Abstract
In the nematode Caenorhabditis elegans, animals mutant in the gene encoding the protein product of the unc-45 gene (UNC-45) have disorganized muscle thick filaments in body wall muscles. Although UNC-45 contains tetratricopeptide repeats (TPR) as well as limited similarity to fungal proteins, no biochemical role has yet been found. UNC-45 reporters are expressed exclusively in muscle cells, and a functional reporter fusion is localized in the body wall muscles in a pattern identical to thick filament A-bands. UNC-45 colocalizes with myosin heavy chain (MHC) B in wild-type worms as well as in temperature-sensitive (ts) unc-45 mutants, but not in a mutant in which MHC B is absent. Surprisingly, UNC-45 localization is also not seen in MHC B mutants, in which the level of MHC A is increased, resulting in near-normal muscle thick filament structure. Thus, filament assembly can be independent of UNC-45. UNC-45 shows a localization pattern identical to and dependent on MHC B and a function that appears to be MHC B–dependent. We propose that UNC-45 is a peripheral component of muscle thick filaments due to its localization with MHC B. The role of UNC-45 in thick filament assembly seems restricted to a cofactor for assembly or stabilization of MHC B.
Keywords: tetracopeptide repeats, CRO1/SHE4, unc-54, myo-3, myogenesis
Introduction
The nematode Caenorhabditis elegans has proven to be an excellent model to study muscle development and function because of its anatomic simplicity, fixed cell lineage, transparency, and facility to genetic analysis ( Waterston 1988; Moerman and Fire 1997). Of the thousand somatic cells in adult nematodes, there are 189 muscle cells in males and 164 in hermaphrodites, of which the 95 body wall muscles and 20 pharyngeal muscle cells are striated muscles ( Waterston 1988; White 1988). The structure and components of C. elegans striated muscles are very similar to vertebrate muscles, composed of thick filaments, thin filaments, M-lines, and dense bodies ( Waterston 1988; Moerman and Fire 1997). In C. elegans, muscle thick filaments are composed principally of myosin, paramyosin, and associated proteins. C. elegans has four myosin heavy chain (MHC) isoforms with MHC A and B in the body wall muscles and other single sarcomere muscles, and MHC C and D exclusively in the pharyngeal muscles ( Ardizzi and Epstein 1987). MHC B is the most abundant isoform, contributing ∼70% of the total MHC protein in the adult ( Waterston 1988).
unc-45 function is required for normal embryonic development of C. elegans, as embryonic lethal alleles at the unc-45 locus have been isolated ( Venolia and Waterston 1990). However, unc-45 was initially characterized based on a temperature-sensitive (ts) viable allele at the locus ( Epstein and Thomson 1974). At the restrictive temperature (20°C or above), the strongest unc-45 (ts) alleles result in animals that are fully paralyzed and have a reduced thick filament number in the body wall muscles. A shift between the permissive and restrictive temperatures during larval development results in the reversal of the phenotype, suggesting that the protein product of the unc-45 gene (UNC-45) may be necessary for thick filament assembly ( Epstein and Thomson 1974). Embryos homozygous for the nonconditional alleles, as well as RNAi phenocopies of the presumed null phenotype, lack all body movements and arrest at the “two-fold” stage of embryogenesis, when most embryonic cell divisions and the early steps in morphogenesis are complete ( Venolia and Waterston 1990; Venolia et al. 1999). The maternal rescue of one of the lethal alleles (st604) and partial maternal rescue of the other two (st601 and st603) indicate that the unc-45 gene product (mRNA or protein) is present in the oocytes ( Venolia and Waterston 1990). Moreover, studies using thick filaments isolated from the unc-45 (ts) worms grown at the restrictive temperature show that MHC A and B are no longer differentially localized and the thick filaments are unstable in vitro ( Barral et al. 1998).
The unc-45 gene has been cloned recently and encodes a predicted protein of 961 amino acids, which contains three tandem tetratricopeptide repeat (TPR) motifs at the NH2 terminus and a region with similarity to the fungal CRO1/SHE4 proteins in the COOH-terminal half ( Venolia et al. 1999). TPR motifs have been found in many proteins with apparently divergent functions ( Goebel and Yanagida 1991), and may be involved in protein–protein interactions ( Das et al. 1998). A TPR-containing cyclophilin has been shown recently to be required for larval muscle development in parasitic and free-living nematode species ( Page and Winter 1998). The CRO1/SHE4 domain of UNC-45 is similar to part of the fungal CRO1 protein and the yeast SHE4 protein. CRO1 protein is required for the transition between the syncytial and cellular states of the filamentous fungus Podospora anserina ( Berteaux-Lecellier et al. 1998). The SHE4 protein of budding yeast is required for the segregation of determinants between mother and daughter cells for proper HO expression, which affects the mating type switching ability of the cell ( Jansen et al. 1996; Wendland et al. 1996). SHE4 may interact with an unconventional myosin Myo4p (SHE1) in this process ( Jansen et al. 1996). The sequence similarity between UNC-45 and CRO1/SHE4 suggests that UNC-45 may also interact with at least one myosin isoform, presumably in the body wall muscles.
Here, we show that unc-45::green fluorescent protein (GFP) reporter fusions are expressed in all muscle cells and a functional UNC-45::GFP fusion is localized to the A-bands of thick filaments in body wall muscles. Immunofluorescence experiments demonstrate that UNC-45 colocalizes with MHC B in the thick filaments of wild-type worms and is also associated with the abnormal thick filaments in unc-45 (ts) mutants grown at the restrictive temperature. In unc-54–null animals, in which MHC B is absent, and in unc-54; sup-3 mutants, in which increased MHC A partially compensates for lack of MHC B, UNC-45 cannot be detected in association with thick filaments. Therefore, we conclude that UNC-45 is a component of muscle thick filaments due to its colocalization with MHC B, but not MHC A, in the body wall muscles. Furthermore, we show that UNC-45 may be added into thick filaments after MHC isoforms.
Materials and Methods
Strains and Genetics
Strains carrying unc-45 (e286), unc-45 (r450), unc-45 (m94), and N2 (wild-type) were obtained from the stock collection of the Medical Research Council Laboratory of Molecular Biology, Cambridge, UK; CB190 (unc-54 [e190]) from the Caenorhabditis Genetics Center, University of Minnesota, Minneapolis, MN; and double mutant RW2329 (unc-54 [e190]; sup-3 [e1407st90st92]) and triple mutant RW2665 (unc-54 [e190]; unc-45 [m94]; sup-3 [e1407st90st92]) from the laboratory of R.H. Waterston (Washington University, St. Louis, MO). The strains were maintained as described ( Wood 1988).
