Abstract
Elevated endogenous cholecystokinin (CCK) release induced by protease inhibitors leads to pancreatic growth. This response has been shown to be mediated by the phosphatase calcineurin, but its downstream effectors are unknown. Here we examined activation of calcineurin-regulated nuclear factor of activated T-cells (NFATs) in isolated acinar cells, as well as in an in vivo model of pancreatic growth. Western blotting of endogenous NFATs and confocal imaging of NFATc1-GFP in pancreatic acini showed that CCK dose-dependently stimulated NFAT translocation from the cytoplasm to the nucleus within 0.5–1 h. This shift in localization correlated with CCK-induced activation of NFAT-driven luciferase reporter and was similar to that induced by a calcium ionophore and constitutively active calcineurin. The effect of CCK was dependent on calcineurin, as these changes were blocked by immunosuppressants FK506 and CsA and by overexpression of the endogenous protein inhibitor CAIN. Parallel NFAT activation took place in vivo. Pancreatic growth was accompanied by an increase in nuclear NFATs and subsequent elevation in expression of NFAT-luciferase in the pancreas, but not in organs unresponsive to CCK. The changes also required calcineurin, as they were blocked by FK506. We conclude that CCK activates NFATs in a calcineurin-dependent manner, both in vitro and in vivo.
INTRODUCTION
An adequate supply of pancreatic digestive enzymes is essential for digestion and absorption of food. Pancreas, a normally quiescent organ, is capable of growth as an adaptive response to environmental challenges such as hyperphagia (McLaughlin et al., 1983), increased dietary protein (Green et al., 1986 1992), or injury (Bentrem and Joehl, 2003). The most firmly established regulators of adult pancreatic growth are gut hormones, in particular cholecystokinin (CCK; Brannon, 1990; Logsdon, 1999). Administration of exogenous CCK (Niederau et al., 1987), its analogue cerulein (Solomon et al., 1983; Berube et al., 1993) or an increase in endogenous CCK secretion (Otsuki et al., 1993; Tashiro et al., 2004) all lead to pancreatic growth in a number of animal models. This trophic response is blocked by coadministration of CCK receptor antagonist (Wisner et al., 1988) and is abolished in CCK or CCKA receptor–deficient mice (Sato et al., 2002; Tashiro et al., 2004). In addition, CCK stimulates proliferation of primary acinar cells in culture (Logsdon, 1986). CCK signals via G-protein–coupled receptors to a number of downstream targets. One important effector is Gq, which couples to phosphoinositide-specific phospholipase C (PLC-β) and thereby elevates intracellular calcium (Williams and Yule, 2006). The initial steps of this pathway are now well understood, but how increased intracellular calcium relates to adaptive growth of the pancreas is not clear.
Ongoing work in a number of systems has focused attention on the calcium/calmodulin-dependent phosphatase calcineurin (also known as PP2B) as a key mediator of adaptive changes in the adult. Calcineurin activates several substrates, most notably a family of four nuclear factor of activated T-cells (NFATc1-c4) transcription factors (Rao et al., 1997). In a basal state NFATs are heavily phosphorylated and sequestered in the cytoplasm, but upon stimulation rapidly translocate into the nucleus due to calcineurin-mediated dephosphorylation (Crabtree and Olson, 2002). This pathway was originally identified and is still by far best-characterized in the immune system (Hogan et al., 2003). Activation of NFATs, however, has recently been implicated as a critical link between a prolonged increase in intracellular Ca2+ and initiation of a transcriptional program leading to adaptive changes in other tissues, including chondrogenesis (Ranger et al., 2000; Tomita et al., 2002), bone remodeling (Matsuo et al., 2004; Asagiri et al., 2005), lung maturation (Dave et al., 2006), cerebellar development (Sato et al., 2005), and skeletal muscle fiber-type switching (Chin et al., 1998). The importance of calcineurin-NFAT signaling in adaptive growth has thus far been investigated primarily in muscle, especially the heart. Cardiac-specific expression of a constitutively active calcineurin or NFATc4, for instance, is sufficient to induce hypertrophy (Molkentin et al., 1998) and results in accelerated heart failure (Wilkins et al., 2004). In addition, genetic and pharmacological inhibition of calcineurin-NFAT axis have shown that this pathway is necessary for a full hypertrophic response in cardiac tissue of rodents. In contrast to the heart, our study is the first to examine calcineurin-NFAT signaling in the course of a physiologically adaptive adult growth that is largely hyperplastic with synthesis of new cells (Crozier et al., 2006).
The functional significance of calcineurin in exocrine pancreas has been studied primarily in the context of stimulus-secretion coupling. Both the regulatory and the catalytic subunits of calcineurin are expressed in the pancreas and colocalize in the cytoplasm (Burnham, 1985; Yokoyama et al., 1990). CCK dose-dependently activates calcineurin in isolated acinar cells and the effect is blocked by specific inhibitors of this phosphatase, immunosuppressants FK506 and cyclosporin A (CsA; Groblewski et al., 1998). Inhibition of calcineurin also leads to a decrease in secretagogue-stimulated protein synthesis (Sans and Williams, 2004). Recently, our laboratory has addressed the role of calcineurin in a model of pancreatic growth driven by increased endogenous secretion of CCK. Chronic elevation in plasma levels of CCK induced by feeding a diet containing a protease inhibitor (PI) camostat led to more than a doubling in pancreatic weight as well as parallel increases in DNA and protein content (Tashiro et al., 2004). These changes were completely blocked by injection of CsA or FK506 without any effect on circulating CCK. The downstream transcriptional effectors of calcineurin, however, have not been systematically evaluated. The current study, therefore, examines exocrine pancreatic expression of calcineurin-regulated NFATc1-c4 and details activation of these transcription factors by CCK. Thus far, the vast majority of work centered on calcineurin-NFAT signaling in adaptation is based on either secondary, transformed cell lines, or in vivo models. Here we combine a detailed, multifaceted examination of growth factor–induced activation of calcineurin-NFAT signaling in well-differentiated primary cells together with a study of a physiological model of growth in vivo. We show that CCK activates NFATs both in vitro and in vivo in a calcineurin-dependent manner and point to NFATs as likely transcriptional effectors of calcineurin in a hormonally regulated, adaptive growth response.
MATERIALS AND METHODS
Materials
Camostat was provided by Ono Pharmaceuticals (Osaka, Japan). TaqMan reverse transcription reagents and Expand high fidelity PCR system were purchased from Roche (Basel, Switzerland). Trizol and PCR primers were obtained from Invitrogen (Carlsbad, CA). Antibodies to NFATc1 (sc-7294), NFATc2 (sc-7296), NFATc3 (sc-8321; polyclonal used in isolated acini) and (sc-8405; monoclonal used mostly in vivo), NFATc4 (sc-1153), GFP (sc-9996),and Lamin A/C (sc-20681) were from Santa Cruz Biotechnology (Santa Cruz, CA); those to cyclophilin A (07-313) from Upstate (Lake Placid, NY); to VDAC (ab15895) and BIP (ab2902) from Abcam (Cambridge, MA); and to Amylase (A-8273) from Sigma (St. Louis, MO). Secondary antibodies and chemiluminescence reagents came from Amersham Pharmacia (Piscataway, NJ). Precast gels, nitrocellulose membrane, and SDS-PAGE markers were from Bio-Rad (Hercules, CA). Collagenase was purchased from Crescent Chemicals (Hauppauge, NY), sulfated CCK-8 peptide from Research Plus (Bayonne, NJ), Bombesin from Bachem (Torrance, CA) and MiniComplete protease inhibitor from Roche. All other chemicals were obtained from Sigma (St. Louis, MO).
