Neurotransmission occurs at synapses, specialized points of contact between presynaptic nerve terminals and postsynaptic neurons. At excitatory synapses, receptors and downstream signaling enzymes are clustered in the postsynaptic density (PSD), a cytoskeletal web beneath the plasma membrane. Transmission at these excitatory synapses is mediated primarily by glutamate acting on two classes of ligand-gated ion channels: AMPA receptors and NMDA receptors. The AMPA receptors are involved in moment-to-moment signaling, whereas NMDA receptors play an important role in initiating synaptic plasticity.
Recent cell biological analyses have emphasized remarkable differences in regulation of the synaptic expression of these two classes of receptors (Malenka and Nicoll 1999; Malinow et al. 2000). NMDA receptors are stable components of the PSD, whereas AMPA receptors cycle on and off the synaptic membrane in a manner that is tightly controlled by neuronal activity (Lüscher et al. 1999; Beattie et al. 2000; Ehlers 2000; Lin et al. 2000). Regulated insertion and removal of AMPA receptors at the synapse provides a mechanism for altering synaptic efficacy, and for storing information in the brain (Malenka and Nicoll 1999; Malinow et al. 2000). This has lead to intensive study of the molecular architecture that recruits and anchors glutamate receptors at the synapse.
A key breakthrough in understanding mechanisms for synapse assembly came from the discovery that proteins containing PSD-95/SAP-90, Discs-large, ZO-1 homologous domian (PDZ) motifs play central roles in scaffolding receptors and signaling elements (Kennedy 1997; Craven and Bredt 1998; Hsueh and Sheng 1998; Garner et al. 2000). The prototypical PDZ protein, PSD-95, is a membrane-associated guanylate kinase (MAGUK) that contains three PDZ domains and associates with NMDA receptors at the synapse. Three other neuronal MAGUKs, PSD-93, synapse-associated protein (SAP)-97, and SAP-102, are also expressed in neurons throughout the brain. The first and second PDZ domains of PSD-95, and the other neuronal MAGUKs, bind to the extreme COOH termini of NMDA receptor subunits and certain other proteins that terminate with a Ser/Thr-X-Val motif. In addition, the second PDZ domain from PSD-95 binds to a PDZ domain at the NH2 terminus of neuronal nitric oxide synthase (Brenman et al. 1996). By bridging the NMDA receptor to neuronal nitric oxide synthase, PSD-95 functions as a scaffold to enhance activation of calmodulin-dependent neuronal nitric oxide synthase activity by Ca2+ influx through the NMDA receptor (Sattler et al. 1999).
Membrane-associated Guanylate Kinases in Synaptic Development
In addition to its three PDZ domains, PSD-95 contains an SH3 motif and a region homologous to guanylate kinase (GK). Classically, SH3 domains associate with proline-containing peptides that conform to the consensus P-X-X-P. Indeed, the SH3 domain of PSD-95 has been shown to associate with two such proline-containing motifs in the kainate receptor subunit 2 (Garcia et al. 1998). However, the SH3 domain of PSD-95 and related MAGUK proteins lacks a critical tyrosine (corresponding to Y136 in src) that is conserved in SH3 domains, and is critical for binding to proline-containing peptides. Therefore, it will be interesting to determine how SH3 domains from PSD-95 or other MAGUKs bind structurally to their targets.
The GK domain of PSD-95 is catalytically inactive, but has been shown to bind to specific neuronal proteins including microtubule associated protein (MAP)1A (Brenman et al. 1998), guanylate kinase–associated kinesin (GAKIN) (Hanada et al. 2000), and guanylate kinase–associated protein (GKAP) (Kim et al. 1997; Takeuchi et al. 1997). As microtubules are not present in dendritic spines or at the PSD, interactions with MAP1A and GAKIN likely participate in transport of PSD-95 in dendrites. On the other hand, GKAP is a postsynaptic protein of the PSD, which links PSD-95 to another large protein complex built around the protein Shank (Naisbitt et al. 1999).
