Abstract
Prior studies have implicated the superior temporal sulcus region for processing various types of biological motion in children and adults. However, no previous research in children compared this activity to that involved in coherent, meaningful, non-biological motion perception to show specificity for biological motion processing. In this study, we used functional magnetic resonance imaging to explore which brain regions were specific for biological motion in 7- to 10-year-old children. We compared brain activity in response to biological motion by a biological figure (a walking human); biological motion by a non-biological figure (a walking robot); disorganized, non-biological motion by a disjointed mechanical figure; and organized, non-biological motion by a grandfather clock. We identified a network of brain regions that had a greater response evoked by biological than by non-biological motion, including the superior temporal sulcus and mirror neuron regions. Additionally, we found a developmental change suggesting increasing specificity for biological motion with age in the superior temporal sulcus region. We discuss these results in the context of research and theory that has emphasized the important role of biological motion perception in the development of theory-of-mind abilities.
INTRODUCTION
Social perception refers to the initial stages of evaluating the intentions of others using gaze direction, body movements, hand gestures, facial expressions, and other types of biological motion cues (Allison, Puce, & McCarthy, 2000). This construct shares a common thread with the notion of an “intentional stance” introduced by the philosopher Dennett (1987) to describe the assumptions and attributions humans tend to make when interpreting and predicting the actions of others. Social perception is part of a domain of cognitive skills variously referred to as social attention, social cognition, mentalizing, and theory of mind (ToM). We and others have argued that basic social perception, such as biological motion perception, is an ontogenetic and phylogenetic prequel to more sophisticated social cognition abilities, particularly ToM (e.g., Baron-Cohen, 1995; Frith & Frith, 1999; Pelphrey & Morris, 2006; Premack and Woodruff, 1978; Saxe, Carey, & Kanwisher, 2004). ToM is a higher-order and later-developing social perception ability that consists of the understanding that another person can have different desires, beliefs, and intentions than oneself and the use of this information to predict the behavior of the other person (Premack & Woodruff, 1978).
Our abilities to understand and anticipate the actions, intentions, thoughts, behaviors, and beliefs of others have long been the subject of psychological and philosophical inquiry. Recently, cognitive neuroscientists have begun to identify the neural circuitry that supports these social perception abilities. Brothers (1990) coined the term “social brain” to refer to the network of neuroanatomical structures thought to be involved in social perception. These structures include: (1) the lateral fusiform gyrus (FFG), or “fusiform face area,” located in the ventral occipitotemporal cortex of humans and thought to be important for rapid face recognition (e.g., Kanwisher, McDermott, & Chun, 1997; Puce, Allison, Asgari, Gore, & McCarthy, 1996). (2) The posterior superior temporal sulcus (STS) region, which has been implicated in the interpretation of the actions and social intentions of others through analysis of biological motion cues (e.g., Bonda, Petrides, Ostry, & Evans, 1996; Grossman & Blake, 2002; Pelphrey, Singerman, Allison, & McCarthy, 2003b; Pelphrey, Viola, & McCarthy, 2004; Saxe, Xiao, Kovacs, Perrett, & Kanwisher, 2004). In humans, the term “STS region” refers to the STS proper, portions of the superior and middle temporal gyri, and areas of the angular gyrus near the ascending limb of the STS (Allison et al., 2000). (3) The amygdala (AMY), a limbic brain structure that is comprised of at least thirteen different nuclei and is highly interconnected with other cortical and subcortical brain structures. This region has been implicated in determining the emotional states of others through analysis of facial expressions (e.g., Morris et al., 1996). (4) The temporo-parietal junction (TPJ), which is a functionally defined region localized to an area of cortex medial and superior to the posterior STS region and that is involved in the attribution and analysis of other people’s mental states (e.g., Saxe & Kanwisher, 2003). (5) The medial prefrontal cortex (mPFC), which is involved in evaluating the mental states of others (see Gallagher & Frith, 2003, for a review) and in representing semantic knowledge about the psychological aspects of other people (e.g., Mitchell, Banaji, & Macrae, 2005; Mitchell, Heatherton, & Macrae, 2002). (6) Portions of the parietal cortex, including the inferior and superior parietal lobules, and the anterior intraparietal sulcus (aIPS), and (7) frontal cortical regions, including the inferior frontal gyrus (IFG) that have been dubbed parts of the “mirror system” in humans because they activate equally to the execution of a motor action and the observation of a motor action by another person (e.g., Buccino et al., 2001; Decety & Chaminade, 2003; Iacoboni, Woods, Brass, Bekkering, Mazziotta, & Rizzolatti, 1999; Iacoboni et al., 2001; Pelphrey, Morris, & McCarthy, 2004; Rizzolatti, Fadiga, Gallese, & Fogassi, 1996; see Iacoboni, 2005, for a recent review).
To date, the majority of research regarding the social brain has been conducted in adult participants. The study of the social brain in school-aged children offers exciting implications for our understanding of the development of social perception and social cognition abilities, including ToM. Studies using functional magnetic resonance imaging (fMRI) and evoked response potentials (ERPs) are beginning to provide some important information. Thus far, the most is known about the face-sensitive area of the FFG. Some degree of FFG specialization for faces is apparent early in development (e.g., Tzourio-Mazoyer, De Schonen, Crivello, Reutter, Aujard, & Mazoyer, 2002), and this specialization continues to develop throughout infancy and into late childhood and adolescence (Aylward et al., 2005; Taylor, Edmonds, McCarthy, & Allison, 2001; Taylor, McCarthy, Saliba, & Degiovanni, 1999). The posterior STS region has been implicated for processing eye-gaze shifts in children between the ages of 7 and 10 years (Mosconi, Mack, McCarthy, & Pelphrey, 2005). However, nothing is known about its role in more general biological motion perception in this age range or whether this region is selective for biological motion in children of any age. Studies have found similar mirror neuron activity in children between 10 and 12 years of age as had been identified previously in adults, but patterns of activity in these areas have not yet been explored in younger children (Dapretto et al., 2006; Ohnishi et al., 2004). In this article, we will focus on one component of the social brain to determine if it is specific for biological motion perception: the STS region.
A primary goal for the emerging field of developmental social neuroscience is to map out the ontogenetic development of the key components and processes of the social brain and to understand how their emergence and refinement allows for more complex social abilities, such as ToM. Frith and Frith (1999) suggested that the ability to distinguish between biological and non-biological figures and their actions is one of the likely evolutionary and developmental precursors to ToM. They also noted that the STS region that has been implicated in biological motion processing is adjacent to regions of the brain used for mentalizing. The ability to infer goal states using the actions of an agent has been called an “intentionality detector” and proposed as a component of the human mind-reading system (Baron-Cohen, 1995). By the age of four months, infants can detect biological motion from impoverished stimuli—point-light walkers—and prefer these movies to those of non-biological motion (Fox & McDaniel, 1982). A study of 8-month-old infants using ERPs suggested that there was activity in the right hemisphere in response to biological motion (Hirai & Hiraki, 2005); however, a more precise neurobiological basis of this social perception ability has not been identified. No imaging study has examined the brain mechanisms for the perception of biological motion compared to non-biological motion in children of any age.
