Abstract
The glucocorticoid receptor (GR) mediates the biological effects of glucocorticoids (GCs) through activation or repression of gene expression, either by DNA binding or via interaction with other transcription factors, such as AP-1. Work in tissue culture cells on the regulation of AP-1–dependent genes, such as collagenase (MMP-13) and stromelysin (MMP-3) has suggested that the antitumor and antiinflammatory activity of GCs is mediated, at least in part, by GR-mediated downmodulation of AP-1. Here, we have identified phorbol ester-induced expression of MMP-3 and MMP-13 in mouse skin as the first example of an in vivo system to measure negative interference between AP-1 and GR in the animal. Cell type-specific induction of these genes by tumor promoters is abolished by GCs. Importantly, this is also the case in GRdim mice expressing a DNA binding-defective mutant version of GR. In contrast, the newly identified target genes in skin, plasma glutathione peroxidase and HSP-27, were induced by GC in wild-type, but not in GRdim mice. Thus, these data suggest that the DNA binding-independent function of the GR is dispensable for repression of AP-1 activity in vivo and responsible for the antitumor promoting activity of GCs.
Keywords: tumor promotion, mouse skin, AP-1, matrix metalloproteinase, collagenase, glucocorticoid receptor, GRdim
Glucocorticoids (GCs) are in widespread medical use to inhibit inflammatory processes. Furthermore, they are known to block experimentally induced tumorigenesis, such as phorbol ester-induced multistep carcinogenesis in mouse skin (Belman and Troll 1972). These biological activities of GCs are mediated by positive or negative regulation of gene expression via binding to the glucocorticoid receptor (GR), a member of the steroid hormone receptor superfamily, which acts as a ligand-induced transcription factor (Beato et al. 1995). Different modes of action of transcriptional regulation by GR have been described. Upon ligand-induced dimerization the GR binds to conserved DNA motifs known as glucocorticoid response elements (GRE) and negative acting GREs (nGREs), respectively, to positively or negatively regulate gene expression (Beato et al. 1995; Karin 1998). Second, mutual interference between GR and other transcription factors, such as AP-1 and NFκB, does not require DNA binding of the GR, but rather is mediated by interaction with these factors (Beato et al. 1995; Karin 1998). The physiological importance of the dimerization- and DNA binding-independent repression function of GR was documented in homozygous mice carrying a DNA binding-defective mutant of GR (GRdim). In these mice, DNA binding and, as a consequence, transcriptional regulation of target genes containing GREs and nGREs was impaired, whereas repression of AP-1–mediated gene expression in primary embryonic fibroblasts was normal (Reichardt et al. 1998). In contrast to GR-deficient mice, GRdim mice survive until adulthood (Cole et al. 1995; Reichardt et al. 1998), suggesting that the capacity of the GR to interfere with other transcription factors plays an essential role for normal functions in the body.
Down-modulation of the activity of transcription factors, such as AP-1 and NFκB, is thought to mediate the antiinflammatory and antitumor promoting activities of GCs. Although different lines of evidence have suggested an important role of both, the transactivation and the antiapoptotic activities of NFκB in cell transformation (Foo and Nolan 1999), a direct role of NFκB in the induction of experimentally induced carcinogenesis in skin, has not yet been demonstrated. In contrast, the lack of malignant progression during skin tumor development in c-fos − /− mice (Saez et al. 1995) strongly underlines the requirement of AP-1–regulated gene expression in this process. High constitutive expression levels of AP-1 target genes, such as interstitial collagenase (MMP-13) and stromelysin-1 (MMP-3), were observed in skin tumors (Basset et al. 1997). In tissue culture cells, repression of interstitial collagenase and stromelysin expression by dexamethasone is mediated through negative interference between AP-1 and GR (Beato et al. 1995; Dumont et al. 1998; Karin 1998). Whether the DNA binding-dependent or -independent function of the GR is required for repression of tumor promotion and phorbol ester-induced gene expression in the animal is still unclear.
