Abstract
The intracellular trafficking of the epidermal growth factor receptor (EGFR) is regulated by a cross-talk between calmodulin (CaM) and protein kinase Cδ (PKCδ). On inhibition of CaM, PKCδ promotes the formation of enlarged early endosomes and blocks EGFR recycling and degradation. Here, we show that PKCδ impairs EGFR trafficking due to the formation of an F-actin coat surrounding early endosomes. The PKCδ-induced polymerization of actin is orchestrated by the Arp2/3 complex and requires the interaction of cortactin with PKCδ. Accordingly, inhibition of actin polymerization by using cytochalasin D or by overexpression of active cofilin, restored the normal morphology of the organelle and the recycling of EGFR. Similar results were obtained after down-regulation of cortactin and the sequestration of the Arp2/3 complex. Furthermore we demonstrate an interaction of cortactin with CaM and PKCδ, the latter being dependent on CaM inhibition. In summary, this study provides the first evidence that CaM and PKCδ organize actin dynamics in the early endosomal compartment, thereby regulating the intracellular trafficking of EGFR.
INTRODUCTION
Calmodulin (CaM) is a ubiquitous small calcium sensor that regulates a variety of cellular processes in a spatial and temporal manner within the cell (Toutenhoofd and Strehler, 2000). Microenvironment variations in the concentration of CaM may be critical to the control of cellular processes that involve specific and high-affinity CaM-binding proteins (Berridge et al., 2000; Carafoli, 2002; Kahl and Means, 2003).
We have previously shown that CaM is crucial to the regulation of the dynamics of endosomes. In particular, in the presence of W13, a highly specific CaM antagonist, trafficking is blocked in early endosomes, thereby interfering with transport toward degradation and recycling pathways. This blockage was associated with a dramatic alteration of the morphology and size of early endosomes (Tebar et al., 2002; Lladó et al., 2004). Furthermore we showed that a cross-talk between CaM and protein kinase Cδ (PKCδ) is involved in the regulation of the budding from the early endocytic compartment (Lladó et al., 2004).
Several lines of evidence implicate the actin cytoskeleton in the CaM- and PKCδ-mediated regulation of vesicular trafficking. Because actin contributes to the maintenance of organelle stability (Apodaca, 2001), swelling and morphological changes observed in response to CaM/PKCδ activity (Lladó et al., 2004) may involve altered actin dynamics. Moreover, several CaM-binding proteins and PKCδ substrates are also actin-binding proteins, including α-actinin, adducin, and myristoylated alanine-rich protein kinase C substrate (MARCKS) (Chakravarthy et al., 1999). Finally, RhoGTPases and CaM-binding proteins responsible for fusion-fission events during vesicular trafficking also depend on actin filaments for pinch-off and vesicular movement (Mayorga et al., 1994; Mu et al., 1995; Colombo et al., 1997; Ridley, 2001; Burgoyne and Clague, 2003; Lawe et al., 2003; Donaldson, 2005).
The actin cytoskeleton has been shown to play a vital role in controlling the multifaceted process of endocytosis, including endocytosis at different ports of entry such as clathrin/caveolae-dependent mechanisms and the fusion of vesicles with early endosomes (Apodaca, 2001; Lanzetti et al., 2001; Taunton, 2001; Stamnes, 2002; Ayscough, 2004; Kaksonen et al., 2006). Molecules such as dynamin, syndapin, Huntingtin-interacting protein 1 related (HIP1R), Abp1, synaptojanin, neural Wiskott-Aldrich syndrome protein (N-WASP), intersectin, and cortactin link the endocytic machinery to the actin cytoskeleton (Qualmann et al., 2000; Jeng and Welch, 2001; Schafer, 2002; da Costa et al., 2003).
More precisely, cortactin activates Arp2/3 complex and regulates the formation of branched actin networks during various cellular processes (Daly, 2004). In trafficking events, cortactin has been involved in clathrin-mediated endocytosis as well as in pinocytosis, regulation of epidermal growth factor receptor (EGFR) degradation, or endosomal positioning (McNiven et al., 2000; Cao et al., 2003, 2005; Lynch et al., 2003; Cabezas et al., 2005; Merrifield et al., 2005; Sauvonnet et al., 2005; Timpson et al., 2005; Zhu et al., 2005; Mettlen et al., 2006; Le Clainche et al., 2007).
Here, we analyze the mechanisms by which CaM regulates membrane trafficking along the endocytic pathway. We show that when CaM is inhibited, PKCδ activation leads to F-actin accumulation around the early endosomes, blocking receptor trafficking. Furthermore, we demonstrate that Arp2/3 complex and cortactin, together with PKCδ, supply the molecular machinery required for the formation of the actin coat associated with enlarged early endosomes. Finally, this study shows a new role for actin, through cortactin and Arp2/3 complex, in the control of membrane trafficking events at the early endocytic compartment.
MATERIALS AND METHODS
Reagents
Mouse receptor-grade epidermal growth factor (EGF) and W13 were from Sigma Chemical (Madrid, Spain). Rottlerin, Y27632, and cytochalasin D were from Calbiochem (Merck Eurolab KGaA, Darmstadt, Germany). Transferrin conjugated with tetramethylrhodamine B isothiocyanate (TRITC) was from Invitrogen (Carlsbad, CA). Primary antibodies used were as follows: mouse monoclonal anti-EGFR (American Type Culture Collection, Manassas, VA), mouse monoclonal anti-actin (Valeant Pharmaceuticals, Costa Mesa, CA), rabbit anti-RhoB (Bethyl Laboratories, Montgomery, TX), mouse monoclonal anti-early endosomal antibody (EEA)1 (BD Biosciences Transduction Laboratories, Erembodegem, Belgium), mouse monoclonal anti-p16-Arc (Synaptic Systems, Göttingen, Germany), rabbit anti-WASP family Verprolin-homologous protein (WAVE) and mouse monoclonal anti-cortactin (Upstate Biotechnology, Charlottesville, VA), rabbit anti-green fluorescent protein (GFP) (Abcam, Cambridge, United Kingdom), rabbit anti-PKCδ (Santa Cruz Biotechnology, Santa Cruz, CA). Peroxidase-labeled antibodies and SDS-polyacrylamide gel electrophoresis (PAGE) molecular weight markers were from Bio-Rad (Hercules, CA). 125I-EGF and calmodulin-Sepharose beads were purchased from GE Healthcare (Chalfont St. Giles, England). Protein A bound to Sepharose was from Pierce Chemical (Rockford, IL). Yellow fluorescent protein (YFP)-actin and GFP-tagged plasmids were from Clontech (Palo Alto, CA). GFP-WA and GFP-WASPΔWA, myc RhoB wt, pSUPER RhoB, GFP-PKCδ (Ling et al., 2004), and flag cortactin (Weed et al., 2000) were kindly provided by M. Way (London Research Institute, London, United Kingdom), A. J. Ridley (Ludwig Institute for Cancer Research and Department of Biochemistry and Molecular Biology, University College London, London, United Kingdom), C. Larsson (Lund University, Lund, Sweden), and S. A. Weed (West Virginia University, Morgantown, WV), respectively.
Cell Culture
Normal rat kidney cells (NRK) were grown in DMEM containing 5% fetal calf serum (FCS), pyruvic acid, antibiotics, and glutamine. DMEM and FCS were purchased from Biological Industries (Beit Haemek, Israel). In some experiments, we also used Green monkey kidney cells (COS1), Vero cells, or HeLa cells.
