Epithelial cells are associated laterally in a sheet, with the apical surface facing the lumina, often covered by microvilli, and the basolateral side underlying connective tissue. Development and maintenance of epithelial polarity require cell–substrate and cell–cell adhesions that promote localized assembly of submembranous cytoskeleton networks and specialized membrane intracellular transport pathways. The assembly of tight junctions (TJs) plays a key role in the formation of structurally and functionally distinct basolateral and apical plasma membrane (PM) domains. How are the PM proteins delivered to specialized and polarized cell surface domains? Even though the involvement of v- and t-soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs) in apical and basolateral PM delivery is now well established, are these proteins sufficient to specify vesicle docking and fusion at sites in the appropriate membrane domains? Recent data highlight TJs as major sites for localization of proteins involved in vesicle docking/targeting and signaling. This review will focus on how TJ constituents contribute to these processes.
Molecular Architecture of TJ, Plasma Membrane Barriers
TJs are specialized PM microdomains that form continuous branching strands around each epithelial cell, separating apical from basolateral sides. By freeze–fracture electron microscopy, they appear as a network of continuous anastomosing fibrils. These fibrils are tightly connected laterally to those in apposing membranes (kisses) to seal the paracellular space between adjacent cells. This architectural organization confers two important properties to TJs: they act as (a) a selective intercellular barrier regulating diffusion of molecules and ions across the paracellular route, and (b) a fence within the lateral PM, thus impairing the intermixing of apical and basolateral membrane proteins and lipids. Significant progress in understanding the functional architecture of TJs was achieved by identifying key components of TJs.
Occludin was the first integral membrane protein found concentrated within TJ fibrils. Several pieces of evidence suggest that occludin is involved in the barrier and fence functions of TJs (Tsukita and Furuse 1999). Overexpression of the chicken occludin in MDCK cells increases transepithelial electrical resistance, whereas COOH-terminal truncated occludin increases paracellular flux of small tracers and induces leakage of N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-S-indacene-3-pentanoyl) sphingosyl phosphocholine (BODIPY)–sphingomyelin from the apical domain (Balda et al. 1996). Introduction of a peptide containing the second extracellular loop of occludin into Xenopus A6 epithelial cells causes a drop in transepithelial electrical resistance and mislocalization of occludin at TJs (Wong and Gumbiner 1997). However, data from several experiments indicate that occludin is not sufficient to generate bona fide TJ strands. For example, differentiated embryonic bodies that were isolated from embryonic stem cells in which the occludin gene was knocked out still developed a normal network of TJ fibrils between adjacent epithelial cells (Saitou et al. 1998). In fact, establishment of TJ strands depends on claudins, which is another recently identified protein family that has at least 18 members. Claudins possess four transmembrane domains and are also localized at the site of close membrane–membrane apposition (kisses) within TJs. Expression of claudins1 and 2 into fibroblasts lacking TJs induces the formation of TJ strands that are morphologically similar to the epithelial TJ strands (Tsukita and Furuse 2000). Analysis of oligodendrocyte-specific protein (OSP/claudin11) KO mice reveals the absence of TJ strands in myelin sheets of oligodendrocytes and sertoli cells (Gow et al. 1999), and paracellin1/claudin16 KO mice show an abnormal paracellular passage of Mg2+ ions (Simon et al. 1999). This finding leads to the proposal that claudins and occludin generate a series of regulated channels within TJ membranes for the passage of ions and small molecules.
The cytoplasmic face of TJs is enriched in many peripheral membrane proteins (Table ). ZO-1, a 220-kD TJ phosphoprotein, is a member of the membrane-associated guanylate kinase domain (GUK) localized at cell–cell contacts (Mitic and Anderson 1998). It contains three PDZ (PSD95, Dlg, and ZO-1), an SH3 domain, and an inactive GUK. PDZ domains are protein–protein interaction modules that recognize motifs of three amino acids at the COOH terminus of transmembrane proteins. ZO-1 may act as a molecular scaffold bringing together many proteins of TJs. ZO-1 binds claudins, occludin, ZO-2, ZO-3, cingulin, and actin (Cordenonsi et al. 1999; Wittchen et al. 1999). It has three PDZ modules that could bind many different protein partners to control the dynamics of TJ assembly. Thus, it is tempting to speculate that the ZO-1/-2/-3 proteins are required for the clustering of claudins and occludin to generate TJ fibrils and, presumably, the pores within these fibrils.
Table 1.