Biological motion perception does not always develop typically. For example, eight- to ten-year-old children with autism were found to perform poorly on a biological motion perception task relative to typically developing children; however, they performed equivalently on a task requiring that they detect a different class of form from motion (Blake, Turner, Smoski, Pozdol, & Stone, 2003). Unlike typically developing individuals, adults with autism perform poorly when asked to attribute mental states to moving figures (Castelli, Frith, Happé, & Frith, 2002). Given these findings suggesting that individuals with autism do not possess the basic building blocks believed to underlie ToM, it is noteworthy that these individuals also show pronounced deficits on ToM tasks (e.g., Baron-Cohen, Leslie, & Frith, 1985; Perner, Frith, Leslie, & Leekham, 1989). Thus, early biological motion detection abilities could allow children to develop the ability to use knowledge about the actions and intentions of others to infer mental states and thus to develop full-fledged ToM abilities.
In the present study, we sought to explore the development of biological motion regions, with a particular focus on the neurofunctional development of the STS region. We used a paradigm that we had previously employed with a sample of adults (Pelphrey, Mitchell, McKeown, Goldstein, Allison, & McCarthy, 2003a). In that study, we sought to determine whether the STS region responded to biological motion more than to other types of complex, meaningful motion or to random motion. The adult participants viewed a virtual scene that included four types of carefully matched, animated stimuli: a walking human, a walking robot, a grandfather clock, and a disjointed mechanical figure. We chose these figures rather than more commonly used stimuli, such as point-light walkers, in order to avoid the use of random motion controls and to better clarify the importance of motion organization and biological figures. Thus, we could use the four figures to explore whether a biological motion pattern (walking) would be processed differently if made by a biological (human) versus non-biological (robot) figure and whether there were differences in brain activation patterns for organized versus disorganized mechanical motion.
In adults, there were no significant differences between the human and the robot or between the clock and the disjointed mechanical figure in the posterior STS region. This region (right hemisphere > left hemisphere) responded more strongly to biological motion (as conveyed by the walking robot or walking human) than to nonmeaningful but complex nonbiological motion (the disjointed mechanical figure) or complex and meaningful nonbiological motion (the movements of the grandfather clock). Importantly, not every brain region showed this pattern of effects. We observed a dissociation of function between the STS region and an area posterior and inferior to the posterior STS region corresponding to the motion-sensitive visual area MT or V5 (MT/V5, e.g., McCarthy, Spicer, Adrignolo, Luby, Gore, & Allison, 1995; Tootell et al., 1995; Zeki, Watson, Lueck, Friston, Kennard, & Frackowiak, 1991). Whereas the STS region “preferred” biological motion, MT/V5 responded equally to all four types of motion.
In the current study, a group of healthy, typically developing children ranging from 7 to 10 years of age watched movements made by a human, a robot, a mechanical figure, and a grandfather clock. Using this paradigm, we could determine whether children showed specialization for biological relative to non-biological motion as well as how that specialization might increase with age. As in our previous study of adults, we tested a hierarchy of questions regarding activity within the STS region. First, we compared responses to the human and the robot in order to determine whether the STS is sensitive to biological motion itself or merely to the superficial characteristics of the stimulus. Then, we tested whether the STS responds more strongly to biological motion (as conveyed by the robot) than to a nonmeaningful but complex motion (the mechanical figure) and/or a complex and meaningful non-biological motion (the grandfather clock). In addition to these primary analyses, we explored whether the children’s degree of specificity for biological motion in the STS would increase with age.
METHODS AND EXPERIMENTAL DESIGN
Subjects
Nine typically developing, healthy children (4 females, 5 males; M = 9.2 years, range = 7.4–10.6 years) participated successfully in this study. All had normal or corrected-to-normal vision and were screened against neurological and psychiatric illnesses. Parents gave informed consent prior to participation. The children were given a toy as a token of our appreciation, and their families were financially compensated for their time. The Institutional Review Board of the Duke University Medical Center approved this project.
Thirteen children were brought in for scans. They were first trained in our mock scanner. The practice sessions are described in more detail below. Of these 13 children, 9 completed the study without excessive head movement (i.e., < 3 mm in the x, y, or z dimensions).
Experimental design
For a previous study in adults (Pelphrey et al., 2003a), we created four animated figures using the Poser 4.0®software program (Curious Labs Inc., Santa Cruz, CA, USA). We used these stimuli for the present study. These were a human, a robot, a mechanical assembly, and a grandfather clock (Figure 1). In an event-related design, the four figures were always present (Figure 1, top panel), and on each trial, one of the four figures moved for 2 s. Trials were separated by a 10-s intertrial interval (ITI), during which all four figures were present on the screen and none were moving. The left-to-right order of the figures varied across runs and the order of the movements was randomized across trials. Over the course of 96 trials, children saw 24 exemplars of each category of motion. The human, viewed in profile, walked in place. In another condition, the robot, composed of a sphere, torus, and four rods that simulated a head, torso/hips, two arms, and two legs, respectively, moved in place to simulate the sweeping of arms and legs and the sway of hips that comprise human walking. Each part of the robot moved the same distance with the same angular rotation and velocity as did its counterpart on the human figure. Thus, while the figures differed markedly in form, their movements were nearly identical. The mechanical assembly was composed of pieces identical to those of the robot, but the configuration of pieces was different, as were their axes of rotation. The amount of movement made by the mechanical assembly was identical to the amount of movement made by the robot and human. Finally, the grandfather clock had two moving hands and a pendulum below. The pendulum was the same size as the leg of the robot, and the amount of movement made by the clock was very similar to the amount of movement made by the other stimulus figures. Hereafter, we use Human, Robot, Mechanical, and Clock as shorthand for the stimulus conditions. We also created biological motion and non-biological motion conditions by averaging the responses of regions to Human and Robot (Biological) and to Clock and Mechanical (Non-biological), given our previous findings in adults suggesting that this is appropriate (Pelphrey et al., 2003a).
Figure 1.
