Abstract
Retinal ganglion cell axons grow towards the optic fissure in close contact with the basal membrane, an excellent growth substratum. One of the ligands of receptor tyrosine phosphatase CRYPα is located on the retinal and tectal basal membranes. To analyze the role of this RPTP and its ligand in intraretinal growth and guidance of ganglion cell axons, we disrupted ligand- receptor interactions on the retinal basal membrane in culture. Antibodies against CRYPα strongly reduced retinal axon growth on the basal membrane, and induced a dramatic change in morphology of retinal growth cones, reducing the size of growth cone lamellipodia. A similar effect was observed by blocking the ligand with a CRYPα ectodomain fusion protein. These effects did not occur, or were much reduced, when axons were grown either on laminin-1, on matrigel or on basal membranes with glial endfeet removed. This indicates that a ligand for CRYPα is located on glial endfeet. These results show for the first time in vertebrates that the interaction of a receptor tyrosine phosphatase with its ligand is crucial not only for promotion of retinal axon growth but also for maintenance of retinal growth cone lamellipodia on basal membranes.
Keywords: receptor protein tyrosine phosphatase, axon growth, actin cytoskeleton, growth cone, integrin
One of the first steps in retinal axon growth occurs early in development in the retina. During the process of intraretinal axon guidance, ganglion cell axons grow towards the optic fissure and exit the eye at the optic nerve head to reach their visual centers in the brain. In the chick embryo, the first retinal ganglion cell axons are found in the central retina at Hamburger-Hamilton (HH) stage 15 (E2-E3), shortly after invagination of the optic vesicle (Mey and Thanos 1992). From the earliest stages onwards these axons grow straight to the optic fissure. As more and more ganglion cells differentiate and send out axons, fascicles form which also head directly to the fissure. In recent years, several mechanisms have been suggested to account for intraretinal axon guidance.
Neurolin, the goldfish homologue of DM-GRASP, is involved in guidance of dorsal retinal axons towards the optic disc (Ott et al. 1998). Neurolin is a member of the immunoglobulin superfamily consisting of five extracellular Ig domains, a transmembrane region, and a short cytoplasmic sequence. In vivo injections of monoclonal antibodies against Ig domains 1 and 3 resulted in defasciculation of retinal axons, whereas antibodies directed against Ig domain 2 led to intraretinal guidance errors of ganglion cell axons (Leppert et al. 1999). The direct growth towards the optic disc also requires other immunoglobulin superfamily members like L1 and N-CAM (Brittis and Silver 1995; Brittis et al. 1995). Injection of Fab fragments against E587, a member of the L1 subfamily, resulted in a delay of fasciculation of young axons as well as defasciculation (Bastmeyer et al. 1995; Giordano et al. 1997).
A role for the FGF receptor (FGFR), a receptor tyrosine kinase, in guidance of retinal ganglion cell axons toward the fissure became evident after adding receptor-specific antibodies to living whole-mount rat retinae. Blocking of the FGFR resulted in defasciculation of retinal axons in the center of the retina and in guidance errors in the retinal periphery (Brittis et al. 1996). In Xenopus retinae, in >40% of retinal ganglion cells expressing a truncated, kinase-defective FGFR, axons were unable to leave the retina (McFarlane et al. 1996). These observations underscore the importance of the FGFR in intraretinal axon guidance and in the exit of retinal axons from the eye. The results are at least partially explained by the function of this receptor in mediating the effects of N-cadherin, NCAM, and L1 (reviewed in Viollet and Doherty 1997), other cell adhesion molecules and components of the ECM.
Chondroitin sulfate proteoglycan, a major component of the ECM, was suggested to act as an inhibitory molecule, preventing growth of retinal axons towards the periphery (Brittis et al. 1992). Having reached the optic disc, axons start to exit from the eye to enter the optic nerve. In the mouse embryo, netrin-1 seems to be responsible for the exit of retinal axons but not for their growth towards the optic disc (Deiner et al. 1997). In the eye, retinal axons grow in close contact to an extracellular matrix structure, the basal lamina or basal membrane (BM) (Rager 1980; Easter et al. 1984; Silver and Rutishauser 1984; Halfter and Boxberg 1992; Halfter 1996). The retinal basal lamina (membrana limitans interna) is an excellent growth substratum for retinal axons and is superior to laminin (Halfter 1996) and, moreover, extremely important during early stages of neural development (Halfter 1998). Its outgrowth promoting activities, however, are not completely characterized. Important molecular components of this structure include laminin, nidogen, collagen IV, agrin, heparan sulfate proteoglycan, chondroitin sulfate proteoglycan, tenascin, and at least 10 unidentified extracellular matrix components (Halfter and Boxberg 1992; Halfter 1996; Faissner 1997). Among these unidentified molecules are probably candidates contributing to the excellent growth-stimulating characteristics of the retinal BM.
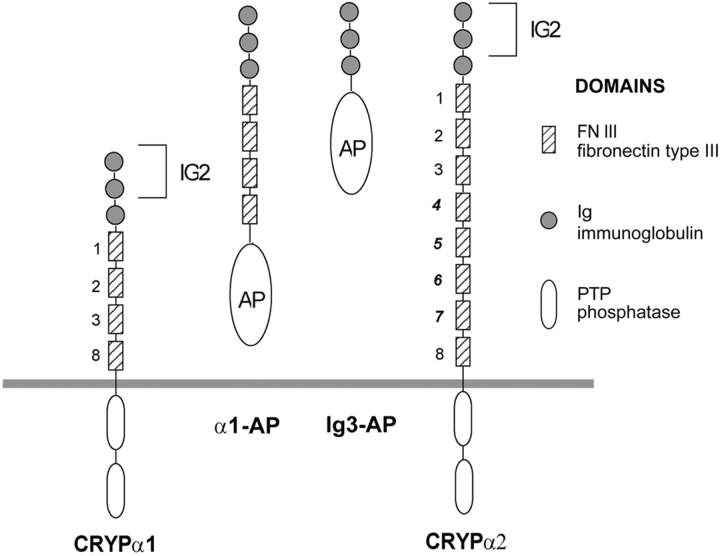
We became interested in studying the retinal basal membrane in more detail because of the recent finding that several receptor protein tyrosine phosphatases (RPTPs)1, including CRYPα (Stoker 1994), CRYP-2 (Bodden and Bixby 1996), and PTPμ (Gebbink et al. 1991), are expressed on retinal axons and growth cones during formation of the retinotectal projection (Stoker et al. 1995a; Ledig et al. 1999). CRYPα is a member of the type IIa subfamily of RPTPs. This subfamily is of particular interest given their structural resemblance to the neural CAMs (see Fig. 1) and their expression pattern in the developing CNS (Schaapveld et al. 1998). This structure suggested that they would have extracellular ligands either on cells or in the extracellular matrix. Although the identity of these ligands remains to be determined, it appears that at least one of these is present on the retinal BM (Haj et al. 1999).
Figure 1.
Schematic diagram of receptor tyrosine phosphatase CRYPα isoforms and the soluble alkaline phosphatase (AP) fusion proteins used in this study.
To date the function of these RPTPs during nervous system development in vertebrates remains largely to be determined (Stoker and Dutta 1998; van Vactor 1998). Most of what we know about the function and signaling mechanisms of RPTPs comes from studies in Drosophila (Desai et al. 1997a). The Drosophila RPTPs, DLAR, DPTP69D, and DPTP99A are required for motor axon guidance (Desai et al. 1996, Desai et al. 1997b; Krueger et al. 1996). Signals downstream of DLAR also control the actin cytoskeleton of axons (Wills et al. 1999a,Wills et al. 1999b). In vertebrates, very recent data (Uetani et al. 1997; Elchebly et al. 1999; Wallace et al. 1999) also indicate that at least PTPσ (CRYPα is the chick orthologue of PTPσ) and PTPδ (Mizuno et al. 1993) are important for proper development of the central nervous system. PTPμ, belonging to the same type II family of PTPs as CRYPα, was also recently described as being important for regulating cadherin-mediated retinal axon outgrowth (Burden-Gulley and Brady-Kalnay 1999).
We have addressed the question of CRYPα function in early retinal axons by attempting to perturb functionally the interactions of CRYPα1 with its ligand in the in vivo substrate, the retinal BM. The presence of a BM ligand was demonstrated in vitro using a fusion protein consisting of the ectodomain of CRYPα1 and the enzyme alkaline phosphatase (α1-AP) (see Fig. 1 for structures). This protein prominently binds to the BM (Haj et al. 1999). To analyze the potential function of the CRYPα ligand, we grew retinal axons on retinal BMs (Halfter et al. 1987) and treated them either with CRYPα specific antibodies or with soluble α1-AP. Both the axonal growth rate and the growth cone morphology of retinal neurons were strongly influenced by these reagents. Significantly, on BMs with endfeet removed (BM-Ef) and on an artificial acellular BM (matrigel), these effects were sharply reduced. We conclude that CRYPα and its ligand on glial endfeet play important roles in promoting retinal axon growth and maintenance of growth cone morphology.
Materials and Methods
Antibodies and Reagents
The generation of the polyclonal antibody anti-CRYPα and its purification have been previously described (Stoker et al. 1995a). The polyclonal anti-NCAM antibody and its function-blocking activity have been characterized (Bixby and Reichardt 1987; Bixby et al. 1987). The monoclonal antibody against β1-integrin was JG22 (Greve and Gottlieb 1982; Tomaselli et al. 1986). The CRYPα1-AP and Ig3-AP fusion proteins were generated by subcloning CRYPα1 fragments spanning amino acids 1–721 and 1–316 into the APtag2 vector (Cheng et al. 1995; Haj et al. 1999). AP was generated by transfecting the APtag4 vector (Cheng et al. 1995). All three vectors were transfected into cos7 or 293T cells using SuperfectTM (Qiagen) and the proteins were collected after 6 d in the conditioned medium. Medium was passed through an anti-AP agarose column (Sigma), and the fusion protein eluted using 0.1 M glycine-HCl, pH 2.5, with immediate buffering to pH 8 with Tris-HCl. The protein was dialyzed against TBS buffer and stored at 4°C. Laminin-1 and matrigel were obtained from Becton Dickinson. N-cadherin was purified as described (Bixby and Zhang 1990).
AP Staining
E6 retinal cryosections were prepared as previously described (Ledig et al. 1999) and incubated with α1-AP. The AP staining method was also previously described (Cheng et al. 1995; Haj et al. 1999).
