Abstract
Regulation of cell polarity is a process observed in all cells. During directed migration, cells orientate their microtubule cytoskeleton and the microtubule-organizing-center (MTOC), which involves integrins and downstream Cdc42 and glycogen synthase kinase-3β activity. However, the contribution of G protein-coupled receptor signal transduction for MTOC polarity is less well understood. Here, we report that the heterotrimeric Gα12 and Gα13 proteins are necessary for MTOC polarity and microtubule dynamics based on studies using Gα12/13-deficient mouse embryonic fibroblasts. Cell polarization involves the Gα12/13-interacting leukemia-associated RhoGEF (LARG) and the actin-nucleating diaphanous formin mDia1. Interestingly, LARG associates with pericentrin and localizes to the MTOC and along microtubule tracks. We propose that Gα12/13 proteins exert essential functions linking extracellular signals to microtubule dynamics and cell polarity via RhoGEF and formin activity.
INTRODUCTION
Polarity is a multifaceted and necessary aspect of cellular function. Directed cell migration during development and differentiation, apicobasolateral polarity in epithelial cells, and axon specification in neuronal cells are only a few examples of the far-reaching impact of cell polarity. These processes require tightly regulated asymmetric signaling of membrane trafficking, cell adhesion, and cytoskeleton dynamics (Fukata et al., 2003). Polarization of the microtubule-organizing-center (MTOC) and microtubule reorganization represent hallmarks of the polarized phenotype of a directionally migrating cell centrally involving the monomeric GTPase Cdc42 (Kiosses et al., 1997; Etienne-Manneville and Hall, 2003).
MTOCs such as the centrosome are potent microtubule nucleators, and they have the ability to organize and polarize microtubules in arrays (Zimmerman et al., 1999). It is suggested that different proteins are involved in organizing and nucleating microtubules at the centrosomes. Pericentrin, one of the first characterized proteins found at the pericentriolar material (Doxsey et al., 1994), forms a complex with γ-tubulin at the centrosome. In this complex, pericentrin is bound to the centrosome lattice and to γ-tubulin complexes, which directly nucleate microtubules (Dictenberg et al., 1998; Luders and Stearns, 2007).
In migrating cells, microtubule dynamics are controlled in a spatiotemporal manner (Akhmanova and Hoogenraad, 2005). In turn, other regulatory components are dependent on microtubule dynamics, such as local activation of RhoA, successful transport of membrane vesicles and focal adhesion turnover (Wittmann and Waterman-Storer, 2001; Moissoglu and Schwartz, 2006; Birkenfeld et al., 2007). These events are likely regulated by proteins on microtubule plus-ends (Galjart and Perez, 2003). A number of microtubule plus-end–binding proteins have been described, such as adenomatosis polyposis coli (APC), cytoplasmatic linker proteins, cytoplasmatic linker protein-associating proteins, dynein/dynactin complex, and EB1 (Galjart and Perez, 2003; Mimori-Kiyosue and Tsukita, 2003). A conserved function of EB1 is to elongate microtubules (Ligon et al., 2003). EB1 can associate with APC (Su et al., 1995) and stabilizes the microtubule lattice seam (Sandblad et al., 2006).
In Drosophila melanogaster Schneider cells, DRhoGEF2 is involved in apical-basal cell polarity; DRhoGEF2 contains a regulator of G protein signaling (RGS) domain that is known to bind activated α subunits of heterotrimeric G proteins. DRhoGEF2 colocalizes with γ-tubulin at the centrosome, and it is transported to the cell cortex along microtubules tips, in an EB1-dependent manner (Rogers et al., 2004).
RhoA has been implicated in cell polarity; genetic studies in D. melanogaster demonstrate that the Drosophila RhoA homologue is required for tissue polarity (Strutt et al., 1997). In Xenopus laevis, activation of RhoA and the formin Daam1 is involved in planar cell polarity during gastrulation (Habas et al., 2001). Furthermore, the RhoA-effector and potent actin nucleator mDia1 has been shown to play essential roles in directional migration of mammalian cells (Faix and Grosse, 2006). Mammalian Dia1 localizes to membrane ruffles (Watanabe et al., 1997) and to the front of migrating fibroblasts in a Gα12/13 protein-dependent manner (Goulimari et al., 2005). In addition, the cytoskeletal scaffold and cell polarity protein IQGAP1 was recently demonstrated to recruit mDia1 to the leading front of migrating cells (Brandt et al., 2007).
The importance of heterotrimeric Gα12/13 proteins for RhoA-mediated actin dynamics, oncogenic transformation, or cancer cell invasion is well established (Gudermann et al., 2000; Kelly et al., 2006a,b); however, their roles in microtubule dynamics and the regulation of the microtubule cytoskeleton have not been investigated. Gα12/13 proteins are essential for development because double-deficient mice die at embryonic day 8.5 (Offermanns, 2003), and suppression of Gα12/13 function disrupts convergence and extension during zebrafish gastrulation (Lin et al., 2005). We previously demonstrated that Gα12/13 proteins are necessary for wound-induced cell migration through localized Rho-mDia1 function (Goulimari et al., 2005). Here, we analyze the role of Gα12/13 in cell polarity by assessing MTOC reorientation and microtubule dynamics in mouse embryonic fibroblasts (MEFs) lacking these G proteins. Our findings demonstrate that Gα12/13 critically regulate microtubule dynamics and MTOC polarization through LARG, which belongs to the family of RhoGEFs containing an RGS domain that directly binds to Gα12/13 proteins (Fukuhara et al., 2000; Tanabe et al., 2004; Kreutz et al., 2007). We find that LARG associates with pericentrin and localizes to the MTOC and along microtubule tracks. These data suggest an important biological role for LARG in the control of cell polarity downstream of Gα12/13.
MATERIALS AND METHODS
Materials
Antibodies used were as follows: α-RhoA (26C4) and α-LARG (N-14) were from Santa Cruz Biotechnology (Santa Cruz, CA); α-EB1, α-Rac, α-Cdc42, α-p140Dia1, and α-glycogen synthase kinase (GSK)-3β were from BD Transduction Laboratories (Lexington, KY); α-pericentrin (MTOC) was from Eurogentec (Seraing, Belgium); α-α tubulin, nocodazole, and anti-c-myc-agarose were from Sigma-Aldrich (St. Louis, MO); and α-GSK-3β (serine 9) and α-β catenin were from BioSource International (Camarillo, CA). The Alexa 350-phalloidin and anti-mouse Alexa 568 were from Invitrogen (Carlsbad, CA). Y27632 compound was from Calbiochem (San Diego, CA), and lysophosphatidic acid (LPA) was from BIOMOL Research Laboratories (Plymouth Meeting, PA). Anti-EE was from Covance (Hiss Diagnostics, Freiburg, Germany). The green fluorescent protein (GFP)-EB1 was a gift from Dr. J. Wehland (Helmholtz Centre of Infection Research, Braunschweig, Germany). All mDia1 and LARG constructs used in this study have been described previously (Goulimari et al., 2005; Rosenfeldt et al., 2006; Brandt et al., 2007; Kitzing et al., 2007). GFP-postsynaptic density 95/disc-large/zona occludens (PDZ)-RGS and GFP-RGS were generated by amplifying the cDNA from LARG encoding amino acids 72–556 and 348–556, respectively, by using the following primers: forward 5′-cgcgcgggatcctgcgtaatcatccagaaagat-3′ and reverse 5′-cgcgcggaattcttattttactcccaaatgcttcat-3′ for PDZ-RGS and forward 5′-cgccgcggatccggacagtgcagctgtttcca-3′ for RGS. The polymerase chain reaction (PCR) products were ligated into an EFplink2-GFP vector.