Transgenic Line
A plasmid (pDP#WA036) that contains the full-length unc-45 cDNA fused in frame to GFP and driven by the unc-45 promoter was described previously ( Venolia et al. 1999). The transgenic line (DP193 edEx74) generated as described ( Mello et al. 1991) contains this construct as part of an extrachromosomal array along with pRF4 (rol6 [su1006dm]). An isolate of this line showing transmission of the array to >95% of the progeny was used in this study. GFP expression was examined using the fluorescein isothiocyanate filter set under a Zeiss Axioskop.
Antibody Production and Affinity Purification
A portion of the unc-45 cDNA, corresponding to a 58-residue region from amino acid 18 to 76 of the predicted UNC-45 protein, was ligated in frame to the glutathione S-transferase coding region in the expression vector pGEX-2T (Amersham Pharmacia Biotech). This glutathione S-transferase::UNC-45 fusion was expressed in E. coli strain BL21(DE3) under standard conditions and purified on glutathione agarose (Amersham Pharmacia Biotech) as described ( Smith and Johnson 1988). Rabbit antiserum to this fusion protein was raised and purified as described ( Ausubel et al. 1991). The preimmune serum did not detect the putative UNC-45 protein on Western blots and did not show any significant staining in immunofluorescence.
Immunoblotting
SDS-soluble nematode protein extract was prepared as described ( Goetinck and Waterston 1994) and separated by SDS-PAGE. The gel was electroblotted onto Hybond-ECL (Amersham Pharmacia Biotech) and blocked in Tris-buffered saline with 1% Triton X-100 (TBST) containing 5% skim milk powder for 1 h at room temperature. Purified anti–UNC-45 antiserum was added at a 1:10,000 dilution and incubated at room temperature for 1 h. HRP-conjugated anti–rabbit IgG (Amersham Pharmacia Biotech) was added at a dilution of 1:2,500. The blot was washed three times in TBST and detected using ECL Western blotting Reagents (Amersham Pharmacia Biotech).
Microscopy
Polarized light microscopy was performed as described ( Waterston et al. 1980; Hobert et al. 1999). For immunofluorescence, young larvae were fixed as described ( Miller and Shakes 1995) using the methanol/acetone fixation method and air-dried after fixation. For whole-mount fixation of older larvae and adults, the method used was originally developed by Finney and Ruvkun 1990 and modified for the study of muscle as described ( Miller and Shakes 1995).
mAbs against MHC isoforms (kindly provided by Dr. David Miller, 2Vanderbilt University, Nashville, TN) were used at 1:100 dilution for DM 5-6 growth media and 1:1,000 dilution for DM 5-8 ascites, respectively. Purified polyclonal antibody 7N5 against UNC-45 was used at 1:500–1:1,000 dilution. The secondary antisera (Sigma Chemical Co.) used were fluorescein isothiocyanate–labeled anti–rabbit (at 1:1,000 dilution) or tetramethylrhodamine B isothiocyanate–labeled anti–mouse (at 1:1,000 dilution) Igs.
Motility Assay
The N2 (wild-type), unc-45 (m94), double mutant RW2329 (unc-54 [e190]; sup-3 [e1407st90st92]), and triple mutant RW2665 (unc-54 [e190]; unc-45 [m94]; sup-3 [e1407st90st92]) strains were grown at 25°C. Five young adult worms of each strain were put in the center of five plates that were completely seeded with a lawn of E. coli cells (OP50). The worms were allowed to crawl for 1 h at 25°C, and the traces were marked with a pen and photographed. All five worms of the same strain showed similar motility.
Results
unc-45::GFP Is Expressed in all Muscle Cells
We have shown previously that the unc-45 promoter drives expression of GFP in muscle cells in C. elegans, but in our initial analysis the transgenic arrays were unstable, only a fraction of the progeny inherited the arrays and only a fraction of the muscle cells in any transgenic animal expressed the GFP reporter ( Venolia et al. 1999). In addition, subcellular localization of the resulting reporter protein, which contains only a small fragment of UNC-45, would not be informative for the native protein. We have since obtained a stable transgenic unc-45 cDNA::GFP line in which GFP is fused in frame to the entire UNC-45 coding region, >95% of the progeny inherit the array, and the array is mitotically stable. This unc-45 cDNA::GFP construct can rescue the unc-45 (ts) mutant phenotype at the restrictive temperature of 25°C assayed by both improved motility and muscle structure ( Fig. 1), indicating that the fusion protein retains UNC-45 function. In this transgenic line, GFP expression is detected in all muscle cells examined, including body wall muscle cells, pharyngeal muscle cells, anal-intestinal muscle cells, gonad sheath muscle cells, and sex-specific muscle cells in both males and hermaphrodites. This supports a general role of UNC-45 in development and function of all muscles.
Figure 1.
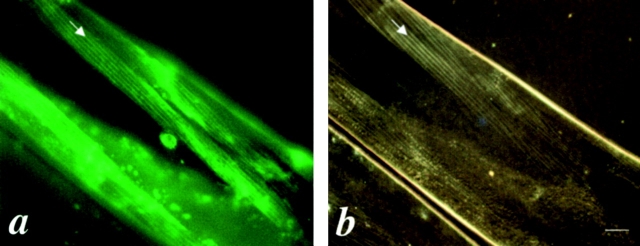
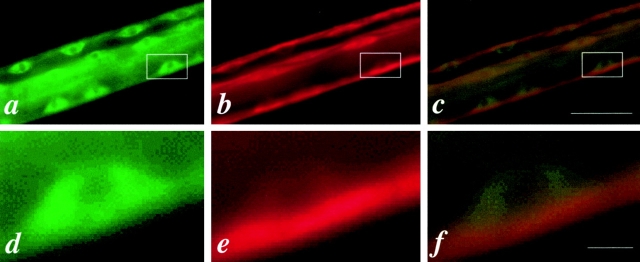
Comparison of the functional UNC-45::GFP protein distribution and the muscle filament pattern under polarized light microscopy in the body wall muscles of the rescued unc-45 (r450). a is the GFP signal associated with the UNC-45::GFP fusion protein. b is the same field showing the muscle filament pattern as visualized by polarized light microscopy. The arrows indicate the thick filaments containing A-bands. Bar, 10 μm.
