Abstract
In dividing cells, expression of mutations is DNA strand symmetric. Of all mutations originating de novo in nondividing cells, only those in the transcribed (noncoding) strand are immediately expressed in mRNA and protein. In contrast, any new mutation in the nontranscribed (coding) strand remains unexpressed until the cells enter S phase and begin proliferation. This previously unrecognized difference enables us to examine the cell cycle-dependent origin of multiple tumorigenic mutations in stem cells. The human p53 gene, which acts as a gatekeeper in the control of G1 to S phase transition, was chosen for the analysis. Of all multiple mutations contained in p53 databases, we have tested in detail CpG transitions. Three features of CpG sites dictate this choice: C → T transitions at methylated mCpG are the direct product of mC deamination and are replication-independent; it is easy to identify the strand bearing a primary mC → T event because C → T on the transcribed strand appears as G → A on the nontranscribed strand; and CpG transitions are the most frequent (as both singular and multiple occurrences) tumor-related p53 mutations. The origin of double nonsilent CpG transitions in nondividing cells predicts a significant excess of the heterostrand (C → T, G → A) doublets over the homostrand (C → T, C → T and G → A, G → A) doublets. For p53, we found such an excess. Based on this result, along with the results of three other tests reported here, we conclude that the majority of multiple p53 mutations from human tumors occurred in quiescent stem cells.
Keywords: DNA strand asymmetry/mCpG sites/p53 tumorigenicity/multiple mutations
The modern mutation theory of carcinogenesis addresses the same classic “Luria–Delbruck vs. Cairns” dilemma (1–4) that has been formulated for so-called “adaptive” mutagenesis in bacteria (5) and yeast (6) cells under stressful conditions. The central point of the dilemma is whether selection for preexisting mutations predominates over an immediate adaptive mutational response. Recent findings (7, 8) partly reconciled these polar views by showing that when bacteria are strongly stressed by starvation, allowing survival but not growth, some cells generate multiple mutations. Similarly, in the absence of growth, multiple tumorigenic mutations may originate (1–3).
Cancer is commonly assumed to result from an accumulation of multiple genetic changes, perhaps including as many as five to seven successive point mutations (9, 10). Consistent with the multihit precondition of malignant development is the age distribution of cancer incidence, with the exception of retinoblastoma caused by two mutational events (11, 12). However, the spontaneous germinal mutation rate is too small to provide this tumor-prone accumulation in a human lifetime (10). This led to the hypothesis (1) that genes that control cell division must be hypermutable in premalignant stem cells.
The best candidates to examine for multiple mutations are tumor suppressor genes, especially p53, in which mutations occur in nearly 50% of human cancers. There are numerous instances of so-called “multiplets” (usually doublets) in which more than one p53 mutation has been found to occur in the same tumor sample (13). In the majority of cases single p53 mutations (singlets) have been observed. Multiplets that include silent p53 mutations are of particular interest because silent mutations represent selectively innocuous events, often being merely hitchhikers with the nonsilent partner, which confers proliferative advantage and which drives tumor growth (2, 14). Silent p53 mutations appear in tumors about 20 times more frequently than might be expected (2). Also, the Poisson model fits the observed data on tumors with one, two, three, and more p53 mutations fairly well (2). All of the above observations indicate that hypermutagenesis operates on the p53 gene in tumor formation.
There is an intriguing analogy (1) between the situation that occurs when mutations in growth-limiting genes of nutritionally stressed bacteria result in a selective advantage and that which occurs when mutations in p53 lead to tumor formation. The p53 gene appears to integrate cellular responses to environmental insults (15), with the wild-type p53 protein acting as a guardian against tumor formation either by arresting cells with DNA damage (mainly in the G1 phase of the cell cycle) or by committing them to apoptosis (16, 17). The mutant p53 gene often fails in these functions, thus opening the gate for the uncontrolled expansion of transformed p53− clones.
The question then arises as to whether hypermutagenesis in the p53 gene occurs mainly during the G1 phase of the cell cycle, which is substantially prolonged in stem cells with wild-type p53, or simply that cells with preexisting p53 mutations are selected for tumor growth after passing the G1–S checkpoint. This question brings us back to the “Luria–Delbruck vs. Cairns” dilemma.
A 34-fold increase in the frequency of hypoxanthine phosphoribosyltransferase (HPRT) mutants was reported (18) for some mismatch repair-deficient human tumor cell lines maintained at high density under growth limitation, i.e., predominantly in pre-S phase (see also ref. 19). However, HPRT is a cancer-irrelevant housekeeping gene. That cancer cells could originate from nondividing stem cells by hypermutagenesis in the p53 tumor suppressor gene is an attractive hypothesis (1–4). To examine this hypothesis, a definitive analytical test is needed to distinguish between multiple mutations that arise in nondividing cells and those that arise in dividing cells. Our test is based on the supposition that, in nondividing cells, only those de novo mutations that occur in the transcribed (noncoding) DNA strand can be expressed rapidly in mRNA and protein.
Most suitable for the test are C → T and G → A transitions in CpG dinucleotides, which comprise about 30% of the p53 databases (13, 20). Five of six hot spots of tumor-associated p53 mutations are CpG sites (codons 175, 245, 248, 273, and 282) located in exons 5–8 encoding the central domain of the p53 protein. By binding to the specific DNA targets, this domain activates downstream reporter genes, which in turn directly regulate cell division (21). Within exons 5–8 of the human p53 gene, all CpG dinucleotides are methylated equally on both DNA strands (22), with the mCpG sites being endogenous mutagenic sites. By relatively frequent spontaneous hydrolytic deamination, mC (5-methylcytosine) converts to T (23, 24). Note that mC → T is a replication-independent change (25). CpG is a palindrome; therefore, CpG → TpG transitions on the transcribed (noncoding) strand appear as complementary CpG → CpA transitions in mRNA and, after replication, on the nontranscribed (coding) strand of DNA (Fig. 1). Thus, for mCpG sites one can identify the strand on which the primary mC deamination event occurred. By using these three advantages of CpG transitions in the p53 gene (replication independence of primary mC deamination events, reliable strand identification, and highest frequency in human tumors, both in singular and multiple configuration), we show that multiple p53 mutations do emerge mainly in quiescent stem cells.
