Abstract
Interferon (IFN)α induces apoptosis via Bak and Bax and the mitochondrial pathway. Here, we investigated the role of known IFNα-induced signaling cascades upstream of Bak activation. By pharmacological and genetic inhibition of the kinases protein kinase C (PKC)δ, extracellular signal-regulated kinase (ERK), and c-Jun NH2-terminal kinase (JNK) in U266-1984 and RHEK-1 cells, we could demonstrate that all three enzymes are critical for the apoptosis-associated mitochondrial events and apoptotic cell death induced by IFNα, at a step downstream of phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR). Furthermore, the activation of JNK was found to occur in a PKCδ/ERK-dependent manner. Inhibition of these kinases did not affect the canonical IFNα-stimulated Janus tyrosine kinase-signal transducer and activator of transcription signaling or expression of IFN-responsive genes. Therefore, enucleated cells (cytoplasts) were examined for IFNα-induced apoptosis, to test directly whether this process depends on gene transcription. Cytoplasts were found to undergo apoptosis after IFNα treatment, as analyzed by several apoptosis markers by using flow cytometry, live cell imaging, and biochemical analysis of flow-sorted cytoplasts. Furthermore, inhibition of mTOR, ERK, and JNK blocked IFNα-induced apoptosis in cytoplasts. In conclusion, IFNα-induced apoptosis requires activation of ERK1/2, PKCδ, and JNK downstream of PI3K and mTOR, and it can occur in a nucleus-independent manner, thus demonstrating for the first time that IFNα induces apoptosis in the absence of de novo transcription.
INTRODUCTION
Interferons (IFNs) comprise a family of pleiotropic polypeptides with the common denominator of inhibiting viral replication, making them indispensable in the innate immunity against viral infections. This group of cytokines also possesses antitumor activities, which have led to their application in the treatment of a variety of cancers (Strander and Einhorn, 1996). Despite their wide use, the mechanisms that underlie these antitumor activities are still largely unclear; hence, the factors responsible for IFN resistance are also unknown (Grander, 2000).
An attractive mechanism for the antitumor activity exerted by IFNα is the induction of apoptosis. We and others have shown that IFNα can induce apoptosis in several transformed cell lines from many different tissues and in primary tumor cells (Manabe et al., 1993; Thyrell et al., 2002). We have also demonstrated that IFNα-induced apoptosis proceeds via the mitochondrial pathway with activation of the Bcl-2 family members Bak and Bax, subsequent release of cytochrome c (cyt c), and activation of caspases (Panaretakis et al., 2003). Many different proapoptotic stimuli activate Bak and Bax, causing the release of cyt c from mitochondria; however, the upstream signaling leading to this common pathway is most probably more agent specific (Wang et al., 2001; Panaretakis et al., 2002). Several signaling pathways are activated by IFNα, including the canonical Janus tyrosine kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, largely responsible for the antiviral activities of IFNα, and a cohort of other pathways whose role for the IFN-induced biological outcomes remains controversial, such as the phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR), the p38 mitogen-activated protein kinase (MAPK), protein kinase C (PKC)δ, and JNK/stress-activated protein kinase (SAPK) pathways (Parmar and Platanias, 2003; Katsoulidis et al., 2005). In a recent publication, we showed that IFNα-induced Bak activation and apoptosis in several tumor cell lines required activation of PI3K and mTOR (Thyrell et al., 2004).
It is generally believed that the cellular responses to IFNs require STAT-mediated changes in gene transcription. The fact that engagement of the IFN receptor also activates other phosphorylation cascades apart from the classical JAK/STAT signaling opens up additional putative ways for IFNs to alter cellular physiology. The function of these accessory pathways in IFN signaling has so far mainly been studied in the context of their ability to modulate STAT-regulated transcription by affecting STAT phosphorylation (Nguyen et al., 2001; Zhao et al., 2005). Our findings that the PI3K/mTOR pathway, normally regulating downstream phosphorylation cascades independently of de novo protein synthesis, is crucial for the IFN-induced apoptosis without affecting STAT signaling, prompted us to investigate the importance of nucleus-independent events in IFNα-induced apoptosis. Instead of using chemical inhibitors of transcription, we decided to apply a method that allows removal of the nucleus from cells while maintaining their viability for a considerable time (Poste, 1972; Mandic et al., 2003). This also allowed us to investigate the role of the kinases extracellular signal-regulated kinase (ERK), PKCδ, and JNK that are activated by the engagement of the IFN-receptor independently of STAT-induced transcription, in IFNα-induced apoptosis. Thus, the aim of the present study was to determine the downstream cytoplasmic signaling cascades used by PI3K and mTOR leading to the activation of Bak to execute the proapoptotic effects of IFNα.
MATERIALS AND METHODS
Cell Lines, Culture Conditions, and Treatments
The multiple myeloma cell line U266-1984 (Nilsson et al., 1970) was cultured in RPMI 1640 medium, and RHEK-1 (Rhim, 1989) cells were cultured in DMEM supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, 2 mM l-glutamine, 50 μg/ml streptomycin, and 50 μg/ml penicillin (Gibco, Berlin, Germany), and they were maintained in a humidified incubator under 5% CO2 at 37°C. Recombinant human IFNα2b (Schering Plough, Kenilworth, NJ), with a specific activity of 2.0 × 108 units/mg and a purity of >99%, was used at a concentration of 300 or 5000 U/ml. SP600125 (10 μM) and rottlerin (2 μM) were purchased from Calbiochem (Darmstadt, Germany), U0126 (10 μM) was from Promega (Madison, WI), and Ly294002 (10 μM) and rapamycin (1 μM) were from Sigma-Aldrich (St. Louis, MO). The concentrations of the kinase inhibitors have been titrated previously (Thyrell et al., 2004; Panaretakis et al., 2005), and they were added 1 h before IFN, in the concentrations specified above.
Assessment of Apoptosis
Apoptotic redistribution of plasma membrane phosphatidylserine (PS) was assessed by annexin V-FLUOS (Roche Diagnostics, Mannheim, Germany). Changes in ΔΨm were detected by incubation of living cells with tetramethylrhodamine ethyl ester (TMRE) (Invitrogen, Carlsbad, CA), activation of caspase-3 was assessed using an antibody against the active conformation of caspase-3 (BD Biosciences PharMingen, San Diego, CA), and activation of Bak was detected using the AM03 antibody (clone TC100; Calbiochem) (Griffiths et al., 1999) and analyzed by flow cytometry, as described previously (Panaretakis et al., 2003). Cytoplasts were stained for active caspase-3 (BD Biosciences PharMingen) and propidium iodide (PI) (50 μg/ml) before analysis on the FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Adenoviral Vectors and Infection of U266 Cells
Generation of the wild-type and kinase dead recombinant PKCδ adenoviruses has been described previously (Carpenter et al., 2001). The kinase dead mutant K376R has been shown to function as an isoform-specific inhibitory kinase (Li et al., 1995). The infection was carried out as described previously (Panaretakis et al., 2005).
Transfections
Transient transfections with the pcDNA3-JNK1-APF and pcDNA3-JNK1-WT plasmids were performed using Lipofectamine 2000 (Invitrogen) according to manufacturer's protocol. We used 7 × 105 cells, 0.75 μg of DNA, and 1.5 μl of Lipofectamine 2000 per transfection. The following day, the cells were reseeded and treated with 5000 U/ml IFNα for 36 h, harvested, and stained for FLAG-expression and caspase activity.
