Abstract
We have used microinjection and time-lapse video microscopy to study the role of cyclin A in mitosis. We have injected purified, active cyclin A/cyclin-dependent kinase 2 (CDK2) into synchronized cells at specific points in the cell cycle and assayed its effect on cell division. We find that cyclin A/CDK2 will drive G2 phase cells into mitosis within 30 min of microinjection, up to 4 h before control cells enter mitosis. Often this premature mitosis is abnormal; the chromosomes do not completely condense and daughter cells fuse. Remarkably, microinjecting cyclin A/CDK2 into S phase cells has no effect on progress through the following G2 phase or mitosis. In complementary experiments we have microinjected the amino terminus of p21Cip1/Waf1/Sdi1 (p21N) into cells to inhibit cyclin A/CDK2 activity. We find that p21N will prevent S phase or G2 phase cells from entering mitosis, and will cause early prophase cells to return to interphase. These results suggest that cyclin A/CDK2 is a rate-limiting component required for entry into mitosis, and for progress through mitosis until late prophase. They also suggest that cyclin A/CDK2 may be the target of the recently described prophase checkpoint.
Keywords: cyclin, CDK, mitosis, p21, GFP
Progress through the cell cycle is regulated by sequential waves of different cyclin/cyclin-dependent kinase (CDK)1 activities. In animal cells, cyclin E/CDK2 is required for the initiation of DNA replication, and cyclin B/CDK1 for the entry into mitosis. Cyclin A is unusual in that it appears to be required for both DNA synthesis and for mitosis (Pagano et al. 1992). With respect to its S phase role, cyclin A may be rate limiting for DNA replication because it can accelerate the entry of G1 phase cells into S phase when overexpressed from a tetracycline-inducible promoter (Resnitzky et al. 1995). Moreover, cyclin A will promote DNA replication in Xenopus egg (Strausfeld et al. 1996) and human cell extracts (D'Urso et al. 1990; Fotedar et al. 1996a; Krude et al. 1997), and has been localized to sites of DNA replication (Cardoso et al. 1993; Sobczak et al. 1993). Conversely, microinjecting anti–cyclin A antibodies, or antisense RNA, will prevent mammalian tissue culture cells from replicating their DNA (Girard et al. 1991; Zindy et al. 1992). Although cyclin A does appear to be necessary for S phase, there is some debate over whether cyclin A is needed to initiate DNA replication, or whether it plays a role in continuing DNA synthesis, perhaps to prevent rereplication (Petersen et al. 1999).
The role of cyclin A at mitosis is also ill defined. The most definitive evidence that cyclin A is required for cells to begin mitosis is the observation that in a Drosophila mutant that lacks cyclin A, cells arrest in G2 phase after the supply of maternal cyclin A is exhausted (Knoblich and Lehner 1993; Lehner and O'Farrell 1989). In support of this, anti–cyclin A antibodies arrest cells before mitosis when injected into tissue culture cells in G2 phase (Pagano et al. 1992). Moreover, cyclin A mRNA alone can induce meiotic maturation when injected into Xenopus oocytes (Swenson et al. 1986). In this assay, and also in genetic analyses using Drosophila mutants that lack cyclin A or cyclin B, or both, it appears that cyclin A acts synergistically with cyclin B to induce mitosis (Devault et al. 1992; Knoblich and Lehner 1993). However, these experiments did not reveal a specific role for cyclin A compared with cyclin B in mitosis. One difference that has been observed is that amino-terminal truncated mutants of clam cyclin B, but not cyclin A, activate ubiquitin-mediated cyclin destruction in vitro (Luca et al. 1991).
The initiation of mitosis is subject to a number of important checkpoint controls to ensure that DNA is completely replicated and undamaged before cell division begins (reviewed in Elledge 1996). Both unreplicated and damaged DNA are able to prevent mitosis by inhibiting the dephosphorylation and consequent activation of cyclin B/CDK1. Recent results have emphasized the importance of the Cds1 and Chk1 protein kinases that are activated by the presence of unreplicated DNA and/or damaged DNA (Fogarty et al. 1997; Furnari et al. 1997; Peng et al. 1997; Sanchez et al. 1997; Boddy et al. 1998; Lindsay et al. 1998). Once activated, Chk1 and Cds1 phosphorylate and inactivate cell division cycle (Cdc)25C, in part by creating phosphorylation-dependent binding sites for 14-3-3 proteins that may sequester Cdc25C (Lopez-Girona et al. 1999). The end result is to prevent Cdc25 from dephosphorylating and activating cyclin B/CDK1, and, therefore, prevent the cell from entering mitosis. In addition to this pathway, there may be a role for the CDK inhibitor p21Cip1/Waf1/Sic1 in preventing mitosis in the presence of DNA damage. p21Cip1/Waf1/Sic1 is able to bind and inhibit directly a number of different cyclin/CDKs, including cyclin E/CDK2 and cyclin A/CDK2, but not cyclin B/CDK1 to a significant extent (Harper et al. 1995). The carboxyl terminus of p21Cip1/Waf1/Sic1 is also able to bind to PCNA (Chen et al. 1995; Goubin and Ducommun 1995; Nakanishi et al. 1995; Warbrick et al. 1995), and this appears to contribute to a DNA damage checkpoint when p21Cip1/Waf1/Sic1 is overexpressed (Tournier et al. 1996; Cayrol et al. 1998). Once cells have entered mitosis, there is another checkpoint in early prophase that will cause a cell to return to an interphase-like state when the chromosomes are damaged (Rieder and Cole 1998), although the nature of the regulator(s) involved has not been defined.
In this paper, we demonstrate that cyclin A/CDK2 activity is a rate-limiting component for entry into mitosis because exogenous active cyclin A/CDK2 will drive cells prematurely into mitosis, effectively eliminating G2 phase. However, cyclin A/CDK2 cannot overcome the unreplicated DNA checkpoint. We further show that cyclin A/CDK activity is required for cells to complete prophase, because inhibiting cyclin A/CDK activity in mid prophase causes cells to return to G2 phase. Cyclin A/CDK becomes dispensable in late prophase at the same time that cyclin B1 translocates into the nucleus. This suggests that the prophase checkpoint may act by inhibiting cyclin A/CDK.
Materials and Methods
Cell Culture and Synchronization
HeLa cells were cultured in DME and synchronized in G2 phase by a thymidine-aphidicolin block (Pines and Hunter 1989). PtK1 cells (European Collection of Cell Cultures no. 91013163) were cultured in Ham's F-12 medium (GIBCO BRL), 100 U/ml penicillin-G, 0.1 mg/ml streptomycin, 1 mM Na pyruvate, 10% foetal bovine serum, and 1% (vol/vol) Fungizone (GIBCO BRL) at 37°C/6% CO2.
Construction and Purification of GST-p21N
Human p21Cip1 (1–90) was generated by PCR from the p21Cip1 cDNA (kind gift of Dr. J. Roberts, Fred Hutchison Cancer Center, Seattle, WA) using the following primers; 5′ GCCGGATCCCCATGTCAGAACCG, and 3′ GCCGGATCCCTATCCCAACTCATCCCGGCCTCG. After digestion with BamHI, the PCR product was subcloned into the BamHI site of pGEX3T (Pharmacia) to yield pGEX-p21N. For expression, pGEX-p21N was used to transform E. coli (BL21) and the GST-p21N fusion protein was purified by glutathione Sepharose chromatography after which it was >90% pure on a Coomassie blue R250–stained SDS–polyacrylamide gel. The concentration of purified GST-p21N was determined by Coomassie blue R250 staining using BSA standards. All constructs were confirmed by sequencing on an automatic sequencer (Department of Biochemistry, University of Cambridge).
