Abstract
During apoptosis, caspases, a family of proteases, disassemble a cell by cleaving a set of proteins. Caspase-3 plays a major role in the disassembly of the nucleus by processing several nuclear substrates. The question is how caspase-3, which is usually cytoplasmic, gains access to its nuclear targets. It was suggested that caspase-3 is actively transported to the nucleus through the nuclear pores. We found that caspase-9, which is activated earlier than caspase-3, directly or indirectly inactivates nuclear transport and increases the diffusion limit of the nuclear pores. This increase allows caspase-3 and other molecules that could not pass through the nuclear pores in living cells to enter or leave the nucleus during apoptosis by diffusion. Hence, caspase-9 contributes to cell disassembly by disrupting the nuclear-cytoplasmic barrier.
Keywords: apoptosis, caspases, nuclear transport, nuclear pores
Introduction
Apoptosis is cell suicide that is induced by various stimuli. During apoptosis, the cell is disassembled by caspases, a family of highly specific proteases (reviewed in Cryns and Yuan 1998; Thornberry and Lazebnik 1998; Budihardjo et al. 1999). Caspases are expressed constitutively as precursors, which are activated by proteolytic processing. The current model is that caspases are activated in a proteolytic cascade, which includes initiator and effector caspases, their inhibitors, and activators. Initiator caspases are activated by autoproteolysis, which is induced by binding to specific activators. Each initiator caspase is activated in response to a subset of cytotoxic stimuli. For example, caspase-8 is activated by binding to DISC, a protein complex, which is formed after stimulation of the death receptors such as Fas and TNFR (reviewed in Ashkenazi and Dixit 1998). Caspase-9 is activated by binding to a complex containing APAF-1 and cytochrome c, which is formed after a number of stimuli, including DNA damage. Importantly, cytochrome c has to be released from mitochondria to participate in caspase-9 activation. The activated initiator caspases activate effector caspases by proteolytic processing. While initiator caspases are specific for each pathway of apoptosis, the effector caspases are shared.
Initiator and effector caspases disassemble a cell by cleaving a set of proteins. This processing results in dismantling of cellular structures, disrupting metabolism, inactivating anti-apoptotic activity of some proteins, and promoting pro-apoptotic activity of others (reviewed in Thornberry and Lazebnik 1998; Porter 1999). Remarkably, only a small fraction of proteins is cleaved, indicating that cell disassembly is highly efficient. Although it is not clear how the processing of caspase substrates leads to cell death, it appears that caspases target key components of cell structures and signaling pathways. For example, during apoptosis, DNA is degraded by CAD, a DNAse, which is constitutively expressed but is bound to its inhibitor ICAD/DFF45 in live cells (Liu et al. 1997; Enari et al. 1998). Caspase-3 releases active CAD by cleaving ICAD/DFF45 at only two sites (Sakahira et al. 1998). In another example, caspases disassemble the nuclear lamina, which is formed by polymers of lamins, by cleaving these proteins at a single site (Lazebnik et al. 1995; Orth et al. 1996; Takahashi et al. 1996).
Interestingly, caspase-3 is primarily cytoplasmic (Chandler et al. 1998; Mancini et al. 1998; Zhivotovsky et al. 1999; this study), while the complex of CAD with ICAD/DFF45 is nuclear (Liu et al. 1998; Samejima and Earnshaw 1998, Samejima and Earnshaw 2000). Therefore, the question is, how does caspase-3 reach ICAD/DFF45 and its other nuclear targets? This question is part of a larger problem, which is how the apoptotic machinery, which is initially activated in the cytoplasm, reaches the rest of the cell (Porter 1999). It was suggested that caspase-3 and perhaps other caspases are translocated into the nucleus by active transport and that transport is required for apoptosis (Yasuhara et al. 1997; Kuwana et al. 1998; Kohler et al. 1999).