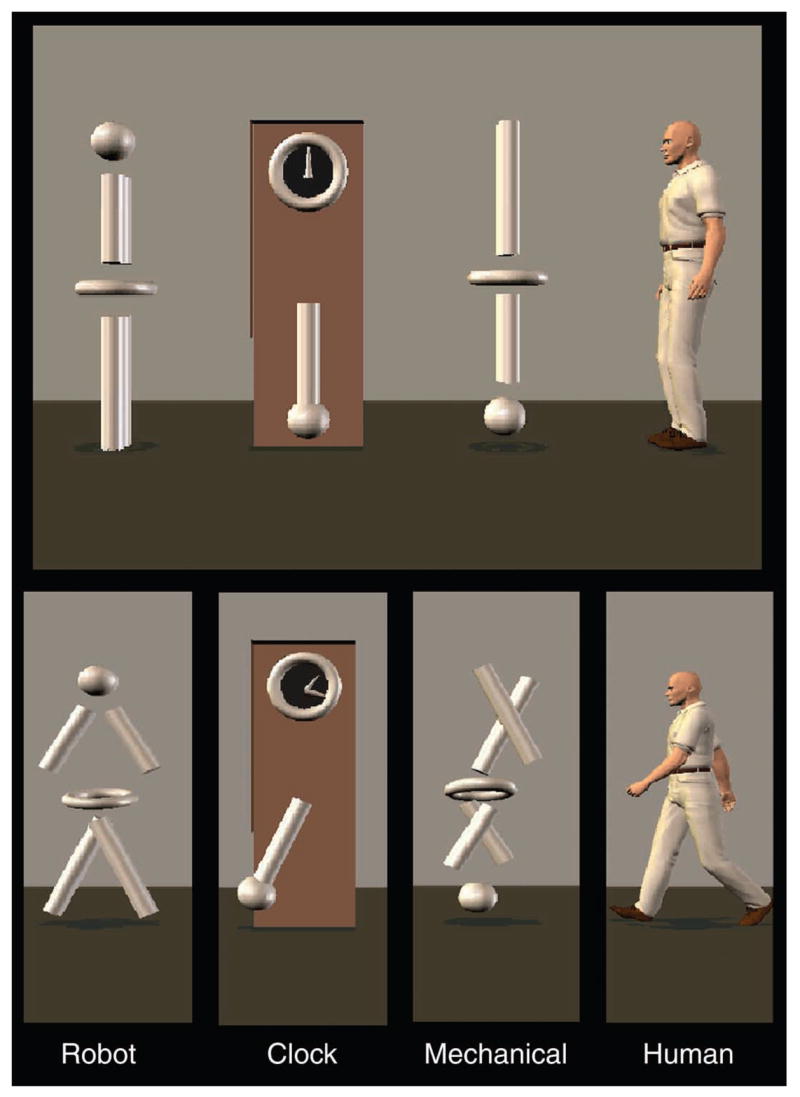
Top: One of the static displays viewed by the children between each trial. Bottom: Examples of the type of movement displayed in each condition.
We used CIGAL (Voyvodic, 1999) to control stimulus presentation. Subjects viewed stimuli at XGA resolution through MRI-compatible LCD goggles. Subjects were instructed only to attend to the screen at all times. Trials were randomized within runs lasting approximately 5 minutes (24 trials per run).
Imaging protocol
Scanning was performed on a General Electric 4T LX NVi MRI scanner system equipped with a quadrature birdcage radio frequency (RF) head coil. Sixty-eight high-resolution anatomical images were acquired using a 3D fast SPGR pulse sequence (Tr = 1500 ms; TE = 20 ms; FOV = 24 cm; image matrix = 2562; voxel size= 0.9375 × 0.9375 × 1.9 mm). Whole-brain functional images were acquired in the same space as the anatomical images using a gradient-recalled inward-spiral pulse sequence (Glover & Law, 2001; Guo & Song, 2003) sensitive to blood oxygenation level-dependent (BOLD) contrast (Tr = 1500 ms; TE = 35 ms; FOV = 24 cm; image matrix = 642; α = 62 degrees; voxel size = 3.75 × 3.75 × 3.8 mm; 34 axial slices). The anterior and posterior commissures were identified in the mid-sagittal slice of a scout image series and were used as landmarks for the prescription of the BOLD contrast images. A semi-automated, high-order shimming procedure ensured global field homogeneity. Runs consisted of the acquisition of 200 successive brain volumes beginning with 4 discarded RF excitations to allow for steady-state equilibrium.
Preparing children for fMRI scans
We used a simulator scanner to help the children to participate successfully in the scanning sessions without excessive anxiety or head motion. We developed a protocol and computer software for use with the MRI simulator to limit head motion by teaching children to remain still during fMRI scanning. Children were “trained” using operant conditioning procedures implemented in custom-written software that received input from a head motion sensor and used that input to direct the operation of a video player. The child watched a favorite movie, and the movie was halted whenever the child exhibited head motion above a progressively stricter threshold.
Data analysis
Image preprocessing was performed with custom programs and SPM 99 modules (Wellcome Department of Cognitive Neurology, UK). Images were time-adjusted to compensate for the interleaved slice acquisition and realigned to the tenth image to correct for head movements between scans. The realigned scans were then spatially normalized to the Montréal Neurologic Institute (MNI) template found in SPM 99 using the standard two-part procedure involving a twelve-parameter affine registration for global normalization followed by a non-linear basis function registration for regional transformations. The functional data were high-pass filtered and spatially smoothed with an 8 mm isotropic Gaussian kernel prior to statistical analysis. These normalized and smoothed data were used in the analysis procedures described below.
The primary analysis involved a random-effects assessment of the differences between the Biological and Non-biological conditions at the expected peak of the hemodynamic response (HDR). This analysis consisted of the following steps: (1) the epoch of image volumes beginning 1.5 s before and 12 s after the onset of each stimulus was excised by condition from the continuous time series of volumes, creating a 1.5 s prestimulus baseline and a poststimulus time window that continued until the onset of the next stimulus. (2) The average intensity of the HDR at expected peak was computed for the time interval ranging from 6 to 9 s by trial type. This segment was chosen to encompass the time at which the amplitude of the HDR reached its maximum for each trial type. A t-statistic was then computed at each voxel within the brain to quantify the HDR differences between the response to the Biological and Non-biological conditions. This process was performed separately for each subject. (3) The Biological > Non-biological t-maps were then subjected to a random-effects analysis that assessed the significance of the difference in response at the peak of the HDR to the Biological > Non-biological conditions across subjects.
To reduce the number of statistical comparisons and thus the false-positive rate, the random-effects analyses were restricted to only those voxels in which an HDR was evoked by either the Biological or Non-biological conditions. These voxels were identified in the following additional steps: (4) The single-trial epochs for each subject were averaged separately for Biological and Non-biological trials, and the averaged BOLD-intensity signal values for each voxel within the averaged epoch were converted to percent signal change relative to the prestimulus baseline. (5) The time waveform for each voxel in the averaged Biological and Non-biological epochs was correlated with a canonical reference waveform and t-statistics were calculated for the correlation coefficients for each voxel. This procedure provided whole-brain t-maps separately for the Biological and Non-biological trial types for each subject in MNI space. (6) The t-maps for each subject and for each trial type were used to calculate an average t-map for each trial type created by the average T method described by Lazar, Luna, Sweeney, and Eddy (2002) with a threshold set at t > 3.6. (7) The difference t-map computed in step 3 above was then masked by the results of step 6. That is, the differences between the Biological and Non-biological trial types were retained only for those voxels that had a significant positive hemodynamic waveform for either the Biological or Non-biological motion trial types. The threshold for significance was set at a voxelwise uncorrected p < .05 (two-tailed) and a spatial extent of six uninterpolated voxels in order to significantly reduce the possibility of false-positive errors (Xiong, Gao, Lancaster, & Fox, 1995). We also used a false discovery rate (Genovese, Lazar, & Nichols, 2002) value of p < .05. (8) We described each cluster of Biological > Non-biological activation by anatomical location, MNI co-ordinates, and Brodmann’s area. Clusters of Biological > Non-biological activity identified in this analysis were used to test a series of nested hypotheses regarding their responses to the Human, Mechanical, Robot, and Clock conditions.
