Abstract
Matrilysin, a matrix metalloproteinase, is expressed and secreted lumenally by intact mucosal and glandular epithelia throughout the body, suggesting that its regulation and function are shared among tissues. Because matrilysin is produced in Paneth cells of the murine small intestine, where it participates in innate host defense by activation of prodefensins, we speculated that its expression would be influenced by bacterial exposure. Indeed, acute infection (10–90 min) of human colon, bladder, and lung carcinoma cells, primary human tracheal epithelial cells, and human tracheal explants with type 1–piliated Escherichia coli mediated a marked (25–50-fold) and sustained (>24 h) induction of matrilysin production. In addition, bacterial infection resulted in activation of the zymogen form of the enzyme, which was selectively released at the apical surface. Induction of matrilysin was mediated by a soluble, non-LPS bacterial factor and correlated with the release of defensin-like bacteriocidal activity. Bacteria did not induce matrilysin in other cell types, and expression of other metalloproteinases by epithelial cells was not affected by bacteria. Matrilysin was not detected in germ-free mice, but the enzyme was induced after colonization with Bacteroides thetaiotaomicron. These findings indicate that bacterial exposure is a potent and physiologically relevant signal regulating matrilysin expression in epithelial cells.
Keywords: metalloproteinase, bacteria, adhesin, defensin, host defense
Introduction
Although mucosal epithelia are specialized to serve set functions among different tissues, these cells share a number of common features related to defense against infection and response to injury. For example, secreted products, such as antimicrobial peptides, provide the first line of innate defense by directly killing bacteria at the mucosal surface of several epithelia. In response to injury, epithelial cells initiate a programmed series of separate yet interdependent responses, such as proliferation, migration, and matrix assembly, to rapidly restore the integrity of damaged tissue. Furthermore, injury and infection are not necessarily distinct events. Indeed, injury provides an opportunity for infection, and infection can lead to injury. Hence, some of the epithelial responses observed to be associated with either of these events may actually be a key component of both responses. The data we present here, along with previous observations in injured and infected tissues, indicate that matrilysin, a matrix metalloproteinase (MMP), functions during both repair and mucosal defense.
Matrilysin (MMP-7) has been demonstrated in vitro to be able to degrade or process a variety of matrix and nonmatrix proteins, including elastin, the core protein of proteoglycans, fibronectin, and serpins, among others (Murphy et al. 1991; Sires et al. 1993; Halpert et al. 1996). Despite lacking the COOH-terminal hemopexin-like domain present in other MMPs, which is thought to restrict substrate specificity (Birkedal-Hansen et al. 1993), matrilysin has the general characteristics of MMPs, such as being secreted in a latent form (28 kD) and being inhibited by tissue inhibitors of metalloproteinases. However, unlike other MMPs, which are expressed or released only in response to injury, disease, or inflammation, matrilysin is expressed by noninjured, noninflamed exocrine and mucosal epithelium in most, if not all, adult tissues. Specifically, matrilysin is produced by all skin and salivary glands, airway ciliated cells, and by the ductal or glandular epithelium of the pancreas, liver, breast, intestine, and urogenital tissues (Wilson et al. 1992; Rodgers et al. 1994; Saarialho-Kere et al. 1995; Wilson et al. 1995). In addition, matrilysin is markedly upregulated by migrating epithelium in the injured airway (Dunsmore et al. 1998) and intestine (Saarialho-Kere et al. 1996), suggesting that this MMP serves a key role in the repair of epithelium. Indeed, reepithelialization of the injured trachea is markedly impaired in matrilysin-null mice (Dunsmore et al. 1998).
Several observations implicate matrilysin in innate host defense among intact epithelia. All tissues in which matrilysin is constitutively expressed open to the environment and are vulnerable to bacterial exposure, and matrilysin is prominently upregulated in tissues with a heavy bacterial load, such as in lungs with severe cystic fibrosis (Dunsmore et al. 1998). In the mouse small intestine, matrilysin is expressed in Paneth cells (Wilson et al. 1995), which are specialized defense cells that secrete defensins, lysozyme, phospholipase A2, and other antimicrobial molecules (Ouellette 1997). Matrilysin-null mice have an impaired ability to activate prodefensins and to kill exogenous bacteria in their small intestine (Wilson et al. 1999). These observations prompted us to study if the interaction of bacteria with host cells regulates the expression of matrilysin.
Bacterial adherence to epithelial host surfaces is needed for establishment of a normal microflora, but it is also a crucial step for infection. The molecules mediating adhesion are adhesins, which are located either directly on the cell surface (nonpilus adhesins) or on the tips of fimbriae (pili) (Hultgren et al. 1993). FimH is a mannose-binding adhesin that mediates the interaction of type 1–piliated bacteria with mannose-containing glycoproteins on eukaryotic cell surfaces (Jones et al. 1995; Sokurenko et al. 1997). In our studies, we used different strains of gram-negative bacteria to assess if the interaction between bacteria and epithelial cells controls the expression of matrilysin. Our data show that bacterial exposure is a potent and relevant process that controls matrilysin expression and activation, suggesting a novel role for microorganisms in the regulation of this metalloproteinase and of mucosal defense mechanisms.
Materials and Methods
Cell and Tissue Culture
The human colon adenocarcinoma cell lines HT29 and WiDr, the human lung carcinoma cell line Calu3, and the human bladder carcinoma cell line J82 were obtained from the American Type Culture Collection. These cell lines were maintained in RPMI 1640 medium supplemented with 10% FBS without antibiotics. Primary human tracheal epithelial cells derived by explant culture were supplied by Dr. Dwight Look, (Washington University, St. Louis, MO), and were grown in RPMI 1640/10% FBS. Normal human skin fibroblasts (ATCC, CRL1905) were grown in DME supplemented with 20% FBS. The human monocytic cell line U937 was differentiated by treatment with 80 nM phorbol-12-myristate 13-acetate (PMA; Sigma Chemical Co.) and 1 μg/ml lipopolysaccharide (LPS; Sigma Chemical Co.) as previously described (Saarialho-Kere et al. 1993). Primary human keratinocytes were isolated from full thickness healthy skin and grown in on collagen-coated dishes as previously described (Sudbeck et al. 1994).
To study directional secretion of matrilysin, human colon epithelial cells were plated at a high density into Transwell inserts (Costar Corp.). After reaching confluency, the integrity of the monolayer was ascertained by measuring the electrical resistance across the epithelial layer as described in Dunsmore et al. 1998.
For organ culture studies, a segment of the proximal end of normal human trachea was obtained from donor lungs destined for transplants and dissected into 1-cm3 pieces. Individual pieces of trachea were placed into the wells of 6-well cluster dishes, so that the lumenal side was exposed, and covered with 2 ml of RPMI 1640 medium supplemented with 0.5% FBS in the absence of antibiotics.
Bacteria, Cytokines, and Other Reagents
Escherichia coli DH5α was obtained from GIBCO (Life Technologies). E. coli ORN103 and AAEC185 are nonpathogenic laboratory strains that do not express pili or adhesins. The recombinant strains ORN103/pSH2 and AAEC185/pSH2 express the complete type 1 pilus operon (type 1+/fimH +) (Orndorff 1987), and ORN103/pUT2002 and AAEC185/pUT2002 (type 1+/fimH −) are the respective fimH − isogenic mutant strains (Minion et al. 1986). NU14 is an E. coli uropathogenic isolate that expresses FimH-containing type 1 pili. NU14-1 is an fimH − mutant in which a chloramphenicol cassette was recombined into the fimH gene (Langermann et al. 1997). The gut isolates, E. coli G167 and EC80, are also type 1–piliated (fimH +) strains. We also used two E. coli strains expressing the Pap operon and distinct adhesins, PagGI or PapGII (Kuehn et al. 1992). The Salmonella typhimurium strains 14028s (mouse virulent wild type) and ms7953s (isogenic mouse-avirulent phoP mutant) were a gift from Dr. Fred Heffron (Oregon Health Sciences University, Portland, OR; Fields et al. 1989). Piliated Bordetella pertussis BC23 was provided by Dr. William Goldman (Washington University, St. Louis, MO). Recombinant human cytokines, interleukins, and growth factors were obtained from R&D Systems, Inc. E. coli (serotypes 055:B5, 0128:B12, and 0111:B4), Klebsiella pneumoniae, and Pseudomonas aeruginosa (serotype 10) LPS, polymyxin B, and p-amino-phenylmercuric acetate (APMA) were obtained from Sigma Chemical Co.
Infection Protocol
Human epithelial cells were seeded onto 6-well plates and were grown to ∼90% confluency. Expression of type 1 pili by bacteria was induced by growing the cells in static broth at 37°C for 48 h and was confirmed by hemagglutination assays as previously described (Langermann et al. 1997). About 4 × 108 bacteria, representing a bacterial to epithelial cell ratio of 300:1, were added in 1 ml of RPMI 1640, 0.5% FBS to each well. Bacteria were incubated with eukaryotic cells for up to 90 min. In some experiments, 100 mM d-mannose, d-glucose, or d-maltose was included during the 90-min infection. Epithelial monolayers were washed extensively with PBS to remove nonadherent bacteria, and the cultures were further incubated up to 48 h in RPMI 1640/0.5% FBS and 50 μg/ml gentamicin to kill the remaining extracellular bacteria. Cycloheximide (10 μg/ml) was added to some cultures during the postinfection incubation. Conditioned media were collected, centrifuged at 12,000 rpm for 10 min to remove debris, and stored at −20°C. Each condition in each experiment was done in duplicate.