Immunofluorescence Staining
Cells grown on coverslips were fixed with freshly prepared 4% para-formaldehyde for 12 min at room temperature and mildly permeabilized with phosphate-buffered saline (PBS) containing 0.1% Triton X-100, 0.1% bovine serum albumin (BSA) at room temperature for 3 min. Coverslips were then incubated in the same buffer, in which Triton X-100 was omitted, at room temperature for 1 h with the primary antibody, washed intensively, and then incubated with adequate secondary antibodies labeled with Alexa Fluor 488, Alexa Fluor 594 (Invitrogen), or cyanine (Cy)5 (Jackson ImmunoResearch Laboratories, Soham Cambridgeshire, United Kingdom). Both primary and secondary antibody solutions were precleared by centrifugation at 14,000 × g for 10 min. In some experiments, phalloidin-conjugated to TRITC (Sigma Chemical) or to Alexa Fluor 350 (Invitrogen) was incubated together with the secondary antibodies. After staining, the coverslips were mounted in Mowiol (Calbiochem). The images were recorded using an inverted epifluorescence Axiovert 200M microscope (Carl Zeiss, Göttingen, Germany) equipped with a photometric coolSNAP HQ camera, all controlled by SlideBook 3.0.10.5 software (Intelligent Imaging Innovation, Denver, CO) or a Leica TCS SL laser scanning confocal spectral microscope (Leica Microsystems, Heidelberg or Mannheim, Germany). Final analysis of deconvolved images was performed using Adobe Photoshop 5.5 (Adobe Systems. Mountain View, CA) and ImageJ (http://rsb.info.nih.gov/ij/).
Colocalization between p16 or cortactin and transferrin-TRITC on endosomes was quantified using the ImageJ program (Wayne Rasband, National Institutes of Health) and the “Highlighting Colocalization” plugin (Pierre Bourdoncle, Institute Jacques Monod, Service Imagerie, Paris, France). The Highlighting Colocalization plug-in generated an image of colocalized points (binary). A threshold image of transferrin-TRITC was created and colocalization was expressed as the ratio between the area of colocalized points and the threshold area of the corresponding image. At least 15 cells were analyzed for each condition.
Generation and Expression of DNA Constructs
The original Flag-tagged human wild-type (wt)-cofilin in the pKEX-2-XR plasmid (a kind gift of Y. Samstag, Ruprecht Karl University, Institute of Immunology, Heidelberg, Germany) has been described previously (Nebl et al., 1996). To obtain a recombinant cofilin S3A mutant fused to YFP, the point mutation was introduced by polymerase chain reaction (PCR) by using a primer containing serine 3 (TCC) mutated to alanine (GCC). Then, a Bgl II/Hind III fragment was excised from the pKEX-2-XR plasmid and ligated into an equally digested pEYFPC3 vector.
Transient expression of all plasmids was performed using Effectene (QIAGEN, Valencia, CA). Cells were used for experiments 24–48 h after transfection.
Suppression of Cortactin Expression by RNA Interference (RNAi)
Knockdown of cortactin was performed as described previously (Timpson et al., 2005). Briefly, 19-nucleotide RNAs were chemically synthesized (Ambion, Austin, TX) based on the sequence 5′-AAGCUGAGGGAGAAUGUCUUGU-3′ (small interfering RNA; [siRNA]1) (Helwani et al., 2004) or 5′-GACUGGUUUUGGAGGCAAAUUUU-3′ (siRNA2) (Engqvist-Goldstein et al., 2004). siRNA against GFP was used as a control. For transfection Oligofectamine (Invitrogen) was used following manufacturer's instructions.
Recycling and Degradation of 125I-EGF
125I-EGF recycling and degradation were measured as described previously (Kornilova et al., 1996). Briefly, cells in six-wells plates were incubated with 5 ng/ml 125I-EGF for 7 min at 37°C and washed in cold DMEM. The 125I-EGF that had not been internalized during 37°C incubation was removed from the cell surface by a 2.5-min acid wash (0.2 M sodium acetate, 0.5 M NaCl, pH 4.5). At this point cells are referred to as “125I-EGF-loaded cells.” Trafficking of 125I-EGF-receptor complexes in these loaded cells was then initiated by incubating the cells in fresh binding medium containing 100 ng/ml unlabeled EGF and dimethyl sulfoxide, W13 (7.5 μg/ml), or cytochalasin D (0.1 μM) at 37°C for 30 min. Excess of unlabeled EGF in the medium and at the surface prevented rebinding and reinternalization of recycled 125I-EGF. At the end of the chase incubation, the medium was collected to measure the amount of intact and degraded 125I-EGF by precipitation with trichloracetic acid (TCA). Then, cells were incubated for 5 min with 0.2 M acetic acid, pH 2.8, containing 0.5 M NaCl at 4°C to determine the amount of surface-bound 125I-EGF. Finally, cells were solubilized in 1 N NaOH to measure the amount of intracellular 125I-EGF. The amount of recycled 125I-EGF was calculated by adding the radioactivity counted on the cell surface and the TCA-precipitated radioactivity in the medium during chase incubation, and the recycling or degradation rate was expressed to the total of EGF molecules.
SDS-PAGE and Western Blotting
SDS-PAGE was carried out as described by Laemmli (1970). The protein content of the samples was measured by the method of Bradford (Bradford, 1976; Bio-Rad). For Western blotting, 10–50 μg of protein per well was transferred electrophoretically at 60 V for 2 h at 4°C to Immobilon-P transfer membranes (Millipore, Billerica, MA), and antigens were identified using different antibodies diluted in Tris-buffered saline with 0.05% Tween 20. Finally, the reaction product was detected using the ECL system (GE Healthcare).
Immunoprecipitation
HeLa cells grown on 100-mm dishes were transfected with flag pcDNA3 cortactin and phosphorylated (p)EGFP or pEGFP-PKCδ and split after 6 h on 60-mm dishes. Twenty hours later, cells were starved for 1 h and treated ± W13 in binding medium (DMEM, 0.1% BSA, 20 mM HEPES, pH 7.3). Cells were then washed in PBS and solubilized by scraping with a rubber policeman in TGH buffer (1% Triton X-100, 10% glycerol, 50 mM NaCl, 50 mM HEPES, pH 7.3, 0.5% sodium deoxycholate, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 10 mg/ml leupeptin, 10 mg/ml aprotinin) followed by gentle rotation for 10 min at 4°C. Lysates were then centrifuged at 14,000 × g for 10 min at 4°C. Supernatants of transfected or not transfected cells were incubated with rabbit polyclonal anti-GFP or monoclonal anti-cortactin antibodies for 2 h at 4°C and then for 60 min after the addition of protein A or protein G-Sepharose, respectively. Immunoprecipitates were washed twice in TGH buffer supplemented with 150 mM NaCl and then once without NaCl. SDS-polyacrylamide gels (12%) were used to separate proteins. Proteins were then transferred to Immobilon-P and immunoblotted using anti-cortactin, anti-PKCδ, or anti-GFP followed by the appropriate peroxidase-conjugated secondary antibody and ECL detection.
Affinity Chromatography with CaM-Sepharose
For pull-down assays with cellular lysates, HeLa cells in a 100-mm dish were serum starved for 1 h, washed twice in ice-cold phosphate-buffered saline, lysed with 0.5 ml of TGH buffer, gently rotated for 10 min at 4°C, and clarified by centrifugation. Lysates (equalized for protein content) were incubated with 30 μl of CaM-Sepharose for 2 h at 4°C in the presence of 1 mM CaCl2 or 5 mM EGTA. The unbound fraction was collected by centrifugation, and the remaining bound fraction was washed twice in TGH buffer containing CaCl2 or EGTA supplemented with 100 mM NaCl and then once without NaCl. An aliquot (25 μl) of the unbound fraction and the total of the bound fraction were analyzed by electrophoresis and Western blotting. A lysate from HeLa cells was always loaded in the same gel as a control for the mobility of each protein.
RESULTS
We have previously reported the effect of the CaM antagonist W13 on the morphology and dynamics of the endocytic pathway. W13 treatment produced large aberrant endosomes containing early endocytic markers and internalized EGF, dextran, and transferrin (Apodaca et al., 1994; Tebar et al., 2002). The blockage in the ongoing trafficking from these early endosomes was PKCδ dependent (Lladó et al., 2004).
CaM and PKCδ Regulates the Formation of an F-Actin Coat Surrounding the Early Endosomes
To explore a possible connection between the actin cytoskeleton and morphologically aberrant early endosomes, we first examined, in NRK cells,1 whether the endosomes produced by W13 contained actin. By means of immunofluorescence, F-actin was labeled with phalloidin-TRITC, and early endosomes were labeled with anti-EEA1. Untreated (Figure 1A) and W12-treated (data not shown) NRK cells showed an abundance of stress fibers and early endosomes in the perinuclear region of the cell. In NRK cells treated with W13, F-actin was found strongly associated with enlarged early endosomes; this observation was concomitant with a significant decrease (28%) in the number of stress fibers in the cytoplasm. The staining with phalloidin also showed F-actin along the plasma membrane (in both W13 and control cells) (Figure 1).
