Abstract
Cdk1 was proposed to compensate for the loss of Cdk2. Here we present evidence that this is possible due to premature translocation of Cdk1 from the cytoplasm to the nucleus in the absence of Cdk2. We also investigated the consequence of loss of Cdk2 on the maintenance of the G1/S DNA damage checkpoint. Cdk2−/− mouse embryonic fibroblasts in vitro as well as regenerating liver cells after partial hepatectomy (PH) in Cdk2−/− mice, arrest promptly at the G1/S checkpoint in response to γ-irradiation due to activation of p53 and p21 inhibiting Cdk1. Furthermore re-entry into S phase after irradiation was delayed in Cdk2−/− cells due to prolonged and impaired DNA repair activity. In addition, Cdk2−/− mice were more sensitive to lethal irradiation compared to wild-type and displayed delayed resumption of DNA replication in regenerating liver cells. Our results suggest that the G1/S DNA damage checkpoint is intact in the absence of Cdk2, but Cdk2 is important for proper repair of the damaged DNA.
INTRODUCTION
Cyclin-dependent kinases (Cdks) are a family of serine/threonine kinases that represent the core of the cell cycle machinery. Successive waves of Cdk activity control initiation and progression through the eukaryotic cell cycle in response to both intracellular and extracellular signals. Cdks form active heterodimeric complexes following binding to cyclins, their regulatory subunits (for review see Morgan, 1997). Cdk4 and Cdk6 are thought to be involved in early G1, whereas Cdk2 was believed to be essential to complete G1 and to initiate S phase. Cdk4 and Cdk6 form active complexes with D-type cyclins to initiate the cell division cycle by phosphorylating the retinoblastoma protein (Rb). Late in the G1 phase, Cdk2/cyclin E complexes complete Rb phosphorylation and drive cells through the restriction point (for review see Weinberg, 1995; Mittnacht, 1998). In this context it was believed that Cdk2 is solely responsible for the G1/S phase transition with cyclin E and later in complex with cyclin A is essential for S phase progression. On the other hand, Cdk1 in association with cyclin B is essential to control entry into and exit from mitosis (Dunphy et al., 1988; Riabowol et al., 1989; Izumi and Maller, 1993; Pan et al., 1993; Labib et al., 1995).
Superimposed on the regulation of the cell cycle are a number of checkpoint mechanisms, which are not essential for cell cycle progression but are critical for the cellular response to stress, including abnormal mitogenic stimuli and DNA damage. In response to DNA damage, cell cycle checkpoint pathways are activated, which results in arrest in G1, regulating progression through S phase and preventing entry into mitosis with unrepaired damage (Murray, 1994; Li and Nicklas, 1995; Elledge, 1996; O'Connor, 1997; Mercer, 1998; Shackelford et al., 1999; Lukas et al., 2004; Sancar et al., 2004; O'Connell and Cimprich, 2005; Houtgraaf et al., 2006). The G1 cell cycle checkpoint is primarily responsible for preventing damaged DNA from being replicated and results among other things in the ataxia telangiectasia mutated (ATM)/ATM- and Rad3-related (ATR)-mediated activation of p53. Activation of p53 inhibits its nuclear export and degradation, thus resulting in increased levels of p53 (Morgan and Kastan, 1997; Schwartz and Rotter, 1998; Chaurvedi et al., 1999; Hall-Jackson et al., 1999; Cuddihy and Bristow, 2004; Helt et al., 2005). p53 then activates one of its downstream targets, p21Cip1/Waf1, which binds to and inhibits Cdk2/cyclin E complexes, thereby arresting cells at the G1/S checkpoint (Adams et al., 1996; Poon et al., 1996; Brugarolas et al., 1999). Analysis of p21-deficient cells revealed that the p21 protein is essential for arresting cells at the G1/S checkpoint because p21−/− cells bypass the G1/S arrest in response to DNA damage induced by irradiation (Deng et al., 1995). On the other hand, DNA damage also induces G2 arrest even in the absence of p21 or p53, but p53 and p21 appear to be essential for prolonged G2/M arrest (Bunz et al., 1998). Cells lacking p53 arrest transiently in G2, with Cdk1 extensively phosphorylated at the inhibitory sites Thr14 and Tyr15 and with both Cdk1 and cyclin B1 restricted to the cytoplasm (Herzinger et al., 1995). In the presence of p53, p21 is induced and inhibits Cdk1, thereby contributing to prolong the G2/M arrest (Bunz et al., 1998). Activation of the DNA damage checkpoint and a transient cell cycle block in response to irradiation allows cells to induce DNA repair. Many proteins that are involved in DNA repair and that are activated by the ATM/ATR pathway are enriched in the nucleus and form DNA damage foci at broken chromosomes. After successful repair, these foci are cleared from the nucleus. These proteins include phosphorylated γ-H2AX, an extensively studied DNA repair protein involved in double-strand break (DSB) repair that in turn promotes the assembly of several checkpoint and DNA repair factors including 53BP1, MDC1, NBS1, and BRCA1 at the site of DNA damage (Schultz et al., 2000; Rappold et al., 2001; Celeste et al., 2002; Stewart et al., 2003; Lee et al., 2005).
Recently it was discovered that Cdk2, the kinase activated by both E-type and A-type cyclins, is dispensable for mouse development. Cdk2−/− embryonic fibroblasts proliferate in culture with a slight delay in S phase entry, indicating that Cdk2 activity is not essential for mitotic cell divisions (Berthet et al., 2003; Ortega et al., 2003). Further studies uncovered that other kinases, especially Cdk1, compensate for Cdk2's loss by driving cells through G1/S transition in association with cyclin E (Aleem et al., 2005). Previous studies have shown that in a wild-type background, Cdk1 translocates to the nucleus during mitosis after nuclear breakdown (Bailly et al., 1989; Moore et al., 1999). If Cdk1 has to execute the G1/S transition in the absence of Cdk2, an earlier translocation of Cdk1 to the nucleus would be required but has not yet been demonstrated. Nevertheless, it cannot be excluded that Cdk1 can compensate for Cdk2 even when localized to the cytoplasm. Therefore, we addressed whether the loss of Cdk2 will affect Cdk1's translocation because Cdk1 has to execute both G1/S and G2/M transitions. In addition, the finding that Cdk1/cyclin E substitutes for Cdk2/cyclin E presents an intriguing twist for the G1/S DNA damage checkpoint and raises several questions. How is the p53 pathway controlled and how is p21 regulated in the absence of its target Cdk2 and will it be induced or not? If p21 is induced, are the cells arrested at the G1/S checkpoint or will they bypass the G1/S checkpoint in the absence of Cdk2 because p21 has no target to inhibit? Furthermore, if the translocation of Cdk1 occurs in the absence of Cdk2, is it affected in response to DNA damage at the G1/S transition? Another important question is, if p21 is able to arrest cells at G1/S even in the absence of Cdk2, will the arrest be sufficient and long enough for the cells to repair their damaged DNA and resume DNA replication? One study has shown that loss of Cdk2 is dispensable for the G1/S arrest in response to radiation damage. In addition, overexpression of either p21 or p27 arrests Cdk2−/− cells at both G1/S and G2/M checkpoints (Martin et al., 2005), but the molecular mechanism behind the G1/S checkpoint maintenance in the absence of Cdk2 has not been explored. Furthermore it was described that in yeast, Cdk1 is required for DSB induced by recombinational repair (Caspari et al., 2002; Ira et al., 2004) but in mammalian cells it has not been determined which Cdk fulfills this function. In addition, a recent study demonstrated that cyclin A1 is essential for DNA DSB repair and the repair function of cyclin A1 depends on Cdk2 (Müller-Tidow et al., 2004). Nevertheless, the effects of irradiation-induced DNA damage and the resulting repair activity in the absence of Cdk2 has not yet been studied in vivo.
In the present study we have investigated the consequence of loss of Cdk2 on the subcellular translocation of Cdk1 at different phases of the cell cycle. In addition, we explored the impact of loss of Cdk2 on the maintenance of the G1/S DNA damage checkpoint and concomitant repair of damaged DNA in response to irradiation in cells as well as in animals. We show that Cdk1 shuttles between cytoplasm and nucleus, and the translocation of Cdk1 from cytoplasm to nucleus occurs earlier in the absence of Cdk2 and was not influenced substantially by irradiation. We also describe that synchronized Cdk2−/− mouse embryonic fibroblasts (MEFs) in vitro as well as regenerating liver cells after partial hepatectomy (PH) in Cdk2−/− mice, arrest at the G1/S checkpoint in response to γ-irradiation. We describe that the p53-p21 pathway is intact in the absence of Cdk2 and the induced p21 inhibits Cdk1 to arrest cells at the G1/S transition. In addition, we found that the arrested Cdk2−/− cells entered into S phase delayed in response to irradiation due to prolonged and impaired DNA repair activity. Our results indicate that Cdk2 contributes to DNA repair in vivo.
MATERIALS AND METHODS
Preparation of MEFs, Cell Culture, and Irradiation of Cells
MEFs were prepared as described previously (Berthet et al., 2003) from littermate 13.5 dpc (days post-coitus) Cdk2+/+ and Cdk2−/− embryos. MEFs were cultured in DMEM medium (Invitrogen, Carlsbad, CA; 10569-010) supplemented with 10% fetal bovine serum (FBS; Gemini Bioproducts, West Sacramento, CA; 100-106) and 1% penicillin streptomycin (Invitrogen; 15140-122) in a CO2 incubator (5% CO2 and 20% O2) at 37°C. Cells were passaged serially in vitro, and P3 cultures were used for this study. MEFs prepared from three separate embryos from three different females were used for each experiment. For experiments involving serum starvation and stimulation, cells were washed twice with phosphate-buffered saline (PBS), collected by trypsinization from P3 cultures, cultured in DMEM medium with 0.1% FBS at a density of 2.5 × 106 cells in 10-cm culture dishes for 96 h, and stimulated with 10% FBS in DMEM medium for the indicated time points.