Here, we have measured phorbol ester- and GC-induced changes in MMP-13 and MMP-3 gene expression in mouse skin to analyze mutual interference between AP-1 and GR in vivo. Both genes contain functional AP-1 sites, which are essential for upregulation by many growth stimulating agents in tissue culture (Angel and Karin 1991). MMP-13 appears to represent the most selective reporter gene to measure AP-1 activity: in c-fos transgenic mice, expression of MMP-13 is enhanced, whereas in c-fos knockout mice, the level of MMP-13 transcripts is reduced (Gack et al. 1994; Porte et al. 1999). Second, phorbol ester-dependent induction of MMP-13 is almost completely lost in fibroblasts from c-fos and c-jun knockout embryos (Hu et al. 1994; Schreiber et al. 1995). Finally, the AP-1 site in the murine MMP-13 promoter is necessary and sufficient for 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-dependent induction (Porte et al. 1999), demonstrating that other transcription factors, including NFκB, whose activities have been found to be affected by phorbol esters (Nelsen et al. 1988), do not contribute to phorbol ester-dependent induction of this gene.
By the analysis of MMP-13 and MMP-3 gene expression in skin of phorbol ester- and GC-treated wild-type and GRdim mice, we could demonstrate that AP-1 activity is repressed by the GR in vivo. Since induction of both genes is considered a hallmark of altered gene expression during mouse skin carcinogenesis, these data strongly suggest that the ability of GR to undergo protein–protein interaction with transcription factors, such as AP-1, is responsible for the antitumor promoting activity of GCs.
Materials and Methods
Animals
The dorsal skin of 7–9-wk-old female C57BL/6J mice (BRL) or GRdim mice (Reichardt et al. 1998) was shaved 4 d before experimentation. Mice were treated topically with 1–10 nmol TPA or with 50 μg dexamethasone (Sigma Chemical Co.) dissolved in 200 μl acetone. The animals were killed 0–6 h after application.
Northern Blot Analysis
Skin tissues were homogenized and RNA was prepared as described previously (Reichardt et al. 1998) and analyzed by Northern blot (Gack et al. 1994; Schreiber et al. 1995). The probes for 18 S rRNA, HSP-27, and plasma glutathione peroxidase-3 (PGX-3) were obtained by reverse transcriptase PCR using a mouse skin RNA preparation.
In Situ Hybridization and Immunohistochemistry
6-μm paraffin sections from skin biopsies were subjected to in situ hybridization using 35S-UTP–labeled sense and antisense probes of MMP-13 and MMP-3 as described in Gack et al. 1995. Immunohistochemistry was performed using a polyclonal rabbit anti–mouse GR antibody (M20; Santa Cruz), followed by an ABC staining procedure (ABC Rabbit IgG Kit; Vector Labs, Inc.) according to the manufacturer's instructions.
cDNA Expression Array Hybridization
Atlas™ mouse cDNA expression array I filters were hybridized with radioactively labeled first strand cDNA following the specifications of the manufacturer (CLONTECH). Differences in expression patterns were analyzed using AIS software and Array Vision software module (Imaging Research).
Results
Rapid Induction of MMP-13 and MMP-3 Gene Expression by TPA in Mouse Skin
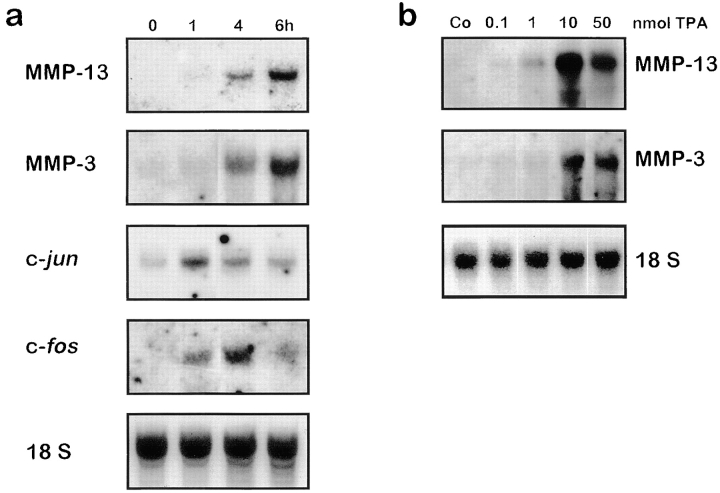
To establish an in vivo experimental system suitable to measure AP-1–dependent gene expression and to study mutual interference between AP-1 and GR in the animal, we first determined expression of MMP-3 and MMP-13 in mouse skin after treatment with TPA. In mock-treated animals, transcription levels of MMP-3 and MMP-13 were barely detectable (Fig. 1 a). Upon application of TPA, an up to 100-fold induction of both genes was observed within four to six hours. Induction of both genes is dose-dependent, reaching maximal levels at 10 nmol, whereas 1 nmol of TPA was not sufficient for potent induction (Fig. 1 b). Furthermore, upregulation was preceded by transcriptional activation of the main regulators of MMP-3 and MMP-13 gene expression, c-jun and c-fos. Induction of these immediate early genes was already detectable within one hour and declined at four hours in the case of c-jun, and between four and six hours for c-fos (Fig. 1 a).
