Abstract
We report the isolation of adherent, clonogenic, fibroblast-like cells with osteogenic and adipogenic potential from the blood of four mammalian species. These cells phenotypically resemble but are distinguishable from skeletal stem cells found in bone marrow (stromal stem cells, “mesenchymal stem cells”). The osteogenic potential of the blood-borne cells was proven by an in vivo transplantation assay in which either polyclonal or single colony–derived strains were transplanted into the subcutis of immunocompromised mice, and the donor origin of the fully differentiated bone cells was proven using species-specific probes. This is the first definitive proof of the existence of circulating skeletal stem cells in mammals.
Keywords: blood cells, tissue culture, adherent colonies, transplantation, osteogenesis
Introduction
The post-natal bone marrow of mammals harbors two populations of stem/progenitor cells that are thought of as part of separate lineages and exhibit distinct fundamental biological properties: (a) hematopoietic stem cells, which are nonadherent and circulating, and (b) stromal stem cells, which are adherent and fibroblastic in habit, and noncirculating (Friedenstein et al. 1968; Bianco and Riminucci 1998). Stromal stem cells can give rise to all major skeletal tissues, such as cartilage, bone, myelosupportive stroma, and associated adipocytes, and fibrous connective tissue (Friedenstein et al. 1966, Friedenstein et al. 1968; Owen 1988). Throughout development, these particular tissues are thought to be formed entirely by extravascular mesodermal progenitors that associate with developing skeletal rudiments via migratory processes that do not rely upon their ability to circulate in blood (Bianco and Riminucci 1998). Other solid-phase mesodermal tissues such as skeletal muscle also fall into the same general category (Cossu et al. 1996; Bianco and Cossu 1999).
Recent data have shown that both muscle and skeletal cells of donor origin differentiate within muscle and bone when marrow-derived cell populations are infused into the systemic circulation under some experimental conditions (Ferrari et al. 1998; Hou et al. 1999). These data provide evidence for at least some degree of systemic transplantability of progenitors of solid-phase mesodermal tissues. Complete reconstitution of the hematopoietic system after the systemic infusion of progenitor/stem cells illustrates the natural properties of the hematopoietic stem cell; hematopoietic stem cells are capable of negotiating the circulation and homing to the bone marrow in normal physiology. Whether any physiological event relevant to extravascular mesodermal tissues calls for the existence of a naturally circulating progenitor cell remains to be determined. Critical to addressing this question would be the demonstration of circulating progenitors for solid-phase mesodermal tissues in the intact organism.
In this study, we isolated adherent and clonogenic cells from whole blood of adult animals of four different species (mouse, rabbit, guinea pig, and human). We demonstrated that some polyclonal strains and several single colony–derived strains formed bone upon in vivo transplantation, the gold standard assay of osteogenic potential. Furthermore, they were capable of forming adipocytes in vitro. These studies prove for the first time that some circulating cells that can form adherent colonies on plastic are competent to form genuine bone tissue in vivo, and establish for the first time the existence of circulating osteogenic stem cells.
Materials and Methods
Blood Collection
All procedures were performed in accordance to specifications of institutionally approved animal protocols. Blood from mice (BALB/cj and Swiss NIH-SW-CR), rabbits (New Zealand), and guinea pigs (Hartley) was collected by cardiac puncture in syringes containing sodium heparin (Fisher Scientific) at a final concentration of 100 U/ml. Human venous blood was collected from healthy volunteers under National Institutes of Health (NIH)-approved protocols, and was mixed with sodium heparin at a final concentration of 100 U/ml. Alternatively, human buffy coat concentrates with citrate (CDPA-1), byproducts of blood donated for transfusion, were also used. Blood was harvested from donors of varying ages as indicated in Table .
Table 1.