Infection of tracheal tissues was done with an initial inoculum of 107 cells per 1-cm3 piece of trachea added in a final volume of 2 ml of RPMI 1640 medium supplemented with 0.5% FBS. After a 90-min incubation at 37°C, the tissues were carefully washed with PBS before the addition of fresh medium containing 50 μg/ml gentamicin. Conditioned media were collected 48 h later as described above.
Immunoblotting
Media samples from the bacterially infected epithelial cells or tissues were concentrated 10-fold by lyophilization. Aliquots of concentrated media were separated by 12% SDS-PAGE and transferred to nitrocellulose membranes (Hybond ECL; Amersham). Blots were incubated with a 1:3,000 dilution of anti-human matrilysin polyclonal antiserum in blocking buffer for 2 h. This antiserum detects both the precursor (28 kD) and mature (19 kD) forms of human matrilysin (Busiek et al. 1992; Halpert et al. 1996). Membranes were subsequently incubated with a 1:3,000 dilution of a peroxide-linked donkey anti–rabbit IgG (Amersham) in blocking buffer for 1 h, washed twice, and developed with the enhanced chemiluminescence system (Amersham). We also used a polyclonal antiserum raised against the recombinant catalytic domain of human matrilysin (gift of Dr. H.G. Welgus, Parke-Davis, Ann Arbor, MI), with similar results.
On our matrilysin Western blots, we often observed an additional, higher molecular mass band migrating at ∼70–80 kD (see Fig. 1 A). To establish the origin of this nonspecific band, we performed infection experiments on live and formalin-fixed epithelial monolayers. Although the precursor and activated forms of matrilysin were produced only by live cells, signal for the higher molecular mass band was also seen in conditioned media from fixed cells (data not shown). This band was not seen in immunoprecipitates of metabolically labeled, bacterial-exposed cells but was detected in media of cells treated with cycloheximide after infection, which blocks matrilysin expression (data not shown; see Fig. 3). The nonspecific band was also seen in mock infections done with bacteria in the absence of epithelial cells and was detected in bacterial broth (data not shown). Based on these various controls, we conclude that the additional band detected by the antimatrilysin antibodies is nonspecific and derives from bacteria culture medium.
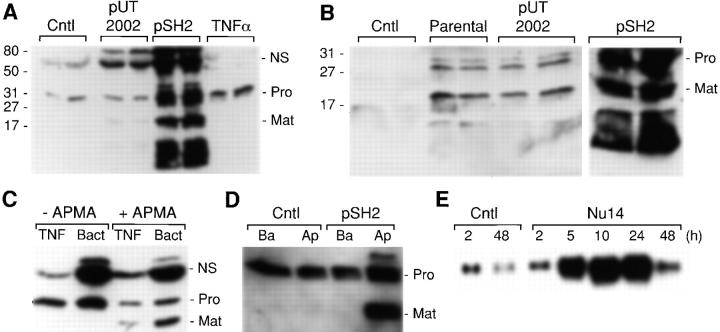
Figure 1.
FimH-dependent induction and activation of matrilysin in epithelial cells. (A) WiDr human colon carcinoma cells were infected for 90 min with E. coli strains ORN103/pUT2002 (type 1+/fimH −) and ORN103/pSH2 (type 1+/fimH +) at a ratio of 300:1 bacteria/epithelial cells. Cultures were washed extensively and re-fed with medium containing 50 μg/ml gentamicin. Some cultures were treated for 48 h with 50 ng/ml of human recombinant TNFα. Conditioned media were collected 48 h after infection and analyzed by Western blotting for matrilysin protein. the 28-kD proform of matrilysin (Pro), the 19-kD activated form of matrilysin (Mat), and autolytic fragments (broad migrating faster below activated matrilysin) were seen in pSH2-infected cells. A nonspecific (NS) band is seen at ∼70 kD. Numbers on the left side of the autoradiogram indicate the migration of molecular mass standards. Samples from two separate cultures were analyzed for each group. (B) J82 human bladder carcinoma cells were infected for 90 min with the parental E. coli strain ORN103 and the recombinant strains ORN103/pUT2002 and ORN103/pSH2. Conditioned media were collected 48 h later, and the expression of matrilysin was analyzed by Western blotting. (C) Aliquots of conditioned media from TNFα-stimulated (TNF) or ORN103/pSH2-infected (Bact) colon epithelial cells were incubated at 37°C for 1 h in the presence or absence of 1 mM APMA, and the products were analyzed by Western blotting. (D) WiDr colon cells were grown on Transwell inserts, and some cultures were infected with ORN103/pSH2. Conditioned media was collected after 48 h from the apical and basal compartments and analyzed by Western blotting. (E) HT29 colon cells were infected for 90 min with E. coli NU14 (300 bacteria per epithelial cell). Total RNA was isolated at the indicated times after the infection. Matrilysin mRNA was detected by Northern hybridization.
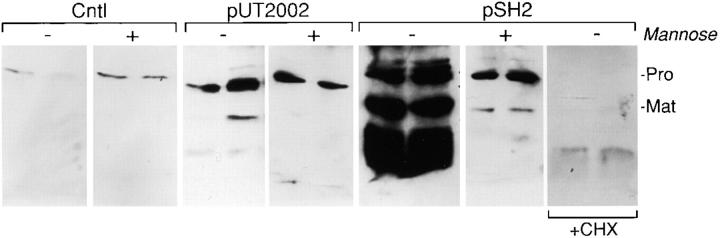
Figure 3.
Mannose inhibits FimH-mediated induction and activation of matrilysin. WiDr human colon epithelial cells were infected for 90 min with the recombinant strains ORN103/pUT2002 (type 1+/fimH −) and ORN103/pSH2 (type 1+/fimH +) at a ratio of 300 bacteria per epithelial cell, in the presence or absence of 100 mM mannose or 10 μg/ml cycloheximide (CHX). Conditioned media were collected after a 48-h incubation and analyzed by Western blotting. (Pro) 28-kD proform of matrilysin; and (Mat) 19-kD activated matrilysin.
Northern Hybridization
About 1.5 × 106 human colon carcinoma cells were infected with 4 × 108 bacteria for 90 min, as described above. At different times after infection, total RNA was isolated, separated by electrophoresis, and blotted onto nylon filters. Gel-purified cDNA probes for human matrilysin, collagenase-1 (MMP-1), stromelysin-1 (MMP-3), and MT1-MMP (MMP-14) were labeled by random priming.
Zymography
Conditioned media samples were collected 48 h after infection and were concentrated 10-fold by lyophilization. Medium samples were resolved at 4°C through 10% nonreducing SDS–polyacrylamide gels impregnated with 1 mg/ml gelatin or casein. Gels were incubated overnight at 37°C in 50 mM Tris, pH 8.0, 5 mM CaCl2 and 0.5 μM ZnCl2. Gelatinolytic and caseinolytic proteins were identified as clear bands on a dark background after staining with 1% Coomassie blue.
Immunohistochemistry
germ-free mice were raised as described (Bry et al. 1996). At 4 mo of age, some germ-free mice were inoculated with B. thetaiotaomicron, a commensal bacterium of the mouse small intestine. 10 d later, age-matched germ-free, monocontaminated ex-germ-free, and conventionally housed mice were killed, and the jejunal segments were isolated and frozen in OPTC. Frozen sections were processed for immunohistochemistry using an HRP detection system (Vectastain ABC Elite kit; Vector Laboratories, Inc.) and TrueBlue as a substrate (Kirkegaard & Perry Laboratories, Inc.) as described previously (Wilson et al. 1997). Affinity-purified anti-mouse matrilysin antibody was used at 1:5,000, and anti-CRS1C prosegment antibody, which reacts with several murine cryptdin-like intestinal α-defensins, was used at 1:500. For negative controls, sections were processed with preimmune serum. Sections were counterstained with contrast red.
Bacterial Broth Studies
To obtain bacterial culture broth, E. coli strains AAEC185, AAEC185/pUT2002 (type 1+/fimH −), and AAEC185/pSH2 (type 1+/fimH +) were grown overnight with shaking at 37°C in RPMI 1640 supplemented with 0.5% FBS. The optical density at 600 nm of the cultures was typically between 1.5 and 2.0, and in these growth conditions, piliation of the bacteria is limited. Bacterial cultures were centrifuged for 10 min at 12,000 rpm, cell pellets were discarded, and supernatants were sterilized by filtering through a 0.2-μm filter (Gelman Sciences). For stimulation of naïve HT29 colon carcinoma cells, bacterial broth was diluted in fresh medium (RPMI 1640/0.5% FBS) and added to 5 × 105 cells per well in 12-well plates. Conditioned media from the HT29 cells were collected after 48 h and analyzed by Western blotting as described above.
Bacteriocidal Assays
Colon epithelial cells were exposed to the ORN103/pSH2 (type 1+/fimH +) strain for 90 min as described above. Bacteria were removed by extensive washing and killed by incubation in fresh medium containing 50 μg/ml gentamicin. After 4 h, monolayers were washed to eliminate traces of the antibiotic, and the cells were allowed to condition fresh media for 48 h. Conditioned media were collected, centrifuged to remove debris, and filtered through a 0.2-μm sterile filter.