RESULTS
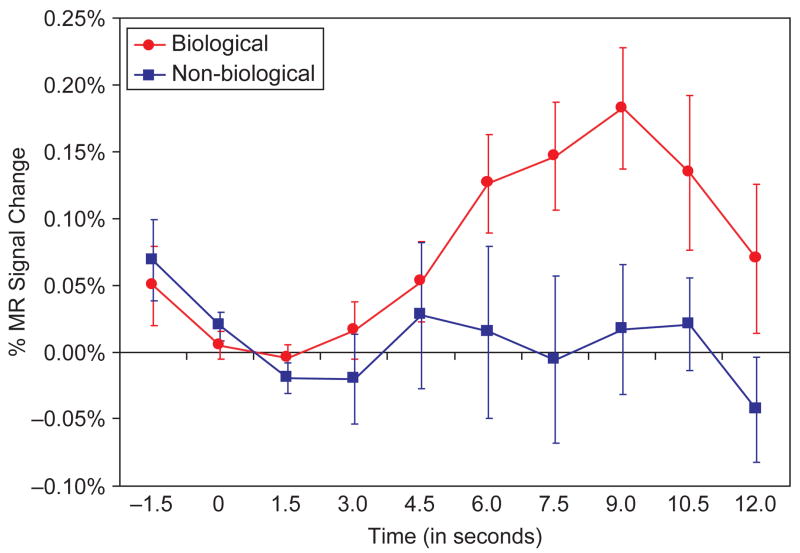
The biological motion stimuli evoked activation in a distributed network of cortical brain regions. As illustrated in Figure 2, the activated areas included: (1) regions of the posterior, middle, ventral-temporal, and occipital-temporal cortices, including the FFG, posterior and middle STS regions, and the parietal-temporal-occipital fossa (PTOF) bilaterally (Figure 2A–D, 2F); (2) frontal cortical regions, including the bilateral IFG, pre-central gyri, and middle and superior frontal gyri (MFG, SFG; Figure 2A–B, 2E); and (3) regions of the parietal cortex, including the postcentral gyri, intraparietal sulci (IPS), and the superior and inferior parietal lobules bilaterally (Figure 2E–F). The red-to-yellow overlay depicts activations evoked during Biological trials, and the blue-to-light blue overlay depicts activation evoked during Non-biological trials. These t-statistic maps reflect the magnitude of the correlation between a canonical reference waveform and the time course of the HDR from each voxel (see analysis step 6 in methods and experimental design). Both Biological and Non-biological trials evoked activation in the lateral occipital temporal cortex and PTOF bilaterally (corresponding to the expected location of area MT/V5), the right FFG, the right inferior parietal lobule, and the right IFG. However, in these areas of overlapping activation, Biological trials generally evoked activation over a greater spatial extent compared to Non-biological trials. The time course of activation was consistent across regions and is illustrated for the right STS region in Figure 3. As can be seen, activity in response to the Biological conditions increased with the onset of the motion, peaked approximately 9 s after the onset, and then began to return to baseline shortly after the offset of the movement.
Figure 2.
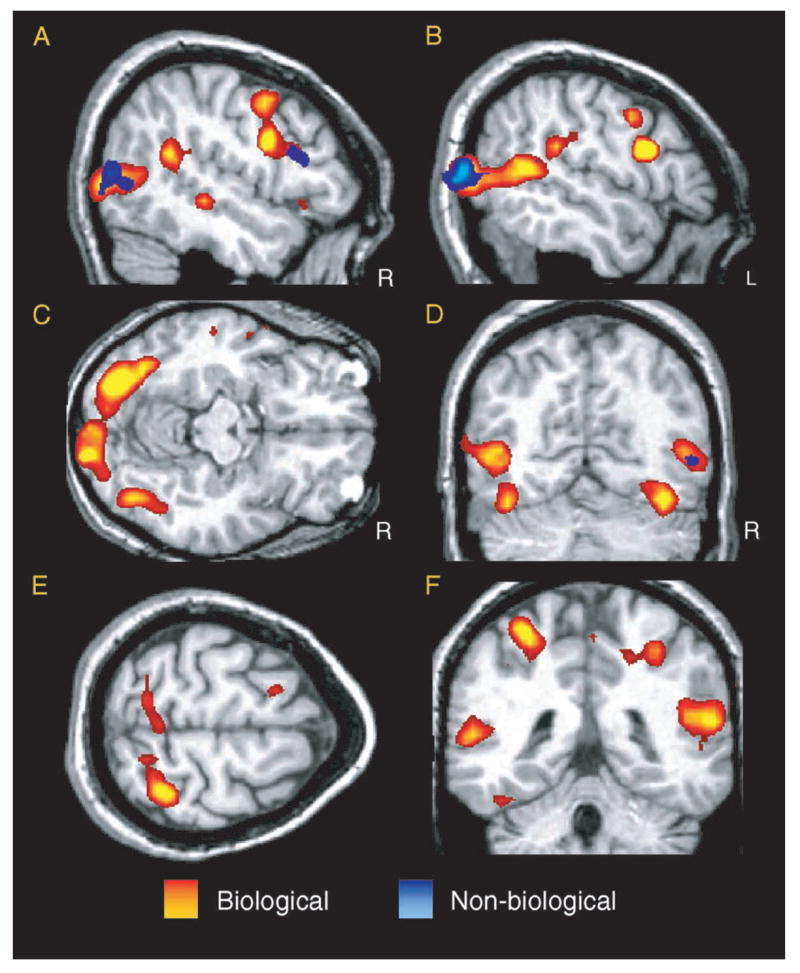
T-statistic activation maps indicating the areas of activity throughout the brain in response to biological motion (red-to-yellow gradient) and non-biological (blue-to-light-blue gradient). The threshold for maps is set at t > 3.6.
Figure 3.
Waveforms showing the percent of MR signal change from the right hemisphere superior temporal sulcus region in response to biological (red) and non-biological (blue) motion.
Although some brain structures showed strong activation to both Biological and Non-biological trial types, several areas, such as the STS region, evinced significantly greater activation during Biological trials than during Non-biological trials. These regions were identified in a random-effects analysis and are illustrated in Figure 4A–E. This contrast identified a network of 15 functional regions of interest (ROIs) across 10 anatomical locations and two hemispheres. The regions of Biological > Non-biological activity included: (1) regions of the right and left FFG, posterior and middle STS region bilaterally, and the PTOF (Figure 4A–F); (2) the right MFG (Figure 4A) and left IFG (Figure 4B); and (3) the intraparietal sulci and the superior and inferior parietal lobules bilaterally (Figure 4D–E). Statistics for these ROIs, including centers of activation in MNI coordinates, Brodmann’s areas, and voxel counts, are listed in Table 1. The regions included regions previously identified in adults as being involved in social perception via the perception of biological motion (e.g., Bonda et al., 1996; Pelphrey et al., 2003a) and regions implicated in the human mirror neuron system (e.g., Iacoboni et al., 1999; Iacoboni, Molnar-Szakacs, Gallese, Buccino, Mazziotta, & Rizzolatti, 2005).
