Abstract
Myogenesis is regulated by cell adhesion receptors, including integrins of the β1 family. We report the identification of a novel muscle-specific β1 integrin binding protein (MIBP). MIBP binds to the membrane-proximal cytoplasmic region shared by β1A and β1D integrins, and the binding occurs in vivo as well as in vitro. Furthermore, we show that MIBP is abundantly expressed by C2C12 myogenic cells before fusion, and the expression of MIBP is dramatically downregulated during subsequent differentiation. Finally, we show that overexpression of MIBP in C2C12 cells resulted in a suppression of fusion and terminal differentiation, suggesting that MIBP may play a key role in controlling the progression of muscle differentiation.
Keywords: integrin binding protein, muscle, myogenic differentiation, signal transduction, myogenin
The formation of muscle fibers from individual myoblasts is a highly orchestrated process that is regulated in part by interactions of cell adhesion macromolecules (McDonald et al. 1995; Sastry and Horwitz 1996). Integrins of the β1 family are cell adhesion receptors that have been implicated in a wide variety of developmental processes (Brakebusch et al. 1997). At present, 12 α subunits are known, of which 9 (α1, α2, α4, α5, α6, α7, α9, α11, and αV) are expressed by skeletal muscle either during differentiation or by mature muscle cells (Gullberg et al. 1998). Several results suggest that integrins of the β1 family play key regulatory roles in muscle development. For example, overexpression of the α5 subunit in quail myoblasts results in continued cell proliferation, whereas overexpression of the α6 subunit promotes muscle differentiation (Sastry et al. 1996). Other studies using chimeric transgenic mice that were α5 integrin−/−;+/+ showed that the α5−/− cells were able to contribute to skeletal muscle, but the myofibers were unstable, resulting in a form of muscular dystrophy (Taverna et al. 1998). Similar results showing a mild muscular dystrophy were obtained with a targeted deletion of the α7 integrin chain (Mayer et al. 1997), and mutations in the human integrin α7 gene lead to a congenital myopathy (Hayashi et al. 1998). Differential splicing of primary transcripts may also be important for the progression of myogenesis, both for the α7 chain (Burkin and Kaufman 1999) and for the β1 chain, which switches during myogenesis from the β1A isoform to the β1D isoform (de Melker and Sonnenberg 1999). Other studies using β1−/− cells obtained from transgenic mice clearly show that the β1 subunit is not essential for muscle differentiation (Hirsch et al. 1998), although similar experiments with β1−/− cells in cardiac muscle show delayed differentiation (Fässler et al. 1996). The β1 integrins, although only one of several receptor families mediating cell adhesion in muscle development, nevertheless are likely to play important regulatory roles in the process.
Considerable progress was made recently by Sastry et al. 1999, who demonstrated that the β1 integrin cytoplasmic domain directly influences myoblast proliferation and differentiation. Since the β1 integrin cytoplasmic domain lacks catalytic activity, this implies a critical role for potential β1 integrin cytoplasmic binding partners in the regulation of myogenic differentiation. Using yeast two-hybrid methodology, we report the identification of a novel muscle integrin binding protein (MIBP) that binds to the membrane-proximal cytoplasmic region shared by β1A and β1D. Furthermore, MIBP expression was dramatically downregulated during C2C12 myogenic differentiation, and overexpression of MIBP in myogenic cells resulted in a suppression of myogenesis. The results suggest that MIBP may play an important role in controlling muscle differentiation.
Materials and Methods
Cells, Antibodies, and Other Reagents
Mouse myoblast cells C2C12 and African green monkey COS-7 cells were from American Type Culture Collection. Cells were maintained in DME supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Rabbit anti–β1 integrin antibody MC231 was kindly provided by Dr. John A. McDonald (Mayo Clinic, Scottsdale, AZ). Mouse monoclonal antimyogenin F5D was obtained from the Developmental Studies Hybridoma Bank.
Yeast Two-Hybrid Assays
A cDNA fragment encoding the cytoplasmic domain of human integrin β1D (residues 749–801) was amplified by PCR and inserted into the EcoRI/XhoI site in the pLexA vector (pLexA/β1D). The bait construct was introduced into EGY48 (p8op-lacZ) yeast cells by transformation. The transformants were used to screen a human heart MATCHMAKER LexA cDNA library (>3 × 106 independent clones) as described previously (Tu et al. 1999). To analyze protein–protein interaction between MIBP and β1 mutants, yeast cells were cotransformed with pB42AD and pLexA expression vectors encoding MIBP and the β1 sequences. The transformants were plated and growth of blue colonies in the leucine-deficient medium indicates a positive interaction (Tu et al. 1999).
Northern Blot
A MIBP cDNA probe was prepared by labeling the full-length human MIBP cDNA using an AlkPhos-direct labeling-detection system (Amersham Pharmacia Biotech). A blot containing equal amounts of polyA+ RNA (2 μg/lane) from different human tissues (Clontech Laboratories, Inc.) was hybridized with the MIBP probe. The hybridized mRNA bands were detected with CDP-Star Detection System (Amersham Pharmacia Biotech).
Immunoblotting of Human Tissues for MIBP
Human fetal tissues were washed twice with PBS, homogenized in lysis buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 10 mM Tris, pH 7.5, 1 mM EDTA, 0.2 mM 4-(2-aminoethyl)benzenesulfonylfluoride, HCl, 10 μg/ml aprotinin, 1 μg/ml pepstatin A, and 5 μg/ml leupeptin), and analyzed by immunoblotting with monoclonal anti-MIBP antibody 5B4.7.