Figure 1.
CaM regulates F-actin dynamics in early endosomes. (A) NRK cells were incubated for 60 min with the specific CaM inhibitor W13 (5 μg/ml) at 37°C. Labeling with phalloidin-TRITC and anti-EEA1 (Alexa Fluor 488) is shown. (B) High magnification of actin patches surrounding endosomes of cells treated with W13 as described in A. (C) NRK cells were microinjected with YFP-actin, DNA expression vector, and incubated 60 min with W13 (5 μg/ml) and 30 min with transferrin-TRITC at 37°C. Bars, 10 μm.
Higher magnification showed that individual early endosomes contained both EEA1 and F-actin. However, there was a discontinuous, patched distribution of these two molecules at the membrane, showing different domains with some areas of colocalization (see insets in Figure 1B).
To examine whether these early endosomes incorporated ectopically expressed actin, NRK cells (n = 200) were microinjected with YFP-actin expression vector and then incubated for 60 min with W13 and for 30 min with transferrin-TRITC at 37°C. Figure 1C shows that fluorescent actin clearly labels those early endosomes filled with transferrin.
Next, to determine whether this F-actin coat was responsible for the arrest of ongoing trafficking, actin filaments were disrupted using two types of depolymerizing agents: cytochalasin D and the expression of constitutively active YFP-cofilin (S3A) (Moriyama et al., 1996). Cytochalasin D induced the disassembly of actin filaments and a concurrent loss of the aberrant and W13-induced early endosomes morphology (Figure 2A). Similar results were obtained in COS1 cells expressing YFP-cofilin S3A (Figure 2B). Both results indicated that, in cells depleted of functional CaM, the formation of the F-actin coat is responsible for the alteration of the morphology of early endosomes and therefore might control the exit (budding) from this compartment.
Figure 2.
Actin may control early endosome exit in cells treated with the CaM antagonist. (A) NRK cells were incubated for 45 min with W13 (5 μg/ml) and 30 min with the actin-depolymerizing agent cytochalasin D (1 μM). Anti-EEA1 (Alexa Fluor 488) and phalloidin-TRITC stainings are shown. Bar, 10 μm. (B) Vero cells transiently expressing constitutively active YFP-cofilin (S3A) were treated with W13 for 60 min at 37°C. After fixation, the cells were immunolabeled with anti-EEA1 (Alexa Fluor 594) and phalloidin-TRITC. Bar, 10 μm.
To further characterize the involvement of actin polymerization on the trafficking events from these early endosomes, recycling and degradation of EGFR was quantified using 125I-EGF, as described in Materials and Methods. Supplemental Table S1, A and B, show the effects of cytochalasin D on the recycling and degradation of the EGF-receptor in control and W13-treated HeLa cells, respectively. In control cells, F-actin disruption by cytochalasin D inhibits recycling (∼20%) and degradation (∼30%) of the receptor (Supplemental Table S1; similar results were obtained with YFP-cofilinS3A expression, data not shown). In addition, cytochalasin D or YFP-cofilinS3A expression (data not shown) significantly restored EGF receptor recycling, but not its degradation, when CaM is inhibited (Supplemental Table S1). These results indicated that actin polymerization participates in the regulation of recycling as well as degradation of EGFR.
We have previously shown that when CaM was inhibited, active PKCδ was necessary to inhibit EGFR recycling from the early endocytic compartment (Lladó et al., 2004). Thus, to determine whether PKCδ was also involved in the formation of the F-actin coat when cells were depleted of CaM, NRK cells were incubated with the PKCδ inhibitor rottlerin. The endocytic compartment was loaded with transferrin-TRITC, and finally cells were double labeled with phalloidin. When both PKCδ and CaM were inhibited, NRK cells showed a significant recovery in the number of stress fibers, their early endosomes were smaller and surrounded by less F-actin (Figure 3, compare W13 + rottlerin [bottom panels] with W13 panels).
Figure 3.
A CaM and PKCδ interplay modulates actin on endosomes. NRK cells were preincubated with W13 (5 μg/ml) for 30 min, treated with the PKCδ-specific inhibitor rottlerin (5 μM) for 30 min, and then 15 min with transferrin-TRITC (50 μg/ml) at 37°C. EEA1 (Alexa Fluor 488), transferrin-TRITC, and phalloidin (Alexa Fluor 350) stainings are shown. Bar, 10 μm.
PKCδ Regulates Endocytic Trafficking in RhoB-overexpressing Cells
GTPases of the Rho family (e.g., Cdc42, Rac1, RhoA, RhoD, and TC10) are important regulators of various membrane trafficking events, but also of actin dynamics (Symons and Rusk, 2003). In particular, RhoB is localized, at least in part, to early endosomes (Adamson et al., 1992; Robertson et al., 1995; Rondanino et al., 2007), and it has been shown that overexpression of its constitutively active mutant slows the transit of receptor-bound EGF between endocytic compartments (Gampel et al., 1999; Fernandez-Borja et al., 2005; Rondanino et al., 2007).
Because of these striking similarities between the effects of RhoB overexpression and CaM inhibition, we investigated whether RhoB could have a role in CaM-regulated endocytic trafficking. Thus, COS1 cells were transfected with myc-RhoBwt and then incubated with EGF, fixed, and triple labeled with anti-myc, phalloidin-TRITC, and anti-EGFR. In support of a link between RhoB and CaM and similar to the results described above (Figures 1 and 2), overexpression of RhoBwt produced large vesicular structures that were surrounded by F-actin (Figure 4A).
Figure 4.
Comparison of RhoB and CaM roles in endosomes. (A) Twenty-four hours after transfection with myc-RhoB wt, COS1 cells were incubated with EGF (100 ng/ml; 15 min) at 37°C, fixed, and triple-stained with anti-myc (Alexa Fluor 488), phalloidin-TRITC and anti-EGFR (Cy5). (B) COS1 cells were transfected with pSUPER RhoB, and, after 72 h of knockdown, cells were incubated with W13 (5 μg/ml; 60 min) at 37°C. EEA1 was detected with specific antibody followed by Alexa Fluor 594. The extent of RhoB depletion is shown by Western blotting. (C) NRK cells were preincubated with W13 (5 μg/ml; 30 min), treated with the ROCK inhibitor Y27632 (20 μM) for the next 45 min and with transferrin-TRITC (50 μg/ml) for the last 15 min at 37°C. After fixation, cells were labeled with phalloidin-Alexa Fluor 350 and anti-EEA1 (Alexa Fluor 488). Bar, 10 μm. (D) COS1 cells were transfected and treated as described in A, adding 5 μM rottlerin for 30 min. Bar, 10 μm. On the right, quantification of the number of RhoB-overexpressing cells presenting 5 or more enlarged endosomes (diameter >350 nm) from two independent experiments (performed as described above; n = 900 cells) is represented. Each data point represents value averaged (±SD). ***p < 0.001 for Student's t test, indicating statistical significance of differences between EGF- and EGF- + rottlerin-treated cells.
To determine the effect of CaM inhibition on endosome morphology upon RhoB knockdown (RhoB depletion is shown in Figure 4B by Western blotting), cells were transfected with pSUPER RhoB (a plasmid encoding for siRNA targeting RhoB). In cells with reduced expression of RhoB, endosomes were similar to those in nontransfected cells (Figure 4B, top). However, in these RhoB-depleted cells, the inhibition of CaM still produced large aberrant early endosomes (Figure 4B, bottom), indicating that RhoB is not involved in the W13-inducible early endosome morphology.
Moreover, to confirm the data obtained upon siRNA-mediated silencing of RhoB, we next investigated whether ROCK, one of the most important RhoB effectors (Ridley, 2006), was involved. To this end, NRK cells were treated with Y27632, a specific Rho kinase (ROCK) inhibitor, and the early endosome morphology and actin cytoskeleton were analyzed (Figure 4C). ROCK inhibition clearly reduced the number of stress fibers but early endosomes still showed normal size and location. As shown in Figure 4C, the presence of Y27632 did not interfere with the effect of W13 on early endosomes, and very large EEA1-positive endosomes surrounded by F-actin were still observed. Therefore, we conclude that RhoB was not necessary for the altered morphology observed in early endosomes in the absence of active CaM.