Figure 1.
TPA induces MMP-13 and MMP-3 in skin. a, TPA (10 nmol) in acetone was applied to the back skin. After 0, 1, 4, and 6 h, mice were killed and RNA from skin was prepared. MMP-13, MMP-3, c-jun, and c-fos expression was analyzed by Northern blot analysis. b, TPA (0.1, 1, 10, and 50 nmol) was applied as described in a, and animals were killed after 6 h and analyzed by Northern blot analysis. Rehybridization with a cDNA fragment of 18 S RNA was performed serving as a loading control.
To identify the specific cell type responsible for enhanced MMP-13 and MMP-3 gene expression in response to TPA, in situ hybridization analysis of parallel transversal sections of skin biopsies was performed. Whereas in untreated skin, no significant signals for the expression of MMP-13 and MMP-3 could be detected (data not shown), both genes were highly induced in a cell type-specific manner upon phorbol ester treatment (Fig. 2). MMP-13 transcripts were observed in the epidermis only in a subset of basal keratinocytes (Fig. 2, a and b). MMP-3 expression was found exclusively in monocytic cells in the dermal compartment (Fig. 2c and Fig. d). Interestingly, in contrast to primary and immortalized skin fibroblasts showing enhanced expression of both genes in response to TPA (Clark et al. 1985; Wilhelm et al. 1987), neither MMP-13 nor MMP-3 transcripts were found in fibroblasts of the dermis (Fig. 2b and Fig. d).
Figure 2.
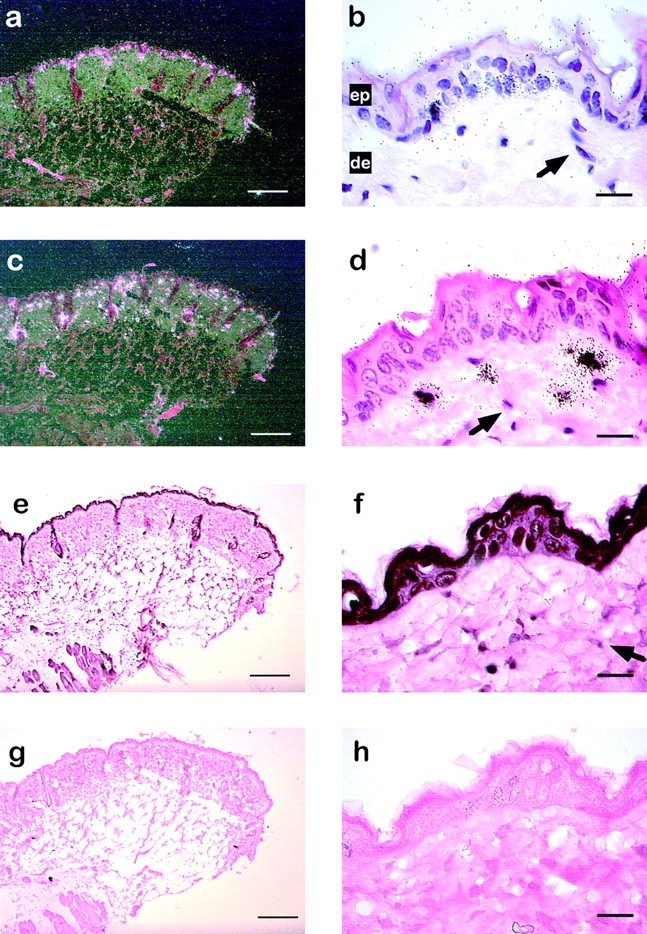
Cell type-specific expression of MMP-13, MMP-3, and GR in skin of TPA-treated mice. In situ hybridization for MMP-13 (a and b) and MMP-3 (c and d) on transverse sections of the skin from female C57BL/6 mice treated for 6 h with TPA. Dark-field (a) and bright-field (b) illumination of a section hybridized with a MMP-13 specific riboprobe. The arrow indicates nuclei from fibroblasts. Dark-field illumination (c) and bright-field (d) illumination of a skin section hybridized with a MMP-3 specific riboprobe. e–h, Immunohistochemical analysis of GR expression using anti-mouse GR antibody (e and g) or secondary anti-rabbit IgG antibody alone (g and h). The arrow indicates nuclei from fibroblasts. ep, Epidermis; de, dermis. Bars: (a, c, e, and g) 100 μm; (b, d, f, and h) 20 μm.
