Abstract
The role of lipid rafts in T cell antigen receptor (TCR) signaling was investigated using fluorescence microscopy. Lipid rafts labeled with cholera toxin B subunit (CT-B) and cross-linked into patches displayed characteristics of rafts isolated biochemically, including detergent resistance and colocalization with raft-associated proteins. LCK, LAT, and the TCR all colocalized with lipid patches, although TCR association was sensitive to nonionic detergent. Aggregation of the TCR by anti-CD3 mAb cross-linking also caused coaggregation of raft-associated proteins. However, the protein tyrosine phosphatase CD45 did not colocalize to either CT-B or CD3 patches. Cross-linking of either CD3 or CT-B strongly induced tyrosine phosphorylation and recruitment of a ZAP-70(SH2)2–green fluorescent protein (GFP) fusion protein to the lipid patches. Also, CT-B patching induced signaling events analagous to TCR stimulation, with the same dependence on expression of key TCR signaling molecules. Targeting of LCK to rafts was necessary for these events, as a nonraft- associated transmembrane LCK chimera, which did not colocalize with TCR patches, could not reconstitute CT-B–induced signaling. Thus, our results indicate a mechanism whereby TCR engagement promotes aggregation of lipid rafts, which facilitates colocalization of LCK, LAT, and the TCR whilst excluding CD45, thereby triggering protein tyrosine phosphorylation.
Keywords: lipid rafts, T cell antigen receptor, LCK, cholera toxin-B, signal transduction
One of the earliest detectable biochemical events after T cell antigen receptor (TCR)1 ligation is the rapid tyrosine phosphorylation of multiple intracellular proteins (Klausner and Samelson 1991). The TCR activates tyrosine phosphorylation by interacting sequentially with two different types of cytoplasmic protein tyrosine kinase (PTK; Weiss and Littman 1994). Membrane-associated Src-family PTKs, LCK and FYN, phosphorylate paired tyrosine residues in the immunoreceptor tyrosine-based activation motifs (ITAMs) of the ζ and CD3 subunits of the TCR (Weiss 1993). The cytoplasmic PTKs, ZAP-70 and SYK, are then recruited to the activated receptor via interaction of their NH2-terminal tandem Src-homology 2 (SH2) domains with phospho-ITAMs, which facilitates their subsequent phosphorylation and activation (Chan and Shaw 1996). As a consequence of TCR activation of these PTKs, numerous intracellular proteins become tyrosine phosphorylated and trigger downstream signaling pathways. These include hydrolysis of inositol-containing phospholipids, Ca2+ mobilization and activation of the Ras/extracellular-regulated kinase (ERK) MAP kinase pathway (Cantrell 1996). Together, these signaling pathways ultimately induce the transcription of genes essential for cell cycle entry, including the T cell growth factor, interleukin-2.
Plasma membranes of many cell types, including T cells, contain microdomains commonly referred to as lipid rafts, which are biochemically distinct from bulk plasma membrane (Simons and Ikonen 1997; Brown and London 1998). These domains are enriched in sphingolipids and cholesterol, which form lateral lipid assemblies in an unsaturated glycerophospholipid environment (Simons and Ikonen 1997). Lipid rafts are resistant to solubilization at low temperature by nonionic detergents, such as Triton X-100, and, due to their low buoyant density, can be isolated by density gradient ultracentrifugation (Brown and Rose 1992; Parton and Simons 1995). In T cells, a number of proteins involved in signal transduction copurify with lipid rafts isolated on sucrose gradients. These include proteins attached to the outer surface of the plasma membrane, such as glycosylphosphatidylinositol (GPI)-linked receptors (Brown and Rose 1992); proteins attached to the inner face of the plasma membrane, such as the dually acylated Src-family kinases LCK and FYN (Rodgers et al. 1994; Shenoy-Scaria et al. 1994); and transmembrane proteins, such as the S-acylated adapter protein LAT (Zhang et al. 1998a). Ligation of GPI-linked receptors triggers transmembrane signal transduction in T cells, via activation of Src-family kinases (Brown 1993). Furthermore, targeting of LCK to lipid rafts is essential for its signaling function in T cells (Kabouridis et al. 1997), and LAT, which is required for TCR signal transduction (Finco et al. 1998), must associate with lipid rafts to couple to TCR-activated PTKs (Zhang et al. 1998a). Together, these data suggest an important role for lipid rafts in TCR signaling.
The possible involvement of lipid rafts in mediating signaling via the TCR has recently been investigated by several laboratories, using sucrose gradient ultracentrifugation to purify detergent-insoluble raft proteins. However, the results of these studies are conflicting. Two laboratories found that T cell activation was accompanied by recruitment of the TCR into rafts where the ζ chain was then phosphorylated and signaling initiated (Montixi et al. 1998; Xavier et al. 1998). Other studies, however, have detected little or no phosphorylated TCR in the rafts, although TCR cross-linking was found to induce tyrosine phosphorylation of a number of other proteins present in this fraction, including LAT (Brdicka et al. 1998; Zhang et al. 1998a; P. Kabouridis, A. Magee, and S. Ley, unpublished observations). One possible reason for these discrepancies may be that the TCR has only a moderate affinity for lipid rafts in vivo that can be disrupted by nonionic detergents. In this study, lipid rafts were visualized in intact cells by confocal microscopy using fluorescently labeled cholera toxin B (CT-B) subunit, which binds glycosphingolipids, and then cross-linked into patches with anti–CT-B antibody (Fra et al. 1994; Harder et al. 1998). This method demonstrated that LCK, LAT, and the TCR were associated with lipid raft patches, but association of the TCR with these patches was not stable to Triton X-100 extraction. Furthermore, T cell stimulation with cross-linked anti-CD3 mAb induced a similar aggregation of lipid raft-associated proteins, including the TCR and LCK. The protein tyrosine phosphatase CD45, which regulates LCK activity, was not colocalized with CT-B or TCR patches. These experiments with intact cells, therefore, suggest a mechanism in which TCR activation involves the aggregation of lipid rafts and segregation of LCK and CD45, mediated by their differential affinity for these structures, which stimulates PTK signaling.
Materials and Methods
Green Fluorescent Protein Constructs, Cell Culture, and Transfection
The mouse LCK gene was inserted upstream from the enhanced green fluorescent protein (EGFP) gene (J. Pines, Wellcome/CRC Institute, Cambridge, UK) and subcloned into the pcDNA3 expression plasmid (Invitrogen Corp.). Site-directed mutagenesis (Transformer System, CLONTECH Laboratories) was used to remove the LCK termination and GFP initiation codons. The pcDNA3-LCK-GFP construct produced an LCK-GFP fusion protein with a six amino acid glycine-rich linker region. The ZAP-70(SH2)2-GFP construct, containing the two SH2 domains of ZAP-70 fused to the NH2 terminus of EGFP (Sloan-Lancaster et al. 1998), was a kind gift from L. Samelson (National Institutes of Health, Bethesda, MD).
E6.1 Jurkat T cells and derivative cell lines were cultured in RPMI medium supplemented with 5% FCS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Transient transfection of DNA constructs was by electroporation, using 10 μg DNA and 107 cells washed twice with serum-free RPMI and resuspended in 250 μl (Bio-Rad gene pulser, 960 μF, 250 V). The cells were then cultured overnight in 5% FCS/RPMI before analysis.
Patching and Immunofluorescence Confocal Microscopy
For all fluorescence microscopy experiments, cells were washed in serum-free RPMI and attached to TESPA-coated coverslips (3-aminopropyltriethoxy silane; Sigma Chemical Co.) by incubation on ice for 45 min (5 × 105 cells/slip). CT-B–rhodamine conjugate (List Biological Laboratories) was used to label endogenous glycosphingolipids, at 10 μg/ml in PBS with 0.1% BSA. Lipid raft aggregation, or patching, was induced after CT-B labeling as described by Fra et al. 1994, by incubating the cells with anti–CT-B antibody (1/250 in PBS/0.1% BSA; Calbiochem-Novabiochem Corp.) for 30 min on ice, and then 20 min at 37°C. For fixation, cells were then treated with 3% paraformaldehyde in PBS for 30 min at room temperature. Patching of the TCR was achieved in the same way, using 10 μg/ml anti-CD3 mAb (UCHT1) followed by 5 μg/ml anti-mouse Texas red conjugate (Nycomed Amersham, Inc.). BODIPY FL-labeled C5-ganglioside GM1 (150 nM in PBS; Molecular Probes, Inc.) was incorporated into live cells attached on coverslips by incubation for 30 min on ice. The cells were then fixed directly or patched with CT-B–rhodamine and anti–CT-B before fixation, as above. No cross-talk was detected between BODIPY and rhodamine fluorescence channels.
For immunofluorescence staining, cells were attached to coverslips and fixed as described, and then blocked with 2% BSA/PBS for 10 min. When intracellular staining was required, blocking was preceded by permeabilization with 0.1% NP-40/PBS for 5 min. Cells were then incubated with primary antibody (1–10 μg/ml in 2% BSA/PBS) for 30 min, washed in PBS, followed by incubation with fluorescein-conjugated secondary antibodies (Nycomed Amersham, Inc.) for 15 min. The primary antibodies used were: LCK1 polyclonal antiserum (Koegl et al. 1994) or LCK 3A5 mAb (Santa Cruz), UCHT1 (anti-CD3 mAb; P. Beverley, Jenner Institute, Compton, Berkshire, UK), anti-LAT antiserum (L. Samelson, National Institutes of Health, Bethesda, MD), antidecay accelerating factor (anti-DAF) mAb IIH6 (M.E. Medof, University Hospital of Cleveland, OH), anti-CD59- and anti-CD45-fluorescein-conjugated mAbs (Serotec Ltd.), antitransferrin receptor (anti-TfR) mAb (Serotec Ltd.), 4G10 antiphosphotyrosine (anti-PTyr) mAb (B. Druker, Oregon University, Portland, OR), and polyclonal anti-PTyr antibody (Upstate Biotechnology Inc.). Where indicated, the TCR was also stained using UCHT1 biotin-conjugated mAb (Sigma Chemical Co.) followed by streptavidin Texas red conjugate (Calbiochem-Novabiochem Corp.). Confocal microscopy was performed with a Leica TCS SP confocal microscope with 63 and 100× objective lenses, using laser excitation at 488 and 568 nm. The widths of fluorescein/GFP and rhodamine/Texas red emission channels were set such that bleed through across channels was negligible.
