Abstract
Epicatechin gallate (ECG) is the third major catechin component in green tea, but it shows strong biological activity in some aspects, including apoptosis, cell growth inhibition, and membrane transport system in various cells. We previously reported that ECG induces activating transcription factor 3 (ATF3), which is involved in pro-apoptosis in HCT-116 cells. In this report, we present a molecular mechanism by which ECG induces ATF3 expression at the transcriptional level. We found that Sp3 contributed to the basal expression of the ATF3 gene, whereas EGR-1 played an important role in ECG-induced ATF3 expression in HCT-116 cells, as assessed by EMSA and co-transfection experiments. These results suggested that EGR-1, a tumour suppressor protein, could substantiate ECG’s role of ATF3 expression in human colorectal cancer cells. We also found that pro-oxidant activity of ECG contributed to ECG-induced ATF3 expression.
Keywords: ATF3, ECG, EGCG, EGR-1
1. Introduction
Green tea (Camellia sinensis) is the most widely consumed beverage in the world, next to water. Tea contains large amounts of flavonoids, and the major flavonoids in green tea are catechins, which include epigallocatechin gallate (EGCG), epigallocatechin (EGC), epicatechin gallate (ECG), and epicatechin (EC). EGCG is the most abundant catechin in green tea, and has been reported to have biological activities, such as anti-oxidative (1), pro-oxidative (2) and anti-inflammatory effects (3, 4) in a variety of experimental models. Although ECG is the third most abundant constituent amongst the green tea catechins, much attention has been focused on its anti-tumourigenic activity, due to distinguished features of ECG compared to EGCG. For example, it has been reported that ECG has anti-angiogenic (5), and anti-oxidation (6) activities. We have recently reported that ECG has an anti-tumourigenic effect; it increased the G1-sub population, cleaved poly (ADP-ribose) polymerase (PARP) in HCT-116 cells (7), and suppressed cyclin D1 and β-catenin pathways in mouse oral SCC7 cancer cells (8). However, the molecular targets that might be involved in ECG-induced anti-tumourigenesis have not yet been reported.
Activating transcription factor 3 (ATF3) is a member of the ATF/CREB family, and is induced upon exposure of cells to a variety of physiological and pathological stimuli (9). This response is thought to have cell-defending effects, such as cell cycle arrest and apoptosis (10, 11). On the other hand, ATF3 is also rapidly induced in regenerating liver (12), or in cells treated with growth-stimulating factors such as serum, epidermal growth factor or fibroblast growth factor (13). These conflicting results may depend on stimuli or cell types used in the studies. In HCT-116 cells, ATF3 is reported to be increased by nonsteroidal anti-inflammatory drugs (NSAID) (14), conjugated linoleic acid (15), LY294002 (16), and 3, 3′-diindolylmethane (17), which are shown to have anti-tumourigenic activity in human colorectal cancer cells. In this study, we focused on the transcriptional regulation of ATF3, affected by green tea catechins. We found that early growth response gene-1 (EGR-1) is involved in ECG-induced ATF3 expression, whereas Sp3 contributed to the basal expression of ATF3 in HCT-116 cells. In addition, ATF3 expression by ECG may result from the oxidative stress generated by ECG in the cell culture media.
2. Materials and methods
2.1. Cell lines, reagents and plasmids
Cell lines were purchased from ATCC (Rockville, MD). HCT-116 and SW480 human colorectal cells were maintained in McCoy’s 5A and RPMI medium, respectively, supplemented with 10% foetal bovine serum and gentamicin (10 μg/ml). Catechins (EGCG, ECG, EGC and EC), glutathione (GSH), H2O2, and catalase were purchased from Sigma (St Louis, MO). NAG-1 (nonsteroidal anti-inflammatory drug-activated gene-1) antibody was described previously (18). ATF3, Actin, EGR-1, Sp1 and Sp3 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The pATF3 −1850/+34 and pATF3 −84/+34 luciferase reporter vectors were provided by Dr. S. Kitajima (Tokyo Medical and Dental University, Tokyo, Japan). For the deletion clones of the ATF3 promoter, pATF3 −1850/+34 was used and serially deleted using the Erase-a-Base System (Promega, WI). The EGR-1, Ftz and CRE sites between the −514 and +34 region of the ATF3 promoter were deleted using the QuickChangeII site directed Mutagenesis Kit (Stratagene, TX). To delete these sites, the following primers were used: del EGR-1 F, 5′-GCTGGTGTGTGTCTCAGTGAGGGAACGCGC-3′; del EGR-1 R, 5′-GCCAGCCCAGGCGCGTTCCCTCACTGAGAC-3′; del Ftz F, 5′-GTTCGGCCGGTTCTCCCGGGTAGCATTACG-3′; del Ftz R, 5′-CCCAGGCTGACGTAATGCTACCCGGGAGAA-3′; del CRE F, 5′-CGGGAAGCTATTAATAGCATGCCTGGGACT-3′; and del CRE R, 5′-CCGTGTTGCCAGTCCCAGGCATGCTATTAA-3′. EGR-1 (pCDNA3.1-EGR-1/NEO) and Sp3 (pCMV4-Sp3flu) expression vectors were previously described (19, 20).
2.2. Reverse transcription- polymerase chain reaction (RT)-PCR
Total RNA was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA), and 5 μg of total RNA was reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). One μl of synthesised cDNA was added to a 25 μl PCR reaction mixture with human ATF3 gene-specific primers (F: 5′-GTTTGAGGATTTTGCTAACCTGAC-3′ and R: 5′-AGCTGCAATTCTTATTTCTTTCTCGT-3′) and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene-specific primers (F: 5′-TCAACGGATTTGGTCGTATT-3′ and R: 5′-CTGTGGTCATGAGTCCTTCC-3′). The thermal cycling conditions were as follows: initial denaturation at 94 °C for 2 min, followed by 30 and 25 cycles (ATF3 and GAPDH, respectively) of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, and final extension at 72 °C for 10 min. The products were electrophoresed on 2% agarose gel and photographed under UV light.