Repression of TPA-induced Genes by Glucocorticoids in Skin
We next wanted to investigate whether GR could interfere with AP-1 activity under these conditions. Since the expression pattern of GR in murine skin has not yet been described, we first wanted to confirm the presence of GR in MMP-3– and MMP-13–expressing cells in the skin by immunohistochemistry. Strong expression of GR protein was observed throughout the epidermis (Fig. 2e and Fig. f) and in mononuclear cells in the dermis (Fig. 2 f). Weaker signals for GR protein were observed in dermal fibroblasts (Fig. 2 f). These data show that GR is expressed by a broad range of cells in the skin, including MMP-13 and MMP-3 positive cells. Therefore, TPA-induced expression of MMP-13 and MMP-3 in skin in the presence or absence of GC is an appropriate system to measure GR-specific inhibition of gene expression in vivo. Consequently, we asked whether TPA-induced expression of MMP-13 and MMP-3 is repressed by dexamethasone in these cells. We found a complete inhibition of induction of both genes in skin after concomitant treatment with TPA and dexamethasone in skin (Fig. 3 a).
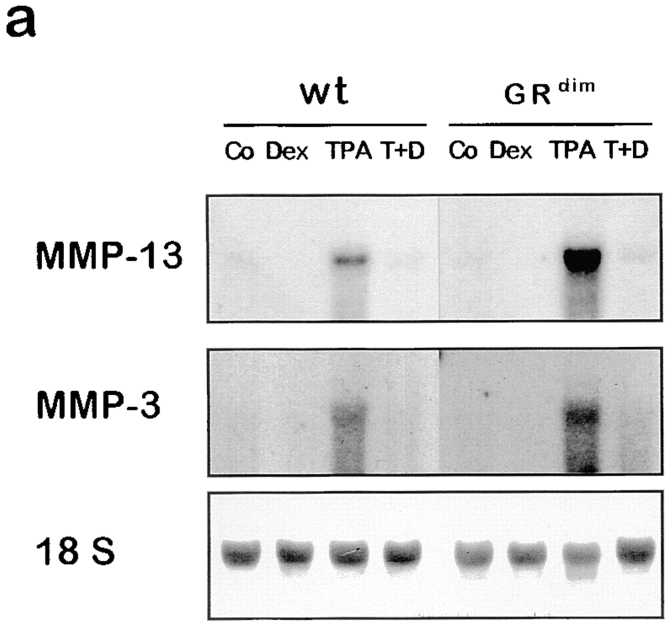
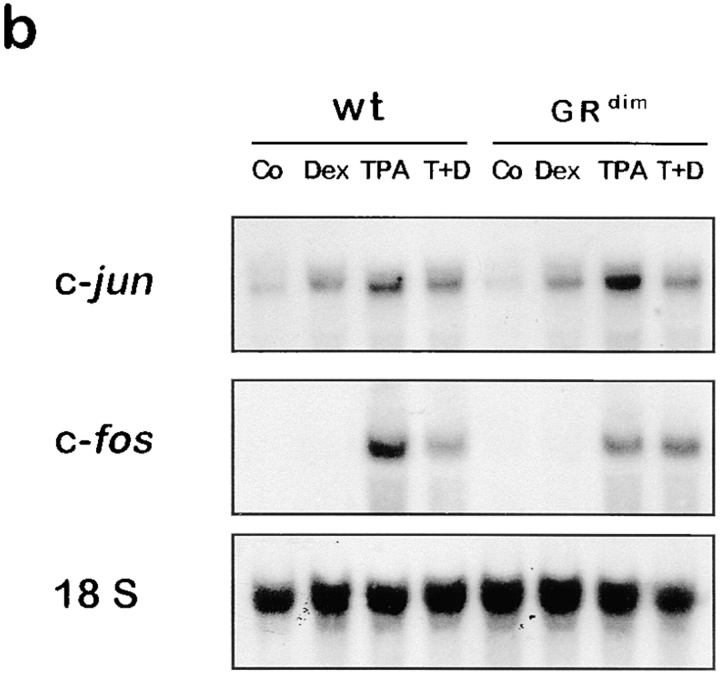
Figure 3.