Tight Junction Proteins
| TJ proteins | Structural features | Function | Known partners |
|---|---|---|---|
| Claudins | 4 TM, COOH-YV | TJ barrier | ZO-11 |
| Occludin | 4 TM | TJ barrier | ZO-1, ZO-2, Vap33, actin1–3 |
| JAM | 1 TM, Ig-like | Monocyte transmigration4 | |
| ZO-1 | 3 PDZ, 1SH3, GUK | Scaffold protein, signaling molecule | Occludin, claudins, ZO-2, -3, cingulin, actin, ZONAB1,2,5,6 |
| ZO-2 | 3 PDZ, 1SH3, GUK | Scaffold protein | ZO-1, actin2 |
| ZO-3 | 3 PDZ, 1SH3, GUK | Scaffold protein | ZO-1, occludin2 |
| AF-6 | PDZ | Scaffold protein | Ras, ZO-17 |
| Dlg (Drosophila) | 3 PDZ, 1SH3, GUK | Scaffold protein, signaling molecule8 | |
| Scribble (Drosophila) | 4 PDZ | Fence/membrane traffic9 | |
| Cingulin | coiled coil | ZO-1, -2, -3, occludin, myosin5 | |
| Symplekin | Signaling molecule10 | ||
| ASIP/Par3 | 3 PDZ | Signaling molecule | PKC ζ11,12,13 |
| Rab3b | GDP/GTP binding | Membrane traffic14 | |
| Rab13 | GDP/GTP binding | Membrane traffic | δ-PDE15,16 |
| Rab8 | GDP/GTP binding | Membrane traffic | G/C kinase17,18, exocyst subunits19 |
| Sec6, Sec8 | Vesicle docking |
Exponent numbers indicate references: 1, Itoh et al. 1999; 2, Wittchen et al., 1999; 3, Lapierre et al., 1999; 4, Martin-Padura et al. 1998; 5, Cordenonsi et al., 1999; 6, Balda and Matter, 2000; 7, Yamamoto et al. 1997; 8, Woods and Bryant, 1991; 9, Bilder and Perrimon, 2000a; 10, Keon et al., 1996; 11, Joberty et al., 2000; 12, Lin et al., 2000; 13, Izumi et al. 1998; 14, Weber et al., 1994; 15, Zahraoui et al., 1994; 16, Marzesco et al. 1998; 17, Huber et al., 1993; 18, Ren et al. 1996; and 19, Grindstaff et al., 1998.
TM, transmembrane.
The complexity of TJ organization is reflected by the growing number of protein partners (Table ). A ZO1-associated nucleic acid–binding protein (ZONAB) associates with the SH3 domain of ZO-1 (Balda and Matter 2000). In contrast, the partners for PDZ and SH3 domains of ZO-2/-3 have not yet been identified. It is possible that these latter domains, and those contained in the other TJ proteins, also participate in targeting the proteins mentioned above, or different proteins, to TJs. Therefore, the presence of multiple PDZ modules in several TJ proteins points to a high degree of complexity of protein–protein associations. It remains to be determined how these proteins are spatially and dynamically arranged to form TJ complexes, and how the interaction at the junctional complex, with the underlying actin cytoskeleton, may regulate TJ functions. Exactly how actin is coupled to TJs is still an open question. A small number of actin filaments anchors at sites of kisses within TJs (Madara 1998), presumably in close contact with occludin/claudins.
Polarized Membrane Traffic in Epithelial Cells
The occurrence of TJs implies that epithelial cells have specific routes to deliver and/or internalize proteins from the apical and basolateral PMs. These routes have been recently reviewed by several authors (Keller and Simons 1997; Yeaman et al. 1999; Mostov et al. 2000) and can be classified into five groups (Fig. 1): (a) A1, the biosynthetic route to the apical PM; (b) A2, the apical recycling; (c) B1, the biosynthetic route to the basolateral PM; (d) B2, the basolateral recycling; and (e) B/A and A/B, the transcytosis from the basolateral to the apical PMs and vice versa, respectively.
Figure 1.
