Rancillac et al., 2006) in conveying signals between neurons and blood vessels has been described recently using several novel approaches. Interneurons have access to parenchymal arterioles but apparently not to arterioles and arteries proximal to the appearance of the Virchow-Robin space (Abadia-Fenoll, 1969; Busija 1993; Lovick et al., 1999). These interneurons may be involved in vascular signaling from either other local neurons or from projections from distant neurons. Virtually the entire parenchymal segment of the cerebral vasculature is surrounded by astroglial end feet, and this anatomical arrangement is one component of the blood-brain barrier (Hawkins and Davis, 2005; Abbott et al., 2006). While the surface of pial cerebral arteries and arterioles are not usually thought to be invested with astroglia (Mercier and Hatton, 2002), they are positioned over the glia limitans (Niermann et al., 2001) and thus can be influenced by glial-derived factors released into the cerebrospinal fluid (CSF) which is in contact with the blood vessels. A recent report indicates that astroglia, detected with immunostaining against glial fibrillary acidic protein (GFAP), are present in surface arterioles and small arteries from the cerebral circulation of piglets (Leffler et al., 2006a). However, additional studies using other approaches are needed to support this unusual finding. The ubiquitous positioning of the astroglia among cerebral blood vessels and neurons and the well known intercellular interactions involving these cells, appear optimal for rapid and integrated control of vascular tone in order to meet metabolic demands (Figure 1). Cortically-derived astroglial cells are avid producers of prostaglandins and thromboxane (Nam et al, 1996; Thore et al., 1994; 1996) and cerebral arteries normally located close to cortical astroglia are responsive to even small concentrations of dilator and constrictor prostanoids (Busija and Leffler, 1987b; Leffler and Busija, 1985; Wagerle and Busija, 1990). In particular, the profile of prostanoids produced by cerebral arteries varies considerably from that produced by cortical astrogial of the same species (Busija, 1997; 2002) and thus the CSF bathing the cerebral arteries contains substantial amounts of vasoactive prostanoids arising from the brain parenchyma. Other studies have shown that astroglia and neurons can produce and release a wide range of vasodilator substances such as nitric oxide (NO) carbon monoxide (CO), adenosine, hydrogen ion, potassium ion, cyclooxygenase, lipoxygenase, and p-450 monooxygenase products (Bhardwaj et al., 2002; Zonta et al., 2003; Filosa et al., 2006; Koehler et al., 2006; Leffler et al., 2006a; Murphy et al., 1994; Ohata et al., 2006). Neuronal- and astroglial-derived factors can act directly on vascular smooth muscle (Domoki et al., 2002; Filosa et al., 2006) or can act indirectly via endothelium-dependent dilator agents (Murphy et al., 1994). In some brain preparations such as cerebellar slices, astrocytes can also release vasoconstrictor agents (Mulligan and MacVicar, 2004; Rancillac et al., 2006). Recent studies in retina indicate that glial cells can produce both constrictor and dilator agents through omega-hydroxylase and cytochrome P450 epoxygenase pathways, repectively (Metea and Newman, 2006; Metea et al., 2007).
Figure 1.
Schematic illustration of likely mechanisms linking activation of neuronal NMDA receptors in the cerebral cortex to dilation of cerebral arteries and arterioles. NMDA or glutamate activates NMDA receptors on neurons, and following intercellular signaling to nNOS containing neurons, nitric oxide (NO) NO is produced which diffuses to arteries and arterioles and causes vasodilation. This intercellular transmission is blocked by tetrodotoxin (TTX), and the NMDA rceptors can be impaired by actions of reactive oxygen species (ROS). It seems likely that other dilator agents in addition to NO such as adenosine and P-450 monooxygenase products also are involved especially in some species. The role of carbon monoxide (CO) derived from astroglia remains controversial.
3. Studies of glutamatergic agonists in intact brain
3.1. Responses during normal conditions
Busija and Leffler (1989) were the first to demonstrate that glutamate and its synthetic analogue, N-methyl-D-aspartate (NMDA) are dilator agents in the intact cerebral circulation (Table 1). Glutamate and NMDA, when applied to the exposed surface of the piglet cerebral cortex, dilated overlying pial arterioles in a dose-dependent fashion and these responses were unaffected by blockade of prostaglandin synthesis. The control mechanisms of the cerebral circulation and stage of cortical neuronal development of the neonatal pig appear to be similar to the human neonate (Busija, 2002). Dilator responses to glutamate and NMDA were repeatable several times in the same preparation following removal of these agents by flushing with artificial cerebrospinal fluid (aCSF) (Busija and Leffler, 1989; Busija et al., 1996; Philip and Armstead, 2003) and even prolonged application to the cortical surface did not appear to damage the cortex in vivo or affect pial arteriolar responses to other, non-glutamatergic dilator stimuli (Busija and Leffler, 1989). These findings concerning repeatable dilator responses to glutamate and NMDA, which imply little or no damage to arterioles or cortex are in contrast to studies on cultured cerebral endothelium (Sharp et al., 2005; Parfenova et al., 2006) or neurons (Nagy et al., 2004) where glutamate or NMDA can be toxic. Numerous studies by several laboratories have repeated the essential features of these response to glutamate and NMDA in piglets (Armstead et al., 2001; Philip and Armstead, 2003; Leffler et al., 2006a). In addition, all of the original studies in piglets with glutamate and NMDA have been replicated numerous times in other species such as the rat (Bhardwaj et al., 2000, Iliff et al., 2003, Pelligrino et al., 1995; 1996; Sun et al., 2005) and rabbit (Faraci and Breese, 1993). Thus, the fundamental finding that glutamate and NMDA elicit cerebrovascular dilation in vivo appears to be a universal finding across species. In contrast to these studies, Huang et al. (1994) has reported that NMDA and glutamate constrict cortical arteries in the rat. The reason for this unusual finding by Huang et al. (1994) is not clear.
Table 1.
Glutamate agonists and cerebral vascular tone
| Year | Authors | Preparation | Species | Agent | Application | Effect | Mechanism |
|---|---|---|---|---|---|---|---|
| 1989 | Busija & Leffler | Cranial Window | Piglet | Glutamate
NMDA |
Topical
Topical |
Dilation
Dilation |
Non-prostanoid
Non-prostanoid |
| 1989 | Hardebo et al. | Isolated arteries | Rat, cow, man | Glutamate | Topical | No response | NA |
| 1992 | Armstead et al. | Cranial Window | Piglet | Glutamate | Topical | Increased metabolism | Not examined |
| 1993 | Faraci & Breese | Cranial Window
Isolated arteries |
Rabbit
Rabbit |
NMDA
NMDA |
Topical
Topical |
Dilation
No response |
NO mediated
Tetrodotoxin-sensitive NA |
| 1993 | Busija & Wei | Cranial Window | Piglet | NMDA | Topical | Dilation | Anoxia-sensitive |
| 1994 | Huang et al. | Cranial Window | Rat | Glutamate
NMDA |
Topical
Topical |
Constriction
Constriction |
Non-prostanoid |
| 1994 | Nakai & Maeda | Microspheres | Rat | NMDA | Injection | Increased CBF | Not examined |
| 1994 | Faraci et al. | Cranial Window | Rabbit | Kainate | Topical | Dilation | NO-mediated |
| 1994 | Faraci & Breese | Cranial Window | Rabbit | NMDA | Topical | Dilation | Non-trigeminal
Non-cholinergic |
| 1994 | Faraci & Heistad | Cranial Window | Rat | NMDA | Topical | Dilation | Unaffected by aging |
| 1995 | Mayhan & Didion | Cranial Window | Rat | NMDA | Topical | Dilation | Reduced by alcohol |
| 1995 | Meng et al. | Cranial Window | Piglet | Glutamate | Topical | Dilation | NMDA receptor mediated
Neuronal NO mediated |
| 1995 | Faraci & Brian | Cranial Window | Rabbits | NMDA | Topical | Dilation | Neuronal NO mediated |
| 1995 | Northington et al. | Microspheres/H Clearance | Sheep | NMDA | Microdialysis | Dilation | NO mediated |
