Abstract
We have prepared antibodies specific for HSET, the human homologue of the KAR3 family of minus end-directed motors. Immuno-EM with these antibodies indicates that HSET frequently localizes between microtubules within the mammalian metaphase spindle consistent with a microtubule cross-linking function. Microinjection experiments show that HSET activity is essential for meiotic spindle organization in murine oocytes and taxol-induced aster assembly in cultured cells. However, inhibition of HSET did not affect mitotic spindle architecture or function in cultured cells, indicating that centrosomes mask the role of HSET during mitosis. We also show that (acentrosomal) microtubule asters fail to assemble in vitro without HSET activity, but simultaneous inhibition of HSET and Eg5, a plus end-directed motor, redresses the balance of forces acting on microtubules and restores aster organization. In vivo, centrosomes fail to separate and monopolar spindles assemble without Eg5 activity. Simultaneous inhibition of HSET and Eg5 restores centrosome separation and, in some cases, bipolar spindle formation. Thus, through microtubule cross-linking and oppositely oriented motor activity, HSET and Eg5 participate in spindle assembly and promote spindle bipolarity, although the activity of HSET is not essential for spindle assembly and function in cultured cells because of centrosomes.
Keywords: mitotic spindle, HSET, Eg5, kinesin, microtubule
Precise segregation of genetic material into daughter cells during cell division is vital to ensure viability of future cellular generations. This process is carried out by the mitotic spindle, a highly organized and dynamic microtubule array whose assembly and disassembly is spatially and temporally regulated during the cell cycle (McIntosh and Koonce 1989; Mitchison 1989; Rieder 1991). Microtubules within the spindle lattice have a defined order that is determined by many factors, including their localized nucleation by centrosomes, the actions of several microtubule associated proteins, and their capture and stabilization by chromosomes (Kirschner and Mitchison 1986; Inoue and Salmon, 1995; Hyman and Karsenti 1996, Hyman and Karsenti 1998; Nicklas 1997; Waters and Salmon 1997; Rieder and Salmon 1998). Overall, the mitotic spindle is a symmetrical and fusiform structure. Its constituent microtubules are oriented with their minus ends focused at the spindle poles and the plus ends extending outwards, either towards the cell cortex or to the equator of the spindle. Defining how this dynamic protein super assembly is constructed and how it conducts the complex task of chromosome separation will require both the identification of its various components and determination of their specific functions.
To further understand mitotic spindle structure and function, we have focused on microtubule organization at mitotic spindle poles (Compton 1998). In somatic cells, centrosomes act as the dominant site for microtubule nucleation. Duplication of centrosomes occurs in a cell cycle regulated manner, and both the duplication and separation of the centrosomes is essential for establishing two spindle poles and generating a bipolar mitotic spindle (McIntosh 1983; Mazia 1984; Sluder and Rieder 1985; Maniotis and Schliwa 1991; Zhang and Nicklas 1995). However, recent experiments have shown that focusing of microtubule minus ends at spindle poles involves noncentrosomal factors in addition to centrosomes. This fact is borne out by several experimental observations, including electron microscopic analysis illustrating that many spindle microtubules are not anchored to centrosomes (Rieder 1981; Nicklas et al. 1982; Wolf and Bastmeyer 1991; McDonald et al. 1992; Mastronarde et al. 1993), the observation that some cell types assemble spindles in the absence of conventional centrosomes (Szollosi et al. 1972; Brenner et al. 1977; Keyer et al. 1984; Mazia 1984; Bastmeyer et al. 1986; Steffen et al. 1986; Theurkauf and Hawley 1992; Rieder et al. 1993; Schatten 1994; Debec et al. 1995; McKim and Hawley 1995; Vernos and Karsenti 1995; de Saint Phalle and Sullivan 1998), and that microtubule organization at spindle poles requires several noncentrosomal structural and motor proteins (Verde et al. 1991; Gaglio et al. 1995, Gaglio et al. 1996, Gaglio et al. 1997; Heald et al. 1996, Heald et al. 1997; Matthies et al. 1996; Walczak et al., 1996, Walczak et al. 1998; Pallazzo et al. 1999).
A variety of microtubule motor proteins have been identified as spindle components required for the assembly and/or maintenance of spindle poles. For example, cytoplasmic dynein, a minus end-directed motor, is necessary to efficiently focus microtubule minus ends at spindle poles in a variety of animal systems (Vaisberg et al. 1993; Gaglio et al. 1996, Gaglio et al. 1997; Heald et al. 1996, Heald et al. 1997; Merdes et al. 1996; Pallazzo et al. 1999). The multiprotein activator of cytoplasmic dynein, dynactin, is also required for spindle pole organization in these systems and cytoplasmic dynein and dynactin appear to act together to both focus microtubule minus ends and to transport the structural protein NuMA to the site of the developing spindle pole (Echeverri et al. 1996; Gaglio et al. 1996; Merdes et al. 1996). In addition to the minus end-directed activity of cytoplasmic dynein, the plus end-directed kinesin-like protein, Eg5, has been shown to contribute to spindle pole organization (Sawin et al. 1992; Heck et al. 1993; Blangy et al. 1995; Gaglio et al. 1996; Wilson et al. 1997). Eg5 is a member of the BimC class of kinesin proteins and forms a homotetrameric, bipolar complex (Kashina et al. 1996). In the absence of Eg5 activity, microtubule minus ends are inefficiently focused, leading to broad spindle poles, and recent experiments have suggested that Eg5 contributes to spindle organization by cross-linking constituent microtubules (Sharp et al. 1999a). Thus, spindle pole organization is a complex problem involving multiple oppositely oriented motor activities. We have shown that the minus end-directed motor activity of cytoplasmic dynein acts antagonistically to the plus end-directed motor activity of Eg5 (Gaglio et al. 1996). To further complicate this process, we also reported that microtubule asters formed efficiently in a cell free system in the complete absence of both Eg5 and cytoplasmic dynein, leading us to the conclusion that a third motor activity was acting to drive aster formation in this system (Gaglio et al. 1996).
In this paper, we examine the role of the minus end-directed kinesin protein, HSET, in spindle assembly in animal cells and in focusing microtubule asters in HeLa cell mitotic extracts. HSET is the human homologue of the KAR3 family of minus end-directed kinesin-like motors (Sawin and Endow 1993; Ando et al. 1994; Barton and Goldstein 1996; Khan et al. 1997; Nakagawa et al. 1997; Hirokawa 1998). One of the best studied members of this family is nonclaret disjunctional (ncd)1 in Drosophila melanogaster. Ncd was first described as a mutation that resulted in chromosome nondisjunction during female meiosis and in early mitotic divisions (Sturtevant 1929; Lewis and Gencarella 1952; Davis 1969; Portin 1978; Nelson and Szauter 1992). Further investigation determined that this phenotype was the result of severely disordered spindles in these mutant flies. The ncd mutation leads to spindles with splayed poles that are frequently split into multiple distinct foci, and spurs of microtubules have been observed to project from the main body of these spindles (Kimble and Church 1983; Hatsumi and Endow 1992a,Hatsumi and Endow 1992b; Endow et al. 1994; Endow and Komma 1996, Endow and Komma 1997). This motor and its homologues are believed to contribute to both the overall structural integrity of the spindle and the efficiency of spindle formation by focusing microtubule minus ends (Matthies et al. 1996; Endow and Komma 1996, Endow and Komma 1997; Walczak et al. 1997), although the precise mechanism of action is unclear. Here we show that HSET localizes between microtubules in the metaphase spindle of human cells, consistent with a cross-linking function. In addition, we show that HSET is essential to establish cohesive poles in mouse meiotic spindles and to generate microtubule asters in vitro, but its role is masked by centrosomes in somatic cells. Finally, we show that the minus end-directed activity of HSET acts antagonistically to the plus end-directed activity of Eg5, both in vitro and in vivo. We propose that these two motor proteins, through cross-linking and oppositely oriented motor activity, generate a well-ordered framework of microtubule bundles within the spindle. This cross-linking activity is important for the overall structural stability of the spindle lattice, although the activity of HSET is dispensable for spindle assembly when centrosomes are present.
Materials and Methods
Cell Culture
The human HeLa cell line and the monkey CV1 cell line were maintained in DME containing 10% FCS, 2 mM glutamine, 100 iU/ml penicillin, and 0.1 mg/ml streptomycin. The human CF-PAC1 cell line was maintained in Iscoves modified DME containing 10% FCS, 2 mM glutamine, 100 iU/ml penicillin, and 0.1 mg/ml streptomycin. Cells were grown at 37°C in a humidified incubator with a 5% CO2 atmosphere.
Antibodies
The HSET-specific antibodies were prepared by immunizing rabbits with recombinant HSET protein expressed in bacteria. A 1365-bp EcoRI fragment from the HSET cDNA ps55 (Ando et al. 1994) was ligated into pGEX-5X-3 at the unique EcoRI site in the multicloning site. This construct results in the fusion of the open reading frames for GST and the COOH-terminal 377 amino acids of HSET. The orientation of the HSET sequence was verified by multiple combinatorial restriction digests and the construct transformed into Escherichia coli BL21 (Stratagene). Expression of the GST–HSET fusion protein was induced by addition of 1 mM IPTG to a liquid culture. Cells were harvested after 6 h, pelleted by centrifugation at 7,000 rpm at 4°C, resuspended in 10 ml PBS containing protease inhibitors (5 mg/ml chymostatin, leupeptin, antipain, pepstatin, and 100 mg/ml phenylmethylsulphonyl fluoride) and sonicated on ice. The lysed cells were then incubated on ice for 30 min with 1% Triton X-100 and the insoluble debris removed by centrifugation at 11,000 rpm for 15 min at 4°C. The soluble fraction was collected and passed over a column of packed glutathione Sepharose-4B (Pharmacia Biotechnology Inc.). The column was washed twice with PBS to remove any nonbound protein, after which the bound GST–HSET protein was eluted by three successive washes with 10 mM reduced glutathione in 50 mM Tris-HCl, pH 8.0. GST–HSET was further purified from the eluate by SDS-PAGE, where the GST–HSET containing band was excised from a polyacrylamide gel, electroeluted from the gel, dialyzed against water, lyophilized, and resuspended in PBS. This pure GST–HSET fraction was used to immunize two rabbits, which produced two similar HSET specific antibodies, HSET-1 and HSET-2.
The remaining antibodies used in these experiments were as follows. αCTP-2, raised against the COOH-terminal tail of XCTK2 (Walczak et al. 1997), was generously donated by Claire Walczak (University of Indiana, Bloomington, IN). NuMA was detected with the rabbit polyclonal antibody (Gaglio et al. 1995). Tubulin was detected using the mAb DM1α (Sigma Chemical Co.). Eg5 was detected using a rabbit polyclonal antibody raised against the central rod domain, expressed as clone M4F (Whitehead and Rattner 1998). Cytoplasmic dynein was detected using a mAb specific for IC74 intermediate chain (mAb 70.1; Steuer et al. 1991). Finally, γ-tubulin was detected using a mouse mAb (Sigma Chemical Co.).
Indirect Immunofluorescence
Indirect immunofluorescence microscopy was performed on cultured cells by immersion in microtubule stabilization buffer (MTSB; 4 M glycerol, 100 mM Pipes, pH 6.9, 1 mM EGTA, and 5 mM MgCl2) for 1 min at room temperature, extraction in MTSB + 0.5% Triton X-100 for 2 min, followed by MTSB for 2 min. Cells were then fixed in −20°C methanol for 10 min. Indirect immunofluorescence microscopy on mitotic asters assembled in the cell free mitotic extract was performed by dilution of 5 μl of the extract into 25 μl of KHM buffer (78 mM KCl, 50 mM Hepes, pH 7.0, 4 mM MgCl2, 2 mM EGTA, 1 mM DTT; Burke and Gerace 1986). The diluted sample was then spotted onto a poly-l-lysine–coated glass coverslip and fixed by immersion in −20°C methanol. Both the fixed cells and mitotic asters were rehydrated in TBS (10 mM Tris-HCl, pH 7.5, 150 mM NaCl) containing 1% albumin and all antibody incubations and washes were performed in TBS + 1% albumin. Each primary antibody was incubated on the coverslip for 30 min, followed by 5 min washes in TBS + 1% albumin, and the bound antibodies were detected using either fluorescein- or Texas red-conjugated species-specific secondary antibodies at dilutions of 1:500 (Vector Labs, Inc.). The DNA was detected using DAPI (4′,6-diamidino-2-phenylindole) at 0.4 μg/ml (Sigma Chemical Co.). After a final wash, the coverslips were mounted in Vectashield FITC-guard mounting medium (Vector Labs, Inc.) and observed on a Nikon Optiphot microscope equipped for epifluorescence.