Cell Culture and Wound Healing Assays
Cell culture procedure and wound-healing assays were described previously (Goulimari et al., 2005). Briefly, confluent monolayers of starved cells were scratched once with a cut 10-μl tip. Cells were monitored using a CTR MIC microscope (Leica, Wetzlar, Germany). Pictures were acquired with a Leica DC 350 FX camera using the Leica software FW4000. For statistical analysis, triplicates of wound distance were measured at three randomly defined wound gap locations in each frame. At least three independent experiments were used.
Knockdown of Mouse RhoA, mDia1, Cdc42, and LARG Induced by Small Interfering RNA (siRNA)
All siRNA sequences used are from IBA GmbH (Goettingen, Germany). The sequence for mDia1 siRNA has been published previously (Arakawa et al., 2003; Goulimari et al., 2005; Eng et al., 2006; Yamana et al., 2006). The siRNA sequence against RhoA was chosen according to Wang et al. (2003). siRNA corresponding to Cdc42 is as follows (5′-GCU GGU CAG AGC CAU GGA U UU-3′). The siRNA against LARG is the following (5′-AAA CCA AAU GUA UAG AGC U TT-3′), and it is located in the 3′ untranslated region (UTR). A control sequence (5′-CGC GUA GAA GAU GAA GUU GTT-3′) was purchased from IBA. Transfection of MEFs with siRNA duplexes (2 μg/μl) was performed using magnet-assisted transfection according to manufacturer's instructions (IBA) as described previously (Goulimari et al., 2005). Cells were analyzed 48 h after siRNA transfection.
Immunofluorescence and Microscopy
Cells were stained as described previously (Grosse et al., 2003), or they were fixed with ice-cold methanol for microtubule and MTOC staining. For calcium depletion, cells were grown in 10% fetal bovine serum (FBS) DMEM without calcium (Invitrogen) with the addition of 4 mM EGTA and 1 mM magnesium chloride, and then they were fixed and stained (Sahai and Marshall, 2002). Immunofluorescence micrographs were obtained with a Leica DC 350 F camera on a Leica DM IRE 2 microscope and Leica IM 50 software. For MTOC stainings, cells were fixed 6 h after wounding, and images were acquired on a Nikon Eclipse 90i upright automated microscope equipped with a Nikon DS-1QM cooled black-and-white charge-coupled device (CCD) camera and NIS Elements AR 3.20 software (Nikon, Tokyo, Japan). Positive MTOCs were scored using a 90° angle relative to the wound. Cells were analyzed 6 h after wounding, unless otherwise stated. Confocal images were acquired at a spinning disk confocal ERS-FRET (PerkinElmer Life and Analytical Sciences, Boston, MA) on a Nikon TE2000 inverted microscope (Nikon, Tokyo, Japan) equipped with a EM-CCD camera (Hamamatsu, Bridgewater, NJ) and UltraVIEW ERS software.
Microinjections
For rescue experiments, cells were scratched and after 4 h they were microinjected with the indicated proteins. Then, 1.5 h later, cells were fixed with 8% formaldehyde (FA) for 10 min at room temperature and stained for MTOC as described above. An Eppendorf Femtojet and Injectman micromanipulator attached to an Axiovert 135 microscope (Carl Zeiss, Jena, Germany) was used for microinjections. Concentration for all constructs was the following: GFP (0.05 μg/μl), wild-type (WT) Gα12 (0.05 μg/μl), WT Gα13 (0.05 μg/μl), GFP-LARG (0.2 μg/μl), Dia1-compl (0.05 μg/μl), ΔDAD (0.05 μg/μl), Δ750-770ΔDAD (0.05 μg/μl), flag-pcDNA3 (0.05 μg/μl), EE-Gα12 (0.2 μg/μl), EE-Gα13 (0.2 μg/μl), GFP-EB3 (0.05 μg/μl), GFP-PDZ-RGS (0.2 μg/μl), and GFP-RGS (0.2 μg/μl).
In Situ Rho Hybridization
For in situ Rho hybridization, cells were incubated with recombinantly purified GFP or GFP-rhotekin-Ras binding domain (RBD) (GFP-RBD). Generation of the probes and in situ Rho affinity assays have been described previously (Goulimari et al., 2005). The following changes were made to the protocol. After fixation and permeabilization, cells were blocked in 5% FBS in phosphate-buffered saline (PBS) for 1 h. After blocking, the cells were preincubated with recombinantly purified monomeric red fluorescent protein (mRFP) (0.02 μg/μl) in 5% FBS/PBS for 1 h to block unspecific binding, followed by incubation with GFP-RBD (0.02 μg/μl). Software analysis (NISElements AR 2.30; Nikon) was used to subtract the mRFP background from the respective GFP-RBD image.
Total Internal Reflection Fluorescence Microscopy (TIRFM) and Analysis of Microtubule Dynamics
For TIRF microscopy, the cells were grown on glass-bottomed dishes (MatTek, Ashland, MA) until confluent. For immunocytochemistry, the cells were subsequently scratched and fixed with ice-cold methanol at the indicated times and stained for EB1 as described above, but they were maintained in a PBS solution, enabling internal reflection to take place. TIRF images were obtained on a Nikon TIRF setup with 488-nm and 561-laser excitation, a 60× PlanApo numerical aperture 1.45 objective on a TE-2000 equipped with an Orca-AG CCD camera (Hamamatsu) and NIS Elements AR 2.30 software (Nikon). For live-cell TIRFM, cells were scratched and 30 min later, leading edge cells were microinjected with GFP-EB1 (0.05 μg/μl) as described above. Time-lapse microscopy was performed 1 h later on the TIRF setup where cells were kept at 37°C with a Tokai Hit heating chamber. To monitor EB1 dynamics, time-lapse sequences were acquired at intervals of 3 s for a period of 5 or 10 min.
For quantification of microtubule advance rates, data were imported into ImageJ (http://rsb.info.nih.gov/ij/), and kymographs were created to visualize the displacement of the EB1 dashes. Kymograph analysis creates a time versus distance plot of intensity along the axis of a rectangular region of interest. A vertical trace indicates no displacement, and traces with shallow slopes indicate high velocity. Advance rates of microtubules were calculated by measuring angles of all distinguishable traces. Maximum projection images from time-lapse sequences were used to directly compare the EB1 tracks as well as manual tracking and velocity quantifications of EB1 and EB3 comets from epifluorescence movies were done with ImageJ.
For analysis of LARG localization with TIRFM, cells were grown on glass-bottomed dishes (MatTek) until confluent. Confluent cells were scratched, and 30 min later they were microinjected with GFP-LARG (0.2 μg/μl), fixed with 8% FA, and stained for α-tubulin.
Pull-Down Assays, Coimmunoprecipitions, and Western Blot Analysis
Lysates from wounded MEFs were subjected to RhoA pull-down assays by using agarose beads bound to glutathione transferase (GST)-rhotekin-RBD as described previously (Grosse et al., 2000; Vogt et al., 2003). Rac and Cdc42 activity assays were performed in parallel to RhoA pull-down by adding 1 μg/ml purified biotinylated Cdc42/Rac interactive binding peptide to the lysis reaction (Price et al., 2003). Proteins were eluted with SDS-sample buffer and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). For Western blot detection of GSK-3β phosphorylation, the total lysates from GTPase activity assays were used in parallel. TAT-C3 treatment (0.2 μM) and treatment with Y27632 (10 μM) were performed for 18 h before cell wounding. Cell monolayers were lysed at the indicated times, and proteins were subjected to SDS-PAGE. Densitometric quantifications were analyzed in Adobe Photoshop CS2 (Adobe Systems, Mountain View, CA).
For interaction studies, human embryonic kidney (HEK) cells were transfected with 1 μg of myc-LARG for 48 h. Cell extracts were then subjected to immunoprecipitation and immunoblotting as described previously (Grosse et al., 2003).