Cyclin-Cdk Expression, Purification, and Injection
Cyclin A/CDK2, cyclin E-CDK2, and cyclin A/CDK2K33R were expressed in and purified from baculovirus-infected Sf9 cells as previously described (Krude et al. 1997), as were cyclin B1-MmGFP and cyclin B1 (Clute and Pines 1999). Proteins were >90% pure on silver-stained gels. Proteins were concentrated in injection buffer (200 mM NaCl, 12.5 mM Tris-Cl, pH 8.0, 2.5 mM DTT, 1 mM EDTA, and 1 mM glutathione) in a Vivaspin 5,000 MW cut-off microconcentrator (Vivascience) and ∼5% of the cell volume injected into cells using an Eppendorf semiautomatic microinjector (Eppendorf) attached to a Leica DMIRBE microscope (Leica). The cDNAs for Cdc25B and Cdc25C, mutant and wild-type (kind gifts of Dr. I. Hoffmann, DKFZ, Germany) were cloned into pCMX under the CMV promoter, and injected into cells as described (Hagting et al. 1998).
Histone H1 Kinase Assays
Histone H1 kinase assays with purified cyclin A/CDK2 or cyclin B1-cdc2 were performed as described (Krude et al. 1997). The amount of 33P incorporated into histone H1 was quantified using a scintillation counter (Beckman).
Immunofluorescence
Cells were fixed in 50:50 vol/vol methanol/acetone and stained with affinity-purified anti–cyclin B1 serum at 1:200 dilution, and with an anti–β tubulin monoclonal antibody (Amersham) at 1:100 as described (Pines 1997). As secondary antibodies, Cy5-labeled anti–rabbit and Cy2-labeled anti–mouse antibody were used at 1:200 and 1:500, respectively.
Time-Lapse Imaging and Analysis
Cells were plated and synchronized in ΔT 0.15-mm dishes (Bioptechs). For microinjection and observation, the culture medium was replaced with CO2-independent medium without phenol red (GIBCO BRL) and overlaid with mineral oil. Dishes were maintained at 37°C using the ΔT system (Bioptechs). Lambda 10-2 filter wheels (Sutter Instruments) with custom filters (Chroma Technology) were used to control the excitation and emission wavelengths (for cyclin B1-GFP imaging). The filter wheel on the camera port also had a polaroid filter to capture DIC images. Images were captured on a Leica DMIRBE with a PentaMax camera (Princeton Instruments), and a PowerWave computer (PowerComputing) or Macintosh 8600 (Apple) computer running IP Lab Spectrum imaging software (Scanalytics Inc.) as described (Karlsson and Pines 1998). To quantify the amount of fluorescence in a cell over time, a region encompassing the cell was drawn manually and copied onto successive fluorescence images of a time series saved in IP Lab Spectrum format. The sum of pixel intensities (total fluorescence) and the number of pixels (area) of this region in successive images was quantified using IP Lab Spectrum. The mean pixel intensity outside the region of cell fluorescence (mean background) was measured and multiplied by the number of pixels (area) of the original cell region. This represented that amount of the total fluorescence in the cell region which was due to background rather than GFP fluorescence. This was subtracted from the total fluorescence of the cell to give a figure representing the total amount of GFP fluorescence, and thus, the amount of GFP-linked protein in the cell (total fluorescence minus background). Captured images were animated with Adobe Premiere and the time to reach mitosis recorded for each cell. Cells were scored as entering mitosis when they had rounded up and condensed their chromosomes. Time-lapse imaging showed that cells were still viable after premature mitosis because cells exited mitosis and flattened out. Images were exported to Adobe Photoshop for printing.
Results
To investigate the role of cyclin A in mitosis, we have used a time-lapse microscopy assay to determine the effect on entry into mitosis of inhibiting or enhancing cyclin A/CDK2 kinase activity. In this assay, cells were first synchronized in S or G2 phase by a thymidine-aphidicolin block and release regime, and then viewed by time-lapse DIC microscopy to assess when they entered, and subsequently exited, mitosis. In each experiment, control cells were simultaneously visualized in the same field as the experimental cells. Cells were demonstrated to be in S or G2 phase by flow cytometry and by the incorporation of BrdU.
Cyclin A/CDK2 Causes G2 Phase but Not S Phase Cells to Enter Mitosis Prematurely
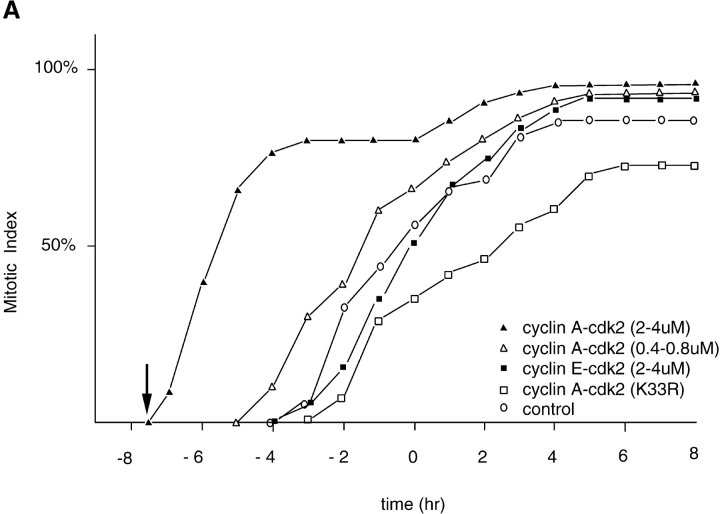
To increase the amount of active cyclin A/CDK2 in a specific phase of the cell cycle we microinjected cyclin A/CDK2 purified from baculovirus-infected Sf9 cells into synchronized HeLa cells. This complex was an active histone H1 kinase and >90% pure on a silver-stained SDS-PAGE gel, as previously described (Krude et al. 1997). We found that injecting a final concentration of 2–4 μM cyclin A/CDK2, which represents ∼20–40-fold excess over the endogenous cyclin A, into early G2 cells (∼7 h after release from an aphidicolin block), caused the cells to begin to enter mitosis 30 min to 1 h after injection (Fig. 1 A). This was at least 4 h before control. Uninjected cells began mitosis. This effect required CDK2 kinase activity, because cyclin A, in a complex with an inactive CDK2K33R mutant, did not promote, indeed it slightly inhibited, entry into mitosis (Fig. 1 A). The effect was specific to cyclin A/CDK2 because microinjecting the same amount of cyclin E-CDK2 did not cause G2 phase cells prematurely to enter mitosis (Fig. 1 A). (We injected an equal amount of cyclin E/CDK2 rather than attempt to inject an equal amount of kinase activity, because no single substrate can be used to compare the physiological specific activities of different cyclin/CDKs. This is due to the substrate-targeting role of the cyclin which causes the apparent activity of a cyclin/CDK to depend on the substrate in the assay. For example, unlike cyclin A/CDK2, cyclin E/CDK2 only weakly phosphorylates histone H1, but it strongly phosphorylates NPAT (Zhao et al. 1998).) The mitosis-promoting activity of cyclin A/CDK2 was also dose dependent, because injecting a final concentration of 0.4–0.8 μM cyclin A/CDK2 (a four- to eightfold excess over endogenous cyclin A/CDK2) caused early G2 phase cells to enter mitosis earlier than control cells, but later than cells injected with 2–4 μM cyclin A/CDK2 (Fig. 1 A). Overall, these results suggested that cyclin A/CDK2 might be a rate-limiting component in G2 phase for the initiation of mitosis.
Figure 1.
