Abstract
Migratory cells including invasive tumor cells frequently express CD44, a major receptor for hyaluronan and membrane-type 1 matrix metalloproteinase (MT1-MMP) that degrades extracellular matrix at the pericellular region. In this study, we demonstrate that MT1-MMP acts as a processing enzyme for CD44H, releasing it into the medium as a soluble 70-kD fragment. Furthermore, this processing event stimulates cell motility; however, expression of either CD44H or MT1-MMP alone did not stimulate cell motility. Coexpression of MT1-MMP and mutant CD44H lacking the MT1-MMP–processing site did not result in shedding and did not promote cell migration, suggesting that the processing of CD44H by MT1-MMP is critical in the migratory stimulation. Moreover, expression of the mutant CD44H inhibited the cell migration promoted by CD44H and MT1-MMP in a dominant-negative manner. The pancreatic tumor cell line, MIA PaCa-2, was found to shed the 70-kD CD44H fragment in a MT1-MMP–dependent manner. Expression of the mutant CD44H in the cells as well as MMP inhibitor treatment effectively inhibited the migration, suggesting that MIA PaCa-2 cells indeed use the CD44H and MT1-MMP as migratory devices. These findings revealed a novel interaction of the two molecules that have each been implicated in tumor cell migration and invasion.
Keywords: MT-MMP, metalloproteinase, motility, CD44, invasion and metastasis
Introduction
CD44 is a multistructual and multifunctional cell adhesion molecule that is involved in cell–cell and cell–matrix interactions (Naot et al. 1997). This family of glycoprotein consists with many isoforms generated by both posttranslational modifications and different use of alternatively spliced exons. The encoded amino acids from variant exons are inserted within the extracellular part between ligand binding globular domain and transmembrane domain (Naot et al. 1997). To date, at least 20 different CD44 transcripts have been described. However, the most abundant form is the standard hematopoietic type, CD44H, which does not have any variant insertions (Naot et al. 1997).
Its adhesion activity to extracellular matrix (ECM) was shown to be located in the NH2-terminal globular domain that contains three disulfide bonds. Through this domain, CD44 binds hyaluronic acid (HA), type I collagen, fibronectin, fibrin, laminin, and chondroitin sulfate (Naot et al. 1997). CD44 has been shown to play roles in many important physiological and pathological processes such as lymphocyte homing, T cell activation, wound healing, angiogenesis, and metastatic spread of cancer cells (Naot et al. 1997). It is expressed in many types of migratory cells and metastatic tumor cells (Gunthert et al. 1991; Naot et al. 1997; Sneath and Mangham 1998) and has been shown to promote miratory potential of these cells (Thomas et al. 1992, Thomas et al. 1993; Henke et al. 1996; Okada et al. 1996; Trochon et al. 1996; Ladeda et al. 1998). However, the mechanism underlying the phenomenon is not clear.
CD44 was shown to be shed from the cell surface by proteolytic processing (Goebeler et al. 1996; Naot et al. 1997; Okamoto et al. 1999). The soluble CD44 (sCD44) has been detected in cell culture supernatants (Goebeler et al. 1996; Okamoto et al. 1999), arthritic synovial fluid (Haynes et al. 1991), and plasma (Haberhauer et al. 1997; Kittl et al. 1997). Also, it has been reported that higher levels of sCD44 were detected in serum from the patients bearing malignant cancer with metastasis (Guo et al. 1994; Masson et al. 1999; Yamane et al. 1999). Thus, generation of sCD44 may reflect certain biological and pathological situations. The shedding was shown to be inhibited by the inhibitors specific for metalloproteinases or serine proteinases (Bazil and Strominger 1994; Okamoto et al. 1999). Recently, inhibition of metalloproteinase but not of serine proteinase was demonstrated to suppress CD44-dependent cell migration (Okamoto et al. 1999), suggesting that CD44-mediated cell migration may require the cell surface processing of CD44 by metalloproteinase. However, the proteinase responsible for the shedding is not identified yet.
When cells migrate in the tissue, ECM located at the migratory direction has to be degraded. Matrix metalloproteinases (MMPs) are a group of the enzymes that is responsible for ECM degradation (Werb 1997; Matrisian 1999; Murphy and Gavrilovic 1999; Nagase and Woessner 1999). To date, 21 mammalian MMP genes were molecularly cloned, and their products can be subgrouped into the soluble-type MMPs and the membrane-type MMPs (MT-MMPs) (Nagase and Woessner 1999; Seiki 1999). Since MT-MMPs are tethered to the plasma membrane either through transmembrane domain (Sato et al. 1994; Cao et al. 1995) or glycosylphosphatidylinositol anchor (Itoh et al. 1999; Kojima et al. 2000), they are well placed for pericellular proteolysis that associates with cell growth, migration, and morphological change of cells in tissue (Nagase and Woessner 1999; Seiki 1999). They have a basic amino acid motif at the end of propeptide that can be recognized and processed by furin or related proteinase for the activation (Nagase and Woessner 1999; Seiki 1999; Yana and Weiss 2000). Thus, they are processed intracellularly and appear on the cell surface as an active form.
Among the MT-MMPs, MT1-MMP is shown to be frequently expressed in migratory cells such as macrophages (Sato et al. 1997), endothelial cells (Hiraoka et al. 1998), and invasive cancer cells (Seiki 1999). MT1-MMP degrades collagen types I, II, and III, fibronectin, laminin 1 and 5, vitronectin, and aggrecan (d'Ortho et al. 1997; Ohuchi et al. 1997; Buttner et al. 1998; Fosang et al. 1998; Koshikawa et al. 2000). It also activates other MMPs such as proMMP-2 (gelatinase A) (Sato et al. 1994) and proMMP-13 (procollagenase 3) (Knauper et al. 1996). Thus, the expression of MT1-MMP on the cell surface is thought to trigger multiple proteinase cascades.
Although the investigations of MT-MMPs have been focused on ECM degradation, it is possible that they also process membrane proteins. Cell surface localization of MT1-MMP, as well as CD44, was reported to be at the edge of the motile cells (lamellipodia), thus there is a substantial possibility that MT1-MMP processes CD44. In this study, we demonstrated that MT1-MMP directly shed CD44H from the cell surface and stimulated cell migration. Identification of the processing sites of CD44H by MT1-MMP enabled us to design the mutant CD44H that is resistant to the processing. Expression of the mutant CD44H could not stimulate cell migration and rather counteracted the cell migration promoted by wild-type CD44H and MT1-MMP. These results provide a novel molecular paradigm of cell migration that may be involved in tumor invasion and metastasis.
Materials and Methods
Expression Vectors for CD44H, Its Mutants, and MT-MMPs
The cDNA encoding CD44H was obtained by reverse transcript PCR (RT-PCR) using total RNA isolated from MIA PaCa-2 cells and subcloned into the mammalian expression vector, pSG5 (Stratagene). The sequence was confirmed to be identical to that of accession no. M24915 by DNA sequencing. The cDNA encoding CD44H, tagged with c-Myc epitope at the NH2 terminus and with a FLAG tag at the COOH terminus, was generated by PCR and subcloned into pSG5. A mutant CD44H that lacks the region between Lys158 to Thr197 (CD44HM), which includes all MT1-MMP cleavage sites, was constructed by PCR. All the PCR-generated fragments were confirmed by DNA sequencing. The cDNAs of human MT1-MMP (accession no. D26512), MT2-MMP (D86331), MT3-MMP (D50477), MT4-MMP (AB021225), and MT5-MMP (AB021227) were subcloned into pSG5. (Sequence data are available from GenBank/EMBL/DDBJ under indicated accession nos.)
RT-PCR and Their Primers
In brief, total RNA (3 μg) was reverse transcribed with 0.3 μg of random primer. Then, a portion of reverse transcript product (1 μl) was amplified with Taq DNA polymerase using a Takara DNA thermal cycler MP (Takara) for 30 cycles (20 cycles for glyceraldehyde-3-phosphate dehydrogenase [GAPDH]). Primers for specific amplification were listed as follows: CD44 (forward primer: 5′-AGACATCTACCCCAGCAAC-3′, reverse primer: 5′-CGTTGAGTCCACTTGGCTTTC-3′); MT1-MMP (forward primer: 5′-GCTTGCAAGTAACAGGCAAA-3′, reverse primer: 5′-AAATTCTCCGTGTCCATCCA-3′); MT2-MMP (forward primer: 5′-TCGACGAAGAGACCAAGGAGT-3′, reverse primer: 5′-CTTGAAGTTGTCAACGTCCT-3′); MT3-MMP (forward primer: 5′-ATGTGCTACAGTCTGCGGAAC-3′, reverse primer: 5′-TATCCACATCACGTTTGCCA-3′); MT4-MMP (forward primer: 5′-TGCGTGCACTCATGTACTAC-3′, reverse primer: 5′-GCCGCATGATGG-AGTGTGCA-3′); MT5-MMP (forward primer: 5′-GGATCAGACAACGATCGAGT-3′, reverse primer: 5′-CAGCTTGAAGTTGTGCGTCT-3′); GAPDH (forward primer: 5′-AAGGCTGAGAACGGGAAGCTTGTCATCAAT-3′, reverse primer: 5′-TTCCCGTCTAGCTCAGGGATGACCTTGCCC-3′).