| 1995 | Kaiser & During | Laser Doppler | Rat | NMDA | Microdialysis | Increased CBF | NO-/adenosine mediated |
| 1995 | Pelligrino et al. | Cranial Window | Rat | NMDA | Topical | Dilation | Tetrodotoxin-sensitive |
| 1995 | Faraci & Brian | Cranial Window | Rabbit | NMDA | Topical | Dilation | Neuronal NO mediated |
| 1995 | Mayhan & Patel | Cranial Window | Rat | NMDA | Topical1 | Dilation | Reduced by hyperglycemia |
| 1996 | Bari et al. | Cranial Window | Piglet | NMDA | Topical | Dilation | Inhibited by superoxide anion |
| 1996 | Nakai & Maeda | Laser Doppler/Microspheres | rats | NMDA | Injection | Increased CBF | Activated central pathway |
| 1996 | Pelligrino et al. | Laser Doppler | Rat | NMDA | Intravenous | Increased CBF | NO-mediated |
| 1996 | Wendling et al. | Arterial rings | Bovine | NMDA
Gltuamate |
Topical
Topical |
No response
No response |
NA
NA |
| 1996 | Weiss et al. | 14C-iodoantipyrine | Rat | NMDA | Topical | Increased CBF | Increased O2 consumption |
| 1996 | Hara et al. | Laser Doppler | Rat | NMDA | ICV | Increased CBF | Increased brain PO2 |
| 1997 | Fergus & Lee | Hippocampus slice | Rat | Glutamate
NMDA AMPA Kainate |
Topical
Topical Topical Topical |
Dilation
Dilation No response Dilation |
NA
Neuronal NO mediated Tetrodotoxin-sensitive NA NA |
| 1997 | Lu et al. | 14C-iodoantipyrine | Rat | Glutamate | Topcial | Increased CBF | Increased O2 consumption |
| 1997 | Bari et al. | Cranial Window | Piglet | Kainate | Topical | Dilation | NO and prostaglandin mediated |
| 1998 | Yang & Chang | Laser Doppler | Rat | NMDA | Topical | Increased CBF | Neuronal NO mediated |
| 1998 | Bari et al. | Cranial Window | Piglet | NMDA | Topical | Dilation | nNOS and NMDA receptors located on different neurons |
| 1998 | Taylor et al. | Doppler Ultrasound | Piglet | NMDA | Injection | Increased CBF | Not examined |
| 1999 | Lovick et al. | Hippocampus slice | Rat | NMDA | Topical | Dilation | Neuronal NO mediated
Blocked by Hb NOS neurons close to arterioles |
| 1999 | Domoki et al. | Cranial Window | Piglet | NMDA | Topical | Dilated | Resistant to cortical depolarization |
| 2000 | Bhardwaj et al. | H Clearance | Rat | NMDA | Microdialysis | Increased CBF | NO-and P-450 monooxygenase mediated |
| 2002 | Domoki et al. | Cranial Window | Piglet | NMDA | Topical | Dilation | Endothelium Independent
NO metabolite in CSF |
| 2002 | Chi et al. | Cranial Window | Rat | NMDA | Topical | Increased CBF | Neuronal NO dependent |
| 2003 | Parfenova et al. | Endothelium | Piglet | Glutamate
cis-ACPD |
Topical
Topical |
CO production
CO production |
Heme oxygenase dependent
Heme oxygenase dependent |
| 2003 | Fuimana et al. | Isolated arteries | Piglet | Glutamate
cis-ACPD |
Topical
Topical |
Dilation
Dilation |
CO-Mediated
CO-Mediated |
| 2003 | Iliff et al. | Cranial Window | Rat | Glutamate | Topical | Dilation | Adenosine-mediated |
| 2004 | Philip & Armstead | Cranial Window | Piglet | NMDA | Topical | Dilation | Inhibited by superoxide anion |
| 2005 | Simandle et al. | Isolated arteries | Piglet | NMDA
Glutamate |
Topical
Topical |
No dilation
No dilation |
NA
NA |
| 2006 | Ayata & Moskowitz | Cranial Window
Laser Doppler |
Mouse | NMDA | Topical | Increased CBF | CSD-mediated
NO-mediated |
| 2006a | Leffler et al. | Cranial Window | Piglet | Glutamate | Topical | Dilation | Astrocyte dependent
CO mediated Tetrodotoxin independent |
| 2006 | Bari et al. | Cranial Window | Piglet | NMDA | Topical | Dilation | Reduced by kynurenic acid |
| 2006 | Ohata et al. | Cranial Window | Rat | AMPA | Topical | Dilation | Adenosine/CO coupled |
Abbreviations: NMDA, N-methyl-D-aspartate; AMPA, alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate, NO, nitric oxide; CO, carbon monoxide, 1-aminocyclopropanecarboxylic acid (ACPD), ACPD, 1-aminocyclopropanecarboxylic acid; Hb, hemoglobin; NA, not applicable.
Faraci and Breese (1993) first demonstrated that dilation to NMDA was eliminated or reduced by treatment with the general NOS inhibitor, Nω-nitro-L-arginine (L-NAME) in rabbits. These results were later been confirmed in the piglet (Meng et al., 1995; Bari et al., 1996) and in the rat (Pelligrino et al., 1995; 1996). Subsequent studies published in the same year for piglet and rabbit showed that administration of 7-nitroindazole (7-NI), the selective inhibitor of neuronal NOS (nNOS), abolished or reduced dilation to NMDA or glutamate (Faraci and Brian, 1995; Meng et al., 1995). Thus, another similar finding in all species studied is that NOS inhibition reduces or eliminates cerebrovascular dilation to NMDA and glutamate. Support for these pharmacological studies was the finding that NMDA application to the cortex resulted in accumulation of NO degradation products in the CSF surrounding the arteries (Domoki et al., 2002). The presence of an underlying vein under the pial arteriole under study reduced its ability to dilate to NMDA compared to an arteriole directly on the cortical surface (Domoki et al., 2002), thereby further reinforcing the concept that diffusion of a vasoactive, possibly gaseous substance from the cortical surface reaching the arteriole caused the dilation. Robinson et al., (2002) have reported that 1-aminocyclopropanecarboxylic acid (ACPD), which they characterized as a NMDA receptor agonist, dilates piglet pial arterioles. However, depending upon the exact configuration of this compound (trans-/cis-/racemic), which was not specified in the paper, it could also act as a glycine receptor agonist and/or a glutamate-site antagonist at NMDA receptors (Nahum-Levy et al., 1999; Peterson et al., 2004). We are unaware of any other in vivo cerebral circulatory studies in any other species using this compound.
Although glutamate has the potential to exert vasoactive influences via activation of any of the three ionotropic glutamate receptors, topical application to the surface of the cerebral cortex appears to predominantly activate the NMDA receptors. Thus, MK-801, a selective NMDA receptor antagonist, blocks most if not all of the cerebral dilator response to glutamate as well as NMDA in rabbits, piglets, and rats (Faraci and Breese, 1993; Meng et al., 1995; Pelligrino et al., 1995;1996). The reason for this preference for the NMDA receptors in the cortex is not known, but may be due to their location in the cerebral cortex or greater affinity for glutamate than kainate or AMPA receptors. Administration of the sodium channel blocker tetrodotoxin, an agent which does not usually affect the endothelium (Emerson and Segal, 2001), completely eliminates NMDA-induced dilation in vivo in piglet (Leffler et al., 2006a), rabbit (Faraci and Breese, 1993), and rat (Pelligrino et al., 1996) cerebral arterioles and arteries. However, dilator responses of cerebral resistance vessels to glutamate were reported to be intact in piglets despite the presence of tetrodotoxin (Leffler et al., 2006a).
Despite several recent reports (Fiumana et al., 2003; Parfenova et al., 2001; 2003), it is questionable that the primary effects of glutamate and NMDA receptor agonists represent direct, endothelium-dependent effects on arteries and arterioles. Isolated cerebral arteries in a number of species have not been found to dilate in response to application of glutamate or NMDA (Faraci and Breese, 1993; Simandle et al., 2005) and endothelial damage in vivo failed to affect NMDA-induced dilation while responses to other endothelium-dependent dilator stimuli were blocked (Domoki et al., 2002). Furthermore, the absence of functional NMDA and AMPA receptors in rat and human cerebromicrovascular endothelial cells has also been demonstrated (Morley et al., 1998).
Consistent with a direct role on parenchyma, glutamate application increased cortical metabolic rate in proportion to calculated changes in cerebral vascular resistance (Armstead et al., 1992). NMDA-induced dilation also apparently does not involve activation of perivascular nerves, since blockade of muscarinic and CGRP receptors does not affect the cerebral vascular dilator response to topical NMDA in rabbits (Faraci and Breese, 1994).
