Abstract
γ-Tubulin is an indispensable component of the animal centrosome and is required for proper microtubule organization. Within the cell, γ-tubulin exists in a multiprotein complex containing between two (some yeasts) and six or more (metazoa) additional highly conserved proteins named gamma ring proteins (Grips) or gamma complex proteins (GCPs). γ-Tubulin containing complexes isolated from Xenopus eggs or Drosophila embryos appear ring-shaped and have therefore been named the γ-tubulin ring complex (γTuRC). Curiously, many organisms (including humans) have two distinct γ-tubulin genes. In Drosophila, where the two γ-tubulin isotypes have been studied most extensively, the γ-tubulin genes are developmentally regulated: the “maternal” γ-tubulin isotype (named γTub37CD according to its location on the genetic map) is expressed in the ovary and is deposited in the egg, where it is thought to orchestrate the meiotic and early embryonic cleavages. The second γ-tubulin isotype (γTub23C) is ubiquitously expressed and persists in most of the cells of the adult fly. In those rare cases where both γ-tubulins coexist in the same cell, they show distinct subcellular distributions and cell-cycle-dependent changes: γTub37CD mainly localizes to the centrosome, where its levels vary only slightly with the cell cycle. In contrast, the level of γTub23C at the centrosome increases at the beginning of mitosis, and γTub23C also associates with spindle pole microtubules. Here, we show that γTub23C forms discrete complexes that closely resemble the complexes formed by γTub37CD. Surprisingly, however, γTub23C associates with a distinct, longer splice variant of Dgrip84. This may reflect a role for Dgrip84 in regulating the activity and/or the location of the γ-tubulin complexes formed with γTub37CD and γTub23C.
INTRODUCTION
The temporal and spatial control of microtubule nucleation and organization are essential for many fundamental cellular processes, including cell shape changes, intracellular transport, cell motility, partitioning of polarity signals during embryonic development, and, perhaps most importantly, the faithful segregation of chromosomes during cell division. In most animal cells, microtubules are nucleated and organized by the centrosome, which is composed of a pair of centrioles surrounded by the amorphous pericentriolar material (PCM) that appears to be the site of microtubule nucleation and anchoring (Gould and Borisy, 1977). Although efforts to generate an inventory of centrosomal components have made great progress in recent years (for example, Andersen et al., 2003), the molecular organization of the centrosome itself is not well understood, and mechanistic descriptions of how centrosomal proteins contribute to microtubule formation and organization are yet to be obtained.
γ-Tubulin (Oakley and Oakley, 1989) is an indispensable component of the animal centrosome required for microtubule nucleation and organization in all eukaryotes (reviewed in Wiese and Zheng, 2006). Human γ-tubulin can restore viability to Schizosaccharomyces pombe lacking endogenous γ-tubulin (Horio and Oakley, 1994), suggesting that γ-tubulins are functionally conserved in phylogenetically distant organisms. Within the cell, γ-tubulin exists in a multiprotein complex containing between two (in the case of Saccharomyces cerevisiae) and five or more (S. pombe, Xenopus laevis, Drosophila melanogaster, and humans) additional proteins named gamma ring proteins (Grips) or gamma complex proteins (GCPs). γ-Tubulin containing complexes isolated from Xenopus eggs or Drosophila embryos appear ring-shaped and have therefore been named γ-tubulin ring complex (γTuRC; Zheng et al., 1995). The non-γ-tubulin γTuRC components are themselves highly conserved (Murphy et al., 1998, 2001; Anders et al., 2006; Tassin et al., 1998; Martin et al., 1998; Haren et al., 2006; Lüders et al., 2006). One of the major subcomplexes of the γTuRC, which has been named the γ-tubulin small complex (γTuSC; Oegema et al., 1999), appears to be a tetramer composed of two molecules of γ-tubulin and one molecule each of two other Grips known as Spc97p and Spc98p in S. cerevisiae, Dgrip84 and Dgrip91 in Drosophila melanogaster, or GCP2 and GCP3 in mammals (reviewed in Wiese and Zheng, 2006). The tetramer was first described in S. cerevisiae (Knop and Schiebel, 1997), where it may be the only soluble γ-tubulin complex. The metazoan γTuRC is thought to be composed of six or seven tetramers held together and capped by several additional proteins that copurify with γ-tubulin from Xenopus egg extracts or Drosophila embryos (Zheng et al., 1995; Oegema et al., 1999; Gunawardane et al., 2003), but subcomplexes between γ-tubulin and non-γTuSC Grips may also contribute to γTuRC structure (Gunawardane et al., 2000).
Consistent with a role for the γTuRC in microtubule nucleation, γ-tubulin and its binding partners are localized to the PCM at the minus ends of the microtubules (Moritz et al., 1995a,b; reviewed in Wiese and Zheng, 2006; Moritz and Agard, 2001). However, the molecular mechanism(s) for the function of the γTuRC in microtubule nucleation are still unclear. In addition to facilitating nucleation of microtubules, γ-tubulin also seems to play a role in interphase microtubule organization (Jung et al., 2001; Horio and Oakley, 2003), spindle assembly (Paluh et al., 2000; Prigozhina et al., 2001; Vogel and Snyder, 2000a,b; Sampaio et al., 2001; Barbosa et al., 2003; Lüders et al., 2006), and spindle assembly checkpoint regulation (Vardy and Toda, 2000; Hendrickson et al., 2001; Vardy et al., 2002; Müller et al., 2006; reviewed by Blagden and Glover, 2003). Little is known to date about how γ-tubulin or the Grips participate in each of these functions.
Curiously, the genomes of several organisms, including humans and flies, encode two distinct γ-tubulin genes (Zheng et al., 1991; Wilson et al., 1997; Tavosanis et al., 1997; Wise et al., 2000). In Drosophila, where the two γ-tubulin isotypes have been studied most extensively, the γ-tubulin genes are developmentally regulated: the “maternal” γ-tubulin isotype (named γTub37CD according to its location on the genetic map) is highly expressed in the ovary and is deposited in the egg, where it is thought to orchestrate the early embryonic cleavages (Wilson et al., 1997; Llamazares et al., 1999). Consistent with this idea, mutation of γTub37CD results in female sterility (Tavosanis et al., 1997). In contrast, γTub23C is essentially ubiquitous and is required for viability, microtubule organization during somatic mitotic divisions, and male meiosis (Sunkel et al., 1995; Sampaio et al., 2001). Although both isotypes are present in early embryos, only γTub37CD is detected at centrosomes in syncytial embryos, whereas punctate structures containing γTub23C distribute throughout the cytoplasm (Wilson et al., 1997). Later during development, both γTub23C and γTub37CD localize to centrosomes in cellularized embryos (Tavosanis et al., 1997; Wilson et al., 1997; Raynaud-Messina et al., 2001). The extent to which each γ-tubulin isotype contributes to the assembly of female meiotic spindles remains to be resolved (Tavosanis et al., 1997; Wilson and Borisy, 1998; Llamazares et al., 1999).
Differences in the timing of their association with the centrosome have also been reported for the two γ-tubulin isotypes in Drosophila tissue culture cells (Raynaud-Messina et al., 2001). In Kc23 cells, the centrosomal levels of γTub37CD have been reported to vary only slightly throughout the cell cycle, whereas centrosomal γTub23C levels increased sharply during late G2. Furthermore, γTub23C was also recruited to the microtubules of the mitotic spindle, whereas γTub37CD was never found on spindle microtubules. This suggests the existence of mechanisms that allow the cell to distinguish between the two γ-tubulin isotypes. Here, we set out to gain a better understanding of the biochemistry of γTub23C. We report that Drosophila γTub23C isolated from tissue culture cells forms bona fide ring complexes that include most of the same Grips as the γTuRCs formed by γTub37CD. However, we were surprised to find that γTub23C and γTub37CD associate with distinct splice variants of Dgrip84/GCP2 that differ by 74 amino acids in their amino termini. We speculate that Dgrip84 may be involved in regulating the subcellular localization of γ-tubulin.