Protein import and export is regulated by a complex machinery, which includes soluble components and nuclear pores (reviewed in Mattaj and Englmeier 1998; Nakielny and Dreyfuss 1999; Talcott and Moore 1999). Nuclear pores are gated channels composed of at least 50 proteins, many of which have not been characterized. Particles of <9 nM in diameter or globular proteins of less than 50–60 kD can enter the nucleus by diffusion, whereas larger objects should be actively transported. To be imported through the nuclear pores, a protein should have a nuclear localization signal (NLS) or be bound to an NLS-containing protein. The NLS is recognized by importins that form a transport complex with the protein. The complex translocates through the pores by a multi-step process, which involves interactions between the complex and nuclear pore components. Once in the nucleus, the transport complex is dissociated upon binding of Ran-GTP to the importins which release the protein. The dimeric complex of importin and Ran-GTP is returned to the cytoplasm, where Ran-GTP is converted into Ran-GDP by the subsequent action of Ran-GAP and Ran-BPs. Ran-GDP is translocated into the nucleus as a complex with NTF2, where it is regenerated into Ran-GTP by RCC1. Nuclear export occurs similarly except that the exported protein forms a complex with exportins and Ran-GTP. According to this model, both import and export of proteins requires a gradient of Ran-GTP in which a majority of the protein is concentrated in the nucleus (Izaurralde et al. 1997).
To understand how the cytoplasmic apoptotic machinery disassembles the nucleus, we investigated how caspase-3 reaches its nuclear substrates. We found that nuclear transport is inactivated after the activation of caspase-9. Furthermore, caspase-9 directly or indirectly increases the diffusion limit of nuclear pores, thereby allowing caspase-3 and other molecules to enter the nucleus by diffusion. Hence, caspase-9 promotes cell disassembly by disrupting the nuclear-cytoplasmic barrier.
Materials and Methods
Cell Culture
MCF-7 cells (American Type Culture Collection) were maintained in MEM media supplemented with 10% fetal bovine serum and nonessential amino acids.
Induction of Apoptosis
Apoptosis in cells was induced with 50 μM cisplatin (Sigma-Aldrich), 1 μM staurosporine, or 35 μg/ml TNF (Pharmingen) plus 10 μg/ml cycloheximide (Sigma-Aldrich) as indicated. Apoptosis was monitored by release of cytochrome c and/or chromatin condensation.
Antibodies
Monoclonal antibodies to caspase-9 (1-2), caspase-8 (1-3), caspase-3 (4-1-18), and caspase-7 (1-11) were described previously (Fearnhead et al. 1998). Monoclonal antibody to caspase-3 used for immunofluorescence was purchased from Transduction Laboratories. Rabbit polyclonal antibody to cytochrome c was prepared against cytochrome c and were affinity purified on cytochrome c agarose. Antibodies to nuclear transport proteins were purchased from Transduction Laboratories, except for RanGap (Zymed Laboratories), mAB414 (Babco), and QE51 (Covance).
Immunofluorescence/Confocal Microscopy
Cells were grown on poly-l-lysine (Sigma-Aldrich)–coated glass coverslips, fixed in PBS containing 4% paraformaldehyde, permeabilized in PBS containing 0.2% Triton and 0.5% BSA, and blocked with 2% BSA and 2% normal goat serum. Primary antibody incubation was performed according to the manufacturers' protocols (exceptions are described below) and was followed by secondary antibody conjugated to Alexa 594 or Alexa 488 (Molecular Probes). Cells were stained with either 4′6-diamidino-2-phenylindole (DAPI) or propidium iodide and coverslips were mounted using Prolong Antifade (Molecular Probes).
Monoclonal antibody to caspase-3 (0.5 μg/ml; Transduction Laboratory) was incubated with 1% acetone powder prepared from MCF-7 cells for 1 h at room temperature, and clarified by centrifugation (10,000 g, 30 min) before using for immunofluorescence.
Images were acquired either on a Zeiss Axiophot microscope equipped with a Photometrics SenSys cooled CCD camera using Image 2.0.5 software (Oncor) or on a confocal laser scanning microscope (LSM410; Carl Zeiss, Inc.).