Application of kainate and AMPA receptor agonists also results in dilation of cerebral arteries in all species studied when applied to the cerebral cortex, but these agents have not been as extensively studied in this context as have glutamate and NMDA. Kainate leads to prolonged dilator responses in piglets that are partially blocked equally by indomethacin and L-NAME, but not by adenosine or NMDA receptor antagonists (Bari et al., 1997). Similarly, kainate-induced dilation of cerebral arterioles is reduced by NOS inhibition in rabbits (Faraci et al., 1994). Garthwaite et al. (1989) have reported that kainate leads to production of NO by brain. It also appears that antagonists or blockers of heme oxygenase also reduce dilation to kainate (Ohata et al., 2006) or to the kainate receptor agonist, (RS)-2-amino-3-(3-hydroxy-5-t-butylisoxazol-4-yl)propionic acid (ATPA) (Robinson et al., 2002). AMPA application leads to cerebrovascular dilation which reportedly is reduced with inhibitors of heme oxygenase (Ohata et al., 2006; Robinson et al., 2002) and adenosine receptor blockade (Ohata et al., 2006). Bhardwaj et al. (1997) has reported that AMPA application leads to cortical production of NO. Thus, activation of all three types of glutamatergic ionotropic receptors in cerebral cortex leads to activation of nNOS and production of NO.
Activation of glutamatergic receptors on neurons and astroglia in the cerebellum also promotes vascular dilation. The relative contribution of the AMPA and NMDA receptors to vasodilation appears to be different than in the cerebral cortex probably due to the regional distribution of these receptor subtypes. Effects of glutamatergic stimuli on the cerebellar arteries and arterioles are discussed in detail in several relevant papers (Akgoren et al., 1997; Rancillac et al., 2006; Yang and Iadecola, 1996; Yang et al., 1999). For example, both electrical stimulation of parallel fibers in the cerebellum, which causes release of glutamate, and the microinjection of glutamate to this region, increases blood flow in part via activation of AMPA receptors and subsequent production and actions of neuronally-derived NO (Yang and Iadecola, 1996). In contrast, activation of stellate cells or application of NMDA dilates cerebellar arteries via production and actions of NO (Rancillac et al., 2006).
3.2 Altered responses during pathological conditions
Maintenance of metabolism/blood flow in the brain may be disrupted by pathological conditions (Table 1). Several groups have found that cerebrovascular dilator responses to glutamatergic agonists can be changed by a variety of experimental conditions in all species studied. For example, cerebrovascular dilation to NMDA is reduced or eliminated in various species following asphyxia (Busija and Wei, 1993), alcohol administration (Mayhan and Didion, 1995), hyperglycemia (Mayhan and Patel, 1995), ischemia/reperfusion (Busija et al., 1996), hypoxia/reoxygenation (Bari et al., 1996;1998), and traumatic brain injury (Armstead, 2000) via mechanisms largely dependent upon production and actions of reactive oxygen species (ROS) such as superoxide anion (Bari et al., 1996; Philip and Armstead, 2003;2004). The primary site of action of the ROS appears to be at the level of the NMDA receptor (Kim et al., 1999; Choi and Lipton, 2000; Guerguerian et al., 2002) since dilator responses of pial arterioles are still intact to exogenous NO after ischemic stress (Busija et al., 1996) (Figure 1). Attenuation of NMDA-induced dilator responses can be prevented by pre-treatment with activators of plasma membrane or mitochondrial KATP channels (Veltkamp et al., 1998; Domoki et al., 1999), cyclooxygenase inhibitors (Busija et al., 1996; Domoki et al., 2001), protein synthesis inhibitors (Veltkamp et al., 1999), and superoxide dismutase (Bari et al., 1996). In contrast to these findings with NMDA, kainate-induced cerebral arteriolar dilation is resistant to ischemic stress (Bari et al., 1997). This finding may indicate that kainate receptors are present on ischemia-resistant neurons and astroglia or that kainate receptors are not readily affected by actions of ROS. We are unaware of studies examining effects of ROS-mediated stresses on AMPA-induced dilation.
4. Mechanisms of dilation
The combined results from many investigators in different species have lead to the proposal that NMDA receptor stimulation in cortical neurons results in calcium influx, membrane depolarization, activation of intracellular nNOS, and the subsequent production of NO, which diffuses to cerebral arteries and arterioles and causes relaxation of vascular smooth muscle without the involvement of astroglia or endothelium (Bari et al., 1998; Domoki et al., 1999; Faraci and Breese, 1993; Faraci and Brian, 1995; Meng et al., 1995; Pelligrino et al.,1996; Veltkamp et al., 1998) (Figure 1).
Immunohistochemistry of the piglet brain revealed a particularly distinct band of NMDA receptors (NMDAR 2A/B) containing neurons in cortical layer II/III interspersed with large nNOS-positive neurons (Bari et al., 1998). NMDAR 2A/B-nNOS double-immunopositive neurons usually were not found, or at least were not prominent at the light microscopy level. Unlike cortical neurons in culture, a situation in which almost all the mature neurons contain NMDA receptors and nNOS (Kim et al., 1999), NMDA receptors are restricted to more limited populations of neurons in cerebral cortex and may or may not be co-localized with nNOS depending upon anatomical location, developmental stage, or species studied (Aoki et al., 1997; Bari et al., 1998; Gracy and Pickel, 1997). In particular, Aoki et al., (1997) detailed the difficulty and uncertainty of fully evaluating dual NMDA receptor and nNOS localization in neurons in the sensory cortex at the light and electron microscopic levels. Thus, it seems likely that additional neurons, or possibly astroglia, are involved in the transmission of the dilator signaling from the activated neurons possessing NMDA receptors to blood vessels. This view is reinforced by pharmacological results from rats (Bhardwaj et al., 1997) indicating that NMDA-induced brain production of NO involved not only neurons with NMDA-receptors but also those with AMPA-receptors. We are unaware of any additional studies which have examined the relationship between NMDA-receptor and nNOS containing neurons in the cerebral cortex. Based upon well known age- and species- differences in cerebrovascular control mechanisms, it is not surprising that part of the dilator responses to NMDA in rats may be due to production and release of other substances such as adenosine (Iliff et al., 2003) or P-450 monooxygenase metabolites (Bhardwaj et al., 2002) (Figure 1). However, we could find no evidence that adenosine (Bari et al., 1998), prostaglandins (Busija and Leffler, 1989), or P-450 monooxygenase products (Domoki et al., 2002) contribute substantially to NMDA-induced cerebral arterial dilation in piglets. Nonetheless, the common finding is that one or more dilator agents, primarily arising from cortical neurons, mediate the dilator responses to NMDA, which is consistent with the probable involvement of multiple neurons or sequential signaling pathways.
The lack of principal involvement of astroglia as initiators of NMDA-induced cerebral dilation is indicated by the ability of tetrodotoxin to totally block the response to topically applied NMDA (Farci and Breese, 1993; Pelligrino et al., 1995; Leffler et al., 2006a) and by the persistence of the dilator response to NMDA despite destruction of the glia limitans (Leffler, CW, personal communication). Tetrodotoxin does not normally impair astroglia-initiated responses or endothelium-dependent dilation (Emerson and Segal, 2001). The ability of astroglia to produce large amounts of vasoactive prostaglandins (Busija 1997; Busija 2003; Nam et al, 1996; Thore et al., 1994; 1996) and the lack of effect of indomethacin on NMDA- or glutamate-induced cerebral arteriolar dilation in piglets (Busija and Leffler, 1989), provides additional evidence that astroglia do not play a role in conveying or modifying signaling between NMDA receptor containing neurons and vascular smooth muscle. Several studies in diverse species (rat, cat, piglet, cow, man) fail to show significant dilator effects of NMDA and glutamate on isolated cerebral arteries (Faraci and Breese, 1993; Hardebo et al., 1989; Simandle et al., 2005; Takayasu and Dacey, 1989; Wendling et al., 1996). Additionally, impairment of endothelial function does not affect pial arterial and arteriolar dilation in vivo to topical application of NMDA (Domoki et al., 2002). Again, the lack of direct effects of NMDA or glutamate in cerebral resistance vessels appears to be a common finding among the species studied.
