Abstract
Neuropilin-1 is a type 1 membrane protein with three distinct functions. First, it can mediate cell adhesion via a heterophilic molecular interaction. Second, in neuronal cells, neuropilin-1 binds the class 3 semaphorins, which are neuronal chemorepellents, and plays a role in the directional guidance of axons. Neuropilin-1 is expected to form complexes with the plexinA subfamily members and mediate the semaphorin-elicited inhibitory signals into neurons. Third, in endothelial cells, neuropilin-1 binds a potent endothelial cell mitogen, vascular endothelial growth factor (VEGF)165, and regulates vessel formation. Though the binding sites in neuropilin-1 for the class 3 semaphorins and VEGF165 have been analyzed, the sites involved in cell adhesion activity of the molecule have not been identified. In this study, we produced a variety of mutant neuropilin-1s and tested their cell adhesion activity. We showed that the b1 and b2 domains within the extracellular segment of neuropilin-1 were required for the cell adhesion activity, and peptides with an 18–amino acid stretch in the b1 and b2 domains were sufficient to induce the cell adhesion activity. In addition, we demonstrated that the cell adhesion ligands for neuropilin-1 were proteins and distributed in embryonic mesenchymal cells but distinct from the class 3 semaphorins, VEGF, or plexins.
Keywords: neuropilin-1, cell adhesion, cell aggregation, semaphorins, mutant protein
Introduction
The identification and characterization of the cell surface receptor proteins that help to trigger the intercellular signals to regulate cell proliferation, differentiation, migration, or cell–cell contact are important if one is to understand the cellular and molecular mechanisms underlying the organization of multicellular tissues or organs.
Neuropilin-1 is a unique membrane protein that is highly conserved among various vertebrate species, including Xenopus frog (Takagi et al. 1987, Takagi et al. 1991), chicken (Takagi et al. 1995), mouse (Kawakami et al. 1996), rat (He and Tessier-Lavigne 1997; Kolodkin et al. 1997), and human (He and Tessier-Lavigne 1997; Kolodkin et al. 1997). Neuropilin-1 is expressed in a variety of neuronal and non-neuronal cells and can mediate at least three distinct intercellular signals to regulate diverse aspects of embryonic development.
First, neuropilin-1 functions as a cell adhesion receptor. When neuropilin-1 is expressed on cell surfaces of the fibroblast cell line, L cells, it interacts with some unknown ligand(s) on the surface of the cells and mediates cell–cell adhesion (Takagi et al. 1995).
Second, neuropilin-1 is expressed in particular classes of neurons (Takagi et al. 1987, Takagi et al. 1991, Takagi et al. 1995; Kawakami et al. 1996), and binds the class 3 semaphorins (He and Tessier-Lavigne 1997; Kolodkin et al. 1997), which are potent neuronal chemorepellents (Luo et al. 1993; Kolodkin et al. 1993; Fan and Raper 1995; Messersmith et al. 1995; Püschel et al. 1995). We have reported that, in neuropilin-1–deficient mutant mice produced by targeted disruption of the neuropilin-1 gene, neuropilin-1–deprived dorsal root ganglion neurons are protected from growth cone collapse elicited by semaphorin 3A (Sema3A/SEMA3A; previously, collapsin-1/semaphorin D/semaphorin III; Semaphorin Nomenclature Committee 1999), and their fibers were misguided (Kitsukawa et al. 1997). These results indicate that neuropilin-1 functions as a receptor or an indispensable component of receptor complex for the class 3 semaphorins to transduce semaphorin-elicited repulsive signals into growth cones (Chen et al. 1997; Feiner et al. 1997; He and Tessier-Lavigne 1997; Kolodkin et al. 1997). More recently, it has been shown that neuropilin-1 forms complexes with other neuronal membrane protein, plexins, and propagates Sema3A signals (Takahashi et al. 1999; Tamagnone et al. 1999).
Third, neuropilin-1 is expressed in endothelial cells (Kitsukawa et al. 1995), and binds an isoform of vascular endothelial growth factor (VEGF), VEGF165 (Soker et al. 1998), which is a major regulator of vasculo-angiogenesis (Ferrara and Henzel 1989; Keck et al. 1989; Leung et al. 1989). Overexpression of neuropilin-1 in mouse embryos resulted in an excess production of blood vessels and malformed hearts (Kitsukawa et al. 1995). In contrast, the neuropilin-1–deficient mouse embryos exhibited severe defects in embryonic vessel formation (Kawasaki et al. 1999). These findings indicate that neuropilin-1 interacts with VEGF165 and plays important roles in vasculo-angiogenesis.
The extracellular segment of neuropilin-1 has a domain combination different from that of any other cell surface receptor, consisting of five domains known as a1, a2, b1, b2, and c, each of which is shared by a wide variety of molecules (Takagi et al. 1991, Takagi et al. 1995; Kawakami et al. 1996). The a1/a2-like domains are shared by the complement components C1r and C1s (Leytus et al. 1986; Mackinnon et al. 1987), the human bone morphogenetic protein-1 (BMP-1; Wozney et al. 1988), the Drosophila dorsal-ventral patterning protein Tolloid (Shimell et al. 1991), and the choroid plexus protein p14 (Lecain et al. 1991). The b1/b2-like domains exist in the coagulation factors V and VIII (Toole et al. 1984; Jenny et al. 1987), a protein component of the milk fat globule membrane (MFGPs; Stubbs et al. 1990), and receptor tyrosine kinase DDR (the discoidin domain receptor; Johnson et al. 1993) and its rat homologue Ptk-3 (Sanchez et al. 1994). The central portion of the c domain designated as the MAM domain is contained in metalloendopeptidases meprins and the receptor protein tyrosine phosphatase (Beckmann and Bork 1993).
The multi-function and multi-domain structure of neuropilin-1 suggests that each function of neuropilin-1 maps to a distinct region within the extracellular segment of the protein. Previous studies have shown that the class 3 semaphorins can bind to the a1/a2 and b1/b2 domains (Giger et al. 1998; Nakamura et al. 1998), and VEGF165 to the b1/b2 domains (Giger et al. 1998). However, the sites that are involved in cell adhesion have not been determined.
Here, we produced cell lines expressing mutant neuropilin-1s in which the extracellular domains had been deleted in various combinations, and tested their cell adhesion activity. Through the analyses, we found that the b1 and b2 domains were essential for the cell adhesion activity of neuropilin-1. And then we produced a variety of recombinant proteins for the b1 and b2 domains, tested their cell adhesion activity, and determined the sites with an 18–amino acid stretch that were sufficient for the cell adhesion activity of neuropilin-1. We showed that cell adhesion ligands for neuropilin-1 were proteins and distributed in embryonic mesenchymal cells. In addition, we tested whether the class 3 semaphorins (SEMA3A, Sema3B, and Sema3C), VEGF165, and the plexinA subfamily members (plexinA1, plexinA2, and plexinA3) can bind to the cell adhesion sites of neuropilin-1 or compete the neuropilin-1–mediated cell adhesion, and obtained a conclusion that these semaphorins, VEGF, or plexins were not the cell adhesion ligands for neuropilin-1.
Materials and Methods
Cell Culture
L cells and embryonic cells (see below) were cultured in a medium consisting of 45% DME, 45% Ham F12 medium (F12), 10% FCS, and antibiotics. COS-7, KB, p19, NIH3T3, and HEK293T cells were cultured in DME containing 10% FCS. Trunk mesenchyme of E13 mouse embryos was treated with 0.1% trypsin in Ca2+- and Mg2+-free Puck's saline G (CMF) containing 1 mM EDTA (CMFE), and dispersed into single cells.