2.3. Western blot analysis
Cells were grown to 60–80% confluency in 60-mm plates followed by serum-starvation for 24 h. The indicated compounds were treated for 24 h and total cell lysates were isolated using RIPA buffer (1 × PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing 1 mM PMSF, 1 μg/mL aprotinin, and 1 μg/mL leupeptin. Proteins (30 μg) were separated by SDS-PAGE and transferred for 1 h on nitrocellulose membrane (Pall Co., Deland, FL). The blots were blocked for 1 h with 5% skim milk in TBS/0.05% Tween 20 (TBS-T), and probed with specific antibodies at 4 °C overnight. After washing with TBS-T, the blots were treated with horseradish peroxidase-conjugated secondary antibody for 1 h and washed four times. Proteins were detected by the Enhanced Chemi-Luminescence system (Pierce, Rockford, IL).
2.4. Transfection using luciferase reporter system
HCT-116 cells were plated in 12-well plates at 2 × 105 cells/well in McCoy’s 5A media. For the co-transfection, 0.25 μg of reporter vector and 0.25 μg of expression vector were transfected with 0.05 μg of pRL-null vector using the Lipofectamine Reagent (Invitrogen, Carlsbad, CA). After transfection, cells were incubated without serum for 24 h, and treated with indicated compounds for 24 h. Cells were then harvested in 1× luciferase lysis buffer, and luciferase activity was determined and normalized to the pRL-null luciferase activity using the Dual Luciferase Assay Kit (Promega, Madison, WI).
2.5. Transfection of antisense oligonucleotides
Antisense oligo (5′-ZECGGGGCGCGGGGAACFOT-3′), and sense EGR-1 oligo (5′-AEZGTTCCCCGCGCCCCGOA-3′) were synthesised by Invitrogen (Carlsbad, CA). HCT-116 cells were transfected with each antisense oligo at a concentration of 100 nM using TransIT-TKO transfection reagent (Mirus, Madison, WI). After transfection for 24 h, the medium was replaced with serum-free medium for 24 h. The cells were incubated for 1 and 24 h with 50 μM of ECG, and total protein was subjected to Western blot analysis.
2.6. Electrophoretic mobility shift analysis
Nuclear extracts were prepared using a Nuclear Extract kit (Active Motif, Carlsbad, CA). For the electrophoretic mobility shift assay, double-stranded oligonucleotides corresponding to the EGR-1 binding site (Top strand, 5′-GTGAGCGAGGGCGGGGGGTGAGCGAGGGCGGGGG-3′; bottom strand, 5′-CCCCCGCCCTCGCTCACCCCCCGCCCTCGCTCAC-3′) and mutant EGR-1 binding site (top strand, 5′-GTGAGCGAAAACGGGGGGTGAGCGAAAACGGGGG-3′; bottom strand, 5′-CCCCCGTTTTCGCTCACCCCCCGTTTTCGCTCAC-3′) were synthesised, and the wild type oligonucleotide was end-labelled with [γ-32P]ATP by the T4 polynucleotide kinase (Promega, Madison, WI). Assays were done by incubating 10 μg of nuclear extracts in binding buffer (Promega, Madison, WI) containing 1 × 105 cpm of labelled probe for 20 min at room temperature. To assure the specific binding of transcription factors to the probe, the probe was competed by 10-, 50-, and 100-fold molar excesses of cold wild type or mutant oligonucleotides. For the supershift experiments, antibodies (0.6 μg each) were incubated with nuclear extracts at room temperature for 10 min before adding to the binding reaction. The samples were then electrophoresed on 5% nondenaturing polyacrylamide gels with 0.5× Tris borate/EDTA, and gels were dried and subjected to autoradiography.
3. Results
3.1. ECG and EGCG increase ATF3 expression in human colorectal cancer cells
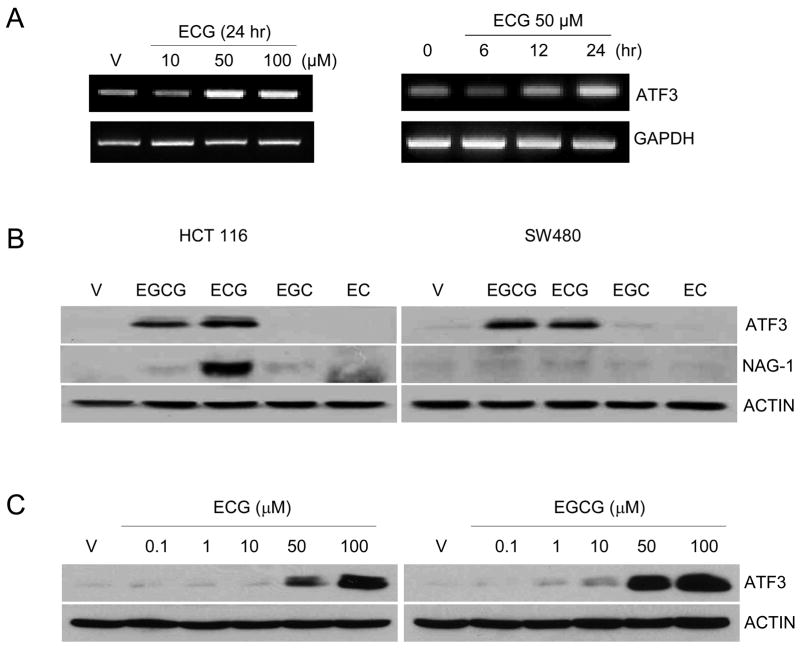
Green tea contains various types of catechins, and each catechin has its own biological effect. During the study of ECG’s effect on anti-tumourigenic activity, we have identified that ATF3 is a highly induced gene in the presence of 50 μM ECG treatment in HCT-116 cells, as assessed by microarray (data not shown). We confirmed ECG-induced ATF3 transcript expression in dose- and time-dependent manners using RT-PCR (Fig. 1A). We also investigated whether other catechins affect ATF3 expression at the protein level. As shown in Fig. 1B, both EGCG and ECG increased ATF3 expression in human colorectal cancer cells, HCT-116 (p53 wildtype) and SW480 (p53 mutated), indicating that p53 expression may not be involved in the catechin-induced ATF3 expression. It has been reported that NAG-1 is also induced by ECG and EGCG in HCT-116 cells (7), and we confirmed ECG increased NAG-1 expression in HCT-116 cells. Finally, both ECG and EGCG at 50 μM began to increase ATF-3 expression with the highest increase at 100 μM (Fig. 1C).