For antimicrobial activity assays, E. coli DH5α and S. typhimurium bacteria were grown to the midexponential phase, washed with PBS, and their concentration was estimated spectrophotometrically. For the liquid growth inhibition assay, 200 μl of the epithelial cell conditioned media, diluted in water or 10 mM sodium phosphate buffer, pH 7.0, were added to 100 μl of bacteria at 1–5 × 106 cells/ml. Salt concentration of conditioned media was altered by the addition of 5 M NaCl, and the effect of the pH was assessed using 50 mM phosphate buffer, pH 7.5, or 50 mM acetate buffer, pH 5.5. At the start of the incubation and after 3 h of incubation at 37°C, the number of CFUs was determined by plating serial dilutions. All incubations were performed at least in duplicate.
Results
Stimulation of Matrilysin by Adherent Bacteria
To evaluate the effect of bacterial infection on the expression of matrilysin in human epithelial cells, we incubated WiDr human colon carcinoma cells with the recombinant strains ORN103/pUT2002 (type 1+/fimH −) and ORN103/pSH2 (type 1+/fim +) and assayed by immunoblotting the levels of enzyme secreted into the medium. Strain ORN103/pSH2 contains the FimH adhesin at the tip of the bacterial pili, whereas strain ORN103/pUT2002 does not. After 90 min, the bacteria were killed with gentamicin, and conditioned media were collected 48 h later. Low levels of promatrilysin protein (28 kD) were detected in the medium from control cells (Fig. 1 A). The amount of matrilysin increased slightly in the medium from cells infected with the fimH − mutant strain pUT2002, and a small level of activated enzyme was detected. In contrast, infection with the adherent type 1–piliated bacteria, pSH2, led to a marked increase in the amount of antimatrilysin material secreted by the epithelial cells (Fig. 1 A). Three specific bands were detected with the antimatrilysin antibodies. The band migrating at ∼28 kD corresponds to promatrilysin and, in these experiments, represents ∼30% of the total secreted enzyme. The band migrating at 19 kD corresponds to activated matrilysin and represents ∼20% of the total secreted enzyme. The fastest migrating form, a broad band between 10–15 kD, represents autolytically cleaved products of matrilysin, which are predictably produced in vitro in any sample with abundant activated matrilysin. This band does not represent the cleaved prodomain because our antibodies do not recognize this region of the protein. Totaling the densitometric values for these three bands indicated that infection with the ORN103/pSH2 strain mediated about a 50-fold increase in the amount of secreted matrilysin compared with the low levels released by noninfected cells (Fig. 1 A).
Because the type 1 pili harboring the adhesin FimH mediate the attachment of uropathogenic E. coli to the mucosal surface of the bladder (Langermann et al. 1997; Mulvey et al. 1998), we assessed if these bacteria affected matrilysin expression by human bladder cells. No matrilysin was detected in the conditioned media from noninfected J82 bladder carcinoma cells (Fig. 1 B). A slight induction and processing of matrilysin was observed in cells exposed to the parental strain ORN103 or to the FimH-deficient strain ORN103/pUT2002 (type 1+/fimH −) (Fig. 1 B). However, infection with the ORN103/pSH2 (type 1+/fimH +) strain potently induced the secretion and activation of matrilysin (Fig. 1 B). Furthermore, the same quantitative stimulation and pattern of matrilysin release and activation were seen with a variety of other human epithelial cell lines tested, including HT29 colon carcinoma cells, Calu3 lung carcinoma cells, as well as with primary human tracheal epithelial cells from normal lung (see Fig. 7 B).
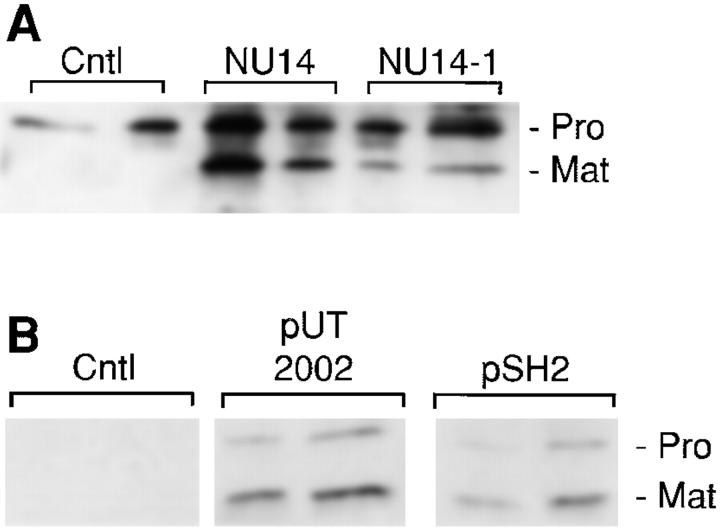
Figure 7.
Ex vivo infection of human tracheal explants and infection of human tracheal epithelial cells. (A) Pieces (1 cm3) of freshly isolated normal adult human trachea were infected with the E. coli clinical isolates NU14 (fimH +) and NU14-1 (fimH −) for 90 min, washed extensively to eliminate nonadherent bacteria, and incubated for 24 h in fresh media containing 50 μg/ml gentamicin. Released matrilysin was detected by Western analysis. (B) Human tracheal primary epithelial cells were infected with the type 1–piliated recombinant strains ORN103/pSH2 (fimH +) and ORN103/pUT2002 (fimH −) for 90 min, and allowed to condition fresh media for 48 h. Matrilysin secretion was assessed by immunoblotting.
To further characterize the bands detected by the antimatrilysin antiserum, we induced the autolytic activation of prometalloproteinases present in the conditioned media from colon epithelial cells. in the conditioned media from cells stimulated with TNFα or exposed to bacteria, APMA treatment led to a reduction in the density of the 28-kD promatrilysin band and a predicted shift to the activated form of 19 kD (Fig. 1 C). Furthermore, matrilysin in the conditioned media samples showed caseinolytic activity in substrate zymograms, and this activity was inhibited by EDTA (data not shown). In contrast, the density of the nonspecific band (NS) was not altered by APMA treatment (Fig. 1 C), suggesting that this band does not represent promatrilysin complexed with other products.
Vectorial Secretion of Matrilysin
Typically, bacteria initially interact with epithelial tissues at the apical surface. To examine the effect of bacterial challenge on the directional secretion of matrilysin, we plated WiDr colon epithelial cells in Transwell inserts and bacteria were added to the upper chamber. The integrity of the epithelial monolayer was verified by measuring the transepithelial electrical resistance, which was unaffected by bacterial infection. Promatrilysin, which was secreted by control cells, was released in nearly equal proportions into the basal and apical chambers (Fig. 1 D). In response to bacterial exposure, matrilysin production and activation were markedly increased, and all of the stimulated zymogen and activated enzyme were released into the upper compartment (Fig. 1 D). These data indicate that in addition to potently augmented production, bacteria regulate the vectorial secretion of matrilysin towards the apical surface.
mRNA Levels
We also examined if adherent bacteria stimulated matrilysin gene expression. HT-29 colon carcinoma cells were infected with NU14, a FimH-expressing, type 1–piliated uropathogenic isolate. At 2 h after a 90-min infection, matrilysin mRNA levels were stimulated slightly above control levels, but by 5 h after infection, matrilysin mRNA levels had increased ∼10-fold and remained elevated at 24 h (Fig. 1 E). The sustained elevated levels of matrilysin mRNA allowed for the increase in total secreted enzyme accumulated over a 48-h postinfection period.
Effect of Other Mediators
We assessed if other host-derived mediators, known either to stimulate matrilysin expression or to be influenced by bacterial exposure, affected matrilysin production by human epithelial cells. TNFα mediated only a 5–7-fold increase in the amount of matrilysin secreted by epithelial cells and only the 28-kD zymogen form of matrilysin was seen (Fig. 1 A; Table ). Most of the factors we used, such as HGF/SF and IL-8, did not influence matrilysin production, and others, such as IL-6, IL-1β, and phorbol ester, stimulated enzyme production modestly. Of note, bacterial LPS did not modulate matrilysin expression by epithelial cells (Table ), although this compound does stimulate enzyme expression in monocytes (Busiek et al. 1992; see Fig. 4 C).
Table 1.
Comparison of the Relative Induction and Activation of Matrilysin by Bacteria, Cytokines, and Other Stimuli
| Condition | Concentration | Fold induction | Matrilysin activation |
|---|---|---|---|
| ng/ml | |||
| No stimulus | − | ||
| Adherent bacteria | 50–75 | + | |
| Nonadherent bacteria | 5–10 | ± | |
| IL-1β | 10–25 | 12 | − |
| IL-6 | 25–50 | 8 | − |
| TNFα | 25–50 | 7 | − |
| EGF | 100 | 3 | − |
| Phorbol ester | 10–50 | 3 | − |
| LPS | 1,000 | ne | − |
| IFN-γ | 50–100 | ne | − |
| IL-4 | 20–50 | ne | − |
| HSF/SF | 30–60 | ne | − |
| GM-CSF | 50 | ne | − |
| IL-8 | 100 | ne | − |
| MCP-1 | 50–100 | ne | − |
| TGF-β1 | 100 | ne | − |
HT29 human colon epithelial cells were plated on 6-well plates and infected with bacteria (300 bacterial/cell) or treated with the indicated concentrations of cytokines and other stimuli. Western blotting was performed on conditioned media collected 48 h after infection or 48 h after addition of the other stimuli. Autoradiograms were analyzed by densitometry, and the results are expressed relative to the values of control cells (ne, no effect). (+) 50% or more activated matrilysin; (±) <50% activated matrilysin; and (−) no activated matrilysin.
