Abstract
Aim:
Determine efficacy of two-year continuous subantimicrobial dose doxycycline (SDD; 20 mg bid) on alveolar bone in postmenopausal osteopenic, estrogen-deficient women undergoing periodontal maintenance in a two-year double-blind, placebo-controlled, randomized clinical trial.
Materials and Methods:
128 subjects randomized to SDD or placebo (n=64 each). Posterior vertical bite-wings taken at baseline, one and two years for alveolar bone density (ABD), using radiographic absorptiometry (RA) and computer-assisted densitometric image analysis (CADIA), and alveolar bone height (ABH). Statistical analyses utilized Generalized Estimating Equations; primary analyses were intent-to-treat (ITT). Results presented as SDD versus placebo.
Results:
Under ITT, there was no statistically-significant effect of SDD on ABD loss (RA: p=0.8; CADIA: p=0.2) or ABH loss (p=0.2). Most sites (81−95%) were inactive. For subgroup analyses, mean CADIA was higher with SDD for non-smokers (p=0.05) and baseline probing depths ≥ 5 mm (p =0.003). SDD was associated with 29% lower odds of more progressive ABH loss in women > 5 years postmenopausal (p=0.05) and 36% lower among protocol-adherent subjects (p =0.03).
Conclusion:
In postmenopausal osteopenic women with periodontitis, SDD did not differ overall from placebo. Based on exploratory subgroup analyses, additional research is needed to determine the usefulness of SDD in non-smokers, subjects > 5 years postmenopausal and in deeper pockets.
Keywords: periodontitis, osteopenia, randomized clinical trial, subantimicrobial dose doxycycline (SDD), postmenopausal, periodontal maintenance, dual-energy x-ray absorptiometry (DEXA), radiographic absorptiometry (RA) method, computer-assisted densitometric image analysis (CADIA).
Clinical Relevance
Scientific Rationale:
Postmenopausal estrogen deficiency is associated with increased oral bone loss. Tetracyclines inhibit collagenase activity and enhance osteoblast activity and collagen production. This two-year clinical trial's goal was to determine the efficacy of subantimicrobial dose doxycycline (SDD) in reducing alveolar bone (AB) density/height loss in postmenopausal, estrogen-deficient osteopenic women with periodontitis.
Principal Findings:
SDD did not significantly alter AB loss overall but, in exploratory subgroup analyses, SDD was associated with statistically-significantly reduced AB loss in non-smokers, subjects > 5 years postmenopausal, protocol-adherent subjects and deeper pockets.
Practical Implications:
In postmenopausal osteopenic women with periodontitis, this clinical trial failed to demonstrate that SDD is useful in altering AB loss overall. Based on exploratory subgroup analyses, additional research is needed to determine the usefulness of SDD in non-smokers, subjects > 5 years postmenopausal and in deeper pockets.
Tetracyclines and their chemically-modified non-antibacterial analogs can inhibit certain host-derived tissue-destructive matrix metalloproteinases such as collagenases and gelatinases (Golub et al. 1983), including those which help mediate bone resorption (Rifkin et al. 1994, Golub et al. 1998). Golub and coworkers have found that these drugs, by a non-antibacterial mechanism, also can enhance osteoblast activity, collagen production and bone formation (Golub et al. 1990, Sasaki et al. 1992, Bain et al. 1997, Craig et al. 1998, Golub et al. 1999). One of the disease conditions found to be beneficially affected by this discovery was osteoporosis during both experimental diabetes (Golub et al. 1990, Sasaki et al. 1992, Bain et al. 1997) and estrogen deficiency (Golub et al. 1999). In addition, Williams et al. (1996) at the National Institute on Aging (NIH) have shown that minocycline can increase bone formation and decrease bone resorption resulting in increased systemic bone density in ovariectomized rats. Estrogen deficiency in postmenopausal women: (a) represents a key factor in the pathogenesis of osteoporosis (Cranney et al. 2002); (b) involves accelerated bone resorption overpowering the rate of bone formation (Riggs & Melton 1986); and (c) has, in recent years, been associated with increased tooth loss (Tezal et al. 2005), and oral bone loss (Payne et al. 1997, Payne et al. 1999). Based on these data, we proposed the following hypothesis: tetracyclines, namely subantimicrobial dose doxycycline (SDD), by a non-antimicrobial property, can reduce alveolar bone loss in estrogen-deficient postmenopausal women with periodontitis and with osteopenia of the lumbar spine or femoral neck. Accordingly, our research team conducted a two-year, double-blind randomized placebo-controlled clinical trial to determine whether a two-year continuous regimen of SDD (20 mg bid) can reduce alveolar bone density loss and alveolar bone height loss in postmenopausal, osteopenic, estrogen-deficient women on periodontal maintenance therapy for moderate to severe chronic periodontitis.
Material and Methods
Participants.
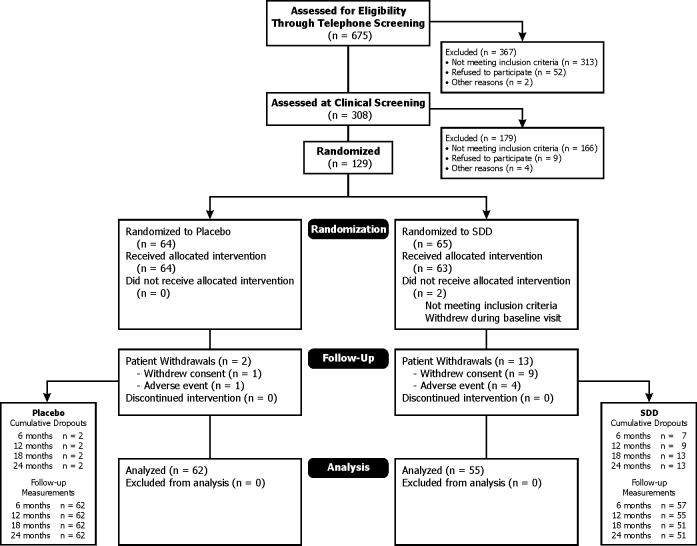
The study protocol was reviewed and approved by the University of Nebraska Medical Center Institutional Review Board and the Stony Brook Institutional Review Board. The eligibility screening process began with a telephone screen (n=675), followed by a clinical screening visit (n=308) for telephone screening-eligible subjects (Figure 1). One hundred thirty-six (44% of clinical screening visit contacts) were eligible based on the clinical screening visit at the University of Nebraska Medical Center College of Dentistry (UNMC COD) and the School of Dental Medicine at Stony Brook University (Stony Brook) and dual-energy x-ray absorptiometry (DEXA) scans of the lumbar spine and femoral neck in medical clinics (Arthritis Center of Nebraska and Osteoporosis Center at Stony Brook). Subjects who provided consent and were eligible based on the clinical screening visit and DEXA scans then were randomized (n=128). An additional subject was randomized who was found to have fewer than 9 posterior teeth at the baseline visit and was, therefore, declared ineligible. This subject did not receive any study medication.
Figure 1.
Patient recruitment and follow-up flowchart showing the flow of subjects through each stage of the clinical trial, from screening through completion of the study protocol and through statistical analyses (intent-to-treat). Sixty subjects satisfied the per-protocol criteria (28 placebo and 32 SDD subjects) at one year and 43 subjects satisfied the per-protocol criteria (19 placebo and 24 SDD subjects) at the two-year time point.
Subjects were recruited from the following sources: private periodontal and general dental practices in Nebraska and Long Island, New York (n=57), the UNMC COD and Stony Brook patient pools (n=48), and advertisements (n=23).