Fig. 1.
ECG induces ATF3 expression in colorectal cancer cells. (A) HCT-116 cells were treated with the indicated concentration of ECG for 24 h. RT-PCR was performed using ATF3- and GAPDH-specific primers as described in the Materials and Methods section. (B) HCT-116 and SW480 cells were treated with 50 μM of EGCG, ECG, EGC and EC, respectively for 24 h. Thirty μg of total cell lysates were loaded, and Western analysis was performed using ATF3 (1:500), NAG-1 (1:1,000) and Actin (1:500) antibodies. (C) HCT-116 cells were treated with 0, 0.1, 1, 10, 50, and 100 μM of ECG and EGCG for 24 h. Thirty μg of total cell lysates were loaded, and Western analysis was performed using ATF3 and Actin antibodies.
3.2. EGR-1 is involved in ECG- and EGCG-induced ATF3 expression
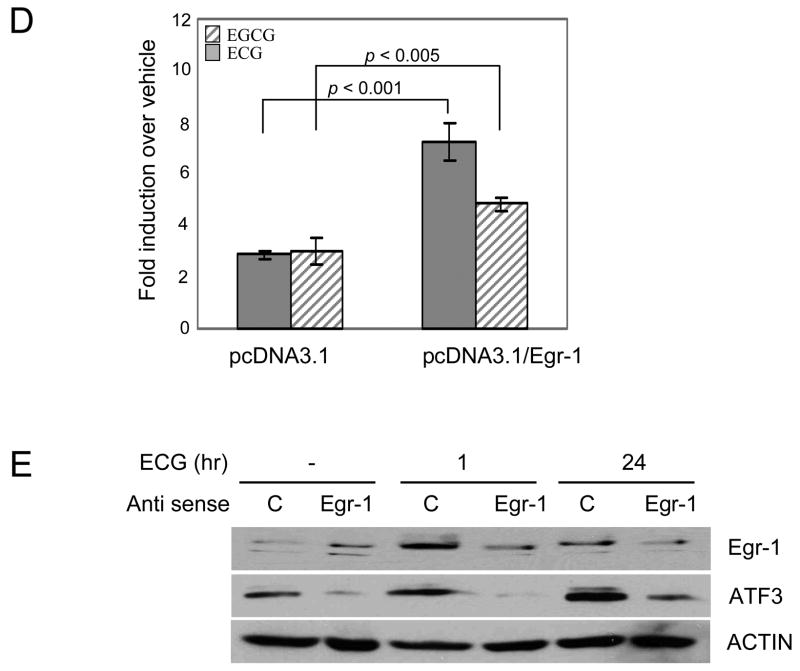
To investigate the molecular mechanism by which catechin induced ATF3 transcriptional regulation, ATF3 promoter clones were used (16). Both ECG and EGCG increased ATF3 promoter activity more than 3-fold using the ATF3 promoter construct containing regions between −1850 and −514, but did not increase luciferase activity using a construct containing the −84 region (Fig. 2A), suggesting that ECG and EGCG-responsible elements might be between the −514 and −85 region of the ATF3 promoter.
Fig. 2.
The EGR-1 binding site is involved in ECG-induced ATF3 expression. (A) Each indicated construct of the ATF3 promoter (0.5 μg) was co-transfected with 0.05 μg of pRL-null vector into HCT-116 cells using Lipofectamine and cells were treated with ECG (50 μM) or EGCG (50 μM) for 24 h. The promoter activity was measured as a ratio of firefly luciferase signal/renilla luciferase signal. The x-axis shows the relative luciferase unit (RLU) of each construct. The results are the means ± S.D. of three replicates. (B) The nucleotide sequence of the −514 to −75 regions in the ATF3 promoter. Underlines represent the binding site of indicated transcription factors and are used for internal deletion clones. (C) Each deletion construct (0.5 μg) of the ATF3 promoter was co-transfected with 0.05 μg of pRL-null vector into HCT-116 cells using Lipofectamine and cells were treated with ECG (50 μM) or EGCG (50 μM) for 24 h. The x-axis shows fold induction over vehicle as 1.0. The results are the means ± S.D. of three replicates. The two-tailed Student’s t-test was used as a statistic. *, P = 0.005; **, P = 0.003 versus vehicle treated cells. (D) Empty vector (pcDNA3.1/NEO) or EGR-1 expression vector (0.25 μg) was co-transfected with pATF3 −514 (0.25 μg) and pRL-null (0.05 μg) into HCT-116 cells. The y-axis shows fold induction over vehicle as 1.0. The results are the means ± S.D. of three replicates. Mean values were compared by the two-tailed Student’s t-test. (E) Antisense oligonucleotide (5′-ZECGGGGCGCGGGGAACFOT-3′), and sense oligonuleotide (5′-AEZGTTCCCCGCGCCCCGOA-3′) were transfected to HCT-116 cells using TransIT-TKO transfection reagent. After ECG treatment at the indicated time point, 30 μg of total cell lysates were subjected to Western analysis using EGR-1 (1:500), ATF3 (1:500) and Actin (1:500) antibodies. Z, phosphorothioate-T; E, phosphorothioate-G; F, phosphorothioate-A; O, phosphorothioate-C; T, Deoxythymidine; A, Deoxyadenine.