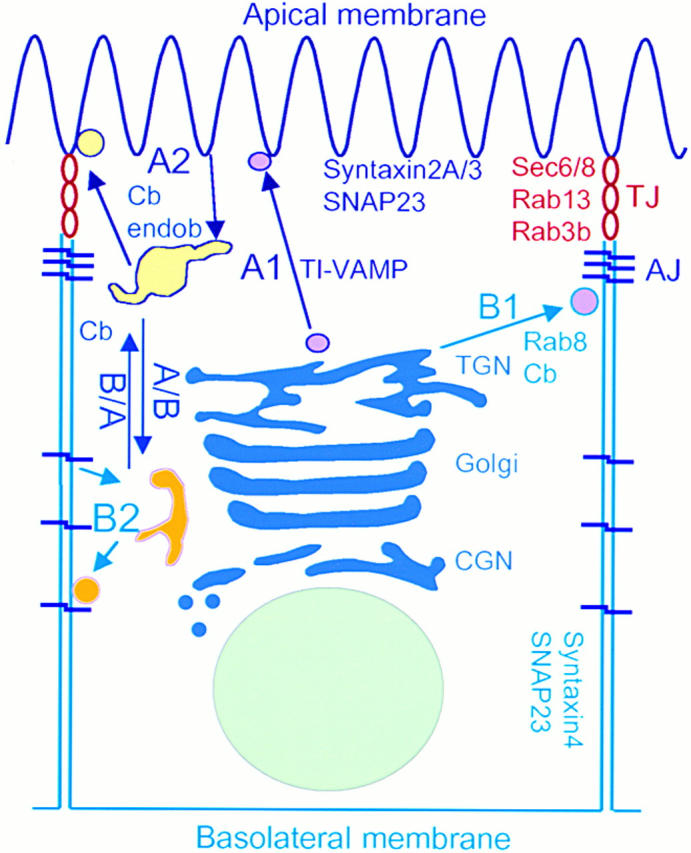
Schematic representation of polarized transport in an epithelial cell. Arrows specify transport from and toward either the apical or the basolateral PM. A1, the biosynthetic route to the apical PM; A2, the apical recycling; B1, the biosynthetic route to the basolateral PM; B2, the basolateral recycling; B/A, the transcytosis from the basolateral to the apical membrane and vice versa. Two distinct populations of postGolgi vesicles (purple filling) are delivered either to the apical or the basolateral PM. Proteins internalized from each PM domain (yellow filling) recycle with apical or basolateral endosomes. Rab3b, Rab13, and Sec6/8 are concentrated in TJ areas (red rings).
Route A1 and B/A coexist in the human adenocarcinoma of the colon (CaCo-2) cell line and in hepatocytes, whereas route A1 is dominant in MDCK cells. There are also crossroads between routes B2, A2, and B/A, which meet in recycling and sorting endosomes in several types of epithelial cells. Endocytotic signals play a role in routes B1 and B2, whereas N- and O-glycosylation and association with raft microdomains have been implicated in route A1 (Keller and Simons 1997; Yeaman et al. 1999; Mostov et al. 2000). Interestingly, ZO-1 and occludin are associated with raft microdomains in T84 cells (Nusrat et al. 2000). Nevertheless, none of these signals seem to establish absolute rules for apical and/or basolateral sorting. Specific adaptors may play crucial roles in the sorting of apical and basolateral proteins. Hence, μ1B, a subunit of an AP-1 coat, is required for the B1 route, and possibly B2 (Folsch et al. 1999).
Two PM Domains—Two SNARE Machineries?
Overwhelming evidence show that SNAREs are key proteins of membrane traffic. It is now clearly established that they provide the core machinery for lipid bilayer fusion (Weber et al. 1998), and that they are involved in all of the trafficking pathways (Bock and Scheller 1999). SNAREs of the exocytotic pathways include the syntaxin (1, 2, 3, and 4), synaptosome-associated protein (SNAP23/25), and brevin/Vesicle-Associated Membrane Protein (VAMP) (1, 2, 3, 7, and 8) families. Three syntaxin isoforms show a polarized localization in epithelial cells. Syntaxins 2A and 3 localize to the apical PM in CaCo-2 and MDCK cells. Syntaxin 4 is specifically present at the basolateral PM. SNAP23, a t-SNARE that interacts with syntaxins 2/3/4, is concentrated at the apical PM, but is also present in the basolateral membrane (Low et al. 1996; Galli et al. 1998; Quiñones et al. 1999; Riento et al. 1998). Syntaxin 3 plays a role in routes A1 and A2, but not BA. SNAP23 is involved in routes A1, B1, B2, and BA (Leung et al. 1998; Low et al. 1998; Lafont et al. 1999). Involvement of syntaxin 2 and 4 in routes B1, B2, and BA has not yet been established. Thus, the relationship between syntaxins and the different exocytotic routes is not yet clearly determined. Strikingly, when MDCK cells lose their polarity, apical t-SNAREs redistribute into the apical vacuolar compartment, and basolateral t-SNAREs redistribute into the basolateral counterpart (Low et al. 2000), thus demonstrating that t-SNARE's polarity is conserved.