RESULTS
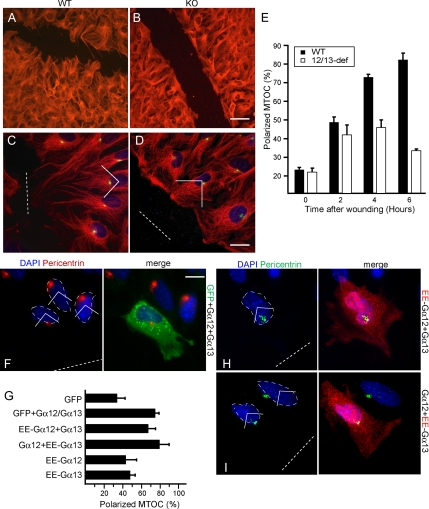
To analyze the microtubule cytoskeleton of Gα12/13-deficient MEFs, we performed tubulin and MTOC stainings on scratch-wounded cell monolayers. Under 10% serum conditions, WT cells displayed polarized microtubules protruding toward the wound gap (Figure 1A) in contrast to Gα12/13-deficient cells (KO) (Figure 1B). We then compared MTOC relocalization in scratched monolayers of WT or Gα12/13-deficient MEFs (Figure 1, C and D). MTOC reorientation, although cell-type specific, typically occurs within 1–6 h (Fukata et al., 2003). Within this time frame, most WT cells reorientated their MTOC in front of the nucleus toward the wound (Figure 1, C and E). However, the ability of the Gα12/13-deficient cells to polarize the MTOC was strongly affected (Figure 1, D and E). This defect could be rescued after reintroduction of untagged or EE-tagged versions of Gα12 and Gα13 (Figure 1, F and G). Gα12 or Gα13 alone could not rescue MTOC reorientation in the KO cells (Figure 1G). EE-Gα12 comicroinjected with Gα13 and vice versa showed a dotty localization pattern (Figure 1, H and I) and efficiently rescued MTOC polarity (Figure 1G). Additional analysis revealed that the serum component LPA can alone promote polarized migration of fibroblasts as expected (Palazzo et al., 2001), but not in cells lacking Gα12/13 (Supplemental Figure 1).
Figure 1.
Gα12/13 is required for MTOC polarization. (A) WT and (B) KO MEFs were stained with α-tubulin (red) antibody to visualize the microtubule cytoskeleton 6 h after wounding. Bar, 30 μm. (C) WT and (D) KO cells were stained with anti-α-tubulin (red), anti-pericentrin to visualize the MTOC (green), and 4,6-diamidino-2-phenylindole (DAPI) to visualize the nucleus (blue). Bar, 20 μm. (E) The percentage of WT (■) and KO MEFs (□) in the front row having their MTOC in the forward-facing quadrant (shown) was assessed at the indicated time after wounding. We scored 200 cells per time point. Data represents means ± SEM of five independent experiments. (F) Representative image of a KO cell microinjected with GFP, WT Gα12, and WT Gα13 and stained for MTOC (green) and DAPI (blue). Bar, 10 μm. (G) Quantification of positive MTOCs in cells expressing the indicated constructs. (H) Representative image of a KO cell expressing EE-Gα12(red) and Gα13 after comicroinjection and stained for DAPI (blue) and pericentrin (green) (I) Representative image of a KO cell expressing EE-Gα13 (red) and Gα12 after comicroinjection and stained for DAPI (blue) and pericentrin (green). Results represent the means ± SEM of three independent experiments (70 cells total). Wound gaps are indicated by dotted lines.
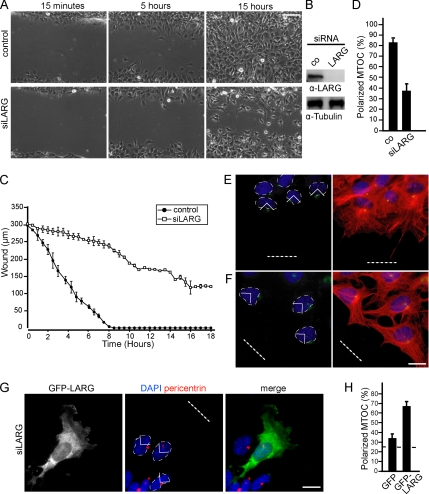
Gα12/13 proteins share a common downstream effector, the RGS domain-containing RhoGEF family of proteins (Fukuhara et al., 2000; Booden et al., 2002; Vogt et al., 2003). There are three known mammalian RGS-containing Rho-GEFs: PDZ-RhoGEF, found abundantly in brain (Swiercz et al., 2002); p115-RhoGEF, which is highly expressed in hematopoietic cells (Whitehead et al., 1996; Girkontaite et al., 2001); and LARG, which seems to be ubiquitously expressed (Swiercz et al., 2002). Interestingly, RNA-mediated knockdown of LARG robustly inhibited wounding-induced cell migration compared with control siRNA-transfected cells (Supplemental Movies 1 and 2 and Figure 2, A–C). This phenotype seemed to be comparable with the migration pattern and overall morphological appearance of Gα12/13-deficient cells, albeit less severe. Next, we investigated whether LARG may also regulate cell polarity. We performed MTOC stainings in WT MEFs transfected with control (Figure 2E) or LARG-specific siRNA against the 3′ UTR of LARG (Figure 2F). Indeed, MTOC reorientation was severely affected in the LARG-knockdown cells compared with control siRNA-treated cells (Figure 2D). Similar data were obtained in NIH3T3 cells (Supplemental Figure 2). Reexpression of full-length LARG in cells treated with siRNA rescued MTOC polarity, confirming the role of LARG in the process (Figure 2, G and H).
Figure 2.
LARG is necessary for directed cell migration and MTOC polarization. (A) Representative phase contrast images from live-cell recordings (Supplemental Movies 1 and 2) of siRNA control transfected MEFs (top) or LARG siRNA transfected MEFs (bottom) at 15 min, 5 h, and 15 h after wounding. Bar, 50 μm. (B) Cell extracts were immunoblotted for LARG. (C) Statistical analysis of scratch-induced migration assays from 30-min interval live cell recordings; •, control; □, siRNA against LARG. (D) Quantification of positive MTOCs in cells transfected with control siRNA or siRNA against LARG. Error bars in C and D represent SEM. Representative images of WT cells after control siRNA (E) or siRNA against LARG treatment (F) and stained for MTOC (green), DAPI (blue), and merged with α-tubulin (red). (G) Representative image of a cell microinjected with GFP-LARG (green; arrow) after the indicated treatment and stained for MTOC (red) and DAPI (blue). (H) Quantification of positive MTOCs in cells microinjected with GFP-LARG after treatment with siRNA against LARG. Data represent means ± SEM of three independent experiments (75 cells total). Bar, 10 μm. Wound gaps are indicated by dotted lines.
The requirement of Gα12/13 and LARG for MTOC polarization led us to investigate the functional role of RhoA in this process. Recently, Rho signaling through Diaphanous was shown to stabilize adherens junctions via catenin–cadherin complex formation (Sahai and Marshall, 2002). However, loss of cell-cell contacts was observed 4 h after wounding in Gα12/13-deficient MEFs (Goulimari et al., 2005), coinciding with MTOC reorientation defects. We therefore compared β-catenin localization, and we found that cell-cell contacts were disorganized and less abundant in Gα12/13-deficient cells (Figure 3, A and B). However, calcium depletion in WT cells resulted in relocalization of β-catenin to the cytoplasm (Figure 3C), but they did not have an effect on MTOC reorientation (Figure 3I).
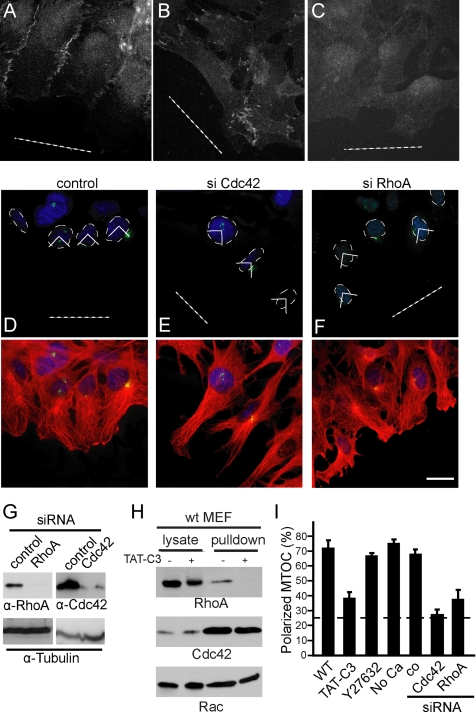
Figure 3.