The DNA binding function of the GR is dispensable for repression of MMP-13 and MMP-3 induction in vivo. Wild-type (wt) or GRdim mice were treated with acetone (Co), 50 μg dexamethasone (Dex), 10 nmol TPA (TPA), or 10 nmol TPA with 50 μg dexamethasone (T+D). Animals were killed after 6 h (a) or 1.5 h (b) and analyzed by Northern blot.
Repression of Gene Transcription by the GR Does Not Require DNA Binding of the Receptor
To analyze in more detail the molecular mechanism responsible for the repression of AP-1–dependent genes by GC in skin, we took advantage of GRdim mice. These mice carry a DNA binding-defective GR. Previously, transient transfection studies in tissue culture cells suggested that the DNA binding function of the GR is not required for transrepression of AP-1–mediated transcription (Heck et al. 1994). However, in light of the lack of induction of MMP-3 and MMP-13 expression by TPA in dermal fibroblasts in vivo (Fig. 2) and the complex regulatory processes present in skin, it is still possible that both DNA binding-dependent and -independent functions of GR are required for repression of TPA-induced expression of MMP-3 and MMP-13 in skin cells. To determine the importance of either one of these functions of GR, we analyzed the expression of both genes in GRdim mice. Expression of both MMP-3 and MMP-13 is dramatically induced upon TPA treatment in GRdim mice, similar to wild-type mice. In situ hybridization analysis confirmed that induction of these genes in GRdim mice originates from basal keratinocytes and monocytic cells (data not shown), resembling the pattern of expression in wild-type mice (Fig. 2). Interestingly, induction was completely repressed by dexamethasone in both wild-type and GRdim mice (Fig. 3 a), which strongly suggests that the DNA binding-dependent function of the GR is not required for repression of TPA-induced MMP gene expression in vivo.
GR has been reported to either activate (Jonat et al. 1990) or repress c-jun expression (Wei et al. 1998) in tissue culture cells, depending on the cell type. To confirm that GR-dependent repression of MMP-3 and MMP-13 cannot be explained by a loss of c-jun and/or c-fos expression upon hormone treatment, we measured the level of c-fos and c-jun transcripts in unstimulated and stimulated mouse skin. Significant basal level of c-jun transcripts can be detected in wild-type and GRdim mice, which became further enhanced upon TPA treatment (Fig. 3 b). Induction of c-fos was even more pronounced. Expression of c-jun, but not c-fos, was significantly induced by dexamethasone in both wild-type and GRdim mice, which might be mediated by the DNA binding-independent, positive function of GR on Jun/Jun homodimers (Miner and Yamamoto 1992; Teurich and Angel 1995) binding to the c-jun promoter. Most importantly, dexamethasone reduced TPA-induced expression of c-fos and c-jun only slightly (Fig. 3 b). The presence of c-jun and c-fos transcripts in TPA- and dexamethasone-treated animals confirmed that repression of phorbol ester-induced MMP-3 and MMP-13 expression is most likely due to inhibition of AP-1 activity by GR and cannot be explained by a loss of expression of the critical AP-1 components c-Jun and c-Fos.
Positive Regulation of PGX-3 and HSP27 by GC in Mouse Skin Is Absent in GRdim Mice
To prove abrogation of GR-mediated transactivation function in GRdim mice, we aimed to detect differences in GC-dependent regulation of GR target genes in skin cells of wild-type and GRdim mice. Therefore, we performed gene expression profiling on a mouse Atlas™ cDNA expression array. Filters containing spotted DNA from 588 known genes were hybridized in parallel with radiolabeled cDNA derived from RNA of skin from untreated and dexamethasone-treated mice. Among the differentially expressed genes (data not shown), two examples, PGX-3 and HSP-27, were analyzed by Northern blot analysis (Fig. 4). In the skin of wild-type mice, expression of both PGX-3 and HSP-27 was significantly upregulated (4.3- and 2.9-fold, respectively) six hours after dexamethasone treatment. Enhanced levels of PGX-3, but not HSP-27, already were detectable after 1.5 hours (data not shown). Importantly, in GRdim mice, almost no elevation in mRNA levels of PGX-3 and HSP-27 is detectable (Fig. 4).
Figure 4.
Induction of HSP-27 and plasma glutathione peroxidase 3 requires the DNA binding function of the GR. Control (Co) and dexamethasone (50 μg; Dex) treated wild-type (wt) or GRdim mice were killed after 6 h and RNA from back skin was analyzed for PGX-3 and HSP-27 expression by Northern blot.