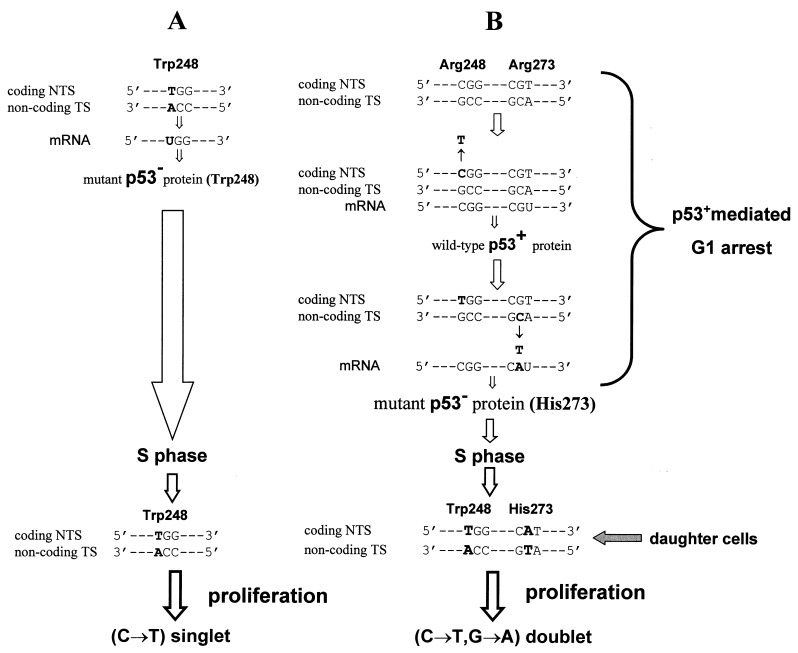
Figure 1.
Expression asymmetry of DNA strands in nondividing cells is illustrated by CpG transitions in p53 hotspot codons 248 and 273 during p53-mediated G1 arrest. (A) Shown is the mutant p53− cell with preexisting CpG → TpG in codon 248. Because of DNA replication, the original C⋅G base pair was substituted for T⋅A before the checkpoint in G1. The cell transcribes the mutant p53 mRNA (C → U), which is in turn translated into an altered protein (Arg248 → Trp). As a result, the cell escapes G1 arrest and is selected for proliferation. This preexisting single CpG transition is a tumor driver. (B) Shown is the wild-type p53+ cell arrested in G1 phase. The first C → T transition originates de novo in codon 248 on the coding nontranscribed strand (NTS). To be expressed, it has to wait for another, necessarily nonsilent, mutation on the transcribed strand (TS), which would release the cell from the G1 arrest. This second mutation is shown as C → T in codon 273, on the transcribed strand. It appears as G → A in mRNA and is translated into His instead of Arg in the p53 protein. As a result, the cell enters S phase, then undergoes cell division and proliferation. The corresponding clone appears in the tumor as a p53 doublet. The first mutation is a hitchhiker, and the second mutation is a tumor driver.
THEORETICAL RATIONALE OF THE TEST
Mutations in dividing cells are replication dependent (Rd), whereas those in nondividing cells are time dependent (Td) (1–4). It was unclear how to estimate Td mutation rate (2). We have developed several tests that distinguish between Td and Rd processes based on the assumption that mutations originating in nondividing cells will show the expression asymmetry of DNA strands.
Expression Asymmetry of DNA Strands.
In prolonged G1 phase, any new mutation in the nontranscribed (coding) strand is not expressed until the cell enters S phase (Fig. 1). This means that such mutations will gain a chance of expression in daughter cells only after DNA replication. On the other hand, nonsilent mutations in the transcribed (noncoding) strand are expressed as soon as mRNA and protein are synthesized (Fig. 1). Clearly, such strand asymmetry is expected only for mutations arising in quiescent cells and not for replication errors in proliferating cells.
As a mediator of G1-arrest, p53 is the ideal gene in which to examine the G1 phase-timed strand asymmetry by analysis of tumors bearing multiple p53 mutations. Cells with a preexisting p53 mutation are “immediately” selected for tumor development (Fig. 1A), as the mutant p53 protein fails to mediate G1 arrest. Subsequent p53 mutations occurring in dividing cells are replication dependent and strand symmetric. Because preexisting p53 mutations had passed through replication, they enter the G1–S checkpoint already strand symmetric. For example, germ-line p53 mutations associated with Li-Fraumeni syndrome certainly belong in this group. Alternatively, if somatic mutations occur in quiescent cells, only nonsilent p53 mutations on the transcribed strand are selected for proliferation (Fig. 1B). Accordingly, even if a nonsilent mutation happens first in the coding strand of p53 gene, it cannot be revealed until cell division, i.e., until a critical nonsilent mutation occurs on the transcribed strand of G1–S transition regulating genes, including p53 (Fig. 1B). Instances in which a transcribed p53 mutation alone is insufficient to abolish the G1 arrest will not appear in tumors. When analyzing the p53 nonsilent singlets, one deals with p53 mutations that were critical for the G1–S transition (26). By using the term “immediate” selection, we imply that all other necessary tumorigenic events have already happened. Independently of the occurrence of these events before or during G1 arrest, the Td hypothesis predicts the strand-asymmetric expression and selection of p53 hypermutations, whereas the Rd hypothesis does not make this prediction.