Inclusion criteria included: 45−70 years of age at telephone screening; postmenopausal for at least 6 months and not receiving hormone replacement therapy (HRT); osteopenia of the lumber spine or femoral neck (T-score of −1.0 to −2.5 inclusive); history of generalized moderate to advanced periodontitis and undergoing periodontal maintenance; and having at least 9 posterior teeth and at least two sites with probing depths ≥ 5 mm together with bleeding on probing, ≥ 5 mm clinical attachment level loss, and radiographic evidence of alveolar bone height loss. Subjects also had to be willing to sign UNMC and Stony Brook Institutional Review Board-approved consent forms and had to be in good general health without co-morbidities that may interfere with adherence to the study protocol, planned follow-up or endpoint measurement.
Exclusion criteria included: allergy or hypersensitivity to tetracyclines; diseases or regular drug therapy that would affect the inflammatory or immune response (e.g., chronic use of non-steroidal anti-inflammatory drugs [NSAIDs]) or bone remodeling (e.g., prescription estrogens, bisphosphonates, calcitonin, and steroids); requirement for antibiotic premedication; diabetes; active periodontal therapy within the past year; normal bone mineral density (BMD) at both the lumbar spine and femoral neck (T-score above −1.0) or osteoporosis of the lumbar spine or femoral neck (T-score less than −2.5).
Demographic data.
The following information was gathered at the clinical screening, baseline and all study visits (every 6 months for two years): subject height and weight; smoking status (former, current or never smoker); smoking dose (packs per day and number of years that subject had smoked); and total number of teeth present. At telephone screening, age, race, ethnicity and the number of years postmenopausal were recorded.
Study design.
This study was a double-blind, placebo-controlled, randomized 2-year clinical trial with each subject instructed to take all study medications daily for two years. Study participants, those administering the interventions and those assessing the outcomes were blinded to group assignment. The study had two treatment arms: 20 mg doxycycline twice daily (low-dose or subantimicrobial dose-doxycycline; SDD) and a placebo look-alike twice daily. Sixty-four eligible subjects were randomized into each treatment arm. All subjects received calcium and vitamin D supplements twice daily (a total of 1200 mg of calcium and 400 IU of vitamin D daily). Subjects were instructed not to take the study drug and calcium/vitamin D at the same time, being certain the supplements were taken at least one hour since taking the study drug. All subjects received periodontal maintenance every 3−4 months throughout the study delivered by the subjects' own dental care providers and not by the study clinicians. The periodontal maintenance was provided at no cost to the subjects.
Randomization and masking.
Subjects were centrally randomized with the randomization stratified by study center (UNMC COD or Stony Brook) and current smoking status (current smoker or not current smoker). The computer-generated randomization list was generated in blocks with size varying randomly among 4, 6, and 8. The treatment code identifying SDD and placebo arms was concealed from study investigators until all patient follow-up had been completed and all outcome measurements had been made.
Treatment assignments were given via telephone and confirmed via fax through a central coordinating center.
Systemic BMD determination.
BMD scans of the lumbar spine and femoral neck were taken using DEXA (Hologic 4500, Waltham, MA) at baseline, one year and 2 years and T-scores were computed (i.e., comparison of individual's BMD relative to the peak bone mass seen in 20−29 year old healthy female subjects) (Looker et al. 1997). BMD values (and not T-scores) were compared between baseline and 1 year and baseline and 2 years. The individual BMD least significant change (LSC) at UNMC for the lumbar spine was +/- 0.025 g/cm2 and for the femoral neck was +/- 0.040 g/cm2. The LSC at Stony Brook for the lumbar spine was +/- 0.026 g/cm2 and at the femoral neck was +/- 0.045 g/cm2. The same DEXA machine was used throughout the study at each institution.
Oral radiographic analyses: radiographic procedures.
Four posterior bitewing radiographs (maxillary and mandibular right and left), each positioned to visualize a posterior sextant in the mouth and centered in the molar-premolar area, were taken at baseline, 1 year and 2 years. All radiographs were taken by a single examiner at each center. The (F-speed) #2 size intraoral film was secured to a bitewing holder containing a bone density reference wedge as a bite block and the film was exposed using an extended geometry method (Payne et al. 1999) introduced by Jeffcoat and coworkers (1987). A Quint Sectograph radiographic unit (Los Angeles, CA) was used for all radiographic images at both clinical centers. All radiographs at each center were developed with a designated film processor. Radiographic analyses were performed centrally at the Longitudinal Radiographic Assessment Facility in San Antonio, TX and the examiner (PVN) was blinded to subject treatment and all data except for bitewing radiographs.
Oral radiographic analyses: image capture and digitization.
After the baseline image was digitized and saved on the computer, the 1-year or 2-year follow-up radiograph was aligned with the baseline image using a real-time subtraction procedure and was digitized in that alignment (Payne et al. 1999). The density and contrast of the baseline and follow-up films were matched using a non-parametric contrast matching program (Rüttimann et al.1986) as implemented in the software package DSR™ (Electro Medical Systems, Richardson, TX). These matched images were used in the bone density analyses.
Radiographic absorptiometry (RA) (primary endpoint).
Quantitative analysis of bone density was determined by the RA method (Kuhl & Nummikoski 2000) at baseline, one year and two years at crestal and subcrestal areas of interest.
Any relative percent change in density between baseline and follow-up areas of interest beyond +/- 25%, which corresponded roughly to the 5th and 95th percentiles of the relative percent change distribution, was re-measured to confirm the change.
Computer-Assisted Densitometric Image Analysis (CADIA) (secondary endpoint).
Semi-quantitative alveolar bone density changes at the one-year and two-year visits relative to baseline were determined at the crestal and subcrestal areas of interest using the CADIA method (Payne et al. 1999).
Any CADIA change value beyond +/- 30, which corresponded roughly to the 5th and 95th percentiles of the CADIA change distribution, was re-measured to confirm the change.
Alveolar Bone Height Measurements (secondary endpoint).
The measurements were performed using the method described by Hausmann and colleagues (1992). Linear measurements between the fixed reference point (cementoenamel junction [CEJ] or restoration margin) and the alveolar crest were made for baseline, 1-year and 2-year radiographs.
Any change in alveolar bone height between baseline and follow-up beyond +/- 0.5 mm, which corresponded roughly to the 5th and 95th percentiles of the alveolar bone height change distribution, was re-measured to confirm the change.
Radiographic quality control.
Continuous radiographic film quality control procedures were implemented for the study. One set of radiographs was shipped within one week of their being taken via overnight mail to the Longitudinal Radiographic Assessment Facility in San Antonio for analysis (the duplicate set remained in the subject's record). If discrepancies in projections or errors in exposure or processing were detected, new radiographs were taken within one month.
Oral radiographic repeatability studies.
Following initial radiographic measures on approximately one-third of the study subjects, films from 13 randomly-chosen subjects were measured a second time to quantify the measurement reliability.
The intra-examiner reproducibility for alveolar bone height and RA and CADIA density measures was quantified by the standard deviation (SD) of the difference between replicate measures across all sites (Osborn et al. 1992).
Assessment of adverse events.
This study was monitored by an independent Data and Safety Monitoring Board (DSMB) appointed by the National Institute of Dental and Craniofacial Research (NIH; Bethesda, MD). Subjects recorded adverse events and concomitant medications each day they participated in this trial in a study diary. All adverse events were recorded regardless of attribution. Each adverse event was evaluated for duration, intensity (mild, moderate or severe), seriousness and relation with the study medication or other causes. Serious adverse events meeting definitions established by the United States Food and Drug Administration also were recorded.