Cell Culture and Transfection of Expression Plasmids
Cell lines from human pancreatic carcinoma (MIA PaCa-2), breast carcinoma (ZR-75-1), and osteosarcoma (MG-63) were obtained from American Type Culture Collection and cultured in RPMI-1640 medium (Life Technologies) supplemented with 10% FBS and kanamycin.
Cells were seeded in six-well plates at 105 cells/well and transfected with plasmid DNA (1 μg) using FuGENE6™ (Roche Molecular Biochemicals) according to the manufacturer's instructions.
Antibodies and Inhibitors
Mouse mAb (2C5) against human CD44 was from R&D Systems; anti–human CD44 rat mAb A020 was from Chemicon International Inc.; mouse anti-FLAG M2 mAb was from Sigma-Aldrich; mouse anti–c-Myc mAb was from Oncogene Research Products; and mouse anti–hMT1-MMP mAb (113-5B7), mouse anti–hMT2-MMP mAb (162-22G5), and mouse anti–MT3-MMP mAb (117-4E) were gifts from Dr. Kazushi Iwata (Fuji Chemical Industries, Toyama, Japan). Polyclonal antibodies specific to MT4-MMP and MT5-MMP were raised in rabbit using recombinant enzymes expressed in Escherichia coli as antigens. Proteinase inhibitors, 4-(2-Aminoethyl)-benzenesulfonyl fluoride hydrochloride (AEBSF), N-[N-(l-3-Trans-carboxirane-2-carbonyl)-l-leucyl]-agmatine (E-64) and soybean trypsin inhibitor were purchased from Roche Molecular Biochemicals. BB94 (Talbot and Brown 1996) was a gift from Dr. Peter D. Brown (British Biotech Pharmaceuticals Ltd., Oxford, UK). Tissue inhibitor of metalloproteinase (TIMP)–1 and TIMP-2 were expressed in HighFive insect cells (Invitrogen) infected with recombinant baculoviruses to express human TIMP-1 and TIMP-2, respectively. Recombinant viruses were made using Bac-To-Bac™ baculovirus expression systems (Life Technologies). TIMPs were purified from culture medium by Green A Dyematrex (Millipore) and gel permeation column on S-200.
Western Blot Analysis
To detect proteins in the culture supernatant, the medium was treated with 10% TCA. Cell lysate and TCA-precipitated proteins were separated by SDS-PAGE, and the proteins in the gel were transferred to a polyvinyldifluoride membrane. After blocking the membrane with 10% fat-free dry milk in Tris-buffered saline, the membrane was probed with the first antibody specific to each antigen. The membrane was further probed with alkaline phosphatase–conjugated goat anti–mouse IgG to visualize bands.
Indirect Immunofluorescence Staining
Transfected cells were seeded on glass coverslips at 3 × 104 cells/well. 16 h later, cells were fixed with 4% paraformaldehyde in PBS (pH 7.5) and stained with rat anti-hCD44 mAb (A020) and mouse anti–hMT1-MMP mAb (113-5B7). Cy3-conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories) and Alexa 488–conjugated goat anti–rat IgG (Molecular Probes) were used as a secondary antibody. F-Actin was stained by Alexa 594–conjugated phalloidin (Molecular Probes). The signals were analyzed using confocal laser microscope (Bio-Rad Laboratories).
HA-binding Assay
ZR-75-1 cells transfected with the expression plasmids were seeded in a 12-well plate at 3 × 104 cells/well. 24 h later, the culture medium was replaced with serum-free medium containing 100 μg/ml fluorescein-conjugated HA (FITC-HA; Seikagaku Kogyo). After a period of incubation at 37°C, the cells were washed three times with PBS, fixed with 4% paraformaldehyde, and analyzed by confocal laser microscope. The fluorescence intensity of the cell-bound HA was measured using LaserSharp processing software (Bio-Rad Laboratories) and a confocal laser microscope.
Expression of rCD44HS and rCD44HSM in E. coli
The cDNA encoding stem region of CD44H (130Thr–268Glu), with FLAG tag at the NH2 terminus and His6 tag at the COOH terminus (rCD44HS), and its deletion mutant rCD44HSM (deleted between Lys158 and Thr197) were generated by PCR and subcloned into pET3a expression vector (Stratagene). All the PCR-generated fragments were confirmed by DNA sequencing. The E. coli strain of BL21 (DE3)pLysS was transformed with these plasmids, and the protein expression was induced by 0.4 mM IPTG. Cells were collected and sonicated in TNC buffer (50 mM Tris-HCl, 150 mM NaCl, 10 mM CaCl2, 0.02% NaN3) containing 2 mM PMSF. Supernatant was collected, and the His6-tagged protein was purified by a chelating sepharose and a gel filtration column using ÄKTA explorer 10S systems (Amersham Pharmacia Biotech).
Determination of the Cleavage Sites of CD44H
To determine the cleavage sites of CD44H, purified rCD44HS was incubated with purified active catalytic domain of MT1-MMP in TNC buffer. The reaction was terminated by addition of EDTA, adjusting final concentration at 50 mM. The generated fragments were separated by reverse phase chromatography on a Sephasil protein C4 5 μm ST 4.6/100 column (Amersham Pharmacia Biotech) using a linear gradient of 10–40% acetonitrile with 0.1% trifluoroacetic acid by ÄKTA explorer 10S systems (Amersham Pharmacia Biotech). The NH2-terminal amino acid sequence of each fragment was determined using the Beckman Coulter LF3000 amino acid sequencer.
Phagokinetic Track Motility Assay
Phagokinetic track motility assay was performed as described previously (Albrecht-Buehler 1977). Colloidal gold-coated coverslips were placed in a 12-well plate, and transfected cells were seeded at 3 × 103/well. After 12-h incubation, the phagokinetic tracks were visualized using dark-field illumination in a confocal laser microscope (Bio-Rad Laboratories). Images were processed and measured using NIH Image software.
Results
Processing of CD44H by MT-MMPs
To examine whether MT-MMPs can shed CD44H, CD44H was coexpressed with different MT-MMPs in human breast carcinoma ZR-75-1 cells that express undetectable levels of both endogenous CD44H and MT1-MMP. Expressed CD44H was detected as a 95-kD protein (Fig. 1 A, Cell, lane 2) and did not show soluble fragment sCD44H in the medium (Med, lane 2). On the other hand, coexpression of MT1-MMP or MT3-MMP resulted in shedding of a 70-kD sCD44H into the media (lanes 3 and 5, respectively), whereas MT2, MT4, and MT5-MMP did not (lanes 4, 6, and 7, respectively). To ensure that the lack of CD44H processing by MT2, MT4, and MT5-MMP is not the result of inefficient delivery of the enzymes to the cell surface, immunoreactivity of FLAG-tagged MT-MMPs on the surface was examined. Relative intensities of cell surface signals were as follows: MT1-MMP (1.0); MT2-MMP (0.32); MT3-MMP (0.36); MT4-MMP (1.08); MT5-MMP (0.29); and that of mock-transfected cells was negligible. Thus, the amount of MT2, MT4, and MT5-MMP on the cell surface is almost comparable to that of MT3-MMP that can cleave CD44H. The cells also showed gelatin-degrading activity upon expression of MT-MMPs in a BB94-sensitive manner (synthetic hydroxamate MMP inhibitor). Relative gelatin-degrading activities by the cells were as follows: MT1-MMP (+3), MT2-MMP (+1), MT3-MMP (+2), MT4-MMP (+1), MT5-MMP (+1).
Figure 1.