The p53 protein functions as a dimer of dimers, and many p53 missense mutations act in a transdominant negative fashion probably because even a single mutant p53 subunit may impose incorrect conformation on the entire tetramer (27, 28). Consequently, unlike the compulsory double-hit inactivation (11, 29) of other cancer-related genes, loss of the wild-type p53 allele in mutant p53− cells is an infrequent event in tumors (28). However, it should be noted that this loss of heterozygosity simply decreases the threshold of p53 activity under which de novo recessive p53 mutations become critical for G1–S transition of nondividing cells. In any case, it does not matter for the test of the Td origin of p53 multiplets whether the second, tumor driving mutation occurs on the transcribed strand of the same or homologous p53 gene.
Strand Asymmetry of CpG Transitions.
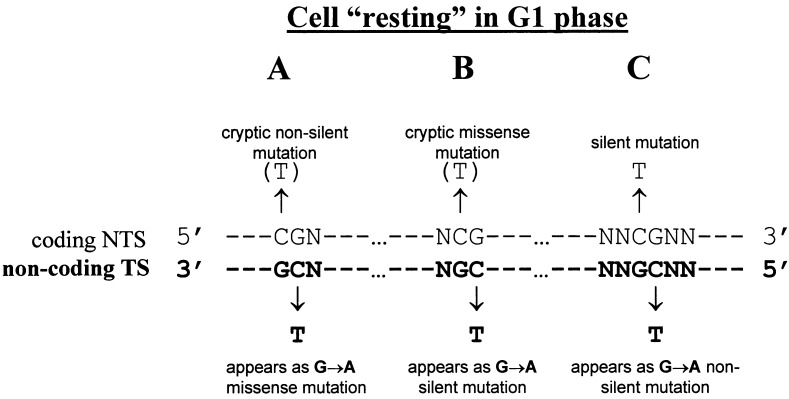
There are three types of CpGs in coding sequences (Fig. 2). In codons CGN both C → T and G → A transitions generate only nonsilent mutations (Fig. 2A), whereas in codons NCG nonsilent mutation caused by C → T transition is mirrored by silent G → A (Fig. 2B), the reverse being the case for intercodon CpGs (Fig. 2C). These genetic code-based differences allow for a number of quantitative tests to distinguish between Td and Rd origins of p53 multiple mutations and to make the following predictions:
Figure 2.
Three types of CpG dinucleotide that can be used for testing the Td mutagenesis in nondividing cells.
(i) p53 doublets composed of nonsilent CpG transitions in codons CGN.
Three types of doublets are possible with regard to the strand on which the two primary mC → T transitions occur. Because G → A on the coding strand originates from C → T on the noncoding strand, we designate these three doublets as homostrand (C → T/C → T and G → A/G → A), and heterostrand (C → T/G → A), respectively. According to our hypothesis, in the absence of strand-specific repair and selection, the representation of the three doublets for the Rd mutation process should be close to a 1(C → T/C → T): 2(C → T/G → A): 1 (G → A/G → A) ratio whereas solely heterostrand doublets (C → T/G → A) are expected for Td mutagenesis (Fig. 1B).
Both repair and selection may bias the 1:2:1 ratio toward an excess of the homostrand (C → T/C → T) doublet. Indeed, preferential repair of T∗G mismatches generated by C → Ts in the transcribed strand during transcription (30) will result in a deficiency of G → A equivalents in the coding strand. A similar tendency toward C → T excess and G → A deficiency is expected because of selection at the p53 protein level (14, 31). By the genetic code, the protein perturbation caused by C → T at each CpG-containing codon exceeds that caused by G → A at the same CpG site (14, 31). Accordingly, a mutant p53 protein altered by C → T transition is, on average, expected to be more tumorigenic than that changed by G → A. Thus, for CpG transitions, repair and selection would further accentuate strand asymmetry in nondividing stem cell mutagenesis. Therefore, excess of p53 heterostrand (C → T/ G → A) doublets, if observed, should point to their Td origin.
(ii) Comparison of germinal singular vs. somatic singular vs. somatic multiple CpG transitions.
In contrast to somatic cells, germ-line cells (at least from the paternal line) are not expected to show evidence for the Td origin of p53 mutations. In somatic cells the Td origin is easier to uncover in the p53 multiplet database because preexisting single p53 mutations “gallop” through the G1 phase. As singlets, both nonsilent CpG transitions, C → T and G → A, can transform cells and drive tumor growth. As multiplets, the hypothesis of their Td origin in nondividing cells assigns that role only to G → A (Fig. 1B). Therefore, the Td hypothesis predicts a progressive increase in the G → A/C → T ratio in “germ-line/singlet vs. somatic/singlet vs. somatic/multiplet” comparisons. To neutralize sequence context-dependent effects as much as possible, it is desirable to test the increase at codon resolution. p53 hotspot CpG sites provide this opportunity.
(iii) p53 doublets with silent hitchhikers.
Silent p53 mutations are invisible to selection and may appear in tumors exclusively as neutral hitchhikers. The corresponding nonsilent cancer-driving mutation arises after a neutral hitchhiker, either in p53 or in any other cancer-related gene. For driving CpG transitions in p53, the Td hypothesis predicts excess of G → A over C → T, whereas their silent p53 partners might be strand-symmetric. However, this is not the case when CpG transitions themselves are silent.
(iv) Silent CpG transitions.
