Abstract
Shiga toxin (Stx) binds to the cell, and it is transported via endosomes and the Golgi apparatus to the endoplasmic reticulum and cytosol, where it exerts its toxic effect. We have recently shown that Stx activates the tyrosine kinase Syk, which in turn induces clathrin phosphorylation and up-regulates Stx uptake. Here, we show that toxin-induced signaling can also regulate another step in intracellular Stx transport. We demonstrate that transport of Stx to the Golgi apparatus is dependent on the mitogen-activated protein kinase p38. Treatment of cells with chemical inhibitors or small interfering RNA targeting p38 inhibited Stx transport to the Golgi and reduced Stx toxicity. This p38 dependence is specific to Stx, because transport of the related toxin ricin was not affected by p38 inhibition. Stx rapidly activated p38, and recruited it to early endosomes in a Ca2+-dependent manner. Furthermore, agonist-induced oscillations in cytosolic Ca2+ levels were inhibited upon Stx stimulation, possibly reflecting Stx-dependent local alterations in cytosolic Ca2+ levels. Intracellular transport of Stx is Ca2+ dependent, and we provide evidence that Stx activates a signaling cascade involving cross talk between Ca2+ and p38, to regulate its trafficking to the Golgi apparatus.
INTRODUCTION
Shiga toxin (Stx) is composed of a cell-binding B-moiety and an enzymatically active A-subunit. The toxin binds to the target cell, and it is subsequently taken up by endocytosis. It is then transported via early endosomes, and the Golgi apparatus to the endoplasmic reticulum (ER), from where it retrotranslocates to its final destination, the cytosol. The toxic effect of Shiga is to inactivate ribosomes and thus inhibit protein synthesis.
It is now accepted, in the case of hormone receptors, that ligand-binding induced changes in receptor structure can stimulate an intrinsic kinase activity or an associated kinase. The signaling cascade induced by receptor stimulation can also regulate endocytosis (Gonzalez-Gaitan and Stenmark, 2003; Polo and Di Fiore, 2006). The importance of kinase-mediated signaling in endocytosis and intracellular transport has been demonstrated by a genome-wide analysis (Pelkmans et al., 2005). Another important finding was that p38 is able to regulate formation of the Rab5–guanine nucleotide dissociation inhibitor (GDI) complex (Cavalli et al., 2001). Furthermore, the Rab5 effectors Rabenosyn-5 and early endosome antigen 1 (EEA1) are substrates for p38 (Mace et al., 2005). Interestingly, mitogen-activated protein (MAP) kinases are known to associate with endosomes (Pol et al., 1998), and this has been demonstrated for p38 as well. p38 has been purified with the endosome fraction in a sucrose gradient (Delcroix et al., 2003), and it also has been observed on endosomal structures, by confocal microscopy (Pelkmans et al., 2005). Recently, it was demonstrated that p38 can be recruited to a signalosome involved in the regulation of the platelet-activating factor-induced clathrin-mediated endocytosis (McLaughlin et al., 2006). Taken together, p38 can be localized on endosomes to phosphorylate downstream effectors from here.
Several studies have shown that Stx, upon binding or entry into cells, is able to trigger signaling cascades (Katagiri et al., 1999; Ikeda et al., 2000; Mori et al., 2000; Cameron et al., 2003; Takenouchi et al., 2004). However, the main focus has been on Stx-induced apoptosis and signaling related to this late event. Interestingly, not only the tyrosine kinases have been shown to be activated upon Stx intoxication (Foster and Tesh, 2002; Smith et al., 2003). However, such kinases are mostly activated upon ribotoxic stress, a cellular response that occurs as a consequence of toxin-induced ribosomal inactivation in the cytosol. We have previously presented data demonstrating that Stx is an active player in its own transport. We have shown that Stx binding activates the tyrosine kinase Syk, and several proteins, one of these proteins being clathrin heavy chain (CHC), are phosphorylated (Lauvrak et al., 2006). For Stx, which binds to a glycolipid and not to a transmembrane protein receptor, one may ask what triggers a signaling cascade in the cytosol. So far, this is not known. However, signaling is not only caused by phosphorylation of receptors but also Ca2+ currents can mediate downstream cytosolic phosphorylations. It has been known for two decades that divalent ions are able to modulate Stx trafficking (Sandvig and Brown, 1987) and that in Madin Darby canine kidney cells, ricin and, to a lesser extent, Stx transport is sensitive to interference of Ca2+ homeostasis (Lauvrak et al., 2002). Furthermore, early cellular events that might precede phosphorylation cascades were detected in B-cells stimulated by Stx. Taga et al. (1997) have shown that exposing Burkitt's lymphoma cells to Stx triggers a Ca2+ influx. These events were, however, linked to apoptotic signaling rather than regulation of transport (Cherla et al., 2003). A link between Ca2+ and p38 activation has been proposed in Vero cells (Ikeda et al., 2000). However, in this study, p38 activation was dependent on entry of the active A subunit, thus presumably caused by ribotoxic stress.
In the present study, we have investigated the importance of the MAP kinase p38 and Ca2+ in the regulation of Stx transport. We show that p38 is rapidly activated by Stx and that its activity is required for transport of Stx from endosomes to the Golgi apparatus. p38-regulated trafficking seems to be specific to Stx, because transport of the related toxin ricin was insensitive to p38 inhibition. In addition, Stx is able to modulate oscillations in Ca2+ levels caused by the addition of ATP or histamine, suggesting a link between Stx trafficking and Ca2+ homeostasis. We further show that cytosolic Ca2+ seems to be necessary for proper targeting of p38 to the endosomes. Taken together, our data support a model in which Stx, by modifying Ca2+ homeostasis, recruits p38 to endosomes to regulate its intracellular transport.
MATERIALS AND METHODS
Cell Lines, Products, and Reagents
HeLa and HEp2 cells were grown under 5% CO2 in DMEM with 10% fetal calf serum supplemented with penicillin at 100 U/ml, streptomycin at 100 U/ml, and l-glutamine at 2 mM (Invitrogen, Carlsbad, CA). SB203580 was from Calbiochem (San Diego, CA), SKF86002 was from Sigma-Aldrich (St. Louis, MO), and 3,4,5-trimethoxybenzoic acid 8-(diethylamino)octyl ester (TMB-8) was from Alexis Biochemicals (Lausen, Switzerland). [3H]Leucine was from PerkinElmer Life and Analytical Sciences (Boston, MA). All cell culture reagents were from Invitrogen (Paisley, United Kingdom). Stx1 was obtained from Nacalai Tesque (Kyoto, Japan), and Stx was a kind gift from J. V. Kozlov (Academy of Sciences of Russia, Moscow, Russia) and J. E. Brown (U.S. Army Medical Research Institute of Chemical Defense, Fort Detrick, MD). The antibodies used in this study were anti-Stx (anti-STX1-13C4; Toxin Technology, Sarasota, FL), anti-ricin (Sigma-Aldrich), anti-p38 (p38α and p38P [pT180/pY182]; BD Biosciences, Palo Alto, CA), anti-p38β2 (Zymed Laboratories, South San Francisco, CA), anti-p38 (Cell Signaling Technology, Beverly, MA), anti-α-tubulin (Sigma-Aldrich), anti-clathrin heavy chain (RDI Division of Fitzgerald Industries International, Concord, MA), and anti-Rab5, anti-EEA1, and anti-annexin (our collection). StxB-Sulf2 expression construct was a kind gift from Dr. B. Goud (Institut Curie, Paris, France). StxB-Sulf2, and ricin-Sulf1 were prepared as described previously (Rapak et al., 1997; Lauvrak et al., 2006).