Functional UNC-45::GFP Is Localized to the A-bands of Body Wall Muscles
It has been suggested that UNC-45 protein may be involved in muscle thick filament assembly ( Epstein and Thomson 1974). UNC-45 could act catalytically in the cytoplasm to modify the thick filament components for assembly, or it could act as a component of the thick filament itself. To distinguish between these alternatives, we examined the expression pattern of this functional UNC-45::GFP in the body wall muscle cells in adult worms. The GFP expression pattern resembles the pattern of A-bands of thick filaments ( Fig. 1 a). To confirm this, the same field was examined under polarized light microscopy and an identical pattern was seen ( Fig. 1 b). This indicates that functional UNC-45::GFP is associated with thick filaments in body wall muscles.
Antiserum Generated against UNC-45 Protein
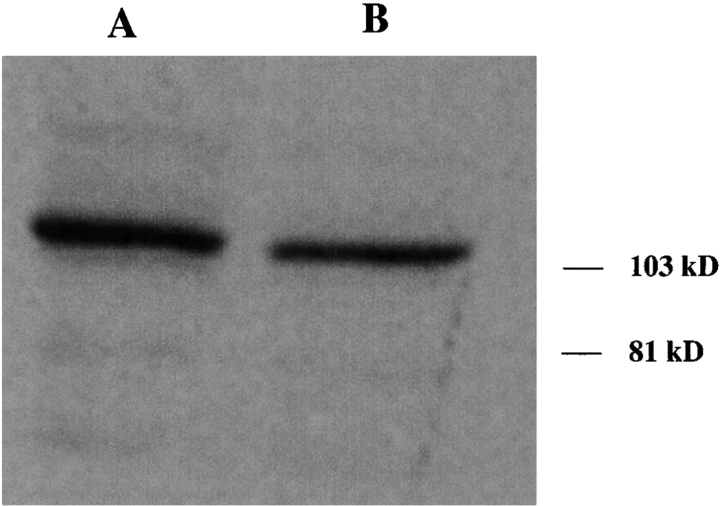
To confirm that this expression pattern of UNC-45::GFP reflects the actual subcellular localization of native UNC-45 in the body wall muscles, antiserum against an epitope of the UNC-45 protein was generated. A region of 58 amino acids, corresponding to the NH2-terminal end of UNC-45, was expressed as a glutathione S-transferase fusion protein. The affinity-purified rabbit antiserum (7N5) to this fusion protein reacts specifically to an ∼105,000 = Mr polypeptide in extracts from wild-type worms ( Fig. 2). The band detected is approximately the same size as the predicted molecular weight for the UNC-45 primary translation product. The preimmune serum did not show any reaction on immunoblotting assays.
Figure 2.
The 7N5 antiserum generated against an NH2-terminal fragment of UNC-45 reacts specifically with a polypeptide of about 105 K (MWr) on Western blots of wild-type worms. The polypeptide detected is consistent with the predicted size of UNC-45 (∼107 kD). The same amount of protein extract was used for each lane. The 7N5 antiserum was used in dilutions of 1:5,000 and 1:10,000, respectively, in lanes A and B. The preimmune serum used at 1:5,000 dilution did not show any specific reaction on Western blots (data not shown).
UNC-45 Is Localized to the Polar Regions of Thick Filaments as MHC B in the Body Wall Muscles of Wild-type Worms
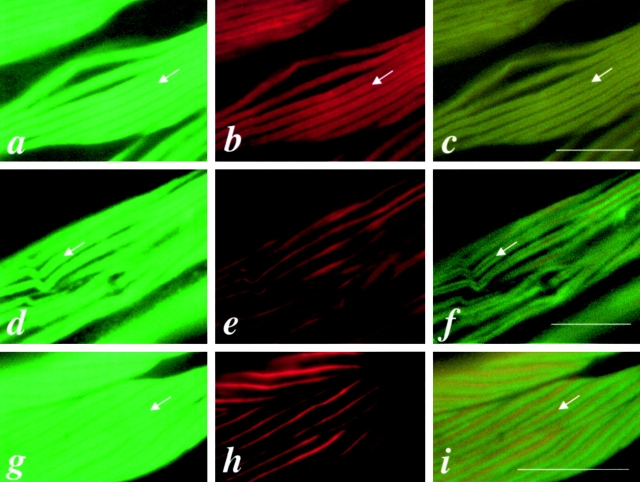
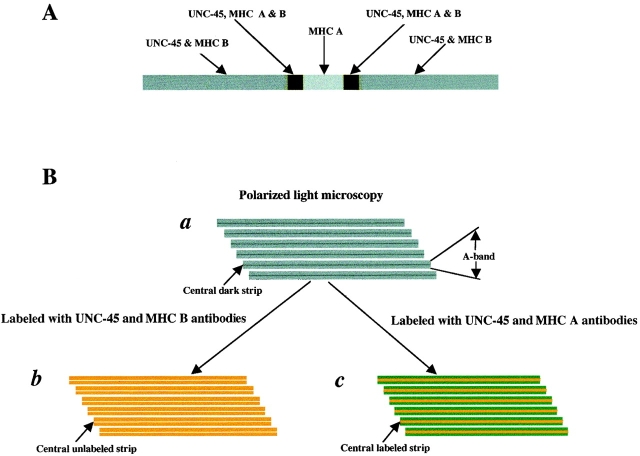
Since the functional UNC-45::GFP fusion protein is localized to the A-bands of body wall muscles, we examined these cells in wild-type adult worms by immunofluorescence. In body wall muscles, there are two myosin isoforms, MHC A and B. MHC A is localized in the central part of thick filaments, whereas MHC B is present in the two polar regions ( Miller et al. 1983). Double-staining with 7N5 and either anti–MHC B antibody or anti–MHC A antibodies showed that UNC-45 expression pattern overlaps the MHC B expression pattern, leaving an unstained central gap ( Fig. 3 c), whereas MHC A expression is localized in the central part of the A-bands and overlaps only slightly bilaterally with UNC-45 ( Fig. 3 f). Fig. 3 i shows the MHC A and B double-staining pattern, which is similar to that of UNC-45 and MHC A double-staining. This indicates that UNC-45 may colocalize with MHC B, but not MHC A, in the body wall muscles. Fig. 4 is a schematic diagram of muscle thick filaments, depicting the localization of UNC-45, MHC A, and B on the thick filament ( Fig. 4 A), and showing thick filaments as visualized by polarized light microscopy ( Fig. 4 B), when labeled with both UNC-45 and MHC B antibodies and labeled with both UNC-45 and MHC A antibodies, respectively.