There are two types of silent CpG transitions: G → A in codons NCG and C → T in intercodon NNCGNN (Fig. 2). In all intercodon CpGs of the human p53 gene, C → T transitions are silent, whereas equally probable symmetric C → Ts on the opposite-transcribed strand appear as nonsilent G → As in mRNA (Fig. 2C). The corresponding mutant p53 proteins fail to prevent cells from entering proliferation. In NCG codons C → Ts on the transcribed strand appear as G → As in mRNA and are invariably silent, but all equally probable mirror C → Ts on the coding strand are also silent as long as the cell remains nondividing (Fig. 2B). Thus, in a nondividing cell, for any length of time, per one CpG site, a silent G → A is twice as likely as a silent C → T to occur.This difference gives us another Td vs. Rd test.
MATERIALS AND METHODS
Data on human tumor-associated p53 mutations were retrieved from the latest available version of the p53 database (13) and were augmented by additional records from ref. 20 that were not documented in ref. 13. The resulting data set contained 379 distinct cases of more than one p53 mutation detected in the same tumor sample, accounting for a total of 808 mutations. The majority of multiplets (333 of 379) are doublets. A total of 1,927 CpG transitions have been analyzed. To apply the Td vs. Rd tests we have subdivided these 1,927 transitions into the following groups: (i) 57 germ-line single CpG transitions, all nonsilent, including 39 associated with Li-Fraumeni syndrome; (ii) 1,679 somatic single nonsilent CpG transitions; (iii) 39 multiplets (32 doublets) containing 2 nonsilent CpG transitions with a total of 78 transitions; (iv) 95 multiplets (83 doublets) containing 1 nonsilent CpG transition; (v) 20 multiplets (as a part of combined groups (iii) and (iv)) with at least 1 silent hitchhiker and a nonsilent CpG transition as a tumor driver; and (vi) 18 silent CpG transitions, 8 of these being a hitchhiking part of p53 doublets, and 10 being singlets most likely driven by tumorigenic mutation(s) beyond the p53 gene.
As a baseline control for Td vs. Rd tests, we used 71 CpG transitions randomly fixed during evolution in four germ-line p53 retropseudogenes (S.N.R., G. Holmquist, and A.S.R., unpublished data), one from Mus musculus and three others from Rattus norvegicus (GenBank accession numbers K02110, U07019, U07020 and L12046, respectively). Because processed pseudogenes are transcriptionally and translationally silent, their evolutionary CpG → TpG/CpG → CpA pattern was shaped by mutagenesis only.
To minimize the impact of transcription-coupled repair on strand asymmetry, we excluded from the tests all CpG-related incidences of UV-induced cyclobutane pyrimidine dimers observed in patients with the repair deficiency disorder xeroderma pigmentosum (XP-C complementation group). XP-C fibroblast cells demonstrate extremely selective repair of the transcribed strand, eventually resulting in strong bias toward C → T transitions (95%) on the nontranscribed coding strand (30).
Standard statistical tests were employed to estimate the significance of the results. Nonparametric tests were applied to the data on homo- and heterostrand doublets (Table 1,C) to determine whether their frequency ratio differed significantly from the 1:2:1 ratio predicted for the Rd mechanism. The data in Table 2 were grouped into nominal categories and subjected to two-level table association analysis. Various χ2 and nominal tests were used to determine whether the NC → T/NG → A ratio changes from germ-line/singlet to somatic/singlet to somatic/multiplet categories, as the Td hypothesis predicts.
Table 1.
Strand asymmetry of CpG transitions in p53 genes: Gene resolution
| CpG transition class | ||
|---|---|---|
| Silent CpG transitions | ||
| Germ line p53 retropseudogenes | ||
| p53 genes in human tumors* | ||
| Nonsilent CpG transitions | ||
| Germinal, including Li–Flaumeni syndrome | ||
| Singlets in human tumors | ||
| Multiplets in human tumors | ||
| Multiplets with silent hitchhikers† | ||
| p53 doublets composed of nonsilent CpG transitions | ↓ Homostrand C → T, C → T | ↘↙ Heterostrand C → T, G → A | ↓ Homostrand G → A, G → A |
|---|---|---|---|
| All cancers in databases (13, 20)‡ | 5 | 26 | 6 |
| Colorectal and skin cancers | 4 | 4 | 2 |
| All but colorectal and skin cancers§ | 1 | 22 | 4 |
Although the sample size is too small for reliable statistical inference, the observed ratio (6 C → T:12 G → A) fits the expected ratio of 1:2 (Td hypothesis) much better than 1:1 (Rd hypothesis).
Three multiplets from “p53 late” colorectal cancers are excluded. Similarly, although the sample size is too small, the observed data do not fit the 1:1 ratio predicted by the Rd hypothesis.
A nonparametric goodness of fit test was performed to ascertain the significance of the deviation from 1:2:1 ratio predicted by the Rd hypothesis. It was found significant at the 93.6% level (0.064 P value), an almost significant deviation from the 1:2:1 ratio.
Same, with 0.005 P value (indicating highly significant deviation from the 1:2:1 ratio).
Table 2.