Small Interfering RNA (siRNA) Design and Transfection
Two different siRNAs targeting different parts of p38α and p38β mRNAs were designed. They were selected according to their physicochemical profiles and their specificity to the target mRNA by BLAST analysis profile (http://www.ncbi.nlm.nih.gov/BLAST/), and they were fitted as closely as possible to the criteria depicted in Reynolds et al. (2004). p38 siRNA target sequences were as follows: p38α, 5-GCUGUUGACUGGAAGAACA-3 and 5-CUGCGGUUACUUAAACAUA-3 (siRNA1 and -2, respectively) and p38β, 5-AAGGACCUGAGCAGCAUCUU-3 and 5-AAGUGUACUUGGUGACCACC-3 (siRNAb1 and -b2, respectively). High-performance liquid chromatography-purified p38 siRNAs were ordered from MWG Biotech (Ebersberg, Germany), and a negative control siRNA was from Eurogentec (Seraing, Belgium). Cells were transiently transfected with the indicated siRNA by using Oligofectamine (Invitrogen) according to the manufacturer's protocol.
Calcium Analysis
Variations in cytosolic calcium concentrations were measured using the calcium probe Fura-2 as described previously (Maturana et al., 2002). Cells were loaded for 20 min at room temperature with the membrane permeant Fura-2 acetoxymethyl ester (AM) in medium containing 140 mM NaCl, 5 mM KCl, 1.2 mM Ca2+, 1 mM MgCl2, 10 mM glucose, and 20 mM HEPES, pH 7.4. Fura-2 fluorescence (excitation, 340/380 nm; emission, 510 nm) was monitored with an imaging system. Loaded cells plated on coverslips were mounted on an IX70 inverted microscope (Olympus, Tokyo, Japan), and measurements were performed at 37°C (Thermoplate from Tokai Hit, Shizuoka, Japan) unless otherwise indicated. Images were captured with a charge-coupled device Orca-ER camera (Hamamatsu, Shizuoka, Japan). The cells were illuminated with a 75-W xenon lamp through a 10% neutral density filter (Omega Optical, Tokyo, Japan), a Lambda 10-2 filter wheel (Sutter Instrument, Novato, CA), and a 60× oil immersion PlanApo objective lens (numerical aperture. 1.4; Olympus). Camera and filter wheel shutter were under the control of the MetaMorph software (Molecular Devices, Sunnyvale, CA).
Endocytosis Assays
Stx internalization was quantified following the procedure described previously (Torgersen et al., 2005).
Sulfation Assays
Transfected or untransfected HeLa cells were washed twice with sulfate-free medium (minimal essential medium 12-126; Lonza Walkersville, Walkersville, MD) before incubation with 0.2 mCi/ml Na235SO4 in the same medium for 3 h at 37°C. Inhibitors were added as indicated, and then they were present in the same medium during the last 30 min of this incubation. Cells were incubated with StxB-Sulf2 for 45 min or ricin-Sulf1 for 3 h. They were then washed twice with cold phosphate-buffered saline (PBS) and lysed in lysis buffer (0.1 M NaCl, 10 mM Na2HPO4, pH 7.4, 1 mM EDTA, 1% Triton X-100, and 60 mM n-octyl-β-glucopyranoside, supplemented with Complete protease inhibitors; Roche Diagnostics, Mannheim, Germany). Cleared lysate was immunoprecipitated with anti-Stx or anti-ricin antibodies. The immunoprecipitated complex was separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to a polyvinylidene difluoride (PVDF) membrane, and investigated by autoradiography. Band intensities were quantified using ImageQuant 5.0 software (Molecular Dynamics, Sunnyvale, CA). Total cellular protein sulfation was measured as the amount of 5% trichloroacetic acid (TCA)-precipitated 35SO42− in the lysates.
Subcellular Fractionation of Endosomes
HEp2 cells were starved in serum-free medium for 1 h before treatment with or without 100 μM TMB-8 for 30 min. The cells were then incubated with or without 250 ng/ml StxB for 20 min in the same medium. Endosomes were purified as described previously (Aniento et al., 1996). Briefly, the cells were homogenized in homogenization buffer (250 mM sucrose and 3 mM imidazole, pH 7.4), and the postnuclear supernatant (PNS) was adjusted to 40% sucrose, 3 mM imidazole, pH 7.4. The PNS was then subjected to equilibrium flotation through layers of 35%, 25%, and 250 mM sucrose solutions in 3 mM imidazole, pH 7.4. The visible bands at the PNS/35%, 35%/25%, and 25%/250 mM sucrose solution interfaces correspond to the “heavy” membrane (HM), early endosome (EE), and late endosome (LE) fractions, respectively (Aniento et al., 1996). The protein concentrations in the purified fractions were measured, and equal amounts of protein were separated by SDS-PAGE and analyzed by Western blotting.
Immunoprecipitation
Detection of phosphorylated proteins after Stx stimulation was performed as follows: HeLa cells were starved in HEPES medium for 2 h and treated with 250 ng/ml StxB for the indicated durations. The cells were then lysed in binding buffer (20 mM Tris-HCl, 10 mM EDTA, 100 mM NaCl, 1% NP-40, and 0.2 mM orthovanadate) supplemented with a Complete protease inhibitor cocktail (Roche Diagnostics). Tyrosine-phosphorylated proteins were immunoprecipitated overnight at 4°C by using 20 μl of the slurry of an anti-phosphotyrosine column (Upstate Biotechnology, Charlottesville, VA) in batch as described previously (Wälchli et al., 2004). The eluate was subjected to SDS-PAGE and transferred onto a PVDF membrane. Immunostaining was performed with the indicated antibodies.
Cytotoxicity Assays
HeLa cells were pretreated as indicated in the figure legends. The cells were washed twice with leucine-free medium, and then they were incubated with increasing concentrations of toxin (Stx or ricin) for 2.5 h. After this, cells were incubated for 30 min in the presence of 2 μCi/ml [3H]leucine, and finally they were extracted twice with 5% TCA. The precipitate was dissolved in 0.1 M KOH, and the associated radioactivity was measured.
Confocal Fluorescence Microscopy
Cells grown on glass coverslips were transfected with siRNA against p38α or p38β, and they were analyzed 2 days after transfection. Alternatively, the cells were treated with or without 30 μM SB203580 for 30 min, and then they were incubated with 2 μg/ml StxB for 20 min. For experiments with ricin, the concentration used was also 2 μg/ml. The cells were fixed with 10% Formalin solution (Sigma-Aldrich), permeabilized with 0.2% Triton X-100 in PBS, and immunostained with appropriate antibodies. Fluorophore-labeled secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). DRAQ5 (Alexis Biochemicals, San Diego, CA) was used to stain the nuclei. The cells were mounted in Mowiol (Calbiochem), and then they were examined by laser scanning confocal microscope LSM 510 META (Carl Zeiss, Jena, Germany). Images were prepared with the LSM Image Browser software (Carl Zeiss).
RESULTS
Retrograde Trafficking of Stx Is Dependent on p38α
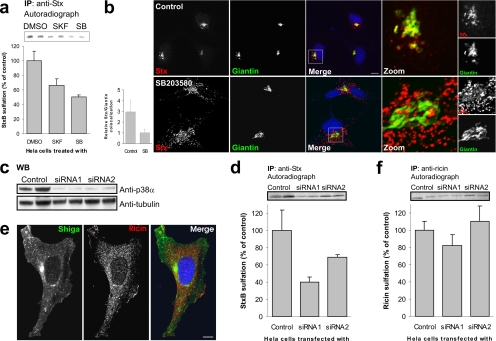
Stx induces early signaling events that can regulate endocytosis, and possibly also intracellular transport, of the toxin (Lauvrak et al., 2006). To analyze the involvement of kinases in Stx transport, we undertook an unbiased screen of specific kinase inhibitors. In this screen, the MAP kinase p38 stood out as a candidate regulator of retrograde Stx transport. We performed a sulfation experiment (Johannes et al., 1997), a biochemical approach that allows quantification of the amount of Stx that reaches the trans-Golgi network (TGN) (see Materials and Methods). The p38 inhibitors SB203580 and SKF86002 reduced Stx sulfation to 50% (Figure 1a), suggesting that p38 activity is required for transport of Stx to the Golgi apparatus. This was confirmed visually by confocal immunofluorescence microscopy. Cells treated with SB203580, displayed a threefold reduction in colocalization between Stx and the Golgi marker Giantin (Figure 1b).