For irradiation studies, P3 MEFs growing in DMEM medium supplemented with 10% FBS were washed two times with PBS, trypsinized, and plated at a density of 2.5 × 106 in 10-cm culture dishes containing DMEM medium with 0.1% FBS. After 96 h of serum starvation cells were washed with PBS, trypsinized, and collected in 12-ml Falcon tubes (Becton Dickinson Labware, Bedford, MA) containing 5 ml DMEM with 10% FBS. The cells were immediately irradiated in a γ-irradiator with doses of either 5 or 10 Gy. After irradiation the cells were replated (10-cm culture dishes) and collected at the indicated time points for cell cycle profiles and whole cell lysate preparations.
AlamarBlue Cell Proliferation Assay
Proliferation of Cdk2+/+ and Cdk2−/− MEFs in response to 5- and 10-Gy irradiation was analyzed in 96-well plates. Triplicates of 1500 cells/well for each sample were seeded and the proliferation rate of the cells was measured by using the AlamarBlue proliferation assay (Biosource, Camarillo, CA; DAL1100; Ahmed et al., 1994). Complete medium with 10% AlamarBlue was added to each well and a fluorescent reading (Novostar, BMG Labtechnologies, Offenburg, Germany) was carried out after 4 h. Triplicate wells were measured every day for up to 7 d.
Immunocytochemistry and Confocal Microscopy
Serum-starved (DMEM medium with 0.1% FBS, 96 h) and stimulated (DMEM medium with 10% FBS) cells from 10-cm culture dishes (with 5-Gy irradiation or without irradiation) were transferred on to coverslips in 12-well plates at a density of 1 × 105 and probed with specific antibodies at 0, 6, 12, 18, 24, and 30 h after stimulation. For immunofluorescence microscopy, the cells were washed twice with cold PBS, fixed with ice-cold acetone-methanol (1:1) for 5 min, and washed three times with ice-cold PBS. The cells were then permeabilized with 0.5% NP-40 for 10 min, washed three times with ice-cold PBS, and incubated with specific primary antibodies at 4°C overnight. The following primary antibodies were used for immunocytochemistry: anti-Cdk2, anti-Cdk1 (Berthet et al., 2003; 1:200 dilution), anti-p21 (Santa Cruz Biotechnology, Santa Cruz, CA, sc-6246, 1:200), anti-phospho-H2AX [Ser139] (Chemicon, Temecula, CA; AB3369 1:100 dilution), and phospho-(Ser/Thr) ATM/ATR substrate antibody (Cell Signaling, Beverly, MA; 2851S, 1:100 dilution). Cells were washed three times with PBS and incubated with either AlexaFluor488 goat anti-mouse (Molecular Probes, Eugene, OR; 47759A) or AlexaFluor568 goat anti-rabbit (Molecular Probes; A11011) secondary antibodies for 30 min at room temperature. The coverslips were washed three times with PBS, air-dried for 10 min, and mounted with fluorescence mounting medium (Vectashield, H-1200; Vector Laboratories, Burlingame, CA) containing 4′,6′-diamidino-2-phenylindole (DAPI). The optical single slice images were captured with a confocal laser-scanning microscope LSM510 (Zeiss, Thornwood, NY). The foci in the images were automatically extracted using an in-house MATLAB based application (Release 7.3, Mathworks, Natick, MA). First, we equalized the histogram of the red channel (or the channel containing the foci) in order to use the complete dynamic range of 256-Gy-level intensities. Next, we suppressed the background noise with a gray-scale morphological closing. Then, we applied a threshold algorithm to identify all pixels whose intensity values were within the range of [(1/3) (2/3)] (max_c − min_c), where max_c and min_c denote the maximum and minimum intensity values after gray-scale morphological closing operation, respectively. In the final step, we removed regions that were smaller than 2 pixels. The anti-phospho-H2AX (Ser139) foci and phospho-(Ser/Thr) ATM/ATR substrate foci per nucleus was counted in a minimum of 200 nuclei at each time point for each sample.
Anti-BrdU-Propidium Iodide Double Staining of Cells Analyzed by Fluorescence-activated Cell Sorting
Cells were pulse-labeled with 10 μM final concentration of BrdU (5-bromo-2′-deoxyuridine, Molecular Probes, B23151) for 2 h before collection, washed once with PBS, trypsinized, and collected by centrifugation at 1000 rpm for 5 min. The cell pellet was resuspended in 1 ml of 1% bovine serum albumin (BSA) in PBS and centrifuged at 1000 rpm for 5 min at room temperature. The pellet was resuspended in 200 μl of PBS, fixed in 5 ml of ice-cold 70% ethanol by adding ethanol drop by drop while vortexing, and incubated on ice for 30 min. The cells were centrifuged at 1000 rpm for 5 min at 4°C and the supernatant was aspirated. The pellet was washed once with 5 ml of PBS/1% BSA and resuspended in 1 ml of 2 N HCl + 0.5% Triton X-100 at room temperature for 20 min. After centrifugation at 1000 rpm for 5 min, the supernatant was aspirated, and the pellet was resuspended in 1 ml of 0.1 M NaB4O7, pH 8.5. The pellet was incubated for 5 min, centrifuged at 1000 rpm for 5 min, washed once with 5 ml PBS/1% BSA, resuspended in 1 ml of 0.5% Tween-20/1% BSA/PBS, and the cell concentration was adjusted to achieve 1 × 106 cells per test. Then the cells were incubated with 1 μg of anti-BrdU-AlexaFluor488 antibodies (Molecular Probes, A21308) for 45 min at room temperature, washed once in 5 ml of 0.5% Tween-20/1% BSA/PBS, centrifuged at 1000 rpm for 5 min, and resuspended in 0.5 ml of PBS + 5 μg (final concentration) of propidium iodide + 10 μl of 10 mg/ml RNase A. All the centrifugation steps were done in an RC-3B refrigerated centrifuge using a HG-4L rotor (Sorvall Instruments, Newton, CT). The serum-stimulated nonirradiated and serum-stimulated irradiated (5 Gy) cells were collected at 12, 18, 24, and 30 h for cell cycle profiles. Flow cytometric analysis was carried out with a FACScan (Becton Dickinson, Franklin Lakes, NJ) equipped with the Cellquest software. The acquisition was analyzed using the FlowJo 8.1.1 software (PowerPC, Intel, Mountain View, CA).
Comet Assay
P3 MEFs were serum-starved (0.1% FBS) for 96 h, trypsinized, and collected in 5 ml DMEM with 10% FBS in 15ml Falcon tubes (2097), and 2.5 × 106 cells per 5 ml of DMEM/10% FBS were irradiated immediately with either 5 or 10 Gy. After irradiation, the cells were replated in 10-cm culture dishes and collected 2 and 18 h after irradiation for comet assays. The nonirradiated serum-starved and stimulated cells served as control. The comet assay (Singh et al., 1988, 1994) was done according to the manufacturer's protocol (CometAssay HT Sample, 4252–040-K, Trevigen, Gaithersburg, MD). The experiments were done in triplicate, and at least 100 cells were analyzed per sample. The amount of DNA damage was scored as high damage or low damage on the basis of the migration of the comet tails.
Mice, Irradiation, and Surgical Procedures
Mice were housed under standard conditions and were maintained on a 12-h light/dark cycle. Mice were fed a standard chow diet containing 6% crude fat and were treated in compliance with the National Institutes of Health guidelines for animal care and use.
For irradiation studies mice were subjected to 11 Gy in a γ-irradiator. Two hours after irradiation the mice were operated together with nonirradiated mice that served as control. Twelve-week-old Cdk2+/+ and Cdk2−/− male mice were used for this study, and all the animals were operated under sterile conditions in the early hours of the day between 9 am and 12 pm, as described previously (Satyanarayana et al., 2003). Mice were anesthetized by a single intraperitoneal injection of avertin and were subjected to 70% PH. The left lateral, left median, and right median lobes were separately isolated and then removed with a single ligature. Care was taken to avoid disruption of the portal vein, biliary tract, or gallbladder. All animals resumed normal activities promptly after recovering from anesthesia. After 2 h of BrdU labeling, mice were euthanized at 24 (n = 3), 36 (n = 3), 48 (n = 4), 72 (n = 4), and 96 (n = 4) h after PH. For BrdU pulse labeling, 10 μl/g body weight labeling reagent (10:1 ratio, 5-bromo-2-deoxyuridine and 5-fluro-20-deoxyuridine, cell proliferation kit, RPN20, Amersham Pharmacia, Piscataway, NJ) was administered intraperitoneally 2 h before they were euthanized. After the mice were euthanized, a portion of the liver lobe was snap-frozen in liquid nitrogen and stored at −80°C for preparation of whole cell lysates. The remaining lobe was fixed in 10% buffered neutral Formalin (Sigma, St. Louis, MO; HT50-1-128) for sectioning and immunohistochemical stainings.
BrdU Immunohistochemical Stainings
Formalin fixed liver sections (5 μm thick) were deparaffinized and then denatured in 4 N HCl for 20 min at 37°C. The sections were briefly rinsed once in “Holme's Boric Acid–Borax Buffer pH 7.6” [(85 ml of boric acid (12.37 g/l) +15 ml borax/sodium borate (19.07 g/l)] and incubated in prewarmed 0.01% trypsin for 3 min at 37°C. After rinsing in PBS, the endogenous peroxidase activity was blocked by incubating in 0.3% hydrogen peroxide in methanol for 30 min. The primary (biotinylated anti-BrdU antibody, DAKO, Carpinteria, CA; M0744) and secondary antibodies (anti-mouse streptavidin-peroxidase antibody) and DAB substrate steps were performed according to the manufacturer's instructions (DAKO, K3954).