RhoA activity is required for MTOC polarization. WT MEFs (A and C) or Gα12/13-deficient fibroblasts (C) were stained for cell junctions by using β-catenin antibody. (C) Cells were maintained in calcium-free medium before wounding to displace β-catenin from the cell-cell contacts (see Materials and Methods). (D–F) Representative images of cells stained for MTOC (green) and DAPI (blue) after transfection with siRNA against the indicated proteins. Merged images with α-tubulin (red) are shown. Dotted white line shows the wound. (G) Cell extracts were immunoblotted for the indicated proteins. (H) WT MEFs were maintained in 10% serum, and they were treated with TAT-C3. Cdc42 or Rac pull-down analysis was performed simultaneously with the Rho assay (as described in Materials and Methods). Western blot analyses of GST-RBD precipitates (pull-down) and of corresponding lysates are shown. (I) Quantification of positive MTOCs after the indicated treatment (see Materials and Methods). Data represent means ± SEM of three independently performed experiments (200 cells/treatment). Bar, 10 μm.
To characterize the role of RhoA in cell polarity, we used the cell-permeable Rho inhibitory toxin TAT-C3 (Figure 3H) and siRNA against RhoA (Figure 3G). As shown in Figure 3I, MTOC polarization was inhibited by both TAT-C3 and siRNA against RhoA but not by 10 μM of the ROCK inhibitor Y27632 (Figure 3, D, F, and I). These results were confirmed in NIH3T3 fibroblasts (Supplemental Figure 2, A–J).
Recently, it was suggested that Cdc42 is not necessary during directed migration (Czuchra et al., 2005). However, in this study in vitro differentiated Cdc42-deficient fibroblasts were used that could have developed compensatory mechanisms. In fact, the specific requirement for Cdc42 in cell polarity was confirmed in MEFs derived from Cdc42−/− embryos (Yang et al., 2006). We tested the involvement of Cdc42 in our cell system by using RNA interference. Of notice, siRNA against Cdc42 confirmed its requirement for MTOC reorientation (Figure 3, E and I) and directed migration (data not shown).
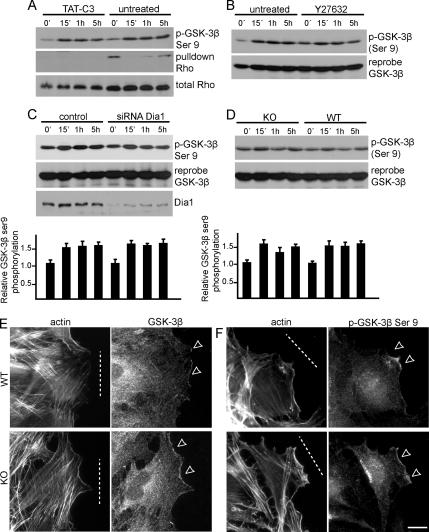
A well described signaling cascade in MTOC reorientation is Cdc42-dependent phosphorylation of GSK-3β at serine 9. This phosphorylation leads to GSK-3β inhibition and subsequent localization at the leading edge of migrating rat astrocytes, where it interacts with APC (Etienne-Manneville and Hall, 2003). Hence, we investigated GSK-3β phosphorylation during scratch-induced migration in WT and Gα12/13-deficient MEFs. Overnight incubation with 0.2 μM TAT-C3 had no effect on serine 9 phosphorylation of GSK-3β in WT cells (Figure 4A), despite the ablation of RhoA activity (Figure 3H). Consistent with this, inhibition of ROCK or down-regulation of mDia1 also had no effect (Figure 4, B and C). Furthermore, we did not observe significant differences in the levels or localizations of GSK-3β or its phosphorylated form in wounded WT or Gα12/13-deficient MEFs (Figure 4, D–F). These data implicate a role for Gα12/13 and RhoA-mDia1 signaling in MTOC reorientation in addition to or in cooperation with Cdc42 and GSK-3β function.
Figure 4.
Gα12/13 and RhoA-mDia1 are not involved in GSK-3β regulation. (A) WT MEFs were treated with TAT-C3, scratch-wounded for the times indicated, and subjected to Rho pull-down analysis. Cell extracts were immunoblotted to visualize RhoA or serine 9 phosphorylation of GSK-3β. (B) Immunoblot analysis of wound-induced GSK-3β serine 9 phosphorylation and reprobe for GSK-3β after treatment of WT cells with Y27632. (C) WT cells transfected with siRNA against mDia1 and immunoblotted for the indicated proteins. (D) Immunoblot analysis of GSK-3β or the serine 9 phosphorylated form in WT and KO cells from a scratch-induced time course experiment. (C and D) Densitometric quantifications of three independent experiments are shown together with representative Western blots; error bars represent SEM. (E) Representative images of actin and GSK-3β localization in wound-edge WT and KO cells (6 h after wounding). (F) Representative images of actin and GSK-3β serine 9-phosphorylated localization in wound-edge WT and KO cells (6 h after wounding). Dotted white lines represent the wound, and white arrows show areas of interest. Bar, 10 μm.
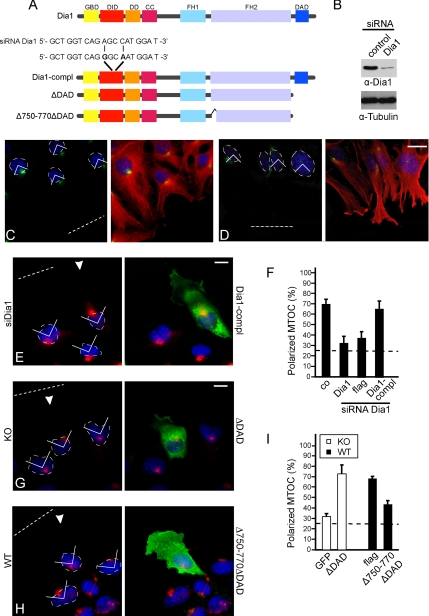
To determine whether the RhoA effector mDia1 might be involved in MTOC polarity, we used siRNA against mDia1 (Figure 5B). Depletion of mDia1 by siRNA resulted in inhibition of MTOC reorientation (Figure 5, C–F) in line with previous observations (Yamana et al., 2006). Furthermore, reintroduction of an mDia1 mutant (Dia1-compl) resistant to the siRNA used (Figure 5A) rescued MTOC reorientation in these cells (Figure 5, E and F). To better characterize the involvement of mDia1 in cell polarity we used mDia1 constructs lacking the Dia-autoinhibitory-domain (DAD) (Figure 5A) that is necessary to maintain the dormant protein conformation. Microinjection of ΔDAD in leading edge Gα12/13-deficient cells promoted MTOC reorientation compared with control (Figure 5, G and I). To investigate whether mDia1-induced actin assembly was necessary, we expressed a mDia1 ΔDAD construct lacking amino acids 750–770 (Figure 5A), a region required for formin homology 2 (FH2) domain homodimerization and subsequent in vivo and in vitro actin polymerization (Copeland and Treisman, 2002; Copeland et al., 2004). Interestingly, this construct inhibited MTOC reorientation in wild-type cells (Figure 5, H and I), and as may be expected inhibited cell migration (data not shown).