Mouse oocytes were permeabilized, fixed, and processed for the immunocytochemical detection of spindle components as described previously (Simerly and Schatten 1993). Cells were labeled with a fluorescein-conjugated secondary antibody to identify the injected antibody, antitubulin, followed by a rhodamine-conjugated secondary antibody and 5 μg/ml Hoechst 33342 for fluorescence DNA localization. Epifluorescent microscopy and photography were performed on a Zeiss Axiophot equipped with appropriate filters for all three fluorochromes.
Immunoblotting
Cultured cells or proteins from the mitotic extracts were solubilized directly with SDS-PAGE sample buffer. The proteins were then separated by size using SDS-PAGE (Laemmli 1970), and transferred to PVDF membrane (Millipore Corp.). The membranes were blocked in TBS containing 5% nonfat milk for 30 min at room temperature, and the primary antibody incubated for 6 h at room temperature in TBS containing 1% nonfat milk. Nonbound primary antibody was removed by washing five times for 3 min each in TBS, and the bound antibody was detected using either HRP-conjugated Protein A or HRP-conjugated goat anti–mouse (Bio-Rad Co.). The nonbound secondary reagent was removed by washing five times for 3 min each in TBS, and the signal detected using enhanced chemiluminescence (Nycomed Amersham Inc.).
Electron Microscopy
To localize HSET by immunogold EM on mitotic spindles in cultured CF-PAC1 cells, the cells were grown on photo-etched alphanumeric glass coverslips (Bellco Glass Co.). The position of mitotic cells was determined by phase-contrast microscopy and noted for subsequent selection for examination by EM. Cells were rinsed in MTSB for 1 min at room temperature, extracted in MTSB + 2% Triton X-100 for 5 min, followed by MTSB for 2 min. Cells on the coverslips were then fixed in 1% glutaraldehyde in 0.1 M Na-cacodylate buffer for 30 min. Detergent extraction of cells before fixation was necessary to remove soluble cytosolic components from cells which obscure visualization of HSET on the spindle. This extraction procedure was not deleterious to spindle structure as judged by the presence of interpolar, kinetochore, and astral microtubules. After fixation, the coverslips were washed twice for 15 min each in 0.1 M Na-cacodylate buffer, twice for 15 min each in PBS, three times for 15 min each in 0.5 mg/ml NaBH4, twice for 5 min each in PBS+1% BSA, and finally, once in TBS+1% BSA for 10 min. The anti-HSET rabbit polyclonal IgG was then added at a concentration of 0.13 mg/ml in TBS+1% BSA and incubated for 1 h. The coverslip was then washed with TBS+1% BSA, and incubated for 4 h with a 1/50 dilution of goat anti–rabbit FAb fragments conjugated with 3-nm gold particles (Nanoprobes Inc.) in TBS+1% BSA. The sample was then washed once with TBS+1% BSA, once in 0.1 M Na- cacodylate buffer, and fixed with 2% glutaraldehyde in 0.1 M Na cacodylate. After final fixation, the cells were rinsed in 0.1 M Na-cacodylate buffer, postfixed with 1% OsO4 in 0.1 M Na-cacodylate buffer for 30 min at room temperature, and en-bloc stained in 2% aqueous uranyl acetate. Cells were dehydrated through a graded series of ethanols and propylene oxide, and flat-embedded in epon (LX112)/araldite (502). The glass coverslip was removed by etching in cold concentrated hydrofluoric acid as described by Moore 1975 and Rieder and Bowser 1987. The area containing the mitotic cells that were previously selected by phase-contrast microscopy was identified with the help of a dissecting microscope, cut out of the flat-embedded rectangle, and remounted onto epoxy blanks. 120–150-nm sections were prepared and stained with 2% uranyl acetate for 45 min at 50°C.
We specifically chose 3-nm gold-conjugated FAb fragments as the secondary reagent for all immuno-EM. These small gold particles are at or near the resolution limit for detection in EM, but were essential for optimal penetration of the dense microtubule structures, and to avoid silver enhancement techniques. This allowed us to fix the specimens with osmium tetroxide, which was important in revealing the electron dense material associated with the spindle microtubules. All electron micrographs were taken at 80 or 100 kV on a JEOL 100CX.
Preparation and Immunodepletion of Mitotic Extracts
Mitotic extracts from HeLa cells were prepared according to Gaglio et al. 1995. HeLa cells were synchronized in the cell cycle by double block with 2 mM thymidine. After release from thymidine block, the cells were allowed to grow for 6 h and then nocodazole was added to a final concentration of 40 ng/ml. The mitotic cells that accumulated over the next 4 h were collected by mitotic shake-off and incubated for 30 min at 37°C with 20 μg/ml cytochalasin B. The cells were then collected by centrifugation at 1,500 rpm and washed twice with cold PBS containing 20 μg/ml cytochalasin B. Cells were washed one last time in cold KHM buffer containing 20 μg/ml cytochalasin B, and finally Dounce homogenized (tight pestle) at a concentration of ∼3 × 107 cells/ml in KHM buffer containing 20 μg/ml cytochalasin B, 20 μg/ml phenylmethylsulfonyl fluoride, and 1 μg/ml each of chymostatin, leupeptin, antipain, and pepstatin. The crude cell extract was then subjected to sedimentation at 100,000 g for 15 min at 4°C. The supernatant was recovered and supplemented with 2.5 mM ATP (prepared as Mg2+ salts in KHM buffer) and 10 μM taxol, and the mitotic asters were stimulated to assemble by incubation at 30°C for 30 min. After incubation, the samples were processed for indirect immunofluorescence microscopy as described above, and the remainder of the extract containing the assembled mitotic asters was subjected to sedimentation at 10,000 g for 15 min at 4°C. The supernatant and pellet fractions were both recovered and solubilized in SDS-PAGE sample buffer for immunoblot analysis.
In all experiments, HSET was perturbed by addition of the HSET-1 antibody at a final concentration of 0.1 mg/ml. Immunodepletions from the extract before aster assembly were carried out using 100 μg of anti-Eg5 affinity-purified rabbit polyclonal IgG, or mAb 70.1, which is specific for the IC74 intermediate chain of cytoplasmic dynein. Each antibody was adsorbed onto ∼25μl of either protein A-conjugated agarose or protein G-conjugated agarose (Boehringer Mannheim, Corp.). The 70.1 mAb against cytoplasmic dynein intermediate chain was coupled to protein A-conjugated agarose using goat anti-murine IgM-specific antibody (Vector Labs, Inc.). The antibody-coupled agarose was washed in KHM buffer and then packed by centrifugation to remove the excess fluid. Efficient depletion of the target protein was routinely achieved by sequential depletion reactions in which the total quantity of packed agarose did not exceed 40 μl per 100 μl of extract. First, half of the antibody-coupled agarose was resuspended with the mitotic extract and incubated with agitation for 1 h at 4°C. After this incubation, the agarose was removed from the extract by sedimentation at 15,000 g for 10 s and saved. Next, the extract was recovered and used to resuspend the other half of the antibody-coupled agarose and another incubation performed with agitation for 1 h at 4°C. After this incubation, the agarose was removed by sedimentation at 15,000 g for 10 s and pooled with the agarose pellet from the initial depletion reaction. In all cases, immunoblot analysis indicates that this depletion protocol results in ∼100% efficient depletion of the target protein in experiments both where only one protein was depleted and when more than one protein was depleted (see Results). The depleted extract was recovered and microtubule polymerization induced by the addition of taxol, ATP, and incubation at 30°C for 30 min. Each depletion experiment was performed at least three times and in all cases the data shown are representative of the microtubule structures we observed.
Microinjection
CF-PAC1 or HeLa cells growing on photo-etched alphanumeric glass coverslips (Bellco Glass Co.) were microinjected following the procedures of Compton and Cleveland 1993, and Capecchi 1980. For the antibody microinjection experiments, interphase cells were microinjected in the cytoplasm with either a preimmune IgG or the immune IgG, and monitored by phase-contrast microscopy as they progressed into mitosis. αHSET-1, αEg5, and the rabbit preimmune IgG's were concentrated in 10 mM KPO4, 100 mM KCl, pH 7.0, at concentrations of 10 mg/ml (αHSET-1 and preimmune) and 1–2 mg/ml αEg5. After injection, cells were followed until they entered mitosis and then processed for immunofluorescence microscopy as detailed in the text.
Mouse oocytes were obtained as described in Simerly et al. 1990. Immature oocytes from outbred IRC mice (Sprague-Dawley) were collected from minced ovaries and the cumulus cells were removed by pipetting. Fully grown oocytes were maintained in a modified Tyrode's solution (TALP; Bavister 1989) with 100 μg/ml dibutyryl cAMP (dbcAMP; Sigma Chemical Co.) to arrest spontaneous development (Wasserman et al. 1976). Meiotic maturation was initiated once the derivatized AMP was removed by rinsing in culture medium. Micropipettes were front-loaded with antibody from a small droplet under mineral oil juxtaposed to the culture medium containing the oocytes. Microinjection was performed by puncturing zona-intact oocytes with a 1-μm beveled micropipette (Sutter Instruments), sucking in a small amount of cytoplasm, and expelling the antibody and cytoplasm (Uehara and Yanagimachi 1976; Thadani 1980). Antibody concentrations used were as described, and ∼5% of the egg volume was microinjected with either preimmune or immune IgGs.
Results
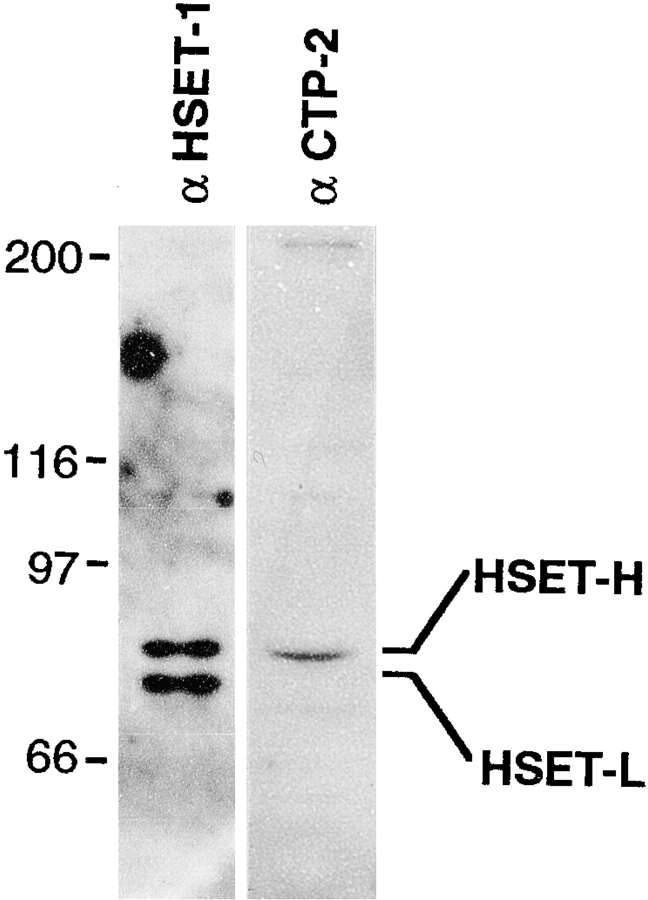
To investigate the role of the minus end-directed kinesin-related protein, HSET, in mitotic spindle assembly in mammalian cells, we raised polyclonal antibodies against the COOH-terminal 377 amino acids of the protein. This segment of HSET was expressed as a GST fusion protein, purified by affinity chromatography as described in Materials and Methods, and used to immunize two rabbits. Both rabbits responded similarly to immunization and immunoblot analysis against total HeLa cell protein, showing that these antibodies, αHSET-1 (Fig. 1) and αHSET-2 (data not shown), specifically recognized two proteins with equal intensity at 80 and 75 kD. The 80-kD protein identified by our antibodies comigrated with the protein identified by αCTP-2, an antibody raised against the COOH-terminal 11 amino acids (CVIGTARANRK) of XCTK-2, the Xenopus laevis HSET homologue (Walczak et al. 1997). Two lines of evidence indicate that the two proteins identified by our antibodies are different isoforms of HSET. First, we have immunoprecipitated sufficient quantities of each protein from a HeLa cell extract to obtain peptide sequence using mass spectrometry. We obtained 12 amino acid peptide sequences from both proteins that were 100% identical to the published HSET sequence. Second, searches of the EST database reveal two classes of HSET cDNAs. The segments of the HSET protein encoded by the two classes of HSET cDNAs are identical, except for the predicted COOH-terminal amino acids. The predicted protein sequence derived from one class of cDNA terminates in the sequence RLPPVSLVRTRGWL, whereas the predicted protein sequence from the other class of cDNA terminates in the sequence NQCVIGTAQANRK. This is consistent with the immunoblot showing that the αCTP-2 antibody is specific for only one of the two isoforms (Fig. 1). The genomic organization of the HSET locus recently reported by Janitz et al. 1999 accounts for only one isoform that terminates in the sequence NQCVIGTAQANRK. Further inspection of the genomic sequence reveals that the COOH terminus of the other isoform is encoded on a distinct exon. We have labeled the 80-kD form HSET-H, and the 75-kD form HSET-L, and with a few specific exceptions as noted, we refer to these proteins collectively as HSET throughout this manuscript. We are currently investigating how the two isoforms are produced and if they differ in any specific functional properties.