Cells and Cell Culture
Pancreatic acini were isolated from 6- to 8-wk-old male ICR mice by collagenase digestion, as previously described (Yule and Williams, 1992). HEK293 and AR42J cells were obtained from ATCC (Rockville, MD) and cultured according to supplied instructions. Jurkat T-cells were a gift of Dr. David M. Markovitz (University of Michigan, Ann Arbor, MI) and were grown in an RPMI-1640 medium (Invitrogen), supplemented with 10% fetal bovine serum and antibiotic/antimycotic.
Animals and Treatment
Male ICR mice (Harlan Sprague Dawley, Indianapolis, IN), weighing ∼30g were used, unless noted otherwise. CCK-deficient mice and appropriate controls were on a C57/Bl6 background, whereas NFAT-luciferase mice and the nontransgenic controls were on FVBN background. The mice were housed at 22–24°C on a 12-h light/dark cycle with free access to water and a standard rodent chow (5001 Rodent Diet; PMI Nutrition International, St. Louis, MO). In acute studies, mice were fasted overnight, refed with powder chow containing 0.1% camostat for 0.5–2 h, and then killed. For long-term growth and in vivo luciferase studies, mice were fed ad libitum either with powder chow containing 0.1% camostat or the same chow without camostat (control) for 3–7 d. Animals treated with FK506 were injected subcutaneously twice a day with either 3 mg/kg FK506 or vehicle (8% ethanol in 0.9% saline). Injections began 2 d before initiating feeding protocols. All studies were approved by the University of Michigan Committee on Use and Care of Animals.
RNA Isolation, RT, and PCR
RNA isolation, cDNA synthesis, and PCR followed the same procedures as described previously (Sans and Williams, 2004). Briefly, total RNA was isolated from kidney, liver, or pancreas using TRIzol and RNeasy spin columns (Qiagen, Valencia, CA). For AR42J cells and isolated acinar cells, RNA was obtained using RNeasy kit according to manufacturer's instructions. RNA quality was evaluated by agarose gel electrophoresis and UV spectrometry. Total RNA, 1 μg, was reverse-transcribed using TaqMAN reagents with random hexamers as primers. PCR was carried out using reagents from High Fidelity PCR system in Eppendorf Epgradient Mastercycler (Fremont, CA). The primers were designed with Primer Express software from Applied Biosystems (Foster City, CA) based on gene sequences retrieved from GenBank NCBI (see Supplementary Table).
Preparation of Whole Cell, Cytoplasmic, and Nuclear Lysates
For whole cell lysates, pancreatic tissue was homogenized with a polytron homogenizer in an ice-cold phosphate-buffered saline (PBS) with MiniComplete protease inhibitor. A brief sonication and centrifugation followed and the supernatant was saved for further analysis. Cytoplasmic/nuclear protein extraction from intact pancreas adhered to the same protocol as already described (Guo et al., 2007). In short, pancreas was removed and homogenized in 1.5–2 ml of a 2 M sucrose buffer with a motor-driven pestle. Nuclei were collected at 30,000 rpm in a Beckman Optima TLX ultracentrifuge (Fullerton, CA) for at 4°C. The pellet was washed with ice-cold PBS, centrifuged, and resuspended in 0.25–0.5 ml of high-salt nuclear lysis buffer, and the original supernatant was saved as a cytoplasmic extract. Cytoplasmic/nuclear protein extraction from primary acinar cells and a Jurkat cell line followed a modified nuclear extraction protocol (Shaw et al., 1988). Cytoplasmic extracts were obtained in the same manner as above, except only 0.5–0.75 ml of high-sucrose buffer was used. The pellet was then washed with ice-cold PBS and resuspended in 350–750 μl of a low-salt resuspension buffer (50 mM HEPES, 50 mM KCl, 0.1 mM EDTA, 10% glycerol, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM benzamidine, and MiniComplete protease inhibitor). Ammonium sulfate (35–75 μl, 3 M) was added to bring its concentration in the buffer up to 0.3 M. The samples were incubated for 30 min on a rocking platform in a cold room and then centrifuged for 15 min at 100,000 rpm in the Beckman ultracentrifuge. To precipitate nuclear proteins, the resulting supernatant was added to an equal volume of 3 M ammonium sulfate and centrifuged for 10 min at 50,000 rpm. The pellet was resuspended in 250–500 μl of low-salt resuspension buffer and used in future experiments. All protein concentrations were determined using protein assay reagent (Bio-Rad, Hercules, CA).
SDS-PAGE and Western Blotting
The samples were loaded onto precast SDS-polyacrylamide gels (Bio-Rad) with 15–60 μg/lane as described in Results. Proteins were then transferred onto nitrocellulose membranes, blocked, and incubated overnight with primary antibody at 4°C. The membranes were washed, incubated with the appropriate secondary antibody, and visualized on an Alpha Ease FC8900 imaging system (Alpha Innotech, San Leandro, CA); background-subtracted raw densitometric value (rdv) quantitation was carried using the same system. NFAT nuclear occupancy in isolated acinar cells was calculated as follows: (rdv of nuclear extract × average fraction of total cellular protein in nucleus)/(rdv of nuclear extract × average fraction of total cellular protein in nucleus + rdv of cytoplasmic extract × average fraction of total cellular protein in cytoplasm).
Adenoviruses
NFATc1-GFP adenovirus was a kind gift of Dr. Martin Schneider (University of Maryland, Baltimore, MD). NFAT-luciferase, FLAG-tagged truncated form containing the calcineurin-inhibitory domain of endogenous inhibitor CAIN (Flag-CAIN) and constitutively active calcineurin (CaCN) adenoviruses were constructed as previously described (Wilkins et al., 2004). An adenovirus expressing bacterial β-galactosidase and EGFP (βgal/GFP) driven by separate cytomegalovirus (CMV) promoters was obtained from Dr. He (John Hopkins Oncology Center, Baltimore, MD) and used as a control. High titer virus was collected using a Vivapure AdenoPACK 100 kit (Vivascience, Hannover, Germany) according to the manufacturer's instructions and titered using the agarose overlay method, as described previously (Chen et al., 2001).
Confocal Fluorescence Microscopy
Secondary cell lines (HEK293 and AR42J) were grown directly on 25-mm glass coverslips in six-well tissue culture plates until near confluence and incubated overnight with 107 PFU/ml NFATc1-GFP or βgal/GFP adenovirus. Acinar cells were isolated in the afternoon and incubated in suspension overnight with an adenovirus. Next day, acini were collected, resuspended in fresh DMEM, placed on glass coverslips treated with Cell-TAK Adhesive (BD Biosciences, Bedford, MA), and allowed to attach for ∼30 min. The coverslips were then mounted as the bottom of a chamber placed on a microscope stage, incubated in clear DMEM, and loaded with 1.5 μl/ml DRAQ5 nuclear-labeling dye for 3 min. Imaging was performed using an Olympus FluoView 500 confocal microscope (Melville, NY). GFP fluorescence was excited with a 488-nm blue argon laser and emission was measured through a 505–565-nm barrier filter. Samples were scanned using a 25× objective and magnified by 1.5–2.0 zoom with FluoView version 5.0 software. Digital images were collected either individually or as multi-TIFF time series and processed using Photoshop v8.0 software (Adobe Systems, Mountain View, CA).