Assessment of adherence.
At each visit, subjects were counseled on the importance of taking the study medications in the prescribed manner. Percent adherence was calculated separately for the randomized study drug and calcium/vitamin D using pill counts and the number of days between study visits.
Statistical analyses.
Sample size justification.
The targeted total sample size was justified based on the primary study endpoint, radiographic evidence of a decrease, of at least two times the standard deviation of replicate measures, in alveolar bone density from baseline. The sample size calculation was adjusted for the correlation among observations sampled within a given subject's mouth and for the correlation among the longitudinal observations using the method suggested by Rochon (1998), based on Generalized Estimating Equations (GEE) analysis (Liang & Zeger, 1986). A total sample size of 102 subjects (51 per treatment group), with an average of 18 tooth- or site-level measures made at 2 follow-up time points, results in 80% power to detect a true difference between the placebo probability of alveolar bone density loss of 14% versus 7% in the SDD arm assuming a two-sided, significance level of 0.05 and an exchangeable correlation parameter of 0.14. The estimated probabilities of bone density loss were based on unpublished pilot data using the CADIA method, as measurements using the RA method were not available in this pilot study. To adjust for an expected 20% dropout rate before the two-year visit, the total number of randomized subjects was 128, or 64 per treatment group.
Analyses of radiographic measures.
The relative RA change from baseline, the change in alveolar bone height from baseline, and the CADIA measures were each coded into 3 categories of change (improvement, no change, disease progression) using thresholds of two times the standard deviation of replicate measures, as described in the oral radiographic repeatability studies section above. The primary outcome is the RA measurement and the CADIA and alveolar bone height measures are secondary outcomes. The thresholds defining changes at the site level for the RA relative change were ≤ −0.19 at a crestal site or ≤ −0.15 at a subcrestal site defining progression, ≥ 0.19 at a crestal site or ≥ 0.15 at a subcrestal site defining improvement, and values in between the thresholds defining no change. The thresholds defining changes at the site level for the CADIA measures were +/− 17 at a crestal site or +/− 14 at a subcrestal site and +/− 0.4 mm for the alveolar bone height measures, using a coding algorithm similar to the RA definitions.
To account for the correlation among measures within a mouth over time, GEE was used to fit cumulative logistic regression models for the categorical responses to compare the odds of more progressive disease (among the ordered categories of improvement, no change, and progression) over the treatment period between the SDD and placebo groups. Covariates in the regression models included time, treatment, and their interaction, where non-significant interaction terms were dropped from subsequent models and average treatment effects across both follow-up time points were reported unless otherwise noted. Randomization stratification factors (baseline smoking status and study center) also were included in the regression models as was the baseline outcome measurement for the RA and alveolar bone height measures. Continuous measures of change relative to baseline at the site level in RA, CADIA, and alveolar bone height also were analyzed, as secondary outcome measures, using GEE to fit linear regression models, as described for the categorical endpoints, keeping in mind the dependence of analyses of mean change on the disease progression rate [Hujoel et al. 1993].
Two-year changes in weight, height and the percent change from baseline in BMD at the lumbar spine and femoral neck, measured at the subject level on a continuous scale, and the odds of tooth loss over the two-year period were compared between treatment groups using linear and logistic regression, respectively, as described for the oral bone density measures.
The primary analysis was based on an intent-to-treat (ITT) paradigm. All measured sites were included in the intent-to-treat analysis with the exception of sites from missing and extracted teeth, and mesial sites of second premolars when the first premolars were missing. Data from all subjects were analyzed according to the randomized treatment assignment regardless of treatment adherence or use of significant concomitant medications (see below).
Primary and secondary analyses were repeated using only data from a “per-protocol” analysis set. The per-protocol analysis set included measurements up to the time point at which a subject's recorded pill adherence count for either the randomized study drug or calcium/vitamin D supplement dropped below 80% and measurements up to the point of initiation of significant concomitant medications, including HRT, chronic NSAID use (defined as 30 days between six-month visits or longer), chronic antibiotic use (more than two courses of antibiotics in a six-month period; each course could be up to 21 days) or any tetracycline use other than the randomized study drug, bisphosphonate use (e.g., Actonel and Fosamax), selective estrogen receptor modulator use (e.g., Evista, Tamoxifen), calcitonin use, steroid use, and thyroid medication use. In addition, the per-protocol analysis set did not include measurements following: 1) periodontal surgery or quadrant root planing with local anesthetic (American Dental Association procedure code 04341); 2) tooth extraction for the extracted tooth and adjacent sites; and 3) new crown placement.
A summary of the number of subjects excluded from the per-protocol analysis set for each drug group by reason is as follows: a) subjects dropping out of the study prior to the one-year visit (placebo: n=2; SDD: n=9); b) subjects not adherent to study drug and calcium/vitamin D based on 80% adherence threshold and not using significant concomitant medications (placebo: n=21; SDD: n=11); c) subjects using significant concomitant medications but adherent to the study drug and calcium/vitamin D (placebo: n=10; SDD: n=10); and d) subjects not adherent to the study drug and calcium/vitamin D and using significant concomitant medications (placebo: n=3; SDD: n=2). No subjects were deleted for active periodontal therapy in either group, although sites as defined above were deleted from the per-protocol set. No subject had more than 7 sites that were deleted for active periodontal therapy during any 6-month period.
Subgroup analyses.
Subgroup analyses were performed for each of the “per-protocol” criteria (adherence, concomitant medication use and active periodontal therapy) separately. In addition, pre-specified subgroup analyses were performed in groups defined by current smoking status (current smoker or not), by time since the onset of menopause (within 5 years of the baseline exam or longer), by adherence to study drug alone (subjects were defined as adherent up to the time point at which they took less than 80% of the study drug and defined as non-adherent thereafter), by study center, by tooth location (maxilla or mandible), by baseline PD of the site (1−4 mm, 5−6 mm and ≥ 7 mm), by baseline alveolar bone height (0 to 2 mm, > 2 to ≤ 4 mm, and > 4 mm from CEJ), high mandibular tori (present or absent), by site location (between the first and second molars, between the first molars and second premolars, or between the premolars), and by regular aspirin use (patients were defined as non-users up to the time point at which they took ≤ 325 mg aspirin per day for at least 90 days and defined as users thereafter). To determine if the treatment effect differed between subgroups over time, an interaction among treatment, time and subgroup effects, as well as corresponding main effects, two-way interactions and terms described above, were included in a GEE regression model. Non-significant interaction terms were dropped from the model. Results are only presented for subgroup comparisons where the treatment by subgroup, with or without time, interaction was significant. No formal adjustment to the alpha level was made for the multiple tests performed (Pocock 1997).
Adverse events.
The adverse event distribution over the entire treatment period was descriptively summarized and compared between SDD and placebo using the Chi-square test or Fisher's exact test if expected cell counts were small.
Results
Participant Flow
Figure 1 shows the flow of subjects through each stage from screening through statistical analysis.
Recruitment
Subjects were recruited and randomized on a rolling-admission basis beginning in June 2002 and ending in October 2003. The last subject completed the clinical trial in October 2005.
Demographic and clinical data
One hundred twenty-eight eligible women aged 45−70 years underwent randomization. The SDD and placebo groups were comparable and well-matched with respect to all baseline characteristics (Table 1). The intent-to-treat sample and per-protocol sample were very similar and, within each analysis set, the placebo and SDD subjects had similar characteristics (data not shown). The study sample reflected the ethnic distribution within the study catchment areas (Nebraska and Long Island).
Table 1.
