Abstract
Podosomes are dynamic actin-rich structures composed of a dense F-actin core surrounded by a cloud of more diffuse F-actin. Src performs one or more unique functions in osteoclasts (OCLs), and podosome belts and bone resorption are impaired in the absence of Src. Using Src−/− OCLs, we investigated the specific functions of Src in the organization and dynamics of podosomes. We found that podosome number and the podosome-associated actin cloud were decreased in Src−/− OCLs. Videomicroscopy and fluorescence recovery after photobleaching analysis revealed that the life span of Src−/− podosomes was increased fourfold and that the rate of actin flux in the core was decreased by 40%. Thus, Src regulates the formation, structure, life span, and rate of actin polymerization in podosomes and in the actin cloud. Rescue of Src−/− OCLs with Src mutants showed that both the kinase activity and either the SH2 or the SH3 binding domain are required for Src to restore normal podosome organization and dynamics. Moreover, inhibition of Src family kinase activities in Src−/− OCLs by Src inhibitors or by expressing dominant-negative SrcK295M induced the formation of abnormal podosomes. Thus, Src is an essential regulator of podosome structure, dynamics and organization.
INTRODUCTION
The nonreceptor tyrosine kinase Src plays multiple roles in integrin signaling, in the regulation of the cell cytoskeleton and in cell migration (Sanjay et al., 2001; Frame, 2004; Playford and Schaller, 2004; Alper and Bowden, 2005). Furthermore, Src activation is associated with the reorganization of actin in specific adhesion structures. Indeed, expression of v-Src, the oncogenic form of Src, induces a rearrangement of the actin cytoskeleton characterized by a switch from stress fibers to podosomes in BHK cells (Tarone et al., 1985). Podosomes are adhesion structures found in highly motile cells such as dendritic cells, macrophages, and osteoclasts (Linder and Aepfelbacher, 2003). They are characterized by a dense central column of F-actin, the actin core, which is surrounded by a loosely organized actin meshwork, called the podosome cloud (Collin et al., 2006), which colocalizes with adaptor and regulatory proteins such as Cbl, Pyk2, paxillin, vinculin, integrins, talin, and Src. These surrounding actin fibers have recently been visualized in high-resolution scanning electron microscopy, revealing that they form domes above and below the central actin core (Destaing et al., 2003; Luxenburg et al., 2007). The distribution of specific regulatory proteins between the core and the cloud varies. For instance, Src and the actin regulatory protein dynamin2 are largely absent from the podosome core, whereas the Arp2/3 complex, Wasp, and cortactin are present both within the actin cloud and the podosome core (Ochoa et al., 2000; Pfaff and Jurdic, 2001; Destaing et al., 2003; Bruzzaniti et al., 2005). Despite these differences between the core and the cloud, fluorescence recovery after photobleaching (FRAP) experiments have shown similar rates of actin flux in the core and in the actin cloud (Destaing et al., 2003). Although podosomes contain proteins that are also present at focal adhesion plaques, they differ in their structure and in their highly dynamic nature. Indeed, live cell imaging of green fluorescent protein (GFP)-actin revealed that podosomes undergo cycles of rapid assembly-disassembly with a life span of 2–4 min (Ochoa et al., 2000; Destaing et al., 2003; Luxenburg et al., 2006).
Deletion of Src leads to reduced motility and alterations of the cytoskeleton in osteoclasts (Sanjay et al., 2001). Thus, Src appears to play an essential role in the dynamics and organization of actin in podosomes. Src is a member of a family of nine closely related tyrosine kinases defined by a common domain structure, including a myristoylated amino-terminal domain that targets Src to membranes, which is followed by two different Src homology (SH) protein-binding domains (SH2 and SH3) that mediate the binding of Src to other proteins, and the tyrosine kinase catalytic domain, or SH1. The SH3 domain interacts with proline-rich PXXP motifs, whereas the SH2 domain interacts with phosphorylated tyrosine residues (Pawson and Gish, 1992; Brown and Cooper, 1996). In the inactive state, Src adopts a “closed” conformation that is stabilized by the intramolecular interaction of the SH2 domain with phosphorylated tyrosine 527 (residue numbers refer to chicken Src), and of the SH3 domain with the linker region between the SH2 and the kinase domains (reviewed in Roskoski, 2004). Activation of Src occurs either when tyrosine 527 is dephosphorylated, allowing an “opening” of the molecule or when the intramolecular interaction of the SH2 or SH3 domains are disrupted by intermolecular interactions with Src binding partners. Activation of Src leads to the autophosphorylation of tyrosine 416 in the activation loop of the kinase domain, which is essential for the full tyrosine kinase activity of Src. Thus, Src has both a tyrosine kinase function, which phosphorylates specific tyrosines in Src substrates, and an adaptor function, mediated by its SH2 and SH3 domains, which localizes the tyrosine kinase activity within the proper molecular complexes.
Despite the fact that Src is ubiquitously expressed, the targeted disruption of its gene in mice leads to only one predominant phenotype: osteopetrosis (failure to resorb bone). This phenotype results from a defect in the function of osteoclasts, a cell that expresses high levels of Src (Soriano et al., 1991; Horne et al., 1992; Lowell et al., 1996). Mature multinucleated osteoclasts are formed in Src−/− mice, but these cells do not organize a sealing zone, the F-actin and integrins adhesion structure essential for bone resorption in vivo and in vitro. This defect and the impaired migratory and resorptive functions of Src−/− osteoclasts (Sanjay et al., 2001) suggested a link between Src function and podosome assembly/disassembly. Detachment and respreading experiments that compared Src+/− and Src−/− OCLs showed that the deletion of Src induces a delay in podosome formation (Sanjay et al., 2001). Thus, osteoclasts provide an excellent cellular model to understand the specific function of Src in the regulation of dynamic actin organization in podosomes.
In this study, we have compared podosome organization and actin dynamics in Src+/− and Src−/− OCLs. Our results indicate that Src regulates at least four elements of dynamic podosome organization: formation, structure, life span, and rate of actin flux within podosomes and that both the tyrosine kinase activity and the interactions mediated by SH2 or SH3 domains are required for proper podosome function.
MATERIALS AND METHODS
Reagents
The Src inhibitor PP1 was purchased from Biosource (Invitrogen, Carlsbad, CA). Recombinant human receptor activator of NF-κB ligand (RANK-L) was a gift from Prostrakan (Romainville, France). Mouse macrophage-colony stimulating factor (M-CSF) was purchased from R&D systems (Minneapolis, MN). Anti-phosphotyrosine antibody (P-Tyr-100) was from Cell Signaling Technology (Danvers, MA). Anti-avian Src antibody (clone GD11) and the Src kinase assay kit were obtained from Upstate Biotechnology (Lake Placid, NY). Polyclonal anti-Src antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rhodamine- or Texas Red-phalloidin was purchased from Molecular Probes (Eugene, OR).