Caspase Activation
Cells treated with 5000 U/ml IFNα for 36 h, and then they were harvested and stained with FAM-VAD-FLICA (Immunochemistry Technologies LLC, Bloomington, MN) according to the manufacturer's protocol and fixed overnight at 4°C. The cells were then permeabilized with 90% methanol on ice for 30 min, stained with anti-FLAG M2 (1:500) and anti-glutathione transferase (GST) antibody (1:50) in phosphate-buffered saline with 0.5% bovine serum albumin for 30 min at room temperature (r.t.) followed by an allophycocyanin-conjugated secondary antibody. GST- and FLAG-expressing cells were detected on the FACSCalibur flow cytometer, and FAM-VAD-FLICA positivity was measured in the positive populations. For quantification and comparison, median fluorescence intensity values were calculated using CellQuest software (BD Biosciences).
Western Blot Analysis
SDS-polyacrylamide gel electrophoresis was performed as described previously (Thyrell et al., 2004) The antibodies used were as follows: rabbit polyclonal immunoglobulin (IgG) against phosphorylated (p)JNK (pTpY183/185; BioSource International, Camarillo, CA), pERK (Thr202/Tyr204; Cell Signaling Technology, Danvers, MA), phospho-STAT1 (Tyr701; Upstate Signaling Solutions, Charlottesville, VA), phospho-STAT1 (Ser727), STAT1, phospho-Stat3 (Ser727), phospho-cJun (Ser63) (Cell Signaling Technology), calreticulin (Calbiochem), PKCδ (Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal IgG against cytoceratine-18 (M30) (PEVIVA, Stockholm, Sweden), and goat polyclonal IgG against Nuclear lamin A/C (Santa Cruz Biotechnology). Secondary horseradish peroxidase-conjugated anti-rabbit antibodies were from Cell Signaling Technology, anti-mouse was from Rockland Immunochemicals (Gilbertsville, PA), and anti-goat was from Dako Denmark (Glostrup, Denmark).
Luciferase Reporter Assay
Luciferase reporter construct containing consecutive copies of the γ activation site (GAS) or IFN-stimulated response element (ISRE) enhancer elements from Clontech (Mountain View, CA) were used for transfections of RHEK-1 cells. The cells were plated at 105 cells per well in a 24-well plate, and they were cotransfected in triplicate with 0.5 μg of luciferase- and 10 ng of TK-Renilla reporter plasmid by using Lipofectamine 2000 (Invitrogen) for 8 h; thereafter, they were treated with 5000 U/ml IFNα for 16 h. Protein lysates were prepared and assayed for luciferase activity using the Dual-Luciferase Reporter Assay System (Promega). Light emission was detected using an Anthos Lucy3 luminometer and analyzed using the Anthos LucySoft software (Anthos Labtech Instruments, Eugendorf, Austria) and Microsoft Excel.
Cytoplast Preparation and Cell Sorting
Enuclated cells were prepared as described previously (Mandic et al., 2003). Briefly, cells were harvested and resuspended in 12.5% Ficoll with cytochalasin B (5 μg/ml) and incubated 60 min at 37°C. One milliliter of the cell suspension was layered onto a density gradient (1 ml of 25%; 1 ml of 17%; 300 μl of 16%, and 300 μl of 15% Ficoll) and centrifuged at 30,000 rpm in a Sorvall Discovery 90SE ultracentrifuge for 60 min, at 32°C. Cytoplasts were aspirated from the interface between the 25 and 17% Ficoll layers, seeded in poly-l-lysine–coated plates, and allowed to recover for 60 min. Thereafter, the cytoplast preparations were cultured in the presence or absence of IFN for 45 min, 16 h, and 32 h respectively, harvested, and incubated with Hoechst 33258 (10 μg/ml) for 20 min at 37°C or fixed with 4% formaldehyde and stained with PI. Nucleated and enucleated cells were separated based on DNA content on the FACSDiVa (BD Biosciences) with a 360-nm Enterprise II excitation laser and 450/20-nm emission filter, and BD FACSDiva software.
Immunocytochemistry
The cells were cytospun onto glass slides, fixed, and stained as described previously (Thyrell et al., 2002) with a polyclonal antibody against insulin receptor substrate (IRS)-1 (Upstate Signaling Solutions) overnight at + 4°C or mouse monoclonal antibody against cyt c (clone 6H2.B4) and a polyclonal anti-active caspase-3 antibody (BD Biosciences PharMingen) for 1 h at room temperature. Images were acquired at r.t. with a Zeiss Axioplan 2 imaging microscope (with Zeiss Plan–NEOFLUAR 63×/1.4 oil or 100×/1.3 oil objective), Zeiss Axio Cam HRm camera, and Axiovision 4.5 software.
Confocal Microscopy and Time-Lapse Recording
For time-lapse recording, cytoplasts/cells were grown in a glass chamber. Mitochondria with an intact ΔΨm were labeled with the potential-dependent dye TMRE (25 nM) (Invitrogen). Cell nuclei were counterstained with 0.1 μg/ml Hoechst 33342 (Invitrogen). To visualize exposure of PS, annexin V-FLUOS (Roche Diagnostics) was added to the medium. Chambers were place in the POC-chamber/CTI Controller/Heating insert P system for live cell imaging. Time-lapse recording of ΔΨm and annexin V binding was acquired at 15-min intervals. Samples were analyzed under Zeiss 510 Meta confocal laser scanning microscopy equipped with an inverted Zeiss Axiovert 200 m microscope.
RESULTS
Involvement of PKCδ, ERK1/2, and JNK Kinases in IFNα-induced Apoptosis
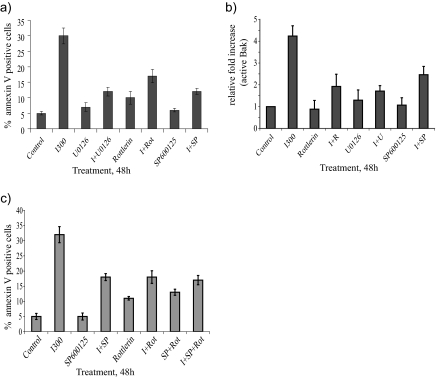
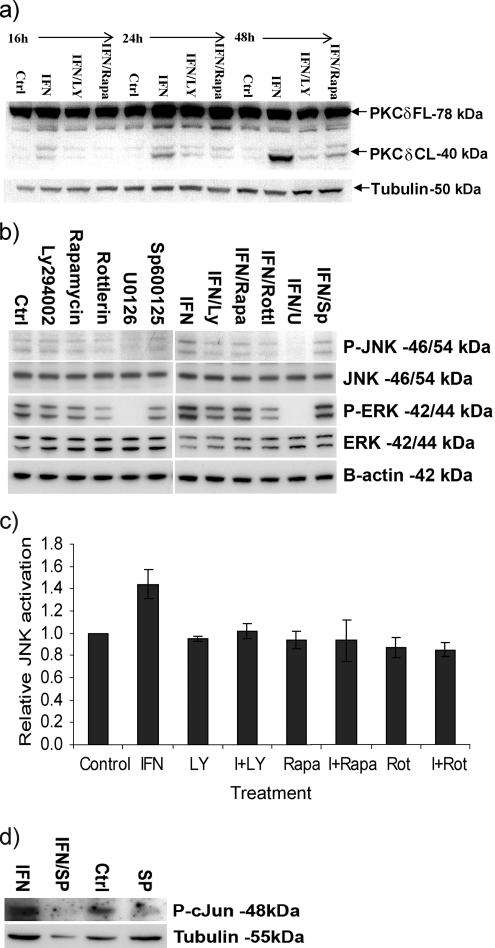
A number of kinases have been shown to be regulated by IFNα, but their involvement in the proapoptotic signaling induced by this cytokine has not been fully elucidated. In this study, a panel of chemical inhibitors was used to identify the kinases that play a key role in IFNα-induced apoptosis. The chemical inhibitors rottlerin (2 μM), U0126 (10 μM), and SP600125 (10 μM), being inhibitors of PKCδ, ERK, and JNK, respectively, were able to potently block the IFNα-mediated proapoptotic response in U266-1984 cells as measured as PS exposure by annexin V stainings (Figure 1a). Furthermore, these inhibitors also similarly blocked the IFNα-induced Bak activation (Figure 1b). The specific inhibition of JNK, PKCδ, and mitogen-activated protein kinase kinase (MEK) activity by SP600125, rottlerin, and U0126 was evaluated by Western blotting as dephosphorylation of cJun (Figure 3c), STAT3 (data not shown), and ERK (Figure 3b), respectively.