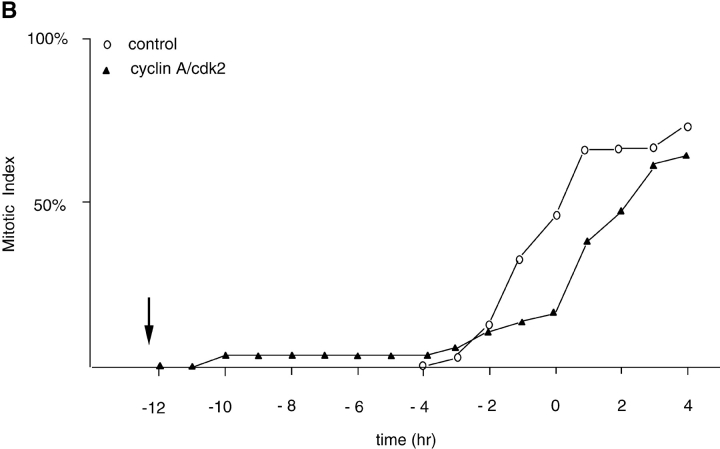
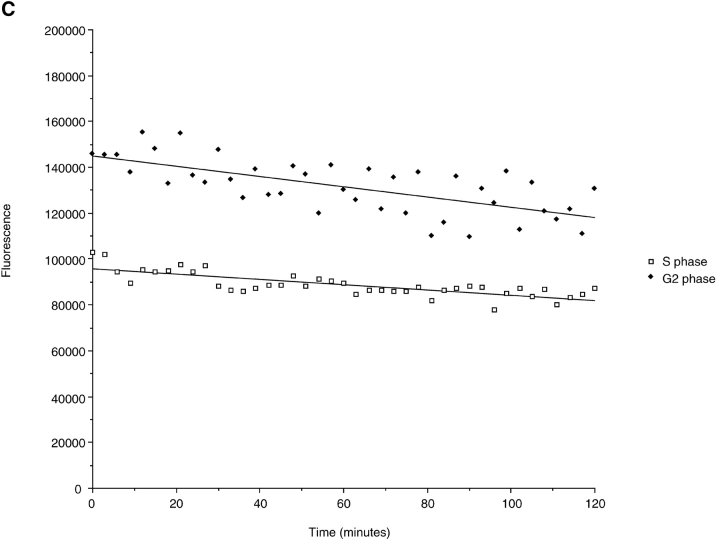
(A) Cyclin A/CDK2 will promote mitosis in G2 phase cells. HeLa cells were synchronized at the beginning of S phase by a thymidine-aphidicolin block and then released. After 7 h cells entered G2 phase and at least 35 cells were injected (time marked by the arrow) with either cyclin A/CDK2 (2–4 μM, filled diamonds; 0.4–0.8 μM, open diamonds), or cyclin E-CDK2 (2–4 μM, filled squares), or cyclin A/CDK2K33R (open squares) or uninjected (open circles). Cells were followed by time-lapse DIC microscopy to determine when they entered mitosis as defined by rounding up and chromosome condensation. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least two independent experiments. (B) Cyclin A/CDK2 does not promote mitosis in S phase cells. HeLa cells were synchronized at the beginning of S phase by a thymidine-aphidicolin block and then released. After 2 h cells were either injected (time marked by the arrow) with cyclin A/CDK2 (2–4 μM; filled diamonds, n = 36) or not (open circles) and followed by time-lapse microscopy to determine when they entered mitosis. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least three independent experiments. (C) Cyclin A/CDK2 is not inactivated in S phase cells by proteolysis. HeLa cells were synchronized at the beginning of S phase or G2 phase by a thymidine-aphidicolin block and release regime. S phase (squares) or G2 phase (diamonds) cells were injected with purified cyclin A-GFP and imaged by DIC and epifluorescence microscopy every 3 min, keeping all parameters (exposure time, magnification and numerical aperture of the lens) constant. The amount of fluorescence in the cells was quantified (see Materials and Methods) and the rate of decrease calculated for S phase (114 pixels per minute) and G2 phase (224 pixels per minute). The effect of bleaching was minimal as most of the decrease was prevented by adding a proteasome inhibitor MG132 (not shown). Values are representative of two independent experiments.
We then asked whether cyclin A was simply able to accelerate cells through G2 phase, or whether it was also able to cause mitosis in cells with unreplicated DNA. Therefore, we injected cyclin A/CDK2 into S phase cells (2 h after release from an aphidicolin block). This caused <10% of the cells to enter mitosis and, furthermore, >90% of the cells had an apparently normal length G2 phase, suggesting either that the exogenous cyclin A/CDK2 had been inactivated, or that the appropriate mitotic substrates were not present in S phase (Fig. 1 B).
Although human cyclin A had been shown to accumulate through S and G2 phases until it was rapidly degraded in mitosis (Pines and Hunter 1990), it was possible that the exogenous cyclin A was inactivated in S phase through proteolysis. To test this, we injected a cyclin A–GFP fusion protein which acts as a proper marker for cyclin A; it binds and activates CDK2, localizes to the nucleus, and is rapidly degraded in mitosis (del Elzen, N., and J. Pines, manuscript in preparation). This fusion protein also has mitosis promoting activity in G2 phase cells. We were able to measure the rate of proteolysis of the protein by measuring the amount of GFP fluorescence (Clute and Pines 1999). There was a low rate of degradation in S and in G2 phases, which increased with larger amounts of protein, but this rate was insufficient to account for the inactivation of cyclin A. Furthermore, the rate was the same in S or G2 phase cells (Fig. 1 C). Given that cyclin A was equally stable whether injected into cells in S phase or G2 phase, this suggested that S phase cells do not inactivate exogenous cyclin A by proteolysis.
Cells that Enter Mitosis Prematurely Do Not Properly Divide
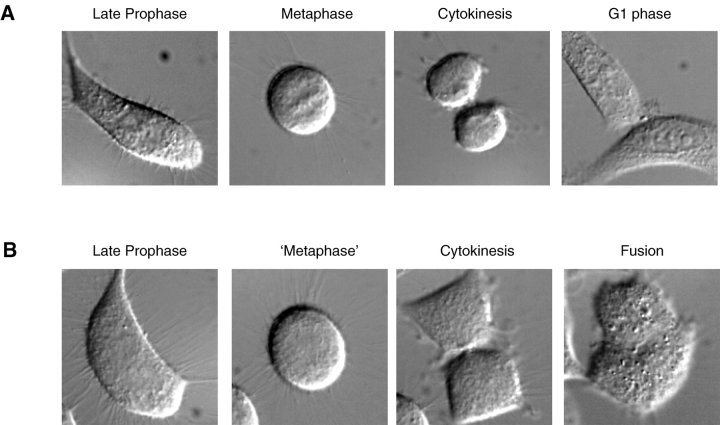
Cells that are forced to enter mitosis without completing DNA synthesis undergo premature chromosome condensation/mitotic catastrophe and die after arresting in mitosis. However, this was not the case for the G2 cells that were forced to enter mitosis prematurely by the injection of cyclin A/CDK2. In many of these cells, mitosis was considerably prolonged, from ∼1 h to between 3 and 4 h. Prophase and prometaphase/metaphase were the most prolonged; anaphase and telophase were of relatively normal duration. In cells injected in mid to late G2 phase the chromosomes clearly condensed and aligned on the metaphase plate, and these cells subsequently divided and remained separate (Fig. 2 A). However, in cells injected in early G2 phase, and in the small minority of S phase cells that prematurely entered mitosis when we injected cyclin A/CDK2, condensed chromosomes were never visible and in these cases the sister cells fused soon after cytokinesis (Fig. 2 B). This phenotype is, therefore, likely to reflect premature condensation of incompletely replicated DNA.
Figure 2.
Cells forced into premature mitosis by cyclin A often fail to condense fully their chromosomes. HeLa cells were synchronized in G2 phase and injected with 2–4 μM cyclin A/CDK2 as in Fig. 1 A. Representative DIC images are shown of a cell that does (A) and a cell that does not (B) fully condense its chromosomes at metaphase. The cell that does not condense its chromosomes divides but the daughter cells fuse.