Retroviral Gene Transduction
cDNAs of interest were cloned into a MarX-IV-neo (caspase-3 and caspase-3–green fluorescent protein, GFP) or MarX-IV-puro (caspase-9) retroviral gene transfer vector (Hannon et al. 1999). Retrovirus was produced by transfection into LinX-A packaging cells (L.Y. Xie, D. Beach, and G.J. Hannon, unpublished observations). Media from transfected LinX cells were supplemented with 8 μg/ml of polybrene, 10% FBS, and nonessential amino acids and added to plates of MCF-7 cells. Plates were centrifuged at 1,000 g for 1 h, and then incubated for 12–18 h at 32°C. Media were then replaced after 2 d and infected cells were selected using 1 μg/ml puromycin or 600 μg/ml Geneticin for 4 or 7 d, respectively. Infected cell lines were maintained in MEM media supplemented with 0.5 μg/ml puromycin or 300 μg/ml Geneticin.
GFP Oligomers, GFP-β-gal and NLS-GFP
Plasmids expressing GFP multimers were generated by cloning in frame one or more GFP cDNAs into pEGFP (CLONTECH Laboratories, Inc.). Plasmid expressing NLS-GFP was generated by subcloning the triplicated SV40 T-Antigen nuclear localization sequence (DPKKKRKV)3 into pEGFP. Plasmid encoding GFP-β-gal was generated by subcloning the beta-galactosidase cDNA (Promega) into pEGFP.
Transient Transfections
Plasmids encoding GFP multimers, GFP-β-Gal, and GFP-NLS were transfected into MCF-7 and MCF-7/C9DN cells using Fugene (Boehringer) according to the manufacturer's instructions. The average transfection efficiency was 30%. Apoptosis was induced 18 h after transfection.
Results and Discussion
Caspase-3 Translocates to the Nucleus during Apoptosis
To investigate how caspase-3 reaches its nuclear substrates during apoptosis, we used a breast carcinoma cell line MCF-7, which does not express caspase-3 because of a mutation in the caspase-3 gene (Janicke et al. 1998). As a result of the mutation, MCF-7 cells undergo apoptosis without typical apoptotic nuclear changes, such as DNA cleavage and condensation of chromatin into distinct round particles, although some changes in chromatin structure are detectable (Woo et al. 1998). Expression of caspase-3 in MCF-7 cells restores both DNA fragmentation and chromatin condensation, providing a model system to study the function of caspase-3 and its derivatives (Janicke et al. 1998).
To monitor caspase-3 localization in live and apoptotic cells, we decided to use a fusion of this protein with GFP, which would eliminate potential problems associated with the detection of proteins in apoptotic cells by immunofluorescence. In particular, we were concerned that chromatin condensation would make nuclear proteins less accessible to the antibodies. First, we investigated whether fusing caspase-3 to GFP affects the function of this protease. We used a retroviral gene transduction system (Hannon et al. 1999) to make MCF-7 cell lines that express caspase-3 (MCF-7/C3) or a fusion of caspase-3 to E-GFP (MCF-7/C3-GFP). Both caspase-3 and the fusion protein were expressed at levels comparable with the levels of caspase-3 in other breast carcinoma cell lines (Fig. 1 A). Consistent with the previous reports, we detected no caspase-3 in MCF-7 cells (Fig. 1 A). Both MCF-7/C3 and MCF-7/C3-GFP cells, but not the parental cell line, underwent typical apoptotic chromatin condensation after treatment with cisplatin, an anticancer drug that induces DNA damage (Fig. 1 B). This indicated that the fusion to GFP did not affect the ability of caspases-3 to induce nuclear changes of apoptosis. This conclusion was also supported by the finding that caspase-3 activity in extracts from apoptotic MCF-7/C3 and MCF-7/C3-GFP cells was similar when measured with DEVD-AFC, a peptide substrate for caspase-3 (data not shown). Consistent with the previous reports (Krajewska et al. 1997; Chandler et al. 1998; Mancini et al. 1998; Zhivotovsky et al. 1999), caspase-3 was found primarily in the cytoplasm in live cells, as was caspase-3-GFP (Fig. 1 C). Because caspase-3 and its fusion to GFP were similar in their activity and localization, and because the fusion protein could be visualized more reliably, we used caspase-3 GFP in this study.