Figure 5.
mDia1 regulates MTOC polarity. (A) Domain organization of mDia1 (GBD, GTPase-binding domain; DID, Dia inhibitory domain; DD, dimerization domain; CC, coiled coil; FH1, formin homology 1 domain). Dia1-compl contains the two point mutations A711G and C714A (positions 10 and 13 in the siRNA target sequence). (B) Cell lysates were immunoblotted for mDia1. Representative image of WT cells treated with control siRNA (C) and siRNA against mDia1 (D) stained for MTOC (green), DAPI (blue) and merged with α-tubulin (red). (E) Representative image of a WT cell treated with siRNA against mDia1 and microinjected with Dia1-compl (green, arrow). Image shows MTOC (red) and DAPI (blue). (F) Quantification of positive MTOCs after expression of the indicated constructs. Data represent means ± SEM of three independent experiments (total of 60 cells). (G) Representative image of a KO cell microinjected with ΔDAD (green, arrow) and stained for MTOC (red) and DAPI (blue). (H) Representative image of a WT cell microinjected with Δ750-770ΔDAD (green; arrow) and stained for MTOC (red) and DAPI (blue). (I) Quantification of polarized MTOCs after expression of the indicated constructs. Data represent means ± SEM of three independent experiments (total of 70–75 cells for each construct). Bar, 10 μm. The wound is indicated by a dotted line.
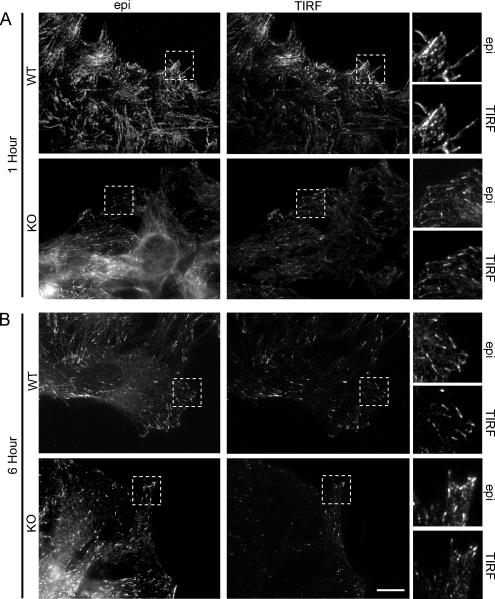
MTOC reorientation is affected in the Gα12/13-deficient cells at 4 h after wounding. However, migrating MEFs must reorganize their microtubule cytoskeleton much earlier, because WT cells move immediately after wounding. To determine early changes in microtubules we studied microtubule dynamics in the Gα12/13-deficient MEFs at 1 h after wounding. First, we analyzed the distribution of endogenous EB1. Both epifluorescence microscopy and TIRF microscopy revealed that there were fewer and less distinct EB1 positive dashes in the Gα12/13-deficient cells compared with the WT cells, in particular, at 1 h after wounding (Figure 6). These data indicate that the microtubule cytoskeleton is deregulated in the Gα12/13-deficient MEFs at time points where MTOC reorientation is not yet affected.
Figure 6.
Comparative study of EB1 localization in WT and Gα12/13-deficient MEFs using epifluorescence and TIRF microscopy. (A) Shown are representative epifluorescence and corresponding TIRF images of WT and KO leading edge cells, respectively, stained with α-EB1 to visualize the plus-end of microtubules at the cell membrane at 1 h after wounding. (B) Epifluorescence and TIRF images of WT and KO cells, respectively, 6 h after wounding. Areas marked within a dotted square are shown in magnification. Bar, 10 μm.
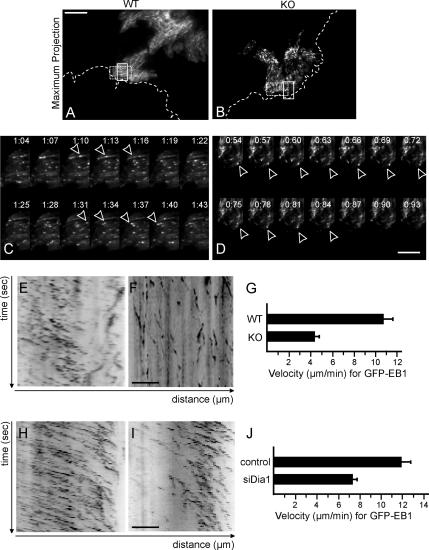
To directly characterize changes in microtubule dynamics, we monitored GFP-EB1 by TIRF microscopy after microinjection. TIRF microscopy shows enhanced sensitivity because it minimizes out-of focus signal from regions inside the cell. This allowed us to image low-expressing cells and to exclude possible microtubule stabilization effects due to overexpression of EB1. Live-cell imaging revealed GFP-EB1 as moving dashes that indicates microtubule-end growth (Supplemental Movies 3–6). Figure 7A shows the maximum projection (see Materials and Methods) of a time-lapse sequence of WT cells. The EB1-positive microtubule plus-ends move in a polarized manner in parallel tracks toward the leading edge (Figure 7A and Supplemental Movie 3). In Gα12/13-deficient MEFs, EB1 dynamics were aberrant (Supplemental Movie 4). Although some EB1 dashes move toward the leading edge (Figure 7B), the majority of dashes move in an unpolarized manner. To look closer at the behavior of EB1-positive microtubules, we analyzed the movies frame by frame. Figure 7C shows that in WT cells, EB1 labeling is visible as distinct, fast-moving dashes, which persist for three to four frames. In comparison, EB1 dashes in Gα12/13-deficient cells are less motile (Figure 7D). Kymographic analysis of the movies allowed for direct assessment of the EB1 tracks and velocity. Figures 7E and 8F show the representative areas, illustrated by the dotted rectangle, analyzed by a kymograph for WT and Gα12/13-deficient cells, respectively. The angle of the EB1 traces in the kymograph is a direct measure for the microtubule advance rate. The resulting velocities show that microtubule plus-ends in Gα12/13-deficient cells move significantly slower, with a mean velocity of 4.4 μm/min ± 0.5 compared with the WT cell velocity of 10.8 μm/min ± 0.8 (Figure 7G).
Figure 7.
EB1 dynamics are disturbed in the Gα12/13-deficient fibroblasts. (A) Maximum intensity projection image of GFP-EB1 in a WT leading edge cell (Supplemental Movie 3). (B) Maximum projection image of GFP-EB1 in a KO cell (Supplemental Movie 4). White dotted line represents the wound. Bar, 10 μm. (C) The sequence of images shows EB1 behavior in a WT cell at a 3-s interval and represents the area within the white rectangle in A. (D) Sequence showing EB1 in a KO cell from the area within the white rectangle in B. Bar, 5 μm. (E) Kymographic analysis of GFP-EB1 in a WT cell showing time (seconds) on the y-axis and distance (micrometers) traveled on the x-axis, representing the area within the dotted rectangle in A. (F) Kymographic analysis of GFP-EB1 in a KO cell from the area within the dotted rectangle in B. Bar, 5 μm. (I) Graph showing the velocity in μm/min of GFP-EB1 in WT and KO cells. Error bars represent SEM (6 cells analyzed in each example). (H) Kymographic analysis of GFP-EB1 in WT cells treated with control siRNA (Supplemental Movie 5). (I) Kymograph of GFP-EB1 in WT cells treated with siRNA against mDia1 (Supplemental Movie 6). (J) Graph showing the velocity of migrating EB1 dashes in WT cells treated with siRNA against the indicated proteins. Error bars show SEM (6 cells analyzed in each example).
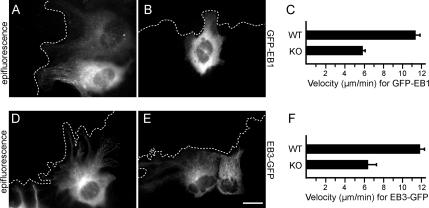
Figure 8.