The Effect of Cyclin A/CDK2 Can Be Blocked by Dominant Negative Mutants of Cdc25B and Cdc25C
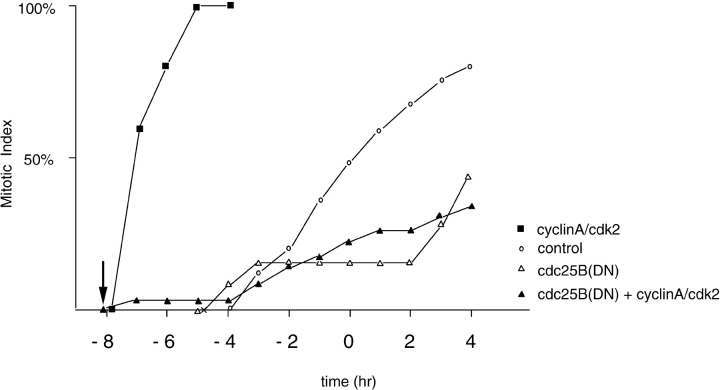
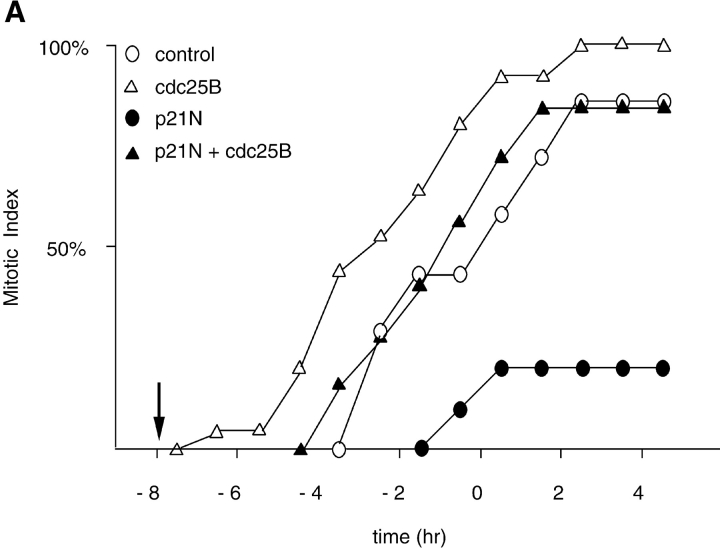
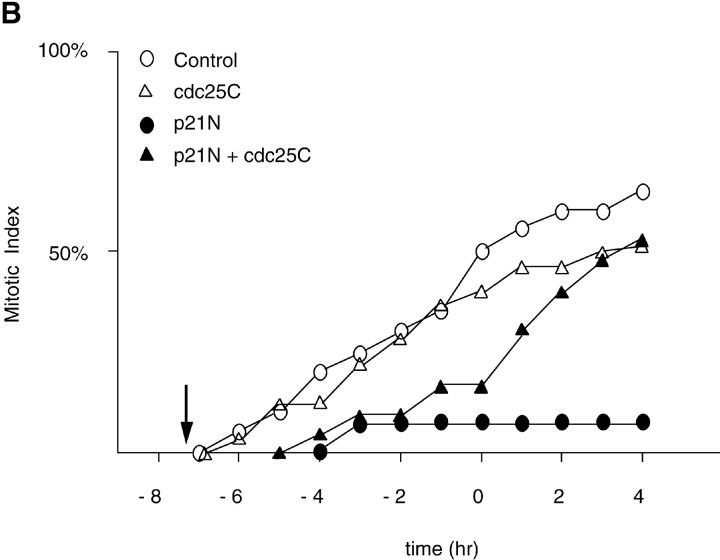
We wished to determine whether the M phase–promoting activity of cyclin A/CDK2 was acting through known regulators of cyclin B1/CDK1. Therefore, we injected cells with expression constructs that encoded inactive mutants of Cdc25B or Cdc25C, which act as dominant negative mutants and arrest cells in G2 phase. A dominant negative mutant of Cdc25B was able to block the effect of injecting cyclin A/CDK2 (Fig. 3), as did a dominant negative Cdc25C, but to a lesser extent than the Cdc25B mutant (data not shown). Cells injected with cyclin A/CDK2 plus the dominant negative forms of Cdc25B or Cdc25C did round up, and their chromosomes partially condensed, but their nuclear envelopes remained intact and they did not divide (data not shown). These results suggested that cyclin A/CDK2 was acting upstream of the activation of cyclin B/CDK1, and that cyclin B1/CDK1 activity was required for nuclear envelope breakdown but not for the initial stages of chromosome condensation (see below).
Figure 3.
A catalytically inactive mutant of Cdc25B prevents cyclin A/CDK2 from promoting premature mitosis. HeLa cells in S phase were microinjected or not with a catalytically inactive form of cdc25B3 cDNA under the CMV promoter at a concentration of 0.1 μg/μl. When cells entered G2 phase (∼4 h later) they were injected or not with cyclin A/CDK2 to a final concentration of ∼2–4 μM. A minimum of 36 cells for each sample were followed by time-lapse DIC microscopy as in Fig. 1 and the results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least two independent experiments.
Inhibiting Cyclin A/CDK2 Prevents Cells from Entering Mitosis
Our results suggested that cyclin A was able to promote mitosis once cells had replicated their DNA, and, therefore, we wished to determine the period in G2 phase for which cyclin A was required to initiate mitosis. To answer this question, we designed a means to inhibit cyclin A/CDK2 activity at specific points in G2 and M phase.
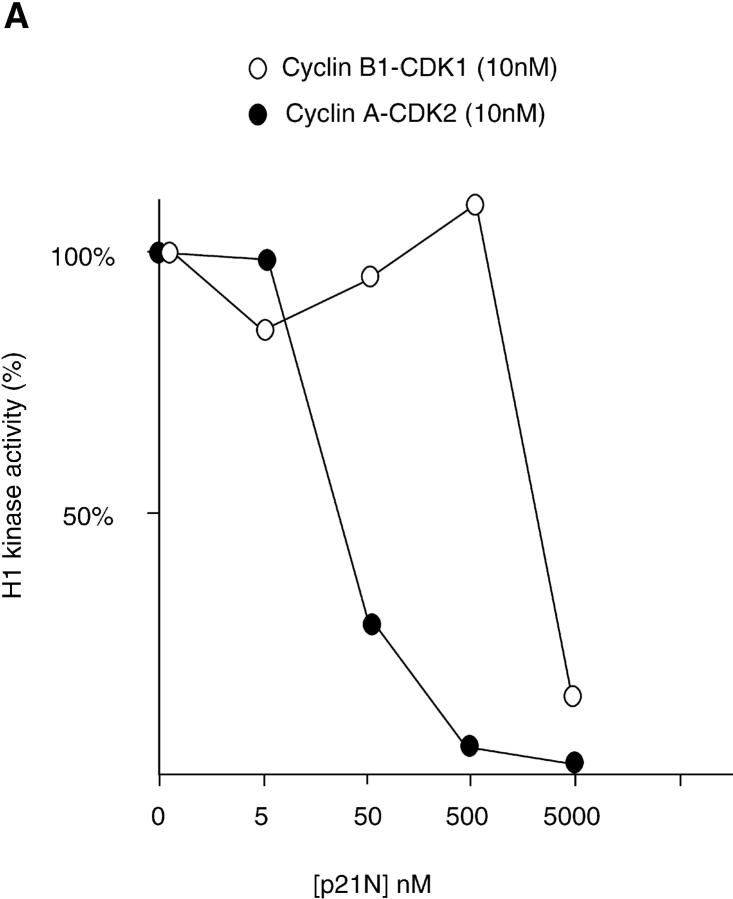
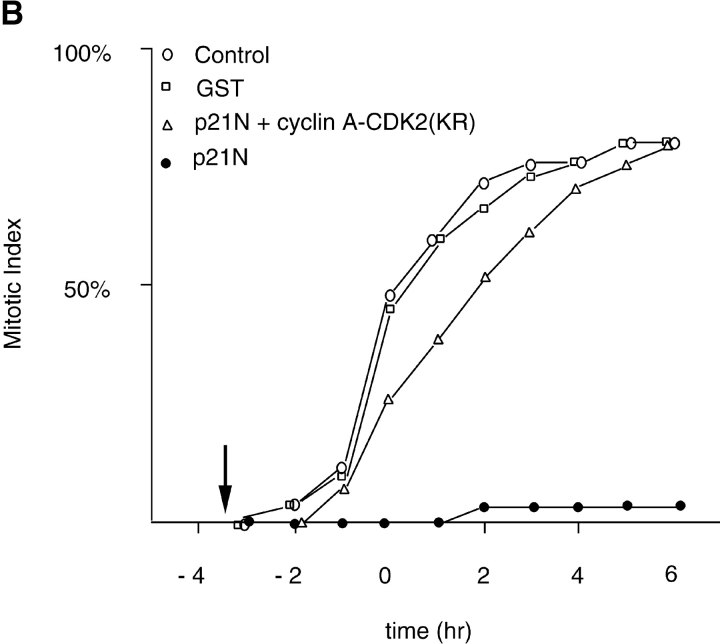
To inhibit cyclin A/CDK2 kinase activity, we chose to microinject the first 90 amino acids of human p21Waf1/Cip1/Sdi1 (hereafter referred to as p21N) purified as a GST-fusion protein from E. coli. This construct contained both a cyclin binding motif (Cy1) and a CDK-inhibitory motif (Chen et al. 1995, Chen et al. 1996; Goubin and Ducommun 1995; Warbrick et al. 1995; Fotedar et al. 1996b), and efficiently inhibited cyclin A/CDK2 more than cyclin B/CDK1 in vitro (Fig. 4 A). The effect of injecting GST-p21N was specific to the p21N moiety because injecting either buffer or GST had no effect on the dynamics of entry into mitosis (Fig. 4 B). Furthermore, coinjection with an equal amount of cyclin A/CDK2 negated the effect of p21N (Fig. 4 B). We did not use full-length p21Cip1/Waf1/Sic1 because we found that this activated a G2 checkpoint (data not shown) related to that activated by overexpressing PCNA, as previously reported (Tournier et al. 1996; Cayrol et al. 1998).