Donor and Cell Culture Characteristics in the Determination of Colony-forming Efficiency of Blood-derived Adherent Cells
| Aminal species | No. of donors | Age range | Plating density × 105/cm2 | Colony-forming efficiency/106 cells | Range between donors | No. of polygonal/ fibroblast-like colonies | Percent polygonal | No. of osteogenic clones/clones transplanted |
|---|---|---|---|---|---|---|---|---|
| Mouse | 37 | 3 wk–2 yr | 0.2–1.4 | 0.93 | 0–3.8 | 5/33 | 13 | 1/8 |
| Rabbit | 5 | 9–10 mo | 1.4–3.2 | 0.18 | 0–0.58 | 6/41 | 13 | 3/19 |
| Guinea pig | 5 | 5–8 mo | 0.5–2.2 | 2.7 | 1.1–3.9 | 15/319 | 5 | 2/4 |
| Human | 10 | 22–60 yr | 1–37.3 | Rare | 0–0.025 | 0/2 | 0 | 1/2 |
Cell Culture
Whole blood from mice, rabbits, and guinea pigs, and whole blood, total buffy coat cells, and mononuclear buffy coat cells from humans, were plated in a range of cell densities (Table ). In most instances, standard plastic culture vessels were used (Becton Dickinson). Some human samples were plated in either fibronectin-coated flasks (Becton Dickinson) or collagen type I–coated dishes (Flexcell International); however, this was not found to increase colony formation. Culture media of several compositions were used based on αMEM (Life Technologies), 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate (Biofluids), with 20% serum (FBS; Equitech-Bio) or human serum blood type AB (Pel-Freez), or a combination of the two), with or without 10−8 M dexamethasone (Sigma-Aldrich) and 10−4 M ascorbic acid phosphate (Wako), or 100 ng/ml of mouse or human leukemia inhibitory factor (Life Technologies, R&D Systems). However, medium composition did not influence the number of colonies formed within an experiment, and consequently the data were pooled. Cells were cultured at 37°C in an atmosphere of 100% humidity and 5% CO2. Complete medium replacement was performed 1 d after plating and twice a week thereafter. Colonies consisting of 50 or more cells were counted between 10 and 41 d, depending on the animal species. 1–3 d later, the cells were released by trypsin-EDTA (Life Technologies). Colonies were detached either simultaneously to generate polyclonal strains (guinea pig), or individually using cloning cylinders to generate single colony–derived strains (mouse, rabbit, and human). Cells were then plated at 0.1–0.2 × 105 cells/cm2. Subsequent passages were performed when cells were approaching confluence. Adipogenic conversion of passaged cells was determined by using medium with 20% rabbit serum (Life Technologies), as previously described (Diascro et al. 1998).
Cultures of Bone Marrow Stromal Cells and Skin Fibroblasts
Long bones of mice, rabbits, and guinea pigs were obtained according to specifications of an approved animal protocol. Fragments of human bone were obtained from patients undergoing corrective surgery in accordance with NIH regulations governing the use of human subjects in research. Single cell suspensions of bone marrow were prepared and cultures of bone marrow stromal cells were established as described previously (Kuznetsov and Gehron Robey 1996). Murine skin fibroblasts and human foreskin fibroblasts were cultured as described previously (Kuznetsov and Gehron Robey 1996; Krebsbach et al. 1997; Satomura et al. 2000).
Phenotypic Characterization
Polycolonal and single colony–derived strains of blood-derived adherent cells and marrow stromal cells from each species were plated in eight-chamber slides (VWR Scientific) at 2 × 104 cells/cm2 for immunohistochemical analysis with a panel of antibodies, as described previously (Gronthos et al. 2000) (see Table ). Bound antibody was localized using either peroxidase-conjugated Ig (ABC kit; Vector Laboratories) or FITC-conjugated Ig (Wako), serotyped according to the species of primary antibody. In other cases, histochemical staining was performed by standard protocols using diagnostic kits for α-naphthyl acetate esterase, acid phosphatase, alkaline phosphatase (kits 91-A, 387-A, and 86-C, respectively; Sigma-Aldrich), and Oil Red O for visualization of lipid accumulation. Slides were viewed using an Axioplan 2 (Carl Zeiss, Inc.), and images were acquired uisng a DMC-1 digital camera (Polaroid Corp.) and Photoshop 5.0 software (Adobe Systems Inc.).
Table 2.