MATERIALS AND METHODS
Buffers and Reagents
The following buffers were used: HB: 50 mM K-HEPES, pH 7.6, 1 mM MgCl2, 1 mM EGTA, 1 mM β-mercaptoethanol (β-ME) and protease inhibitor stock (1:200 final dilution; see below). H100: HB plus 100 mM NaCl; H200: HB plus 200 mM NaCl; and H500: HB plus 500 mM NaCl. Elution buffer (EB): H200 plus 100 μM GTP and 1 mg/ml Dros23C or Dros37C peptide. Homogenization buffer (for embryos): H100 plus 10%glycerol, 1 mM PMSF and protease inhibitor stock (1:100 final dilution). Cell lysis buffer (for S2 and Kc cells): H200 plus 0.5% NP-40, 1 mM GTP, and 1 mM PMSF. Protease inhibitor stock: 10 mM benzamidine-HCl, 1 mg/ml aprotinin, 1 mg/ml leupeptin, and 1 mg/ml pepstatin A in ethanol. LPC: 10 mg/ml leupeptin,10 mg/ml pepstatin A, and 10 mg/ml chymostatin dissolved in DMSO. Mounting medium: 20 mM Tris-Cl, pH 9.0, 90% glycerol, and 0.1% p-phenylenediamine. Sample buffer: 250 mM Tris, pH 6.8, 12% SDS wt/vol, 20% β-ME vol/vol, and 40% glycerol vol/vol. Tubulin was purified from the bovine brain and labeled with tetramethylrhodamine as described (http://skye.med.harvard.edu). For RNA work, aqueous solutions were treated with 0.05% diethyl pyrocarbonate (DEPC; Research Products International, Mount Prospect, IL) and autoclaved for 30 min.
Antibody Production
Dgrip84 antibodies were a kind gift from Y. Zheng (Carnegie Institution of Washington, Baltimore, MD) and are described in Oegema et al. (1999). Synthetic peptides (made by the Protein Synthesis facility of the University of Wisconsin, Madison Biotech Center) corresponding to the COOH-terminal 17 amino acids of γTub23C (Cys-PVDSKSEDSRSVTSAGS; GenBank Accession no. P23257) or γTub37CD (Cys-QIDYPQWSPAVEASKAG; GenBank Accession no. P42271) were used to raise and affinity purify rabbit polyclonal antibodies as described elsewhere (Field et al., 1998). Peptides contained an additional N-terminal cysteine residue to facilitate coupling to KLH for antibody production, or to SulfoLink coupling gel (Pierce Biotechnology, Rockford, IL) for affinity purification. Specific antibodies were purified as described (Kellogg and Alberts, 1992), except that before affinity purification of the antisera with the immunogenic peptide, each serum was passed over a column corresponding to the nonimmunogenic γ-tubulin isotype. This procedure depleted potential cross-reacting antibodies and thus ascertained the specificity of the affinity-purified antibodies.
Drosophila S2 Cell Tissue Culture
Schneider S2 cells (Schneider, 1972) were cultured in Schneider's Drosophila medium as described by the supplier (Invitrogen, Carlsbad, CA). Cells to be used for extract preparation were grown for 4 d at 27°C in ∼70 ml Drosophila medium in T-150 flasks. Cells were collected by centrifugation and washed twice with PBS, the volume of the cell pellet was estimated, and the cells were stored at −80°C until use.
PEG Precipitation
PEG (polyethylene glycol P-2139; average mol wt 5–8000; Sigma Chemical Co., St. Louis, MO) was added to a final concentration of 1–5% (from a 30% stock in H100) to clarified Drosophila embryo or S2 cell extract. The mixture was incubated on ice for 20 min, spun at 17,000 rpm for 10 min in a JA20 rotor (Beckman, Fullerton, CA), and the supernatant was removed. The pellets were resuspended in a volume corresponding the original extract volume of H200 plus 0.05% NP-40 and 100 μM GTP and were clarified at 17,000 rpm for 10 min in a JA20 rotor. Equivalent portions of supernatant and pellet fractions were then analyzed by sucrose gradient fractionation, SDS-PAGE, and Western blotting.
Immunoisolation of γ-Tubulin–containing Complexes from Drosophila Embryo Extract
Drosophila embryo extract was prepared by homogenizing Drosophila embryos in homogenization buffer as described (Moritz et al., 1998; Oegema et al., 1999). Embryo extract was frozen in aliquots in liquid nitrogen, stored at −80°C, and used within 2 wk of preparation. Clarified extract was prepared by centrifugation of thawed crude extract for 1 h at 50,000 rpm in a rotor (SW55; Beckman) at 4°C. γ-Tubulin complexes were immunoprecipitated from the supernatant essentially as described (Oegema et al., 1999), except that the PEG precipitation step was omitted. Briefly, anti-γ-tubulin antibody (23C or 37CD; ∼8 μg/ml) was incubated with the supernatant at 4°C for 1 h with gentle rotation. Per 12 ml of clarified extract, 35 μl of protein A affiprep beads (Bio-Rad Laboratories, Hercules, CA) were equilibrated in wash buffer (H200 plus 0.05% NP-40, 100 μM GTP, and 1 mM PMSF) and added to the antibody-extract mix. After a further incubation for 1 h with gentle rotation, the beads were collected and washed six times with wash buffer and were incubated for 16–18 h at 4°C with an equal volume of EB containing the respective peptide. γ-Tubulin complexes were collected by loading an additional 40 μl of EB onto the beads and collecting the supernatant.
Immunoisolation of γ-Tubulin–containing Complexes from Drosophila Tissue Culture Cells
Drosophila tissue culture cell extract was prepared by resuspending thawed S2 cell pellets in 3 vol of cell lysis buffer, brief vortexing, and incubation on ice for 10 min. Cell debris was removed by a 10-min spin (at 4°C) at 2400 rpm in a Beckman table-top centrifuge. The supernatant was then incubated with antibody and processed as described above for the immunoisolation of γ-tubulin complexes from embryo extract.
Sucrose Gradient Sedimentation
Sucrose gradients (5–40%) were poured and run as described by Oegema et al. (1999). Briefly, gradients were poured by hand as step gradients (five steps of equal volume) in H100 plus 1 mM GTP and allowed to diffuse into continuous gradients. After centrifugation at 50,000 rpm in a Beckman TLS-55 rotor for 4.5 h at 4°C, gradients were fractionated by hand (16 fractions plus pellet). Fractions were separated on a 10% SDS-PAGE gel and analyzed by Western blot or Coomassie stain, as indicated. For some experiments, each fraction was precipitated by addition of 10% trichloroacetic acid (TCA) and centrifugation (30 min) in an Eppendorf centrifuge after a 30-min incubation on ice. Pellets were resuspended in SDS-sample buffer and analyzed by SDS-PAGE and Western blot.
Electron Microscopy
Negative staining electron microscopy of peptide-eluted complexes was described elsewhere (Zheng et al., 1995; Wiese and Zheng, 2000). Samples were viewed at 80 kV in a Philips Tecnai 12 microscope (Mahwah, NJ) equipped with a cooled CCD camera.
RT-PCR to Detect Dgrip84/GCP2 Isoforms
Total RNA was extracted from 100 μl of frozen 0–4 h Drosophila embryos or ∼107 S2 cells by homogenizing the cells in Trizol reagent (Invitrogen) and extracting the RNA according to the manufacturer's recommendations.
The following primers were used to reverse transcribe Dgrip84 mRNA (all isoforms) and amplify specific regions of the gene to distinguish the isoforms: the first strand cDNA template was synthesized from the extracted RNA using the ImPromII-Reverse Transcription System (Promega, Madison, WI) according to the manufacturer's recommendations and using the Dgrip84/GCP2 gene-specific primer, 5′-CTCTACACACATCTTGTC-3′. Isoform-specific PCR primers used to differentiate the Dgrip84 isoforms were as follows: To distinguish the 5′ end of the “A”-isoform from B/C using three PCR primers: forward primer 5′-AAAGAGCTCAATTGCC-3′ (specific for the 5′ untranslated region of isoform A) combined with reverse primer 5′-TCGAGCAGCATACTC-3′ (amplifies all isoforms) gives an 885-base pair band if isoform A is present, no product for isoform B or C; forward primer 5′-CAGTCGATCTAGCCGTTG-3′(specific for isoforms B and C, because this primer targets a region that is spliced out in isoform A) with reverse primer 5′-TCGAGCAGCATACTC-3′(amplifies all isoforms) gives an 855-base pair band if isoform B or C is present. To test for the presence of the 3′ insertion that distinguishes isoform C from isoforms A and B, we used forward primer 5′-GCTAGTCTCTATCGAG-3′ and reverse primer 5′-CTGTTGATCTGCTTGAGG-3′. This primer pair amplifies a 500-base pair band if the insert is present, or a 400-base pair band if the insert is absent. PCR products were cloned and sequenced to confirm their identity.