Figure 4.
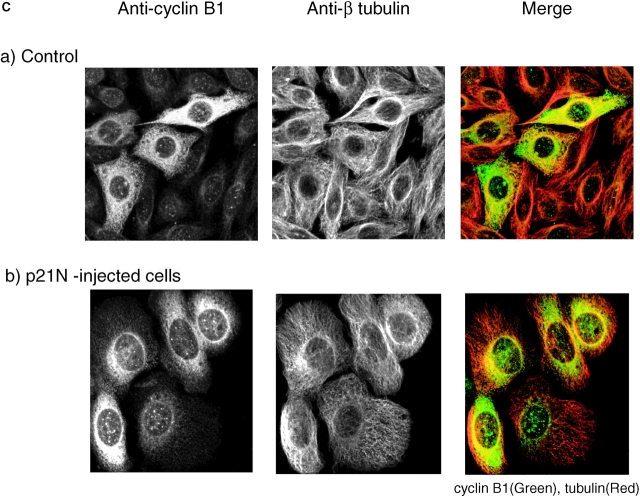
p21N inhibits cyclin A/CDK2 and blocks cells in G2 phase. (A) p21N inhibits cyclin A/CDK2 better than cyclin B-cdc2 in vitro. Cyclin A/CDK2 and cyclin B-cdc2 were produced and purified from baculovirus infected cells and assayed for histone H1 kinase activity with γ-[33P]ATP. GST-p21N was produced and purified from E. coli and added to the cyclin-Cdk incubations at the indicated concentrations. At a 40-fold excess of p21N, cyclin A/CDK2 is almost completely inhibited but cyclin B1-CDK1 is unaffected. (B) p21N prevents cells from entering mitosis. G2 phase HeLa cells were injected or not with a final concentration of 2 μM p21N (filled circles, representing ∼40-fold excess over cyclin A/CDK2), or p21N preincubated with cyclin A/CDK2K33R (open diamonds), or with GST (open squares) and their entry into mitosis followed by time-lapse microscopy. A minimum of 25 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (C) Cells arrested by p21N arrest in G2 phase. G2 phase HeLa cells were injected or not with a final concentration of 2 μM p21N and fixed and stained for immunofluorescence. Cells were stained with anti–cyclin B1 as a marker of G2 phase, and anti-tubulin to visualize any mitotic spindles. (a) Control cells that have mostly reentered G1 phase; (b) p21N-injected cells fixed at a time when all the control cells had completed mitosis.
We found that injecting ∼2 μM p21N, which represents a ∼40-fold excess over the endogenous cyclin A (data not shown), at 10 h after release from an aphidicolin block caused cells to arrest in G2 phase (Fig. 4 B). (We injected excess p21N to ensure that we completely blocked cyclin A/CDK2 activity.) Previous studies in which full-length p21 was overexpressed have found that cyclin B1 accumulated in the nucleus (Bates et al. 1998; Dulic et al. 1998). Therefore, we stained cells blocked in G2 phase by p21N with anti–cyclin B1 antibodies and found that cyclin B1 remained cytoplasmic (Fig. 4 C). Thus, full-length p21Cip1/Waf1/Sic1 does not cause cyclin B1 to accumulate in the nucleus by inhibiting cyclin A/CDK kinase activity.
Cdc25s and Active Cyclin B/CDK1 Can Overcome the Block Imposed by p21N
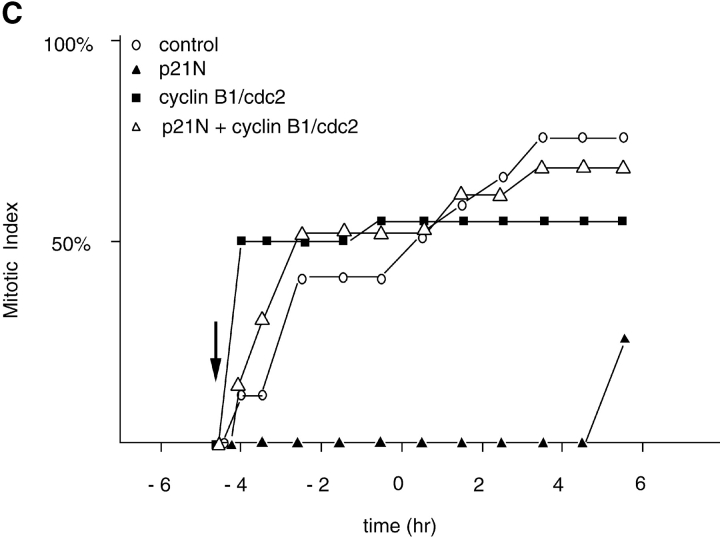
Our previous results showed that dominant negative mutants of Cdc25B and Cdc25C blocked the mitosis-promoting activity of cyclin A/CDK2. Therefore, we assayed whether overexpressing Cdc25B and C, or microinjecting active cyclin B1/CDK1, could promote mitosis when cyclin A/CDK2 was inhibited with p21N. Injecting the cDNAs for Cdc25B (Fig. 5 A) or Cdc25C (Fig. 5 B), into cells blocked in G2 phase by injection of p21N caused cells to enter mitosis, although passage through mitosis was delayed (Fig. 5). We were also able to force cells into mitosis with active cyclin B1/CDK1 purified from baculovirus-infected Sf9 cells (Fig. 5 C). However, this differed from the effect of cyclin A/CDK2 because the time to entry into mitosis did not depend on the dose of the cyclin B1/CDK1 kinase, and the nuclear envelope broke down as soon as the cells rounded up. Thus, it appeared that the function of cyclin A/CDK2 to promote mitosis was bypassed by directly activating cyclin B1/CDK1, suggesting that cyclin A/CDK2 acted upstream of cyclin B/CDK1. These results also showed that p21N did not inhibit cyclin B/CDK1 in vivo.
Figure 5.