Immunoblotting
Whole cell lysates from P3 MEFs and frozen liver samples were prepared as described previously (Berthet et al., 2003). For Western blotting, 50 μg of protein was separated on 12.5% precast gels (Bio-Rad, Richmond, CA), transferred onto Immobilon-P transfer membrane (Millipore, Bedford, MA, IPVH00010) and probed with specific primary antibodies. The primary antibodies used were as follows: Cdk2, Cdk1 (as described previously; Berthet et al., 2003), p53 (Cell Signaling, 2524), p21 (Santa Cruz, sc-6246), and phospho-Cdk1 [Tyr15] (Cell Signaling, 91115). All the antibodies were used at a dilution of 1:1000. For coimmunoprecipitations, (Cdk2-p21, Cdk1-p21), 250 μg of protein from cell lysates or 500 μg of protein from liver extracts and 7 μl of Cdk2-coupled agarose A beads (as described; Berthet et al., 2003) and Cdk1-coupled agarose A beads (Santa Cruz, sc-54AC) were used.
RESULTS
Cdk1 Translocates Prematurely to the Nucleus in the Absence of Cdk2
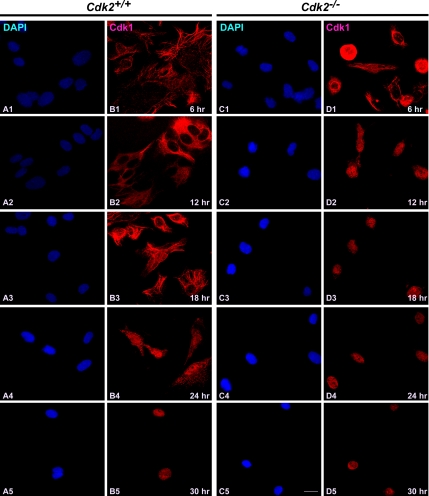
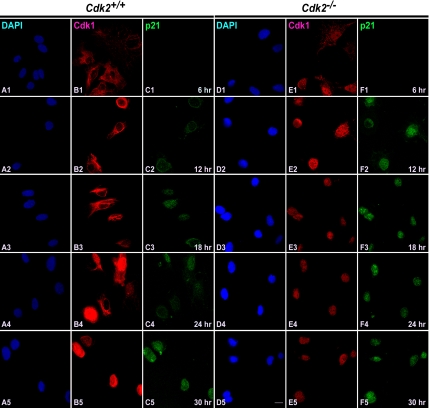
One of the aspects of mitotic regulation is the activation and translocation of Cdk1/cyclin complexes and their regulatory enzymes between specific subcellular compartments that in turn regulates the timing of entry into mitosis (Pines, 1995). Cdk1/cyclin B1 complexes associate with microtubules during interphase but translocate into the nucleus at the G2/M transition to allow phosphorylation of nuclear substrates critical for mitosis (Bailly et al., 1989, 1992). According to the current model, signals elicited by DNA damage in S and G2 phases prevent mitotic entry by inhibiting both activation and nuclear import of Cdk1/cyclin B1 (Jin et al., 1998; Yang and Kornbluth, 1999). On the basis of recent finding that Cdk1 substitutes for Cdk2 function at the G1/S transition (Aleem et al., 2005), it is interesting to explore whether the trafficking of Cdk1 is affected in order to execute the G1/S transition in the absence of Cdk2. To better understand the translocation of Cdk1 in the absence of Cdk2, we determined the localization of Cdk1 in serum-starved and stimulated wild-type and Cdk2−/− MEFs at different stages of cell cycle by immunofluorescence (Figure 1). Our study revealed that in wild-type cells, Cdk1 was present mainly in the cytoplasm in the serum-starved cells. At 6, 12, and 18 h after serum stimulation, when cells are mainly in the G1 phase, G1/S transition, and the initiation of S phase of cell cycle, Cdk1 remained in the cytoplasm (Figure 1, B1–B3). Twenty-four hours after serum stimulation, which represent the peak of S phase, a fraction of cells (∼20%) displayed nuclear localization of Cdk1, and 30 h after stimulation Cdk1 was localized to the nucleus in the majority of wild-type cells (∼65%; Figure 1, B4 and B5).
Figure 1.
Cdk1 translocates early to the nucleus in the absence of Cdk2. Immunocytochemical staining of Cdk1 in Cdk2+/+ and Cdk2−/− MEFs at different time points after serum stimulation. Localization of Cdk1 was detected by rabbit anti-Cdk1 antibodies followed by AlexaFluor568 goat anti-rabbit antibodies (red; B1–B5 and D1–D5), and the nuclei were counterstained with DAPI (blue; A1–A5 and C1–C5). The images were captured with a laser confocal microscope (63×). The representative pictures are from one of three independent stainings. Scale bar, 10 μm in every panel.
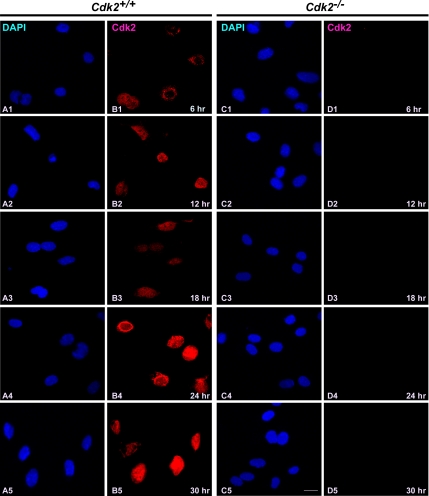
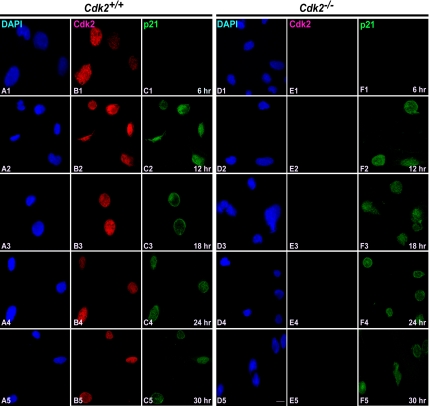
In contrast to the trafficking of Cdk1 in wild-type cells, Cdk2−/− cells showed a different translocation pattern of Cdk1 (Figure 1). Most of the cells (∼60%) displayed nuclear localization of Cdk1 as early as 12 h after serum stimulation, and in a fraction of cells (∼15%) Cdk1 nuclear localization was observed as early as 6 h after serum stimulation in Cdk2−/− cells (Figure 1, D1 and D2). At later time points (18, 24, and 30 h), Cdk1 was found predominantly in the nucleus (in ∼75% of cells; Figure 1, D3–D5). This indicates that Cdk1 translocates to the nucleus earlier in the absence of Cdk2, which enables Cdk1 to compensate for the loss of Cdk2 and execute the G1/S transition. In addition, immunofluorescence studies revealed that Cdk2 was present predominantly in the nucleus (between 6 and 30 h after serum stimulation) in wild-type cells (Figure 2, B1–B5; Moore et al., 1999) and was not detected in Cdk2−/− cells irrespective of the cell cycle stage as expected (Figure 2, D1–D5).
Figure 2.
Cdk2 resides predominantly in the nucleus irrespective of cell cycle stage. Immunofluorescence staining of Cdk2 in serum-stimulated Cdk2+/+ and Cdk2−/− MEFs at different time points. Cdk2 localization was detected by rabbit anti-Cdk2 antibodies followed by AlexaFluor568 goat anti-rabbit antibodies (red; B1–B5 and D1–D5) and the nuclei were counterstained with DAPI (blue; A1–A5 and C1–C5). The images were captured with a laser confocal microscope (63×). The representative pictures are from one of three independent stainings. Scale bar, 10 μm in every panel.
Cdk2 Is Dispensable for G1/S DNA Damage Checkpoint Arrest
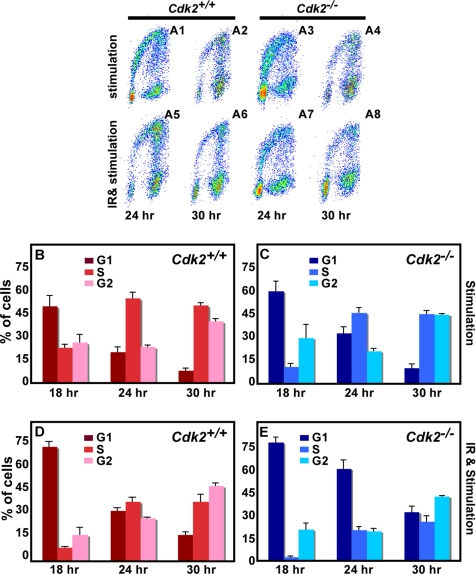
All eukaryotic cells have evolved a multifaceted response to counteract the potentially deleterious effects of DNA damage. In response to DNA damage, checkpoints (G1, S, and G2/M) are activated to arrest cell cycle progression allowing time for repair (Kaufmann, 1995; O'Connor, 1997; Lukas et al., 2004; Sancar et al., 2004; Houtgraaf et al., 2006). The G1 cell cycle checkpoint prevents damaged DNA from being replicated, and central to this checkpoint is the activation of the p53-p21 pathway (Morgan and Kastan, 1997; Schwartz and Rotter, 1998). The activated p53 induces p21, which binds to and suppresses Cdk2/cyclin E activity, thereby resulting in G1 arrest (Brugarolas et al., 1999; Maeda et al., 2002). To identify the consequence of loss of Cdk2 on the maintenance of the G1/S checkpoint arrest, serum-starved Cdk2−/− and wild-type cells were γ-irradiated, immediately stimulated by 10% FBS, and cell cycle progression was measured by BrdU incorporation at different time points (0–30 h after stimulation). In response to serum starvation, ∼90% of the cells were in the G0 phase of the cell cycle in both wild-type and Cdk2−/− cultures (data not shown). At 12 h after serum stimulation only 3% of wild-type and 1% of Cdk2−/− nonirradiated cells displayed BrdU incorporation. In contrast, few cells were detected in S phase in wild-type and Cdk2−/−-irradiated cells 12 h after serum stimulation (data not shown). After 18 h of serum stimulation, nonirradiated Cdk2−/− cells displayed a slightly delayed S phase entry in comparison to wild-type cells (Figure 3B and C), as has been described (Berthet et al., 2003). On the other hand, after γ-irradiation few Cdk2−/− cells entered S phase with concomitant accumulation of cells in G1 phase, whereas wild-type cells already started to enter S phase (Figure 3, D and E). At later time points (24 and 30 h after serum stimulation), we observed that both wild-type and Cdk2−/− cells were progressing through S phase after radiation damage but it appeared that the G2/M progression was delayed with more cells accumulating in the G2 phase of the cell cycle (Figure 3, A5–A8, D, and E). In addition, careful analysis of the cell cycle profile in irradiated cells revealed that even though both wild-type and Cdk2−/− cells were reentering S phase after radiation damage, DNA replication appeared to be further delayed in Cdk2−/− cells (Figure 3, D and E). This analysis indicates that Cdk2 is not necessary for cells to arrest at the G1/S DNA damage checkpoint in response to radiation damage but Cdk2 might be involved in DNA repair.
