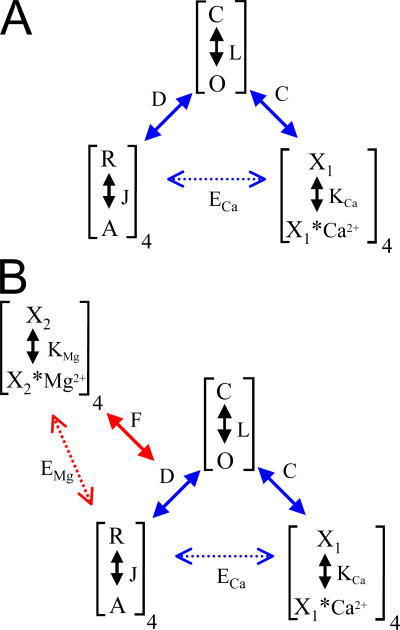
A signature feature of large conductance, calcium- and voltage-activated K+ channels (now usually termed “BK” channels) is their dual regulation by two physiological signals, cytosolic Ca2+ and membrane voltage. This dual-sensing capacity of BK channels distinguishes them from other voltage-dependent K+ channels in terms of the physiological roles they can play, allowing their voltage-sensing function to be dynamically regulated by variations in submembrane [Ca2+]. Furthermore, as illustrated abundantly in various past articles of this journal (Cox et al., 1997; Horrigan and Aldrich, 1999; Horrigan et al., 1999; Rothberg and Magleby, 1999; Cui and Aldrich, 2000; Rothberg and Magleby, 2000), this dual-sensing ability of BK channels has provided an almost unparalleled model for investigation of ion channel allosteric gating mechanisms. This line of investigation led to the basic idea developed robustly by Horrigan and Aldrich (2002) that the regulation of BK gating by Ca2+ and voltage arises from three coupled equilibria, a voltage-sensor equilibrium (J), a ligand-binding equilibrium (K), and the channel pore domain open–closed equilibrium (L). These coupled equilibria are schematized in Fig. 1 A (Horrigan and Aldrich, 2002), with the coupling between voltage sensor and gate opening given by D, coupling between ligand binding and gate opening by C, and between voltage sensor and ligand binding by E.
Figure 1.
Diagram of basic allosteric schemes describing regulation of BK channels by voltage, Ca2+, and Mg2+. In A, the scheme tested by Horrigan and Aldrich to describe BK gating contains three coupled allosteric equilibria: L, the closed–open conformational equilibrium, J, the voltage sensor equilibrium, and KCa, the Ca2+ binding equilibrium (Horrigan and Aldrich, 2002). D describes coupling factor linking the voltage sensor equilibrium to gate opening, C, the coupling factor linking Ca2+ binding to channel opening, and ECa, the coupling factor linking Ca2+ binding to voltage sensor movement. Values for coupling factors are: D ∼ 25; C ∼ 8; and ECa ∼ 2.4. Since D and C correspond to the contribution of a single sensor to gate opening, voltage sensor movement can shift the gate opening equilibrium up to D4 (∼400,000), while Ca2+ binding increase gate opening up to C4 (∼4,096). In B, the scheme developed by Ma and Horrigan to account for allosteric regulation by Mg2+ (Horrigan and Ma, 2007) is integrated with the scheme in A. The Mg2+ binding equilibrium is given by KMg, which coupled (coupling constant F) to an increase in the coupling strength between the voltage sensor equilibrium (J) and gating (L). Mg2+ binding also weakly influences the voltage sensor equilibrium, J, as defined by coupling constant EMg. F is determined to be ∼2, which given the four Mg2+ binding sites and four voltage sensors, results in an overall effective coupling between J and L defined by F4D4 or 6.25*106.
In order for BK channels to play their role as dual sensors of Ca2+ and voltage in appropriate physiological ranges, it is essential that neither signal be so strongly coupled to gate opening that it negates the role of the other sensor. As a consequence, BK channels are well suited for teasing apart interactions among multiple allosterically coupled equilibria. Furthermore, once having such equilibria quantitatively defined, it allows for detailed analysis of the structural determinants that contribute to the energetics of the equilibria and their coupling, as has been done for the analysis of the role of various charged residues in the BK S1–S4 domain in voltage sensing (Ma et al., 2006). BK channels thus differ from channels specialized to be regulated by primarily a single physiological signal, whether voltage or ligand, where the coupling between sensor and gate opening is often so tight as to negate simple separation of distinct conformational equilibrium with the channel protein.