We then searched for the transcription factor binding sites within this region using programmes (Gene Regulation, TFSEARCH, and Transcription Element Search System). As shown in Fig. 2B, we found three cis-acting elements, EGR-1, CRE, and Ftz, in this region. We have previously reported that EGR-1 phosphorylation enhances ATF3 expression in colorectal cancer cells (16). Tamura and colleagues reported that the c-myc complex at the CRE site of the ATF3 gene promoter plays a role in mediating the serum response (21).
The Fushi tarazu (Ftz) site, a factor that defines Drosophila’s segmental regions (22), was also identified in this region (Fig. 2B). To identify the role of each cis-acting element, each site was deleted from the pATF3 −514/+34 construct and transfected into HCT-116 cells. When the EGR-1 site was deleted at positions −245 to −237, ATF3 promoter activity was significantly decreased. However, the deletion of Ftz and CRE sites did not affect ATF3 transcription (Fig. 2C). This result indicated that the EGR-1 site at positions −245 to −237 is an important region in ECG- and EGCG-induced ATF3 expression. To elucidate EGR-1’s role in catechin-induced ATF3 expression, EGR-1 expression vector and antisense EGR-1 oligo were applied to further experiments. When EGR-1 expression vector was co-transfected with pATF3 −514/+34, the promoter activity by ECG or EGCG was increased (Fig. 2D). Furthermore, the suppression of endogenous EGR-1 mRNA by antisense oligo decreased ECG-induced ATF3 expression (Fig. 2E). These results demonstrated that EGR-1 is a responsible element in ATF3 gene expression after ECG and EGCG treatment.
3.3. EGR-1 and Sp3 bind to the ATF3 promoter
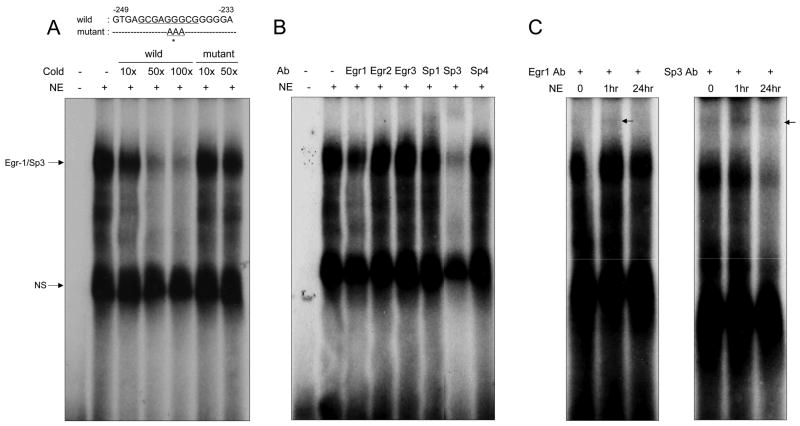
To determine whether EGR-1 can interact with the ATF3 promoter, an electrophoretic mobility shift assay (EMSA) was performed using the oligonucleotides containing two copies of the EGR-1 binding sequence at positions −245 to −237 in the ATF3 promoter (Fig. 3A, top panel). Nuclear extracts were isolated from vehicle- or ECG-treated HCT-116 cells for 1 and 24 h. When nuclear extracts from 1 h ECG-treated cells mixed with oligonucleotide, a shift band was observed (Fig. 3A). This specific band was decreased by the addition of 10-, 50-, and 100-fold cold wild type oligonucleotides. However, when the mutant oligonucleotides were added, the band intensity did not decrease, indicating that this site is able to bind with a specific protein. Next, to confirm whether EGR-1 indeed binds to this site, we carried out a supershift assay. Since the Sp transcription factors could bind to this GC-rich site as well, we also used Sp family antibodies. As shown in Fig. 3B, Sp3 clearly binds to this site, and EGR-1 binding was also observed in the long exposed film (data not shown and Fig. 3C). Interestingly, the supershifted EGR-1 band was seen only in the sample from the 1 h treated nuclear extract, but the density was decreased in the 24 h treated nuclear extract (Fig. 3C, left panel). On the other hand, supershifted Sp3 bands were seen in all the nuclear extracts tested here. These results suggested EGR-1 interplay with Sp3 proteins in the presence of ECG at the ATF3 promoter.
Fig. 3.
EGR-1 and Sp3 bind to the EGR-1 site in the ATF3 promoter. (A) Top panel, comparison of wild type and mutant sequences between −249 and −233 of the ATF3 promoter region. Asterisk indicates mutated bases. Bottom panel, 10 μg of nuclear extract from HCT-116 cells were incubated with 32P-labelled double-stranded oligonucleotides corresponding to the EGR-1 site (−245~−237) ranging from the −249 to −233 regions of the ATF3 promoter. Competitions were done in the 10, 50, 100 or 10, 50 molar excess of non-radiolabelled wild or mutant oligonucleotides, respectively. The binding reactions were resolved by 5% non-denaturing acrylamide electrophoresis. NS indicates non specific binding. (B) Supershift assays were performed by 10 min preincubation of the reaction mixture with 0.6 μg of each antibody prior to the addition of radiolabelled probe. Arrow indicates supershifted band. (C) Supershift assays were performed using three different nuclear extracts from ECG treated HCT-116 cells. Arrow indicates supershift band.