Finally, to address whether RhoB acts together with PKCδ, COS1 cells were transfected with RhoB (as in Figure 4A) but now adding rottlerin, for 30 min. Triple labeling in Figure 4D showed that the inhibition of PKCδ restored the size of early endosomes enlarged by the overexpression of RhoB. Quantification (in ∼900 cells) showed that treatment with rottlerin reduced the number of RhoB-transfected cells that contained more than five aberrant early endosomes (with a diameter >350 nm) from 71 to 42%. Therefore, RhoB overexpression and CaM inhibition converge in PKCδ to regulate exit from early endosomes.
Arp2/3 Complex Participates in the Formation of the Actin Coat in Early Endosomes
The key steps regulating actin dynamics, nucleation, and elongation are catalyzed by a discrete number of proteins (and/or protein complexes) (Revenu et al., 2004). Among these, we have identified, by means of immunocytochemistry, several actin-binding proteins present in these large early endosomes: annexins A1 and A2, α-actinin, and MARCKS (Supplemental Figure S1).
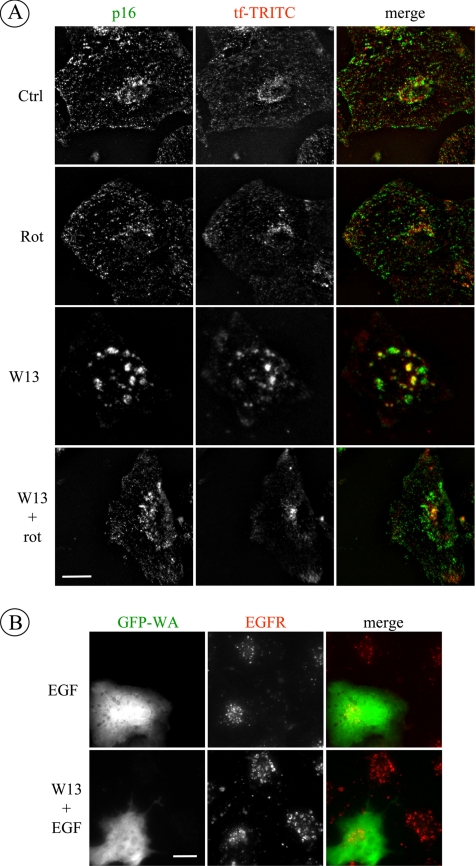
One possible explanation for the accumulation of F-actin around early endosomes, in the absence of CaM, is an induced local polymerization of actin. For this process, the actin related protein (Arp)2/3 complex activity is crucial (Goley and Welch, 2006). The complex is inactive by itself, and it can be activated through interaction with members of the N-WASP/WAVE family, which are regulated by small GTPases such as Cdc42 and Rac1 (Stradal et al., 2004; Stradal and Scita, 2006). To address the possible involvement of Arp2/3 complex in the accumulation of F-actin in endosomes, we first examined whether Arp2/3 complex was present in the large W13-induced early endosomes. NRK cells were preincubated with W13 and then treated with rottlerin. In cells treated with W13, the anti-p16 (a subunit of Arp2/3 complex) (Welch et al., 1997) specifically localized in large vesicular structures, some of them containing internalized transferrin-TRITC (24.4%) (Figure 5A). However, in the presence of rottlerin (±W13) the staining of endosomes was similar to that in control cells (14%).
Figure 5.
Arp2/3 participates in the CaM and PKCδ regulated actin dynamics. (A) NRK cells were preincubated with W13 (5 μg/ml; 30 min) and treated with rottlerin (5 μM) and transferrin (50 μg/ml) for 30 min at 37°C. p16, a subunit of the Arp2/3 complex (Alexa Fluor 488) and transferrin-TRITC labeling are shown. (B) Twenty-four hours after transfection with the WA domain of N-WASP tagged with GFP (which binds and sequesters Arp2/3), COS1 cells were incubated with W13 (5 μg/ml; 90 min) and EGF (100 ng/ml; 15 min) at 37°C. Next, cells were fixed and stained with anti-EGFR (Alexa Fluor 594). Bar, 10 μm.
To examine the role of Arp2/3 complex in the morphological effect of W13 on endosomes, we transfected COS1 cells with the GFP-tagged WA domain of N-WASP, which acts in a dominant-negative manner as it binds and sequesters Arp2/3 complex (Higgs et al., 1999; Moreau et al., 2000; Marchand et al., 2001; Shao et al., 2006). COS1 cells were incubated with W13 and EGF, fixed, and labeled with anti-EGFR. In cells expressing the WA domain of N-WASP, W13 did not affect the enlargement of early endosomes (Figure 5B).
We have previously shown that W13 inhibits the recycling of EGFR (and transferrin) (Tebar et al., 2002; Lladó et al., 2004). Thus, to assess whether the blockage of Arp2/3 complex upon WA overexpression restored the trafficking toward the recycling pathway, cells were transfected with the WA construct, incubated with 125I-EGF for 7 min, and then treated with W13. In cells transfected with the WA construct (efficiency of transfection ∼60%), the recycling of EGF-EGFR was partially but significantly recovered (25%) (Supplemental Table S2A). In addition, overexpression of the WA domain in controls significantly inhibited the recycling of EGF-EGFR (Supplemental Table S2A). This seems like a moderate effect (11%), but considering the incomplete transfection efficiency, the statistical significance of these findings suggests a physiological role of Arp2/3 complex in receptor recycling.
Different pathways involving the actin nucleation promoting factors (NPFs) converge for the activation of Arp2/3-mediated actin polymerization, including N-WASP, WAVE, and cortactin (Goley and Welch, 2006). To determine which of these three is operative when CaM is inhibited, we first used a dominant negative of N-WASP (N-WASPΔWA-GFP) (Moreau et al., 2000). The expression of this N-WASP mutant, which does not bind to Arp2/3 complex, did not interfere with the effect of W13 on the formation of aberrant early endosomes (Figure 6A) or in the blockage of the recycling of 125I-EGF (Supplemental Table S2B).
Figure 6.
N-WASP or WAVE are not involved in W13 effect. (A) COS1 cells transiently expressing GFP-N-WASP-ΔWA were incubated with W13 (5 μg/ml) for 90 min and EGF (100 ng/ml) for the last 15 min at 37°C, fixed, and immunolabeled with anti-EGFR (Alexa Fluor 594). Bar, 10 μm. (B) NRK cells were preincubated with W13 (7.5 μg/ml) for 60 min and then treated with transferrin-TRITC (50 μg/ml) for 30 min at 37°C. Next, cells were fixed and stained with anti-WAVE (Alexa Fluor 488). Bar, 10 μm.
In addition, when NRK cells were labeled with anti-WAVE together with transferrin-TRITC, WAVE was predominantly localized at the plasma membrane even after the treatment with W13. Large endocytic and transferrin-TRITC–positive structures produced by the W13 treatment were not labeled with anti-WAVE (Figure 6B). Therefore, neither of these two NPFs seemed to participate in the activation of Arp2/3 complex.
Cortactin Is Involved in the Regulation of EGF Recycling from Early Endosomes
Cortactin is a multidomain protein consisting of an NH2-terminal acidic region that binds to the Arp2/3 complex, a fourth repeat that binds to filamentous actin and a COOH-terminal Src homology 3 domain, which recruits a variety of other cellular proteins. Cortactin can stimulate the actin-nucleation activity of the Arp2/3 complex alone or in combination with N-WASP (Daly, 2004).
To address whether cortactin is involved in CaM- and PKCδ-dependent pathways regulating endocytic transport, we first studied the cellular location of cortactin in NRK cells incubated with transferrin-TRITC. In untreated or rottlerin-treated cells, cortactin was mainly located at the plasma membrane and in some extent in transferrin-positive vesicles (8.2%) (Figure 7A). However, inhibition of CaM by W13 resulted in an increased localization of cortactin in enlarged endocytic structures, colocalizing with transferrin-TRITC (18.2%). W13 and rottlerin together restored the normal cortactin pattern (9.1%), which indicates that the endosomal localization of cortactin is controlled by CaM and PKCδ signaling.
Figure 7.