Figure 1.
p38 inhibition reduces transport of StxB, but not ricin, to the TGN. (a) HeLa cells were incubated with radioactive sulfate for 3 h, and the indicated inhibitors (SKF86002 and SB203580; 30 μM) or the carrier (dimethyl sulfoxide [DMSO]; 0.1% final concentration) were present for the last 30 min. StxB was then added, and the incubation was continued for 45 min. StxB was immunoprecipitated from the lysates, and its degree of sulfation was analyzed by SDS-PAGE and autoradiography. The band intensities were quantified and the average plotted with error bars showing deviations. The experiment was performed three times with duplicates. (b) HeLa cells were incubated with or without SB203580 for 30 min, before addition of and further incubation with 2 μg/ml StxB for 20 min. The cells were then fixed and permeabilized before staining with the indicated antibodies. DRAQ5 was used for nuclear staining. Bar, 10 μm. Colocalization of StxB with the Golgi marker Giantin was quantified using Zeiss LSM Image Browser. Bars represent SD; n = 10. (c) We analyzed 1/100 of the Stx-IP supernatant (from D) for p38α by Western blot. α-Tubulin was used as loading control. (d) As in a, but in this case cells were transfected with 100 nM of the indicated siRNA and incubated for 48 h before StxB-Sulf2 treatment. These experiments were repeated at least three times with duplicates. (e) Cells were incubated for 20 min with 2 μg/ml Stx and 2 μg/ml ricin before fixation and permeabilization. They were then stained with antibodies as indicated. Bar, 10 μm. (f) As in d, but with 90-min incubation of ricin sulf-1 instead of StxB-Sulf2.
Next, we designed siRNAs that specifically targeted the p38α or p38β isoforms. Two days after transfection with p38α siRNA, when p38α knockdown was almost complete as determined by Western immunoblot (Figure 1c), transport of Stx to the Golgi was reduced to a similar extent as observed with the chemical inhibitors against p38 (Figure 1d). Because the available antibodies did not allow us to detect p38β by Western immunoblot, we took advantage of confocal microscopy to verify the siRNA-mediated knockdown of p38β. As shown in Supplemental Figure S1A, p38β was knocked down to a similar extent as p38α with the respective siRNAs (compare bottom and top panels). Transfection of cells with siRNAs targeting p38β did not affect the level of Stx sulfation, that is, the transport of Stx to the TGN (Supplemental Figure S1B). These observations suggest a role for the p38α isoform only, in regulation of Stx transport. We routinely check the total levels of protein sulfation under the various experimental conditions, and no significant differences between inhibitor- or siRNA-treated cells and control cells were observed (data not shown).
To determine whether the effect of p38α-inhibition/p38α-knockdown on retrograde transport to the TGN is specific for Stx, or whether it is involved in transport to the TGN in general, we investigated the transport of the related toxin ricin. In contrast to Stx, which binds specifically to the glycosphingolipid Gb3, ricin binds to both glycolipids and glycoproteins with terminal galactose. Ricin and Stx are thus endocytosed by different mechanisms, and their nonoverlapping endosomal localization is shown in Figure 1e. Analogous to what was done for Stx, we investigated the retrograde transport of a sulfation site-containing version of ricin (Rapak et al., 1997). Treatment of cells with either SB203580 (data not shown) or p38α siRNA (Figure 1f) had no significant effect on ricin sulfation. Thus, p38α is specifically involved in TGN transport of Stx, and not in general transport to the Golgi apparatus.
Stx Endocytosis Is Independent of p38
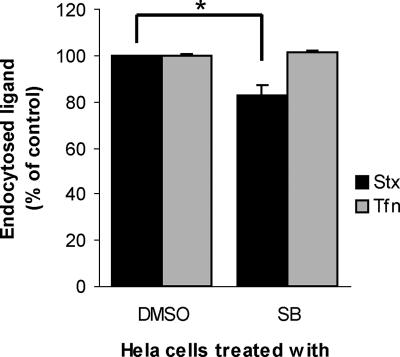
The observed reduction in Stx transport to the Golgi apparatus may be due to inhibition of a specific transport step between endosomes and TGN, or to inhibition of earlier events, like binding or endocytosis. Long-term inhibition of p38 by SB203580 has been shown previously to reduce binding of Stx to human brain endothelial cells (Stricklett et al., 2005). We therefore tested the effect of the two p38 inhibitors, SB203580 and SKF86002, on binding and endocytosis of Stx in our cells. Neither of the inhibitors affected toxin binding (data not shown). However, a slight decrease in Stx uptake was observed in cells treated with SB203580 (Figure 2, black bars). This effect was not due to a general block in clathrin-dependent endocytosis, because the transferrin endocytosis was unaffected by the inhibitor, both after 5 and 20 min (data not shown; Figure 2, gray bars). Importantly, the siRNAs targeting p38α did not reduce binding or endocytosis of Stx (data not shown). From these data, we conclude that the main regulatory activity of p38α on Stx transport is on the step from endosomes to the Golgi apparatus.
Figure 2.
Effect of p38 inhibition on endocytosis. HeLa cells were incubated with 30 μM SB203580 (SB) or carrier (DMSO; 0.1% final concentration) for 30 min at 37°C before Stx-SS-Biotin (black bars) or transferrin-SS-Biotin (Tfn; gray bars) was added to the medium. The incubation was continued for 20 min. Cells were 2-mercaptoethane sulphonate sodium treated, and internalized Stx- or transferrin-SS-Biotin labeled with Ru(II)-tag either directly (transferrin) or via antibody (Stx) was fished out from cell lysates by streptavidin-coated magnetic beads and measured by electrochemiluminescence. This experiment was repeated three times with duplicates; error bars represent deviations. *p ≤ 0.005, determined by the paired Student's t test.
Depletion of p38α Protects Cells against Stx Toxicity
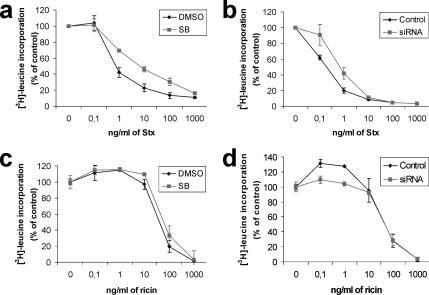
Because knockdown of p38α resulted in a strong reduction in endosome to Golgi transport of Stx, we wanted to study the effect on Stx transport to the cytosol. To this end, we performed a toxicity assay. As shown in Figure 3, a and b, the p38 inhibitor SB203580 and siRNA against p38α were able to decrease the toxicity of Stx four- to fivefold. This is in agreement with the sulfation and immunofluorescence data, and it further shows that p38α is required for proper Stx transport. We have reported previously that toxins are sometimes able to overcome a block in their trafficking (Llorente et al., 2003), apparently by using compensatory transport pathways up-regulated by the cell. However, for p38, even after long-term inhibition (siRNA treatment), the cells did not seem to be able to compensate for the lack of the p38α kinase in trafficking. To further confirm that the observed effects were specific for Stx transport, we performed analogous experiments for ricin. In agreement with data from sulfation experiments, ricin toxicity was not affected by treatment with p38 inhibitor or siRNA (Figure 3, c and d). Together, these results confirm that p38 is required for proper trafficking of Stx, but not ricin.