Comparative study of EB1 and EB3 dynamics with epifluorescence time-lapse imaging. (A) Epifluorescence image of a WT cell expressing GFP-EB1 after microinjection. The image corresponds to Supplemental Movie 7. (B) Epifluorescence image of a KO cell expressing GFP-EB1 after microinjection corresponding to Supplemental Movie 8. (C) Graph shows the mean velocity of GFP-EB1 comets as measured with manual tracking (see Materials and Methods) in micrometers per minute. (D) Epifluorescence image of a WT cell microinjected with EB3-GFP. The image corresponds to Supplemental Movie 9. (E) Epifluorescence image of a KO cell expressing EB3-GFP after microinjection corresponding to Supplemental Movie 10. (F) Graph shows the mean velocity of EB3-GFP comets in micrometers per minute. Five cells were analyzed for each experiment. Bar, 3 μm.
To study the role of mDia1 in microtubule dynamics, we looked at EB1 dynamics after treatment with siRNA against mDia1. Leading edge cells depleted of mDia1 were microinjected with GFP-EB1 and monitored with TIRF (Supplemental Movies 5 and 6), and representative kymographs show that microtubule advance was slower than in the control siRNA-treated cells (Figure 7, H–J). Together, these data indicate that Gα12/13 proteins regulate microtubule dynamics during directed cell migration.
To confirm our findings, we followed GFP-EB1 dynamics in live WT (Figure 8A) and KO cells (Figure 8B) with epifluorescence microscopy. Dynamic imaging revealed GFP-EB1 moving dashes indicative of microtubule-end growth (Supplemental Movies 7 and 8). To calculate the velocity of the migrating EB1 comets, we used manual tracking, which confirmed that EB1 comets migrate slower in the Gα12/13-deficient cells (5.8 μm/min), compared with the WT cells (11.2 μm/min) (Figure 8C).
To verify the differences observed in microtubule dynamics, we analyzed another microtubule tip-binding protein, EB3-GFP, that was previously used to visualize microtubule growth (Stepanova et al., 2003). Time-lapse imaging of EB3-GFP in WT (Figure 6D and Supplemental Movie 9) and KO cells (Figure 8E and Supplemental Movie 10) showed that EB3 comets migrate slower in the Gα12/13-deficient cells (6.2 μm/min) than in the WT cells (11.8 μm/min) (Figure 8F).
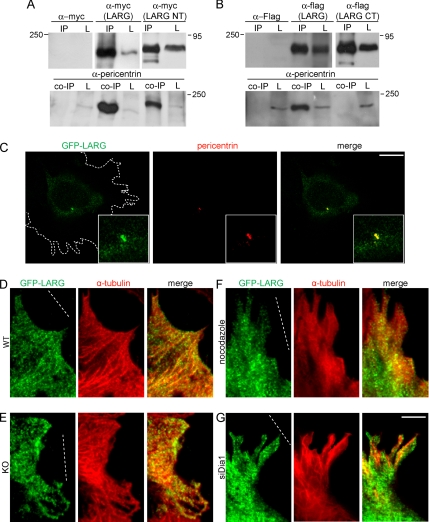
A recent study in Drosophila S2 cells reported that DRhoGEF2 is localized at the centrosome and that it is transported to the cell cortex along microtubules in an EB1-dependent manner. We hypothesized that the mammalian homologue LARG may be regulated in a similar manner. To test this, we performed immunoprecipitation studies and found that LARG efficiently coimmunoprecipitated with pericentrin (Figure 9A) but not with EB1 (data not shown), suggesting that LARG regulates MTOC polarity by interactions with pericentrin. Immunoprecipitations with either the N terminus of LARG containing the first 820 amino acids (LARG-NT) or the C terminus of LARG containing amino acids 1131–1544 (LARG-CT) revealed that the interaction occurs within the N-terminal half of LARG (Figure 9, A and B). Interestingly, using spinning disk confocal microscopy we observed LARG colocalization with pericentrin in wounded, polarized fibroblasts (Figure 9C).
Figure 9.
LARG associates with pericentrin, and it is localized along microtubule tracks. (A) HEK cells were transfected with myc, myc-LARG, or myc-LARG NT, and they were subjected to immunoprecipitation (IP). Input is shown to the right (L, lysate) and represents 10% of total cell lysate (top lane). Coimmunoprecipitation with endogenous pericentrin (coIP) is shown in the bottom lane. Fifty percent of total IP was loaded on gel for each example shown. (B) HEK cells were transfected with flag, flag-LARG, or flag-LARG CT, and they were subjected to IP. Input is shown to the right (L) and represents 10% of total cell lysate (top lane). CoIP endogenous pericentrin is shown in the bottom lane. Fifty percent of total IP was loaded on gel for each example shown. Molecular weight marker (kilodaltons) is shown to the left or right of each panel. (C) WT MEFs were microinjected with GFP-LARG. Representative image of a 0.3-μm Z section from a spinning disk confocal sequence showing GFP-LARG, pericentrin (red) staining, and merged is shown. The outline of the cell is shown in a dotted line. Insets show the area of colocalization in a higher magnification. Bar, 1 μm. Leading edge WT (D) and KO (E) were microinjected with GFP-LARG. Shown are representative TIRF images of GFP-LARG, α-tubulin (red), and merged images. (F) Leading WT MEFs were microinjected with GFP-LARG and immediately treated with 2 μM nocodazole for 1.5 h. Shown are representative TIRF images of GFP-LARG, α-tubulin staining (red), and merged. (G) WT MEFs were treated with siRNA against mDia1. Leading edge cells were microinjected with GFP-LARG and representative images as shown with α-tubulin (red) and merged. Bar, 3 μm. The wound is indicated by a dotted line. In total, seven cells were analyzed in each example.
Next, we microinjected leading edge WT and Gα12/13-deficient MEFs with GFP or GFP-LARG, stained for α-tubulin, and then analyzed the cells by TIRF microscopy, which allows for analysis close to the cell cortex. This revealed that GFP-LARG showed a pattern reminiscent of microtubule tracks extending toward the cell front in WT cells (Figure 9D). However, this was not observed when GFP-tagged constructs containing either the PDZ-RGS or the RGS domain alone were used (Supplemental Figure 3). When we looked at GFP-LARG localization in Gα12/13-deficient MEFs, some tracks were still visible, albeit less distinct, and not generally toward the front of the cell (Figure 9E). LARG localization along microtubule tracks was also sensitive to nocodazole treatment or RNA interference against mDia1 (Figure 9, F and G).
DISCUSSION
In this study, we identified a Gα12/13 signal transduction pathway involving LARG and mDia1 that regulates microtubule-based cell polarity in mammalian cells. The presence of serum components activating Gα12/13-coupled receptors such as LPA may, therefore, be important in regulating microtubule dynamics in directional cell movement.
How could Gα12/13 activation control microtubules? Our data suggest a model where the Gα12/13-regulated RhoGEF LARG is associated with the microtubule cytoskeleton, which may localize the RhoGEF for activation at the cell cortex. Interestingly, LARG has recently been shown to associate with mDia1 in a feedback module, suggesting a tight mechanistic control of both proteins for cell morphological events (Kitzing et al., 2007). Our data suggest that mDia1 may influence microtubule behavior by locally controlled actin assembly. This is supported by the fact that a ΔDAD-mDia1 mutant deficient in actin polymerization inhibits MTOC orientation in wild-type cells, whereas the active variant ΔDAD promotes polarity in Gα12/13-deficient cells. The role of mDia1 in cell polarity is further emphasized by the fact that it directly associates with the microtubule-regulating and MTOC polarizing protein IQGAP1 (Fukata et al., 2002; Watanabe et al., 2004), which also recruits active mDia1 to the front of migrating cells for localized actin polymerization (Brandt and Grosse, 2007; Brandt et al., 2007). However, the involvement of mDia1-mediated actin rearrangement could also be due to its requirement for the production of extensions needed for orientation toward the wound and subsequent microtubule rearrangement.