Figure 3.
Cells arrest at the G1/S checkpoint in vitro in the absence of Cdk2. (A) Flow cytometric analysis of Cdk2+/+ and Cdk2−/− MEFs after serum stimulation alone and 5-Gy γ-irradiation and serum stimulation after 24 and 30 h. The cells were stained with propidium iodide and anti-BrdU AlexaFluor488 antibodies. Representative dot plots are from one of three independent cell cycle profiles. (B) Bar graph representing the percentage of Cdk2+/+ MEFs in G1 (red), S (fuchsia), and G2 (pink) phases of the cell cycle after serum stimulation at the indicated time points. (C) Bar graph presenting the percentage of Cdk2−/− MEFs in G1 (navy blue), S (blue), and G2 (aqua) phases of the cell cycle after serum stimulation at the indicated time points. (D) Bar graph presenting the percentage of Cdk2+/+ MEFs in G1 (red), S (fuchsia), and G2 (pink) phases of the cell cycle after 5-Gy irradiation and serum stimulation at the indicated time points. (E) Bar graph presenting the percentage of Cdk2−/− MEFs in G1 (navy blue), S (blue), and G2 (aqua) phases of the cell cycle after 5-Gy irradiation and serum stimulation at the indicated time points. For all graphs the average and SDs were acquired from three independent experiments.
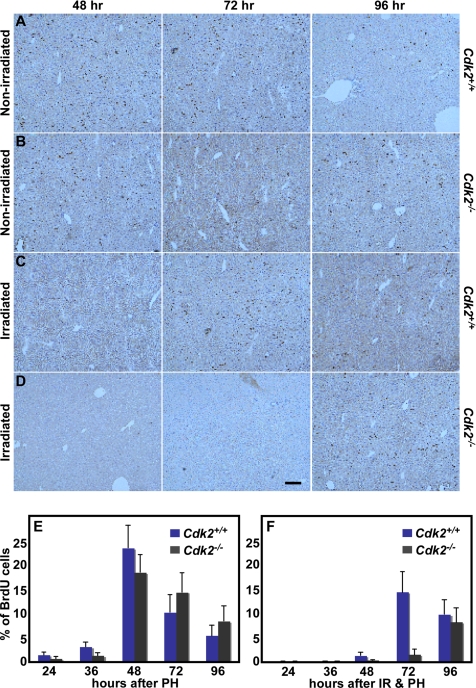
We aimed to explore the DNA damage response in vivo to confirm and extend our results in MEFs. To this end, we used liver regeneration as a model system to study cell cycle initiation and progression in the regenerating liver of nonirradiated and 11 Gy whole body irradiated wild-type and Cdk2−/− mice after PH. The liver is a mitotically inactive organ, with more than 90% of the cells in the G0 phase of the cell cycle. In response to PH, liver cells reenter the cell cycle in a highly synchronized manner and regenerate lost mass by one to two rounds of replication within a week, thus representing one of the best models to study in vivo cell cycle initiation and progression (Fausto, 2000; Kountouras et al., 2001). In response to PH, S phase initiation occurs at 24 h and peaks at 48 h, and the first round of replication is completed at approximately 72 h (Fausto, 2000; Satyanarayana et al., 2004). We monitored the initiation and progression of the cell cycle between 24 and 96 h after PH and observed that the onset of S phase is slightly delayed in nonirradiated Cdk2−/− mice compared with wild-type mice (Figure 4), similar to MEFs (see Figure 3). The initiation and the peak of S phase were not altered, but the percentage of BrdU-positive cells was lower at 24, 36, and 48 h after PH in Cdk2−/−-regenerating livers compared with wild-type livers (Figure 4, A, B, and E). As a result of this delay, increased numbers of BrdU-positive cells were observed at 72 and 96 h after PH in Cdk2−/− mice (Figure 4, A, B, and E). In response to 11-Gy irradiation and PH, both wild-type and Cdk2−/− mice displayed substantial differences in the initiation of S phase in the regenerating livers. Few BrdU-labeled cells were detected at 24 and 36 h after PH in both genotypes (Figure 4F). The initiation of S phase occurred at 48 h, and the S phase peak was observed at 72 h after PH in wild-type regenerating livers (Figure 4, C and F). In contrast to the irradiated wild-type mice, we detected a reduced number of cells in S phase 48 and 72 h after PH in Cdk2−/− mice, and the resumption of S phase was observed at 96 h (Figure 4, D and F). This observation demonstrates that liver cells are arresting at the G1/S transition during liver regeneration in both wild-type and Cdk2−/− mice. In addition, Cdk2−/− liver cells require additional time (∼24 h more) compared with wild-type liver cells to enter S phase after arresting at the G1/S checkpoint in response to radiation damage. In this context it is important to analyze the consequence of loss of Cdk2 on the activity of the p53-p21 pathway because the G1/S DNA damage checkpoint is maintained by an inhibitory interaction between p21 and Cdk2/cyclin E complexes in wild-type mice and cells.
Figure 4.
Cells arrest at the G1/S checkpoint in vivo in the absence of Cdk2. Representative photographs of immunohistochemical BrdU staining pattern in the regenerating livers of nonirradiated Cdk2+/+ mice (A), nonirradiated Cdk2−/− mice (B), irradiated Cdk2+/+ mice (C), and irradiated Cdk2−/− mice (D) at the indicated time points. Scale bar, 100 μm in all panels. (E) Bar graph showing the percentage of BrdU-positive liver cells at the indicated time points in the regenerating livers of Cdk2+/+ (navy blue) and Cdk2−/− (black) mice after 70% PH. (F) Bar graph indicating the percentage of BrdU-positive liver cells at different time points in the whole body–irradiated and regenerating livers of Cdk2+/+ (navy blue) and Cdk2−/− (black) mice after 70% PH. The average and SDs were calculated after counting a minimum of 2500 nuclei from several low power fields per sample from each genotype. For each time point 3 (24 and 36 h) or 4 (48, 72, and 96 h) Cdk2+/+ and Cdk2−/− mice were used.
Activation of the p53-p21 Pathway in Response to Radiation Damage Is Unaffected by the Loss of Cdk2
The ATM/ATR-mediated activation of p53 and the concomitant induction of p21 is a well-described molecular event in response to radiation-induced DNA damage (for review see Kaufmann, 1995; Morgan and Kastan, 1997; Lukas et al., 2004). Induction of p21 inhibits Cdk2/cyclin E activity, thereby arresting cells at the G1/S checkpoint (Brugarolas et al., 1995; Brugarolas et al., 1999). To explore whether the loss of Cdk2 affects the activation of the p53-p21 pathway, we monitored the timing of induction of p53 and p21 at protein level in irradiated MEFs and in regenerating liver of wild-type and Cdk2−/− mice (Figure 5). We did not detect any significant difference in the timing of induction of p53 between wild-type and Cdk2−/− MEFs after irradiation and serum stimulation (Figure 5B). Even though p53 induction was similar in wild-type and Cdk2−/− cells, it appears that p21 induction was slightly delayed in Cdk2−/− cells (Figure 5B, third panel from top). In contrast to the irradiated and serum-stimulated cells, we detected no significant induction of either p53 or p21 in nonirradiated serum-stimulated cells as expected (Figure 5A, third panel from top). Similar to in vitro observations, we also found activation of the p53 pathway in vivo in response to irradiation and PH. Induced expression of p53 was observed in the regenerating livers of both wild-type and Cdk2−/− mice in response to 11-Gy whole body irradiation (Figure 5D, third panel from top). Increased levels of p21 were observed at 36–96 h after irradiation and PH with similar results for both genotypes (Figure 5D, fourth panel from top). In addition, we detected a slight induction of p21 in the regenerating livers of nonirradiated mice (Figure 5C, fourth panel from top). This is in accordance with the previous results describing the induction of p21 during liver regeneration after PH, which is essential for controlled growth of the remnant liver mass (Albrecht et al., 1997). Furthermore, immunofluorescence staining revealed that the induction and localization of p21 was similar in wild-type and Cdk2−/− cells (Figure 6). After irradiation and serum stimulation, p21 expression was observed between 12 and 30 h in both wild-type and Cdk2−/− cells and was exclusively present in the nucleus (Figure 6, C2–C5 and F2–F5). We did not detect induction of p53 or p21 in the nonirradiated wild-type and Cdk2−/− cells at any time point after serum stimulation alone by immunofluorescence (data not shown). This observation indicates that the loss of Cdk2 does not alter the activation of the p53-p21 pathway in vitro as well as in vivo in response to radiation-induced DNA damage, and the induction of p21 can arrest cells at G1/S transition even in the absence of Cdk2.