An important reason for having a well-defined allosteric gating mechanism describing BK channel behavior is that it provides an essential tool for understanding mechanistically how other kinds of regulatory elements may influence BK channel function. In addition to Ca2+ and voltage, the gating behavior of BK channels can be modulated by a variety of other factors, most notably accessory β subunits (Nimigean and Magleby, 1999; Xia et al., 1999; Cox and Aldrich, 2000; Orio et al., 2006; Wang et al., 2006) and cytosolic Mg2+ (Shi and Cui, 2001; Zhang et al., 2001), but also including pH (Avdonin et al., 2003), heme (Horrigan et al., 2005), phosphorylation (Tian et al., 2001), and other soluble messengers. The power of having a viable allosteric model for BK channels is that the underlying mechanisms by which each of these regulatory factors alters BK gating can be determined in an energetically meaningful fashion— is a modulator affecting only voltage sensor function, modifying the Ca2+ regulatory mechanism, directly acting to gate or close the channel, or acting in some general fashion that influences multiple aspects of gating? By evaluating regulatory effects in terms of the defined allosteric model, this provides the promise that the structural elements and energetic basis for such regulation can be determined. Although this is the potential power of such an approach, rarely has this promise been achieved. Now two recent papers, one from Frank Horrigan's lab in this issue of the journal (see Horrigan and Ma on p. 13) and the other jointly coauthored by the labs of Jianmin Cui and Horrigan (Yang et al., 2007), apply this approach with remarkable success to investigation of the mechanism by which cytosolic Mg2+ activates BK channels. Together these papers not only establish that the underlying physical basis for the Mg2+ action involves specific repulsive electrostatic interactions between Mg2+ and a key basic residue in the S4 voltage sensor (Yang et al., 2007) but define the energetic consequences of these interactions on the parameters describing the BK channel gating behavior (Horrigan and Ma, 2007). Knowing both the identity of the charged loci involved in the electrostatic effects, and how they impact on allosteric behavior, has allowed Horrigan and Ma (2007) to propose an intriguing and simple explanation of Mg2+ action based on the inverse relation between the distance separating a Mg2+ bound to the cytosolic domain from R213 in the S4 voltage sensor and the electrostatic force between them, which tend to stabilize both the voltage sensor and the cytosolic domain (see details below) in their activated conformations. Energetically, it appears that this explanation applies, irrespective of the specific conformational positions of voltage sensor and Mg2+ site. Somewhat ironically, our understanding of the physical details of how Mg2+ regulates BK channel function, in some aspects, has vaulted ahead of our understanding of the physical details of either the voltage regulation or Ca2+ regulation of BK channels.
Before highlighting the key findings from these new papers, some background regarding Mg2+ regulation of BK channels is warranted. The story begins ∼20 years ago when two separate labs (Golowasch et al., 1986; Oberhauser et al., 1988) described the differential ability of divalent cations to influence activity of BK channels. In particular, millimolar concentrations of Mg2+ (and other divalent cations) were observed to enhance activation of BK channels by Ca2+, while having little ability to directly activate BK channels. The basis for the Mg2+-dependent effects on BK channels and its physiological significance then remained dormant for more than a decade, a period during which significant progress was made in the cloning of BK channel α subunits (Butler et al., 1993) and the first identification of potential high-affinity Ca2+ binding sites on the BK channel α subunits (Schreiber and Salkoff, 1997). Our mechanistic understanding of BK channel gating also advanced considerably during this time with the development of allosteric gating models based on the implicit symmetry of tetrameric channels containing four voltage sensors and four Ca2+ binding sites (Cui et al., 1997; Horrigan and Aldrich, 1999; Horrigan et al., 1999; Rothberg and Magleby, 1999). Such models have provided a meaningful framework for analysis of the mechanistic basis of BK channel regulation by ligands and voltage.