The magnitude of the increase in NO production upon NMDA application probably is underestimated by measurements of degradation products in perivascular CSF (Domoki et al., 2002) because a high percentage of the NO generated by neurons is prevented from reaching the pial vessels by reactions with iron containing substances such as hemoglobin and by other cellular components. This assumption is supported by the finding that arteriolar segments being “shielded” from the parenchyma by an underlying large vein show significantly smaller dilatory responses to NMDA (Domoki et al., 2002). Nonetheless, it appears that NO is able to diffuse considerable distances in healthy cortical tissue to exert vascular effects.
It has been suggested that endothelium-derived NO plays a permissive role in carbon monoxide (CO)-mediated dilator responses to glutamate and NMDA receptor agonists in the piglet cerebral circulation (Barkoudah et al., 2004; Leffler et al., 2006b). Therefore, L-NAME may not only block NO-dependent responses but those involving CO as well. In our studies using 7-NI, which blocked both glutamate and NMDA-induced dilator responses in arterioles (Meng et al., 1995), endothelial NOS would still be functionally intact and capable of generating substantial amounts of vascular NO from resistance and capacitance vessels as well as capillaries and thus provide the necessary background levels of NO for CO production. If NO is necessary to allow the expression of CO-mediated dilation to glutamatergic receptor agonists, then is unclear why 7-NI abolishes cerebral vascular dilation to glutamate and NMDA. In contrast to the results in piglets, CO has been shown to be a tonic suppressor of NO-dependent dilation in the rat cerebral circulation via inhibition of NOS activity (Ishikawa et al., 2005). Ishikawa et al. (2005) also found a similar mechanism operating in cultured porcine endothelial cells.
5. Controversies
Two recent publications have challenged the concept that glutamate- or NMDA-mediated dilator responses involve sequential stimulation of neuronal NMDA receptors, activation of nNOS, and direct relaxation of vascular smooth muscle by NO. First, Ayata and Moskowitz (2006) have reported that NMDA application elicits cortical spreading depression (CSD) which complicates the elucidation of mechanism involved in NMDA-induced cerebral vasodilation. Second, Fiumana et al., (2003) have reported that glutamate and a NMDA receptor agonist are able to dilate isolated arteries via production and actions of CO.
5.1. Contribution of cortical spreading depression to NMDA-mediated responses
Ayata and Moskowitz (2006) have suggested an alternative mechanism concerning NMDA-induced cerebrovascular responses in mice. Based upon electrophysiological recordings, they propose that the cerebrovascular responses to topical NMDA represents a complex response due to overlapping vasoactive influences associated with CSD-related effects and direct effects of NMDA on individual neurons (Ayata and Moskowitz, 2006). Such a mechanism is plausible since it was shown earlier by other authors (Lauritzen and Hansen, 1992; Lauritzen, 1994) that propagation of CSD is promoted by neuronal release of glutamate and subsequent activation of NMDA receptors on neighboring neurons. Whether a similar complex interaction between CSD and direct effects on neurons by NMDA also occurs in other species with lissencephalic brains such as rats and rabbits in unclear. L-NAME treatment in rats fails to affect cerebrovascular responses to CSD (Fabricius et al., 1995; Shimizu et al., 2002) while dilator responses to topical NMDA are reduced or eliminated following acute NOS blockade in this species (Pelligrino et al., 1996). Similarly, in rabbits, nNOS-derived NO and calcitonin gene-related peptide released from perivascular nerves contribute equally to CSD-induced dilation of pial arterioles (Colonna et al., 1993; 1994; 1997) while topical application of 100 μM NMDA is completely abolished by inhibition of NOS (Faraci and Breese, 1993).
CSD probably does not occur in piglet brain or in other gyrencephalic brains from other species in response to glutamate or NMDA for several reasons. Cortical spreading depression is not easily invoked in piglet cortex or in the neonatal brain in general and the pattern of dilation to NMDA is not consistent with that seen with CSD (Busija and Leffler, 1989; Faraci and Breese, 1993; Shibata et al., 1990). Additionally, the application of NMDA to the large area of the cortex present under the cranial window as used in piglets (and rabbits), probably affected all of the exposed neurons simultaneously and therefore would not be expected to initiate a CSD-like episode that would progress across the cortical surface under observation. While it is possible that widespread cortical depolarization, rather than selective depolarization of NMDA-receptor containing neurons, occurs under the cranial window with glutamate or NMDA application, at least in the piglet, the arteriolar effects of cortical depolarization (Domoki et al., 1999b) are completely different than observed with application of glutamatergic agonists in this species (Busija and Leffler, 1989; Meng et al., 1995). Additionally, while blockade of KATP channels with glibenclamide enhances dilator effects to CSD in rats (Shimizu et al., 2000), glibenclamide attenuates cerebral vascular responses to NMDA (Philip and Armstead, 2004). Thus, it appears that in mice, but probably not in other species under normal conditions, NMDA decreases cerebrovascular resistance via mechanisms that are not related to initiation or propagation of CSD, but rather involve the selective, direct activation of NMDA-containing neurons.
5.2. Direct effects of glutamate and NMDA receptor agonists on cerebral arteries
Fiumana et al., (2003) reported that both glutamate and 1-aminocyclopentane-cis-1,3-dicarboxylic acid (cis-ACPD), the latter a putative NMDA receptor agonist, dilated isolated arteries up to 15–20% via generation and actions of CO. Thus, cell types specific to the vasculature rather than neurons were the apparent targets of glutamate and cis-ACPD and the dilator agents were produced solely within the vascular wall. This study contrasts with all other studies done in isolated cerebral arteries from a variety of species. Additionally, these authors indicated an essential role of the endothelium since glutamate-induced dilation of isolated piglet arteries was reversed to constriction following endothelial denudation. Whether cis-ACPD continued to dilate these arteries following endothelium denudation was not reported. Unfortunately, our laboratory has been unable to replicate the results for glutamate or NMDA on isolated piglet arteries and arterioles, not only at physiological doses, but at pharmacological doses as well (Simandle et al., 2005). Furthermore, we have shown that NMDA-induced dilation in vivo was still intact following impairment of endothelial function (Domoki et al., 2002). Other authors similarly have shown that NMDA does not dilate isolated cerebral arteries from rabbit (Faraci and Breese, 1993), rat (Hardebo et al., 1989; Takayasu and Dacey, 1989), cat (Hardebo et al., 1989), cow (Wendling et al., 1996) and man (Hardebo et al., 1989) (Table 1). In addition, cerebral vascular dilator responses to glutamate, when present, may not be specific for the R- and L- isomers of glutamate (Wendling et al., 1996), thereby indicating a lack of specific action of this amino acid against NMDA or other glutamatergic receptors. The reasons for these differences could involve use of different NMDA-receptor agonists or other aspects of the experimental approach. While cis-ACPD has been reported to be a NMDA receptor agonist, this agent apparently has not been used by other groups and its potency and specificity for NMDA receptors has not been examined in the cerebral circulation.
It is also possible that some aspects of our experimental protocol prevented the detection of significant NMDA or glutamate dilator responses in isolated arteries of piglets. For example, Leffler et al. (2006a) have reported the presence of positive GFAP-immunostaining of piglet pial arterioles and arteries (diameters up to ~200 μM), and suggested that the presence of functionally intact astroglia associated with the vessel wall was necessary for the dilator responses to glutamate in pressurized, isolated cerebral vessels. Thus, based upon this publication (Leffler et al., 2006a) and an earlier one (Fiumana et al., 2003) in which it was reported that endothelium damage prevented dilation to glutamate, functionally intact cerebral vascular astroglia and endothelium apparently are both required for glutamate-induced dilation of isolated arteries. This finding concerning the presence of astroglia in surface cerebral vessels is in contrast to previous reports which limit projections of the astroglia to the glia limitans, which apparently does not penetrate the pia and arachnoid layers of the meninges where the pial blood vessels are located, and to the exterior of parenchymal blood vessels distal to the Virchow-Robins space (Mercier and Hatton, 2002; Niermann et al., 2001). The surface resistance vessels of the cerebral cortex are tightly bound by the pia to the underlying glia limitans and it is possible that positive GFAP immunostaining is due to extravascular contamination by attached cortical astroglia during removal of the arteries and arterioles. However, it seems unlikely that astroglia pulled off the cortical surface along with the arteries and arterioles could be functionally intact following procedures and conditions involved in the preparation and study of pressurized cerebral arteries in vitro. Nonetheless, this interesting finding concerning the presence of functionally intact astroglia in surface cerebral arteries and arterioles should be confirmed by another laboratory.