Route A1 involves tetanus neurotoxin (TeNT) insensitive-(TI)VAMP (also called VAMP7). Indeed, TI-VAMP forms SNARE complexes with syntaxin 3, and antibodies against TI-VAMP inhibit delivery of HA to the apical PM (Galli et al. 1998; Lafont et al. 1999). The v-SNARE involved in route B1 has not yet been identified, but transport of VSV-G to the basolateral PM is partially inhibited by TeNT (Ikonen et al. 1995), so it is likely to involve cellubrevin, the only TeNT-sensitive brevin yet characterized in epithelial cells (Galli, T., unpublished observation). Epithelial cells could package together two different v-SNAREs to perform routes AB and BA, one binding to a basolateral syntaxin and one to an apical syntaxin distinct from syntaxin3. Alternatively, they could use one v-SNARE capable of binding both basolateral and apical syntaxins. In contrast to fibroblasts, cellubrevin does not colocalize with transferrin receptor in CaCo-2 cells (Galli et al. 1994, Galli et al. 1998), but it recycles with both the apical and basolateral PMs in MDCK cells (Steegmaier et al. 2000), and it colocalizes with transcytosed proteins, such as IgA in hepatocytes (Calvo et al. 2000). Therefore, cellubrevin is a good candidate for being the v-SNARE of routes BA and/or AB. Endobrevin/VAMP8 recycles with the apical PM in MDCK cells (Steegmaier et al. 2000) so it is expected to play a role in route A2 in these cells. In other epithelial cells, the v-SNARE of A2 is likely to be cellubrevin, because H+ secretion in collecting duct cells (Alexander et al. 1997) and recycling of the H+-ATPase with the apical PM in epididymis and vas deferens (Breton et al. 2000) are sensitive to TeNT. Whereas several of the above data suggest that v- and t-SNAREs function in polarized traffic, the complex case of cellubrevin, which is implicated in different routes, could imply that other factors define cognate docking and fusion sites. For instance, VAP-33, a protein localized in TJs that interacts with VAMP/synaptobrevins and with the cytoplasmic COOH-terminal of occludin (Lapierre et al. 1999), could participate in this process.
TJ, a Spatial Landmark for Vesicle Docking
Could TJs be sites of vesicle docking? It has been shown that aminopeptidase N is delivered to cell–cell contacts before its final localization at the cell apex in MDCK cells (Louvard 1980). Although the molecular mechanisms involved are still poorly understood, there is increasing evidence that TJs are a site of action for several proteins required for vesicle targeting and docking. Among these proteins are the small GTPases of the Rab family, which regulate different steps of exocytotic and endocytotic pathways (Chavrier and Goud 1999). Rab8 is involved in vesicle transport to the basolateral domain. It is found on Golgi-derived vesicles and on the basolateral PM, including TJs, of epithelial MDCK cells (Huber et al. 1993). Two other small GTPases, Rab3b and Rab13, are localized to TJs. Recruitment of Rab13 to TJs requires both the assembly and integrity of TJs (Weber et al. 1994; Zahraoui et al. 1994). Although the exact role of Rab proteins is still unclear, they may control the assembly of protein complexes necessary for docking of transport vesicles with appropriate target membranes (Schimmoller et al. 1998). Rab8 and Rab13 share high amino acid sequence identity with the Saccharomyces cerevisiae Sec4, a small GTPase required for polarized delivery of cargo vesicles to the PM during the budding process. Sec4 may control the assembly of the exocyst, a complex composed of at least eight proteins that concentrates at sites of vesicle fusion in yeast (Guo et al. 1999). Mammalian homologues of the exocyst subunits (except Sec3) have been identified (Hazuka et al. 1999). In polarized epithelial MDCK cells, components of the exocyst, Sec6 and Sec8, concentrate at TJs. Like Rab13, Sec6/8 are recruited to the PM from a cytosolic pool after cell–cell contact formation. Antibodies against Sec8 inhibit basolateral transport of low density lipoprotein receptors, but not the apical transport of p75NTR (Grindstaff et al. 1998). Taken together, these results strongly suggest that TJs may provide the machinery required for docking/fusion of transport vesicles. Recruitment of the small GTPases Rab 3b and 13 and Sec6/8 to TJs may specify a spatial landmark on the lateral PM where subsets of basolateral, apical, and/or junctional membrane proteins are preferentially delivered. As a consequence, the restriction of Rabs and Sec6/8 complex to TJs may enhance the specificity and efficiency of the targeting/docking of transport vesicles to their appropriate surface microdomains.