Culture of Retinal Explants: The Basal Membrane Assay
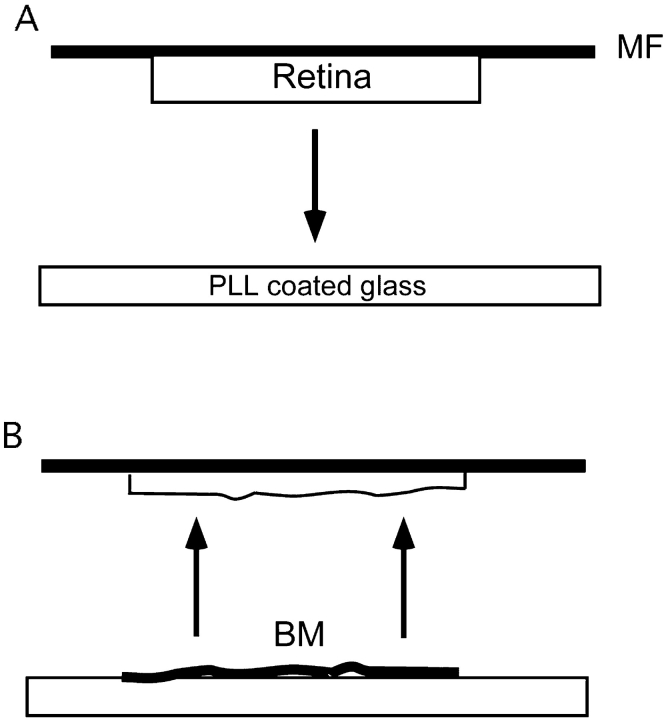
The basal membrane assay was performed as described in Halfter et al. 1987. The basal lamina was prepared from E7 retina. In the first step, the retina is flatmounted on a nitrocellulose filter (Sartorius AG). The filter with the attached retina is then put upside down on a poly-l-lysine (PLL)-coated glass coverslip (see Fig. 2 A). Two small metal bars are put on the filter before it is incubated for 10 min at 37°C. Afterwards the filter is lifted up while the basal lamina sticks to the glass surface (see Fig. 2 B). To remove the glial endfeet from the basal lamina, glass coverslips were washed with 2% Triton X-100 for 5 min. The Triton was removed after three 10-min wash steps with Hank's solution. For experiments with laminin as a growth substrate glass coverslips were coated with laminin at 37°C for 2 h and afterwards washed with Hank's. Coating of glass coverslips with matrigel was carried out according to the manufacturer's protocol. For experiments with N-cadherin as a growth substrate, PLL-precoated glass coverslips were coated with N-cadherin at 37°C for 3 h, resulting in a PLL/N-cadherin substrate mix. Retinal explants as outgrowth source were prepared from E6 retinae as previously described (Ledig et al. 1999). Cultures were incubated for 24 h at 37°C in 1 ml F12 medium containing methylcellulose and various amounts of preadded different antibody in a humidified chamber (5% CO2).
Figure 2.
The basal membrane assay. An E6 retina was first dissected and flatmounted on a nitrocellulose membrane filter (MF), and then put upside down onto a PLL-coated glass coverslip, weightened, and incubated for 10 min (A). After lifting the filter up the basal membrane (BM) sticks to the surface of the glass coverslip (B) (Scheme after Kroeger 1989).
Staining of Retinal Cultures
After incubation cultures were immediately fixed in 4% paraformaldehyde (dissolved in 0.1 M phosphate buffer) for 10 min at room temperature. They were permeabilized with 0.1% Triton X-100 in PBS and blocked by 1% BSA in PBS. Stainings with Alexa-labeled phalloidin were performed according to the manufacturer's protocol (Molecular Probes Inc.). The fixed cultures were finally covered with moviol.
Evaluation of Data
Analysis was done using an Axiophot (Zeiss) fluorescence microscope using a Sony CCD-camera together with the Analysis program (SIS). To quantify outgrowth real-time pictures were taken and directly measured on the screen. The average axon length of a culture was determined as the distance from the explant to the region reached by at least 60% of the axons (longer and shorter neurites were not considered) (see Fig. 3 A). Growth cone morphology was analyzed by measuring two diameters of a growth cone using a 100× objective (see Fig. 3 B). The d1 parameter represents the length of the growth cone in μm, measured from the growth cone-axon neck to the border of the leading lamellae, and the d2 parameter represents its width, characterized mainly by the extension of its lamellipodia. The data were analyzed by regression analysis, ANOVA, and Student's t test using the EXCEL 98 program (Microsoft) and StatView 4.5 (Abacus Concepts Inc.).
Figure 3.
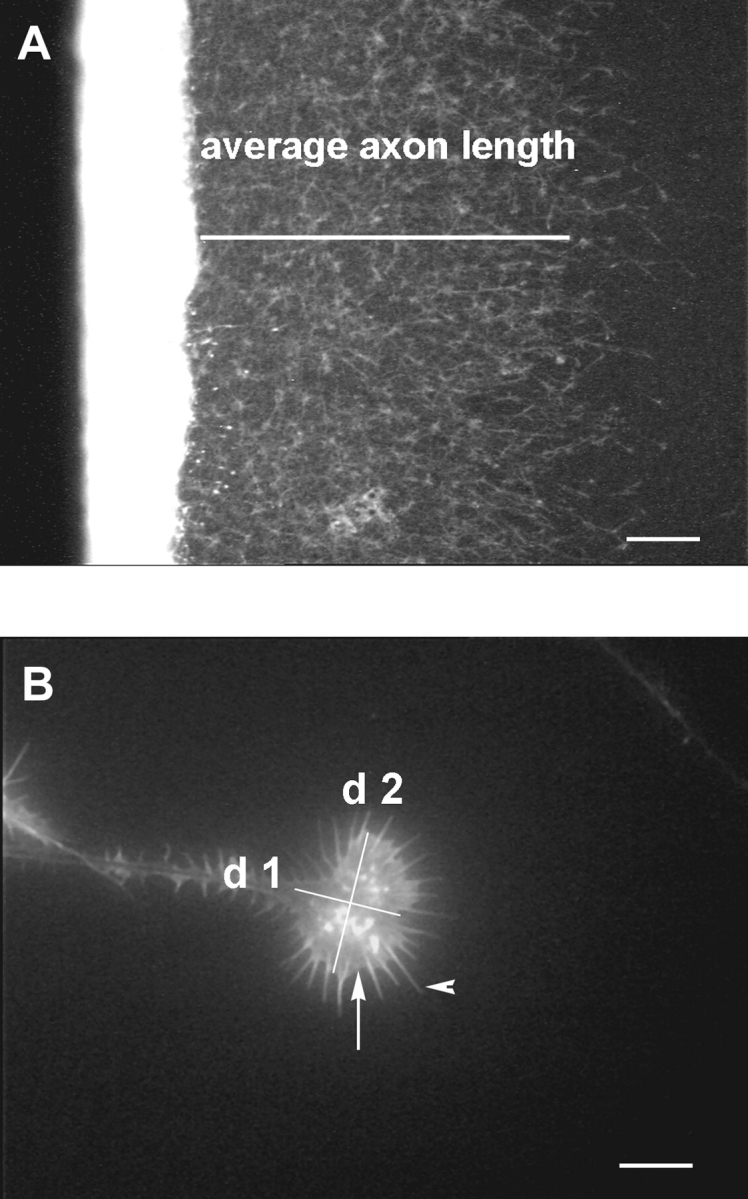
Criteria for data analysis. A shows a control culture on BM after a 24-h incubation time. We defined the average axon outgrowth as the distance between the explant and the growth front covered by at least 60% of the axons. B demonstrates how growth cone data were measured. A growth cone grown on LN was characterized by its two diameters d1 and d2. The arrow indicates lamellipodia and the arrowhead filopodia of the growth cone. Bars: (A) 0.1 mm; (B) 0.01 mm.
Results
The CRYPα Ligand Is Expressed in the Retinal Basal Membrane
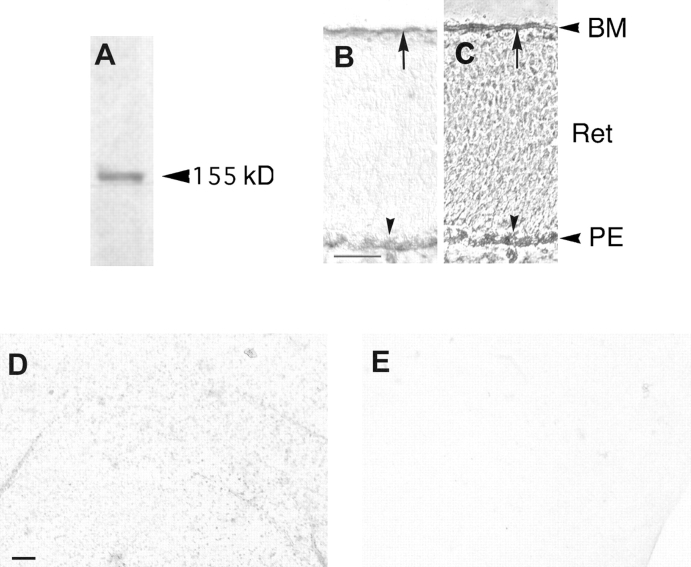
Immunostaining with CRYPα-specific antibodies strongly labels retinal axons inside the retina, optic nerve, and tract, and in the optic tectum of the chick embryo (Stoker et al. 1995b; Ledig et al. 1999). CRYPα is already present at E4 on retinal axons and growth cones. Two isoforms of CRYPα, CRYPα1 and α2 (Fig. 1), were described, both containing 3 Ig-like domains but differing in the number of fibronectin type III-like domains (FNIII). α1 has four and α2 has eight FNIII repeats, respectively (Stoker et al. 1995b). Until E7, only CRYPα1 is expressed in retinal ganglion cells, whereas CRYPα2 is coexpressed by E8 (Haj et al. 1999). We suggest, therefore, that for the early stages of retinal optic fiber growth, CRYPα1 is the relevant isoform. To examine the expression of potential ligands of this PTP, the extracellular domain of the CRYPα1 isoform was fused to the enzyme alkaline phosphatase (Fig. 1) and the fusion protein α1-AP was used to analyze the localization of the ligands in the developing chick retinotectal system (Haj et al. 1999). Using α1-AP (see Fig. 4 A), a ligand was detected at E6 in the basal membrane of the retina (see Fig. 4 B), the optic stalk and the optic chiasm (Haj et al. 1999) and in many basal membranes throughout the embryonic brain (Stoker, A.W., unpublished observations).
Figure 4.
A shows the α1-AP fusion protein in cos7 cell supernatant, immunodetected with IG2 sera. α1-AP consists of the extracellular domain of CRYPα, which has a molecular weight of ∼90 kD together with the alkaline phosphatase of 65 kD. B and C show binding of the α1-AP fusion protein to a section of the E6 retina, visualized with AP staining. C is the corresponding phase contrast view. Arrows indicate staining in the BM of the retina. The arrowheads indicate the pigment epithelium. D shows binding of the α1-AP fusion protein to a BM from a E7 retina, visualized with AP staining. The stained dots represent glial endfeet. There is no binding of α1-AP to an E7 detergent-washed endfeet-free BM detectable as can be seen in E. Abbreviations: BM, basal membrane; Ret, retinal cell layer; PE, pigmented epithelium. Bars: (B and C) 0.1 mm; (D and E) 0.01 mm.