Baseline demographic and clinical characteristics of each group
| Characteristic | Placebo (n=64) | SDD (n = 64) | |
|---|---|---|---|
| Age (years) | 57.94 (5.70) | 58.53 (5.95) | |
| Ethnicity | |||
| Hispanic or Latino | 4 (6%) | 1 (2%) | |
| Not Hispanic or Latino | 60 (94%) | 63 (98%) | |
| Race | |||
| Asian | 2 (3%) | 1 (2%) | |
| Black or African American | 1 (2%) | 1 (2%) | |
| White | 61 (95%) | 62 (97%) | |
| Years Postmenopausal | |||
| 5 or fewer years | 23 (36%) | 25 (39%) | |
| More than 5 years | 41 (64%) | 39 (61%) | |
| Smoker | |||
| Current | 13 (20%) | 13 (20%) | |
| Former | 18 (28%) | 22 (34%) | |
| Never | 33 (52%) | 29 (45%) | |
| Weight (pounds) | 165.30 (34.57) | 158.70 (26.00) | |
| Height (inches) | 64.11 (2.36) | 63.96 (2.51) | |
| Number of Teeth | 25.69 (2.62) | 26.14 (2.54) | |
| Alveolar Bone Density | 12.48 (4.10) | 12.41 (3.98) | |
| (Radiographic Absorptiometry Method) (mg/mm2) | |||
| Alveolar Bone Height (mm) | 3.11 (1.30) | 3.32 (1.42) | |
| Probing Depth (mm) | 3.83 (1.19) | 3.83 (1.14) | |
| Lumbar Spine | |||
| Bone Mineral Density (g/cm2) | 0.91 (0.074) | 0.92 (0.086) | |
| T-score | −1.28 (0.67) | −1.18 (0.79) | |
| Femoral Neck | |||
| Bone Mineral Density (g/cm2) | 0.71 (0.079) | 0.69 (0.061) | |
| T-score | −1.29 (0.67) | −1.46 (0.55) |
Data are expressed as count (%) for categorical variables and mean (SD) for continuous measures. SD for alveolar bone density, alveolar bone height and probing depth was estimated using a linear mixed model.
Over the 2-year treatment period, 1 (2%) SDD subject lost 2 teeth, 8 (16%) lost 1 tooth and 42 (82%) lost no teeth compared to 5 (8%) losing two teeth, 7 (11%) losing one tooth, and 50 (81%) losing no teeth in the placebo group, where the odds of losing at least one tooth did not differ between treatment groups (p=0.9). In summary, seventeen teeth were lost in the placebo group and ten teeth were lost in the SDD group over the two-year clinical trial. No significant difference between groups with respect to changes in height or weight was observed over the two-year clinical trial.
Reliability studies
The standard deviations of replicate measurements for the RA method, CADIA and alveolar bone height, respectively, were as follows: 9.3% for the crestal location and 7.6% for the subcrestal location; 8.6 for the crestal location and 7.0 for the subcrestal location; and 0.2 mm.
Outcomes measures: Intent-to treat analyses
The vast majority of sites for oral radiographic outcomes did not show significant change at one year or two years, either improvement or disease progression (81−95% depending on the time point and measurement: RA, CADIA, and alveolar bone height).
Based on regressing modeling, the odds of more progressive disease did not differ significantly between groups based on the categorical RA measure [OR=1.04 (SDD relative to placebo), 95% confidence interval [CI]: 0.80 to 1.34, p=0.8] nor the categorical CADIA measure [OR=0.84 (SDD relative to placebo), 95% CI: 0.65 to 1.08, p=0.2].
The mean relative RA change was 0.0031 units (or 0.31%) greater for subjects receiving SDD relative to placebo, which was not statistically significant [95% CI: −0.0058 to 0.012, p=0.5]. However, the mean CADIA value was 0.99 units greater for subjects receiving SDD relative to placebo, which was marginally statistically significant [95%CI: −0.045 to 2.02, p=0.06].
The odds of more progressive disease (alveolar bone height loss) based on categorical alveolar bone height change measures were estimated to be 18% lower for subjects receiving SDD relative to placebo, which was not statistically significant [OR=0.82 (SDD relative to placebo), 95% CI: 0.62 to 1.08, p=0.2].
BMD percentage change from baseline at the lumbar spine and femoral neck are summarized in Table 2. Mean BMD % change values were similar between the study groups for the femoral neck (p=0.5) and lumbar spine (p=0.5) and extremely small (less than 0.4% per year).
Table 2.
Percent change in lumbar spine and femoral neck over 2 years
| Measurement | Study drug | 2-Year BMD % change | Treatment group comparison | |||||
|---|---|---|---|---|---|---|---|---|
| n | Median | Mean | SD | Difference in mean change (SDD-placebo)* | 95% CI | p-value | ||
| Lumbar spine | ||||||||
| Placebo | 62 | −0.60 | −0.60 | 3.96 | ||||
| SDD | 51 | −0.34 | −0.29 | 4.34 | ||||
| 0.46 | −1.03, 1.95 | 0.5 | ||||||
| Femoral neck | ||||||||
| Placebo | 62 | −0.80 | −0.38 | 4.72 | ||||
| SDD | 51 | −0.80 | −0.77 | 4.51 | ||||
| −0.54 | −2.17, 1.08 | 0.5 | ||||||
The difference in the mean change over time was estimated using a linear regression model adjusted for baseline BMD, baseline smoking status, study center, and treatment.
Per-protocol analyses
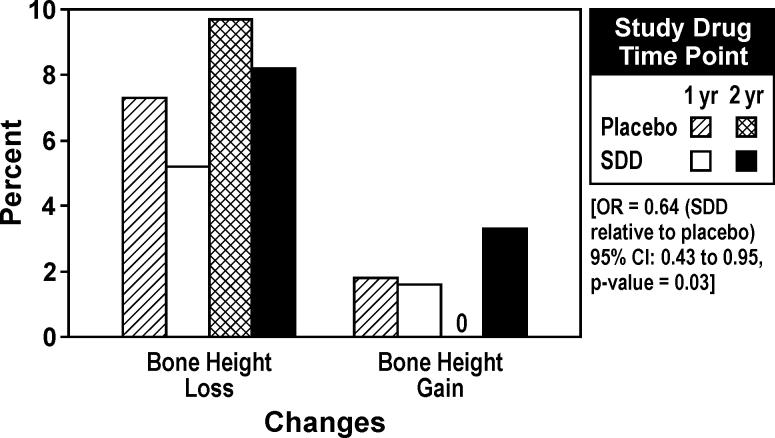
Sixty subjects satisfied the per-protocol criteria (28 placebo and 32 SDD subjects) at one year and 43 subjects satisfied the per-protocol criteria (19 placebo and 24 SDD subjects) at the two-year time point. Based on regression modeling, the odds of more progressive alveolar bone height loss were estimated to be 36% lower for subjects receiving SDD relative to placebo, which was significant (p =0.03) (Figure 2). In fact, no sites among the placebo per-protocol sample showed alveolar bone height gain over two years, while 3.3% of SDD sites manifested alveolar bone height gain (at least 0.4 mm). No significant change was noted between groups with respect to the per-protocol sample for the RA (OR=1.04, 95% CI: 0.66 to 1.62, p=0.9) and CADIA (OR=0.73, 95% CI: 0.50 to 1.09, p=0.1) outcomes.
Figure 2.
Percentage of sites demonstrating alveolar bone height changes over one and two years based on per-protocol analysis (subjects who adhered to the protocol). Threshold for change was ± 0.4 mm, based on two times the standard deviation of replicate measurements.