A crucial question is what maintains the correct distribution of targeted proteins to one PM domain? A possible answer is suggested by recent studies on Scrib, Dlg, and Lgl, three Drosophila proteins localized to the epithelial septate junctions (the analogue of vertebrate TJs). Loss of function of any of these genes leads to the disruption of cell polarity. These genes show strong genetic interactions, suggesting they are involved in a common pathway to control both cell growth and polarity. Furthermore, Scrib, Dlg, and Lgl are mutually dependent for proper localization, raising the possibility that they physically interact (Bilder et al. 2000). In the Scrib mutants, adherens junction proteins, including armadillo (a β-catenin homologue), are mislocated and found around the cell periphery, and apical transmembrane proteins exhibit unrestricted distribution to both apical and basolateral domains (Bilder and Perrimon 2000). Thus, Scrib is required for maintaining apical membrane proteins at the apical domain, and it may play a role in polarized targeting of vesicles charged with apical proteins. In agreement with this hypothesis, the Lgl homologues in yeast (Sro7) and mammals (tomosyn) bind to PM t-SNAREs Sec9p and syntaxin 1, respectively, which directly promote fusion of transport vesicles with the PM (Fujita et al. 1998; Lehman et al. 1999). An attractive model for the function of Scrib, Dlg, and Lgl could be that the PDZ domains of Scrib and Dlg bind to transmembrane proteins and organize cell surface asymmetry, whereas Lgl locally promotes the assembly of SNARE complexes. The localized assembly of SNAREs at specific sites of the PM would restrict vesicle docking and fusion at these sites.
Scrib, like other leucine-rich repeats and PDZ domain (LAP) proteins, could also bind through its leucine-rich repeats to small GTPases of the Ras family known to play an important role in intracellular transport. Accordingly, PDZ-containing proteins may interact (directly or indirectly) with the Rab/exocyst and contribute to the specificity and accuracy of vesicle-targeting events. We speculate also that the interactions of Rab/exocyst/Lgl/Scrib/Dlg define a checkpoint site at TJs where misrouted proteins could be identified and rerouted to their correct destination.
TJ, a Signal Transduction Site
Various signaling molecules, such as protein kinase C (PKC), heterotrimeric G-protein, and phospholipase C (PLC), that affect the organization of the actin cytoskeleton and regulate membrane traffic are implicated in TJ functions. Recent findings show that Cdc42 and Rac, involved in the dynamics of actin cytoskeleton and cell polarity, bind to a protein complex containing Par6, Par3/ASIP, and atypical PKCζ isoform. In the nematode Caenorhabditis elegans, Par-3 and Par-6 are implicated in asymmetric cell division. For example, Par-3 mutations cause mislocalization of several asymmetrically distributed proteins, disruption of mitotic spindle orientation and disruption of asymmetric cell division. In epithelial cells, Par3/ASIP localizes to TJs. Overexpression of either Par6, the NH2-terminal part of Par3, or the activated form of Cdc42 leads to mislocalization of ZO-1, indicating that the Cdc42/Par6/Par3/aPKCζ complex may be involved in maintaining cell–cell contact (Joberty et al. 2000; Lin et al. 2000). The specific binding of activated Cdc42/Rac1 to Par6 links Par6/Par3/aPKCζ complex to signaling pathways regulating actin cytoskeleton and vesicle targeting. Interestingly, microinjection of Cdc42T22N, a dominant-negative form of Cdc42, into MDCK cells leads to mislocalization of the basolateral membrane protein, gp58 (Kroschewski et al. 1999). Given the ability of Cdc42 to control actin cytoskeleton, it is possible that the Cdc42 mutation causes localized alterations in cortical actin filaments, leading to the loss of gp58's basolateral polarity. This result also suggests that the actin cytoskeleton may help to immobilize a Cdc42-interacting protein required to target basolateral membrane proteins. Control of the dynamics of F-actin rearrangement by the small GTPases Rho, Rac, and Cdc42 points to a role for these GTPases in TJ structure. Indeed, overexpression of activated forms RhoV14 and RacV12 induces the disorganization of TJ strands and a chaotic distribution of ZO-1 and occludin. ZO-1, as well as occludin staining, extends to the base of the cells expressing RhoV14 and RacV12 (Jou et al. 1998). One explanation is that RhoV14 and RacV12 cause dramatic changes in actin–myosin dynamics, leading to an alteration in TJ protein–protein interaction. Hence, the Rho-protein family, by regulating actin organization, could participate in targeting vesicles to the appropriate sites on the PM. Signal transduction at TJs could regulate membrane traffic by phosphorylation of Rab effectors, SNARE proteins, or their partners. For instance, these signaling pathways could play a major role in allowing exocytotic vesicles to reach the apical domain.