Expression and Purification of Recombinant Maltose Binding Protein, Glutathione S-transferase–, and His-tagged Fusion Proteins
DNA constructs encoding maltose binding protein (MBP)–MIBP (pMAL-c2/MIBP), glutathione S-transferase (GST)–β1D (pGEX-5x-1/β1D), and His–MIBP (pET-15b/MIBP) fusion proteins were generated by inserting cDNAs encoding full-length or partial sequences of human MIBP and β1D integrin into the corresponding vectors. The recombinant vectors were used to transform Escherichia coli cells, and the recombinant proteins were purified with glutathione–Sepharose 4B beads, amylose-agarose beads, and His-BindR Resin (Novagen), respectively.
Production and Characterization of a Monoclonal Anti-MIBP Antibody
Mouse monoclonal anti-MIBP antibody was generated using purified His–MIBP recombinant protein as an antigen (Tu et al. 1999). Hybridoma supernatants were screened for anti-MIBP antibody activity by ELISA and immunoblotting. One mAb (clone 5B4.7) recognized MBP–MIBP and His–MIBP but not MBP or irrelevant His-tagged proteins.
Coprecipitation Assays
For direct binding assays, glutathione–Sepharose 4B beads were preincubated with affinity-purified GST fusion protein containing the β1D cytoplasmic domain (GST–β1D) or GST as a control (5 μg/30 μl beads), and then mixed with His–MIBP (5 μg) and incubated at 4°C for 1 h. After washing, His–MIBP coprecipitated with GST–β1D was detected by immunoblotting with anti-MIBP antibody 5B4.7. To perform GST fusion protein pull down assays using cell lysates, C2C12 cells were washed once with cold PBS and lysed with the lysis buffer (PBS, 1% Triton X-100, 0.2 mM 4-(2-aminoethyl)benzenesulfonylfluoride, HCl, 10 μg/ml aprotinin, 1 μg/ml pepstatin A and 5 μg/ml leupeptin). The cell lysates (500 μg) were incubated with equal amounts (10 μg) of GST–β1D, or GST alone as a negative control, and the GST fusion proteins were precipitated with glutathione–Sepharose 4B beads. MIBP in the precipitates was detected by immunoblotting with anti-MIBP antibody 5B4.7.
Coimmunoprecipitation Assays
The full-length MIBP cDNA was inserted into the HindIII/SalI site of pFLAG-CMV2 vector (Kodak). COS-7 cells were transfected with pFLAG-MIBP, or pFLAG-CMV2 as a control, using Lipofectamine Plus (Life Technologies, Inc.). 48 h after transfection, the cells were lysed using lysis buffer. Cell lysates (500 μg protein) were incubated with agarose beads conjugated with mouse monoclonal anti-FLAG antibody M2 (50 μl) or protein A–agarose beads coupled with an irrelevant mouse IgG (50 μl) at 4°C for 1 h. The beads were washed and FLAG-MIBP and β1 integrin were detected in precipitates by immunoblotting with anti-FLAG antibody M5 and anti–β1 integrin antibody MC231, respectively.
Myogenic Differentiation
C2C12 cells were cotransfected with pFLAG-MIBP (or FLAG-CMV2 as a control) and a vector containing a neomycin-resistant marker pEGFP-c2; Clontech Laboratories Inc., using Lipofectamine Plus. The transfectants were selected with 0.5 mg/ml G418 and cloned. Five clones (E3.11, D9.8, B3, C4, and D4) that stably express FLAG-MIBP were obtained. The expression of FLAG-MIBP by the transfectants was analyzed by immunofluorescence staining and immunoblotting with anti-FLAG antibody M5. To analyze the effect of MIBP overexpression on myogenic differentiation, C2C12 cells stably expressing FLAG-MIBP, FLAG control transfectants, and the parental C2C12 cells were grown in DME containing 10% FBS in 24-well collagen-coated plates (Becton Dickinson) until confluence was reached. Myogenic differentiation was induced by switching the medium to DME containing 2% horse serum. Myogenin was detected by immunoblotting with monoclonal antimyogenin antibody F5D.
Results
Identification and Cloning of a Novel MIBP
We have used yeast two-hybrid screens to identify β1 integrin cytoplasmic binding proteins. A bait construct (pLexA/β1D) encoding the β1D integrin cytoplasmic domain (residues 749–801) was used to screen a human heart LexA cDNA library (>3 × 106 independent clones). 15 positive clones were obtained. DNA sequencing showed that plasmids from 3 out of the 15 positive clones contained an open reading frame encoding a novel protein that we termed muscle integrin binding protein or MIBP (Fig. 1 A). The binding of MIBP to the β1D cytoplasmic domain was confirmed by yeast two-hybrid binding assays using purified pB42AD encoding MIBP (Table ). In control experiments, elimination of either the β1D or MIBP sequence failed to activate the reporter genes. In addition, replacement of the integrin cytoplasmic sequences with those of irrelevant proteins (e.g., lamin C) abolished the interaction (Table ), further confirming the specificity of the interaction. We also found that MIBP interacts with the β1A cytoplasmic domain (Table ). Northern blot analysis of human tissues revealed that MIBP mRNA is predominantly expressed in skeletal and cardiac muscle (Fig. 1 B). No expression was detected in brain, placenta, lung, liver, kidney, or pancreas (Fig. 1 B).
Figure 1.