In addition to binding exogenous ligands, the SH3 and GK domains of MAGUKs associate with each other in an intramolecular fashion (McGee and Bredt 1999; Shin et al. 2000). This is reminiscent of regulatory interactions in Src family kinases, in which the SH3 domain autoinhibits the kinase. The precise role for SH3–GK binding in MAGUKs is unclear. However, many previously identified genetic mutations of MAGUKs in invertebrates occur in the SH3 or GK domains, and all of these mutations disrupt intramolecular SH3–GK binding. Furthermore, mutations that block the SH3–GK interaction prevent receptor clustering by PSD-95 (Shin et al. 2000). Interestingly, calmodulin binds to a conserved basic amphipathic α-helix between the SH3 and GK domains of SAP-102, and promotes multimerization of SAP-102 (Masuko et al. 1999). It will be important to understand the details of these regulatory mechanisms in order to elucidate how they may participate in receptor clustering by MAGUK proteins.
MAGUK proteins PSD-95 and PSD-93 can also multimerize via sequences at their NH2 termini (Kim et al. 1996). Mutagenic analysis revealed that two critical cysteine residues at positions 3 and 5 of PSD-95 are essential for protein multimerization and for receptor clustering (Hsueh et al. 1997). These cysteines are also modified by palmitate, a 16-carbon fatty acid that is linked by thioester bonds to PSD-95 (Topinka and Bredt 1998). Palmitoylation is essential for postsynaptic clustering of PSD-95, presumably because this lipid modification targets PSD-95 to endosomal vesicles that are trafficked to the synapse (Craven et al. 1999; El-Husseini et al. 2000a). Protein palmitoylation is a reversible process, akin to phosphorylation, that is dynamically regulated by specific stimuli. Indeed, activation of glutamate receptors appears to rapidly depalmitoylate PSD-95 at the synapse (Husseini, A., E. Schnell, S. Dakoji, R.A. Nicoll, and D.S. Bredt, unpublished observations). Future studies should elucidate how palmitate cycling on PSD-95 regulates its synaptic functions.
By acting as a molecular scaffold, PSD-95 and related MAGUKs participate in synapse development and plasticity. Mutations in Drosophila-Discs large alter postsynaptic structure and plasticity of larval neuromuscular junctions (Lahey et al. 1994). Consistent with this developmental role, overexpression of PSD-95 in hippocampal neurons drives maturation of excitatory synapses, as evidenced by enhanced synaptic clustering and function of AMPA receptors (El-Husseini et al. 2000b). Interestingly, PSD-95 overexpression also mediates a retrograde signal to enhance maturation and function of presynaptic nerve terminals that synapse upon neurons overexpressing PSD-95 (El-Husseini et al. 2000b). This retrograde signal may be mediated via the neuronal adhesion protein neuroligin, which binds to PSD-95 and can trigger formation of presynaptic nerve terminals by linking to the presynaptic protein neurexin (Scheiffele et al. 2000). Overexpressing the PSD-95 binding protein Shank also promotes pre- and postsynaptic development in a manner that is enhanced by Homer, a protein that interacts with metabotropic glutamate receptors (Sala et al. 2001). Future studies are needed to establish the interdependence and hierarchies of these postsynaptic protein complexes in mediating synaptic development.
Targeted disruption of PSD-95 in mice alters synaptic plasticity, such that long-term potentiation (LTP) is enhanced, and long-term depression (LTD) is eliminated, fitting with roles for PSD-95 in mediating signaling downstream of NMDA. Although one might have expected that disrupting PSD-95 would alter synaptic development, no abnormalities in synaptic structure were detected in PSD-95 mutant mice (Migaud et al. 1998). This normalcy may be explained by molecular redundancy, as four MAGUKs are expressed at the PSD of neurons. Furthermore, several additional PDZ proteins interact with NMDA at synapses including synaptic scaffolding molecule (Hirao et al. 1998) and mouse homologues of Lin-7/MALS (Jo et al. 1999). Genetic analysis of these complex families of postsynaptic scaffolding proteins will likely require multiple compound knockouts.