Luciferase Reporter Assay
Isolated acini were incubated in suspension with 5 × 106 PFU of NFAT-luciferase adenovirus for 30 min and then distributed evenly in 22-mm 12-well plates. The cells were preincubated for 1 h and were then stimulated for 5.5 h. Acini were collected by centrifugation, washed with PBS, and lysed in 200 μl of reporter lysis buffer (Promega, Madison, WI). Luciferase activity was measured with beetle luciferin as substrate (Luciferase Assay Kit, Promega) and raw data collected with a Berthold luminometer (Wildbad, Germany). When using FK506 or CsA, acini were pretreated with these inhibitors for 30 min before adding NFAT-luciferase, whereas for CaCN or Flag-CAIN, acini were incubated with the virus overnight. Each condition was assayed using cells from at least three independent acinar cell preparations with four replicate wells per assay. The data were normalized to protein concentration in the lysate.
Transgenic Mice
NFAT-luciferase transgenic mice were generated as previously described (Wilkins et al., 2004). Briefly, nine copies of an NFAT binding site from the IL-4 promoter were positioned 5′ to a minimal promoter from the alpha-myosin heavy-chain gene and inserted upstream of a luciferase reporter gene in pGL-3 Basic (Promega). The animals were phenotypically normal, were bred with wild-type FVBN mice, and were genotyped by PCR using a tail-clipping method and primers listed in the Supplementary Table. To measure luciferase expression, 50 mg of pancreas, liver, or kidney was excised and homogenized in 800 μl of ice-cold PBS with 0.25 mM EDTA. The samples were sonicated and centrifuged and 20 μl of the supernatant was used for analysis.
Statistics
Multiple comparison data were analyzed by one-way ANOVA followed by Dunnett's posttest carried out on Graphpad Prism (Hearne Scientific, Auckland, New Zealand) and expressed as means ± SE. Normalized luciferase data from NFAT-luciferase transgenic mice were analyzed by a two-tailed Student's t test.
RESULTS
NFATc1-c4 Expression in Exocrine Pancreas
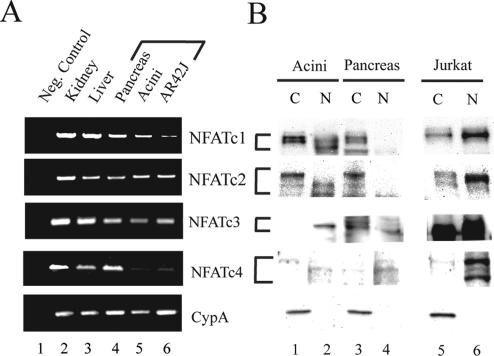
Expression of calcineurin-regulated NFAT genes (NFATc1-c4) was examined at the mRNA level using semiquantitative RT-PCR and at the protein level using Western blotting. All four NFAT genes were strongly expressed in the intact pancreas. Isolated pancreatic acini and the acinar-cell–derived AR42J cell line, however, strongly expressed NFATc1-c3, whereas NFATc4 was expressed weakly, if at all (Figure 1A). The amplicons were the same size as those from kidney and liver, two organs previously reported to express all four NFAT genes (Hoey et al., 1995). Parallel endogenous NFAT expression was observed at the protein level, with Western blots showing all four NFAT genes in the pancreas, but primarily NFATc1-c3 in isolated acinar cells (Figure 1B). On the basis of these findings, we concluded that NFATc1-c3 were main calcineurin-regulated NFAT genes in exocrine pancreas and hence all subsequent work was limited to these three genes.
Figure 1.
Expression pattern of calcineurin-regulated NFAT genes (NFATc1-c4) in exocrine pancreas. (A) Semiquantitative RT-PCR using primer pairs specific for NFATc1-c4 genes, as described in Materials and Methods. Lane 1, RT control; lane 2, kidney; lane 3, liver; lane 4, pancreas, lane 5, pancreatic acini, and lane 6, acinar cell-derived AR42J cell line. Cyclophillin A was included as a control. (B) Western blot analysis of NFAT expression at the protein level. Lanes 1 and 2, cytoplasmic [C] and nuclear [N] extracts of pancreatic acini (30 μg/lane); Lanes 3 and 4, C and N extracts of whole pancreas (30 μg/lane); lanes 5 and 6, C and N extracts of Jurkat T-cell line (positive control, 15 μg/lane), separated slightly for clarity. Cyclophilin A (CypA) was used as a loading control.
Stimulation with CCK Leads to Increased Nuclear NFAT Occupancy
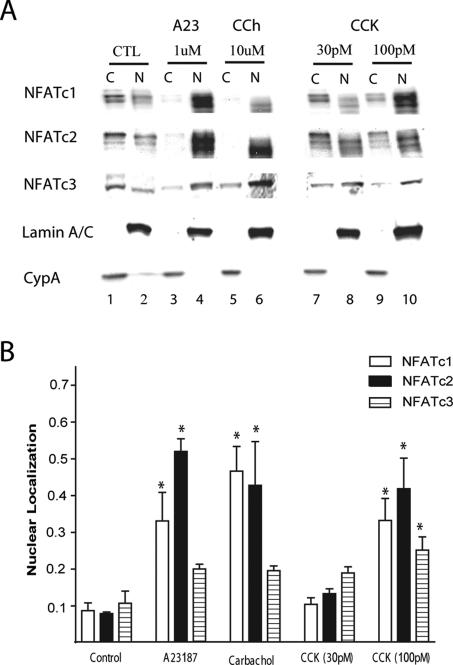
Nuclear translocation is a hallmark of NFAT activation. To examine the effect of CCK on the fraction of these transcription factors that reside in the nucleus, we performed Western blotting using cytoplasmic and nuclear lysates of isolated acinar cells (Figure 2, A and B). One-hour incubation with 100 pM CCK resulted in a significant 3–5-fold increase in nuclear NFATc1 and c2 and a lower increase in NFATc3. Similar results were obtained in response to the calcium ionophore A23187 (2 μM, positive control) as well as the muscarinic agonist carbachol (10 μM). The purity of the extracts was confirmed by Western blotting and showed more than 95% enrichment of cytosolic marker cyclophilin A and nuclear marker lamin A/C (Figure 2A) in their respective fractions. Western blots against markers of cytoplasmic organelles, for zymogen granules (amylase), endoplasmic reticulum (BIP), and mitochondria (VDAC), all produced markedly stronger cytoplasmic signal and only amylase appeared in the nuclear fraction (data not shown). In addition, the increase in nuclear NFATs could not be attributed to increased expression, because short-term treatment with CCK did not increase total NFATc1-c3 within the whole cell lysates (Supplementary Figure 1).
Figure 2.
Stimulation by CCK leads to an increase in nuclear occupancy of endogenous NFATs. (A) Isolated acinar cells were left untreated (CTL) or treated with 1 μM of A23187, 10 μM of carbachol and 30 or 100 pM of CCK for 1 h. Cytoplasmic [C] and nuclear [N] extracts were prepared, loaded at 30 μg/lane and analyzed by Western blotting, using antibodies for NFATc1-c3. Representative blots, along with nuclear (lamin A/C) loading control are shown. Blots of CCK-treated extracts (lanes 7–10) were separated for clarity. (B) Quantitation of nuclear occupancy based on densitometry measurements of Western blots such as the one shown in A and adjusted for total protein as described in Materials and Methods. Values are the mean and SE for 3–6 experiments. *p ≤ 0.05 versus control.