Several membrane-associated GUKs are involved in organizing signal transduction at TJs. In Drosophila, the Dlg mutation interferes with proliferation control, apical–basal polarity, development of septate junctions, and the ability of cells to differentiate (Woods and Bryant 1991). In subconfluent epithelial cells, ZO-1, as well as symplekin, can be found in the nucleus, which raises the possibility that both proteins may have a role in regulating transcription (Gottardi et al. 1996; Keon et al. 1996). Recently, Balda and Matter 2000 have identified ZONAB, a protein homologous to Y-box transcription factors, which localizes to the nucleus and TJs. ZO-1 and ZONAB control endogenous ErbB-2 expression and regulate the paracellular permeability. These results indicate that ZO-1 is implicated in a signal transduction pathway that leads to the activation of specific gene expression. Expression of two NH2-terminal mutants of ZO-1 (ZO-11–422 and ZO-11–794) cause a dramatic change in cell morphology, reminiscent of an epithelium to mesenchyme transition (Ryeom et al. 2000), suggesting that ZO-1 is involved in regulating epithelial cell differentiation. In addition, it was recently reported that introduction of occludin into Raf-1–transformed Pa-4 epithelial cells results in reacquisition of epithelial phenotype, with formation of functionally intact TJs (Li and Mrsny 2000). These data also suggest that occludin is a key molecule in Raf-1–transformation signaling. Taken together, the results imply that the TJ is a site of signaling.
Based on the studies reviewed here, TJs emerge as a platform used to coordinate multiple cellular processes. TJs function at crossroads of the secretory and signaling routes. They may serve both as a docking domain and probably as a switch station for subsets of proteins and lipids destined to the basolateral and/or the apical PM. At least some of the basolateral and/or apical membrane proteins could be delivered to TJs via the Rab/exocyst/Scrib/Lgl complex. A major challenge for future TJ studies will be to investigate its potential role in organizing and orienting polarized membrane trafficking.
Acknowledgments
We are grateful to Drs. Margaret Butler and Ray Golsteyn for a critical review of the manuscript.
This work was supported by grants from the Association pour la Recherche sur le Cancer (ARC 9923) to A. Zahraoui, and from the Ministère de la Recherche et des Technologies: ACI-Jeunes Chercheurs (no. 5254) to T. Galli.
Footnotes
T. Galli's present address is Group of Membrane Traffic and Neuronal Plasticity, INSERM U536, Institut du Fer-à-Moulin, 75005 Paris, France.
Abbreviations used in this paper: CaCo, adenocarcinoma of the colon; GUK, guanylate kinase domain; PKC, protein kinase C; PM, plasma membrane; SNAP, synaptosome-associated protein; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; TeNT, tetanus neurotoxin; TI, tetanus neurotoxin insensitive; TJ, tight junction; VAMP, vesicle-associated membrane protein; ZONAB, ZO1-associated nucleic acid–binding protein.
References
- Alexander E.A., Shih T., Schwartz J.H. H+ secretion is inhibited by clostridial toxins in an inner medullary collecting duct cell line. Am. J. Physiol. Renal.Physiol. 1997;42:F1054–F1057. doi: 10.1152/ajprenal.1997.273.6.F1054. [DOI] [PubMed] [Google Scholar]
- Balda M.S., Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO (Eur. Mol. Biol. Organ.) J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M.S., Whitney J.A., Flores C., Gonzalez S., Cerejido M., Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical–basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D., Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Bilder D., Li M., Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bock J.B., Scheller R.H. SNARE proteins mediate lipid bilayer fusion. Proc. Natl. Acad. Sci. USA. 1999;96:12227–12229. doi: 10.1073/pnas.96.22.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton S., Nsumu N.N., Galli T., Sabolic I., Smith P.J., Brown D. Tetanus toxin-mediated cleavage of cellubrevin inhibits proton secretion in the male reproductive tract. Am. J. Physiol. Renal. Physiol. 2000;278:F717–F725. doi: 10.1152/ajprenal.2000.278.5.F717. [DOI] [PubMed] [Google Scholar]