Cortactin regulates recycling in the early endosomes. (A) NRK cells were preincubated for 30 min with W13 (5 μg/ml), treated with rottlerin (5 μM) for 30 min, and then with transferrin (50 μg/ml) for 15 min at 37°C. Cortactin (Alexa Fluor 488) and transferrin-TRITC staining are shown. Bar, 10 μm. (B) HeLa cells were transfected for 72 h with cortactin siRNA duplex 1 and 2 or GFP siRNA. Then, cells internalized 125I-EGF (5 ng/ml; 7 min), and they were treated with W13 (7.5 μg/ml) for 30 min at 37°C to measure recycling and degradation. Each data point in the histogram represents the mean of a minimum of six replicates from two independent experiments for each siRNA (±SD). *p < 0.05, **p < 0.01, ***p < 0.001 for Student's t test, indicating statistical significances of differences between GFP and cortactin siRNA-transfected cells. The degree of down-regulation of cortactin is demonstrated by Western blotting.
To confirm the involvement of cortactin in the regulation of EGFR recycling from early endosomes, cells were transfected with two different cortactin siRNAs (Engqvist-Goldstein et al., 2004; Helwani et al., 2004) to knock down the endogenous protein (Figure 7B). In both experiments, EGFR recycling was recovered in cells treated with W13 (from 22 to 32%, consistent with the degree of cortactin depletion shown by Western blotting). In control cells, knock down of cortactin did not affect recycling rates (Figure 7B). However, cortactin knock down inhibited EGFR degradation in controls without modifying the W13 effect. Interestingly, these results mimic the previously reported effects on EGFR trafficking and degradation observed when PKCδ was inhibited (Lladó et al., 2004).
Because these findings suggested that cortactin could be regulated by CaM and PKCδ, we analyzed whether cortactin binds to CaM and/or to PKCδ. Figure 8A shows a representative pull-down, using Sepharose-CaM, and it can be observed that in the presence of calcium, cortactin binds to CaM. Under the same conditions, RhoB was not found to interact with CaM (Figure 8A). Finally, the interaction between cortactin and PKCδ was analyzed by means of coimmunoprecipitation. Cells, ectopically expressing cortactin and GFP-PKCδ or GFP (as control), were immunoprecipitated using a polyclonal antibody against GFP. Cortactin was coimmunoprecipitated with GFP-PKCδ, and this interaction was increased by W13 treatment (Figure 8B). Reciprocally, endogenous cortactin was immunoprecipitated using a monoclonal specific antibody and the coimmunoprecipitated endogenous PKCδ was increased after inhibition of CaM (Figure 8C). Whether the interaction of cortactin and PKCδ modulated by CaM has any functional significance in actin dynamics remains to be investigated.
Figure 8.
CaM and PKCδ interact with cortactin. (A) Cellular lysates from HeLa cells were incubated with CaM-Sepharose in the presence of Ca2+ or EGTA as described in Materials and Methods. All bound fraction and 25 μl of the unbound fraction or lysate was loaded and the presence of cortactin and RhoB was analyzed by Western blotting. (B) HeLa cells were cotransfected with cortactin and GFP or GFP-PKCδ. After 24-h transfection, cells were incubated for 30 min with or without W13 (10 μg/ml) at 37°C. HeLa cell extracts were incubated with anti-GFP polyclonal antibodies, and the immunocomplex was pulled down by using protein A-Sepharose. The presence of cortactin and GFP or GFP-PKCδ in the immunoprecipitates was analyzed by Western blotting by using anti-cortactin and anti-GFP antibodies, respectively. (C) HeLa cells were treated for 30 min ± W13 (10 μg/ml) at 37°C. Cell extracts were incubated with anti-cortactin monoclonal antibody and pulled down using protein G-Sepharose. The presence of PKCδ and cortactin in the immunoprecipitates was analyzed by Western blotting.
Together, the data presented in this study suggest that the interplay of CaM/PKCδ might regulate actin polymerization through cortactin-mediated activation of Arp2/3 complex in early endosomes. Consequently, this might significantly affect the trafficking along the recycling pathway.
DISCUSSION
In previous studies, we demonstrated the functional relationship between CaM and PKCδ in the control of EGFR and transferrin trafficking in the early endocytic compartment. Now, we report that when CaM is inhibited, PKCδ signaling promotes the formation of an actin coat surrounding the endosomes, which is critical for the inhibition of the ongoing traffic from these structures. In particular, we provide evidence that PKCδ, through Arp2/3 complex and cortactin, generates an F-actin coat in early endosomes.
CaM and Actin Dynamics in the Early Endosomes
CaM may regulate the actin cytoskeleton through its effectors, thereby controlling endo- and exocytic pathways (Guerin and de Chastellier, 2000; Taunton et al., 2000; Qualmann and Kessels, 2002; Merrifield et al., 2004; Chang et al., 2005). The assembly of actin coats on different populations of endosomes and vacuoles has been reported as a result of a variety of stimuli in distinct cell types throughout the endocytic compartment. For example, the expression of Arf6 Q67L (constitutively active) or phosphatidylinositol 4-phosphate 5-kinase (PIP5K) blocks membrane traffic after endocytosis and is characterized by enlarged vacuolar structures that are phosphatidylinositol bisphosphate (PIP2)-enriched and contain actin-coated membranes (Brown et al., 2001). In addition, there are specific physiological situations such as infection by Leishmania donovani promastigotes that lead to inhibition of phagosome maturation and the accumulation of periphagosomal F-actin in macrophages. Moreover, dominant-negative Cdc42N17 inhibits LPG-mediated phagosomal accumulation of F-actin and retention of Arp2/3 complex and myosin (Lodge and Descoteaux, 2005a,b). In addition, the deficiency of PIP2 5-phosphatase in Lowe Syndrome, leads to an elevation of PIP2, which affects actin polymerization. Cells from patients with this syndrome show a decrease of long actin stress fibers but an increase in punctuate F-actin staining (Suchy and Nussbaum, 2002). In Dictyostelium amoebae, it has also been demonstrated that an actin coat prevents the clustering of late endosomes and their aggregation (Drengk et al., 2003). Also, Gauthier et al. (2007) demonstrated that sorting from GPI-activator protein–enriched early endosomal compartments to late endosomes requires dynamic F-actin structures on early endosomes. In this study, we observed a strong recruitment of F-actin to the early endocytic compartment, responsible for receptor recycling blockage, upon inhibition of CaM. Therefore, actin polymerization and depolymerization must be tightly regulated to guarantee the correct endocytic membrane transport. Along these lines, in the present study the use of actin depolymerizing agents (cytochalasin D, constitutively active cofilin), demonstrates the involvement of F-actin in EGFR recycling and degradation in normal cells. Moreover, F-actin disruption by these agents, significantly restores EGFR recycling but not its degradation when CaM is inhibited. The inability to rescue the W13 effect on degradation indicates that there might exist additional effects of CaM inhibition, not mediated via actin, on the EGFR degradative pathway. These data confirm the physiological relevance of the actin cytoskeleton to preserve morphological characteristics and receptor trafficking from the endosomal compartment.
Furthermore, an F-actin coat surrounding early endosomes has also been described in cells overexpressing the GTPase RhoB. RhoB is localized in the early and late endocytic compartment and overexpression of the wild-type protein or a constitutively active mutant inhibits EGFR recycling and degradation (Gampel et al., 1999; Fernandez-Borja et al., 2005; Rondanino et al., 2007). Here, we demonstrated a requirement for PKCδ downstream of RhoB in the formation of an actin coat around enlarged endosomes. However, upon RhoB depletion, inhibition of CaM still resulted in a blockage of EGFR trafficking and the production of enlarged endosomes labeled with phalloidin-TRITC. These results, together with the lack of interaction between CaM and RhoB in pull-down, indicate that although the mechanisms by which RhoB overexpression and CaM inhibition induce actin-coated enlarged endosomes are different, they both exhibit a PKCδ dependency.