Blocking CRYPα-Ligand Interactions Reduces Retinal Axon Length
The expression of the putative ligand in the retinal basal membrane prompted us to use this membrane as a growth substrate for retinal axons and to analyze what role the interaction of CRYPα with its ligand has in retinal axon growth. To this end, retinal basal membranes were isolated according to a published method (Halfter et al. 1987) and retinal strips were explanted. Retinal axons were grown on retinal basal membranes or on laminin for 24 h either in the presence of polyclonal antibodies directed against the two outermost Ig-like domains of CRYPα (IG2 antibody; Fig. 1), or in the presence of α1-AP, thereby disturbing ligand-receptor interactions. Importantly, the IG2 antibody is known to block the binding of α1-AP to the basal membrane ligand (see Haj et al. 1999) and therefore is predicted to block CRYPα ligand interactions in general. A fusion protein containing the 3 Ig domains of CRYPα (Fig. 1; Ig3-AP) was used as a negative control, as previous experiments suggested that this protein does not to bind to the CRYPα ligand (Haj et al. 1999). To analyze the effect of disturbing the CRYPα-ligand interaction, the average length of retinal axons leaving the explant was measured from the edge of the explant to the front of the majority of axons (Fig. 3 A).
The IG2 antibody and α1-AP strongly reduced the length of retinal axons, in a dose-dependent manner on BM (Fig. 5A and Fig. C). We obtained the same result in preliminary studies with IG2 antibodies on the tectal membrane stripe assay (Walter et al. 1987) with tectal membrane preparations (Ledig, M.M., and B.K. Mueller, unpublished data). Outgrowth of both nasal and temporal axons was reduced, but there was no effect on the decision behavior (data not shown). 125 pmol of IG2 reduced retinal axon length by 63% on basal membrane and by 25% on LN (Fig. 5A and Fig. B). 26 pmol α1-AP reduced retinal axon length on BM by ∼50% (Fig. 5 C). No comparable effect was observed with Ig3-AP on BM (data not shown) or with α1-AP or Ig3-AP when retinal axons were grown on LN (Fig. 5 D). A slight dose-dependent effect, caused by the alkaline phosphatase (AP), was observed on both LN and retinal BM, but only when used at high levels (compare Fig. 5C and Fig. D, with controls in A and B). 125 pmol of NCAM antibody was used as a control for antibody binding and did not influence retinal axon length on LN or BM (Fig. 5E and Fig. F, respectively). Moreover, there were also no obvious effects on fasciculation by IG2 or by the polyclonal control antibody NCAM (data not shown).
Figure 5.
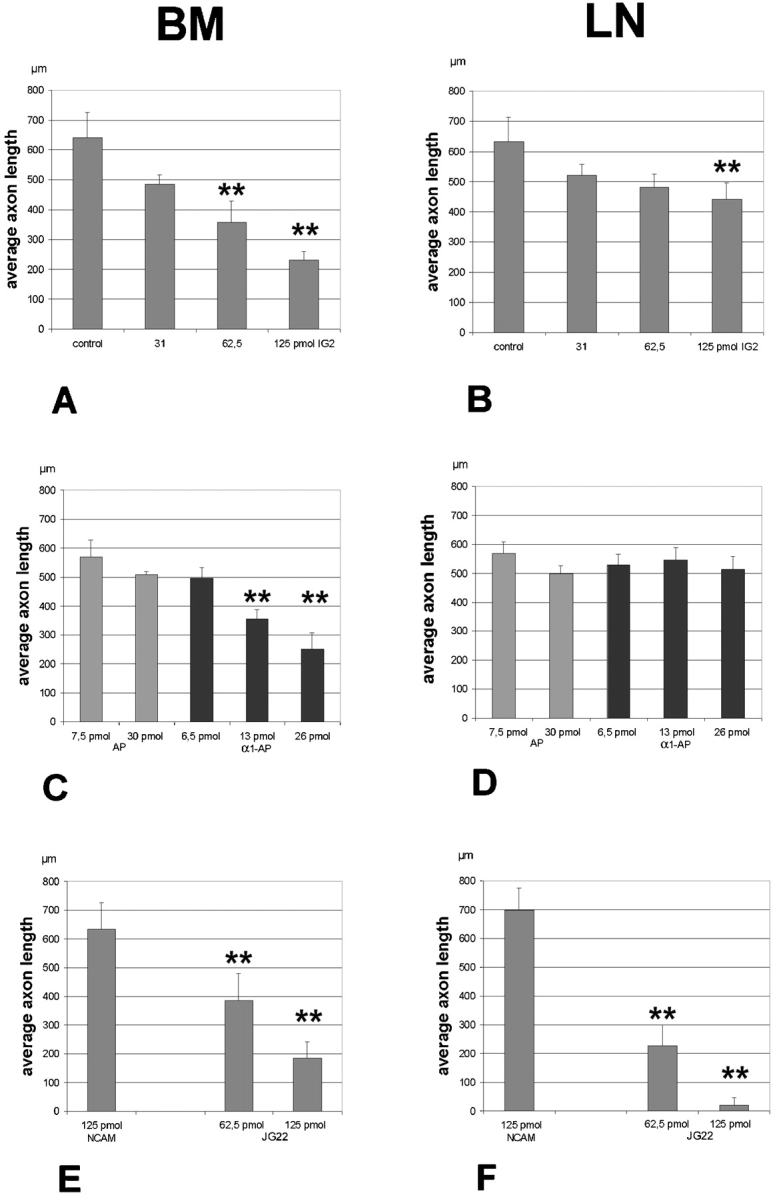
Quantification of the effect of IG2 and α1-AP on neurite outgrowth on LN and BM. E6 retinal explants were cultured on either LN or BM with the amounts of antibody indicated below the bars. IG2 and α1-AP reduced the average length of axon outgrowth on BM in a dose-dependent fashion (A and C). IG2 also showed a significant effect on LN (B). No effect was observed for α1-AP on LN (D). To address the possibility of an effect induced by antibody cross-linking we used anti-NCAM as a control. No effect of 20 μg anti-NCAM was observed on either LN or BM (E and F). Anti-β-integrin JG22 was used as a comparison for IG2. On BM neurite outgrowth was affected in a dose-dependent manner by JG22 (E) while on LN outgrowth was almost completely blocked by an amount of 20 μg antibody (F). One datapoint represents a measurement of one retinal strip from an independent experiment performed with at least one retinal strip. Double asterisks indicate P < 0.0001.
A second antibody, directed against the β1-integrin chain (JG22), was used to compare the effect of CRYPα1 to one of the major receptors for ECM molecules. Using this antibody significantly reduced retinal axon length in a dose-dependent manner on both substrates. 125 pmol of JG22 blocked retinal axon growth on LN almost completely (Fig. 5 F). On BM retinal axon length was reduced by 71% (Fig. 5 E). This suggests the presence of different outgrowth promoting activities in basal membranes, mediating their effects by at least two different receptor systems, the integrins, and the RPTP CRYPα.
Blocking CRYPα Ligand Interactions Alters Retinal Growth Cone Morphology
The d1 and d2 parameters were used to measure growth cone extensions (Fig. 3 B). Retinal axons growing on the retinal basal membrane or on laminin exhibit a morphology with few filopodia but elaborate lamellipodia (Fig. 6 A). In the presence of increasing amounts of IG2 or α1-AP, lamellipodia are gradually retracted and the number of long filopodia is increased (Fig. 6, B–D and F–H). This striking transition from a lamellipodial to a filopodial growth cone morphology was most evident after adding 125 pmol IG2 or 26 pmol of α1-AP (Fig. 6D and Fig. H) (Table ). Significantly, this was only observed on retinal BM (Fig. 6, A–H, and 9 A) but not on LN (Fig. 6, I–P, and 9 B). The transition in growth cone morphology was reflected in a >40% reduction of the growth cone width (d2) without any significant change of its length (d1) (Fig. 9A and Fig. B) (Table ). Neither control antibody anti-NCAM (125 pmol) nor AP (30 pmol) influenced retinal growth cone morphology on either substrate (Fig. 6E and Fig. M, Fig. 8D and Fig. E, and Fig. 9C and Fig. D). A similar change in growth cone morphology was observed after adding 62.5 or 125 pmol of the β1 integrin antibody JG22 (Fig. 8J and Fig. M, and Fig. 9 C). In contrast to inhibition of the CRYPα ligand interaction, the morphological change seen with JG22 was not restricted to growth cones on BM but was also found for growth cones growing on LN (Fig. 8K and Fig. N, and Fig. 9 D; Table ).
Figure 6.
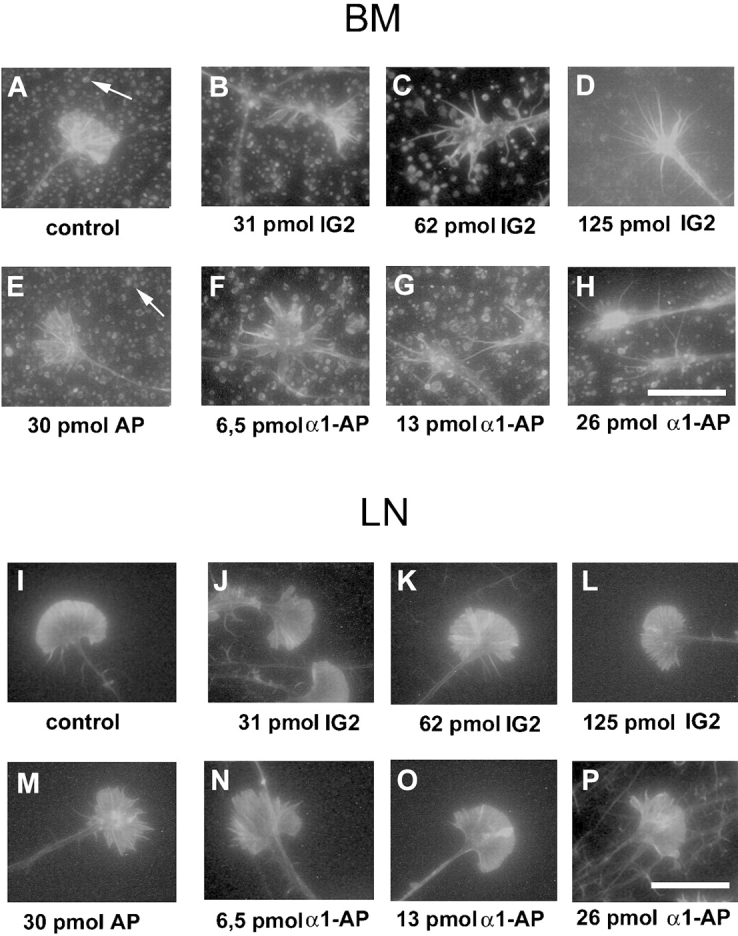
The morphology of the growth cone is affected by treatment with IG2 antibody or α1-AP fusion protein in a substrate-dependent manner. Effects on morphology were seen on BM (B–D and F–H), but not on LN (J–L and N–P). On BM growth cone lamellipodia were retracted in a dose-dependent manner when adding either IG2 (A–D) or α1-AP (E–H). Arrows indicate glial endfeet structures. Bar, 0.02 mm.