CCK Promotes Nuclear Translocation of NFATc1-GFP
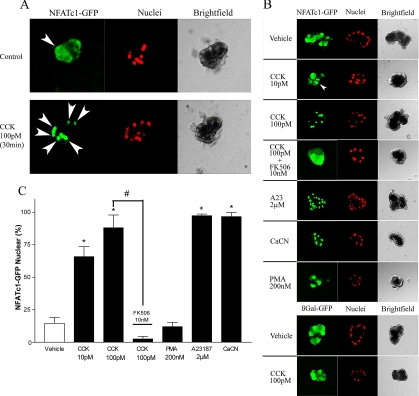
To more directly visualize changes in subcellular localization of NFATs, we used an adenoviral vector to express NFATc1-GFP fusion protein. Isolated acini were incubated overnight with either NFATc1-GFP or a control virus expressing βgal and green fluorescent protein (GFP) and were then imaged with a confocal microscope. As in our previous experience with adenovirus-mediated expression in acini (Chen et al., 2003), more than 95% of individual acinar cells displayed GFP fluorescence. In addition, nuclear marker DRAQ5 was taken up into the cells within 3 min of incubation and rapidly produced a detectable signal with no observable cytotoxicity, at least in the short term (up to 4 h).
In unstimulated acini, NFATc1-GFP showed a broadly cytoplasmic distribution, with a low but detectable nuclear signal in some cells. On 30-min treatment with 100 pM CCK, there was a change to an almost exclusively nuclear signal (Figure 3A; high magnification overlay in Supplementary Figure 2A). This translocation was rapid and reached a sustained peak between 30 and 60 min (Supplementary Figure 2B). Nuclear shuttling of NFATc1-GFP was also characterized in response to a variety of other agents, as shown in images of representative acini after 30 min of treatment (Figure 3B) and quantitation of these results as “% nuclear NFATc1-GFP” by colocalization of GFP and DRAQ5 signals (Figure 3C). CCK-mediated translocation of NFATc1-GFP occurred in a dose-dependent manner, because 10 pM CCK produced a lower degree of nuclear localization than 100 pM. The response was also dependent on calcineurin, because 30-min preincubation with FK506, a specific inhibitor of this enzyme, completely blocked CCK-driven nuclear shuttling. In contrast, acini treated with either 107 PFU/ml constitutively active calcineurin (CaCN) or 1 μM A23187 exhibited an almost exclusively nuclear NFATc1-GFP signal. In acini treated with PMA, an agent that does not elevate intracellular calcium, the localization of the fusion protein was the same as in vehicle treated cells. Lastly, acini incubated with adenovirus expressing GFP showed a diffusely cytoplasmic fluorescence that was not altered by 30-min treatment with 100 pM CCK. We also carried out experiments with acinar-cell derived AR42J cell line and HEK293 cells. AR42J cells responded to CCK with a nearly complete nuclear translocation at 1 nM, whereas HEK cells which do not express CCK receptor, responded to A23187 and CaCN, but not CCK. Western blots against GFP in acini incubated overnight with NFATc1-GFP likewise showed a similar shift in nuclear localization upon stimulation with 100pM CCK as that observed for endogenous NFATc1 in Figure 2A (data not shown).
Figure 3.
CCK promotes nuclear translocation of NFATc1-GFP. Isolated mouse acini were incubated overnight with 107 PFU/ml either NFATc1-GFP or βgal/GFP adenovirus, loaded with DRAQ5 nuclear-labeling dye and imaged on a Cell-Tak–coated glass coverslip using an Olympus FluoView 500 confocal microscope. (A) Time course of a single isolated mouse acinus before (top) and after (bottom) 30-min treatment with 100 pM CCK. (B) Sample images of acini treated for 30 min with agents as listed. In experiments using FK506 and CaCN, acini were pretreated for 30 min and overnight, respectively. (C) Randomly chosen medium-sized (5–20 cell) DRAQ5-loaded acini expressing GFP (approx. 95% of all acinar cells) were used to quantitate % of nuclei showing NFATc1 signal, using colocalization of DRAQ5 and GFP. Data are a mean ± SE of 3–5 independent acinar isolations with averaged counts from at least 10 acini per preparation. *p < 0.01 compared with vehicle-treated control.
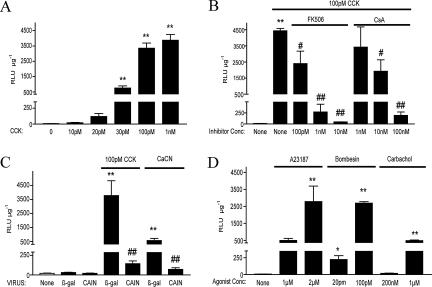
CCK Leads to NFAT Activation in a Calcineurin-dependent Manner
To examine the functional consequences of NFAT translocation, we incubated isolated acinar cells with 5 × 106 PFU/ml of an adenovirus where expression of luciferase gene is controlled by nine tandem NFAT binding sites from the IL-4 promoter. Transcriptional activation of these elements in response to NFATs has been well-documented (Szabo et al., 1993; Sanna et al., 2005). CCK dose-dependently activated luciferase expression, which plateaus at 100 pM with an ∼500-fold increase compared with unstimulated cells (Figure 4A). CCK-mediated NFAT activation was dependent on calcineurin, as it was dose-dependently inhibited by two different inhibitors, FK506 and CsA (Figure 4B). This finding is further supported by an adenoviral overexpression of a well-established endogenous calcineurin inhibitor CAIN (Lai et al., 1998). Overnight incubation with 107 PFU/ml adenovirus expressing CAIN blocked both CCK- and CaCN-induced increase in NFAT-luciferase activity (Figure 4C). Lastly, we demonstrated that the reporter dose-dependently responded to a range of agonists that elevate intracellular calcium and are thereby known to activate calcineurin, including A23187, bombesin, and carbachol (Figure 4D); agents that signal via other pathways, such as PMA and VIP, did not have any effect (data not shown).
Figure 4.
CCK activates NFATs in isolated acinar cells (in vitro). Isolated acinar cells were incubated for 6.5 hours with 5 × 106 PFU/ml NFAT-luciferase (NFAT-luc) adenovirus, treated, lysed, and assayed for luciferase activity. For adenoviruses (CaCN, FLAG-CAIN, or βgal/GFP), treatments were carried out overnight with 107 PFU/ml; for all other agents, cells were treated for 5.5 h. (A) Dose-response activation of NFAT-luc reporter with 10 pM–1 nM of CCK. (B) Dose-response inhibition of CCK-induced NFAT-luc activation by specific calcineurin inhibitors FK506 (100 pM–10 nM) and CsA (1 nM–100 nM). (C) Inhibition of CCK- and CaCN-induced NFAT-luc activation by overexpression of truncated form of endogenous calcineurin inhibitor CAIN (Flag-CAIN). (D) Dose-response activation of NFAT-luciferase with A23187, bombesin, and carbachol. Relative luciferase activity (RLU) was normalized to protein concentration in the lysates. Data represent a mean ± SE of 3–8 independent acinar isolations with averaged counts of four wells per preparation. *p < 0.05 or **p < 0.01 compared with vehicle-treated controls and #p < 0.05 or ##p < 0.01 when compared with agonist (100 pM CCK)-treated controls.