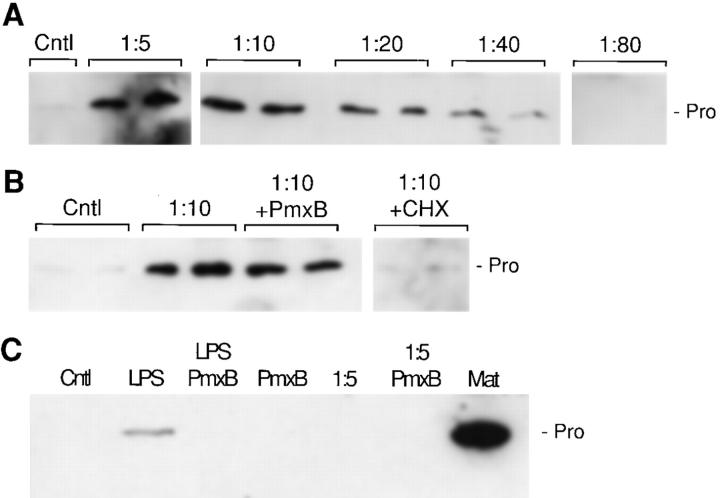
Figure 4.
Inductive activity in bacterial conditioned medium. (A) HT29 human colon cells were treated with different dilutions of bacterial broth from the strain AAEC185/pSH2 (type 1+/fimH +) for 48 h, and matrilysin was assayed by Western blotting. (Cntl) Unstimulated cells; and (Pro) 28-kD promatrilysin. (B) HT29 cell cultures were incubated in duplicate with a 1:10 dilution of bacterial broth from the strain AAEC185/pSH2, in the presence or absence of 103 U/ml polymyxin B (Pmx B) and 10 μg/ml cycloheximide (CHX). (C) U937 human monocytic cells were incubated in the presence of 1 μg/ml E. coli LPS, and a 1:5 dilution of bacterial broth, in the presence or absence of 103 U/ml polymyxin B. MMP-7 denotes an aliquot of conditioned media from HT29 cells stimulated with bacterial broth.
Time and Dose Response
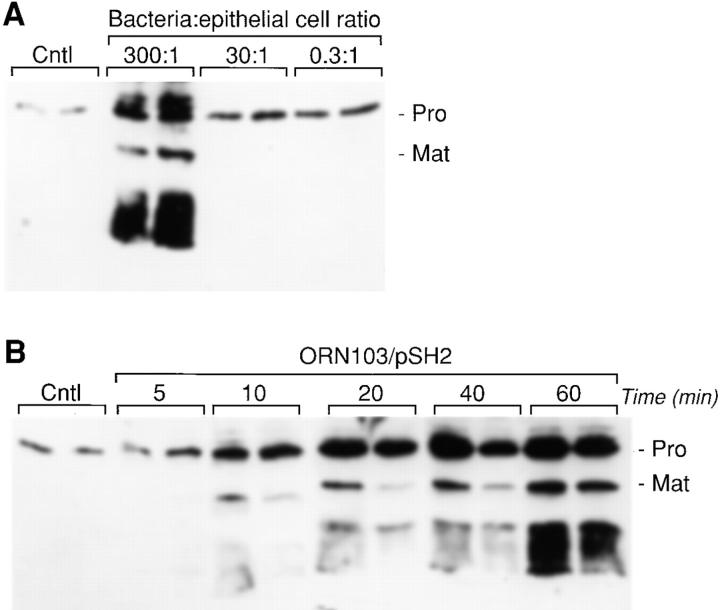
The accumulation of matrilysin in the conditioned media of infected HT29 cells was dependent on the number of bacteria added to the culture (Fig. 2 A). Demonstrating that epithelial cells are extremely sensitive to bacterial infection, a 5- to 10-fold increase in secreted matrilysin was detected even when the ratio of bacterial-to-epithelial cells was <1 (Fig. 2 A). We also assessed the effect of infection time on matrilysin secretion. Similar to that seen with a 90-min infection (Fig. 1A and Fig. B), matrilysin secretion and activation were markedly stimulated by a 60-min exposure to adherent bacteria (Fig. 2 B), although shorter incubations also upregulated matrilysin secretion (Fig. 2 B). With infections longer than 90 min, extensive cell death was seen (data not shown).
Figure 2.
Dose dependence and time-course analysis of bacterially induced upregulation of matrilysin. (A) HT29 human colon epithelial cells were infected for 90 min with E. coli strain ORN103/pSH2 (type 1+/fimH +) at the indicated bacterial/epithelial cell ratio. (B) HT29 cells were infected with the recombinant strain ORN103/pSH2, at a ratio of 300 bacteria per epithelial cell, for different periods of time, from 5 to 60 min. conditioned medium samples were collected at 48 h after infection and analyzed by Western blotting. (Pro) 28-kD proform of matrilysin; and (Mat) 19-kD activated matrilysin.
Mannose Inhibition
adhesion of FimH-containing type 1 pili to epithelial cells can be inhibited by d-mannose and its derivatives (Firon et al. 1987). To investigate if the induction of matrilysin expression triggered by FimH-expressing bacteria was sensitive to mannose, we infected WiDr cells with the recombinant strains ORN103/pUT2002 (type 1+/fimH −) and ORN103/pSH2 (type 1+/fimH +) in the presence or absence of 100 mM mannose. Addition of mannose effectively blocked the FimH-mediated increase in matrilysin release, but had no effect on the smaller stimulation seen in cells infected with the FimH-deficient strain (Fig. 3). other sugars, such as glucose or maltose, did not affect the bacterial-mediated stimulation of matrilysin (data not shown). In addition, as demonstrated by the complete lack of detectable protein in the presence of cycloheximide, the bacterial-mediated stimulation of matrilysin required new protein synthesis (Fig. 3).
Inductive Factor Is Released by Bacteria
Because expression of matrilysin was induced by bacteria even in the presence of mannose, it is likely that a bacterial soluble factor affects enzyme production. To investigate this possibility, we grew strains of E. coli bacteria to obtain sterile liquid broth filtrates, which were diluted in fresh cell culture medium, and added to HT29 colon carcinoma cells. bacterial broth from the strain AAEC185/pSH2 (type 1+/fimH +) induced matrilysin expression by epithelial cells in a dose-dependent fashion (Fig. 4 A). A 7–10-fold accumulation of promatrilysin was observed with a 1:5 dilution of the liquid broth, although no processing of matrilysin to the active form was observed. Similar results were obtained with bacterial broths from E. coli AAEC185 and AAEC185/pUT2002 (type 1+/fimH −) (data not shown).
Although different preparations of commercial LPS, which are essentially devoid of protein, had no effect on matrilysin expression by HT29 colon cells (Table ), we could not rule out the possibility that the LPS-containing complexes naturally released by the bacteria may be partially responsible for the upregulation of matrilysin. In this respect, the addition of polymyxin B, an antibiotic which specifically binds LPS and has anti-endotoxin properties, did not block the induction of matrilysin in response to bacterial broth filtrates (Fig. 4 B). These findings suggest that the inductive factor is unrelated to LPS. In addition, cycloheximide totally abrogated accumulation of matrilysin, indicating that de novo protein synthesis is required (Fig. 4 B). Predictably, polymyxin B blocked LPS-induced accumulation of matrilysin in U937 human monocytic-like cells (Fig. 4 C). However, matrilysin was not induced in either basal or differentiated U937 cells in response to bacterial broth filtrates (Fig. 4 C; data not shown). Together, these results indicate that different bacterial factors and host cell pathways regulate matrilysin expression in epithelial cells and monocytic/macrophages.
Other Bacteria
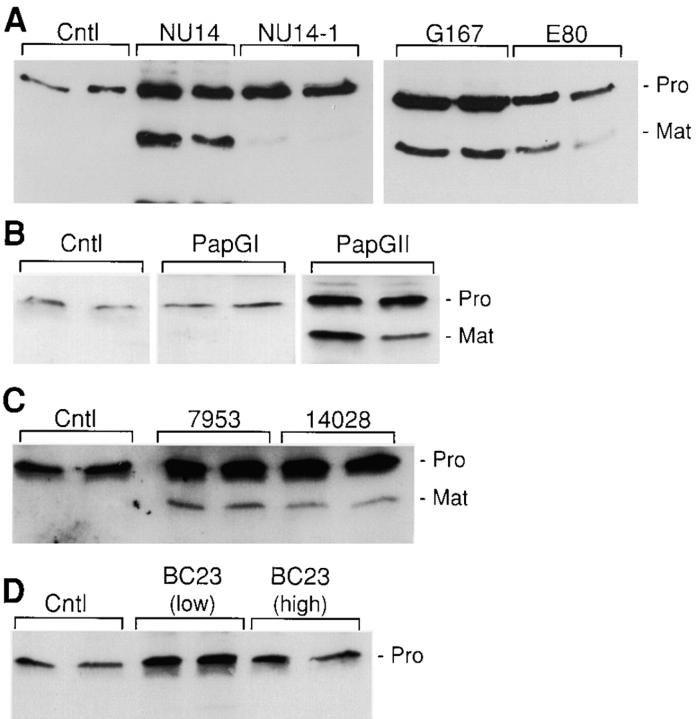
We assessed if other bacteria induce matrilysin expression. WiDr colon carcinoma cells were infected with clinical isolates of uropathogenic E. coli grown under conditions that favor expression of type 1 pili (Langermann et al. 1997). Infection with the NU14 isolate strongly induced the expression and processing of matrilysin, whereas the isogenic FimH-deficient strain, NU14-1, had a much reduced effect (Fig. 5 A). Demonstrating further that FimH-expressing strains induce matrilysin expression in epithelial cells, infection with the isolates G167 and EC80, both of which express FimH-containing pili, also stimulated matrilysin release and activation (Fig. 5 A).