RESULTS
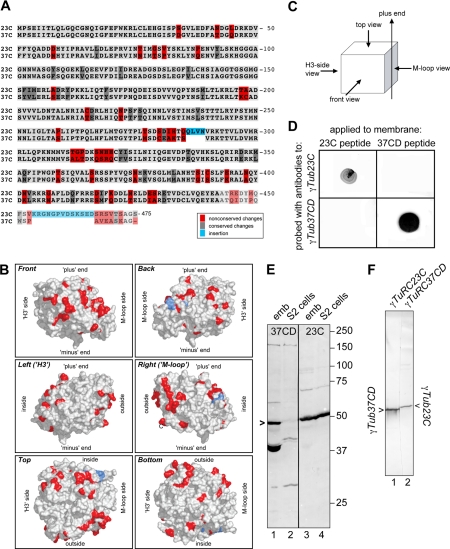
To analyze γTub23C and its interacting proteins we began by raising antibodies specific for γTub23C or γTub37CD (Figure 1). Sequence alignments and molecular modeling (Figure 1, A and B) revealed that the differences between γTub23C and γTub37CD (a total of 81 amino acids) did not cluster to defined regions within the γ-tubulin molecule but were distributed across most of the surfaces (Figures 1B and Supplementary Figure S1). The biggest difference between γTub23C and γTub37CD was a 14-amino acid insertion near the C-terminus of γTub23C. We took advantage of this difference to generate γ-tubulin isotype-specific antibodies by immunizing rabbits with synthetic peptides corresponding to the C-terminal 17 amino acids of each γ-tubulin isotype (see Materials and Methods). Dot-blot analysis confirmed that the affinity-purified antibodies were specific for their respective immunogenic peptides and did not cross-react with the nonimmunogenic peptide (Figure 1D). As expected, the antibodies recognized bands of ∼50 kDa (γTub37CD antibodies) and ∼52 kDa (γTub23C antibodies), respectively, on Western blots of embryo extracts (Figure 1E) or of purified γ-tubulin complexes (Figure 1F). In addition, the γTub23C antibodies recognized a band of ∼50 kDa in S2 (Drosophila tissue culture) cell extracts, whereas no band corresponding to γ-tubulin was recognized by the γTub37CD antibodies in S2 cell extracts (Figure 1E). This suggests that our S2 cell line does not express γTub37CD, or only undetectably low levels.
Figure 1.
Analysis of Drosophila γTub23C and γTub37CD and isotype-specific antibodies. (A) Sequence alignment between γTub37CD and γTub23C reveals that nonconserved differences between the two isotypes are scattered throughout the primary sequence. Light gray, identities; medium gray, conserved changes; red, differences that change the character of the amino acid; blue, amino acid insertions in γTub23C relative to γTub37CD; the transparent amino acids near the C-termini of the molecules are not represented in the structural model. (B) A hypothetical structural surface model of γTub23C (generated by the structure prediction program SwissModel; Peitsch, 1995; Guex and Peitsch, 1997; Schwede et al., 2003) is based on the crystal structure for human γ-tubulin (Aldaz et al., 2005). To highlight the residues not conserved between the isotypes, the backbone of the residues whose character is significantly different between γTub23C and γTub37CD are colored in red and the M-loop insertion in γTub23C that is not present in γTub37CD is colored in blue, as in A. Snapshot images of different faces of the molecule are shown in each panel, as indicated in the upper left-hand corner. The orientation of the snapshots are based on the observation that γ-tubulin can roughly be modeled as a cube, and on the orientation of α and β tubulin within the microtubule lattice, as illustrated in (C). The snapshots illustrate two important points: first, the differences between the two γ-tubulins are not randomly distributed throughout the molecule but map mainly to the surfaces, presumably leaving the core of the structure largely unaffected (see also Supplementary Figure S1). Second, the differences map to all six faces of the cube. Corresponding ribbon models can be found in the online supplementary material, Figure S1. (D) Dot-blot analysis of affinity-purified antibodies. Aqueous solutions of each peptide were spotted onto a piece of nitrocellulose membrane, and the membrane was probed with each antibody, as indicated. The antibodies do not cross-react with each other. (E) Western blot of total extracts from embryos (emb, lanes 1 and 3) or S2 cells (S2 cells, lanes 2 and 4) probed with antibodies to γTub37CD (lanes 1 and 2) or γTub23C (lanes 3 and 4). Positions of molecular weight markers are indicated on the right. (F) Western blot of purified γ-tubulin complexes. Lane 1, γTub23C complexes probed with γTub37CD antibodies; lane 2, γTub37CD complexes probed with γTub23C antibodies. The ∼40-kDa band recognized by the γTub37C antibodies in embryo extracts probably shares an epitope with γTub37CD, as this band cross-reacts with antisera produced by several different rabbits (our unpublished observation); however, this protein apparently does not coimmunoprecipitate with the γTuRC and thus did not interfere with the analysis of the purified complex.
Dgrip84 Migrates Differently in Drosophila Embryos and Tissue Culture Cells
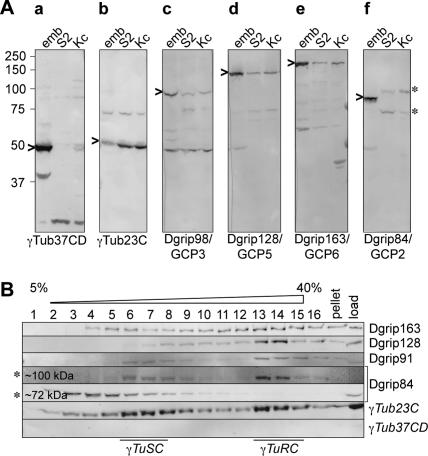
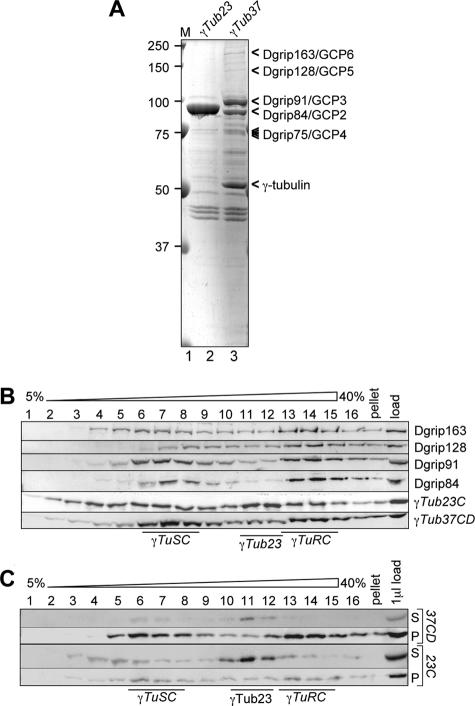
We next wanted to understand whether γTub23C formed γTuRC-like complexes. For this, we first asked whether the Dgrips that interact with γTub37CD in early Drosophila embryos also exist in cells that only express γTub23C. Extracts of Drosophila tissue culture cells (Schneider S2 or Kc) or embryos were Western blotted and probed for individual γTuRC proteins (Figure 2A). This analysis revealed that Dgrip163/GCP6, Dgrip128/GCP5, Dgrip98/GCP3, and γTub23C migrated similarly in extracts made from embryos or tissue culture cells. In contrast, Dgrip84/GCP2 behaved differently in embryos and tissue culture cells: no band corresponding to the embryonic Dgrip84 was found in the tissue culture cell lines. Instead, two novel bands appeared that migrated at ∼72 and ∼100 kDa (Figure 2Af). To determine which, if either, of the novel bands might be a subunit of the γTuRC, we analyzed extracts of S2 (Figures 2B and 3B) or Kc (Figure 3C) cells on 5–40% sucrose gradients and probed Western blots of gradient fractions for various γTuRC subunits. This analysis showed that the ∼72-kDa band migrated near the top of the sucrose gradient and thus appeared to be unrelated to the γTuRC or the γTuSC. Whether or how the ∼72-kDa band is related to Dgrip84 is not yet clear. In contrast, the 100-kDa band migrated similar to γTub23C in tissue culture cells (Figure 2B) and like Dgrip84 in embryos (Figure 3D). Thus, our results strongly suggested that the ∼100-kDa band may be the Dgrip84 of somatic cells.