Plasmids
pEGFP-Actin Vector was from Clontech (Palo Alto, CA). The avian Src constructs (Src WT, SrcK295M, and SrcK295M/Y527F) were generous gifts from Dr. P. Schwartzberg (National Institutes of Health, Bethesda, MD) and Dr. J. Brugge (Harvard Medical School, Boston, MA). pBKSrcY527F, pBKSrcW118K, and pBKSrcR175L constructs were made using site-directed mutagenesis kit (Stratagene La Jolla, CA) using the following primers: SrcY527F Forward: aca gag ccc cag ttc cag cct gga gag aac; Reverse: gtt ctc tcc agg ctg gaa ctg ggg ctc tat; Src R175L Forward: gga acc ttc ttg gtc ctg gag agc gag acg aca aaa; Reverse: ttt tgt cgt ctc gct ctc cag gac caa gaa ggt tcc; Src W118K Forward: aac aac acg gaa ggt gac aag tgg ctg ctg gct cat tcc; and Reverse: gga atg agc cag cca ctt gtc acc ttc cgt gtt gtt. The mutations were confirmed by sequencing. The cDNA encoding glutathione S-transferase (GST)-conjugated Src SH3 domain, SH2 domain, or both the SH3 and SH2 domains were described previously (Sanjay et al., 2001). GFP-paxillin was a generous gift from Dr K. Rottner (German Research Centre for Biotechnology, Braunschweig, Germany).
Osteoclast Differentiation
Spleen leukocytes were differentiated in vitro in the presence of RANK-L (80 ng/ml) and M-CSF (20 ng/ml) for 7 d to produce a population of osteoclasts in which podosome organization is homogenous and where the comparison and quantification is highly reproducible (Destaing et al., 2003). Spleen leukocytes from 6- to 8-wk-old male mice were purified with lymphocyte separation medium (MP Biomedicals, Solon, OH) following the manufacturer's recommendations. Cells were seeded at 2500 cells/mm2 and were cultured for 8 d on coverslips in differentiation medium (α-MEM medium [Invitrogen, Carlsbad, CA] containing 10% fetal calf serum [FCS; Invitrogen], 20 ng/ml M-CSF, and 80 ng/ml soluble recombinant RANK-L).
Microinjection
Mouse spleen cell-derived osteoclasts were transferred to observation medium (α-MEM without bicarbonate [ref. 11900, Invitrogen] containing 10% fetal calf serum, 20 mM HEPES, 20 ng/ml M-CSF and 20 ng/ml soluble recombinant RANK-L). Microinjection of cDNAs (0.2 mg/ml in water) into one nucleus per osteoclast was performed at room temperature on a IX 71 inverted microscope (Olympus, Tokyo, Japan) using an InjectMan NI2 micromanipulator and an Eppendorf Femtojet microinjector (Hamburg, Germany). After microinjection, cells were maintained at 37°C and 5% CO2 for at least 6 h in differentiation medium before imaging.
Time-Lapse and Confocal Microscopy
Osteoclasts were differentiated in 35-mm glass-bottom Petri dishes and then transferred to observation medium. After microinjection, the dishes were placed on a 37°C heated stage (Carl Zeiss Microimaging, Inc., Thornwood, NY) and cells were imaged with a Zeiss Axiovert 200M containing a 40× (NA 1.0) Zeiss Plan-Apochromat objective and a 63× (NA 1.4) Plan Neo Fluor objective. Meta Imaging Series 6.0 (Universal Imaging, Downington, PA) was used to mount .avi movies from image stacks. Extracted images from stacks were processed with Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA) and Image J (http://rsb.info.nih.gov/ij/). Potential differences of podosome life span were statistically analyzed in Excel by the Student's t test (p < 0.05 was considered significant). At least 500 podosomes in 6–9 cells in 3–5 independent experiments were analyzed for each condition. Significance of the differences between standard deviations was also analyzed in Excel with a “single ANOVA” test or F-test. For immunofluorescence, cells were fixed with 4% para-formaldehyde in phosphate-buffered saline (PBS), pH 7.4, processed as described (Ory et al., 2000), and imaged with a Zeiss LSM Meta using a 63× (NA 1.4) Plan Neo Fluor objective. To ensure that only one fluorochrome was detected at any one time, each channel was imaged sequentially using the multitrack recording module before merging, using the same settings to allow comparisons. FRAP experiments were performed on OCLs prepared as for regular videomicroscopy experiments using the same confocal equipment. For actin cloud analysis, the fluorescence intensity of rhodamine-phalloidin–stained F-actin podosomes and associated actin clouds was quantified using the Image J “3D Surface Plot” plug-in program (created by Kai Uwe Barthel), which expresses pixel intensity as height in three-dimensional plots.
FRAP Quantification
Image analysis was performed with Meta Imaging Series (Universal Imaging). Bleaching time (3.2 s) and bleaching area were the same for all experiments. The fluorescence recovery was measured only within podosome cores that existed during the whole recovery time. Fluorescence intensity at each time point, I(t), was normalized to the starting fluorescence intensity, I(0), (prebleach). To analyze and compare recovery kinetics, FRAP measurements were fitted to a single exponential curve, I(t) = I(0) + k1e−k2t (performed with Igor Pro4.0; WaveMetrics, Lake Oswego, OR as described;Meyvis et al., 1999) to determine the characteristic time of recovery (τ), which is the inverse of k2. Potential differences of characteristic time of recovery have been statistically analyzed in Excel using the Student's t test (p < 0.05 was considered significant).
GST Pulldown Assay
GST fusion proteins were expressed in BL21 cells (Novagen, San Diego, CA) transformed with the pGEX-4-T1 Src constructs. Specifically, 500 ml of culture was induced with 1 mM isopropyl-β-d-thiogalactopyranoside for 3 h. Clarified bacterial lysates containing the fusion proteins were obtained by a standard procedure described elsewhere (Bartkiewicz et al., 1999). The binding of the fusion proteins to glutathione-Sepharose-4B resin (Amersham Biosciences, Piscataway, NJ) was performed in batch-wise manner for 2 h at 4°C, with 0.1% Triton X-100 and 150 mM NaCl included in the buffer to reduce nonspecific binding. The amounts of glutathione-Sepharose–bound purified proteins were evaluated visually on NuPAGE gels (Invitrogen, Carlsbad, CA) stained with Coomassie Brilliant Blue R-250 (Sigma, St. Louis, MO), comparing with a bovine serum albumin standard.
293-VnR cells were lysed in buffer containing 50 mM Tris-Cl, pH 7.5, 1% Triton X-100, and 150 mM NaCl. Clarified lysate protein, 75 μg, was incubated with 3 μg of the desired GST fusion protein bound to glutathione-Sepharose-4B beads (Amersham Biosciences, Piscataway, NJ) for 2 h at 4°C. Beads were washed three times with 1.5 ml of lysis buffer. The samples were then subjected to SDS-PAGE and Western blot analysis to detect Cbl. Before immunostaining, membranes were stained with Ponceau S (Sigma) to verify equal loading of the GST fusion proteins.
Src Immunoprecipitation and In Vitro Kinase Assay
Cell cultures were washed twice with ice-cold PBS and lysed in buffer containing 20 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 0.05% IGEPAL CA-630, 10% glycerol, 10 mM EDTA, 1 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM phenylmethanesulfonyl fluoride. After 30 min on ice, cell lysates were cleared by centrifugation at 12,000 × g for 20 min. Src immunoprecipitation was performed by incubating 500 μg of total cell lysate protein with 2 μg of antibody for 2 h on ice and then adding 20 μl of protein G-agarose. After incubation for 1 h at 4°C with end-over-end mixing, the immune complex was recovered by centrifugation and washed four times with buffer containing 20 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 0.02% IGEPAL CA-630, 10 mM EDTA, 1 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM phenylmethanesulfonyl fluoride. The immunoprecipitates were then subjected to SDS-PAGE and Western blot analysis. In vitro kinase activity was determined using the Src assay kit from Upstate Biotechnology as previously reported (Sanjay et al., 2001). To ensure equal immunoprecipitation of Src, aliquots of beads were subjected to Western blot analysis.