Figure 1.
HSET is expressed as two isoforms in HeLa cells. Total HeLa cell protein was separated by SDS-PAGE and then was blotted with the HSET-specific antibody (αHSET-1) or a COOH-terminal peptide antibody (αCTP-2; Walczak et al. 1997). The two isoforms of HSET detected by αHSET-1 are designated HSET-H and HSET-L. Migration positions of myosin (200), β-galactosidase (116), phosphorylase B (97), and albumin (66) are shown in kD.
HSET Cross-links Microtubules within the Mitotic Spindle
To localize HSET at high resolution within the mitotic spindle, we performed immunogold EM of human CF-PAC1 cells at metaphase. The protocol we have developed involves extraction with a microtubule stabilizing buffer, followed by fixation with glutaraldehyde. This process removes the soluble components of the cells, allowing good penetration of the antibodies, but preserves spindle structure including astral microtubules, centrosomes/centrioles, kinetochore fibers, and chromosomes (Fig. 2 A). Immunofluorescence microscopy showed that HSET localization on the spindle was not detectably different between cells extracted before or after fixation, indicating that its localization was not altered by extraction (data not shown). Using this technique, we obtained good labeling within the metaphase spindle counting 768 gold particles in sections through the long (pole to pole) axis of the half spindle of four different mitotic cells. This labeling was specific because >90% of the gold particles were spindle associated (Table ) and no gold labeling was observed when the αHSET-1 antibody was replaced with preimmune antibody (data not shown).
Figure 2.
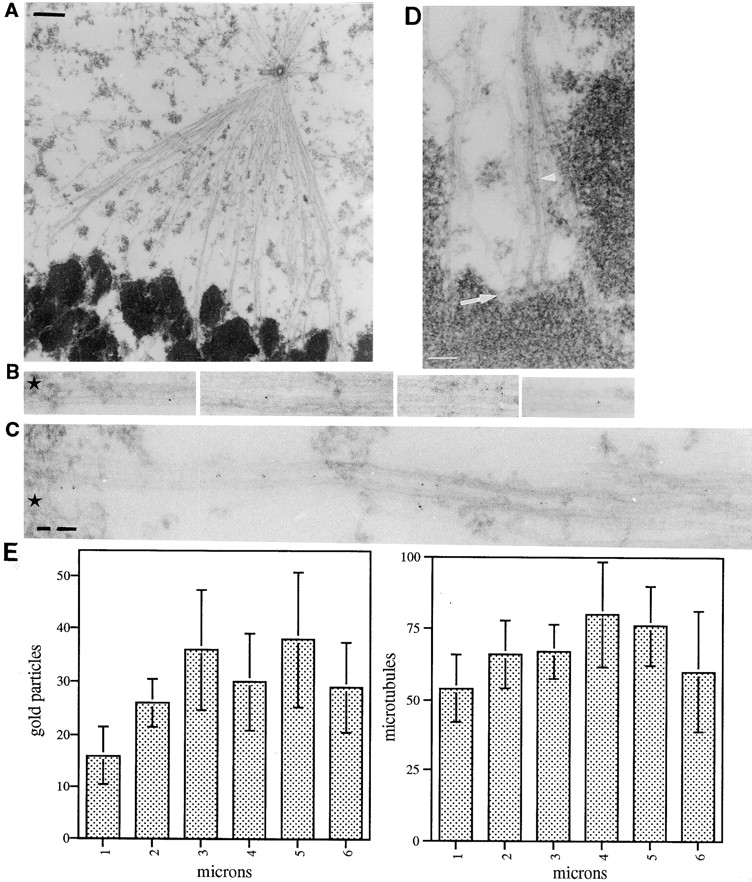
HSET predominately localizes between parallel microtubules in the mitotic spindle of human CF-PAC-1 cells. Cultured CF-PAC-1 cells were fixed and processed for immunogold EM as described in Materials and Methods. A, Low magnification image of a cell processed for EM under these conditions. Bar, 1 μm. B–D, High magnification images showing typical HSET localization between microtubules within the spindle. Frequently, HSET localized to microtubules that terminated within a mass of chromatin (B and C; black star) or within a kinetochore (D; arrow). Arrowhead in D indicates a gold particle. Bars, 0.1 μm. E, Sections through the half spindle of four independent cells were divided into 1-μm regions perpendicular to the long axis of the spindle, with the first region (1 μm) spanning the centrosome and the last region (6 μm) close to the chromosomes. The total number of microtubules and gold particles (generated by immunolabeling for HSET) were counted in each section. The total values were averaged over the number of sections, and the average number of gold particles and microtubules plotted as a function of the region of the spindle.
Table 1.
Immunogold Localization Shows that HSET Predominately Associates between Microtubules in the Metaphase Mitotic Spindle of CF-PAC1 Cells
| Location | Gold Particles (% total) |
|---|---|
| n | |
| Between microtubules | 376 (48.96) |
| Microtubule side wall | 260 (33.85) |
| Astral microtubules | 67 (8.72) |
| Not spindle-associated | 65 (8.46) |
We quantified the localization of the gold particles obtained by staining for HSET in two ways. First, we divided the half spindle into 1-μm sections perpendicular to the long axis of the spindle. We then counted the number of gold particles and microtubules in each of these sections (Fig. 2 E). The average number of microtubules per section is relatively constant, although there are fewer microtubules nearest the centrosome, consistent with previous work (e.g., Brinkley and Cartwright 1971). The average number of gold particles was also relatively constant, although there are fewer gold particles near the pole. These data indicate that HSET is concentrated within the main body of the half spindle and contrasts sharply with the concentration of NuMA at the spindle pole that we observed previously, using a similar technique (Dionne et al. 1999).
Second, we quantified the position of each individual gold particle relative to the microtubules (Table ). More than 82% of the gold particles were localized within the main body of the spindle with <18% being either not spindle-associated or associated with the astral microtubules. Nearly half of all the gold particles were found to be located between adjacent microtubules (Table and Fig. 2B and Fig. C). Individual gold particles can be seen in the high magnification images in Fig. 2 B and C, with HSET's association with spindle microtubules most clearly depicted in Fig. 2 C. This image shows an uninterrupted length of a pair of microtubules that have several gold particles in the intermicrotubule space. Many of the microtubules labeled for HSET terminate within a mass of chromatin (Fig. 2B and Fig. C; black star) or at a kinetochore (Fig. 2 D, arrow). This indicated that the microtubule polymers in those specific images are oriented parallel to one another with respect to their plus and minus ends. Thus, while our data do not address if HSET localized between antiparallel microtubules, they show that a fraction of HSET is localized between parallel microtubules within the spindle during metaphase. These results, in combination with various in vitro data showing that members of this class of kinesin-related protein have two (or more) microtubule binding domains and are capable of bundling microtubules (McDonald et al. 1990; Meluh and Rose 1990; Chandra et al. 1993; Kuriyama et al. 1995; Sharp et al. 1997; Walczak et al. 1997; Karabay and Walker 1999), suggest that HSET plays a role in cross-linking microtubules within the mammalian metaphase spindle.
Centrosomes Mask HSET Activity during Mitosis in Living Cells
To determine if the HSET-specific antibodies were capable of disrupting mitotic spindle assembly in living cells, we microinjected αHSET-1 into both HeLa and CF-PAC1 cells. Injected cells were then monitored as they progressed through the cell cycle, fixed, and processed for immunofluorescence either in mitosis or after mitosis was completed. The fixed cells were stained for tubulin and for the injected rabbit antibody. Immunofluorescence analysis of 41 mitotic cells showed that 26 had normal bipolar mitotic spindles. The remaining 15 mitotic cells appeared to have abnormal spindles (data not shown), however, the abnormality was subtle in that the spindles were somewhat barrel-shaped with slightly broader poles than usual. This abnormality did not impede normal transit through mitosis, since 100% (32 out of 32) of αHSET-1–injected cells completed mitosis and formed typical pairs of G1 cells within a typical one hour time frame. This efficiency was similar to values obtained with the preimmune control antibody, where ∼90% of injected cells completed mitosis normally (n = 18). These results suggest that either our antibody is not effective at perturbing HSET function in vivo or that inhibition of HSET has no severely deleterious effect on spindle morphology or function in vivo.