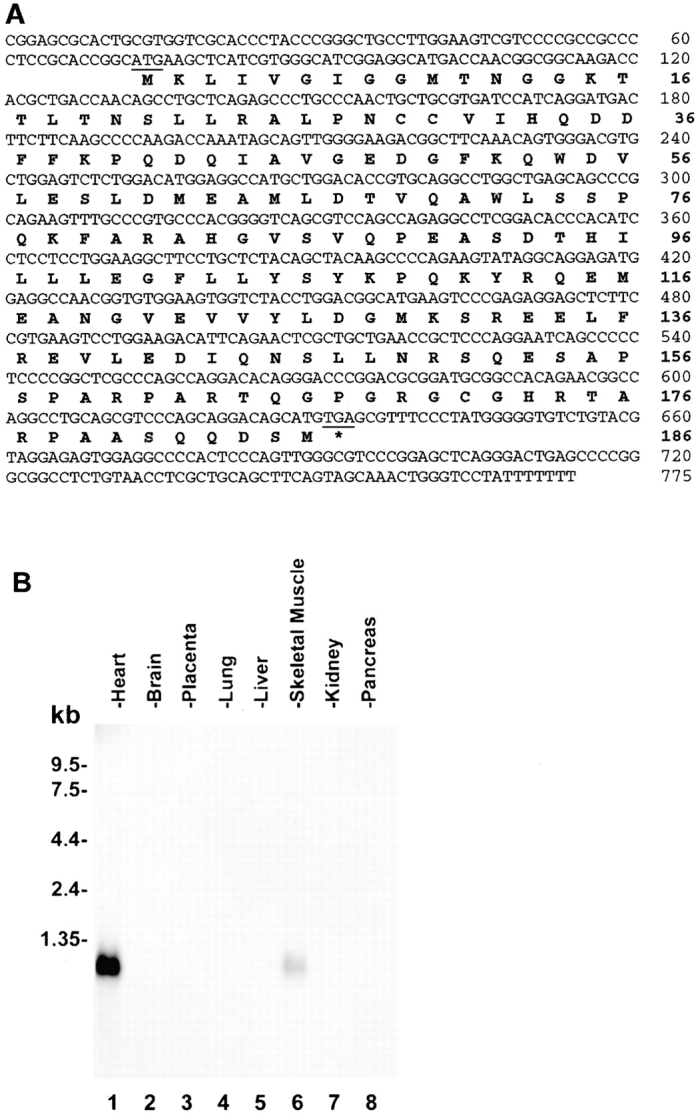
Primary structure and tissue distribution of MIBP. (A) Nucleotide and deduced amino acid sequences of human MIBP. The amino acid sequence is shown below the nucleotide sequence, and the asterisk indicates the stop codon (sequence data available from EMBL/GenBank/DDBJ under accession no. AF 190819). (B) Northern blot of MIBP mRNA in human tissues. 2 μg of polyA+ RNA from human tissues were hybridized with a cDNA probe specific for MIBP.
Table 1.
Interactions of MIBP with Cytoplasmic Domains of β1 Integrins in Yeast Two-Hybrid Binding Assay
| pB42AD construct | pLexA construct | Reporter gene | |
|---|---|---|---|
| LEU2 | LacZ | ||
| pB42AD-MIBP | pLexA-β1D | + | + |
| pB42AD-MIBP | pLexA-β1A | + | + |
| pB42AD-MIBP | pLexA | − | − |
| pB42AD-MIBP | pLexA-lamin C | − | − |
| pB42AD | pLexA-β1D | − | − |
| pB42AD | pLexA-β1A | − | − |
Interactions between proteins encoded by the pLexA and pB42AD constructs were determined by the activation of the reporter genes (LEU2 and LacZ). pLexA-β1D contains residues 749–801 of the β1D integrin cytoplasmic domain. pLexA-β1A contains residues 749–798 of the β1A integrin cytoplasmic domain.
MIBP Is Predominantly Expressed in Skeletal Muscle
To facilitate studies on MIBP, we generated a monoclonal anti-MIBP antibody (5B4.7) using His–MIBP fusion protein as an antigen. mAb 5B4.7 recognizes both His–MIBP (Fig. 2 A, lane 3) and MBP–MIBP (Fig. 2 A, lane 2), but not MBP (Fig. 2 A, lane 1) or an irrelevant His-tagged protein (Fig. 2 A, lane 8). Moreover, analyses of mAb 5B4.7 with a series of MIBP deletion mutants revealed that it recognizes an epitope located within the NH2-terminal region (residues 1–109) of MIBP (Fig. 2 A, lanes 4–7). To test whether mAb 5B4.7 recognizes endogenous MIBP expressed by mammalian cells, we probed mouse C2C12 myoblast lysates. The results showed that it recognizes a single protein band with an apparent molecular mass of ∼19 kD, which is similar to the predicated mass of MIBP (Fig. 2 B, lane 2). Furthermore, binding of the antibody to endogenous 19-kD protein was completely inhibited by an excess of MBP–MIBP (Fig. 2 B, lanes 3–6), but not of MBP (Fig. 2 B, lane 7). We conclude that mAb 5B4.7 specifically recognizes mammalian MIBP as well as recombinant MIBP proteins.
Figure 2.