Expression of Mutant Neuropilin-1 Proteins in L Cells
Truncated mouse neuropilin-1 cDNAs that lack regions encoding the a1-a2 domains (amino acid [aa] 22–271), a1-b2 domains (aa 22–587), b1-b2 domains (aa 272–587), c domain (aa 588–811), or b1-c domains (aa 272–811) (see Fig. 1 A and 2 A) were constructed as follows. The cDNA fragments upstream and downstream of the domains that would be deleted were amplified by PCR and an EcoRI site added to the 3′-end of the upstream and 5′-end of the downstream sequences. After being checked for PCR errors these sequences were ligated at the EcoRI site and inserted into the eukaryotic expression vector Miw (Suemori et al. 1990). As a result of the ligation, the deleted domains were replaced by two amino acids, glutamic acid and phenylalanine, which were translated from the EcoRI sequence GAATTC. To construct NP-abcp (see Fig. 1 A), the cDNA encoding the 1205–1279 aa of Xenopus plexin (aa 1230–1257, the transmembrane domain; see Ohta et al. 1995) was amplified by PCR and replaced with the transmembrane-cytoplasmic region of neuropilin-1. The myc tag sequence GGEQKLISEEDL in the NP-abcm, NP-abm and NP-am constructs (see Fig. 2 A) was introduced as follows. An XbaI site was added to the 3′-end of the coding region of neuropilin-1 by PCR, and then the XbaI-myc tag-stop codon adapter was ligated. In all mutant neuropilin-1 cDNAs, the signal sequence was retained intact. Construction of the vector for the full-length neuropilin-1 was reported elsewhere (Kawakami et al. 1996). To isolate cells that stably express truncated neuropilin-1, L cells, a mouse fibroblastic cell line, were cotransfected with the truncated neuropilin-1 cDNAs and pST-neoB (Katoh et al. 1987) according to the calcium phosphate method (Chen and Okayama 1987) and selected with GENETICIN (GIBCO-BRL). The sequence data for mouse neuropilin-1 and Xenopus plexin are available from GenBank/EMBL/DDBJ under the accession numbers D50086 and D38175, respectively.
Figure 1.
Cell adhesion activity of transfectants which express mutant neuropilin-1 proteins (Part 1). (A) A schematic representation of mutant neuropilin-1s. NP-full, intact neuropilin-1; NP-bc, neuropilin-1 lacking the a1 and a2 domains; NP-c, neuropilin-1 protein lacking the a1, a2, b1, and b2 domains; NP-abcp, neuropilin-1 whose transmembrane and cytoplasmic regions are replaced by that of the Xenopus plexin. (B) Immunoblot of transfectants expressing intact and mutant neuropilin-1 proteins, by using an antibody raised against the b1-c domains. The number followed by the name of the construct represents the clone number. (C) Quantification of cell aggregation activity of the mutant neuropilin-1s. The degree of aggregation of transfectants is expressed by the index Nt/N0, where Nt and N0 are the total particle number at incubation times t and 0, respectively. White and black bars represent N30/N0 and N60/N0, respectively. (D–F) Cell aggregation at 60 min in gyration, detected by phase contrast (D and E) or fluorescence microscope (F). Parental L cells do not show cell aggregability (D). In contrast, fluorescein-labeled parental L cells and transfectants expressing NP-full 86 form mixed cell aggregates (E and F). E and F show the same field. Bar, 100 μm.
Figure 2.
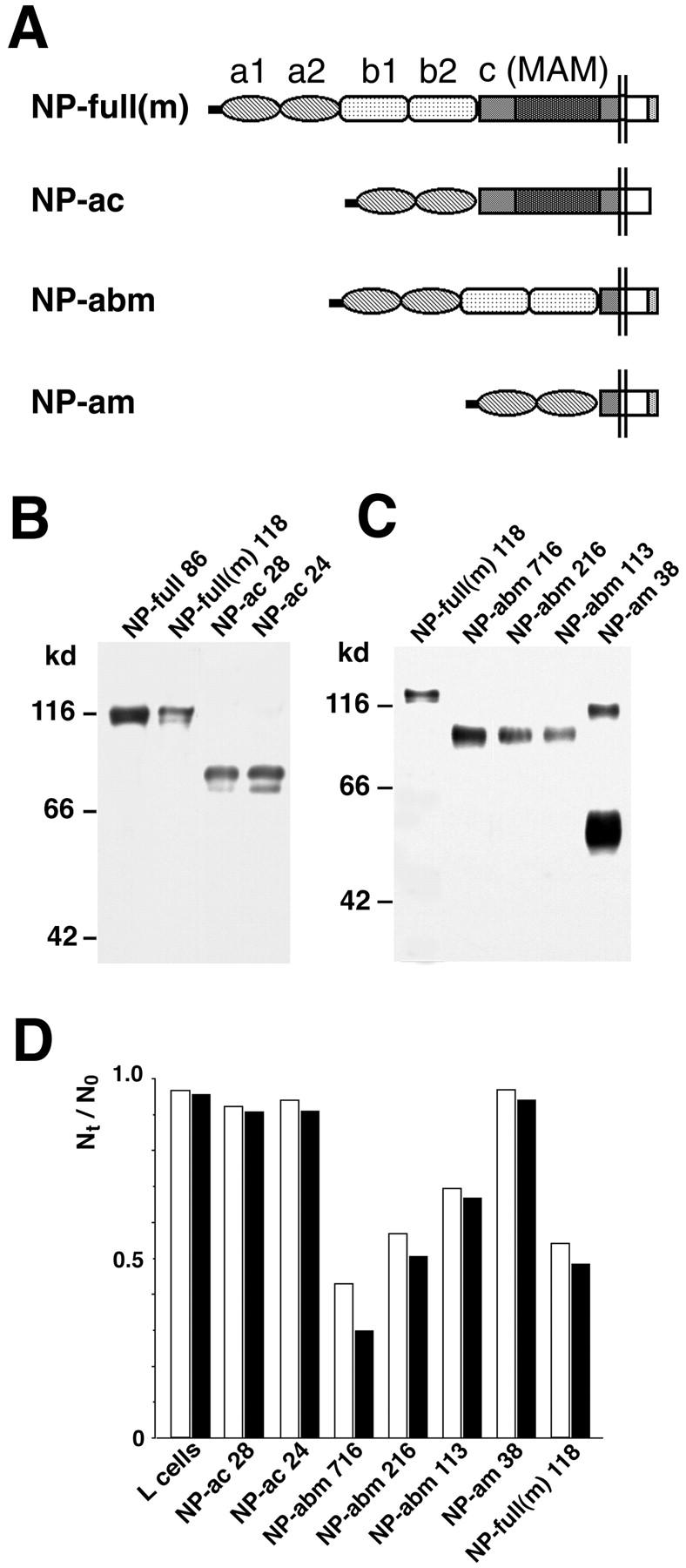
Cell adhesion activity of transfectants which express mutant neuropilin-1 proteins (Part 2). (A) A schematic representation of mutant neuropilin-1s; NP-full(m), full-length neuropilin-1 containing myc tag at the COOH-terminal end; NP-ac, neuropilin-1 lacking the b1 and b2 domains; NP-abm, neuropilin-1 lacking the c domain (myc-tagged); NP-am, neuropilin-1 lacking the b1, b2, and c domains (myc-tagged). (B and C) Immunoblot of the transfectants with the antibody raised against the b1-c domains (B) and anti-myc antibody (C). The number followed by the name of the construct represents the clone number. A band at the 100-kD position in the lane of NP-am appears to be dimerized proteins. (D) Quantification of cell aggregation activity of the mutant neuropilin-1s. White and black bars represent N30/N0 and N60/N0, respectively.
Production of Fc-tagged Recombinant Neuropilin-1 Proteins
cDNAs encoding the full-length of neuropilin-1 extracellular segment (aa 1–852), or the b1-b2 domain-deleted one, were ligated into the expression vector pEF-Fc (Mizushima and Nagata 1990; Nishimura et al. 1987) with the adapter of a splicing donor (see Fig. 3 A). The vector was transfected into COS-7 cells using a calcium phosphate precipitation technique. After transfection, cells were grown in GIT medium (Wako) for 4 d. The Fc-tagged neuropilin-1 proteins in culture supernatant were purified by MAPS-II kit (Bio-Rad).
Figure 3.