A key advance from these studies was the recognition that voltage and Ca2+ each regulate BK channel activation relatively independently, formalizing the idea that a voltage sensor equilibrium and Ca2+-binding equilibrium each couple via separate pathways to the closed–open conformational equilibrium of the pore domain (Fig. 1 A). These ideas culminated in the notable paper by Horrigan and Aldrich that provided explicit definition of the key allosteric constants for a full model incorporating the idea that BK gating involves these three independent allosteric equilibria (Horrigan and Aldrich, 2002). Bolstered by these advances, two laboratories revisited the question of how Mg2+ might regulate BK channel gating and described the ability of a range of Mg2+ concentrations to regulate the activation of BK macroscopic conductance over a range of voltages and [Ca2+] (Shi and Cui, 2001; Zhang et al., 2001). These studies showed that Mg2+- dependent effects involve a low-affinity, relatively nonselective divalent cation site that regulates BK gating independently from regulation mediated by the high- affinity sites involved in Ca2+-dependent regulation. Furthermore, it was suggested that Mg2+ might directly regulate the channel closed–open equilibrium, perhaps in a fashion similar to regulation of the closed–open equilibrium by Ca2+, although through a distinct binding site.
Around the same time, Rod MacKinnon's lab published the initial structure of a cytosolic domain involved in ligand-dependent regulation of the bacterial Escherichia coli 6TM K+ channel (Jiang et al., 2001). Such so-called RCK domains (for regulator of conductance for K+) are now recognized as a common feature of a large family of bacterial transporters and channels (Lingle, 2007). Remarkably, RCK domains share homology with a pair of similar regulatory domains on each BK channel α subunit (Jiang et al., 2001). Additional work on the RCK-containing, Ca2+-regulated MthK bacterial K+ channel by Youxing Jiang, both while in the MacKinnon laboratory (Jiang et al., 2002) and after establishing his own laboratory (Dong et al., 2005; Ye et al., 2006), showed that the MthK cytosolic domain arises from a set of four distinct homodimers assembled in an octameric gating ring. A Ca2+-dependent conformational change in the gating ring structure is thought to provide the energy required for channel opening by tugging on the linkers connecting the gating ring to the pore domain inner helices (Jiang et al., 2002). This view of gating in the MthK channel now guides much of the thinking about the role of the cytosolic domain of BK channels, which is conjectured to involve a similar octamer of RCK-containing modules (see Lingle, 2007). Although the relevance of these structural ideas to BK channels still requires additional validation, the presence of putative RCK domains in BK channels immediately suggested to investigators of BK channels that such domains may contain sites involved in ligand-dependent regulation.
Following this lead, both the Cui and Lingle laboratories independently found that Mg2+-dependent regulation of BK channels could be largely removed by mutation of particular residues in the putative first RCK domain (RCK1). In particular, two residues, E374 (Shi et al., 2002) and E399 (Xia et al., 2002), each independently removed essentially all regulation of BK gating by Mg2+ at concentrations of 10 mM and below. Furthermore, using homology modeling between the E. coli 6TM RCK domain and the BK channel sequence, the Cui group also generated a possible structure of a portion of the BK RCK1 domain that might coordinate Mg2+ binding (Shi et al., 2002). Although direct structural information demonstrating ligation of Mg2+ remains unavailable, this analysis suggested that E374, E399, and potentially Q397 might participate in coordination of Mg2+ binding. Subsequent work using a more thorough mutagenesis screen provided further support for the idea that both E374 and E399 contribute to Mg2+ binding, whereas the Q297 position may influence the ability of Mg2+ to bind at E374 and E399 (Yang et al., 2006).