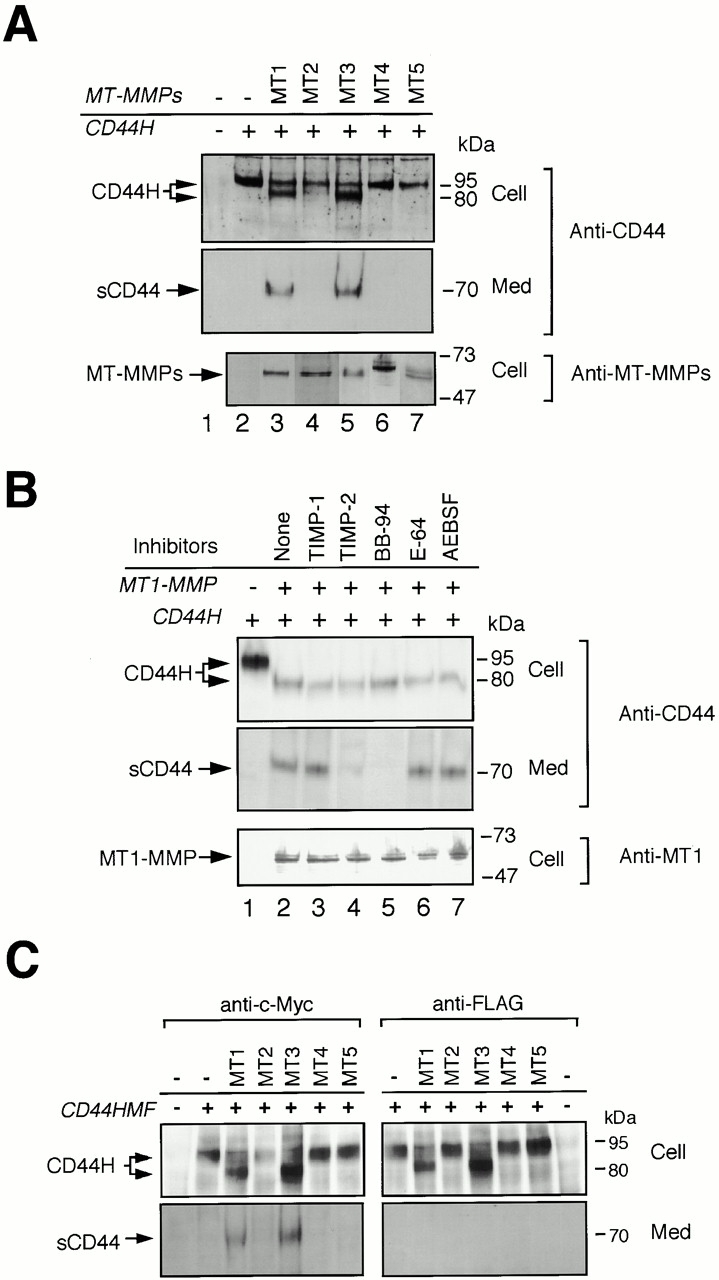
Shedding of CD44H by MT-MMPs. (A) CD44H was coexpressed with each of the MT-MMPs, as indicated by transient transfection of the expression plasmids into ZR-75-1 cells, and incubated in the serum-free media. After 48 h, cell lysates and medium fractions were collected and subjected to Western Blot analyses using monoclonal anti-CD44 and specific antibodies against each MT-MMP. (B) ZR-75-1 cells were transiently transfected with the expression plasmids for CD44H and MT1-MMP and cultured in serum-free media in the presence or absence of various proteinase inhibitors as indicated. After 48 h, cell lysates and medium fractions were collected and subjected to Western blot analyses. (C) CD44H with NH2-terminal c-Myc tag and COOH-terminal FLAG tag was coexpressed with each of the MT-MMPs, as indicated by transient transfection of the expression plasmids into ZR-75-1 cells, and analyzed the same as in A. The antibody against FLAG and c-Myc were used to determine the integrity of the peptide core of CD44H for the Western blot as indicated.
The shedding by MT1-MMP was inhibited by TIMP-2 and BB94, but not by TIMP-1 or a serine proteinase inhibitor, AEBSF (Fig. 1 B). TIMP-2 but not TIMP-1 is known to inhibit MT1-MMP, whereas all soluble MMPs including MMP-2 and MMP-13 can be inhibited by both TIMPs (Nagase and Woessner 1999; Seiki 1999). Also, endogenous MMP-2 was not detected in the culture supernatant of ZR-75-1 by zymography (data not shown). Thus, CD44H is thought to be processed directly by MT1-MMP rather than by some other soluble MMPs activated by MT1-MMP. Similar results were obtained with MT3-MMP.
Upon coexpression of either MT1-MMP or MT3-MMP with CD44H, CD44H with a lower molecular mass (80 kD) was detected in the cell fraction in addition to the 95-kD CD44H (Fig. 1 A). To examine the integrity of the NH2- and COOH-terminal ends of the molecule, we constructed CD44H, tagged with c-Myc epitope at the NH2 terminus and FLAG tag at the COOH terminus, and subjected it to Western Blotting using specific antibodies against these tags. Consequently, the 80-kD CD44H in the cell lysate appeared to retain both tags (Fig. 1 C), suggesting that it is not a processing product of 95-kD CD44H and the polypeptide core is intact. Thus, posttranslational modifications of CD44H, most likely glycosylations, might be affected by the coexpression of these MT-MMPs, although the reason is not clear. Also, the 70-kD sCD44H detected in culture medium was confirmed to be the NH2-terminal part of CD44HMF retaining the NH2-terminal Myc tag but not the COOH-terminal FLAG tag (Fig. 1 C).
Effect of CD44H Processing on Ligand-binding Capacity and Morphology of the Cells
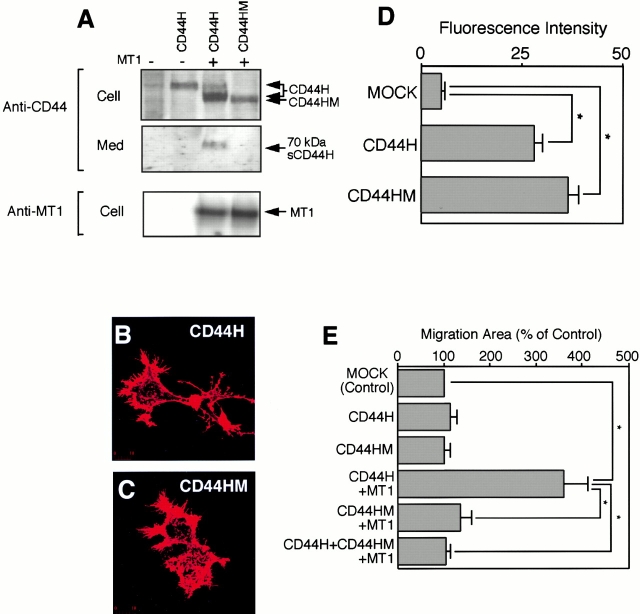
HA is the major ligand for CD44 on the cell surface. Therefore, we examined the effect of processing of CD44H by MT1-MMP on the ability of the cells to bind HA. Transfected ZR-75-1 cells were incubated with increasing amount of FITC-HA, and amount of the bound HA was quantified by fluorescence scanning using a confocal laser microscope (Fig. 2 A). The expression of CD44H greatly increased the binding of FITC-HA compared with the mock-transfected cells. The binding was saturable, and an addition of 100-fold excess nonlabeled HA competed the binding and decreased it to the level of nontransfected cells (Mock). Coexpression of MT1-MMP with CD44H reduced the HA binding activity significantly by 40% (Fig. 2 B, compare CD44H+MT1 with CD44H). On the other hand, when the transfected cells were cultured in the presence of BB94 to inhibit the shedding of CD44H, the amount of bound HA was significantly increased compared with samples without BB94 (Fig. 2 B, compare CD44H+MT1/BB94 with CD44H+MT1). Thus, expression of MT1-MMP surely downregulates the net HA-binding activity as a result of CD44H processing.
Figure 2.
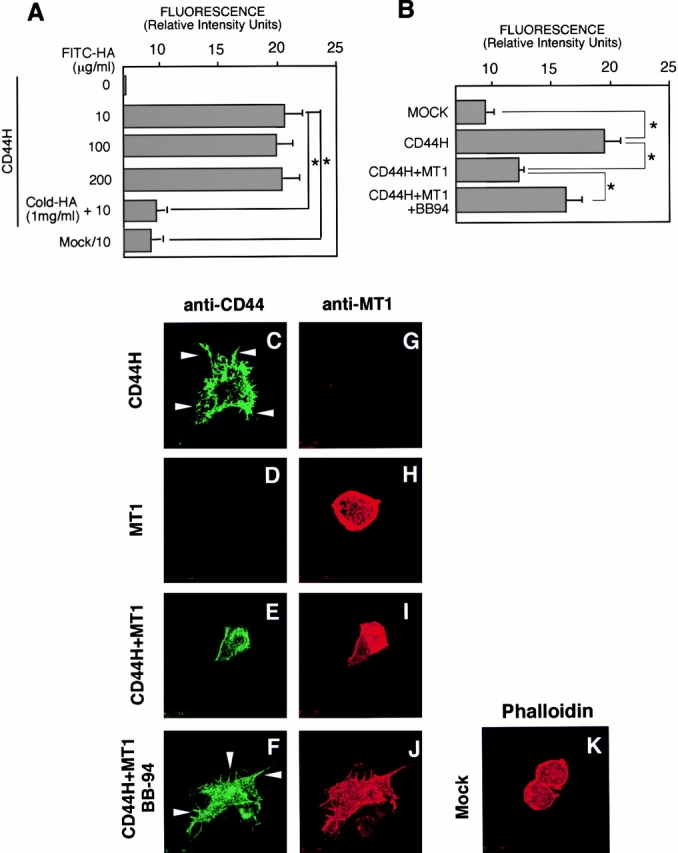
Effect of CD44 shedding on HA-binding activity and cell morphology. (A) ZR-75-1 cells were transfected with expression plasmid for CD44. After 24 h, the cells were incubated with increasing concentrations of FITC-labeled HA for 60 min at 37°C. After washing unbound FITC-HA, the relative intensity of green fluorescence of the transfected cells was analyzed by confocal laser microscopy. The average value of 40 individual cells was plotted (mean ± SEM). A 100-fold excess amount of cold-HA was used to compete FITC-HA binding. (B) ZR-75-1 cells were transfected with expression plasmids for CD44 and/or MT1-MMP. FITC-HA binding was analyzed similarly. ZR-75-1 cells were transfected with control vector (Mock), CD44H cDNA (CD44H), CD44H and MT1-MMP cDNAs (CD44H+MT1), CD44H and MT1-MMP cDNAs cultured in the presence of BB94 (CD44H+MT1/BB94). (C–K) ZR-75-1 cells transfected with expression plasmids indicated were cultured on glass slides. The cells were stained with rat anti–human CD44 and mouse anti–human MT1-MMP mAbs without permeabilization. Signals were visualized by further probing with Alexa 488–conjugated anti–rat IgG or Cy3-conjugated anti–mouse IgG and analyzed by a confocal laser microscope. Representative pictures are presented. Cells express CD44H (C and G), MT1-MMP (D and H), CD44H and MT1-MMP (E and I), CD44H and MT1-MMP cultured in the presence of BB94 (F and J), and mock-transfected cells (K). Cells were stained with ant-CD44 mAb (C–F) or anti–MT1-MMP (G–J). Mock cells were stained for F-actin by Cy3-conjugated phalloidin (K). *P < 0.05 by Student's t test.