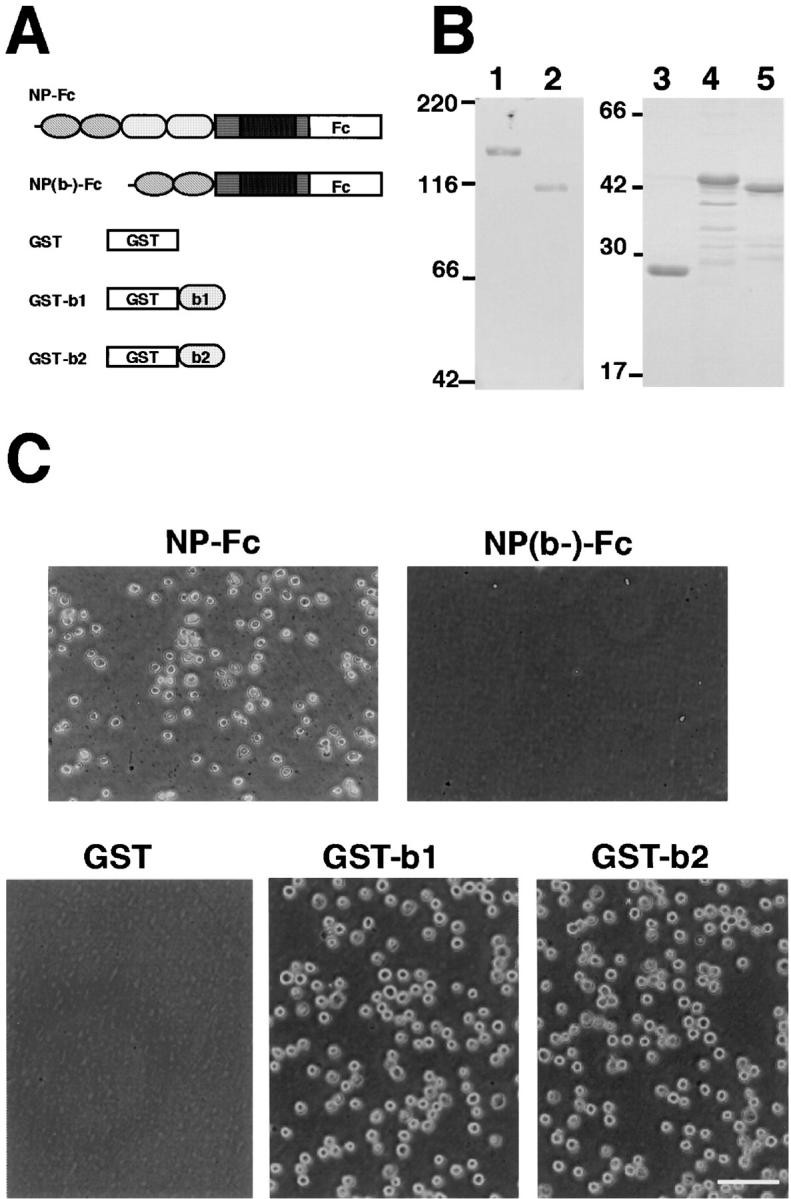
Cell adhesion activity of the recombinant neuropilin-1 proteins. (A) A schematic representation of the Fc-tagged full-length neuropilin-1 extracellular segment (NP-Fc) and the b1-b2 domains–deleted one [NP(b-)-Fc], and GST-tagged recombinant b1 (GST-b1) and b2 (GST-b2) domains. (B) SDS-PAGE of the recombinant proteins stained with Coomassie brilliant blue G-250. Lanes 1–5 correspond to NP-Fc, NP(b-)-Fc, GST, GST-b1, and GST-b2 recombinant proteins, respectively. (C) Cell adhesion to the recombinant proteins immobilized on nitrocellulose-coated culture dishes. L cells adhere to Fc-NP, GST-b1, and GST-b2 but not NP(b-)-Fc and GST. Bar, 100 μm.
Production of GST-tagged Recombinant Neuropilin-1 Proteins
To produce glutathione-S-transferase (GST)-tagged recombinant proteins, cDNAs for the b1 or b2 domains (see Fig. 3 A) or the deletants of these domains (see Fig. 4 A and 5 A) were amplified by PCR and inserted into an expression vector, pGEX4T-1 (Amersham Pharmacia Biotech). The expression plasmids were transfected to E. coli (strain BL21). The expression of recombinant proteins was induced with 0.1 mM isopropyl-β-d-thiogalactoside for 3 h at 37°C. The recombinant proteins, GST-b1 and GST-b2, were recovered as inclusion bodies after sonication of the cells. The inclusion bodies were washed several times with 0.1% PBS containing 0.1% (vol/vol) Triton X-100 (PBST) and solubilized with a solution containing 8 M urea, 25 mM 3-cyclohexylamino-1-propanesulfonic acid (CAPS), pH 10.7, and 10 mM EDTA. The denatured proteins were refolded by stepwise dialysis against the following buffers A to E. Buffer A included 1 M urea, 25 mM CAPS, pH 9.8, 2 mM reduced glutathione, 0.02 mM oxidized glutathione, 0.005% Tween 80. Buffer B included 1 M urea, 50 mM Tris, pH 9.0, 2 mM reduced glutathione, 0.02 mM oxidized glutathione, 0.005% Tween 80. Buffer C was identical to buffer B but was at pH 8.5. Buffer D replaced the urea of buffer C with 150 mM NaCl. Buffer E contained PBS, pH 8.0. All GST-tagged b1 and b2 deletants were soluble, and purified using glutathione Sepharose 4B (Amersham Pharmacia Biotech). The affinity-purified proteins were dialyzed against PBS.
Figure 4.
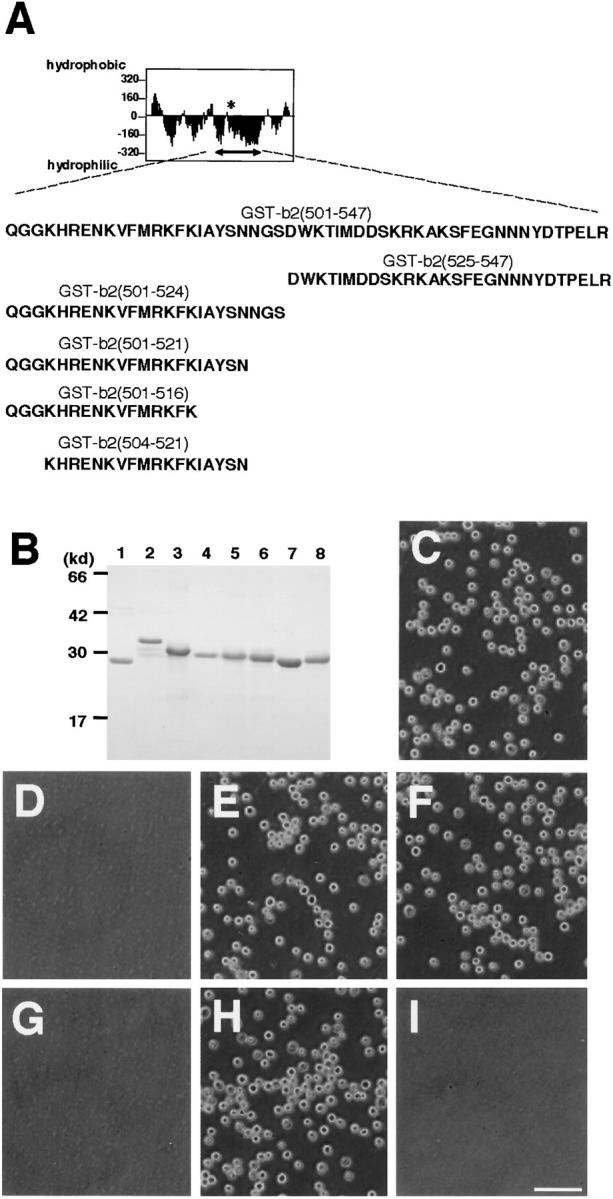
Determination of cell adhesion sites in the b2 domain. (A) A list of synthesized peptides in the b2 domain. The upper figure represents the Kyte-Doolittle hydoropathy plot of the b2 domain. An asterisk indicates a putative glycosylation site. An arrow indicates the region to which a series of GST-tagged recombinant proteins are synthesized. The number in the name of each recombinant protein indicates the position of the amino acid residues. (B) SDS-PAGE of the affinity-purified recombinant proteins. Lane 1, GST; lane 2, GST-b2(501–547); lane 3, GST-b2(525–547); lane 4, GST-b2(501–524); lane 5, GST-b2(501–521); lane 6, GST-b2(501–516); lane 7, GST-b2(504–521); and lane 8, GST-b1(347–364; see the legend for Fig. 5). The gel was stained with Coomassie brilliant blue G-250. (C–I) Adhesion of L cells to the immobilized recombinant proteins at 30 min. C, GST-b2(501–547); D, GST-b2(525–547); E, GST-b2(501–524); F, GST-b2(501–521); G, GST-b2(501–516); H, GST-b2(504–521). Trypsin-treated L cells did not adhere to GST-b2(504–521) (I). Bar, 100 μm.
Immunoblot
Proteins were separated by SDS-PAGE (on 10% acrylamide gel in the presence of 2-mercaptoethanol) and transferred onto nitrocellulose membranes as described previously (Towbin et al. 1979). The nitrocellulose membranes were reacted with the rabbit anti–neuropilin-1 antibodies (Kawakami et al. 1996) or anti-myc antibodies (the culture supernatant of hybridoma 9E10; Evan et al. 1985), and then with HRP-conjugated anti–rabbit IgG (Jackson ImmunoResearch) or anti–mouse IgG (Amersham Pharmacia Biotech). Immunoreactivity was detected by the ECL system (Amersham Pharmacia Biotech).