3.4. Catechins alter ATF3 expression mediated by EGR-1
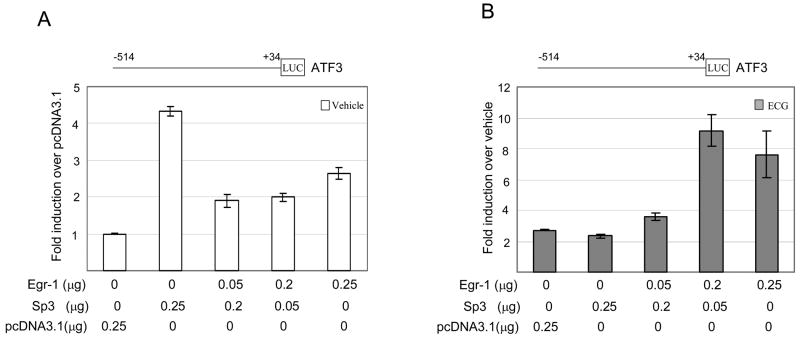
To examine whether Sp3 and EGR-1 coordinate or compete for ATF3 gene expression, we co-transfected with Sp3 and EGR-1 expression vectors with pATF3−514/+34 construct. At the basal level of expression, only Sp3 transfection increased the ATF3 promoter activity 4-fold, whereas EGR-1 expression suppressed Sp3-induced ATF3 expression (Fig. 4A). Interestingly, in the ECG-treated condition, Sp3 did not affect the activity of the ATF3 promoter, indicating that Sp3 might play an important role in the ATF3 expression at the basal level. Furthermore, EGR-1 expression caused the induction of ECG-induced ATF-3 expression (Fig. 4B), indicating that EGR-1 plays an important role in ECG-induced ATF3 expression. Co-transfection of EGR-1 with Sp1 construct produced similar results as the Sp3 co-transfection experiment (data not shown). This result showed that Sp3 contributed to the basal expression of the ATF3 gene, whereas EGR-1 contributed to the ECG-induced ATF3 expression.
Fig. 4.
Sp3 regulates basal ATF3 promoter activity. The pATF3 −514 construct (0.25 μg) was co-transfected with empty, EGR-1, or Sp3 expression vector into HCT-116 cells in the presence of pRL-null vector (0.05 μg). The promoter activity was measured as a ratio of firefly luciferase signal/renilla luciferase signal. (A) The y-axis shows fold induction over pcDNA3.1 empty vector as 1.0. (B) The y-axis is fold induction over vehicle as 1.0. The results are the means ± S.D. of three replicates.
3.5. ECG-induced ATF3 expression is associated with oxidative stress
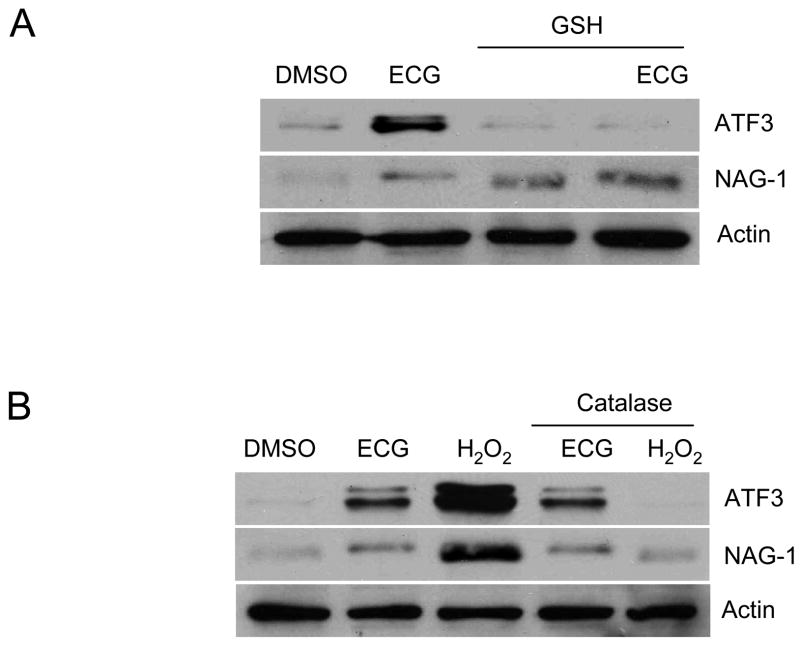
Catechins can function not only as antioxidants, but also as pro-oxidants (23). To test whether ATF3 expression is related to oxidative stress after ECG treatment in the cell culture system, HCT-116 cells were pre-treated with GSH, an intracellular antioxidant, followed by ECG treatment. ATF3 induction was not seen in the GSH pre-treated sample (Fig. 5A), indicating that oxidative stress is involved in ECG-induced ATF3 expression. ECG is also an inducer of NAG-1 in HCT-116 cells (Fig. 1B), but the oxidative stress by ECG does not contribute to NAG-1 expression (Fig. 5A). Because EGCG treatment causes a production of H2O2 in human bronchial cells (24), we sought to identify whether the H2O2 acts as an oxidative stress source in ECG-induced ATF3 expression in the HCT-116 cells. Catalase, which catalyses H2O2 to water and oxygen, was added to the media before ECG-treatment. The pre-treatment of catalase did not block the activation of either ATF3 or NAG-1, but had a clear inhibitory effect on H2O2 (Fig. 5B). Taken together, these results suggest that ECG-induced ATF3 expression is mediated by ECG-generated oxidative stress, but not by H2O2-generated oxidative stress, in the media.