RESULTS
Src Controls Podosome Formation
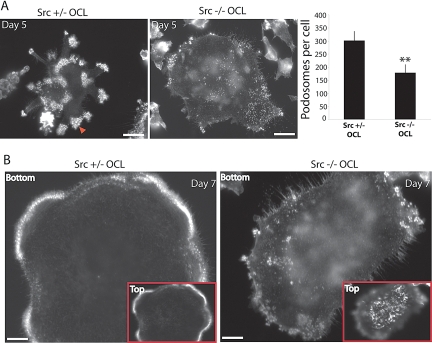
Deleting the Src gene or inhibiting Src activity by chemical inhibitors or by overexpressing kinase-dead, dominant negative Src reduces the bone-resorbing activity and the motility of the osteoclasts. To understand the specific function of Src in podosomes, we examined their organization in Src+/− and Src−/− osteoclasts, visualized by F-actin staining with phalloidin. (No phenotypic difference has been reported between Src+/+ and Src+/− cells and animals [Schwartzberg et al., 1997; Sanjay et al., 2001].) During normal osteoclast differentiation in culture, podosomes exhibit three types of organization: 1) podosome clusters, 2) expanding podosome rings, and 3) stable peripheral podosome belts. Early in the differentiation (day 4–5), podosomes are grouped into clusters which can evolve to form small and expanding dynamic rings. In mature cells, these rings are stabilized at the cell periphery to form a large peripheral belt (Destaing et al., 2003). Despite the absence of Src, Src−/− OCL formed podosomes (Figure 1A). However, podosomes in Src−/− OCLs failed to organize beyond the cluster stage. After 7 d of differentiation in vitro, when podosomes of Src+/− osteoclasts had already formed belts, Src−/− podosomes were less numerous and still organized into clusters (Figure 1B). We therefore used OCLs at day 5 of differentiation, when podosomes are still organized into clusters in both Src+/− and Src−/− OCLs, for further comparisons between Src+/− and Src−/− cells. At this stage, in OCLs with 3–12 nuclei, the number of podosomes was nearly 50% lower in the absence of Src (Figure 1A). In spite of this change, other dynamic actin structures, such as actin ruffles at the top of the cells (Figure 1B), were present in Src−/− OCLs. Thus, Src has a selective effect on the formation and organization of podosomes.
Figure 1.
Src controls podosome formation and actin organization in osteoclasts. (A) F-actin staining with phalloidin revealed that Src−/− OCLs form fewer podosomes. Podosome numbers were determined by counting 20 different cells per condition (cell size normalized to the number of nuclei). Phalloidin staining of Src+/− OCLs revealed the presence of the actin cloud around podosome cores (indicated by red arrow). (B) Src−/− OCLs do not form podosome belts but form actin ruffles at the top of the cell (4 μm above the cell substrate interface), which are absent from Src+/− OCLs (scale bar, 10 μm).
Src Activity Regulates the Formation of the Actin Cloud
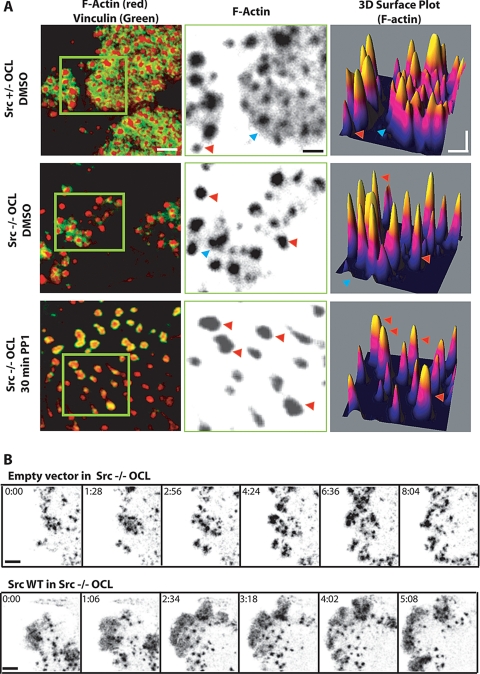
Notwithstanding the reduced number of podosomes and the failure of podosome clusters to reorganize into a belt, and later a sealing zone, the podosomes in the Src−/− OCLs still exhibited the typical actin core surrounded by adhesion molecules such as paxillin and vinculin that colocalized with the actin cloud. However, the extent of the cloud (as visualized with phalloidin) appeared markedly reduced from the extensive intercore staining seen in the Src+/− OCLs (Figure 2A). The density and intensity of phalloidin staining of the podosomes was quantified by confocal microscopy (Figure 2A). In Src+/− OCLs (top panels), the presence of the intense actin cloud in the podosome clusters appears with a broad base in 3D Surface Plot images (blue arrow, Figure 2A). In Src−/− OCLs (middle panels), the actin cloud was reduced (blue arrow) or absent (red arrow), although the podosome cores were as prominent as those of Src+/− cells. These results were confirmed in live Src−/− OCLs and Src−/− OCLs reconstituted with wild-type Src (SrcWT) after transfection with GFP-actin. Re-expression of SrcWT restored normal podosome number and accumulation of GFP-actin into the cloud (Figure 2B). To determine if other Src family members also contributes to podosome organization, Src family kinase activities were inhibited by treating Src−/− OCLs with 10 μM PP1 for 1 h. This led to the formation of mostly single podosomes that consistently lacked the actin cloud (red arrow), suggesting that one or more PP1-sensitive Src family members compensate partially for the absence of Src. The progressive reduction of the actin cloud in Src+/−, Src−/−, and PP1-treated Src−/− OCLs was accompanied by a progressive decrease in vinculin staining surrounding the podosome cores. In PP1-treated cells, vinculin, which normally colocalizes with the actin cloud, was detected within the podosome cores (left panels, Figure 2A). Thus, it appears that the normal formation of the cloud, but not of the core, requires the presence of Src and that at least one other Src family member can partially compensate for the absence of Src.
Figure 2.
Src activity regulates the actin cloud. (A) Left panels, Src+/− osteoclasts, Src−/− osteoclasts, and Src−/− osteoclasts treated with the Src family kinase inhibitor PP1 were fixed and stained for vinculin (green) and F-actin (red). The actin channel (center panels, magnification of areas in green square in left panels) clearly shows the extensive presence of the less dense actin cloud between individual podosome cores in the Src+/− cells. Processing the actin images with the “3D surface plot” plug-in (right panels) revealed many podosomes with an associated cloud (blue arrow) and some without (red arrow). The actin clouds were present but much less extensive in the Src−/− cells, and treatment with PP1 essentially eliminated the clouds. (B) Time series sequence extracted from observation of Src−/− OCLs microinjected with GFP-actin and empty vector (top) or GFP-actin and SrcWT (bottom, scale bar, 3 μm).