Subgroup analyses
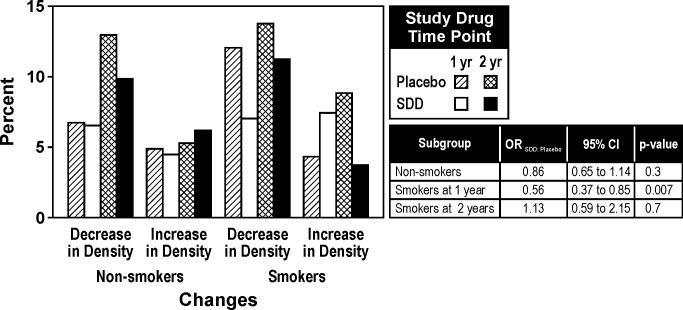
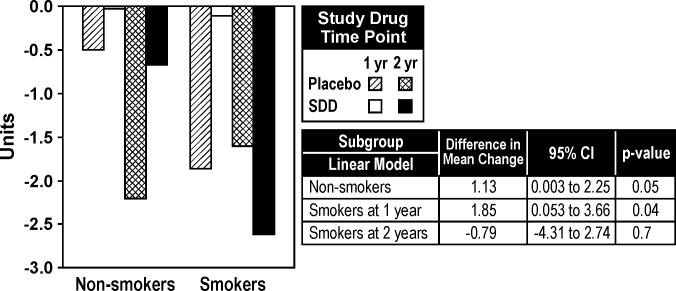
There was significant evidence based on the categorical CADIA measure and the continuous CADIA measure that the treatment effect over time differed by smoking status (time by treatment by subgroup interaction, p< 0.01 for each endpoint). Among smokers, SDD was associated with reduced alveolar bone density loss at one-year relative to placebo while no significant association was seen at two years (Figures 3 and 4). Based on the continuous CADIA measure, SDD was associated with reduced alveolar bone density loss relative to placebo among non-smoking subjects (Figure 4).
Figure 3.
Percentage of sites demonstrating CADIA density changes over one and two years in non-smokers and smokers (subgroup analysis). For the placebo group, 49 non-smokers and 13 smokers were included in the analysis. For the SDD group, 44 non-smokers and 11 smokers were included in the analysis. Thresholds for a decrease or increase in alveolar bone density were ± 17 units for crestal CADIA change and ± 14 units for subcrestal CADIA change. These thresholds were based on two times the standard deviation of replicate measurements.
Figure 4.
Mean CADIA density changes over one and two years in non-smokers and smokers (subgroup analysis). For the placebo group, 49 non-smokers and 13 smokers were included in the analysis. For the SDD group, 44 non-smokers and 11 smokers were included in the analysis. Differences in mean change are presented as SDD minus placebo.
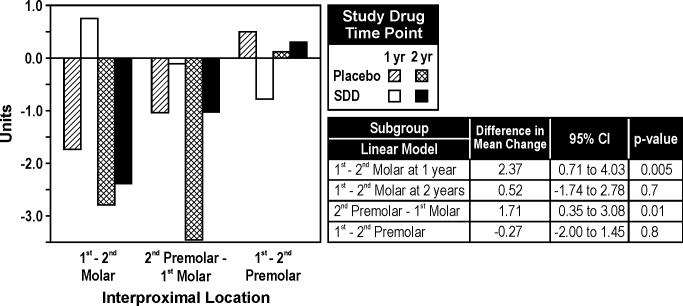
There was significant evidence, based on the continuous measure of CADIA change, that the treatment effect over time differed by site location (p=0.008). Among the first molar-second molar locations, SDD was associated with an average gain in alveolar bone density at one year relative to placebo which was not sustained through two years (Figure 5). Among the second premolar-first molar locations, SDD was associated with reduced alveolar bone density loss relative to placebo.
Figure 5.
Mean CADIA density changes over one and two years by posterior interproximal location (subgroup analysis). For the placebo and SDD groups, respectively, 62 and 55 subjects contributed site location subgroup data. Differences in mean change are presented as SDD minus placebo.
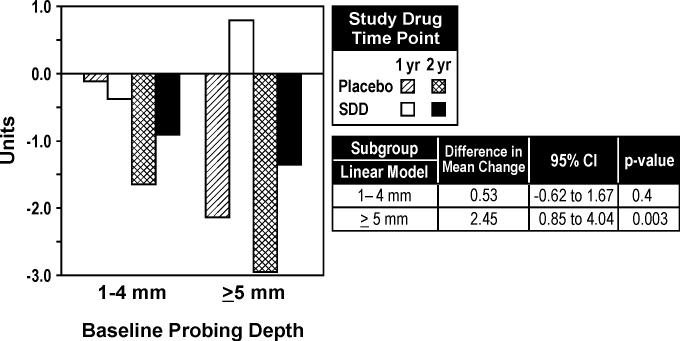
Based on the continuous measure of CADIA change, there was a significant interaction among study drug, time, and baseline probing depth (p=0.03). Among sites with a baseline probing depth of ≥ 5 mm, SDD was associated with reduced alveolar bone density loss relative to placebo (Figure 6).
Figure 6.
Mean CADIA density changes over one and two years based on baseline probing depth (1−4 mm and ≥ 5 mm; subgroup analysis). Differences in mean change are presented as SDD minus placebo. For the placebo group, 62 and 61 subjects were included in the analyses for 1−4 mm probing depths and ≥ 5 mm probing depths, respectively. For the SDD group, 55 subjects and 51 subjects were included in the analyses for 1−4 mm probing depths and ≥ 5 mm probing depths, respectively
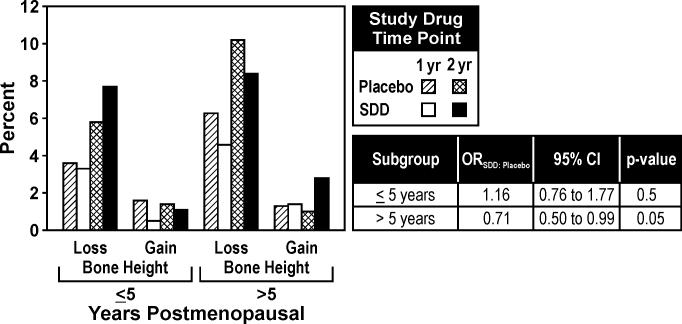
There was significant evidence that the effect of study drug differed by time after menopause for alveolar bone height change (drug by menopause interaction, p=0.04). Among subjects who were beyond 5 years of menopause, SDD was associated with a 29% reduction in the odds of more progressive disease (bone height loss) (Figure 7).
Figure 7.
Percentage of sites demonstrating alveolar bone height changes over one and two years based on number of years postmenopausal (≤ 5 years and > 5 years; subgroup analysis). For the placebo group, 22 and 40 subjects were included in the analyses for women ≤ 5 years postmenopausal and > 5 years postmenopausal, respectively, while for the SDD group, 22 and 33 subjects were included in the analyses for women ≤ 5 years postmenopausal and > 5 years postmenopausal, respectively. Threshold for change was ± 0.4 mm, based on two times the standard deviation of replicate measurements.
There was no significant evidence of any other subgroup effects based on the test of the interaction between treatment and subgroup status.
Subject adherence
Across all follow-up periods, 11−15% of the placebo subjects took less than 80% of the prescribed study drug compared to 4−14% of the SDD subjects.
Across all follow-up periods, 20−27% of the placebo subjects took less than 80% of the calcium/vitamin D compared to 16−20% of the SDD subjects.