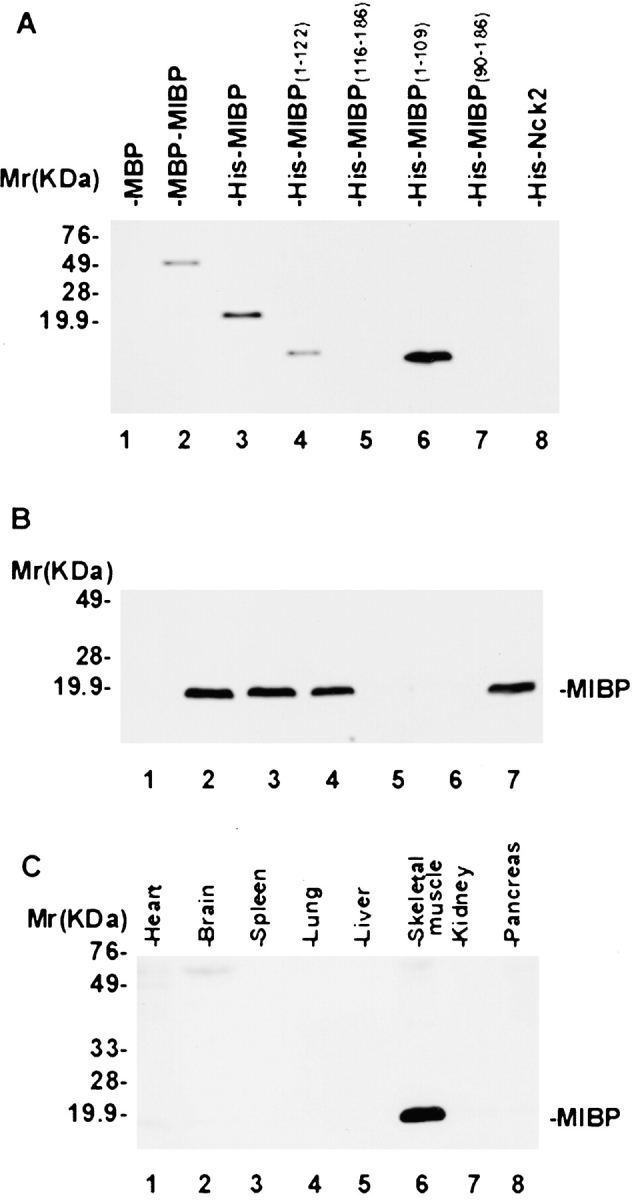
MIBP is predominantly expressed in skeletal muscle. (A) Immunoblot with mAb 5B4.7. Each lane was loaded 10 ng of recombinant proteins. Lane 1, MBP; lane 2, MBP–MIBP; lane 3, His–MIBP; lanes 4–7, His fusion proteins containing partial MIBP sequences as indicated in the figure; and lane 8, His fusion protein containing an irrelevant protein Nck2. (B) Immunoblot showing mAb 5B4.7 specifically recognizes mammalian MIBP. Equal amounts of C2C12 cell extracts (5 μg/lane) were probed with 3.3 nM irrelevant control mouse IgG (lane 1), 3.3 nM mAb 5B4.7 (lane 2), 3.3 nM mAb 5B4.7 preincubated with 1.65 nM (lane 3), 3.3 nM (lane 4), 16.5 nM (lane 5), or 33 nM (lane 6) MBP–MIBP, or 3.3 nM mAb 5B4.7 preincubated 33 nM MBP (lane 7). Note that binding of mAb 5B4.7 to mammalian MIBP was blocked by an excess amount of MBP–MIBP (lanes 5 and 6) but not of MBP (lane 7). (C) MIBP is predominantly expressed in skeletal muscle. Equal amounts (15 μg/lane) of human fetal tissues were analyzed by immunoblotting with mAb 5B4.7.
Next, we analyzed the expression of MIBP protein in different human tissues using the monoclonal anti-MIBP antibody. Consistent with the results from Northern blotting (Fig. 1 B), MIBP protein was detected in skeletal muscle and heart, but not in other tissues (Fig. 2 C). However, although abundant MIBP mRNA was detected in the heart (Fig. 1 B), the level of MIBP protein in the heart was significantly lower than in skeletal muscle (Fig. 2 C), suggesting that the tissue-specific expression of MIBP may be controlled at the translational as well as the transcriptional level.
MIBP Binds to β1 Integrins In Vitro and In Vivo
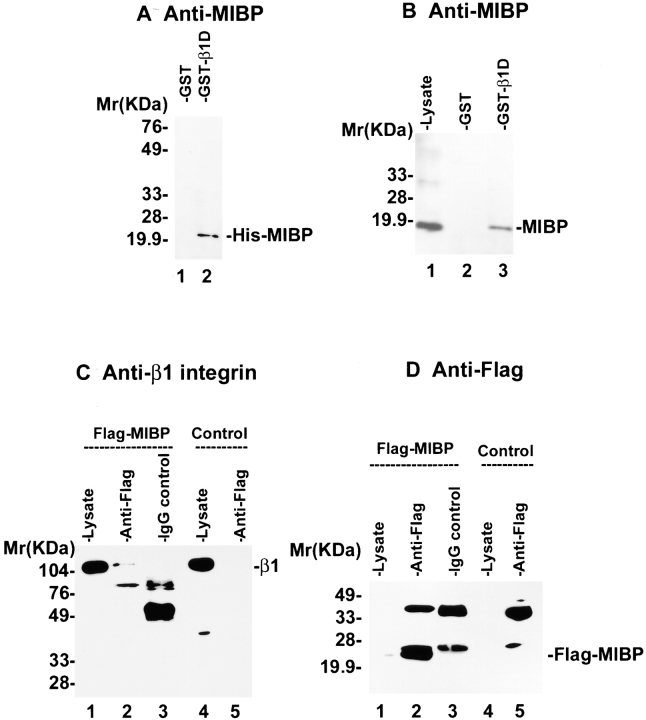
To test whether MIBP can directly bind to the β1 integrin cytoplasmic domain, we expressed and purified a GST fusion protein containing the β1D cytoplasmic domain. GST–β1D (Fig. 3 A, lane 2), but not GST (Fig. 3 A, lane 1), readily interacted with the purified recombinant His-tagged MIBP, indicating that the two proteins can directly interact with each other in the absence of other proteins. In addition, mammalian MIBP protein expressed by the C2C12 myoblasts was coprecipitated with GST–β1D fusion protein (Fig. 3 B, lane 3) but not GST (Fig. 3 B, lane 2). Thus, both mammalian and recombinant MIBP proteins interact with the β1 integrin cytoplasmic domain in vitro. To test whether MIBP associates with β1 integrins in vivo, we expressed a FLAG-tagged MIBP in mammalian cells (Fig. 3 D, lane 1). Coimmunoprecipitation experiments with a monoclonal anti-FLAG antibody showed that the β1 integrins (Fig. 3 C, lane 2) were specifically coprecipitated with FLAG-MIBP (Fig. 3 D, lane 2) from the lysate of the FLAG-MIBP transfectants, but not from that of the control transfectants (Fig. 3 C, lane 5). In additional control experiments, no β1 integrins were precipitated from the FLAG-MIBP lysates with a control mouse IgG (Fig. 3C and Fig. D, lane 3). Thus, MIBP forms a complex with the β1 integrins in mammalian cells as well as in vitro.