Figure 1.
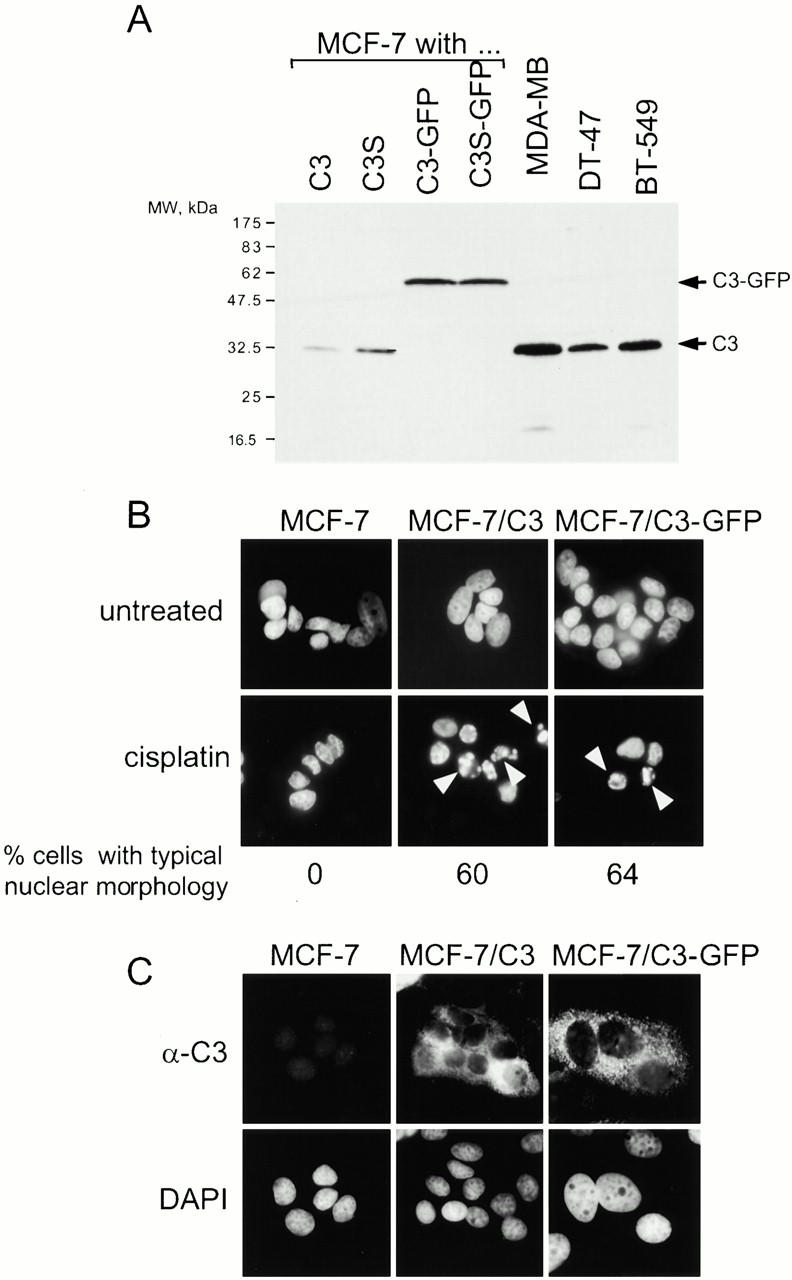
Caspase-3 and caspase-3-GFP have similar function and localization in MCF-7 cells. (A) Caspase-3 and caspase-3-GFP are ectopically expressed in MCF-7 at levels similar to the levels of caspase-3 in other breast carcinoma cell lines. Total cell lysates prepared from MCF-7/C3, MCF-7/C3S (catalytically inactive C3 mutant), MCF-7/C3-GFP, and MCF-7/C3S-GFP, and the three indicated breast carcinoma cell lines were analyzed by immunoblotting using a monoclonal antibody to caspase-3. 50 μg of lysate were loaded per lane. (B) MCF-7 cells that express either caspase-3 or caspase-3-GFP undergo typical apoptotic chromatin condensation. MCF-7, MCF-7/C3, and MCF-7/C3-GFP cells were treated with 50 μM cisplatin for 12 h, fixed, stained with DAPI to visualize chromatin, and scored by fluorescence microscopy for typical apoptotic chromatin condensation (indicated with white arrows). 60% of cells expressing C3 and 64% of cells expressing C3-GFP had typical apoptotic nuclear morphology, which was not observed in MCF-7 cells. (C) Caspase-3 and caspase-3-GFP have similar, cytoplasmic localization. Caspase-3 in MCF-7, MCF-7/C3, and MCF-7/C3-GFP cells was visualized by fluorescence microscopy using a monoclonal antibody to caspase-3, as described in Materials and Methods. As expected, no caspase-3 was detected in MCF-7 cells.
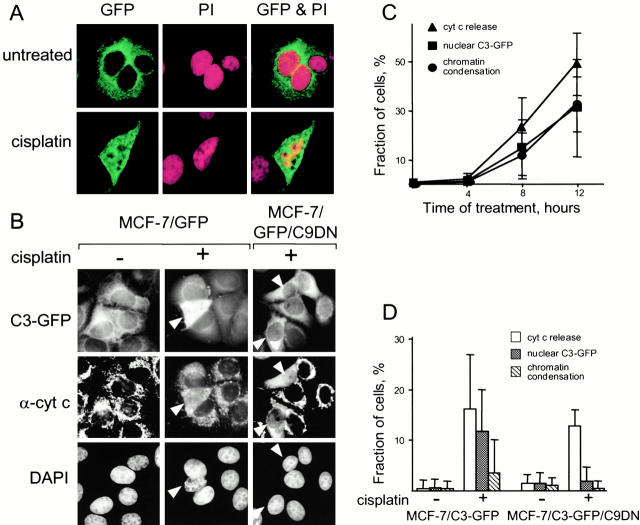
To confirm that caspase-3-GFP enters the nucleus during apoptosis (Nakagawara et al. 1997; Posmantur et al. 1997), we treated MCF-7/C3-GFP with cisplatin. We found that caspase-3-GFP was no longer excluded from the nucleus as detected by confocal (Fig. 2 A) and fluorescence (B) microscopy.
Figure 2.