Cell Aggregation Assay
Transfectants and parental L cells were dissociated into single cells with CMFE and suspended in 10 mM Hepes-buffered CMF (HCMF). To label parental L cells with fluorescent dye, 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes; 10 μM) was added to the cell suspension. The fluorescein-labeled parental L cells (2.5 × 105 cells in 250 μl) and unlabeled transfectants (2.5 × 105 cells in 250 μl) were mixed and placed in 24-well culture dishes (SUMILON). To prevent cells from adhering to the wells, the dishes were preincubated with culture medium containing FCS for 1 h. The cell suspensions were agitated at 80 rpm for 30 min or 60 min at 37°C, and then fixed with 4% paraformaldehyde in PBS. The particles were enumerated by hemocytometer. The extent of aggregation was represented by the index Nt/N0, where Nt and N0 are the total particle number at incubation times t and 0, respectively (Takeichi 1977).
Cell Substrate Adhesion Assay
The recombinant neuropilin-1 proteins (65 and 500 μg/ml for the Fc-tagged and GST-tagged recombinant proteins, respectively) were spotted on nitrocellulose-coated culture dishes (Nunc 150288) for 30 min at room temperature as reported (Lemmon et al. 1989; Ohta et al. 1995). The dishes were blocked with 5% skimmed milk in PBS at 4°C overnight, and washed several times with CMFE containing 100 μg/ml bovine serum albumin (CMFEB). Cells were dissociated with CMFE and suspended in CMFEB (1 × 106 cells/ml). 2 ml of the cell suspension was applied to the dishes and kept at room temperature for 30 min. To test whether semaphorins or VEGF inhibit the neuropilin-1–mediated cell adhesion, the culture dishes with immobilized neuropilin-1 proteins were preincubated with 1.0 ml of affinity-purified myc-tagged chick semaphorin 3A (SEMA3A, previously collapsin-1 [Kobayashi et al. 1997]; a gift from Drs. Raper and Kobayashi) or human VEGF165 (PEPRO TECH EC) at room temperature for 30 min, and then added L cell suspension (2 × 106 cells in 1 ml) and kept at room temperature for 30 min.
Semaphorin-binding Assay
The neuropilin-1 expressing transfectants and parental L cells were incubated with the culture medium containing alkaline phosphatase (AP)-tagged SEMA3A (SEMA3A-AP; gifted from Drs. Raper and Kobayashi) at 37°C for 90 min, washed several times with CMF, and then fixed with paraformaldehyde for 1 h. The dishes were washed with PBST and incubated at 65°C for 10 min to inactivate endogenous phosphatase. After a rinse with PBST, the cells were stained in coloring buffer at 37°C for 10 h (Cheng and Flanagan 1994). To test the binding of semaphorins to the recombinant neuropilin-1 proteins, the culture media containing SEMA3A-AP, Sema3B-AP or Sema3C-AP (Takahashi et al. 1998; Sema3B-AP and Sema3C-AP; a gift from Drs. Takahashi and Strittmatter) were applied to the dishes with immobilized neuropilin-1 proteins at 37°C for 90 min. The dishes were washed several times with the culture medium and fixed with paraformaldehyde for 1 h. They were then washed with PBST and incubated in the coloring buffer at 37°C for 10 h or more.
Plexin Binding Assay
The expression vectors for the mouse plexinA subfamily members (plexinA1, plexinA2, and plexinA3; Kameyama et al. 1996a,Kameyama et al. 1996b) were constructed as follows. The plexin cDNAs whose native signal sequences were replaced to the one of the Sema3A (aa 1–25) and myc-tag sequences were inserted into the expression vector, pCAGGS (Niwa et al. 1991). The expression vector was transfected into the COS-7 cells by LIPOFECTAMINE (GIBCO-BRL) following the manufacturer's protocol. After 1.5 d, the mixture of recombinant proteins [GST-b1(347–364) and GST-b2(504–521)] were added to the culture medium, and incubated at 37°C for 1 h. After washing with the culture medium, the cells were fixed with paraformaldehyde for 1.5 h at room temperature. After the rinsing with PBST, the dishes were treated with 0.1% H2O2 in PBST to inactivate the endogenous peroxidase. The recombinant proteins bound to the cell surfaces were detected by the combination of the anti-GST monoclonal antibody (CLONTECH), HRP-conjugated second antibody, and DAB. The sequence data for the mouse plexinA1, plexinA2, and plexinA3 are available from GenBank/EMBL/DDBJ under the accession numbers D86948, D86949, and D86950, respectively.
Image Acquisition and Analysis
Fluorescence images were obtained using a charged-coupled device (CCD) camera (Photometrics), digitized, and processed by background subtraction and contrast enhancement using IP Lab Spectrum (Scanalytics). Light transmission or phase contrast microscopic images were obtained using a HC-2500 CCD camera system (FUJIFILM) and digitized by the photolab-2500 software (FUJIFILM).
Results
Identification of Cell Adhesion Domains of Neuropilin-1
To identify the domains of the neuropilin-1 protein involved in cell adhesion, we first analyzed the cell adhesion activity of the transfectants which express mutant neuropilin-1 lacking the a1-a2 domains (Fig. 1 A; NP-bc) or the a1-b2 domains (Fig. 1 A; NP-c), and the protein in which the transmembrane and cytoplasmic domains were replaced with the transmembrane domain and NH2-terminal 22 amino acids of the cytoplasmic domain of Xenopus plexin (Fig. 1 A; NP-abcp). We isolated several transfectants that expressed different amounts of mutant neuropilin-1 proteins (Fig. 1 B). All of the transfectants were immunoreactive with an antibody generated against the b2-c domains of mouse neuropilin-1(Kawakami et al. 1996) in living condition (data not shown), indicating that the mutant proteins were expressed on cell surfaces.
The cell adhesion activity of the mutant neuropilin-1 proteins was determined by cell aggregation assay; a 1 to 1 mixture of transfectants and fluorescein-labeled parental L cells was agitated at 80 rpm in CMF, and then particles were enumerated at 30 or 60 min. Parental L cells did not form aggregates (Fig. 1C and Fig. D), indicating that L cells do not show cell adhesiveness in this conditions. In contrast, transfectants expressing intact neuropilin-1 protein (NP-full) had cell adhesion activity and made mixed cell aggregates with parental L cells (Fig. 1C, Fig. E, and Fig. F). As L cells whose cell surface proteins had been digested with 0.1% trypsin lost cell adhesion activity for the neuropilin-1–expressing transfectants (data not shown), the cell adhesion ligand(s) for neuropilin-1 on L cells appears to be a protein. The strength of cell adhesion shown by the transfectants expressing the a1-a2 domain deleted neuropilin-1 (NP-bc) was similar to that of the transfectants with full-length neuropilin-1 (Fig. 1 C). Mutant neuropilin-1 in which the transmembrane and cytoplasmic region had been replaced by the corresponding regions of Xenopus plexin (NP-abcp) also showed cell adhesion activity in proportion to the expression level of the mutated proteins (compare Fig. 1B with C). In contrast, the transfectants expressing the c domain but not a1-b2 domains (NP-c) showed no cell adhesion activity (Fig. 1 C), even though sufficient amounts of truncated proteins were expressed (Fig. 1 B). These results suggest that the b1-b2 domains but not a1-a2 or transmembrane-cytoplasmic domains are involved in the heterophilic cell adhesion.
To confirm further the involvement of the b1-b2 domains in cell adhesion, we produced other lines of transfectant in which the b1-b2, c, or b1-c domain were deleted (Fig. 2 A). As the anti–neuropilin-1 antibody was generated against the b2-c domains (Kawakami et al. 1996), the antibody was inadequate to detect mutant neuropilin-1s lacking these domains. Therefore, we introduced myc tag into the COOH-terminal end of the mutant neuropilin-1 proteins. Each transfectant expressed mutant neuropilin-1 proteins of expected molecular size (Fig. 2B and Fig. C). Cell aggregation analysis demonstrated that the transfectants expressing the b1-b2 domain–deleted neuropilin-1 (NP-ac) or the b1-c domain deleted neuropilin-1 (NP-am) did not show cell adhesion activity (Fig. 2 D). On the other hand, the transfectants expressing the c domain–deleted neuropilin-1 (NP-abm) showed cell adhesion activities, proportional to the expression levels of the mutant proteins (Fig. 2B, Fig. C, and Fig. D).