Figure 5.
Induction of matrilysin by adherent clinical isolates of E. coli and other bacteria. (A) WiDr human colon epithelial cells were infected for 60 min at a ratio of 300 bacteria per epithelial cell. The bacteria used were the type 1–piliated strains NU14, the isogenic FimH-deficient strain NU14-1, and the isolates G167 and EC80. (B) HT29 cells were infected with E. coli expressing type P pili and either the papGI or papGII adhesin. (C) HT29 cells were infected with S. typhimurium strains 14028s and ms7953s. (D) HT29 cells were infected with B. pertussis BC23 at two different ratios of bacteria to epithelial cell: 50:1 (low) and 500:1 (high). Western blotting was done with media collected 48 h after infection.
To assess if piliated bacteria containing other adhesins induce matrilysin expression, we infected colon cells with the E. coli expressing type P pili, which contains PapG adhesins (Kuehn et al. 1992). Infection with the bacteria expressing the PapGI adhesin had no effect on matrilysin production, whereas bacteria expressing the PapGII adhesin stimulated matrilysin release similar to FimH bacteria (Fig. 5 B). Avirulent (ms7953s) and virulent (14028s) strains of S. typhimurium, which can adhere quite efficiently to human colon cells (Finlay and Falkow 1990), also stimulated matrilysin expression and activation in HT-29 human colon carcinoma cells (Fig. 5 C), although at a lower level than seen with some of the E. coli strains. Infection of colon epithelial cells with B. pertussis BC23 at two different multiplicities of infection did not affect matrilysin expression (Fig. 5 D).
Cell Specificity
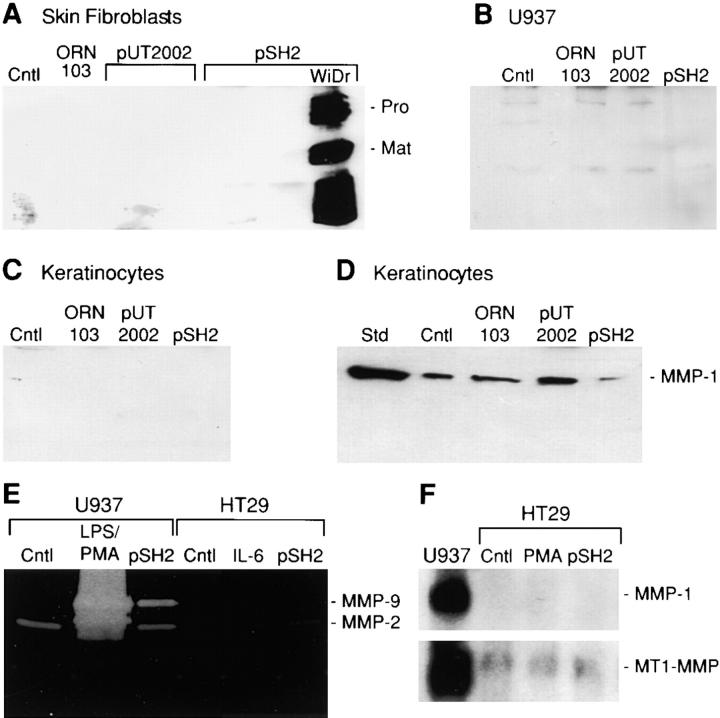
Matrilysin is predominantly expressed by mucosal epithelial cells in normal and injured tissues (Saarialho-Kere et al. 1995; Wilson et al. 1995; Dunsmore et al. 1998), as well as by tumor cells of epithelial origin (McDonnell et al. 1991; Newell et al. 1994; Saarialho-Kere et al. 1996; Wilson et al. 1997). To investigate if acute bacterial exposure stimulates matrilysin expression in other cell types, we infected normal human skin fibroblasts, basal and differentiated U937 monocytic-like cells, and primary human keratinocytes with type 1–piliated E. coli strains. Each of these cell types produces a variety of MMPs, however, matrilysin was not detected in any of these cells after infection with parental or type 1–piliated bacteria (Fig. 6, A–C). In contrast, a predictably strong matrilysin response was observed with WiDr colon epithelial cells exposed to FimH-expressing E coli.
Figure 6.
Cell type specificity of bacterial induction of matrilysin and lack of induction of other MMPs in epithelial cells. Human skin fibroblasts (A), U937 human monocytic-like cells (B), and primary human keratinocytes (C) were infected with the parental strain ORN103 and the type 1–piliated strains ORN103/pUT2002 (type 1+/fimH −) and ORN103/pSH2 (type 1+/fimH +). Western blotting for matrilysin (A, B, and C; MMP-7) and collagenase-1 (MMP-1; D) was performed on conditioned media collected 48 h after infection. WiDr human colon epithelial cells infected with ORN103/pSH2 were included as positive controls (A). (Cntl) Uninfected cells; and (Std) purified human collagenase-1. (E) Gelatin zymography was performed on serum-free conditioned media samples from infected U937 and HT29 cells. Zones of substrate clearing correspond to the activities of gelatinase-A (MMP-2) and gelatinase-B (MMP-9). A strong induction of gelatinase-B was observed in U937 cells treated for 24 h with 1 μg/ml E. coli LPS and 80 nM PMA. No gelatinolytic activity was seen in gels incubated in the presence of 50 mM EDTA (data not shown). (F) Northern blot analysis for collagenase-1 (MMP-1) and MT1-MMP mRNAs was performed using total RNA isolated from HT29 cells at 6 h after infection. RNA from U937 cells stimulated with LPS and PMA was used as a positive control for the expression of these MMPs. Ethidium bromide staining verified equal loading among lanes before and after transfer.
Furthermore, bacteria did not influence the production of other MMPs by epithelial cells. The expression of collagenase-1 (MMP-1) by primary keratinocytes was not altered by bacterial exposure (Fig. 6 D). As determined by substrate zymography, gelatinase-B (MMP-9) was not released by HT-29 cells (Fig. 6 E). In contrast, acute bacterial infection stimulated slightly (about fivefold) the release of gelatinase-B by U937 cells, but still at levels that were markedly less than those stimulated by PMA/LPS treatment (Fig. 6 E). Production of gelatinase-A (MMP-2) by U937 or HT-29 cells was not affected by bacteria (Fig. 6 E). Similarly, bacterial infection did not induce expression of stromelysin-1 (data not shown) or collagenase-1 (MMP-1) or stimulate the low levels of MT1-MMP mRNA in colon carcinoma cells (Fig. 6 F).
Ex Vivo and In Vivo Stimulation
To assess if bacteria regulate matrilysin expression in a complex tissue environment, we infected freshly isolated pieces of normal adult human trachea. Tracheal explants were exposed to type 1–piliated bacteria for 90 min, and the expression of matrilysin was determined by immunoblotting 24 h later. A low level of promatrilysin was detected in the medium from noninfected explants (Fig. 7 A). Exposure to fimH + bacteria (NU14) mediated a significant increase in matrilysin release and activation, whereas the fimH − mutant bacteria caused a noticeable but reduced stimulation (Fig. 7 A). Similarly, infection with piliated E. coli strains induced matrilysin expression in primary human tracheal epithelial cells (Fig. 7 B). Matrilysin was not produced by control tracheal epithelial cells (Fig. 7 B), even though these cells produce the enzyme in intact, normal tissue (Fig. 7 A; Dunsmore et al. 1998). Because the cells were isolated and grown under sterile conditions, the absence of bacteria may have eliminated the agent that mediates gene expression in vivo.
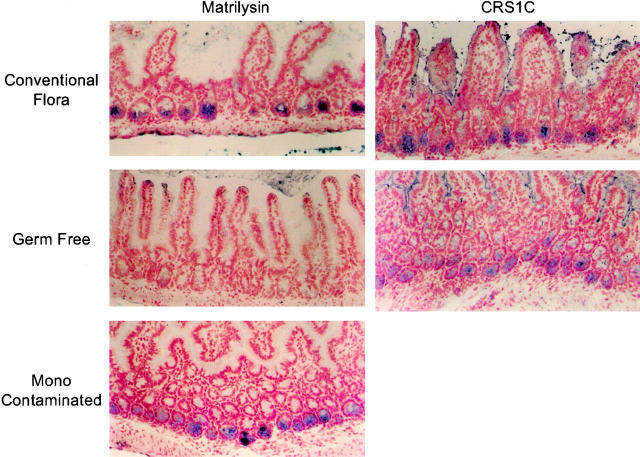
in vivo, matrilysin is produced in many epithelia, possibly because of prior or continued contact with pathogenic or commensal bacteria. In the postnatal mouse, matrilysin is prominently expressed by the epithelial Paneth cells located at the base of the small intestinal crypts (Fig. 8; Wilson et al. 1995). To assess if the expression of matrilysin by Paneth cells is regulated by bacterial exposure, we compared the production of this MMP in the small intestine of germ-free and ex-germ-free mice belonging to the NMRI inbred strain (Bry et al. 1996). Matrilysin protein was not detected in the Paneth cells of germ-free mice lacking an intestinal microflora (Fig. 8), but it was induced within 10 d after monocontamination with the commensal species B. thetaiotaomicron (Fig. 8). In contrast, expression of the α-defensin peptide proCRS1C, a marker of Paneth cell differentiation (Ouellette et al. 1994), was not affected (Fig. 8). These in vivo findings suggest that bacterial exposure is a key, physiologically relevant regulator of matrilysin expression.