Phenotypic Characterization of Multicolony-derived Strains of Human Marrow Stromal Cells and Guinea Pig Blood-derived Adherent Cells, and of Single Colony–derived Strains of Human and Rabbit Blood-derived Adherent Cells
| Marker | Rabbit polygonal non-osteogenic | Rabbit fibroblast-like osteogenic | Guinea pig osteogenic | Human fibroblast-like non-osteogenic | Human fibroblast-like osteogenic | Human marrow stromal cells | |
|---|---|---|---|---|---|---|---|
| Fibroblastic | |||||||
| Col I (LF41) | ++ | + | ++ | ++ | ++ | ++ | |
| Col III (LF71) | −/+ | −/+ | + | + | + | ++ | |
| Fibronectin (rabbit; ICN Biomedicals) | ++ | ++ | |||||
| Fibronectin (guinea pig, MsGP13; Serotec) | ++ | ||||||
| CD44 (H9H11) | ++ | ++ | ++ | ||||
| β1 integrin subunit (MAB1959, Chemicon) | ++ | +/++ | ++ | ||||
| Osteogenic | |||||||
| ON (BON-1) | ++ | +/++ | ++ | −/+ | ++ | ++ | |
| OPN (LF123) | +/++ | + | ++ | −/+ | −/+ | −/+ | |
| BSP (LF83) | +/++ | + | + | − | − | −/+ | |
| OC (LF32) | −/+ | −/+ | −/+ | ||||
| Adipocytic | |||||||
| CEBPα (Santa Cruz Biotechnology, Inc.) | −/+ | −/+ | −/+ | ||||
| PPARγ2 (Santa Cruz Biotechnology, Inc.) | − | − | − | − | − | ||
| Endothelial | |||||||
| CD34 (Dako) | − | − | − | ||||
| Muc-18 | − | − | + | ||||
| Factor VIII (Dako) | − | − | − | ||||
| Endoglin | − | − | + | ||||
| PAL-E (rabbit; Accurat Chemical Scientific) | − | − | |||||
| EN4 (guinea pig; Accurat Chemical Scientific) | − | ||||||
| Marrow stromal cells | |||||||
| Stro-1 | − | − | −/+ | ||||
| CD106 (VCAM-1) (human, MCA907; Serotec) | ++ | ++ | + | ||||
| Smooth muscle | |||||||
| α-Smooth muscle actin (1A4; Dako) | + | ++ | ++ | ++ | ++ | −/+/++ | |
| Smooth muscle (rabbit and guinea pig, ACL-10002; Accurat Chemical Scientific) | −/+ | + | +/++ | ||||
| Skeletal muscle | |||||||
| MyoD (M318; Dako) | − | − | − | ||||
| Monocyte/macrophage | |||||||
| CD14 (human, TUK4; Dako) | − | − | − | ||||
| RAM11 (rabbit; Dako) | − | − | |||||
| MR-1 (guinea pig; Serotec) | − | ||||||
| Leukocyte | |||||||
| CD45 (human, 2B11/PD7-26; Dako) | − | − | − | ||||
| CD45 (rabbit, L12/201; Serotec) | − | − | |||||
| CD45 (guinea pig, 1H-1; Serotec) | − | ||||||
| Nerve | |||||||
| Neurofilament (2F11; Dako) | − | − | − | ||||
| Histochemical | |||||||
| Alkaline phosphatase | − | − | − | − | − | −/+/++ | |
| Acid phosphatase | + | + | + | + | + | + | |
| α-Naphthyl acetate esterase | − | − | +/++ | +/++ | + | ||
| Oil Red O (lipid accumulation) | |||||||
| 20% FBS | −/+ | − | −/+ | −/+ | + | −/+ | |
| 20% Rabbit serum | +/++ | −/+/++ | ++ | ++ | ++ | ++ | |
++, intensive staining; +, weak staining; −, negative staining.
In Vivo Transplantation Assay
An in vivo transplantation assay was employed to study the osteogenic potential of the blood-derived adherent cells (see Fig. 1) along with marrow stromal cells (as a positive control) and murine and human fibroblasts (as negative controls), as previously reported (Krebsbach et al. 1997). Cells were trypsinized, pelleted, resuspended in 1 ml of culture medium, and 1.5–3.0 × 106 cells were mixed with 40 mg of hydroxyapatite/tricalcium phosphate ceramic powder (particle size 0.5–1.0 mm, generously provided by Zimmer, Inc.). After a 90-min incubation at 37°C with rotation, the particles with attached cells were collected by a brief centrifugation. A secondary matrix was formed by mixing 15 μl of mouse fibrinogen (3.2 mg/ml in PBS) and 15 μl of mouse thrombin (25 U/ml in 2% CaCl2; Sigma-Aldrich) such that a solid fibrin gel was formed. The transplants were placed in subcutaneous pockets in the backs of 8–15-wk-old immunocompromised female mice (bg-nu/nu-xid; Harlan Sprague Dawley). The transplants were recovered 8 wk later, fixed in 4% phosphate-buffered formalin (Sigma-Aldrich), decalcified in 10% EDTA, pH.8.0 (Quality Biological, Inc.), and embedded in paraffin or glycol methacrylate. 5- or 7-μm sections were prepared from each block and either stained with hematoxylin and eosin (paraffin sections) or Goldner's modified trichrome stain (glycol methacrylate), or used for in situ hybridization (paraffin).
Figure 1.