Collectively, the results obtained in cell aggregation analysis indicate that the b1-b2 domains play an important role in neuropilin-1–mediated heterophilic cell adhesion, and suggest that the cell binding site(s) is located within the domains.
Cell Adhesion Activity of Recombinant Neuropilin-1 Proteins
To determine whether the b1 and b2 domains possess cell adhesion activity, we produced Fc-tagged recombinant proteins for the full-length of neuropilin-1 ectodomain (NP-Fc) and the b1-b2 domains–deleted one [NP(b-)-Fc] by COS-7 cells and GST-tagged recombinant proteins for the b1 (GST-b1) and b2 (GST-b2) domains in E. coli, and tested their cell adhesion activity by cell substrate adhesion assay. Each recombinant protein was absorbed on nitrocellulose-coated culture dishes, and then L cells were applied to the dishes.
As shown in Fig. 3 C, L cells adhered to the NP-Fc-, but not NP(b-)-Fc, absorbed culture dishes. L cells also adhered to the immobilized GST-b1 or GST-b2 but not GST; Fig. 3 C used as a control substrate. These results were well coincided with the results obtained by the cell aggregation assay described above, and further suggest that neuropilin-1 has at least two cell adhesion sites, one in the b1 domain and the other in the b2 domain.
Determination of Cell Adhesion Sites within the b Domain
To identify cell adhesion sites within the b domain, a series of peptides for these domains were synthesized, and tested their cell adhesion activity by cell substrate adhesion assay.
The hydropathy profile of the b2 domain indicated that the central region around a putative glycocylation site (aa 501–547) was most hydrophilic (Fig. 4 A). Therefore, we thought that cell adhesion sites might exist within this region, and tested cell adhesion activity of the GST-tagged recombinant protein for this region. As expected, the GST-tagged peptide for aa 501–547 within the b2 domain (GST-b2[501-547]) showed cell adhesion activity (Fig. 4 C), indicating that a cell adhesion motif of the b2 domain is located within this region. We next produced GST-tagged peptides in which the NH2 and COOH termini of this region were stepwise deleted (Fig. 4A and Fig. B) and tested their cell adhesion activity. As shown in Fig. 4D–H, 18 amino acid residues (aa 504–521; GST-b2[504–521]) were sufficient to mediate cell adhesion. Trypsin-treated L cells did not adhere to GST-b2(504–521) (Fig. 4 I), indicating that the cell adhesion ligand(s) recognized by the peptide is a protein.
On the assumption that the cell adhesion site within the b1 domain exists in the region homologous to the cell adhesion site within the b2 domain, we prepared a GST-tagged peptide for aa 347–364 of the b1 domain (Fig. 5 A and Fig. 4 B, lane 8), and tested its cell adhesion activity. As shown in Fig. 5 B, L cells adhered to the immobilized GST-tagged peptide, GST-b1(347–364). Trypsin-treated L cells did not adhere to the recombinant protein (Fig. 5 C). These results indicate that the cell adhesion activity in the b1 domain also exists in the homologous 18–amino acid sequences in the b2 domain.
Figure 5.
Determination of cell adhesion sites in the b1 domain. (A) Amino acid alignment of the b1 and b2 domains. Shaded boxes indicate consensus sequences. An open box indicates the cell adhesion sites in the b2 domain and its homologous region (347–364 aa) in the b1 domain. (B and C) Cell adhesion activity of the GST-tagged b1(347–364) recombinant protein [GST-b1(347–364)]. L cells (B) but not trypsin-treated L cells (C) adhere to the immobilized GST-b1(347–364). Bar, 100 μm.
Adhesion of Embryonic Cells and Line Cells to Recombinant Neuropilin-1 Proteins
To examine which types of cell adhere to neuropilin-1, we performed cell substrate adhesion assay for primary embryonic cells and line cells derived from different origins, including HEK293T (human embryonic kidney), COS-7 (monkey kidney), HeLa (human cervix carcinoma), p19 (mouse embryonic carcinoma), KB (human epidermoid carcinoma), and NIH3T3 (mouse embryo).
Trunk mesenchymal cells from E13 mouse embryos that had been dissociated from the primary culture with EDTA adhered to the GST-b1(347–364) and GST-b2(504–521) recombinant proteins (Fig. 6A and Fig. C). In contrast, when the cells were dissociated with trypsin, they did not adhere to the substrates (Fig. 6B and Fig. D). These results suggest that a cell adhesion ligand(s) for neuropilin-1 exists on the surfaces of embryonic cells and is a protein. All of the line cells examined adhered to the GST-b1(347–364) and GST-b2(504–521) recombinant proteins (data not shown), suggesting that the cell adhesion ligand(s) for neuropilin-1 is a ubiquitous membrane protein.
Figure 6.
Adhesion of embryonic mesenchymal cells to the recombinant proteins for the cell adhesion sites of neuropilin-1. Trunk mesenchymal cells from E13 mouse embryos adhere to the immobilized GST-b1(347–364) (A and B) and GST-b2(504–521) (C and D) recombinant proteins. Trypsin-treated mesenchymal cells do not adhere to the recombinant proteins (B and D). Bar, 100 μm.
Interaction of Semaphorins, VEGF, and Plexins to the Cell Adhesion Sites of Neuropilin-1
Neuropilin-1 is shown to interact with the class 3 semaphorins (Chen et al. 1997; Feiner et al. 1997; He and Tessier-Lavigne 1997; Kolodkin et al. 1997), VEGF165 (Soker et al. 1998), and the plexinA subfamily members (Takahashi et al. 1999; Tamagnone et al. 1999). Therefore, we tested whether these molecules can interact with the cell adhesion sites of neuropilin-1 and mediate cell adhesion.
First, we tested the binding of SEMA3A-AP to the transfectants expressing mutant neuropilin-1s. The transfectants were incubated with the culture supernatant containing SEMA3A-AP (20 collapse units). Strong AP staining was observed in the transfectants expressing NP-full (Fig. 7 A), NP-ac (Fig. 7 C), and NP-abm (Fig. 7 D). In contrast, SEMA3A-AP bound very weakly to the transfectants expressing NP-bc (Fig. 7 B) or NP-am (Fig. 7 E), even though sufficient amounts of the truncated neuropilin-1 proteins were expressed in these transfectants (see Fig. 1 B and 2 C). No SEMA3A-AP binding was observed for the cells expressing NP-c (Fig. 7 F) or parental L cells (data not shown). These results indicate that semaphorin 3A can bind to the b1 and b2 domains which contain the cell adhesion sites of neuropilin-1, as well as the a1-a2 domains.
Figure 7.
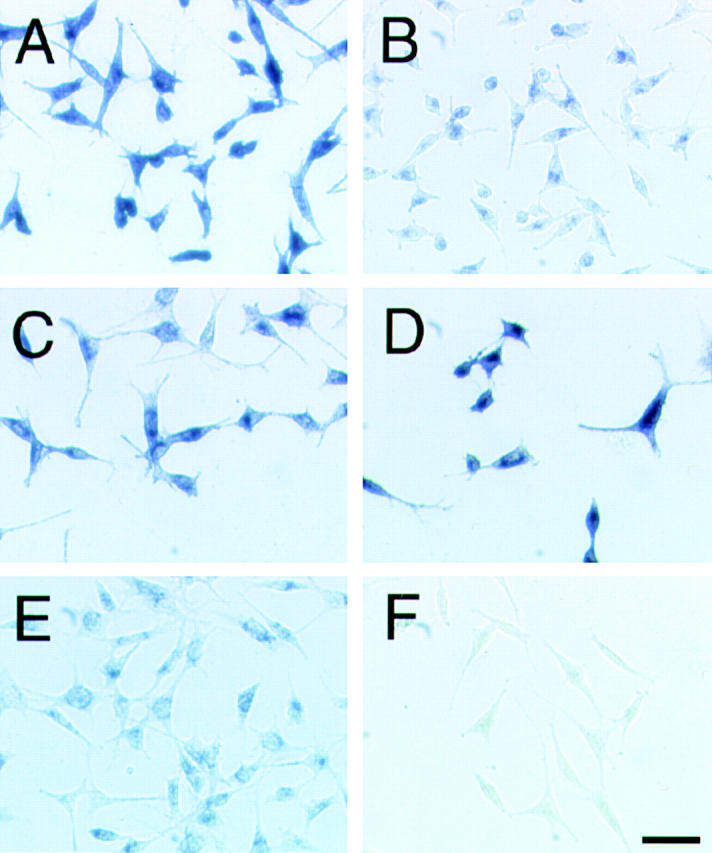
Binding of AP-tagged semaphorin 3A (SEMA3A-AP) to mutant neuropilin-1 proteins. Binding of SEMA3A-AP to the transfectants expressing NP-full 86 (A; see Fig. 1 B), NP-bc 30 (B; see Fig. 1 B), NP-ac 24 (C; see Fig. 2 B), NP-abm 716 (D; see Fig. 2 C), NP-am 38 (E; see Fig. 2 C), and NP-c 68 (F; see Fig. 1 B). SEMA3A-AP bound to cell surface was visualized with NBT/BCIP. Bar, 100 μm.