Western Blotting Analysis and Immunoprecipitation
For stimulation by CT-B patching, 106 cells/100 μl were treated in microfuge tubes using the same conditions described for immunofluorescence, using unconjugated CT-B (Calbiochem-Novabiochem Corp.). Where indicated, the equivalent number of cells were stimulated with 2 μg/ml OKT3 anti-CD3 antibody (Fab′)2 fragments for 5 min at 37°C, or with 100 nM phorbol 12-myristate 13-acetate (PMA; Sigma Chemical Co.) for 10 min at 37°C. After treatment, the cells were washed in cold PBS and harvested in lysis buffer at 106 cells/100 μl (50 mM Hepes, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 20 mM NaF, 10 mM sodium pyrophosphate, 1 mM PMSF, 1 mM sodium orthovanadate, and 5 μg/ml each of chymostatin, leupeptin, and pepstatin). After 5 min on ice, cell debris was removed by centrifugation. Cleared lysates were assayed for protein content (Bio-Rad protein assay reagent) and samples, normalized for total protein, were analyzed by SDS-PAGE and transferred to nitrocellulose. Blots were probed with appropriate antibodies and detected with HRP-conjugated secondary antibodies and chemiluminescence (New England Nuclear Life Sciences). Antibodies used for Western blots were: 4G10 anti-PTyr mAb, ZAP-4 anti-ZAP-70 antiserum (Huby et al. 1995), anti-LAT antiserum (M. Turner, Babraham Institute, Cambridge, UK), anti-ERK-2 antiserum (C. Marshall, ICR, London, UK), and antisera specific for activated ERK1/2 (PhosphoPlus p44/42 antibody, New England Biolabs). ZAP-4 was also used for immunoprecipitation of ZAP-70 from lysates, by coupling to protein A–Sepharose (Pharmacia Biotech, Inc.) with dimethylpimelimidate (DMP; Sigma Chemical Co.; Schneider et al. 1982) and incubation with 250 μl of lysate at 4°C for 2 h. Similarly, GST-Grb2 pulldown experiments were performed by incubating cell lysates with 5 μg of GST fusion protein that was immobilized by binding to glutathione-Sepharose (Pharmacia Biotech, Inc.). Precipitated protein was recovered by centrifugation, washed three times in lysis buffer, and analyzed by SDS-PAGE and Western blotting.
Calcium Flux Analysis
RPMI-washed cells were incubated with 2 μM Indo 1-AM (Molecular Probes, Inc.) at 5 × 106 cells/ml for 30 min at 37°C, washed in RPMI, and kept on ice. CT-B patching of cells was as described. Ca2+ flux was monitored at 37°C using an LS50 Perkin-Elmer luminescence spectrometer, with excitation at 355 nm and emission measured at 480 and 405 nm, representing free versus Indo-1-associated intracellular Ca2+, respectively, to give an absorbance ratio. To confirm Indo-1 loading, ionomycin was added to a sample of each set of cells used and the Ca2+ flux monitored.
Nuclear Factor of Activated T Cells–Luciferase Assay
107 cells/point were transfected with 10 μg of pBR322-3XNFAT-Luc vector (G. Crabtree, Stanford University, CA), as described above, and incubated for 2 h at 37°C. The cells were then stimulated as indicated with CT-B patching or with anti-CD3 antibodies, as described above. After culturing overnight, control and treated cells were lysed in 120 μl of cell culture lysis reagent (Promega) for 10 min on ice, and centrifuged. 50 μl of the cleared supernatant was assayed for luciferase using the Promega luciferase assay kit with a Clinilumat (Berthold) luminometer. All treatments were performed in duplicate and the results shown are mean ± SEM.
Results
Colocalization of LCK-GFP to Cross-linked Cholera Toxin-B Patches in Live Jurkat T Cells
To localize LCK without the need to permeabilize cells with detergent, which could disrupt membrane organization, a construct was generated in which GFP was fused onto the COOH terminus of full length LCK (Cubitt et al. 1995). When expressed in LCK-deficient JCam-1.6 cells (Straus and Weiss 1992), the LCK-GFP fusion protein was able to phosphorylate the TCR ζ chain after CD3 cross-linking and reconstitute the ability of the TCR to induce nuclear factor of activated T cells (NFAT), indicating that it retained wild-type LCK signaling function (data not shown). Sucrose gradient centrifugation of Triton X-100 solubilized cell lysates prepared from transiently transfected E6.1 Jurkat T cells also revealed that a significant percentage of LCK-GFP partitioned into the low density raft fraction (data not shown), similar to the endogenous protein (Kabouridis et al. 1997). Transient transfection of LCK-GFP into Jurkat T cells revealed a predominantly homogenous localization at the plasma membrane (Fig. 1 A), with some intracellular staining probably representing late endosomes, similar to the distribution of the endogenous protein detected by immunofluorescence (Ley et al. 1994; and Fig. 1 B).
Figure 1.
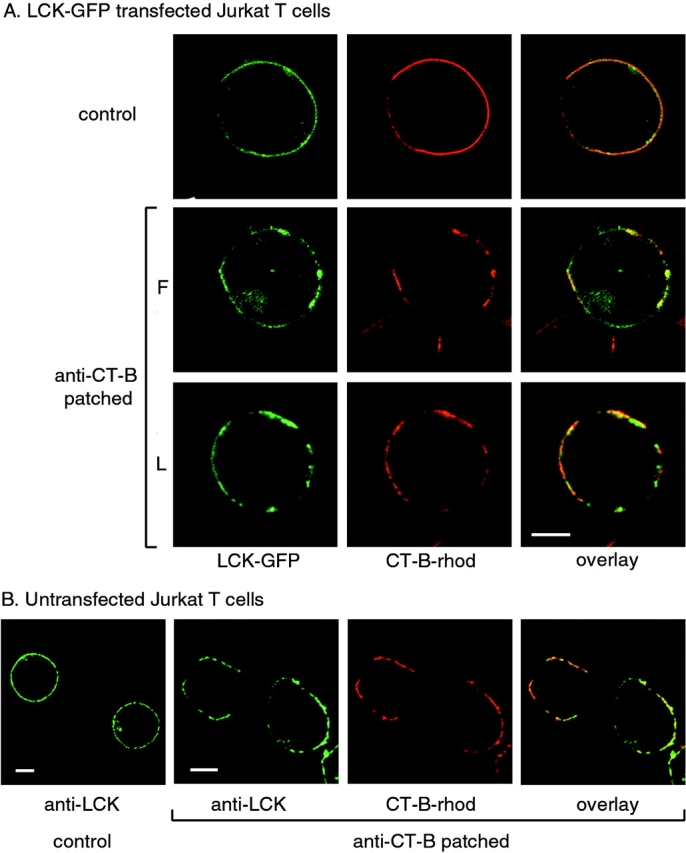
Subcellular localization of LCK and CT-B–rhodamine in Jurkat T cells. A, Cells transiently transfected with LCK-GFP were incubated with CT-B–rhodamine and left untreated (control) or treated by cross-linking with anti–CT-B antibody, as described in Materials and Methods. Single confocal sections were taken of either fixed (F) or live (L) cells using fluorescence in GFP and rhodamine channels. B, Control and anti–CT-B patched cells were fixed and stained with anti-LCK antibody for endogenous protein and visualized by confocal microcopy as in A. Bars, 5 μm.
To study the distribution of LCK-GFP with respect to lipid rafts, Jurkat T cells were stained with rhodamine-labeled CT-B. This reagent binds to glycosphingolipids, with a strong affinity for GM1 and lower affinity for other gangliosides (Parton 1994), and therefore, can be used as a marker for lipid rafts, which are enriched in glycosphingolipids (Fra et al. 1994; Harder et al. 1998), although nonraft GM1 will also be detected. Staining of live Jurkat T cells with CT-B–rhodamine demonstrated a homogeneous distribution of GM1 at the plasma membrane (Fig. 1), similar to the distribution of LCK-GFP. This suggested that raft microdomains may be too small to visualize by light microscopy, which has a resolution of ∼200 nm, consistent with a recent study that estimated lipid rafts are <70 nm in diameter (Varma and Mayor 1998). The homogeneous distribution of CT-B was detected despite the fact that it binds GM1 pentavalently and could therefore potentially cause GM1 aggregation. However, when the CT-B was cross-linked with anti–CT-B antibody, staining became concentrated to distinct patches within the membrane. Significantly, a substantial fraction of transfected LCK-GFP was associated with the CT-B–stained patches (Fig. 1 A), both in fixed (F) and live (L) cells, although the extent of colocalization varied between cells, as shown. Colocalization of endogenous LCK with CT-B patches was also confirmed by immunofluorescence (Fig. 1 B). These results indicate that LCK is preferentially localized to glycosphingolipid-rich domains within unpermeabilized plasma membrane, supporting recent experiments using permeabilized cells (Harder and Simons 1999).
CT-B Cross-linked Patches Exhibit Properties Similar to Lipid Rafts Isolated Biochemically
GPI-linked receptors are strongly associated with lipid rafts isolated biochemically by virtue of their Triton X-100 insolubility and low density (Brown and Rose 1992; Rodgers et al. 1994), whilst other membrane proteins, such as the transferrin receptor (TfR) and the tyrosine phosphatase CD45, do not copurify (Rodgers and Rose 1996; Montixi et al. 1998). To compare CT-B patches with known biochemical properties of lipid rafts, Jurkat T cells were stained for the GPI-linked proteins CD59 and DAF (CD55), and for CD45 and the TfR, in both CT-B–patched and control cells. Consistent with biochemical analyses, CD59 (Fig. 2) and DAF (data not shown) were both substantially concentrated in CT-B patches, compared with a more uniform distribution in unpatched cells. The linker molecule LAT, which is essential for TCR signaling and copurifies with lipid rafts isolated biochemically from T cells (Zhang et al. 1998a), also colocalized with CT-B patches (Fig. 2, bottom row). The TfR and CD45, however, remained uniformly distributed after CT-B patching. Indeed, CD45 even appeared to be partially excluded in some cells (Fig. 2, fourth row). To confirm the ability of CT-B to cross-link the bulk of cell surface GM1, Jurkat T cells were labeled with GM1-BODIPY and the cells were then treated with CT-B plus anti–CT-B antibody. Unpatched GM1-BODIPY showed an even distribution at the plasma membrane. However, after CT-B/anti–CT-B treatment, almost all detectable label was restricted to patches (Fig. 2, top row).
Figure 2.