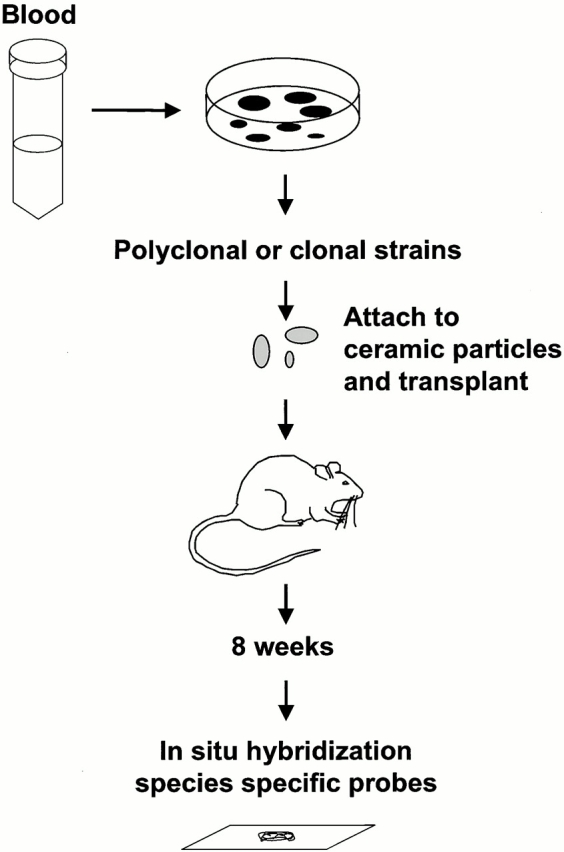
Identification of skeletal stem cells in peripheral blood. Blood obtained from mice, rabbits, guinea pigs, and humans was plated in vitro. In all four species, adherent clonogenic colonies were formed. After ex vivo expansion, they were attached to ceramic particles and transplanted in the subcutis of immunocompromised mice. After 8 wk, the transplants were examined for bone formation. The donor origin of bone in guinea pig and human transplants was determined using species-specific repetitive DNA sequences.
In Situ Hybridization for Species-specific Repetitive DNA Sequences
In situ hybridization for species-specific DNA sequences was used to determine the origin of bone formed in the transplants of guinea pig– and human blood–derived adherent cells. A 3,878-bp long guinea pig repetitive DNA sequence (GenBank accession no. AF318129; provided by Prof. J. Spencer, Queen's University, Kingston, Ontario, Canada, and Dr. J. Fredenburgh, Hamilton Civic Hospitals Research Center, Hamilton, Ontario, Canada) was evaluated, and revealed no homology with either mouse or human genomic DNA in a BLAST search. Several sets of primers were created, and one was chosen based on its performance in PCR and, subsequently, in in situ hybridization. This set was sense: 5′-CTCCTGTCCTGCATCCACT-3′ and antisense: 5′-GGATATGAGAGACAGTGGTG-3′. Two consecutive PCR amplifications were used to generate digoxigenin-labeled guinea pig–specific probes. In the first PCR, Platinum Taq DNA Polymerase kit (Life Technologies) was used with 1 μg of specific primers and 1 μg of either guinea pig or mouse genomic DNA. Using this primer set, one intense band (345 bp) was formed with guinea pig but not mouse genomic DNA. The band was excised and purified using the Qiaex II Gel Extraction Kit (QIAGEN) according to the manufacturer's recommendations. The product was reamplified in the second PCR using the same Platinum Taq DNA Polymerase kit except that 0.1 mM dNTP was substituted with 0.1 mM dATP, 0.1 mM dCTP, 0.1 mM dGTP, 0.065 mM dTTP (PerkinElmer), and 0.035 mM digoxigenin-11-dUTP (Boehringer). The digoxigenin-labeled probe specific for human repetitive alu sequence was created using primers and PCR conditions reported previously (Kuznetsov et al. 1997). The guinea pig– and human-specific digoxigenin-labeled probes were used for in situ hybridization according to a technique specifically modified for decalcified sections of hydroxyapatite-containing transplants (Kuznetsov et al. 1997). Digoxigenin-labeled DNA was detected by immunohistochemistry using antidigoxigenin alkaline phosphatase-conjugated Fab fragments (Boehringer).