Caspase-3 translocates to the nucleus during apoptosis after caspase-9 activation. (A) Caspase-3 translocates into the nucleus during apoptosis. MCF-7/C3-GFP cells were treated with 50 μM cisplatin for 8 h, fixed, and stained with propidium iodide (PI). Caspase-3-GFP (GFP, green) and the nuclei (PI, red), were visualized by confocal microscopy. An arrow indicates a cell in which chromatin begins to condense. Cells at later stages of apoptosis were difficult to observe because they shrink and detach. (B–D) Translocation of caspase-3 into the nucleus during apoptosis requires caspase-9 activity. (B) MCF-7/C3-GFP and MCF-7/C3-GFP cells expressing a dominant-negative mutant of caspase-9 (MCF-7/C3-GFP/C9DN) were treated with 50 μM cisplatin for 8 h. The cells were stained with DAPI to visualize the chromatin and with a cytochrome c antibody to detect the release of this protein from mitochondria. GFP, DAPI, and cytochrome c were visualized by fluorescence microscopy. White arrows indicate cells with released cytochrome c. (C) The rate of caspase-3 translocation into the nucleus is similar to that of chromatin condensation, and of cytochrome c release from mitochondria. MCF-7/C3-GFP cells were treated and stained as in B, except cells were fixed at the indicated time. The incidence of C3-GFP translocation to the nucleus, cytochrome c release, and change in chromatin structure was scored. (D) Dominant-negative mutant of caspase-9 (C9DN) prevents C3-GFP translocation to the nucleus and chromatin condensation. MCF-7/C3-GFP and MCF-7/C3-GFP/C9DN cells were treated and stained as in B. The incidence of C3-GFP translocation to the nucleus, cytochrome c release and change in chromatin structure was scored. 200–250 cells were counted for each measurement in all experiments. Average values of three experiments are presented in C and D.