A possible role of CO in conveying or modifying the initial NO signal from NMDA receptor containing neurons in the cortex to vascular smooth muscle cannot be excluded especially when glutamate is administered. Histological examination of the cortical areas underlying pial arterioles indicate that NMDA receptors and brain NOS are located on different neurons (Bari et al., 1998) and thus a multi-cellular sequence of events involving adjacent neurons and perhaps astroglia is likely required for the generation of dilator agents responsible for the vascular response. Glutamate in particular can have effects on multiple cell types in brain either via actions on metabotropic and inotropic receptors, or as a consequence of uptake and metabolism by astroglia (Schousboe and Waagepetersen, 2005). Further evidence for a multi-cellular mechanism also is suggested by studies by Phililp and Armstead (2004b), where blockade of ATP-sensitve potassium (KATP) channels or calcium-activated potassium (KCa) channels both reduce dilation to NMDA application, although the contribution of KATP channels is much greater. There are other examples of multiple cell types mediating cerebrovascular dilation to physiological stimuli. For example, we have shown that in CSD-induced cerebrovascular dilation in rabbits, cortically derived NO, perivascular nerve derived calcitonin gene-related peptide, and prostaglandins from parenchyma and/or blood vessels all contribute to the final blood flow response (Colonna et al., 1993; 1994; 1997).
Interpretation of data from recent studies in piglet by Leffler and colleagues (Leffler et al., 2005; 2006a) leads to the suggestion that nitric oxide availability and related intracellular actions represent mandatory components linking activation of glutamatergic receptors to CO-mediated cerebral vascular dilation. In support of this theory are findings that indicate that the NO donor, sodium nitroprusside, increases CO levels in piglet cerebral microvessels to the same extent as glutamate (Leffler et al., 2005) and that CO apparently dilates piglet pial arterioles via activation of smooth muscle KCa channels (Barkoudah et al., 2004). On the other hand, Armstead (1997) has reported that the KCa channel inhibitor, iberiotoxin, which should block CO-mediated smooth muscle responses, does not affect pial arteriolar dilation following sodium nitroprusside administration in piglets. Production of CO also appears to occur following activation of AMPA receptors in rat and piglet cerebral cortex (Ohata et al., 2006; Robinson et al., 2002). On the other hand, there are contrasting findings in which it was shown that CO inhibits NOS activity in rat cerebral arteries and cultured porcine endothelial cells (Ishikawa et al., 2005). Additionally, a role of CO in control of the adult cerebral circulation, especially in the rat, has been discounted by recent experiments (Andressen et al., 2006). Thus, this interesting area needs additional research in order to more precisely define the possible role of CO in glutamatergic receptor-induced vasodilation in the cerebral circulation.
6. Conclusions
Activation of all three types of ionotropic receptors on neurons by glutamatergic agonists leads to dilation of cerebral resistance vessels and provides the foundation for tight coupling between metabolic demand and blood flow in brain. Glutamate appears to preferentially activate NMDA receptors in cortex, and the preponderance of evidence indicates that NMDA receptor activation leads to depolarization of cortical neurons and enhanced production of NO from those or adjacent neurons. The NO produced by neurons then diffuses relatively long distances to cerebral arterioles and arteries and relaxes vascular smooth muscle apparently without the contribution of vasoactive substances from endothelium, perivascular nerves, or astroglia. While NO appears to be the predominant, perhaps initiating component of the cascade of events leading to cerebrovascular dilator responses following NMDA-receptor activation of cortical neurons, it appears likely that additional vasoactive agents such as adenosine and cytochrome p-450 monooxygenase products also make important contributions, especially in rats. However, the potential contribution of astroglial- or endothelial-derived CO especially in the neonate in response to glutamate deserves further consideration.
Acknowledgments
Supported by NIH grants HL-07731, HL-030260, DK-062372, and HL-065380, Y. F. Wu Research and Education Fund, WFUSM Venture Fund, and K.G. Phillips Fund for the Prevention and Treatment of Heart Disease, WFUSM Interim Funding, and by the Hungarian Science Foundation (OTKA K 63401 and IN 69967). We thank Nancy Busija, M.A., for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abadia-Fenoll F. Structure of the leptomininx and cerebral vessels of the cat. II. Constituent elements of the intracerebral vessels wall (analogy with the meninges) Angiology. 1969;20:535–562. doi: 10.1177/000331976902000908. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Akgoren N, Mathiesen C, Rubin I, Lauritzen M. Laminar analysis of activity- dependent increases of CBF in rat cerebellar cortex: dependence on synaptic strength. Am J Physiol. 1997;273:H1166–1176. doi: 10.1152/ajpheart.1997.273.3.H1166. [DOI] [PubMed] [Google Scholar]
- Aoki C, Rhee J, Lubnin M, Dawson TM. NMDA-R1 subunit of the cerebral cortex co-localizes with neuronal nitric oxide synthase at pre- and postsynaptic sites and in spines. Brain Res. 1997;750:25–40. doi: 10.1016/s0006-8993(96)01147-x. [DOI] [PubMed] [Google Scholar]
- Armstead WM. Role of activation of calcium-sensitive K+ channels in NO- and hypoxia-induced pial artery vasodilation. Am J Physiol Heart Circ Physiol. 1997;272:H1785–H1790. doi: 10.1152/ajpheart.1997.272.4.H1785. [DOI] [PubMed] [Google Scholar]
- Armstead WM. NOC/oFQ contributes to age-dependent impairment of NMDA-induced cerebrovasodilation after brain injury. Am J Physiol Heart Circ Physiol. 2000;279:H2188–2195. doi: 10.1152/ajpheart.2000.279.5.H2188. [DOI] [PubMed] [Google Scholar]
- Armstead WM. Age-dependent vasopressinergic modulation of NOC/oFQ-induced impairment of NMDA cerebrovasodilation after brain injury. J Neurotrauma. 2001;18:615–623. doi: 10.1089/089771501750291855. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Mirro R, Zuckerman S, Busija DW, Leffler CW. The influence of opioids on local cerebral glucose utilization in the newborn pig. Brain Res. 1992;571:97–102. doi: 10.1016/0006-8993(92)90513-9. [DOI] [PubMed] [Google Scholar]
- Ayata C, Moskowitz MA. Cortical spreading depression confounds concentration-dependent pial arteriolar dilation during N-methyl-D-aspartate superfusion. Am J Physiol Heart Circ Physiol. 2006;290:H1837–1841. doi: 10.1152/ajpheart.01102.2005. [DOI] [PubMed] [Google Scholar]
- Barkoudah E, Jaggar JH, Leffler CW. The permissive role of endothelial NO in CO-induced cerebrovacular dilation. Am J Physiol Heart Circ Physiol. 2004;287:H1459–1465. doi: 10.1152/ajpheart.00369.2004. [DOI] [PubMed] [Google Scholar]
- Bari F, Errico RA, Louis TM, Busija DW. Differential effects of short-term hypoxia and hypercapnia on N-methyl-D-aspartate-induced cerebral vasodilatation in piglets. Stroke. 1996;27:1634–1639. doi: 10.1161/01.str.27.9.1634. [DOI] [PubMed] [Google Scholar]
- Bari F, Louis TM, Busija DW. Kainate-induced cerebrovascular dilation is resistant to ischemia in piglets. Stroke. 1997;28:1272–1276. doi: 10.1161/01.str.28.6.1272. [DOI] [PubMed] [Google Scholar]
- Bari F, Thore CR, Louis TM, Busija DW. Inhibitory effects of hypoxia and adenosine on N-methyl-D-aspartate-induced pial arteriolar dilation in piglets. Brain Res. 1998;780:237–244. doi: 10.1016/s0006-8993(97)01196-7. [DOI] [PubMed] [Google Scholar]