Figure 3.
p38 inhibition protects against Stx, but not ricin, cytotoxicity. HeLa cells were treated with 30 μM SB or carrier (DMSO; 0.1% final concentration) for 30 min (a and c) or transfected with p38α siRNA1 or a control siRNA 48 h before the experiment (b and d). After this, the cells were incubated with increasing concentrations of Stx (a and b) or ricin (c and d) for 2.5 h. Protein synthesis was measured by [3H]leucine incorporation. All experiments were repeated at least twice with duplicates. Error bars represent standard deviations.
p38 Is Activated by Stx
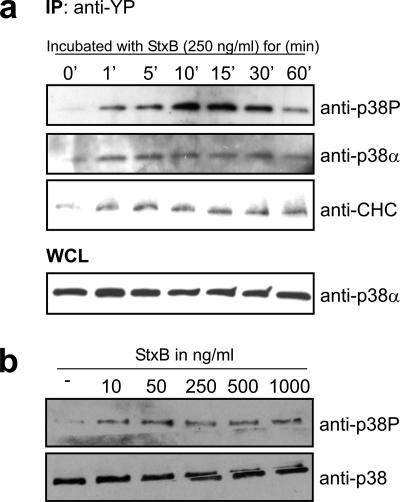
Previous reports have shown that the MAP kinase p38 is a regulator of endosomal transport (Cavalli et al., 2001; Delcroix et al., 2003; Fratti et al., 2003; Mace et al., 2005; Pelkmans et al., 2005). Therefore, we aimed at testing whether Stx can activate p38. Lysates of cells treated with Stx for different times were passed through an anti-phosphotyrosine (anti-YP) column, and eluted proteins were separated by SDS-PAGE and transferred to a PVDF membrane for immunoblot analysis. As shown, activated (Tyr- and Thr-phosphorylated) p38 (p38P) bound to the anti-YP column already after 1 min of Stx stimulation of the cells, and a peak was observed after 10–15 min (Figure 4a, top). The membrane was also probed for p38α (Figure 4a, second panel from the top). The observed binding of this isoform to the anti-YP column suggests that at least part of the p38P bands represent activated p38α. However, due to poor antibodies we were not able to detect p38β in immunoblots, and one can therefore not exclude that the p38P bands also contain activated p38β. p38P shows a peak that seems to be a bit delayed compared with p38α. The p38P-antibody recognizes the doubly phosphorylated (Thr and Tyr) protein, whereas the column binds Tyr-phosphorylated proteins. The p38α antibody does not distinguish between the different phosphorylation states of the protein, and this might therefore explain the seemingly different phosphorylation kinetics for p38P and p38α. As a control, the membrane was reprobed with anti-CHC antibody, because we have previously shown that CHC becomes phosphorylated upon Stx binding (Figure 4a, third panel from the top; Lauvrak et al., 2006). As shown, also the kinetics of CHC phosphorylation is rapid. As a control of equal loading to the columns, the whole cell lysate (WCL) was probed against p38α (Figure 4a, bottom). We also tested the sensitivity of the p38 response to different Stx concentrations. p38 activation (Tyr and Thr phosphorylation) was analyzed after 5-min incubation with increasing concentrations of Stx (Figure 4b). Whole cell lysates were subjected to SDS-PAGE and analyzed by Western immunoblot. As presented, Stx concentrations as low as 10 ng/ml were able to stimulate p38 phosphorylation.
Figure 4.
StxB is able to activate p38α upon binding. (a) HeLa cells were starved for 2 h in HEPES medium before incubation with 250 ng/ml StxB for the indicated times. Cells were lysed, and cell lysate was passed through an anti-YP column. The eluate was then analyzed by Western immunoblotting with the indicated antibodies. One percent of the WCL was analyzed by SDS-PAGE and Western immunoblot to serve as control of equal loading on the column. (b) HeLa cells were incubated with increasing concentrations of StxB for 5 min at 37°C. Lysates were prepared and run for Western blot analysis by using the indicated antibodies. These experiments were repeated three times.
Stx Modifies the Intracellular Ca2+ Response to Agonists
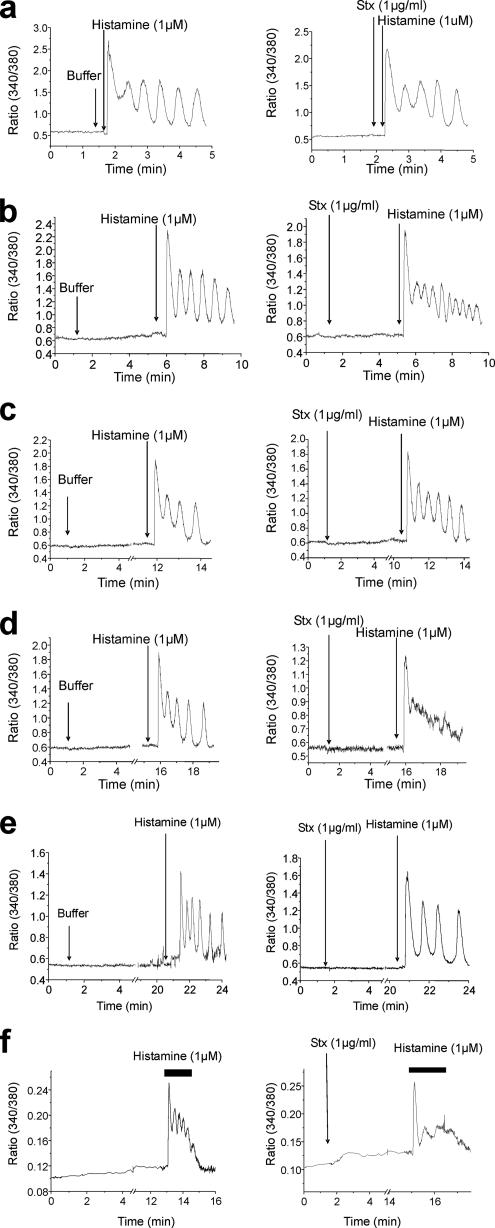
Because cytosolic Ca2+ levels affect Stx transport (Sandvig and Brown, 1987; Chen et al., 2002; Lauvrak et al., 2002), we analyzed the effect of Stx binding on Ca2+ homeostasis. In contrast to data obtained from studies in B cells (Taga et al., 1997), binding of Stx itself (unlike addition of ionomycin) did not induce any observable Ca2+ response in HeLa cells (Supplemental Figure S2A). The same was observed when purified StxB was added to the cells (Supplemental Figure S2D, left). This may be due to a local effect of Stx signaling, not measurable with a cytosolic calcium probe such as Fura-2AM, or, alternatively, a negative response. To test whether Stx addition modifies Ca2+ fluxes, we investigated the effect of Stx on histamine- or ATP-induced oscillations in Ca2+ levels. Stx was added to the cells, and histamine was then added after different time points (20 s, and 4, 10, and 15 min; Figure 5, a–d, respectively). No significant effect on the cytosolic Ca2+ oscillations induced by histamine could be observed at the earliest time points (Figure 5, a–c). After 15 min, however, the cytosolic Ca2+ oscillations induced by histamine were strongly attenuated, despite the fact that an intracellular Ca2+ increase was still observed (Figure 5d). The same was observed after ATP stimulation (Supplemental Figure S2B) and for StxB (Supplemental Figure S2C). These results suggest that Stx must be taken up by the cell to exert its effect on cytosolic Ca2+ levels. We next investigated whether the observed attenuation of cytosolic Ca2+ oscillations is a result of Stx localization to early endosomes per se. To this end, we performed the experiment at 18°C, a temperature that leads to Stx accumulation in early endosomes (Mallard et al., 1998). The histamine-induced Ca2+ oscillations were not affected by a 20-min Stx incubation at this temperature (Figure 5e), suggesting that the Stx-positive endosomes must reach, and perhaps fuse with, another compartment to affect the cytosolic Ca2+ oscillations caused by histamine. We also wanted to investigate whether the effect of Stx on oscillations in Ca2+ levels is p38 dependent. Cells were preincubated with SB203580 for 30 min before the experiment was performed as described. Under these experimental conditions, Stx treatment still inhibited the histamine-induced Ca2+ oscillations (Figure 5f), as seen without the inhibitor (Figure 5d). Interestingly, the basal level of cytosolic Ca2+ was increased in the presence of SB203580 (Figure 5f). In summary, these data show that Stx is able to act on Ca2+ homeostasis by affecting the intracellular Ca2+ cycling and that this is dependent on Stx internalization, but not on p38 activity. Because the effects of holotoxin and purified B-chain were similar, one can use the B-chain alone to investigate this phenomenon further.