Caspase-3 Entry into the Nucleus Requires Active Caspase-9
Apoptosis can be considered as a two-step process in which the first step is a preparation for activating caspases and the second is the activation and its consequences. We asked whether caspase-3 translocation to the nucleus occurs before activation of caspases or requires their activity. Because we used a drug that damages DNA and because apoptosis induced by DNA damage is often mediated by caspase-9, we tested whether cisplatin kills MCF-7 cells through the caspase-9 pathway. Treatment with cisplatin released cytochrome c from mitochondria, indicating that the caspase-9 pathway is initiated. The release of cytochrome c correlated with translocation of caspase-3 into the nucleus and with typical chromatin condensation (Fig. 2B and Fig. C). To test whether caspase-9 activity is required, we used retroviral gene transduction to make a cell line that expressed caspase-9 dominant-negative mutant (C9DN) (MCF-7/C3-GFP/C9DN). This mutant prevents the activation of caspase-9, but not the preceding steps of apoptosis by competing with the endogenous caspase-9 for binding to APAF-1. Consistent with previous reports (Fearnhead et al. 1998), expression of C9DN did not affect release of cytochrome c from mitochondria, but prevented chromatin condensation (Fig. 2B and Fig. D) and detachment of the cells (data not shown). These observations were consistent with the notion that cisplatin induces apoptosis in MCF-7 by activating caspase-9. The entry of caspase-3-GFP into the nucleus during apoptosis was blocked in MCF-7/C3-GFP/C9DN cells (Fig. 2B and Fig. C), indicating that caspase-9 activity is required for caspase-3 translocation. To determine whether caspase-3 activity or processing were required for translocating this caspase, we made cell lines that express various caspase-3 mutants, including those with mutated catalytic cysteine, lacking some or all processing sites (D9, D28, D175), or missing the prodomain (M1 to D28). All the mutants translocated into the nucleus similarly to caspase-3 (data not shown). Hence, caspase-9 activity is required for caspase-3 to enter the nucleus, whereas caspase-3 activity is not.
The Nuclear Transport Is Altered during Apoptosis
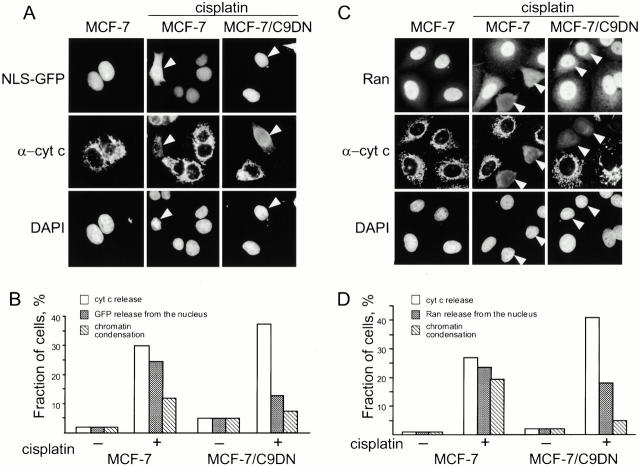
Previous reports suggested that caspase-3 is translocated into the nucleus by active transport (Yasuhara et al. 1997). Active transport typically concentrates a protein in a particular compartment. However, we observed that caspase-3 concentrations in the nucleus and cytoplasm equilibrated. Therefore, we tested whether active nuclear transport functions during apoptosis. A common assay to monitor nuclear transport is to use GFP fused to three copies of the SV40 T-antigen nuclear localization signal (GFP-NLS), a soluble molecule that accumulates in the nucleus of living cells (Roberts and Goldfarb 1998). As expected, GFP-NLS transiently expressed in MCF-7 cells localized to the nucleus (Fig. 3A and Fig. B). However, after treatment with cisplatin, GFP-NLS was distributed throughout the cell. The release of GFP-NLS from the nucleus, like the equilibration of caspase-3 between the nucleus and cytoplasm, was prevented in MCF-7 cells that expressed caspase-9 dominant-negative mutant (Fig. 3A and Fig. B). These observations were consistent with the notion that active transport is altered during apoptosis and that this alteration is caused by the activity of caspase-9.