Figure 5.
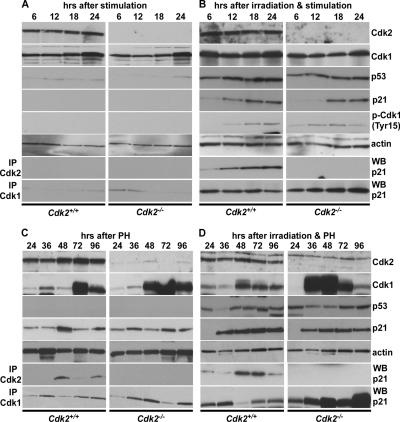
p21 inhibits Cdk1 to arrest cells at the G1/S checkpoint in the absence of Cdk2. Representative Western blots displaying the expression of Cdk2, Cdk1, p53, p21, phospho-Cdk1-(Tyr15), actin (1st to 6th panel from top), and Cdk2-p21 and Cdk1-p21 coimmunoprecipitations (7th and 8th panel) in nonirradiated Cdk2+/+ (left panel) and Cdk2−/− (right panel) MEFs (A) and 5-Gy irradiated Cdk2+/+ (left column) and Cdk2−/− (right column) MEFs (B) and from nonirradiated Cdk2+/+ (left column) and Cdk2−/− (right column) regenerating livers (C) and from irradiated Cdk2+/+ (left column) and Cdk2−/− (right column) regenerating livers (D) at the indicated time points. Actin (5th panel) was used as a loading control.
Figure 6.
Loss of Cdk2 does not influence the induction of p21. Immunofluorescence costaining of Cdk2 and p21 in 5-Gy irradiated and serum-stimulated Cdk2+/+ and Cdk2−/− MEFs at the indicated time points. Cdk2 localization was detected by rabbit anti-Cdk2 antibodies followed by AlexaFluor568 goat anti-rabbit antibodies in Cdk2+/+ cells (red; B1–B5) and in Cdk2−/− cells (red; E1–E5). p21 was detected by mouse anti p21 antibodies followed by AlexaFluor488 goat anti-mouse antibodies in Cdk2+/+ cells (green; C1–C5) and Cdk2−/− cells (green; F1–F5). The nuclei were counterstained with DAPI (blue; A1–A5 and D1–D5). The images were captured with a laser confocal microscope (63×). The representative pictures are from one of three independent stainings. Scale bar, 10 μm in all panels.
p21/Cdk1 Complexes Keep the G1/S DNA Damage Checkpoint Intact
Our present study indicates that Cdk2−/− cells arrest at the G1/S checkpoint in response to irradiation not only in vitro (MEFs in culture) but also in vivo (liver cells during regeneration; Figures 3 and 4). We have described that the p53-p21 pathway is not perturbed in Cdk2−/− cells. A recent study reported that Cdk2−/− cells arrest at the G1/S checkpoint in response to either irradiation or overexpression of p21 (Martin et al., 2005). Nevertheless, the molecular mechanism behind the p21-mediated G1/S arrest in Cdk2−/− cells has not yet been determined. The ability of cells to arrest at the G1/S checkpoint in response to irradiation in the absence of p21's primary target Cdk2 raises the question how are Cdk2−/− cells arrested by p21 and how do they maintain the G1/S DNA damage checkpoint intact? Recent findings that Cdk1 complexes with cyclin E, compensates the functions of Cdk2 in its absence, and drives cells through the G1/S transition (Aleem et al., 2005) suggest that p21 might be able to inhibit Cdk1 and arrest cells at the G1/S checkpoint. To test this hypothesis, first we analyzed the subcellular localization of Cdk1 and p21 in response to irradiation in wild-type and Cdk2−/− MEFs. After irradiation and serum stimulation Cdk1 was predominantly localized in the cytoplasm after 6–24 h in wild-type cells (Figure 7, B1–B4). p21 was detected from 12 h onward and was predominantly present in the nucleus (Figure 7, C2–C5). This indicates that Cdk1 might not be accessible to p21 at the G1/S transition in wild-type cells. On the other hand because of earlier translocation of Cdk1 to the nucleus in Cdk2−/− cells (Figures 1 and 7, E1–E5), both Cdk1 and p21 are present in the nucleus between 12 and 30 h after serum stimulation (Figure 7, E2–E5 and F2–F5). Because the presence of two proteins in the same compartment cannot prove a possible interaction between them, we further analyzed the interaction between Cdk1-p21 both in vitro and in vivo by coimmunoprecipitation experiments.
Figure 7.
Cdk1 and p21 localize to the nucleus in the absence of Cdk2. Immunocytochemical costaining of Cdk1 and p21 in 5-Gy irradiated and serum-stimulated Cdk2+/+ and Cdk2−/− MEFs at the indicated time points. Cdk1 localization was detected by rabbit anti-Cdk1 antibodies followed by AlexaFluor568 goat anti-rabbit antibodies in Cdk2+/+ cells (red; B1–B5) and in Cdk2−/− cells (red; E1–E5). p21 was detected by mouse anti-p21 antibodies followed by AlexaFluor488 goat anti-mouse antibodies in Cdk2+/+ cells (green; C1–C5) and Cdk2−/− cells (green; F1–F5). The nuclei were counterstained with DAPI (blue; A1–A5 and D1–D5). The images were captured with a laser confocal microscope (63×). The representative pictures are from one of three independent stainings. Scale bar, 10 μm in all panels.
Immunoprecipitations revealed that in MEFs p21 was binding to Cdk1 in the absence of Cdk2 between 6 and 24 h after irradiation and serum stimulation (Figure 5B, bottom panel). This time point typically represents the G1 and G1/S transition of the cell cycle after serum starvation and stimulation. In wild-type cells, we found binding of p21 to Cdk1, but the amount of p21 interacting with Cdk1 was decreased compared with the Cdk1-p21 interaction in Cdk2−/− cells, with the expression level of Cdk1 comparable in wild-type and Cdk2−/− cells (Figure 5B, bottom panel). As expected, we did not detect any significant interaction between Cdk1 and p21 in nonirradiated serum-stimulated cells. This is mainly due to the lack of induction of p21 in these cells (Figure 5A, fourth panel from top). When we extended the studies further and explored the same phenomenon in vivo, we found similar p21-Cdk1 interactions during liver regeneration in response to irradiation in Cdk2−/− mice. We did not detect any significant difference in the expression level of Cdk2 in the regenerating nonirradiated and irradiated livers. In both nonirradiated and irradiated livers, premature induction and overexpression of Cdk1 was observed in the absence of Cdk2 (Figure 5C, D, second panel from top), which differed from our results in MEFs. The interaction between Cdk1 and p21 was observed in both conditions (irradiated and nonirradiated) and in both genotypes (Cdk2+/+ and Cdk2−/−), but the amount of p21 binding to Cdk1 was increased in Cdk2−/− cells in response to irradiation compared with wild-type cells (Figure 5C, D, bottom panel). Furthermore we analyzed the inhibitory phosphorylation of Cdk1 (Tyr15) at the G1/S checkpoint in response to irradiation to identify whether this mechanism also contributed to maintain the G1/S checkpoint in Cdk2−/− cells. In response to DNA damage, Chk1 phosphorylates and inhibits Cdc25C, which in turn prevents the dephosphorylation of inhibitory sites in Cdk1, and this pathway was identified to be one of the major mechanisms to arrest cells at the G2 checkpoint (Herzinger et al., 1995; Bunz et al., 1998). In our study, we could not detect significant differences in the inhibitory phosphorylation of Tyr15 of Cdk1 between wild-type and Cdk2−/− cells (Figure 5, A and B). This indicates that the Cdc25C regulated inhibitory phosphorylation of Cdk1 does not play an important role in maintaining the G1/S checkpoint in Cdk2−/− cells. Our study demonstrates that p21 binds to Cdk1 in the absence of Cdk2 to arrest cell cycle progression in response to irradiation. Our immunofluorescence and immunoprecipitation experiments provide evidence that p21 inhibits Cdk1 both in vitro and in vivo, resulting in cells arresting at the G1/S transition in the absence of Cdk2.