Results and Discussion
Clonogenic adherent cells were observed in cultures of peripheral blood from all species investigated, albeit with significant variation in colony forming efficiency (number of colonies per 106 nucleated cells) across animal species, and from one individual donor to another (Table ). In all species except human (in which a total of two colonies were obtained), two distinct types of cell morphology were observed. The majority of the colonies were composed of cells with fibroblastic morphology, but a number of colonies consisted of cells that exhibited a distinctive polygonal shape (Fig. 2). However, upon characterization of cloned strains of both types with a broad panel of markers, the pattern was virtually identical (Table and Fig. 3). This was noted for the lack of expression of hematopoietic (CD45, CD14) and endothelial (endoglin, CD34, Factor VIII–related antigen, Muc-18, PAL-E, EN4) markers, variable expression of osteogenic makers (osteopontin and bone sialoprotein), and the consistent expression of collagen types I and III, fibronectin, osteonectin, α-smooth muscle actin, CD44, VCAM-1, and the β1 integrin subunit. The phenotypic profile of blood-borne adherent cells overall was nearly identical between species (rabbit, guinea pig, and human) and quite similar to that of marrow stromal cells derived from each species. However, the human blood-derived adherent cells were negative for the human marrow stromal marker, Stro-1, as well as for endoglin and Muc-18, all of which are expressed in human marrow stromal cells (Gronthos et al. 2000). In addition, blood-derived cells from all species were negative for alkaline phosphatase, an enzyme commonly expressed in marrow stromal cells from mouse, guinea pig, and humans.
Figure 2.
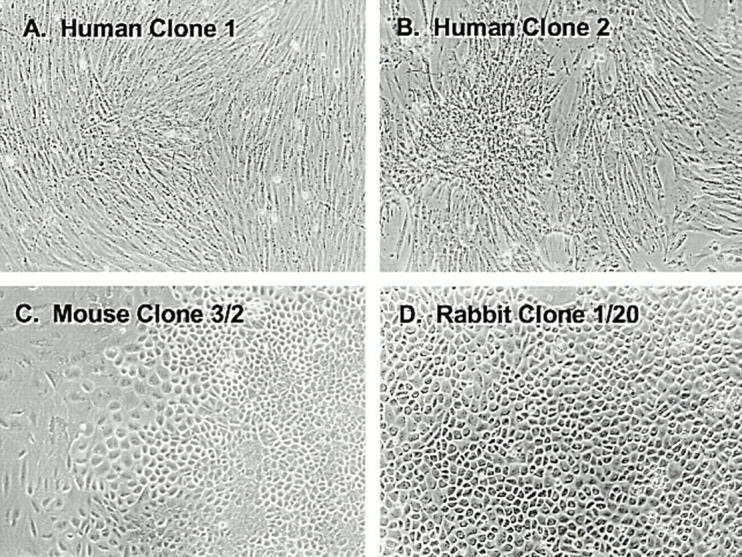
Morphological features of blood-derived adherent clonogenic cells. The majority of clones that developed from all animal species, including the two human colonies (A and B) consisted of cells with a fibroblastic morphology. However, between 5 and 13% of the colonies, depending on the species, had a distinctly polygonal morphology in mouse (C), rabbit (D), and guinea pig (not shown).
Figure 3.
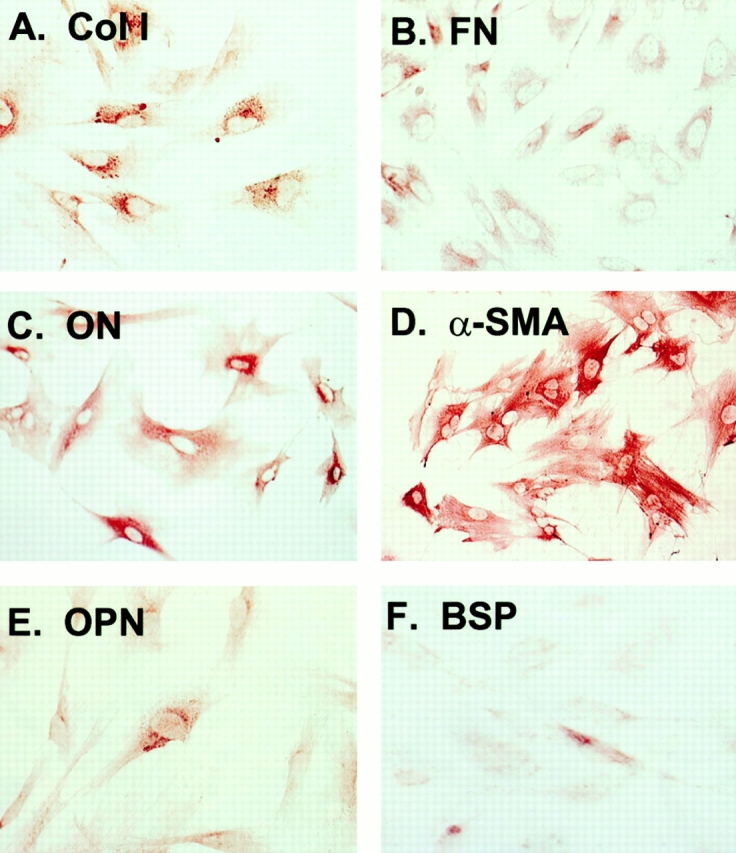
Representative immunophenotype of blood-derived adherent cells. Although two morphologically distinct types of cells were detected in three of the four animal species, their immunophenotype was identical, and this did not vary significantly from one species to another. Their similarity to marrow stromal cells is demonstrated by their expression of (A) type I collagen, (B) fibronectin, (C) osteonectin, and (D) α-smooth muscle actin, and variable expression of (E) osteopontin and (F) bone sialoprotein.