CpG transition strand asymmetry in the p53 gene: Codon resolution
| Codon | Germ line
single
|
Tumor single
|
Tumor multiple†
|
|
|---|---|---|---|---|
| NC → T/NG → A* transitions | NC → T/NG → A transitions | NC → T/NG → A transitions (I) | NC → T/NG → A transitions (II) | |
| Transactivation domain | ||||
| 10–11 GTCGAG | — | — | 0/1 | — |
| 30–31 AACGTT | — | — | 0/1 | — |
| 36 CCG | — | — | 0/3 | — |
| 47 CCG | — | 2/0 | — | — |
| 82 CCG | 1/0 | — | — | — |
| Central domain | ||||
| 110 CGT | — | 3/0 | — | — |
| 125 ACG | — | 2/0 | 0/1 | — |
| 152 CCG | 1/0 | 15/0 | 6/6 | 4/6 |
| 153–154 CCCGGC | — | 0/2 | 3/1 | — |
| 156 CGC | — | 2/2 | — | — |
| 156–157 CGCGTC | — | 0/4 | 0/2 | — |
| 158 CGC | — | 5/23 | 1/3 | 0/3 |
| 158–159 CGCGCC | — | 0/3 | 2/1 | — |
| 170 ACG | — | 2/0 | 2/2 | 1/2 |
| 175 CGC | 0/8 | 4/284 | 6/16‡ | 2/16‡ |
| 181 CGC | 1/1 | 8/12 | 0/2 | — |
| 196 CGA | — | 61/1 | 6/2 | 3/2 |
| 202 CGT | — | 1/2 | 1/0 | — |
| 213 CGA | 2/0 | 72/8 | 7/6‡ | 4/6 |
| 222 CCG | — | — | 1/0 | — |
| 244–245 GGCGGC | 0/6 | 0/102 | 0/14 | — |
| 248 CGG | 11/8 | 189/234 | 12/22 | 2/22‡ |
| 267 CGG | 0/1 | 8/1 | 1/1 | — |
| 273 CGT | 4/4 | 190/224 | 7/20 | 3/20‡ |
| 282 CGG | 6/0 | 141/8 | 15/6‡ | 8/6‡ |
| 283 CGC | — | 6/2 | 1/1 | — |
| 290 CGC | — | 3/2 | 0/4 | — |
| 297–298 CACGAG | — | — | 1/1 | — |
| Tetramerization domain | ||||
| 306 CGA | 1/0 | 22/0 | 1/0 | — |
| 337 CGC | 1/1 | 4/0 | — | — |
| 342 CGA | — | 25/0 | 1/1 | — |
NC→T/NG→A is a ratio of putative CpG→TpG deamination-induced transitions on the nontranscribed strand to the ones that occurred on the transcribed strand. Five hotspot CpG sites are shown in boldface type.
Column (I) shows total NC→T at each CpG, while in column (II) the C→Ts that are hitchhiking parts of the heterostrand doublets (C→T G→A) are excluded. This allows testing the Td vs. Rd origin of tumor-driving p53 mutations by comparing the strand asymmetry in germ–line/singlet, somatic/singlet, and somatic/multiplet groups.
Based on the nominal cross tabulation, various statistical tests were performed to ascertain whether the observed differences of the NC→T/NG→A value between the three groups were statistically significant. The confidence levels of more than 99% were obtained for codons 175, 213, 248, 273, and 282, thus indicating nonrandomness of the differences. Although the remaining codons also fit the Td hypothesis, they were not statistically tested because of small sample size.
RESULTS
No strand asymmetry was found for CpG transitions in rodent p53 retropseudogenes (Table 1). This lack of asymmetry substantiates our basic postulate that primary mC deamination occurs with the same probability on either of the p53 DNA strands. We have also tested the alignment of CpG-rich Alu repeats within germ-line p53 introns of primate species (32). Again, no evidence of the strand asymmetry for CpG transitions was found in these translationally silent Alu sequences.
Table 1 shows that the number of nonsilent heterostrand doublets (C → T, G → A) significantly exceeded the total of nonsilent homostrand doublets (C → T, C → T) and (G → A, G → A). In about the same proportion, the doublets in which a nonsilent G → A transition drives a silent hitchhiker outnumber the doublets with a nonsilent C → T driver. When CpG transitions are silent, G → A again occurs twice as frequently as C → T, despite the fact that potentially mutable intercodon CpGs (Fig. 2C) outnumber intracodon CpGs (Fig. 2B) 11 to 9 in the human p53 gene.
Heterostrand nonsilent doublets (C → T, G → A) are underrepresented in colorectal and skin cancers (Table 1). Among the different genes (APC, ras, DCC, and p53) contributing to colorectal cancer, mutations in p53 occur and are selected for at a later time, probably after malignant transformation (15). In contrast, skin cancer p53 mutations occur very early, most likely in as-yet-untransformed cells (33). Consistent with our hypothesis, “p53-late” colorectal cancers show no evidence for an excess of p53 heterostrand doublets (C → T, G → A). In “p53-early” skin cancer, a distinctive feature is a preferential repair of the transcribed strand (30). It is commonly thought that the G1 arrest serves to repair DNA damage. This may be true for many genes except p53, because G1 arrest is p53-mediated. Furthermore, the Td hypothesis predicts that p53 mutations originating de novo on the transcribed strand have very limited, if any, time to be repaired during G1 phase, because mutant p53 proteins cannot prevent cells from proliferation. Therefore, we suggest that transcription-coupled repair may play a minimal role for nonsilent p53 multiple mutations of Td origin. P53 multiplets from skin cancers contain 10 CpG → TpG transitions for 2 CpG → CpA transitions. This imbalance shows that most skin cancer p53 multiplets are indeed of Rd origin. Not surprisingly, elimination of colorectal and skin cancer data resulted in a statistically significant excess of heterostrand (C → T, G → A) doublets (Table 1, § footnote).
All CpG transitions are shown in Table 2 at codon resolution. In accordance with the Td hypothesis, for the majority of CGN codons, the incidence of G → A compared with C → T gradually increases from germinal singlet through somatic singlet to somatic multiplet groups. The increase is particularly pronounced at codons 248 and 273, the hottest mutational spots recorded in all cancers. The Td origin predicts that C → T transitions appear in multiplets only as hitchhikers. Indeed, for codons 248 and 273, most of their C → Ts have been found in heterostrand (C → T, G → A) doublets (compare columns I and II in Table 2). Thus, a tumor-driving role of G → A transitions in these two codons is convincingly apparent in multiplets because of their Td origin in nondividing cells.