Molecular Machinery Involved in the Formation of the Actin Coat and the Consequent Inhibition of Endosome Traffic
The involvement of CaM and PKCδ in the regulation of the actin cytoskeleton in early endosomes was investigated by means of immunolocalization, expression of dominant-negative proteins and RNAi knockdown techniques. After inhibition of CaM, we identified a group of actin-binding proteins associated with early endosomes, which are possible candidates to participate in the formation of the actin coat. Among them are α-actinin, annexins A1 and A2, MARCKS, Arp2/3 complex, and cortactin. Conversely, we did not detect endogenous cofilin, WAVE, or adducin proteins (data not shown). Interestingly, the PKCδ/CaM-mediated loss of MARCKS from the plasma membrane could explain the reduction in number of stress fibers observed in these cells. MARCKS is an important factor to anchor actin cytoskeleton to the plasma membrane and also to regulate the structure of actin fibers at this cellular location (Aderem, 1992; Hartwig et al., 1992). Consequently, the PKCδ-triggered dissociation of MARCKS from the plasma membrane, after W13 treatment, could induce the detachment of microfilaments from the plasma membrane.
Moreover, it has been described that the regulatory domain of PKCδ inhibits RhoA by an unknown mechanism and decreases the level of stress fibers in neuronal cells (Ling et al., 2004). Cdc42, and to a lesser extent Rac, are important downstream factors in the pathway through which PKC might mediate morphological and cytoskeletal effects, including stress fibers loss (Troller and Larsson, 2006).
Interestingly, the presence of MARCKS, annexin A1, and α-actinin in endosomes relies on PKCδ activity when CaM is inhibited (Supplemental Figure S1). Then, cortactin might not be the only candidate involved in the W13-mediated inhibition of membrane traffic. In fact, when the expression of cortactin is down-regulated, we could not observe a full recovery of EGF recycling. It is plausible that several proteins could act in conjunction to produce the W13-altered endosomes; therefore, further investigation is necessary to address the involvement of these other candidates.
Our observations suggest that the Arp2/3 complex might be responsible for the F-actin coat assembly when CaM is not functional; p16, a member of the Arp2/3 complex, was found in the endosomal membranes of W13-treated cells. Moreover, when Arp2/3 complex is sequestered in the cytosol by the overexpressed WA domain of N-WASP or by N-WASPΔGBD (Supplemental Figure S2), the morphology of the endosomes is restored. The quantitative analysis of recycling of EGF-EGFR demonstrated the participation of Arp2/3 complex to guarantee the correct recycling of the EGF receptor from this endosomal compartment.
The activity of the Arp2/3 complex depends on NPFs such as N-WASP and suppressor of cAMP receptor (SCAR)/WAVE. These proteins provide a link between Cdc42 and Rac1 activation, respectively, and regulation of actin polymerization (Machesky and Insall, 1999; Miki et al., 2000; Higgs and Pollard, 2001). Indeed, the N-WASP/SCAR/WAVE family of proteins have been implicated in promoting motility of endosomes and other organelles (Taunton et al., 2000; Southwick et al., 2003). Moreover, cortactin also has NPF activity, binds F-actin and has been implicated in the motility of endosomes (Daly, 2004, and references therein). Thus, having demonstrated Arp2/3 complex involvement, we attempted to identify which NPF was upstream of Arp2/3 complex and therefore responsible for the blockage of trafficking in W13-treated cells. The expression of a dominant-negative mutant of N-WASP did not impair the formation of actin-coated endosomes, indicating that N-WASP was not involved. In addition, after CaM inhibition, WAVE was not detected in endosomes. However, in W13-treated cells, cortactin was increased in this compartment (in a PKCδ-dependent manner) and a significant and consistent recovery of EGFR recycling, but not EGFR degradation, was observed upon RNAi-mediated knockdown of cortactin. Besides, depletion of cortactin inhibits EGFR degradation in control cells. These effects were similar to the previously described when PKCδ was inhibited (Lladó et al., 2004).
There are some similarities and implications between the participation of cortactin in the W13-induced enlargement of endosomes and inhibition of membrane traffic to other reported situations. Expression of PIP5K in differentiated 3T3L1 adipocytes results in the formation of enlarged vacuole-like structures coated with F-actin, cortactin, dynamin, and N-WASP (Kanzaki et al., 2004). It has also been shown that cortactin localizes to the bacterial vacuole formed upon infection of epithelial cells with Chlamydia trachomatis (Fawaz et al., 1997), Moreover, a role of the cortactin–dynamin complex in the regulation of post-Golgi transport has been also reported previously (Cao et al., 2005). Thus, cortactin may act at different membrane organelles to regulate formation and scission of transport vesicles.
In addition, it has been shown that cortactin is involved in the accumulation of actin filaments at coated pits and in impairing vesicle release when cells are depleted of Hip1R. Hip1R negatively regulates cortactin-stimulated actin polymerization to guarantee a transient and productive interaction between coated pits and actin during vesicle internalization (Engqvist-Goldstein et al., 2004; Le Clainche et al., 2007). In fact, this same proposal could be formulated in our study on CaM/cortactin and vesicles formation in endosomes. It has been described that CaM regulates budding-exit from endosomes (de Figueiredo and Brown, 1995; Lladó et al., 2004), and here we have shown that cortactin is also responsible for the accumulation of actin filaments at endosomes when CaM is inhibited. Therefore, calcium-bound CaM may regulate cortactin-stimulated actin polymerization levels to achieve vesicle formation in endosomes.
Somewhat unexpectedly, we found that cortactin knockdown inhibits EGFR degradation in HeLa cells, whereas we previously reported that depletion of cortactin facilitated EGFR degradation in cortactin-overexpressing cancer cell lines (Timpson et al., 2005). Although this might seem controversial, levels of cortactin before and after knockdown are very different in both model systems, and high/low cortactin expression levels seem to modulate EGFR trafficking differently in common cell lines, such as HeLa, and cancer cells. In our previous study using head and neck cancer cell lines expressing very high levels of cortactin, its knockdown reduced levels to those found in most cell lines. In the present study, we strongly downregulated low endogenous cortactin expression levels in a commonly used cell line.
Our model proposes that the dynamic assembly and disassembly of F-actin is required for optimal endocytosis and trafficking. Thus, perturbation of this process via elevated (through CaM inhibition, cortactin overexpression or Hip1R knockdown) or diminished actin polymerization (e.g., cytochalasin D, cortactin knockdown in normal cells) can block trafficking events.
Interestingly, we described here for the first time, a novel interaction between cortactin and PKCδ, which is regulated by CaM. The fact that this interaction was increased by W13 can be explained by the competitive binding of CaM and PKCδ to cortactin. Actually, in a variety of substrates (MARCKS, adducin, GAP 43), PKC and CaM compete for the same binding domains (Chakravarthy et al., 1999). After CaM inhibition, active PKCδ is responsible for the accumulation of actin, through cortactin and Arp2/3 complex, around endosomes. In this scenario, we hypothesize that CaM may control the interaction between PKCδ and cortactin. To some extent their activity regulates the actin cytoskeleton in early endosomes and consequently, endocytic fate.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maria Calvo and Anna Bosch for assistance in the confocal imaging (Unitat Microscopia Confocal, Serveis Cientificotècnics, Universitat de Barcelona-Institut d'Investigacions Biomèdiques August Pi i Sunyer [IDIBAPS]); Anne J. Ridley, Christer Larsson, Scott A. Weed, Yvonne Samstag, and Michael Way for donating plasmids; Maria Molinos for technical assistance; and Eulalia Rius for microinjection support. We also thank Gustavo Egea and Miguel Morales for valuable discussions. This study was supported by grants BFU2006-01151 and GEN2003-20662-C07-01 (to C.E.), BMC2003-09496 and BFU2006-15474 (to F.T.), BFU2005-01716 and GEN2003-20662) (to A.P.), and by the Ramón y Cajal Research Program (to F.T.) from Ministerio de Educación y Ciencia (Spain). T.G. is supported by the Gretl Raymond Foundation and the National Heart Foundation of Australia grant G06S2559. R.J.D. is funded by the National Health and Medical Research Council of Australia and the Cancer Council New South Wales. A.L. is supported by Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo (Spain) and by the collaborative grant to C.E. and T.G. of Universitat de Barcelona and University of New South Wales (UB/2006). J.M. is recipient of an IDIBAPS predoctoral fellowship.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0411) on October 24, 2007.
To better visualize the actin cytoskeleton or due to the different efficiency of transfection or siRNA knockdown, we used the following cell lines: NRK, COS1, Vero, and HeLa. W13 or rottlerin have the same effect in all of the above-mentioned cell lines.