Figure 1.
PKCδ, ERK, and JNK are involved in IFNα-induced apoptosis. U266-1984 cells were treated with or without 300 U/ml IFNα for 48 h in the presence or absence of rottlerin, U0126, SP600125, or a combination of inhibitors. To assess apoptosis, cells were stained for annexin V (a and c) and for Bak activation (b) and analyzed by flow cytometry. The percentages of annexin V- and active Bak-positive cells were analyzed by flow cytometry. The bars represent the mean value of three independent experiments.
Figure 3.
PKCδ, ERK, and JNK are sequentially activated downstream of PI3K and mTOR. (a) U266-1984 cells were treated for the indicated time points with 5000 U/ml IFNα in the presence or absence of Ly294002 (10 μM) or rapamycin (1 μM), respectively. The cleavage of PKCδ was detected with immunoblotting. PKCδ-CL: PKCδ cleaved. Tubulin was used as loading control. (b) Immunoblot analysis of IFNα-stimulated phosphorylation of ERK1/2 and JNK1/2 after treatment of U266-1984 cells with Ly294002 (10 μM), rapamycin (1 μM), rottlerin (2 μM), U0126 (10 μM), or SP600125 (10 μM) in the presence or absence of IFNα 5000 U/ml for 16 h. β-Actin was used as a loading control. (c) To further confirm JNK activation as being most proximal to the apoptotic machinery, cells were cultured in the absence or presence of IFNα, with or without pre-incubation with the indicated kinase inhibitors and stained for the phosphorylated form of JNK (Thr183/Tyr185) and analyzed by flow cytometry. The percentages of pJNK-positive cells were quantified using CellQuest software (BD Biosciences) and expressed as relative induction compared with the control cells. (d) Immunoblot analysis of cJun phosphorylation on the serine (S63) residue after treatment of U266-1984 cells with SP600125 (10 μM) in the presence or absence of 5000 U/ml IFNα for 16 h. Tubulin was used as loading control. All results shown are representative of two independent experiments.
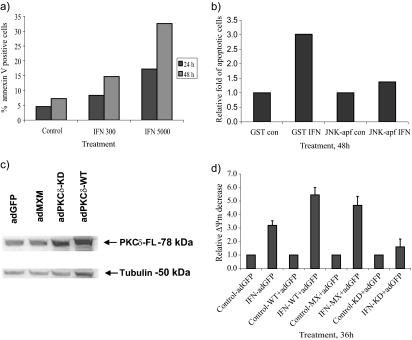
The RHEK-1 cell line undergoes apoptosis with similar kinetics and signaling as U266-1984 cells (Figure 2a; Thyrell et al., 2004). Pretreatment with U0126 and SP600125 yielded comparable results in RHEK-1 as in U266-1984, whereas the PKCδ inhibitor rottlerin was toxic to RHEK-1 cells; thus, its effect could not be evaluated (data not shown). Together, these data suggest that PKCδ, ERK, and JNK are actively involved in the IFNα-induced apoptotic signaling cascade and that they act upstream of the mitochondria.
Figure 2.
Dominant-negative mutants of PKCδ and JNK inhibit IFNα-induced apoptosis. (a) RHEK-1 cells were treated for 24 and 48 h with the indicated amounts of IFNα, and the percentage of annexin V-positive cells was quantified by using the CellQuest software (BD Biosciences). (b) RHEK-1 cells were transiently transfected with GST (control) or JNK1-APF and the amount of FLICA-positive cells in response to IFNα (5000 U/ml; 48 h) was analyzed by flow cytometry. The results shown are representative of two independent experiments. (c and d) U266-1984 cells were coinfected with adGFP plus adPKCδ-WT or adPKCδ-KD or adMXM, and the PKCδ protein expression and the effect on interferon-induced mitochondrial depolarization in GFP-positive cells (d) was analyzed. PKCδ-FL, PKCδ full length. The bars represent the mean value of two independent experiments.
JNK inhibition was also achieved by transient transfection of RHEK-1 cells with a dominant-negative form of JNK1 (dnJNK). Expression of the dnJNK in RHEK-1 cells abolished the IFNα-induced caspase activity as assayed by FLICA positivity in individual cells, further demonstrating that JNK activation is involved in apoptosis induced by IFNα and that JNK activity is required upstream of the mitochondrial events (Figure 2b).
Furthermore, we confirmed the involvement of PKCδ in IFNα-induced apoptosis by using an adenoviral construct encoding a catalytically inactive dominant inhibitory form of PKCδ (adPKCδ-KD) (Li et al., 1995). U266-1984 cells were infected for 6 h with adenoviruses encoding either wild-type PKCδ (adPKCδ-WT), a dominant-negative kinase dead PKCδ (adPKCδ-KD), or a mock vector (adMXM), and expression of PKCδ protein was analyzed by immunoblotting (Figure 2c). AdPKCδ-WT and adPKCδ-KD were abundantly expressed in U266-1984 cells infected with these constructs compared with the cells infected with either adGFP or with adMXM alone, which expressed endogenous levels of PKCδ. AdPKCδ-WT–, adPKCδ-KD–, and adMXM-infected U266-1984 cells were coinfected with adGFP, and the green fluorescent protein (GFP)-positive cell population was assayed for mitochondrial depolarization (loss of ΔΨm). The amount of TMRE fluorescence reflects the integrity of mitochondrial inner membrane potential. We have previously shown that loss of TMRE positivity fully correlate with apoptotic cell death, as measured by other markers such as caspase-3 activity, PS exposure, and TMRE release in U266-1984 cells (Panaretakis et al., 2002). In line with the effect induced by rottlerin, the U266-1984 cells infected with the adPKCδ-KD demonstrated a clear decrease in IFNα-induced apoptosis compared with mock and adPKCδ-WT virus-infected cells (Figure 2d). The RHEK-1 cell line is not infectable with adenovirus and was therefore not included in this experiment.