Fig. 5.
ECG-induced ATF3 expression is associated with oxidative stress. (A) Effect of antioxidant on ECG-induced ATF3 expression. HCT-116 cells were plated at 1×106 cells in a 60-mm plate. Reduced GSH (5 mM) was added into the media for 1 h before 50 μM ECG treatment. After 24 h, total cell lysates were harvested for Western blot. (B) Role of H2O2 in ECG-induced oxidative stress. Three thousand U/ml of catalase were added into the media for 30 min before 50 μM ECG or 500 μM H2O2 treatment. Thirty μg of total cell lysates were loaded and Western analysis was performed using ATF3, NAG-1 and Actin antibodies.
4. Discussion
Catechins are a group of compounds that naturally occur in some plants. These compounds are characterised as containing two or more aromatic rings, each bearing at least one aromatic hydroxyl, and are found abundantly in green tea. EGCG is the most abundant catechin, and we believe that EGCG plays an important role in green tea’s effect on biological functions. Recently, we and others reported that ECG, another catechin in green tea, also showed strong biological activity including apoptosis, cell growth inhibition, and membrane transporter (7, 25, 26). However, molecular targets of ECG have not been studied in detail, compared to EGCG. To find a potential target of ECG at the transcriptional level, we performed a microarray analysis using ECG-treated HCT-116 cells, and we found ATF3 is the second most highly expressed gene by ECG (9-fold induction, data not shown). This is consistent with our previous result, showing ECG induces ATF3 expression at the protein level (7).
The ATF3 promoter contains a variety of response elements, including the activating protein-1, ATF/CRE, NF-κB, E2F, and Myc/Max binding sites (27), and is regulated by a variety of transcription factors, including NF-κB (28), EGR-1 (14) and ATF/CRE (21). Although two EGR-1 binding sites in the ATF3 promoter are involved in ATF3 expression by sulindac sulfide and troglitazone, the detailed molecular mechanisms were not previously presented. In this paper, we examined the green tea catechins’ activation of EGR-1 expression as well as the EGR-1 binding affinity to the −245 to −237 regions of the ATF3 promoter (Fig. 3C). Expression of EGR-1 and its role in cancer is complicated and may either inhibit or stimulate growth depending on the cellular context in which it takes place (29). EGR-1 has been reported to inhibit apoptosis and enhance tumour growth (30, 31) in addition to inducing metastasis-related factors in vitro, such as the vascular endothelial growth factor (VEGF) receptor Flt-1 and matrix metalloproteinase (MMP) (32, 33), indicating that EGR-1 might act as a master protein in directing invasion and metastasis during cancer progression. On the other hand, a number of reports also indicated that EGR-1 acts as a tumour suppressor gene. EGR-1 is down-regulated in several types of neoplasia as well as in an array of tumour cell lines. It induces cell growth arrest and apoptosis (29, 34–37), and is an important factor involved in neuronal apoptosis (37). EGR-1 acts as a pro-apoptotic protein by directly binding to p53 (36), NAG-1 (19) and PTEN promoters (38). These reports suggested that EGR-1 might act as a tumour suppressor protein. While these results indicated that EGR-1 plays a significant role in growth suppression, the consequences of EGR-1 over or under expression might be different, depending on cell context. These varieties may be dependent on expression of other members of the EGR-1 family; Sp transcription factors, EGR-1 binding repressors, or factors yet to be identified.
The Sp family members contain three zinc fingers (DNA binding) close to the C-terminus, and glutamine-rich domains adjacent to the serine/threonine stretch in the N-terminal region. They bind to and act through the GC-boxes (GGGGCGGGG), to which transcription factors containing the zinc finger domain are able to bind. Sp1 has been described as a positive regulator of transcription, whereas Sp3 has been shown to either activate or repress transcription in different cell types (39, 40). We found that Sp3 specifically binds to the GC box in the ATF3 promoter (Fig. 3C), as a positive regulator. This is another example of Sp and EGR-1 competition in the same binding site. Indeed, we have shown that Sp and EGR-1 compete in sulindac sulfide-induced NAG-1 expression (19). Thus, the mechanisms involved in the competition of two different transcription factors in the same site need to be considered as a novel mechanism to control the genes that are involved in compound-induced transactivation. However, further experiments may be needed.
Hydrogen peroxide, hydroxyl radicals, peroxide anions, and superoxide anion are collectively known as reactive oxygen species (ROS), which accelerate membrane damage, DNA base oxidation, DNA strand breaks and chromosome aberration involved in the carcinogenesis process (41). These ROS are removed by superoxide dismutase, catalase and glutathione peroxidase. Catechins act as antioxidants in vitro by scavenging ROS and nitrogen species, and chelating redox-active transition metal ions. For example, EGCG inhibits xanthine oxidase (XO), the major source of ROS, to produce uric acid and acts as a scavenger of superoxide in human leukemia cells (42). On the contrary, recent studies have demonstrated catechins contribute pro-oxidative activity in cell cultures. Nakagawa and colleagues reported that EGCG produces H2O2 in human Jurkat cells, and this hydrogen peroxide reduces Fe(II) to Fe(III). This reduction produces highly toxic hydroxyl radicals such as •OH and OH−, which in turn induce apoptotic cell death (43). Our data also indicated that ROS contributes to ECG-induced ATF3 expression, but that ROS does not result from H2O2 (Fig. 5). Although further mechanisms are required, our data clearly showed that pro-oxidative activity of ECG contributed to the ECG-induced ATF3 expression but not ECG-induced NAG-1 expression. It is also necessary to take into consideration the dose of ECG used for our study. 50 μM of ECG is likely to be the highest dose that can be attained physiologically in the body. The concentration of green tea catechins reach no higher than 1 μM in human plasma even after consumption of larger amounts of beverage (44). However, if we assume complete extraction, with gastric fluid volume of 100 to 500 ml, the ECG concentration in lumen may range between 75 and 300 μM (26). It is also reported that the efficacy of 50% inhibition in cell culture varied, but was generally between 22 to 130 μM (45). Taken together with our study, a 50 μM catechin in cell culture reflects a higher range of plasma concentration; however, to know the exact effective concentration in the cell culture, it should be considering the bioability, degradation, as well as metabolite effects of catechins in in vitro system.