Expression of CD44H in ZR-75-1 significantly altered cell morphology, possibly by changing the adherent nature of the cells. As shown in Fig. 2 C, expression of CD44H resulted in the formation of numerous small and large protrusions (arrowheads) at the adherent edge of the cells compared with the mock-transfected cells (Fig. 2 K). When MT1-MMP was coexpressed with CD44H, on the other hand, these protrusions were not formed (Fig. 2E and Fig. I). The expression of MT1-MMP alone did not change cell shape (Fig. 2 H) compared with the mock-transfected cell (Fig. 2 K). The effect of MT1-MMP on the CD44H-expressing cells (Fig. 2E and Fig. I) appeared to be the result of CD44H processing by MT1-MMP, as many protrusions were formed when the cells were cultivated in the presence of BB94 (Fig. 2F and Fig. J).
Effect of CD44H and MT1-MMP on Cell Migration
Next, we examined the effect of CD44H and MT1-MMP on the migration of the cells. As shown in Fig. 3A and Fig. B, the expression of either CD44H or MT1-MMP alone had no effect on the cell migration. However, coexpression of CD44H and MT1-MMP significantly increased motility of the cells. Addition of BB94 to these transfectants, which inhibits the processing of CD44H by MT1-MMP (Fig. 1 C), suppressed the increased cell migration. A similar result was also obtained with the MG-63 osteosarcoma cell line, which expresses low levels of CD44H (Fig. 3C and Fig. D).
Figure 3.
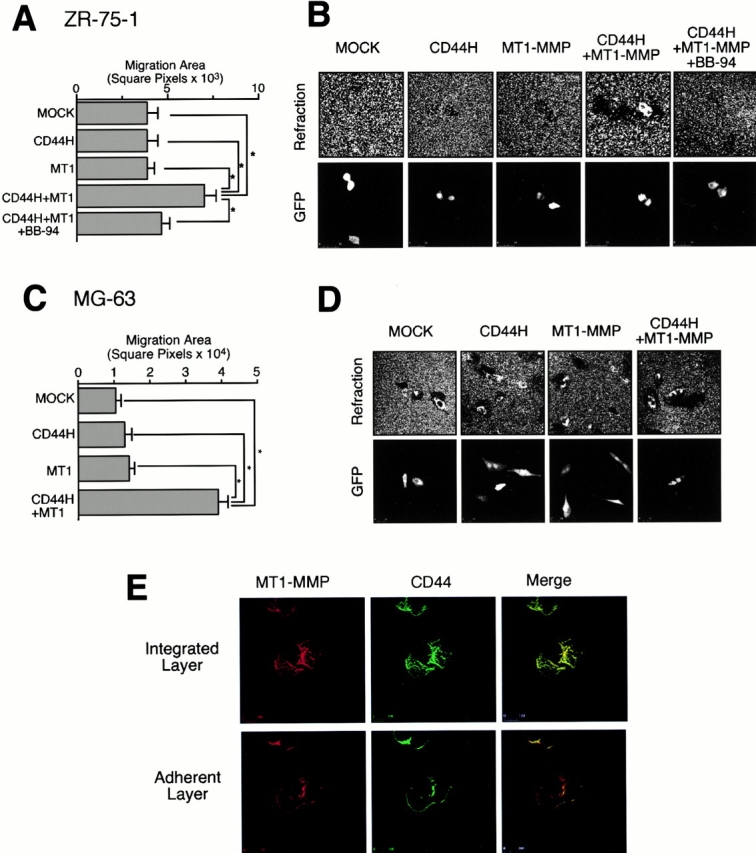
Effect of the shedding of CD44H by MT1-MMP on the cell motility. (A) ZR-75-1 cells were transfected with the expression plasmids for CD44H and/or MT1-MMP together with the one for GFP. The motility of GFP-positive cells was analyzed by phagokinetic track assay on colloidal gold–coated coverslips. The migrated area of the cell was visualized under darkfield illumination, and migration area was measured using NIH Image. The average of 30 cells ± SEM is shown. (B) Representative phagokinetic track of the migrating cell was visualized under darkfield illumination (Refraction). Transfected cells were indicated as GFP-positive cells (GFP). (C) Osteosarcoma MG-63 cells were analyzed as above. (D) Representative phagokinetic track of the migrating cell was visualized under darkfield illumination (Refraction). Transfected cells were indicated as GFP-positive cells (GFP). (E) MT1-MMP and CD44H expressing ZR-75-1 cells on glass coverslip were immunostained for CD44H and MT1-MMP. The signal was analyzed by confocal microscope. The combined image from all sections (top, Combined Sections) and one section from the cell attachment site is shown (bottom, Adherent Section). *P < 0.05 by Student's t test.
Since the expression plasmids were introduced into the cells by transient transfection together with green fluorescent protein (GFP) plasmid as a transfection marker, we could compare motility of both transfected and nontransfected cells in the same field. Stimulation of cell migration was observed only with the transfected cells expressing CD44H and MT1-MMP, but not with the surrounding nontransfected cells, indicating that the shed CD44H fragment itself does not have activity to stimulate cell motility (Fig. 3 D). We also examined the cell motility using coloidal gold glass coverslips coated with HA instead of serum-coated coverslips, but there are no differences in the data.
When cell surface localization of both CD44H and MT1-MMP were analyzed by confocal microscope, both molecules appeared to distribute over the cell surface (Fig. 3 E, Integrated Image). However, colocalization was prominent at the adherent edge of the transfected cells (Fig. 3 E, Adherent Layer).
Determination of the Cleavage Site of CD44H by MT1-MMP
According to the molecular size of sCD44H, the cleavage site of CD44H by MT1-MMP was expected to locate within the stem region of CD44H (Thr130-Gln265) that is between the globular and transmembrane domain (Fig. 4 A). Therefore, we expressed the fragment corresponding to that region (rCD44HS) in E. coli and incubated it with recombinant MT1-MMP catalytic domain (rMT1-CAT) to determine the putative cleavage site. Incubation of rCD44HS with rMT1-CAT generated 28- and 26-kD bands in a time-dependent manner on SDS-PAGE (data not shown). To isolate these fragments, the reaction mixture was subjected to reverse phase chromatography, and four fragment peaks were recovered (Fig. 4 B). These peak fractions were collected and subjected to NH2-terminal amino acid sequence analyses. The NH2-terminal sequence of peak 2 corresponded to that of the original fragment, and those of peaks 1, 3, and 4 were mapped to 163Thr (TNPED), 193Tyr (YIFYT), and 187Ser (SSTSG), respectively (Fig. 4 C). From their molecular sizes and height of the peaks, cleavage between the Gly192–Tyr bond (corresponding to peak 3) and Arg186–Ser bond (corresponding to peak 4) are likely to generate 26- and 28-kD fragments, respectively. A mutant CD44H fragment that lacks the 40 internal amino acids, which include all the three cleavage sites (158Lys to 197Thr), was constructed, and it was confirmed to be resistant to cleavage by rMT1-CAT (data not shown).
Figure 4.