Taken together, negative interference of AP-1 by GR does not require the DNA binding function in vivo, since in wild-type and in GRdim mice, MMP-13 and MMP-3 are equally repressed, whereas genes positively regulated are activated by GC only in wild-type mice, but not in GRdim mice.
Discussion
We established an experimental system to induce transcription of the AP-1–dependent genes MMP-13 (collagenase-3) and MMP-3 (stromelysin-1) in skin by the application of TPA. In line with the critical role of de novo synthesis of c-Jun and c-Fos for full transcriptional activation of interstitial collagenases (Angel and Karin 1991), we found a rapid upregulation of c-jun and c-fos mRNA upon TPA treatment in skin (Kennard et al. 1995).
In contrast to the coordinate induction of both genes in tissue culture cells (Angel and Karin 1991), the expression pattern of MMP-13 and MMP-3 genes in the skin was restricted to distinct cell types. MMP-13 was expressed upon TPA treatment in basal keratinocytes, which are the sites of expression of MMP-13 observed during wound healing in mice (Madlener et al. 1998). TPA-induced MMP-3 expression was mainly found in monocytic cells in the dermal compartment. However, we could not detect expression of MMP-3 in the basal layer of the epidermis by in situ hybridization, although others have observed MMP-3 expression in fractionated keratinocytes derived from TPA-treated epidermis (Krieg et al. 1988). During experimental wound healing, induction of MMP-3 has been found in some cells of the mesenchymal compartment of the skin and in basal keratinocytes after 24 hours (Madlener et al. 1998). Thus, most likely MMP-3 induced by TPA treatment only partially reflects the expression pattern observed during wounding. Surprisingly, in contrast to fibroblasts in tissue culture (Wilhelm et al. 1987; Madlener et al. 1998), we found no induction of either MMP-13 or MMP-3 in dermal fibroblasts upon TPA treatment of skin. The specific microenvironment composed of soluble factors and components of the extracellular matrix obviously does not allow the responsiveness of these cells to TPA by induced expression of MMPs. The molecular mechanism controlling cell type-specific upregulation of MMP-13 and MMP-3 transcripts in the skin remains to be elucidated.
Importantly, the phorbol ester-induced expression of MMP-13 and MMP-3 was efficiently inhibited by GCs in a cell type-specific manner. To analyze if DNA binding of the GR is involved in this downregulation of MMP-13 and MMP-3 in vivo, we made use of GRdim mice. To address the question whether gene activation by GCs is indeed impaired in GRdim mice, we identified GC-induced genes in the skin using a high-density filter screening approach. PGX-3 and HSP27 were among the strongest induced genes and as yet have not been described to be responsive to GCs. For both PGX-3 and HSP27, we could show a failure of GC-mediated upregulation in GRdim mice.
In contrast to loss of GC-dependent gene activation GRdim mice, TPA-mediated expression of AP-1 target genes, such as MMP-13, MMP-3, and MMP-9 (gelatinase B; data not shown) is efficiently downregulated by dexamethasone. Obviously, the DNA binding function of GR and subsequent transcriptional activation of GRE-dependent genes is not required for transrepression of AP-1 in vivo. The presence of c-fos and c-jun transcripts upon cotreatment with TPA and dexamethasone suggests that the de novo synthesis of both AP-1 components is not abolished by GCs. Specifically, induction of c-jun by TPA, which requires the activity of MAP kinases, such as JNK, to hyperphosphorylate preexisting c-Jun protein, was not repressed by GCs. Thus, inhibition of the JNK pathway by GR, which was described in tissue culture cells (Caelles et al. 1997), may not play a major role in vivo, at least in skin.
In transgenic mice, overexpression of c-jun and c-fos, as well as tissue-specific expression of the AP-1 target genes, human collagenase and stromelysin, induces, or at least enhances, tumorigenesis (Wang et al. 1991, Wang et al. 1995; D'Armiento et al. 1995; Sternlicht et al. 1999). Downregulation of AP-1 target gene expression has been proposed to be a crucial event in GC-mediated inhibition of tumor formation in the multiple stage model of carcinogenesis in skin (Jonat et al. 1990). Here, we demonstrate that TPA, one of the most potent and best characterized tumor promoters in mouse skin carcinogenesis, induces MMP-13 and MMP-3 very rapidly at the same dosage, which is, when applied periodically, optimal to mediate tumor formation (Fürstenberger and Kopp-Schneider 1995). The critical role of AP-1 in MMP-13 and MMP-3 expression and the downmodulation of expression of both genes by GCs strongly suggest that transrepression of AP-1 activity is a key feature of antitumor promoting activity of GCs. The finding that this activity of GR does not require the DNA binding function might be highly valuable in the search for better therapeutical strategies of GC application in skin carcinogenesis and related diseases.