Figure 3.
MIBP interacts with β1 integrins in vitro and in vivo. (A) MIBP directly interacts with the β1 integrin cytoplasmic domain in vitro. His–MIBP was incubated with glutathione–Sepharose beads, to which GST (lane 1) or GST–β1D fusion protein was bound (lane 2). His–MIBP bound to GST–β1D was detected by immunoblotting with anti-MIBP antibody 5B4.7. (B) Coprecipitation of mammalian MIBP with GST–β1D fusion protein. C2C12 lysates were incubated with beads coupled to GST (lane 2) or GST–β1D (lane 3). MIBP bound to the GST fusion protein was detected by immunoblotting with anti-MIBP antibody 5B4.7. Lane 1 was loaded with 5 μg C2C12 lysate. (C and D) β1 integrins associate with MIBP in mammalian cells. Anti-FLAG (lanes 2 and 5) or control mouse IgG (lane 3) immunoprecipitates were prepared using lysates of FLAG-MIBP transfectants (lanes 2 and 3) or FLAG control transfectants (lane 5) as described in Materials and Methods. Lanes 1 and 4 were loaded with 3 μg of the FLAG-MIBP and FLAG control lysates, respectively. β1 integrins (C) and FLAG-MIBP (D) were detected by immunoblotting with anti–β1 integrin antibody MC231 and anti-FLAG antibody M5, respectively.
The Membrane-proximal Region of the β1 Integrin Cytoplasmic Domains Mediates the Interaction with MIBP
The cytoplasmic domains of β1D and β1A share a common membrane-proximal region. Since MIBP binds to both β1D and β1A cytoplasmic domains (Table ), it most likely recognizes a site located within this region. To test this, we generated a series of β1D/β1A mutants and analyzed their ability to interact with MIBP in yeast two-hybrid binding assays. The results showed that MIBP specifically interacts with the membrane-proximal region of the β1D or β1A cytoplasmic domain (Table ).
Table 2.
MIBP Binds to the Membrane-proximal Region of the β1 Integrin Cytoplasmic Domain
| β1D integrin cytoplasmic domain deletion mutants | β1D integrin sequence | MIBP binding |
|---|---|---|
| Int β1D (749–801) | LIWKLLMIIHDRREFAKFEKEKMNAKW | + |
| DTQENPIYKSPINNFKNPNYGRKAGL | ||
| ΔBDMT1 (749–795) | LIWKLLMIIHDRREFAKFEKEKMNAKW | + |
| DTQENPIYKSPINNFKNPNY | ||
| ΔBDMT2 (749–785) | LIWKLLMIIHDRREFAKFEKEKMNAKW | + |
| DTQENPIYKS | ||
| ΔBDMT3 (749–777) | LIWKLLMIIHDRREFAKFEKEKMNAKW | + |
| DT | ||
| ΔBDMT4 (773–801) | ------------------------AKW | − |
| DTQENPIYKSPINNFKNPNYGRKAGL |
Interactions between proteins encoded by the pLexA and pB42AD constructs were determined by the activation of the reporter genes LEU2 and LacZ.