Figure 2.
Drosophila tissue culture cells express a slower migrating version of Dgrip84. (A) Western blot analysis of γTuRC components in embryos, S2 cells, and Kc cells, as indicated above the lanes. Probes are indicated below the blots and are as follows: (a) γTub37CD, (b) γTub23C, (c) Dgrip98/GCP3, (d) Dgrip128/GCP5, (e) Dgrip163/GCP6, and (f) Dgrip84/GCP2. The embryonic “cognate” proteins are indicated by arrowheads on the left of each panel. Asterisks in f denote two bands (∼100 and ∼75 kDa) in tissue culture cells that are recognized by the Dgrip84 antibody. Note the absence in the tissue culture cells of a band corresponding to γTub37CD in panel a, and the absence of a band corresponding to embryonic Dgrip84 in panel f. (B) Sucrose gradient analysis of S2 cell extracts reveals that the slower migrating protein recognized by the Dgrip84 antibodies migrates similarly to γTub23C. Sucrose gradient fractions were probed for γTuRC components as indicated on the right. The positions of γTuSC and γTuRC are indicated below the blot. Asterisks on the left denote the two proteins in tissue culture cell extracts that are recognized by the Dgrip84 antibody. Only the ∼100-kDa protein migrates like the embryonic Dgrip84 (compare with Figure 3).
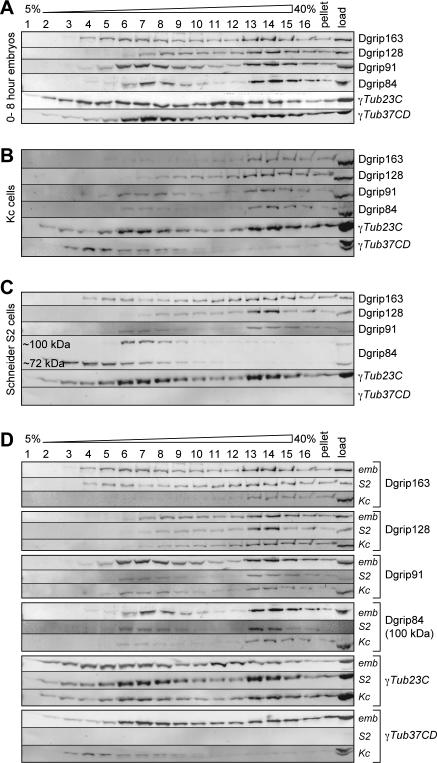
Figure 3.
Sucrose gradient analysis of γ-tubulins and Dgrips in embryos and tissue culture cells. (A) Embryo extracts, 0–8 h, were analyzed on 5–40% sucrose gradients. Gradient fractions were probed for Dgrips and γ-tubulins as indicated on the right. (B and C) Extracts made from Kc cells (B) or S2 cells (C) were analyzed on sucrose gradients as described for A. D. Individual panels from A to C were regrouped to allow a more direct comparison for each γTuRC component between the migration patterns in embryos and tissue culture cells. γTub23C migrates differently in embryos, but in S2 and Kc cells resembles the behavior of γTub37CD. For Dgrip84, only the ∼100-kDa bands are shown in S2 and Kc cells.
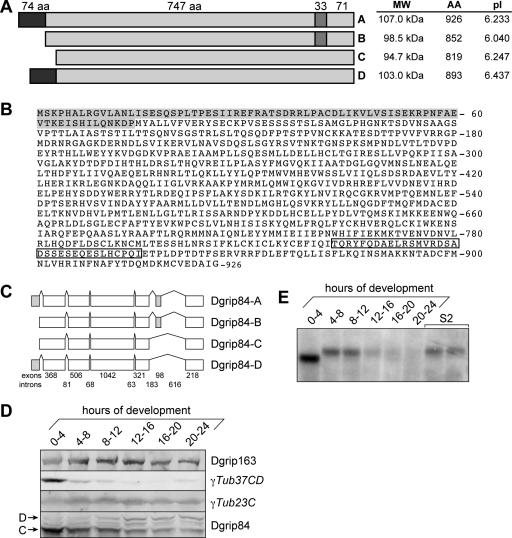
Two possibilities exist to explain the increased size of Dgrip84 in tissue culture cells: the protein might be differentially modified by secondary modifications, or different splice variants of the protein might be expressed. Database analysis revealed the existence of three potential splice variants of Dgrip84 (diagramed in Figure 4). The shortest isoform, Dgrip84-C (Accession no. NP_523409), differed from the longest isoform, Dgrip84-A (Accession no. NP_728264), in two ways: a 33-aa insertion near the C-terminus in Dgrip84-A, and a 74-aa extension at its N-terminus. Isoform Dgrip84-B (Accession no. NP_728265) was intermediate in that it had the C-terminal insertion but lacked the N-terminal extension. A fourth configuration, lacking the C-terminal insertion but having the N-terminal extension, was also possible but was not reported in the database. We named this configuration Dgrip84-D (Figure 4, A–C).
Figure 4.
Dgrip84 isoforms in Drosophila. (A) Diagram of the relationships between different Dgrip84 isoforms. The position of the 33-amino acid C-terminal insertion and the 74-amino acid N-terminal extension are indicated. Theoretical molecular weights, number of amino acids, and pI are indicated on the right for each isoform (A–D). Isoforms A–C are currently present in the database (Accession no. NP_728264, NP_728265, and NP_523409, respectively). The isoform found in S2 cells (see below) has the extension but not the insertion. We named this the “D” isoform. (B) Amino acid sequence of Dgrip84 isoform A. The N-terminal extension and C-terminal insertion are indicated by gray shading and a box, respectively. (C) Gene structure of the Dgrip84 isoforms. Numbers below the diagram indicate the number of nucleotides in each exon (top row) or intron (bottom row). (D) Western blot of staged embryos. Drosophila embryos were collected for 4 h and were then aged for the times indicated above the blots. Maximum age was 24 h. Embryos were then homogenized, loaded onto SDS-PAGE gels, and Western blotted. The blots were probed with antibodies to Dgrip163, γTub37CD, γTub23C, or Dgrip84, as indicated on the right. The positions of the slower migrating (“C”) and faster-migrating (“D”) Dgrip84 bands are indicated on the left. (E) Northern blot of staged embryos probed with a Dgrip84 probe common to all isoforms. Embryos were collected and aged as in D. Early embryos (0–4 h) express a shorter mRNA than later embryos or S2 cells. To determine the identity of the isoforms, we performed RT-PCR and sequenced the relevant regions of cDNAs recovered form early embryos or S2 cells (see Supplementary Figure S3). This analysis shows that early embryos express the “C” isoform, whereas S2 cells express the “D” isoform. The reduction in signal for the older embryos (starting with 12–16-h embryos) may be due to sample degradation, although our loading control (rp49; DeZazzo et al., 2000) remained unchanged.
To gain a better understanding of which of the Dgrip84 isoforms was expressed in embryos and tissue culture cells, we examined Dgrip84 mRNA and protein during early fly development. For this, we collected staged embryos (4-h time intervals for 24 h) or unsynchronized S2 cells and made extracts for Western blot analysis (Figure 4D). Alternatively, we used the extracts to isolate total mRNA for Northern blot analysis (Figure 4E) or RT-PCR (Supplementary Figure S2).
Western blots of staged embryos probed with anti-Dgrip84 antibodies showed that Dgrip84 (marked “D” in Figure 4D) migrated more slowly in older embryos compared with younger ones. The slower-migrating form of Dgrip84 was detectable within 8–12 h of development, and its relative levels increased as the levels of the shorter isoform (marked “C” in Figure 4D) slowly declined.