Although these earlier studies establish the existence of a low-affinity Mg2+ regulatory site, the mechanistic basis for regulation by Mg2+ required further clarification. The first hint that the low-affinity Mg2+ site (also activated by mM Ca2+) might act in a fashion distinct from the high-affinity Ca2+-dependent site was the observation of a differential ability of the low-affinity Mg2+ sites and the high-affinity Ca2+ sites to increase channel open probability (Po) at negative voltages, where voltage sensors are in resting positions (Horrigan and Aldrich, 2002). The idea here is that the effects of ligands on Po at negative potentials allows specific determination of the ability of a ligand to directly modulate the C-O equilibrium (defined by the equilibrium constant L in Fig. 1 A). For BK channels, concentrations of Ca2+ that act at the high-affinity sites markedly increase Po at negative potentials (∼4,000-fold), because the Ca2+ binding equilibrium directly couples to the C-O equilibrium. The inability of the low-affinity site to increase Po at negative potentials therefore suggests that the low-affinity site must be altering some other aspect of BK gating distinct from effects on L. A potential role for the involvement of the voltage sensor in Mg2+ effects was then given support in a subsequent paper from the Cui and Horrigan labs (Hu et al., 2003), which examined the ability of mutations in the BK S4 segment and S4–S5 linker to influence either gating regulation by either micromolar Ca2+ or millimolar Mg2+. The results clearly demonstrated that mutations in the C-terminal half of S4 and in the S4–S5 linker had little or no effect on activation by micromolar Ca2+. However, mutation of R213 completely abolished activation by mM Mg2+, while other residues on the cytosolic side of position R213 had milder effects on activation by Mg2+. Horrigan's lab then established that, among several basic residues in the S4 segment, R213 is the primary contributor to gating charge movements (Ma et al., 2006). These results clearly indicated that the voltage sensor is critical for Mg2+-dependent activation, but left open two key questions: first, what is the mechanism by which Mg2+ binding might interact with the voltage-sensor and, second, how does Mg2+ binding lead to enhanced channel activation?
Now two recent papers (Horrigan and Ma, 2007; Yang et al., 2007), one of them in this issue, provide a major leap in our understanding. One paper shows that the energetic basis for the Mg2+-dependent effects involves a specific electrostatic interaction between bound Mg2+ and the R213 residue in the S4 voltage sensor (Yang et al., 2007). The second paper (Horrigan and Ma, 2007) provides an insightful evaluation of the consequences of this electrostatic interaction and reveals that Mg2+ primarily increases the strength of coupling between voltage sensors and channel activation, an effect that arises from the ability of Mg2+ to favor the stabilization of channels in states with both activated voltage sensors and open gates. In both papers, a rich variety of experimental tests are employed to support the final conclusions.
What are the results supporting the idea that Mg2+ binding facilitates BK activation by a simple electrostatic interaction between Mg2+ and the voltage-sensing residue, R213 (Yang et al., 2007)? First, Mg2+-dependent activation is sensitive to ionic strength in a fashion consistent with the effect arising from an electrostatic interaction. Importantly, Ca2+-dependent activation exhibits little dependence on ionic strength. Second, charge manipulations at position Q397, which has been shown to be positioned close to the site of Mg2+ binding (Shi et al., 2002; Yang et al., 2006), influence gating in a fashion consistent with a simple electrostatic interaction. Specifically, the Q397K and Q397R mutations mimic the ability of Mg2+ to shift activation to more negative potentials, whereas Q397E and Q397D shift activation to more positive potentials. Third, introduction of net positive charge on the Q397C residue by reaction with MTSET resulted in an MTSET-dependent shift in gating similar in magnitude to that produced by Mg2+, and the shift in activation produced by the Q397K mutation is sensitive to ionic strength in a fashion similar to the Mg2+ effect. Finally, the effects of net positive charge at the Q397 position was shown to produce functional effects on gating current and channel Po similar to those produced by Mg2+.
To test whether these effects involve a specific interaction of Mg2+ with R213, the ability of 10 mM Mg2+ to shift gating was examined in a series of constructs in which all the potentially cytosolically exposed charged residues in the voltage sensor domain (S1–S4 and S4–S5 linker) were mutated. Only R213 abolished the ability of Mg2+ to shift gating; furthermore, the R213Q mutation also abolished the ability of Q397R to shift activation. Reintroduction of positive charge by MTSEA in R213C restored the ability of 10 mM Mg2+ to shift activation.
Together these results compellingly argue that the energetic basis for the ability of Mg2+ to shift activation arises from a simple electrostatic repulsion between bound Mg2+ and R213 in the S4 voltage sensor. An implication of the results is that the voltage sensor and the Mg2+-binding site must be sufficiently close to allow such interactions. Based on consideration of the known structures of the cytosolic domain of the MthK K+ channel (Jiang et al., 2002) and the position of voltage sensors in the Kv1.2 channel structure (Long et al., 2005a,b), the authors point out that both the Mg2+ binding site and the R213 residue in BK channels may be positioned at comparable lateral distances from the axis of the permeation pathway (Yang et al., 2007).