Loss of Cdk2 Is Associated with Increased Radiosensitivity and Results in Impaired DNA Repair
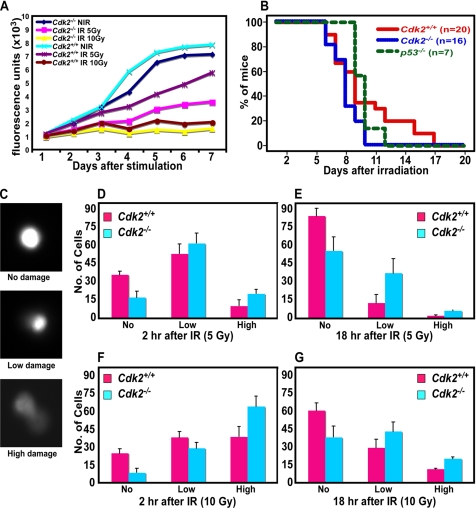
The primary function of the activation of the G1/S checkpoint is to arrest cells transiently in response to DNA damage in order to provide cells sufficient time to repair their DNA and resume DNA replication. It has been previously described that inhibition of Cdk2 by p21 is sufficient to arrest cells in response to different genotoxic stresses until they repair their damaged DNA and resume replication (Poon et al., 1996; Brugarolas et al., 1999). In the present study, we have demonstrated that in the absence of Cdk2, p21 inhibits Cdk1 to arrest cells at the G1/S checkpoint. This raises the question whether Cdk1 inhibition by p21 is sufficient and strong enough to keep the cells in the arrested state until their damaged DNA is repaired? Both in vitro and in vivo cell cycle profiles after irradiation indicate that Cdk2−/− cells are arresting for longer periods of time similar to that of wild-type cells. Surprisingly, we found that Cdk2−/− MEFs as well as liver cells displayed delayed resumption of DNA replication after arresting at the G1/S checkpoint in comparison to wild-type cells (Figures 3 and 4). This observation prompted us to analyze Cdk2−/− cells further for their sensitivity toward radiation damage. Therefore, we measured proliferation rates of wild-type and Cdk2−/− MEFs after 5- and 10-Gy irradiation for longer periods of time (7 d after irradiation and stimulation), which indicates how well the cells are proliferating after entering S phase in response to radiation damage. Our analysis indicated that after 10-Gy irradiation, both wild-type and Cdk2−/− cells exhibited low rates of proliferation (Figure 8A). In contrast, in response to 5-Gy irradiation, there was a significant difference in the proliferation rate between wild-type and Cdk2−/− cells, with wild-type cells proliferating at a higher rate than Cdk2−/− cells (Figure 8A). To identify radiosensitivity at organismal level, wild-type and Cdk2−/− mice were whole body irradiated, and the survival of the animals was monitored. Interestingly, we found that wild-type mice survived longer than Cdk2−/− mice. All Cdk2−/− mice died within 10 d after whole body irradiation, whereas wild-type mice survived to a maximum of 16 d (p = 0.023). Irradiated Cdk2−/− mice died even earlier than p53−/− mice (p = 0.014; Figure 8B). Our in vitro and in vivo analyses indicate that Cdk2−/− MEFs and mice display increased radiosensitivity in response to irradiation.
Figure 8.
Enhanced radiosensitivity in Cdk2−/− cells. (A) Line graph displaying the proliferation rate of Cdk2+/+ and Cdk2−/− nonirradiated and irradiated (5 and 10 Gy) MEFs. (B) Kaplan-Meier survival curve of Cdk2+/+ (n = 20), Cdk2−/− (n = 16), and p53−/− (n = 7) mice in response to 11-Gy whole body irradiation. p53−/− mice were used as a control. (C) Representative photographs of comet migration tails showing high, low, and no damage. Bar graph presenting the number of cells with different level of DNA damage as measured by migration of Comet tails in Cdk2+/+ (red) and Cdk2−/− (blue) cells 2 h after 5-Gy irradiation (D), 18 h after 5-Gy irradiation (E), 2 h after 10-Gy irradiation (F), and 18 h after 10-Gy irradiation (G). For each time point a minimum of 100 nuclei were scored from each sample. Each sample was determined in triplicates, and the averages and SDs were calculated from three independent experiments with three independent batches of MEF cells.
In addition to the analysis at cell and organismal level, measurement of DSBs in individual nuclei by Comet assays (Singh et al., 1988, 1994) revealed that the migration tails were more prominent in Cdk2−/− cells than in wild-type cells (Figure 8, D–G). Two hours after 5-Gy irradiation there were more nuclei with DSBs, as measured by migration of comet tails, in Cdk2−/− than in wild-type cells (Figure 8D). At 18 h after irradiation, a significant number of Cdk2−/− nuclei (∼45%) still displayed low to high levels of DSBs, whereas only about ∼10% of wild-type cells displayed DSBs (Figure 8E). More severe consequences were observed when cells were exposed to 10-Gy irradiation, with more than 90% of Cdk2−/− cells displaying low or high levels of DSBs 2 h after irradiation, and in turn these cells failed to repair their DNA, which was corroborated by the persistence of low or high levels of DSBs even 18 h after irradiation (Figure 8, F and G). In contrast, it appeared that wild-type cells repaired the damaged DNA after irradiation, because ∼60% of the nuclei showed no signs of DNA damage 18 h after 10-Gy irradiation, though initially ∼75% of the cells displayed some levels of damage (Figure 8, F and G). This analysis suggests that the DSBs are more prominent in Cdk2−/− cells after radiation damage, and furthermore these cells are characterized by delayed DNA repair activity.
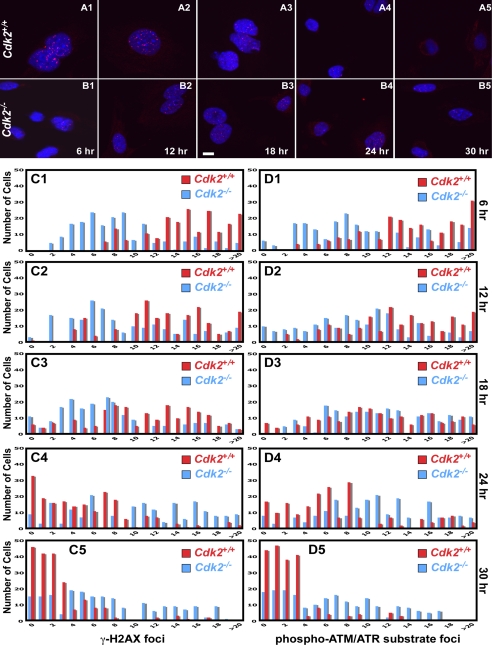
From the above observation, we were tempted to speculate that although Cdk2 is not necessary to maintain the G1/S checkpoint, it might be affecting DNA repair. To explore the hypothesis that Cdk2−/− cells might display impaired DNA repair activity in response to radiation damage, we measured γ-H2AX foci formation after 5-Gy irradiation. Recent studies indicated that several proteins actively participating in DNA repair are activated through the ATM/ATR pathway and form foci at the site of DNA damage (Schultz et al., 2000; Rappold et al., 2001; Celeste et al., 2002; Stewart et al., 2003; Lee et al., 2005). One of these proteins is phophorylated γ-H2AX, which is involved in DSB repair by recruiting several checkpoint and DNA repair proteins to the site of damage, and once the damage is repaired these proteins are cleared from the nucleus (Rappold et al., 2001; Celeste et al., 2002; Stewart et al., 2003). To visualize DNA repair activity in Cdk2−/− and wild-type cells, we analyzed the formation and clearance of γ-H2AX foci by immunofluorescence staining after 5-Gy irradiation and serum stimulation. Our study indicated, that most of the Cdk2−/− cells displayed decreased numbers of nuclear foci compared with wild-type cells between 6 and 18 h after irradiation (Figure 9, A1–A3, B1–B3, and C1–C3). At later time points (24 h after irradiation), the nuclear DNA damage foci started to disappear from wild-type nuclei, and after 30 h the foci were completely cleared from the nuclei (Figure 9, A4–A5 and C4–C5). In contrast to wild-type cells, most of the Cdk2−/− cells displayed fewer γ-H2AX foci for prolonged periods of time, which were not cleared from the nucleus even after 30 h (Figure 9, B4–B5 and C4–C5). In addition to γ-H2AX, we used a polyclonal antibody, which is specific for phosphorylated substrates of ATM/ATR (see Materials and Methods), to analyze the DNA repair activity in Cdk2−/− cells. These immunofluorescence studies revealed an impaired and prolonged appearance of DNA damage foci in Cdk2−/− cells compared with wild-type cells (Figure 9, D1–D5). Therefore, Cdk2 belongs to the small group of proteins that when deleted will affect foci formation in DNA damaged cells.
Figure 9.
Cdk2−/− cells have impaired DNA repair activity. (A and B) Immunofluorescence staining of γ-H2AX in 5-Gy irradiated and serum-stimulated Cdk2+/+ and Cdk2−/− MEFs at the indicated time points. γ-H2AX foci were detected by rabbit anti-γ-H2AX antibodies followed by AlexaFluor568 goat anti-rabbit antibodies in Cdk2+/+ (red; A1–A5) and in Cdk2−/− (red; B1–B5) cells. The nuclei were counter-stained with DAPI and the merged AlexaFluor568 and DAPI pictures were presented (scale bar, 10 μm). Bar graphs (red bars: Cdk2+/+, blue bars: Cdk2−/−) displaying the total number of cells on the Y axis and the number of γ-H2AX foci (C) or phospho-(Ser/Thr) ATM/ATR substrate foci (D) per cell on the X axis at the indicated time point. Number of foci was counted from (single plane images) a minimum of 200 cells per genotype at each time point.
DISCUSSION
Until recently it was thought that Cdk2 is a master regulator of the G1/S transition and S phase progression of the cell cycle. Knockout mouse models and short hairpin RNA experiments have demonstrated that Cdk2 is not necessary for the mitotic cell cycle in mammalian cells (Berthet et al., 2003; Ortega et al., 2003; Tetsu and McCormick, 2003). After extensive investigation, Cdk1 and Cdk4 were identified to compensate for Cdk2 functions (Berthet et al., 2006; Berthet and Kaldis, 2007). It was described that Cdk1/cyclin E complexes drive cells through the G1/S transition in the absence of Cdk2 (Aleem et al., 2005). In wild-type cells, Cdk1 is present predominantly in the cytoplasm during G0, G1, and S phase and is imported to the nucleus with cyclin B1 after nuclear breakdown in mitosis (Bailly et al., 1989, 1992). This timely translocation of Cdk1 to the nucleus poses an additional level of cell cycle regulation because earlier import of Cdk1 to the nucleus would cause premature initiation of mitosis. In this context it was important to investigate how the loss of Cdk2 affects Cdk1 translocation. If Cdk1 substitutes for Cdk2, it needs to be in the nucleus at the time of the G1/S transition; otherwise it cannot execute S phase initiation and progression. Although it was described that Cdk1/cyclin E complexes drive cells through G1/S in the absence of Cdk2 (Aleem et al., 2005), it has not been determined whether Cdk1 translocates to the nucleus at the beginning of S phase in order to perform this function. In this study, we presented evidence that Cdk1 translocates to the nucleus much earlier (before the beginning of S phase) in the absence of Cdk2 in order to execute the G1/S transition. Once Cdk1 is imported into the nucleus it remains localized in the nucleus until the completion of mitosis. Even though Cdk1 is imported into the nucleus much earlier in Cdk2−/− cells compared with wild-type cells, no premature initiation of mitosis is evident as indicated by the cell cycle profiles in Cdk2−/− cells. This could be due to the fact that Cdk1/cyclin E complexes can drive cells through the G1/S transition but unlike Cdk1/cyclin B1 complexes cannot phosphorylate substrates, which are necessary for the initiation of mitosis.