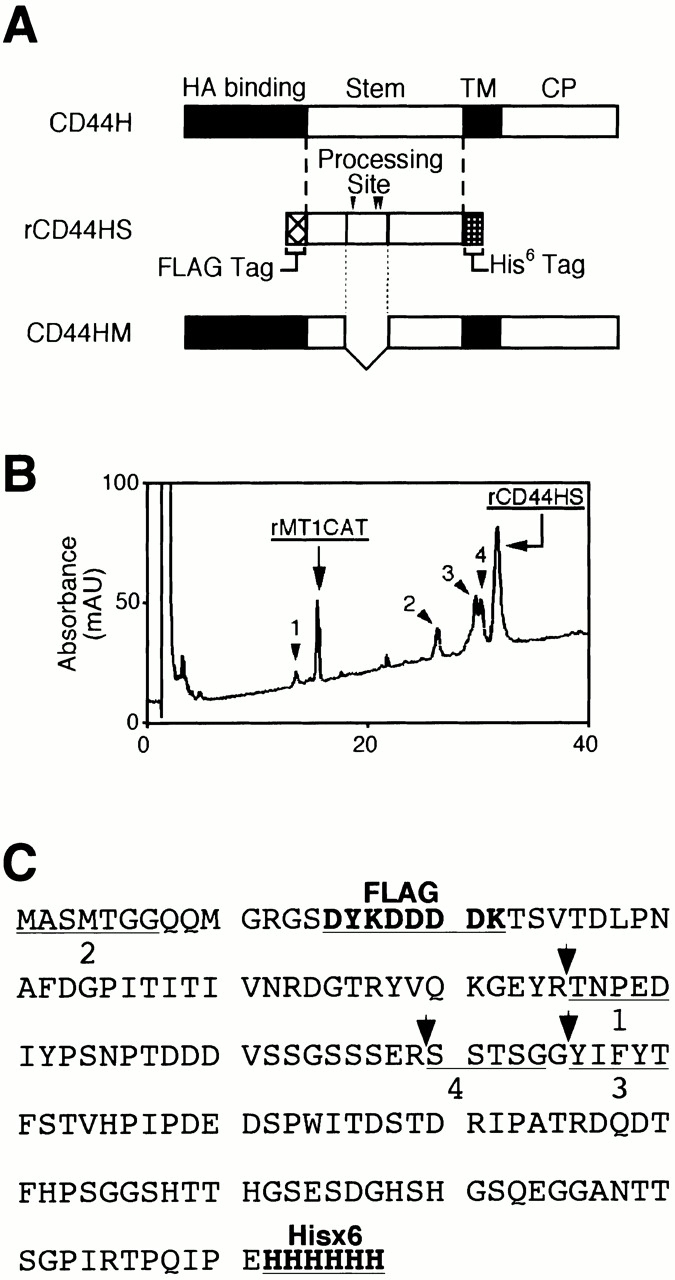
Processing of CD44H by MT1-MMP in vitro. (A) Schematic illustration of CD44H, the stem fragment (rCD44HS) expressed in E. coli, and the mutant lacking the processing sites by MT1-MMP (CD44HM). Fragments expressed in E. coli were tagged with FLAG at the NH2 terminus and His6 at the COOH terminus, as indicated. Processing sites (C) are indicated by the arrowheads. (B) rCD44HS (96 μg) was incubated with 3.6 μg of the catalytic fragment of MT1-MMP (rMT1CD) at 37°C for 180 min. Reaction products were separated by reverse-phase chromatography. Peaks indicated (peaks 1–4) were collected and subjected to automatic amino acid sequencer (Beckman Coulter LF3000). (C) Amino acid sequence of rCD44HS is presented. Thick letters at the NH2 terminus are the FLAG tag, and those at the COOH terminus are the His6 tag. The sequence between the tags corresponds to T130-E268 of CD44H. The determined NH2-terminal sequences of the peaks are underlined. Stem, the region between the HA-binding globular domain and transmembrane domain; TM, transmembrane domain; CP, cytoplasmic tail.
CD44H Processing Is Critical for Cell Migration
Although BB94 inhibits cell migration driven by CD44H and MT1-MMP, it does not necessarily mean that the processing of CD44H is the absolute requirement for cell migration. To examine this, we constructed the mutant CD44H that can not be processed by MT1-MMP. Deletion of the 40 internal amino acids (158Lys–197Thr) was introduced into CD44H (Fig. 4 A, CD44HM), and it was expressed in ZR-75-1 cells. CD44HM was not shed by MT1-MMP under the condition where the wild-type CD44H was shed into the media (Fig. 5 A). To confirm that the mutant CD44HM retains comparable ligand-binding ability to the wild-type CD44H, binding of FITC-HA to the cells was analyzed. The cells expressing CD44HM showed comparable HA-binding activity (Fig. 5 D) and similar morphological change of the cells to that of the wild-type CD44H-expressing cells (Fig. 5B and Fig. C). On the other hand, coexpression of MT1-MMP with CD44HM did not promote migration of the cells (Fig. 5 E, CD44HM+MT1). Furthermore, CD44HM inhibited migration of the cells promoted by CD44H and MT1-MMP (Fig. 5 E, compare CD44H+ CD44HM+MT1 with CD44H+MT1), suggesting a dominant-negative effect against the wild-type CD44H. These data indicate that processing of CD44H is an essential step for CD44H and MT1-MMP–promoted cell migration.
Figure 5.
Dominant-negative effect of CD44HM on cell migration stimulated by CD44H and MT1-MMP. (A) Either wild-type CD44H or the mutant CD44HM was expressed in ZR-75-1 cells together with MT1-MMP. Cell lysate and medium fractions were subjected to Western blot analyses as described in the legend to Fig. 2. (B and C) CD44H- or CD44HM-expressing ZR-75-1 cells were stained with rat anti–human CD44 mAb and analyzed by confocal microscopy. (D) HA-binding activity of CD44H- or CD44HM-expressing ZR-75-1 were examined as described in the legend to Fig. 2. Transfected cells were incubated with FITC-HA for 3 h at 37°C, and fluorescence associated to the cells was measured. (E) Transfected ZR-75-1 cells were subjected to the migration assay as described in the legend to Fig. 3. *P < 0.05 by Student's t test.
Proteinases Responsible for CD44H Shedding in a Human Pancreatic Tumor Cell Line
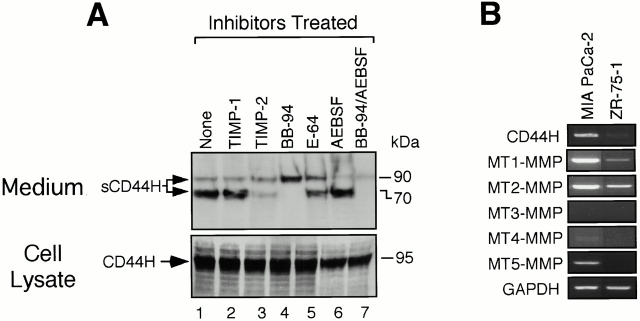
A human pancreatic tumor cell line, MIA PaCa-2, expresses high levels of CD44, which is spontaneously shed into the culture medium (Fig. 6 A). The major form of CD44 expressed in MIA PaCa-2 cells was confirmed to be CD44H by RT-PCR using a set of specific primers that can amplify all the splicing variants (Fig. 6 B).
Figure 6.
Shedding of endogenous CD44H in human pancreatic tumor cell line, MIA PaCa-2. (A) MIA PaCa-2 cells (3 × 105) were cultured in a six-well plate in serum-free medium in the presence or absence of the proteinase inhibitors as indicated. After 48 h, the cell lysate (bottom) and the conditioned medium (top) were subjected to Western blot analyses. CD44 was detected by the mouse anti–human CD44 mAb. Concentrations of the inhibitors were adjusted as follows: 50 nM for TIMP-1, 50 nM for TIMP-2, 10 μM for BB94, 1.0 μM for E-64, and 1.0 mM for AEBSF. (B) Expression of genes for CD44 and MT-MMPs were examined by RT-PCR using specific primers as described in Materials and Methods. Sizes of the amplified fragments were 461 bp for CD44, 589 bp for MT1-MMP, 578 bp for MT2-MMP, 461 bp for MT3-MMP, 334 bp for MT4-MMP, 564 bp for MT5-MMP, and 500 bp for GAPDH. PCR product of CD44 indicates that CD44 expressed in MIA PaCa-2 is CD44H.
Using an anti-CD44 mAb, 95-kD CD44H was detected in the cell lysate as a major form (Fig. 6 A, lane 1). The cells shed 90- and 70-kD sCD44H spontaneously into the medium (Fig. 6 A, lane 1). To examine the types of proteinases that are responsible for the shedding, we tested proteinase inhibitors selective for metalloproteinase (BB94), serine proteinases (AEBSF), and cystein proteinases (E-64) (Fig. 6 A). BB94 completely inhibited the shedding of the 70-kD fragment, selectively (Fig. 6 A, lane 5). In contrast, AEBSF inhibited the shedding of the 90-kD but not the 70-kD fragment (Fig. 6 A, lane 7). The combination of BB94 and AEBSF inhibited the shedding of the both fragments (Fig. 6 A, lane 8). Thus, the 70-kD fragment is processed by metalloproteinases, and the 90-kD fragment, by serine proteinases. Since inhibition of serine poroteinase did not inhibit the shedding of the 70-kD form, the shedding event by these different types of proteinase occurred independently rather than in a sequential manner. The shedding of the 70-kD fragment was inhibited by TIMP-2 but not by TIMP-1 (Fig. 6 A, lanes 2 and 3) and the size of the fragment is similar to that of the one processed by MT1-MMP upon coexpression with CD44H. Such different sensitivity to TIMP-1 and TIMP-2 is characteristic of MT-MMPs (Nagase and Woessner 1999; Seiki 1999). Thus, the metalloproteinase responsible for the shedding of the 70-kD fragment in MIA PaCa-2 cells is likely to be either MT1-MMP or MT3-MMP as they have the ability to process CD44H (Fig. 1). Transcripts for MT-MMPs were analyzed by RT-PCR, and MIA Paca-2 cells were found to express MT1-MMP but not MT3-MMP (Fig. 6 B). Expression of MT1-MMP was also confirmed by Western blotting (data not shown).