A study by Eng et al. (2006) showed that mDia1 can regulate the Par6-aPKC target GSK-3β. We studied GSK-3β regulation in WT, Gα12/13-deficient, and RhoA- and mDia1-depleted MEFs to determine whether there is cross talk between the GSK-3β pathway and Gα12/13 signaling in our cell system, but we found no significant differences. This independence of RhoA signaling is in line with previous studies in astrocytes (Etienne-Manneville and Hall, 2003). However, we cannot exclude the possibility that Gα12/13-Rho-mDia1 signaling is influenced by GSK-3β.
When we assessed the influence of Gα12/13 on the dynamics of EB1 in Gα12/13-deficient MEFs, we observed that EB1 localization at the plus-ends of growing microtubules was sparse during cell migration. Dynamic imaging of GFP-EB1 positive microtubules revealed that the advancement rate was slower and less directed toward the wound edge in Gα12/13-deficient cells as early as 90 min after wounding. We speculate that these altered microtubule dynamics could account for the failure to reorient the MTOC in the Gα12/13-deficient cells, which becomes evident at later time points. A recent study in Caenorhabditis elegans supports this scenario, demonstrating how growing microtubules come into direct contact with specific sites at the cortex before depolymerizing, and computational analysis showed that the generated forces are able to pull the mitotic spindle into the right position (Kozlowski et al., 2007). The altered microtubule advancement rates in Gα12/13-deficient or mDia1-depleted cells point to a role of EB1 beyond its role in APC-mediated microtubule stabilization. This is also suggested by a recent study showing that EB1 colocalizes only partially and to a very small extend to APC at the tip of microtubules and that this interaction occurs only transiently (Kita et al., 2006). In addition, EB1 depletion studies in both mouse fibroblasts and Drosophila S2 cells suggest that EB1 promotes microtubule dynamics independent of APC (Rogers et al., 2002; Kita et al., 2006). We see that EB1 localization and dynamics are regulated by Gα12/13 signaling.
The RGS domain-containing protein LARG has been implicated in mediating RhoA activation downstream of GPCRs and Gα12/13 through its ability to directly interact with Gα subunits (Reuther et al., 2001; Booden et al., 2002). Nevertheless, its biological importance has remained poorly understood. We used RNA interference against LARG and revealed its involvement in cell polarity. Initial evidence for the involvement of RGS-domain containing RhoGEFs in cell polarity came from studies in Drosophila S2 cells where the LARG homologue DRhoGEF2 was shown to act downstream of the trimeric G protein Concertina to induce shape change during gastrulation and morphogenesis (Parks and Wieschaus, 1991; Barrett et al., 1997; Halsell et al., 2000). In the present study, we show that in mammalian cells LARG interacts and colocalizes with pericentrin and may be dynamically regulated to the cell cortex via microtubules, suggesting a comparable scenario like in Drosophila cells, where DRhoGEF2 is transported to the cell cortex in an EB1-dependent manner (Rogers et al., 2004). However, different mechanisms must be at work in mammalian cells because we did not observe interaction of LARG with EB1. Interestingly, we found that mDia1 is required for the localization of LARG along microtubules. Hence, the function of mDia1 in microtubule dynamics may feed back into the efficient localized distribution of LARG during directional cell movement. Clearly, further studies are necessary to reveal the precise nature of the interaction between LARG and the microtubule cytoskeleton and to identify the proteins that might be involved in its transport to the cortex.
Cell polarity during migration is a very dynamic event involving a range of proteins and mechanisms. Our data implicate that Gα12/13 regulate microtubule dynamics and cell polarity during migration in mammalian cells in a RhoGEF and formin-dependent manner.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Di Ventura and E. T. Bodor for critical reading of the manuscript. We are grateful to S. Gutkind (NIH, Bethesda, MD) for providing GFP-LARG and N. Galjart (Erasmus Medical Centre, Rotterdam, The Netherlands) for EB3-GFP. We thank A. Rippberger for technical assistance and the Nikon Imaging Center at the University of Heidelberg for providing microscope equipment. This work was supported by the Emmy Noether Program of the Deutsche Forschungsgemeinschaft GR 2111/1-2 (to R.G.).
Abbreviations used:
- EB1
end binding protein 1
- FH2
formin homology 2 domain
- LARG
leukemia associated RhoGEF
- MEF
mouse embryonic fibroblast
- MTOC
microtubule-organizing center
- TIRF
total internal reflection fluorescence.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-11-1045) on October 24, 2007.
REFERENCES
- Akhmanova A., Hoogenraad C. C. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr. Opin. Cell Biol. 2005;17:47–54. doi: 10.1016/j.ceb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Arakawa Y., Bito H., Furuyashiki T., Tsuji T., Takemoto-Kimura S., Kimura K., Nozaki K., Hashimoto N., Narumiya S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J. Cell Biol. 2003;161:381–391. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K., Leptin M., Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91:905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- Birkenfeld J., Nalbant P., Bohl B. P., Pertz O., Hahn K. M., Bokoch G. M. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev. Cell. 2007;12:699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booden M. A., Siderovski D. P., Der C. J. Leukemia-associated Rho guanine nucleotide exchange factor promotes G alpha q-coupled activation of RhoA. Mol. Cell Biol. 2002;22:4053–4061. doi: 10.1128/MCB.22.12.4053-4061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt D. T., Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–1023. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt D. T., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 2007;178:193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J. W., Copeland S. J., Treisman R. Homo-oligomerization is essential for F-actin assembly by the formin family FH2 domain. J. Biol. Chem. 2004;279:50250–50256. doi: 10.1074/jbc.M404429200. [DOI] [PubMed] [Google Scholar]
- Copeland J. W., Treisman R. The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization. Mol. Biol. Cell. 2002;13:4088–4099. doi: 10.1091/mbc.02-06-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuchra A., Wu X., Meyer H., van Hengel J., Schroeder T., Geffers R., Rottner K., Brakebusch C. Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells. Mol. Biol. Cell. 2005;16:4473–4484. doi: 10.1091/mbc.E05-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg J. B., Zimmerman W., Sparks C. A., Young A., Vidair C., Zheng Y., Carrington W., Fay F. S., Doxsey S. J. Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S. J., Stein P., Evans L., Calarco P. D., Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Eng C. H., Huckaba T. M., Gundersen G. G. The Formin mDia regulates GSK3{beta} through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol. Biol. Cell. 2006;17:5004–5016. doi: 10.1091/mbc.E05-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- Faix J., Grosse R. Staying in shape with formins. Dev. Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Fukata M., Nakagawa M., Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr. Opin. Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Fukata M., Watanabe T., Noritake J., Nakagawa M., Yamaga M., Kuroda S., Matsuura Y., Iwamatsu A., Perez F., Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Chikumi H., Gutkind J. S. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 2000;485:183–188. doi: 10.1016/s0014-5793(00)02224-9. [DOI] [PubMed] [Google Scholar]
- Galjart N., Perez F. A plus-end raft to control microtubule dynamics and function. Curr. Opin. Cell Biol. 2003;15:48–53. doi: 10.1016/s0955-0674(02)00007-8. [DOI] [PubMed] [Google Scholar]
- Girkontaite I., Missy K., Sakk V., Harenberg A., Tedford K., Potzel T., Pfeffer K., Fischer K. D. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat. Immunol. 2001;2:855–862. doi: 10.1038/ni0901-855. [DOI] [PubMed] [Google Scholar]
- Goulimari P., Kitzing T. M., Knieling H., Brandt D. T., Offermanns S., Grosse R. Galpha12/13 is essential for directed cell migration and localized Rho-Dia1 function. J. Biol. Chem. 2005;280:42242–42251. doi: 10.1074/jbc.M508690200. [DOI] [PubMed] [Google Scholar]