One of the widely accepted concepts in cell cycle progression is that in response to different genotoxic stresses Cdk2 becomes the major target of inhibition at the G1/S checkpoint. In response to several genotoxic insults, ATM/ATR-mediated activation of p53 induces p21, which in turn arrests cells at the G1/S checkpoint by inhibiting its primary target Cdk2 (Brugarolas et al., 1999). In this simplified context, loss of Cdk2 might present an intriguing situation for the induced p21. It was well established that in the absence of p21, cells bypass the G1/S checkpoint (Deng et al., 1995), because the target (Cdk2) is present, but the inhibitor (p21) is absent. What happens if the target (Cdk2) is absent and inhibitor (p21) is present needs to be explored. Therefore, we investigated whether the p53-p21 pathway can be activated or not in the absence of Cdk2. Will the cells bypass the G1/S checkpoint in the absence of Cdk2, similar to p21 deficient cells? Our study revealed that the activation of p53-p21 pathway is not perturbed in the absence of Cdk2. The timing of induction of both p53 and p21 is similar in the absence or presence of Cdk2. In addition, our study indicates that in vitro (MEFs) and in vivo (liver cells during regeneration) cells are arresting promptly at the G1/S checkpoint in the absence of Cdk2 in response to radiation-induced DNA damage. This indicates that p21 is able to arrest cells even in the absence of Cdk2. This observation suggests that either Cdk2 is not the primary target of p21 or alternatively p21 can block cell cycle progression by interacting with molecules other than Cdk2. Assuming the latter possibility, the most likely molecule to compensate for Cdk2 is Cdk1. In accordance with this, we detected premature translocation of Cdk1 to the nucleus, which was not affected by radiation-induced DNA damage. As a result of premature translocation of Cdk1 to the nucleus, both Cdk1 and the induced p21 are present in the nucleus, and as a result Cdk1 is accessible to p21 for inhibition. Because presence of two proteins in the same compartment does not prove a direct interaction between two molecules, we further explored the direct binding of p21 to Cdk1 by coimmunoprecipitations. We detected direct interactions between Cdk1 and p21 at the G1/S checkpoint in response to radiation-induced DNA damage. This indicates that in the absence of Cdk2, Cdk1 is driving the cells through the G1/S transition and the molecular mechanism behind the G1/S checkpoint maintenance in response to irradiation is due to an interaction between Cdk1 and p21 at the G1/S transition. The prime concern here is whether the inhibition of Cdk1 by p21 at the G1/S checkpoint is sufficient and persistent to maintain the cells in the arrested state until they repair their damaged DNA and resume DNA replication. Our cell cycle profiles indicated that Cdk2−/− MEFs as well as the proliferating liver cells after PH in Cdk2−/− mice arrested for prolonged periods of time, similar to that of wild-type in response to irradiation. Surprisingly, we found that in Cdk2−/− mice the resumption of DNA replication took additional 24 h after irradiation compared with wild-type liver cells. Two possible mechanisms could explain this phenomenon: the first is that Cdk2−/− cells accumulated more DSBs in response to radiation-induced DNA damage in comparison to wild-type cells. Another possibility is that Cdk2 might have a role in DNA repair, and as a result Cdk2−/− cells displayed delayed resumption of DNA replication because of impaired repair activity. In accordance with this hypothesis, we found that DSBs are more prominent in Cdk2−/− cells than in wild-type cells in response to radiation-induced DNA damage. Comet assays indicated that Cdk2−/− cells accumulated extensive DNA damage compared with wild-type cells in response to irradiation. In addition, the damage was not repaired for prolonged periods of time in Cdk2−/− cells in comparison to wild-type cells where damaged DNA was mostly repaired. Furthermore, not only at cellular level but also at organismal level, Cdk2−/− mice proved to be radiosensitive, and in turn these mice die earlier than wild-type mice in response to lethal doses of γ-irradiation, similar to that of p53−/− mice. This indicates that Cdk2 might play a role in DSB repair induced by irradiation. Our results agree with a recent report that cyclin A1 functions in DSB repair, and this repair function depends on Cdk2 activity (Müller-Tidow et al., 2004). In addition a recent study suggested that inhibition of Cdk2 by Roscovitine (which also inhibits a number of other Cdks; see Berthet et al., 2007) led to blunted activation of Chk1 due to reduced phosphorylation (Deans et al., 2006). Chk1 is one of the downstream targets of the ATM/ATR pathway whose phosphorylation and activation is one of the necessary steps in the DNA damage signal transduction cascade because activated Chk1 in turn activates and promotes some of the DNA repair proteins to the sites of damage (Westphal, 1997; Hammond et al., 2003; Sancar et al., 2004). From the above studies it appears that Cdk2 might play an important role in more than one DNA repair pathway. In accordance with these studies, we found delayed and impaired DNA repair activity in Cdk2−/− cells. In our study we determined that Cdk2−/− cells do not completely fail to form DNA repair foci (γ-H2AX, ATM/ATR substrates) at the sites of damage, but rather they exhibited lower numbers of foci for prolonged periods of time. This suggests that Cdk2 has a function upstream of the ATM/ATR pathway in addition to the one well-known function downstream. In wild-type cells, large numbers of foci were observed immediately after irradiation, and they are mostly cleared from the nucleus within 24 h after irradiation. In contrast, we found that the formation of foci is delayed in Cdk2−/− cells, and at the same time they exhibited a persistence of lower numbers of foci, which are not cleared even 30 h after irradiation. Two possible mechanisms could explain the impaired repair activity of Cdk2−/− cells. In the presence of Cdk2, the Cdk2/cyclin E or the Cdk2/cyclin A complex promotes the DNA repair function by activating a DNA repair complex, and in the absence of Cdk2 this complex cannot be fully activated, resulting in impaired repair of damaged DNA. Alternatively in the absence of Cdk2, although the formation of Cdk1/cyclin E is able to initiate the G1/S checkpoint, it might not be able to maintain the checkpoint that in turn leads to premature and progressive termination of the checkpoint, including the activation of DNA repair activity. Thus the presence of Cdk1/cyclin E might be interfering with the formation of active DNA repair complexes.
Our study indicates that Cdk1 translocates early to the nucleus to take over the function of Cdk2 in its absence, which in turn is inhibited by p21 in response to DNA damage to maintain the G1/S checkpoint. In addition, we have demonstrated that the presence of Cdk2 is either directly or indirectly influencing the repair activity, and DNA repair is delayed and impaired in the absence of Cdk2 in cells and animals.
ACKNOWLEDGMENTS
We thank Matt McCollum and Angie Smith for animal care; Rodney Wiles for helping with the irradiation; and Donna Butcher, Roberta Smith, and the Pathology/Histotechnology Laboratory for tissue sectioning and staining. We thank Prabhakar Gudla for helping with confocal microscopy. We appreciated the help of the Kaldis lab members for discussions and support. We are thankful to Weimin Li and Kasim Diril for comments on the manuscript. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-06-0525) on October 17, 2007.