At first glance, it might seem natural to assume that the repulsive influence from Mg2+ simply “pushes” on R213, thereby favoring the tendency of voltage sensors to move to an activated position. Now, as reported in this issue, Horrigan and Ma address this question in detail, by defining the specific effects of Mg2+ on the various allosteric constants defining activation of BK channels (Horrigan and Ma, 2007). The analysis leads to a somewhat surprising conclusion. Namely, the primary effect of Mg2+ is to increase the strength of coupling (allosteric constant: D) between voltage sensor movement and channel activation, whereas Mg2+ has only minor direct effects on the voltage sensor equilibrium (J). (The overall scheme that integrates the allosteric effects of Mg2+ with those of voltage- and Ca2+-dependent regulation of the closed–open equilibrium is given in Fig. 1 B.) As described in more detail below, an analysis of the kinetic and equilibrium effects of Mg2+ leads to the conclusion that the effects of Mg2+ are markedly state dependent. The ability of Mg2+ to increase activation therefore arises from a relative stabilization of channels in which both voltage sensors and cytosolic gating ring are in activated positions. Since the electrostatic interactions between R213 and Mg2+ are apparently larger when voltage sensors are in resting positions or when the cytosolic domain is in a closed conformation, the effect of Mg2+ can be thought of as the consequence of the relative minimization of the electrostatic energy between R213 and Mg2+ when both voltage sensors and gating ring are in activated conformations. This general picture of Mg2+ action therefore corresponds to a rather novel mechanism in which electrostatic repulsion acts to modulate the effective coupling between two other coupled allosteric equilibria, in this case, voltage sensor movement and channel activation.
So what are the observations that lead to these conclusions? Within the framework of the Horrigan-Aldrich model (Fig. 1 A), the ability of Mg2+ to activate gating might arise in any of several mechanistically distinct ways: (a) Mg2+ might alter the closed–open equilibrium (L), (b) Mg2+ might shift the voltage sensor equilibrium (J), favoring voltage sensor activation at a given voltage, or (c) Mg2+ might alter the coupling (D) between voltage sensor movement (J) and the closed–open (C-O) equilibrium (L). Horrigan and Ma systemically address these possibilities to reach the conclusion that effects on D account for most of the actions of Mg2+.
Possible effects of Mg2+ on the C-O equilibrium were considered first. Examination of the ability of Mg2+ to increase channel open probability at negative potentials, where voltage sensors are inactive, showed that, whereas 2 μM Ca2+ markedly increases Po at negative potentials, Mg2+ was without effect. This was consistent with the earlier results showing that the effective Po at negative potentials was not increased by Ca2+ concentrations acting on the low-affinity site (Horrigan and Aldrich, 2002). Furthermore, in constructs with mutations that shift the voltage sensor equilibrium either to more negative (R207Q) or more positive (R167E) voltages, the effects of Mg2+ are not intrinsically voltage dependent, but are coupled to the activation range of the voltage sensors. This also argues that Mg2+ binding is not directly coupled to regulation of the C-O equilibrium (L). Then, to test for possible effects on J, Horrigan and Ma determined the effects of Mg2+ on the voltage dependence of gating current movements along with determinations of the fast time constant of gating current decay (τgFast). Their results showed that the voltage sensor equilibrium is shifted about −17 mV at 10 mM Mg2+, with a small slowing of τgFast at more negative potentials and a faster τgFast at more positive potentials. Importantly, the ability of 10 mM Mg2+ to shift the voltage sensor equilibrium is reduced by ∼50% by mutation of E374 and E399, supporting the idea that the E374/E399 Mg2+ binding site accounts for the effects of Mg2+ on J. However, despite these effects on J, the authors calculate that the observed −17-mV shift in the gating current equilibrium would only account for a shift in activation Po of similar magnitude. This contrasts markedly with the approximately −67-mV shift in GV curves produced by 10 mM Mg2+ and shows that the effects of Mg2+ on J alone are insufficient to account for the activation by Mg2+.