Acknowledgments
We are grateful to Dr. N. Keon and Dr. L. Ringrose for critical reading of the manuscript. We thank Dr. B. Sorg for the generous gift of TPA.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (An 182/6-3 and D.10049270/SFB 405), the European Community (ERB4061PL95-078, ERB FMRX-CT98-0197, PL 96-0179, and PL 96-3505), and a Ph.D. Fellowship from the Boehringer-Ingelheim Fonds (J. Tuckermann).
Footnotes
Jan P. Tuckermann's present address is Division of Molecular Biology of the Cell I, Deutsches Krebsforschungszentrum, D-69120 Heidelberg, Germany.
Abbreviations used in this paper: GCs, glucocorticoids; GR, glucocorticoid receptor; GRdim, mice homozygous for a mutation in the endogenous GR gene; GRE, GR responsive element; MMP, matrix metalloproteinase; PGX-3, plasma glutathione peroxidase-3; TPA, 12-O-tetradecanoyl-phorbol-13-acetate.
References
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Basset P., Okada A., Chenard M.P., Kannan R., Stoll I., Anglard P., Bellocq J.P., Rio M.C. Matrix metalloproteinases as stromal effectors of human carcinoma progressiontherapeutic implications. Matrix Biol. 1997;15:535–541. doi: 10.1016/s0945-053x(97)90028-7. [DOI] [PubMed] [Google Scholar]
- Beato M., Herrlich P., Schutz G. Steroid hormone receptorsmany actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Belman S., Troll W. The inhibition of croton oil-promoted mouse skin tumorigenesis by steroid hormones. Cancer Res. 1972;32:450–454. [PubMed] [Google Scholar]
- Caelles C., Gonzalez-Sancho J.M., Munoz A. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 1997;11:3351–3364. doi: 10.1101/gad.11.24.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S.D., Wilhelm S.M., Stricklin G.P., Welgus H.G. Coregulation of collagenase and collagenase inhibitor production by phorbol myristate acetate in human skin fibroblasts. Arch Biochem. Biophys. 1985;241:36–44. doi: 10.1016/0003-9861(85)90358-3. [DOI] [PubMed] [Google Scholar]
- Cole T.J., Blendy J.A., Monaghan A.P., Krieglstein K., Schmid W., Aguzzi A., Fantuzzi G., Hummler E., Unsicker K., Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- D'Armiento J., DiColandrea T., Dalal S.S., Okada Y., Huang M.T., Conney A.H., Chada K. Collagenase expression in transgenic mouse skin causes hyperkeratosis and acanthosis and increases susceptibility to tumorigenesis. Mol. Cell. Biol. 1995;15:5732–5739. doi: 10.1128/mcb.15.10.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont A., Hehner S.P., Schmitz M.L., Gustafsson J.A., Liden J., Okret S., van der Saag P.T., Wissink S., van der Burg B., Herrlich P. Cross-talk between steroids and NF-kappa Bwhat language? Trends Biochem. Sci. 1998;23:233–235. doi: 10.1016/s0968-0004(98)01212-2. [DOI] [PubMed] [Google Scholar]
- Foo S.Y., Nolan G.P. NF-kappaB to the rescueRELs, apoptosis and cellular transformation. Trends Genet. 1999;15:229–235. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- Fürstenberger G., Kopp-Schneider A. Malignant progression of papillomas induced by the initiation–promotion protocol in NMRI mouse skin. Carcinogenesis. 1995;16:61–69. doi: 10.1093/carcin/16.1.61. [DOI] [PubMed] [Google Scholar]
- Gack S., Vallon R., Schaper J., Ruther U., Angel P. Phenotypic alterations in fos-transgenic mice correlate with changes in Fos/Jun-dependent collagenase type I expression. J. Biol. Chem. 1994;269:10363–10369. [PubMed] [Google Scholar]
- Gack S., Vallon R., Schmidt J., Grigoriadis A., Tuckermann J., Schenkel J., Weiher H., Wagner E.F., Angel P. Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ. 1995;6:759–767. [PubMed] [Google Scholar]
- Heck S., Kullmann M., Gast A., Ponta H., Rahmsdorf H.J., Herrlich P., Cato A.C. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E., Mueller E., Oliviero S., Papaioannou V.E., Johnson R., Spiegelman B.M. Targeted disruption of the c-fos gene demonstrates c-fos–dependent and –independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H.J., Park K.K., Cato A.C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammationdown-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Karin M. New twists in gate regulation by glucocorticoid receptoris DNA binding dispensable? Cell. 1998;93:487–490. doi: 10.1016/s0092-8674(00)81177-0. [DOI] [PubMed] [Google Scholar]