Figure 4.
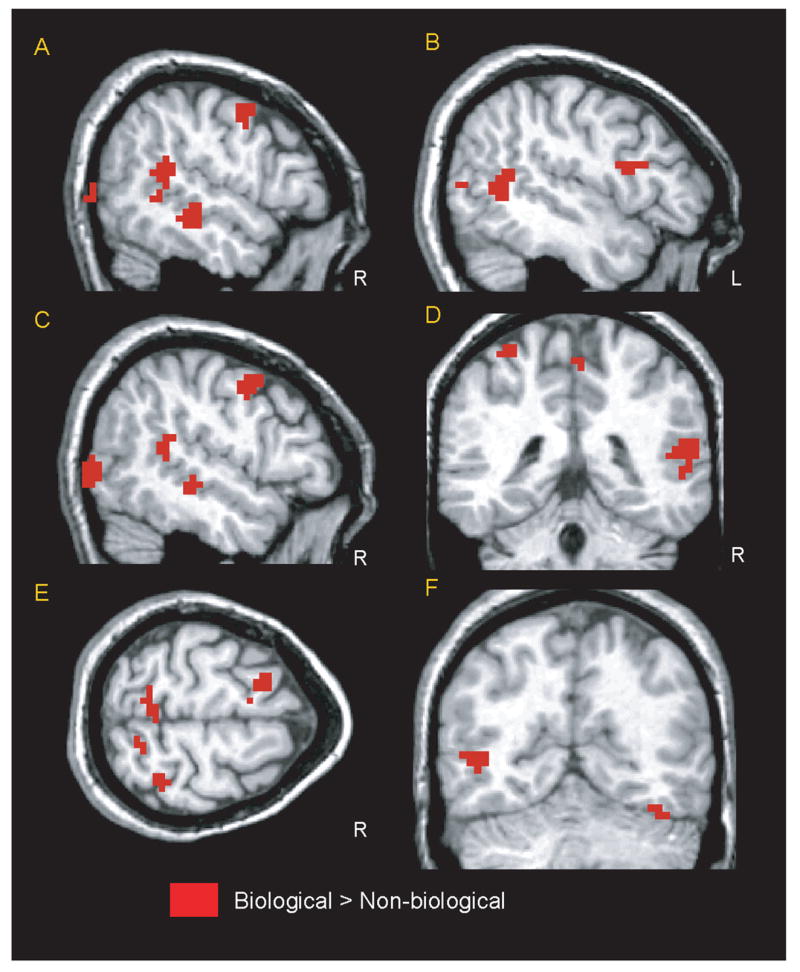
The areas of the brain that showed a greater amount of activity in response to biological than to non-biological motion in a random-effects analysis. The map has a threshold of a voxelwise uncorrected p <.05 (two-tailed) and a spatial extent of six contiguous voxels.
TABLE 1.
Summary of observed regions of biological > non-biological motion activation
| Region | Side | x | y | z | Nvox | BA |
|---|---|---|---|---|---|---|
| Fusiform/middle occipital gyri | R | 25 | 35 | 44 | 1368 | 18 |
| Superior temporal sulcus region | R | 57 | −28 | −4 | 144 | 21 |
| Superior temporal sulcus region | L | −45 | −58 | 12 | 172 | 39 |
| Superior temporal sulcus region | R | 60 | −44 | 18 | 232 | 13 |
| Middle temporal gyrus | L | −53 | −78 | 11 | 44 | 19 |
| Cuneus | R | 28 | −89 | 32 | 376 | 19 |
| Inferior frontal gyrus | L | −53 | 7 | 23 | 88 | 44 |
| Cingulate gyrus | R | 17 | −26 | 48 | 44 | 31 |
| Precentral gyrus | R | 53 | −2 | 51 | 128 | 6 |
| Precentral gyrus | L | −42 | −9 | 59 | 68 | 6 |
| Paracentral lobule | R | 6 | −50 | 65 | 140 | 5 |
| Postcentral gyrus | L | −14 | −59 | 67 | 32 | 7 |
| Postcentral gyrus | L | −33 | −49 | 70 | 44 | 5 |
| Superior frontal gyrus | R | 18 | 6 | 71 | 88 | 6 |
| Superior frontal gyrus | R | 10 | −21 | 77 | 48 | 6 |
Note: Nvox = number of voxels in the ROI; x, y, and z refer to the stereotaxic MNI co-ordinates of the center of activation within an ROI; r = right hemisphere; L = left hemisphere; BA = Brodmann’s area. The threshold for significance of the clusters reported here was set at a voxelwise uncorrected p < .05 and a spatial extent of six functional voxels.
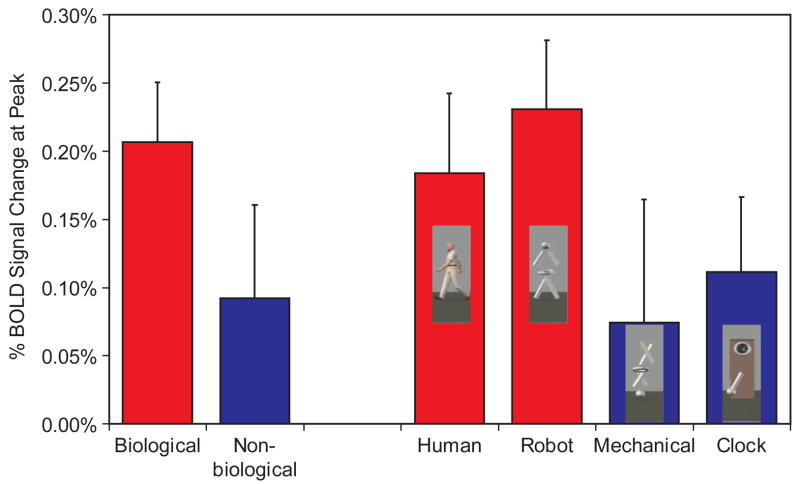
As shown in the two left most columns of Figure 5, the right STS region responded over twice as much to the Biological (M = 0.21%, SE = 0.05%) compared to the Non-biological (M = 0.09%, SE = 0.07%) condition, t(8) = 2.65, p = .03 (two-tailed). Using the peak amplitude scores averaging across voxels in this region (see right four columns of Figure 5), we tested a hierarchy of questions with three additional paired-sample t-tests. First we compared responses to the Human (M = 0.19%, SE = 0.06%) and Robot (M = 0.23%, SE = 0.05%). Equivalent responses to these two conditions indicated that the STS was responding to the biological motion conveyed by the figure and not to the form of the figure, t(8) = −0.69, p = .51 (two-tailed). After we had established that the Robot and Human evoked equivalent responses from the STS, we used the Robot as a representative of biological motion and evaluated whether the STS region responded more strongly to biological motion (Robot) than to a meaningless but complex non-biological motion (Mechanical) or a coherent complex meaningful non-biological motion (Clock). The STS responded more strongly to Robot than to Clock (M = 0.11%, SE = 0.06%); t(8) = 2.61; p = .01 (one-tailed), or Mechanical (M = 0.07%, SE = 0.10%); t(8) = 2.19; p = .03 (one-tailed). This pattern of effects was virtually identical when we considered the posterior and middle STS ROIs in isolation, when we performed the same analyses for the left STS region, and when we collapsed across the left and right STS regions.