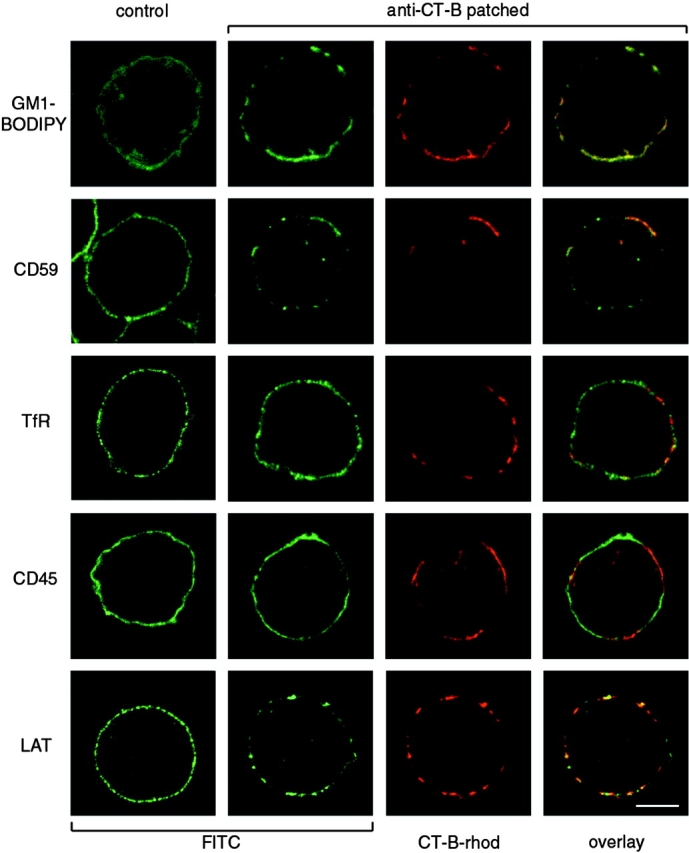
Localization of lipid raft and nonlipid raft markers with respect to CT-B patches. Top row, Jurkat T cells were incubated with fluorescent GM1-BODIPY and then left untreated (control) or treated with CT-B/anti–CT-B patching. Fluorescence of GM1-BODIPY (fluorescein channel) and CT-B–rhodamine was visualized by confocal microscopy. Other rows, Control cells or cells treated with CT-B–rhodamine and anti–CT-B cross-linking were fixed and stained with antibodies against the cell surface proteins CD59, TfR, CD45, and LAT using FITC-conjugated secondary antibodies. Single confocal sections show fluorescence in fluorescein and rhodamine channels. Bar, 5 μm.
Since lipid rafts are characteristically detergent-resistant, the association of LCK and GPI-linked receptors with the CT-B patches was determined after extraction by Triton X-100. Immunofluorescence showed that LCK-GFP, CD59 (Fig. 3 A), and DAF (data not shown) concentrated in the CT-B patches were insensitive to detergent extraction. However, the TfR, which was not concentrated in CT-B patches, was effectively removed. Also, LCK-GFP that was not in patches appeared to be more sensitive to detergent treatment, whereas colocalization of LCK-GFP with lipid patches typically became more pronounced after Triton X-100 extraction (Fig. 3 A, top row).
Figure 3.
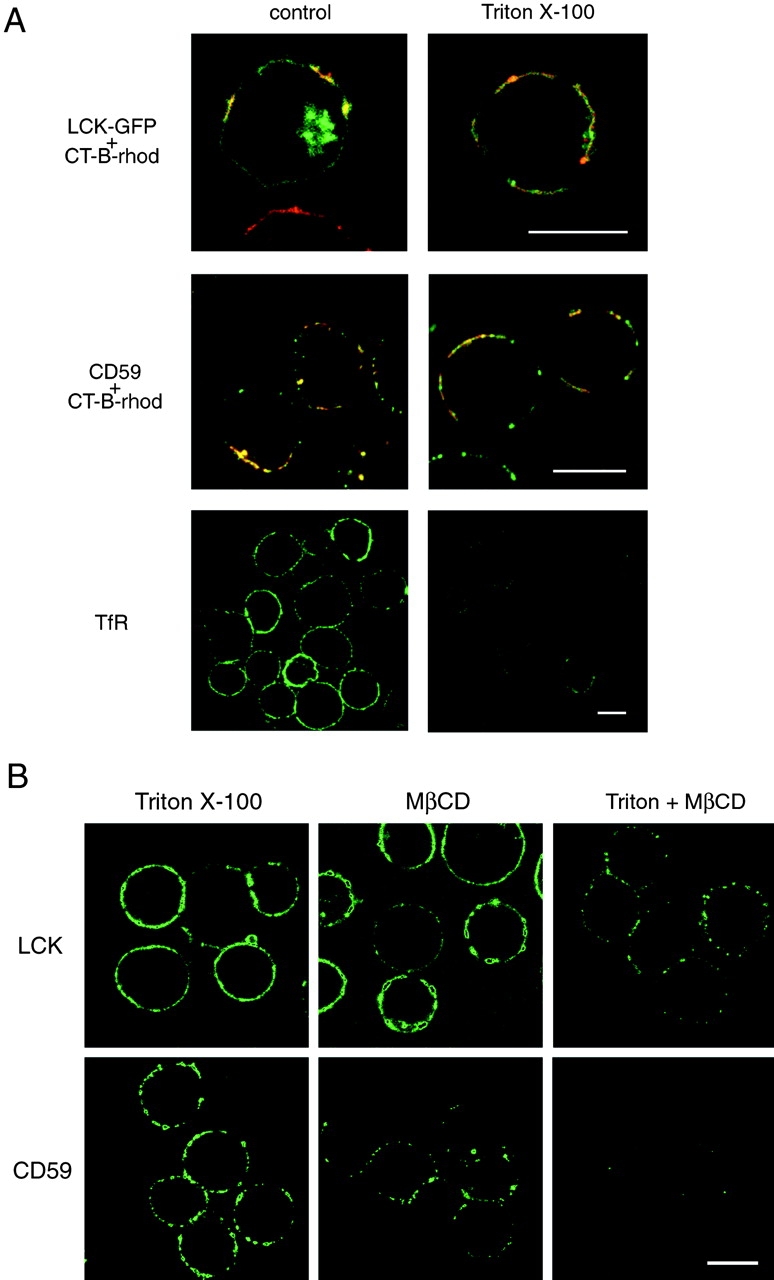
A, Detergent-resistance of proteins associated with CT-B patches. Jurkat cells transfected with LCK-GFP (top row) or left untransfected (other rows) were patched with CT-B–rhodamine plus anti–CT-B. They were then either directly fixed (control) or extracted with 1% Triton X-100 for 5 min on ice before fixation, as indicated. Untransfected cells were stained for CD59 or CD45, using FITC-conjugated secondary antibodies. Fluorescence of GFP and fluorescein (green) or rhodamine (red) was visualized by confocal microscopy, with identical settings for control versus treated samples. For images showing LCK and CD59 localization, red and green images were overlaid to reveal regions of colocalization (appearing yellow) with CT-B. Bars, 10 μm. B, Disruption of detergent-insoluble CT-B–patched proteins by MβCD. Jurkat cells were patched with CT-B/anti–CT-B and then treated with either 1% Triton X-100 for 10 min on ice, 10 mM MβCD for 15 min at 37°C, or MβCD followed by Triton X-100 extraction. The cells were then fixed and stained for LCK or CD59. Confocal images of the various treatments were taken with identical settings to allow comparison of staining. Bar, 10 μm.
Depletion of cellular cholesterol impairs the ability of GPI-anchored proteins to associate with the detergent-insoluble membrane raft fraction (Hanada et al. 1995). To examine whether there was a similar requirement for the association of GPI receptors and LCK with CT-B–induced patches, Jurkat T cells, prepatched with CT-B, were treated with 10 mM methyl-β-cyclodextrin (MβCD) to deplete cellular cholesterol (Scheiffele et al. 1997). This treatment alone did not disrupt CT-B–induced patches or their association with LCK, CD59 (Fig. 3 B), or DAF (data not shown), although CD59 staining was somewhat reduced. However, MβCD treatment did cause the CT-B patches to become detergent-sensitive, as patches of colocalized proteins were severely disrupted (LCK) or completely lost (CD59) after extraction with Triton X-100 in MβCD-treated cells, but not control cells. Together, therefore, the data in this section indicate that the CT-B–induced patches correspond to aggregated lipid rafts and are consistent with a previous study analyzing lipid domain structure in BHK and Jurkat T cells using cross-linked CT-B (Harder et al. 1998).
Association of the T Cell Antigen Receptor with Lipid Raft Patches
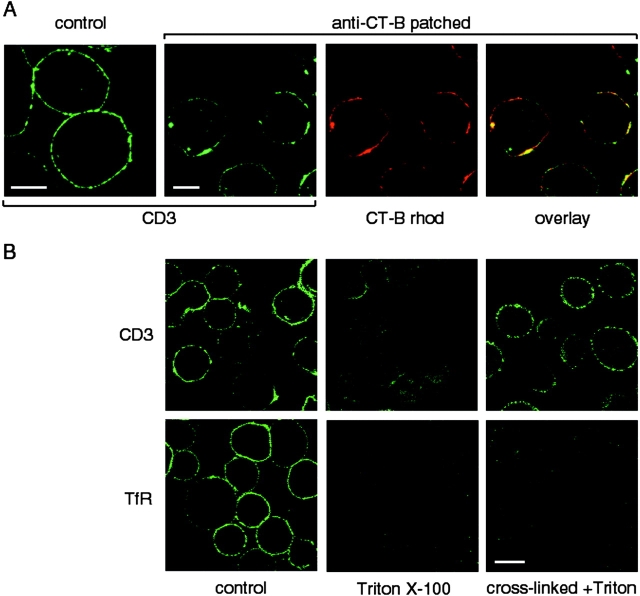
TCR association with lipid rafts is not detectable in our experiments using detergent insolubility and sucrose gradient ultracentrifugation (Kabouridis, P., T. Magee, and S. Ley, unpublished observations). However, as stated in the introduction, detergent extraction may disrupt the association of proteins that interact weakly with lipid rafts. To examine the possible association of the TCR with lipid rafts without using detergent, Jurkat T cells were patched with cross-linked CT-B and, after fixing, stained with an anti-CD3 mAb. The TCR was found to clearly colocalize with the CT-B–induced patches, whereas TCR staining was more evenly distributed when cells were fixed before CT-B staining (Fig. 4 A). Colocalization was evident within 2 min after CT-B cross-linking (data not shown). Triton X-100 treatment of CT-B–patched cells resulted in an almost complete loss of CD3 staining from CT-B patches (Fig. 4 B), unlike the other CT-B colocalizing proteins (e.g., LCK, CD59; see Fig. 3 A), suggesting a weak association of the TCR with lipid rafts. However, cross-linking of the receptor with anti-CD3 mAb before extraction stabilized the association of the receptor with lipid patches in the presence of detergent. In contrast, cross-linking of the TfR had no effect on TfR detergent sensitivity (Fig. 4 B, bottom row).
Figure 4.
The TCR colocalizes with CT-B patches in Jurkat T cells. A, Cells were treated by patching of CT-B–rhodamine as in earlier figures, and then fixed and stained with anti-CD3 mAb and FITC-conjugated secondary antibody. Bars, 5 μm. B, Cells were CT-B patched, and then fixed directly (control), extracted with 1% Triton X-100 for 5 min on ice, or incubated for 30 min on ice with antibodies against CD3 or TfR to cross-link the receptors before Triton extraction, as indicated. After fixation, the cells were stained for CD3 (top) or TfR (bottom), and confocal images were taken with identical settings to allow comparison of staining before and after treatment. Bar, 10 μm.