Table 1.
Statistical Analysis of Growth Cone Morphology
| Substrate + antibody | n | Growth cone diameter ± SEM | P | ||
|---|---|---|---|---|---|
| μm | |||||
| BM | Control | d1 | 60 | 12.86 ± 2.59 | vs. BM-Ef, <0.0001 |
| d2 | 60 | 11.24 ± 2.08 | vs. BM-Ef, <0.0001 | ||
| BM | 20 μg NCAM | d1 | 40 | 12.22 ± 1.63 | NS |
| d2 | 40 | 10.98 ± 1.49 | NS | ||
| BM | 20 μg IG2 | d1 | 40 | 11.74 ± 1.36 | NS |
| d2 | 40 | 5.14 ± 1.36 | <0.0001 | ||
| BM | 20 μg β-integrin | d1 | 40 | 7.07 ± 4.93 | <0.0001 |
| d2 | 40 | 3.75 ± 2.85 | <0.0001 | ||
| LN | Control | d1 | 40 | 15.22 ± 2.23 | vs. BM, 0.0014 |
| d2 | 40 | 13.23 ± 2.54 | vs. BM, 0.0016 | ||
| LN | 20 μg NCAM | d1 | 40 | 13.99 ± 2.22 | NS |
| d2 | 40 | 14.96 ± 1.95 | NS | ||
| LN | 20 μg IG2 | d1 | 40 | 12.95 ± 2.83 | 0.0059 |
| d2 | 40 | 12.96 ± 3.31 | NS | ||
| LN | 20 μg β-integrin | d1 | 40 | Not detectable | |
| d2 | 40 | Not detectable | |||
| BM-Ef | Control | d1 | 40 | 17.44 ± 1.96 | vs. LN, 0.0002 |
| d2 | 40 | 16.41 ± 2.88 | vs. LN, <0.0001 | ||
| BM-Ef | 20 μg NCAM | d1 | 40 | 17.61 ± 2.94 | NS |
| d2 | 40 | 16.26 ± 2.26 | NS | ||
| BM-Ef | 20 μg IG2 | d1 | 40 | 15.32 ± 2.39 | 0.0163 |
| d2 | 40 | 15.35 ± 2.08 | NS | ||
| BM-Ef | 20 μg β-integrin | d1 | 40 | 5.40 ± 5.96 | <0.0001 |
| d2 | 40 | 2.59 ± 2.84 | <0.0001 |
n, Number of measured growth cones (10 growth cones from 4/6 independent experiments were analyzed); P, values from single-factor ANOVA analyzing the influence of IG2 on the same substrate and the influence of the different substrates themselves, 99% confidence interval NS indicates, no significance (statistical values > 0.05).
Figure 9.
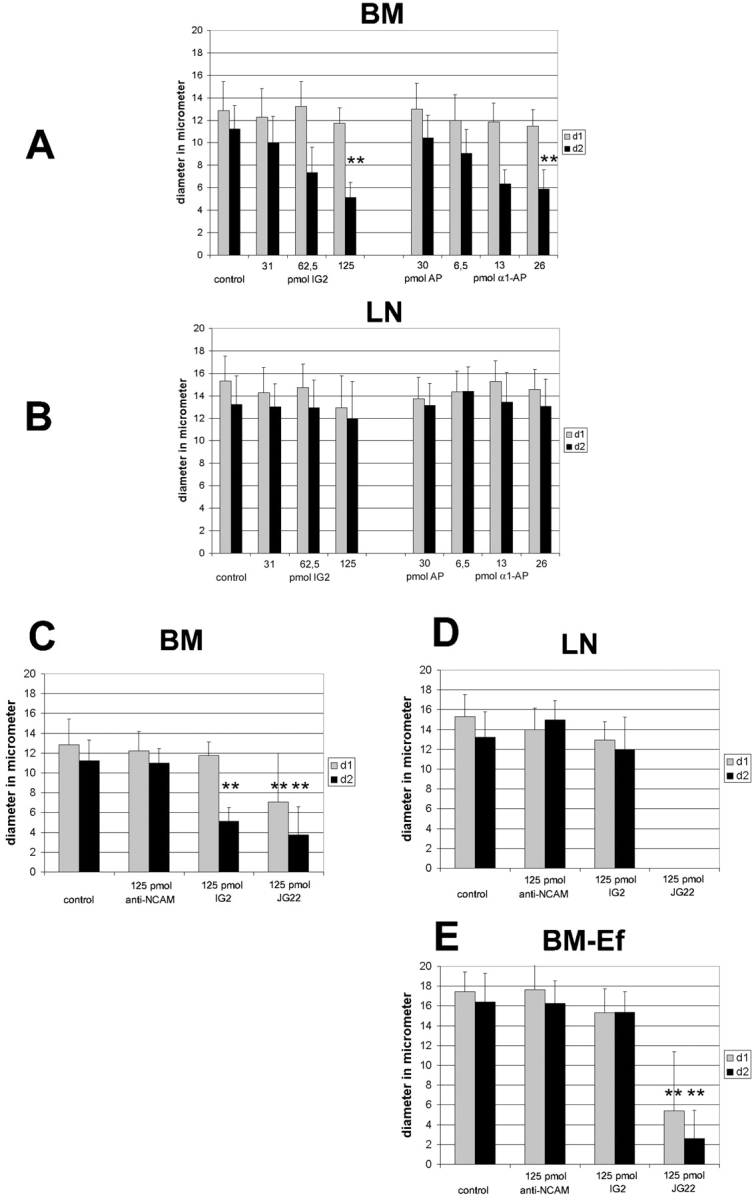
Quantification of growth cone morphology on different substrates. On BM both IG2 and α1-AP affect growth cone morphology. D2 is significantly reduced in a dose-dependent manner while d1 is not affected at all (A). There was no effect on LN (B). Comparing the influence of both antibody and growth substrates it is remarkable that growth cones on LN and BM-Ef are larger in their overall size compared with ones grown on BM (C–E). Moreover, treatment with IG2 only had a significant effect on d2 of growth cones grown on BM (C), but not on LN (D) and BM-Ef (E). When using 125 pmol JG22 there were not any growth cones detectable on LN (D). Growth cone morphology was less, but still heavily affected on BM-Ef (E) and BM (C) (** indicate statistical significance P < 0.0001). See Table for data.
Figure 8.
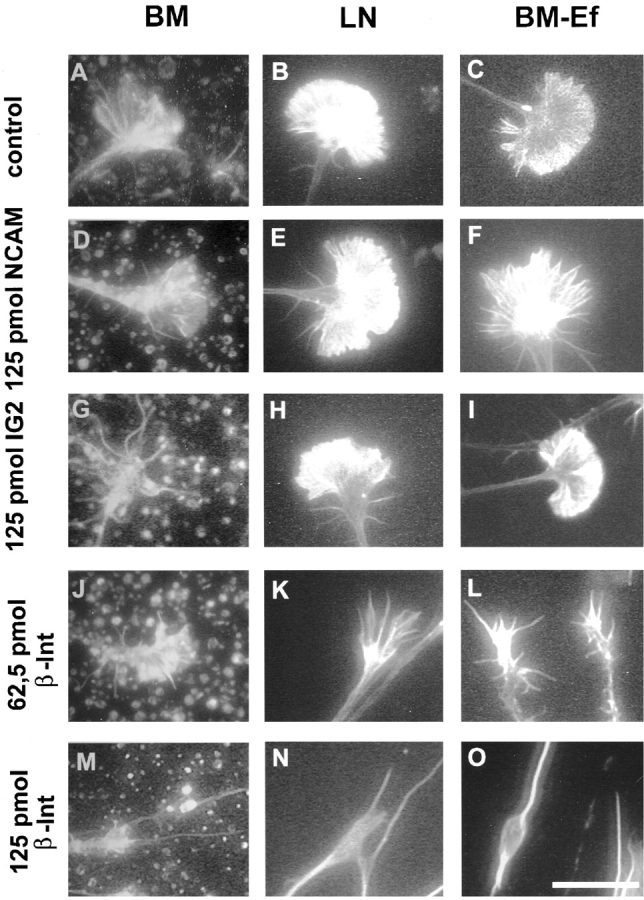
Comparison of the effect of IG2 on different growth substrates and to the change in growth cone morphology induced by blocking the integrin receptor. Anti-NCAM was used as polyclonal control antibody (D–F). After removal of glial endfeet IG2 no longer has an effect on growth cone morphology (compare A–C with G–I), meaning that the ligand must be located on the glial endfeet. Axon growth is largely mediated via the integrin receptor. Laminin, one of the integrin ligands is located in the ECM. Therefore, blockage of the integrin receptor β-subunit results in an effect on all substrates (J–O) that is dose-dependent (compare J–L with M–O). Interestingly growth cones are less affected in the presence of glial endfeet (J and M). Bar, 0.02 mm.
Therefore, blocking the interaction of the RPTP CRYPα with its ligand from either the receptor or the ligand side, not only reduced retinal axon length but induced a significant shift in the morphology of retinal growth cones to a more filopodial appearance. This suggests that CRYPα action influences the maintenance of lamellipodia when growth cones are migrating on the intact retinal BM substrate.
The CRYPα Ligand Is Found on Glial Endfeet
The retinal basal membrane contains the endfeet of the Mueller glia cells. These endfeet are visible under microscopic bright field illumination and are stained by Alexa-phalloidin (Fig. 6). Using such a staining procedure, endfeet appear as dot like structures with a diameter of ∼2 μm and with a ring-like F-actin organization. Incubating the α1-AP protein on E7 basal membranes resulted in staining of only these glial endfeet (Fig. 4 D). This suggests that the putative ligand for CRYPα1 is located on the glial endfeet. We tested this possibility by removing the endfeet from the retinal BM. Removal of the endfeet was performed by washing the retinal basal membrane with 2% Triton X-100 in PBS according to a published protocol (Halfter et al. 1987) and resulted in a completely transparent basal membrane (BM-Ef). In accordance with our hypothesis, there was no detectable staining of BM-Ef using the α1-AP protein (Fig. 4 E).