Processing Site–deleted Mutant, CD44HM, Inhibits Migration of the Pancreatic Tumor Cells
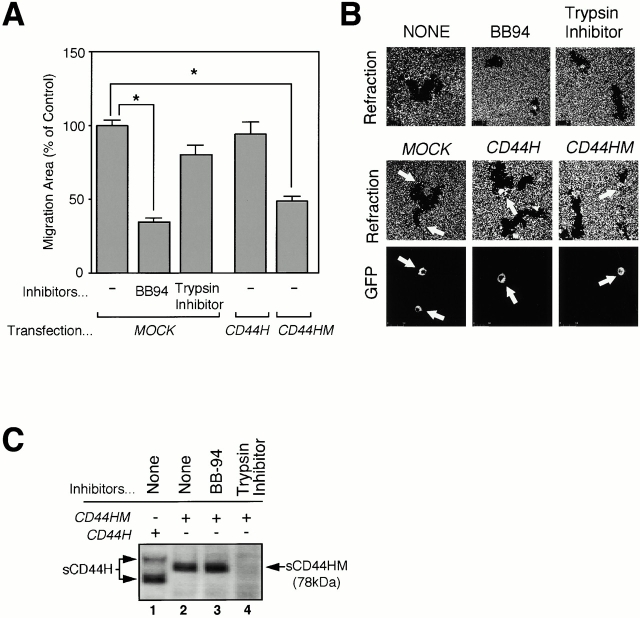
MIA PaCa-2 cells showed spontaneous motile activity on the slide glass coated with coloidal gold (see below). Thus, we asked whether this motility is attributed to the shedding of endogenous CD44H by MT1-MMP (Fig. 7A and Fig. B). BB94, which inhibits shedding of 70-kD sCD44H, suppressed the motility by 65%, whereas the serine proteinase inhibitor (trypsin inhibitor) had no effect. In addition, expression of CD44HM inhibited the motility by 40%, whereas expression of full-length CD44H had no effect. Since CD44HM plasmid was transfected transiently, both transfected and nontransfected cells were found in the same field (transfected cells with arrow). In spite of the spontaneous shedding of 70 kD sCD44 from surrounding cells, inhibition of the motility was observed with the cells expressing CD44HM (Fig. 7 B, CD44HM). This suggests that processing event but not its product (70-kD sCD44) is important to promote migration.
Figure 7.
BB94 and CD44HM inhibits motile activity of MIA PaCa-2. (A) MIA PaCa-2 cells were transfected with the expression plasmid for the FLAG-tagged CD44H or CD44HM together with the one for GFP. After 48 h, cells were subjected to the migration assay as described in the legend to Fig. 4. Mock- or CD44HM-transfected cells were cultured in the presence or absence of BB94 or trypsin inhibitor. (B) Representative phagokinetic track of the migrating cell was visualized under darkfield illumination (Refraction). Transfected cells were indicated as GFP-positive cells (GFP; arrow). (C) Medium fractions from same transfectants were subjected to Western blot analyses using anti-FLAG M2 mAb as described in the legend to Fig. 1. *P < 0.05 by Student's t test.
CD44HM is resistant to MT1-MMP–dependent processing but still susceptible to the serine proteinase, as it was shed as a 78-kD fragment that was inhibited by AEBSF (data not shown) or trypsin inhibitor (Fig. 7 C, lane 4). Since CD44HM inhibited the migration of the cells, this further strengthened the idea that MT1-MMP–dependent shedding of CD44H promotes cell migration, whereas the shedding by a serine proteinase has no effect on the motile phenotype of the cells.
Discussion
Processing of ECM Receptors and Cell Migration
Organized ECM–cell interaction is essential for cell migration (Keely et al. 1998; Sheetz et al. 1998), and CD44 is one of the ECM receptors that is thought to mediate cell migration (Naot et al. 1997; Sneath and Mangham 1998; Bourguignon et al. 2000). CD44 is a primary receptor for HA, which is abundantly present in many tissue (Naot et al. 1997). However, such interaction itself may limit cell movement in tissue. In addition, CD44 binds other solid ECM components such as collagen I or fibrin as well (Naot et al. 1997). Therefore, it appears critical to regulate detachment and attachment in an organized manner in order to accomplish cell migration
In this study, we demonstrated that CD44H stimulated cell migration when it is coexpressed with MT1-MMP. This phenomenon was accompanied by the shedding of CD44H from the cell surface by MT1-MMP. Although the precise mechanism to stimulate the cell motility is still unknown, the shedding event appears to be important, as the expression of the mutant CD44HM that can not be processed by MT1-MMP did not stimulate the migration (Fig. 5 E). It is possible to speculate that the shed 70-kD fragment stimulates cell motility in an autocrine and paracrine manner. However, this is not plausible because the effect of CD44H and MT1-MMP was not observed with the surrounding nontransfected cells in the same field (Fig. 3 D). In addition, expression of CD44HM suppressed motility of the transfected MIA PaCa-2 cells, however the surrounding nontransfected cells continuously generate the 70-kD fragment (Fig. 7 B). There are two possible mechanisms for the promotion of cell migration. One is that cleavage of CD44H by MT1-MMP may be required for the cells to detach from the ECM to migrate, and the other is that the COOH-terminal portion of the fragment remaining on the cell may generate some signals to stimulate motility. We prefer the former possibility because CD44HM acted dominant negatively against the wild-type CD44H in the cells expressing MT1-MMP (Fig. 5). It is interesting that an unknown serine proteinase also processes CD44H, but this shedding exerts no effect on the cell motility. These results may indicate that the processing of CD44H by two proteinase systems is regulated independently in space and timing. Although we do not know the localization of the serine proteinase, it may be different from that of MT1-MMP. CD44H is concentrated at the adherent edge, together with MT1-MMP, but it also distributes over the surface of the cells (Fig. 3 E). Thus, we prefer the idea that spatial and timely processing of CD44H at the adherent edge by MT1-MMP is critical for stimulation of the migration, allowing cells to be detached from the ECM to move on to the different site. Alternatively, shedding of CD44H by a serine proteinase may occur on the cell body where CD44H is not interacting with the foothold ECM. Although many reports have shown that expression of CD44 promotes cell migration (Thomas et al. 1992, Thomas et al. 1993; Henke et al. 1996; Okada et al. 1996; Trochon et al. 1996; Ladeda et al. 1998), the mechanisms that explain this phenomenon have been obscure. This is the first report to our knowledge that discloses the specific molecular interaction underlying this phenomenon.
Recombinant CD44H fragment was processed by MT1-MMP at three sites. However, the fragment produced in E. coli lacks glycosylations that may affect accessibility of the enzyme. Thus, there is a possibility that processing sites on the cell surface may differ from these sites determined in vitro. If CD44H (95 kD) on the cell surface is cleaved at these three sites, expected molecular mass of the sCD44H would be 73–76 kD, which is close to the 70-kD fragment we observed. However, due to heterogeneous glycosylation of the molecule, we could not confirm that the 70-kD fragment contains three different cleavage products. Nevertheless, we think that the actual cutting site by MT1-MMP contains at least one of the identified sites because deletion mutant CD44HM was not processed.
The role of CD44H during cell migration may resemble that of L-selectin during leukocyte rolling on endothelial cells. At the early stage of inflammation, circulating neutrophils start to interact with endothelial cells, and then extravasate to the site of inflammation (Lasky 1992). The interaction occurs by two steps. First, there is a loose interaction through cell surface carbohydrates and their receptors such as L-, E- and P-selectins. Then, tighter interaction mediated by VLA4 and VCAM-1 follows (Ebnet and Vestweber 1999). Recently, L-selectin was reported to be processed by TIMP-3–sensitive metalloproteinases (Borland et al. 1999). In the presence of metalloproteinase inhibitor, processing of L-selectin and leukocyte rolling were inhibited (Walcheck et al. 1996). Both CD44 and L-selectin are the receptors for carbohydrates and play a role in cell adhesion collaborating with integrins. Thus, cooperation of carbohydrate receptors and integrins may be a general apparatus for cell adhesion during migration. It is known that integrin-dependent adhesion can be released by intracellular signals (Kolanus and Seed 1997; Kumar 1998). On the other hand, the adhesion through carbohydrate receptor including CD44 and L-selectin may require extracellular processing by specific proteinases for the detachment.