Next, we tested the binding of SEMA3A-AP, Sema3B-AP, and Sema3C-AP to the immobilized GST-tagged neuropilin-1 proteins, GST-b1, GST-b2, GST-b1(347–364), and GST-b2(504–521). Fc-tagged neuropilin-1 ectodomain (NP-Fc) and GST were used as positive and negative controls, respectively. As shown in Fig. 8 A, these three semaphorins bound to NP-Fc, GST-b1, and GST-b2, but not GST-b1(347–364) or GST-b2(504–521), which are the cell adhesion sites of neuropilin-1. These results suggest that the class 3 semaphorins do not interact with the cell adhesion sites of neuropilin-1. This was further confirmed by the competition assay. The presence of SEMA3A (6.7 nM) did not inhibit the adhesion of L cell to NP-Fc (Fig. 8 B). In addition, we also showed that VEGF165 (100 nM) did not interfere with the cell adhesion activity of neuropilin-1 (Fig. 8 C).
Figure 8.
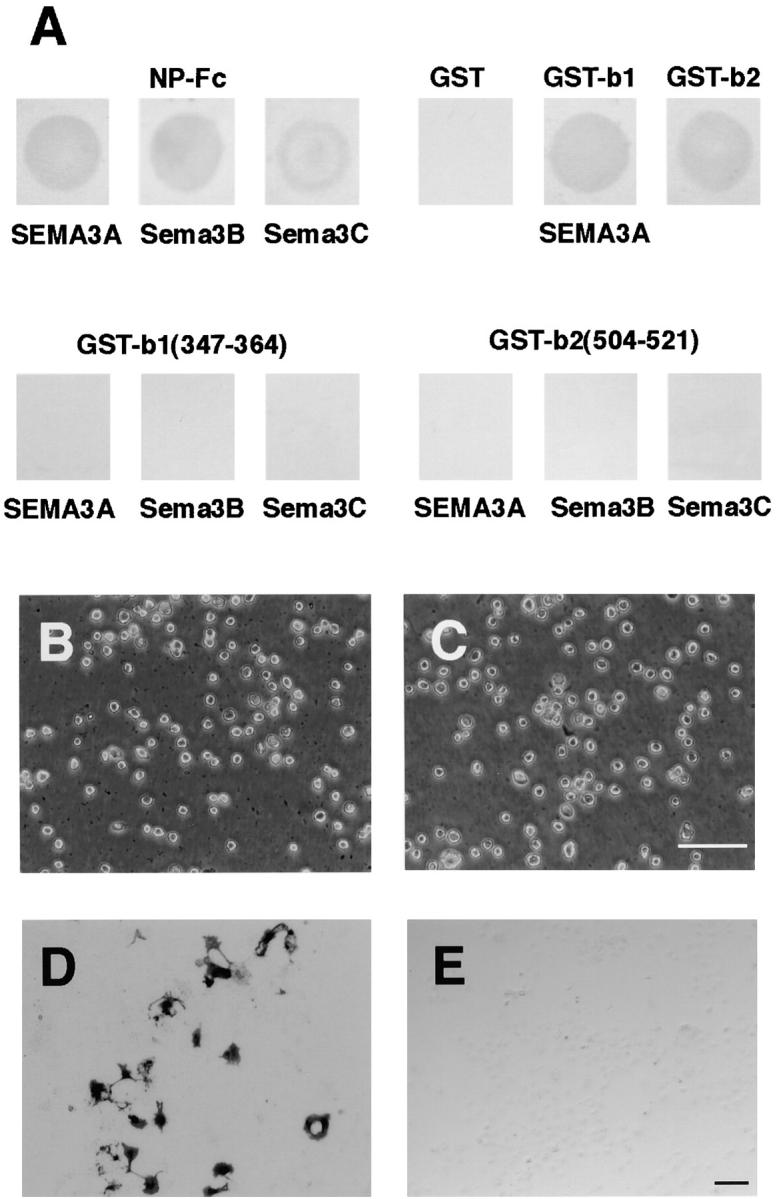
Binding of semaphorins, VEGF, and plexins to the recombinant neuropilin-1 proteins. (A) Bindings of SEMA3A-AP, Sema3B-AP and Sema3C-AP to the immobilized NP-Fc, GST, GST-b1, GST-b2, GST-b1(347–364), and GST-b2(504–521) were visualized with NBT/BCIP. (B and C) Adhesion of L cells to the immobilized NP-Fc in the presence of SEMA3A (C) and VEGF165 (D). SEMA3A and VEGF165 do not interfere with the binding of L cells to NP-Fc. (D and E) Binding of GST-tagged recombinant proteins for the cell adhesion sites of neuropilin-1 to myc-tagged plexinA3 expressed in COS-7 cells. The plexinA3 was visualized by immunohistochemistry with anti-myc antibody (D). GST-b1(347–364) and GST-b2(504–521) bound to the cells were detected by immunohistochemistry with anti-GST antibody. Bars, 100 μm.
Finally, we examined whether the cell adhesion sites of neuropilin-1 interact with the plexinA subfamily members. We expressed the mouse plexinA1, plexinA2, and plexinA3 in COS-7 cells, applied the mixture of GST-b1(347–364) and GST-b2(504–521) recombinant proteins (45 μg/ml each), and monitored the binding of the recombinant proteins by anti-GST antibody. We did not observe prominent binding of GST-b1(347–364) and GST-b2(504–521) to the plexinA3-expressing cells (Fig. 8D and Fig. E), or to plexinA1- and plexinA2-expressing cells (data not shown).
Collectively, these results suggest that the class 3 semaphorins, VEGF, or the plexinA subfamily members do not interact with the cell adhesion sites of neuropilin-1, and are not cell adhesion ligands for neuropilin-1.
Discussion
Neuropilin-1 is a molecule with multi-domains and multi-functions. The present structure-function analyses on neuropilin-1 identified the sites involved in cell adhesion.
Our previous study had indicated that L cells acquire cell adhesiveness when they are transfected with neuropilin-1 cDNA and express the proteins on their surface (Takagi et al. 1995). However, it was not known whether the cytoplasmic segment of neuropilin-1 is required for the cell adhesion activity as it is in cadherins (Nagafuchi and Takeichi 1988). In this study, we constructed a mutant neuropilin-1 cDNA in which the transmembrane and cytoplasmic regions had been replaced by the corresponding regions of Xenopus plexin, transfected L cells, and isolated cell lines expressing the mutant neuropilin-1 protein, NP-abcp. Cell aggregation analysis showed that the NP-abcp–expressing cells adhered as well as the cells expressing the full-length of neuropilin-1. Plexin is a cell adhesion molecule but its cytoplasmic segment is not required for the cell adhesion activity (Ohta et al. 1995). In addition, this study showed that the Fc-tagged recombinant protein for the extracellular segment of neuropilin-1 retained cell adhesion activity. Therefore, we can conclude that the extracellular part of neuropilin-1 is sufficient to mediate cell adhesion.
The present cell aggregation analyses using L cells in which the domains in the extracellular segment of neuropilin-1 had been deleted showed that the b1/b2, but not a1/a2 or c, domains were essential to the cell adhesion activity of neuropilin-1. As L cells adhered to the recombinant proteins for the b1 and b2 domains, but not the b1-b2 domains–deleted protein, these two domains can mediate cell adhesion independently. Furthermore, cell substrate adhesion assays for a series of recombinant proteins for the b1 and b2 domains clarified that there are two cell adhesion sites with a stretch of 18 amino acid residues in the central part of these domains.