To determine whether the appearance of the slower-migrating Dgrip84 protein could be explained by a switch to a longer Dgrip84 isoform, we examined the isoforms expressed in staged embryos by Northern blot analysis. Northern blots showed that the shorter isoform of Dgrip84 expressed in early embryos (0–4 h) was replaced by a longer isoform later in development that was also present in S2 cells (Figure 4E). To identify the specific isoforms present in early embryos and S2 cells, we first designed a strategy to identify the isoform by RT-PCR using specific primers (diagramed in Supplementary Figure S2). Our results were then confirmed by direct sequencing of the RT-PCR product. Consistent with results from previous work (Oegema et al., 1999; Gunawardane et al., 2000), we found that the isoform present in early embryos lacked both the N-terminal extension and the C-terminal insertion. This suggested that the embryos expressed mainly the “C”-isoform of Dgrip84. S2 cells, on the other hand, expressed mRNAs that contained the N-terminal extension. However, we were surprised to find no evidence for the C-terminal insertion. This suggested that the major isoform of Dgrip84 expressed in S2 cells is the previously unknown, novel “D” splice variant of Dgrip84 (Figure 4A). Together, these results suggested that the expression of Dgrip84 splice variants is developmentally regulated.
To confirm that the longer Dgrip84 splice variant is indeed a component of the γ-tubulin complex, we used our γ-tubulin isoform-specific or Dgrip84 antibodies to immunoprecipitate protein complexes from embryos or S2 cells and compared these by Coomassie-stained SDS-PAGE gels and Western blot (Figure 5). With the exception of the band that corresponds to Dgrip84, similar sets of proteins were precipitated from S2 tissue culture cells and from 0- to 3-h embryos using antibodies directed against Dgrip84 (Figure 5Aa), and their protein profile closely resembled the γTub37CD and γTub23C complexes isolated from embryos and S2 cells, respectively (Figure 5Ab; Oegema et al., 1999). This suggested that both Dgrip84 slice variants are components of γ-tubulin complexes. One important difference between S2 and embryonic γ-tubulin complexes was the absence of the band corresponding to the embryonic Dgrip84 and a concomitant appearance of a ∼100-kDa band in the S2 γ-tubulin complexes. Thus, consistent with the findings described above, the γ-tubulin complexes isolated from embryos or S2 cells differed by the associated Dgrip84 splice variant.
Figure 5.
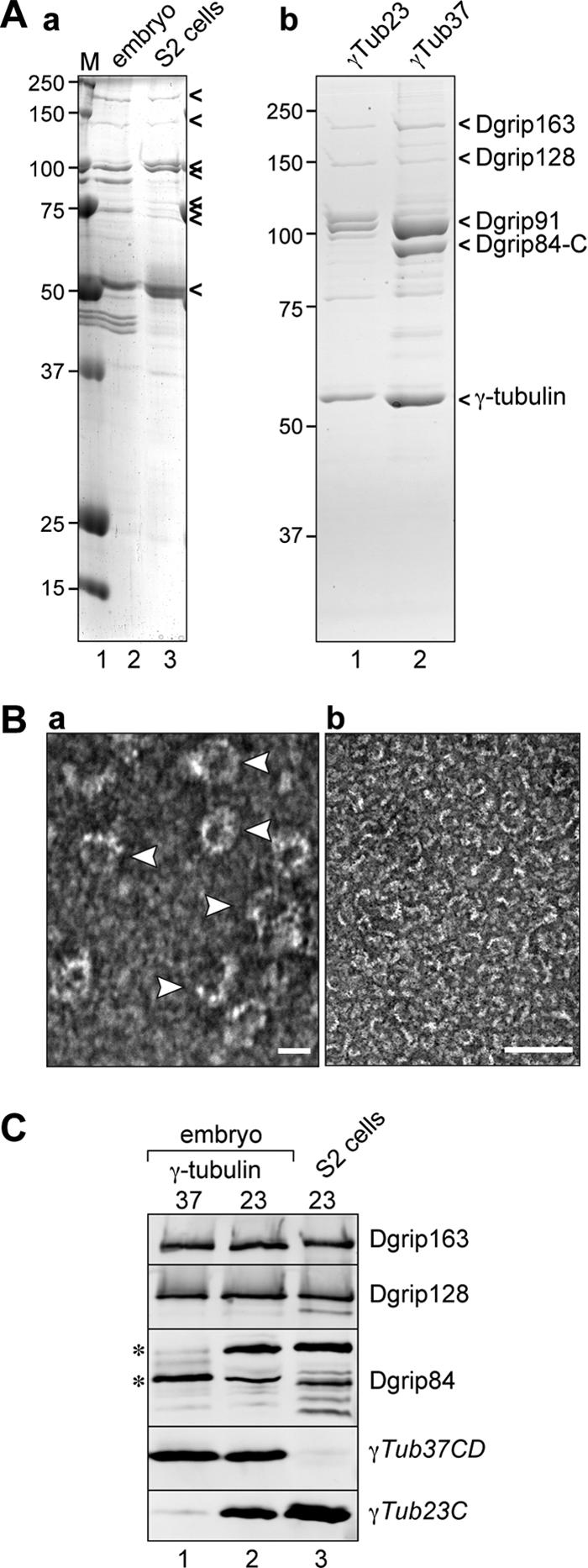
Dgrip84-D is a component of the γTuRC. (A) Comparison between protein complexes immunoprecipitated with various antibodies. Panel a, Coomassie-stained gel of proteins that coimmunoprecipitate with Dgrip84 antibodies from embryo (lane 2) or S2 cell (lane 3) extracts. γTuRC proteins are highlighted by arrowheads on the right, positions of molecular-weight markers are indicated on the left. Panel b, γ-tubulin complexes isolated from S2 cells using antibodies to γTub23C (lane 1), or from 0-to 8-h embryos using antibodies to γTub37CD (lane 2). Complexes isolated from S2 cells with antibodies to Dgrip84 or γTub23C resemble each other, as do complexes isolated from embryos by antibodies to Dgrip84 or γTub37CD. Complexes isolated from S2 cells lack a band corresponding to the embryonic Dgrip84; however, a new band appears above Dgrip91. (B) Negative-staining electron microscopy of γTub23C complexes isolated from S2 cells. Arrowheads in the left panel point to individual ring complexes. Scale bars, 25 nm in the left panel, 100 nm in the right panel. (C) Western blot of proteins that copurify with γTub37CD from early (0–8 h) embryos (lane 1), with γTub23C from late (8–16 h) embryos (lane 2), or with γTub23C from S2 cells (lane 3). Antibodies to Dgrips used to probe the Western blot are indicated on the right. The asterisks mark the two Dgrip84 isoforms that copurify with γTub37CD or γTub23C. The multiple bands in lane 3 might represent additional Dgrip84 isoforms or degradation products.
Next, we wanted to understand the structure of the S2 cell γ-tubulin complexes in greater detail. Using the γTub23C antibody, we adapted the strategy for isolating γTub37CD complexes from early embryo extracts (Oegema et al., 1999) to isolate γ-tubulin complexes from S2 cell extracts (Figure 5Ab). In this strategy, γ-tubulin complexes are immunoprecipitated using the affinity-purified peptide antibodies, and the complexes are then eluted from the affinity beads with the specific synthetic peptide against which the antibodies were raised. This allowed us to examine the complexes by negative staining electron microscopy (Figure 5B), which showed that the purified γTub23C complexes appeared as ∼25-nm rings that closely resembled the embryonic γTuRC37CD (Zheng et al., 1995; Oegema et al., 1999). We therefore concluded that γTub23C formed a “canonical” γTuRC in S2 cells.
The purified complexes were further analyzed by Western blotting with antibodies directed against embryonic Dgrips (Figure 5C). The Dgrips we tested (163/GCP6, 128/GCP5, and 98/GCP3) all copurified with γTub23C complexes isolated from late embryos (8–16 h) or S2 cells and migrated similarly on a 10% SDS-PAGE gel as their early embryonic counterparts (Figure 5C). Consistent with the results described above, the γ-tubulin complexes contained distinct but immunologically related Dgrip84 isoform(s), depending on which γ-tubulin isotype was present: γTub37CD correlated with a faster migrating Dgrip84, whereas the presence of γTub23C correlated with a slower migrating isoform. Interestingly, a small amount of γTub23C copurified with γTub37CD and vice versa (Figure 5C; see also Figures 1F and 6C), suggesting that some γTuRCs may contain both γ-tubulin isotypes.
Figure 6.