PI3K and mTOR Activity Is Required for IFNα-induced PKCδ, ERK, and JNK Activation
The proapoptotic cleavage of PKCδ is commonly used as a marker of its activation (Koriyama et al., 1999). IFNα induced cleavage of PKCδ, starting from the 16-h time point (Figure 3a). We have previously shown that IFNα-activated PI3K/mTOR signaling is required for the induction of apoptosis (Thyrell et al., 2004). To determine whether PKCδ activation is downstream of the PI3K/mTOR pathway or whether it is part of a separate pathway, U266-1984 cells were cotreated with IFNα and the PI3K-inhibitor Ly294002 (10 μM) or the mTOR-inhibitor rapamycin (1 μM), for the indicated time points, and their effect on PKCδ cleavage was investigated. Both Ly294002 and rapamycin potently blocked the IFNα-induced apoptosis and PKCδ cleavage (Figure 3a). These data indicate that PI3K and mTOR are acting upstream in the cascade, leading to the IFN-induced activation of PKCδ.
After the potent inhibition of IFNα-induced apoptosis by the MEK inhibitor U0126 (Figure 1a), we also examined the relationship between PI3K/mTOR and IFNα-induced ERK phosphorylation. IFNα induced ERK phosphorylation after 8 and 16 h of treatment of U266-1984 (data not shown; Figure 3b). This phosphorylation was inhibited by U0126 (10 μM), Ly294002 (10 μM), rapamycin (1 μM), and rottlerin (2 μM), but not by SP600125 (10 μM). These results indicate that ERK activation is dependent on functional PI3K, mTOR and PKCδ signaling, but not on JNK activation.
IFNα-induced PKCδ and ERK Act Upstream of JNK
JNK activation is mediated by phosphorylation on key threonine and tyrosine residues (Davis, 2000). Because IFNα-induced apoptosis requires JNK activation, the position of this kinase in the IFNα-induced proapoptotic signaling cascade was also investigated. IFNα-induced JNK phosphorylation after 8 h (data not shown) and 16 h of treatment of U266-1984 cells was measured by Western blotting and flow cytometry (Figure 3, b and c). IFN-induced JNK phosphorylation was inhibited by Ly294002 (10 μM), rapamycin (1 μM), rottlerin (2 μM), and U0126 (10 μM), indicating that JNK is activated downstream of PI3K, mTOR, PKCδ and ERK (Figure 3, b and c). In addition, SP600125 was not able to block ERK activation (Figure 3b) or IFNα-induced PKCδ cleavage (data not shown). IFNα-induced JNK activity was also demonstrated as enhanced phosphorylation of cJun, a downstream target of the SAPK/JNK pathway, after 16 h of treatment. The specific inhibition of JNK activity by SP600125 at a concentration of 10 μM was confirmed by dephosphorylation of cJun (Figure 3d). These data point toward a linear signaling cascade induced by IFNα, and they are further supported by the fact that the combination of SP600125 and rottlerin do not synergize in inhibiting IFNα-induced apoptosis (Figure 1c).
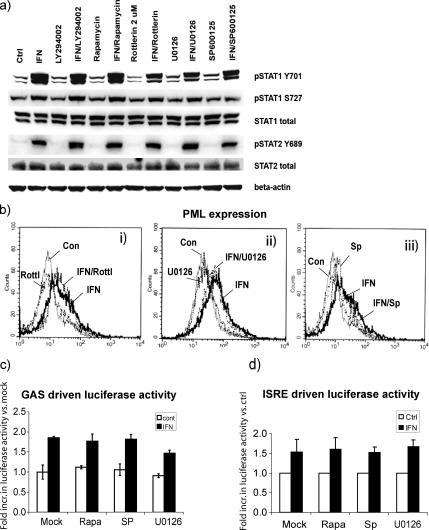
PKCδ, ERK, and JNK Activation Does Not Affect JAK/STAT Signaling
To examine whether the kinase inhibitors rottlerin, U0126, and SP600125 act by blocking JAK/STAT signaling, we performed immunoblot analysis of the phosphorylation states of STAT1 and STAT2, and flow cytometric analysis and immunoblotting for the expression of known interferon-stimulated genes (ISGs). IFNα-induced serine and tyrosine phosphorylation of STAT1 was not inhibited by either Ly294002, rapamycin, rottlerin, U0126, or SP600125 (Figure 4a). In addition, expression of known IFNα stimulated genes, including promyelocytic leukemia protein (PML), interferon regulated factor-9 and Mx, was not altered by the presence of kinase inhibitors (Figure 4b; data not shown). In line with these data, IFN-induced GAS and ISRE regulated reporter activity in RHEK-1 cells was not affected by either rapamycin or SP600125. U0126 did cause a moderate reduction of GAS-, but not ISRE-, regulated luciferase reporter activity, suggesting that the ERK signaling pathway might be partially involved in IFNα-dependent gene regulation (Figure 4, c and d). In summary, the canonical JAK/STAT signaling that results in the activation of gene expression was not affected by the inhibition of PKCδ, ERK, or JNK.
Figure 4.
Rottlerin, U0126, and SP600125 do not affect IFNα-induced JAK/STAT signaling. (a) Immunoblot analysis of phosphorylation on residue tyrosine (Y701) and serine (S727) of STAT1 and residue tyrosine (Y689) of STAT2 after 30 min of 300 U/ml IFNα treatment in the presence or absence of inhibitors: Ly294002 (10 μM), rapamycin (1 μM), rottlerin (2 μM), SP600125 (10 μM), or U0126 (10 μM). (b) Flow cytometric analysis of the PML protein levels after 300 U/ml IFNα treatment for 24 h. Gray thin line, control cells; black bold line, IFNα-treated cells. i, black dash and dot, IFNα + rottlerin-treated cells; black dashed line, rottlerin-treated cells. ii, black dash and dot, IFNα + U0126-treated cells; black dashed line, U0126-treated cells. iii, black dash and dot, IFNα + SP600125-treated cells; black dashed line, SP600125-treated cells. The results shown are representative of two independent experiments. (c) IFN-induced transcription of a GAS-regulated luciferase construct in the presence and absence of kinase inhibitors rapamycin (1 μM), SP600125 (10 μM), and U0126 (10 μM). (d) IFN-induced transcription of an ISRE regulated luciferase construct in the presence and absence of kinase inhibitors rapamycin (1 μM), SP600125 (10 μM), and U0126 (10 μM).
Cytoplasts Maintain the Ability to Respond to IFNα
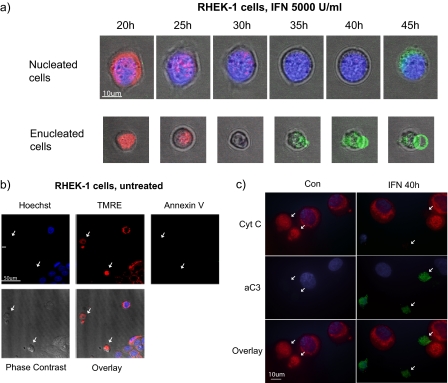
The kinases identified above activate signaling cascades with both cytoplasmic and nuclear targets. To further investigate the role of cytoplasmic events that are independent of de novo gene expression in IFNα-induced apoptosis, we prepared enucleated cells, cytoplasts. For the cytoplast preparation, RHEK-1 cells were used because U266-1984 multiple myeloma cells have a large nucleus and a small cytoplasm, and upon removal of the nucleus the cells do not maintain a functional structure, resulting in nonviable cytoplasts. Cytoplasts prepared from RHEK-1 cells, in contrast, could be kept in culture for at least 48 h without any major loss in viability as shown by morphology, mitochondrial integrity, and absence of PS exposure (Figure 6b).