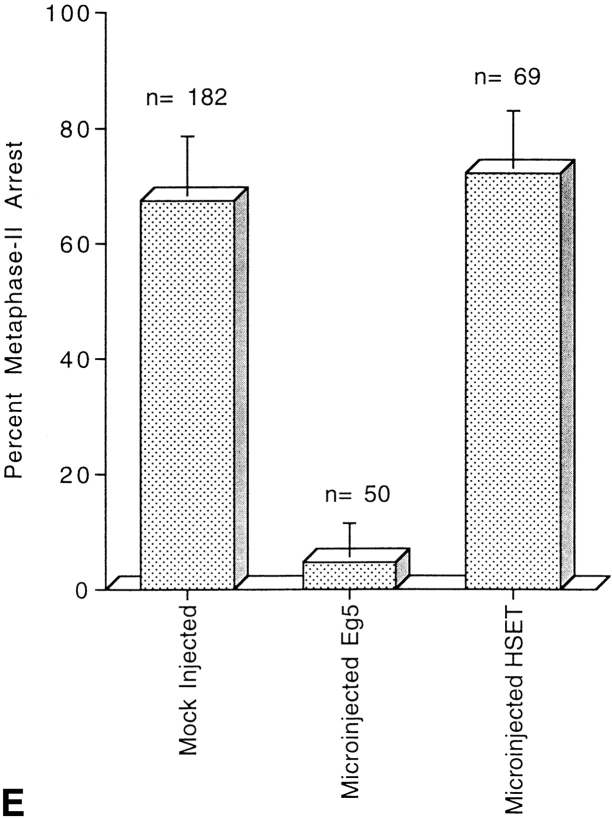
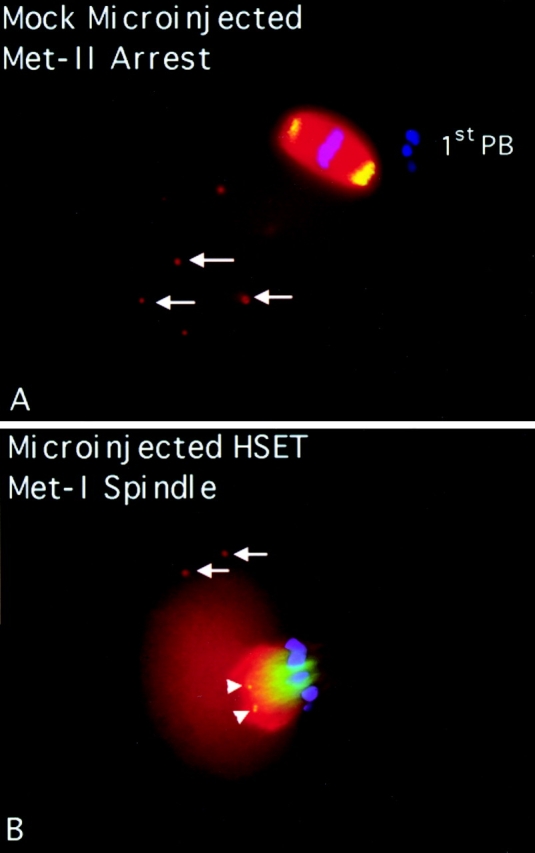
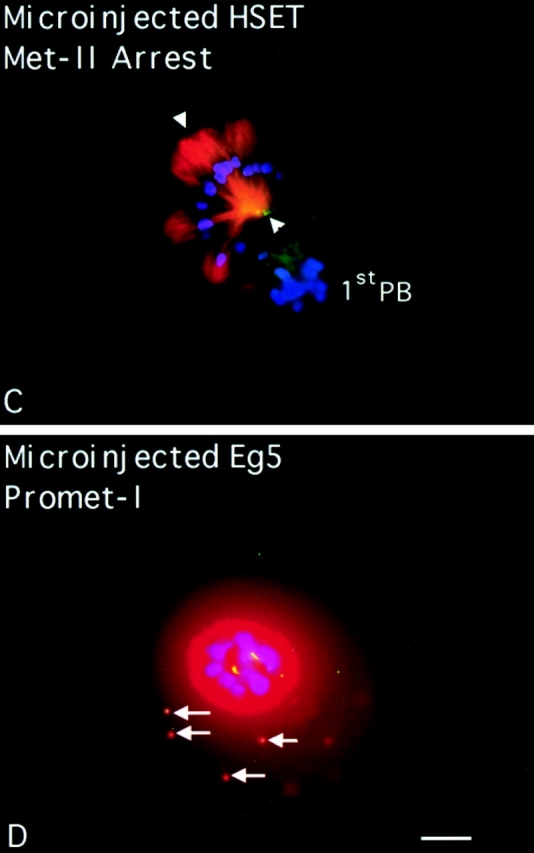
Previous work on this class of kinesin-related motor had shown it to be involved in meiotic spindle assembly and function (Lewis and Gencarella 1952; Davis 1969; Endow and Komma 1996, Endow and Komma 1997; Matthies et al. 1996). To test if perturbation of HSET function blocked meiotic spindle assembly and function in mammalian cells, we injected αHSET-1 antibodies into mouse oocytes (Fig. 3). Immunoblot analysis of total protein from mouse oocytes showed that HSET-H is the predominant isoform in these cells, and that our antibodies were specific for HSET-H in this cell type (data not shown). The oocytes were injected at the germinal vesicle stage and allowed to mature for 16 h, after which metaphase II arrest would normally occur. Injected oocytes were then processed for indirect immunofluorescence where we stained for chromatin, tubulin, and the injected antibody. In mock-injected oocytes, which completed meiosis and arrested at metaphase II, the meiotic spindles were typically barrel-shaped with broad poles and few astral microtubules. Eg5 localized strongly to the spindle poles of these cells (Fig. 3 A) and HSET localized along the length of the body of the spindle (data not shown). There were also numerous cytoplasmic asters (cytasters) scattered throughout the cytoplasm (Fig. 3 A, arrows), but these did not immunostain for HSET or Eg5. Mock injection of oocytes did not affect the progression of meiosis, as ∼70% of cells proceeded through meiosis and arrested at metaphase II within the expected time frame (Fig. 3 E). Oocytes injected with αHSET-1 also progressed through meiosis in the expected time frame, with >70% of αHSET-1–injected oocytes eliciting a first polar body and arresting at metaphase II (Fig. 3 E). Oocytes injected with αHSET-1 and fixed during the first meiotic metaphase showed bipolar spindles in the center of the cell with spindle poles that were broader than in control cells (Fig. 3 B). HSET was observed throughout the length of the spindle, as well as in small aggregates near the microtubule minus ends (Fig. 3 B; arrowheads). The morphology of metaphase II spindles in αHSET-1–injected oocytes, however, was dramatically disrupted compared with either mock-injected cells or metaphase I spindles (Fig. 3 C). The spindle poles were splayed, microtubule minus ends appeared to have lost cohesion, and overall, the spindles had lost bipolarity (Fig. 3 C). In these injected cells, HSET was predominately localized in small aggregates near the microtubule minus ends (Fig. 3 C, arrowheads). To verify that antibody injection was capable of blocking meiosis before metaphase II arrest, we injected antibodies specific to the motor Eg5. Injection of Eg5 antibodies into germinal vesicle stage oocytes blocked the formation of the first bipolar meiotic spindle, with >90% of cells arresting at prometaphase I (Fig. 3 E). In these cells, an astral array of microtubules was assembled around the condensed maternal chromosomes (Fig. 3 D). Therefore, perturbation of Eg5 function blocked the maturation of oocytes at meiosis I. In contrast, perturbation of HSET, while causing obvious defects in the structure of the metaphase I spindle, did not block progression of meiosis until metaphase II. Collectively, these data show that HSET is important for the formation of spindle poles in mammalian oocytes, although the loss of HSET activity was more deleterious to cells in metaphase II than metaphase I of meiosis.
Figure 3.
HSET is essential for microtubule organization in metaphase II spindles in mouse oocytes. Mouse oocytes at the germinal vesicle stage were either mock injected (A), injected with antibodies specific for HSET (Β and C), or injected with antibodies specific for Eg5 (D). Injected oocytes were then matured in vitro until metaphase I (7 h; B) or until metaphase II arrest (16 h; A, C, and D). Oocytes were processed for indirect immunofluorescence using antibodies specific for tubulin (red), DNA (blue), and either Eg5 (A) or the injected antibody (B–D; green). Arrows indicate cytoplasmic asters; arrowheads indicate foci of HSET antigen; and the first polar bodies are marked when discernible (1stPB). E, Percentage of microinjected oocytes that mature to metaphase II arrest after 16 h of maturation in vitro postinjection. Oocytes were either mock injected, injected with antibodies specific for Eg5 antibody, or injected with antibodies specific for HSET, as indicated. Bar, 10 μm.
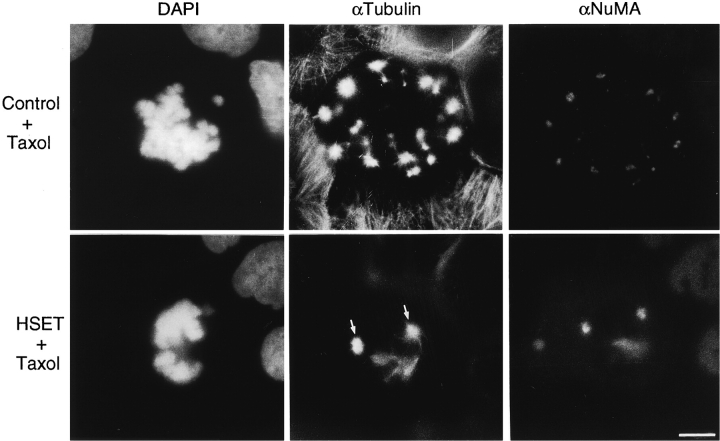
The results of these experiments indicate that our antibodies were capable of perturbing meiotic spindle assembly in mouse oocytes, but that they did not alter the normal assembly and function of the mitotic spindle in cultured cells. Mouse oocytes assemble spindles in the absence of conventional centrosomes (Szollosi et al. 1972), and we suspected that centrosomes provide additional structural stability to microtubule minus ends at spindle poles that masked any deleterious effect when HSET motor activity was inhibited in cultured cells. To test this hypothesis, we microinjected HSET-specific antibodies into cultured cells and treated those cells with taxol to induce microtubule aster formation. We reasoned that, if centrosomes stabilize the spindle so that HSET function was nonessential, then microtubule asters induced with taxol (many of which lack centrosomes) should be disrupted by our antibodies. For this experiment, we microinjected cells with either the preimmune antibody (control) or αHSET-1, treated the cells with 10 μM taxol, fixed, and processed the cells for immunofluorescence after they entered mitosis (Fig. 4). We stained these cells with antibodies specific for tubulin and NuMA to highlight aster morphology. In cells injected with the preimmune antibody, multiple microtubule asters (13.7 ± 2.6 asters/cell, n = 10) were observed scattered throughout the cell cytoplasm (Fig. 4, control). Each of these asters had NuMA concentrated at the core, consistent with taxol-induced asters in uninjected cells, indicating that mitotic aster formation was unaffected by microinjection. In contrast, cells injected with αHSET-1 display disorganized microtubule bundles extending throughout the cell cytoplasm, and only a few mitotic asters (1.5 ± 1.3 asters/cell, n = 10) after taxol treatment (Fig. 4, HSET). NuMA associated with both the asters and the microtubule bundles in these cells, and staining with a human centrosome-specific autoimmune serum (courtesy of J.B. Rattner, University of Calgary, Calgary, Alberta, Canada) verified that each aster observed in these cells contained a centrosome (data not shown). Thus, HSET is essential for meiotic spindle assembly under acentrosomal conditions, but HSET is not essential for spindle assembly in cultured cells because any functional role that it plays is covered by the presence of centrosomes.
Figure 4.
HSET is required for the assembly of taxol-induced asters in cultured CF-PAC1 cells. Cultured CF-PAC1 cells were microinjected with either a preimmune antibody (control) or the HSET-specific antibody (HSET) and then treated with 10 μM taxol to induce the assembly of cytoplasmic microtubule asters. The injected cells were monitored by phase-contrast microscopy until they entered mitosis and then analyzed by indirect immunofluorescence microscopy using antibodies specific for tubulin (αTubulin), NuMA (αNuMA), and the DNA-specific dye DAPI, as indicated. Bar, 10 μm.