- Bari F, Nagy K, Guidetti P, Schwarcz R, Busija DW, Domoki F. Kynurenic acid attenuates NMDA-induced pial arteriolar dilation in newborn pigs. Brain Res. 2006;1069:39–46. doi: 10.1016/j.brainres.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Northington FJ, Ichord RN, Hanley DF, Traystman RJ, Koehler RC. Characterization of ionotropic glutamate receptor-mediated nitric oxide production in vivo in rats. Stroke. 1997;28:850–856. doi: 10.1161/01.str.28.4.850. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Northington FJ, Carhuapoma JR, Falck JR, Harder DR, Traystman RJ, Koehler RC. P-450 epoxygenases and NO synthase inhibitors reduce cerebral blood flow response to N-methyl-D-aspartate. Am J Physiol. 2000;279:H1616–1624. doi: 10.1152/ajpheart.2000.279.4.H1616. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Sibson N, Pellerin L. Does glutamate image your thoughts? Trends Neurosci. 2002;25:359–364. doi: 10.1016/s0166-2236(02)02168-9. [DOI] [PubMed] [Google Scholar]
- Busija DW. The role of central neural pathways in the regulation of cerebral blood flow. In: Phillis JW, editor. The regulation cerebral blood flow. New York: CRC Press; 1993. pp. 65–77. [Google Scholar]
- Busija DW. Eicosanoids and cerebrovascular control. In: Welch KMA, Caplan LR, Reis DJ, Siesjö BK, Weir B, editors. Primer on Cerebrovascular Diseases. Academic Press; 1997. pp. 93–96. [Google Scholar]
- Busija DW. Prostaglandins and other Eicosanoids. In: Edvinsson L, Krause D, editors. Cerebral blood flow and metabolism. Lippincott Williams and Wilkins; 2002. pp. 325–338. [Google Scholar]
- Busija DW, Leffler CW. Hypothermia reduces cerebral metabolic rate and cerebral blood flow in newborn pigs. Am J Physiol. 1987;22:H869–H873. doi: 10.1152/ajpheart.1987.253.4.H869. [DOI] [PubMed] [Google Scholar]
- Busija DW, Leffler CW. Eicosanoid synthesis elicited by norepinephrine in piglet parietal cortex. Brain Res. 1987;403:243–248. doi: 10.1016/0006-8993(87)90061-8. [DOI] [PubMed] [Google Scholar]
- Busija DW, Leffler CW. Dilator effects of amino acid neurotransmitters on piglet pial arterioles. Am J Physiol. 1989;257:H1200–1203. doi: 10.1152/ajpheart.1989.257.4.H1200. [DOI] [PubMed] [Google Scholar]
- Busija DW, Wei M. Altered cerebrovascular responsiveness to N-methyl-D-aspartate after asphyxia in piglets. Am J Physiol. 1993;265:H389–394. doi: 10.1152/ajpheart.1993.265.1.H389. [DOI] [PubMed] [Google Scholar]
- Busija DW, Leffler CW, Pourcyrous M. Hyperthermia increases cerebral metabolic rate and blood flow in neonatal pigs. Am J Physiol. 1988;24:H343–H346. doi: 10.1152/ajpheart.1988.255.2.H343. [DOI] [PubMed] [Google Scholar]
- Busija DW, Meng W, Bari F, McGough PS, Errico RA, Tobin JR, Louis TM. Effects of ischemia on cerebrovascular responses to N-methyl-D-aspartate in piglets. Am J Physiol. 1996;270:H1225–H1230. doi: 10.1152/ajpheart.1996.270.4.H1225. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YB, Lipton SA. Redox modulation of the NMDA receptor. Cell Mol Life Sci. 2000;57:1535–1541. doi: 10.1007/PL00000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna DM, Meng W, Deal DD, Gowda W, Busija DW. Neuronal NO promotes cerebral cortical hyperemia during cortical spreading depression in rabbits. Am J Physiol. 1997;272:H1315–1322. doi: 10.1152/ajpheart.1997.272.3.H1315. [DOI] [PubMed] [Google Scholar]
- Colonna DM, Meng W, Deal DD, Busija DW. Calcitonin gene-related peptide promotes cerebrovascular dilation during cortical spreading depression in rabbits. Am J Physiol. 1993;266:H1095–H1102. doi: 10.1152/ajpheart.1994.266.3.H1095. [DOI] [PubMed] [Google Scholar]
- Colonna DM, Deal DD, Meng W, Busija DW. Nitric oxide promotes arteriolar dilation during cortical spreading depression in rabbits. Stroke. 1994;25:2463–2470. doi: 10.1161/01.str.25.12.2463. [DOI] [PubMed] [Google Scholar]
- Chi OZ, Liu X, Weiss HR. Effects of inhibition of neuronal nitric oxide synthase on NMDA-induced changes in cerebral blood flow and oxygen consumption. Exp Brain Res. 2002;148:256–260. doi: 10.1007/s00221-002-1310-7. [DOI] [PubMed] [Google Scholar]
- Domoki F, Perciaccante JV, Veltkamp R, Bari F, Busija DW. Mitochondrial potassium channel opener diazoxide preserves neuronal-vascular function after cerebral ischemia in newborn pigs. Stroke. 1999a;30:2713–2718. doi: 10.1161/01.str.30.12.2713. [DOI] [PubMed] [Google Scholar]
- Domoki F, Veltkamp R, Bari F, Busija DW. Cerebrovascular reactivity remains intact after cortical depolarization in newborn piglets. Pediatr Res. 1999b;45:834–837. doi: 10.1203/00006450-199906000-00009. [DOI] [PubMed] [Google Scholar]
- Domoki F, Perciaccante JV, Puskar M, Bari F, Busija DW. Cyclooxygenase-2 inhibitor NS398 preserves neuronal function after hypoxia/ischemia in piglets. Neuroreport. 2001;12:4065–4068. doi: 10.1097/00001756-200112210-00041. [DOI] [PubMed] [Google Scholar]
- Domoki Fm, Perciaccante JV, Shimizu K, Puskar M, Busija DW, Bari F. N-methyl-D-aspartate-induced vasodilation is mediated by endothelium-independent nitric oxide release in piglets. Am J Physiol. 2002;282:H1404–H1409. doi: 10.1152/ajpheart.00523.2001. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol. 2001;280:H160–H167. doi: 10.1152/ajpheart.2001.280.1.H160. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Akgoren N, Lauritzen M. Arginine-nitric oxide pathway and cerebrovascular regulation in cortical spreading depression. Am J Physiol. 1995;269:H23–29. doi: 10.1152/ajpheart.1995.269.1.H23. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR. Nitric oxide mediates vasodilation in response to activation of N-methyl-D-aspartate receptors in brain. Circ Res. 1993;72:476–480. doi: 10.1161/01.res.72.2.476. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR. Dilatation of cerebral arterioles in response to N-methyl-D-aspartate: role of CGRP and acetylcholine. Brain Res. 1994;640:93–97. doi: 10.1016/0006-8993(94)91860-0. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Brian JE., Jr 7-Nitroindazole inhibits brain nitric oxide synthase and cerebral vasodilatation to N-methyl-D-aspartate. Stroke. 1995;26:2172–2175. doi: 10.1161/01.str.26.11.2172. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Responses of cerebral arterioles to N-methyl-D-aspartate and activation of ATP-sensitive potassium channels in old rats. Brain Res. 1994;654:349–351. doi: 10.1016/0006-8993(94)90499-5. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR, Heistad DD. Responses of cerebral arterioles to kainate. Stroke. 1994;25:2080–2083. doi: 10.1161/01.str.25.10.2080. [DOI] [PubMed] [Google Scholar]
- Fellin T, Sul JY, D’Ascenzo M, Tkano H, Pascual O, Haydon PG. Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP. Novartis Found Symp. 2006;276:208–217. doi: 10.1002/9780470032244.ch16. [DOI] [PubMed] [Google Scholar]
- Fergus A, Lee KS. Regulation of cerebral microvessels by glutamatergic mechanisms. Brain Res. 1997;754:35–45. doi: 10.1016/s0006-8993(97)00040-1. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nature Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol. 2003;284:H1073–1079. doi: 10.1152/ajpheart.00881.2002. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signaling in the nervous system. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Andert M, Neurocheon J. A kainate receptor linked to nitric oxide synthase from arginine. J Neurochem. 1989;53:1952–1954. doi: 10.1111/j.1471-4159.1989.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Guerguerian A-M, Brambrink AM, Traystman RJ, Huganir RL, Martin LJ. Altered expression and phosphorylation of N-methyl-D-aspartate receptors in piglet striatum after hypoxia–ischemia. Mol Brain Res. 2002;104:66–80. doi: 10.1016/s0169-328x(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Pickel VM. Ultrastructural localization and comparative distribution of nitric oxide synthase and N-methyl-D-aspartate receptors in the shell of the rat nucleus accumbens. Brain Res. 1997;747:259–272. doi: 10.1016/s0006-8993(96)01249-8. [DOI] [PubMed] [Google Scholar]