Figure 5.
Stx inhibits cytosolic Ca2+ oscillations. Cytosolic Ca2+ was measured in HeLa cells, loaded with the calcium probe Fura-2AM. Stx (1 μg/ml) was added to the medium, as indicated, after initiation of Ca2+ measurements. Histamine (1 μM) was added 20 s (a), 4 min (b), 10 min (c), or 15 min (d) after Stx addition. For c and d, the traces are cut between 5 and 10 or 15 min, respectively. The left traces represent cells without toxin. Traces show the Fura-2AM fluorescent ratio (340 nm/380 nm). (e) After Fura-2AM loading, HeLa cells were incubated at 18°C for measurements. Stx was added as indicated. Histamine (1 μM) was added 20 min after the Stx addition. Traces presented are cut between 5 and 20 min. (f) Cells were pretreated with 2 μM SB203580 for 30 min. The cells were then loaded with Fura-2AM. Stx was added 2 min after the measurements started. Histamine (1 μM) was added 15 min after the Stx addition. Each trace is a representative Ca2+ response for 18–30 individual cells per experimental condition.
Intracellular Trafficking of Stx in HeLa Cells Is Sensitive to Ca2+ Levels: Nonadditive Effects of TMB-8 and p38 Inhibition
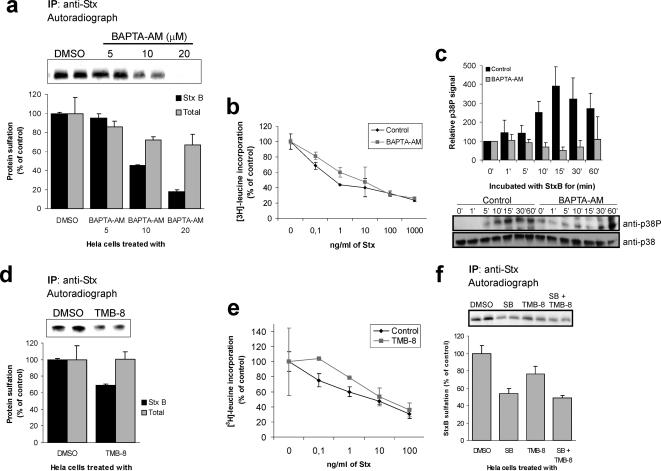
Cross talk between Ca2+ signaling and p38 activation has been proposed previously (Chao et al., 1992; Ikeda et al., 2000; Takeda et al., 2004; Fazal et al., 2005), and we set out to investigate whether there is a link between the requirements for Ca2+ and p38 in Stx transport. First, endocytosis assays were performed in the presence of Ca2+ chelators. As reported by Chen et al. (2002), none of these chelators seemed to affect Stx uptake to any large extent (data not shown). However, we noticed that 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM, a membrane-permeant Ca2+ chelator, became cytotoxic at concentrations higher than 20 μM (data not shown). We therefore tested the effect of different concentrations on toxin sulfation. 20 μM BAPTA-AM reduced Stx sulfation by >80% compared with that of the control (Figure 6a, black bars), whereas total protein sulfation was reduced by 30% at this concentration (Figure 6a, gray bars). The effect on total protein sulfation is in agreement with the results of Chen et al. (2002) showing that also anterograde, ER-to-Golgi, transport is sensitive to removal of Ca2+. In the further studies, we chose to work with 10 μM BAPTA-AM, a concentration that gave strong reduction in Stx sulfation, but only moderately affected total protein sulfation (Figure 6a). To confirm these data, we performed Stx toxicity experiments on cells treated with 10 μM BAPTA-AM. Under these conditions, we observed a 15-fold protection against Stx (average ± deviation, 14.8 ± 2.4; n = 2) (Figure 6b).
Figure 6.
StxB transport to the TGN is sensitive to Ca2+ variations. (a) HeLa cells were incubated with BAPTA-AM at the indicated concentrations or the carrier (DMSO; 0.1% final concentration) for 30 min before incubation with StxB for 45 min and lysis of the cells. StxB was immunoprecipitated from the lysates, and its degree of sulfation analyzed by SDS-PAGE and autoradiography. The band intensities were calculated and plotted as average of parallels. Total cellular proteins sulfation was measured after TCA precipitation and plotted relative to the control. This experiment was performed twice with duplicates; error bars show deviations. (b) Cells were incubated with or without 10 μM BAPTA-AM for 30 min before addition of Stx. The experiment was then performed as described in Figure 3. This experiment was performed twice with duplicates; error bars show deviations. (c) Cells were serum starved for 1 h, and then they were treated with or without 10 μM BAPTA-AM for 30 min before StxB was added. Cell lysates were separated by SDS-PAGE and analyzed by Western blot. Quantification of three separate experiments is shown in the histogram. (d) Cells were incubated with 100 μM TMB-8 or the carrier (DMSO; 0.1% final concentration) for 30 min before incubation with StxB-Sulf2 for 45 min. The experiment was then carried out as described in A. (e) Cells were incubated with or without 100 μM TMB-8 for 30 min before addition of Stx. The experiment was then performed as described in Figure 3. This experiment was repeated three times with duplicates. Error bars show deviations. (f) As in d, but in this assay cells were also treated or not with 30 μM SB203580 before addition of StxB-Sulf2. This experiment was repeated twice with duplicates. Error bars show deviations.
We also investigated the Stx-induced activation of p38 in the presence of BAPTA-AM. As shown in Figure 6c, BAPTA-AM efficiently inhibited p38 phosphorylation.
To confirm the results from experiments with BAPTA-AM–treated cells, we also tested the effect of TMB-8, an inhibitor of intracellular Ca2+ release (Bencherif et al., 1995). This inhibitor reduced Stx sulfation with 30% compared with that of the control cells, whereas total protein sulfation remained unaffected (Figure 6d). In agreement with this, we observed a 2.5 ± 1.1 (n = 3) protection against Stx in cells treated with TMB-8 (Figure 6e). Thus, these data indicate that the cytosolic Ca2+ level, and perhaps even a local change in Ca2+ concentration, is important for proper transport of Stx to the Golgi apparatus.
Next, we wanted to study the link between Ca2+ and the requirement for active p38 for proper Stx transport. Because inhibitors of both p38 and Ca2+ release reduce Stx transport, we reasoned that if p38 and Ca2+ act on the same pathway, cotreatment with the two inhibitors should not cause further reduction. As shown in Figure 6f, no additive effect on Stx sulfation was observed in cells treated with both SB203580 and TMB-8 compared with cells treated with SB203580 alone. This suggests that p38 activation and the level of Ca2+ together regulate one step in the Stx transport.