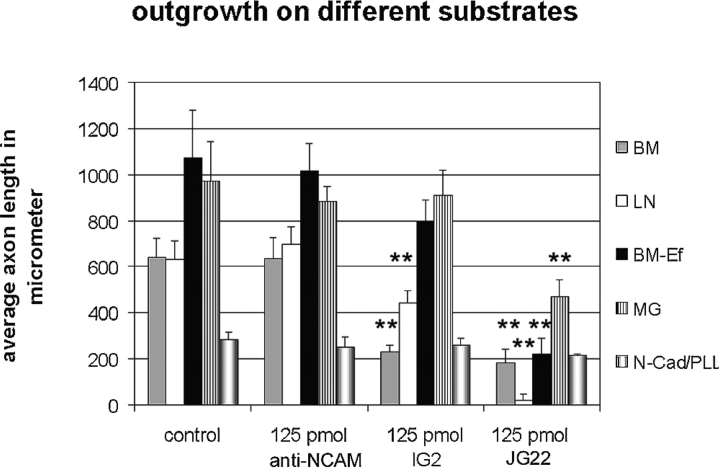
Washing away the endfeet demonstrated the enormous outgrowth-promoting potential of the ECM. Whereas the average axon length in controls was almost the same on LN and BM, removal of the endfeet caused an increase in the average axon length of >40% (Fig. 7 and Table ). However, growth on BM-Ef was much less susceptible to inhibition with IG2. IG2 inhibited axon growth on complete BM by 63%, but inhibition of growth on BM-Ef was slight and similar to that seen on LN (25–30%; Fig. 7). As an additional control for specificity of inhibition by IG2 antibodies, we tested matrigel, an acellular basement membrane preparation from EHS sarcoma cells that lacks detectable CRYPα ligand (McKinnell, I., and A. Stoker, unpublished work). This was considered appropriate because most brain BMs at stage E6 are ligand-positive, and other BMs are very difficult to isolate. Axon growth on matrigel was as prolific as on BM-Ef, and was completely unaffected by IG2 (Fig. 7). Finally, anti N-CAM antibody had no influence on retinal axon growth on any substrate (Fig. 7 and Table ). These results suggest that the ligand of the PTP CRYPα is located predominantly on retinal glial endfeet structures.
Figure 7.
Quantification of the influence of the growth substrate. Removal of the glial endfeet from BM resulted in strong increase in average axon length. The effect of IG2 is strongest on BM, nearly equal on BM and LN. Both effects are statistically significant (P < 0.0001). There was almost no effect of IG2 on matrigel and on N-cadherin. In the presence of glial endfeet JG22 exhibits weaker effects on axon outgrowth than on matrigel, BM-Ef, or LN. Anti-NCAM had no effect on any substrates. Double asterisks indicate P < 0.0001. See Table for data.
Table 2.
Statistical Analysis of the Average Axon Length
| Substrate + antibody | n | Average axon length ± SEM | P | ||
|---|---|---|---|---|---|
| P1 | P2 | ||||
| μm | |||||
| BM | Control | 19 | 642.0 ± 83.4 | — | vs. LN, NS |
| BM | 20 μg NCAM | 6 | 634.0 ± 93.7 | NS | vs. LN, NS |
| BM | 20 μg IG2 | 16 | 232.4 ± 27.3 | <0.0001 | vs. LN, <0.0001 |
| BM | 20 μg β-integrin | 9 | 184.2 ± 57.0 | <0.0001 | vs. LN, 0.0001 |
| LN | Control | 14 | 635.5 ± 85.5 | — | vs. BM-Ef, <0.0001 |
| LN | 20 μg NCAM | 8 | 698.2 ± 76.1 | NS | vs. BM-Ef, NS |
| LN | 20 μg IG2 | 9 | 442.8 ± 53.1 | <0.0001 | vs. BM-Ef, <0.0001 |
| LN | 20 μg β-integrin | 5 | 20.4 ± 25.8 | <0.0001 | vs. BM-Ef, 0.0004 |
| BM-Ef | Control | 8 | 1073.6 ± 209.3 | — | vs. BM, <0.0001 |
| BM-Ef | 20 μg NCAM | 8 | 1014.8 ± 119.4 | NS | vs. BM, <0.0001 |
| BM-Ef | 20 μg IG2 | 13 | 796.6 ± 94.3 | 0.0015 | vs. BM, <0.0001 |
| BM-Ef | 20 μg β-integrin | 6 | 220.6 ± 69.7 | <0.0001 | vs. BM, NS |
| MG | Control | 9 | 970.4 ± 173.3 | — | vs. BM-Ef, NS |
| MG | 20 μg NCAM | 3 | 883.8 ± 65.7 | NS | vs. BM-Ef, NS |
| MG | 20 μg IG2 | 11 | 909.8 ± 109.7 | NS | vs. BM-Ef, 0.014 |
| MG | 20 μg β-integrin | 7 | 470.5 ± 74.4 | <0.0001 | vs. BM-Ef, 0.001 |
| NC/PLL | Control | 9 | 281.5 ± 35.9 | — | — |
| NC/PLL | 20 μg NCAM | 3 | 256.8 ± 30.9 | NS | — |
| NC/PLL | 20 μg IG2 | 6 | 252.7 ± 41.3 | NS | — |
| NC/PLL | 20 μg β-integrin | 3 | 213.4 ± 5.8 | 0.0014 | — |
n, Number of independent experiments with at least two retinal strips; P1, values from single-factor ANOVA (ANOVA was used because most of the data show a normal distribution) analyzing the influence of different antibodies on the same substrate, 99% confidence interval; P2, values from single-factor ANOVA analyzing the influence of the growth substrate when treated with the same antibody, 99% confidence interval (the substrate that was used for the comparison is indicated); NS indicates no significance (statistical values > 0.05).
On intact BMs, the anti-β1-integrin antibody (JG22) reduced outgrowth by ∼70%, suggesting that, in addition to CRYP-α interactions, ECM/integrin interactions are important regulators of axon growth on this tissue. As expected, inhibition of axon growth by JG22 was even greater when BM-Ef was tested, and outgrowth on LN was completely blocked. In contrast, axon growth on matrigel was inhibited only 48% by JG22. This result suggests that matrigel contains growth-promoting substances in addition to the well-characterized ECM components that are completely susceptible to inhibition by integrin antibodies (Tomaselli et al. 1986). PLL/N-cadherin mediated outgrowth was not affected by either IG2 or JG22 (Fig. 7 and Table ).
Comparison of the morphology of retinal growth cones on the three different substrates (LN, BM, and BM-Ef) revealed that growth cones on LN (Fig. 8 B and 9 D) and BM-Ef (Fig. 8 C and 9 E) are more elaborate, possessing larger d1 and d2 parameters, than growth cones on BM (Fig. 8 A and 9 C). Blocking the interaction of the RPTP CRYPα with its ligand-affected growth cone morphology on BM (Fig. 8 G and 9 C) but not on BM-Ef (Fig. 8 I and 9 E), LN (Fig. 8 H and 9 D), or matrigel (data not shown). Growth cone morphology on BM-Ef was affected by JG22 in the same way as on LN (compare Fig. 8L and Fig. O with K and N). For statistical data see Table .
Discussion
In recent years, evidence has accumulated that RPTPs play important roles in axon growth and guidance (reviewed in Chien 1996; Desai et al. 1997a; Gershon et al. 1998; Wills et al. 1999b). Nearly all of this evidence comes from studies in invertebrates, especially from Drosophila and leech. Far less is known about the role of RPTPs in the nervous system of vertebrates. Here we present data from in vitro experiments in which we examined the role of the RPTP CRYPα in the growth of retinal axons towards the optic fissure.
Previous experiments have shown that CRYPα is expressed at very early stages on retinal axons and growth cones (Stoker et al. 1995a; Ledig et al. 1999). The finding that a currently unknown ligand is present on basal membranes of the eye, optic nerve, optic tract, and tectum, pointed to an important role of this receptor and its ligand in intraretinal axon growth and during growth of retinal axons towards their target, the tectum opticum (Haj et al. 1999). Blocking the CRYPα-ligand interaction from both the receptor (IG2 antibodies) and the ligand side (α1-AP) induced dramatic changes in growth cone morphology and retinal axon length on the in vivo-like BM substrate. No effects on growth cone morphology and only weak effects on outgrowth were observed on a laminin substratum, on the physiological ECM substratum matrigel and on detergent-washed, endfeet-free basal membranes. We also observed no changes in outgrowth or growth cone morphology when we used Ig3-AP, a truncated form of CRYPα with no ligand binding capacity (Ledig, M., unpublished work; Haj et al. 1999). Taken together with the α1-AP staining pattern on BM we suggest that the elusive CRYPα ligand is found predominantly on the surface of endfeet of Müller glia cells. The ligand itself exerts growth promoting and growth cone lamellipodia-stimulating activities.
We have demonstrated that the glial endfeet of the retinal BM contain a balance of positive and negative cues. For example, removal of the glial endfeet leads to an enormous increase in axon outgrowth on BMs, whereas only on intact BMs do antibodies to CRYPα and the CRYPα1-AP fusion protein induce dramatic reductions in outgrowth. Previous data have demonstrated that the net outcome of these factors is indeed permissive for retinal axon growth (Stier and Schlosshauer 1999). In the presence of these endfeet, the disturbance of CRYPα-ligand interactions causes a strong decrease of axon outgrowth. This suggests that during normal, intraretinal axon outgrowth, the growth-facilitating or -promoting role of CRYPα is in constant balance with negative influences on the growth cone. Therefore, this work places the RPTPs in a critical position in the regulation of intra-growth cone phosphotyrosine levels and maintenance of axon outgrowth (Cummings 1999).
Glial endfeet are present on retinal basal membranes (Bauch et al. 1998), but their molecular components are not completely characterized. The laminin/nidogen complex is present in the BM (Cohen et al. 1987). It was recently shown that this complex binds in vitro to the fifth fibronectin type III domain of the long isoform of LAR (O'Grady et al. 1998), an RPTP structurally related to CRYPα2. However, this isoform of LAR is not expressed in the nervous system. We would not expect a similar interaction of CRYPα1 with laminin, as in vitro binding assays failed to detect an interaction of CRYPα1 or CRYPα2 with laminin 1 or 2 (Haj et al. 1999; McKinnell, I.W., and A.W. Stoker, unpublished work). In the same in vitro assays, matrigel did not bind to CRYPα. Recent work also suggests that CRYPα does not interact homophilically (Haj et al. 1999). These data underline our idea that the CRYPα ligand is a currently unknown transmembrane, membrane-anchored, or membrane-associated molecule at the surface of the endfeet of Mueller glia cells (Fig. 10).