Activators of cyclin B-CDK2 and active cyclin B-CDK2 itself overcome the p21 block. (A) G2 phase HeLa cells were microinjected or not at the indicated time with p21 to a final concentration of 2–4 μM, and the cDNA encoding cdc25B3 under the CMV promoter at a concentration of 0.1μg/μl. Entry into mitosis was followed by time-lapse microscopy and a minimum of 21 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (B) G2 phase HeLa cells were microinjected or not at the indicated time with p21N to a final concentration of 2–4 μM, and the cDNA encoding cdc25C under the CMV promoter at a concentration of 0.1 μg/μl. Entry into mitosis was followed by time-lapse microscopy and a minimum of 23 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (C) G2 phase HeLa cells were microinjected or not at the indicated time with p21N to a final concentration of 2–4 μM, and purified active cyclin B1-CDK1 (∼2 μM). Entry into mitosis was followed by time-lapse microscopy and a minimum of 20 cells was followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments.
p21N Will Cause Prophase Cells to Return to Interphase
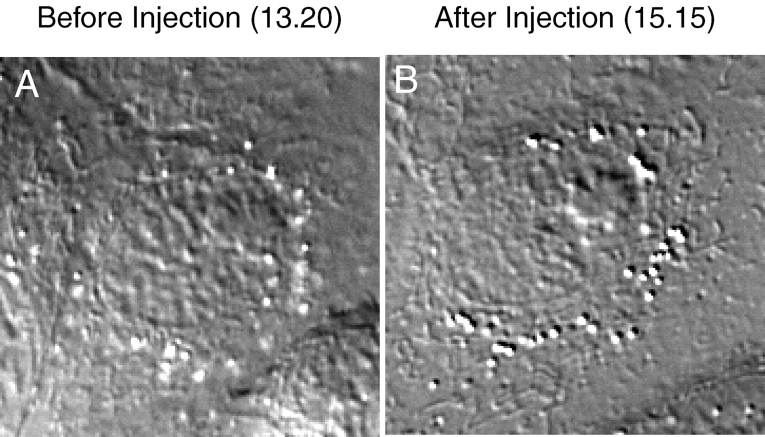
To define the period of the cell cycle for which cyclin A/CDK2 kinase was required for the initiation of mitosis, we injected p21N into late G2 phase and mitotic cells. As expected, we found that late G2 phase cells remained in G2 phase. Prometaphase cells that had undergone nuclear envelope breakdown continued to progress through mitosis (10/10 cells), and this was further evidence that p21N did not block cyclin B1/CDK1. However, when we injected p21N into prophase HeLa cells that had not yet reached NEBD, these cells did not continue through mitosis but decondensed their chromosomes, flattened out and arrested, apparently in interphase again (6/6 cells, and see below). Control prophase cells injected with GST completed mitosis (data not shown). After 2–3 h, cells injected with p21N began to re-enter mitosis and this time completed cell division. This observation was reminiscent of the prophase checkpoint that had recently been identified by Rieder and Cole 1998, who found that PtK1 cells in early prophase, >30 min before NEBD, would return to interphase after nuclear irradiation. Therefore, we microinjected p21N into prophase PtK1 cells. We found that prophase PtK1 cells returned to interphase up to the time when the nucleoli disappeared in mid prophase (17/17 cells Fig. 6 B). p21N could not prevent cells later in prophase from progressing through mitosis; these cells underwent NEBD ∼10–20 min after microinjection (8/8 cells). Thus, microinjecting p21N mimicked the effect of the prophase checkpoint.
Figure 6.
p21N will cause a prophase cell to return to G2 phase. PtK1 cells in prophase were microinjected with purified GST-p21N to a final concentration of 2–4 μM and visualized by time-lapse DIC microscopy. (A) PtK1 cells just before microinjection at 13.20. The condensed chromosomes are clearly visible. (B) The same PtK1 cell 115 min after injection of p21N. The chromosomes have decondensed and the nucleolus has reformed. Images are representative of 17 cells in at least five independent experiments.
p21N Cannot Prevent Cells from Completing Mitosis after Cyclin B1/CDK1 Has Translocated into the Nucleus
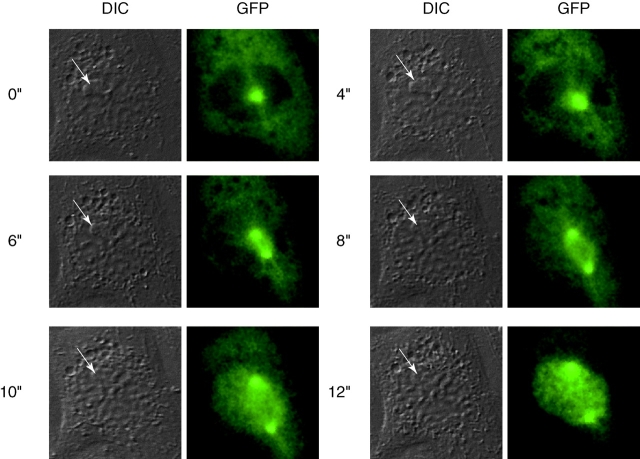
From our studies, and from those of Rieder and Cole 1998, there appeared to be a point in mid to late prophase after which the cells are committed to NEBD. From our results, this point correlated with the time when the nucleoli disappeared. One event that occurs at approximately this time is the translocation of cyclin B1/CDK1 into the nucleus, therefore, we reasoned that the translocation of cyclin B1/CDK1 might mark the point of no return for mitosis. We have previously shown that a cyclin B1-GFP fusion protein acts as a proper marker for the endogenous cyclin B1 (Hagting et al. 1998, Hagting et al. 1999). Therefore, we correlated the behavior of a cyclin B1-GFP chimaera in PtK1 cells with the morphology of prophase nuclei under DIC microscopy. We found an excellent agreement between the time at which the nucleoli disappeared, which marks the point when p21N is unable to return the cells to interphase, and the time when cyclin B1-GFP translocated into the nucleus (Fig. 7).
Figure 7.
Cyclin B1-GFP translocates into the nucleus at the end of prophase just after the nucleolus disappears. PtK1 cells in late G2 phase were injected with purified cyclin B1-GFP. Cells were imaged by time-lapse fluorescence and DIC microscopy and images captured at the indicated times. DIC and fluorescence images show that cyclin B1-GFP translocates into the nucleus just after the nucleolus disappears between the 8″ and 10″ time points (the nucleolus is indicated by an arrow). Images are representative of two independent experiments in this study, and confirmed by several other studies in this laboratory (Hagting et al. 1999).
Discussion
Cyclin A/CDK2 Can Promote Mitosis
In this paper we have used time-lapse DIC microscopy to study the requirement for cyclin A/CDK2 at mitosis. In our experiments we used cyclin A/CDK2 because this is the major form of cyclin A/CDK activity in G2 phase in HeLa cells (Pines and Hunter 1990). We have demonstrated that cyclin A/CDK2 activity appears to be rate limiting for the initiation of mitosis in G2 phase because purified, active cyclin A/CDK2 kinase will initiate mitosis within 30 min of microinjection if cells have finished DNA replication. This role for CDK2 kinase in the initiation of mitosis is consistent with experiments using Xenopus extracts, where CDK2 was shown to be a positive regulator of cyclin B/CDK1 (Guadagno and Newport 1996). However, in these embryonic extracts CDK2 was partnered with cyclin E, whereas we found that cyclin E/CDK2 was unable to promote mitosis. Thus, it appears that the role of the CDK2 kinase in promoting mitosis is conserved in adult, mammalian cells but that the cyclin partner has altered to cyclin A. We have also used the amino terminus of p21 as a specific inhibitor of cyclin A/CDK2 to demonstrate that cyclin A/CDK2 activity is not only required for cells to enter mitosis, but also to progress through mitosis until the middle of prophase. If cyclin A/CDK2 is inhibited in early or mid prophase this causes the cell to return to interphase. However, cyclin A/CDK2 becomes dispensable once cyclin B has translocated into the nucleus.
Cyclin A/CDK as a Target of the Cell Cycle Checkpoint Machinery
Active cyclin A/CDK2 is able to accelerate entry into mitosis by at least 4 h, effectively eliminating G2 phase. However, if cyclin A/CDK2 is injected into cells that are still replicating their DNA, this has no effect on the timing of mitosis in >90% of the injected cells. This may be because the appropriate mitotic substrates are not present, or are inaccessible, in S phase cells. Alternatively, S phase cells may be able to inactivate the mitosis-promoting activity of excess cyclin A/CDK2. Thus, in addition to the inhibition of Cdc25 by Cds1 and Chk1 (Boddy et al. 1998), the negative regulation of cyclin A/CDK2 may underlie the observation that S phase nuclei are able to delay the entry of G2 cells into mitosis (Rao and Johnson 1970). We have shown that any inactivation is not through degrading cyclin A, therefore, it could be through cyclin A/CDK being bound by an excess of CDK inhibitors such as p21Cip1, or p27Kip1 or p57Kip2. However, recent results have shown that there is little free p21Cip1 after S phase begins (Cai and Dynlacht 1998) and p27Kip1 levels fall substantially through ubiquitin-dependent proteolysis in late G1 phase (Pagano et al. 1995). By analogy with the inactivation of cyclin B-CDK1 after DNA damage, cyclin A/CDK2 might be inactivated by phosphorylating CDK2 on threonine 14 (T14) and/or tyrosine 15 (Y15). Interestingly, when we microinjected cyclin A/CDK2 into cells that were arrested in G2 phase by UV irradiation, we found that the cells rounded up but then flattened out again without breaking down their nuclear envelopes (data not shown). Our interpretation of this is that cyclin A/CDK2 was not inactivated, but it could not drive cells further than prophase because cyclin B1/CDK1 was inhibited by the UV treatment.