- Calvo M., Pol A., Lu A., Ortega D., Pons M., Blasi J., Enrich C. Cellubrevin is present in the basolateral endocytic compartment of hepatocytes and follows the transcytotic pathway after IgA internalization. J. Biol. Chem. 2000;275:7910–7917. doi: 10.1074/jbc.275.11.7910. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Goud B. The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 1999;11:466–475. doi: 10.1016/S0955-0674(99)80067-2. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M., D'Atri F., Hammar E., Parry D.A., Kendrick-Jones J., Shore D., Citi S. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J. Cell Biol. 1999;147:1569–1582. doi: 10.1083/jcb.147.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H., Ohno H., Bonifacino J.S., Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Shirataki H., Sakisaka T., Asakura T., Ohya T., Kotani H., Yokoyama S., Nishioka H., Matsuura Y., Mizoguchi A., Scheller R.H., Takai Y. Tomosyna syntaxin-1–binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- Galli T., Chilcote T., Mundigl O., Binz T., Niemann H., De Camilli P. Tetanus toxin–mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor–containing vesicles in CHO cells. J.Cell Biol. 1994;125:1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli T., Zahraoui A., Vaidyanathan V.V., Raposo G., Tian J.M., Karin M., Niemann H., Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol.Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi C.J., Arpin M., Fanning A.S., Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell–cell contacts. Proc. Natl. Acad. Sci. USA. 1996;93:10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow A., Southwood C.M., Li J.S., Pariali M., Riordan G.P., Brodie S.E., Danias J., Bronstein J.M., Kachar B., Lazzarini R.A. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- Grindstaff K.K., Yeaman C., Anandasabapathy N., Hsu S.C., Rodriguez-Boulan E., Scheller R.H., Nelson W.J. Sec6/8 complex is recruited to cell–cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuka C.D., Foletti D.L., Hsu S.C., Kee Y., Hopf F.W., Scheller R.H. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurol. 1999;19:1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L.A., Pimplikar S., Parton R.G., Virta H., Zerial M., Simmons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J. Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E., Tagaya M., Ullrich O., Montecucco C., Simons K. Different requirements for NSF, SNAP, and rab proteins in apical and basolateral transport in MDCK cells. Cell. 1995;81:571–580. doi: 10.1016/0092-8674(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M., Morita K., Kubota K., Saoto M., Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Hirose T., Tamai Y., Hirai S., Nagashima Y., Fujimoto T., Tabuse Y., Kemphues K.J., Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G., Peterson C., Gao L., Macara I.G. The cell polarity protein Par6 links Par3 and an atypical protein kinase C to Cdc42. Nat. Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Jou T.S., Schneeberger E.E., Nelson W.J. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P., Simons K. Post-Golgi biosynthetic trafficking. J. Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Keon B.H., Schafer S., Kuhn C., Grund C., Franke W.W. Symplekin, a novel type of tight junction plaque protein. J. Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R., Hall A., Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat. Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Lafont F., Verkade P., Galli T., Wimmer C., Louvard D., Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc. Natl. Acad. Sci. USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre L.A., Tuma P.L., Navarre J., Goldenring J.R., Anderson J.M. VAP-33 localizes to both an intracellular vesicle population and with occludin at the tight junction. J. Cell Sci. 1999;112:3723–3732. doi: 10.1242/jcs.112.21.3723. [DOI] [PubMed] [Google Scholar]
- Lehman K.G., Rossi J.E., Adamo, Brenwald P. Yeast homologues of Tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol. 1999;146:125–140. doi: 10.1083/jcb.146.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S.M., Chen D., DasGupta B.R., Whiteheart S.W., Apodaca G. SNAP-23 requirement for transferrin recycling in streptolysin-O-permeabilized Madin-Darby canine kidney cells. J. Biol. Chem. 1998;273:17732–17741. doi: 10.1074/jbc.273.28.17732. [DOI] [PubMed] [Google Scholar]
- Li D., Mrsny R.J. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J. Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Edwards A.S., Fawcett J.P., Mbamalu G., Scott J.D., Pawson T. A mammalian PAR3–PAR6 complex implicated in Cdc42/Rac1 and aPKC signaling and cell polarity. Nat. Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- Louvard D. Apical membrane aminopeptidase appears at site of cell–cell contact in cultured kidney epithelial cells. Proc. Natl. Acad. Sci.USA. 1980;77:4132–4136. doi: 10.1073/pnas.77.7.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S.H., Chapin S.J., Weimbs T., Komuves L.G., Bennett M.K., Mostov K.E. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S.H., Roche P.A., Anderson H.A., vanIjzendoorn S.C., Zhang M., Mostov K.E., Weimbs T. Targeting of SNAP-23 and SNAP-25 in polarized epithelial cells. J. Biol. Chem. 1998;273:3422–3430. doi: 10.1074/jbc.273.6.3422. [DOI] [PubMed] [Google Scholar]