To investigate whether the blood-borne adherent cells had an osteogenic differentiation potential, polyclonal strains from guinea pig and clonal strains from mouse, rabbit, and human cultures were transplanted with a ceramic-based carrier into the subcutis of immunocompromised mice (Fig. 1 and Fig. 4). Histology-proven bone was demonstrated in 12–50% of the strains transplanted, depending on the animal species (Table and Fig. 4). All transplants that contained bone were generated by cells that displayed a fibroblastic morphology in vitro. However, it was not possible to completely rule out an osteogenic potential for the polygonal cells due to their overall lower frequency. A variable proportion of the bone-containing transplants also demonstrated the formation of a complete hematopoietic marrow, including adipocytes. Control transplants of murine and human skin fibroblasts were consistently devoid of bone, as has been reported previously (Krebsbach et al. 1997; Kuznetsov et al. 1997; Satomura et al. 2000) (data not shown). Positive controls using marrow stromal cells from all species consistently generated an ossicle complete with bone and hematopoietic marrow and adipocytes, as has been described (Krebsbach et al. 1997; Kuznetsov et al. 1997) (data not shown).
Figure 4.
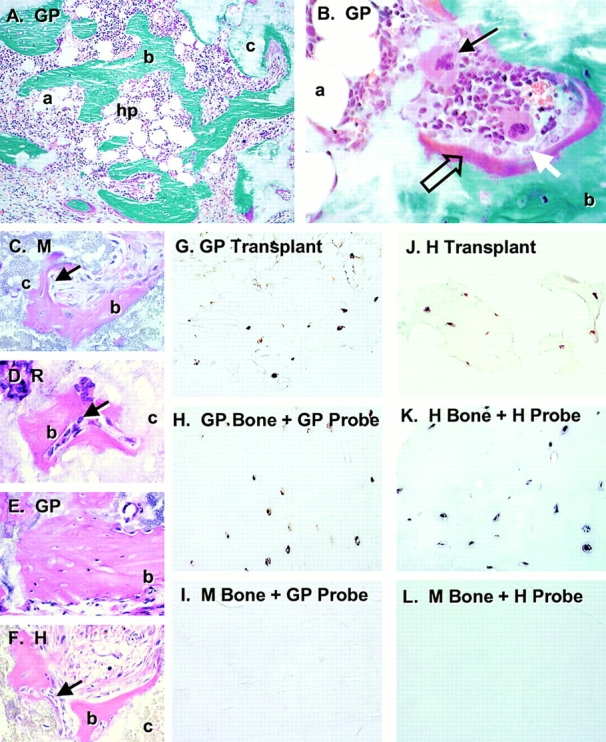
Bone formation by in vivo transplantation of blood-derived adherent cells. Strains of blood-derived adherent cells from guinea pig (GP, in A, B, and E), mouse (M, in C), rabbit (R, in D), and human (H, in F) were attached to ceramic particles (c) and transplanted into the subcutis of immunocompromised mice. After 8 wk, the transplants were harvested and sections were stained with Goldner's to distinguish between bone (b, green in A and B) and osteoid (open arrow, red in B). In all species, bone was deposited on the surfaces of the ceramic particles by mature osteoblasts (white arrow in B, arrows in C, D, and F). In transplants in which there was a significant amount of bone formed, a fully functional hematopoietic marrow (hp) was also established (A and B), complete with megakaryocytes (arrow in B) and adipocytes (a). Sections of paraffin-embedded transplants generated by guinea pig (G) and human (J) blood-derived adherent cells were used for in situ hybridization using a guinea pig–specific sequence (G, H, and I) and a human-specific alu sequence (J, K, and L) as probes. Osteocytes completely embedded in bone were easily identifiable in both guinea pig and human transplants, indicating the donor nature of the cells that formed the bone. Specificity of the probes was demonstrated by the positive reaction of the guinea pig probe with guinea pig bone (H) and negative reaction with mouse bone (I), and by positive reaction of the human probe with human bone (K) and negative reaction with mouse bone (L).