Stx Regulates the Recruitment of p38α to Endocytic Membranes in a Ca2+-dependent Manner
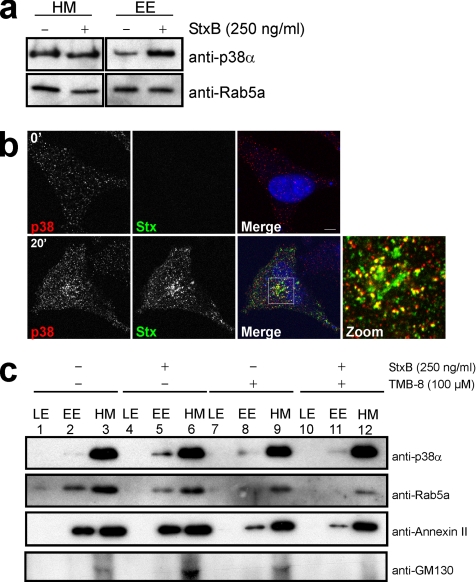
In neurons, MAP kinase p38 has been shown to be targeted to transport vesicles (Delcroix et al., 2003), and it has been suggested to be implicated in regulation of neurotransmitter transport. We therefore tested whether Stx binding was able to regulate the targeting of p38α to endosomes. To facilitate the studies, we used HEp2 cells, which, in contrast to HeLa cells, contain vesicles easily separable in a sucrose gradient. As a control, HEp2 cells were tested in Stx sulfation experiments in the presence of p38α siRNA (Supplemental Figure S3A) and SB203580 or TMB-8 (Supplemental Figure S3B), and they were shown to respond as HeLa cells. Cellular fractions were purified as described previously (Aniento et al., 1996). Equal amounts of the purified endosomal fractions were analyzed by SDS-PAGE and Western immunoblot. p38α was found in the early endosomal fraction, and recruitment to this fraction occurred in an Stx-dependent manner (Figure 7a). In agreement with this finding, we observed increased vesicular p38 staining pattern in HeLa cells treated with Stx, compared with control cells, as shown by immunofluorescence microscopy (Figure 7b). And, indeed, a fraction of the Stx-positive endosomes were positive for p38 (Figure 7b) and the early endosome marker EEA1 (Supplemental Figure S4). Importantly, we did not observe translocation of p38 to the nucleus upon Stx stimulation, suggesting a local activation of p38 on endosomes (see Discussion).
Figure 7.
p38 is recruited to early endosomes by a Ca2+-dependent mechanism upon Stx binding. (a) HEp2 cells were starved for 1 h before incubation with 250 ng/ml StxB for 20 min. Endosome fractions were prepared as described in Materials and Methods, and equal amounts of proteins were separated by SDS-PAGE and analyzed by Western immunoblotting with the indicated antibodies. (b) HeLa cells were incubated with 2 μg/ml StxB for 0 (top) or 20 min (bottom) before fixation and permeabilization. Antibodies against p38 and Stx were used. Bar, 5 μm. (c) As in a, but cells were treated with or without TMB-8 for 30 min before stimulation with 250 ng/ml StxB. Equal amounts of all fractions were separated by SDS-PAGE and analyzed by Western blot.
To test a possible influence of variations in Ca2+ levels on p38 recruitment to endosomes, we repeated the endosome purification in the presence of TMB-8. As shown in Figure 7c, this inhibitor blocked p38α recruitment to early endosomal membranes (top, compare lanes 5 and 11). In agreement with previous studies (Cavalli et al., 2001), the early endosomal marker Rab5a was redistributed upon p38 activation (Figure 7c). Rab5a was also sensitive to Ca2+ depletion (Figure 7c). We therefore used annexin II as an additional internal marker (Figure 7c), because it has been shown to be only partially sensitive to Ca2+ depletion (Jost et al., 1997; Konig and Gerke, 2000). Despite the fact that a fraction of annexin II was detached from the endosomes upon TMB-8 treatment, the protein did not seem to be sensitive to Stx binding, and it could therefore be used as a marker for the stability of our system in respect to Stx. Finally, we used GM130 as a marker for Golgi membranes to show the purity of our endosome fractions (Figure 7c). These data suggest that the Ca2+ requirement for endosome to Golgi transport of Stx can be connected to the Ca2+ dependent recruitment of p38 to endosomes. Furthermore, the ability of the toxin to promote this recruitment may be related to the Stx-dependent modification of Ca2+ fluxes.
DISCUSSION
We have previously shown that Stx is an active player in mediating its own transport. Stx activates the tyrosine kinase Syk, which then specifically regulates cell entry of the toxin (Lauvrak et al., 2006). In the present article, we elucidate further on the ability of Stx to promote its own transport. We show that endosome to Golgi transport of Stx is regulated by the MAP kinase p38 and Ca2+. Endocytosis of Stx induces a change in cytosolic Ca2+ levels, which in turn leads to activation of p38 and its recruitment to early endosomes.
The role of p38 in endosome to Golgi transport has not previously been investigated, but p38 has been found to regulate endocytosis and transport from endosomes. Fratti et al. (2003) have demonstrated that the activation of p38 by Mycobacterium tuberculosis is important for correct sorting of the pathogen (Fratti et al., 2003), and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking is improved by p38 (Huang et al., 2004). More recently, p38α has been implicated in the endocytosis of μ opioid receptor (Mace et al., 2005), and together with p38β, in epidermal growth factor receptor internalization (Vergarajauregui et al., 2006).
In the present study, inhibition of p38 activity, or knockdown of p38α, impaired transport of Stx from endosomes to the Golgi apparatus and protected the cells against the toxin. Importantly, the phenotypes observed by the two different methods used to validate the implication of p38α in intracellular trafficking of Stx, were similar, even though the incubation times differed significantly for the chemical inhibitors and siRNA. The requirement for p38 in retrograde transport seems to be specific for Stx, because there was no effect on transport of the related toxin ricin upon inhibition of p38 activity. These data are in agreement with the findings that p38 regulates sorting from early endosomes by phosphorylation of Rab5-GDI, EEA1, and Rabenosyn-5 (Cavalli et al., 2001; Fratti et al., 2003; Huang et al., 2004; Mace et al., 2005). It was recently suggested that Stx retrograde transport is independent of Rab5 (Fuchs et al., 2007). Future studies are needed to clarify these questions.
Previous reports show that p38 inhibitors protect different cell lines against Stx (Ikeda et al., 2000; Smith et al., 2003; Stricklett et al., 2005). However, these were studies of apoptotic cell death after long-term incubation with the toxin (24 h), and p38 activation was in these cells (Vero and brain endothelial cells) a late event induced by entry of active A-chain into cytosol. In contrast, we show that Stx rapidly activates p38 in HeLa cells. Also, as shown, after only 2.5 h of incubation with the toxin, there is a strong decrease in Stx-induced toxicity in cells treated with p38 inhibitor. A similar protection could be observed even after 90 min (data not shown). The strong protection might be due to a combination of two effects, the p38-dependent trafficking described in the present article, and ribotoxic stress attenuation described in previous reports (Foster and Tesh, 2002; Smith et al., 2003). Nevertheless, ricin, which also triggers ribotoxic stress (Iordanov et al., 1997; Foster and Tesh, 2002), was not affected by p38 inhibition, suggesting that Stx-related p38 activity is mainly dedicated to Stx trafficking in HeLa cells. The strong effect on Stx toxicity after p38 inhibition may indicate that p38 regulates transport from the Golgi apparatus to the ER as well. It would, therefore, be interesting to test whether p38α is also recruited to the Golgi membrane upon Stx binding.
The importance of Ca2+ homeostasis for Stx transport has been demonstrated, but signaling proteins linked to this Ca2+ requirement have not previously been characterized (Sandvig and Brown, 1987; Ikeda et al., 2000; Chen et al., 2002). Here, we have shown that Stx is able to trigger changes in Ca2+ levels induced by ATP or histamine. This effect was not observed under conditions where Stx was retained in the early endosomes, suggesting that endosome fusion with a later compartment might be required for the Stx-induced change in histamine-induced cytosolic Ca2+ oscillations. However, this finding does not exclude that Stx is able to induce Ca2+ changes in proximity to endosomes without fusion to another compartment, and with different kinetics. We further show that modification in Ca2+ levels is necessary for activation of p38 and its recruitment to early endosomes after Stx stimulation. p38 inhibition did not affect the Stx-induced change in Ca2+ oscillations, supporting that p38 activation and recruitment to endosomes may occur after Ca2+ signaling.