CCK Promotes Calcineurin-dependent Increase in Nuclear NFATs In Vivo
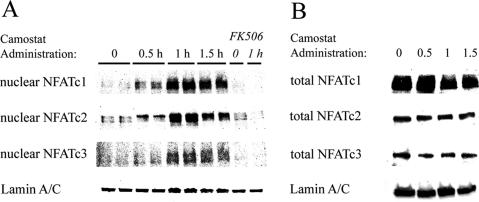
The data presented thus far clearly show that CCK can promote calcineurin-dependent nuclear translocation and activation of NFATs in isolated acinar cells. Given the evidence in vitro, we went on to examine whether the same holds true in vivo. Male ICR mice were fed with a diet containing the synthetic trypsin inhibitor camostat, which results in increased secretion of CCK and elevation in plasma levels of this hormone from a basal level of 0.5–1.5 pM to ∼10 pM (Yamamoto et al., 2003; Tashiro et al., 2004). Nuclear extracts from mice fed camostat showed an increase in nuclear NFAT signal with a peak at 1 h, as shown by representative Western blots in Figure 5A. Densitometric measurements of four independent Western blots of NFATc1, showed a 2.3 ± 0.7-fold increase compared with basal at 0.5 h, a 4.9 ± 2.0 fold increase at 1 h, and a 4.0 ± 1.3-fold increase at 1.5 h. The effect was completely abolished by injection of FK506, with the nuclear NFATc1 signal declining to 0.4 ± 0.1-fold of basal. Similar changes were seen for NFATc2 and c3. As in isolated acini, the increase in nuclear NFATs could not be attributed to increased expression of these proteins, since short-term camostat treatment did not alter total NFATc1-c3 within the whole cell pancreatic lysates (Figure 5B).
Figure 5.
Elevation in endogenous CCK secretion promotes increase in nuclear NFATs in vivo. (A) Mice adapted to powdered diet were fasted overnight and fed with a chow containing 0.1% camostat for 0–1.5 h. Animals treated with FK506 were injected with 3 mg/kg twice a day for 2 d before fasting. Nuclear protein was extracted and analyzed by Western blotting. Blots shown are representative of at least three independent experiments for NFATc1-c3, along with nuclear (lamin A/C) loading control. (B) Short-term treatment with camostat-containing diet does not alter total NFAT protein expression in the pancreas. Whole cell extracts were loaded with 60 μg/lane and analyzed by Western blotting using antibodies for NFATc1-c3. Representative blots, along with loading control (Lamin A/C), are shown.
Treatment with protease inhibitors such as camostat, is known to affect other GI hormones, most notably secretin (Watanabe et al., 1992). To address the importance of CCK to nuclear NFAT shuttling in our in vivo model, we performed Western blotting using nuclear lysates from CCK-deficient mice (Lacourse et al., 1999) fed a camostat-containing diet for 1 h. Nuclear NFATs in these genetically modified animals were not appreciably increased compared with fasted controls as well as to strain and age-matched wild-type mice (Supplementary Figure 3A). In addition, in order to control the timing and to compel rapid feeding, mice fed with a camostat-containing diet were fasted overnight. Food intake in previously fasted animals increases plasma CCK from a basal of 1 pM to 3–5 pM (Sans and Williams, 2004; Tashiro et al., 2004), but this small change is not enough to produce pancreatic growth and also does not lead to an increase in nuclear NFATs (Supplementary Figure 3B).
CCK Promotes Calcineurin-dependent Transcriptional Activation of NFATs In Vivo
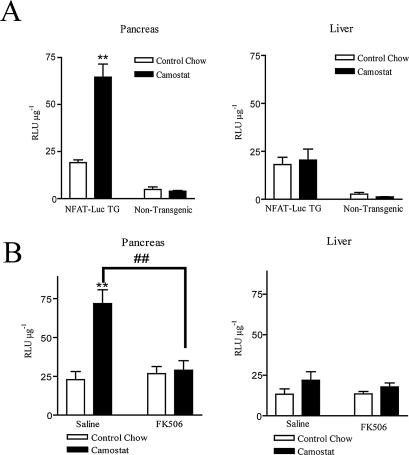
To examine the functional consequences of increased nuclear NFATs in vivo, we used a transgenic mouse model expressing the same NFAT-luciferase reporter as the one used in the isolated acinar cell experiments described above. Because the mice were on an inbred FVBN background, we first examined the parameters of pancreatic growth response of this strain. Similar to previously evaluated ICR and C57BL/6 mouse strains (Sato et al., 2002; Tashiro et al., 2004), FVBN mice exhibited a robust pancreatic growth in response to camostat-containing diet, with more than a twofold increase in total wet pancreatic weight and relative pancreatic weight as well as significant increases in total pancreatic protein and DNA (Table 1). When they were fed camostat, both transgenic mice and their littermate controls showed the same degree of pancreatic growth, but relative luciferase activity was significantly increased only in the pancreases of transgenic mice (Figure 6A). In liver, an organ that we would not expect to be affected by an elevation in endogenous CCK, there was no difference in reporter expression due to camostat feeding in either transgenic or nontransgenic mice. Moreover, the camostat-induced increase in NFAT-driven luciferase activity was dependent on calcineurin, because injection of FK506 completely blocked camostat-induced increase in NFAT-luciferase reporter expression in the pancreases of NFAT-luc transgenic mice. Liver expression of luciferase was not affected by FK506 (Figure 6B).
Table 1.
Camostat increases pancreatic growth in FVBN mice
| None | 4 d | 7 d | |
|---|---|---|---|
| Body weight (g) | 24.7 ± 0.6 | 24.3 ± 0.8 | 23.8 ± 2.5 |
| Pancreatic weight (mg) | 227 ± 27 | 349 ± 13* | 490 ± 112** |
| Relative pancreatic weight (PW/BW, mg/g) | 7.97 ± 0.9 | 14.4 ± 0.7** | 20.6 ± 2.3** |
| Protein content (mg/pancreas) | 38.3 ± 5.2 | 56.2 ± 8.6 | 106 ± 21** |
| DNA content (mg/pancreas) | 1.76 ± 0.4 | 1.96 ± 0.2 | 2.68 ± 0.3** |
Male mice adapted to the powder diet were fed either a control chow or a chow containing 0.1% camostat for 4 or 7 d. The excised pancreases were weighed and homogenized, and the protein and DNA content were determined as described in Materials and Methods. Results are means ± SD for 4–5 mice.
* p < 0.05 or ** p < 0.01 vs. control chow (no camostat).
Figure 6.
Elevation in endogenous CCK leads to NFAT activation in vivo. (A) FVBN male NFAT-luciferase mice and their nontransgenic littermates were fed with either a control chow or a chow containing 0.1% camostat for 7 d. The mice were then killed, and their livers and pancreases were quickly excised and homogenized. Animals treated with FK506 were injected with 3 mg/kg twice a day for 2 d before and throughout the time of treatment with camostat. (A) Feeding camostat-containing diet increases NFAT-luciferase reporter expression in the pancreases of transgenic NFAT-luc mice but not in wild-type littermate controls; in the liver there is no difference in reporter expression due to camostat treatment in either group. (B) Injection of FK506 completely blocked the camostat-induced increase in NFAT-luciferase reporter expression in the pancreases of NFAT-luc transgenic mice. Liver expression was not affected. n = 3–8 animals per group. **p < 0.01 compared with the control chow-fed group; ##p < 0.01 compared with the saline-injected, camostat-fed group.