Regulation of Synaptic AMPA Receptors by PDZ Proteins
Whereas PDZ domains associate firmly with NMDA receptors at the PSD, PDZ interactions with AMPA receptors can be dynamically regulated and appear to participate in synaptic plasticity. The most studied forms of synaptic plasticity are LTP and LTD, in which brief repetitive stimulation of excitatory synapses results in either a long-lasting increase or decrease in synaptic strength, depending on the pattern of stimulation. Both LTP and LTD depend on activation of NMDA receptors and a rise in spine calcium; the magnitude of the rise dictates whether LTP or LTD occurs.
Whereas NMDA receptors clearly control induction of LTP, the mechanisms underlying expression of LTP have been more difficult to define. Considerable experimental evidence indicates that LTP involves postsynaptic enhancement of AMPA receptor responses (Malenka and Nicoll 1999; Malinow et al. 2000). However, a postsynaptic locus for LTP expression seemed inconsistent with the fact that LTP induction decreases the synaptic failure rate, which typically indicates an increase in transmitter release, a presynaptic change. A solution to this paradox was the discovery of silent synapses, which possess NMDA receptors, but no functional AMPA receptors (Malenka and Nicoll 1999; Malinow et al. 2000). Importantly, LTP turns on or “AMPAfies” silent synapses, which explains the decreased failure rate.
How does NMDA receptor–dependent LTP control synaptic activity of AMPA receptors? Considerable evidence suggests that Ca2+ influx via NMDA receptors activates calmodulin-dependent kinase (CaMK)II. This leads to the phosphorylation of the intracellular COOH terminus (Ser831) of GluR1, an AMPA receptor subunit, and increases the single channel conductance (Scannevin and Huganir 2000; Soderling 2000). However, this mechanism does not explain activation of silent synapses, which would seem to require rapid insertion of AMPA receptors. In support of this receptor–insertion model, it was noted that manipulations that interfere with membrane-fusion events block LTP (Lledo et al. 1998). Furthermore, using two photon imaging tags as well as an electrophysiological tag, Malinow et al. 2000 found that AMPA receptor subunits move into dendritic spines (Shi et al. 1999) and are inserted into the synaptic membrane during LTP (Hayashi et al. 2000).
Recent studies indicate that PDZ proteins play a major role in this regulated insertion of AMPA receptors during LTP. AMPA receptors are tetramers assembled from four subunits, GluR1–GluR4; most AMPA receptors contain at least a GluR1 and a GluR2 subunit. The COOH terminus of GluR1 contains a PDZ-binding motif that interacts with the second PDZ domain of SAP-97 (Leonard et al. 1998). Despite the high sequence homology between PDZ domains of SAP-97 and PSD-95, GluR1 interacts only with SAP-97. This COOH-terminal PDZ-binding site on GluR1 appears critical for CaMKII-dependent insertion of AMPA receptors, as mutations of the site block plasticity in hippocampal slice cultures. As CaMKII does not directly phosphorylate this site (Thr887), it is postulated that CaMKII phosphorylates an intermediary protein with a PDZ domain, and binding of this protein to GluR1 is critical for receptor delivery to the synapse (Hayashi et al. 2000).
The COOH terminus of GluR2 binds to a pair of multi-PDZ proteins, GRIP/APB (Dong et al. 1997; Srivastava et al. 1998), and also to a single PDZ protein, PICK1 (Xia et al. 1999). Interestingly, phosphorylation of the GluR2 PDZ-binding site by protein kinase (PK)C selectively disrupts binding of GluR2 to GRIP/ABP, but leaves PICK1 binding intact (Matsuda et al. 1999; Chung et al. 2000). These PDZ interactions with GluR2 can differentially participate in synaptic plasticity. In sensory relay neurons of the spinal cord, interaction of AMPA receptors with GRIP appears to be necessary for recruitment of AMPA receptors to silent synapses (Li et al. 1999). On the other hand, induction of LTD in cerebellar Purkinje cells may involve GluR2 interaction with PICK1, as disrupting GluR2–PICK1 interactions inhibits LTD in cerebellum (Xia et al. 2000). Therefore, PKC-mediated induction of LTD in cerebellum may be mediated by phosphorylation of the PDZ- binding site of GluR2. In addition to this PDZ binding, the COOH terminus of GluR2 interacts with NSF (Nishimune et al. 1998; Osten et al. 1998; Song et al. 1998; Noel et al. 1999), a major mediator of membrane fusion. This NSF interaction appears to regulate expression of AMPA receptors at the synapse, as disrupting NSF binding decreases AMPA receptor responses at synapses and occludes LTD (Lüscher et al. 1999; Lüthi et al. 1999).