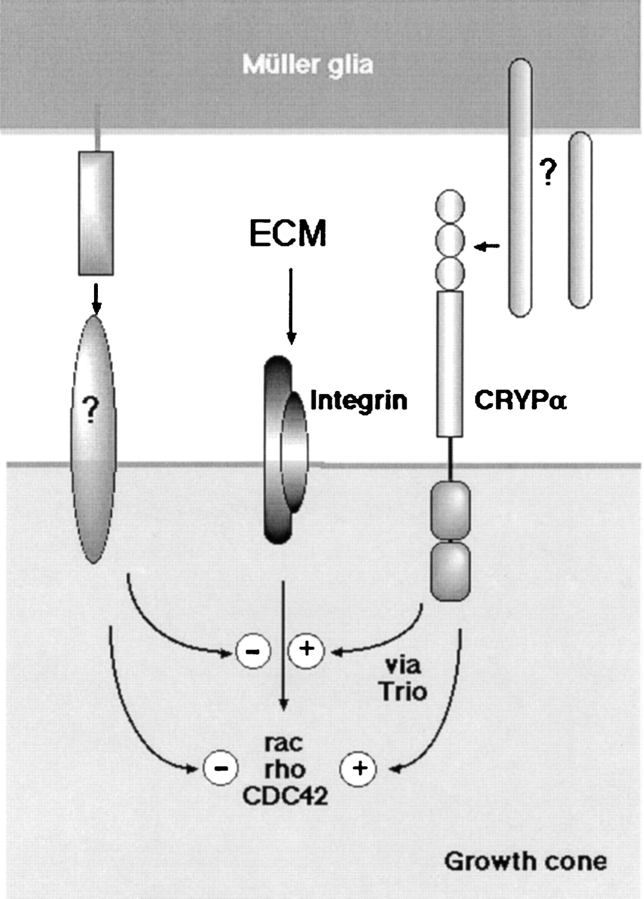
Figure 10.
Our data indicate that there is a yet unknown ligand located on or associated with glial endfeet of retinal Müller glia cells. Signaling into the growth cone is mediated via the CRYPα1 receptor. These signals are important for outgrowth and the actin cytoskeleton. According to the work from other groups, it is likely that downstream signaling is mediated via the Trio protein, which is capable of activating rac and rho. The integrin receptor mediates the laminin signal from the ECM into the growth cone. It has been previously shown that integrin downstream activates Rac and Cdc42. So both receptors mediate signals, that are important for axon outgrowth and growth cone morphology. The CRYPα1-ligand interaction is growth-promoting and could balance a negative interaction between the growth cone and Müller glia cells. The glial endfeet, therefore, appear to have a strict growth controlling function containing a balance of growth promoting and inhibiting factors on their surfaces.
On BM-Ef, neurite growth signals are mainly integrated via β1 integrin receptors (Sakaguchi and Radke 1996). Blocking the β1-subunits of the integrin receptors results in an enormous reduction of outgrowth. Due to the difficulties in isolating a native, ligand-negative basal membrane, we chose matrigel as a physiological control substrate that lacks detectable α1-AP binding activity. The results obtained on matrigel are largely the same than on BM-Ef, except that outgrowth on matrigel is less affected by blocking the integrin receptor. This suggests, unsurprisingly, that matrigel matrix contains additional growth-promoting components aside from laminin and the putative CRYPα ligand.
Blocking the interaction of CRYPα with its ligand as well as the integrin receptor resulted in a dose-dependent loss of growth cone lamellipodia and in a transition towards a more filopodial morphology. Although this could relate to the reduced growth rate in both treatments, this does not imply that a filopodial morphology is always disadvantageous for growth cone migration (e.g., Bovolenta and Mason 1987). The data also indicate that both integrins and CRYPα can both influence lamellipodial dynamics, either independently or synergistically. What could be the link between CRYPα and lamellipodia?
Well-known molecular regulators of lamellipodia and filopodia in fibroblasts are the small GTP-binding proteins of the Rho family, Rac and Cdc42, respectively (Nobes and Hall 1995, Nobes and Hall 1999; Hall 1998). Available evidence suggests that they exert similar functions in neuronal growth cones (reviewed in Mueller 1999). Control of the growth cone actin cytoskeleton by RPTPs could be achieved by interacting adapter proteins, like Trio. Trio binds to the intracellular domain of the CRYPα family member, LAR, and has two guanine exchange factor domains specific for Rac and Rho (Debant et al. 1996; Bellanger et al. 1998). Furthermore, the Caenorhabditis elegans Trio homologue, UNC-73, was shown to be required for axon growth and guidance (Steven at al., 1998). The phenotypic similarities of null mutants for DLAR and the Drosophila Rac suggest their interaction in guidance of intersegmental nerve b (Kaufmann et al. 1998). Further evidence for an important function of RPTPs in controlling the actin cytoskeleton comes from two recent papers showing that profilin is regulated by DLAR involving the cytoplasmic tyrosine kinase abl and its substrate enabled (Wills et al. 1999a,Wills et al. 1999b). Trio could also provide a connection between DLAR and profilin via Rho and phosphatidylinositol 4-phosphate 5-kinase (Chong et al. 1994).
From our data that blocking of CRYPα-ligand interactions on BM leads to changes in the actin cytoskeleton and to lamellipodia formation, it seems most likely that the CRYPα downstream signaling pathway also involves Rac. This may be related to a similar process as shown for integrins (Keely et al. 1997; Clark et al. 1998; Kuhn et al. 1998) (Fig. 10). There is likely to be considerable cross-talk between RPTPs and other extracellular receptors. This is especially important for recognition of multiligand complexes consisting of cell adhesion molecules and other growth cone guidance activities (Holland et al. 1998). The downstream signaling pathways of both CRYPa and integrin receptors seem to converge as well. There are several examples for an interplay of RPTPs and integrins (Shenoi et al. 1999; Su et al. 1999), and it is quite likely that the same is true for CRYPα. The LAR-interacting protein (LIP) localizes type II RPTPs to focal adhesions (Serra-Pages et al. 1995, Serra-Pages et al. 1998; Pulido et al. 1995), and, therefore, brings them into the close neighborhood of integrins. An interplay between RPTPs and integrin could then happen on the level of the small GTPases and Src-family of cytoplasmic kinases (Schlaepfer and Hunter 1998), both of which are shared in downstream pathways. This could result in the joint control of adhesive and deadhesive properties of cells or growth cones (Helmke et al. 1998). Such a relationship has been shown, for example, with the T and B cell RPTP CD45 and integrins (Roach et al. 1997; Shenoi et al. 1999).
Based on the data we have presented, we can formulate a basic model for some of the signaling processes downstream of CRYPα and integrins in retinal growth cones (Fig. 10). It is clear that laminin-integrin interactions alone can stimulate growth cone migration and lamellipodia formation in the absence of other incoming signals (Tomaselli et al. 1986). On a complete BM, however, we suggest there is a positive, CRYPα-dependent signal and a balancing negative signal(s) coming from an independent ligand-receptor complex. The latter signal could partly suppress integrin-promoted outgrowth and lamellipodia formation, by downregulating, directly or indirectly, the Rac pathway. This is counteracted by the positive CRYPα signal. Thus, loss of CRYPα signal in our experiments induces a significant block in neurite outgrowth. The modest influence of the IG2 antibody on a pure laminin substrate could be partly explained by a low level of activity in both the CRYPα and negative signals, in the absence of their cognate ligands. CRYPα antibodies would then cause a only slight suppression of integrin-mediated outgrowth, without grossly altering lamellipodia. This model needs to be tested further, but possible candidates for the negative signals would include nongraded Eph receptors, and their ephrin ligand, which are known to be present on glial endfeet (Braisted et al. 1997). Another interesting possibility could be that there are some cis-interactions between integrins and CRYPα on the growth cone.
PTPμ, another membrane tyrosine phosphatase, was recently shown to mediate homophilic trans-interactions (Gebbink et al. 1995; Zondag et al. 1995), and to be involved in retinal axon growth (Burden-Gulley and Brady-Kalnay 1999). PTPμ interacts with three different, calcium-dependent cell adhesion molecules, N-, R-, and E-cadherin (Brady-Kalnay et al. 1998; Burden-Gulley and Brady-Kalnay 1999). It was shown that N- and R-cadherin promote outgrowth of neurites (e.g., Bixby and Zhang 1990; Redies and Takeichi 1993). Used as a substrate, PTPμ stimulated neurite outgrowth of E8 chick RGC neurites and on an N-cadherin substrate; downregulation of this phosphatase resulted in a significant decrease of the retinal neurite length, suggesting that the phosphatase activity of PTPμ is important for growth of RGC neurites on N-cadherin (Burden-Gulley and Brady-Kalnay 1999).
RPTPβ/PTPζ is another outgrowth-inducing RPTP (Peles et al. 1995; Sakurai et al. 1997). It binds to other members of cell surface recognition complexes, among them contactin/F11 (Brümmendorf et al. 1998) and Nr-CAM, enabling signal transduction into the growth cone (Peles et al. 1998). But the relevance of this RPTP for the development of the retinotectal system remains to be elucidated.
During formation of the retinotectal map, there now appear to be, besides the integrins and N-cadherin, at least two different RPTPs involved in outgrowth of retinal ganglion cell axons: CRYPα and its yet undefined ligand, and PTPμ. Our results suggest that besides the RTKs (Drescher et al. 1997; Mueller et al. 1996), RPTPs such as CRYPα play an extremely important, complementary role in promoting formation of the visual system. It remains to be seen if gene deletion of RPTPs such as the mammalian orthologue of CRYPα, RPTPσ (Elchebly et al. 1999; Wallace et al. 1999), results in disturbance of the retinotectal projection.
Acknowledgments
We thank F. Bonhoeffer for supporting the work and for critical comments on the manuscript. We owe many thanks to P. Macchi and S. Wahl for critically reading the manuscript.
B.K. Mueller was supported by the DFG (Sönderforschungsbereich 430). The work of M. Ledig was supported by the DFG Graduiertenkolleg Neurobiologie (GKN) Tübingen and by a short-term fellowship from the Federal European Biochemical Society (FEBS). A.W. Stoker was funded by The Royal Society (UK) and by the Wellcome Trust (grant 046188). J.L. Bixby was funded by the National Institutes of Health and the National Science Foundation.
Footnotes
1.used in this paper: AP, alkaline phosphatase; BM, basal membrane, BM-Ef, basal membranes with endfeet removed; ECM, extracellular matrix; LN, laminin; PLL, poly-l-lysine; RPTP, receptor protein tyrosine phosphatase
Dr. Haj's present address is Department of Surgery, Beth Israel Deaconess Medical Center and Harvard Medical School, 330 Brookline Avenue, Boston, MA 02215.