We have presented data showing that there is a link between Ca2+, p38 and Stx trafficking on the endosomal level. MAP kinases were reported to be found on endosomes even before any connection to trafficking was demonstrated (Pol et al., 1998). In our assay, we observed that a small fraction of p38 is activated upon Stx binding, suggesting that a small pool of p38 is sufficient to regulate toxin transport. One can speculate that this pool has to be targeted to the endosomal membrane in order to regulate Stx trafficking. In other words, in contrast to EGF receptor internalization (Vergarajauregui et al., 2006; Zwang and Yarden, 2006), activation of p38 in itself may not be sufficient to improve Stx trafficking. The endosomal recruitment is likely to be dependent on the presence of scaffold proteins (Morrison and Davis, 2003; Kolch, 2005). Such proteins have been shown to be essential for the recruitment of extracellular signal-regulated kinase to the Golgi (Morrison and Davis, 2003; Wang et al., 2005) or to the late endosome/lysosome (Wunderlich et al., 2001; Teis et al., 2002). It was recently shown that p38 requires β-arrestin-1 recruitment for proper activation in platelet-activating factor-stimulated cells (McLaughlin et al., 2006). For the present story, it is, therefore, tempting to speculate on the existence of a Ca2+-dependent scaffolding protein that is involved in recruitment of p38 to endosomes. Interestingly, it has been shown that scaffold proteins can be sufficient to activate p38α (Ge et al., 2002), suggesting that the proper targeting can bypass an upstream signaling cascade. Together, these results support a critical role for a yet unidentified scaffold protein that would link p38 to endosomes in a Ca2+-dependent manner.
In previous studies, we have observed that compensatory trafficking events can take place upon inhibition or knockdown of proteins essential for trafficking (Llorente et al., 2003; Utskarpen et al., 2006). For p38, there were no indications of such, supporting that this kinase has a unique role in the endosome to Golgi and ER route of Stx. Thus, in contrast to the reduced Stx transport after Rab6A inhibition, which can be overcome by upregulation of Rab6A' (Del Nery et al., 2006; Utskarpen et al., 2006), there is an absolute requirement for p38α activity for proper trafficking of Stx.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to E. Rolén and A.-G. Myrann for expert technical assistance. We acknowledge Prof. R. J. Davis (University of Massachusetts, Worcester, MA) for the p38, MKK3, and MKK6 cDNA constructs. Prof. N. Kurebayashi (Juntendo University School of Medicine, Tokyo, Japan) kindly let us use a microscope for calcium measurements. We are grateful to Dr. K. Pattni and H. Raa for critical reading of the manuscript. This study was supported by The Norwegian Radium Hospital, The University of Oslo, The Norwegian Cancer Society, The Norwegian Research Council and Humanities, The Novo Nordisk Foundation, The Jahre Foundation, and Jeanette and Søren Bothners Legacy. S.W. was in 2003 supported by Federation of European Biochemical Societies and Fond National Suisse de la Recherche Scientifique postdoctoral fellowships.
Abbreviations used:
- Stx
Shiga toxin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-06-0565) on October 24, 2007.
REFERENCES
- Aniento F., Gu F., Parton R. G., Gruenberg J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif M., Eisenhour C. M., Prince R. J., Lippiello P. M., Lukas R. J. The “calcium antagonist” TMB-8 [3,4,5-trimethoxybenzoic acid 8- (diethylamino)octyl ester] is a potent, non-competitive, functional antagonist at diverse nicotinic acetylcholine receptor subtypes. J. Pharmacol. Exp. Ther. 1995;275:1418–1426. [PubMed] [Google Scholar]
- Cameron P., Smith S. J., Giembycz M. A., Rotondo D., Plevin R. Verotoxin activates mitogen-activated protein kinase in human peripheral blood monocytes: role in apoptosis and proinflammatory cytokine release. Br. J. Pharmacol. 2003;140:1320–1330. doi: 10.1038/sj.bjp.0705560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V., Vilbois F., Corti M., Marcote M. J., Tamura K., Karin M., Arkinstall S., Gruenberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol. Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- Chao T. S., Byron K. L., Lee K. M., Villereal M., Rosner M. R. Activation of MAP kinases by calcium-dependent and calcium-independent pathways. Stimulation by thapsigargin and epidermal growth factor. J. Biol. Chem. 1992;267:19876–19883. [PubMed] [Google Scholar]
- Chen J. L., Ahluwalia J. P., Stamnes M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J. Biol. Chem. 2002;277:35682–35687. doi: 10.1074/jbc.M204157200. [DOI] [PubMed] [Google Scholar]
- Cherla R. P., Lee S.-Y., Tesh V. L. Shiga toxins and apoptosis. FEMS Microbiol. Lett. 2003;228:159–166. doi: 10.1016/S0378-1097(03)00761-4. [DOI] [PubMed] [Google Scholar]
- Del Nery E., Miserey-Lenkei S., Falguieres T., Nizak C., Johannes L., Perez F., Goud B. Rab6A and Rab6A' GTPases play non-overlapping roles in membrane trafficking. Traffic. 2006;7:394–407. doi: 10.1111/j.1600-0854.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- Delcroix J. D., Valletta J. S., Wu C., Hunt S. J., Kowal A. S., Mobley W. C. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- Fazal N., Choudhry M. A., Sayeed M. M. Inhibition of T cell MAPKs (Erk 1/2, p38) with thermal injury is related to down-regulation of Ca2+ signaling. Biochim. Biophys. Acta. 2005;1741:113–119. doi: 10.1016/j.bbadis.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Foster G. H., Tesh V. L. Shiga toxin 1-induced activation of c-Jun NH(2)-terminal kinase and p38 in the human monocytic cell line THP-1, possible involvement in the production of TNF-alpha. J. Leukoc. Biol. 2002;71:107–114. [PubMed] [Google Scholar]
- Fratti R. A., Chua J., Deretic V. Induction of p38 mitogen-activated protein kinase reduces early endosome autoantigen 1 (EEA1) recruitment to phagosomal membranes. J. Biol. Chem. 2003;278:46961–46967. doi: 10.1074/jbc.M305225200. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Haas A. K., Spooner R. A., Yoshimura S., Lord J. M., Barr F. A. Specific Rab GTPase-activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. J. Cell Biol. 2007;177:1133–1143. doi: 10.1083/jcb.200612068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge B., Gram H., Di Padova F., Huang B., New L., Ulevitch R. J., Luo Y., Han J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M., Stenmark H. Endocytosis and signaling: a relationship under development. Cell. 2003;115:513–521. doi: 10.1016/s0092-8674(03)00932-2. [DOI] [PubMed] [Google Scholar]
- Huang C. C., You J. L., Wu M. Y., Hsu K. S. Rap1-induced p38 mitogen-activated protein kinase activation facilitates AMPA receptor trafficking via the GDI.Rab5 complex. Potential role in (S)-3,5-dihydroxyphenylglycene-induced long term depression. J. Biol. Chem. 2004;279:12286–12292. doi: 10.1074/jbc.M312868200. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Gunji Y., Yamasaki S., Takeda Y. Shiga toxin activates p38 MAP kinase through cellular Ca(2+) increase in Vero cells. FEBS Lett. 2000;485:94–98. doi: 10.1016/s0014-5793(00)02204-3. [DOI] [PubMed] [Google Scholar]