A New Role of MT1-MMP in Tumor Invasion
MT1-MMP was originally identified as an activator of proMMP-2 on the surface of invasive tumor cells (Sato et al. 1994). MMP-2 is thought to be responsible for the degradation of basement membrane as it degrades type IV collagen (Liotta et al. 1991; Stetler-Stevenson et al. 1993; Tryggvason et al. 1993). The rate of activation of MMP-2 in tumor tissue is well-correlated to the expression levels of MT1-MMP and to the tumor spread (Nomura et al. 1995; Tokuraku et al. 1995; Nakamura et al. 1999), thus MT1-MMP is believed to be the in vivo proMMP-2 activator during cancer cell invasion. MT1-MMP also activates proMMP-13 (Knauper et al. 1996), and directly degrades type I, II, and III collagens, fibronectin, vitronectin (Ohuchi et al. 1997), and laminin 1 (Ohuchi et al. 1997) and 5 (Koshikawa et al. 2000). Recently, it was shown that pericellular collgen I degrading activity of MT1-MMP expressed in MDCK cells was shown to be pronounced to effect on cellular invasiveness, whereas overexpression of soluble collagenases (MMP-1 and MMP-13) did not affect it at all (Hotary et al. 2000). It was also reported that the specific processing of laminin 5 γ chain by MT1-MMP itself or MMP-2 activated by MT1-MMP stimulated motility of the breast cancer cells cultured on a laminin 5–coated dish (Koshikawa et al. 2000). Thus, MT1-MMP is in fact a powerful tool for cancer cells to migrate through basement membrane and the interstitial stromal tissue by triggering direct as well as indirect ECM proteolysis on the cell surface (Basbaum and Werb 1996; Seiki 1999).
In addition to the previous knowledge, we demonstrate here a new role of MT1-MMP in cell migration in conjunction with CD44H. MT1-MMP is frequently expressed in a variety of human cancers (Seiki 1999) and is the major type of MT-MMP expressed in tumors (Ueno et al. 1997; Nakamura et al. 1999). CD44H is also expressed frequently and abundantly in human cancer cells (Naot et al. 1997). Thus, there is a substantial chance for the molecular cooperation between MT1-MMP and CD44H in cancer cells, as demonstrated by the pancreatic cancer cell line, MIA PaCa-2 in this work.
Processing Enzymes for CD44
Although CD44 was reported to be shed by both serine proteases and metalloproteinase (Bazil and Strominger 1994, Okamoto et al. 1999), little was known about the responsive proteases and the shed fragments. In this report, we demonstrated that CD44H can be shed as a 90-kD fragment by unknown serine proteinases and a 70-kD fragment by MT1-MMP in MIA PaCa-2 cells. Although the 70-kD CD44H fragment can be shed by MT1-MMP, MT1-MMP may not be the only enzyme to generate the 70-kD fragment in other cells. Indeed, another pancreatic tumor cell line, PANC-1, expresses very low levels of MT1-MMP, but the level of shed 70-kD sCD44H was similar to that of MIA-PaCa 2. In PANC-1, the shedding was inhibited by TIMP-1, which cannot inhibit MT1-MMP (data not shown). Okamoto et al. 1999 also reported CD44 shedding by unknown TIMP-1-sensitive metalloproteinase in U251MG human glioma cells. Thus, there is a possibility that some soluble MMPs or ADAM family member(s) cleave CD44 at similar sites to those by MT1-MMP in these cell lines. We examined whether MMP-2, MMP-7, and MMP-9 could digest the rCD44HS in vitro, however none of these cleaved the fragment (data not shown). It is interesting that MMP-9 could not process the fragment, as it is reported to associate with CD44 (Bourguignon et al. 1998; Yu and Stamenkovic 1999).
The processing of CD44H by MT1-MMP occurs immediately upstream of the insertion site for variable regions by alternative splicing, and therefore the efficiency of the processing may be affected by these insertions. Since biological activities of these variants have been reported to vary (Gunthert et al. 1991), it is of interest to consider how the processing and its effect on migration are affected by the insertions. Bartolazzi et al. 1995 demonstrated that CD44 splice variants that contain variable exons 6–10, 7–10, and 8–10 are shed more efficiently than the standard form in malignant lymphoma cells. Although the type of responsible proteinase in this case is not known, we are now investigating the effect of different variant forms of CD44 on cell migration.
In conclusion, our study shows a novel molecular interaction between CD44H and MT1-MMP that promotes cell migration. Since CD44 and MT1-MMP are frequently expressed in motile cells, including many cancer cells, this may be a part of general mechanism for cell migration and invasion in the tissue. Therefore, the dominant-negative CD44H (CD44HM) may become a potential tool to treat cancer cell invasion and metastasis.
Acknowledgments
We thank Drs. Hong Zhu and Erik Thompson for the critical reading of the manuscript, Dr. Kazuhiro Chida for discussion, Dr. Shinobu Ohmi for peptide sequence, and Ms. Noriko Itoh and Akiko Takamura for excellent technical support.
This work was supported by the Special Coordination Fund for Promoting Science and Technology from the Ministry of Science and Technology of Japan and by a grant-in-aid for Cancer Research from the Ministry of Education, Science, and Culture of Japan.
Footnotes
Abbreviations used in this paper: AEBSF, 4-(2-Aminoethyl)-benzenesulfonyl fluoride hydrochloride; E-64, N-[N-(l-3-Trans-carboxirane-2-carbonyl)-l-leucyl]-agmatine; ECM, extracellular matrix; FITC-HA, fluorescein-conjugated HA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; HA, hyaluronic acid; MMP, matrix metalloproteinase; MT-MMP, membrane-type MMP; RT-PCR, reverse transcript PCR; sCD44, soluble CD44; TIMP, tissue inhibitor of metalloproteinases.
References
- Albrecht-Buehler G. The phagokinetic tracks of 3T3 cells. Cell. 1977;11:395–404. doi: 10.1016/0092-8674(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Bartolazzi A., Jackson D., Bennett K., Aruffo A., Dickinson R., Shields J., Whittle N., Stamenkovic I. Regulation of growth and dissemination of a human lymphoma by CD44 splice variants. J. Cell Sci. 1995;108:1723–1733. doi: 10.1242/jcs.108.4.1723. [DOI] [PubMed] [Google Scholar]
- Basbaum C.B., Werb Z. Focalized proteolysisspatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr. Opin. Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- Bazil V., Strominger J.L. Metalloprotease and serine protease are involved in cleavage of CD43, CD44, and CD16 from stimulated human granulocytes. Induction of cleavage of L-selectin via CD16. J. Immunol. 1994;152:1314–1322. [PubMed] [Google Scholar]
- Borland G., Murphy G., Ager A. Tissue inhibitor of metalloproteinases-3 inhibits shedding of L- selectin from leukocytes. J. Biol. Chem. 1999;274:2810–2815. doi: 10.1074/jbc.274.5.2810. [DOI] [PubMed] [Google Scholar]
- Bourguignon L.Y., Gunja-Smith Z., Iida N., Zhu H.B., Young L.J., Muller W.J., Cardiff R.D. CD44v(3,8-10) is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J. Cell Physiol. 1998;176:206–215. doi: 10.1002/(SICI)1097-4652(199807)176:1<206::AID-JCP22>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Bourguignon L.Y., Zhu H., Shao L., Chen Y.W. CD44 interaction with tiam1 promotes Rac1 signaling and hyaluronic acid-mediated breast tumor cell migration. J. Biol. Chem. 2000;275:1829–1838. doi: 10.1074/jbc.275.3.1829. [DOI] [PubMed] [Google Scholar]
- Buttner F.H., Hughes C.E., Margerie D., Lichte A., Tschesche H., Caterson B., Bartnik E. Membrane type 1 matrix metalloproteinase (MT1-MMP) cleaves the recombinant aggrecan substrate rAgg1mut at the ‘aggrecanase’ and the MMP sites. Characterization of MT1-MMP catabolic activities on the interglobular domain of aggrecan. Biochem. J. 1998;333:159–165. doi: 10.1042/bj3330159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Sato H., Takino T., Seiki M. The C-terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for pro-gelatinase A activation. J. Biol. Chem. 1995;270:801–805. doi: 10.1074/jbc.270.2.801. [DOI] [PubMed] [Google Scholar]
- d'Ortho M.P., Will H., Atkinson S., Butler G., Messent A., Gavrilovic J., Smith B., Timpl R., Zardi L., Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur. J. Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- Ebnet K., Vestweber D. Molecular mechanisms that control leukocyte extravasationthe selectins and the chemokines. Histochem. Cell Biol. 1999;112:1–23. doi: 10.1007/s004180050387. [DOI] [PubMed] [Google Scholar]
- Fosang A.J., Last K., Fujii Y., Seiki M., Okada Y. Membrane-type 1 MMP (MMP-14) cleaves at three sites in the aggrecan interglobular domain. FEBS Lett. 1998;430:186–190. doi: 10.1016/s0014-5793(98)00667-x. [DOI] [PubMed] [Google Scholar]
- Goebeler M., Kaufmann D., Brocker E.B., Klein C.E. Migration of highly aggressive melanoma cells on hyaluronic acid is associated with functional changes, increased turnover and shedding of CD44 receptors. J. Cell Sci. 1996;109:1957–1964. doi: 10.1242/jcs.109.7.1957. [DOI] [PubMed] [Google Scholar]