References
- Bastmeyer M., Ott H., Leppert C.A., Stuermer C.A. Fish E587 glycoprotein, a member of the L1 family of cell adhesion molecules, participates in axonal fasciculation and the age-related order of ganglion cell axons in the goldfish retina. J. Cell Biol. 1995;130:969–976. doi: 10.1083/jcb.130.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch H., Stier H., Schlosshauer B. Axonal versus dendritic outgrowth is differentially affected by radial glia in discrete layers of the retina. J. Neurosci. 1998;18:1774–1785. doi: 10.1523/JNEUROSCI.18-05-01774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger J.M., Lazaro J.B., Diriong S., Fernandez A., Lamb N., Debant A. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene. 1998;16:147–152. doi: 10.1038/sj.onc.1201532. [DOI] [PubMed] [Google Scholar]
- Bixby J.L., Zhang R.J. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J. Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J.L., Reichardt L.F. Effects of antibodies to N-CAM on the differentiation of neuromuscular contacts between ciliary ganglion neurons and myotubes in vitro. Dev. Biol. 1987;119:363–372. doi: 10.1016/0012-1606(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Bixby J.L., Pratt R., Lilien J., Reichardt L.F. Neurite outgrowth on muscle cell surfaces involves extracellular matrix receptors as well as Ca2+-dependent and -independent cell adhesion molecules. Proc. Natl. Acad. Sci. USA. 1987;84:2555–2559. doi: 10.1073/pnas.84.8.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodden K., Bixby J.L. CRYP-2A receptor-type tyrosine phosphatase selectively expressed by developing vertebrate neurons. J. Neurobiol. 1996;31:309–324. doi: 10.1002/(SICI)1097-4695(199611)31:3<309::AID-NEU4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bovolenta P., Mason C. Growth cone morphology varies with position in the developing mouse visual pathway from retina to first targets. J. Neurosci. 1987;7:1447–1460. doi: 10.1523/JNEUROSCI.07-05-01447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay S.M., Mourton T., Nixon J.P., Pietz G.E., Kinch M., Chen H., Brackenbury R., Rimm D.L., Del Vecchio R.L., Tonks N.K. Dynamic interaction of PTPμ with multiple cadherins in vivo. J. Cell Biol. 1998;141:287–296. doi: 10.1083/jcb.141.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay S.M., Tonks N.K. Protein tyrosine phosphatases as adhesion receptors. Curr. Opin. Cell Biol. 1995;7:650–657. doi: 10.1016/0955-0674(95)80106-5. [DOI] [PubMed] [Google Scholar]
- Braisted J.E., McLaughlin T., Wang H.U., Friedman G.C., Anderson D.J., O'Leary D.D.M. Graded and lamina-specific distributions of ligands of EphB receptor tyrosine kinases in the developing retinotectal system. Dev. Biol. 1997;191:14–28. doi: 10.1006/dbio.1997.8706. [DOI] [PubMed] [Google Scholar]
- Brittis P.A., Silver J., Walsh F.S., Doherty P. Fibroblast growth factor receptor function is required for the orderly projection of ganglion cell axons in the developing mammalian retina. Mol. Cell. Neurosci. 1996;8:120–128. doi: 10.1006/mcne.1996.0051. [DOI] [PubMed] [Google Scholar]
- Brittis P.A., Lemmon V., Rutishauser U., Silver J. Unique changes of ganglion cell growth cone behavior following cell adhesion molecule perturbationsa time-lapse study of the living retina. Mol. Cell. Neurosci. 1995;6:433–449. doi: 10.1006/mcne.1995.1032. [DOI] [PubMed] [Google Scholar]
- Brittis P.A., Silver J. Multiple factors govern intraretinal axon guidancea time-lapse study. Mol. Cell. Neurosci. 1995;6:413–432. doi: 10.1006/mcne.1995.1031. [DOI] [PubMed] [Google Scholar]
- Brittis P.A., Canning D.R., Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T., Kenwrick S., Rathjen F.G. Neural cell recognition molecule L1from cell biology to human hereditary brain malformations. Curr. Opin. Neurobiol. 1998;8:87–97. doi: 10.1016/s0959-4388(98)80012-3. [DOI] [PubMed] [Google Scholar]
- Burden-Gulley S.M., Brady-Kalnay S.M. PTPμ regulates N-cadherin-dependent neurite outgrowth. J. Cell Biol. 1999;144:1323–1336. doi: 10.1083/jcb.144.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.J., Nakamoto M., Bergemann A.D., Flanagan J.G. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- Chien C.B. PY in the fly receptor-like tyrosine phosphatases in axonal pathfinding. Neuron. 1996;16:1065–1068. doi: 10.1016/s0896-6273(00)80131-2. [DOI] [PubMed] [Google Scholar]
- Chong L.D., Traynor-Kaplan A., Bokoch G.M., Schwartz M.A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Clark E.A., King W.G., Brugge J.S., Symons M., Hynes R.O. Integrin-mediated signals regulated by members of the rho family of GTPases. J. Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Burne J.F., McKinlay C., Winter J. The role of laminin and the laminin/fibronectin receptor complex in the outgrowth of retinal ganglion cell axons. Dev. Biol. 1987;122:407–418. doi: 10.1016/0012-1606(87)90305-8. [DOI] [PubMed] [Google Scholar]
- Cummings F.W. Spatial patterning via PTP adhesive phosphatases. J. Theor. Biol. 1999;196:19–26. doi: 10.1006/jtbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- Debant A., Serra-Pages C., Seipel K., O'Brien S., Tang M., Park S.H., Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc. Natl. Acad. Sci. USA. 1996;93:5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner M.S., Kennedy T.E., Fazeli A., Serafini T., Tessier-Lavigne M., Sretavan D.W. Netrin-1 and DCC mediate axon guidance locally at the optic discloss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Desai C.J., Sun Q., Zinn K. Tyrosine phosphorylation and axon guidanceof mice and flies Curr. Opin. Neurobiol. 7 1997. 70 74a [DOI] [PubMed] [Google Scholar]
- Desai C.J., Krueger N.X., Saito H., Zinn K. Competition and cooperation among receptor tyrosine phosphatases control motoneuron growth cone guidance in Drosophila Development. 124 1997. 1941 1952b [DOI] [PubMed] [Google Scholar]
- Desai C.J., Gindhart J.G., Jr., Goldstein L.S., Zinn K. Receptor tyrosine phosphatases are required for motor axon guidance in the Drosophila embryo. Cell. 1996;84:599–609. doi: 10.1016/s0092-8674(00)81035-1. [DOI] [PubMed] [Google Scholar]
- Drescher U., Bonhoeffer F., Mueller B.K. The Eph family in retinal axon guidance. Curr. Opin. Neurobiol. 1997;7:75–80. doi: 10.1016/s0959-4388(97)80123-7. [DOI] [PubMed] [Google Scholar]
- Easter S.S., Jr, Bratton B., Scherer S.S. Growth-related order of the retinal fiber layer in goldfish. J. Neurosci. 1984;4:2173–2190. doi: 10.1523/JNEUROSCI.04-08-02173.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M., Wagner J., Kennedy T.E., Lanctot C., Michaliszyn E., Itie A., Drouin J., Tremblay M.L. Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase sigma. Nat. Genet. 1999;21:330–333. doi: 10.1038/6859. [DOI] [PubMed] [Google Scholar]
- Faissner A. The tenascin gene family in axon growth and guidance. Cell Tissue Res. 1997;290:331–341. doi: 10.1007/s004410050938. [DOI] [PubMed] [Google Scholar]
- Gebbink M.F., Zondag G.C., Koningstein G.M., Feiken E., Wubbolts R.W., Moolenaar W.H. Cell surface expression of receptor protein tyrosine phosphatase RPTP mu is regulated by cell-cell contact. J. Cell Biol. 1995;131:251–260. doi: 10.1083/jcb.131.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink M.F., van Etten I., Hateboer G., Suijkerbuijk R., Beijersbergen R.L., Geurts van Kessel A., Moolenaar W.H. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991;290:123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- Gershon T.R., Baker M.W., Nitabach M., Macagno E.R. The leech receptor protein tyrosine phosphatase HmLAR2 is concentrated in growth cones and is involved in process outgrowth. Development. 1998;125:1183–1190. doi: 10.1242/dev.125.7.1183. [DOI] [PubMed] [Google Scholar]
- Giordano S., Laessing U., Ankerhold R., Lottspeich F., Stuermer C.A. Molecular characterization of E587 antigenan axonal recognition molecule expressed in the goldfish central nervous system. J. Comp. Neurol. 1997;377:286–297. doi: 10.1002/(sici)1096-9861(19970113)377:2<286::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Greve J.M., Gottlieb D.I. Monoclonal antibodies which alter the morphology of cultured chick myogenic cells. J. Cell. Biochem. 1982;18:221–229. doi: 10.1002/jcb.1982.240180209. [DOI] [PubMed] [Google Scholar]
- Halfter W. Disruption of the retinal basal lamina during early embryonic development leads to a retraction of vitreal end feet, an increased number of ganglion cells, and aberrant axonal outgrowth. J. Comp. Neurol. 1998;397:89–104. doi: 10.1002/(sici)1096-9861(19980720)397:1<89::aid-cne7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Halfter W. The behavior of optic axons on substrate gradients of retinal basal lamina proteins and merosin. J. Neurosci. 1996;16:4389–4401. doi: 10.1523/JNEUROSCI.16-14-04389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W., Boxberg Y. Axonal growth on solubilized and reconstituted matrix from the embryonic chicken retina inner limiting membrane. Eur. J. Neurosci. 1992;4:840–852. doi: 10.1111/j.1460-9568.1992.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Halfter W., Reckhaus W., Kröger S. Nondirected axonal growth on basal lamina from avian embryonic neural retina. J. Neurosci. 1987;7:3712–3722. doi: 10.1523/JNEUROSCI.07-11-03712.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the actin cytoskeleton. Science. 1998;279:509–514. [Google Scholar]
- Haj F., McKinnell I.W., Stoker A.W. Retinotectal ligands for the receptor tyrosine phosphatase CRYalpha. Mol. Cell. Neurosci. 1999;14:225–240. doi: 10.1006/mcne.1999.0785. [DOI] [PubMed] [Google Scholar]
- Helmke S., Lohse K., Mikule K., Wood M.R., Pfenninger K.H. SRC binding to the cytoskeleton, triggered by growth cone attachment to laminin, is protein tyrosine phosphatase-dependent. J. Cell Sci. 1998;111:2465–2475. doi: 10.1242/jcs.111.16.2465. [DOI] [PubMed] [Google Scholar]
- Holland S.J., Peles E., Pawson T., Schlessinger J. Cell-contact-dependent signaling in axon growth and guidanceEph receptor tyrosine kinases and receptor protein tyrosine phosphatase beta. Curr. Opin. Neurobiol. 1998;8:117–127. doi: 10.1016/s0959-4388(98)80015-9. [DOI] [PubMed] [Google Scholar]
- Kaufmann N., Wills Z.P., Van Vactor D. Drosophila Rac1 controls motor axon guidance. Development. 1998;125:453–461. doi: 10.1242/dev.125.3.453. [DOI] [PubMed] [Google Scholar]
- Keely P.J., Westwick J.K., Whitehead I.P., Der C.J., Parise L.V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Kroeger, S. 1989. Schichtenbildung im Tektum opticum des Hühnerembryos am Beispiel der Trennung vom retino-tectaler und tecto-bulbärer Projektion. Dissertation Universität Tübingen.