Adverse events
Of the 64 subjects assigned to placebo, 8 (13%) reported a serious adverse event (SAE). Among the 64 subjects assigned to SDD, 7 (11%) reported an SAE (Table 3). Overall, adverse event experiences were similar between the two groups (Table 4). However, significantly fewer SDD subjects experienced a dermatologic adverse event (including rash, itchy skin, acne, rosacea, hives and nail fungus) at some time during the clinical trial compared to placebo subjects (2% versus 17%, p = 0.002).
Table 3.
Serious adverse events during the clinical trial
| SDD | Placebo |
|---|---|
| Diverticulitis | Appendicitis |
| Pneumonia and kidney obstruction | Recurrent breast cancer |
| Breast cancer | Colon cancer |
| Collapsed lung resulting in hospitalization | Acute pancreatitis and cholelithiasis |
| Broken arm requiring surgery | Hospitalization for hypertension |
| Gallbladder removal (2) | Squamous cell carcinoma of skin |
| Basal cell carcinoma of skin | Hospitalization for knee surgery |
| Acute pancreatitis |
Table 4.
Summary of patients experiencing each type of adverse event over the entire treatment period by study drug
| Count (% of patients) | |||
|---|---|---|---|
| Type of Adverse Event | Study Drug Group |
p-value comparing percentages | |
| Placebo (n=64) | SDD (n=64) | ||
| Ache/Pain | 35 (55%) | 33 (52%) | 0.7 |
| Arthritis/Inflammation | 5 (8%) | 7 (11%) | 0.5 |
| Cancer | 3 (5%) | 2 (3%) | >0.9 |
| Cardiac: BP, Cholesterol, MI | 5 (8%) | 4 (6%) | >0.9 |
| Cold/Cough/Respiratory | 28 (44%) | 34 (53%) | 0.3 |
| Dermatologic | 11 (17%) | 1 (2%) | 0.002* |
| GI Upset | 14 (22%) | 15 (23%) | 0.8 |
| Hearing/Vision | 5 (8%) | 0 (0%) | 0.06 |
| Infection | 22 (34%) | 14 (22%) | 0.1 |
| Injury | 5 (8%) | 5 (8%) | >0.9 |
| Minor Surgery | 7 (11%) | 6 (9%) | 0.8 |
| Oral Events and Lesions | 3 (5%) | 3 (5%) | >0.9 |
| Osteoporosis | 0 (0%) | 3 (5%) | 0.2 |
| Psychological/Sleep/Neurological | 6 (9%) | 8 (12%) | 0.6 |
| Other | 3 (5%) | 7 (11%) | 0.2 |
Significantly fewer SDD subjects experienced a dermatologic adverse event (including rash, itchy skin, acne, rosacea, hives, and nail fungus) compared to placebo subjects. No other differences were significant.
DISCUSSION
Most sites (81−95%) in both the SDD and placebo-treated groups did not show significant change over time, based on thresholds for RA, CADIA and alveolar bone height change, and no overall treatment effects were observed based on the ITT analyses, perhaps because either SDD was not effective overall in this population or because the overall group showed stable (non-progressive) disease. Therefore, there were relatively few actively-destructive sites available to respond to treatment. The relative periodontal stability of this patient population is not surprising in light of the treatment rendered to the two groups. First, patients in both groups received periodontal maintenance every three to four months throughout the two-year clinical trial. These periodontal maintenance visits were provided to the patients at no cost. Therefore, subjects were extremely compliant with periodontal maintenance; in fact, over 90% of the subjects in each group had at least one periodontal maintenance visit between each of the semi-annual study visits throughout the clinical trial. Periodontal maintenance has been shown to be extremely effective in maintaining periodontal stability (Kaldahl et al. 1996). Second, all subjects received 1200 mg of calcium/400 IU vitamin D supplements daily throughout the clinical trial, as this regimen represents the standard of care for postmenopausal women (Position Statement, North American Menopause Society 2006). Based on a recent publication by Hildebolt's research group (2004), calcium and vitamin D may have beneficial effects on alveolar bone in postmenopausal women (Hildebolt et al. 2004). Finally, in addition to regular periodontal maintenance, study participants had separate study visit appointments where periodontal measurements were made every six months. Therefore, all study subjects may have had a heightened awareness of their periodontal status and a heightened interest in their periodontal health.
However, in spite of the above comments, a significant treatment effect was observed in the alveolar bone height per-protocol analysis and several subgroup analyses. As noted by Pocock (1997), secondary outcome measures should be analyzed and presented with appropriate caution in interpretation, particularly when a number of secondary outcome measures have been analyzed. He further states that formal adjustments to the p-values are “usually of limited value” except as informal guides for interpretation, noting in particular concerns about the conservative nature of formal methods of adjustment like the Bonferroni correction, which may result in reduced power. Therefore, no formal adjustment was made to the alpha level.
Based on the CADIA measure, SDD was associated with statistically-significantly reduced alveolar bone density loss over two years in non-smokers (Figure 4). In addition, SDD was associated with statistically-significantly reduced alveolar bone density loss at the twelve month visit in smokers (Figure 3 and 4). The negative influence of smoking on alveolar bone is well established (Hildebolt et al. 2000, Payne et al. 2000). While SDD was associated with reduced alveolar bone density loss in non-smokers over two years, the overwhelming negative impact of smoking on alveolar bone density may have eliminated any potential treatment effect in smokers over 24 months.
The effect of SDD also had site-specificity; for example, among the second premolar-first molar locations, the mean CADIA value (i.e., relative alveolar bone density) was statistically-significantly higher in the SDD group then the placebo group (Figure 5). In addition, statistically-significantly higher mean CADIA values were seen at the twelve-month visit at first molar-second molar interproximal sites. The site specificity of these findings are in agreement with a previous publication from our group (Payne et al. 1997), whereby we observed increased alveolar bone density loss in the molar-molar and molar-premolar interproximal sites relative to premolar-premolar interproximal sites in estrogen-deficient postmenopausal women. Although SDD is a systemically administered drug, site specificity can still be expected, as individual sites behave independently due to, for example, differential root anatomy and differential access for personal and professional plaque removal. The more vulnerable the site, the greater opportunity SDD may have to improve radiographic outcomes.
With respect to alveolar bone height over two years, SDD was associated with reduced odds of progressive bone loss in subjects who were > 5 years postmenopausal at baseline (Figure 7) and for subjects who adhered to the protocol (Figure 2). The increased alveolar bone height observed at some sites in this clinical trial has been reported previously in response to HRT (Civitelli et al. 2002) and the biological basis for this apparent alveolar bone height gain may be a result of an increase in alveolar bone density at some sites that projects as an increase in alveolar bone height. The reduced odds of progressive alveolar bone height loss in response to SDD in our clinical trial are in agreement with the study by Ciancio and Ashley (1998). They showed in a chronic periodontitis population that placebo subjects experienced, on average, alveolar bone height loss over a six-month period, while SDD subjects showed no evidence of alveolar bone height loss. Their clinical trial included only 20 subjects per group and, to our knowledge, is the only other trial in which alveolar bone represented a measured outcome in response to SDD, as most clinical studies have focused on the effects of SDD on soft tissue measurements only (Caton et al. 2000, Caton et al. 2001, Preshaw et al. 2004). The data published by Ciancio and Ashley (1998) are consistent with a previous study by Golub et al. (1997) in which SDD treatment in chronic periodontitis subjects significantly reduced pyridinoline crosslinked carboxyterminal telopeptide fragments of type I collagen (ICTP) levels in gingival crevicular fluid. ICTP is a diagnostic marker of active bone resorption during metabolic bone diseases (Risteli et al. 1993) and periodontitis (Giannobile et al. 1995). Finally, the most significant postmenopausal bone loss occurs within five to seven years of menopause (Lindsay et al. 1980, Pacifici et al. 1989). Our finding that SDD was effective only in subjects who were beyond five years of menopause at baseline suggests that a more potent doxycycline formulation would be necessary to mitigate bone loss in the early postmenopausal period of higher bone turnover while SDD may be more appropriate later in menopause.