Figure 3.
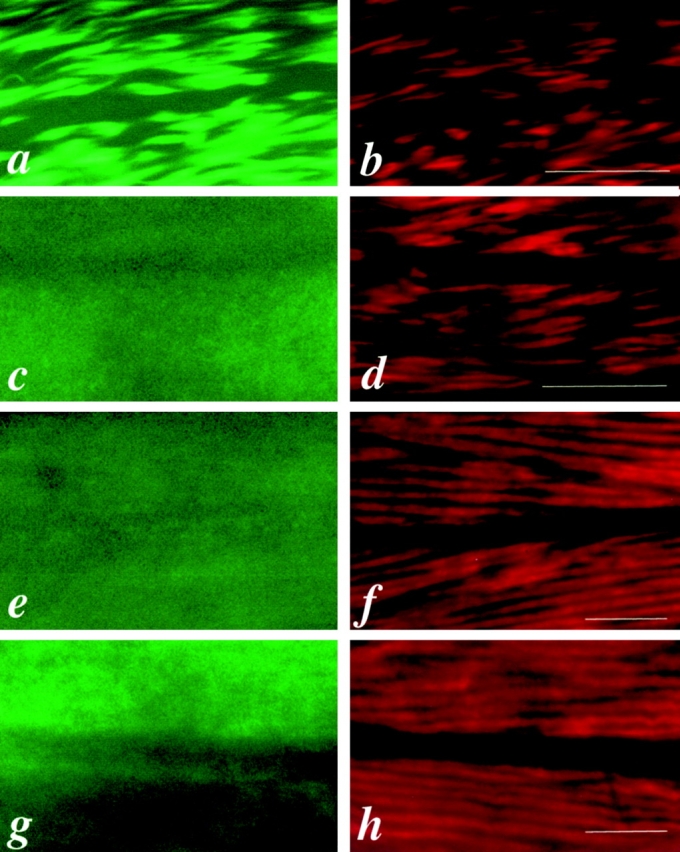
UNC-45 colocalizes with MHC B in the thick filaments of body wall muscles of wild-type adult worms. a, b, and c are the same field of body wall muscle labeled with anti–UNC-45 (7N5), anti–MHC B (DM 5-8), or double-labeled with 7N5 and DM 5-8, respectively. d, e, and f are the same field of body wall muscle labeled with 7N5, anti–MHC A (DM 5-6), or double-labeled with 7N5 and DM 5-6, respectively. g, h, and i are the same field of body wall muscle labeled with DM 5-8, DM 5-6, or double-labeled with DM 5-8 and DM 5-6, respectively. Note that DM 5-8 reacts with MHC B, which is localized on the two polar regions of the A-bands, and DM 5-6 reacts with MHC A, which is localized in the middle of the A-bands. c shows the overlapped pattern of UNC-45 and MHC B at the two polar regions of the thick filaments. f and i show a similar pattern with a central labeled strip when double-labeled with UNC-45 and DM 5-6, or DM 5-8 and DM 5-6, respectively. The arrows indicate the central unlabeled strip for UNC-45 (a and d) and for MHC B (b and g), the central unlabeled strip for double-staining (c) as shown in Fig. 4 B, panel b, and the central labeled strip for double-staining (f and i) in Fig. 4 B, panel c. Bars, 10 μm.
Figure 4.
Schematic diagram of muscle thick filaments. (A) Thick filament, depicting the localization of UNC-45, MHC A, and B. (B, panel a) Schematic view of thick filaments as visualized by polarized light microscopy, showing A-band and the central dark strip in the A-band that corresponds to the region containing only the thick filaments but not thin filaments. (B, panel b) Schematic view of thick filaments when labeled with both UNC-45 (green) and MHC B (red) antibodies as shown in Fig. 3 c. The color appears as light yellow, since the UNC-45 and MHC B are completely overlapped. The central unlabeled strip corresponds to the region containing only MHC A, but not MHC B or UNC-45. (B, panel c) Schematic view of thick filaments when labeled with both UNC-45 (green) and MHC A (red) antibodies as shown in Fig. 3 f. The central labeled strip corresponds to the whole region containing MHC A and appears as dark yellow due to the overlapping parts of MHC A and UNC-45 and the central part containing only MHC A.
Mutant UNC-45 Is Still Associated with Thick Filaments in unc-45 (ts) Mutant Worms Grown at the Restrictive Temperature
unc-45 (ts) mutants grown at the restrictive temperature (20°C or above) are paralyzed, and show a reduced number of thick filaments that are present in a disorganized arrangement ( Epstein and Thomson 1974). To test if mutant UNC-45 protein is still associated with thick filaments in the unc-45 (ts) mutants grown under restrictive conditions, we examined worms doubly stained with UNC-45 and MHC A or MHC B antisera. We chose the conditional mutants unc-45 (r450) and unc-45 (e286), which are both missense alleles resulting in different amino acid substitutions in the CRO1/SHE4 domain ( Barral et al. 1998) and should therefore still react with the polyclonal antisera. For the worms grown at both 22 and 25°C, the mutant UNC-45 protein is still associated with the disorganized thick filaments. The UNC-45 staining pattern ( Fig. 5 a) is similar to the MHC A staining pattern ( Fig. 5 b) as well as the MHC B staining pattern (data not shown). These results suggest that the association of UNC-45 with the thick filaments may not be affected in these mutants, but that this assembly or association is not sufficient to provide wild-type UNC-45 activity. In this case, it cannot be determined whether UNC-45 still differentially localizes with MHC B or MHC A, since the two myosins are scrambled in the unc-45 (ts) mutant worms grown at the restrictive temperature ( Barral et al. 1998), and their localization is no longer differential.
Figure 5.