Figure 5.
The total percent signal change in the BOLD response at peak caused by biological, non-biological, human, robot, mechanical, and clock movements in the right superior temporal sulcus region.
In contrast to the STS region, a left-hemisphere region localized posterior and inferior to the STS region, corresponding to the expected location of area MT/V5 (Dumoulin et al., 2000), exhibited a very different response profile. This region responded equivalently to the Biological (M = 0.37%, SE = 0.08%) and Non-biological (M = 0.49%, SE = 0.10%) conditions, t(8) = 1.92; p = .09 (two-tailed). Thus, we observed a dissociation between the response profiles of the STS region and area MT/V5 in school-aged children.
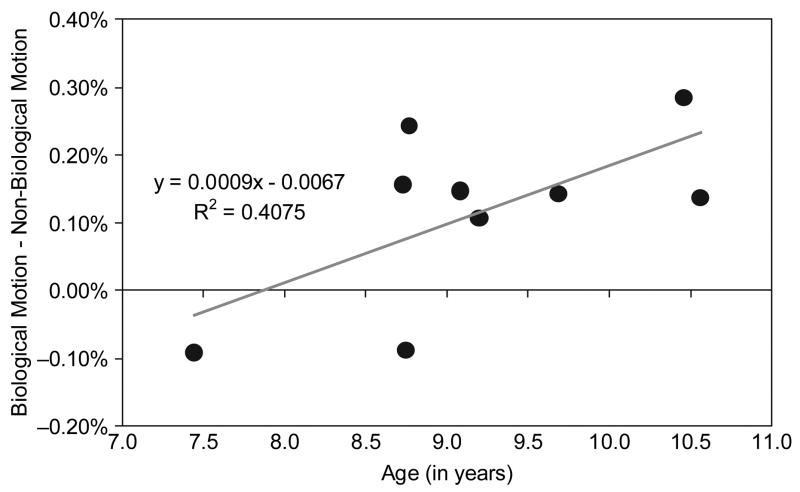
On average, activity in the STS region clearly differentiated biological from non-biological motion, but individual differences in the magnitude of the differentiation were apparent when we visually inspected findings from the individual subjects. To explore possible developmental trends in the functional development of the STS region, we correlated age (in years) with the magnitude of Biological > Non-biological differentiation at the peak of the HDR in the right STS region. We assumed that higher levels of Biological > Non-biological activity (i.e., greater Biological–Non-biological difference scores) were indicative of a more maturely functioning system. Consequently, we anticipated a positive correlation between the specificity of the STS region for biological motion and age. As illustrated in Figure 6, the magnitude of the Biological > Non-biological difference score was positively correlated with age in the right STS region (r = .64, p = .03, two-tailed), with age accounting for approximately 41% of the variance in the Biological > Non-biological difference scores. This correlation was similar (r = .52, p = .15, two-tailed), but of lesser magnitude, in the left STS, with age accounting for 25% of the variance in the Biological > Non-biological difference scores. We conducted this analysis for the other regions of Biological > Non-biological activity identified in the random-effects analysis (see Table 1) and found no other significant correlations or strong trends. These findings suggest that the degree of Biological > Non-biological differentiation in the STS region increases with age in typically developing children across the 7- to 10-year age range.
Figure 6.
A scatter plot of biological motion minus non-biological motion difference scores from the right superior temporal sulcus region as a function of age of the participant. Age accounted for 41% of the variance in the difference scores.
DISCUSSION
In this study, we sought to explore the development of social brain regions involved in the perception of biological motion and particularly to test the specificity of the response from the STS region in children to biological motion compared to non-biological motion. We used a paradigm that we had previously employed to demonstrate in adults that the STS response was specific to biological compared to non-biological motion (Pelphrey et al., 2003a). A group of typically developing children ranging from 7 to 10 years of age watched movements made by a human, a robot, a mechanical figure, and a grandfather clock. This design allowed us to determine whether the STS region in children exhibited specialization for biological relative to non-biological motion as well as how that specialization might increase with age. We hypothesized that the children’s degree of specificity for biological motion in the STS region would be somewhat less than that of adults, but would increase to more adult-like levels with age.
We identified a network of social brain regions that were preferentially engaged for biological motion over non-biological motion perception. These regions included sectors of the posterior and middle STS bilaterally, FFG bilaterally, the PTOF, the right MFG, the left IFG, the IPS bilaterally, and the superior and inferior parietal lobules bilaterally. Some of these regions (e.g., the STS, MFG, and FFG) have been implicated previously in biological motion processing in adults (e.g., Bonda et al., 1996, Pelphrey et al., 2003a, 2004; Saxe et al., 2004). Other regions (e.g., the IPS and IFG) have been identified as components of the mirror neuron system (e.g., Iacoboni et al., 1999, 2005). These findings from children clearly replicate our prior findings (Pelphrey et al., 2003a). In that study of adults, the STS region was sensitive to biological motion, and it was more responsive to biological motion, whether made by the human (a biological figure) or the robot (a non-biological figure), than by the non-biological motion made by the mechanical figure or the clock. Unfortunately, the previous study was limited in its ability to identify regions of activity in the frontal cortex because the pulse sequence used to acquire the imaging data was limited to more posterior regions of the brain.
Overall, the STS regions of 7- to 10-year-old children showed the same pattern of differentiation between biological and non-biological motion as those of adults in our previous study. However, there were important differences. First, our analysis of whether this pattern changed with age suggested that the younger children had less differentiation between the two motion types in the STS region than did the older children. Previous research has suggested protracted development in certain social brain regions (e.g., the FFG; Aylward et al., 2005; Taylor et al., 1999, 2001), but not others (e.g., the mirror neuron system; Dapretto et al., 2006; Ohnishi et al., 2004). Of all of the regions that were activated in our paradigm, the only area that showed a developmental change was the STS. Given these findings, it seems possible that the specificity of this region for the perception of biological motion increases with age. This increase in specificity could be due to a number of factors, including maturation, additional experience with biological motion, or an overall increase in social skills. We note, however, that the current study was cross-sectional and limited to a relatively small number of children; thus, conclusions regarding the presence or cause of developmental change are necessarily tentative. Future research should examine this further in the context of a longitudinal design that would allow inferences to be made about individual development.