The CADIA method detected differences between the two treatment groups within subgroups of subjects with respect to changes in alveolar bone density over time. However, there were no differences noted using the RA method. It had been originally anticipated, based on the publication by Kuhl & Nummikoski (2000), that the threshold for significant alveolar bone density loss would be 10% using the RA method. The threshold, based on two times the standard deviation of replicate measurements, for this clinical trial was higher: 19% for the crestal area of interest and 15% for the subcrestal area of interest. These increased thresholds resulted from increased variability introduced by the calibration wedge. As a result, the higher thresholds likely precluded the detection of both positive and negative alveolar bone density changes.
This trial included subjects with osteopenia of either the lumbar spine or femoral neck. These subjects had reduced bone mass, but not osteoporosis. In this clinical trial, over a two-year period, mean progressive BMD loss at the lumbar spine and femoral neck was minimal in both groups, on the order of tenths of one percent per year. Bone density is maintained close to peak levels until menopause, at which time it declines by an average of 1% per year, although bone loss declines even more rapidly within the first five to seven years following menopause (Riggs & Melton 1986). Therefore, relative to the expected average yearly rate of systemic BMD loss, the subjects in this clinical trial were essentially non-progressive, similar to the lack of progression of periodontitis in most of the pocket sites in these osteopenic subjects (see above). This cohort was likely stable over two years systemically because all subjects received calcium and vitamin D supplements. Adequate calcium intake has been shown to reduce bone loss and fractures in postmenopausal women (Position Statement, North American Menopause Society 2006).
For ethical reasons, osteoporotic subjects were not included in the clinical trial. Osteoporotic subjects need to be treated with conventional therapies that affect bone metabolism such as bisphosphonates, selective estrogen receptor modulators, or parathyroid hormone (teriparatide). It is likely that osteoporotic subjects would have shown greater alveolar bone loss or systemic BMD loss than osteopenic subjects. To include osteoporotic subjects in future clinical trials, it is suggested that more potent doxycycline derivatives (e.g., once daily, controlled release of 40 mg doxycycline or a once daily administration of a chemically-modified tetracycline) be given to subjects as an adjunct to conventional therapies listed above as opposed to a stand-alone treatment. In addition, once daily dosing with these newer generation doxycyclines should improve subject compliance.
This clinical trial demonstrated the safety of SDD over a two-year period when compared to placebo, as adverse events among SDD subjects were similar to placebo. However, significantly fewer SDD subjects experienced a dermatologic adverse event than placebo subjects, which is in agreement with recent literature that demonstrates the effectiveness of SDD in the treatment of acne and rosacea (Skidmore et al. 2003, Sanchez et al. 2005). In addition, this is the longest duration clinical trial that has examined the safety and efficacy of SDD.
In conclusion, the vast majority of posterior interproximal sites in both groups did not manifest significant positive or negative alveolar bone change over the two-year clinical trial and there were no overall treatment effects based on the ITT analyses. However, in spite of the overall stability of the patient population, subgroup analyses suggest that the use of SDD, over a two-year period, was associated with statistically-significantly reduced alveolar bone density loss in non-smokers, at first molar-second premolar interproximal sites, and for sites with baseline probing depths ≥ 5 mm. In addition, the use of SDD, over a two-year period, was associated with statistically-significantly reduced alveolar bone height loss in women who were more than 5 years postmenopausal and in subjects who adhered to the protocol. These data suggest that SDD may be a useful adjunct to periodontal maintenance therapy in subjects with less rapid systemic bone turnover (subjects more than 5 years postmenopausal), in subjects who do not smoke, and for sites with deeper probing depths.
Acknowledgements
We acknowledge and thank the following individuals for their hard work and dedication to this clinical trial: Eugene Boilesen as database administrator, Alison Lahners for her role as project assistant and registrar, Julie Layton as clinical research assistant, Trudy Meinberg for subject recruitment, Mary Morris as project manager, and Marian Schmid for her role as research technologist. We thank the Nebraska Periodontitis Referral Network (PRN) for referring subjects for this clinical trial. We also acknowledge Teresa Powell for administrative support throughout the clinical trial, Deborah Dalton for manuscript preparation and Kim Theesen for preparation of graphics. SDD and placebo tablets were provided by CollaGenex Pharmaceuticals, Inc. (Newtown, PA).
Source of Funding
The project was supported by Grant Number R01DE012872 from the National Institute of Dental & Craniofacial Research (Dr. Jeffrey Payne, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health.
Footnotes
Conflict of Interest Statement
Lorne M. Golub is listed as an inventor on several patents for the drug mentioned in this publication and these patents have been fully assigned to his institution, State University of New York at Stony Brook.
Lorne M. Golub is a consultant to CollaGenex Pharmaceuticals, Inc. and the Fund for Autoimmune Diseases Research.
REFERENCES
- Bain S, Ramamurthy NS, Impeduglia T, Scolman S, Golub LM, Rubin C. Tetracycline prevents cancellous bone loss and maintains near-normal rates of bone formation in streptozotocin-diabetic rats. Bone. 1997;21:147–153. doi: 10.1016/s8756-3282(97)00104-x. [DOI] [PubMed] [Google Scholar]
- Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J, Walker C. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. Journal of Periodontology. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J, Walker C. Subantimicrobial dose doxycycline as an adjunct to scaling and root planing: post-treatment effects. Journal of Clinical Periodontology. 2001;28:782–789. doi: 10.1034/j.1600-051x.2001.280810.x. [DOI] [PubMed] [Google Scholar]
- Ciancio S, Ashley R. Safety and efficacy of sub-antimicrobial-dose doxycycline therapy in patients with adult periodontitis. Advances in Dental Research. 1998;12:27–31. doi: 10.1177/08959374980120011501. [DOI] [PubMed] [Google Scholar]
- Civitelli R, Pilgram TK, Dotson M, Muckerman J, Lewandowski N, Armamento-Villareal R, Yokoyama-Crothers N, Kardaris EE, Hauser J, Cohen S, Hildebolt CF. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement therapy: a randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2002;162:1409–1415. doi: 10.1001/archinte.162.12.1409. [DOI] [PubMed] [Google Scholar]
- Craig RG, Yu Z, Xu L, Barr R, Ramamurthy N, Boland J, Schnier M, Golub LM. A chemically modified tetracycline inhibits streptozotocin-induced diabetic depression of skin collagen synthesis and steady-state type I procollagen mRNA. Biochimica et Biophysica Acta. 1998;1402:250–260. doi: 10.1016/s0167-4889(98)00008-1. [DOI] [PubMed] [Google Scholar]
- Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C, Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocrine Reviews. 2002;23:570–578. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Lynch SE, Denmark RG, Paquette DW, Fiorellini JP, Williams RC. Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis. A pilot study in beagle dogs. Journal of Clinical Periodontology. 1995;22:903–910. doi: 10.1111/j.1600-051x.1995.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, Giannobile WV. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflammation Research. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, Ramamurthy NS. Minocycline reduces gingival collagenolytic activity during diabetes: Preliminary observations and a proposed new mechanism of action. Journal of Periodontal Research. 1983;18:516–526. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Advances in Dental Research. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- Golub LM, Ramamurthy NS, Kaneko H, Sasaki T, Rifkin B, McNamara TF. Tetracycline administration prevents diabetes-induced osteopenia in the rat: initial observations. Research Communications in Chemical Pathology and Pharmacology. 1990;68:27–40. [PubMed] [Google Scholar]
- Golub LM, Ramamurthy NS, Llavaneras A, Ryan ME, Lee HM, Liu Y, Bain S, Sorsa T. A chemically-modified nonantimicrobial tetracycline (CMT-8) inhibits gingival matrix metalloproteinases, periodontal breakdown, and extra-oral bone loss in ovariectomized rats. Annals of the New York Academy of Sciences. 1999;878:290–310. doi: 10.1111/j.1749-6632.1999.tb07691.x. [DOI] [PubMed] [Google Scholar]
- Hausmann E, Allen K, Carpio L, Christersson LA, Clerehugh V. Computerized methodology for detection of alveolar crestal bone loss from serial intraoral radiographs. Journal of Periodontology. 1992;63:657–662. doi: 10.1902/jop.1992.63.8.657. [DOI] [PubMed] [Google Scholar]
- Hildebolt CF, Pilgram TK, Dotson M, Armamento-Villareal R, Hauser J, Cohen S, Civitelli R. Estrogen and/or calcium plus vitamin D increase mandibular bone mass. Journal of Periodontology. 2004;75:811–816. doi: 10.1902/jop.2004.75.6.811. [DOI] [PubMed] [Google Scholar]
- Hildebolt CF, Pilgram TK, Yokoyama-Crothers N, Vannier MW, Dotson M, Muckerman J, Hauser J, Cohen S, Kardaris EE, Hanes P, Shrout MK, Civitelli R. Alveolar bone height and postcranial bone mineral density: negative effects of cigarette smoking and parity. Journal of Periodontology. 2000;71:683–689. doi: 10.1902/jop.2000.71.5.683. [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Baab DA, DeRouen TA. Measures of treatment efficacy. Journal of Clinical Periodontology. 1993;20:601–605. doi: 10.1111/j.1600-051x.1993.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Jeffcoat MK, Reddy MS, Webber RL, Williams RC, Ruttimann U. Extra oral control of geometry for digital subtraction radiography. Journal of Periodontal Research. 1987;22:396–402. doi: 10.1111/j.1600-0765.1987.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK. Long-term evaluation of periodontal therapy: II. Incidence of sites breaking down. Journal of Periodontology. 1996;67:103–108. doi: 10.1902/jop.1996.67.2.103. [DOI] [PubMed] [Google Scholar]
- Kuhl ED, Nummikoski PV. Radiographic absorptiometry method in measurement of localized alveolar bone density changes. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 2000;89:375–381. doi: 10.1016/s1079-2104(00)70105-3. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lindsay R, Hart DM, Forrest C, Baird C. Prevention of spinal osteoporosis in oophorectomised women. Lancet. 1980;2:1151–1154. doi: 10.1016/s0140-6736(80)92592-1. [DOI] [PubMed] [Google Scholar]
- Looker AC, Orwoll ES, Johnston CC, Jr., Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low femoral bone density in older U.S. Adults from NHANES III. Journal of Bone and Mineral Research. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- North American Menopause Society The role of calcium in peri- and postmenopausal women: 2006 position statement of the North American Menopause Society. Menopause. 2006;13:862–877. doi: 10.1097/01.gme.0000243566.25205.0b. [DOI] [PubMed] [Google Scholar]
- Osborn JB, Stoltenberg JL, Huso BA, Aeppli DM, Pihlstrom BL. Comparison of measurement variability in subjects with moderate periodontitis using a conventional and constant force periodontal probe. Journal of Periodontology. 1992;63:283–289. doi: 10.1902/jop.1992.63.4.283. [DOI] [PubMed] [Google Scholar]
- Pacifici R, Rifas L, McCracken R, Vered I, McMurtry C, Avioli LV, Peck WA. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2398–402. doi: 10.1073/pnas.86.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JB, Reinhardt RA, Nummikoski PV, Dunning, David G, Patil KD. The association of cigarette smoking with alveolar bone loss in postmenopausal females. Journal of Clinical Periodontology. 2000;27:658–664. doi: 10.1034/j.1600-051x.2000.027009658.x. [DOI] [PubMed] [Google Scholar]
- Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporosis International. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil KD. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. Journal of Periodontology. 1997;68:24–31. doi: 10.1902/jop.1997.68.1.24. [DOI] [PubMed] [Google Scholar]
- Pocock SJ. Clinical trials with multiple outcomes: A statistical perspective on their design, analysis and interpretation. Controlled Clinical Trials. 1997;18:530–545. doi: 10.1016/s0197-2456(97)00008-1. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Hefti AF, Novak MJ, Michalowicz BS, Pihlstrom BL, Schoor R, Trummel CL, Dean J, Van Dyke TE, Walker CB, Bradshaw MH. Subantimicrobial dose doxycycline enhances the efficacy of scaling and root planing in chronic periodontitis: a multicenter trial. Journal of Periodontology. 2004;75:1068–1076. doi: 10.1902/jop.2004.75.8.1068. [DOI] [PubMed] [Google Scholar]
- Rifkin BR, Vernillo AT, Golub LM, Ramamurthy NS. Modulation of bone resorption by tetracyclines. Annals of the New York Academy of Sciences. 1994;732:165–180. doi: 10.1111/j.1749-6632.1994.tb24733.x. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ. Involutional osteoporosis. New England Journal of Medicine. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Risteli J, Elomaa I, Niemi S, Novamo A, Risteli L. Radioimmunoassay for the pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen: a new serum marker of bone collagen degradation. Clinical Chemistry. 1993;39:635–640. [PubMed] [Google Scholar]
- Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Statistics in Medicine. 1998;17:1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rüttimann UE, Webber RL, Schmidt E. A robust digital method for film contrast correction in dental radiographs. Journal of Periodontal Research. 1986;21:486–495. doi: 10.1111/j.1600-0765.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Sanchez J, Somolinos AL, Almodovar PI, Webster G, Bradshaw M, Powala C. A randomized, double-blind, placebo-controlled trial of the combined effect of doxycycline hyclate 20-mg tablets and metronidazole 0.75% topical lotion in the treatment of rosacea. Journal of the American Academy of Dermatology. 2005;53:791–797. doi: 10.1016/j.jaad.2005.04.069. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ramamurthy NS, Golub LM. Tetracycline administration increases collagen synthesis in osteoblasts of streptozotocin-induced diabetic rats: a quantitative autoradiographic study. Calcified Tissue International. 1992;50:411–419. doi: 10.1007/BF00296771. [DOI] [PubMed] [Google Scholar]
- Skidmore R, Kovach R, Walker C, Thomas J, Bradshaw M, Leyden J, Powala C, Ashley R. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. Archives of Dermatology. 2003;139:459–464. doi: 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]
- Tezal M, Wactawski-Wende J, Grossi SG, Dmochowski J, Genco RJ. Periodontal disease and the incidence of tooth loss in postmenopausal women. Journal of Periodontology. 2005;76:1123–1128. doi: 10.1902/jop.2005.76.7.1123. [DOI] [PubMed] [Google Scholar]
- Williams S, Wakisaka A, Zeng QQ, Barnes J, Martin G, Wechter WJ, Liang CT. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone. 1996;19:637–644. doi: 10.1016/s8756-3282(96)00302-x. [DOI] [PubMed] [Google Scholar]