REFERENCES
- Adamson P., Paterson H. F., Hall A. Intracellular localization of the P21rho proteins. J. Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A. Signal transduction and the actin cytoskeleton: the roles of MARCKS and profilin. Trends Biochem. Sci. 1992;17:438–443. doi: 10.1016/0968-0004(92)90016-3. [DOI] [PubMed] [Google Scholar]
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- Apodaca G., Enrich C., Mostov K. E. The calmodulin antagonist, W-13, alters transcytosis, recycling, and the morphology of the endocytic pathway in Madin-Darby canine kidney cells. J. Biol. Chem. 1994;269:19005–19013. [PubMed] [Google Scholar]
- Ayscough K. R. Endocytosis: actin in the driving seat. Curr. Biol. 2004;14:R124–R126. [PubMed] [Google Scholar]
- Berridge M. J., Lipp P., Bootman M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown F. D., Rozelle A. L., Yin H. L., Balla T., Donaldson J. G. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Clague M. J. Calcium and calmodulin in membrane fusion. Biochim. Biophys. Acta. 2003;1641:137–143. doi: 10.1016/s0167-4889(03)00089-2. [DOI] [PubMed] [Google Scholar]
- Cabezas A., Bache K. G., Brech A., Stenmark H. Alix regulates cortical actin and the spatial distribution of endosomes. J. Cell Sci. 2005;118:2625–2635. doi: 10.1242/jcs.02382. [DOI] [PubMed] [Google Scholar]
- Cao H., Orth J. D., Chen J., Weller S. G., Heuser J. E., McNiven M. A. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol. Cell Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Weller S., Orth J. D., Chen J., Huang B., Chen J. L., Stamnes M., McNiven M. A. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat. Cell Biol. 2005;7:483–492. doi: 10.1038/ncb1246. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M. I., Beron W., Stahl P. D. Calmodulin regulates endosome fusion. J. Biol. Chem. 1997;272:7707–7712. doi: 10.1074/jbc.272.12.7707. [DOI] [PubMed] [Google Scholar]
- Chakravarthy B., Morley P., Whitfield J. Ca2+-calmodulin and protein kinase Cs: a hypothetical synthesis of their conflicting convergences on shared substrate domains. Trends Neurosci. 1999;22:12–16. doi: 10.1016/s0166-2236(98)01288-0. [DOI] [PubMed] [Google Scholar]
- Chang F. S., Han G. S., Carman G. M., Blumer K. J. A WASp-binding type II phosphatidylinositol 4-kinase required for actin polymerization-driven endosome motility. J. Cell Biol. 2005;171:133–142. doi: 10.1083/jcb.200501086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa S. R., Okamoto C. T., Hamm-Alvarez S. F. Actin microfilaments et al.–the many components, effectors and regulators of epithelial cell endocytosis. Adv. Drug Deliv. Rev. 2003;55:1359–1383. doi: 10.1016/j.addr.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Daly R. J. Cortactin signalling and dynamic actin networks. Biochem. J. 2004;382:13–25. doi: 10.1042/BJ20040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo P., Brown W. J. A role for calmodulin in organelle membrane tubulation. Mol. Biol. Cell. 1995;6:871–887. doi: 10.1091/mbc.6.7.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G. Arfs, phosphoinositides and membrane traffic. Biochem. Soc. Trans. 2005;33:1276–1278. doi: 10.1042/BST0331276. [DOI] [PubMed] [Google Scholar]
- Drengk A., Fritsch J., Schmauch C., Ruhling H., Maniak M. A coat of filamentous actin prevents clustering of late-endosomal vacuoles in vivo. Curr. Biol. 2003;13:1814–1819. doi: 10.1016/j.cub.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Zhang C. X., Carreno S., Barroso C., Heuser J. E., Drubin D. G. RNAi-mediated Hip1R silencing results in stable association between the endocytic machinery and the actin assembly machinery. Mol. Biol. Cell. 2004;15:1666–1679. doi: 10.1091/mbc.E03-09-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawaz F. S., van Ooij C., Homola E., Mutka S. C., Engel J. N. Infection with Chlamydia trachomatis alters the tyrosine phosphorylation and/or localization of several host cell proteins including cortactin. Infect. Immun. 1997;65:5301–5308. doi: 10.1128/iai.65.12.5301-5308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Borja M., Janssen L., Verwoerd D., Hordijk P., Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J. Cell Sci. 2005;118:2661–2670. doi: 10.1242/jcs.02384. [DOI] [PubMed] [Google Scholar]
- Gampel A., Parker P. J., Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase rhoB. Curr. Biol. 1999;9:955–958. doi: 10.1016/s0960-9822(99)80422-9. [DOI] [PubMed] [Google Scholar]
- Gauthier N. C., Monzo P., Gonzalez T., Doye A., Oldani A., Gounon P., Ricci V., Cormont M., Boquet P. Early endosomes associated with dynamic F-actin structures are required for late trafficking of H. pylori VacA toxin. J. Cell Biol. 2007;177:343–354. doi: 10.1083/jcb.200609061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley E. D., Welch M. D. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- Guerin I., de Chastellier C. Disruption of the actin filament network affects delivery of endocytic contents marker to phagosomes with early endosome characteristics: the case of phagosomes with pathogenic mycobacteria. Eur. J. Cell Biol. 2000;79:735–749. doi: 10.1078/0171-9335-00092. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Helwani F. M., Kovacs E. M., Paterson A. D., Verma S., Ali R. G., Fanning A. S., Weed S. A., Yap A. S. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J. Cell Biol. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs H. N., Blanchoin L., Pollard T. D. Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization. Biochemistry. 1999;38:15212–15222. doi: 10.1021/bi991843+. [DOI] [PubMed] [Google Scholar]
- Higgs H. N., Pollard T. D. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- Jeng R. L., Welch M. D. Cytoskeleton: actin and endocytosis–no longer the weakest link. Curr. Biol. 2001;11:R691–R694. doi: 10.1016/s0960-9822(01)00410-9. [DOI] [PubMed] [Google Scholar]
- Kahl C. R., Means A. R. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr. Rev. 2003;24:719–736. doi: 10.1210/er.2003-0008. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Kanzaki M., Furukawa M., Raab W., Pessin J. E. Phosphatidylinositol 4,5-bisphosphate regulates adipocyte actin dynamics and GLUT4 vesicle recycling. J. Biol. Chem. 2004;279:30622–30633. doi: 10.1074/jbc.M401443200. [DOI] [PubMed] [Google Scholar]
- Kornilova E., Sorkina T., Beguinot L., Sorkin A. Lysosomal targeting of epidermal growth factor receptors via a kinase-dependent pathway is mediated by the receptor carboxyl-terminal residues 1022–1123. J. Biol. Chem. 1996;271:30340–30346. doi: 10.1074/jbc.271.48.30340. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanzetti L., Di Fiore P. P., Scita G. Pathways linking endocytosis and actin cytoskeleton in mammalian cells. Exp. Cell Res. 2001;271:45–56. doi: 10.1006/excr.2001.5369. [DOI] [PubMed] [Google Scholar]
- Lawe D. C., Sitouah N., Hayes S., Chawla A., Virbasius J. V., Tuft R., Fogarty K., Lifshitz L., Lambright D., Corvera S. Essential role of Ca2+/calmodulin in Early Endosome Antigen-1 localization. Mol. Biol. Cell. 2003;14:2935–2945. doi: 10.1091/mbc.E02-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clainche C., Pauly B. S., Zhang C. X., Engqvist-Goldstein A. E., Cunningham K., Drubin D. G. A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. EMBO J. 2007;26:1199–1210. doi: 10.1038/sj.emboj.7601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling M., Troller U., Zeidman R., Lundberg C., Larsson C. Induction of neurites by the regulatory domains of PKCdelta and epsilon is counteracted by PKC catalytic activity and by the RhoA pathway. Exp. Cell Res. 2004;292:135–150. doi: 10.1016/j.yexcr.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Lladó A., Tebar F., Calvo M., Moreto J., Sorkin A., Enrich C. Protein kinase Cdelta-calmodulin crosstalk regulates epidermal growth factor receptor exit from early endosomes. Mol. Biol. Cell. 2004;15:4877–4891. doi: 10.1091/mbc.E04-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge R., Descoteaux A. Leishmania donovani promastigotes induce periphagosomal F-actin accumulation through retention of the GTPase Cdc42. Cell Microbiol. 2005a;7:1647–1658. doi: 10.1111/j.1462-5822.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- Lodge R., Descoteaux A. Modulation of phagolysosome biogenesis by the lipophosphoglycan of leishmania. Clin. Immunol. 2005b;114:256–265. doi: 10.1016/j.clim.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Lynch D. K., Winata S. C., Lyons R. J., Hughes W. E., Lehrbach G. M., Wasinger V., Corthals G., Cordwell S., Daly R. J. A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J. Biol. Chem. 2003;278:21805–21813. doi: 10.1074/jbc.M211407200. [DOI] [PubMed] [Google Scholar]