Figure 3.
Nuclear-cytoplasmic barrier is disrupted during apoptosis. (A) Activation of caspase-9 leads to release of NLS-GFP during apoptosis. MCF-7 and MCF-7/C9DN cells were transiently transfected with NLS-GFP. 18 h after transfection, the cells were treated with 50 μM cisplatin for 10 h, fixed, and stained with a polyclonal antibody to cytochrome c and DAPI. White arrows indicate cells that have released cytochrome c. Cells were scored for the release of cytochrome c, release of nuclear GFP, and change in chromatin structure (B). Note that expression of C9DN causes the decrease in the ratio between the cells that release cytochrome c and NLS-GFP. 200–250 cells were counted for each measurement in all experiments. Shown is a representative of three experiments. (C) Activation of caspase-9 leads to release of Ran from the nucleus. MCF-7 and MCF-7/C9DN cells were treated as in A, fixed, and stained with a monoclonal antibody to Ran, a polyclonal antibody to cytochrome c, and DAPI to visualize nuclei. The antibodies were visualized with Alexa488-conjugated anti–mouse and Alexa596-conjugated anti–rabbit secondary antibodies. White arrows indicate cells with released cytochrome c. Cells were scored for the release of cytochrome c, loss of nuclear Ran staining, and change in chromatin structure (D). Note that expression of C9DN causes the decrease in the ratio between the cells that release cytochrome c and Ran. 200–250 cells were counted for each measurement in all experiments. Shown is a representative of three experiments.
A prerequisite of active nuclear transport is the nuclear-cytoplasmic gradient of Ran-GTP, in which a majority of the protein is concentrated in the nucleus (Izaurralde et al. 1997). We found that Ran, like GFP-NLS, was released from the nucleus during apoptosis and distributed throughout the cell (Fig. 3C and Fig. D). As we observed with GFP-NLS, expression of the caspase-9 dominant-negative mutant prevented Ran release. The dissipation of the Ran gradient and the release of NLS-GFP were both consistent with the idea that nuclear transport is inactivated in apoptosis. Hence, caspase-3 would have to enter the nucleus by other means.
Caspase-3 could be actively exported from the nucleus in living cells. In this case, inactivating nuclear transport would lead to diffusion of caspase-3 into the nucleus. We have not identified any obvious nuclear export signals (NES) in caspase-3. Deletion of one motif that might be an NES (I20 to D34, L. Englmeier, unpublished observations) had no effect on caspase-3 localization (data not shown). Therefore, we considered an alternative possibility, that caspase-3 is excluded from the nucleus because of the diffusion limit of the nuclear pores. Although caspase-3 is a 32-kD protein, which could diffuse into the nucleus, it is conceivable that this caspase may form complexes with other proteins, such as caspase inhibitors, or it may oligomerize. We reasoned that if caspase-3 or caspase-3–containing complexes are excluded because of their size, then the diffusion limit of the nuclear pores would have to increase for caspase-3 to enter the nucleus during apoptosis.