Cross-linking of the TCR with anti-CD3 mAb stimulates the rapid tyrosine phosphorylation of intracellular proteins and activation of downstream signaling pathways (Weiss and Littman 1994). To examine whether TCR signal transduction involves its association with lipid rafts, Jurkat T cells were incubated with anti-CD3 mAb and cross-linked with anti-Ig antibody. This treatment induced patching of the TCR at the cell surface. The TCR patches were colocalized with both LCK-GFP and CD59, but not with CD45 (Fig. 5 A), indicating that CD3 cross-linking stimulates a similar aggregation of proteins, as seen in CT-B patches. In the converse experiment, antibody-mediated cross-linking of CD59 (Fig. 5 B) also resulted in copatching of CD3. Cross-linking of CD45 with antibodies, which also induced its patching at the plasma membrane, had no effect on lipid rafts, as indicated by LCK-GFP distribution (data not shown). Thus, anti-CD3 patching induces aggregation of lipid rafts which concentrates the TCR with LCK, but not the protein tyrosine phosphatase CD45.
Figure 5.
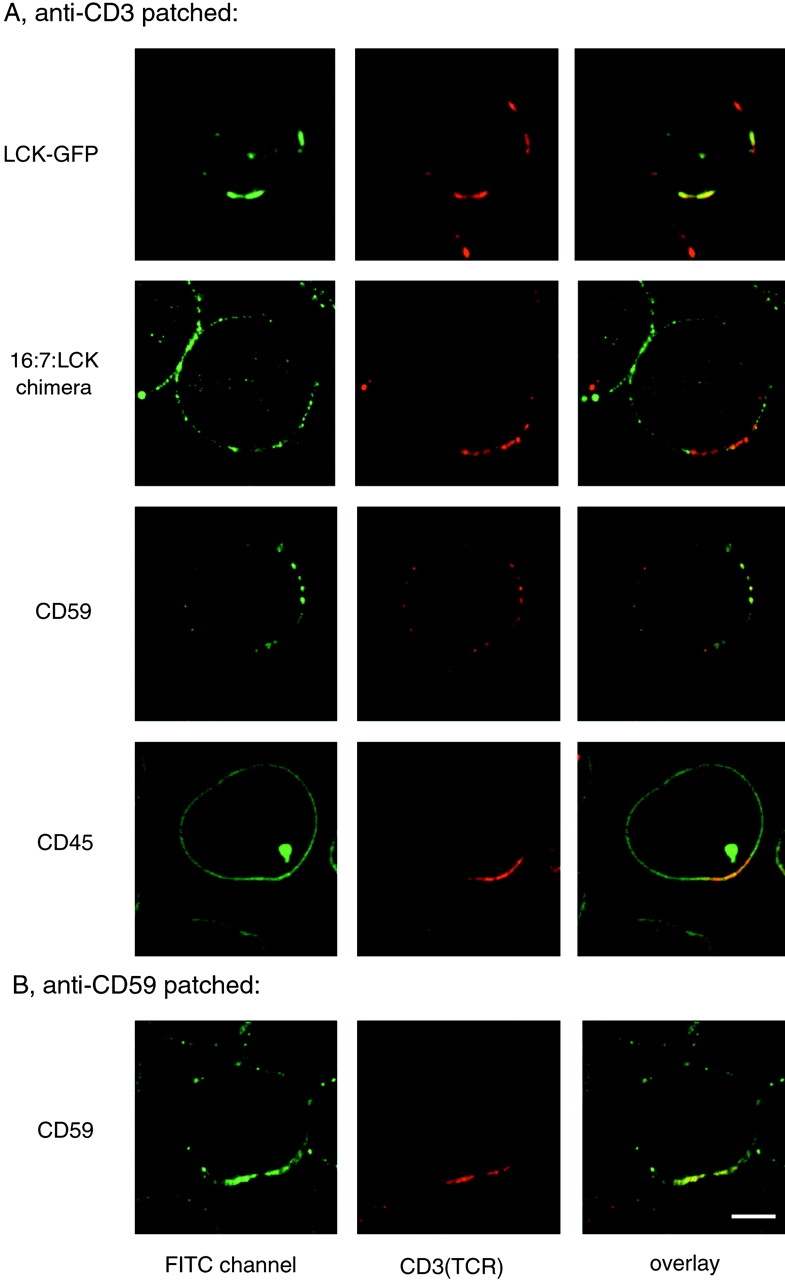
TCR patches resemble lipid raft patches in protein content. A, Cells expressing LCK-GFP (top row), 16:7:LCK (second row), or untransfected Jurkat cells (other rows) were incubated with anti-CD3 mAb followed by anti-Ig Texas red to induce receptor patching. Cells were then fixed and stained as indicated with anti-LCK antiserum followed by FITC-conjugated secondary antibody, or with FITC-conjugated mAbs against CD59 or CD45, to avoid cross-reactivity with anti-CD3 mAb. B, Jurkat cells were incubated with FITC-conjugated anti-CD59 mAb followed by anti-Ig to induce receptor patching. The patched cells were then stained with biotin-conjugated anti-CD3 mAb followed by streptavidin Texas red. Bar, 5 μm.
To investigate the specificity of the LCK-TCR coaggregation, we analyzed JCam-1.6 T cells that lack endogenous LCK, but have been transfected to express LCK fused to the extracellular domain of CD16 and the transmembrane domain of CD7 (16:7:LCK; Kolanus et al. 1993). This LCK chimera is excluded from rafts isolated biochemically and does not properly reconstitute TCR signaling in JCam-1.6 cells (Kabouridis et al. 1997). Staining for LCK in 16:7:LCK-expressing cells after patching of the TCR showed that the LCK chimera did not colocalize with the patches (Fig. 5 A, second row), unlike LCK-GFP (Fig. 5 A, top row) or endogenous LCK stained in normal Jurkat T cells (not shown). This indicates that colocalization of LCK with CD3 patches is dependent on the correct targeting of LCK to lipid rafts.
Cross-linking of the TCR or CT-B Induces Tyrosine Phosphorylation and TCR Activation in Lipid Raft Patches
TCR ligation stimulates tyrosine phosphorylation at the cell cortex, coincident with the inner face of the plasma membrane (Ley et al. 1994). To investigate if TCR cross-linked patches contained tyrosine-phosphorylated proteins, Jurkat T cells were patched with anti-CD3 mAb and, after fixing, stained with anti-PTyr antiserum. The TCR-induced patches were strongly stained for PTyr, compared with unstimulated cells (Fig. 6 A, top row). Significantly, aggregation of lipid rafts by CT-B cross-linking also stimulated strong PTyr staining coincident with the patches (Fig. 6 A, middle row), and this was visible as early as 1 min after cross-linking (data not shown). CT-B–induced tyrosine phosphorylation was dependent on LCK, as no increase in PTyr staining was induced by raft aggregation in JCam-1.6 cells lacking LCK expression (Fig. 6 A, bottom row). However, TCR association with aggregated lipid rafts was clearly not dependent on LCK, since CD3 staining colocalized with CT-B patches in JCam-1.6 cells (Fig. 6 B). Thus, aggregated lipid rafts, induced by CD3 or CT-B cross-linking, appear to be sites of PTK signaling.
Figure 6.
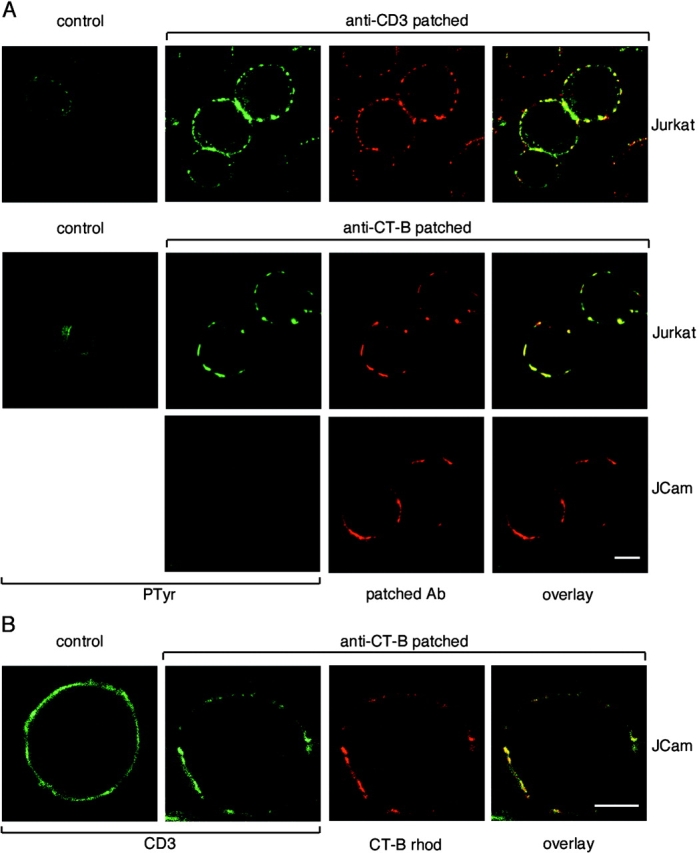
A, TCR and CT-B patching induces tyrosine phosphorylation. Jurkat cells were treated with anti-CD3 mAb and anti-Ig Texas red (top row) or with CT-B–rhodamine plus anti–CT-B (middle row), to induce patching. LCK-deficient JCam-1.6 cells (bottom row) were also patched with CT-B–rhodamine and anti–CT-B. Both patched and control cells were fixed and stained with anti-PTyr polyclonal antibody (top row) or mAb (other rows). Confocal images of CD3-patched or CT-B–patched cells were taken with identical settings to allow comparison of both control versus treated cells and of Jurkat versus JCam-1.6 cells. B, TCR colocalization with CT-B is independent of LCK. Control and CT-B–patched JCam-1.6 cells were fixed and stained with anti-CD3 antibody and visualized by confocal microscopy. Bars, 5 μm.
CD3 cross-linking induces phosphorylation of ITAMs of the TCR by LCK and subsequent recruitment of ZAP-70 PTK (Weiss and Littman 1994). These signaling events may be monitored in intact cells using a chimeric ZAP-70 double SH2 protein linked to GFP, which translocates from the cytoplasm to the plasma membrane upon binding to phospho-ITAMs (Sloan-Lancaster et al. 1998). To investigate whether aggregated lipid rafts were the sites of phosphorylation of the TCR, Jurkat T cells were transiently transfected with the ZAP-70(SH2)2-GFP construct. The cells were then patched with anti-CD3 mAb, anti–CT-B, or anti-TfR mAbs and fixed. Both CD3 and CT-B patching induced ZAP-70(SH2)2-GFP to relocalize to the plasma membrane and become concentrated in the lipid raft patches (Fig. 7). In contrast, cross-linking of the TfR had no effect on ZAP-70(SH2)2-GFP distribution, compared with control cells (Fig. 7, bottom). The TCR, therefore, appears to become tyrosine phosphorylated specifically in the environment of aggregated lipid rafts.
Figure 7.