The Expression of MIBP Is Downregulated during Myoblast Differentiation
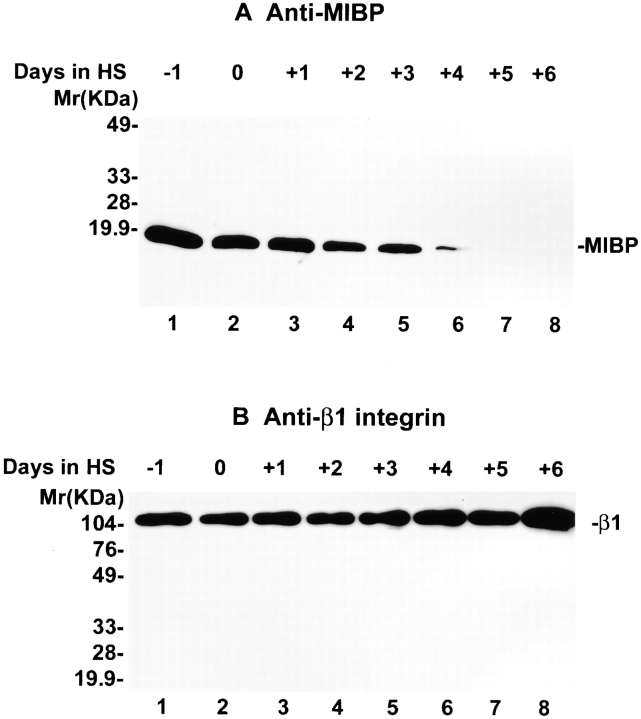
To begin to investigate the role of MIBP in myogenic differentiation, we analyzed MIBP expression during myogenic differentiation using the mouse C2C12 myoblast line as a model system. The results showed that abundant MIBP protein is expressed before terminal differentiation of C2C12 myoblasts (Fig. 4 A, lane 1). Myogenic differentiation was induced by switching the culture medium to DME containing 2% horse serum. Myotubes were observed within the first 2 d of induction, and >80% of the cells were fused into multinucleated myotubes on day 4. The MIBP expression level was decreased upon induction of myogenic differentiation (Fig. 4 A, lanes 2–8). Less than 10% of MIBP was expressed 4 d after the induction of differentiation (Fig. 4 A, compare lanes 2 and 6), and the expression of MIBP was further decreased beyond detection after day 5. In control experiments, the same membrane was stripped and reprobed with an anti–β1 integrin antibody (MC231, which recognizes both β1A and β1D integrins). The β1 integrins (β1A and/or β1D) were detected at all stages of C2C12 differentiation (Fig. 4 B, lanes 1–8).
Figure 4.
Downregulation of MIBP expression during myogenesis. (A) Mouse C2C12 cells were induced to differentiate by switching the medium to DME containing 2% horse serum at day 0. Cells were harvested from 1 d before induction to 6 d after induction. Equal amounts (5 μg) of the cell extracts were loaded onto each lane. MIBP was detected by immunoblotting with mAb 5B4.7. (B) Expression of β1 integrins during C2C12 differentiation. The membrane in A was stripped and reprobed with antibody MC231 that recognizes both β1A and β1D integrins.
Overexpression of MIBP in Myoblasts Inhibits Myogenic Differentiation
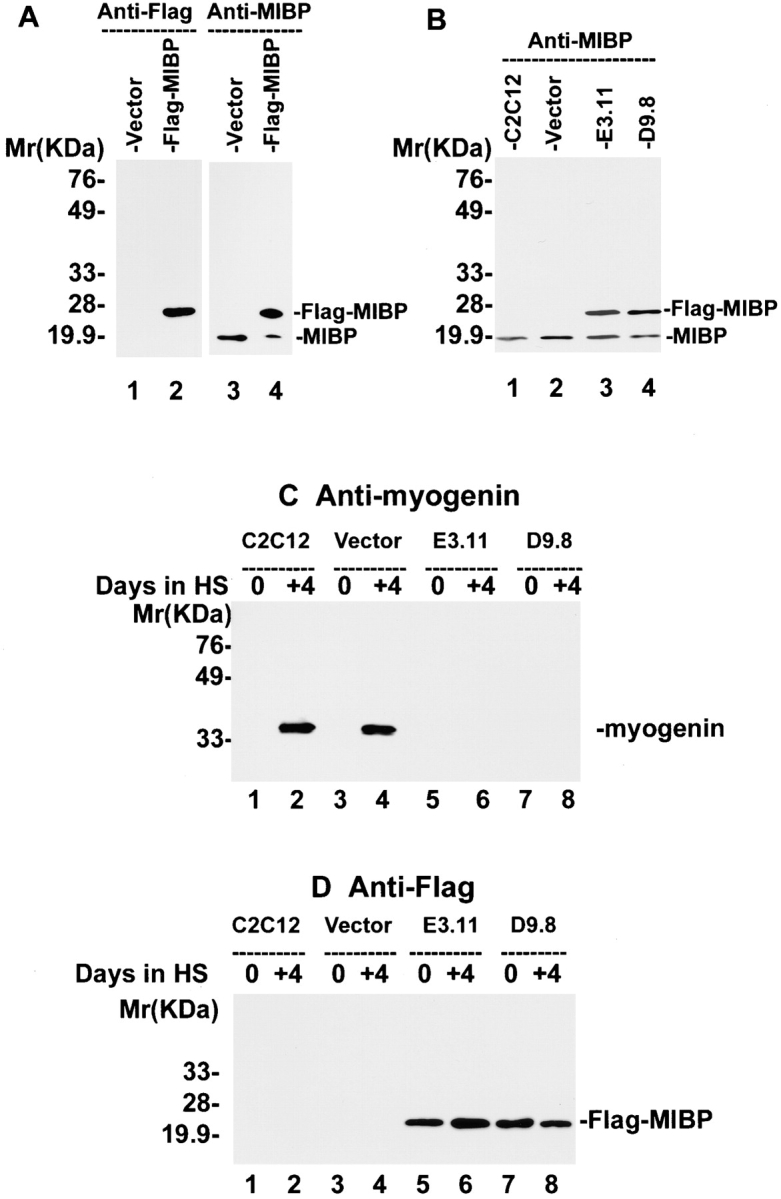
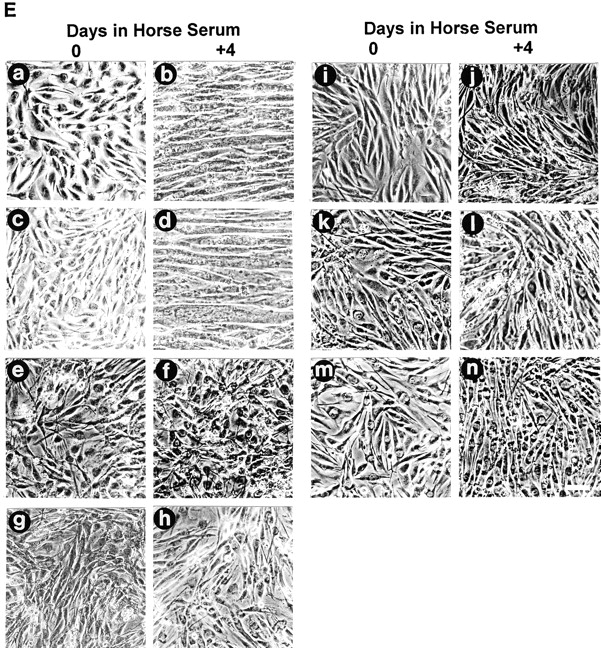
The striking downregulation of MIBP during myogenic differentiation suggests that a higher MIBP expression level may prevent myoblasts from undergoing terminal differentiation. To test this, we overexpressed FLAG-tagged MIBP in C2C12 myoblasts. The expression of FLAG-tagged MIBP in the FLAG-MIBP transfectants, but not C2C12 cells transfected with a vector lacking the MIBP sequence, was confirmed by immunoblotting with an anti-FLAG antibody (Fig. 5 A, lanes 1 and 2) and the anti-MIBP antibody 5B4.7 (Fig. 5 A, lanes 3 and 4). Two independently isolated C2C12 clones (E3.11 and D9.8) that express FLAG-MIBP at a level comparable to that of endogenous MIBP in the proliferating myoblasts (Fig. 5 B) were selected for further analysis. As expected, myogenin (a biochemical marker for myogenic differentiation; Andrés and Walsh 1996) (Fig. 5 C) and abundant multinucleated myotubes (Fig. 5 E) were detected in both the parental C2C12 cells and the vector control transfectants after induction of myoblast differentiation. In contrast, no multinucleated