- Iordanov M. S., Pribnow D., Magun J. L., Dinh T. H., Pearson J. A., Chen S. L., Magun B. E. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L., Tenza D., Antony C., Goud B. Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 1997;272:19554–19561. doi: 10.1074/jbc.272.31.19554. [DOI] [PubMed] [Google Scholar]
- Jost M., Zeuschner D., Seemann J., Weber K., Gerke V. Identification and characterization of a novel type of annexin-membrane interaction: Ca2+ is not required for the association of annexin II with early endosomes. J. Cell Sci. 1997;110:221–228. doi: 10.1242/jcs.110.2.221. [DOI] [PubMed] [Google Scholar]
- Katagiri Y. U., Mori T., Nakajima H., Katagiri C., Taguchi T., Takeda T., Kiyokawa N., Fujimoto J. Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J. Biol. Chem. 1999;274:35278–35282. doi: 10.1074/jbc.274.49.35278. [DOI] [PubMed] [Google Scholar]
- Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- Konig J., Gerke V. Modes of annexin-membrane interactions analyzed by employing chimeric annexin proteins. Biochim. Biophys. Acta. 2000;1498:174–180. doi: 10.1016/s0167-4889(00)00094-x. [DOI] [PubMed] [Google Scholar]
- Lauvrak S. U., Llorente A., Iversen T. G., Sandvig K. Selective regulation of the Rab9-independent transport of ricin to the Golgi apparatus by calcium. J. Cell Sci. 2002;115:3449–3456. doi: 10.1242/jcs.115.17.3449. [DOI] [PubMed] [Google Scholar]
- Lauvrak S. U., Walchli S., Iversen T. G., Slagsvold H. H., Torgersen M. L., Spilsberg B., Sandvig K. Shiga toxin regulates its entry in a Syk-dependent manner. Mol. Biol. Cell. 2006;17:1096–1109. doi: 10.1091/mbc.E05-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente A., Lauvrak S. U., van Deurs B., Sandvig K. Induction of direct endosome to endoplasmic reticulum transport in Chinese hamster ovary (CHO) cells (LdlF) with a temperature-sensitive defect in epsilon-coatomer protein (epsilon-COP) J. Biol. Chem. 2003;278:35850–35855. doi: 10.1074/jbc.M303425200. [DOI] [PubMed] [Google Scholar]
- Mace G., Miaczynska M., Zerial M., Nebreda A. R. Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid receptor endocytosis. EMBO J. 2005;24:3235–3246. doi: 10.1038/sj.emboj.7600799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F., Antony C., Tenza D., Salamero J., Goud B., Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana A., Van Haasteren G., Piuz I., Castelbou C., Demaurex N., Schlegel W. Spontaneous calcium oscillations control c-fos transcription via the serum response element in neuroendocrine cells. J. Biol. Chem. 2002;277:39713–39721. doi: 10.1074/jbc.M200464200. [DOI] [PubMed] [Google Scholar]
- McLaughlin N. J., Banerjee A., Kelher M. R., Gamboni-Robertson F., Hamiel C., Sheppard F. R., Moore E. E., Silliman C. C. Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J. Immunol. 2006;176:7039–7050. doi: 10.4049/jimmunol.176.11.7039. [DOI] [PubMed] [Google Scholar]
- Mori T., et al. Globotriaosyl ceramide (CD77/Gb3) in the glycolipid-enriched membrane domain participates in B-cell receptor-mediated apoptosis by regulating lyn kinase activity in human B cells. Exp. Hematol. 2000;28:1260–1268. doi: 10.1016/s0301-472x(00)00538-5. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Davis R. J. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Fava E., Grabner H., Hannus M., Habermann B., Krausz E., Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- Pol A., Calvo M., Enrich C. Isolated endosomes from quiescent rat liver contain the signal transduction machinery. Differential distribution of activated Raf-1 and Mek in the endocytic compartment. FEBS Lett. 1998;441:34–38. doi: 10.1016/s0014-5793(98)01517-8. [DOI] [PubMed] [Google Scholar]
- Polo S., Di Fiore P. P. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Rapak A., Falnes P. O., Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc. Natl. Acad. Sci. USA. 1997;94:3783–3788. doi: 10.1073/pnas.94.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W. S., Khvorova A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Brown J. E. Ionic requirements for entry of Shiga toxin from Shigella dysenteriae 1 into cells. Infect. Immun. 1987;55:298–303. doi: 10.1128/iai.55.2.298-303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. E., Kane A. V., Campbell S. T., Acheson D. W., Cochran B. H., Thorpe C. M. Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells. Infect. Immun. 2003;71:1497–1504. doi: 10.1128/IAI.71.3.1497-1504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricklett P. K., Hughes A. K., Kohan D. E. Inhibition of p38 mitogen-activated protein kinase ameliorates cytokine up-regulated shigatoxin-1 toxicity in human brain microvascular endothelial cells. J. Infect. Dis. 2005;191:461–471. doi: 10.1086/427188. [DOI] [PubMed] [Google Scholar]
- Taga S., et al. Intracellular signaling events in CD77-mediated apoptosis of Burkitt's lymphoma cells. Blood. 1997;90:2757–2767. [PubMed] [Google Scholar]
- Takeda K., Matsuzawa A., Nishitoh H., Tobiume K., Kishida S., Ninomiya-Tsuji J., Matsumoto K., Ichijo H. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 2004;5:161–166. doi: 10.1038/sj.embor.7400072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi H., Kiyokawa N., Taguchi T., Matsui J., Katagiri Y. U., Okita H., Okuda K., Fujimoto J. Shiga toxin binding to globotriaosyl ceramide induces intracellular signals that mediate cytoskeleton remodeling in human renal carcinoma-derived cells. J. Cell Sci. 2004;117:3911–3922. doi: 10.1242/jcs.01246. [DOI] [PubMed] [Google Scholar]
- Teis D., Wunderlich W., Huber L. A. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell. 2002;3:803–814. doi: 10.1016/s1534-5807(02)00364-7. [DOI] [PubMed] [Google Scholar]
- Torgersen M. L., Lauvrak S. U., Sandvig K. The A-subunit of surface-bound Shiga toxin stimulates clathrin-dependent uptake of the toxin. FEBS J. 2005;272:4103–4113. doi: 10.1111/j.1742-4658.2005.04835.x. [DOI] [PubMed] [Google Scholar]
- Utskarpen A., Slagsvold H. H., Iversen T. G., Walchli S., Sandvig K. Transport of Ricin from endosomes to the Golgi apparatus is regulated by Rab6A and Rab6A'. Traffic. 2006;7:663–672. doi: 10.1111/j.1600-0854.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- Vergarajauregui S., San Miguel A., Puertollano R. Activation of p38 mitogen-activated protein kinase promotes epidermal growth factor receptor internalization. Traffic. 2006;7:686–698. doi: 10.1111/j.1600-0854.2006.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. Y., Weng J., Anderson R. G. OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science. 2005;307:1472–1476. doi: 10.1126/science.1107710. [DOI] [PubMed] [Google Scholar]
- Wunderlich W., Fialka I., Teis D., Alpi A., Pfeifer A., Parton R. G., Lottspeich F., Huber L. A. A novel 14-kilodalton protein interacts with the mitogen-activated protein kinase scaffold mp1 on a late endosomal/lysosomal compartment. J. Cell Biol. 2001;152:765–776. doi: 10.1083/jcb.152.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wälchli S., Espanel X., Harrenga A., Rossi M., Cesareni G., Hooft van Huijsduijnen R. Probing protein-tyrosine phosphatase substrate specificity using a phosphotyrosine-containing phage library. J. Biol. Chem. 2004;279:311–318. doi: 10.1074/jbc.M307617200. [DOI] [PubMed] [Google Scholar]
- Zwang Y., Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J. 2006;25:4195–4206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.