Stargazin Is Needed for Functional AMPA Receptors in Cerebellar Granule Cells
Stargazin, which is mutated in epileptic stargazer mice (Letts et al. 1998), is the first transmembrane protein found to interact with glutamate receptors (Chen et al. 2000). In addition to suffering epilepsy, stargazer mice exhibit cerebellar ataxia. Physiological studies showed that synaptic AMPA receptor currents are selectively absent from synapses on cerebellar granule cells, though NMDA-mediated responses are normal at these same synapses (Chen et al. 1999; Hashimoto et al. 1999). Despite this lack of functional AMPA receptors, the levels of the AMPA receptor subunits GluR2 and GluR4 appear normal in stargazer cerebellum. The lack of synaptic AMPA responses reflects a block in AMPA receptor trafficking to the synapse, as electron microscopic analysis with immunogold labeling shows a profound loss of synaptic AMPA receptors (Chen et al. 2000). In addition to disrupting synaptic AMPA receptors, lack of stargazin also abolishes AMPA receptor responses at extrasynaptic sites (Chen et al. 2000).
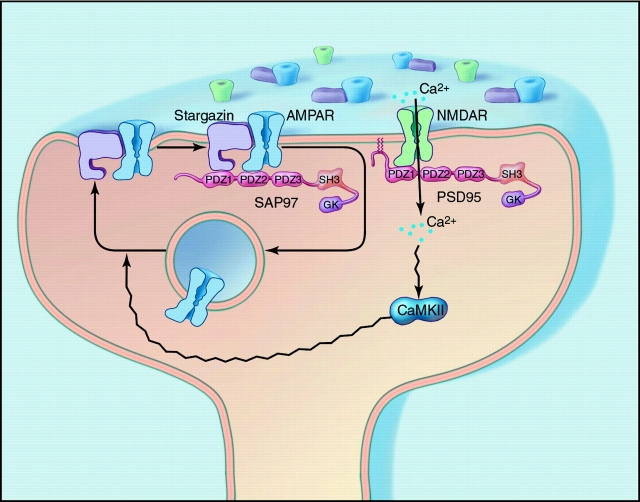
The structure of stargazin helps explain these dual roles in regulation of AMPA receptors. Stargazin is a tetraspanin, a protein with four transmembrane domains (Letts et al. 1998). The cytosolic COOH-terminal tail of stargazin contains a PDZ-binding site, which binds to type 1 PDZ domains from PSD-95 and related proteins. This PDZ-binding site is critical for synaptic function of AMPA receptors, as transfecting mutant granule cells with a stargazin construct lacking the extreme COOH terminus (stargazinΔC) does not rescue synaptic responses. Interestingly, stargazinΔC, which also binds to AMPA receptors, does rescue extrasynaptic AMPA receptor responses (Chen et al. 2000). These data suggest that stargazin mediates two distinct steps in trafficking AMPA receptors to the synapse (Fig. 1). First, the transmembrane domains of stargazin interact with AMPA receptors and regulate the delivery of AMPA receptors to the cell surface. Second, interaction of the COOH terminal PDZ-binding site of stargazin with PSD-95 or a related PDZ protein targets the AMPA receptors to synapses.
Figure 1.
Differential regulation of synaptic glutamate receptors by PDZ proteins. NH2-terminal palmitoylation anchors PSD-95 at the postsynaptic density, and PDZ domains from PSD-95 associate with the COOH termini of NMDA receptor subunits. By contrast, AMPA receptor delivery to the plasma membrane requires stargazin. Synaptic targeting of AMPA receptors requires stargazin binding to PDZ proteins such as SAP-97, which additionally binds the COOH terminus of the GluR1 subunit of AMPA receptors. Whereas NMDA receptors are firmly anchored at the postsynaptic membrane, synaptic expression of AMPA receptors is dynamically regulated by neuronal activity. During intense synaptic stimulation, calcium influx through NMDA receptors activates CaMKII, which mediates insertion of synaptic AMPA receptors.