Src Regulates Both the Life Span and Rate of Actin Polymerization of Podosomes
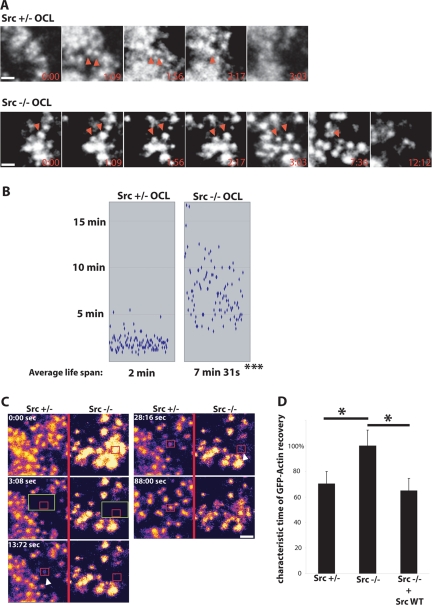
Even though podosome number and the presence of the actin cloud were reduced in the absence of Src, podosomes were still formed in Src−/− OCLs. We hypothesized that the extent of the actin cloud was related to the rate of actin flux within podosomes. To measure both the rate of actin polymerization and the life span of podosomes, nuclei of Src+/− and Src−/− OCLs were microinjected with GFP-actin cDNA, and live cells were imaged on an inverted microscope 6 h later. As shown in Figure 3A, the average podosome life span in Src+/− OCLs was 2 ± 0.8 min, consistent with our previous findings (Destaing et al., 2003). In contrast, the life span of Src−/− podosomes was markedly longer, reaching 7.52 ± 2.7 min (Figure 3B). Moreover, the podosome life span was more heterogeneous in Src−/− OCLs (shown by the increase in SD: 0.8 min for Src+/− podosomes and 2.7 min for Src−/− podosomes, p < 0.01).
Figure 3.
Src regulates podosome life span and the actin flux in podosomes. (A) Time series (in min) extracted from representative observations of Src+/− (top) or Src−/− (bottom) OCLs expressing GFP-actin. Red arrows indicate individual podosomes. The absence of c-Src induced the presence of long-lived podosomes (scale bar, 4 μm). (B) Distribution of podosome life span for Src+/− and Src−/− OCLs. The life spans of a total 500 podosomes were measured in 3–5 independent experiments (*p < 0.001). (C) FRAP analysis was performed on Src+/− and Src−/− OCLs expressing GFP-actin. The change in fluorescence intensity of a podosome (red square) within the bleached area (green square) was quantified over 2 min, and the characteristic time of recovery (τ) was determined. GFP-actin fluorescence recovered faster in the core of the Src+/− podosome (arrow, τ = 13.72 s) than in the core of Src−/− podosomes (arrow, τ = 28.16 s; scale bar 2 μm). (D) 15–20 τ values for Src+/− OCLs and Src−/− OCLs expressing GFP-actin + empty vector or GFP-actin + SrcWT were determined in two independent experiments. Values are presented as a percentage of the average τ of Src−/− podosomes (*p < 0.05).
The turnover of actin within podosomes was studied by FRAP analysis, as previously described (Ochoa et al., 2000; Destaing et al., 2003). Actin incorporation into Src−/− podosome cores was observed, indicating that these structures are indeed true podosomes (Figure 3C). However, the recovery of GFP-actin in Src−/− podosomes was slower (Figure 3C). Although the characteristic time of recovery (τ) of GFP-actin in the podosome core for Src+/− podosomes ranged between 15 and 55 s (average τ = 44 ± 6 s) and was comparable to τ values previously reported, τ values in Src−/− podosomes were 40% greater (average τ = 62 ± 5.5 s). Thus, the absence of c-Src caused the actin flux into the podosome core to decrease. Re-expression of SrcWT in Src−/− OCLs restored a normal τ value (Figure 3D).
Thus, Src regulates several aspects of actin organization and dynamics in podosomes: it stimulates the initial nucleation and the rate of flux of actin into the core, promotes the formation of the actin cloud and the podosome belt, and reduces the life span of individual podosomes, all aspects that may be interrelated. We next sought to determine which domain(s) of Src are involved in these regulatory functions.
Src Kinase Activity Is Essential for the Regulation of Podosome Organization
In addition to its catalytic domain, Src has two protein-binding domains, SH2 and SH3, that function as adaptors in its subcellular targeting and in the regulation of its tyrosine kinase activity. It was suggested that the adaptor function of Src is sufficient to rescue the effect of the absence of Src in osteoclast functions in vivo (Schwartzberg et al., 1997), although a requirement for the kinase activity was later demonstrated (Miyazaki et al., 2004). To determine the relative contributions of the catalytic domain and the SH2 and SH3 domains to podosome regulation, we microinjected cDNA encoding a series of Src mutants (Supplementary Figure 1A) into Src−/− OCLs and determined the ability of each mutant to restore normal podosome formation and life span. Kinase activity was disabled by mutating the catalytic site lysine 295 to methionine (SrcK295M), whereas constitutively active Src was generated by mutating the inhibitory tyrosine 527 to phenylalanine (SrcY527F). A Src mutant harboring both mutations, SrcK295M/Y527F, was also generated. This mutant lacks kinase activity but has SH2 and SH3 binding activity, due to the absence of the intramolecular SH2-pTyr527 interaction (Moarefi et al., 1997; Young et al., 2001). SH2 binding activity was inactivated by mutating arginine 175 to leucine (SrcR175L, as reported in Xing et al., 2001). The SH3 domain was inactivated by mutating tryptophan 118 to lysine (SrcW118K) and the inactivation was confirmed by the loss of binding to Cbl, a major Src binding partner (Supplementary Figure 1B).
The effect of each mutation on the catalytic activity of Src was tested by expressing mutant proteins in 293 VNR cells and monitoring the autophosphorylation of tyrosine 416 (a marker of the intrinsic kinase activity of Src) by Western blot (Supplementary Figure 1C). These results were further confirmed by kinase assays with a peptide substrate on immunoprecipitated Src from transfected 293 VnR cells (Supplementary Figure 1D). The tyrosine kinase activities of SrcK295M and SrcK295M/Y527F were similar to the empty vector control. In contrast, other Src mutants had kinase activities that were significantly greater than the vector control although not all equivalent to SrcWT, reflecting the loss of autoinhibitory intramolecular interactions.
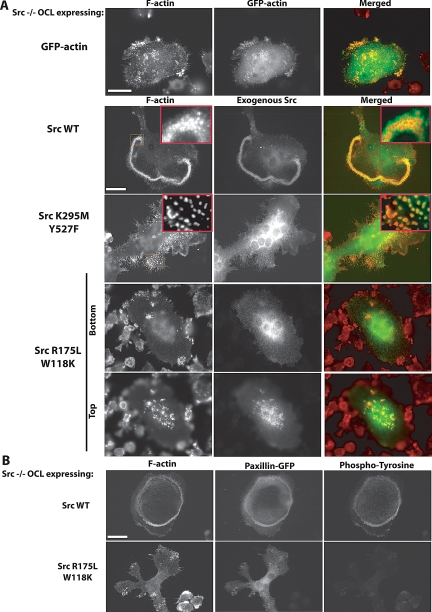
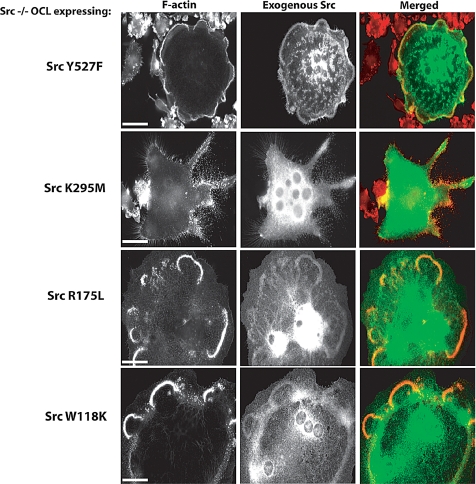
Src mutants were then expressed in Src−/− osteoclasts by intranuclear microinjection of their cDNAs. Three phenotypes were observed. SrcWT or constitutively active SrcY527F increased the number of podosomes and induced the formation of podosome belts, comparable to actin organization in Src+/− OCLs (Figures 4A and 5). In addition, the dorsal actin ruffles characteristic of Src−/− cells were no longer observed (data not shown). In contrast, the expression of SrcK295M (kinase-dead) or SrcK295M/Y527F (kinase-dead, open conformation) not only failed to reduce the Src−/− OCL phenotype, but eliminated the residual actin cloud (Figures 4A and 5), similar to the effect of PP1 treatment on Src−/− OCLs, suggesting that kinase-dead Src mutants act in a dominant-negative manner against other, partially redundant, endogenous Src family kinases.