UNC-45 and MHC A staining in mutant worms. a and b are the same field of body wall muscle from unc-45 (r450) mutant animals grown at the restrictive temperature (22°C) labeled with 7N5 and DM 5-6, respectively. c and d are the same field of body wall muscle from unc-54 (e190) mutants labeled with 7N5 and DM 5-6, respectively. e and f are the same field of body wall muscle from unc-54 (e190); sup-3 (e1407st90st92) mutants labeled with 7N5 and DM 5-6, respectively. g and h are the same field of body muscle from unc-54 (e190); unc-45 (m94); sup-3 (e1407st90st92) mutants labeled with 7N5 and DM 5-6, respectively. Note that UNC-45 is localized to thick filaments in unc-45 (ts) mutants (a), but not in unc-54 (0) mutants (c) in which MHC B is absent and unc-54 (0); sup-3 mutants (e and g) in which MHC B is absent, but the level of MHC A is increased. Bars, 10 μm.
UNC-45 Is Not Localized to Thick Filaments in unc-54–null Mutants in which MHC B Is Absent
If UNC-45 colocalizes only with MHC B but not MHC A in thick filaments in wild-type worms, it is expected that UNC-45 will not be localized to thick filaments in mutants homozygous for the unc-54–null allele in which MHC B protein is absent. unc-54 (e190) is a null mutant that produces viable but paralyzed adults with disorganized thick filaments in which no MHC B is detectable ( Epstein et al. 1974; Dibb et al. 1985; Bejsovec and Anderson 1988). Immunofluorescence shows that UNC-45 is indeed not localized to these disorganized thick filaments ( Fig. 5 c), whereas MHC A is still present ( Fig. 5 d). Therefore, we conclude that UNC-45 does not associate with MHC A in the body wall muscles of worms lacking MHC B.
UNC-45 Is Not Localized to Thick Filaments in unc-54 (0); sup-3 Mutants in which MHC B Is Absent, but the Amount of MHC A Is Increased
sup-3 is an unusual allele of myo-3 (encoding MHC A), which is a strong suppressor of unc-54–null alleles as well as unc-15 missense alleles ( Riddle and Brenner 1978; Waterston 1988; Maruyama et al. 1989). The double mutant RW2329 (unc-54 [e190]; sup-3 [e1407st90st92]) has improved structure of muscle thick filaments and much better movement than unc-54 (e190) ( Waterston 1988). This improvement is due to the increased expression of MHC A encoded by the myo-3/sup-3 locus ( Maruyama et al. 1989). To determine if UNC-45 activity is still required in this sup-3 background, the motility of animals from strains RW2329 (unc-54 [e190]; sup-3 [e1407st90st92]) and RW2665 (unc-54 [e190]; unc-45 [m94]; sup-3 [e1407st90st92]) was assayed at the restrictive temperature (25°C). As shown in Fig. 6, there is no apparent difference between the motility of the double and triple mutants. This indicates that full UNC-45 activity is not required in an unc-54; sup-3 background for assembly of functional thick filaments. In addition, immunofluorescence also shows that UNC-45 is not localized to thick filaments in these mutants ( Fig. 5e and Fig. g). MHC A is still present in thick filaments ( Fig. 5f and Fig. h), which have much better organization than those seen in the unc-54 (e190)–null allele. These results suggest that the assembly of thick filaments containing only MHC A is UNC-45–independent, at least in adult animals.
Figure 6.
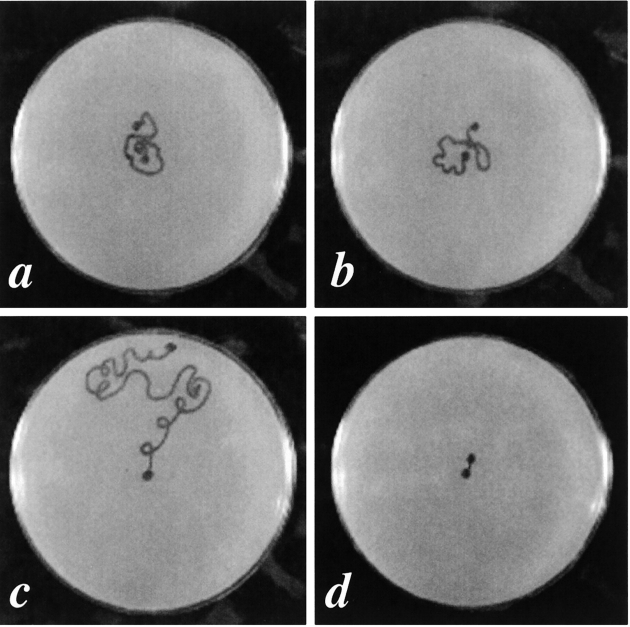
Motility assay for RW2329 (unc-54 [e190]; sup-3 [e1407st90st92]) and RW2665 (unc-54 [e190]; unc-45 [m94]; sup-3 [e1407st90st92]) mutants. a, b, c, and d are plates showing the traces of worms after crawling for 1 h at 25°C for RW2665 (unc-54 [e190]; unc-45 [m94]; sup-3 [e1407st90st92]), RW2329 (unc-54 [e190]; sup-3 [e1407st90st92]), N2, and unc-45 (m94), respectively. The dots in the centers of the plates are the start points and the others are the end points. N2 and unc-45 (m94) were used as controls. Five young adults were assayed for each strain and all showed similar motility. There is no apparent difference for the motility between RW2329 and RW2665.
UNC-45 May Be Added to Thick Filaments after MHC Isoforms Are Assembled
The specific mechanism by which C. elegans muscle thick filaments are assembled remains unclear. In the nematode, it has been proposed that body wall muscle thick filaments are composed of a core structure and an outer layer ( Epstein et al. 1985, Epstein et al. 1988; Deitiker and Epstein 1993). The core structure contains paramyosin as its major protein and at least three other proteins, α-, β-, and γ-filagenins ( Deitiker and Epstein 1993; Epstein et al. 1995; Liu et al. 1998). The outer layer is composed of two differentially localized myosin isoforms, MHC A and B, and associated myosin light chains in body wall muscles. MHC A and B may be involved in the initiation and termination of the assembly, respectively, suggested by their differential localization on thick filament in the wild-type worms ( Miller et al. 1983). In early larval stages, we have observed that MHC B has been assembled into the thick filaments near the cell membrane along the face of the muscle cell that is adjacent to the hypodermis, whereas UNC-45 is still mainly diffuse in the cytoplasm ( Fig. 7). This indicates that UNC-45 may be added to thick filaments after the assembly of MHC A and B. Although the stoichiometry of UNC-45 and MHC B in these cells at this stage cannot be accurately measured, this temporal difference in localization to the thick filament suggests that while UNC-45 is a thick filament component in adult muscles, it does not form a scaffold in the embryo upon which MHC A and B are assembled.