Why are AMPA receptor defects in stargazer mice restricted to cerebellar granule cells? This may be explained by redundancy, as at least two close homologues of stargazin, γ-3 and γ-4, are expressed in neurons. Cerebellar granule cells appear unique in expressing only stargazin, which may explain the selective AMPA-receptor defect in these cells. Indeed, stargazin-like mechanisms appear to regulate AMPA receptors in hippocampus, as transfecting hippocampal neurons with stargazinΔC selectively reduces the amplitude of AMPA receptor synaptic currents (Chen et al. 2000). Targeted disruption of γ-3 and γ-4 homologues of stargazin should help determine the essential roles for this pathway in regulating AMPA receptors in forebrain.
The Stargazin Family Is Related to Claudins
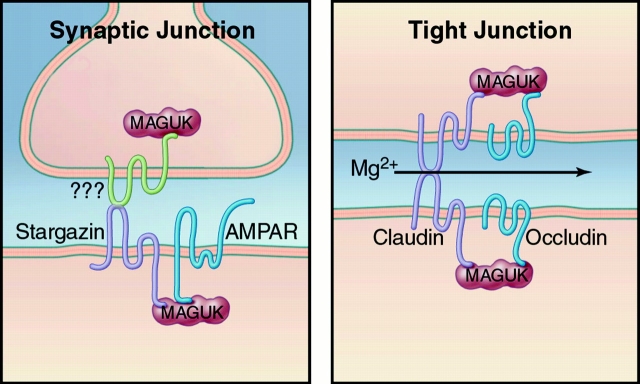
In addition to having high homology to γ-3 and γ-4, stargazin is more distantly related to the γ-1 subunit of the skeletal muscle, voltage-dependent calcium channel, and to a large family of claudin proteins. Claudin1 was originally isolated as a major constituent of liver-tight junctions (Furuse et al. 1998). Subsequent molecular cloning has identified >20 claudin isoforms, which are found at sites of cell–cell contact in a variety of tissues. By analogy to COOH-terminal PDZ-binding site on stargazin, many claudin isoforms terminate with the residues Tyr-Val. This COOH-terminal site on claudins binds to the first PDZ domain of the zonula occludens-1, an epithelial cell MAGUK protein that is closely related to PSD-95 (Itoh et al. 1999). These data point to a general role for claudin (stargazin)–MAGUK protein complexes in regulating or constructing sites of cell–cell contact. Whereas epithelial tight junctions and neuronal synapses serve very different functions, evolution appears to have selected certain similar structural elements to construct these two types of cellular junctions (Fig. 2).
Figure 2.
Tetraspanin–MAGUK complexes regulate both synapses and tight junctions. At neuronal synapses, the tetraspanin, stargazin associates with AMPA receptors and interacts with PDZ domains from neuronal MAGUKs. At tight junctions, a related protein complex is formed between claudin and epithelial cell MAGUKs, which also bind to occludin. Extracellular interactions of claudin proteins bridge cells at tight junctions, and can regulate paracellular flux of Mg2+. Whether stargazin participates in analogous transsynaptic interactions is uncertain.
Might the homology between stargazin and claudins provide some insight concerning the functions of these related tetraspanins? Mutation of claudin-11, an oligodendrocyte-specific protein, disrupts tight junctions in compact myelin (Gow et al. 1999), whereas mutations of claudin-16 disrupt Mg2+ resorption across tight junctions of tubule cells in kidney (Simon et al. 1999). And, very recent studies show that claudin-14 mutations cause hereditary deafness (Wilcox et al. 2001). Freeze fracture reveals that claudins form the strands of intramembranous protein particles at tight junctions, indicating a role for claudins in cell adhesion (Furuse et al. 1998). Whereas electron microscopic studies indicate a grossly normal structure for granule cell synapses in stargazer mice (Chen et al. 2000), an intriguing possibility is that stargazin and its homologues might participate in aspects of adhesion at neuronal synapses. Alternatively, claudins may regulate receptor trafficking in epithelial cells.