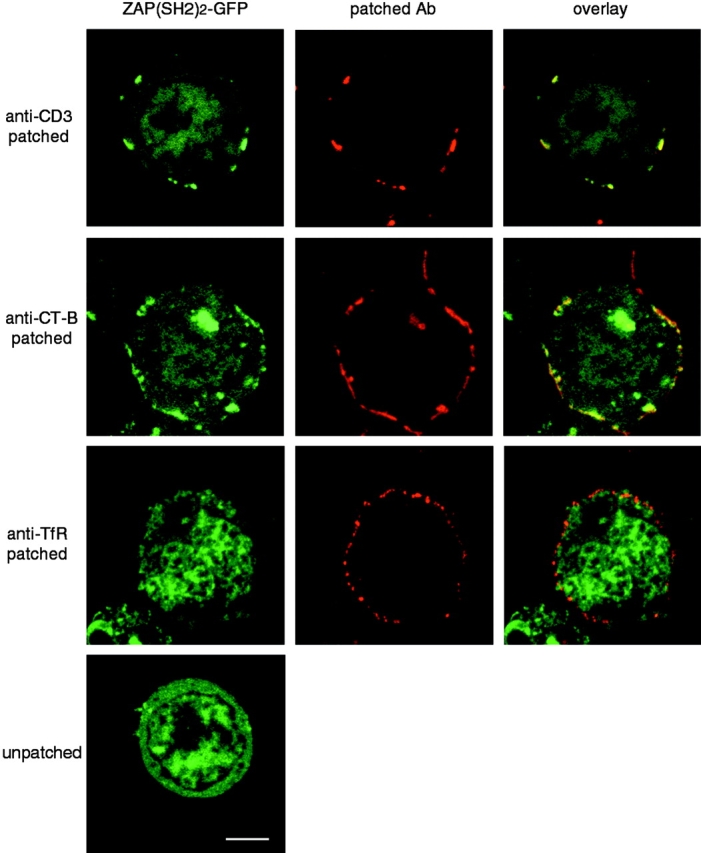
Cytoplasmic ZAP-70(SH2)2-GFP is relocalized to TCR- and CT-B–cross-linked membrane patches. Jurkat cells were transiently transfected with ZAP-70(SH2)2-GFP and incubated overnight to allow expression. They were then patched with anti-CD3 mAb plus anti-Ig Texas red, CT-B–rhodamine plus anti–CT-B, or anti-TfR mAb plus anti-Ig Texas red, as indicated. After fixation, the cells were analyzed by confocal microscopy. Bar, 5 μm.
Cross-linking of Lipid Rafts with CT-B Activates Signaling Pathways in Jurkat T Cells
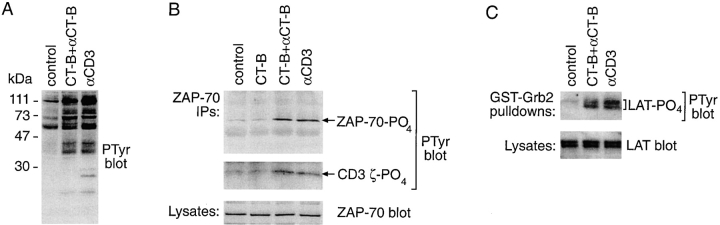
The stimulation of tyrosine phosphorylation and association of ZAP-70(SH2)2-GFP induced by cross-linking either the TCR or CT-B–labeled rafts (above) suggested that lipid raft aggregation might be sufficient to stimulate TCR signaling. To investigate this further, lysates were prepared from cells after CD3- or CT-B–cross-linking. Western blotting with anti-PTyr antibody revealed that CT-B cross-linking triggered tyrosine phosphorylation of a similar set of proteins to those stimulated by anti-CD3 mAb (Fig. 8 A). Analysis of ZAP-70 immunoprecipitates from cell lysates also showed that cross-linked CT-B, but not treatment with CT-B alone, induced ZAP-70 tyrosine phosphorylation and association with the phosphorylated TCR ζ chain (Fig. 8 B). CT-B cross-linking also stimulated phosphorylation of the LAT linker protein, as measured by the induced association of LAT with a GST-Grb2 (Buday et al. 1994) fusion protein and detection with PTyr antibody (Fig. 8 C). These are also proximal signaling events triggered by TCR ligation (Fig. 8B and Fig. C; Chan et al. 1992; Zhang et al. 1998b).
Figure 8.
CT-B patching induces early TCR signaling events. A, Jurkat cells were treated with CT-B followed by anti–CT-B antibody to induce patching, or stimulated for 5 min with anti-CD3 antibody. Lysates of control and treated cells were analyzed by Western blotting with anti-PTyr antibody. The 25-kD band, seen only in the anti-CD3 treated lane, probably represents cross-reactivity of the mouse secondary antibody with the stimulating mAb. B, Jurkat cells were treated as in A, or with CT-B alone, and lysed. ZAP-70 was immunoprecipitated from lysates of control or treated cells, and immunoprecipitates were analyzed by Western blotting with anti-PTyr antibody. ZAP-70 content of lysates was also compared by Western blotting with anti-ZAP-70 antibody (bottom). C, Lysates of Jurkat cells, treated as in A, were incubated with GST-Grb2 fusion protein to precipitate tyrosine phosphorylated LAT, and then analyzed by Western blotting with anti-PTyr antibody. LAT content of lysates was also compared by Western blotting with anti-LAT antibody (bottom).
The ability of CT-B cross-linking to induce other signaling pathways downstream of the TCR was also investigated. CT-B cross-linking was found to induce a rapid increase in intracellular-free Ca2+, although less efficiently than stimulation with anti-CD3 mAb (Fig. 9 A). Secondly, CT-B cross-linking potently induced activation of the ERK MAP kinases, as measured by Western blotting with an antiphospho-ERK antibody, to a similar level as anti-CD3 mAb (Fig. 9 B). CT-B–induced ERK activation was detectable within 1 min of cross-linking, similar to that observed with CD3 cross-linking (data not shown). Activation of the Ras-ERK pathway and of Ca2+ fluxing are together required to stimulate the T cell-specific transcription factor NFAT, which regulates interleukin-2 expression (D'Ambrosio et al. 1994). Therefore, NFAT activity was assayed in Jurkat T cells transiently transfected with a construct containing the luciferase reporter gene under the control of three copies of the NFAT regulatory element (Verweij et al. 1990) and cultured for 24 h. CT-B cross-linking clearly stimulated the production of NFAT, although less efficiently than that achieved with anti-CD3 mAb (Fig. 9 C). Thus, aggregation of lipid rafts by CT-B cross-linking activates signaling pathways similar to those induced by TCR ligation.
Figure 9.
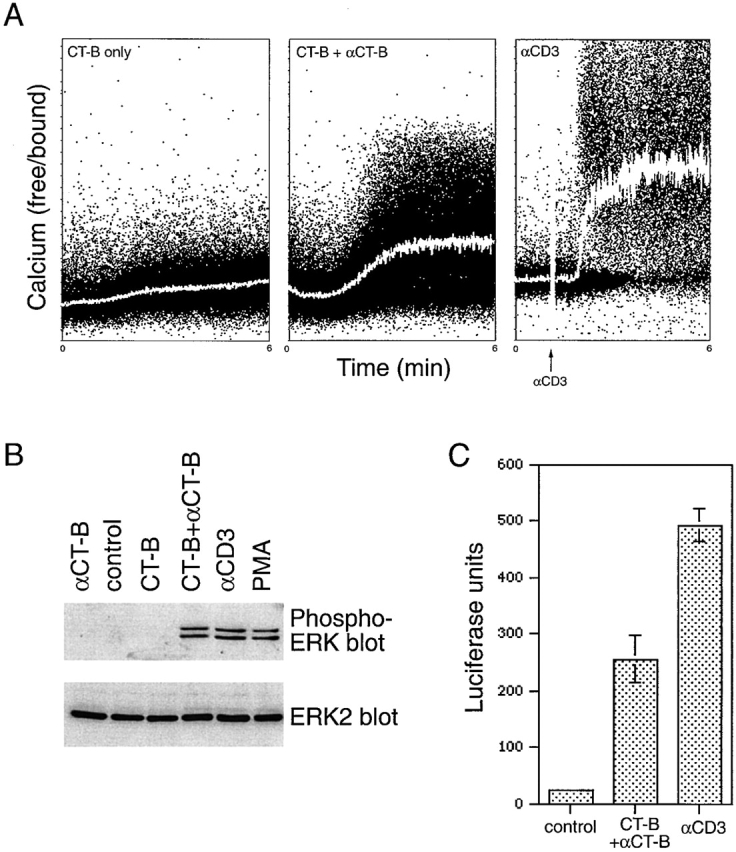
CT-B patching induces signaling pathways downstream from the TCR. A, Ca2+ flux. Jurkat cells were loaded with the Ca2+-binding agent Indo 1, and then treated on ice with CT-B alone (control) or followed by anti–CT-B. Levels of intracellular free Ca2+ were monitored with time by FACS analysis as the cells were warmed to 37°C to induce patching. Indo 1-loaded cells were also stimulated with anti-CD3 mAb as a positive control. B, ERK activation. Jurkat cells were treated with CT-B followed by anti–CT-B, with both agents alone, or stimulated for 5 min with anti-CD3 mAb or PMA. Lysates of control and treated cells were analyzed by Western blotting with antibodies specific for phosphorylated (active) ERK-1 and ERK-2 (top), or for total ERK-2 as a control (bottom). C, NFAT stimulation. Jurkat cells transiently transfected with an NFAT-luciferase reporter construct were treated with CT-B and anti–CT-B to induce lipid raft patching, or stimulated with anti-CD3 antibody. Control and treated cells were incubated overnight before lysis, and lysates were assayed for luciferase activity. The mean of duplicate treatments are shown (± SEM).