myotubes were detected in E3.11 and D9.8 cells overexpressing FLAG-MIBP under identical experimental conditions (Fig. 5 E). Similar results were obtained with all other FLAG-MIBP–overexpressing C2C12 clones that were analyzed (Fig. 5 E). Consistent with the suppression of myotube formation, no myogenin was detected in the cells overexpressing FLAG-MIBP (Fig. 5 C, lanes 6 and 8). The expression of FLAG-MIBP in the FLAG-MIBP transfectants but not the parental C2C12 or the vector control transfectants, before and after the induction of myoblast differentiation was confirmed by immunoblotting with an anti-FLAG antibody (Fig. 5 D). We conclude from these experiments that overexpression of FLAG-tagged MIBP in C2C12 myoblasts suppresses terminal myogenic differentiation.
Figure 5.
Overexpression of FLAG-MIBP inhibits C2C12 myogenesis. (A) Lysates (5 μg/lane) of the FLAG-MIBP (lanes 2 and 4) and the vector only control (lanes 1 and 3) transfectants were immunoblotted with anti-FLAG antibody M5 (lanes 1 and 2) or anti-MIBP antibody 5B4.7 (lanes 3 and 4). (B) Lysates (5 μg/lane) of parental C2C12 cells (lane 1), the vector-only control (lane 2), FLAG-MIBP–expressing E3.11 (lane 3), and D9.8 (lane 4) clones were immunoblotted with anti-MIBP antibody 5B4.7. (C and D) Cells were harvested immediately before induction of differentiation or 4 d after the induction as indicated in the figure. The expression of myogenin (C) and FLAG-MIBP (D) was analyzed by immunoblotting with monoclonal antimyogenin antibody F5D and anti-FLAG antibody M5, respectively. Each lane was loaded with 10 μg of the cell extracts. E shows the morphology of proliferating myoblasts (panels a, c, e, g, i, k, and m) and myoblasts that were induced to differentiate for 4 d (panels b, d, f, h, j, l, and n). Panels a and b are parental C2C12 cells, c and d are control-transfected cells, e–n are FLAG-MIBP transfected clones E3.11 (panels e and f), D9.8 (panels g and h), B3 (panels i and j), C4 (panels k and l), and D4 (panels m and n). Bar, 200 μm.
Discussion
In this study, we have identified and cloned a novel muscle β1 integrin binding protein, MIBP, and provided strong evidence for an important role of MIBP in the regulation of terminal myogenesis. Using C2C12 myogenic cells as a model system, we show that MIBP is abundantly expressed in proliferating myogenic cells. The expression level of MIBP decreases upon induction of terminal myogenic differentiation, and becomes undetectable after the majority of the myoblasts have fused to form multinucleated myotubes. This striking downregulation of MIBP suggests that the amount of MIBP may be a crucial element in the decision-making process of fusion versus proliferation during myogenic differentiation. In support of this, overexpression of an epitope-tagged MIBP under a promoter that is not subject to regulation in muscle cells resulted in a complete suppression of the terminal differentiation of C2C12 cells.