DISCUSSION
Calcineurin-NFAT signaling, originally characterized as a key to cytokine expression, has since been shown to regulate a range of adaptive responses outside the immune system (Horsley and Pavlath, 2002). Our study explores a novel role of this pathway in adaptive hyperplastic growth. We show that NFATc1-c3 are the predominant NFATs expressed in pancreatic acini. All three are known to be regulated by calcineurin, but the differences between them as far as affinity and kinetics of their interactions with calcineurin, dephosphorylation sites, nuclear translocation mechanisms, and binding affinity with DNA and transcriptional partners remain obscure. Although the main focus of this study is not the activation patterns of individual NFATs, based on their robust response and high level of expression, we postulate that NFATc1 and c2 are the most likely to play a functional role in response to CCK. Confirming this prediction awaits further studies using pancreas-specific, NFAT-deficient animals.
Although calcineurin-independent NFAT activation has been reported (Ghosh et al., 1996; Fujii et al., 2005), the most firmly established model of NFAT activation consists of calcineurin-mediated dephosphorylation, nuclear translocation, and transcriptional activation (Molkentin, 2000). CCK rapidly activates calcineurin in exocrine pancreas as shown by an in vitro phosphatase assay (Burnham, 1985) and dephosphorylation of CHRSP-24, a known enzymatic target of calcineurin in vivo (Groblewski et al., 1998). On the basis of imaging of live acinar cells expressing NFATc1-GFP, we show that CCK-stimulated shuttling of NFAT proteins begins within minutes and reaches a sustained peak between 0.5 and 1 h. Although we have not modeled the kinetics of NFATc1-GFP, we made similar observations as those in a recent report by Shen et al. (2005), including accumulation of nuclear NFATc1 into foci (so called “NFATc1 bodies,” as pointed out by the small white arrows in Supplementary Figure 3A) and differences in time course of translocation depending on the stimulatory agent or even varying doses of the same agent. Rich heterogeneity of downstream signals, such as nuclear translocation of transcription factors, may be an important means whereby calcium transients are able to regulate a diverse range of short- and long-term responses (Johnson and Chang, 2000). Subcellular distribution of endogenous NFATs examined by Western blotting closely mirrors imaging experiments with a significant shift from primarily cytoplasmic to nuclear localization within 0.5–1 h. The slight discrepancies between the two methods may relate to detection sensitivities, signal-noise ratio limitation of imaging GFP (Goldman and Spector, 2005), or artifacts of overexpression. In addition, several reports have linked the multiple NFAT bands on Western blots with phosphorylation state of these proteins (Feske et al., 2001; Im and Rao, 2004). The lowest electrophoretic mobility band has been described as corresponding to basal, hyperphosphorylated NFATs, whereas each subsequent higher mobility band as increasingly dephosphorylated protein. Our Western blots of fractionated acinar extracts show a relatively stronger signal of a low electrophoretic mobility band in cytoplasm and higher mobility bands in the nucleus. In addition, treatment with λ-phosphatase led to a dose-dependent downward shift of cytoplasmic NFATc1 banding pattern, reflecting change in a phosphorylation state of the protein (data not shown). This observation further supports the model of calcineurin-mediated dephosphorylation and nuclear translocation of NFATs in response to CCK.
Once in the nucleus, NFATs act to initiate expression of numerous target genes. Although there is a clear spatial, temporal, and functional association, the precise link between nuclear translocation and transcriptional activation remains unclear (Hogan et al., 2003). The latter, for instance, has been shown to be regulated by modifications of the transactivation domains independent of the residues required for translocation (Okamura et al., 2000; Ortega-Perez et al., 2005). To specifically examine CCK-mediated transcriptional activation, we used an NFAT-driven luciferase reporter, introduced into our primary cells by an adenoviral vector. We show that CCK dose-dependently activates NFATs similarly to positive controls, calcium ionophore A23187 and constitutively active calcineurin. Two distinct inhibitors of calcineurin, CsA and FK506, dose-dependently ablate transcriptional activation of NFATs. The striking specificity of these agents has been addressed at length (Ho et al., 1996), but we confirmed our findings by overexpressing the endogenous calcineurin inhibitor CAIN, which unlike the two drugs, does not require association with immunophilins to be effective (Lai et al., 1998). In agreement with Okamura et al. (2000), the magnitude of the inhibition suggests calcineurin is the critical determinant of transcriptional activation of NFATs.
Perhaps the most interesting finding of this study is CCK-mediated activation of calcineurin-NFAT signaling in vivo. As shown here, in other strains of mice (Tashiro et al., 2004) and in rats (Otsuki et al., 1993; Yamamoto et al., 2003), feeding a camostat-containing diet leads to a dramatic, more than twofold increase in total and relative pancreatic weight. Our results also establish that this trophic response is accompanied by an increase in both nuclear NFATs and NFAT-driven luciferase reporter. These effects, like pancreatic growth itself, are mediated by calcineurin, because they are completely blocked by FK506. Although this does not prove a direct cause-and-effect relationship, it suggests that NFATs are likely the key functional transcriptional effectors of calcineurin in CCK-regulated growth. Elevation in endogenous CCK leads to proliferation of pancreatic acini in rodents, but the steps involved in turning on the machinery to allow the cells to enter the cell cycle are not well characterized. It has been shown that calcineurin can regulate cyclinD1 in growth-stimulated fibroblasts (Kahl and Means, 2004) and that NFATc4 can induce cyclinD1 in epidermal cl 41 cells (Ding et al., 2006). Whether analogous mechanisms take place in acinar cells in response to CCK remain to be seen, but preliminary studies have shown increases in cyclin D1 and D2 mRNA within 12–24 h of camostat feeding (Guo and Williams, unpublished data). Aside from directly regulating the cell cycle, NFATs may promote growth by inducing expression of a class of late early-response genes, which then in turn regulate cell proliferation. In addition, recent work in our laboratory has shown that mitogen-activated protein kinase–regulated early response transcription factors of the AP-1 family are activated in pancreatic growth (Guo et al., 2007) within the same time course as shown here for NFATs. The AP-1 family is known to regulate gene expression in concert with NFATs (Macian et al., 2001) as well as to increase the affinity and stabilize the binding of NFATs to DNA (Peterson et al., 1996; Ramirez-Carrozzi and Kerppola, 2001).
The calcineurin-NFAT pathway has been identified as a target for therapeutic intervention in regeneration of skeletal muscle (Sakuma et al., 2003) and β-cells (Heit et al., 2006). Given the importance of calcineurin in hormonally regulated pancreatic growth, manipulation of this pathway could potentially be used to promote regeneration of this organ following chronic pancreatitis or pancreatic resection. Protease inhibitor induced elevation of CCK and digestive enzyme secretion have been shown to take place in humans (Friess et al., 1998) and camostat has been used as an adjunct treatment of pancreatitis in Japan (Brackmann and Rosemeyer, 1984). Future translational applications, however, depend on a better understanding of the role of this pathway and its downstream effectors both in adaptive growth and pathological processes such as pancreatic cancer (Buchholz et al., 2006). Identification of CCK-mediated calcineurin-dependent activation of NFATs as a strong initial signal of this homeostatic growth response is a first step toward that goal.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Linda Samuelson (University of Michigan) for CCK-deficient mice, Dr. Stephen I. Lenz for his expert help with imaging, Dr. Sergio De Frutos for advice on reagents, and Dr. Jessica Schwartz for critical reading of the manuscript. This research was supported by National Institutes of Health Grants DK 59578 (J.A.W.), P30 DK-34933 (Michigan Gastrointestinal Peptide Center), and the Systems and Integrative Biology Training Grant (732 GM) This work also utilized the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center funded by NIH5P60 DK20572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0430) on October 31, 2007.