Figure 6.
IFNα induces cell death in enucleated cells. Live cell imaging by time-lapse microscopy. RHEK-1 cytoplasts and nucleated cells were either treated with 5000 U/ml IFN for 45 h (a) or left untreated (b). Images were taken every 15 min, and the indicated time points were selected to demonstrate changes in the apoptotic markers. RHEK-1 cytoplasts and nucleated cells were stained for nuclear morphology (Hoechst, blue) in combination with TMRE (red) and annexin V (green). Cytoplasts are indicated by arrows. (c) Immunocytochemical analysis of caspase-3 activation (green) and cyt c (red). Release of cyt c manifests as absence of mitochondrial staining. Cytoplasts are indicated by arrows.
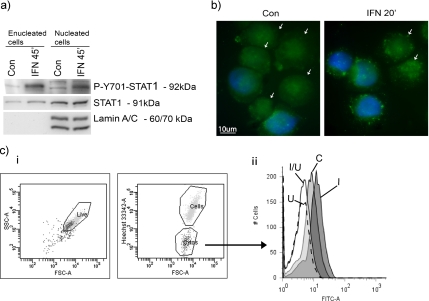
To verify that cytoplasts can function as a valid model-system to study IFNα-effects, we confirmed that IFN-receptor signaling was intact after enucleation. STAT1 tyrosine phosphorylation could clearly be detected upon IFNα treatment as analyzed by Western blotting of protein extracts from fluorescence-activated cell sorting (FACS)-sorted cytoplasts and from nucleated cells (Figure 5a). Nuclear lamin A/C was used as a control of the purity of the cytoplast fraction.
Figure 5.
IFNα activated signaling is intact in enucleated cells. (a) IFNα-receptor signaling is intact in enucleated cells. Immunoblot analysis of the FACS-sorted RHEK-1 cytoplasts and nucleated cells treated with 5000 U/ml IFN for 45 min for the tyrosine 701 phosphorylated STAT1, total STAT1, and lamin A/C. These data are representative of two independent experiments. (b) Immunostaining of RHEK-1 cytoplasts and nucleated cells treated with 5000 U/ml IFN for 20 min and stained for total IRS-1. Cytoplasts are indicated by arrows. (c) Flow cytometric analysis of JNK phosphorylation in enucleated cells after 8 h of IFNα. Hoechst staining was used to separate enucleated from nucleated. The histogram shows phosphorylated JNK in the cytoplast population only. C, control; U, U0126 (10 μM); I, IFN (5000 U/ml); and I/U, IFN (5000 U/ml) + U0126 (10 μM).
IRS-1 is an adaptor protein commonly involved in growth factor receptor signaling. IRS-1 is also activated through tyrosine phosphorylation by JAKs in response to IFNα (Burfoot et al., 1997), and it contains a Scr homology 2-domain that recruits p85, the regulatory subunit of PI3K, to the receptor, thus leading to the activation of PI3K (Shoelson et al., 1992). To verify that the link between the IFN receptor and PI3K was unaffected by the cytoplast preparation, we also examined whether IRS-1 translocates from the cytoplasm to the plasma membrane in response to IFNα treatment. Immunostaining shows that a clear IFNα-induced IRS-1 translocation from the cytoplasm to the plasma membrane occurs both in cytoplast and nucleated cells, (Figure 5b). In addition, we confirmed that the kinase cascade activated in nucleated cells was also activated in cytoplasts. We chose to study JNK activation because we found this kinase to be activated most proximal to the mitochondrial apoptotic machinery and dependent on PI3K, mTOR, PKCδ, and ERK activity. Cells and cytoplasts were stained with Hoechst to allow separation of enucleated cells from nucleated cells by electronic gating and an antibody recognizing the phosphorylated form of JNK (Thr183/Tyr185). The flow cytometric analysis showed a clear activation of JNK in cytoplasts (Figure 5c) and in nucleated cells (data not shown). JNK activation was also confirmed in FACS-sorted cytoplasts by Western blotting (data not shown).
In conclusion, we demonstrated that cytoplasts are viable and have an intact signaling response to IFNα. Thus, cytoplasts were hereafter used as a tool to study the importance of cytoplasmic events, in the absence of de novo transcription, in IFNα-induced apoptosis.
IFNα Induces Cell Death in Enucleated Cells
The morphology and rate of death of the cytoplast preparation, cultured in the presence or absence of IFNα, was followed using time-lapse microscopy (Figure 6, a and b). Around 25 h after IFNα exposure, the RHEK-1 cytoplasts and nucleated cells displayed signs of apoptotic mitochondrial depolarization, shown as loss of TMRE positivity. Loss of ΔΨm was followed by blebbing of the plasma membrane and thereafter exposure of PS, as shown by annexin V-FLUOS positivity (Figure 6a). Control cytoplasts, cultured in medium alone, were viable and maintained their morphology, ΔΨm and annexin V negativity at this time point (Figure 6b). Although photobleaching is a potential risk with the time-lapse method, it is highly unlikely to have an impact on the results in this study. The loss of TMRE correlates with annexin V positivity and morphological changes, which both occur at late time points. Also, if the TMRE stain was bleached, it would be lost in all cells simultaneously, whereas in our time-lapse TMRE negativity is a stochastic event that occurs in concert with the appearance of annexin V positivity and alteration in morphology of that particular cell.
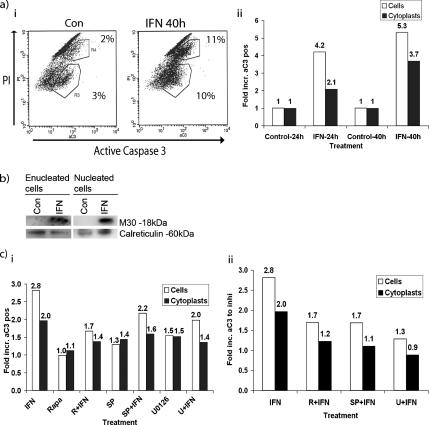
Apoptosis was also studied in individual cytoplasts by immunocytochemistry (Figure 6c) and flow cytometry (Figure 7a), to obtain more quantitative results. Activation of caspase-3 was assessed by flow cytometry by using the antibody that specifically recognizes activated caspase-3. The samples were costained with PI to distinguish cytoplasts from contaminating nucleated cells (∼10-fold higher PI fluorescence), allowing analysis of both populations separately by electronic gating (as shown in Figure 5c). IFNα treatment was found to cause a significant increase in the amount of cells staining positive for active caspase-3 both in gated cytoplasts and in nucleated cells (t = 0.012 and t = 0.006 respectively), although the proportion of apoptotic cells was generally less in the IFN-treated cytoplasts compared with the nucleated cells (Figures 6c and 7a). The results were also verified using FLICA (pan-caspase activity) and annexin V (data not shown). The numbers in Figure 7a represent percentage of the total population staining positive for active caspase-3.
Figure 7.