No less impressive is the same tendency illustrated by the two “warm spot” codons, 196 and 213. In singular status (both in germinal and somatic groups), the nonsense mutation CGA → TGA is much more frequent in tumors than the mirror missense mutation CGA → CAA (Arg → Gln). In multiple status, according to our Td hypothesis, it is not the nonsense (CGA → TGA) on the nontranscribed strand but the missense Arg → Gln caused by CpG → TpG transition on the transcribed strand that might release a nondividing cell from G1 arrest. Indeed, an increase of G → A at codon 196 and particularly at codon 213 in p53 multiplets has been observed (Table 2).
Hotspot codon 175 definitely deviates from the above common pattern. However, we expected this because in germ-line and somatic singular status, codon 175 shows a great excess of Arg → His (CpG → CpA) substitution over the strand mirror Arg → Cys (CpG → TpG). This excess is observed even though by the genetic code Arg → His is functionally a more conservative substitution than Arg → Cys (14, 31). This is because in some sites of the p53 central domain tumorigenic selection favors missense mutations that endow the p53 protein with a gain of oncogenic function that, in contrast to loss of tumor suppressor function, is not necessarily related to the degree of protein alteration (14). Arg → His at codon 175 belongs to this class of gain-of-function mutations (34, 35). The strand mirror Arg → Cys at codon 175 has a good chance of appearing in tumors, but predominantly as a hitchhiking part of multiplets (Table 2, see also ref. 14). This is what the Td hypothesis predicts for p53 hypermutagenesis in G1-arrested stem cells. Thus, codon 175 may serve as an exception confirming the rule. The same exception, although statistically less robust, may be codon 158 (Table 2).
DISCUSSION
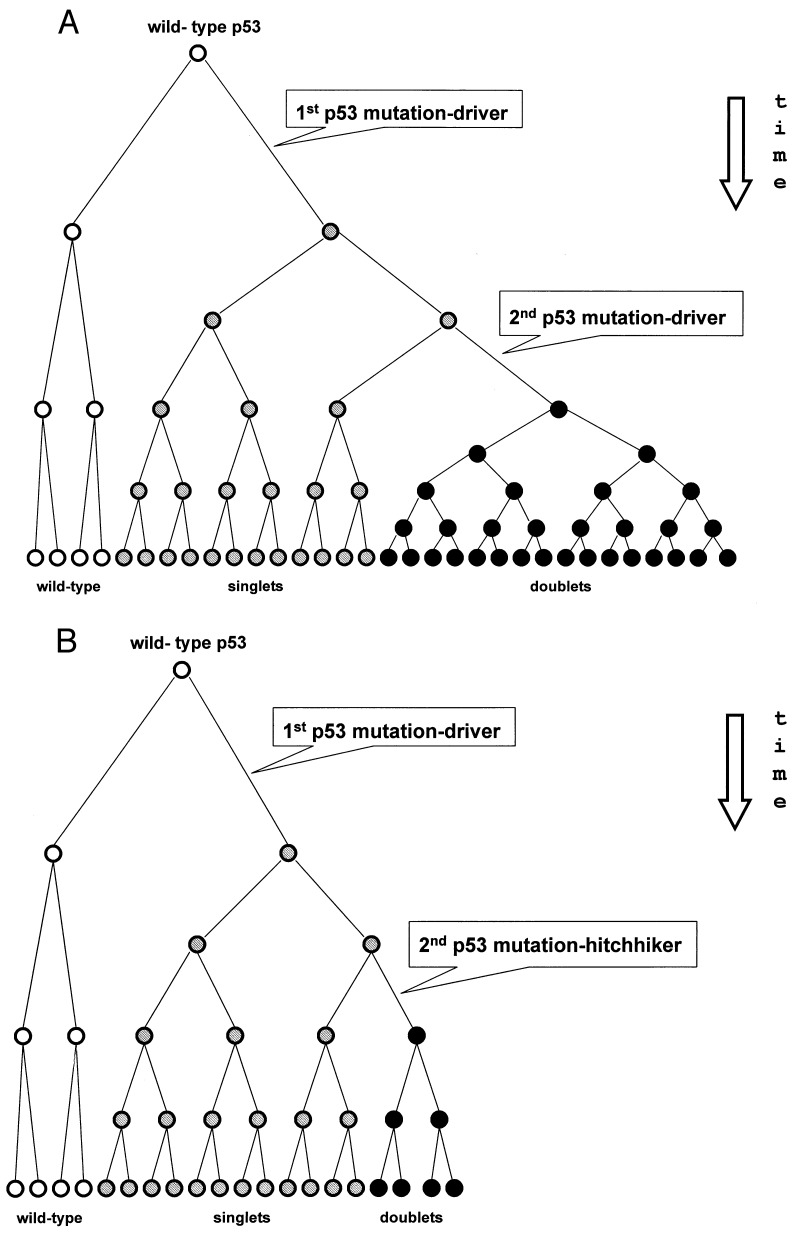
The described strand asymmetry fits the Td hypothesis of p53 hypermutagenesis in nondividing stem cells reasonably well. For most p53 multiplets, this suggests a unicellular origin of driving and hitchhiking mutations. The unicellular origin in turn explains subsequent concerted proliferation of multiple p53 mutations, i.e., it easily resolves the problem that confuses Rd mutagenesis models. All Rd schemes depict tumor development as successive waves of mutant clone expansion directed by stepwise progressive selection (29, 36, 37). This necessarily postulates that every new p53 mutation should confer a proliferative advantage over not only the original wild-type p53 but also over all p53 mutations that emerged earlier in the same clone (Fig. 3A). Otherwise, these new p53 mutations would rarely be observed in most, if not all, cells of the same tumor (Fig. 3B).