Figure 7.
UNC-45 may be added into thick filaments after MHC isoforms. a, b, and c are the same part of a wild-type worm at the L1 larval stage labeled with 7N5, DM 5-8, or double-labeled with 7N5 and DM 5-8, respectively. d, e, and f are enlarged for the boxed areas of a, b, and c, respectively, showing one muscle cell. Note that UNC-45 is diffuse in the cytoplasm (d), whereas MHC B has been assembled into thick filaments near the cell membrane along the face of the muscle cell that is adjacent to the hypodermis (e). Bars, 10 μm.
Discussion
UNC-45 Is a Component of Muscle Thick Filaments
In wild-type nematode, the body wall muscle thick filament is ∼9.7 μm long. MHC A is localized in the central 1.8-μm region and MHC B in the two 4.4-μm polar regions, with two 0.45-μm regions of overlap between MHC A and B ( Miller et al. 1983). Our results show that UNC-45 colocalizes with MHC B in the two polar regions of the thick filament in adult body wall muscles. In unc-45 (ts) mutant animals grown at the restrictive temperature, UNC-45 staining is still associated with disorganized thick filaments. However, this localization to thick filament remnants is lost in unc-54–null mutants, suggesting that UNC-45 localization is dependent on the presence of MHC B. Based on these results, we propose that UNC-45 is a component of thick filaments and that UNC-45 is associated with MHC B in the outer layer of thick filaments in wild-type worms. Immunoblotting experiments demonstrated that the accumulation of MHC B, but not MHC A, is decreased in unc-45 (ts) mutant worms grown at the restrictive temperature ( Barral et al. 1998). This indicates that the accumulation of MHC B, but not MHC A, requires wild-type UNC-45 activity. The overlap of UNC-45 immunolocalization with MHC B, but not MHC A, in the thick filaments is consistent with this.
Is the Assembly of MHC A Independent of UNC-45?
The stable incorporation of MHC A into thick filaments appears to be independent of UNC-45 by several lines of evidence. First, MHC A is localized in the central part of the thick filament, where polarized myosin assembly is thought to initiate ( Waterston 1989) and embryos homozygous for unc-45 lethal alleles begin MHC A assembly into thick filaments ( Venolia and Waterston 1990). Second, unlike MHC B, the steady-state accumulation of MHC A in adult muscle is not dependent on UNC-45 ( Barral et al. 1998). Third, unc-45 (ts); sup-3 mutants show muscle structure that is no better than in unc-45 (ts) mutants, but much worse structure than in unc-54 (0); unc-45 (ts); sup-3 mutants, indicating that MHC B, but not MHC A, is deleterious in an unc-45 background ( Waterston 1988). Moreover, our immunofluorescence studies have shown that UNC-45 does not colocalize with MHC A either in wild-type worms or in the sup-3 background, where MHC A is the only myosin in the body wall muscle thick filaments.
However, there is genetic evidence that UNC-45 may have some role in MHC A assembly or stability earlier in the embryo. Indirectly, unc-45 lethal alleles (st601 and st603) and an MHC A–null mutant allele (myo-3 [st378]) have a similar embryonic lethal phenotype, but unc-54–null alleles are viable ( Dibb et al. 1985; Waterston 1989; Venolia and Waterston 1990). Also, increasing the level of MHC A (as in some sup-3 alleles) can antagonize the maternal rescue seen in some of the unc-45 lethal alleles ( Venolia and Waterston 1990). It is possible that UNC-45 may interact with MHC A for the initiation of thick filament assembly, but this interaction may only be required at an early stage in development and the unc-45 (ts) alleles still maintain sufficient UNC-45 activity for that interaction.
A Model for UNC-45 Function
At least one of the roles of UNC-45 seems to be to ensure the stability of MHC B in the thick filament of the adult worm. Since MHC B is the major myosin in the nematode and it covers most of the thick filament of body wall muscles (except a narrow central region which is occupied by MHC A [ Miller et al. 1983]), the assembly and stability of MHC B would be expected to play a major role in the stability of the thick filament as a whole. In wild-type worms, UNC-45 colocalizes with MHC B and the thick filaments are normal and stable, whereas in unc-45 (ts) mutants grown at the restrictive temperature, even though mutant UNC-45 still colocalizes with MHC B, the thick filaments are short and disorganized. MHC B levels are reduced in these worms, suggesting that unassembled or misassembled MHC B is degraded ( Barral et al. 1998). UNC-45 shows no thick filament localization in MHC B–null mutants. The simplest explanation for these results is that unc-45 (ts) mutant animals are defective for an activity that stabilizes MHC B in long, polarized thick filaments. The lack of this activity leads (a) to the instability of the thick filament, and (b) directly or indirectly to the lack of a polarized arrangement of MHC A and B. unc-45 (ts) mutants are caused by missense mutations within the CRO1/SHE4 domain of UNC-45 ( Barral et al. 1998). This suggests that full activity of the CRO1/SHE4 domain is critical for the MHC B stabilizing function of UNC-45. To test this hypothesis, one would need to be able to remove all UNC-45 function from a muscle cell once the filaments have assembled normally, and examine the stability of the thick filament with time.
Although we have only detected colocalization of UNC-45 and MHC B staining, and the disorganized muscle structure and function resulting from unc-45 (ts) alleles can be suppressed by removing all MHC B and increasing MHC A levels, UNC-45 must be playing a larger role in muscle patterning. As discussed earlier, UNC-45 may have some role with MHC A for the initiation of thick filament assembly, since the sup-3 alleles of MHC A cannot suppress or ameliorate the phenotype of the lethal alleles of unc-45 ( Venolia and Waterston 1990). Also, in embryos homozygous for lethal alleles of unc-45, MHC A assembly begins, but MHC B assembly into thick filaments (even disorganized ones) is not seen ( Venolia and Waterston 1990). This lack of MHC B assembly is similar to that seen in strong myo-3 alleles, where MHC B remains dispersed until late in embryogenesis ( Waterston 1989). Thus, UNC-45 appears to be necessary for the early stages of myosin assembly, even though it does not appear to be concentrated in or around the thick filament at that time. Later, in the adult, it is also necessary for filament stability, and thick filament localization is seen.