Figure 8.
Bacterial-mediated induction of matrilysin in mouse small intestine. Immunostaining for mouse matrilysin and proCRS1C was done on frozen sections of the small intestine prepared from conventionally housed, germ-free, and ex-germ-free NMRI mice. Positive staining for matrilysin (blue) was seen in the Paneth cells located at the base of the intestinal crypts in mice with a normal intestinal microflora but was not detected in the small intestine of germ-free mice. Paneth cell expression of matrilysin was induced within 10 d after contamination with B. thetaiotaomicron. No changes in the staining pattern or intensity of procryptdin-related sequences (proCRS1C), a marker of Paneth cell differentiation, were seen in any samples studied.
Antimicrobial Activity
Human epithelial cells produce a variety of antimicrobial molecules, including defensins and cathelicidins (Zanetti et al. 1995; Stolzenberg et al. 1997), and we have recently reported that matrilysin is needed to activate murine intestinal α-defensins (Wilson et al. 1999). On its own, matrilysin has no bacteriostatic or bacteriocidal properties (Wilson et al. 1999). We assessed if bacterial-mediated induction and activation of matrilysin correlated with the generation of defensin-like bacteriocidal activity. Media collected from control and infected HT29 human colon cells were incubated with E. coli DH5α and with two strains of Salmonella typhimurium, ms7953s and 14028s. Conditioned media from infected HT29 cells caused a marked decrease (∼10,000-fold at a 1:5 dilution) in the number of viable bacteria of all three strains (Fig. 9 A, filled symbols), whereas conditioned media from noninfected epithelial cells had no effect on bacterial survival (Fig. 9 A, open symbols). Bacteriocidal activity was time- and dose-dependent, and essentially the same results were obtained using logarithmic or stationary growth phase bacteria (Fig. 9 A and data not shown).
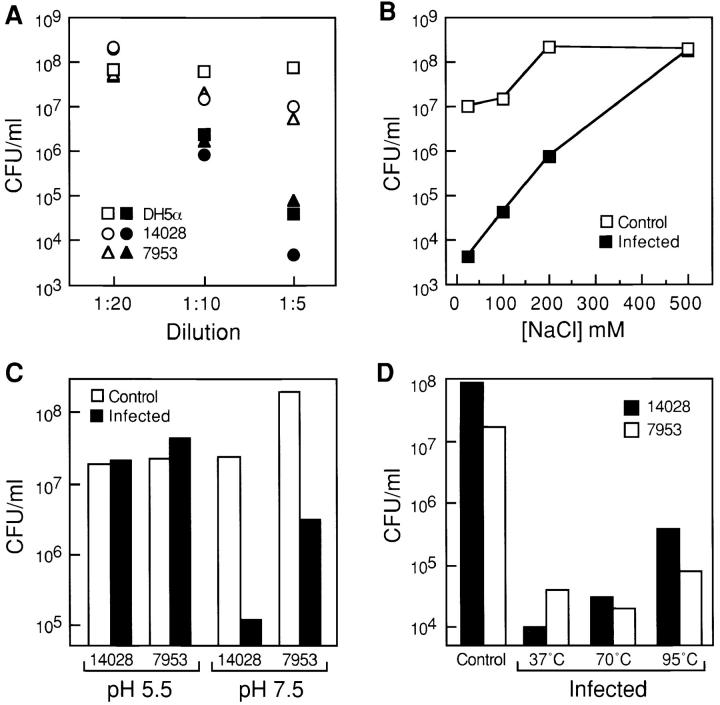
Figure 9.
Characterization of bacteriocidal activity in the epithelial cell conditioned media. (A) E. coli DH5α and S. typhimurium strains 14028s and ms7953s were incubated for 3 h at 37°C in the presence of different dilutions of conditioned medium from HT29 human colon epithelial cells, which were previously infected with the ORN103/pSH2 (type 1+/fimH +) strain (solid symbols), and from uninfected HT29 cells (open symbols). The number of colony forming units (CFU) was determined by plating serial dilutions of the cultures. The input number of bacteria was 2 × 107 (DH5α), 2 × 106 (14028s), and 106 (ms7953s) CFU/ml, respectively. (B) DH5α bacteria (2 × 106 CFU/ml) were incubated for 3 h at 37°C in a 1:5 dilution of conditioned medium from bacterially infected or control HT29 cells containing increasing concentrations of NaCl. Serial dilutions were plated, and the number of colonies was determined after an overnight incubation at 37°C. (C) S. typhimurium 14028s (107 CFU/ml) and ms7953s (2.6 × 107 CFU/ml) cells were incubated in the presence of a 1:5 dilution of conditioned medium from bacterially infected HT29 cells at pH 7.5 (50 mM phosphate buffer) or pH 5.5 (50 mM acetate buffer). (D) S. typhimurium 14028s and ms7953s cells were incubated with a 1:5 dilution of HT29 conditioned medium that was preincubated at 70°C for 30 min or at 95°C for 5 min.
Antimicrobial activity decreased as the NaCl concentration was increased, and was essentially abolished at NaCl concentrations over 200 mM (Fig. 9 B). In addition, bacteriocidal activity was reduced at low pH, but was present at physiologic pH (Fig. 9 C). In contrast, the temperature did not markedly affect the bacteriocidal activity. Preincubation of conditioned media from bacterially infected cells at 70°C for 30 min did not result in a significant loss of the antimicrobial activity and preincubating at 95°C blocked <50% of the activity (Fig. 9 D). The biochemical properties of the antimicrobial activity in conditioned medium, namely sensitivity to salt and pH and resistance to temperature, are characteristic features of activated defensin peptides (Bals et al. 1998a,Bals et al. 1998b; Porter et al. 1997).
Discussion
Our data demonstrate that expression of matrilysin, a member of the MMP family, is strongly induced in human and murine mucosal epithelial tissues, cells, and cell lines by bacterial exposure. This induction was remarkably potent and sensitive, requiring relatively short exposure and <10 bacteria per epithelial cell in the initial inoculum (Fig. 2). Large amounts of matrilysin protein, in both its zymogen and activated forms, were released from infected cells, and strictly toward the apical surface (Fig. 1 D). Based on mRNA levels, matrilysin expression increased as early as 2 h after infection, and remained elevated at 24 h after infection (Fig. 1 E). In addition, bacteria-mediated stimulation of matrilysin was specific to mucosal epithelial cells, and expression of no other metalloproteinases examined was influenced by bacterial exposure (Fig. 6). Along with several other studies on the production of cytokines and chemokines and on changes in cytoplasmic signaling and cytoarchitecture (Finlay and Cossart 1997), our findings indicate that epithelial cells are well tuned to respond to bacterial infection.
In this work, we used E. coli bacteria producing type 1 pili and containing the FimH adhesin as a paradigm of bacterial adherence to host cells and tissues. The binding of molecules such as adhesins to specific receptors on host target cells constitutes a critical step in the establishment of bacterial infection and virulence (Hultgren et al. 1993). Many pathogenic bacteria invade cells in tissue culture, as shown by the ability of the internalized bacteria to survive the antibiotic gentamicin (Miller 1995). However, we observed only negligible rates of bacterial invasion in colon epithelial cells under our experimental conditions, even for the more adherent E. coli strains. In addition, pretreatment of epithelial cells with cytochalasin D, which blocks bacterial invasion by disrupting the host cell actin cytoskeleton, had no effect on the induction of matrilysin (data not shown). Thus, bacterial invasion clearly was not required for induction of matrilysin expression.
Bacteria could initiate matrilysin expression by adhesion to a host receptor, by a soluble factor acting on host cells, or by a combination of these two mechanisms. Several observations indicate that a bacterially derived soluble factor may be the predominant means by which matrilysin is regulated. First, although mannose markedly attenuated induction by fimH + E. coli, this sugar did not affect the stimulation of matrilysin mediated by the nonadherent strains (Fig. 3). Similarly, NU14-1, the fimH − mutant of clinical isolate NU14, stimulated matrilysin expression but not as greatly as did the adherent fimH + strain (Fig. 5). Second, matrilysin was not detected in intestinal Paneth cells of germ-free mice, but it was induced after inoculation with B. thetaiotaomicron (Fig. 8). This normal inhabitant of the murine and human distal intestine does not appear to colonize the crypts of Lieberkühn (Garabedian et al. 1997; Falk et al. 1998). These in vivo results suggest that a factor or factors released by Bacteroides into the intestinal lumen influenced matrilysin gene expression in Paneth cells at the bottom of the crypts. Third, and most compelling, bacterially conditioned broth induced expression of matrilysin (Fig. 4). Thus, our results indicate strongly that a soluble factor or factors released by bacteria regulate matrilysin expression. Similarly, several bacterial factors, or modulins, stimulate host cell cytokine production and other effects (Henderson et al. 1996; Wilson et al. 1998). Although the nature of this putative modulin(s) is currently unknown, it is probably unrelated to bacterial lipopolysaccharide. After all, LPS from several different strains did not affect matrilysin expression, and polymyxin B did not block induction by conditioned medium (Table and Fig. 4).