In summary, we have found that catechins may produce oxidative stress in the cell culture media, followed by the increased expression of EGR-1. This results in the induction of ATF3 protein to protect cells from the extracellular stress signal.
Acknowledgments
We thank Misty Bailey for her critical reading of this manuscript. This work was supported by NIH grant R21CA109423 to SJB.
Footnotes
Conflict of interest statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saffari Y, Sadrzadeh SM. Green tea metabolite EGCG protects membranes against oxidative damage in vitro. Life Sci. 2004;74(12):1513–8. doi: 10.1016/j.lfs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24(8):1369–78. doi: 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- 3.Dona M, Dell’Aica I, Calabrese F, et al. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003;170(8):4335–41. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- 4.Sartor L, Pezzato E, Garbisa S. (−)Epigallocatechin-3-gallate inhibits leukocyte elastase: potential of the phyto-factor in hindering inflammation, emphysema, and invasion. J Leukoc Biol. 2002;71(1):73–9. [PubMed] [Google Scholar]
- 5.Ghosh KS, Maiti TK, Mandal A, Dasgupta S. Copper complexes of (−)-epicatechin gallate and (−)-epigallocatechin gallate act as inhibitors of Ribonuclease A. FEBS Lett. 2006;580(19):4703–8. doi: 10.1016/j.febslet.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Huang CC, Fang JY, Wu WB, Chiang HS, Wei YJ, Hung CF. Protective effects of (−)-epicatechin-3-gallate on UVA-induced damage in HaCaT keratinocytes. Arch Dermatol Res. 2005;296(10):473–81. doi: 10.1007/s00403-005-0540-5. [DOI] [PubMed] [Google Scholar]
- 7.Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004;25(12):2425–32. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]
- 8.Lim YC, Lee SH, Song MH, et al. Growth inhibition and apoptosis by (−)-epicatechin gallate are mediated by cyclin D1 suppression in head and neck squamous carcinoma cells. Eur J Cancer. 2006;42(18):3260–3266. doi: 10.1016/j.ejca.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7(4–6):321–35. [PMC free article] [PubMed] [Google Scholar]
- 10.Yin T, Sandhu G, Wolfgang CD, et al. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272(32):19943–50. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- 11.Cai Y, Zhang C, Nawa T, et al. Homocysteine-responsive ATF3 gene expression in human vascular endothelial cells: activation of c-Jun NH(2)-terminal kinase and promoter response element. Blood. 2000;96(6):2140–8. [PubMed] [Google Scholar]
- 12.Hsu JC, Laz T, Mohn KL, Taub R. Identification of LRF-1, a leucine-zipper protein that is rapidly and highly induced in regenerating liver. Proc Natl Acad Sci U S A. 1991;88(9):3511–5. doi: 10.1073/pnas.88.9.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohn KL, Laz TM, Hsu JC, Melby AE, Bravo R, Taub R. The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Mol Cell Biol. 1991;11(1):381–90. doi: 10.1128/mcb.11.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottone FG, Jr, Moon Y, Alston-Mills B, Eling TE. Transcriptional regulation of activating transcription factor 3 involves the early growth response-1 gene. J Pharmacol Exp Ther. 2005;315(2):668–77. doi: 10.1124/jpet.105.089607. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Yamaguchi K, Kim JS, et al. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis. 2006;27(5):972–81. doi: 10.1093/carcin/bgi268. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi K, Lee SH, Kim JS, Wimalasena J, Kitajima S, Baek SJ. Activating transcription factor 3 and early growth response 1 are the novel targets of LY294002 in a phosphatidylinositol 3-kinase-independent pathway. Cancer Res. 2006;66(4):2376–84. doi: 10.1158/0008-5472.CAN-05-1987. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Kim JS, Yamaguchi K, Eling TE, Baek SJ. Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun. 2005;328(1):63–9. doi: 10.1016/j.bbrc.2004.12.138. [DOI] [PubMed] [Google Scholar]
- 18.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59(4):901–8. [PubMed] [Google Scholar]
- 19.Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol. 2005;67(2):356–64. doi: 10.1124/mol.104.005108. [DOI] [PubMed] [Google Scholar]
- 20.Baek SJ, Horowitz JM, Eling TE. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3. J Biol Chem. 2001;276(36):33384–92. doi: 10.1074/jbc.M101814200. [DOI] [PubMed] [Google Scholar]
- 21.Tamura K, Hua B, Adachi S, et al. Stress response gene ATF3 is a target of c-myc in serum-induced cell proliferation. Embo J. 2005;24(14):2590–601. doi: 10.1038/sj.emboj.7600742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gindhart JG, Jr, King AN, Kaufman TC. Characterization of the cis-regulatory region of the Drosophila homeotic gene Sex combs reduced. Genetics. 1995;139(2):781–95. doi: 10.1093/genetics/139.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CS, Hong J, Hou Z, Sang S. Green tea polyphenols: antioxidative and prooxidative effects. J Nutr. 2004;134(11):3181S. doi: 10.1093/jn/134.11.3181S. [DOI] [PubMed] [Google Scholar]