γTub23C migrates as a large complex in early embryos that is distinct from the embryonic γTuRC. (A) Coomassie gel of proteins that precipitate from early embryos with antibodies to γTub23C (lane 2) or γTub37CD (lane 3). Positions of γTuRC components are indicated on the right. Lane 1, molecular-weight markers (sizes of markers are indicated on the left). (B) Sucrose gradient analysis of extracts made from 0- to 8-h Drosophila embryos. Western blot of 0–8 h embryo extracts fractionated on a sucrose gradient probed with antibodies specific for each γ-tubulin isotype or Dgrips 163, 128, 98, and 84, as indicated on the right. The positions of the γTuRC (fractions 13–15), the γTuSC (fractions 6–8), and the peak fractions for γTub23C (fractions 11 and 12) are indicated below the blot. The gel is loaded such that the lighter sucrose fractions (starting at 5%) are on the left, and the heavier fractions (up to 40%) are on the right. Fraction numbers are indicated above the blots. Load, 1 μl of starting material was loaded as a control. (C) γTub23C and γTub37CD can be distinguished by their sensitivity to polyethyleneglycol (PEG). PEG (2.5%) was added to extracts, and precipitated proteins were separated from soluble proteins by centrifugation. Supernatant (S) and resuspended pellet (P) fractions were then fractionated on sucrose gradients, run on a SDS-PAGE gel, and blotted for γTub23C or γTub37CD as indicated on the right.
Our analysis also revealed important differences between γTub23C present in early embryos and γTub23C present in late embryos or S2 cells (Figures 5 and 6). Although the γTub23C antibodies immunoprecipitated a γTuRC-like complex from late embryos (Figure 5C) or S2 cells (Figure 5, Ab and C), they failed to precipitate γTub23C, or a γTuRC-like complex, from early embryos (Figure 6A). To understand whether γTub23C exists in a γTuRC-like complex in early embryos, we examined embryo extracts (0–8 h) by sucrose gradient analysis and Western blotting (Figure 6B). Under the conditions used (100 mM salt, 5–40% sucrose; 16 fractions), γTub37CD distributed mainly in two peaks that corresponded to the previously characterized γTuRC (fractions 13–15) and γTuSC (fractions 6–8; Oegema et al., 1999). γTub23C showed a distinct (albeit overlapping) distribution: only a small portion of γTub23C was found in the same fractions as the γTuRC37CD. Instead, γTub23C peaked in fractions 11 and 12 and was distributed broadly in fractions 4–9 without forming a distinct peak (Figure 6B). Surprisingly, the “large” γTub23C complex (fractions 11 and 12) was noticeably smaller than the γTuRC37CD. Similarly, the broad distribution of γTub23C in the lower fractions (4–9) suggested that γTub23C might form a smaller complex, perhaps resembling the γTuSC, but this complex may be distinct from and/or more heterogeneous than the γTuSC37CD. We concluded that γTub23C migrated as a large complex in early embryos, but that this complex is mostly distinct from the γTuRC (Figure 6, A and B). These conclusions were further supported by experiments using differential precipitation of extract proteins with increasing concentrations of polyethylene glycol (PEG). γTub37CD and its associated proteins precipitated nearly quantitatively in the presence of 2.5% PEG, whereas a significant portion of γTub23C remained in the supernatant under these conditions. Sucrose gradient analysis of the supernatant and pellet fractions from the 2.5% PEG precipitation showed that the γTub37CD and γTub23C complexes could be separated from one another under these conditions (Figure 6C). Thus, we concluded that although both γ-tubulin isotypes are present in complexes in early embryos, γTub23C complexes are mostly distinct from the γTuRC37CD.
In contrast, γTub23C comigrated with the Dgrips in tissue culture cells (Figure 3; see also Figure 2B), consistent with the observation that it forms a γTuRC in these cells (see above). Although it is not yet clear at which developmental stage γTub23C switches from the “embryonic” complex to become part of the γTuRC, or how this process is regulated, these findings support the idea that the formation of γTuRCs containing γTub23C is developmentally controlled.
DISCUSSION
Distinct patterns of recruitment to the centrosome and spindle microtubules exhibited by γTub23C and γTub37CD in tissue culture cells and syncytial embryos (Sunkel et al., 1995; Wilson et al., 1997; Raynaud-Messina et al., 2001, 2004) prompted us to examine the molecular compositions of the complexes formed by each of the two Drosophila γ-tubulin isotypes. We reasoned that differences in their localization might be regulated by intrinsic differences between the γ-tubulin isotypes or their binding partners, or both.
We analyzed γ-tubulin complexes in two different systems: in Drosophila embryos, where γTub37CD is the major γ-tubulin isotype, and in Schneider S2 tissue culture cells, where γTub23C is the major γ-tubulin isotype and γTub37C plays only a minor role, if any (Raynaud-Messina et al., 2004). Based on the observation that γ-tubulin and its interacting proteins are highly conserved among distantly related species, it was reasonable to suspect that γTub23C forms complexes in Drosophila cells that closely resemble the γTuRC37CD. Here, we provide the first experimental evidence that this is indeed the case. We show that previously identified γ-tubulin–interacting proteins (Dgrips) copurify with γTub23C isolated from tissue culture cells, and together these proteins form a ring-shaped complex that closely resembles the γTuRC37CD when viewed by negative staining electron microscopy. Our studies revealed two unexpected results: 1) γTub23C forms a large complex in early embryos that is distinct from the γTuRC, and 2) expression of different Dgrip84 isoforms is developmentally regulated and correlates with expression of different γ-tubulin isoforms. These findings support the notion that the two Drosophila γ-tubulins interact with different binding partners and perhaps interact differently with similar binding partners.
What Is the Role of the Embryonic γTub23C Complex?
In embryos, γTub23C distributes in punctate patterns not associated with centrosomes, whereas γTub37CD localizes to centrosomes and spindle poles during early embryonic divisions (Wilson et al., 1997). It has been postulated that only γTub37CD is utilized at centrosomes during embryonic cleavages (Wilson et al., 1997). Consistent with this, female meiosis and nuclear divisions during early embryogenesis specifically require γTub37CD, and γTub37CD mutants are female sterile despite the presence of γTub23C in the embryos (Tavosanis et al., 1997; Tavosanis and Gonzales, 2003). This suggests that the γ-tubulin isotypes are not functionally redundant during embryogenesis. Our results provide a potential biochemical explanation for these observations. We propose that the embryonic γTub23C complex may represent a type of ‘storage form’ of γTub23C. This is suggested by the observation that γTub23C could not be immunoprecipitated from early embryos, indicating that the epitope recognized by the antibody was not accessible. We speculate that the storage particle might include molecular chaperones of the TriC protein family, which have been shown to interact with and aid in the folding of γ-tubulin (Melki et al., 1993). We found that the large γTub23C complexes persisted for at least the first 8 h of development, suggesting that the availability of γTub23C is very tightly regulated during early fly development. Full analysis of the embryonic γTub23C complex awaits methods to purify the complex that do not rely on antibody affinity chromatography. How the association of γTub23C with the putative storage particle is regulated and how γTub37CD escapes recruitment to the particle remains a mystery.
What Is the Importance of the Different Isoforms of Dgrip84/GCP2 in Drosophila?
To date, Drosophila Dgrip84 is the only member of the GCP2 protein family that has been reported to exist as more than one splice variant. Database analysis reveals that at least two Drosophila species possess the 74-amino acid N-terminal extension, D. melanogaster and D. pseudoobscura. It is possible that unique structural features of one or the other Dgrip84 isoform facilitate the particularly rapid centrosome assembly of the early Drosophila embryo, as has previously been postulated for γTub37CD (Wilson et al., 1997). Consistent with this hypothesis, Dgrip84 has recently been implicated in centrosome assembly and separation (Colombié et al., 2006). GCP2 (and GCP3) family members have also been implicated in γTuRC recruitment to the spindle pole body in S. cerevisiae and to the centrosome in metazoa (Knop and Schiebel, 1997, 1998; Nguyen et al., 1998; Barbosa et al., 2000; Vinh et al., 2002; Takahashi et al., 2002; Zimmerman et al., 2004; Kawaguchi and Zheng, 2004; Vérollet et al., 2006). The precise role of GCP2 family members in γTuRC function and recruitment remains to be elucidated.