- Hara S, Mukai T, Kuriiwa F, Iwata N, Kanao S, Endo T. Local changes in oxygen tension and blood flow in the brain under hyperthermia induced by intracerebroventricular NMDA in rats. Brain Res. 1996;737:339–342. doi: 10.1016/0006-8993(96)00966-3. [DOI] [PubMed] [Google Scholar]
- Hardebo JE, Wieloch T, Kahrstrom J. Excitatory amino acids and cerebrovascular tone. Acta Physiol Scand. 1989;136:483–485. doi: 10.1111/j.1748-1716.1989.tb08690.x. [DOI] [PubMed] [Google Scholar]
- Hawkins BR, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer HW, Enager P, Thomsen KJ, Lauritzen MJ. Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600372. in press. [DOI] [PubMed] [Google Scholar]
- Huang QF, Gebrewold A, Zhang A, Altura BT, Altura BM. Role of excitatory amino acids in regulation of rat pial microvasculature. Am J Physiol. 1994;266:R158–163. doi: 10.1152/ajpregu.1994.266.1.R158. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, D’Ambrosio R, Ngai AC, Winn HR. Adenosine receptors mediate glutamate-evoked arteriolar dilation in the rat cerebral cortex. Am J Physiol. 2003;284:H1631–1637. doi: 10.1152/ajpheart.00909.2002. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Goda N, Yamaguchi T, Sekizuka E, Suematsu M. Carbon monoxide from heme oxygenase-2 is a tonic regulator against NO-dependent dilation in the adult rat cerebral microcirculation. Circ Res. 2005;97:e104–114. doi: 10.1161/01.RES.0000196681.34485.ec. [DOI] [PubMed] [Google Scholar]
- Kaiser MG, During MJ. Combining laser Doppler flowmetry with microdialysis: a novel approach to investigate the coupling of regional cerebral blood flow to neuronal activity. J Neurosci Methods. 1995;60:165–173. doi: 10.1016/0165-0270(95)00008-i. [DOI] [PubMed] [Google Scholar]
- Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94:4121–4130. doi: 10.1152/jn.00448.2005. [DOI] [PubMed] [Google Scholar]
- Kim WK, Choi YB, Rayudu PV, Das P, Asaad WA, Arnelle DR, Stamler JS, Lipton SA. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion NO- Neuron. 1999;24:461–469. doi: 10.1016/s0896-6273(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117:199–210. doi: 10.1093/brain/117.1.199. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nature Rev Neurosci. 2005;6:77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Hansen AJ. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J Cereb Blood Flow Metab. 1992;12:223–229. doi: 10.1038/jcbfm.1992.32. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Busija DW. Prostanoids in cortical subarachnoid cerebrospinal fluid and pial arterial diameter in newborn pigs. Circ Res. 1985;57:689–694. doi: 10.1161/01.res.57.5.689. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Balabanova L, Fedinec AL, Parfenova H. Nitric oxide increases carbon monoxide production by piglet cerebral microvessels. Am J Physiol Heart Circ Physiol. 2005;289:H1442–H2447. doi: 10.1152/ajpheart.00464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol. 2006a;291:H2897–904. doi: 10.1152/ajpheart.00722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen suflfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol. 2006b;100:1065–1076. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Morales M, Vicente MA, Herreras O. Glutamate receptors of the kainate type and synaptic transmission. Trends Neurosci. 1997;20:9–12. doi: 10.1016/S0166-2236(96)20055-4. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Brown LA, Key BJ. Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience. 1999;92:47–60. doi: 10.1016/s0306-4522(98)00737-4. [DOI] [PubMed] [Google Scholar]
- Lu X, Sinha AK, Weiss HR. Effects of excitatory amino acids on cerebral oxygen consumption and blood flow in rat. Neurochem Res. 1997;22:705–711. doi: 10.1023/a:1027354110563. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Didion SP. Acute effects of alcohol on responses of cerebral arterioles. Stroke. 1995;26:2097–2101. doi: 10.1161/01.str.26.11.2097. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Patel KP. Acute effects of glucose on reactivity of cerebral microcirculation: role of activation of protein kinase C. Am J Physiol Heart Circ Physiol. 1995;269:H1297–1302. doi: 10.1152/ajpheart.1995.269.4.H1297. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Rev. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- Meng W, Tobin JR, Busija DW. Glutamate-induced cerebral vasodilation is mediated by nitric oxide through N-methyl-D-aspartate receptors. Stroke. 1995;26:857–862. doi: 10.1161/01.str.26.5.857. [DOI] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. Immunocytochemical basis for a meningeo-glial network. J Comp Neurol. 2002;420:445–465. doi: 10.1002/(sici)1096-9861(20000515)420:4<445::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanim of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci. 2007;27:2468–2471. doi: 10.1523/JNEUROSCI.3204-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley P, Small DL, Murray CL, Mealing GA, Poulter MO, Durkin JP, Stanimirovic DB. Evidence that functional glutamate receptors are not expressed on rat or human cerebromicrovascular endothelial cells. J Cereb Blood Flow Metab. 1998;18:396–406. doi: 10.1097/00004647-199804000-00008. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Murphy S, Rich G, Orgren KI, Moore SA, Faraci FM. Astrocyte-derived lipoxygenase product evokes endothelium-dependent relaxation of the basilar artery. J Neurosci Res. 1994;38:314–318. doi: 10.1002/jnr.490380309. [DOI] [PubMed] [Google Scholar]
- Nagy K, Kis B, Rajapakse NC, Bari F, Busija DW. Diazoxide preconditioning protects against neuronal cell death by attenuation of oxidative stress upon glutamate stimulation. J Neurosci Res. 2004;76:697–704. doi: 10.1002/jnr.20120. [DOI] [PubMed] [Google Scholar]
- Nahum-Levy R, Fossom LH, Skolnick P, Benveniste M. Putative partial agonist 1-aminocyclopropanecarboxylic acid acts concurrently as a glycine-site agonist and a glutamate-site antagonist at N-methyl-D-aspartate receptors. Mol Pharmacol. 1999;56:1207–1218. doi: 10.1124/mol.56.6.1207. [DOI] [PubMed] [Google Scholar]
- Nakai M, Maeda M. Cerebrovasodilatation of metabolic and non-metabolic origin elicited by chemical stimulation of the lateral periaqueductal gray matter in anaesthetized rats. Neuroscience. 1994;58:785–791. doi: 10.1016/0306-4522(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Nakai M, Maeda M. Vasodilatation and enhanced oxidative metabolism of the cerebral cortex provoked by the periaqueductal gray matter in anaesthetized rats. Neuroscience. 1996;72:1133–1140. doi: 10.1016/0306-4522(96)00013-9. [DOI] [PubMed] [Google Scholar]
- Nam MJ, Thore CR, Busija DW. Effects of protein kinase C activation on prostaglandin production and cyclooxygenase mRNA levels in ovine astroglia. Prostaglandins. 1996;51:203–213. doi: 10.1016/0090-6980(96)00004-4. [DOI] [PubMed] [Google Scholar]
- Niermann H, Amiry-Moghaddam M, Holthoff K, Witte OW, Ottersen OP. A novel role of vasopressin in the brain: modulation of activity-dependent water flux in the neocortex. J Neurosci. 2001;21:3045–3051. doi: 10.1523/JNEUROSCI.21-09-03045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington FJ, Tobin JR, Koehler RC, Traystman RJ. In vivo production of nitric oxide correlates with NMDA-induced cerebral hyperemia in newborn sheep. Am J Physiol. 1995;269:H215–221. doi: 10.1152/ajpheart.1995.269.1.H215. [DOI] [PubMed] [Google Scholar]