Although our data argue in favor of a soluble factor (modulin) controlling matrilysin expression, they do not diminish the importance of bacterial adhesion. Clearly, the most potent stimulation and induction of matrilysin were obtained with epithelial cells exposed to adherent bacteria (Fig. 1, Fig. 3, and Fig. 5). bacterial adhesion could provide a mechanism to effectively increase the concentration of a soluble factor at the host cell surface. Furthermore, because adhesion mediates new gene expression in bacteria (Falkow 1997), additional modulins could be produced once a bacterium contacts a host cell. Together, our data suggest that both bacterial adhesion and soluble factors are at play in the regulation of matrilysin, and synergy between two different mechanisms could be responsible for the higher levels of induction and activation observed in cells exposed to adherent bacteria.
As with all metalloproteinases, matrilysin is produced as an inactive zymogen, and bacterial adhesion may also be involved in regulating the activation of promatrilysin. In our experiments, we detected an abundance of activated (19 kD) matrilysin in the medium of cells exposed to adherent bacteria (Fig. 1, Fig. 3, and Fig. 5). Typically, but not always, only promatrilysin (28 kD) was released by cells exposed to nonadherent mutant strains (Fig. 1 and Fig. 5 A) or to bacterial conditioned culture broth (Fig. 4). Because bacteria were removed or killed and the medium was changed before the start of the 48-h conditioning period, cleavage of promatrilysin was not mediated by a bacterial product. Still, activation of an epithelial cleavage mechanism may require bacterial adhesion or, at least, exposure. For example, the presence of activated matrilysin released from human tracheal explants exposed to either fimH − or fimH + bacteria (Fig. 7) supports this idea.
we recently reported that nearly equal amounts of both pro and active matrilysin are stored in Paneth cells granules in intact small intestine (Wilson et al. 1999). In addition, about half of the Paneth cell granule defensins, which are substrates for matrilysin, were also stored in a cleaved, activated state (Wilson et al. 1999). These findings provide in vivo evidence indicating that proteolytic processing of matrilysin is required for catalytic activity. Thus, the activation we report here may represent a physiologically relevant process. However, promatrilysin has a propensity to autoactivate and degrade itself, in a concentration-dependent manner. Thus, the presence of activated and degraded matrilysin seen in our immunoblots could be products of autolysis expedited by the high levels of enzyme released in response to adherent bacteria.
The expression of matrilysin in noninjured, normal epithelium suggests that this enzyme serves a common homeostatic function among epithelia. In intact epithelium and in polarized monolayers, matrilysin is preferentially secreted to the apical surface (Wilson et al. 1997; Dunsmore et al. 1998). As we show here (Fig. 1 D), bacterial-stimulated matrilysin is released to the apical surface. In contrast, matrilysin is released toward the basal compartment by epithelium migrating over injured airway and by phorbol ester–stimulated pneumocytes (Dunsmore et al. 1998). These observations indicate that the vectorial secretion of matrilysin is regulated in response to different events and stimuli, and suggest that the proteinase may serve different roles at the lumenal and basal surfaces.
Secretory epithelia, such as upper airway and sweat and salivary glands, that express matrilysin open to the outside environment and, hence, would eventually be exposed to microorganisms. Thus, the widespread expression of matrilysin in glandular exocrine epithelium may have been initially induced and subsequently sustained by continual, low level bacterial exposure. Consistent with this idea, matrilysin is seen in adult tissues, but is not detected in developed human sweat glands in utero (Karelina et al. 1994) or in fetal mouse tissues (Wilson et al. 1995). Our in vivo observations that matrilysin protein is not produced in adult germ-free mice but is detected in mice with a conventional microflora (Fig. 8) directly support the concept that bacterial exposure regulates the expression of this proteinase. As we show here (Fig. 9), both matrilysin and defensin-like activity, which are functionally related (see below), are stimulated in response to challenge by pathogenic bacteria, suggesting common regulatory control of mucosal defense components in differentiated cells.
The levels of matrilysin produced after bacterial infection were considerably higher than those detected after treatment with TNFα, IL-1β, and other cytokines or chemokines that stimulate matrilysin in epithelial cells (Klein et al. 1997). Many of these agents, such as TNFα and several interleukins, which stimulated matrilysin expression, are themselves upregulated in epithelial cells in response to bacterial infection (Jung et al. 1995). A primary function of these factors in response to challenge by pathogenic bacteria is thought to be their ability to signal leukocyte recruitment to the site of infection. In contrast, other host proteins, such as antimicrobial peptides, which are upregulated or released in response to infection, function to directly counter bacterial exposure. Thus, the presence of commensal bacteria or transient, low level exposure to pathogenic bacteria may induce a noninflammatory response, possibly reflected by the constitutive expression of matrilysin in healthy, noninflamed tissues. In contrast, infection and colonization with pathogenic bacteria mediates expression of proinflammatory cytokines and chemokines, and stimulation or upregulation of matrilysin may reflect an enhanced capacity for mucosal defense.
In accordance with the suggested role of epithelial cells as sensors for microbial infection (Kagnoff and Eckmann 1997), stimulation of matrilysin expression may be part of a more general activation response to bacteria. It is tempting to speculate that the expression of matrilysin in epithelial cells, along with an ever increasing display of epithelial defense molecules, may play a direct role in the first line of defense against infectious microorganisms. In contrast, other MMPs, such as collagenases and stromelysin-1, whose expression can be stimulated by inflammation (Matrisian 1992) and specific products of pathogenic bacteria (DeCarlo et al. 1998), may participate in localized tissue destruction, such as in periodontal disease (Birkedal-Hansen 1993). a growing body of evidence suggests that most epithelial cells can produce molecules with antimicrobial properties (McCray and Bentley 1997; Stolzenberg et al. 1997; Bals et al. 1998c; Krisanaprakornkit et al. 1998), many of which, like matrilysin, are upregulated by bacterial infection (Tarver et al. 1998). Indeed, defensins, cathelicidins, and others are produced as propeptides that require some degree of proteolytic processing to attain full antimicrobial activity (Lehrer and Ganz 1996).
Our work demonstrates that bacterial infection of epithelial cells results in the generation of a bacteriocidal activity against different species of gram negative bacteria concomitantly with the induction of the expression and activation of matrilysin (Fig. 9). Because matrilysin does not have a direct bacteriocidal effect, we hypothesize that this enzyme modulates the activity of defense-related molecules. Indeed, we have recently demonstrated that cryptdins (enteric α-defensins), a group of small cationic peptides with broad antimicrobial activity (Ouellette et al. 1994; Ouellette and Selsted 1996), are processed to their active forms by matrilysin and that a deficiency in this enzyme results in impaired bacteriocidal activity in vivo (Wilson et al. 1999). We speculate that matrilysin may play a similar role in other epithelia, thus, contributing to mechanisms of innate host defense at mucosal surfaces.
Acknowledgments
The authors thank Dr. Howard Welgus for providing the antimatrilysin antibodies, Dr. Jouko Lohi for many constructive discussions and the Northern analysis, Drs. William Goldman and Fred Heffron for supplying bacterial strains, Dr. Michael Caparon for his critical input and suggestions, Dr. Matthew Mulvey for many helpful suggestions, Dr. Dwight Look for providing human primary tracheal cells, Dr. Andre Ouellette for providing the proCRS1C antibody, Ms. Jill Roby for the immunohistochemistry data, and Ms. Heidi Haagen for technical assistance.
This work was supported by the National Institutes of Health grants HL55479 and HL29594 (to W.C. Parks) and DK51406 and AI29549 (S.J. Hultgren).
Footnotes
Abbreviations used in this paper: LPS, lipopolysaccharide; MMP, matrix metalloproteinase.