Figure 4.
Src kinase activity is essential for normal podosome organization. (A) Microinjection of the cDNA coding for GFP-actin did not affect podosome organization in Src−/− OCLs. Re-expression of SrcWT in Src−/− OCLs restored normal podosome organization. In contrast, expression of kinase-dead Src (SrcK295M/Y527F) induced the formation of isolated podosomes with little or no actin cloud (see inset). The Src mutant with both SH2 and SH3 disabled, SrcW118K/R175L, did not restore podosome organization, and was localized in the actin ruffles at the top of the Src−/− OCLs, not around podosomes (scale bar, 10 μm). (B) To determine whether the SrcW118K/R175L mutant can phosphorylate substrates in vivo, Src−/− OCLs were comicroinjected with paxillin-GFP (as a reporter of microinjected cell) and SrcWT or SrcW118K/R175L. After 6 h, cells were fixed and stained for phosphotyrosine. Expression of SrcWT induced an increase of phosphotyrosine within the cell, whereas SrcW118KR175L did not (scale bar, 10 μm).
Figure 5.
Re-expression of SrcY527F, SrcW118K, or SrcR175L in Src−/− OCLs restored normal podosome organization. In contrast, expression of kinase-dead Src (SrcK295M) induced the formation of isolated podosomes (scale bar, 10 μm).
Disabling only the SH2 domain or the SH3 domain had no detectable effects on the ability of Src to restore normal podosome belts. In contrast, a Src mutant that lacked both SH2 and SH3 domain binding activities, but retained at least partial catalytic activity (SrcW118K/R175L), failed to rescue the podosome organization in Src−/− OCLs. As shown by immunofluorescence microscopy, this mutant failed to localize to podosomes, although it still localized to dorsal actin ruffles (Figure 4A), which were not present in cells that overexpressed SrcR175L or SrcW118K (data not shown). To determine the ability of this mutant to phosphorylate substrates in vivo, SrcWT or SrcW118K/R175L and paxillin-GFP (used as a reporter for microinjected cells) were coexpressed in Src−/− OCLs, and the cells were fixed and stained for F-actin and phosphotyrosine (Figure 4B). As expected, the expression of SrcWT increased phosphotyrosine immunoreactivity. SrcW118K/R175L expression, however, did not produce such an increase, despite its ability to phosphorylate a Src substrate peptide.
Collectively, these results indicate that the SH2 and the SH3 domains have critical but redundant functions in targeting Src to the podosome complex. We conclude that the ‘adaptor’ function of Src is required but not sufficient to rescue the podosome phenotype of Src−/− OCLs, and that the SH2 and the SH3 domain of Src are each sufficient to target Src to the podosome complex, thereby allowing the kinase to act in the proper molecular environment.
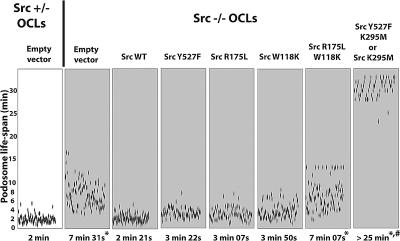
Src Kinase Activity Is Essential to Regulate Podosome Life Span and Structure
Because catalytically active Src with at least one functional binding domain was able to restore normal podosome organization, we asked if the same features regulated podosome life span (Figure 6). Expression of both SrcWT and SrcY527F restored the life span of podosomes to normal (approx. 2–4 min) in Src−/− OCLs. Expression of SrcW118K and SrcR175L also restored a normal life span, confirming that the adaptor functions of the SH2 and SH3 domains are redundant with regard to their effect on podosomes. In contrast, SrcW118K/R175L expression failed to rescue the increased podosome life span of Src−/− OCLs, in agreement with the lack of effect of this construct on podosome formation and intracellular tyrosine phosphorylation. Expression of the two Src kinase-dead mutants not only failed to restore the increased podosome half-life to normal, but actually markedly worsened it (to more than 25 min), mimicking the effect of PP1 treatment on Src−/− podosomes (data not shown) and consistent with results reported by other investigators (Luxenburg et al., 2006). These results further suggest that the dominant-negative Src mutants also antagonize other members of the Src kinase family of proteins that also contribute to podosome regulation.
Figure 6.
Src kinase activity is essential to rescue dynamic podosome properties in Src−/− OCLs. GFP-actin was comicroinjected into Src−/− OCLs with the indicated Src mutant. The life spans of an average of 500 podosomes per condition in 6–9 cells from two to four independent experiments were determined. Expression of SrcWT, SrcY527F, SrcR175L, or SrcW118K restored podosome life span to values comparable to those of podosomes observed in Src+/− OCLs. SrcW118K/R175L did not affect life span of podosomes in Src−/− OCLs. In contrast, expression of Src kinase-dead mutants induced formation of long-lived podosomes, with an average life span greater than 25 min (*p < 0.001 relative to Src+/−; #p < 0.001 relative to Src−/−).
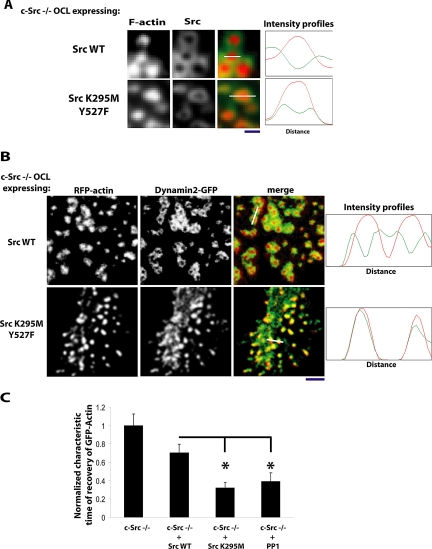
We had observed that inhibiting Src with PP1 resulted in the complete loss of the cloud and the relocalization of vinculin, which normally colocalizes with the cloud, into the outer part of the core (Figure 2B). Because Src also normally localizes with the cloud (Figure 4A, SrcWT insets), the absence of the cloud when the dominant-negative Src mutants were expressed led us to examine the precise localization of these Src mutants. We found that SrcK295M/Y527F colocalized within the outer part of the podosome core (Figure 7A), much as we had observed for vinculin in the PP1-treated cells. We then asked if other molecules, especially actin regulators, were similarly relocalized into the podosome core in response to dominant-negative Src or treatment with PP1 in Src−/− OCLs. We examined dynamin2, a regulator of actin organization that is in the actin cloud (Bruzzaniti et al., 2005; Wheeler et al., 2006). As previously observed, dynamin2 encircled the podosome core in the Src−/− cells (Figure 7B, top panels). In cells coexpressing SrcK295M/Y527F and RFP-actin, dynamin2-GFP no longer formed a ring around the podosomes, but rather colocalized in the core of these long lasting podosomes, confirming the modification of the podosome structure (Figure 7B). Some interpodosome areas still showed dynamin fluorescence, despite the absence of actin fluorescence, indicating that dynamin also associates with other molecules in the region of the podosomes, probably clathrin-containing structures, which we and others have observed in close proximity to podosomes (Luxenburg et al., 2007 and our unpublished observations). This relocalization of dynamin2 into the podosome core led us to determine if the change of podosome structure is associated with a change in net flux of actin into the core. Unexpectedly, FRAP analysis showed that the actin flux was greatly increased in the core of these stable podosomes (Figure 7C). Thus, increased podosome life span is not always correleated with a decreased rate of actin turnover in the podosome, indicating that these two characteristics of podosomes are not directly linked.