Conclusion
In summary, it has become clear that PDZ-containing proteins play a central role in assembling receptors and associated signaling enzymes at synapses and other sites of cell–cell contract. By directly binding the COOH termini of NR2 subunits receptors, PDZ proteins firmly anchor NMDA receptors at the PSD. The coupling of AMPA receptors to the synaptic PDZ scaffold is less tight and can involve an accessory protein, stargazin. Might stargazin interactions with AMPA receptors participate in synaptic plasticity? Since it is well accepted that CaMKII is an essential mediator of LTP and that other protein kinases, such as PKA and PKC, may play important modulatory roles, it will be of interest to determine if stargazin is a target for phosphorylation by these various kinases and if so, whether this phosphorylation plays a role in synaptic plasticity.
Acknowledgments
R.A. Nicoll is a member of the Keck Center for Integrative Neuroscience, and the Silvo Conte Center for Neuroscience Research. D.S. Bredt is an established investigator for the American Heart Association.
Our research is supported by grants from the National Institutes of Health (to D.S. Bredt and R.A. Nicoll), the Howard Hughes Medical Institute Research Resources Program (to D.S. Bredt), and the Human Frontier Research Program (to D.S. Bredt).
Footnotes
Abbreviations used in this paper: CaMK, calmodulin-dependent kinase; GAKIN, guanylate kinase–associated kinesin; GK, guanylate kinase; GKAP, guanylate kinase–associated protein; LTD, long-term depression; LTP, long-term potentiation; MAGUK, membrane-associated guanylate kinase; MAP1A, microtubule-associated protein; PDZ, PSD-95/SAP-90, Discs-large, ZO-1 homologous domain; PK, protein kinase; PSD, postsynaptic density; SAP, synapse-associated protein; stargazinΔC, stargazin construct lacking the extreme COOH terminus.
References
- Beattie E.C., Carroll R.C., Yu X., Morishita W., Yasuda H., von Zastrow M., Malenka R.C. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat. Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Brenman J.E., Chao D.S., Gee S.H., McGee A.W., Craven S.E., Santillano D.R., Huang F., Xia H., Peters M.F., Froehner S.C. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α-1 syntrophin mediated by PDZ motifs. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Brenman J.E., Topinka J.R., Cooper E.C., McGee A.W., Rosen J., Milroy T., Ralston H.J., Bredt D.S. Localization of postsynaptic density-93 to dendritic microtubules and interaction with microtubule-associated protein 1A. J. Neurosci. 1998;18:8805–8813. doi: 10.1523/JNEUROSCI.18-21-08805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Bao S., Qiao X., Thompson R.F. Impaired cerebellar synapse maturation in waggler, a mutant mouse with a disrupted neuronal calcium channel gamma subunit. Proc. Natl. Acad. Sci. USA. 1999;96:12132–12137. doi: 10.1073/pnas.96.21.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Chetkovich D.M., Petrailia R.S., Sweeney N.T., Kawaski Y., Wenthold R.J., Bredt D.S., Nicoll R.A. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chung H.J., Xia J., Scannevin R.H., Zhang X., Huganir R.L. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J. Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven S.E., Bredt D.S. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93:495–498. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- Craven S.E., Husseini A.E., Bredt D.S. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Dong H., O'Brien R.J., Fung E.T., Lanahan A.A., Worley P.F., Huganir R.L. GRIPa synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Ehlers M.D. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- El-Husseini A.E., Craven S.E., Chetkovich D.M., Firestein B.L., Schnell E., Aoki D., Bredt D.S. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering J. Cell Biol. 148 2000. 159 172a [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini A.E., Schnell E., Chetkovich D.M., Nicoll R.A., Bredt D.S. PSD-95 involvement in maturation of excitatory synapses Science. 290 2000. 1364 1368b [PubMed] [Google Scholar]