IFNα induces caspase activation in cytoplasts. (a) The cytoplast preparation from RHEK-1 cells was either left untreated or treated with 5000 U/ml IFN for 40 h. The caspase-3 activity in relation to DNA content was assessed by flow cytometry by using an antibody that specifically recognizes active caspase-3, and PI, respectively. i, nucleated RHEK-1 cells (with ∼10-fold higher PI fluorescence, in the top part of the dot plot) represent a contamination of the cytoplast preparation. Numbers represent percentage of the total population staining positive for active caspase-3. Left, untreated cytoplasts; right, cytoplasts treated with 5000 U/ml IFNα for 40 h. The shift to the right in the dot plot represents an increase in active caspase-3–specific immunofluorescence. ii, RHEK-1 cytoplasts and nucleated cells were treated with IFNα for 24 and 40 h. The levels of active caspase-3 were analyzed by flow cytometry. One representative experiment, shown here as -fold increase of active caspase-3 relative to control. (b) After treatment with IFNα for 40 h, the RHEK-1 cytoplasts were separated from nucleated cells by using Hoechst staining and then sorted by using FACSDiVa. The isolated cytoplasts were analyzed by Western blotting, and the cleavage of cytokeratin-18 was detected using M30 antibody. Calreticulin was used as loading control and cytoplasmic marker. (c) Rapamycin, SP600125, and U0126 inhibit IFNα induced caspase cleavage in cytoplasts. RHEK-1 cytoplasts and nucleated cells were pretreated with the indicated inhibitors for 1 h, followed by IFNα for another 40 h. The levels of active caspase-3 were analyzed by flow cytometry, and they are presented as -fold relative to control (i). SP600125 and U0126 had a moderate toxicity in control cells. For clarity, ii shows the same experiment as in i, presented as -fold increase of active caspase-3 by IFNα relative to treatment with respective inhibitor in the absence of IFNα.
The immunocytochemistry demonstrated that no mitochondrial cyt c could be detected in cytoplasts or nucleated cells with active caspase-3 (Figure 6c), which is expected because caspase-3 is generally activated succeeding mitochondrial depolarization and release of cyt c.
Apart from staining for active caspase-3 in individual cells, the M30 apoptosense assay was used to detect apoptosis induced by IFNα biochemically by immunoblotting. Because the cytoplast preparation also contains a fraction of nucleated cells the samples were first FACS sorted to allow biochemical analysis of cytoplasts only. Briefly, the M30 antibody recognizes an epitope of cytokeratin-18, which is only exposed after this protein has been cleaved by caspase-3 and can thus be used as a specific marker of caspase-dependent apoptotic cell death (Leers et al., 1999). Western blotting with the M30 antibody on extracts from flow sorted cytoplasts showed the appearance of the 18-kDa cleaved fragment of cytokeratin-18 after IFNα-treatment for 40 h (Figure 7b).
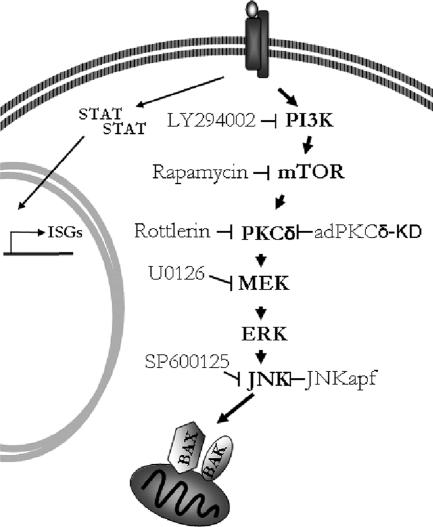
Cytoplasts were also pretreated with chemical inhibitors of MEK, mTOR, and JNK followed by IFNα, to define whether these kinases remain critical for the execution of IFNα-induced apoptosis in enucleated cells. The inhibitors had a similarly protective role in cytoplasts as in nucleated cells, further supporting that cytoplasmic phosphorylation cascades are crucial for the proapoptotic function of IFNα (Figure 7c, i and ii). Figure 8 shows our proposed model for activation of noncanonical signaling, which is essential for the proapoptotic effects of IFNα in this system.
Figure 8.
Proposed model of IFNα-induced activation of noncanonical signaling, shown to be essential for the proapoptotic effects of IFNα.
DISCUSSION
We have recently found that the activity of PI3K and mTOR, two kinases otherwise mainly associated with prosurvival signals, were required for the IFNα-induced activation of the mitochondrial apoptotic pathway in several tumor cell lines (Thyrell et al., 2004). This finding raised two major questions, namely, what signaling downstream of PI3K and mTOR would lead to the activation of the proapoptotic Bak, and is there a requirement for STAT-induced changes in gene transcription for the apoptotic process induced by IFNα in this system? In the present study, we have identified three kinases; PKCδ, the stress-related JNK/SAPK, and the ERK/MAPK, to be activated downstream of PI3K/mTOR and we have also defined the importance of their involvement in IFNα-induced apoptosis.
A key event in the induction of the proapoptotic activity of PKCδ is its caspase-mediated cleavage to a 40-kDa fragment (Koriyama et al., 1999), and overexpression of this catalytic fragment rapidly leads to apoptosis (Ghayur et al., 1996). We found that IFNα induced PKCδ cleavage in U266-1984 myeloma cells. Furthermore, inhibition of both PI3K and mTOR completely blocked this cleavage, indicating that this activation occurs downstream of PI3K and mTOR. It has been previously shown that PKCδ is a direct/indirect downstream target of PI3K and mTOR in other systems (Kumar et al., 2000; Baek et al., 2001). PKCδ activation has been shown to occur as the result of cleavage, and by phosphorylation (Kikkawa et al., 2002), although the exact relationship between these events has not been clearly established. A PI3K/mTOR-dependent phosphorylation of PKCδ may make it amenable for the efficient cleavage to the active proapoptotic 40-kDa fragment. Alternatively, it is possible that PI3K and/or mTOR directly or indirectly phosphorylate/activate a caspase/protease responsible for the cleavage of PKCδ in U266-1984 cells, thus leading to its conversion to its active proapoptotic form.
Although ERK activation has mainly been associated with survival and proliferation, the ERK module is also involved in apoptosis in some systems, such as apoptosis induced by cisplatin and asbestos (Hayakawa et al., 1999; Persons et al., 1999). Inhibition of PKCδ by rottlerin led to a significant inhibition of ERK phosphorylation and reduced apoptosis in response to IFNα. Indeed, PKCδ has been shown to activate MEK/ERK signaling and through this pathway to promote 12-O-tetradecanoylphorbol-13-acetate-induced apoptosis (Ueda et al., 1996).
JNK has previously been reported to be activated in response to IFNα (Caraglia et al., 1999; Takada et al., 2005). However, the relationship of JNK to the mitochondrial proapoptotic events elicited by IFNα and to the other kinases involved in this response has not been analyzed. We found that JNK activation is required for IFN′s proapoptotic activity downstream of PKCδ and ERK, but upstream of the mitochondria. In response to stress, JNK activation commonly occurs via a stepwise phosphorylation on serine/threonine and tyrosine residues by the MAPK kinase module Mkk4/7 (Davis, 2000). Notably, after infrared treatment, PKCδ has been shown to induce the activation of Mkk7, which in turn phosphorylated and activated JNK (Mitsutake et al., 2001), making a similar scenario plausible in response to IFNα, although this remains to be established. Among the kinases analyzed in the present study, JNK activation was found to be the most proximal to the activation of Bak. There are several hypotheses regarding the mechanisms behind Bak activation. A widely accepted model involves the BH3-only proteins (BOPs), which are either directly activating Bak or may sequester antiapoptotic Bcl-2 family members and therefore lower the barrier for efficient Bak activation. One of the BOPs capable of directly binding and activating Bak is Bim. It was recently shown that JNK is able to phosphorylate and release Bim from its interaction with dynein, which in turn activates Bax and Bak (Lei and Davis, 2003). Whether Bim or another BOP is responsible for IFNα-induced Bak activation remains to be established.