Analysis of Signaling in Mutant Jurkat T Cell Lines in Response to CT-B Aggregation of Lipid Rafts
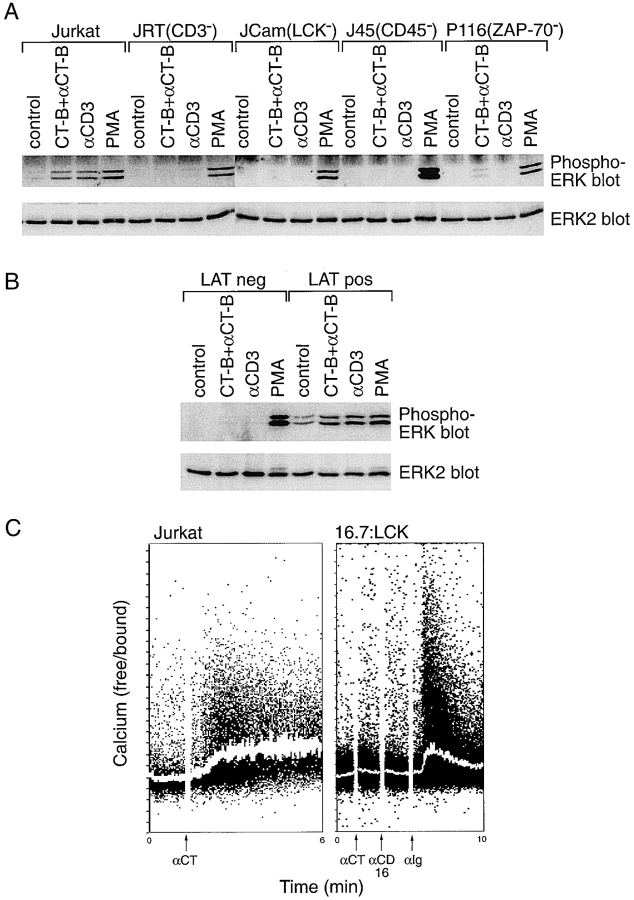
A panel of somatic mutants of the Jurkat T cell line was used to determine the genetic requirements for signaling induced by cross-linked CT-B, using ERK phosphorylation as a readout assay. This analysis revealed that cells deficient in expression of the TCR/CD3 complex (Weiss and Stobo 1984), CD45 (Koretzky et al. 1991), LCK (Straus and Weiss 1992), and ZAP-70 (Williams et al. 1998) displayed poor activation of ERK after CT-B cross-linking, compared with parental Jurkat cells (Fig. 10 A). Anti-CD3 mAb-induced activation of ERK was also inhibited in these cell lines (Fig. 10 A). Similarly, these gene products were necessary for activation of both Ca2+ fluxing and NFAT production after CT-B patching (data not shown). Control experiments demonstrated that cross-linked CT-B induced patching of lipid rafts in all of the cell lines tested and, with the exception of the TCR-negative Jurkat T cells, these patches were associated with the TCR (data not shown). Similarly, in JCam2 cells, which are deficient in LAT expression (Finco et al. 1998), no activation of ERK was detected after CT-B or CD3 cross-linking, in contrast to PMA stimulation (Fig. 10 B, LAT neg). However, both CT-B and CD3 cross-linking increased ERK activity in LAT-reconstituted JCam2 cells (Fig. 10 B, LAT pos). Thus, the genetic requirements for signaling induced by aggegation of lipid rafts with cross-linked CT-B are similar to those for the TCR.
Figure 10.
Analysis of CT-B patch-induced signaling in mutant Jurkat cell lines. A, The Jurkat-derived cell lines J.RT3-T3.5 (JRT, TCR/CD3 deficient), JCam-1.6 (JCam, LCK deficient), J45.01 (J45, CD45 deficient), and P116 (ZAP-70 deficient) were compared with parental Jurkat cells for ERK activation after treatment. Cells were treated by CT-B/anti–CT-B patching, with anti-CD3 antibody, or with phorbol ester (PMA) as a positive control. Control and treated cells were assayed for ERK activation as in Fig. 9 B. B, LAT-deficient JCam2 cells (LAT neg) and cells reconstituted with exogenous LAT (LAT pos) were assayed for ERK activation after CT-B or CD3 cross-linking, or PMA stimulation as in A. C, JCam-1.6 cells stably expressing the CD16:CD7:LCK transmembrane chimera (16:7:LCK) and Jurkat cells were loaded with Indo 1 and incubated with CT-B on ice. They were then monitored for intracellular Ca2+ flux, as in Fig. 9 A. Goat anti–CT-B was added to induce CT-B patching of both cell lines at the time shown. For 16:7:LCK cells, mouse anti-CD16 and goat anti–mouse antibodies were then added, followed by rabbit anti–goat (αIg) to cross-link the chimera with CT-B.
The CD16:CD7:LCK transmembrane chimera (16:7:LCK) does not target correctly to rafts isolated biochemically (Kabouridis et al. 1997) or to TCR-patched rafts in intact cells (Fig. 5). Furthermore, cells expressing this chimera are deficient in signaling in response to TCR cross-linking (Kabouridis et al. 1997). Therefore, to determine if CT-B–induced signaling is dependent on targeting of LCK to rafts, Ca2+ flux in cells expressing the 16:7:LCK chimera was investigated in response to CT-B patching. Unlike normal Jurkat cells, no Ca2+ flux was observed after addition of anti–CT-B cross-linking antibody (Fig. 10 C). Likewise, aggregation of the LCK chimera with anti-CD16 antibody had no effect. However, when the CT-B patches were cocross-linked with the LCK chimera using anti-Ig antibodies, a transient flux was induced (Fig. 10 C). This indicates that not only is LCK expression required for signaling stimulated by lipid raft aggregation (Fig. 10 A), but its correct targeting to these lipid rafts is also necessary.
Discussion
This study provides several lines of evidence that membrane patches formed by cross-linking CT-B correspond to regions of aggregated lipid rafts in intact cells, and that in T cells these membrane subdomains are enriched in key signaling molecules and represent active sites of signaling. Thus, CT-B–labeled membrane patches displayed characteristics consistent with biochemically isolated lipid rafts, including colocalization with the GPI-linked proteins CD59 and CD55, and also the T cell signaling proteins LCK and LAT (Fig. 1 and Fig. 2), all of which copurify with lipid rafts biochemically. In addition, LCK-GFP colocalized with CT-B patches in nonpermeabilized cells, ruling out the possibility of artifacts from detergent treatment, and confirming results with permeabilized cells (Fig. 1; Harder and Simons 1999). In contrast, nonraft associated proteins, such as the TfR and CD45, did not colocalize with CT-B patches. Furthermore, whereas TfR staining was lost after Triton X-100 extraction, LCK and CD59 association with CT-B patches was resistant to Triton X-100 (Fig. 3), indicative of their raft association. However, pretreatment of cells with MβCD to deplete membrane cholesterol rendered these proteins Triton X-100-extractable, similar to the MβCD-induced release of these proteins from the raft fraction isolated biochemically from lymphocyte cells (Ilangumaran and Hoessli 1998; Kabouridis, P., J. Janzen, A. Magee, and S. Ley, unpublished observations). This is also consistent with effects of cholesterol depletion in disrupting the clustered distribution of GPI-linked proteins (Rothberg et al. 1990) and the interaction of the IgE receptor and the Src-family kinase LYN with rafts (Sheets et al. 1999).
While GM1 is concentrated in lipid rafts isolated biochemically (Fra et al. 1994), staining has also revealed colocalization with caveolae (Parton 1994), which are small plasma membrane invaginations found in cells expressing caveolin proteins that share the nonionic detergent-insolubility and low density characteristics of lipid rafts (reviewed by Anderson 1998). Caveolins have been proposed to function as scaffolding proteins for signaling molecules and could determine their localization (reviewed by Okamoto et al. 1998). However, no expression of the three caveolin family members is detectable in Jurkat T cells (P. Janes, S. Ley, and A. Magee, unpublished data; Scherer et al. 1997), or other lymphocyte cell lines (Fra et al. 1994). Also, Jurkat T cells contain no caveolae structures, even with exogenous expression of caveolin-1 at levels similar to MDCK cells that contain caveolae (P. Janes, S. Ley, and A. Magee, unpublished data). Therefore, the localization of T cell signaling proteins to lipid rafts appears to be independent of caveolins and caveolae.
Our results showing association of the TCR with CT-B cross-linked lipid rafts contrasts with biochemical studies analyzing lipid raft composition and highlights the limitations of using detergent insolubility as the only criterion to monitor the association of a particular protein with lipid rafts. Although CD3 staining was clearly concentrated in CT-B patches, this association was completely lost after Triton X-100 extraction. Similarly, the association of VSV-G with lipid rafts is not preserved after Triton X-100 extraction (Harder et al. 1998). It is likely that only proteins that are strongly associated with lipid rafts are Triton X-100 insoluble, whereas weakly associated proteins are extracted. The contradictory results using detergent insolubility to determine whether the TCR is associated with lipid rafts (Brdicka et al. 1998; Montixi et al. 1998; Xavier et al. 1998; Zhang et al. 1998a) may be explained by such a weak association. The TCR also colocalized with CT-B cross-linked lipid raft patches in LCK-deficient JCam-1.6 cells (Fig. 6 B) and in Jurkat cells pretreated with the Src family kinase inhibitor PP1 (data not shown), indicating that the association of the TCR with lipid rafts does not require activity of Src PTKs which are essential for TCR signaling (Karnitz et al. 1992; Straus and Weiss 1992). These observations suggest that the receptor may be constitutively associated with lipid rafts, rather than being actively recruited to these structures. TCR aggregation induced by CT-B patching may, therefore, simply occur as a result of coalescence of lipid microdomains with which the TCR is already associated. The reported increased association of the TCR with detergent-insoluble lipid rafts detected after CD3 cross-linking (Montixi et al. 1998; Xavier et al. 1998) may reflect stabilization of this association by antibody cross-linking, as demonstrated in Fig. 4 B, rather than movement into rafts.
Signal transduction via the TCR is initiated after antibody cross-linking, suggesting that signaling is triggered by receptor oligomerization. Accordingly, TCR signaling is triggered by oligomers of soluble MHC molecules bound to cognate peptide, but not by monomers (Boniface et al. 1998). However, the precise mechanism by which oligomerization triggers TCR signaling is unclear. The results in this study suggest that TCR oligomerization may be important in driving the formation of aggregates of lipid rafts with which it associates. Thus, TCR cross-linking (Fig. 5 A) and CT-B–mediated lipid raft aggregation (Fig. 1 and Fig. 2) caused a similar redistribution of raft-associated proteins including LCK and the TCR, but not the protein tyrosine phosphatase CD45. Raft aggregation may therefore facilitate tyrosine phosphorylation of TCR ITAMs by increasing the concentration of the TCR and LCK in close proximity, whilst excluding CD45. Consistent with this hypothesis, both a general increase in tyrosine phosphorylation (Fig. 6) and specific tyrosine phosphorylation of TCR ITAMs (Fig. 7) occurred in aggregated lipid rafts after either TCR or CT-B patching. Furthermore, CT-B cross-linking induced a similar pattern of protein tyrosine phosphorylation and stimulated the same signaling pathways as the TCR (Fig. 8 and Fig. 9), with the same genetic requirements (Fig. 10). Previous work also supports this view, since CT-B–induced calcium flux in Jurkat T cells has been shown to be markedly reduced in cells lacking the TCR β chain (Gouy et al. 1994).
Redistribution of CT-B–labeled GM1 was not detectable after anti-CD3 mAb cross-linking (data not shown), consistent with a previous study (Viola et al. 1999). This may reflect the presence of a significant fraction of GM1 outside of rafts (Fra et al. 1994). Our results suggest that the raft-associated proteins investigated above (e.g., CD59) may be better markers for these domains, and that TCR patching alone is sufficient to drive lipid raft aggregation, although this is likely to be enhanced by coaggregation with other lipid raft-associated proteins. Consistent with this, Viola et al. 1999 found that stimulation of T cells with low anti-CD3 mAb concentration was enhanced by simultaneous treatment with CT-B or anti-CD59 antibody, suggesting that cross-linking of rafts provided costimulation. Furthermore, cross-linking CD3 with the coreceptor CD28, which increases T cell activation, also enhanced raft aggregation, as indicated by a visible redistribution of CT-B–labeled GM1 (Viola et al. 1999). In the present study, lipid raft aggregation induced by CT-B cross-linking was found to activate TCR signaling less efficiently than direct cross-linking of the TCR by anti-CD3 mAb in some assays (Ca2+ flux, NFAT activation; Fig. 9), possibly due to dilution of the rafts by nonraft-associated GM1. However, lipid raft aggregation is likely to facilitate T cell stimulation induced by TCR ligation, both alone and in concert with coreceptor ligation.