To examine the possible effects of Mg2+ on the coupling, D, between voltage sensors and channel activation, Horrigan and Ma then use a particularly clever and insightful set of experiments. In previous work, these investigators found that mutation of the specific residues, R207Q and R210C, in the S4 voltage sensor resulted in channels in which voltage sensor activation was shifted to more negative potentials (Ma et al., 2006). For such constructs, a range of voltages could be identified over which voltage sensors are essentially locked in activated positions, but channel Po is still less than maximal. Taking advantage of this opportunity to examine effects of Mg2+ on Po under conditions in which the voltage sensors are activated, Horrigan and Ma show that Mg2+ still facilitates opening, both in R207Q and R210C, when voltage sensors are constitutively activated. Since it was already established that Mg2+ is not directly influencing the C-O equilibrium, this argues that that the major effect of Mg2+ is to increase D, the effectiveness of coupling between voltage sensor movement and channel opening. An important implication of these results is that the ability of Mg2+ to increase Po of R210C channels clearly means that, irrespective of what the mechanism may be, the effects of Mg2+ must be mediated by something distinct from alteration of the voltage sensor equilibrium itself.
The idea that Mg2+ enhances voltage sensor/gate coupling is given further support by an examination of the voltage dependence of Po in wild-type channels, both in the absence and presence of Mg2+. The key task here was to determine the relative shift in half activation of voltage sensors either for closed (Vhc) or open (Vho) channels. Although direct measurements of the gating charge equilibrium for open channels are difficult, in previous work Horrigan has established procedures for an approximation of the open channel gating current equilibrium (Ma et al., 2006; Horrigan and Ma, 2007). Using this approach, the present work shows that 10 mM Mg2+ results in an approximately −50-mV additional shift in the open channel voltage sensor half- activation potential compared with the shift observed for closed channels. Whereas the small effect (−17 mV) of Mg2+ on the closed channel voltage sensor equilibrium reflects the small effect of Mg2+ on J, the larger shift observed for the shift of the open channel voltage sensor equilibrium directly reflects an effect of Mg2+ on D, the strength of coupling between J and L. Thus, analysis of effects of Mg2+ on wild-type gating confirms the results obtained with channels with constitutively activated voltage sensors. Satisfyingly, these effects of Mg2+ on D are abolished with mutation of E374 and E399, confirming that it is the binding site for Mg2+ on RCK1 that is critical for these effects.
To provide additional mechanistic insight into how the interactions between Mg2+ and R213 alter BK gating, Horrigan and Ma also examine effects of Mg2+ on kinetic properties of BK channels at negative potentials. It has been previously shown that the effects of Mg2+ on BK channel activation are associated with a pronounced slowing of BK channel tail currents at negative potentials, whereas activation rates at positive potentials are largely unaltered (Zhang et al., 2001; Zeng et al., 2005). Such effects might seem at odds with the result described above, that Mg2+ has no significant effect on BK channel Po when voltage sensors are in resting states. To address this conundrum, single channel open and closed time durations were examined in patches expressing the R167E construct, so that channels could be studied at modest voltages while voltage sensors are still in resting states. As expected, 10 mM Mg2+ did not change Po. However, 10 mM Mg2+ increased the durations of both open times and closed times, thereby accounting for the observation that Po at negative potentials is unchanged by 10 mM while deactivation rates are still slowed.
On balance, then, the results reveal that the effects of Mg2+ on BK activation arise largely from an increased strength of coupling (D) between the voltage sensor and gate, with a smaller contribution from direct effects on J. In addition, Mg2+ exerts small effects on rates of voltage sensor movement (τgFAST), while also affecting the rates of transition between open and closed states at voltages where voltage sensors are inactive. Is there a unified physical model that might account for all this distinct observations, all of which are mediated by Mg2+ acting at the E374/E399 site on the RCK1 domain? The explanation developed by Horrigan and Ma and presented in Fig. 8 B of their paper is based on two key ideas: first, that both voltage sensors and the cytosolic gating ring undergo conformational changes during gating and, second, that the effects of Mg2+ seem to involve a preferential stabilization of channels in which both voltage sensors and gating ring is activated. The basis for the dual movements of both voltage sensors and the Mg2+ site reasonably arises from our understanding of voltage sensor movements in KvAP and KV1.2 and gating ring expansion in the MthK channel. In the presence of Mg2+, channels can exist in four possible conformations: one with inactive voltage sensors and inactive gating ring, one with active voltage sensors and active gating ring, and two with one sensor active and the other sensor inactive. The key idea encapsulated in the Horrigan and Ma model (their Fig. 8 B) is that, as a consequence of state-dependent changes in the relative positions of Mg2+ and R213, the repulsive electrostatic energies are minimized when both voltage sensors and the gating ring are in activated state positions. In all other states a closer positioning between R213 and Mg2+ results in increasing electrostatic interactions that tend to destabilize these states. Horrigan and Ma are justifiably cautious regarding their model, particularly given the absence of specific structural information regarding the relative positioning of the Mg2+ binding site and the voltage sensor in different states. However, the model provides a valuable framework for thinking about how state-dependent changes in the strength of a “simple” electrostatic interaction may result in differential stabilization of particular states relative to others. A particularly important point concerning the Horrigan and Ma model is the following. Irrespective of the specific conformational changes that one might propose to account for the kinetic and Po effects, the effect of Mg2+ results from a state-dependent minimization of repulsive electrostatic energies between Mg2+ and R213, leading to stabilization of activated voltage sensors and activated gating ring.