References
- Bals R., Goldman M.J., Wilson J.M. Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract Infect. Immun. 66 1998. 1225 1232a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R., Wang X., Wu Z., Freeman T., Bafna V., Zasloff M., Wilson J.M. Human β-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung J. Clin. Invest. 102 1998. 874 880b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R., Wang X., Zasloff M., Wilson J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface Proc. Natl. Acad. Sci. USA 95 1998. 9541 9546c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J. Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W.G.I., Bodden M.K., Windsor L.J., Birkedal-Hansen B., DeCarlo A., Engler J.A. Matrix metalloproteinasesa review. Crit. Rev. Oral Biol. Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Bry L., Falk P.G., Midvedt T., Gordon J.I. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273:1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- Busiek D.F., Ross F.P., McDonnell S., Murphy G., Matrisian L.M., Welgus H.G. The matrix metalloprotease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J. Biol. Chem. 1992;13:9087–9092. [PubMed] [Google Scholar]
- DeCarlo A.A., Grenett H.E., Harber G.J., Windsor L.K., Bodden M.K., Birkedal-Hansen B., Birkedal-Hansen H. Induction of matrix metalloproteinases and collagen-degrading phenotype in fibroblasts and epithelial cells by secreted Porphyromonas givgivalis proteinase. J. Periodont. Res. 1998;33:408–420. doi: 10.1111/j.1600-0765.1998.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Dunsmore S.E., Saarialho-Kere U.K., Roby J.D., Wilson C.L., Matrisian L.M., Welgus H.G., Parks W.C. Matrilysin function and expression in airway epithelium. J. Clin. Invest. 1998;102:1321–1331. doi: 10.1172/JCI1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk P.G., Hooper L.V., Midtvedt T., Gordon J.I. Creating and maintaining the gastrointestinal ecosystemwhat we know and need to know from gnotobiology. Microbiol. Mol. Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S. Invasion and intracellular sorting of bacteriasearching for bacterial genes expressed during host-pathogen interactions. J. Clin. Invest. 1997;100:239–243. doi: 10.1172/JCI119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P.I., Groisman E.A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Finlay B.B., Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J. Infect. Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- Finlay B.B., Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- Firon N., Ashkenazi S., Mirelman D., Ofek I., Sharon N. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect. Immun. 1987;55:472–476. doi: 10.1128/iai.55.2.472-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian E.M., Roberts L.J.J., McNevin M.S., Gordon J.I. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J. Biol. Chem. 1997;272:23729–23740. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- Halpert I., Roby J.D., Sires U.I., Potter-Perigo S., Wight T.N., Welgus H.G., Shapiro S.D., Wickline S.A., Parks W.C. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc. Natl. Acad. Sci. USA. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Poole S., Wilson M. Bacterial modulinsa novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol. Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S.J., Abraham S., Caparon M., Falk P., St J.W., Geme, Normark S. Pilus and nonpilus bacterial adhesinsassembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- Jones C.H., Pinkner J.S., Roth R., Heuser J., Nicholes A.V., Abraham S.N., Hultgren S.J. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae . Proc. Natl. Acad. Sci. USA. 1995;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.C., Eckmann L., Yang S.K., Panja A., Fierer J., Morzycka-Wroblewska E., Kagnoff M.F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M.F., Eckmann L. Epithelial cells as sensors for microbial infection. J. Clin. Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina T.V., Goldberg G.I., Eisen A.Z. Matrilysin (PUMP) correlates with dermal invasion during appendageal development and cutaneous neoplasia. J. Invest. Dermatol. 1994;103:482–487. doi: 10.1111/1523-1747.ep12395596. [DOI] [PubMed] [Google Scholar]
- Klein R.D., Borchers A.H., Sundareshan A.H., Bougelet P.C., Berkman M.R., Nagle R.B., Bowden G.T. Interleukin-1β secreted from monocytic cells induces the expression of matrilysin in the prostatic cell line LNCaP. J. Biol. Chem. 1997;272:14188–14192. doi: 10.1074/jbc.272.22.14188. [DOI] [PubMed] [Google Scholar]
- Krisanaprakornkit S., Weinberg A., Perez C.N., Dale B.A. Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect. Immun. 1998;66:4222–4228. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn M.J., Heuser J., Normark S., Hultgren S.J. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature. 1992;356:252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- Langermann S., Palaszynski S., Barnhart M., Auguste G., Pinkner J.S., Burlein J., Barren P., Koenig S., Leath S., Jones C.H., Hultgren S.J. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- Lehrer R.I., Ganz T. Endogenous vertebrate antibiotics. Defensins, protegrins, and other cysteine-rich antimicrobial peptides. Ann. NY Acad. Sci. 1996;797:228–239. doi: 10.1111/j.1749-6632.1996.tb52963.x. [DOI] [PubMed] [Google Scholar]
- Matrisian L.M. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- McCray P.B., Jr., Bentley L. Human airway epithelia express a beta-defensin. Am. J. Respir. Cell. Mol. Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- McDonnell S., Navre M., Coffrey R.J., Matrisian L.M. Expression and localization of the matrix metalloproteinase Pump-1 (MMP-7) in human gastric and colon carcinomas. Mol. Carcinogenesis. 1991;4:527–533. doi: 10.1002/mc.2940040617. [DOI] [PubMed] [Google Scholar]
- Miller V.L. Tissue-culture invasionfact or artifact? Trends Microbiol. 1995;3:69–71. doi: 10.1016/s0966-842x(00)88878-2. [DOI] [PubMed] [Google Scholar]
- Minion F.C., Abraham S.N., Beachey E.H., Goguen J.D. The genetic determinant of adhesive function in type 1 fimbriae of Escherichia coli is distinct from the gene encoding the fimbrial subunit. J. Bacteriol. 1986;165:1033–1036. doi: 10.1128/jb.165.3.1033-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M.A., López-Boado Y.S., Wilson C.L., Roth R., Parks W.C., Heuser J., Hultgren S.J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli . Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M.I., Ward R.V., Docherty A.J.P. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 75 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP) Biochem. J. 1991;277:277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell K.J., Witty J.P., Rodgers W.H., Matrisian L.M. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol. Carcinog. 1994;10:199–206. doi: 10.1002/mc.2940100404. [DOI] [PubMed] [Google Scholar]
- Orndorff P.E. Genetic study of piliation in Escherichia coliimplications for understanding microbe-host interactions at the molecular level. Pathol. Immunopathol. Res. 1987;6:82–92. doi: 10.1159/000157050. [DOI] [PubMed] [Google Scholar]
- Ouellette A.J. Paneth cells and innate immunity in the crypt microenvironment. Gastroenterology. 1997;113:1779–1784. doi: 10.1053/gast.1997.v113.pm9352884. [DOI] [PubMed] [Google Scholar]
- Ouellette A.J., Selsted M.E. Paneth cell defensinsendogenous peptide components of intestinal host defense. FASEB (Fed. Am. Soc. Exp. Biol.) J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- Ouellette A.J., Hsieh M.M., Nosek M.T., Cano-Gauci D.F., Huttner K.M., Buick R.N., Selsted M.E. Mouse Paneth cell defensinsprimary structures and antibacterial activities of numerous cryptdin isoforms. Infect. Immun. 1994;62:5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter E.M., van Dam E., Valore E.V., Ganz T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect. Immun. 1997;65:2396–2401. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W.H., Matrisian L.M., Giudice L.C., Dsupin B., Cannon P., Svitek C., Gorstein F., Osteen K.G. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J. Clin. Invest. 1994;94:946–953. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U.K., Crouch E.C., Parks W.C. Matrix metalloproteinase matrilysin is constitutively expressed in human exocrine epithelium. J. Invest. Dermatol. 1995;105:190–196. doi: 10.1111/1523-1747.ep12317104. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U.K., Vaalamo M., Karjalainen-Lindsberg M.-L., Airola K., Parks W.C., Puolakkainen P. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am. J. Pathol. 1996;148:519–526. [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U.K., Welgus H.G., Parks W.C. Divergent mechanisms regulate interstitial collagenase and 92 kDa gelatinase expression in human monocytic-like cells exposed to bacterial endotoxin. J. Biol. Chem. 1993;268:17354–17361. [PubMed] [Google Scholar]
- Sires U.I., Griffin G.L., Broekelmann T., Mecham R.P., Murphy G., Chung A.E., Welgus H.G., Senior R.M. Degradation of entactin by matrix metalloproteinases. Susceptibility to matrilysin and identification of cleavage sites. J. Biol. Chem. 1993;268:2069–2074. [PubMed] [Google Scholar]
- Sokurenko E.V., Chesnokova V., Doyle R.J., Hasty D.L. Diversity of the Escherichia coli type 1 fimbrial lectin. Differential binding to mannosides and uroepithelial cells. J. Biol. Chem. 1997;272:17880–17886. doi: 10.1074/jbc.272.28.17880. [DOI] [PubMed] [Google Scholar]
- Stolzenberg E.D., Anderson G.M., Ackermann M.R., Whitlock R.H., Zasloff M. Epithelial antibiotic induced in states of disease. Proc. Natl. Acad. Sci. USA. 1997;94:8686–8690. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbeck B.D., Parks W.C., Welgus H.G., Pentland A.P. Collagen-mediated induction of keratinocyte collagenase is mediated by tyrosine kinase and protein kinase C activities. J. Biol. Chem. 1994;269:30022–30029. [PubMed] [Google Scholar]
- Tarver A.P., Clark D.P., Diamond G., Russell J.P., Erdjument-Bromage H., Tempst P., Cohen K.S., Jones D.E., Sweeney R.W., Wines M., Hwang S., Bevins C.L. Enteric beta-defensinmolecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection. Infect. Immun. 1998;66:1045–1056. doi: 10.1128/iai.66.3.1045-1056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C.L., Heppner K.J., Rudolph L.A., Matrisian L.M. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol. Biol. Cell. 1995;6:851–869. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C.L., Heppner K.J., Labosky P.A., Hogan B.L.M., Matrisian L.M. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc. Natl. Acad. Sci. USA. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C.L., Ouellette A.J., Satchell D.P., Ayabe T., López-Boado Y.S., Stratman J.L., Hultgren S.J., Matrisian L.M., Parks W.C. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- Wilson M., Seymour R., Henderson B. Bacterial perturbation of cytokine networks. Infect. Immun. 1998;66:2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.J., Garcia B., Woodson M., Sinha A.A. Metalloproteinase activities expressed during development and maturation of the rat prostatic complex and seminal vesicles. Biol. Reprod. 1992;47:683–691. doi: 10.1095/biolreprod47.5.683. [DOI] [PubMed] [Google Scholar]
- Zanetti M., Gennaro R., Romeo D. Cathelicidinsa novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS (Fed. Eur. Biochem Soc.) Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]