Figure 3.
Scheme illustrating the paradox of detecting multiple p53 mutations of Rd origin in the same tumor. (A) In application to the p53 gene, the classic scheme of clonal Rd evolution in neoplasia (29, 36, 37) implies that the second p53 mutation confers some additional proliferative advantage over that of the first p53 mutation within the same clone; only in this case, because of intensifying selection, the daughter clone bearing the p53 doublet would eventually prevail over those clones bearing singlets and therefore would be detectable in tumor samples. However, this scheme is inconsistent with the fact that one of the mutations in p53 doublets is a hitchhiker (14). (B) The paradox in clonal Rd evolution is that the final frequency of neutral hitchhikers would be rather small (reflecting only the rate of Rd mutations). As a consequence, there is a minimal chance to detect (by routine survey) the p53 doublet clone compared with its singlet precursor in the same tumor. There is no such problem for the Td unicellular origin of p53 doublets (Fig. 1B).
p53-early skin and p53-late colorectal cancers may serve as particular examples of Rd clonal evolution. However, this is not the general case for p53 multiplets. By comparing CpG transition frequency in singlets and doublets at codon resolution of p53 without strand identification, we have proved recently (14) that not only silent but also most nonsilent p53 mutations in doublets represent tumors of unicellular origin. Moreover, in doublets in which both partners are nonsilent p53 mutations, only one of the two mutations needs to be a tumor driver, while the other must be a hitchhiker (14). Unlike any Rd model (Fig. 3), it is exactly what our Td model of G1 phase-timed origin of p53 multiplets predicts (Fig. 1). In addition, our test of Td mutagenesis in nondividing cells clarifies that most hitchhiking components of p53 multiplets originate first on the nontranscribed strand and are then drawn into proliferation by other mutation that originate secondarily on the transcribed strand, within or outside the p53 gene. In the latter case, CpG → TpG transitions of the p53 coding strand remain singular. To quantify these singlets, we need to develop an explicit probabilistic theory of Td evolution.
Cancer development can be divided phenomenologically into two major stages associated with drastic changes in stem cell kinetics. During the initiation stage, stem cells are “resting” in a prolonged G1 phase. For the p53 gene, our test indicated that this phase is hypermutable. On accumulation in the Td regime of some critical number of cancer-prone mutations, stem cells begin to actively proliferate. This is the promotion stage, characterized by the Rd origin of new mutations and by a shortened G1 phase, which implies a limited time for repair. In the absence of either apoptosis or differentiation, this would lead to irreversible malignant growth.
In terms of the role of the G1–S transition in stem cell life, we did not fail to notice the haziness of the definition of “stem cell.” According to a minimalist definition (38), stem cells are distinguished by their potential to realize the inherent repertoire of differentiation. However, for many progenitor cells their “stem” pluripotency can be maintained either by mitotically extensive self-renewal or, alternatively, by a “simple” persistence in nondividing quiescent form (38). The problem here is that stem cell capacity for self-renewal is measured only indirectly (39), by “suicide” methods in which S phase-specific agents selectively kill colony-forming stem cells. Truly quiescent stem cells remain inaccessible to such “suicide” techniques and there are no methods whatsoever to identify nondividing cells (4). The G1 phase-timed origin, strand-asymmetric expression and selection of tumorigenic mutations are essential attributes of quiescent stem cells that, in principle, make it possible to distinguish them from self-renewing “twins.”
Our tests permit exploration of genetic details of the “quiescence vs. self-renewal or proliferation” choice. Although the network of known and presumed interactions between the genes involved in the control of G1–S transition is quite intricate (15), by testing mutations on Td vs. Rd origin we could identify the genes that negatively and positively act, respectively, on the probability of cells entering the S phase. Indeed, the hypothesis of Td mutagenesis predicts an excess of CpG → CpA over mirror CpG → TpG, especially in multiple status, for tumor suppressor genes such as p53, Rb, and p16. p53 multiplet data support this hypothesis. On the contrary, mutations in S phase-promoting genes will consolidate cells in p53-mediated G1 arrest. Therefore, in tumors one would expect to find these genes amplified and/or overexpressed rather than inactivated by mutations. This was shown to be the case for a number of oncogenes including MDM2, a negative regulator of p53, and cyclin D, cyclin E, and in some cancers, cdk-4 (15). However, for each particular gene, the ratio of its mutant to amplified or overexpressed states in tumors depends on the balance of its positive and negative interactions with other genes in the network.
In conclusion, the strand asymmetry of CpG transitions, as the indicator of G1 phase-dependent hypermutagenesis in stem cells, is not of purely academic interest. If the rate of tumorigenic mutations in stem cells can be reduced, the knowledge of when and how tumor suppressor genes gain and express multiple mutations will help in cancer prevention and treatment (4). Another prospect is that, complementary to the approach published in ref. 14, our tests for Td mutagenesis define more accurately which of the mutations in p53 multiplets are tumor drivers and which are only hitchhikers. This knowledge allows us to measure the separate roles that mutagenesis and selection play in tumorigenesis and, accordingly, to build a prognostic p53 map of mutation cancer risks at codon resolution.
Acknowledgments
We thank G. Holmquist, I. Tsyrlova, S. Ohno, E. Roberts, S. Smith, A. Riggs, and J. Kovach for stimulating discussions, and S. Bates for reading the manuscript. We are especially grateful to anonymous reviewers for critical comments and valuable suggestions. This work was supported by National Institutes of Health Grant CA76573.