Our results may be relevant to the observations of Bunz et al. 1998, who showed that in addition to phosphorylating and inactivating cyclin B/CDK1, cells require p21Cip1 to maintain a G2 arrest after DNA damage. We would suggest that p21Cip1 is required to inhibit the M-phase–promoting activity of cyclin A/CDK in G2 phase. Interestingly, when the p53 target gene GADD45 is overexpressed in primary human fibroblasts, they arrest as rounded up cells with condensed DNA and stain strongly for MPM-2 epitopes, but still have intact nuclear envelopes (Wang et al. 1999). This arrest point corresponds to that of cells with active cyclin A/CDK2 but inactive cyclin B1/CDK1. Thus, GADD45 appears to inhibit the step(s) between the initiation of prophase by cyclin A/CDK, and the activation of cyclin B1/CDK1 to initiate nuclear envelope breakdown. Indeed, GADD45 has been shown to bind and inactivate cyclin B/CDK complexes (Zhan et al. 1999).
In most cases, we observed that the early entry into mitosis promoted by excess cyclin A/CDK2 was accompanied by a delayed metaphase, but eventually the cells did divide properly and remained separate. In other cases, mostly in early G2 phase cells and in the small minority of S phase cells that could be forced into mitosis by cyclin A/CDK2, the chromosomes never fully condensed, probably because DNA replication was not complete. These cells had a prolonged mitosis and the daughter cells fused after division. The cell fusion may have been caused by incompletely replicated chromosomes, leading to the presence of chromatin in the region of the cytokinetic furrow, which has been shown to cause the furrow to regress and the daughter cells to fuse (Mullins and Biesele 1977).
Cyclin A/CDK2 Acts Upstream of Cyclin B/CDK1
Cyclin A/CDK2 appears to act upstream of cyclin B1/CDK1 activation. We were able to block the mitosis-promoting effects of cyclin A/CDK2 by expressing a phosphatase-inactive mutant of Cdc25B or, less effectively, by a catalytically inactive form of Cdc25C. (The partial block by the phosphatase-dead form of Cdc25C is likely to be because it is less effective at competing against the pool of the endogenous protein compared with the mutant Cdc25B, given that Cdc25C is a more stable protein than Cdc25B.) The dominant negative Cdc25s blocked cells in prophase, before nuclear envelope breakdown. Consistent with these results, overexpressing either form of Cdc25, or injecting active cyclin B1/CDK1, was able to overcome the block to mitosis imposed by inhibiting cyclin A/CDK2 with p21N. These results suggest that cyclin A/CDK activity is able to drive cells through most of prophase, but that cyclin B/CDK activity is required at the end of prophase for nuclear envelope breakdown.
Our experiments have revealed that cyclin A/CDK activity, rather than cyclin B1/CDK1 activity, is likely to be responsible, directly or indirectly, for a number of changes in the cell as it enters mitosis. Cells that return to interphase when cyclin A/CDK is inactivated by p21N have already begun to condense their chromosomes, to disassemble their nucleoli and to round up. Rieder and Cole 1998 showed that a number of mitosis-specific MPM-2 antigens and phosphorylated histone H3 are also present in cells at this point in mitosis. However, once cyclin B1/CDK1 has translocated into the nucleus, inhibiting cyclin A cannot force cells back into interphase, and very soon after this (∼10 min later) the cells undergo NEBD. Moreover, NEBD marks the point at which cyclin A begins to be degraded (den Elzen, N., and J. Pines, manuscript in preparation). Thus, we can view the entry into (mammalian) mitosis as a two stage event; cyclin A–associated kinase is required for cells to progress into and through prophase, i.e., to condense chromosomes, for the disassembly of the nucleoli, and for the activation and or translocation of cyclin B/CDK1. Once cyclin B1/CDK1 has been activated it is required for progress into metaphase, i.e., for completing chromosome condensation, for nuclear envelope breakdown and completing formation of the mitotic spindle. We note that the centrosomes begin to separate just before cyclin B1 enters the nucleus, making it possible for either cyclin A or cyclin B1 to initiate centrosome separation, although our observation that p21N does not block cells from completing mitosis after the nucleolus disappears suggests that cyclin A/CDK activity is not required to form the spindle itself. Nevertheless, determining the precise time at which cyclin B1/CDK1 is activated, i.e., before or after translocation to the nucleus, is critical for a proper understanding of the irreversible commitment to mitosis. In this respect, studies on maturing starfish oocytes have shown that cyclin B-associated histone H1 kinase activity appears ∼10 min before cyclin B1 enters the nucleus at meiosis I (Ookata et al. 1992). It will also be interesting to determine whether there is another cyclin, perhaps a mammalian cyclin B3, that is subsequently required for some of the events in anaphase and/or telophase.
Cyclin A/CDK May Be the Target of the Prophase Checkpoint
Our studies also suggest that cyclin A/CDK could be the target of the DNA damage checkpoint in prophase. Rieder and Cole 1998 noted that prophase PtK1 cells would return to interphase if they were damaged >30 min before NEBD. In our experiments, p21N causes PtK1 cells to return to mitosis if they were >10–20 min before NEBD. The difference in timing may reflect the time needed to transduce the signal from damaged DNA in Rieder and Cole's studies, compared with our experiments in which we directly introduce an effector molecule. Should a CKI inhibitor be the natural effector molecule we would predict that p21Cip1−/− (or p21Cip1−/−/p27Kip1−/−) cells will have a defective prophase checkpoint.
Acknowledgments
We are grateful to Dr. Jim Roberts (Fred Hutchison Cancer Center, Seattle, WA) for the p21Cip1 cDNA and to Ingrid Hoffmann and Christina Karlsson for the Cdc25B and Cdc25C cDNAs. We also thank Dr. Conly Rieder for helpful advice on why daughter cells sometimes fuse.
N. Furuno was supported by a JSPS/Royal Society exchange fellowship, N. den Elzen is a Commonwealth Universities Scholar, and this work was supported by a Cancer Research Campaign programme grant to J. Pines.
Footnotes
1.used in this paper: CDK, cyclin-dependent kinase; Cdc, cell division cycle
Dr. Furuno's present address is Department of Biology, Kyushu University, Japan.