HSET and Eg5 Act Antagonistically during the Assembly of Microtubule Asters In Vitro and Mitotic Spindles In Vivo
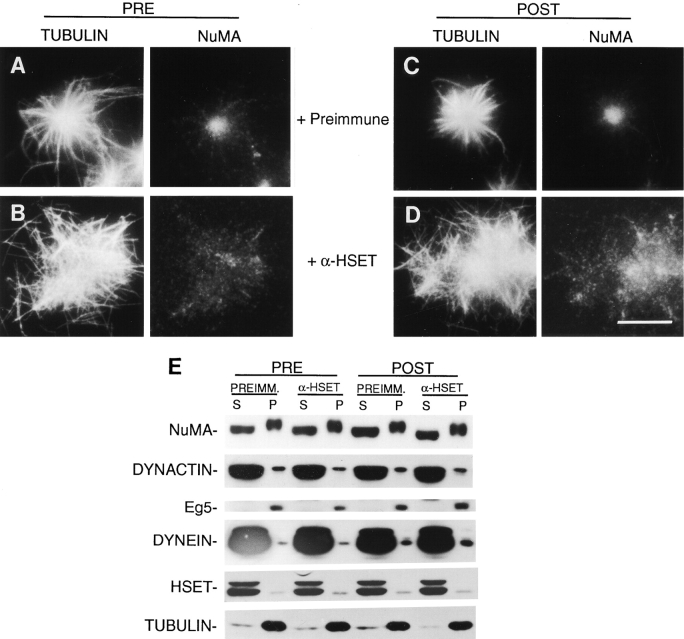
Previously, we have shown that microtubules induced to polymerize with taxol in extracts prepared from mitotic HeLa cells organize into aster-like arrays (Gaglio et al. 1995). The organization of microtubule asters in this system requires the motor activity of cytoplasmic dynein and Eg5, and we proposed that a third motor activity was involved, based on the fact that microtubule asters formed in the complete absence of both cytoplasmic dynein and Eg5 (Gaglio et al. 1996). To determine if HSET has a functional role in organizing microtubule asters in this system, we used our antibodies to specifically perturb HSET activity. Initially, we attempted this by immunodepletion using HSET-specific antibodies. Unfortunately, for reasons that we do not understand, our antibodies maximally depleted only 30% of HSET. This was true regardless of the quantity of antibody used (up to 1 mg). In lieu of immunodepletion, we perturbed the function of HSET by adding our antibodies to the extract. Addition of the preimmune antibody (0.1 mg/ml final concentration) had no effect on the organization of microtubules into asters or on the concentration of NuMA at the aster cores (Fig. 5A and Fig. C). In contrast, addition of αHSET-1 (0.1 mg/ml final concentration) to the mitotic extract before or after the induction of microtubule asters blocked the formation of organized aster-like structures. Under these conditions, microtubules were not well-organized and were loosely aggregated in large, disorganized arrays with NuMA diffusely distributed throughout the microtubule aggregates (Fig. 5B and Fig. D). In addition to these morphological analyses, we separated the mitotic extract into soluble and insoluble fractions and examined the behavior of known aster components by immunoblot analysis (Fig. 5 E). These blots showed HSET to be a bona fide aster component because a small percentage of HSET-L consistently associated with the insoluble aster-containing fraction. These blots also show that there was no difference in the efficiency with which any of the known aster components associate with the soluble or aster-containing insoluble fractions in the presence of αHSET-1. Thus, consistent with the data of Walczak et al. 1997 showing that XCTK2 is required for spindle assembly in vitro using extracts prepared from frog eggs, HSET is a component of microtubule asters assembled in this cell free system, and is required for both the formation and maintenance of aster-like arrays.
Figure 5.
HSET is required for the formation and maintenance of microtubule asters in a cell free mitotic extract. Either the HSET-specific antibody (+αHSET-1; B and D) or a preimmune antibody (+Preimmune; A and C) were added to the mitotic extract either before (PRE) or after (POST) the formation of microtubule asters. The resulting structures were analyzed by indirect immunofluorescence microscopy using antibodies specific for tubulin and NuMA as indicated. E, These extracts were also separated into 10,000 g soluble (S) and insoluble (P) fractions and subjected to immunoblot analysis using antibodies specific for NuMA, dynactin, Eg5, cytoplasmic dynein, HSET, and tubulin as indicated. Bar, 10 μm.
To determine how HSET, cytoplasmic dynein, and Eg5 coordinate microtubule aster formation in this system, we used specific antibodies to perturb the function of each motor individually, as well as to perturb the function of every possible combination of two motors, and all three motors together (Fig. 6). In this experiment, antibodies specific for Eg5 and cytoplasmic dynein were used to deplete those proteins from the mitotic extract, either alone or simultaneously. These depleted extracts, as well as a control extract, were then supplemented with either a preimmune antibody (Fig. 6, control) or αHSET-1 (Fig. 6, +HSET Ab). Microtubule assembly was then induced with taxol, and the resulting structures were fixed and processed for immunofluorescence microscopy using antibodies specific for tubulin and NuMA. The extracts were also separated into soluble, insoluble, and immune pellet fractions, and the behavior of HSET, Eg5, and cytoplasmic dynein within these fractions determined by immunoblot (Fig. 6 I). These immunoblots show that both dynein and Eg5 were depleted to ∼100% in each case. These blots also show that none of these motors coimmunoprecipitated with any of the other motors, consistent with our previously published results (Gaglio et al. 1996). Finally, the immunoblots show that neither the removal of Eg5 and cytoplasmic dynein, nor the addition of αHSET-1, had a detectable effect on the efficiency with which the other motors (Fig. 6 I) or NuMA and dynactin (data not shown) associated with the insoluble microtubule pellet fraction.
Figure 6.
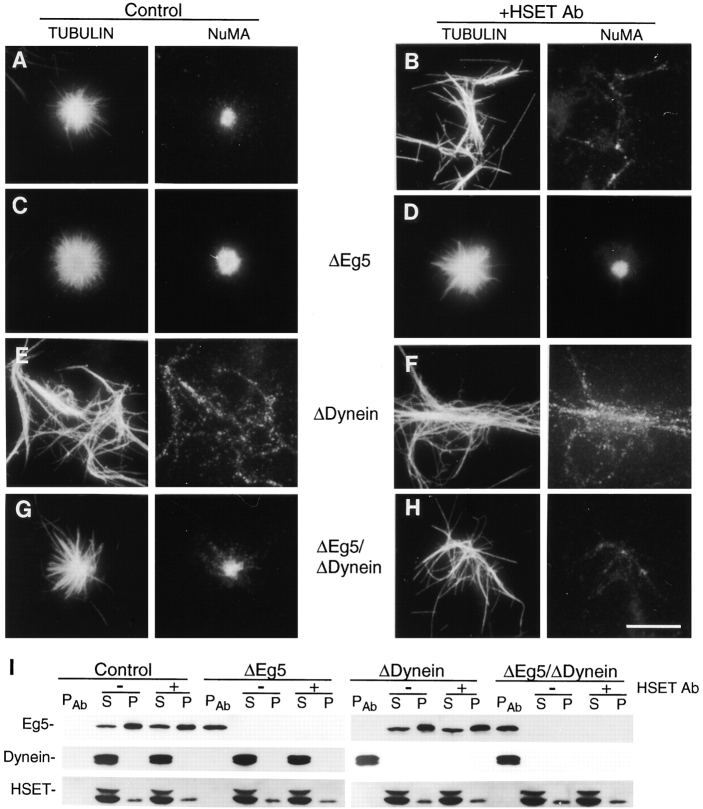
HSET, Eg5, and cytoplasmic dynein are required for the formation of microtubule asters in a cell free mitotic extract. Specific antibodies were used to immunodeplete either Eg5 (ΔEg5; C and D), cytoplasmic dynein (ΔDynein; E and F), or both Eg5 and cytoplasmic dynein (ΔEg5/ΔDynein; G and H) from a HeLa cell mitotic extract. Untreated extracts (A and B) or the depleted extracts were then supplemented with preimmune antibodies (control) or HSET-specific antibodies (+HSET Ab). The formation of microtubule asters was stimulated by the addition of taxol, ATP, and by incubation at 31°C. The resulting structures were analyzed by indirect immunofluorescence using antibodies specific for tubulin and NuMA, as indicated. I, Eg5-, Dynein-, and Eg5/Dynein-depleted mitotic extracts supplemented with preimmune or HSET-specific antibodies were separated into 10,000 g soluble (S) and insoluble (P) components and the immune pellet fraction (PAb), and were subjected to immunoblot analyses using antibodies specific for Eg5, cytoplasmic dynein, and HSET, as indicated. Bar, 10 μm.
As shown previously, addition of preimmune antibody to the extract had no effect on aster assembly, but addition of αHSET-1 prevented the assembly of mitotic asters (Fig. 5 and Fig. 6A and Fig. B). Depletion of Eg5 resulted in microtubule asters that were less tightly focused than the controls (Fig. 6 C; Gaglio et al. 1996). The central core of asters assembled in the Eg5 depleted extract (4.5 ± 0.3 μm, n = 12) were also expanded, relative to the central core of asters in the control extract (2.3 ± 0.4 μm, n = 12), as judged by staining for NuMA. Addition of αHSET-1 to an Eg5 depleted extract resulted in microtubule asters that greatly resemble asters formed under control conditions (compare Fig. 6A and Fig. D). Asters formed in the absence of Eg5 and the presence of αHSET-1 were tightly focused, with NuMA well-concentrated at the central core (2.5 ± 0.4 μm, n = 12). This result shows that, while addition of αHSET-1 alone prevented microtubule aster formation (Fig. 6 B and 5), the HSET antibody did not block aster formation if Eg5 was absent (Fig. 6 D). This result is consistent with the view that microtubule aster formation in this system requires a balance of forces (Gaglio et al. 1996). When HSET alone is perturbed, the balance of forces is upset so that asters cannot form. Microtubule aster formation can be restored under conditions where HSET is perturbed if the balance of forces is equilibrated by also removing the motor activity of Eg5. This result indicates that the minus end-directed activity of HSET antagonizes the plus end-directed activity of Eg5 during microtubule aster assembly in this system.
We next tested the effect on microtubule aster assembly if both minus end-directed motors were perturbed. In the absence of cytoplasmic dynein, microtubules fail to organize into aster-like arrays and were randomly dispersed with NuMA distributed along the length of many of the microtubule polymers (Fig. 6 E; Gaglio et al. 1996). Addition of αHSET-1 to the cytoplasmic dynein depleted extract yielded no microtubule asters and only random microtubule distributions (Fig. 6 F). These results show that microtubule asters fail to form in the absence of HSET alone, cytoplasmic dynein alone, or both HSET and cytoplasmic dynein.
The data presented in Fig. 6B and Fig. D, indicate that the activities of HSET and Eg5 act antagonistically in driving microtubule aster formation in this system. This antagonism is similar to the relationship that we showed previously for cytoplasmic dynein and Eg5 (Gaglio et al. 1996), which is reproduced here in Fig. 6E and Fig. G. These results show that both of these two minus end-directed motors antagonize Eg5 during microtubule aster formation. However, these results do not discriminate between the possibilities that these two motors act together to antagonize Eg5, or that these two motors act independently, with each antagonizing Eg5. To distinguish between these possibilities, we perturbed the function of all three of these motors, reasoning that if these two minus end-directed motors act together, then the perturbation of both minus end motors in an Eg5 depleted extract should yield results similar to the perturbation of either minus end-directed motor alone in an Eg5 depleted extract (i.e., mitotic asters should form). The results of perturbing HSET in a cytoplasmic dynein and Eg5 depleted extract show that aster-like arrays did not form, and that the microtubules were randomly dispersed (Fig. 6 H). The lack of microtubule aster formation in the absence of all three motors is in stark contrast to the microtubule asters that form in the absence of either Eg5 and HSET (Fig. 6 D) or Eg5 and cytoplasmic dynein (Fig. 6 G). This demonstrates that these two motors act independently of each other in antagonizing Eg5 activity in this system.
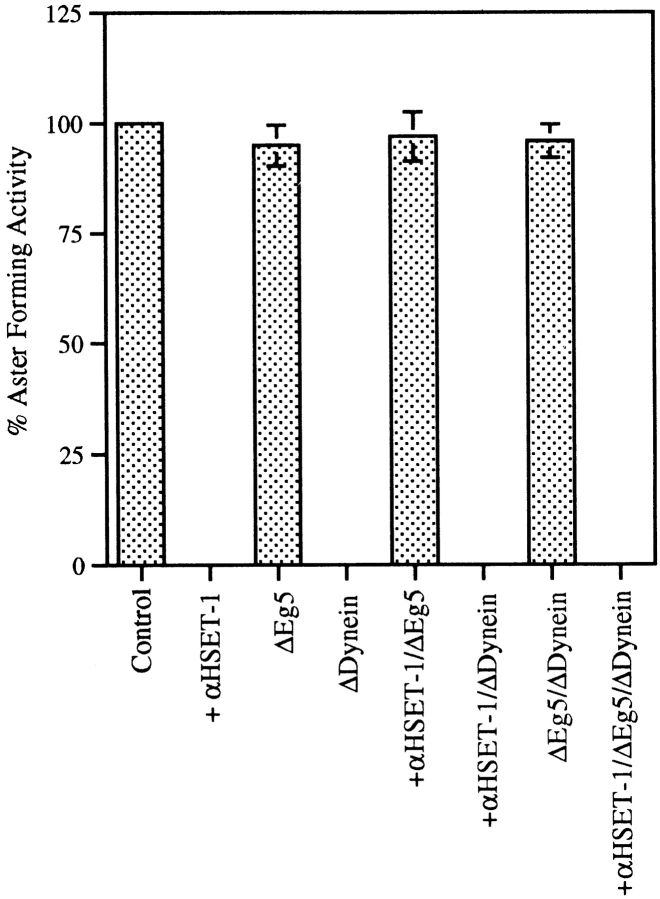
We estimated the microtubule aster forming capacity of the mitotic extracts during these various depletion experiments by counting the total number of microtubule asters in 20 randomly selected microscope fields (Fig. 7). These counts demonstrate that the microtubule aster forming capacity of the extracts depleted for Eg5, Eg5 and cytoplasmic dynein, or Eg5 with the addition of the HSET antibody were comparable to that of a control extract. On the other hand, if the extract was depleted of cytoplasmic dynein, or if the HSET antibody was added to the extract alone, extract depleted of cytoplasmic dynein, or extract depleted of both cytoplasmic dynein and Eg5, then virtually no microtubule asters were observed. Thus, the images shown in Fig. 6 are representative of the populations of microtubule structures observed under each condition tested.