- Furuse M., Fujita K., Hiiragi T., Fujimoto K., Tsukita S. Claudin-1 and -2novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E.P., Mehta S., Blair L.A.C., Wells D.G., Shang J., Fukushima T., Fallon J., Garner C.C., Marshall J. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Garner C.C., Nash J., Huganir R.L. PDZ domains in synapse assembly and signaling. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- Gow A., Southwood C.M., Li J.S., Pariali M., Riordan G.P., Brodie S.E., Danias J., Bronstein J.M., Kachar B., Lazzarini R.A. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- Hanada T., Lin L., Tibaldi E.V., Reinherz E.L., Chishti A.H. GAKIN, a novel kinesin-like protein associates with the human homologue of the Drosophila discs large tumor suppressor in T lymphocytes. J. Biol. Chem. 2000;275:28774–28784. doi: 10.1074/jbc.M000715200. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Fukaya M., Qiao X., Sakimura K., Watanabe M., Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J. Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Shi S.H., Esteban J.A., Piccini A., Poncer J.C., Malinow R. Driving AMPA receptors into synapses by LTP and CaMKIIrequirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hirao K., Hata Y., Ide N., Takeuchi M., Irie M., Yao I., Deguchi M., Toyoda A., Sudhof T.C, Takai Y. A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins. J. Biol. Chem. 1998;273:21105–21110. doi: 10.1074/jbc.273.33.21105. [DOI] [PubMed] [Google Scholar]
- Hsueh Y.P., Kin E., Sheng M.M. Disulfide-linked head-to-head multimerization in the mechanism of ion channel clustering by PSD-95. Neuron. 1997;18:803–814. doi: 10.1016/s0896-6273(00)80319-0. [DOI] [PubMed] [Google Scholar]
- Hsueh Y.P., Sheng M. Anchoring of glutamate receptors at the synapse. Prog. Brain Res. 1998;116:123–131. doi: 10.1016/s0079-6123(08)60434-3. [DOI] [PubMed] [Google Scholar]
- Itoh M., Furuse M., Morita K., Kubota K., Saitou M., Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo K., Derin R., Li M., Bredt D.S. Characterization of MALS/Velis-1, -2, and -3a family of mammalian LIN-7 homologs enriched at brain synapses in association with the postsynaptic density-95/NMDA receptor postsynaptic complex. J. Neurosci. 1999;19:4189–4199. doi: 10.1523/JNEUROSCI.19-11-04189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.B. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- Kim E., Cho K.-O., Rothschild A., Sheng M. Heteromultimerization and NMDA receptor clustering activity of chapsyn-110, a novel member of the PSD-95 family of synaptic proteins. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- Kim E., Naisbitt S.S., Hsueh Y.P., Rao A., Rothschild A., Craig A.M., Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J. Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey T., Gorczyca M., Jia X.X., Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard A.S., Davare M.A., Horne M.C., Garner C.C., Hell J.W. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J. Biol. Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- Letts V.A., Felix R., Biddlecome G.H., Arikkath J., Mahaffey C.L., Valenzuela A., Bartlett F.S., 2nd, Mori Y., Campbell K.P., Frankel W.N. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat. Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- Li P., Kerchner G.A., Sala C., Wei F., Huettner J.E., Sheng M., Zhuo M. AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nat. Neurosci. 1999;2:972–977. doi: 10.1038/14771. [DOI] [PubMed] [Google Scholar]
- Lin J.W., Ju W., Foster K., Lee S.H., Ahmadian G., Wyszynski M., Wang Y.T., Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat. Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Lledo P.M., Zhang X., Südhof T.C., Malenka R.C., Nicoll R.A. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- Lüscher C., Xia H., Beattie E.C., Carroll R.C., von Zastrow M., Malenka R.C., Nicoll R.A. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Lüthi A., Chittajallu R., Duprat F., Palmer M.J., Benke T.A., Kidd F.L., Henley J.M., Isaac J.T., Collingridge G.L. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron. 1999;24:389–399. doi: 10.1016/s0896-6273(00)80852-1. [DOI] [PubMed] [Google Scholar]