Additional evidence for developmental change is the difference in lateralization patterns between children and adults: adults have strongly right-lateralized activity (e.g., Pelphrey et al., 2003a, 2004), whereas children’s activity appeared to be more bilateral. This finding appears to parallel a recent fMRI study of language processing in children. Holland and colleagues (2001) found that the degree of left hemisphere lateralization of language increases with age between 7 and 18 years. In addition to supporting increasing specialization of other brain regions in the middle childhood ages we studied, these findings are particularly interesting because the STS region and IFG activations observed for biological motion in the right hemisphere are strikingly similar in position to the left-lateralized regions corresponding to Broca’s and Wernicke’s areas observed by Holland and colleagues (Holland, Plante, Weber Byars, Strawsburg, Schmithorst, & Ball, 2001). We speculate that the development of right-hemisphere brain regions supporting social perception could parallel the left-hemisphere areas used for language. It is possible that the social perception and language systems overlap in early childhood and draw upon the same set of brain regions bilaterally. Then, as children develop, these systems might become more lateralized and exhibit less overlap. This seems plausible, as an elegant body of behavioral work on language development indicates that language abilities and social perception abilities might require the same underlying skills, including joint attention and intentionality assessment (e.g., Charman, Baron-Cohen, Swettenham, Baird, Cox, & Drew, 2001; Flavell, 1999; Tomasello & Farrar, 1986).
In an earlier fMRI study (Mosconi et al., 2005), we investigated the brain regions involved in the understanding of the intentions underlying gaze shifts in 7- to 10-year-olds. The children watched an animated character shift her gaze either towards (congruent condition) or away from (incongruent condition) a flashing target after it appeared in her visual field. Consistent with a prior study of adults (Pelphrey et al., 2003b), the incongruent condition evoked more activity in the right posterior STS region, suggesting that this area is involved in both processing eye movements and analyzing the intentions that provoke them. While providing important information regarding continuity in the social brain from childhood to adulthood, this study did not clarify whether the STS region in children processed biological motion more generally or how the domain specificity of this region might develop because there was no comparison of biological and non-biological motion conditions. Here we demonstrate that by the age of 7 years, the STS region responds more during the perception of biological compared to non-biological motion, and the specificity of the response to biological motion increases in typically developing children between the ages of 7 and 10 years.
Baron-Cohen (1995) proposed four neuropsychological mechanisms comprising a brain system that supports “mindreading.” Of these, the most basic is the intentionality detector (ID), which “interprets motion stimuli in terms of the primitive volitional mental states of goal and desire” (p. 32). Thus, anything with self-propelled motion can be defined as a potential agent. The ID works together with the eye-direction detector (EDD) to perceive the presence of eyes and the direction of their shifting gaze and attributes the mental state of seeing to the owner of the eyes. Next, the shared attention mechanism (SAM) integrates the information about the agency and intentions of others with the eye direction data and uses these to create joint attention, in which the understanding that another being is purposely looking at something directs the viewer to follow that other person’s attention. Lastly, the theory of mind mechanism (ToMM) integrates all of the information from the ID, EDD, and SAM in support of an individual’s assessment that another person has intentions and beliefs based on his or her own knowledge that are not necessarily in accord with reality. Information concerning the role of the STS region in children’s processing of biological motion is particularly significant for Baron-Cohen’s model because two components of the mindreading system, the EDD and the SAM, rely on normal biological motion perception (particularly the processing of eye movements), and both biological and non-biological motion perception are important for the ID in order to identify movement and analyze goal-directedness. The development and functioning of the ToMM, in turn, relies upon data from these two components. Consistent with a prominent role for biological motion in mindreading, Blakemore and Decety (2001) suggested that one must identify biological motion in order to monitor the presence of potential predators, mates, and prey in the environment and be able to create expectations about their goals and future directions in order to survive. The importance of biological motion perception for ToM is further underscored by humans’ ability to use extremely deprived stimuli (the moving dots of point-light walkers) to detect locomotion (e.g., Johannson, 1973) and other complex actions and even to identify gender, personality, and emotion information (e.g., Barclay, Cutting, Kozlowski, 1978; Cutting & Kozlowski, 1977; de Gelder, Snyder, Greve, Gerard, & Hadjikhani, 2004; Kozlowski & Cutting, 1977).
Individuals with autism show a wide range of social perception problems. One of the most striking and well-documented deficits is their inability to perform ToM tasks (e.g., Baron-Cohen, 1995; Baron-Cohen et al., 1985; Happé, 1995; Perner et al., 1989). If biological motion perception is a necessary precursor for the development of these higher-level social cognition skills, then it follows that these individuals might also have dysfunctional biological motion perception. Blake and colleagues (2003) examined biological motion and global form detection. Children with and without autism viewed moving dot patterns representing either human motion or random movement. Children without autism were able to properly identify which patterns were humans; children with autism were unable to make this differentiation. However, both groups of children performed equivalently when they had to detect a moving shape among a large number of jittering line segments. This suggests that children with autism do not have a problem detecting global form from motion, but rather possess a specific deficit in identifying biological motion. This study provided a striking demonstration of behavioral dysfunction, but did not address the neural substrates underlying this deficit. In an fMRI study of mirror neuron functioning in children with autism, Dapretto and colleagues (1 found that, unlike children without autism, children with autism did not show IFG activity during observation and imitation of emotional facial expressions. The degree of activity in the children with autism was inversely correlated with the degree of social dysfunction. While this study implicated the IFG component of the biological motion perception system, Pelphrey, Morris, and McCarthy (2005) implicated the STS region as an area of dysfunction in autism. Using the congruent and incongruent gaze paradigm described above (Mosconi et al., 2005), we compared the degree to which the activation provoked by incongruent gaze shifts differed from that by congruent gaze shifts. In typically developing adults, there was significantly greater activity in response to the incongruent gaze shifts than to the congruent shifts. However, there was no significant difference between conditions in the adults with autism, suggesting that their STS regions did not differentiate based on the intentionality inherent in gaze shifts. Furthermore, the degree of differentiation in the affected adults strongly correlated with their social functioning ability. To date, there have been no studies comparing biological and non-biological motion perception directly in children with autism. From the previous research, we would predict that there is less selectivity for biological motion in these children. Preliminary data from our laboratory (Carter, Mosconi, & Pelphrey, 2005) using the same paradigm as the current study suggest that there is less differentiation between biological and non-biological motion in the STS region of children with autism than is present in children without autism.
There are a few limitations to this study. For example, there is a small sample size and a limited age range. The nature of fMRI research makes it difficult to study awake, behaving children; most research has focused on older children (ages 8 and above). To our knowledge, only one fMRI experiment has been successfully performed in 4-year-old children (Cantlon, Brannon, Carter, & Pelphrey, 2006). However, expanding the age range to include younger children would make it possible to look at brain changes during the transition in performance on ToM tasks that occurs around four years of age (e.g., Wellman, Cross, & Watson, 2001). It would also be particularly interesting to do a longitudinal study of younger children to see how behavioral changes in social performance might relate to changes in brain activation patterns.
The present study suggested the possibility of developmental changes in the functioning of the STS region during biological motion perception across middle childhood. Out of an extensive network of brain regions involved in the perception of biological motion, only the STS region appeared to exhibit developmental change. Much of what develops in regards to social cognitive abilities in childhood likely involves changes in connections among the social brain regions that allow for increasingly higher-order social perception and mentalizing abilities. Future research should be conducted to explore the development of connectivity to and from the STS region. For example, it might be the case that what appears to be a relative increase in differentiation in the STS region could represent changes in its inputs and outputs. Thus, it could either be a function of tuning in a visual region—the STS—or a development in the input by top-down regions to the STS. This could be examined more closely in concert with functional connectivity (to explore when regions work together) and diffusion tensor imaging (to determine the structural connections between these regions).