Figure 7.
Microtubule aster forming capacity of mitotic extracts depleted of various components. The average number of microtubule asters in 20 randomly selected microscope fields (400×) from three separate experiments is shown and each is normalized to 100% using the control extract.
Collectively, the results from the experiments presented in Fig. 6 and Fig. 7 lead to three conclusions. First, the minus end-directed activity of HSET opposes the plus end-directed activity of Eg5 in a way that is similar to the opposition between cytoplasmic dynein and Eg5. Second, the minus end-directed activities of HSET and cytoplasmic dynein oppose the plus end-directed activity of Eg5 independently of each other. Third, although we cannot rule out a minor role played by other motors, the lack of microtubule organization in the absence of all three of these motors indicates that HSET, Eg5, and cytoplasmic dynein are most likely the primary motors responsible for building microtubule asters in this system.
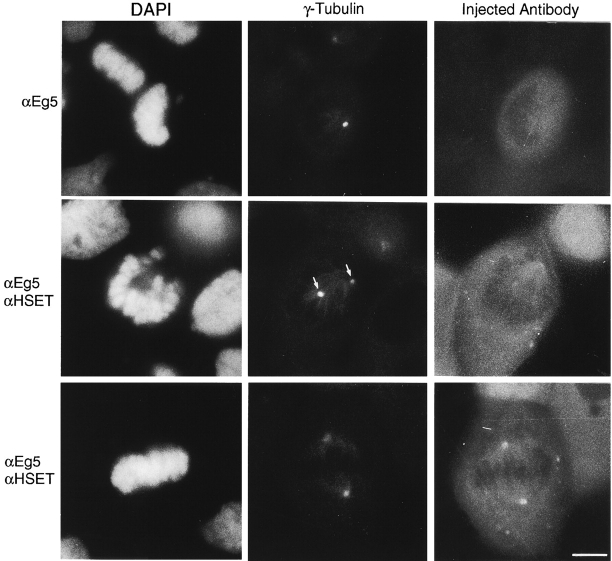
Finally, we tested if HSET functionally opposes Eg5 activity in vivo. For this experiment, we microinjected human CF-PAC1 cells with either antibodies specific for Eg5 or a combination of HSET antibodies and Eg5 antibodies. We monitored the injected cells and fixed and processed them for indirect immunofluorescence using antibodies specific for γ-tubulin to detect centrosomes and for the injected rabbit antibody. Cells injected with the Eg5 antibody alone formed monopolar spindles and arrested in mitosis (Fig. 8, αEg5; Blangy et al. 1995; Gaglio et al. 1996). More than 75% of Eg5-injected cells had centrosomes that had not separated to any measurable degree (Table ). In contrast, >68% of cells injected with both HSET and Eg5 antibodies displayed separated centrosomes (Table ). Many of the double injected cells did not have a symmetric spindle at the time of fixation, as judged by the lack of a well-organized metaphase plate (Fig. 8, middle) or the location of both centrosomes on the same side of the chromosomes. The centrosomes in these cells were clearly separated, but were frequently in different focal planes within the cell, which accounts for the variable intensity of each centrosome shown in Fig. 8. In some instances, symmetric, bipolar spindles formed under these conditions (Fig. 8, αEg5/αHSET, and Table ), and we observed a small fraction of cells (4%) complete mitosis normally, forming pairs of G1 cells with recognizable midbodies (data not shown). These results show a statistically significant (x2 = 50.19, P ≤ 0.0001) increase in centrosome separation in cells injected with antibodies to both HSET and Eg5, compared with cells injected with Eg5 antibodies only. Thus, with respect to centrosome separation, these data indicate that HSET and Eg5 oppose each other in vivo. Furthermore, these results indicate that centrosome separation can proceed under conditions where Eg5 function is blocked.
Figure 8.
HSET antagonizes the plus end-directed activity of Eg5 during centrosome separation in vivo. Cultured CF-PAC1 cells were injected with antibodies specific for Eg5 (αEg5) or simultaneously with two antibodies, one specific for HSET and the other specific for Eg5 (αEg5/αHSET). Cells were monitored by phase-contrast microscopy until they entered mitosis, after which they were fixed and processed for indirect immunofluorescence using antibodies specific for γ-tubulin, the injected antibody/antibodies, and with the DNA-specific dye DAPI, as indicated. Arrows indicate separated centrosomes observed in double injected cells. Bar, 10 μm.
Table 2.
Centrosome Separation Can Occur When the Activities of HSET and Eg5 Are Inhibited Simultaneously In Vivo by Antibody Microinjection
| Centrosome (% total injected population) | |||
|---|---|---|---|
| Injected Antibody | Unseparated | Separated | Bipolar |
| n | n | n | |
| αEg5 | 99 (75.6) | 25 (19.1) | 7 (5.3) |
| αEg5 and αHSET | 41 (31.8) | 66 (51.16) | 22 (17.05) |
Discussion
Previous examination of mitotic spindles in cultured cells by EM has revealed a significant amount of bundling among spindle microtubules (Brinkley and Cartwright 1971; McIntosh 1974; Rieder 1981, Rieder 1982; McDonald et al. 1992; Mastronarde et al. 1993). Also, numerous articles in the literature have reported the visualization of structures cross-linking microtubules in spindles (Wilson 1969; Hepler et al. 1970; Brinkley and Cartwright 1971; McIntosh 1974; Rieder and Bajer 1977; Witt et al. 1981). Consistent with these early descriptive reports, we show here that the kinesin-related protein, HSET, is distributed throughout the main body of the spindle and localizes between microtubules in the metaphase spindle of cultured human cells. This localization, coupled with reports that this class of kinesin protein possesses two (or more) microtubule binding sites (Meluh and Rose 1990; Chandra et al. 1993; Kuriyama et al. 1995; Karabay and Walker 1999), is capable of generating extensive parallel microtubule bundles when expressed in Sf9 cells (Sharp et al. 1997), and induces microtubule bundles in Xenopus egg extracts (Walczak et al. 1997), indicates that HSET most likely participates in spindle assembly and function by promoting microtubule bundling through a cross-linking function.
What role does microtubule cross-linking by HSET play during spindle assembly? HSET is a member of the kinesin-related proteins that possess minus end-directed motor activity. Coupling this motor activity to microtubule cross-linking activity generates a molecule with the potential to slide one microtubule relative to another. As proposed previously (Verde et al. 1991), molecules that combine such unidirectional microtubule motor and cross-linking activities have the potential to promote specific microtubule end convergence, and given that HSET is a minus end-directed motor, it would foster microtubule minus end convergence. During spindle assembly, this activity would participate in focusing microtubule minus ends at the poles, a view supported by the fact that spindle poles are poorly organized when HSET (or its homologues) are perturbed (this report; Kimble and Church 1983; Hatsumi and Endow 1992a,Hatsumi and Endow 1992b; Endow et al. 1994; Endow and Komma 1996, Endow and Komma 1997; Matthies et al. 1996; Walczak et al. 1997). This function for HSET would overlap that of cytoplasmic dynein, which also promotes microtubule minus end focusing at spindle poles (Gaglio et al. 1996; Heald et al. 1996; Merdes et al. 1996). The work presented here, along with results from frog egg extracts (Walczak et al. 1998), show that both of these minus end-directed motors participate in focusing microtubule minus ends. However, this function for HSET is only essential to spindles (or microtubule asters) assembled under acentrosomal conditions. Evidence presented here indicates that centrosomes compensate for HSET, rendering it nonessential for mitotic spindle assembly in cultured cells. This distinction in whether HSET activity is essential depending on the presence or absence of centrosomes is similar to the observation that flies carrying mutant ncd alleles show more severe spindle defects during female meiosis, compared with mitosis (Kimble and Church 1983; Hatsumi and Endow 1992a,Hatsumi and Endow 1992b; Endow et al. 1994; Matthies et al. 1996; Endow and Komma 1996, Endow and Komma 1997).
HSET and Eg5 Act Antagonistically in Animal Cells
We report here that the minus end-directed activity of HSET opposes the plus end-directed activity of Eg5 during microtubule aster assembly in vitro, and centrosome separation and spindle assembly in vivo. This antagonistic relationship is analogous to that seen for members of the KAR3 and bimC families of kinesin-related proteins in budding yeast (Saunders and Hoyt 1992; Saunders et al. 1997), fission yeast (Pidoux et al. 1996), filamentous fungi (O'Connell et al. 1993), and Drosophila (Sharp et al. 1999b). Our work represents the first demonstration of such an antagonistic relationship between these classes of kinesin-related protein in a mammalian system.
In cultured cells, frog egg extracts, Drosophila embryos, and fungi, centrosomes (spindle pole bodies) do not separate in the absence of bimC motor activity, and monopolar spindles result (Saunders and Hoyt 1992; Sawin et al. 1992; Heck et al. 1993; O'Connell et al. 1993; Blangy et al. 1995; Gaglio et al. 1996; Pidoux et al. 1996; Saunders et al. 1997; Sharp et al. 1999a,Sharp et al. 1999b). In each of these experimental systems tested so far, the failure in centrosome separation in the absence of the bimC motor can be relieved by simultaneously perturbing the function of the KAR3 motor (this report; Saunders and Hoyt 1992; O'Connell et al. 1993; Pidoux et al. 1996; Saunders et al. 1997; Sharp et al. 1999b). As originally proposed by Saunders and Hoyt 1992, a likely explanation for how these oppositely oriented motor activities establish and/or maintain centrosome (spindle pole body) separation involves the cross-linking of antiparallel microtubules projecting from each centrosome (spindle pole body). Sliding of these cross-linked antiparallel microtubules relative to each other by the plus end-directed motor (bimC) would push centrosomes apart, while the minus end-directed motor (KAR3) would draw the two centrosomes toward each other. When these two forces become unbalanced in the absence of plus end-directed motor activity, centrosome separation fails, due to the uncontested inward force generated by the minus end-directed motor. Centrosome separation can be restored in the absence of plus end-directed motor activity by reestablishing a balance to the forces acting on centrosomes (spindle pole bodies) by eliminating the activity of the minus end-directed motor.
While this model fits much of the experimental data, there are features of centrosome separation in animal cells that are not fully consistent with such a hypothesis (see Ault and Rieder 1994). First, Sharp et al. 1999a have found that centrosomes separate during prophase in Drosophila embryos before the release of KLP61F (Eg5) from the nuclear compartment. Furthermore, they reported that when KLP61F activity is perturbed by antibody microinjection, centrosomes separate efficiently during prophase, but subsequently collapse upon each other during later stages of mitosis, leading to the characteristic monopolar spindles (Sharp et al. 1999b). This suggests that the process of centrosome separation in Drosophila embryos, and perhaps animal cells, might have two distinct phases, an initial separation phase and a subsequent maintenance phase. These data also suggest that KLP61F is critical for the maintenance phase of centrosome separation, but not essential for the initial separation phase (we note, however, that Whitehead and Rattner 1998 express the opposite view). The initial phase of centrosome separation may be driven by means involving other motor molecules that have been implicated in this process (Vaisberg et al. 1993; Boleti et al. 1996).