Beyond the elucidation of the fundamental mechanism by which BK channels can be regulated by Mg2+, this pair of papers establish the important general point that voltage-dependent channels can be modulated by factors that directly influence the voltage-sensing part of the channel protein. One could imagine that in other channels there may be different strategies that also regulate the coupling of voltage sensor status and channel activation in a similar fashion, whether via auxiliary subunits that might place charges in positions that influence voltage sensors or via enzymatic modifications that alter the local charge in the vicinity of voltage sensors. How local charge might influence the voltage sensor equilibrium or coupling to channel activation would depend on the specific positioning of charges relative to the positions of the voltage sensors in resting and activated states. Though such details may vary in different cases, an important contribution of these papers from the Cui and Horrigan labs is that they demonstrate the physical plausibility of a new type of mechanism by which activity of voltage-gated channels can be regulated.
References
- Avdonin, V., X. Tang, and T. Hoshi. 2003. Stimulatory action of internal protons on Slo1 BK channels. Biophys. J. 84:2969–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, A., S. Tsunoda, D.P. McCobb, A. Wei, and L. Salkoff. 1993. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 261:221–224. [DOI] [PubMed] [Google Scholar]
- Cox, D., and R. Aldrich. 2000. Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J. Gen. Physiol. 116:411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D.H., J. Cui, and R.W. Aldrich. 1997. Allosteric gating of a large conductance Ca-activated K+ channel. J. Gen. Physiol. 110:257–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, J., and R.W. Aldrich. 2000. Allosteric linkage between voltage and Ca2+-dependent activation of BK-type mslo1 K+ channels. Biochemistry. 39:15612–15619. [DOI] [PubMed] [Google Scholar]
- Cui, J., D.H. Cox, and R.W. Aldrich. 1997. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J. Gen. Physiol. 109:647–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J., N. Shi, I. Berke, L. Chen, and Y. Jiang. 2005. Structures of the MthK RCK domain and the effect of Ca2+ on gating ring stability. J. Biol. Chem. 280:41716–41724. [DOI] [PubMed] [Google Scholar]
- Golowasch, J., A. Kirkwood, and C. Miller. 1986. Allosteric effects of Mg2+ on the gating of Ca2+-activated K+ channels from mammalian skeletal muscle. J. Exp. Biol. 124:5–13. [DOI] [PubMed] [Google Scholar]
- Horrigan, F., and R. Aldrich. 2002. Coupling between voltage-sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 120:267–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan, F.T., and R.W. Aldrich. 1999. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca2+. J. Gen. Physiol. 114:305–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan, F.T., J. Cui, and R.W. Aldrich. 1999. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca2+. J. Gen. Physiol. 114:277–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan, F.T., S.H. Heinemann, and T. Hoshi. 2005. Heme regulates allosteric activation of the Slo1 BK channel. J. Gen. Physiol. 126:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan, F.T., and Z. Ma. 2007. Mg2+ enhances voltage-sensor/gate coupling in BK channels. J. Gen. Physiol. In press. [DOI] [PMC free article] [PubMed]
- Hu, L., J. Shi, Z. Ma, G. Krishnamoorthy, F. Sieling, G. Zhang, F.T. Horrigan, and J. Cui. 2003. Participation of the S4 voltage sensor in the Mg2+-dependent activation of large conductance (BK) K+ channels. Proc. Natl. Acad. Sci. USA. 100:10488–10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Pico, M. Cadene, B.T. Chait, and R. MacKinnon. 2001. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 29:593–601. [DOI] [PubMed] [Google Scholar]