UNC-45 is also required outside the body wall muscles. Strong UNC-45 reporter gene expression and immunofluorescence is seen in the pharyngeal muscles, where thick filaments are composed of MHC C and D. No disorganization of pharyngeal muscle structure has yet been reported in unc-45 mutants, but unc-45 lethal alleles result in embryos where no pharyngeal pumping is seen, whereas embryos homozygous for null alleles of MHC A and B do not have apparent pharyngeal defects ( Venolia and Waterston 1990). Thus, UNC-45 may play a role in assembly or stability of thick filaments in pharyngeal muscles (and other muscles) as well as in body wall muscles. Examination of pharyngeal muscle structure using EM may be necessary to address this hypothesis.
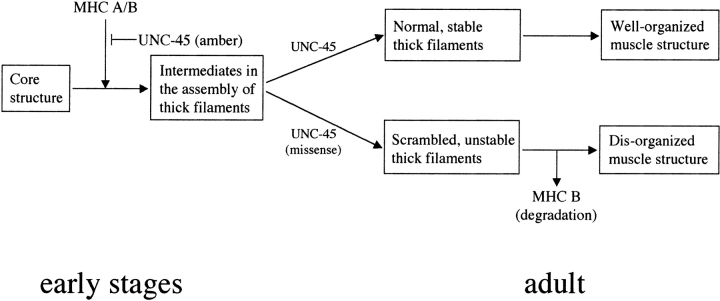
A model for the function of UNC-45 in body wall muscles is shown in Fig. 8. In the early stages of development, UNC-45 is required for the initiation of assembly of MHC A and B onto the thick filament core structure. Although it is not associated with thick filaments in these stages, it may function as a catalyst to modify MHC B (and/or MHC A) before assembly or directly participate in the assembly itself. For unc-45 lethal alleles st601 and st603 (which encode mutant UNC-45 containing stop codons between the TPR domain and the CRO1/SHE4 domain [ Barral et al. 1998]), loss of UNC-45 function results in embryonic lethality. In the larval stages, UNC-45 becomes localized, perhaps onto an intermediate in the assembly of thick filaments and associated with MHC B. In the adult, UNC-45 is a component of thick filaments and functions as a stabilizer for MHC B. In the case of unc-45 missense mutations e286 and r450 (which encode mutant UNC-45 carrying amino acid changes within the CRO1/SHE4 domain), MHC B may not be modified properly in the early stages, so that it cannot be distinguished from MHC A, and their localization on the thick filament is no longer differential. Moreover, MHC B is unstable on the filament and shows reduced accumulation, resulting in disorganized muscle structure. UNC-45 may function in a similar fashion with MHC C and D in the pharyngeal muscles. This model makes some key predictions that can be tested: (a) MHC A assembly into thick filaments in early embryos should initiate, but not proceed far in unc-45 (null) embryos; (b) UNC-45 should physically interact with some form of MHC B, but not MHC A in vitro; and (c) UNC-45 should purify with thick filaments isolated from adult worms, and should be stoichiometrically related to the level of MHC B in the preparation. To fully understand UNC-45 function, especially its role in the initiation of assembly in the early stages, we will need a better understanding of the nature of the MHC A and B molecules in the earliest stages of thick filament assembly in the embryo.
Figure 8.
A model for UNC-45 function. UNC-45 is required for the initiation of thick filament assembly in the early stages of development. It may function as a catalyst to modify MHC B (and/or MHC A) before assembly or directly participate in the assembly itself. In the case of UNC-45 amber mutations, UNC-45 cannot function properly and causes embryonic lethality. Later, UNC-45 is localized to thick filaments in the same patterns as MHC B. In the adult, UNC-45 normally functions as a stabilizer for MHC B. In animals carrying UNC-45 missense mutations in the CRO1/SHE4 domain at the restrictive temperature, MHC B turnover is increased, either due to inherent MHC B instability on the thick filament or an inability to properly assemble MHC B into the thick filament, followed by increased turnover of unassembled MHC B.
Acknowledgments
We are grateful to Dr. David Miller for providing antibodies and to Dr. Andrew Fire for GFP vector pPD95.79. We thank Drs. Pam Hoppe, Don Moerman, and Lee Venolia for critically reading the manuscript, anonymous reviewers for helpful suggestions, and Heather Lemon, Shawna Maguire, and Janette Berg for help in generating the UNC-45 antibody and immunoblotting. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources (NCRR).
This work was supported by Medical Research Council of Canada and the Alberta Heritage Foundation for Medical Research.
Footnotes
Abbreviations used in this paper: GFP, green fluorescent protein; MHC, myosin heavy chain; TPR, tetratricopeptide repeats; (ts), temperature-sensitive; UNC-45, protein product of the unc-45 gene.
References
- Ardizzi J.P., Epstein H.F. Immunochemical localization of myosin heavy chain isoforms and paramyosin in developmentally and structurally diverse muscle cell types of the nematode C. elegans . J. Cell Biol. 1987;105:2763–2770 . doi: 10.1083/jcb.105.6.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.A. Smith, J.G. Seidman, and K. Struh, editors. 1991. Current Protocols in Molecular Biology. John Wiley and Sons, Inc., New York. 1,600 pp.