Another striking difference between centrosome separation in fungal and animal systems was identified by Waters et al. 1993. They showed that the forces acting to separate the mitotic asters in cultured cells are intrinsic to each aster, and that each aster moved independently from the other. This data contradicts the idea that the cross-linking and subsequent sliding of antiparallel microtubules projecting from the two centrosomes is involved in separating centrosomes in these cells. Here, we suggest an alternative viewpoint for the maintenance phase of centrosome separation which involves forces that motors, principally Eg5 and HSET, could exert along microtubules that are oriented parallel to one another within the spindle, and would therefore be contained within each centrosomal aster (half spindle). For example, Eg5 could associate with kinetochore fibers where microtubules have parallel orientations. Most microtubules in kinetochore fibers have their plus and minus ends anchored at the kinetochores and spindle poles, respectively. Other microtubules within these fibers, however, are not anchored to kinetochores, spindle poles, or both (Rieder 1982; McDonald et al. 1992). The cross-linking and plus end-directed activities of Eg5 could generate a net poleward movement on the subset of unanchored microtubules within kinetochore fibers. This microtubule sliding would exert a force on the centrosome away from the chromosome, and consequently, away from the other centrosome. HSET would antagonize the activity of Eg5 in this context through an analogous mechanism using its minus end-directed activity. Whether HSET exerts a poleward (as for spindle pole organization discussed previously) or away from the pole force on unanchored microtubules within the spindle would depend on the orientation with which this asymmetric motor molecule cross-links microtubules (this class of kinesin-related protein exists as homodimers with both motor domains and ATP-insensitive microtubule binding domains located at opposite ends of the molecule; Chandra et al. 1993). The idea that these two motors act on parallel microtubules, while counter to prevalent models, is supported by the localization of subsets of both HSET (this report) and Eg5 (our unpublished data; Sharp et al. 1999a) in the main body of each half spindle among microtubules with parallel orientations. In the end, the cross-linking and oppositely oriented motor activities of these two kinesin-related proteins may act to maintain centrosome separation in animal cells using mechanisms that involve forces generated on microtubules with both parallel and antiparallel orientations.
Acknowledgments
The authors would like to thank Claire Walczak for generously providing the CTP-2 antibody. Also, we would like to thank Clark Whitehead and J.B. Rattner for providing the M4F clone, which was used to prepare the Eg5-specific antibody, and the centrosome-specific human autoimmune serum.
This work was supported by grants from the National Institutes of Health to G. Schatten (R37WD12913) and D.A. Compton (GM51542).
Footnotes
1.used in this paper: MTSB, microtubule stabilization buffer; ncd, nonclaret disjunctional
References
- Ando A., Kikuti Y., Kwata H., Okamoto N., Imai T., Eki T., Yokoyama K., Soeda E., Ikemura T., Abe K., Inoko H. Cloning of a new kinesin-related gene located at the centromeric end of the human MHC region. Immunogenetics. 1994;39:194–200. doi: 10.1007/BF00241260. [DOI] [PubMed] [Google Scholar]
- Ault J.G., Rieder C.L. Centrosome and kinetochore movement during mitosis. Curr. Opin. Cell Biol. 1994;6:41–49. doi: 10.1016/0955-0674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Barton N.R., Goldstein L.S.B. Going mobilemicrotubule motors and chromosome segregation. Proc. Natl. Acad. Sci. USA. 1996;93:1735–1742. doi: 10.1073/pnas.93.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastmeyer M., Steffen W., Fuge H. Immunostaining of spindle components in tipulid spematocytes using a serum against pericentriolar material. Eur. J. Cell Biol. 1986;42:305–310. [PubMed] [Google Scholar]
- Bavister B.D. A consistently successful procedure for in vitro fertilization of golden hamster eggs. Gamete Res. 1989;23:139–158. doi: 10.1002/mrd.1120230202. [DOI] [PubMed] [Google Scholar]
- Blangy A., Lane H.A., d'Herin P., Harper M., Kress M., Nigg E.A. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Boleti H., Karsenti E., Vernos I. Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996;84:49–59. doi: 10.1016/s0092-8674(00)80992-7. [DOI] [PubMed] [Google Scholar]
- Brenner S., Branch A., Meredith S., Berns M.W. The absence of centrioles from spindle poles of rat kangaroo (PtK2) cells undergoing meiotic-like reduction division in vitro. J. Cell Biol. 1977;72:368–379. doi: 10.1083/jcb.72.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B.R., Cartwright J. Ultrastructural analysis of mitotic spindle elongation in mammalian cells in vitrodirect microtubule counts. J. Cell Biol. 1971;50:416–431. doi: 10.1083/jcb.50.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Gerace L. A cell free system to study the reassembly of the nuclear envelope at the end of mitosis. Cell. 1986;44:639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Capecchi M.R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980;22:479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Chandra R., Salmon E.D., Erickson H.P., Lockhart A., Endow S.A. Structural and functional domains of the Drosophila ncd microtubule motor protein. J. Biol. Chem. 1993;268:9005–9013. [PubMed] [Google Scholar]
- Compton D.A. Focusing on spindle poles. J. Cell Sci. 1998;111:1477–1481. doi: 10.1242/jcs.111.11.1477. [DOI] [PubMed] [Google Scholar]
- Compton D.A., Cleveland D.W. NuMA is required for the proper completion of mitosis. J. Cell Biol. 1993;120:947–957. doi: 10.1083/jcb.120.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D.G. Chromosome behavior under the influence of claret-nondisjunctional in Drosophila melanogaster . Genetics. 1969;61:577–594. doi: 10.1093/genetics/61.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint Phalle B., Sullivan W. Spindle assembly and mitosis without centrosomes in parthenogenetic Scaria embryos. J. Cell Biol. 1998;141:1383–1391. doi: 10.1083/jcb.141.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debec A., Détraves C., Montmory C., Géraud G., Wright M. Polar organization of gamma-tubulin in acentriolar mitotic spindles of Drosophila melanogaster cells. J. Cell Sci. 1995;108:2645–2653. doi: 10.1242/jcs.108.7.2645. [DOI] [PubMed] [Google Scholar]
- Dionne M.A., Howard L., Compton D.A. NuMA is a component of an insoluble matrix at mitotic spindle poles. Cell. Motil. Cytoskel. 1999;42:189–203. doi: 10.1002/(SICI)1097-0169(1999)42:3<189::AID-CM3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Echeverri C.J., Paschal B.M., Vaughan K.T., Vallee R.B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow S.A., Komma D.J. Centrosome and spindle function of the Drosophila Ncd microtubule motor visualized in live embryos using Ncd–GFP fusion proteins. J. Cell Sci. 1996;109:2429–2442. doi: 10.1242/jcs.109.10.2429. [DOI] [PubMed] [Google Scholar]
- Endow S.A., Komma D.J. Spindle dynamics during meiosis in Drosophila oocytes. J. Cell Biol. 1997;137:1321–1336. doi: 10.1083/jcb.137.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow S.A., Chandra R., Komma D.J., Yamamoto A.H., Salmon E.D. Mutants of the Drosophila ncd microtubule motor protein cause centrosomal and spindle defects in mitosis. J. Cell Sci. 1994;107:859–867. doi: 10.1242/jcs.107.4.859. [DOI] [PubMed] [Google Scholar]
- Gaglio T., Saredi A., Compton D.A. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J. Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T., Saredi A., Bingham J.B., Hasbani M.J., Gill S.R., Schroer T.A., Compton D.A. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J. Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T., Dionne M.A., Compton D.A. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsumi M., Endow S.A. Mutants of the microtubule motor protein, nonclaret disjunctional, affect spindle structure and chromosome movement in meiosis and mitosis J. Cell Sci. 101 1992. 547 559a [DOI] [PubMed] [Google Scholar]
- Hatsumi M., Endow S.A. The Drosophila ncd microtubule motor protein is spindle-associated in meiotic and mitotic cells J. Cell Sci. 103 1992. 1013 1020b [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Blank T., Sandaltzopoulos R., Beker P., Hyman A., Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Habermann A., Karsenti E., Hyman A. Spindle assembly in Xenopus egg extractsrespective roles of centrosomes and microtubule self-organization. J. Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck M.M.S., Pereira A., Pesavento P., Yannoni Y., Spradling A.C., Goldstein L.S.B. The kinesin-like protein KLP61F is essential for mitosis in Drosophila . J. Cell Biol. 1993;123:665–679. doi: 10.1083/jcb.123.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P.K., McIntosh J.R., Cleland S. Intermicrotubule bridges in the mitotic spindle apparatus. J. Cell Biol. 1970;45:438–444. doi: 10.1083/jcb.45.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hyman A.A., Karsenti E. Morphogenetic properties of microtubules and mitotic spindle assembly. Cell. 1996;84:401–410. doi: 10.1016/s0092-8674(00)81285-4. [DOI] [PubMed] [Google Scholar]
- Hyman A.A., Karsenti E. The role of nucleation in patterning microtubule networks. J. Cell Sci. 1998;111:2077–2083. doi: 10.1242/jcs.111.15.2077. [DOI] [PubMed] [Google Scholar]
- Inoué S., Salmon E.D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janitz K., Wild A., Beck S., Savasta S., Beluffi G., Ziegler A., Volz A. Genomic organization of the HSET locus and the possible association of HLA-linked genes with immotile cilia syndrome (ICS) Immunogenetics. 1999;49:644–652. doi: 10.1007/s002510050660. [DOI] [PubMed] [Google Scholar]
- Karabay A., Walker R.A. Identification of microtubule binding sites in the ncd tail domain. Biochemistry. 1999;38:1838–1849. doi: 10.1021/bi981850i. [DOI] [PubMed] [Google Scholar]
- Kashina A.S., Baskin R.J., Cole D.G., Wedaman K.P., Saxton W.M., Scholey J.M. A bipolar kinesin. Nature. 1996;379:270–272. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyer G., Ris H., Borisy G.G. Centriole distribution during tripolar mitosis in chinese hamster ovary cells. J. Cell Biol. 1984;98:2222–2229. doi: 10.1083/jcb.98.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.L.A., Gogonea C.B., Siddiqui Z.K., Ali M.Y., Kikuno R., Nishikawa K., Siddiqui S.S. Molecular cloning and expression of the Caenorhabditis elegans klp-3, an ortholog of C terminus motor kinesins Kar3 and ncd. J. Mol. Biol. 1997;270:627–639. doi: 10.1006/jmbi.1997.1112. [DOI] [PubMed] [Google Scholar]
- Kimble M., Church K. Meiosis and early cleavage in Drosophila melanogaster eggseffects of the claret-non-disjunctional mutation. J. Cell Sci. 1983;62:301–318. doi: 10.1242/jcs.62.1.301. [DOI] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T.J. Beyond self-assemblyfrom microtubule to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kuriyama R., Kofron M., Essner R., Kato T., Dragas-Granoic S., Omoto C.K., Khodjakov A. Characterization of a minus end-directed kinesin-like motor protein from cultured mammalian cells. J. Cell Biol. 1995;129:1049–1059. doi: 10.1083/jcb.129.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during assembly at the head of the bacteriophage T4. Nature. 1970;373:630–632. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis E.B., Gencarella W. Claret and non-disjunction in Drosophila melanogaster . Genetics. 1952;37:600–601. [Google Scholar]
- Maniotis A., Schliwa M. Microsurgical removal of centrosomes blocks cell reproduction and centriole generation in BSC-1 cells. Cell. 1991;67:495–504. doi: 10.1016/0092-8674(91)90524-3. [DOI] [PubMed] [Google Scholar]