REFERENCES
- Asagiri M., et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentrem D. J., Joehl R. J. Pancreas: healing response in critical illness. Crit. Care Med. 2003;31:S582–589. doi: 10.1097/01.CCM.0000081428.35729.73. [DOI] [PubMed] [Google Scholar]
- Berube F. L., Benrezzak O., Vanier M., Morisset J. Effects of cerulein and epidermal growth factor on pancreatic growth in the reserpinized rat model. J. Pediatr. Gastroenterol. Nutr. 1993;17:39–48. doi: 10.1097/00005176-199307000-00006. [DOI] [PubMed] [Google Scholar]
- Brackmann P., Rosemeyer D. Oral foy in the treatment of chronic pancreatitis. Ric. Clin. Lab. 1984;14:435–437. [PubMed] [Google Scholar]
- Brannon P. M. Adaptation of the exocrine pancreas to diet. Annu. Rev. Nutr. 1990;10:85–105. doi: 10.1146/annurev.nu.10.070190.000505. [DOI] [PubMed] [Google Scholar]
- Buchholz M., Schatz A., Wagner M., Michl P., Linhart T., Adler G., Gress T. M., Ellenrieder V. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25:3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham D. B. Characterization of Ca2+-activated protein phosphatase activity in exocrine pancreas. Biochem. J. 1985;231:335–341. doi: 10.1042/bj2310335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Huber B. T., Grand R. J., Li W. Recombinant adenovirus coexpressing covalent peptide/MHC class II complex and B7- 1, in vitro and in vivo activation of myelin basic protein-specific T cells. J. Immunol. 2001;167:1297–1305. doi: 10.4049/jimmunol.167.3.1297. [DOI] [PubMed] [Google Scholar]
- Chen X., Ernst S. A., Williams J. A. Dominant negative Rab3D mutants reduce GTP-bound endogenous Rab3D in pancreatic acini. J. Biol. Chem. 2003;278:50053–50060. doi: 10.1074/jbc.M309910200. [DOI] [PubMed] [Google Scholar]
- Chin E. R., Olson E. N., Richardson J. A., Yang Q., Humphries C., Shelton J. M., Wu H., Zhu W., Bassel-Duby R., Williams R. S. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R., Olson E. N. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Crozier S. J., Sans M. D., Guo L., D'Alecy L. G., Williams J. A. Activation of the mTOR signalling pathway is required for pancreatic growth in protease-inhibitor-fed mice. J. Physiol. 2006;573:775–786. doi: 10.1113/jphysiol.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave V., Childs T., Xu Y., Ikegami M., Besnard V., Maeda Y., Wert S. E., Neilson J. R., Crabtree G. R., Whitsett J. A. Calcineurin/Nfat signaling is required for perinatal lung maturation and function. J. Clin. Invest. 2006;116:2597–2609. doi: 10.1172/JCI27331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Zhang R., Li J., Xue C., Huang C. Involvement of nuclear factor of activated T cells 3 (NFAT3) in cyclin D1 induction by B[a]PDE or B[a]PDE and ionizing radiation in mouse epidermal Cl 41 cells. Mol. Cell Biochem. 2006;287:117–125. doi: 10.1007/s11010-005-9087-1. [DOI] [PubMed] [Google Scholar]
- Feske S., Giltnane J., Dolmetsch R., Staudt L. M., Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- Friess H., Kleeff J., Isenmann R., Malfertheiner P., Buchler M. W. Adaptation of the human pancreas to inhibition of luminal proteolytic activity. Gastroenterology. 1998;115:388–396. doi: 10.1016/s0016-5085(98)70205-7. [DOI] [PubMed] [Google Scholar]
- Fujii T., et al. Galpha12/13-mediated production of reactive oxygen species is critical for angiotensin receptor-induced NFAT activation in cardiac fibroblasts. J. Biol. Chem. 2005;280:23041–23047. doi: 10.1074/jbc.M409397200. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Sica A., Cippitelli M., Subleski J., Lahesmaa R., Young H. A., Rice N. R. Activation of nuclear factor of activated T cells in a cyclosporin A-resistant pathway. J. Biol. Chem. 1996;271:7700–7704. doi: 10.1074/jbc.271.13.7700. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Spector D. L. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. Live Cell Imaging: A Laboratory Manual. [Google Scholar]
- Green G. M., Jurkowska G., Berube F. L., Rivard N., Guan D., Morisset J. Role of cholecystokinin in induction and maintenance of dietary protein-stimulated pancreatic growth. Am. J. Physiol. 1992;262:G740–G746. doi: 10.1152/ajpgi.1992.262.4.G740. [DOI] [PubMed] [Google Scholar]
- Green G. M., Levan V. H., Liddle R. A. Plasma cholecystokinin and pancreatic growth during adaptation to dietary protein. Am. J. Physiol. 1986;251:G70–G74. doi: 10.1152/ajpgi.1986.251.1.G70. [DOI] [PubMed] [Google Scholar]
- Groblewski G. E., Yoshida M., Bragado M. J., Ernst S. A., Leykam J., Williams J. A. Purification and characterization of a novel physiological substrate for calcineurin in mammalian cells. J. Biol. Chem. 1998;273:22738–22744. doi: 10.1074/jbc.273.35.22738. [DOI] [PubMed] [Google Scholar]
- Guo L., Sans M. D., Gurda G. T., Lee S. H., Ernst S. A., Williams J. A. Induction of early response genes in trypsin inhibitor-induced pancreatic growth. Am. J. Physiol. Gastrointest Liver Physiol. 2007;292:G667–677. doi: 10.1152/ajpgi.00433.2006. [DOI] [PubMed] [Google Scholar]
- Heit J. J., Apelqvist A. A., Gu X., Winslow M. M., Neilson J. R., Crabtree G. R., Kim S. K. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- Ho S., Clipstone N., Timmermann L., Northrop J., Graef I., Fiorentino D., Nourse J., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- Hoey T., Sun Y. L., Williamson K., Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Hogan P. G., Chen L., Nardone J., Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Horsley V., Pavlath G. K. NFAT: ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 2002;156:771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S. H., Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol. Cell. 2004;18:1–9. [PubMed] [Google Scholar]
- Johnson J. D., Chang J. P. Function- and agonist-specific Ca2+ signalling: the requirement for and mechanism of spatial and temporal complexity in Ca2+ signals. Biochem. Cell Biol. 2000;78:217–240. [PubMed] [Google Scholar]
- Kahl C. R., Means A. R. Calcineurin regulates cyclin D1 accumulation in growth-stimulated fibroblasts. Mol. Biol. Cell. 2004;15:1833–1842. doi: 10.1091/mbc.E03-10-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourse K. A., Swanberg L. J., Gillespie P. J., Rehfeld J. F., Saunders T. L., Samuelson L. C. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am. J. Physiol. 1999;276:G1302–G1309. doi: 10.1152/ajpgi.1999.276.5.G1302. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Burnett P. E., Wolosker H., Blackshaw S., Snyder S. H. Cain, a novel physiologic protein inhibitor of calcineurin. J. Biol. Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D. Stimulation of pancreatic acinar cell growth by CCK, epidermal growth factor, and insulin in vitro. Am. J. Physiol. 1986;251:G487–494. doi: 10.1152/ajpgi.1986.251.4.G487. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D., editor. Totowa, NJ: Humana Press; 1999. Role of Cholecystokinin in Physiologic and Pathophysiologic Growth of the Pancreas. [Google Scholar]