It is generally believed that the cellular effects of IFNα are exerted by the expression of IFN-stimulated genes, regulated through the JAK/STAT pathway (Ihle and Kerr, 1995; Darnell, 1997). We and others have previously shown that the IFNα-induced activation of the PI3K/mTOR module, that in the present study was found to act upstream of PKCδ, ERK, and JNK, acts independently of STAT activation (Thyrell et al., 2004; Kaur et al., 2005). Conversely, it has previously been noted that both type I and type II IFN-induced PKCδ activation can be responsible for the phosphorylation of STAT1 on Ser727 and for STAT-dependent gene expression in some systems (Uddin et al., 2002; Kaur et al., 2005). However, our data do not support a role for PKCδ in STAT activation in the present system, because pretreatment with rottlerin did not alter STAT1 serine or tyrosine phosphorylation or the induction of several known IFN-stimulated genes. Similarly, inhibition of JNK, which resulted in a clear block of apoptosis, did not affect IFNα-induced STAT phosphorylation or induction of known ISGs. This implies that STAT activation is not sufficient for executing IFN-induced apoptosis. This fact, together with strong evidence for the cytoplasmic phosphorylation cascades being of a major importance, prompted us to explore the possibility that IFNα-induced apoptosis might occur in the absence of nuclear events.
To answer this question we generated enucleated RHEK-1 cells that we demonstrated were proficient in responding to IFNα, as judged by both STAT1 tyrosine phosphorylation and IRS-1 translocation to the membrane. By using a variety of techniques including time-lapse microscopy, immunoblotting and flow cytometry we showed that cytoplasts undergo apoptosis in response to IFNα treatment. This was also substantiated by the use of an array of morphological and biochemical apoptotic markers including cell morphology, mitochondrial transmembrane potential, release of cyt c and annexin V positivity, showing that the time of the apoptotic onset in the cytoplasts was similar to the kinetics of apoptotic cell death in nucleated cells. Thus, our data demonstrate that death by IFNα can occur efficiently also in the absence of nucleus.
Furthermore, mTOR, JNK and ERK inhibitors blocked IFN-induced apoptosis in RHEK-1 cytoplasts, suggesting that IFN-activated apoptosis is indeed mediated through these cytoplasmic components. Our data thus provide strong evidence that the proapoptotic program activated by IFNα does not require de novo transcription. However, due to a partial reduction in cell death seen in cytoplasts as compared with nucleated cells, it cannot be fully excluded that the apoptosis induced by IFNα in intact nucleated cells may also include a nucleus dependent component.
In summary, the present investigation demonstrates for the first time that IFN-induced cytoplasmic cascades initiated by PI3K and mTOR upstream of PKCδ, JNK, and ERK signaling are specifically involved in IFNα-induced apoptosis at a step upstream of Bak activation. Importantly, the findings also show the IFNα-induced activation and proapoptotic outcome of these kinase cascades to be independent of STAT-regulated de novo transcription of ISGs. The requirement of stress-related signaling in response to IFNα is surprisingly similar to the pathways used by various types of DNA-damaging agents, such as doxorubicin (Panaretakis et al., 2005). The finding of the involvement of specific signaling cascades in the proapoptotic activities of IFNα will ultimately aid in delineating the mechanisms of IFN action and will also help to optimize clinical use of IFN in malignant and viral diseases.
ACKNOWLEDGMENTS
We thank Dr. Trevor Biden (Garvan Institute of Medical Research, Faculty of Medicine, The University of New South Wales, Australia) for generously providing the adenoviral constructs adPKCd-wt, adPKCd-KD, and adMXM and Dr. R. J. Davis (Howard Hughes Medical Institute Research Laboratories, University of Massachusetts Medical Center, Worcester, MA) for sharing the pcDNA3-JNK1-WT and pcDNA3-JNK1-APF constructs. Birgitta Wester is gratefully acknowledged for excellent technical assistance with cell sorting. This study was supported by the Swedish Cancer Society, the Cancer Society of Stockholm, the Swedish Research Council, and the Schering Plough Corporation.
Abbreviations used:
- BOP
BH3-only protein
- cyt c
cytochrome c
- ERK
extracellular signal-regulated kinase
- IFN
interferon
- IRS
insulin receptor substrate
- ISG
interferon-stimulated gene
- JAK
Janus tyrosine kinase
- MEK
mitogen-activated protein kinase kinase
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositol 3-kinase
- PS
phosphatidylserine
- STAT
signal transducer and activator of transcription
- TMRE
tetramethylrhodamine ethyl ester.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-04-0358) on October 17, 2007.
REFERENCES
- Baek S. H., Lee U. Y., Park E. M., Han M. Y., Lee Y. S., Park Y. M. Role of protein kinase Cdelta in transmitting hypoxia signal to HSF and HIF-1. J. Cell. Physiol. 2001;188:223–235. doi: 10.1002/jcp.1117. [DOI] [PubMed] [Google Scholar]
- Burfoot M. S., Rogers N. C., Watling D., Smith J. M., Pons S., Paonessaw G., Pellegrini S., White M. F., Kerr I. M. Janus kinase-dependent activation of insulin receptor substrate 1 in response to interleukin-4, oncostatin M, and the interferons. J. Biol. Chem. 1997;272:24183–24190. doi: 10.1074/jbc.272.39.24183. [DOI] [PubMed] [Google Scholar]
- Caraglia M., et al. Interferon-alpha induces apoptosis in human KB cells through a stress-dependent mitogen activated protein kinase pathway that is antagonized by epidermal growth factor. Cell Death Differ. 1999;6:773–780. doi: 10.1038/sj.cdd.4400550. [DOI] [PubMed] [Google Scholar]
- Carpenter L., Cordery D., Biden T. J. Protein kinase Cdelta activation by interleukin-1beta stabilizes inducible nitric-oxide synthase mRNA in pancreatic beta-cells. J. Biol. Chem. 2001;276:5368–5374. doi: 10.1074/jbc.M010036200. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Davis R. J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Ghayur T., et al. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J. Exp. Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grander D. How does interferon-alpha exert its antitumour activity in multiple myeloma? Acta Oncol. 2000;39:801–805. doi: 10.1080/028418600750063532. [DOI] [PubMed] [Google Scholar]
- Griffiths G. J., Dubrez L., Morgan C. P., Jones N. A., Whitehouse J., Corfe B. M., Dive C., Hickman J. A. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa J., Ohmichi M., Kurachi H., Ikegami H., Kimura A., Matsuoka T., Jikihara H., Mercola D., Murata Y. Inhibition of extracellular signal-regulated protein kinase or c-Jun N-terminal protein kinase cascade, differentially activated by cisplatin, sensitizes human ovarian cancer cell line. J. Biol. Chem. 1999;274:31648–31654. doi: 10.1074/jbc.274.44.31648. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Kerr I. M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- Katsoulidis E., Li Y., Mears H., Platanias L. C. The p38 mitogen-activated protein kinase pathway in interferon signal transduction. J. Interferon Cytokine Res. 2005;25:749–756. doi: 10.1089/jir.2005.25.749. [DOI] [PubMed] [Google Scholar]