A search of the databases shows that the cell adhesion sites within the b1 and b2 domains are highly conserved among Xenopus (Takagi et al. 1991), chicken (Takagi et al. 1995), mouse (Kawakami et al. 1996), rat (He and Tessier-Lavigne 1997; Kolodkin et al. 1997), and human (He and Tessier-Lavigne 1997; Kolodkin et al. 1997) neuropilin-1s (Fig. 9 A), suggesting that cell adhesion activity is a universal function of neuropilin-1. Another member of the neuropilin family, neuropilin-2, also possesses a similar domain structure to neuropilin-1 (Chen et al. 1997). However, the amino acid sequences of the cell adhesion sites of neuropilin-1 do not closely resemble the corresponding regions of neuropilin-2 (Fig. 9 B). Therefore, it is an open question whether neuropilin-2 can mediate cell adhesion as neuropilin-1 does.
Figure 9.
Comparison of amino acid sequences in cell adhesion sites among vertebrate neuropilin-1s (A) or the mouse neuropilin family (B). The amino acid sequences in the cell adhesion sites are highly conserved among the mouse (mNP), rat (rNP), human (hNP), chicken (cNP), and Xenopus (xNP) neuropilin-1s. On the other hand, only about half of the amino acid residues of the cell adhesion site of mouse neuropilin-1 and of mouse neuropilin-2 are identical.
Domains similar to the b1/b2 of neuropilin-1 are shared by various molecules, including coagulation factors V and VIII (Toole et al. 1984; Jenny et al. 1987), DDR/Ptk-3 (Johnson et al. 1993; Sanchez et al. 1994), and MFGP (Stubbs et al. 1990; Larocca et al. 1991). However, the amino acid sequences in the cell adhesion sites of neuropilin-1 are unique. Furthermore, the cell adhesion sites with an 18–amino acid stretch do not contain any motifs reported so far, suggesting that neuropilin-1 is an unique cell adhesion receptor. Interestingly, the cell adhesion sites in the b1 and b2 domains show little homology (see Fig. 5 A). It is likely that each site can interact with distinct cell adhesion ligands or distinct regions of the same ligand.
Several studies have reported that neuropilin-1 can bind the class 3 semaphorins (He and Tessier-Lavigne 1997; Kolodkin et al. 1997; Chen et al. 1997; Feiner et al. 1997). The NH2-terminal Sema domain of the class 3 semaphorins binds to the a1/a2 domains, and the COOH-terminal basic region to the b1/b2 domains or the junction of the a and b domains (Giger et al. 1998; Nakamura et al. 1998). These results that semaphorin 3A (SEMA3A) bound strongly to the transfectants expressing NP-full, NP-abm, and NP-ac, but only weakly to the transfectants expressing NP-bc or NP-am, and not at all to the transfectants expressing NP-c or parental L cells, are mostly consistent with the foregoing results and confirm that semaphorin 3A can bind to the a1/a2 and b1/b2 domains but not the c (MAM) domain of neuropilin-1. The strong binding of SEMA3A to NP-ac and very weak binding to NP-am suggest that the c domain enhances the binding of SEMA3A to the a1/a2 domains, even though the c domain itself does not bind SEMA3A.
The transfectants that expressed the b1-b2 domains–deleted neuropilin-1 (NP-ac) lacked cell adhesion activity but showed strong semaphorin-binding activity, while the NP-bc–expressing transfectants kept cell adhesion activity but lost semaphorin-binding activity. Moreover, the cell substrate adhesion assay using GST-tagged recombinant neuropilin-1 proteins demonstrated that SEMA3A bound to both the b1 and b2 domains but not the GST-tagged cell adhesion sites within the b domains. Other members of the class 3 semaphorin, Sema3B and Sema3C, also did not bind to the cell adhesion sites. In addition, SEMA3A did not inhibit the attachment of L cells to NP-Fc. These findings suggest that the cell adhesion activity and the semaphorin-binding activity are independent functions of neuropilin-1, and that the cell adhesion sites are different to the binding sites for the class 3 semaphorins, even though both sites are mapped within the b domains. VEGF165 can bind to neuropilin-1 (Soker et al. 1998), and the VEGF165 -binding site is localized within the b domains (Giger et al. 1998). This study showed that VEGF165 did not interfere with the neuropilin-1–mediated cell adhesion, suggesting that the binding site for VEGF is different to the cell adhesion sites. As VEGF165 functions as an antagonist for the binding of semaphorin 3A to neuropilin-1 (Giger et al. 1998) and, vice versa, semaphorin 3A inhibits the binding of VEGF165 to neuropilin-1 (Miao et al. 1999), it is likely that VEGF165 and semaphorin 3A share the same binding sites within the b domains of neuropilin-1. In this study, we also showed that the GST-tagged recombinant proteins for the cell adhesion sites did not bind to the plexinA subfamily members (plexinA1, plexinA2, and plexinA3) expressed on COS-7 cells, suggesting that the binding sites for the plexinA subfamily members are different to the cell adhesion site. Collectively, the results obtained in this study suggest that the class 3 semaphorins, VEGF or plexinA subfamily members do not function as cell adhesion ligands for neuropilin-1.
Embryonic mesenchymal cells adhered to the recombinant proteins for the cell adhesion sites of neuropilin-1. This observation suggests that the cell adhesion activity of neuropilin-1 plays roles in embryonic development. The PNS efferent fibers express neuropilin-1 (Kawakami et al. 1996) and grow through embryonic mesenchymal cells. Therefore, it is likely that the embryonic mesenchymal cells with cell adhesion ligands for neuropilin-1 provide substrates along which the neuropilin-1–expressing PNS fibers elongate. Though the abnormal guidance of PNS efferent fibers in the neuropilin-1 knockout mouse embryos is induced primarily by the lack of neuropilin-1–mediated semaphorin 3A signals (Kitsukawa et al. 1997), the reduction of cell adhesiveness between the PNS fibers and surrounding mesenchymal cells may attribute in part to the disorganization of the pathways. Endothelial cells in embryos strongly express neuropilin-1 (Kitsukawa et al. 1995). The formation of the capillary network is severely regressed in the neuropilin-1 knockout mouse embryos, mainly due to the deficiency of neuropilin-1–mediated VEGF signals (Kawasaki et al. 1999). However, it is also likely that the cell adhesion of endothelial cells to mesenchymal cells plays some role in the capillary invasion of mesenchymal tissues. Neuropilin-1 is expressed in mesenchymal cells in various parts of the embryos, including the wall of the digestive tract and vessels and the limb buds (Kitsukawa et al. 1995), suggesting that the cell adhesion activity of neuropilin-1 is involved in the organization of embryonic mesenchyme. The induction of extra toes in the mouse embryos with the gain-of-function of neuropilin-1 (Kitsukawa et al. 1995) is hard to explain by the increase of semaphorin 3A signals or VEGF signals, but may be attributed to the increase in adhesion of mesenchymal cells.
Pretreatment of parental L cells with trypsin abolished the adhesion of L cells to neuropilin-1–expressing cells (Takagi et al. 1995; this study) and to the recombinant proteins for the cell adhesion sites, GST-b1(347–364) or GST-b2(504–521) (this study), suggesting that cell adhesion ligand(s) is a membrane protein. As various line cells of diverse origins adhered to the recombinant proteins for the cell adhesion sites of neuropilin-1, the cell adhesion ligands may be shared by a wide range of cells. To gain further insight into the function of neuropilin-1–mediated cell adhesion activity, identification of the cell adhesion ligands for neuropilin-1 is essential.
Acknowledgments
We thank Drs. Raper and Kobayashi (University of Pennsylvania) for SEMA3A (Collapsin-1)-AP and myc-tagged SEMA3A; Drs. Strittmatter and Takahashi (University of Yale) for Sema3B-AP and Sema3C-AP; Dr. Nagata (Osaka University) for pEF-Fc; and Dr. Miyazaki (Osaka University) for pCAGGS.
This work was supported by a grant from CREST (Core Research for Evolutional Science and Technology) of Japan Science and Technology Corporation (JST) and a grant from the Ministry of Education, Science and Culture (Japan). M. Shimizu was supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
Footnotes
Abbreviations used in this paper: aa, amino acids; GST, glutathione-S-transferase; VEGF, vascular endothelial growth factor.