- Malenka R.C., Nicoll R.A. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R., Mainen Z.F., Hayashi Y. LTP mechanismsfrom silence to four-lane traffic. Curr. Opin. Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Masuko N., Makino K., Kuwahara H., Fukunaga K., Sudo T., Araki N., Yamamoto H., Yamada Y., Miyamoto E., Saya H. Interaction of NE-dlg/SAP102, a neuronal and endocrine tissue-specific membrane-associated guanylate kinase protein, with calmodulin and PSD-95/SAP90. A possible regulatory role in molecular clustering at synaptic sites. J. Biol. Chem. 1999;274:5782–5790. doi: 10.1074/jbc.274.9.5782. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Mikawa S., Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J. Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- McGee A.W., Bredt D.S. Identification of an intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. J. Biol. Chem. 1999;274:17431–17436. doi: 10.1074/jbc.274.25.17431. [DOI] [PubMed] [Google Scholar]
- Migaud M., Charlesworth P., Dempster M., Webster L.C., Watabe A.M., Makhinson M., He Y., Ramsay M.F., Morris R.G., Morrison J.H., O'Dell T.J., Grant S.G. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Naisbitt S., Kim E., Tu J.C., Xiao B., Sala C., Valtschanoff J., Weinberg R.J., Worley P.F., Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Nishimune A., Isaac J.T., Molnar E., Noel J., Nash S.R., Tagaya M., Collingridge G.L., Nakanishi S., Henley J.M. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Noel J., Ralph G.S., Pickard L., Williams J., Molnar E., Uney J.B., Collingridge G., Henley J.M. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- Osten P., Srivastava S., Inman G.J., Vilim F.S., Khatri L., Lee L.M., States B.A., Einheber S., Milner T.A., Hanson P.I., Ziff E.B. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- Sala C., Piech V., Wilson N.R., Passafaro M., Liu G., Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;In press doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Sattler R., Xiong Z., Lu W.-Y., Hafner M., MacDonald J.F., Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- Scannevin R.H., Huganir R.L. Postsynaptic organization and regulation of excitatory synapses. Nat. Rev. Neurosci. 2000;1:133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- Scheiffele P., Fan J., Chioh J., Fetter R., Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development of contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Shi S.H., Hayashi Y., Petralia R.S., Zaman S.H., Wenthold R.J., Svoboda K., Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shin H., Hsueh Y.P., Yang F.C., Kim E., Sheng M. An intramolecular interaction between Src homology 3 domain and guanylate kinase-like domain required for channel clustering by postsynaptic density-95/SAP90. J. Neurosci. 2000;20:3580–3587. doi: 10.1523/JNEUROSCI.20-10-03580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D.B., Lu Y., Choate K.A., Velazquez H., Al-Sabban E., Praga M., Casari G., Bettinelli A., Colussi G., Rodriguez-Soriano J., McCredie D., Milford D., Sanjad S., Lifton R.P. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- Soderling T.R. CaM-kinasesmodulators of synaptic plasticity. Curr. Opin. Neurobiol. 2000;10:375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Song I., Kamboj S., Xia J., Dong H., Liao D., Huganir R.L. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Osten P., Vilim F.S., Khatri L., Inman G., States B., Daly C., DeSouza S., Abagyan R., Valtschanoff J.G., Weinberg R.J., Ziff E.B. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Hata Y., Kirao K., Toyoda A., Irie M., Takai Y. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J. Biol. Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- Topinka J.R., Bredt D.A. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel, Kv1.4. Neuron. 1998;20:125–134. doi: 10.1016/s0896-6273(00)80440-7. [DOI] [PubMed] [Google Scholar]
- Wilcox E.R., Burton Q.L., Naz S., Riazuddin S., Smith T.N., Ploplis B., Belyantseva I., Ben-Yosef T., Liburd N.A., Morell R.J. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- Xia J., Zhang X., Staudinger J., Huganir R.L. Clustering of AMPA Receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Xia J., Chung H.J., Wihler C., Huganir R.L., Linden D.J. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]