- Kaur S., et al. Role of protein kinase C-delta (PKC-delta) in the generation of the effects of IFN-alpha in chronic myelogenous leukemia cells. Exp. Hematol. 2005;33:550–557. doi: 10.1016/j.exphem.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Matsuzaki H., Yamamoto T. Protein kinase C delta (PKC delta): activation mechanisms and functions. J. Biochem. 2002;132:831–839. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- Koriyama H., Kouchi Z., Umeda T., Saido T. C., Momoi T., Ishiura S., Suzuki K. Proteolytic activation of protein kinase C delta and epsilon by caspase-3 in U937 cells during chemotherapeutic agent-induced apoptosis. Cell Signal. 1999;11:831–838. doi: 10.1016/s0898-6568(99)00055-8. [DOI] [PubMed] [Google Scholar]
- Kumar V., Pandey P., Sabatini D., Kumar M., Majumder P. K., Bharti A., Carmichael G., Kufe D., Kharbanda S. Functional interaction between RAFT1/FRAP/mTOR and protein kinase cdelta in the regulation of cap-dependent initiation of translation. EMBO J. 2000;19:1087–1097. doi: 10.1093/emboj/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leers M. P., et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J. Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lei K., Davis R. J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Yu J. C., Shin D. Y., Pierce J. H. Characterization of a protein kinase C-delta (PKC-delta) ATP binding mutant. An inactive enzyme that competitively inhibits wild type PKC-delta enzymatic activity. J. Biol. Chem. 1995;270:8311–8318. doi: 10.1074/jbc.270.14.8311. [DOI] [PubMed] [Google Scholar]
- Manabe A., Yi T., Kumagai M., Campana D. Use of stroma-supported cultures of leukemic cells to assess antileukemic drugs. I. Cytotoxicity of interferon alpha in acute lymphoblastic leukemia. Leukemia. 1993;7:1990–1995. [PubMed] [Google Scholar]
- Mandic A., Hansson J., Linder S., Shoshan M. C. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J. Biol. Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- Mitsutake N., Namba H., Shklyaev S. S., Tsukazaki T., Ohtsuru A., Ohba M., Kuroki T., Ayabe H., Yamashita S. PKC delta mediates ionizing radiation-induced activation of c-Jun NH(2)-terminal kinase through MKK7 in human thyroid cells. Oncogene. 2001;20:989–996. doi: 10.1038/sj.onc.1204179. [DOI] [PubMed] [Google Scholar]
- Nguyen H., Ramana C. V., Bayes J., Stark G. R. Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J. Biol. Chem. 2001;276:33361–33368. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Ponten J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp. Immunol. 1970;7:477–489. [PMC free article] [PubMed] [Google Scholar]
- Panaretakis T., Laane E., Pokrovskaja K., Bjorklund A. C., Moustakas A., Zhivotovsky B., Heyman M., Shoshan M. C., Grander D. Doxorubicin requires the sequential activation of caspase-2, protein kinase C{delta}, and c-Jun NH2-terminal kinase to induce apoptosis. Mol. Biol. Cell. 2005;16:3821–3831. doi: 10.1091/mbc.E04-10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaretakis T., Pokrovskaja K., Shoshan M. C., Grander D. Activation of Bak, Bax, and BH3-only proteins in the apoptotic response to doxorubicin. J. Biol. Chem. 2002;277:44317–44326. doi: 10.1074/jbc.M205273200. [DOI] [PubMed] [Google Scholar]
- Panaretakis T., Pokrovskaja K., Shoshan M. C., Grander D. Interferon-alpha-induced apoptosis in U266 cells is associated with activation of the proapoptotic Bcl-2 family members Bak and Bax. Oncogene. 2003;22:4543–4556. doi: 10.1038/sj.onc.1206503. [DOI] [PubMed] [Google Scholar]
- Parmar S., Platanias L. C. Interferons: mechanisms of action and clinical applications. Curr. Opin. Oncol. 2003;15:431–439. doi: 10.1097/00001622-200311000-00005. [DOI] [PubMed] [Google Scholar]
- Persons D. L., Yazlovitskaya E. M., Cui W., Pelling J. C. Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clin. Cancer Res. 1999;5:1007–1014. [PubMed] [Google Scholar]
- Poste G. Enucleation of mammalian cells by cytochalasin B. I. Characterization of anucleate cells. Exp. Cell Res. 1972;73:273–286. doi: 10.1016/0014-4827(72)90049-3. [DOI] [PubMed] [Google Scholar]
- Rhim J. S. Neoplastic transformation of human epithelial cells in vitro. Anticancer Res. 1989;9:1345–1365. [PubMed] [Google Scholar]
- Shoelson S. E., Chatterjee S., Chaudhuri M., White M. F. YMXM motifs of IRS-1 define substrate specificity of the insulin receptor kinase. Proc. Natl. Acad. Sci. USA. 1992;89:2027–2031. doi: 10.1073/pnas.89.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strander H., Einhorn S. Interferons and the tumor cell. Biotherapy. 1996;8:213–218. doi: 10.1007/BF01877207. [DOI] [PubMed] [Google Scholar]
- Takada E., Shimo K., Hata K., Abiake M., Mukai Y., Moriyama M., Heasley L., Mizuguchi J. Interferon-beta-induced activation of c-Jun NH2-terminal kinase mediates apoptosis through up-regulation of CD95 in CH31 B lymphoma cells. Exp. Cell Res. 2005;304:518–530. doi: 10.1016/j.yexcr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Thyrell L., Erickson S., Zhivotovsky B., Pokrovskaja K., Sangfelt O., Castro J., Einhorn S., Grander D. Mechanisms of Interferon-alpha induced apoptosis in malignant cells. Oncogene. 2002;21:1251–1262. doi: 10.1038/sj.onc.1205179. [DOI] [PubMed] [Google Scholar]
- Thyrell L., Hjortsberg L., Arulampalam V., Panaretakis T., Uhles S., Dagnell M., Zhivotovsky B., Leibiger I., Grander D., Pokrovskaja K. Interferon alpha-induced apoptosis in tumor cells is mediated through the phosphoinositide 3-kinase/mammalian target of rapamycin signaling pathway. J. Biol. Chem. 2004;279:24152–24162. doi: 10.1074/jbc.M312219200. [DOI] [PubMed] [Google Scholar]
- Uddin S., Sassano A., Deb D. K., Verma A., Majchrzak B., Rahman A., Malik A. B., Fish E. N., Platanias L. C. Protein kinase C-delta (PKC-delta) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J. Biol. Chem. 2002;277:14408–14416. doi: 10.1074/jbc.M109671200. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Hirai S., Osada S., Suzuki A., Mizuno K., Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J. Biol. Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- Wang G. Q., Gastman B. R., Wieckowski E., Goldstein L. A., Gambotto A., Kim T. H., Fang B., Rabinovitz A., Yin X. M., Rabinowich H. A role for mitochondrial Bak in apoptotic response to anticancer drugs. J. Biol. Chem. 2001;276:34307–34317. doi: 10.1074/jbc.M103526200. [DOI] [PubMed] [Google Scholar]
- Zhao K. W., Li D., Zhao Q., Huang Y., Silverman R. H., Sims P. J., Chen G. Q. Interferon-alpha-induced expression of phospholipid scramblase 1 through STAT1 requires the sequential activation of protein kinase Cdelta and JNK. J. Biol. Chem. 2005;280:42707–42714. doi: 10.1074/jbc.M506178200. [DOI] [PubMed] [Google Scholar]