Our analysis of Dgrip84 isoforms showed that the isoform expressed in embryos corresponds to the shortest splice variant, whereas the isoform expressed in older embryos and S2 cells corresponds to a previously unreported variant of Dgrip84 that has an N-terminal extension but lacks the reported C-terminal insertion. To date, we have been unable to find experimental evidence for the existence of Dgrip84 isoforms that possess the C-terminal insertion (isoforms A and B). It is possible that these isoforms are expressed only at very low levels or that they are expressed only in certain tissues. The roles of the extension or the insertion in Dgrip84 function are not yet clear, but we noted that the developmental switch to the longer isoform of Dgrip84 correlated with the appearance of γTuRCs composed of γTub23C. Although thus far this is merely a correlation, it is tempting to speculate that the N-terminal Dgrip84 extension aids in folding or assembly of the somatic γTuRC, or is required for incorporation of γTub23C into the γTuRC. Experiments to address these questions are currently underway.
Chimeric γTuRCs to Fine-Tune Microtubule Organization?
The metazoan γTuRC contains 12–15 γ-tubulin molecules (Zheng et al., 1995; reviewed in Jeng and Stearns, 1999; Wiese and Zheng, 2006), most of which are thought to be arranged in tetrameric subcomplexes (γTuSCs) that contain two copies of γ-tubulin. Having two γ-tubulin isotypes in the same cell prompts a number of important questions: can both γ-tubulin isotypes coexist in the same γTuSC? Can both isotypes coexist in the same γTuRC? How does the cell distinguish between the γ-tubulin isotypes? Do the γ-tubulin isotypes perform different functions? How does the cell transition from using primarily one isotype to using the other, and how does the activity of a chimeric γTuRC (for which we found evidence by both sucrose gradient analysis and analysis of purified complexes) or γTuSC differ from each of the homogenous versions? The results presented here are beginning to provide answers to some of these questions, but gaining a true understanding of how γ-tubulin complexes that differ only slightly in their composition are perhaps used to fine-tune microtubule organization will be the subject of future studies.
Supplementary Material
ACKNOWLEDGMENTS
I thank M. Tremaine and P. Dobrzyn for their contributions to the experiments described here, L. O'Brien and L. Liu for critical reading of the manuscript, Y. Zheng for kind gifts of Dgrip antibodies, J. Dubnau (Cold Spring Harbor Laboratory) for the rp49 plasmid, K. Mansoorabadi (UW-Madison) for affinity purifying the γTub37CD antibodies, S. Chitteni-Pattu for the negative staining EM, and A. Steinberg and L. Vanderploeg for help with figure preparation. C.W. is a Scholar of the Sidney Kimmel Foundation for Cancer Research. Work in the Wiese lab was further supported by funds from UW-Madison WISELI, the UW-Madison Graduate School, and the Department of Biochemistry.
Abbreviations used:
- γtub
γ-tubulin
- γTuRC
γ-tubulin ring complex
- γTuSC
γ-tubulin small complex
- MT
microtubule.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-08-0801) on November 14, 2007.
REFERENCES
- Anders A., Lourenco P. C., Sawin K. E. Noncore components of the fission yeast γ-tubulin complex. Mol. Biol. Cell. 2006;17:5075–5093. doi: 10.1091/mbc.E05-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- Aldaz H., Rice L. M., Stearns T., Agard D. A. Insights into microtubule nucleation from the crystal structure of human γ-tubulin. Nature. 2005;435:523–527. doi: 10.1038/nature03586. [DOI] [PubMed] [Google Scholar]
- Barbosa V., Yamamoto R. R., Henderson D. S., Glover D. Mutation of a Drosophila γ-tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization. Genes Dev. 2000;14:3126–3139. doi: 10.1101/gad.182800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa V., Gatt M., Rebollo E., Gonzalez C., Glover D. M. Drosophila dd4 mutants reveal that γTuRC is required to maintain juxtaposed half spindles in spermatocytes. J. Cell Sci. 2003;116:929–941. doi: 10.1242/jcs.00295. [DOI] [PubMed] [Google Scholar]
- Blagden S. P., Glover D. M. Polar expeditions—provisioning the centrosome for mitosis. Nat. Cell Biol. 2003;5:505–511. doi: 10.1038/ncb0603-505. [DOI] [PubMed] [Google Scholar]
- Colombie N., Verollet C., Sampaio P., Moisand A., Sunkel C. E., Bourbon H.-M., Wright M., Raynaud-Messina B. The Drosophila γ-tubulin small complex subunit Dgrip84 is required for structural and functional integrity of the spindle apparatus. Mol. Biol. Cell. 2006;17:272–282. doi: 10.1091/mbc.E05-08-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZazzo J., Sandstrom D., de Belle S, Velinzon K., Smith P., Grady L., DelVecchio M., Ramaswami M., Tully T. nalyot, a mutation of the Drosophila myb-related Adf1 transcription factor, disrupts synapse formation and olfactory memory. Neuron. 2000;27:145–158. doi: 10.1016/s0896-6273(00)00016-7. [DOI] [PubMed] [Google Scholar]
- Field C. M., Oegema K., Zheng Y., Mitchison T. J., Walczak C. E. Purification of cytoskeletal proteins using peptide antibodies. Meth. Enzym. 1998;298:525–541. doi: 10.1016/s0076-6879(98)98043-0. [DOI] [PubMed] [Google Scholar]
- Gould R. R., Borisy G. G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J. Cell Biol. 1977;73:601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane R. N., Martin O. C., Cao K., Zhang L., Dej K., Iwamatu A., Zheng Y. Characterization and reconstitution of Drosophila γ-tubulin ring complex subunits. J. Cell Biol. 2000;151:1513–1523. doi: 10.1083/jcb.151.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane R. N., Martin O. C., Zheng Y. Characterization of a new γTuRC subunit with WD repeats. Mol. Biol. Cell. 2003;14:1017–1026. doi: 10.1091/mbc.E02-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N., Peitsch M. C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Haren L., Remy M. H., Bazin I., Callebaut I., Wright M., Merdes A. NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 2006;172:505–515. doi: 10.1083/jcb.200510028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson T. W., Yao J., Bhadury S., Corbett A. H., Joshi H. C. Conditional mutations in γ-tubulin reveal its involvement in chromosome segregation and cytokinesis. Mol. Biol. Cell. 2001;12:2469–2481. doi: 10.1091/mbc.12.8.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T., Oakley B. R. Human γ-tubulin functions in fission yeast. J. Cell Biol. 1994;126:1465–1473. doi: 10.1083/jcb.126.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T., Oakley B. R. Expression of Arabidopsis γ-tubulin in fission yeast reveals conserved and novel functions of γ-tubulin. Plant Physiol. 2003;33:1926–1934. doi: 10.1104/pp.103.027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng R., Stearns T. γ-Tubulin complexes: size does matter. Trends Cell Biol. 1999;9:339–342. doi: 10.1016/s0962-8924(99)01621-9. [DOI] [PubMed] [Google Scholar]
- Jung M. K., Prigozhina N., Oakley C. E., Nogales E., Oakley B. R. Alanine-scanning mutagenesis of Aspergillus γ-tubulin yields diverse and novel phenotypes. Mol. Biol. Cell. 2001;12:2119–2136. doi: 10.1091/mbc.12.7.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S., Zheng Y. Characterization of a Drosophila centrosome protein CP309 that shares homology with Kendrin and CG-NAP. Mol. Biol. Cell. 2004;15:37–45. doi: 10.1091/mbc.E03-03-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. R., Alberts B. M. Purification of a multiprotein complex containing centrosomal proteins from the Drosophila embryo by chromatography with low-affinity polyclonal antibodies. Mol. Biol. Cell. 1992;3:1–11. doi: 10.1091/mbc.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M., Schiebel E. Spc98p and Spc97p of the yeast gamma-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J. 1997;16:6985–6995. doi: 10.1093/emboj/16.23.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamazares S., Tavosanis G., Gonzalez C. Cytological characterization of the mutant phenotypes produced during early embryogenesis by null and loss-of-function alleles of the γ-Tub37C gene in Drosophila. J. Cell Sci. 1999;112:659–667. doi: 10.1242/jcs.112.5.659. [DOI] [PubMed] [Google Scholar]
- Lüders J., Patel U. K., Stearns T. GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 2006;8:137–147. doi: 10.1038/ncb1349. [DOI] [PubMed] [Google Scholar]