REFERENCES
- Adams P. D., Sellers W. R., Sharma S. K., Wu A. D., Nalin C. M., Kaelin W. G. Identification of a cyclin-Cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol. Cell. Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S. A., Gogal R. M., Jr, Walsh J. E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]-thymidine incorporation assay. J. Immunol. Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Albrecht J. H., Meyer A. H., Hu M. Y. Regulation of cyclin-dependent kinase inhibitor p21WAF1/Cip1/Sdi1 gene expression in hepatic regeneration. Hepatology. 1997;25:557–563. doi: 10.1002/hep.510250311. [DOI] [PubMed] [Google Scholar]
- Aleem E., Kiyokawa H., Kaldis P. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 2005;7:831–836. doi: 10.1038/ncb1284. [DOI] [PubMed] [Google Scholar]
- Bailly E., Dorée M., Nurse P., Bornens M. p34Cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989;8:3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly E., Pines J., Hunter T., Bornens M. Cytoplasmic accumulation of cyclin B1 in human cells: association with a detergent-resistant compartment and with the centrosome. J. Cell Sci. 1992;101:529–545. doi: 10.1242/jcs.101.3.529. [DOI] [PubMed] [Google Scholar]
- Berthet C., Aleem E., Coppola V., Tessarollo L., Kaldis P. Cdk2 knockout mice are viable. Curr. Biol. 2003;13:1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Berthet C., Kaldis P. Cell-specific responses to loss of cyclin-dependent kinases. Oncogene. 2007;26:4469–4477. doi: 10.1038/sj.onc.1210243. [DOI] [PubMed] [Google Scholar]
- Berthet C., Klarmann K. D., Hilton M. B., Suh H. C., Keller J. R., Kiyokawa H., Kaldis P. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev. Cell. 2006;10:563–573. doi: 10.1016/j.devcel.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Berthet C., Rodriguez-Galan M. C., Hodge D. L., Gooya J., Pascal V., Young H. A., Keller J., Bosselut R., Kaldis P. Hematopoiesis and thymic apoptosis are not affected by the loss of Cdk2. Mol. Cell. Biol. 2007;27:5079–5089. doi: 10.1128/MCB.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J., Chandrasekaran C., Gordon J. I., Beach D., Jacks T., Hannon G. J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Brugarolas J., Moberg K., Boyd S. D., Taya Y., Jacks T., Lees J. A. Inhibition of cyclin-dependent kinase 2 by p21 is necessary for retinoblastoma protein-mediated G1 arrest after gamma-irradiation. Proc. Natl. Acad. Sci. USA. 1999;96:1002–1007. doi: 10.1073/pnas.96.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunz F., Dutriaux A., Lengauer C., Waldman T., Zhou S., Brown J. P., Sedivy J. M., Kinzler K. W., Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Caspari T., Murray J. M., Carr A. M. Cdc2-cyclin B kinase activity links Crb2 and Rqh1-topoisomerase III. Genes Dev. 2002;16:1195–1208. doi: 10.1101/gad.221402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A., et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurvedi P., et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- Cuddihy A. R., Bristow R. G. The p53 protein family and radiation sensitivity: yes or no? Cancer Metastasis Rev. 2004;23:237–257. doi: 10.1023/B:CANC.0000031764.81141.e4. [DOI] [PubMed] [Google Scholar]
- Deans A. J., Khanna K. K., McNees C. J., Mercurio C., Heierhorst J., McArthur G. A. Cyclin-dependent kinase 2 functions in normal DNA repair and is a therapeutic target in BRCA1-deficient cancers. Cancer Res. 2006;66:8219–8226. doi: 10.1158/0008-5472.CAN-05-3945. [DOI] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988;54:423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Elledge S. J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration. J. Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Hall-Jackson C. A., Cross D. A., Morrice N., Smythe C. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene. 1999;18:6707–6713. doi: 10.1038/sj.onc.1203077. [DOI] [PubMed] [Google Scholar]
- Hammond E. M., Dorie M. J., Giaccia A. J. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J. Biol. Chem. 2003;278:12207–12213. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- Helt C. E., Cliby W. A., Keng P. C., Bambara R. A., O'Reilly M. A. Ataxia telangiectasia mutated (ATM) and ATM and Rad3-related protein exhibit selective target specificities in response to different forms of DNA damage. J. Biol. Chem. 2005;280:1186–1192. doi: 10.1074/jbc.M410873200. [DOI] [PubMed] [Google Scholar]
- Herzinger T., Funk J. O., Hillmer K., Eick D., Wolf D. A., Kind P. Ultraviolet B irradiation-induced G2 cell cycle arrest in human keratinocytes by inhibitory phosphorylation of the cdc2 cell cycle kinase. Oncogene. 1995;11:2151–2156. [PubMed] [Google Scholar]
- Houtgraaf J. H., Versmissen J., van der Giessen W. J. A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovasc. Revasc. Med. 2006;7:165–172. doi: 10.1016/j.carrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Ira G., et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T., Maller J. L. Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol. Biol. Cell. 1993;4:1337–1350. doi: 10.1091/mbc.4.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P., Hardy S., Morgan D. O. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J. Cell Biol. 1998;141:875–885. doi: 10.1083/jcb.141.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann W. K. Cell cycle checkpoints and DNA repair preserve the stability of the human genome. Cancer Metastasis. Rev. 1995;14:31–41. doi: 10.1007/BF00690209. [DOI] [PubMed] [Google Scholar]
- Kountouras J., Boura P., Lygidakis N. J. Liver regeneration after hepatectomy. Hepatogastroenterology. 2001;48:556–562. [PubMed] [Google Scholar]
- Labib K., Craven R. A., Crawford K., Nurse P. Dominant mutants identify new roles for p34cdc2 in mitosis. EMBO J. 1995;14:2155–2165. doi: 10.1002/j.1460-2075.1995.tb07209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. C., Fernandez-Capetillo O., Pisupati V., Jackson S. P., Nussenzweig A. Specific association of mouse MDC1/NFBD1 with NBS1 at sites of DNA-damage. Cell Cycle. 2005;4:177–182. doi: 10.4161/cc.4.1.1354. [DOI] [PubMed] [Google Scholar]
- Li X., Nicklas B. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- Lukas J., Lukas C., Bartek J. Mammalian cell cycle checkpoints: signalling pathways and their organization in space and time. DNA Repair. 2004;3:997–1007. doi: 10.1016/j.dnarep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Maeda T., Chong M. T., Espino R. A., Chua P. P., Cao J. Q., Chomey E. G., Luong L., Tron V. A. Role of p21Waf-1 in regulating the G1 and G2/M checkpoints in ultraviolet-irradiated keratinocytes. J. Invest. Dermatol. 2002;119:513–521. doi: 10.1046/j.1523-1747.2002.01828.x. [DOI] [PubMed] [Google Scholar]
- Martin A., Odajima J., Hunt S. L., Dubus P., Ortega S., Malumbres M., Barbacid M. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27Kip1 and p21Cip1. Cancer Cell. 2005;7:591–598. doi: 10.1016/j.ccr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Mercer W. E. Checking on the cell cycle. J. Cell Biochem. Suppl. 1998;30–31:50–54. [PubMed] [Google Scholar]
- Mittnacht S. Control of pRB phosphorylation. Curr. Opin.Genet. Dev. 1998;8:21–27. doi: 10.1016/s0959-437x(98)80057-9. [DOI] [PubMed] [Google Scholar]
- Moore J. D., Yang J., Truant R., Kornbluth S. Nuclear Import of Cdk/cyclin complexes: identification of distinct mechanisms for import of Cdk2/cyclin E and Cdc2/cyclin B1. J. Cell Biol. 1999;144:213–224. doi: 10.1083/jcb.144.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Morgan S. E., Kastan M. B. p53 and ATM: cell cycle, cell death, and cancer. Adv. Cancer Res. 1997;71:1–25. doi: 10.1016/s0065-230x(08)60095-0. [DOI] [PubMed] [Google Scholar]
- Müller-Tidow C., et al. The cyclin A1-Cdk2 complex regulates DNA double-strand break repair. Mol. Cell. Biol. 2004;24:8917–8928. doi: 10.1128/MCB.24.20.8917-8928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. Cell cycle checkpoints. Curr. Biol. 1994;6:872–876. doi: 10.1016/0955-0674(94)90059-0. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Cimprich K. A. G2 damage checkpoints: what is the turn-on? J. Cell Sci. 2005;118:1–6. doi: 10.1242/jcs.01626. [DOI] [PubMed] [Google Scholar]
- O'Connor P. M. Mammalian G1 and G2 phase checkpoints. Cancer Surv. 1997;29:151–182. [PubMed] [Google Scholar]
- Ortega S., Prieto I., Odajima J., Martin A., Dubus P., Sotillo R., Barbero J. L., Malumbres M., Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- Pan Z. Q., Amin A., Hurwitz J. Characterization of the in vitro reconstituted cyclin A or B1-dependent Cdk2 and Cdc2 kinase activities. J. Biol. Chem. 1993;268:20443–20451. [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem. J. 1995;308:697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon R. Y., Jiang W., Toyoshima H., Hunter T. Cyclin-dependent kinases are inactivated by a combination of p21 and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage. J. Biol. Chem. 1996;271:13283–13291. doi: 10.1074/jbc.271.22.13283. [DOI] [PubMed] [Google Scholar]
- Rappold I., Iwabuchi K., Date T., Chen J. Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways. J. Cell Biol. 2001;153:613–620. doi: 10.1083/jcb.153.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989;57:393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Sancar A., Lindsey-Boltz L. A., Unsal-Kacmaz K., Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Satyanarayana A., Geffers R., Manns M. P., Buer J., Rudolph K. L. Gene expression profile at the G1/S transition of liver regeneration after partial hepatectomy in mice. Cell Cycle. 2004;3:1405–1417. doi: 10.4161/cc.3.11.1212. [DOI] [PubMed] [Google Scholar]
- Satyanarayana A., Wiemann S. U., Buer J., Lauber J., Dittmar K. E., Wustefeld T., Blasco M. A., Manns M. P., Rudolph K. L. Telomere shortening impairs organ regeneration by inhibiting cell cycle re-entry of a subpopulation of cells. EMBO J. 2003;22:4003–4013. doi: 10.1093/emboj/cdg367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz L. B., Chehab N. H., Malikzay A., Halazonetis T. D. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J. Cell Biol. 2000;151:1381–1390. doi: 10.1083/jcb.151.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D., Rotter V. p53-dependent cell cycle control: response to genotoxic stress. Semin. Cancer Biol. 1998;8:325–336. doi: 10.1006/scbi.1998.0095. [DOI] [PubMed] [Google Scholar]
- Shackelford R. E., Kaufmann W. K., Paules R. S. Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ. Health Perspect. 1999;107(Suppl 1):5–24. doi: 10.1289/ehp.99107s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. P., McCoy M. T., Tice R. R., Schneider E. L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Singh N. P., Stephens R. E., Schneider E. L. Modifications of alkaline microgel electrophoresis for sensitive detection of DNA damage. Int. J. Radiat. Biol. 1994;66:23–28. doi: 10.1080/09553009414550911. [DOI] [PubMed] [Google Scholar]
- Stewart G. S., Wang B., Bignell C. R., Taylor A. M., Elledge S. J. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature. 2003;421:961–966. doi: 10.1038/nature01446. [DOI] [PubMed] [Google Scholar]
- Tetsu O., McCormick F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell. 2003;3:233–245. doi: 10.1016/s1535-6108(03)00053-9. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Westphal C. H. Cell-cycle signaling: Atm displays its many talents. Curr. Biol. 1997;7:R789–R792. doi: 10.1016/s0960-9822(06)00406-4. [DOI] [PubMed] [Google Scholar]
- Yang J., Kornbluth S. All aboard the cyclin train: subcellular trafficking of cyclins and their Cdk partners. Trends Cell Biol. 1999;9:207–210. doi: 10.1016/s0962-8924(99)01577-9. [DOI] [PubMed] [Google Scholar]