The donor origin of tissues formed in the transplants was documented for guinea pig and human by in situ hybridization using species-specific DNA-repetitive sequences as probes (Fig. 4). This demonstrated that genuine osteoblasts and osteocytes were of donor origin, thus they originated from clonogenic cells that had been expanded in cultures of blood cells. The hematopoietic tissue (particularly prominent in transplants generated by guinea pig cells) was of recipient origin, as expected.
It has been previously documented that osteogenic cells also form marrow adipocytes upon in vivo transplantation. Because of the difficulty in visualizing the nuclei of adipocytes, which are rarely intercepted in tissue sections, we sought to determine the adipogenic potential of the cell strains in vitro. All strains, including marrow stromal cells, were negative for PPARγ2, the adipogenic transcription factor. However, CEBPα was detected by immunohistochemistry in both human clonal strains, and adipogenesis was observed in cells from all species upon culture with rabbit serum, a known inducer of adipogenesis in marrow stromal osteoprogenitor cells (Fig. 5) (Diascro et al. 1998).
Figure 5.
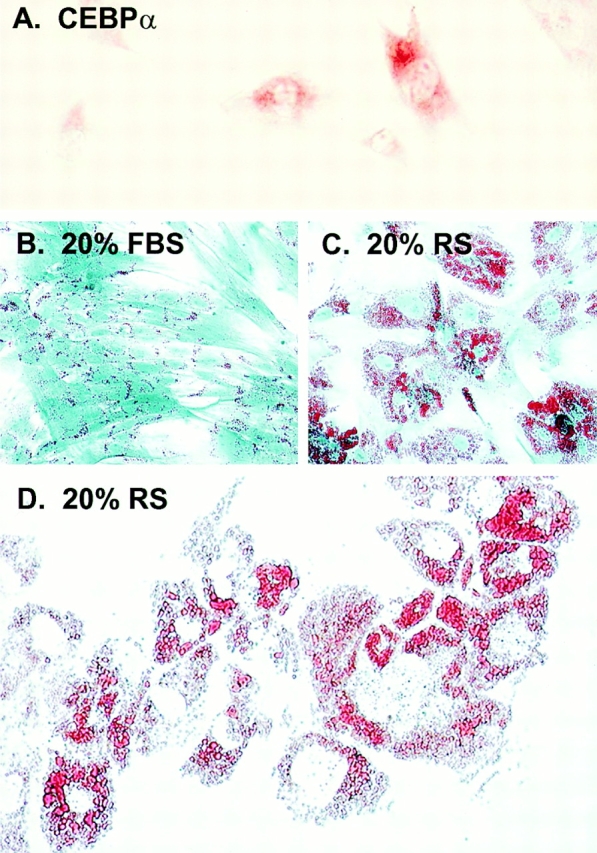
Adipocytic conversion of human blood-derived adherent cells in vitro. Immunohistochemistry of human blood-derived adherent cells revealed expression of the adipocytic transcription factor, CEBPα (A). The ability of blood-derived cells to form adipocyte-like cells was further investigated by assessing lipid accumulation as demonstrated by Oil Red O staining after culture with rabbit serum. As has been seen in marrow stromal cells and in most blood-derived cell types, small lipid-containing structures are present in cultures grown in 20% FBS (B). In cultures grown in 20% rabbit serum, there is a dramatic increase in the number and size of lipid vacuoles (C), which begin to coalesce in vitro (D).
These data show that clonogenic adherent cells of fibroblastic growth habit can be isolated in culture from the adult peripheral blood in a variety of species. A subset of these cells proved to be osteogenic using the best in vivo assay available to date for probing the osteogenic capacity of putative skeletal stem cells. These cells are also capable of accumulating lipid, turning into adipocyte-like cells, upon defined culture conditions in vitro, as do marrow stromal cells in vitro and in vivo (Bianco et al. 1988; Beresford et al. 1992). The formation of a hematopoietic marrow in some transplants in vivo also indicated that a myelosupportive reticular stroma was formed. Thus, blood-borne adherent cells share with marrow stromal stem cells some critical functional features, such as the ability to generate at least three phenotypes of the so-called stromal system (osteoblasts, adipocytes, and reticular cells).