ABBREVIATIONS
- Td
time dependent
- Rd
replication dependent
References
- 1. Strauss B S. Cancer Res. 1992;52:249–253. [PubMed] [Google Scholar]
- 2.Strauss B S. Carcinogenesis. 1997;18:1445–1452. doi: 10.1093/carcin/18.8.1445. [DOI] [PubMed] [Google Scholar]
- 3.Strauss B S. Genetics. 1998;148:1619–1636. doi: 10.1093/genetics/148.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeb L A, Christians F C. Mutat Res. 1996;350:279–286. doi: 10.1016/0027-5107(95)00117-4. [DOI] [PubMed] [Google Scholar]
- 5.Cairns J, Overbaugh J, Miller S. Nature (London) 1988;335:142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- 6.Hall B G. Proc Natl Acad Sci USA. 1992;89:4300–4303. doi: 10.1073/pnas.89.10.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster P L. J Bacteriol. 1997;179:1550–1554. doi: 10.1128/jb.179.5.1550-1554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torkelson J, Harris R S, Lombardo M-J, Nagendran J, Thulin C, Rosenberg S M. EMBO J. 1997;16:3303–3311. doi: 10.1093/emboj/16.11.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeb L A. Cancer Res. 1991;51:3075–3079. [PubMed] [Google Scholar]
- 10.Stein W D. Adv Cancer Res. 1991;56:161–213. doi: 10.1016/s0065-230x(08)60481-9. [DOI] [PubMed] [Google Scholar]
- 11.Knudson A G J. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knudson A G J. Adv Hum Genet. 1977;8:1–66. doi: 10.1007/978-1-4615-8267-0_1. [DOI] [PubMed] [Google Scholar]
- 13.Beroud C, Soussi T. Nucleic Acids Res. 1998;26:200–204. doi: 10.1093/nar/26.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodin S N, Holmquist G P, Rodin A S. Int J Mol Med. 1998;1:191–199. doi: 10.3892/ijmm.1.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 16.Linke S P, Clarkin K C, Wahl G M. Cancer Res. 1997;57:1171–1179. [PubMed] [Google Scholar]
- 17.Polyak K, Xia Y, Zweler J L, Kinzler K W, Vogelstein B. Nature (London) 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 18.Richards B, Zhang H, Phear G, Meuth M. Science. 1997;277:1523–1526. doi: 10.1126/science.277.5331.1523. [DOI] [PubMed] [Google Scholar]
- 19.Holmquist G P. Mutat Res. 1998;400(1–2):59–68. doi: 10.1016/s0027-5107(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 20.Hollstein M, Shomer B, Greenblatt M, Soussie T, Hovig E, Montesano R, Harris C C. Nucleic Acids Res. 1996;24:141–146. doi: 10.1093/nar/24.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho Y, Gorina S, Jeffrey P, Pavletich N P. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 22.Tornaletti S, Pfeifer G P. Science. 1994;263:1436–1438. doi: 10.1126/science.8128225. [DOI] [PubMed] [Google Scholar]
- 23.Rideout W M, Coetzee G A, Olumi A F, Jones P A. Science. 1990;249:1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- 24.Yang A S, Jones P A, Shibata A. Epigenetic Mechanisms of Gene Regulation. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. pp. 77–94. [Google Scholar]
- 25.Smith S S, Kaplan B E, Sowers L C, Newman E M. Proc Natl Acad Sci USA. 1992;89:4744–4746. doi: 10.1073/pnas.89.10.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinzler K W, Vogelstein B. Nature (London) 1996;379:19–20. doi: 10.1038/379019a0. [DOI] [PubMed] [Google Scholar]
- 27.Brachmann R K, Vidal M, Boeke J D. Proc Natl Acad Sci USA. 1996;93:4091–4095. doi: 10.1073/pnas.93.9.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casey G, Lopez M E, Ramos J C, Plumer S J, Arboleda M J, Shaughnessy M, Karlan B, Slamon D J. Oncogene. 1996;13:1971–1981. [PubMed] [Google Scholar]
- 29.Ohno S. Physiol Rev. 1971;51:496–526. doi: 10.1152/physrev.1971.51.3.496. [DOI] [PubMed] [Google Scholar]
- 30.Evans M K, Taffe B G, Harris C C, Bohr V A. Cancer Res. 1993;53:5377–5381. [PubMed] [Google Scholar]
- 31.Rodin S N, Ohno S. Proc Natl Acad Sci USA. 1997;94:5183–5188. doi: 10.1073/pnas.94.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang A S, Gonzalo M L, Zingg J-M, Millar R P, Buckley J D, Jones P A. J Mol Biol. 1996;258:240–250. doi: 10.1006/jmbi.1996.0246. [DOI] [PubMed] [Google Scholar]
- 33.Ziegler A, Jonason A S, Leffell D J, Simon J A, Sharma H W, Kimmelman J, Remington L, Jacks T, Brash D E. Nature (London) 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 34.Dittmer D, Pati S, Zambetti G, Chu S, Teresky A K, Moore M, Finlay C, Levine A J. Nat Genet. 1993;4:42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- 35.Hinds P W, Finlay C A, Quartin R S, Baker S J, Fearon E R, Vogelstein B, Levine A J. Cell Growth Differ. 1990;1:571–580. [PubMed] [Google Scholar]
- 36.Nowell P C. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 37.Nowell P C. Adv Cancer Res. 1993;62:1–17. doi: 10.1016/s0065-230x(08)60313-9. [DOI] [PubMed] [Google Scholar]
- 38.Morrison S J, Shah N M, Anderson D J. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 39.Wu A M. Cell Tissue Kinet. 1981;14:39–52. doi: 10.1111/j.1365-2184.1981.tb00509.x. [DOI] [PubMed] [Google Scholar]