References
- Beckmann G., Bork P. An adhesive domain detected in functionally diverse receptors. Trends Biochem. Sci. 1993;18:40–41. doi: 10.1016/0968-0004(93)90049-s. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chédotal A., He Z., Goodman C.S., Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Cheng H.J., Flanagan J.G. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Evan G.I., Lewis G.K., Ramsay G., Bishop J.M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Raper J.A. Localized collapsing cues can steer growth cones without inducing their full collapse. Neuron. 1995;14:263–274. doi: 10.1016/0896-6273(95)90284-8. [DOI] [PubMed] [Google Scholar]
- Feiner L., Koppel A.M., Kobayashi H., Raper J.A. Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron. 1997;19:539–545. doi: 10.1016/s0896-6273(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Henzel W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Giger R.J., Urquhart E.R., Gillespie S.K., Levengood D.V., Ginty D.D., Kolodkin A.L. Neuropilin-2 is a receptor for semaphorin IVinsight into the structural basis of receptor function and specificity. Neuron. 1998;21:1079–1092. doi: 10.1016/s0896-6273(00)80625-x. [DOI] [PubMed] [Google Scholar]
- He Z., Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- Jenny R.J., Pittman D.D., Toole J.J., Kriz R.W., Aldape R.A., Hewick R.M., Kaufman R.J., Mann K.G. Complete cDNA and derived amino acid sequence of human factor V. Proc. Natl. Acad. Sci. USA. 1987;84:4846–4850. doi: 10.1073/pnas.84.14.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.D., Edman J.C., Rutter W.J. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc. Natl. Acad. Sci. USA. 1993;90:5677–5681. doi: 10.1073/pnas.90.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama T., Murakami Y., Suto F., Kawakami A., Takagi S., Hirata T., Fujisawa H. Identification of plexin family molecules in mice Biochem. Biophys. Res. Commun. 226 1996. 396 402a [DOI] [PubMed] [Google Scholar]
- Kameyama T., Murakami Y., Suto F., Kawakami A., Takagi S., Hirata T., Fujisawa H. Identification of a neuronal cell surface molecule, plexin, in mice Biochem. Biophys. Res. Commun. 226 1996. 524 529b [DOI] [PubMed] [Google Scholar]
- Katoh K., Takahashi Y., Hayashi S., Kondoh H. Improved mammalian vectors for high expression of G418 resistance. Cell Struct. Funct. 1987;12:575–580. doi: 10.1247/csf.12.575. [DOI] [PubMed] [Google Scholar]
- Kawakami A., Kitsukawa T., Takagi S., Fujisawa H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J. Neurobiol. 1996;29:1–17. doi: 10.1002/(SICI)1097-4695(199601)29:1<1::AID-NEU1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Kitsukawa T., Bekku Y., Matsuda Y., Sanbo M., Yagi T., Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4873–4884. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- Keck P.J., Hauser S.D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D.T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T., Shimono A., Kawakami A., Kondoh H., Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T., Shimizu M., Sanbo M., Hirata T., Taniguchi M., Bekku Y., Yagi T., Fujisawa H. Neuropilin-semaphorin III/D–mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Koppel A.M., Luo Y., Raper J.A. A role for collapsin-1 in olfactory and cranial sensory axon guidance. J. Neurosci. 1997;17:8339–8352. doi: 10.1523/JNEUROSCI.17-21-08339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin A.L., Matthes D.J., Goodman C.S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- Kolodkin A.L., Levengood D.V., Rowe E.G., Tai Y.T., Giger R.J., Ginty D.D. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Larocca D., Peterson J.A., Urrea R., Kuniyoshi J., Bistrain A.M., Ceriani R.L. A M r 46,000 human milk fat globule protein that is highly expressed in human breast tumors contains factor VIII-like domains. Cancer Res. 1991;51:4994–4998. [PubMed] [Google Scholar]
- Lecain E., Zelenika D., Laine M.C., Rhyner T., Pessac B. Isolation of a novel cDNA corresponding to a transcript expressed in the choroid plexus and leptomeninges. J. Neurochem. 1991;56:2133–2138. doi: 10.1111/j.1471-4159.1991.tb03476.x. [DOI] [PubMed] [Google Scholar]
- Lemmon V., Farr K.L., Lagenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Leung D.W., Cachianes G., Kuang W.-J., Goeddel D.V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Leytus S.P., Kurachi K., Sakariassen K.S., Davie E.W. Nucleotide sequence of the cDNA coding for human complement C1r. Biochemistry. 1986;25:4855–4863. doi: 10.1021/bi00365a020. [DOI] [PubMed] [Google Scholar]
- Luo Y., Raible D., Raper J.A. Collapsina protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Mackinnon C.M., Carter P.E., Smyth S.J., Dunbar B., Fothergill E. Molecular cloning of cDNA for human complement component C1s. The complete amino acid sequence. Eur. J. Biochem. 1987;169:547–553. doi: 10.1111/j.1432-1033.1987.tb13644.x. [DOI] [PubMed] [Google Scholar]
- Messersmith E.K., Leonardo E.D., Shatz C.J., Tessier-Lavigne M., Goodman C.S., Kolodkin A.L. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Miao H.Q., Soker S., Feiner L., Alonso J.L., Raper J.A., Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motilityfunctional competition of collapsin-1 and vascular endothelial growth factor-165. J. Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO (Eur. Mol. Biol. Organ.) J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura F., Tanaka M., Takahashi T., Kalb R.G., Strittmatter S.M. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Yokoyama M., Araki K., Ueda R., Kudo A., Watanabe T. Recombinant human-mouse chimeric monoclonal antibody specific for common acute lymphocytic leukemia antigen. Cancer Res. 1987;47:999–1005. [PubMed] [Google Scholar]
- Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Ohta K., Mizutani A., Kawakami A., Murakami Y., Kasuya Y., Takagi S., Tanaka H., Fujisawa H. Plexina novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron. 1995;14:1189–1199. doi: 10.1016/0896-6273(95)90266-x. [DOI] [PubMed] [Google Scholar]
- Püschel A.W., Adams R.H., Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Sanchez M.P., Tapley P., Saini S.S., He B., Pulido D., Barbacid M. Multiple tyrosine protein kinases in rat hippocampal neuronsisolation of Ptk-3, a receptor expressed in proliferative zones of the developing brain. Proc. Natl. Acad. Sci. USA. 1994;91:1819–1823. doi: 10.1073/pnas.91.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaphorin Nomenclature Committee Unified nomenclature for the semaphorins/collapsins. Cell. 1999;97:551–552. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- Shimell M.J., Ferguson E.L., Childs S.R., O'Connor M.B. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell. 1991;67:469–481. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- Soker S., Takashima S., Miao H.Q., Neufeld G., Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Stubbs J.D., Lekutis C., Singer K.L., Bui A., Yuzuki D., Srinivasan U., Parry G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc. Natl. Acad. Sci. USA. 1990;87:8417–8421. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suemori H., Kadokawa Y., Goto K., Araki I., Kondoh H., Nakatsuji N. A mouse embryonic stem cell line showing pluripotency of differentiation in early embryos and ubiquitous β-galactosidase expression. Cell Differ. Dev. 1990;29:181–186. doi: 10.1016/0922-3371(90)90120-l. [DOI] [PubMed] [Google Scholar]
- Takagi S., Tsuji T., Amagai T., Takamatsu T., Fujisawa H. Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies. Dev. Biol. 1987;122:90–100. doi: 10.1016/0012-1606(87)90335-6. [DOI] [PubMed] [Google Scholar]
- Takagi S., Hirata T., Agata K., Mochii M., Eguchi G., Fujisawa H. The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron. 1991;7:295–307. doi: 10.1016/0896-6273(91)90268-5. [DOI] [PubMed] [Google Scholar]
- Takagi S., Kasuya Y., Shimizu M., Matsuura T., Tsuboi M., Kawakami A., Fujisawa H. Expression of a cell adhesion molecule, neuropilin, in the developing chick nervous system. Dev. Biol. 1995;170:207–222. doi: 10.1006/dbio.1995.1208. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nakamura F., Jin Z., Kalb R.G., Strittmatter S.M. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat. Neurosci. 1998;1:487–493. doi: 10.1038/2203. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Fournier A., Nakamura F., Wang L.H., Murakami Y., Kalb R.G., Fujisawa H., Strittmatter S.M. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell Biol. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L., Artigiani S., Chen H., He Z., Ming G.I., Song H., Chédotal A., Winberg M.L., Goodman C.S., Poo M. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Toole J.J., Knopf J.L., Wozney J.M., Sultzman L.A., Buecker J.L., Pittman D.D., Kaufman R.J., Brown E., Shoemaker C., Orr E.C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984;312:342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheetsprocedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J.M., Rosen V., Celeste A.J., Mitsock L.M., Whitters M.J., Kriz R.W., Hewick R.M., Wang E.A. Novel regulators of bone formationmolecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]