Figure 7.
Complete inhibition of Src family kinase activities in Src−/− OCLs induces the collapse of the components of the cloud into the podosome core. (A) Src−/− OCLs were microinjected with cDNAs encoding SrcWT or SrcK295M/Y527F and stained for F-actin (with phalloidin) or Src (anti-avian Src). In the cells expressing SrcWT (top panel), podosome cores were surrounded by a well-formed cloud, and Src staining was restricted to the cloud. In the cells expressing SrcK295M/Y527F(bottom panel), no clouds were detected and Src was localized in the outer part of the podosome cores (scale bar, 1 μm). Right panels, fluorescence intensities of F-actin (red) and Src (green) measured along length of the white line indicated in the merged figures. (B) Src−/− OCLs were injected with cDNAs encoding dynamin2-GFP, RFP-actin, and either SrcWT or SrcK295M/Y527F and fixed. In the cells expressing SrcWT (top panel), podosome cores were surrounded by a well-formed cloud, and dynamin2-GFP fluorescence was largely outside the core. In the cells expressing SrcK295M/Y527F(bottom panel), clouds were not detected and dynamin2-GFP was colocalized with the podosome cores, although it was also present in some areas between cores where no actin was detected (scale bar, 5 μm). Right panels, fluorescence intensities of RFP-actin (red) and dynamin2-GFP (green) measured along length of the white line indicated in the merged figures. (C) Fluorescence recovery was normalized and quantified by measuring the characteristic time of recovery (τ). GFP-actin fluorescence recovers extremely rapidly in the core of long-lasting podosomes. τ values were measured in two independent experiments for Src−/− OCLs expressing GFP-actin + empty vector, or GFP-actin + SrcWT, GFP-actin + SrcK295M/Y527F, or GFP-actin + empty vector treated with PP1 10 μM (p < 0.05).
DISCUSSION
Although a link between catalytically active Src and the formation of podosomes has been established (Tarone et al., 1985; Gavazzi et al., 1989; Ochoa et al., 2000; Sanjay et al., 2001; Miyazaki et al., 2004; Luxenburg et al., 2006), the precise role of Src in podosome structure, formation, maintenance, and disassembly has not been systematically investigated. In the present study, we have used Src−/− osteoclasts to investigate the role(s) of c-Src in regulating podosome dynamics. We found that Src−/− OCLs have fewer podosomes than normal cells and that these podosomes have a longer life span, suggesting that Src promotes both the initiation of podosome formation and podosome disassembly. In addition, the rate of constitutive actin flux within the podosome and the amount of actin cloud surrounding the podosome cores are decreased when Src is absent. Src is also required for the organization of podosomes into the peripheral belt, as previously reported (Lakkakorpi et al., 2001; Sanjay et al., 2001; Miyazaki et al., 2004; Luxenburg et al., 2006).
Src's regulation of all of these podosome properties is dependent on Src tyrosine kinase activity, because expressing kinase-dead Src mutants in Src−/− OCLs did not restore normal podosome organization and dynamics. Thus, Src's adaptor function alone is not able to restore the altered properties of podosomes in Src−/− OCLs, consistent with genetic (Xing et al., 2001) and in vitro analysis (Miyazaki et al., 2004) showing that osteoclast function requires Src kinase activity. Our current findings now demonstrate that Src kinase activity is required, at least in part, to regulate podosome assembly and life span and the turnover of actin within the podosome. However, although the kinase activity of Src is essential, it is not sufficient, as shown by the failure of the SrcW118K/R175L mutant, in which both the SH2 and SH3 binding domains are disabled, to restore normal podosome organization and dynamics. Both the SH2 and the SH3 domains mediate the proper intracellular localization of Src, and either is sufficient to properly target Src to podosomes, whereas the myristoylated amino-terminal domain of Src, which functions as a membrane targeting domain, is not. The ability of the SH2-disabled SrcR175L to restore the podosome life span to normal appears to be inconsistent with an earlier report (Luxenburg et al., 2006) that disabling the SH2 domain abolishes the dominant-negative effect of Src251 on podosome life span. This apparent inconsistency may be due to the different experimental design (we examined the rescue of the Src−/− phenotype and the other report examined the effect of a dominant-negative Src). It may also be related to the reported unique role of the SH2 domain in localizing Src to focal adhesions (Yeo et al., 2006). The ability of both the SH2 and the SH3 domain to properly target Src in the absence of the other domain suggests either 1) that there are two (or more) independent binding sites in the podosome complex that bind Src and are individually sufficient to ensure adequate localization of the kinase activity or 2) that the critical substrate of Src in the regulation of podosome function is a protein that can bind independently to Src SH2 and SH3 domains. Two examples of such proteins are Cbl, which is phosphorylated by Src and regulates osteoclast bone resorption activity (Tanaka et al., 1996; Sanjay et al., 2001; Miyazaki et al., 2004) and AFAP-110 (actin filament-associated protein of 110 kDa), which can activate Src kinase activity, disorganize stress fibers and induce podosome formation (Baisden et al., 2001; Gatesman et al., 2004).
Overexpressing the dominant-negative Src proteins (SrcK295M, SrcY527F/K295M) or treating with the Src family kinase inhibitor PP1 further increased podosome life span, consistent with an earlier report (Luxenburg et al., 2006) and further decreased the extent of the cloud in the Src−/− OCLs, indicating that other Src family kinase activities are able to contribute to the regulation of these aspects of normal podosome function. Most interestingly, deleting Src and overexpressing SrcK295M had opposite effects on the rate of actin flux in the podosome, decreasing and increasing, respectively, the rate relative to that observed in the Src+/− osteoclasts. This suggests that the dominant-negative Src is interfering with the functions of proteins that oppose the effect of Src on actin turnover within the podosome. These are most likely other Src family kinases, but we cannot rule out the possibility that they are other SH2- or SH3-containing proteins with binding specificities similar to Src.
Recent studies have shown that the podosome core is surrounded by a diffuse accumulation of actin filaments, termed the actin cloud (Destaing et al., 2003; Collin et al., 2006; Luxenburg et al., 2007). The function of the actin cloud is unclear, but differences in physical characteristics of the extracellular matrix correlate with changes in the density of actin filaments within the cloud (Collin et al., 2006), suggesting that the cloud may play a role in the mutual regulation of adhesion and the cytoskeleton. The combination of high-resolution scanning electron microscopy with fluorescence microscopy on individual and organized podosomes demonstrated that the cloud is composed of a dome-like radial network of less densely packed fibers that extends from the podosome core and appears to anchor it to the membrane (Luxenburg et al., 2007). We have now found that the extent of the cloud is markedly reduced in Src−/− osteoclasts and that Src kinase activity is required to restore the actin cloud to normal, in agreement with the observation that Src (Figure 4; Luxenburg et al., 2006), other Src family members (Poincloux et al., 2006), and Src-binding partners (Szymkiewicz et al., 2004; Bruzzaniti et al., 2005) are present mainly around, rather than within, the podosome core.