References
- Bates S., Ryan K.M., Phillips A.C., Vousden K.H. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene. 1998;17:1691–1703. doi: 10.1038/sj.onc.1202104. [DOI] [PubMed] [Google Scholar]
- Boddy M.N., Furnari B., Mondesert O., Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- Bunz F., Dutriaux A., Lengauer C., Waldman T., Zhou S., Brown J.P., Sedivy J.M., Kinzler K.W., Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Cai K., Dynlacht B.D. Activity and nature of p21(WAF1) complexes during the cell cycle. Proc. Natl. Acad. Sci. USA. 1998;95:12254–12259. doi: 10.1073/pnas.95.21.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso M.C., Leonhardt H., Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replicationcyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- Cayrol C., Knibiehler M., Ducommun B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene. 1998;16:311–320. doi: 10.1038/sj.onc.1201543. [DOI] [PubMed] [Google Scholar]
- Chen J., Jackson P.K., Kirschner M.W., Dutta A. Separate domains of p21 involved in the inhibition of cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- Chen J., Saha P., Kornbluth S., Dynlacht B.D., Dutta A. Cyclin-binding motifs are essential for the function of p21Cip1 . Mol. Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute P., Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1999;1:82–87. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- D'Urso G., Marracino R.L., Marshak D.R., Roberts J.M. Cell cycle control of DNA replication by a homologue from human cells of the p34cdc2 protein kinase. Science. 1990;250:786–791. doi: 10.1126/science.2173140. [DOI] [PubMed] [Google Scholar]
- Devault A., Fesquet D., Cavadore J.C., Garrigues A.M., Labbe J.C., Lorca T., Picard A., Philippe M., Doree M. Cyclin A potentiates maturation-promoting factor activation in the early Xenopus embryo via inhibition of the tyrosine kinase that phosphorylates cdc2. J. Cell Biol. 1992;118:1109–1120. doi: 10.1083/jcb.118.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V., Stein G.H., Far D.F., Reed S.I. Nuclear accumulation of p21Cip1 at the onset of mitosisa role at the G2/M-phase transition. Mol. Cell. Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S.J. Cell cycle checkpointspreventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Fogarty P., Campbell S.D., Abu Shumays R., Phalle B.S., Yu K.R., Uy G.L., Goldberg M.L., Sullivan W. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 1997;7:418–426. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- Fotedar A., Cannella D., Fitzgerald P., Rousselle T., Gupta S., Doree M., Fotedar R. Role for cyclin A-dependent kinase in DNA replication in human S phase cell extracts J. Biol. Chem. 271 1996. 31627 31637a [DOI] [PubMed] [Google Scholar]
- Fotedar R., Fitzgerald P., Rousselle T., Cannella D., Doree M., Messier H., Fotedar A. p21 contains independent binding sites for cyclin and Cdk2both sites are required to inhibit Cdk2 kinase activity Oncogene 12 1996. 2155 2164b [PubMed] [Google Scholar]
- Furnari B., Rhind N., Russell P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Fernandez A., Lamb N.J.C. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Goubin F., Ducommun B. Identification of binding domains on the p21Cip1 cyclin-dependent kinase inhibitor. Oncogene. 1995;10:2281–2287. [PubMed] [Google Scholar]
- Guadagno T.M., Newport J.W. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell. 1996;84:73–82. doi: 10.1016/s0092-8674(00)80994-0. [DOI] [PubMed] [Google Scholar]
- Hagting A., Jackman M., Simpson K., Pines J. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr. Biol. 1999;9:680–689. doi: 10.1016/s0960-9822(99)80308-x. [DOI] [PubMed] [Google Scholar]
- Hagting A., Karlsson C., Clute P., Jackman M., Pines J. MPF localisation is controlled by nuclear export. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4127–4138. doi: 10.1093/emboj/17.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J.W., Elledge S.J., Keyomarsi K., Dynlacht B., Tsai L.H., Zhang P., Dobrowolski S., Bai C., Connell Crowley L., Swindell E. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C., Pines J. Green fluorescent protein. In: Celis J., editor. Cell BiologyA Laboratory Handbook. Vol. 4. Academic Press; San Diego: 1998. pp. 246–252. [Google Scholar]
- Knoblich J.A., Lehner C.F. Synergistic action of Drosophila cyclins A and B during the G2-M transition. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude T., Jackman M.R., Pines J.N., Laskey R.A. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;87:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- Lehner C.F., O'Farrell P.H. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989;56:957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay H.D., Griffiths D.J., Edwards R.J., Christensen P.U., Murray J.M., Osman F., Walworth N., Carr A.M. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe . Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona A., Furnari B., Mondesert O., Russell P. Nuclear localization of Cdc25 regulated by DNA damage and 14-3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- Luca F.C., Shibuya E.K., Dohrmann C.E., Ruderman J.V. Both cyclin A delta 60 and B delta 97 are stable and arrest cells in M-phase, but only cyclin B delta 97 turns on cyclin destruction. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J.M., Biesele J.J. Terminal phase of cytokinesis in D-98s cells. J. Cell Biol. 1977;73:672–684. doi: 10.1083/jcb.73.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M., Robetorye R.S., Pereira Smith O.M., Smith J.R. The C-terminal region of p21SDI1/WAF1/CIP1 is involved in proliferating cell nuclear antigen binding but does not appear to be required for growth inhibition. J. Biol. Chem. 1995;270:17060–17063. doi: 10.1074/jbc.270.29.17060. [DOI] [PubMed] [Google Scholar]
- Ookata K., Hisanaga S., Okano T., Tachibana K., Kishimoto T. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:1763–1772. doi: 10.1002/j.1460-2075.1992.tb05228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Tam S.W., Theodoras A., Beer-Romero P., Del Sal G., Chau V., Yew R., Draetta G., Rolfe M. Role of the ubiquitin proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Graves P.R., Thoma R.S., Wu Z., Shaw A.S., Piwnica Worms H. Mitotic and G2 checkpoint controlregulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Petersen B.O., Lukas J., Sorensen C.S., Bartek J., Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. Localization of cell cycle regulators by immunofluorescence. Methods Enzymol. 1997;283:99–113. doi: 10.1016/s0076-6879(97)83010-8. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNAevidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2 . Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60, and behaves differently from cyclin B. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Rao P.N., Johnson R.T. Mammalian cell fusion studies on the regulation of DNA synthesis and mitosis. Nature. 1970;225:159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Hengst H., Reed S.I. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1 . Mol. Cell. Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Cole R.W. Entry into mitosis in vertebrate somatic cells is guarded by a chromosome damage checkpoint that reverses the cell cycle when triggered during early but not late prophase. J. Cell Biol. 1998;142:1013–1022. doi: 10.1083/jcb.142.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Wong C., Thoma R.S., Richman R., Wu Z., Piwnica Worms H., Elledge S.J. Conservation of the Chk1 checkpoint pathway in mammalslinkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Sobczak T.J., Harper F., Florentin Y., Zindy F., Brechot C., Puvion E. Localization of cyclin A at the sites of cellular DNA replication. Exp. Cell Res. 1993;206:43–48. doi: 10.1006/excr.1993.1118. [DOI] [PubMed] [Google Scholar]
- Strausfeld U.P., Howell M., Descombes P., Chevalier S., Rempel R.E., Adamczewski J., Maller J.L., Hunt T., Blow J.J. Both cyclin A and cyclin E have S phase promoting (Spf) activity in Xenopus egg extracts. J. Cell Sci. 1996;109:1555–1563. doi: 10.1242/jcs.109.6.1555. [DOI] [PubMed] [Google Scholar]
- Swenson K., Farrell K.M., Ruderman J.V. The clam embryo protein cyclin A induces entry into M-phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986;47:861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Tournier S., Leroy D., Goubin F., Ducommun B., Hyams J.S. Heterologous expression of the human cyclin-dependent kinase inhibitor p21Cip1 in the fission yeast, Schizosaccharomyces pombe reveals a role for PCNA in the chk1+ cell cycle checkpoint pathway. Mol. Biol. Cell. 1996;7:651–662. doi: 10.1091/mbc.7.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Zhan Q., Coursen J.D., Khan M.A., Kontny H.U., Yu L., Hollander M.C., O'Connor P.M., Fornace A.J., Jr., Harris C.C. GADD45 induction of a G2/M cell cycle checkpoint. Proc. Natl. Acad. Sci. USA. 1999;96:3706–3711. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick E., Lane D.P., Glover D.M., Cox L.S. A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr. Biol. 1995;5:275–282. doi: 10.1016/s0960-9822(95)00058-3. [DOI] [PubMed] [Google Scholar]
- Zhan Q., Antinore M.J., Wang X.W., Carrier F., Smith M.L., Harris C.C., Fornace A.J., Jr. Association with Cdc2 and inhibition of Cdc2/cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999;18:2892–2900. doi: 10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]
- Zhao J., Dynlacht B., Imai T., Hori T., Harlow E. Expression of NPAT, a novel substrate of cyclin E-CDK2, promotes S-phase entry. Genes Dev. 1998;12:456–461. doi: 10.1101/gad.12.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F., Lamas E., Chenivesse X., Sobczak J., Wang J., Fesquet D., Henglein B., Brechot C. Cyclin A is required in S phase in normal epithelial cells. Biochem. Biophys. Res. Commun. 1992;182:1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]