- Martin O. C., Gunawardane R. N., Iwamatsu A., Zheng Y. Xgrip109: a γ-tubulin-associated protein with an essential role in γ-tubulin ring complex (γTuRC) assembly and centrosome function J. Cell Biol. 1998;141:675–687. doi: 10.1083/jcb.141.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R., Vainberg I. E., Chow R. L., Cowan N. J. Chaperonin-mediated folding of vertebrate actin-related protein and γ-tubulin. J. Cell Biol. 1993;122:1301–1310. doi: 10.1083/jcb.122.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Braunfeld M. B., Sedat J. W., Alberts B. M., Agard D. A. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature. 1995a;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Moritz M., Braunnfeld M. B., Fung J. C., Sedat J. W., Alberts B. M., Agard D. A. Three-dimensional structural characterization of centrosomes from early Drosophila embryos. J. Cell Biol. 1995b;130:1149–1159. doi: 10.1083/jcb.130.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Zheng Y., Alberts B. M., Oegema K. Recruitment of the γ-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol. 1998;142:775–786. doi: 10.1083/jcb.142.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Agard D. A. γ-Tubulin complexes and microtubule nucleation. Curr. Opin. Struct. Biol. 2001;11:174–181. doi: 10.1016/s0959-440x(00)00187-1. [DOI] [PubMed] [Google Scholar]
- Müller H., Fogeron M. L., Lehmann V., Lehrach H., Lange B. M. A centrosome-independent role for γ-TuRC proteins in the spindle assembly checkpoint. Science. 2006;314:654–657. doi: 10.1126/science.1132834. [DOI] [PubMed] [Google Scholar]
- Murphy S. M., Urbani L., Stearns T. The mammalian γ-tubulin complex contains homologues of the yeast spindle pole body components Spc97p and Spc98p. J. Cell Biol. 1998;141:663–674. doi: 10.1083/jcb.141.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. M., Preble A. M., Patel U. K., O'Connel K. L., Dias D. P., Moritz M., Agard D., Stults J. T., Stearns T. GCP5 and GCP 6, two new members of the human γ-tubulin complex. Mol. Biol. Cell. 2001;12:3340–3352. doi: 10.1091/mbc.12.11.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Vinh D. B., Crawford D. K., Davis T. N. A genetic analysis of interactions with Spc110p reveals distinct functions of Spc97p and Spc98p, components of the yeast γ-tubulin complex. Mol. Biol. Cell. 1998;9:2201–2216. doi: 10.1091/mbc.9.8.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley C. E., Oakley B. R. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Oegema K., Wiese C., Martin O. C., Milligan R. A., Iwamatsu E., Mitchison T. J., Zheng Y. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 1999;144:721–733. doi: 10.1083/jcb.144.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh J. L., Nogales E., Oakley B. R., McDonald K., Pidoux A. L., Cande W. Z. A mutation in γ-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein Pkl1p. Mol. Biol. Cell. 2000;11:1225–1239. doi: 10.1091/mbc.11.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch M. C. Protein modeling by E-mail. Bio/Technology. 1995;13:658–660. [Google Scholar]
- Prigozhina N. L., Walker R. A., Oakley C. E., Oakley B. R. γ-Tubulin and the C-terminal motor domain kinesin-like protein, KLPA, function in the establishment of spindle bipolarity in Aspergillus nidulans. Mol. Biol. Cell. 2001;12:3161–3174. doi: 10.1091/mbc.12.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud-Messina B., Debec A., Tollon Y., Garès M., Wright M. Differential properties of the two Drosophila γ-tubulin isotypes. Eur. J. Cell Biol. 2001;80:643–649. doi: 10.1078/0171-9335-00195. [DOI] [PubMed] [Google Scholar]
- Raynaud-Messina B., Mazzolini L., Moisand A., Cirinesi A.-M., Wright M. Elongation of centriolar microtubule triplets contributes to the formation of the mitotic spindle in γ-tubulin-depleted cells. J. Cell Sci. 2004;117:5497–5507. doi: 10.1242/jcs.01401. [DOI] [PubMed] [Google Scholar]
- Sampaio P., Rebollo E., Varmark H., Sunkel C. E., Gonzalez C. Organized microtubule arrays in γ-tubulin-depleted Drosophila spermatocytes. Curr. Biol. 2001;11:1788–1793. doi: 10.1016/s0960-9822(01)00561-9. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 1972;27:353–365. [PubMed] [Google Scholar]
- Schwede T., Kopp J., Guex N., Peitsch M. C. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkel C. E., Gomes R., Sampaio P., Perdigao J., Gonzalez C. γ-Tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO J. 1995;14:28–36. doi: 10.1002/j.1460-2075.1995.tb06972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Yamagiwa A., Nishimura T., Mukai H., Ono Y. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring γ-tubulin ring complex. Mol. Biol. Cell. 2002;13:3235–3245. doi: 10.1091/mbc.E02-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin A. M., Celati C., Moudjou M., Bornens M. Characterization of the human homolog of the yeast Spc98p and its association with γ-tubulin. J. Cell Biol. 1998;141:689–701. doi: 10.1083/jcb.141.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavosanis G., Llamazares S., Goulielmos G., Gonzalez C. Essential role for γ-tubulin in the acentriolar female meiotic spindle of Drosophila. EMBO J. 1997;16:1809–1819. doi: 10.1093/emboj/16.8.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavosanis G., Gonzalez C. γ-Tubulin function during female germ-cell development and oogenesis in Drosophila. Proc. Natl. Acad. Sci. 2003;100:10263–11026. doi: 10.1073/pnas.1731925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy L., Toda T. The fission yeast γ-tubulin complex is required in G(1) phase and is a component of the spindle assembly checkpoint. EMBO J. 2000;19:6098–6111. doi: 10.1093/emboj/19.22.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy, Fujita L. A., Toda T. The γ-tubulin complex protein Alp4 provides a link between the metaphase checkpoint and cytokinesis in fission yeast. Genes Cells. 2002;73:65–73. doi: 10.1046/j.1365-2443.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- Vérollet C., Colombie N., Daubon T., Bourbon H. M., Wright M., Raynaud-Messina B. Drosophila melanogaster γTuRC is dispensable for targeting γ-tubulin to the centrosome and microtubule nucleation. J. Cell Biol. 2006;172:517–528. doi: 10.1083/jcb.200511071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh D.B.N., Kern J. W., Hancock W. O., Howard J., Davis T. N. Reconstitution and characterization of budding yeast γ-tubulin complex. Mol. Biol. Cell. 2002;13:1144–1157. doi: 10.1091/mbc.02-01-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Snyder M. γ-Tubulin of budding yeast. Curr. Top. Dev. Biol. 2000a;49:75–104. doi: 10.1016/s0070-2153(99)49005-2. [DOI] [PubMed] [Google Scholar]
- Vogel J., Snyder M. The carboxy terminus of Tub4p is required for γ-tubulin function in budding yeast. J. Cell Sci. 2000b;113:3871–3882. doi: 10.1242/jcs.113.21.3871. [DOI] [PubMed] [Google Scholar]
- Wiese C., Zheng Y. A new function for the γ-tubulin ring complex as a microtubule minus-end cap. Nat. Cell Biol. 2000;2:358–364. doi: 10.1038/35014051. [DOI] [PubMed] [Google Scholar]
- Wiese C., Zheng Y. Microtubule nucleation: γ-tubulin and beyond. J. Cell Sci. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- Wilson P. G., Borisy G. G. Maternally expressed γTub37CD in Drosophila is differentially required for female meiosis and embryonic mitosis. Dev. Biol. 1998;199:273–290. doi: 10.1006/dbio.1998.8900. [DOI] [PubMed] [Google Scholar]
- Wilson P. G., Zheng Y., Oakley C. E., Oakley B. R., Borisy G. G., Fuller M. T. Differential expression of two γ-tubulin isoforms during gametogenesis and development in Drosophila. Dev. Biol. 1997;184:207–221. doi: 10.1006/dbio.1997.8545. [DOI] [PubMed] [Google Scholar]
- Wise D. O., Krahe R., Oakley B. R. The γ-tubulin gene family in humans. Genomics. 2000;67:164–170. doi: 10.1006/geno.2000.6247. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Jung M. K., Oakley B. R. γ-Tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wong M. L., Alberts B., Mitchison T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman W. C., Sillibourne J., Rosa J., Doxsey S. J. Mitosis-specific anchoring of γ-tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell. 2004;15:3642–3657. doi: 10.1091/mbc.E03-11-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.