- Ohata H, Cao S, Koehler RC. Contribution of adenosine A2A and A2B receptors and heme oxygenase to AMPA-induced dilation of pial arterioles in rats. Am J Physiol. 2006 doi: 10.1152/ajpregu.00757.2005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova H, Neff RA, III, Alonso JS, Shlopov BV, Jamal CN, Sarkisova SA, Leffler CW. Cerebral vascular endothelial heme oxygenase: expression, localization, and activation by glutamate. Am J Physiol Cell Physiol. 2001;281:C1954–C1963. doi: 10.1152/ajpcell.2001.281.6.C1954. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Fedinec A, Leffler CW. Ionotrophic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metabol. 2003;23:190–197. doi: 10.1097/01.WCB.000004823561824.C4. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Basuroy S, Bhattachary S, Tsheranova D, Qu Y, Regan RF, Leffler CW. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol Cell Physiol. 2006;290:C1399–1410. doi: 10.1152/ajpcell.00386.2005. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Wang Q, Loenig HM, Abrecht RF. Role of nitric oxide, adenosine, and N-methyl-D-aspartate receptors, and neural activation in hypoxia-induced pial arteriolar dilation in rats. Brain Res. 1995;704:61–70. doi: 10.1016/0006-8993(95)01105-6. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Gay RL, III, Baughman VL, Wang Q. NO synthase inhibition modulate NMDA-induced changes in cerebral blood flow and EEG activity. Am J Physiol. 1996;271:H990–995. doi: 10.1152/ajpheart.1996.271.3.H990. [DOI] [PubMed] [Google Scholar]
- Peterson SL, Purvis RS, Griffith JW. Differential neuroprotecive effects of the NMDA receptor-associated glycine site partial agonists 1-aminocyclopropanecarboxylic acid (ACPC) and D-cycloserine in lithium-pilocarpine status epilepticus. Neurotoxicology. 2004;25:835–847. doi: 10.1016/j.neuro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Philip S, Armstead WM. Newborn pig nocicieptin/orphanin FQ activates protein tyrosine kinase and mitogen activated protein kinase to impair NMDA cerebrovasodilation after ischemia. Neuroreport. 2003;14:201–203. doi: 10.1097/00001756-200302100-00008. [DOI] [PubMed] [Google Scholar]
- Philip S, Armstead WM. Differential role of PTK, ERK and p38 MAPK in superoxide impairment of NMDA cerebrovasodilatation. Brain Res. 2004a;979:98–103. doi: 10.1016/s0006-8993(03)02879-8. [DOI] [PubMed] [Google Scholar]
- Philip S, Armstead WM. NMDA dilates pial arteries by KATP and Kca channel activation. Brain Res. 2004b;63:127–131. doi: 10.1016/j.brainresbull.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Rancillac A, Guille M, Tong XK, Geoffroy H, Arbault S, Hamel E, Amatore C, Rossier J, Cauli B. Stellate cells release NO and induce vasodilation of intraparenchymal blood vessels in rat cerebral slices. J Appl Physiol. 2006;100:1059–1064. [Google Scholar]
- Robinson JS, Fedinec AL, Leffler CW. Role of carbon monoxide in glutamate receptor-induced dilation of newborn pig pial arterioles. Am J Physiol Heart Circ Physiol. 2002;282:H2371–2376. doi: 10.1152/ajpheart.00911.2001. [DOI] [PubMed] [Google Scholar]
- Roy C, Sherrington C. On the regulation of the blood supply of the brain. J Physiol. 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A, Waagepetersen HS. Role of astrocytes in glutamate homeostatis: Implications for excitotoxicity. Neurotox Res. 2005;8:221–225. doi: 10.1007/BF03033975. [DOI] [PubMed] [Google Scholar]
- Sharp CD, Houghton J, Elrod JW, Warren A, Jackson TH, IV, Jawahar A, Nanda A, Minagar A, Alexander JS. N-methyl-D-aspartate receptor activation in human cerebral endothelium promotes intracellular oxidant stress. Am J Physiol Heart Circ Physiol. 2005;288:H1893–1899. doi: 10.1152/ajpheart.01110.2003. [DOI] [PubMed] [Google Scholar]
- Shibata M, Leffler CW, Busija DW. Cerebral hemodynamics during cortical spreading depression in rabbits. Brain Res. 1990;530:267–274. doi: 10.1016/0006-8993(90)91294-q. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Miller AW, Erdos B, Bari F, Busija DW. Role of endothelium in hyperemia during cortical spreading depression in the rat. Brain Res. 2002;22:40–49. doi: 10.1016/s0006-8993(01)03352-2. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Bari F, Busija DW. Glibenclamide enhances cortical spreading depression-associated hyperemia in the rat. NeuroReport. 2000;11:2103–2106. doi: 10.1097/00001756-200007140-00009. [DOI] [PubMed] [Google Scholar]
- Simandle S, Kerr BA, Lacza Z, Eckman D, Busija DW, Bari F. Piglet pial arteries respond to NMDA in vivo but not in vitro. Microvasc Res. 2005;70:76–83. doi: 10.1016/j.mvr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Sun H, Mayhan WG. Sex difference in nitric oxide synthase-dependent dilatation of cerebral arterioles during long-term alcohol consumption. Alcohol Clin Exp Res. 2005;29:430–436. doi: 10.1097/01.alc.0000156117.87892.22. [DOI] [PubMed] [Google Scholar]
- Takayasu M, Dacey RG., Jr Effects of inhibitory and excitatory amino acid neurotransmitters on isolated cerebral parenchymal arterioles. Brain Res. 1989;482:393–396. doi: 10.1016/0006-8993(89)91207-9. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Barnewolt CE, Dunning PS. Excitotoxin-induced cerebral hyperemia in newborn piglets: regional cerebral blood flow mapping with contrast-enhanced power Doppler US. Radiology. 1998;208:73–79. doi: 10.1148/radiology.208.1.9646795. [DOI] [PubMed] [Google Scholar]
- Thore CR, Nam M, Busija D. Phorbol ester-induced prostaglandin production in piglet cortical astroglia. Am J Physiol. 1994;267:R34–R37. doi: 10.1152/ajpregu.1994.267.1.R34. [DOI] [PubMed] [Google Scholar]
- Thore CR, Nam MJ, Busija DW. Immunofluorescent localization of constitutive and inducible prostaglandin H synthase in ovine astroglia. J Comp Neurol. 1996;367:1–9. doi: 10.1002/(SICI)1096-9861(19960325)367:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Tong XK, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: A means for direct regulation of local cerebral blood flow. J Comp Neurol. 2000;421:161–171. [PubMed] [Google Scholar]
- Veltkamp R, Domoki F, Bari F, Busija DW. Potassium channel activators protect the N-Methyl-D-Aspartate—induced cerebral vascular dilation after combined hypoxia and ischemia in piglets. Stroke. 1998;29:837–843. doi: 10.1161/01.str.29.4.837. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Domoki F, Bari F, Louis TM, Busija DW. Inhibitors of protein synthesis preserve the N-methyl-D-aspartate-induced cerebral arteriolar dilation after ischemia in piglets. Stroke. 1999;30:148–152. doi: 10.1161/01.str.30.1.148. [DOI] [PubMed] [Google Scholar]
- Wagerle LC, Busija DW. Effect of thromboxane A2/Endoperoxide antagonist SQ29548 on the contractile response to acetylcholine in the newborn piglet cerebral arteries. Circ Res. 1990;6:824–831. doi: 10.1161/01.res.66.3.824. [DOI] [PubMed] [Google Scholar]
- Weiss HR, Sinha AK, Lu X. Effect of up-regulation of NMDA receptors on cerebral O2 consumption and blood flow in rat. Brain Res. 1996;730:193–198. doi: 10.1016/0006-8993(96)00446-5. [DOI] [PubMed] [Google Scholar]
- Weiss HR, Doshi D, Sinha AK, Liu X, Chi OZ. 17Beta-estradiol blocks NMDA-induced increases in regional cerebral O2 consumption. Brain Res. 2002;951:177–182. doi: 10.1016/s0006-8993(02)03158-x. [DOI] [PubMed] [Google Scholar]
- Wendling WW, Chen D, Daniels FB, Monteforte MR, Fischer MB, Harakal C, Carlsson C. The effects of N-methyl-D-aspartate agonists and antagonists on isolated bovine cerebral arteries. Anesth Analg. 1996;82:264–268. doi: 10.1097/00000539-199602000-00009. [DOI] [PubMed] [Google Scholar]
- Yang ST, Chang HH. Nitric oxide of neuronal origin mediates NMDA-induced cerebral hyperemia in rats. Neuroreport. 1998;9:415–418. doi: 10.1097/00001756-199802160-00011. [DOI] [PubMed] [Google Scholar]
- Yang G, Iadecola C. Glutamate microinjections in cerebellar cortex reproduce cerebrovascular effects of parallel fiber stimulation. Am J Physiol. 1996;271:R1568–1575. doi: 10.1152/ajpregu.1996.271.6.R1568. [DOI] [PubMed] [Google Scholar]
- Yang G, Chen G, Ebner TJ, Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am J Physiol. 1999;277:R1760–1770. doi: 10.1152/ajpregu.1999.277.6.R1760. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]