MIBP is shown to bind to the β1 integrin cytoplasmic domain, which is known to play a key role in controlling myoblast proliferation and differentiation (Sastry et al. 1999). In addition to suppression of myogenic differentiation, our preliminary results indicate that after switching to differentiation medium, expression of FLAG-MIBP in C2C12 myoblasts enhances cell proliferation (Li, J., R. Mayne, and C. Wu, unpublished observations). Taken together, our results suggest that MIBP most likely functions in the regulation of myogenesis via its interaction with the β1 integrin cytoplasmic domain. In this regard, it is particularly interesting to note that the MIBP-binding site is located within the membrane-proximal region of the β1 integrin cytoplasmic domain, a region likely to play an important role in integrin activation (Hughes et al. 1996). It has been shown that the ligand binding affinity of α5β1 integrin in myoblasts is downregulated during myogenesis (Boettiger et al. 1995). Moreover, this inactivation of α5β1 integrin is functionally important to myogenesis (Boettiger et al. 1995) and could potentially contribute to the matrix switch (from a fibronectin-rich matrix to a laminin-rich matrix) that accompanies myogenic differentiation (Gullberg et al. 1998). Thus, one mechanism whereby MIBP potentially functions is by regulating integrin activation, and consequently, matrix deposition and cell adhesion. In addition, MIBP could modulate signal transduction from integrins to other downstream targets such as focal adhesion kinase and paxillin (Sastry et al. 1999), and thereby influence the decision of myoblasts to fuse and undergo terminal differentiation.
Acknowledgments
This work was supported by National Institutes of Health grant DK54639 (to C. Wu), research project grant #98-220-01-CSM from the American Cancer Society (to C. Wu), and the V Foundation for Cancer Research (to C. Wu). C. Wu is a V Foundation Scholar.
Footnotes
Abbreviations used in this paper: GST, glutathione S-transferase; MBP, maltose binding protein; MIBP, muscle integrin binding protein.
References
- Andrés V., Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D., Enomoto-Iwamoto M., Yoon H.Y., Hofer U., Menko A.S., Chiquet-Ehrismann R. Regulation of integrin alpha 5 beta 1 affinity during myogenic differentiation. Dev. Biol. 1995;169:261–272. doi: 10.1006/dbio.1995.1142. [DOI] [PubMed] [Google Scholar]
- Brakebusch C., Hirsch E., Potocnik A., Fässler R. Genetic analysis of β1 integrin functionconfirmed, new and revised roles for a crucial family of cell adhesion molecules. J. Cell Sci. 1997;110:2895–2904. doi: 10.1242/jcs.110.23.2895. [DOI] [PubMed] [Google Scholar]
- Burkin D.J., Kaufman S.J. The α7β1 integrin in muscle development and disease. Cell Tissue Res. 1999;296:183–190. doi: 10.1007/s004410051279. [DOI] [PubMed] [Google Scholar]
- de Melker A.A., Sonnenberg A. Integrinsalternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays. 1999;21:499–509. doi: 10.1002/(SICI)1521-1878(199906)21:6<499::AID-BIES6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Fässler R., Georges-Labouesse E., Hirsch E. Genetic analyses of integrin function in mice. Curr. Opin. Cell Biol. 1996;8:641–646. doi: 10.1016/s0955-0674(96)80105-0. [DOI] [PubMed] [Google Scholar]
- Gullberg D., Velling T., Lohikangas L., Tiger C.F. Integrins during muscle development and in muscular dystrophies. Front. Biosci. 1998;3:D1039–D1050. doi: 10.2741/a344. [DOI] [PubMed] [Google Scholar]
- Hayashi Y.K., Chou F.-L., Engvall E., Ogawa M., Matsuda C., Hirabayashi S., Yokochi K., Ziober B.L., Kramer R.H., Kaufman S.J. Mutations in the integrin α7 gene cause congenital myopathy. Nat. Genet. 1998;19:94–97. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- Hirsch E., Lohikangas L., Gullberg D., Johansson S., Fässler R. Mouse myoblasts can fuse and form a normal sarcomere in the absence of β1 integrin expression. J. Cell Sci. 1998;111:2397–2409. doi: 10.1242/jcs.111.16.2397. [DOI] [PubMed] [Google Scholar]
- Hughes P.E., Diaz-Gonzalez F., Leong L., Wu C., McDonald J.A., Shattil S.J., Ginsberg M.H. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J. Biol. Chem. 1996;271:6571–6574. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- Mayer U., Saher G., Fässler R., Bornemann A., Echtermeyer F., von der Mark H., Miosge N., Pöschl E., von der Mark K. Absence of integrin α7 causes a novel form of muscular dystrophy. Nat. Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- McDonald K.A., Horwitz A.F., Knudsen K.A. Adhesion molecules and skeletal myogenesis. Semin. Dev. Biol. 1995;6:105–116. [Google Scholar]
- Sastry S.K., Horwitz A.F. Adhesion-growth factor interactions during differentiationan integrated biological response. Dev. Biol. 1996;180:455–467. doi: 10.1006/dbio.1996.0319. [DOI] [PubMed] [Google Scholar]
- Sastry S.K., Lakonishok M., Thomas D.A., Muschler J., Horwitz A.F. Integrin α subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J. Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry S.K., Lakonishok M., Wu S., Truong T.Q., Huttenlocher A., Turner C.E., Horwitz A.F. Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J. Cell Biol. 1999;144:1295–1309. doi: 10.1083/jcb.144.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna D., Disatnik M.H., Rayburn H., Bronson R.T., Yang J., Rando T.A., Hynes R.O. Dystrophic muscle in mice chimeric for expression of α5 integrin. J. Cell Biol. 1998;143:849–859. doi: 10.1083/jcb.143.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Li F., Goicoechea S., Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol. Cell Biol. 1999;19:2425–2434. doi: 10.1128/mcb.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]