- Kennard M.D., Kang D.C., Montgomery R.L., Butler A.P. Expression of epidermal ornithine decarboxylase and nuclear proto-oncogenes in phorbol ester tumor promotion-sensitive and -resistant mice. Mol. Carcinog. 1995;12:14–22. doi: 10.1002/mc.2940120104. [DOI] [PubMed] [Google Scholar]
- Krieg P., Finch J., Fustenberger G., Melber K., Matrisian L.M., Bowden G.T. Tumor promoters induce a transient expression of tumor-associated genes in both basal and differentiated cells of the mouse epidermis. Carcinogenesis. 1988;9:95–100. doi: 10.1093/carcin/9.1.95. [DOI] [PubMed] [Google Scholar]
- Madlener M., Parks W.C., Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp. Cell. Res. 1998;242:201–210. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- Miner J.N., Yamamoto K.R. The basic region of AP-1 specifies glucocorticoid receptor activity at a composite response element. Genes Dev. 1992;6:2491–2501. doi: 10.1101/gad.6.12b.2491. [DOI] [PubMed] [Google Scholar]
- Nelsen B., Hellman L., Sen R. The NF-kappa B-binding site mediates phorbol ester-inducible transcription in nonlymphoid cells. Mol. Cell. Biol. 1988;8:3526–3531. doi: 10.1128/mcb.8.8.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D., Tuckermann J., Becker M., Baumann B., Teurich S., Higgins T., Owen M.J., Schorpp-Kistner M., Angel P. Both AP-1 and Cbfa1-like factors are required for the induction of interstitial collagenase by parathyroid hormone. Oncogene. 1999;18:667–678. doi: 10.1038/sj.onc.1202333. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M., Kaestner K.H., Tuckermann J., Kretz O., Wessely O., Bock R., Gass P., Schmid W., Herrlich P., Angel P., Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Saez E., Rutberg S.E., Mueller E., Oppenheim H., Smoluk J., Yuspa S.H., Spiegelman B.M. c-fos is required for malignant progression of skin tumors. Cell. 1995;82:721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- Schreiber M., Baumann B., Cotten M., Angel P., Wagner E.F. Fos is an essential component of the mammalian UV response. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:5338–5349. doi: 10.1002/j.1460-2075.1995.tb00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht M.D., Lochter A., Sympson C.J., Huey B., Rougier J.P., Gray J.W., Pinkel D., Bissell M.J., Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teurich S., Angel P. The glucocorticoid receptor synergizes with Jun homodimers to activate AP-1–regulated promoters lacking GR binding sites. Chem. Senses. 1995;20:251–255. doi: 10.1093/chemse/20.2.251. [DOI] [PubMed] [Google Scholar]
- Wang Z.Q., Grigoriadis A.E., Mohle-Steinlein U., Wagner E.F. A novel target cell for c-fos–induced oncogenesisdevelopment of chondrogenic tumours in embryonic stem cell chimeras. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:2437–2450. doi: 10.1002/j.1460-2075.1991.tb07783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.Q., Liang J., Schellander K., Wagner E.F., Grigoriadis A.E. c-fos–induced osteosarcoma formation in transgenic micecooperativity with c-jun and the role of endogenous c-fos. Cancer Res. 1995;55:6244–6251. [PubMed] [Google Scholar]
- Wei P., Inamdar N., Vedeckis W.V. Transrepression of c-jun gene expression by the glucocorticoid receptor requires both AP-1 sites in the c-jun promoter. Mol. Endocrinol. 1998;12:1322–1333. doi: 10.1210/mend.12.9.0158. [DOI] [PubMed] [Google Scholar]
- Wilhelm S.M., Collier I.E., Kronberger A., Eisen A.Z., Marmer B.L., Grant G.A., Bauer E.A., Goldberg G.I. Human skin fibroblast stromelysinstructure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc. Natl. Acad. Sci. USA. 1987;84:6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]