The observation that LCK is enriched in patched lipid rafts with the TCR, but not CD45, led us to investigate the importance of specific targeting of LCK to these rafts using a transmembrane LCK chimera (16:7:LCK). This chimera is not associated with rafts isolated biochemically and does not properly reconstitute signaling in LCK-deficient cells in response to TCR cross-linking (Kabouridis et al. 1997). The 16:7:LCK chimera did not localize to TCR-patched rafts (Fig. 5 A), and was unable to induce Ca2+-signaling by CT-B–mediated raft aggregation (Fig. 10), until forced to interact with CT-B–labeled rafts by antibody cocross-linking. These results show that specific targeting of LCK to rafts is essential for raft-mediated TCR signaling. This targeting is likely to be driven by acylation of LCK, which is necessary for its signaling function (Kabouridis et al. 1997), since the 10 amino acid LCK NH2 terminus containing the three acylation sites is sufficient to target GFP to lipid rafts isolated biochemically (Janes, P., C. Jackson, S. Ley, and T. Magee, unpublished data). Similar results have been reported for other acylated proteins, including G protein α-subunits (Galbiati et al. 1999).
The activity of LCK in rafts purified biochemically is markedly reduced compared with LCK in the nonraft fraction (Rodgers and Rose 1996). This is presumed to be due to inaccessibility of raft-associated LCK to CD45, which dephosphorylates the negative regulatory tyrosine 505 of LCK (Ostergaard et al. 1989; Pingel and Thomas 1989; Koretzky et al. 1990). However, in our study, tyrosine phosphorylation of the TCR, which is mediated by LCK (Straus and Weiss 1992), occurred in raft patches identified by confocal microscopy, and PTyr stimulation was dependent on LCK expression (Fig. 6 and Fig. 7). Therefore, LCK appeared to be active in lipid raft patches in which it was concentrated, despite lack of colocalized CD45. A possible explanation for this paradox is that active LCK is more sensitive to detergent extraction, and is removed from rafts isolated biochemically, similar to the TCR. If LCK is active in rafts, this suggests either that LCK retains activity for a time after exclusion from CD45, or that CD45 may not be absolutely required for LCK activity. In support of the latter hypothesis, LCK activity is substantially increased in T cell lines and thymocytes that lack CD45, despite hyperphosphorylation of Tyr 505 (D'Oro and Ashwell 1999). Similarly, in B cells, the fraction of the Src-family PTK LYN that is associated with the B cell receptor displays increased activity in the absence of CD45 expression (Katagiri et al. 1999). In these experiments, both the positive and negative regulatory tyrosines of each kinase were hyperphosphorylated, suggesting that CD45 dephosphorylates the positive regulatory site in addition to the negative regulatory site, thereby inhibiting kinase activity. In addition, CD45 may also dephosphorylate LCK substrates (Gervais and Veillette 1997) and/or the TCR ζ chain (Furukawa et al. 1994), resulting in reduced signaling. Indeed, when CD45 and the TCR are cocross-linked, TCR signaling is strongly downregulated (Ledbetter et al. 1988; Turka et al. 1992). Therefore, exclusion of CD45 from the TCR and LCK, facilitated by lipid raft aggregation, could potentially stimulate tyrosine phosphorylation and T cell activation.
Interestingly, it has been suggested that T cell activation by antigen-presenting cells (APCs) may involve formation of zones of CD45 exclusion, in which TCR tyrosine phosphorylation may be facilitated (Shaw and Dustin 1997). This prediction was based on steric considerations from the predicted size of the CD45 extracellular domain, compared with the TCR/MHC complex (Davis and van der Merwe 1996). Certainly, there is segregation of molecules within APC-T cell contact sites, with TCRs becoming concentrated centrally within a ring of LFA-1/ICAM-1 coreceptors, termed supramolecular activation clusters, or SMACs (Monks et al. 1998; Grakoui et al. 1999), which is consistent with size exclusion (see also Dustin and Shaw 1999). Our data are in keeping with this model of T cell activation, suggesting that differential affinity for lipid rafts may result in a similar segregation of the TCR and CD45 after stimulation with anti-CD3 mAb, and this may facilitate steric segregation in APC-T cell interactions. However, whereas SMAC formation occurs over 30–60 min and is apparently dependent on cytoskeletal rearrangement (Wulfing and Davis 1998), the events described here probably correspond to more immediate signaling responses. Thus, the coaggregation of the TCR with CT-B–labeled lipid rafts, and the stimulation of tyrosine phosphorylation after CD3 or CT-B cross-linking were visible within one to two minutes, as was stimulation of ERK activity. Also, disruption of actin polymerization with cytochalasin D does not inhibit CT-B–induced copatching of CD3 (Janes, P., S. Ley, and A. Magee, unpublished results), or PTyr stimulation by cross-linking of CT-B (Harder and Simons 1999) or CD3 (Huby et al. 1998), whereas actin accumulation to CT-B patches is kinase-dependent (Harder and Simons 1999). In future studies, it will be important to determine whether lipid rafts are also involved in the formation of organized zones of signaling proteins at contacts between T cells and APCs.
Ligation of cell-surface GPI-anchored proteins triggers transmembrane signal transduction in T cells (Brown 1993). However, as GPI-linked proteins are associated only with the outer leaflet of the lipid bilayer, the mechanism of signaling has remained obscure. It is possible that these molecules interact with transmembrane proteins which transmit a signal, or, alternatively, GPI-linked protein association with lipid rafts, which contain Src-family kinases required for their signaling, may be important (Brown 1993). The results of this study suggest that both explanations may be correct. Thus, CD59 and DAF were present in lipid raft patches with the TCR and LCK, and antibody cross-linking of CD59 caused coaggregation of the TCR. This suggests that ligation of GPI receptors may trigger TCR signaling by aggregating lipid rafts. Consistent with this hypothesis, induction of lymphokine production by GPI-linked molecules requires expression of the TCR at the cell surface (Sussman et al. 1988; Korty et al. 1991; Deckert et al. 1995). Thus, the mechanism of signaling after antibody stimulation of the TCR or GPI-linked receptors in T cells may be very similar.
Aggregation of the IgE receptor Fc∈R1 activates the associated Src-family kinase LYN, initiating a signaling cascade that culminates in degranulation (Field et al. 1997). Clustering of Fc∈R1 results in its association with patches enriched in GM1 (Stauffer and Meyer 1997) and its copurification with detergent-insoluble lipid rafts (Field et al. 1997). Furthermore, only the receptor that copurifies with lipid rafts serves as a substrate for LYN (Field et al. 1997). Activation of PTK signaling by Fc∈R1, therefore, appears to involve its association with aggregated lipid rafts, similar to the TCR. Many hematopoietic cell receptors signal via Src kinase-mediated phosphorylation of conserved tyrosine residues in their cytoplasmic domains (Isakov 1997). It is possible that these receptors also utilize lipid rafts to initiate transmembrane signal transduction.
In conclusion, this study demonstrates that stimulation of Jurkat T cells by cross-linking the TCR induces aggregation of lipid rafts in which the TCR and LCK, but not CD45, are concentrated, producing an environment where tyrosine phosphorylation is favored and signaling is triggered. Accordingly, TCR stimulation can be mimicked by direct aggregation of rafts with CT-B cross-linking, which induces a similar redistribution of molecules and similar signaling events. In future studies, it will be important to characterize the structural features of the TCR, which allow it to associate with rafts and would be expected to be critical for signal transduction.
Acknowledgments
We would like to thank the colleagues listed under Materials and Methods for reagents used in this study, and A. Weiss and R. Abrahams for derivative Jurkat E6.1 T cell lines. We are also grateful to S. Pagakis for help with confocal imaging, to P. Kabouridis, J. Janzen, and C. Jackson for reagents and helpful input, and to the National Institute for Medical Research Photo-Graphics department for help with figures.
This work was supported by the Medical Research Council.