- Machesky L. M., Insall R. H. Signaling to actin dynamics. J. Cell Biol. 1999;146:267–272. doi: 10.1083/jcb.146.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J. B., Kaiser D. A., Pollard T. D., Higgs H. N. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat. Cell Biol. 2001;3:76–82. doi: 10.1038/35050590. [DOI] [PubMed] [Google Scholar]
- Mayorga L. S., Beron W., Sarrouf M. N., Colombo M. I., Creutz C., Stahl P. D. Calcium-dependent fusion among endosomes. J. Biol. Chem. 1994;269:30927–30934. [PubMed] [Google Scholar]
- McNiven M. A., Kim L., Krueger E. W., Orth J. D., Cao H., Wong T. W. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield C. J., Perrais D., Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Merrifield C. J., Qualmann B., Kessels M. M., Almers W. Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur. J. Cell Biol. 2004;83:13–18. doi: 10.1078/0171-9335-00356. [DOI] [PubMed] [Google Scholar]
- Mettlen M., Platek A., Van Der Smissen P., Carpentier S., Amyere M., Lanzetti L., de Diesbach P., Tyteca D., Courtoy P. J. Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic. 2006;7:589–603. doi: 10.1111/j.1600-0854.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Miki H., Yamaguchi H., Suetsugu S., Takenawa T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature. 2000;408:732–735. doi: 10.1038/35047107. [DOI] [PubMed] [Google Scholar]
- Moreau V., Frischknecht F., Reckmann I., Vincentelli R., Rabut G., Stewart D., Way M. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat. Cell Biol. 2000;2:441–448. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- Moriyama K., Iida K., Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells. 1996;1:73–86. doi: 10.1046/j.1365-2443.1996.05005.x. [DOI] [PubMed] [Google Scholar]
- Mu F. T., Callaghan J. M., Steele-Mortimer O., Stenmark H., Parton R. G., Campbell P. L., McCluskey J., Yeo J. P., Tock E. P., Toh B. H. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Nebl G., Meuer S. C., Samstag Y. Dephosphorylation of serine 3 regulates nuclear translocation of cofilin. J. Biol. Chem. 1996;271:26276–26280. doi: 10.1074/jbc.271.42.26276. [DOI] [PubMed] [Google Scholar]
- Qualmann B., Kessels M. M. Endocytosis and the cytoskeleton. Int. Rev. Cytol. 2002;220:93–144. doi: 10.1016/s0074-7696(02)20004-2. [DOI] [PubMed] [Google Scholar]
- Qualmann B., Kessels M. M., Kelly R. B. Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 2000;150:111F–116F. doi: 10.1083/jcb.150.5.f111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C., Athman R., Robine S., Louvard D. The co-workers of actin filaments: from cell structures to signals. Nat. Rev. Mol. Cell Biol. Nat. Rev. Mol. Cell Biol. 2004;5:635–646. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- Ridley A. J. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2:303–310. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- Ridley A. J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Robertson D., Paterson H. F., Adamson P., Hall A., Monaghan P. Ultrastructural localization of ras-related proteins using epitope-tagged plasmids. J. Histochem. Cytochem. 1995;43:471–480. doi: 10.1177/43.5.7537292. [DOI] [PubMed] [Google Scholar]
- Rondanino C., Rojas R., Ruiz W. G., Wang E., Hughey R. P., Dunn K. W., Apodaca G. RhoB-dependent modulation of postendocytic traffic in polarized Madin-Darby canine kidney cells. Traffic. 2007;8:932–949. doi: 10.1111/j.1600-0854.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- Sauvonnet N., Dujeancourt A., Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of gammac cytokine receptor. J. Cell Biol. 2005;168:155–163. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A. Coupling actin dynamics and membrane dynamics during endocytosis. Curr. Opin. Cell Biol. 2002;14:76–81. doi: 10.1016/s0955-0674(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Shao D., Forge A., Munro P. M., Bailly M. Arp2/3 complex-mediated actin polymerisation occurs on specific pre-existing networks in cells and requires spatial restriction to sustain functional lamellipod extension. Cell Motil. Cytoskeleton. 2006;63:395–414. doi: 10.1002/cm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick F. S., Li W., Zhang F., Zeile W. L., Purich D. L. Actin-based endosome and phagosome rocketing in macrophages: activation by the secretagogue antagonists lanthanum and zinc. Cell Motil. Cytoskeleton. 2003;54:41–55. doi: 10.1002/cm.10083. [DOI] [PubMed] [Google Scholar]
- Stamnes M. Regulating the actin cytoskeleton during vesicular transport. Curr. Opin. Cell Biol. 2002;14:428–433. doi: 10.1016/s0955-0674(02)00349-6. [DOI] [PubMed] [Google Scholar]
- Stradal T. E., Rottner K., Disanza A., Confalonieri S., Innocenti M., Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14:303–311. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Stradal T. E., Scita G. Protein complexes regulating Arp2/3-mediated actin assembly. Curr. Opin. Cell Biol. 2006;18:4–10. doi: 10.1016/j.ceb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Suchy S. F., Nussbaum R. L. The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am. J. Hum. Genet. 2002;71:1420–1427. doi: 10.1086/344517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M., Rusk N. Control of vesicular trafficking by Rho GTPases. Curr. Biol. 2003;13:R409–R418. doi: 10.1016/s0960-9822(03)00324-5. [DOI] [PubMed] [Google Scholar]
- Taunton J. Actin filament nucleation by endosomes, lysosomes and secretory vesicles. Curr. Opin. Cell Biol. 2001;13:85–91. doi: 10.1016/s0955-0674(00)00178-2. [DOI] [PubMed] [Google Scholar]
- Taunton J., Rowning B. A., Coughlin M. L., Wu M., Moon R. T., Mitchison T. J., Larabell C. A. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol. 2000;148:519–530. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar F., Villalonga P., Sorkina T., Agell N., Sorkin A., Enrich C. Calmodulin regulates intracellular trafficking of epidermal growth factor receptor and the MAPK signaling pathway. Mol. Biol. Cell. 2002;13:2057–2068. doi: 10.1091/mbc.01-12-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpson P., Lynch D. K., Schramek D., Walker F., Daly R. J. Cortactin overexpression inhibits ligand-induced down-regulation of the epidermal growth factor receptor. Cancer Res. 2005;65:3273–3280. doi: 10.1158/0008-5472.CAN-04-2118. [DOI] [PubMed] [Google Scholar]
- Toutenhoofd S. L., Strehler E. E. The calmodulin multigene family as a unique case of genetic redundancy: multiple levels of regulation to provide spatial and temporal control of calmodulin pools? Cell Calcium. 2000;28:83–96. doi: 10.1054/ceca.2000.0136. [DOI] [PubMed] [Google Scholar]
- Troller U., Larsson C. Cdc42 is involved in PKCepsilon- and delta-induced neurite outgrowth and stress fibre dismantling. Biochem. Biophys. Res. Commun. 2006;349:91–98. doi: 10.1016/j.bbrc.2006.07.200. [DOI] [PubMed] [Google Scholar]
- Weed S. A., Karginov A. V., Schafer D. A., Weaver A. M., Kinley A. W., Cooper J. A., Parsons J. T. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M. D., DePace A. H., Verma S., Iwamatsu A., Mitchison T. J. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J. Cell Biol. 1997;138:375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Zhou K., Hao J. J., Liu J., Smith N., Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J. Cell Sci. 2005;118:807–817. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.