- Grosse R., Copeland J. W., Newsome T. P., Way M., Treisman R. A role for VASP in RhoA-Diaphanous signalling to actin dynamics and SRF activity. EMBO J. 2003;22:3050–3061. doi: 10.1093/emboj/cdg287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse R., Roelle S., Herrlich A., Hohn J., Gudermann T. Epidermal growth factor receptor tyrosine kinase mediates Ras activation by gonadotropin-releasing hormone. J. Biol. Chem. 2000;275:12251–12260. doi: 10.1074/jbc.275.16.12251. [DOI] [PubMed] [Google Scholar]
- Gudermann T., Grosse R., Schultz G. Contribution of receptor/G protein signaling to cell growth and transformation. Naunyn Schmiedebergs Arch. Pharmacol. 2000;361:345–362. doi: 10.1007/s002109900208. [DOI] [PubMed] [Google Scholar]
- Habas R., Kato Y., He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Halsell S. R., Chu B. I., Kiehart D. P. Genetic analysis demonstrates a direct link between rho signaling and nonmuscle myosin function during Drosophila morphogenesis. Genetics. 2000;155:1253–1265. doi: 10.1093/genetics/155.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P., Moeller B. J., Juneja J., Booden M. A., Der C. J., Daaka Y., Dewhirst M. W., Fields T. A., Casey P. J. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA. 2006a;103:8173–8178. doi: 10.1073/pnas.0510254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P., Stemmle L. N., Madden J. F., Fields T. A., Daaka Y., Casey P. J. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J. Biol. Chem. 2006b;281:26483–26490. doi: 10.1074/jbc.M604376200. [DOI] [PubMed] [Google Scholar]
- Kiosses W. B., McKee N. H., Kalnins V. I. Relationship between the distribution of stress fibers and centrosomes in endothelial cells of the rat aorta. Cell Motil. Cytoskeleton. 1997;36:228–235. doi: 10.1002/(SICI)1097-0169(1997)36:3<228::AID-CM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kita K., Wittmann T., Nathke I. S., Waterman-Storer C. M. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol. Biol. Cell. 2006;17:2331–2345. doi: 10.1091/mbc.E05-06-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzing T. M., Sahadevan A. S., Brandt D. T., Knieling H., Hannemann S., Fackler O. T., Grosshans J., Grosse R. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 2007;21:1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski C., Srayko M., Nedelec F. Cortical microtubule contacts position the spindle in C. elegans embryos. Cell. 2007;129:499–510. doi: 10.1016/j.cell.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Kreutz B., Hajicek N., Yau D. M., Nakamura S., Kozasa T. Distinct regions of Galpha13 participate in its regulatory interactions with RGS homology domain-containing RhoGEFs. Cell Signal. 2007;19:1681–1689. doi: 10.1016/j.cellsig.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Ligon L. A., Shelly S. S., Tokito M., Holzbaur E. L. The microtubule plus-end proteins EB1 and dynactin have differential effects on microtubule polymerization. Mol. Biol. Cell. 2003;14:1405–1417. doi: 10.1091/mbc.E02-03-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Sepich D. S., Chen S., Topczewski J., Yin C., Solnica-Krezel L., Hamm H. Essential roles of G{alpha}12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J. Cell Biol. 2005;169:777–787. doi: 10.1083/jcb.200501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders J., Stearns T. Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 2007;8:161–167. doi: 10.1038/nrm2100. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Tsukita S. “Search-and-capture” of microtubules through plus-end-binding proteins (+TIPs) J. Biochem. 2003;134:321–326. doi: 10.1093/jb/mvg148. [DOI] [PubMed] [Google Scholar]
- Moissoglu K., Schwartz M. A. Integrin signalling in directed cell migration. Biol. Cell. 2006;98:547–555. doi: 10.1042/BC20060025. [DOI] [PubMed] [Google Scholar]
- Offermanns S. G-proteins as transducers in transmembrane signalling. Prog. Biophys. Mol. Biol. 2003;83:101–130. doi: 10.1016/s0079-6107(03)00052-x. [DOI] [PubMed] [Google Scholar]
- Palazzo A. F., Joseph H. L., Chen Y. J., Dujardin D. L., Alberts A. S., Pfister K. K., Vallee R. B., Gundersen G. G. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 2001;11:1536–1541. doi: 10.1016/s0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Parks S., Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 1991;64:447–458. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- Price L. S., Langeslag M., ten Klooster J. P., Hordijk P. L., Jalink K., Collard J. G. Calcium signaling regulates translocation and activation of Rac. J. Biol. Chem. 2003;278:39413–39421. doi: 10.1074/jbc.M302083200. [DOI] [PubMed] [Google Scholar]
- Reuther G. W., Lambert Q. T., Booden M. A., Wennerberg K., Becknell B., Marcucci G., Sondek J., Caligiuri M. A., Der C. J. Leukemia-associated Rho guanine nucleotide exchange factor, a Dbl family protein found mutated in leukemia, causes transformation by activation of RhoA. J. Biol. Chem. 2001;276:27145–27151. doi: 10.1074/jbc.M103565200. [DOI] [PubMed] [Google Scholar]
- Rogers S. L., Rogers G. C., Sharp D. J., Vale R. D. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 2002;158:873–884. doi: 10.1083/jcb.200202032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. L., Wiedemann U., Hacker U., Turck C., Vale R. D. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr. Biol. 2004;14:1827–1833. doi: 10.1016/j.cub.2004.09.078. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt H., Castellone M. D., Randazzo P. A., Gutkind J. S. Rac inhibits thrombin-induced Rho activation: evidence of a Pak-dependent GTPase crosstalk. J. Mol. Signal. 2006;1:8. doi: 10.1186/1750-2187-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E., Marshall C. J. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat. Cell Biol. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- Sandblad L., Busch K. E., Tittmann P., Gross H., Brunner D., Hoenger A. The Schizosaccharomyces pombe EB1 homolog Mal3p binds and stabilizes the microtubule lattice seam. Cell. 2006;127:1415–1424. doi: 10.1016/j.cell.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Stepanova T., Slemmer J., Hoogenraad C. C., Lansbergen G., Dortland B., De Zeeuw C. I., Grosveld F., van Cappellen G., Akhmanova A., Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J. Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D. I., Weber U., Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Su L. K., Burrell M., Hill D. E., Gyuris J., Brent R., Wiltshire R., Trent J., Vogelstein B., Kinzler K. W. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Swiercz J. M., Kuner R., Behrens J., Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- Tanabe S., Kreutz B., Suzuki N., Kozasa T. Regulation of RGS-RhoGEFs by Galpha12 and Galpha13 proteins. Methods Enzymol. 2004;390:285–294. doi: 10.1016/S0076-6879(04)90018-3. [DOI] [PubMed] [Google Scholar]
- Vogt S., Grosse R., Schultz G., Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J. Biol. Chem. 2003;278:28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- Wang H. R., Zhang Y., Ozdamar B., Ogunjimi A. A., Alexandrova E., Thomsen G. H., Wrana J. L. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Madaule P., Reid T., Ishizaki T., Watanabe G., Kakizuka A., Saito Y., Nakao K., Jockusch B. M., Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Wang S., Noritake J., Sato K., Fukata M., Takefuji M., Nakagawa M., Izumi N., Akiyama T., Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev. Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Whitehead I. P., Khosravi-Far R., Kirk H., Trigo-Gonzalez G., Der C. J., Kay R. Expression cloning of lsc, a novel oncogene with structural similarities to the Dbl family of guanine nucleotide exchange factors. J. Biol. Chem. 1996;271:18643–18650. doi: 10.1074/jbc.271.31.18643. [DOI] [PubMed] [Google Scholar]
- Wittmann T., Waterman-Storer C. M. Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 2001;114:3795–3803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- Yamana N., et al. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol. Cell Biol. 2006;26:6844–6858. doi: 10.1128/MCB.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Wang L., Zheng Y. Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol. Biol. Cell. 2006;17:4675–4685. doi: 10.1091/mbc.E06-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman W., Sparks C. A., Doxsey S. J. Amorphous no longer: the centrosome comes into focus. Curr. Opin. Cell Biol. 1999;11:122–128. doi: 10.1016/s0955-0674(99)80015-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.