Acknowledgments
This study was funded by a Career Development Award from the National Institute of Mental Health (K01 MH071284), a John Merck Scholars Award, and a grant from the National Alliance for Autism Research/Autism Speaks to KAP.
We thank Heather Lucas for help collecting, analyzing, and discussing these data. We thank the children and their families who generously gave their time and effort to make this research possible.
References
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Science. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Park JE, Field KM, Parsons AC, Richards TL, Cramer SC, et al. Brain activation during face perception: Evidence of a developmental change. Journal of Cognitive Neuroscience. 2005;17(2):308–319. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- Barclay CD, Cutting JE, Kozlowski LT. Temporal and spatial factors in gait perception that influence gender recognition. Perception and Psychophysics. 1978;23(2):145–152. doi: 10.3758/bf03208295. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychological Science. 2003;14(2):151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Decety J. From the perception of action to the understanding of intention. Nature Reviews Neuroscience. 2001;2(8):561–567. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. Journal of Neuroscience. 1996;16:3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L. The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. European Journal of Neuroscience. 2001;13(2):400–404. [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA. Functional imaging of numerical processing in adults and 4-year-old children. Public Library of Science: Biology. 2006;4(5):844–854. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EJ, Mosconi M, Pelphrey KA. Neural basis of biological motion perception in children with and without autism. Presented at the annual meeting of the Society for Neuroscience; Washington, DC, USA. 2005. [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A. Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development. 2001;15(4):481–498. [Google Scholar]
- Cutting JE, Kozlowski LT. Recognizing friends by their walk: Gait perception without familiarity cues. Bulletin of the Psychonomic Society. 1977;9:353–356. [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Chaminade T. When the self represents the other: A new cognitive neuroscience view on psychological identification. Conscious Cognition. 2003;12:577–596. doi: 10.1016/s1053-8100(03)00076-x. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Snyder J, Greve D, Gerard G, Hadjikhani N. Fear fosters flight: A mechanism for fear contagion when perceiving emotion expressed by a whole body. Proceedings of the National Academy of Sciences USA. 2004;101(47):16701–16706. doi: 10.1073/pnas.0407042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennett DC. The intentional stance. Cambridge, MA: MIT Press; 1987. [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G, et al. A new anatomical landmark for reliable identification of human area V5/MT: A quantitative analysis of sulcal patterning. Cerebral Cortex. 2000;10(5):454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Flavell JH. Cognitive development: Children’s knowledge about the mind. Annual Review of Psychology. 1999;50:21–45. doi: 10.1146/annurev.psych.50.1.21. [DOI] [PubMed] [Google Scholar]
- Fox R, McDaniel C. The perception of biological motion by human infants. Science. 1982;218(4571):486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TA. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuro-Image. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance Medicine. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35:1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Guo H, Song AW. Single-shot spiral image acquisition with embedded z-shimming for susceptibility signal recovery. Journal of Magnetic Resonance Imaging. 2003;18(3):389–395. doi: 10.1002/jmri.10355. [DOI] [PubMed] [Google Scholar]
- Happé FG. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Development. 1995;66(3):843–855. [PubMed] [Google Scholar]
- Hirai M, Hiraki K. An event-related potentials study of biological motion perception in human infants. Cognitive Brain Research. 2005;22:301–304. doi: 10.1016/j.cogbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Current Opinion in Neurobiology. 2005;15(6):632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, et al. Reafferent copies of imitated actions in the right superior temporal cortex. Proceedings of the National Academy of Sciences USA. 2001;98(24):13995–13999. doi: 10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. PloS Biology. 2005;3(3):1–7. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Johannson G. Visual perception of biological motion and a model for its analysis. Perception and Psychophysics. 1973;14:201–211. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extra-striate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Cutting JE. Recognizing the sex of a walker from a dynamic point-light display. Perception and Psychophysics. 1977;21:575–580. [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: A survey of methods for statistical pooling of information. NeuroImage. 2002;16:538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Spicer M, Adrignolo A, Luby M, Gore J, Allison T. Brain activation associated with visual motion studied by functional magnetic resonance imaging in humans. Human Brain Mapping. 1995;2:234–243. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Mack PB, McCarthy G, Pelphrey KA. Taking an “intentional stance” on eye-gaze shifts: A functional neuroimaging study of social perception in children. NeuroImage. 2005;27(1):247–252. doi: 10.1016/j.neuroimage.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Moriguchi Y, Matsuda H, Mori T, Hirakata M, Imabayashi E, et al. The neural network for the mirror system and mentalizing in normally developed children: An fMRI study. Neuroreport. 2004;15(9):1483–1487. doi: 10.1097/01.wnr.0000127464.17770.1f. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G. Brain activity evoked by the perception of human walking: Controlling for meaningful coherent motion. Journal of Neuroscience. 2003a;23(17):6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP. The role of the superior temporal sulcus region in interpreting the actions of others. Current Directions in Psychological Science. 2006;15(3):136–140. doi: 10.1111/j.0963-7214.2006.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: The perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. Journal of Cognitive Neuroscience. 2004;16(10):1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128(5):1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: The influence of context. Neuropsychologia. 2003b;41(2):156–170. doi: 10.1016/s0028-3932(02)00146-x. [Erratum appeared 2003 in Neuropsychologia, 41(11), 1561–1562] [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass. Psychological Science. 2004;15(9):598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Perner J, Frith U, Leslie AM, Leekam SR. Exploration of the autistic child’s theory of mind: Knowledge, belief, and communication. Child Development. 1989;60(3):688–700. [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? The Behavioral and Brain Sciences. 1978;3:515–536. [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16(16):5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Research: Cognitive Brain Research. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: Linking developmental psychology and functional neuroimaging. Annual Review of Psychology. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42(11):1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Edmonds GE, McCarthy G, Allison T. Eyes first! Eye processing develops before face processing in children. Neuroreport. 2001;12(8):1671–1676. doi: 10.1097/00001756-200106130-00031. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, McCarthy G, Saliba E, Degiovanni E. ERP evidence of developmental changes in processing of faces. Clinical Neurophysiology. 1999;110(5):910–915. doi: 10.1016/s1388-2457(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Farrar MJ. Joint attention and early language. Child Development. 1986;57(6):1454–1463. [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Dale AM, Look RB, Sereno MI, Malach R, et al. Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature. 1995;375(6527):139–141. doi: 10.1038/375139a0. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, De Schonen S, Crivello F, Reutter B, Aujard Y, Mazoyer B. Neural correlates of woman face processing by 2-month-old infants. NeuroImage. 2002;15(2):454–461. doi: 10.1006/nimg.2001.0979. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: The truth about false belief. Child Development. 2001;72(3):655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. Real-time fMRI integrating paradigm control, physiology, behavior and on-line statistical analysis. NeuroImage. 1999;10:91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao J, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping. 1995;3(4):287–301. [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. Journal of Neuroscience. 1991;11(3):641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]