- Krueger N.X., Van Vactor D., Wan H.I., Gelbart M.W., Goodman C.S., Saito H. The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila . Cell. 1996;84:611–622. doi: 10.1016/s0092-8674(00)81036-3. [DOI] [PubMed] [Google Scholar]
- Kuhn T.B., Brown M.D., Bamburg J.R. Rac1-dependent actin filament organization in growth cones is necessary for beta1-integrin-mediated advance but not for growth on poly-D-lysine. J. Neurobiol. 1998;37:524–540. doi: 10.1002/(sici)1097-4695(199812)37:4<524::aid-neu3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ledig M.M., McKinnell I.W., Mrsic-Flogel T., Wang J., Alvares C., Mason I., Bixby J.L., Mueller B.K., Stoker A.W. Expression of receptor tyrosine phosphatases during development of the retinotectal projection of the chick. J. Neurobiol. 1999;39:81–96. doi: 10.1002/(sici)1097-4695(199904)39:1<81::aid-neu7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Leppert C.A., Diekmann H., Paul C., Laessing U., Marx M., Bastmeyer M., Stuermer C.A. Neurolin Ig domain 2 participates in retinal axon guidance and Ig domains 1 and 3 in fasciculation. J. Cell Biol. 1999;25:339–349. doi: 10.1083/jcb.144.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane S., Cornel E., Amaya E., Holt C.E. Inhibition of FGF receptor activity in retinal ganglion cell axons causes errors in target recognition. Neuron. 1996;17:245–254. doi: 10.1016/s0896-6273(00)80156-7. [DOI] [PubMed] [Google Scholar]
- Mey J., Thanos S. Development of the visual system of the chick--a review. J. Hirnforsch. 1992;33:673–702. [PubMed] [Google Scholar]
- Mizuno K., Hasegawa K., Katagiri T., Ogimoto M., Ichikawa T., Yakura H. MPTPδ, a putative murine homologue of HPTPδ, is expressed in specialized regions of the brain and in the B cell lineage. Mol. Cell. Biol. 1993;13:5513–5523. doi: 10.1128/mcb.13.9.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B.K. Growth cone guidancefirst steps towards a deeper understanding. Annu. Rev. Neurosci. 1999;22:351–388. doi: 10.1146/annurev.neuro.22.1.351. [DOI] [PubMed] [Google Scholar]
- Mueller B.K., Bonhoeffer F., Drescher U. Novel gene families involved in neural pathfinding. Curr. Opin. Genet. Dev. 1996;6:469–474. doi: 10.1016/s0959-437x(96)80069-4. [DOI] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho, rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- O'Grady P., Thai T.C., Saito H. The laminin-nidogen complex is a ligand for a specific splice isoform of the transmembrane protein tyrosine phosphatase LAR. J. Cell Biol. 1998;141:1675–1684. doi: 10.1083/jcb.141.7.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott H., Bastmeyer M., Stuermer C.A. Neurolin, the goldfish homologue of DM-GRASP, is involved in retinal axon pathfinding to the optic disk. J. Neurosci. 1998;18:3363–3372. doi: 10.1523/JNEUROSCI.18-09-03363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E., Schlessinger J., Grumet M. Multi-ligand interactions with receptor-like protein tyrosine phosphatase betaimplications for intercellular signaling. Trends Biochem. Sci. 1998;23:121–124. doi: 10.1016/s0968-0004(98)01195-5. [DOI] [PubMed] [Google Scholar]
- Peles E., Nativ M., Campbell P.L., Sakurai T., Martinez R., Lev S., Clary D.O., Schilling J., Barnea G., Plowman G.D. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Pulido R., Serra-Pages C., Tang M., Streuli M. The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatasesmultiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc. Natl. Acad. Sci. USA. 1995;92:11686–11690. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager G.H. Development of the retinotectal project in the chicken. Adv. Anat. Embryol. Cell Biol. 1980;63:1–92. [PubMed] [Google Scholar]
- Redies C., Takeichi M. N- and R-cadherin expression in the optic nerve of the chicken embryo. Glia. 1993;8:161–171. doi: 10.1002/glia.440080304. [DOI] [PubMed] [Google Scholar]
- Roach T., Slater S., Koval M., White L., McFarland E.C., Okumura M., Thomas M.L., Brown E.J. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr. Biol. 1997;7:408–417. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi D.S., Radke K. Beta 1 integrins regulate axon outgrowth and glial cell spreading on a glial-derived extracellular matrix during development and regeneration. Brain Res. Dev. Brain Res. 1996;97:235–250. doi: 10.1016/s0165-3806(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Lustig M., Nativ M., Hemperly J.J., Schlessinger J., Peles E., Grumet M. Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase beta. J. Cell Biol. 1997;136:907–918. doi: 10.1083/jcb.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaapveld R.Q., Schepens J.T., Bachner D., Attema J., Wieringa B., Jap P.H., Hendriks W.J. Developmental expression of the cell adhesion molecule-like protein tyrosine phosphatases LAR, RPTPδ and RPTPσ in the mouse. Mech. Dev. 1998;77:59–62. doi: 10.1016/s0925-4773(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hunter T. Integrin signaling and tyrosine phosphorylationjust the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Serra-Pages C., Medley Q.G., Tang M., Hart A., Streuli M. Liprins, a family of LAR transmembrane protein-tyrosine phosphatase-interacting proteins. J. Biol. Chem. 1998;273:15611–15620. doi: 10.1074/jbc.273.25.15611. [DOI] [PubMed] [Google Scholar]
- Serra-Pages C., Kedersha N.L., Fazikas L., Medley Q., Debant A., Streuli M. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:2827–2838. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoi H., Seavitt J., Zheleznyak A., Thomas M.L., Brown E.J. Regulation of integrin-mediated T cell adhesion by the transmembrane protein tyrosine phosphatase CD45. J. Immunol. 1999;162:7120–7127. [PubMed] [Google Scholar]
- Silver J., Rutishauser U. Guidance of optic axons in vivo by a preformed adhesive pathway on neuroepithelial endfeet. Dev. Biol. 1984;106:485–499. doi: 10.1016/0012-1606(84)90248-3. [DOI] [PubMed] [Google Scholar]
- Steven R., Kubiseski T.J., Zheng H., Kulkarni S., Mancillas J., Ruiz Morales A., Hogue C.W., Pawson T., Culotti J. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans . Cell. 1998;92:785–795. doi: 10.1016/s0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- Stier H., Schlosshauer B. Cross-species collapse activity of polarized radial glia on retinal ganglion cell axons. Glia. 1999;25:143–153. doi: 10.1002/(sici)1098-1136(19990115)25:2<143::aid-glia5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Stoker A.W., Dutta R. Protein tyrosine phosphatases and neural development. Bioessays. 1998;20:463–472. doi: 10.1002/(SICI)1521-1878(199806)20:6<463::AID-BIES4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Stoker A.W., Gehrig B., Haj F., Bay B.-H. Axonal localisation of the CAM-like tyrosine phosphatase CRYPαa signaling molecule of embryonic growth cones Development. 121 1995. 1833 1844a [DOI] [PubMed] [Google Scholar]
- Stoker A.W., Gehrig B., Newton M.R., Bay B.-H. Comparative localisation of CRYPa, a CAM-like tyrosine phosphatase, and NgCAM in the developing chick visual system Dev. Brain Res. 90 1995. 129 140b [DOI] [PubMed] [Google Scholar]
- Stoker A.W. Isoforms of a novel cell adhesion molecule-like protein tyrosine phosphatase are implicated in neural development. Mech. Dev. 1994;46:201–217. doi: 10.1016/0925-4773(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Su J., Muranjan M., Sap J. Receptor protein tyrosine phosphatase alpha activates src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 1999;20:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- Tomaselli K.J., Reichardt L.F., Bixby J.L. Distinct molecular interactions mediate neuronal process outgrowth on non-neuronal cell surfaces and extracellular matrices. J. Cell Biol. 1986;103:2659–2672. doi: 10.1083/jcb.103.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetani N., Asano M., Mizuno K., Yakura H., Iwakura Y. Targeted disruption of the murine protein tyrosine phosphatase delta (MPTPd) results in growth retardation and behavioral abnormalities. In: Yakura H., editor. Kinases and Phosphatases in Lymphocyte and Neuronal Signaling. Springer-Verlag; Berlin: 1997. pp. 313–314. [Google Scholar]
- van Vactor D. Protein tyrosine phosphatases in the developing nervous system. Curr. Opin. Cell Biol. 1998;10:174–181. doi: 10.1016/s0955-0674(98)80139-7. [DOI] [PubMed] [Google Scholar]
- Viollet C., Doherty P. CAMs and the FGF receptoran interacting role in axonal growth. Cell Tissue Res. 1997;290:451–455. doi: 10.1007/s004410050952. [DOI] [PubMed] [Google Scholar]
- Wallace M.J., Batt J., Fladd C.A., Henderson J.T., Skarnes W.C., Rotin D. Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPσ. Nat. Genet. 1999;21:334–338. doi: 10.1038/6866. [DOI] [PubMed] [Google Scholar]
- Walter J., Kern-Veits B., Huf J., Stolze B., Bonhoeffer F. Recognition of position-specific properties of tectal cell membranes by retinal axons in vitro. Development. 1987;101:685–996. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- Wills Z., Marr L., Zinn K., Goodman C.S., Van Vactor D. Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo Neuron 22 1999. 291 299a [DOI] [PubMed] [Google Scholar]
- Wills Z., Bateman J., Korey C.A., Comer A., Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance Neuron. 22 1999. 301 312b [DOI] [PubMed] [Google Scholar]
- Zondag G.C., Koningstein G.M., Jiang Y.P., Sap J., Moolenaar W.H., Gebbink M.F. Homophilic interactions mediated by receptor tyrosine phosphatases mu and kappa. A critical role for the novel extracellular MAM domain. J. Biol. Chem. 1995;270:14247–14250. doi: 10.1074/jbc.270.24.14247. [DOI] [PubMed] [Google Scholar]