- Barral J.M., Bauer C.C., Ortiz I., Epstein H.F. Unc-45 mutations in Caenorhabditis elegans implicates a CRO1/She4p-like domain in myosin assembly. J. Cell Biol. 1998;143:1215–1225 . doi: 10.1083/jcb.143.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejsovec A., Anderson R.P. Myosin heavy chain mutations that disrupt C. elegans thick filament assembly. Genes Dev. 1988;2:1307–1317 . doi: 10.1101/gad.2.10.1307. [DOI] [PubMed] [Google Scholar]
- Berteaux-Lecellier V., Zickler D., Debuchy R., Panvier-Adoutte A., Thompson-Coffe C., Picard M. A homologue of the yeast SHE4 gene is essential for the transition between the syncytial and cellular stages during sexual reproduction of the fungus Podospora anserina . EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1248–1258 . doi: 10.1093/emboj/17.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A.K., Cohen P.T.W., Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5implications for TPR-mediated protein-protein interactions. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1192–1199 . doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitiker P.R., Epstein H.F. Thick filament substructures in Caenorhabditis elegansevidence of two populations of paramyosin. J. Cell Biol. 1993;123:303–311 . doi: 10.1083/jcb.123.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb N.J., Brown D.M., Karn J., Moerman D.G., Bolten S.L., Waterson R.H. Sequence analysis of mutations that affect the synthesis, assembly and enzymatic activity of the unc-54 myosin heavy chain of Caenorhabditis elegans . J. Mol. Biol. 1985;183:543–551 . doi: 10.1016/0022-2836(85)90170-6. [DOI] [PubMed] [Google Scholar]
- Epstein H.F., Thomson J.N. Temperature-sensitive mutation affecting myofilament assembly in C. elegans . Nature. 1974;250:579–580 . doi: 10.1038/250579a0. [DOI] [PubMed] [Google Scholar]
- Epstein H.F., Waterston R.H., Brenner S. A mutant affecting the heavy chain of myosin in Caenorhabditis elegans . J. Mol. Biol. 1974;90:291–300 . doi: 10.1016/0022-2836(74)90374-x. [DOI] [PubMed] [Google Scholar]
- Epstein H.F., Miller D.M., Ortiz I., Berlier G.C. Myosin and paramyosin are organized about a newly identified core structure. J. Cell Biol. 1985;100:904–915 . doi: 10.1083/jcb.100.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H.F., Berlier G.C., Casey D.L., Ortiz I. Purified thick filaments from the nematode Caenorhabditis elegansevidence for multiple proteins associated with core structure. J. Cell Biol. 1988;106:1085–1095 . doi: 10.1083/jcb.106.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H.F., Liu G.Y., Deitiker P.R., Ortiz I., Schmid M.F. Preliminary three-dimension model for nematode thick filament core. J. Struct. Biol. 1995;155:163–174 . doi: 10.1006/jsbi.1995.1041. [DOI] [PubMed] [Google Scholar]
- Finney M., Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans . Cell. 1990;63:895–905 . doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- Goebel M., Yanagida M. The TPR snap helixa novel protein repeat motif from mitosis to transcription. Trends Biochem. Sci. 1991;16:173–177 . doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Goetinck S., Waterston R.H. The Caenorhabditis elegans muscle-affecting gene unc-87 encodes a novel thin filament-associated protein. J. Cell Biol. 1994;127:79–93 . doi: 10.1083/jcb.127.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., Moerman D.G., Clark K.A., Beckerle M.C., Ruvkun G. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans . J. Cell Biol. 1999;144:45–57 . doi: 10.1083/jcb.144.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R., Dowzer C., Michaelis C., Galova M., Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin Myo4p and other cytoplasmic proteins. Cell. 1996;84:687–697 . doi: 10.1016/s0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- Liu F., Bauer C.C., Ortiz I., Cook R.G., Schmid M.F., Epstein H.F. β-Filagenin, a newly identified protein coassembling with myosin and paramyosin in Caenorhabditis elegans . J. Cell Biol. 1998;140:347–353 . doi: 10.1083/jcb.140.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama I.N., Miller D.M., Brenner S. Myosin heavy chain gene amplification as a suppressor mutation in C. elegans . Mol. Gen. Genet. 1989;219:113–118 . doi: 10.1007/BF00261165. [DOI] [PubMed] [Google Scholar]
- Mello C., Kramer J.M., Stinchcomb D., Ambros V. Efficient gene transfer in C. elegansextrachromosomal maintenance and integration of transforming sequences. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:3959–3970 . doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.M., Shakes D.C. Immunofluorescence microscopy. In: Epstein H.F., Shakes D.C., editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. Academic Press, Inc.; San Diego, CA : 1995. pp. 365–394. [Google Scholar]
- Miller D.M., Ortiz I., Berliner G.C., Epstein H.F. Differential localization of two myosins within nematode thick filaments. Cell. 1983;34:477–490 . doi: 10.1016/0092-8674(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Moerman D.G., Fire A. Musclestructure, function and development. In: Riddle D.L., Blumenthal T., Meyer B.J., Priess J.R., editors. C. Elegans II. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York : 1997. pp. 417–470. [PubMed] [Google Scholar]
- Page A.P., Winter A.D. A divergent multi-domain cyclophilin is highly conserved between parasitic and free-living nematode species and is important in larval muscle development. Mol. Biochem. Parasitol. 1998;95:215–227 . doi: 10.1016/s0166-6851(98)00096-6. [DOI] [PubMed] [Google Scholar]
- Riddle D.L., Brenner S. Indirect suppression in Caenorhabditis elegans . Genetics. 1978;89:299–314 . doi: 10.1093/genetics/89.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.B., Johnson K.S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione-S-transferase. Gene. 1988;67:31–40 . doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Venolia L., Waterston R.H. The unc-45 gene of C. elegans is an essential muscle-affecting gene with maternal expression. Genetics. 1990;126:345–354 . doi: 10.1093/genetics/126.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venolia L., Ao W., Kim S., Kim C., Pilgrim D. unc-45 gene of Caenorhabditis elegans encodes a muscle-specific tetratricopeptide repeat-containing protein. Cell Motil. Cytoskelet. 1999;42:163–177 . doi: 10.1002/(SICI)1097-0169(1999)42:3<163::AID-CM1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Waterston R.H. Muscle. In: Wood W.B., editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York : 1988. pp. 281–335. [Google Scholar]
- Waterston R.H. The minor myosin heavy chain, mhcA, of Caenorhabditis elegans is necessary for the initiation of thick filament assembly. EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:3429–3436 . doi: 10.1002/j.1460-2075.1989.tb08507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston R.H., Thomson J.N., Brenner S. Mutants with altered muscle structure in C. elegans . Dev. Biol. 1980;77:271–302 . doi: 10.1016/0012-1606(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Wendland B., McCaffery J.M., Xiao Q., Emr S.D. A novel fluorescence-activated cell sorter–based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J. Cell Biol. 1996;135:1485–1500 . doi: 10.1083/jcb.135.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. The anatomy. In: Wood W.B., editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York : 1988. pp. 81–122. [Google Scholar]
- Wood W.B. The Nematode Caenorhabditis elegans 1988. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York: pp. 680 pp [Google Scholar]