- Low S.H., Miura M., Roche P.A., Valdez A.C., Mostov K.E., Weimbs T. Intracellular redirection of plasma membrane trafficking after loss of epithelial cell polarity. Mol. Biol. Cell. 2000;11:3045–3060. doi: 10.1091/mbc.11.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J.L. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I., Lostaglio S., Schneemann M., Williams L., Romano M., Fruscella P., Panzeri C., Stoppacciaro A., Ruco L., Villa A., Simmons D., Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzesco A.M., Galli T., Louvard D., Zahraoui A. The rod cGMP phosphodiesterase δ subunit dissociates the small GTPase Rab13 from membranes. J. Biol. Chem. 1998;273:22340–22345. doi: 10.1074/jbc.273.35.22340. [DOI] [PubMed] [Google Scholar]
- Mitic L.L., Anderson J.M. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Mostov K.E., Verges M., Altschuler Y. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Nusrat A., Parkos C.A., Verkade P., Foley C.S., Liang T.W., Innis-Whitehouse W., Eastburn K.K., Madara J.L. Tight junctions are membrane microdomains. J. Cell Sci. 2000;113:1771–1781. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- Quiñones B., Riento K., Olkkonen V.M., Hardy S., Bennett M.K. Syntaxin 2 splice variants exhibit differential expression patterns, biochemical properties and subcellular localizations. J. Cell Sci. 1999;112:4291–4304. doi: 10.1242/jcs.112.23.4291. [DOI] [PubMed] [Google Scholar]
- Ren M., Zeng J., De L., Chiarandini C., Rosenfeld M., Adesnik M., Sabatini D.D. In its active form, the GTP-binding protein rab8 interacts with a stress-activated protein kinase. Proc. Natl. Acad. Sci. USA. 1996;93:5151–5155. doi: 10.1073/pnas.93.10.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K., Galli T., Jansson S., Ehnholm C., Lehtonen E., Olkkonen V.M. Interaction of munc-18-2 with syntaxin 3 controls the association of apical SNAREs in epithelial cells. J.Cell Sci. 1998;111:2681–2688. doi: 10.1242/jcs.111.17.2681. [DOI] [PubMed] [Google Scholar]
- Ryeom S.W., Paul D., Goodenough D.A. Truncation mutants of the tight junction protein ZO-1 disrupt corneal epithelial cell morphology. Mol. Biol. Cell. 2000;11:1687–1696. doi: 10.1091/mbc.11.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M., Fujimoto K., Doi Y., Itoh M., Fujimoto T., Furuse M., Takano H., Noda T., Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmoller F., Simon I., Pfeffer S.R. Rab GTPases, directors of vesicle docking. J. Biol. Chem. 1998;273:22161–22164. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- Simon D.B., Lu Y., Choate K.A., Velazquez H., Al-Sabban E., Praga M., Casari G., Bettinelli A., Colussi G., Rodriguez-Soriano J., McCredie D., Milford D., Sanjad S., Lifton R.P. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- Steegmaier M., Lee K.C., Prekereis R., Scheller R.H. SNARE protein trafficking in polarized MDCK cells. Traffic. 2000;1:553–560. doi: 10.1034/j.1600-0854.2000.010705.x. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Furuse M. Occludin and claudins in tight-junction strandsleading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Furuse M. Pores in the wallclaudins constitute tight junction strands containing aqueous pores. J. Cell Biol. 2000;149:13–16. doi: 10.1083/jcb.149.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E., Berta G., Tousson A., St John P., Green M.W., Gopalokrishnan U., Jilling T., Sorscher E.J., Elton T.S., Abrahamson D.R., Kirk K.L. Expression and polarized targeting of a rab3 isoform in epithelial cells. J. Cell Biol. 1994;125:583–594. doi: 10.1083/jcb.125.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B.V., McNew J.A., Westermann B., Gmachl M., Parlati F., Sollner T.H., Rothman J.E. SNAREpinsminimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wittchen E.S., Haskins J., Stevenson B.R. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J. Biol. Chem. 1999;274:35179–35185. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- Wong V., Gumbiner B.M. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J. Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D.F., Bryant P.J. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Harada N., Kano K., Taya S., Canaani E., Matsuura Y., Mizoguchi A., Ide C., Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J. Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K.K., Nelson W.J. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Zahraoui A., Joberty G., Arpin M., Fontaine J.J., Hellio R., Tavitian A., Louvard D. A small rab GTPase is distributed in cytoplasmic vesicles in nonpolarized cells but colocalizes with the tight junction marker ZO-1 in polarized epithelial cells. J. Cell Biol. 1994;124:101–115. doi: 10.1083/jcb.124.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]