- 24.Yang GY, Liao J, Li C, et al. Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000;21(11):2035–9. doi: 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- 25.Okabe S, Suganuma M, Hayashi M, Sueoka E, Komori A, Fujiki H. Mechanisms of growth inhibition of human lung cancer cell line, PC-9, by tea polyphenols. Jpn J Cancer Res. 1997;88(7):639–43. doi: 10.1111/j.1349-7006.1997.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaidyanathan JB, Walle T. Cellular uptake and efflux of the tea flavonoid (−)epicatechin-3-gallate in the human intestinal cell line Caco-2. J Pharmacol Exp Ther. 2003;307(2):745–52. doi: 10.1124/jpet.103.054296. [DOI] [PubMed] [Google Scholar]
- 27.Liang G, Wolfgang CD, Chen BP, Chen TH, Hai T. ATF3 gene. Genomic organization, promoter, and regulation. J Biol Chem. 1996;271(3):1695–701. doi: 10.1074/jbc.271.3.1695. [DOI] [PubMed] [Google Scholar]
- 28.Kaszubska W, Hooft van Huijsduijnen R, Ghersa P, et al. Cyclic AMP-independent ATF family members interact with NF-kappa B and function in the activation of the E-selectin promoter in response to cytokines. Mol Cell Biol. 1993;13(11):7180–90. doi: 10.1128/mcb.13.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Rangnekar VM, Adamson E, Mercola D. Suppression of growth and transformation and induction of apoptosis by EGR-1. Cancer Gene Ther. 1998;5(1):3–28. [PubMed] [Google Scholar]
- 30.Cao X, Mahendran R, Guy GR, Tan YH. Protein phosphatase inhibitors induce the sustained expression of the Egr-1 gene and the hyperphosphorylation of its gene product. J Biol Chem. 1992;267(18):12991–7. [PubMed] [Google Scholar]
- 31.Eid MA, Kumar MV, Iczkowski KA, Bostwick DG, Tindall DJ. Expression of early growth response genes in human prostate cancer. Cancer Res. 1998;58(11):2461–8. [PubMed] [Google Scholar]
- 32.Haas TL, Stitelman D, Davis SJ, Apte SS, Madri JA. Egr-1 mediates extracellular matrix-driven transcription of membrane type 1 matrix metalloproteinase in endothelium. J Biol Chem. 1999;274(32):22679–85. doi: 10.1074/jbc.274.32.22679. [DOI] [PubMed] [Google Scholar]
- 33.Vidal F, Aragones J, Alfranca A, de Landazuri MO. Up-regulation of vascular endothelial growth factor receptor Flt-1 after endothelial denudation: role of transcription factor Egr-1. Blood. 2000;95(11):3387–95. [PubMed] [Google Scholar]
- 34.Muthukkumar S, Han SS, Rangnekar VM, Bondada S. Role of Egr-1 gene expression in B cell receptor-induced apoptosis in an immature B cell lymphoma. J Biol Chem. 1997;272(44):27987–93. doi: 10.1074/jbc.272.44.27987. [DOI] [PubMed] [Google Scholar]
- 35.Muthukkumar S, Nair P, Sells SF, Maddiwar NG, Jacob RJ, Rangnekar VM. Role of EGR-1 in thapsigargin-inducible apoptosis in the melanoma cell line A375-C6. Mol Cell Biol. 1995;15(11):6262–72. doi: 10.1128/mcb.15.11.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair P, Muthukkumar S, Sells SF, Han SS, Sukhatme VP, Rangnekar VM. Early growth response-1-dependent apoptosis is mediated by p53. J Biol Chem. 1997;272(32):20131–8. doi: 10.1074/jbc.272.32.20131. [DOI] [PubMed] [Google Scholar]
- 37.Pignatelli M, Luna-Medina R, Perez-Rendon A, Santos A, Perez-Castillo A. The transcription factor early growth response factor-1 (EGR-1) promotes apoptosis of neuroblastoma cells. Biochem J. 2003;373(Pt 3):739–46. doi: 10.1042/BJ20021918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virolle T, Adamson ED, Baron V, et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol. 2001;3(12):1124–8. doi: 10.1038/ncb1201-1124. [DOI] [PubMed] [Google Scholar]
- 39.Kumar AP, Butler AP. Transcription factor Sp3 antagonizes activation of the ornithine decarboxylase promoter by Sp1. Nucleic Acids Res. 1997;25(10):2012–9. doi: 10.1093/nar/25.10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marin M, Karis A, Visser P, Grosveld F, Philipsen S. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell. 1997;89(4):619–28. doi: 10.1016/s0092-8674(00)80243-3. [DOI] [PubMed] [Google Scholar]
- 41.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227(4685):375–81. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 42.Lin JK, Chen PC, Ho CT, Lin-Shiau SY. Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL-60 cells by theaflavin-3,3′-digallate, (−)-epigallocatechin-3-gallate, and propyl gallate. J Agric Food Chem. 2000;48(7):2736–43. doi: 10.1021/jf000066d. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa H, Hasumi K, Woo JT, Nagai K, Wachi M. Generation of hydrogen peroxide primarily contributes to the induction of Fe(II)-dependent apoptosis in Jurkat cells by (−)-epigallocatechin gallate. Carcinogenesis. 2004;25(9):1567–74. doi: 10.1093/carcin/bgh168. [DOI] [PubMed] [Google Scholar]
- 44.Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7(4):351–4. [PubMed] [Google Scholar]
- 45.Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr. 2000;130(2S Suppl):472S–478S. doi: 10.1093/jn/130.2.472S. [DOI] [PubMed] [Google Scholar]