Interestingly, the loss of the actin cloud is progressively induced by first deleting Src and then treating with the Src family kinase inhibitor or expressing the dominant-negative Src. This actin cloud reduction appears to be more the retraction of the cloud material into the podosome core than the disassembly of the actin filaments and associated proteins in the cloud. Using confocal microscopy to compare the relative locations of the intense F-actin signal of the core with several proteins that are normally most highly localized in the cloud and relatively excluded from the core demonstrated that the cloud-associated proteins (vinculin, Src, dynamin2) remained associated with the podosome and became localized within the punctate actin signal (Figures 2 and 7). This suggests that the actin cytoskeletal complexes in the cloud are not disassembled when Src and other Src family kinases are absent or inhibited, but rather assume a more dense packing characteristic of the podosome core.
Although all of the individual properties of podosomes that we monitored were regulated in part by Src, it appears that these several functions are at least somewhat independent of each other and are regulated by different mechanisms. Thus, we found that the turnover of actin within the podosomes was slowed by eliminating Src, but increased by the Src family kinase inhibitor and the dominant-negative SrcK295M, even though all three treatments increased podosome life span, albeit to differing degrees. In a similar vein, deleting Pyk2, the focal adhesion kinase that complexes with Src and Cbl downstream of αvβ3 integrin activation (Sanjay et al., 2001; Miyazaki et al., 2004), slowed the turnover of actin within the podosome, similar to the effect of deleting Src, but had little effect on the podosome life span (Gil-Henn et al., 2007).
The key Src substrates in the mechanisms that control podosome dynamics and organization remain to be identified. We considered Cbl to be an excellent candidate for being a critically important downstream effector of Src in the regulation of podosome function, given that Cbl is required for normal osteoclast motility (Sanjay et al., 2001), that the recruitment of phosphatidylinositol 3-kinase by Cbl is reported to play a critical role in macrophage motility (Meng and Lowell, 1998), and that mutating the Src-phosphorylated tyrosine residue that serves as the binding site for phosphatidylinositol 3-kinase creates a protein (CblY731F) that has a potent dominant-negative effect on in vitro osteoclast bone resorbing activity (Miyazaki et al., 2004). However, we saw no effect of overexpressing CblY731F on podosome dynamics, indicating that at least this Src-catalyzed phosphorylation event is not coupling to the regulation of dynamic podosome function.
Luxenburg et al. (2006) have proposed that the main signaling pathway controlling podosome life span involves the Src-catalyzed phosphorylation of cortactin. Indeed, others have shown that Src-catalyzed phosphorylation of cortactin decreases cortactin's ability to stimulate actin polymerization (Huang et al., 1997; Martinez-Quiles et al., 2004), and depleting cortactin in osteoclasts causes the loss of podosomes (Tehrani et al., 2006). Cortactin that lacks the identified Src-phosphorylated tyrosines is unable to substitute for wild-type cortactin (Tehrani et al., 2006), but overexpressing this mutated cortactin appears to reduce podosome life span (Luxenburg et al., 2006), the opposite of what would be expected if Src were directly regulating podosome life span by phosphorylating cortactin.
There are a number of other potential downstream effectors of Src in regulating podosome function, including the actin regulatory proteins N-Wasp (Torres and Rosen, 2003; Park et al., 2005) and dynamin2 (Ochoa et al., 2000; Werbonat et al., 2000). Src can also modulate actin polymerization by regulating the interaction of the PI(4,5)P2-synthesizing enzyme PIPKI γ-90 with talin (Lee et al., 2005). In addition, Src interacts with formin homology (FH) proteins or formins, which have been implicated in actin nucleation independently of Arp2/3 (Wallar and Alberts, 2003). The formin mDIA2 is expressed in osteoclasts and regulates podosome organization (Destaing et al., 2005). Src binds to the FH1 region of the formins (Uetz et al., 1996; Tominaga et al., 2000; Gasman et al., 2003) and expression of kinase-dead or constitutively active Src mutants disrupts mDIA-mediated signaling (Tominaga et al., 2000). Finally, Src can regulate actin polymerization by activating other signaling pathways. It has been shown that Src regulates the formation and dynamics of filopodia, an actin-dependent structure, in growth cones by activating a Cdc42-PAK pathway (Robles et al., 2005). Determining which of these or other Src substrates mediate the effects of Src on the various properties of podosomes and the mechanisms by which the Src substrate proteins act will be an important goal of future research.
In conclusion, our study demonstrates that Src regulates several dynamic properties of podosomes: initial podosome formation, podosome life span, the turnover of actin within the podosomes and the extent of the actin cloud that surrounds the podosome core. Src kinase activity is essential but the proper SH2- and/or SH3-dependent localization of Src to podosomes is also required for the normal regulation of these dynamic podosome properties. These results provide further insights into the structure of the podosome and the contribution of c-Src to the regulation of podosome and actin dynamics.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to K. Ford for helping with animal care. This work was supported by grants from the National Institutes of Health to R.B. (AR42927, AR54450) and P.D.C. (NS36251, CA46128, DK45735, DA018343).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-03-0227) on October 31, 2007.
REFERENCES
- Alper O., Bowden E. T. Novel insights into c-Src. Curr. Pharm. Des. 2005;11:1119–1130. doi: 10.2174/1381612053507576. [DOI] [PubMed] [Google Scholar]
- Baisden J. M., Gatesman A. S., Cherezova L., Jiang B.-H., Flynn D. C. The intrinsic ability of AFAP-110 to alter actin filament integrity is linked with its ability to also activate cellular tyrosine kinases. Oncogene. 2001;20:6607–6616. doi: 10.1038/sj.onc.1204802. [DOI] [PubMed] [Google Scholar]
- Bartkiewicz M., Houghton A., Baron R. Leucine zipper-mediated homodimerization of the adaptor protein c-Cbl. A role in c-Cbl's tyrosine phosphorylation and its association with epidermal growth factor receptor. J. Biol. Chem. 1999;274:30887–30895. doi: 10.1074/jbc.274.43.30887. [DOI] [PubMed] [Google Scholar]
- Brown M. T., Cooper J. A. Regulation, substrates and functions of src. Biochim. Biophys. Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- Bruzzaniti A., Neff L., Sanjay A., Horne W. C., De Camilli P., Baron R. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol. Biol. Cell. 2005;16:3301–3313. doi: 10.1091/mbc.E04-12-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin O., Tracqui P., Stephanou A., Usson Y., Clement-Lacroix J., Planus E. Spatiotemporal dynamics of actin-rich adhesion microdomains: influence of substrate flexibility. J. Cell Sci. 2006;119:1914–1925. doi: 10.1242/jcs.02838. [DOI] [PubMed] [Google Scholar]
- Destaing O., Saltel F., Geminard J. C., Jurdic P., Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O., Saltel F., Gilquin B., Chabadel A., Khochbin S., Ory S., Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell Sci. 2005;118:2901–2911. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- Frame M. C. Newest findings on the oldest oncogene; how activated src does it. J. Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- Gasman S., Kalaidzidis Y., Zerial M. RhoD regulates endosome dynamics through Diaphanous-related Formin and Src tyrosine kinase. Nat. Cell Biol. 2003;5:195–204. doi: 10.1038/ncb935. [DOI] [PubMed] [Google Scholar]