- Lingle, C.J. 2007. Gating rings formed by RCK domains: keys to gate opening. J. Gen. Physiol. 129:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S.B., E.B. Campbell, and R. Mackinnon. 2005. a. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 309:897–903. [DOI] [PubMed] [Google Scholar]
- Long, S.B., E.B. Campbell, and R. Mackinnon. 2005. b. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 309:903–908. [DOI] [PubMed] [Google Scholar]
- Ma, Z., X.J. Lou, and F.T. Horrigan. 2006. Role of charged residues in the S1-S4 voltage sensor of BK channels. J. Gen. Physiol. 127:309–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean, C.M., and K.L. Magleby. 1999. The β subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J. Gen. Physiol. 113:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser, A., O. Alvarez, and R. Latorre. 1988. Activation by divalent cations of a Ca2+-activated K+ channel from skeletal muscle membrane. J. Gen. Physiol. 92:67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio, P., Y. Torres, P. Rojas, I. Carvacho, M.L. Garcia, L. Toro, M.A. Valverde, and R. Latorre. 2006. Structural determinants for functional coupling between the β and α subunits in the Ca2+-activated K+ (BK) channel. J. Gen. Physiol. 127:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg, B.S., and K.L. Magleby. 1999. Gating kinetics of single large-conductance Ca2+-activated K+ channels in high Ca2+ suggest a two-tiered allosteric gating mechanism. J. Gen. Physiol. 114:93–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg, B.S., and K.L. Magleby. 2000. Voltage and Ca2+ activation of single large-conductance Ca2+-activated K+ channels described by a two-tiered allosteric gating mechanism. J. Gen. Physiol. 116:75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, M., and L. Salkoff. 1997. A novel calcium-sensing domain in the BK channel. Biophys. J. 73:1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J., and J. Cui. 2001. Intracellular Mg2+ enhances the function of BK-type Ca2+-activated K+ channels. J. Gen. Physiol. 118:589–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J., G. Krishnamoorthy, Y. Yang, L. Hu, N. Chaturvedi, D. Harilal, J. Qin, and J. Cui. 2002. Mechanism of magnesium activation of calcium-activated potassium channels. Nature. 418:876–880. [DOI] [PubMed] [Google Scholar]
- Tian, L., R.R. Duncan, M.S. Hammond, L.S. Coghill, H. Wen, R. Rusinova, A.G. Clark, I.B. Levitan, and M.J. Shipston. 2001. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J. Biol. Chem. 276:7717–7720. [DOI] [PubMed] [Google Scholar]
- Wang, B., B.S. Rothberg, and R. Brenner. 2006. Mechanism of β4 subunit modulation of BK channels. J. Gen. Physiol. 127:449–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, X.M., J.P. Ding, and C.J. Lingle. 1999. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 19:5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, X.-M., X.-H. Zeng, and C.J. Lingle. 2002. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 418:880–884. [DOI] [PubMed] [Google Scholar]
- Yang, H., L. Hu, J. Shi, and J. Cui. 2006. Tuning magnesium sensitivity of BK channels by mutations. Biophys. J. 91:2892–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H., L. Hu, J. Shi, K. Delaloye, F.T. Horrigan, and J. Cui. 2007. Mg2+ mediates interaction between the voltage-sensor and cytosolic domain to activate BK channels. Proc. Natl. Acad. Sci. USA. 104:18270–18275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, S., Y. Li, L. Chen, and Y. Jiang. 2006. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell. 126:1161–1173. [DOI] [PubMed] [Google Scholar]
- Zeng, X.-H., X.-M. Xia, and C.J. Lingle. 2005. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J. Gen. Physiol. 125:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., C.R. Solaro, and C.J. Lingle. 2001. Allosteric regulation of BK channel gating by Ca2+ and Mg2+ through a non-selective, low affinity divalent cation site. J. Gen. Physiol. 118:607–635. [DOI] [PMC free article] [PubMed] [Google Scholar]