- Macian F., Lopez-Rodriguez C., Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- Matsuo K., et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004;279:26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. L., Baile C. A., Peikin S. R. Hyperphagia during lactation: satiety response to CCK and growth of the pancreas. Am. J. Physiol. 1983;244:E61–E65. doi: 10.1152/ajpendo.1983.244.1.E61. [DOI] [PubMed] [Google Scholar]
- Molkentin J. D. Calcineurin and beyond: cardiac hypertrophic signaling. Circ. Res. 2000;87:731–738. doi: 10.1161/01.res.87.9.731. [DOI] [PubMed] [Google Scholar]
- Molkentin J. D., Lu J. R., Antos C. L., Markham B., Richardson J., Robbins J., Grant S. R., Olson E. N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau C., Liddle R. A., Williams J. A., Grendell J. H. Pancreatic growth: interaction of exogenous cholecystokinin, a protease inhibitor, and a cholecystokinin receptor antagonist in mice. Gut. 1987;28(Suppl):63–69. doi: 10.1136/gut.28.suppl.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H., Aramburu J., Garcia-Rodriguez C., Viola J. P., Raghavan A., Tahiliani M., Zhang X., Qin J., Hogan P. G., Rao A. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol. Cell. 2000;6:539–550. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Ortega-Perez I., Cano E., Were F., Villar M., Vazquez J., Redondo J. M. c–Jun N-terminal kinase (JNK) positively regulates NFATc2 transactivation through phosphorylation within the N-terminal regulatory domain. J. Biol. Chem. 2005;280:20867–20878. doi: 10.1074/jbc.M501898200. [DOI] [PubMed] [Google Scholar]
- Otsuki M., Tani S., Fujii M., Nakamura T., Okabayashi Y., Koide M. Differential effects of proteinase inhibitor camostat on exocrine pancreas in fed and fasted rats. Am. J. Physiol. 1993;265:R896–R901. doi: 10.1152/ajpregu.1993.265.4.R896. [DOI] [PubMed] [Google Scholar]
- Peterson B. R., Sun L. J., Verdine G. L. A critical arginine residue mediates cooperativity in the contact interface between transcription factors NFAT and AP-1. Proc. Natl. Acad. Sci. USA. 1996;93:13671–13676. doi: 10.1073/pnas.93.24.13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Carrozzi V. R., Kerppola T. K. Dynamics of Fos-Jun-NFAT1 complexes. Proc. Natl. Acad. Sci. USA. 2001;98:4893–4898. doi: 10.1073/pnas.091095998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger A. M., Gerstenfeld L. C., Wang J., Kon T., Bae H., Gravallese E. M., Glimcher M. J., Glimcher L. H. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J. Exp. Med. 2000;191:9–22. doi: 10.1084/jem.191.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A., Luo C., Hogan P. G. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Sakuma K., Nishikawa J., Nakao R., Watanabe K., Totsuka T., Nakano H., Sano M., Yasuhara M. Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathol. (Berl.) 2003;105:271–280. doi: 10.1007/s00401-002-0647-0. [DOI] [PubMed] [Google Scholar]
- Sanna B., Bueno O. F., Dai Y. S., Wilkins B. J., Molkentin J. D. Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol. Cell. Biol. 2005;25:865–878. doi: 10.1128/MCB.25.3.865-878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans M. D., Williams J. A. Calcineurin is required for translational control of protein synthesis in rat pancreatic acini. Am. J. Physiol. Cell Physiol. 2004;287:C310–C319. doi: 10.1152/ajpcell.00534.2003. [DOI] [PubMed] [Google Scholar]
- Sato M., Suzuki K., Yamazaki H., Nakanishi S. A pivotal role of calcineurin signaling in development and maturation of postnatal cerebellar granule cells. Proc. Natl. Acad. Sci. USA. 2005;102:5874–5879. doi: 10.1073/pnas.0501972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Suzuki S., Kanai S., Ohta M., Jimi A., Noda T., Takiguchi S., Funakoshi A., Miyasaka K. Different effects of oral administration of synthetic trypsin inhibitor on the pancreas between cholecystokinin-A receptor gene knockout mice and wild type mice. Jpn. J. Pharmacol. 2002;89:290–295. doi: 10.1254/jjp.89.290. [DOI] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. [PubMed] [Google Scholar]
- Solomon T. E., Vanier M., Morisset J. Cell site and time course of DNA synthesis in pancreas after caerulein and secretin. Am. J. Physiol. 1983;245:G99–G105. doi: 10.1152/ajpgi.1983.245.1.G99. [DOI] [PubMed] [Google Scholar]
- Szabo S. J., Gold J. S., Murphy T. L., Murphy K. M. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells: roles for NF-Y and NF-ATc. Mol. Cell. Biol. 1993;13:4793–4805. doi: 10.1128/mcb.13.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Samuelson L. C., Liddle R. A., Williams J. A. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am. J. Physiol. Gastrointest Liver Physiol. 2004;286:G784–G790. doi: 10.1152/ajpgi.00446.2003. [DOI] [PubMed] [Google Scholar]
- Tomita M., Reinhold M. I., Molkentin J. D., Naski M. C. Calcineurin and NFAT4 induce chondrogenesis. J. Biol. Chem. 2002;277:42214–42218. doi: 10.1074/jbc.C200504200. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Takeuchi T., Chey W. Y. Mediation of trypsin inhibitor-induced pancreatic hypersecretion by secretin and cholecystokinin in rats. Gastroenterology. 1992;102:621–628. doi: 10.1016/0016-5085(92)90111-b. [DOI] [PubMed] [Google Scholar]
- Wilkins B. J., Dai Y. S., Bueno O. F., Parsons S. A., Xu J., Plank D. M., Jones F., Kimball T. R., Molkentin J. D. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Yule D. I. Stimulus-secretion coupling in the pancreatic acinus. In: Johnson L. R., editor. Physiology of the Gastrointestinal Tract. New York: Academic Press; 2006. pp. 1337–1369. [Google Scholar]
- Wisner J. R., Jr, McLaughlin R. E., Rich K. A., Ozawa S., Renner I. G. Effects of L-364,718, a new cholecystokinin receptor antagonist, on camostat-induced growth of the rat pancreas. Gastroenterology. 1988;94:109–113. doi: 10.1016/0016-5085(88)90617-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Otani M., Jia D. M., Fukumitsu K., Yoshikawa H., Akiyama T., Otsuki M. Differential mechanism and site of action of CCK on the pancreatic secretion and growth in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G681–G687. doi: 10.1152/ajpgi.00312.2002. [DOI] [PubMed] [Google Scholar]
- Yokoyama N., Furuyama S., Wang J. H. Demonstration of calmodulin-stimulated phosphatase isozymes by monoclonal antibodies. J. Biol. Chem. 1990;265:8170–8175. [PubMed] [Google Scholar]
- Yule D. I., Williams J. A. U73122 inhibits Ca2+ oscillations in response to cholecystokinin and carbachol but not to JMV-180 in rat pancreatic acinar cells. J. Biol. Chem. 1992;267:13830–13835. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.