- Mastronarde D.N., McDonald K.L., Ding R., McIntosh J.R. Interpolar spindle microtubules in PTK cells. J. Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies H.J.G., McDonald H.B., Goldstein L.S.B., Theurkauf W.E. Anastral meiotic spindle morphogenesisrole of the non-claret disjunctional kinesin-like protein. J. Cell Biol. 1996;134:455–464. doi: 10.1083/jcb.134.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D. Centrosomes and mitotic poles. Exp. Cell Res. 1984;153:1–15. doi: 10.1016/0014-4827(84)90442-7. [DOI] [PubMed] [Google Scholar]
- McDonald H.B., Stewart R.J., Goldstein L.S.B. The kinesin-like ncd protein of Drosophila is a minus end-directed microtubule motor. Cell. 1990;63:1159–1165. doi: 10.1016/0092-8674(90)90412-8. [DOI] [PubMed] [Google Scholar]
- McDonald K.L., O'Toole E.T., Mastronarde D.N., McIntosh J.R. Kinetochore microtubules in PTK cells. J. Cell Biol. 1992;118:369–383. doi: 10.1083/jcb.118.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J.R. Bridges between microtubules. J. Cell Biol. 1974;61:166–187. doi: 10.1083/jcb.61.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J.R. The centrosome as organizer of the cytoskeleton. Modern Cell Biol. 1983;2:115–142. [Google Scholar]
- McIntosh J.R., Koonce M.P. The mitotic spindle. Science. 1989;246:622–628. doi: 10.1126/science.2683078. [DOI] [PubMed] [Google Scholar]
- McKim K.S., Hawley R.S. Chromosomal control of meiotic cell division. Science. 1995;270:1595–1601. doi: 10.1126/science.270.5242.1595. [DOI] [PubMed] [Google Scholar]
- Meluh P.B., Rose M.D. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell. 1990;60:1023–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- Merdes A., Ramyar K., Vechio J.D., Cleveland D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Mitchison T.J. Mitosisbasic concepts. Curr. Opin. Cell Biol. 1989;1:67–74. doi: 10.1016/s0955-0674(89)80039-0. [DOI] [PubMed] [Google Scholar]
- Moore M.J. Removal of glass coverslips from cultures flat embedded in epoxy resins using HF. J. Microsc. 1975;104:205–207. doi: 10.1111/j.1365-2818.1975.tb04018.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Tanaka Y., Matsuoka E., Kondo S., Okada Y., Noda Y., Kanai Y., Hirokawa N. Identification and classification of 16 new kinesin superfamily (KIF) proteins in mouse genome. Proc. Natl. Acad. Sci. USA. 1997;94:9654–9659. doi: 10.1073/pnas.94.18.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.R., Szauter P. Timing of mitotic chromosome loss caused by the ncd mutation of Drosophila melanogaster . Cell Motil. Cytoskel. 1992;23:34–44. doi: 10.1002/cm.970230105. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B., Kubai D.F., Hays T.S. Spindle microtubules and their mechanical associations after micromanipulation in anaphase. J. Cell Biol. 1982;95:91–104. doi: 10.1083/jcb.95.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M.J., Meluh P.B., Rose M.D., Morris N.R. Suppression of the bimC4 mitotic spindle defect by deletion of klpA, a gene encoding a KAR3-related kinesin-like protein in Aspergillus nidulans . J. Cell Biol. 1993;120:153–162. doi: 10.1083/jcb.120.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallazzo R.E., Vaisberg E.A., Weiss D.G., Kuznetsov S.A., Steffen W. Dynein is required for spindle assembly in cytoplasmic extracts of Spisula solidissima oocytes. J. Cell Sci. 1999;112:1291–1302. doi: 10.1242/jcs.112.9.1291. [DOI] [PubMed] [Google Scholar]
- Pidoux A.L., LeDizet M., Cande W.Z. Fission yeast plk1 is a kinesin-related protein involved in mitotic spindle function. Mol. Biol. Cell. 1996;7:1639–1655. doi: 10.1091/mbc.7.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portin P. Studies on gynandromorphs induced with the claret-nondisjunctional mutation of Drosophila melanogaster. An approach to the timing of chromosome loss in cleavage mitoses. Heredity. 1978;41:193–203. [Google Scholar]
- Rieder C.L. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma. 1981;84:145–158. doi: 10.1007/BF00293368. [DOI] [PubMed] [Google Scholar]
- Rieder C.L. The formation, structure and composition of the mammalian kinetochore and kinetochore fiber. Int. Rev. Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Rieder C.L. Mitosistowards a molecular understanding of chromosome behavior. Curr. Opin. Cell Biol. 1991;3:59–66. doi: 10.1016/0955-0674(91)90166-v. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Bajer A. Heat induced reversible hexagonal packing of spindle microtubules. J. Cell Biol. 1977;74:717–725. doi: 10.1083/jcb.74.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Bowser S. Correlative LM and EM on the same epoxy section. In: Hyat M.A., editor. Correlative Microscopy in Biology. Academic Press; NY: 1987. pp. 249–277. [Google Scholar]
- Rieder C.L., Salmon E.D. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Ault J.G., Eicenlaub-Ritter U., Sluder G. Morphogenesis of the mitotic and meiotic spindleconclusions obtained from one system are not necessarily applicable to the other. In: Vig B.K., Kappas A., editors. Chromosome Segregation and Aneuploidy. Springer-Verlag; NY: 1993. pp. 183–197. [Google Scholar]
- Saunders W., Hoyt M.A. Kinesin-related proteins required for structural integrity of the mitotic spindle. Cell. 1992;70:451–458. doi: 10.1016/0092-8674(92)90169-d. [DOI] [PubMed] [Google Scholar]
- Saunders W., Lengyel V., Hoyt M.A. Mitotic spindle function in Saccharomyces cerevisiae requires a balance between different types of kinesin-related motors. Mol. Biol. Cell. 1997;8:1025–1033. doi: 10.1091/mbc.8.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K.E., Endow S.A. Meiosis, mitosis and microtubule motors. Bioessays. 1993;15:399–407. doi: 10.1002/bies.950150606. [DOI] [PubMed] [Google Scholar]
- Sawin K.E., LeFuellec K., Philippe M., Mitchison T.J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- Schatten G. The centrosome and its mode of inheritancethe reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev. Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- Sharp D.J., Kuriyama R., Essner R., Bass P.W. Expression of a minus-end-directed motor protein induces Sf9 cells to form axon-like processes with uniform microtubule polarity orientation. J. Cell Sci. 1997;110:2373–2380. doi: 10.1242/jcs.110.19.2373. [DOI] [PubMed] [Google Scholar]
- Sharp D.J., McDonald K.L., Brown H.M., Matthies H.J., Walczak C., Vale R.D., Mitchison T.J., Scholey J.M. The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles J. Cell Biol. 144 1999. 125 138a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., Yu K.R., Sisson J.C., Sullivan W., Scholey J.M. Antagonistic microtubule-sliding motors position mitotic centrosomes in Drosophila early embryos Nature Cell Biol 1 1999. 51 54b [DOI] [PubMed] [Google Scholar]
- Simerly C., Schatten G. Techniques for localization of specific molecules in oocytes and embryos. Meth. Enzymol. 1993;225:516–553. doi: 10.1016/0076-6879(93)25035-z. [DOI] [PubMed] [Google Scholar]
- Simerly C., Balczon R., Brinkley B.R., Schatten G. Microinjected kinetochore antibodies interfere with chromosome movement in meiotic and mitotic mouse oocytes. J. Cell Biol. 1990;111:1491–1504. doi: 10.1083/jcb.111.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G., Rieder C.L. Experimental separation of pronuclei in fertilized sea urchin eggschromosomes do not organize a spindle in the absence of centrosomes. J. Cell Biol. 1985;100:897–903. doi: 10.1083/jcb.100.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen W., Fuge H., Dietz R., Bastmeyer M., Müller G. Aster-free spindle poles in insect spermatocytesevidence for chromosome-induced spindle formation. J. Cell Biol. 1986;102:1679–1687. doi: 10.1083/jcb.102.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer E.R., Schroer T.A., Wordeman L., Sheetz M.P. Cytoplasmic dynein localizes to mitotic spindles and kinetochores. Nature. 1991;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Sturtevant A.H. The claret mutant type of Drosophila simulansa study of chromosome elimination and of cell lineage. Z. Wiss. Zool. 1929;135:323–365. [Google Scholar]
- Szollosi D., Calarco P., Donahue R.P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 1972;11:521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Thadani V.M. A study of hetero-specific sperm–egg interactions in the rat, mouse, and deer mouse using in vitro fertilization and sperm injection. J. Exp. Zool. 1980;212:435–453. doi: 10.1002/jez.1402120316. [DOI] [PubMed] [Google Scholar]
- Theurkauf W.E., Hawley R.S. Meiotic spindle assembly in Drosophila femalesbehavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-related protein. J. Cell Biol. 1992;116:1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T., Yanagimachi R. Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biol. Reprod. 1976;15:467–470. doi: 10.1095/biolreprod15.4.467. [DOI] [PubMed] [Google Scholar]
- Vaisberg E.A., Koonce M.P., McIntosh J.R. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Berrez J.-M., Antony C., Karsenti E. Taxol-induced microtubule asters in mitotic extracts of Xenopus eggsrequirement for phosphorylated factors and cytoplasmic dynein. J. Cell Biol. 1991;112:1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernos I., Karsenti E. Chromosomes take the lead in spindle assembly. Trends Cell Biol. 1995;5:297–301. doi: 10.1016/s0962-8924(00)89045-5. [DOI] [PubMed] [Google Scholar]
- Walczak C.E., Mitchison T.J. Kinesin-related proteins at mitotic spindle polesfunction and regulation. Cell. 1996;85:943–946. doi: 10.1016/s0092-8674(00)81295-7. [DOI] [PubMed] [Google Scholar]
- Walczak C.E., Verma S., Mitchison T.J. XCTK2A kinesin-related protein that promotes mitotic spindle assembly in Xenopus laevis egg extracts. J. Cell Biol. 1997;136:859–870. doi: 10.1083/jcb.136.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak C.E., Vernos I., Mitchison T.J., Karsenti E., Heald R. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 1998;8:903–913. doi: 10.1016/s0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- Wasserman P.M., Josefowicz W.J., Letourneau G.E. Meiotic maturation of mouse oocytes in vitroinhibition of maturation at specific stages of nuclear progression. J. Cell Sci. 1976;22:531–545. doi: 10.1242/jcs.22.3.531. [DOI] [PubMed] [Google Scholar]
- Waters J.C., Cole R.C., Rieder C.L. The force-producing mechanism for centrosome separation during spindle formation in vertebrates is intrinsic to each aster. J. Cell Biol. 1993;122:361–372. doi: 10.1083/jcb.122.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J.C., Salmon E.D. Pathways of spindle assembly. Curr. Opin. Cell Biol. 1997;9:37–43. doi: 10.1016/s0955-0674(97)80149-4. [DOI] [PubMed] [Google Scholar]
- Whitehead C.M., Rattner J.B. Expanding the role of HsEg5 within the mitotic and post-mitotic phases of the cell cycle. J. Cell Sci. 1998;111:2551–2561. doi: 10.1242/jcs.111.17.2551. [DOI] [PubMed] [Google Scholar]
- Wilson H.J. Arms and bridges on microtubules in the mitotic apparatus. J. Cell Biol. 1969;40:854–859. doi: 10.1083/jcb.40.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P.G., Fuller M.T., Borisy G.G. Monastral bipolar spindlesimplications for dynamic centrosome organization. J. Cell Sci. 1997;110:451–464. doi: 10.1242/jcs.110.4.451. [DOI] [PubMed] [Google Scholar]
- Witt P.L., Ris H., Borisy G.G. Structure of kinetochore fibersmicrotubule continuity and inter-microtubule bridges. Chromosoma. 1981;83:523–540. doi: 10.1007/BF00328277. [DOI] [PubMed] [Google Scholar]
- Wolf K.W., Bastmeyer M. Cytology of Lepidoptera. V. The microtubule cytoskeleton in eupyrene spermatocytes of Ephistia kuehniella (Pyralidae), Inachis io (Nymphlidae) and Orgyia antiqua (Lymantriidae) Eur. J. Cell Biol. 1991;55:225–237. [PubMed] [Google Scholar]
- Zhang D., Nicklas R.B. The impact of chromosomes and centrosomes on spindle assembly as observed in living cells. J. Cell Biol. 1995;129:1287–1300. doi: 10.1083/jcb.129.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]