- Gatesman A., Walker V. G., Baisden J. M., Weed S. A., Flynn D. C. Protein kinase Cα activates c-Src and induces podosome formation via AFAP-110. Mol. Cell Biol. 2004;24:7578–7597. doi: 10.1128/MCB.24.17.7578-7597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi I., Nermut M. V., Marchisio P. C. Ultrastructure and gold-immunolabelling of cell-substratum adhesions (podosomes) in RSV-transformed BHK cells. J. Cell Sci. 1989;94:85–99. doi: 10.1242/jcs.94.1.85. [DOI] [PubMed] [Google Scholar]
- Gil-Henn H., et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2−/− mice. J. Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne W. C., Neff L., Chatterjee D., Lomri A., Levy J. B., Baron R. Osteoclasts express high levels of pp60c-src in association with intracellular membranes. J. Cell Biol. 1992;119:1003–1013. doi: 10.1083/jcb.119.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Tandon N. N., Greco N. J., Ni Y., Wang T., Zhan X. Proteolysis of platelet cortactin by calpain. J. Biol. Chem. 1997;272:19248–19252. doi: 10.1074/jbc.272.31.19248. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi P. T., Nakamura I., Young M., Lipfert L., Rodan G. A., Duong L. T. Abnormal localisation and hyperclustering of αVβ3 integrins and associated proteins in Src-deficient or tyrphostin A9-treated osteoclasts. J. Cell Sci. 2001;114:149–160. doi: 10.1242/jcs.114.1.149. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Voronov S., Letinic K., Nairn A. C., Di Paolo G., De Camilli P. Regulation of the interaction between PIPKIγ and talin by proline-directed protein kinases. J. Cell Biol. 2005;168:789–799. doi: 10.1083/jcb.200409028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S., Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Lowell C. A., Niwa M., Soriano P., Varmus H. E. Deficiency of the Hck and Src tyrosine kinases results in extreme levels of extramedullary hematopoiesis. Blood. 1996;87:1780–1792. [PubMed] [Google Scholar]
- Luxenburg C., Parsons J. T., Addadi L., Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J. Cell Sci. 2006;119:4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- Luxenburg C., Geblinger D., Klein E., Anderson K., Hanein D., Geiger B., Addadi L. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS ONE. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Quiles N., Ho H.-Y. H., Kirschner M. W., Ramesh N., Geha R. S. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol. Cell Biol. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Lowell C. A. A β1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 1998;17:4391–4403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyvis T.K.L., De Smedt S. C., Van Oostveldt P., Demeester J. Fluorescence recovery after photobleaching: a versatile tool for mobility and interaction measurements in pharmaceutical research. Pharm. Res. 1999;16:1153–1162. doi: 10.1023/a:1011924909138. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Sanjay A., Neff L., Tanaka S., Horne W. C., Baron R. Src kinase activity is essential for osteoclast function. J. Biol. Chem. 2004;279:17660–17666. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- Moarefi I., LaFevre-Bernt M., Sicheri F., Huse M., Lee C.-H., Kuriyan J., Miller W. T. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- Ochoa G.-C., et al. A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory S., Munari-Silem Y., Fort P., Jurdic P. Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J. Cell Sci. 2000;113:1177–1188. doi: 10.1242/jcs.113.7.1177. [DOI] [PubMed] [Google Scholar]
- Park S. J., Suetsugu S., Takenawa T. Interaction of HSP90 to N-WASP leads to activation and protection from proteasome-dependent degradation. EMBO J. 2005;24:1557–1570. doi: 10.1038/sj.emboj.7600586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992;71:359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Pfaff M., Jurdic P. Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin αvβ3. J. Cell Sci. 2001;114:2775–2786. doi: 10.1242/jcs.114.15.2775. [DOI] [PubMed] [Google Scholar]
- Playford M. P., Schaller M. D. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Poincloux R., Vincent C., Labrousse A., Castandet J., Rigo M., Cougoule C., Bordier C., Le Cabec V., Maridonneau-Parini I. Re-arrangements of podosome structures are observed when Hck is activated in myeloid cells. Eur. J. Cell Biol. 2006;85:327–332. doi: 10.1016/j.ejcb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Robles E., Woo S., Gomez T. M. Src-dependent tyrosine phosphorylation at the tips of growth cone filopodia promotes extension. J. Neurosci. 2005;25:7669–7681. doi: 10.1523/JNEUROSCI.2680-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr Src protein-tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun. 2004;324:1155–1164. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- Sanjay A., Houghton A., Neff L., Didomenico E., Bardelay C., Antoine E., Levy J., Gailit J., Bowtell D., Horne W. C., Baron R. Cbl associates with Pyk2 and Src to regulate Src kinase activity, αvβ3 integrin-mediated signaling, cell adhesion, and osteoclast motility. J. Cell Biol. 2001;152:181–195. doi: 10.1083/jcb.152.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P. L., Xing L., Hoffmann O., Lowell C. A., Garrett L., Boyce B. F., Varmus H. E. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 1997;11:2835–2844. doi: 10.1101/gad.11.21.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Szymkiewicz I., Destaing O., Jurdic P., Dikic I. SH3P2 in complex with Cbl and Src. FEBS Lett. 2004;565:33–38. doi: 10.1016/j.febslet.2004.03.100. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Amling M., Neff L., Peyman A., Uhlmann E., Levy J. B., Baron R. c-Cbl is downstream of c-Src in a signalling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- Tarone G., Cirillo D., Giancotti F. G., Comoglio P. M., Marchisio P. C. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp. Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Tehrani S., Faccio R., Chandrasekar I., Ross F. P., Cooper J. A. Cortactin has an essential and specific role in osteoclast actin assembly. Mol. Biol. Cell. 2006;17:2882–2895. doi: 10.1091/mbc.E06-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T., Sahai E., Chardin P., McCormick F., Courtneidge S. A., Alberts A. S. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol. Cell. 2000;5:13–25. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- Torres E., Rosen M. K. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol. Cell. 2003;11:1215–1227. doi: 10.1016/s1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Uetz P., Fumagalli S., James D., Zeller R. Molecular interaction between limb deformity proteins (formins) and Src family kinases. J. Biol. Chem. 1996;271:33525–33530. doi: 10.1074/jbc.271.52.33525. [DOI] [PubMed] [Google Scholar]
- Wallar B. J., Alberts A. S. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 2003;13:435–446. doi: 10.1016/s0962-8924(03)00153-3. [DOI] [PubMed] [Google Scholar]
- Werbonat Y., Kleutges N., Jakobs K. H., van Koppen C. J. Essential role of dynamin in internalization of M2 muscarinic acetylcholine and angiotensin AT1A receptors. J. Biol. Chem. 2000;275:21969–21974. doi: 10.1074/jbc.M001736200. [DOI] [PubMed] [Google Scholar]
- Wheeler A. P., Smith S. D., Ridley A. J. CSF-1 and PI 3-kinase regulate podosome distribution and assembly in macrophages. Cell Motil. Cytoskelet. 2006;63:132–140. doi: 10.1002/cm.20111. [DOI] [PubMed] [Google Scholar]
- Xing L., Venegas A. M., Chen A., Garrett-Beal L., Boyce B. F., Varmus H. E., Schwartzberg P. L. Genetic evidence for a role for Src family kinases in TNF family receptor signaling and cell survival. Genes Dev. 2001;15:241–253. doi: 10.1101/gad.840301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo M. G., Partridge M. A., Ezratty E. J., Shen Q., Gundersen G. G., Marcantonio E. E. Src SH2 arginine 175 is required for cell motility: specific focal adhesion kinase targeting and focal adhesion assembly function. Mol. Cell. Biol. 2006;26:4399–4409. doi: 10.1128/MCB.01147-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. A., Gonfloni S., Superti-Furga G., Roux B., Kuriyan J. Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell. 2001;105:115–126. doi: 10.1016/s0092-8674(01)00301-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.