Footnotes
1.used in this paper: APC, antigen-presenting cell; CT-B, cholera toxin B subunit; DAF, decay accelerating factor; ERK, extracellular-regulated kinase; GFP, green fluorescent protein; GPI, glycosylphosphatidylinositol; ITAM, immunoreceptor tyrosine-based activation motif; MβCD, methyl-β-cyclodextrin; NFAT, nuclear factor of activated T cells; PTK, protein tyrosine kinase; PTyr, phosphotyrosine; SH2, Src-homology 2; TCR, T cell antigen receptor; TfR, transferrin receptor
S. Ley, National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, UK. Tel.: 44-181-959-3666. Fax: 44-181-906-4477. E-mail: t-magee@nimr.mrc.ac.uk or s-ley@nimr.mrc.ac.uk
References
- Anderson R.G.W. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Boniface J.J., Rabinowitz J.D., Wulfing C., Hampl J., Reich Z., Altman J.D., Kantor R.M., Beeson C., McConnell H.M., Davis M.M. Initiation of signal transduction through the T cell receptor requires the peptide multivalent engagement of MHC ligands. Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- Brdicka T., Cerny J., Horejsi V. T cell receptor signalling results in rapid tyrosine phosphorylation of the linker protein LAT present in detergent-resistant membrane microdomains. Biochem. Biophys. Res. Commun. 1998;248:356–360. doi: 10.1006/bbrc.1998.8857. [DOI] [PubMed] [Google Scholar]
- Brown D. The tyrosine kinase connectionhow GPI-anchored proteins activate T cells. Curr. Opin. Immunol. 1993;5:349–354. doi: 10.1016/0952-7915(93)90052-t. [DOI] [PubMed] [Google Scholar]
- Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Buday L., Egan S.E., Viciana P.R., Cantrell D.A., Downward J. A complex of GRB2 adaptor protein, Sos exchange factor, and 36 kDa membrane bound tyrosine phosphoprotein is implicated in Ras activation in T cells. J. Biol. Chem. 1994;269:9019–9023. [PubMed] [Google Scholar]
- Cantrell D. T cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- Chan A.C., Iwashima M., Turck C.W., Weiss A. ZAP-70a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Chan A.C., Shaw A.S. Regulation of antigen receptor signal transduction by protein tyrosine kinases. Curr. Opin. Immunol. 1996;8:394–401. doi: 10.1016/s0952-7915(96)80130-0. [DOI] [PubMed] [Google Scholar]
- Cubitt A.B., Heim R., Adams S.R., Boyd A.E., Gross L.A., Tsien R.Y. Understanding, improving and using green fluorescent proteins. Trends Biochem. Sci. 1995;20:448–455. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio D., Cantrell D.A., Frati L., Santoni A., Testi R. Involvement of p21ras activation in T cell CD69 expression. Eur. J. Immunol. 1994;24:616–620. doi: 10.1002/eji.1830240319. [DOI] [PubMed] [Google Scholar]
- D'Oro U., Ashwell J.D. The CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J. Immunol. 1999;162:1879–1883. [PubMed] [Google Scholar]
- Davis S.J., van der Merwe P.A. The structure and ligand interactions of CD2implications for T cell function. Immunol. Today. 1996;17:177–187. doi: 10.1016/0167-5699(96)80617-7. [DOI] [PubMed] [Google Scholar]
- Deckert M., Ticchioni M., Mari B., Mary D., Bernard A. The glycosylphosphatidylinositol-anchored CD59 protein stimulates both T cell receptor ζ/ZAP-70-dependent and -independent signaling pathways in T cells. Eur. J. Immunol. 1995;25:1815–1822. doi: 10.1002/eji.1830250704. [DOI] [PubMed] [Google Scholar]
- Dustin M., Shaw A.S. Costimulationbuilding an immunological synapse. Science. 1999;283:649–650. doi: 10.1126/science.283.5402.649. [DOI] [PubMed] [Google Scholar]
- Field K.A., Holowka D., Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J. Biol. Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- Finco T.S., Kadlecek T., Zhang W., Samelson L.E., Weiss A. LAT is required for TCR-mediated activation of PLC-γ1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- Fra A.M., Williamson E., Simons K., Parton R.G. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- Furukawa T., Itoh M., Krueger N.X., Streuli M., Saito H. Specific interaction of the CD45 protein tyrosine phosphatase with tyrosine phosphorylated CD3 ζ chain. Proc. Natl. Acad. Sci. USA. 1994;91:10928–10932. doi: 10.1073/pnas.91.23.10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F., Volonte D., Meani D., Milligan G., Lublin D.M., Lisanti M.P., Parenti M. The dually acylated NH2-terminal domain of Gi1α is sufficient to target a green fluorescent protein reporter to caveolin-enriched plasma membrane domains. J. Biol. Chem. 1999;274:5843–5850. doi: 10.1074/jbc.274.9.5843. [DOI] [PubMed] [Google Scholar]
- Gervais F.G., Veillette A. Reconstitution of interactions between protein tyrosine phosphatase CD45 and tyrosine protein kinase p56lck in nonlymphoid cells. J. Biol. Chem. 1997;272:12754–12761. doi: 10.1074/jbc.272.19.12754. [DOI] [PubMed] [Google Scholar]
- Gouy H., Deterre P., Debre P., Bismuth G. Cell calcium signaling via GM1 cell surface gangliosides in the human Jurkat T cell line. J. Immunol. 1994;152:3271–3281. [PubMed] [Google Scholar]
- Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S., Allen P.M., Dustin M.L. The immunological synapsea molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Hanada K., Nishijima M., Akamatsu Y., Pagano R.E. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes. J. Biol. Chem. 1995;270:6254–6260. doi: 10.1074/jbc.270.11.6254. [DOI] [PubMed] [Google Scholar]
- Harder T., Simons K. Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cellsaccumulation of actin regulated by local tyrosine phosphorylation. Eur. J. Immunol. 1999;29:556–562. doi: 10.1002/(SICI)1521-4141(199902)29:02<556::AID-IMMU556>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Harder T., Scheiffele P., Verkade P., Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huby R.D.J., Carlile G.W., Ley S.C. Interactions between the protein-tyrosine kinase, ZAP-70, the proto-oncoprotein Vav and tubulin in Jurkat T cells. J. Biol. Chem. 1995;270:30241–30244. doi: 10.1074/jbc.270.51.30241. [DOI] [PubMed] [Google Scholar]
- Huby R.D.J., Weiss A., Ley S.C. Nocodazole inhibits signal transduction by the T cell antigen receptor. J. Biol. Chem. 1998;273:12024–12031. doi: 10.1074/jbc.273.20.12024. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S., Hoessli D.C. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 1998;335:433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakov N. Immunoreceptor tyrosine-based activation motif (ITAM), a unique module linking antigen and Fc receptors to their signalling cascades. J. Leuk. Biol. 1997;61:6–16. doi: 10.1002/jlb.61.1.6. [DOI] [PubMed] [Google Scholar]
- Kabouridis P.S., Magee A.I., Ley S.C. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO (Eur. Mol. Biol. Organ) J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnitz L., Sutor S.L., Toshihiko T., Reed J.C., Bell M.P., McKean D.J., Leibson P.J., Abraham R.T. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2 dependent cytotoxic T cell line. Mol. Cell. Biol. 1992;12:4521–4530. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T., Ogimoto M., Hasegawa K., Arimura Y., Mitomo K., Okada M., Clark M.R., Mizuno K., Yakura H. CD45 negatively regulates Lyn activity by dephosphorylating both positive and negative regulatory tyrosine residues in immature B cells. J. Immunol. 1999;163:1321–1326. [PubMed] [Google Scholar]
- Klausner R.D., Samelson L.E. T cell antigen receptor activation pathwaysthe tyrosine kinase connection. Cell. 1991;64:875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Koegl M., Zlatkine P., Ley S.C., Courtneidge S.A., Magee A.I. Palmitoylation of multiple Src-family kinases at a homologous N-terminal motif. Biochem. J. 1994;303:749–753. doi: 10.1042/bj3030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanus W., Romeo C., Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993;74:171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- Koretzky G.A., Picus J., Thomas M.L., Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidylinositol pathway. Nature. 1990;346:66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- Koretzky G.A., Picus J., Schultz T., Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc. Natl. Acad. Sci. USA. 1991;88:2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korty P.E., Brando C., Shevach E.M. CD59 functions as a signal-transducing molecule for human T cell activation. J. Immunol. 1991;146:4092–4098. [PubMed] [Google Scholar]
- Ledbetter J.A., Tonks N.K., Fischer E.H., Clark E.A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc. Natl. Acad. Sci. USA. 1988;85:8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley S.C., Marsh M., Bebbington C.R., Proudfoot K., Jordan P. Distinct intracellular localization of Lck and Fyn protein tyrosine kinases in human T lymphocytes. J. Cell Biol. 1994;125:639–649. doi: 10.1083/jcb.125.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks C.R.F., Freiberg B.A., Kupfer H., Sciaky N., Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Montixi C., Langlet C., Bernard A.-M., Thimonier J., Dubois C., Wurbel M.-A., Chauvin J.-P., Pierres M., He H.-T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Schlegel A., Scherer P.E., Lisanti M.P. Caveolins, a family of scaffolding proteins for organising “preassembled signalling complexes” at the plasma membrane. J. Biol. Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Ostergaard H.L., Shakelford D.A., Hurley T.R., Johnson P., Hyman R., Sefton B.M., Trowbridge I.S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc. Natl. Acad. Sci. USA. 1989;86:8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R.G. Ultrastructural localization of gangliosidesGM1 is concentrated in caveolae. J. Histochem. Cytochem. 1994;42:155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- Parton R.G., Simons K. Digging into caveolae. Science. 1995;269:1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- Pingel J., Thomas M.L. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989;58:1055–1065. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W., Rose J.K. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J. Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W., Crise B., Rose J.K. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol. Cell. Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K.G., Ying Y.-S., Kamen B.A., Anderson R.G.W. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J. Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P., Roth M.G., Simons K. Interaction of influenza virus haemagglutinin with sphingolipid–cholesterol membrane domains via its transmembrane domain. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer P.E., Lewis R.Y., Volonte D., Engelman J.A., Galbiati F., Couet J., Kohtz S., van Donselaar E., Peters P., Lisanti M.P. Cell-type and tissue-specific expression of caveolin-2. J. Biol. Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- Schneider C., Newman R.A., Sutherland D.R., Asser U., Greaves M.F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J. Biol. Chem. 1982;257:10766–10769. [PubMed] [Google Scholar]
- Shaw A.S., Dustin M.L. Making the T cell receptor go the distancea topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- Sheets E.D., Holowka D., Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of Fc∈RI and their association with detergent-resistant membranes. J. Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A.M., Dietzen D.J., Kwong J., Link D.C., Lublin D.M. Cysteine3 of Src family protein tyrosine kinases determines palmitoylation and localization in caveolae. J. Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Presley J., Ellenberg J., Yamazaki T., Lippincott-Schwartz J., Samelson L.E. ZAP-70 association with T cell receptor ζ (TCRζ)fluorescence imaging of dynamic changes upon cellular stimulation. J. Cell Biol. 1998;143:613–624. doi: 10.1083/jcb.143.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer T.P., Meyer T. Compartmentalized IgE receptor-mediated signal transduction in living cells. J. Cell Biol. 1997;139:1447–1454. doi: 10.1083/jcb.139.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D.B., Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Sussman J.J., Saito T., Shevach E.M., Germain R.N., Ashwell J.D. Thy-1 and Ly-6-mediated lymphokine production and growth inhibition of a T cell hybridoma require co-expression of the T cell antigen receptor complex. J. Immunol. 1988;140:2520–2526. [PubMed] [Google Scholar]
- Turka L.A., Kanner S.B., Schieven G.L., Thompson C.B., Ledbetter J.A. CD45 modulates T cell receptor/CD3-induced activation of human thymocytes via regulation of tyrosine phosphorylation. Eur. J. Immunol. 1992;22:551–557. doi: 10.1002/eji.1830220238. [DOI] [PubMed] [Google Scholar]
- Varma R., Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Verweij C.L., Guidos C., Crabtree G.R. Cell type specificity and activation requirements for NFAT1 (nuclear factor of activated T cells) transcriptional activity determined by a new method using transgenic mice to assay transcriptional activity of an activated nuclear factor. J. Biol. Chem. 1990;265:15788–15795. [PubMed] [Google Scholar]
- Viola A., Schroeder S., Sakakibara Y., Lanzavecchia A. T lymphocyte costimulation mediated by reorganisation of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- Weiss A. T cell antigen receptor signal transductiona tale of tails and cytoplasmic protein-tyrosine kinases. Cell. 1993;73:209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- Weiss A., Stobo J.D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J. Exp. Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Littman D.R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Williams B.L., Schreiber K.L., Zhang W., Wange R.L., Samelson L.E., Leibson P.J., Abraham R.T. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptorreconstitution studies in a ZAP-70-deficient Jurkat T cell line. Mol. Cell. Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfing C., Davis M.M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible R.P., Samelson L.E. LAT palmitoylationits essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation Immunity. 9 1998. 239 246a [DOI] [PubMed] [Google Scholar]
- Zhang W., Sloan-Lancaster J., Kitchen J., Trible R.P., Samelson L.E. LATthe ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation Cell. 92 1998. 83 92b [DOI] [PubMed] [Google Scholar]