- Gunthert U., Hofmann M., Rudy W., Reber S., Zoller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Guo Y.J., Liu G., Wang X., Jin D., Wu M., Ma J., Sy M.S. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res. 1994;54:422–426. [PubMed] [Google Scholar]
- Haberhauer G., Kittl E.M., Skoumal M., Hubl W., Wagner E., Bayer P.M., Bauer K., Dunky A. Increased serum levels of soluble CD44-isoform v5 in rheumatic diseases are restricted to seropositive rheumatoid arthritis. Acta Med. Austriaca. 1997;24:23–25. [PubMed] [Google Scholar]
- Haynes B.F., Hale L.P., Patton K.L., Martin M.E., McCallum R.M. Measurement of an adhesion molecule as an indicator of inflammatory disease activity. Up-regulation of the receptor for hyaluronate (CD44) in rheumatoid arthritis. Arthritis Rheum. 1991;34:1434–1443. doi: 10.1002/art.1780341115. [DOI] [PubMed] [Google Scholar]
- Henke C.A., Roongta U., Mickelson D.J., Knutson J.R., McCarthy J.B. CD44-related chondroitin sulfate proteoglycan, a cell surface receptor implicated with tumor cell invasion, mediates endothelial cell migration on fibrinogen and invasion into a fibrin matrix. J. Clin. Invest. 1996;97:2541–2552. doi: 10.1172/JCI118702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N., Allen E., Apel I.J., Gyetko M.R., Weiss S.J. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- Hotary K., Allen E., Punturieri A., Yana I., Weiss S.J. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J. Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Kajita M., Kinoh H., Mori H., Okada A., Seiki M. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J. Biol. Chem. 1999;274:34260–34266. doi: 10.1074/jbc.274.48.34260. [DOI] [PubMed] [Google Scholar]
- Keely P., Parise L., Juliano R. Integrins and GTPases in tumour cell growth, motility and invasion. Trends Cell Biol. 1998;8:101–106. doi: 10.1016/s0962-8924(97)01219-1. [DOI] [PubMed] [Google Scholar]
- Kittl E.M., Haberhauer G., Ruckser R., Selleny S., Rech-Weichselbraun I., Hinterberger W., Bauer K. Serum levels of soluble CD44 variant isoforms are elevated in rheumatoid arthritis. Rheumatol. Int. 1997;16:181–186. doi: 10.1007/BF01330293. [DOI] [PubMed] [Google Scholar]
- Knauper V., Will H., Lopez-Otin C., Smith B., Atkinson S.J., Stanton H., Hembry R.M., Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J. Biol. Chem. 1996;271:17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- Kojima S., Itoh Y., Matsumoto S., Masuho Y., Seiki M. Membrane-type 6 matrix metalloproteinase (MT6-MMP, MMP-25) is the second glycosyl-phosphatidyl inositol (GPI)-anchored MMP. FEBS Lett. 2000;480:142–146. doi: 10.1016/s0014-5793(00)01919-0. [DOI] [PubMed] [Google Scholar]
- Kolanus W., Seed B. Integrins and inside-out signal transductionconverging signals from PKC and PIP3. Curr. Opin. Cell Biol. 1997;9:725–731. doi: 10.1016/s0955-0674(97)80127-5. [DOI] [PubMed] [Google Scholar]
- Koshikawa N., Giannelli G., Cirulli V., Miyazaki K., Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar C.C. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- Ladeda V., Aguirre Ghiso J.A., Bal de Kier Joffe E. Function and expression of CD44 during spreading, migration, and invasion of murine carcinoma cells. Exp. Cell Res. 1998;242:515–527. doi: 10.1006/excr.1998.4094. [DOI] [PubMed] [Google Scholar]
- Lasky L.A. Selectinsinterpreters of cell-specific carbohydrate information during inflammation. Science. 1992;258:964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Liotta L.A., Steeg P.S., Stetler-Stevenson W.G. Cancer metastasis and angiogenesisan imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Masson D., Denis M.G., Denis M., Blanchard D., Loirat M.J., Cassagnau E., Lustenberger P. Soluble CD44quantification and molecular repartition in plasma of patients with colorectal cancer. Br. J. Cancer. 1999;80:1995–2000. doi: 10.1038/sj.bjc.6690633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L.M. Cancer biologyextracellular proteinases in malignancy. Curr. Biol. 1999;9:R776–R778. doi: 10.1016/S0960-9822(00)80011-1. [DOI] [PubMed] [Google Scholar]
- Murphy G., Gavrilovic J. Proteolysis and cell migrationcreating a path? Curr. Opin. Cell Biol. 1999;11:614–621. doi: 10.1016/s0955-0674(99)00022-8. [DOI] [PubMed] [Google Scholar]
- Nagase H., Woessner J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Ueno H., Yamashita K., Shimada T., Yamamoto E., Noguchi M., Fujimoto N., Sato H., Seiki M., Okada Y. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human papillary thyroid carcinomas. Cancer Res. 1999;59:467–473. [PubMed] [Google Scholar]
- Naot D., Sionov R.V., Ish-Shalom D. CD44structure, function, and association with the malignant process. Adv. Cancer Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- Nomura H., Sato H., Seiki M., Mai M., Okada Y. Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res. 1995;55:3263–3266. [PubMed] [Google Scholar]
- Ohuchi E., Imai K., Fujii Y., Sato H., Seiki M., Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Okada H., Yoshida J., Sokabe M., Wakabayashi T., Hagiwara M. Suppression of CD44 expression decreases migration and invasion of human glioma cells. Int. J. Cancer. 1996;66:255–260. doi: 10.1002/(SICI)1097-0215(19960410)66:2<255::AID-IJC20>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Okamoto I., Kawano Y., Tsuiki H., Sasaki J., Nakao M., Matsumoto M., Suga M., Ando M., Nakajima M., Saya H. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene. 1999;18:1435–1446. doi: 10.1038/sj.onc.1202447. [DOI] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Sato T., del Carmen Ovejero M., Hou P., Heegaard A.M., Kumegawa M., Foged N.T., Delaisse J.M. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J. Cell Sci. 1997;110:589–596. doi: 10.1242/jcs.110.5.589. [DOI] [PubMed] [Google Scholar]
- Seiki M. Membrane-type matrix metalloproteinases. APMIS. 1999;107:137–143. doi: 10.1111/j.1699-0463.1999.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Sheetz M.P., Felsenfeld D.P., Galbraith C.G. Cell migrationregulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998;8:51–54. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- Sneath R.J., Mangham D.C. The normal structure and function of CD44 and its role in neoplasia. Mol. Pathol. 1998;51:191–200. doi: 10.1136/mp.51.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W.G., Aznavoorian S., Liotta L.A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Talbot D.C., Brown P.D. Experimental and clinical studies on the use of matrix metalloproteinase inhibitors for the treatment of cancer. Eur. J. Cancer. 1996;32A:2528–2533. doi: 10.1016/s0959-8049(96)00398-x. [DOI] [PubMed] [Google Scholar]
- Thomas L., Byers H.R., Vink J., Stamenkovic I. CD44H regulates tumor cell migration on hyaluronate-coated substrate. J. Cell Biol. 1992;118:971–977. doi: 10.1083/jcb.118.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Etoh T., Stamenkovic I., Mihm M.C., Jr., Byers H.R. Migration of human melanoma cells on hyaluronate is related to CD44 expression. J. Invest. Dermatol. 1993;100:115–120. doi: 10.1111/1523-1747.ep12462776. [DOI] [PubMed] [Google Scholar]
- Tokuraku M., Sato H., Murakami S., Okada Y., Watanabe Y., Seiki M. Activation of the precursor of gelatinase A/72 kDa type IV collagenase/MMP-2 in lung carcinomas correlates with the expression of membrane-type matrix metalloproteinase (MT-MMP) and with lymph node metastasis. Int. J. Cancer. 1995;64:355–359. doi: 10.1002/ijc.2910640513. [DOI] [PubMed] [Google Scholar]
- Trochon V., Mabilat C., Bertrand P., Legrand Y., Smadja-Joffe F., Soria C., Delpech B., Lu H. Evidence of involvement of CD44 in endothelial cell proliferation, migration and angiogenesis in vitro. Int. J. Cancer. 1996;66:664–668. doi: 10.1002/(SICI)1097-0215(19960529)66:5<664::AID-IJC14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Hoyhtya M., Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res. Treat. 1993;24:209–218. doi: 10.1007/BF01833261. [DOI] [PubMed] [Google Scholar]
- Ueno H., Nakamura H., Inoue M., Imai K., Noguchi M., Sato H., Seiki M., Okada Y. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997;57:2055–2060. [PubMed] [Google Scholar]
- Walcheck B., Kahn J., Fisher J.M., Wang B.B., Fisk R.S., Payan D.G., Feehan C., Betageri R., Darlak K., Spatola A.F., Kishimoto T.K. Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature. 1996;380:720–723. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysisregulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Yamane N., Tsujitani S., Makino M., Maeta M., Kaibara N. Soluble CD44 variant 6 as a prognostic indicator in patients with colorectal cancer. Oncology. 1999;56:232–238. doi: 10.1159/000011970. [DOI] [PubMed] [Google Scholar]
- Yana I., Weiss S.J. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol. Biol. Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]