This is, to the best of our knowledge, the first indication that cells with true osteogenic potential, directly demonstrated by the formation of histology-proven bone in vivo, can circulate. Our data clearly demonstrate that cells with multiple differentiation potential similar to that of post-natal marrow stromal stem cells (osteo-adipo-fibrogenic) can negotiate the circulation. Indication of the ability of stromal or other extravascular tissue progenitor cells (e.g., myogenic) to home to their respective peripheral tissues when infused into the circulation has recently been provided (Ferrari et al. 1998; Hou et al. 1999). Our data provide the complementary notion that stem cells of extravascular mesodermal tissues exist (physiologically) in the circulating peripheral blood. The origin of these cells, the mode by which they gain access to the blood stream, and their physiological role are all issues that remain to be properly addressed. However, establishment of their existence provides a significant opportunity for further characterization of their differentiation potential, a novel and accessible source of progenitor cells of solid-phase mesodermal tissues, and an interesting glimpse of a hitherto unrecognized level of circulation-mediated, systemic integration of skeletal physiology.
Acknowledgments
The authors acknowledge Prof. J. Spencer, Dr. J. Fredenburgh, Dr. Larry W. Fisher, and Zimmer, Inc.
This work was supported by the Telethon Fondazione Onlus (grant E1029 to P. Bianco).
References
- Beresford J.N., Bennett J.H., Devlin C., Leboy P.S., Owen M.E. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J. Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- Bianco P., Cossu G. Uno, nessuno e centomilasearching for the identity of mesodermal progenitors. Exp. Cell Res. 1999;251:257–263. doi: 10.1006/excr.1999.4592. [DOI] [PubMed] [Google Scholar]
- Bianco P., Costantini M., Dearden L.C., Bonucci E. Alkaline phosphatase positive precursors of adipocytes in the human bone marrow. Br. J. Haematol. 1988;68:401–403. doi: 10.1111/j.1365-2141.1988.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Bianco P., Riminucci M. The bone marrow stroma in vivoontogeny, structure, cellular composition and changes in disease. In: Beresford J.N., Owen M., editors. Marrow Stromal Cell Cultures. Cambridge University Press; Cambridge, UK: 1998. pp. 10–25. [Google Scholar]
- Cossu G., Tajbakhsh S., Buckingham M. How is myogenesis initiated in the embryo? Trends Genet. 1996;12:218–223. doi: 10.1016/0168-9525(96)10025-1. [DOI] [PubMed] [Google Scholar]
- Diascro D.D., Vogel R.L., Johnson T.E., Witherup K.M., Pitzenberger S.M., Rutledge S.J., Prescott D.J., Rodan G.A., Schmidt A. High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. J. Bone Miner. Res. 1998;13:96–106. doi: 10.1359/jbmr.1998.13.1.96. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Cusella-De Angelis G., Coletta M., Paolucci E., Stornaiuolo A., Cossu G., Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Filshie R.J., Zannettino A.C., Makrynikola V., Gronthos S., Henniker A.J., Bendall L.J., Gottlieb D.J., Simmons P.J., Bradstock K.F. MUC18, a member of the immunoglobulin superfamily, is expressed on bone marrow fibroblasts and a subset of hematological malignancies. Leukemia. 1998;12:414–421. doi: 10.1038/sj.leu.2400922. [DOI] [PubMed] [Google Scholar]
- Fisher L.W., Stubbs J.T., III, Young M.F. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop. Scand. 1995;266:61–65. [PubMed] [Google Scholar]
- Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic transplants of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- Friedenstein A.J., Piatetzky-Shapiro I.I., Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci .USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Nguyen Q., Frenkel B., Nilsson S.K., Milne M., van Wijnen A.J., Stein J.L., Quesenberry P., Lian J.B., Stein G.S. Osteoblast-specific gene expression after transplantation of marrow cellsimplications for skeletal gene therapy. Proc. Natl. Acad. Sci. USA. 1999;96:7294–7299. doi: 10.1073/pnas.96.13.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebsbach P.H., Kuznetsov S.A., Satomura K., Emmons R.V., Rowe D.W., Robey P.G. Bone formation in vivocomparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S., Gehron Robey P. Species differences in growth requirements for bone marrow stromal fibroblast colony formation in vitro. Calcif. Tissue Int. 1996;59:265–270. doi: 10.1007/s002239900121. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S.A., Krebsbach P.H., Satomura K., Kerr J., Riminucci M., Benayahu D., Robey P.G. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J. Bone Miner. Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- Owen M. Marrow stromal stem cells. J. Cell Sci. 1988;10:63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- Satomura K., Krebsbach P., Bianco P., Ghernon Robey P. Osteogenic imprinting upstream of marrow stromal cell differentiation. J. Cell Biochem. 2000;78:391–403. [PubMed] [Google Scholar]
- Simmons P.J., Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Zannettino A.C., Rayner J.R., Ashman L.K., Gonda T.J., Simmons P.J. A powerful new technique for isolating genes encoding cell surface antigens using retroviral expression cloning. J. Immunol. 1996;156:611–620. [PubMed] [Google Scholar]