Nuclear Diffusion Limit Is Increased during Apoptosis
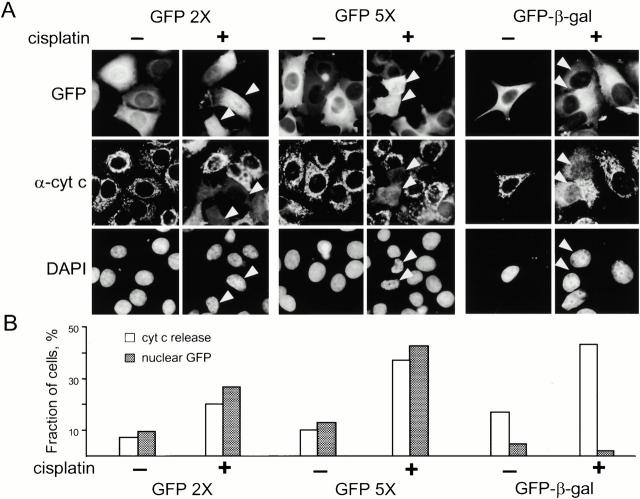
To determine whether the diffusion limit of nuclear pores is altered during apoptosis, we transiently expressed GFP oligomers ranging in size from a single GFP molecule (28 kD) to a GFP pentamer (140 kD). In addition, to test a large molecule, we used a fusion of GFP with bacterial β-galactosidase, a soluble enzyme that is used as a tracer or reporter in mammalian cells (GFP-β-Gal), although we have no direct proof that GFP-β-Gal can freely diffuse in the cell. Considering that-β-Gal is a tetramer, the predicted size of GFP-β-Gal is ∼580 kD. As expected, in untreated cells, GFP was present throughout the cell, whereas the GFP dimer (56 kD), GFP pentamer, and GFP-β-Gal were excluded from the nucleus (Fig. 4A and Fig. B). In apoptotic cells, the GFP dimer and pentamer were observed in both the nucleus and the cytoplasm, whereas GFP-β-Gal remained in the cytoplasm. We also found that GFP-β-Gal containing a nuclear localization signal remained in the nucleus of apoptotic cells (data not shown). Considering that the GFP oligomers are not substrates of the nuclear transport machinery, it is reasonable to conclude that they enter the nucleus during apoptosis by diffusion. Therefore, the permeability of the nuclear pores increases during apoptosis, allowing even large proteins and complexes to freely enter the nucleus during apoptosis. Hence, caspase-3 could enter the nucleus simply because the orifice of the nuclear pore increases.
Figure 4.
Nuclear pore size increases during apoptosis. (A) MCF-7 cells were transiently transfected with GFP dimer (GFP2X), pentamer (GFP5X), or a fusion of GFP with β-galactosidase (GFP-β-gal). 18 h after transfection, cells were treated with 50 μM cisplatin for 10 h, fixed, and stained with anti-cytochrome c antibody and DAPI. White arrows indicate cells with released cytochrome c. Cells were scored for the release of cytochrome c and the presence of GFP in the nucleus (B). Three experiments were performed with GFP2× and GFP5× and two with GFP-β-gal. 200–250 cells were scored for each experiment. Shown is a representative of one such experiment.
It is conceivable that the nuclear pores remain intact but the permeability is increased by perforation of the nuclear membranes. However, the observations that nuclear membranes remain intact during apoptosis argue against this model (Wyllie et al. 1980; Allen 1987). Consistent with these reports, we found no evidence of membrane perforations in apoptotic MCF-7 cells that expressed C3-GFP by electron microscopy (data not shown). The observation that the nuclear-cytoplasmic barrier remains impermeable for GFP-β-Gal also argues against the perforation model. Another possibility is that the collapse of the nuclear lamina during apoptosis contributes to the increase in nuclear pore permeability. Lamins, which form the lamina, are thought to be cleaved by caspase-6, which is processed and activated by caspase-3 (Slee et al. 1999). Consistent with this, we found that lamin B1 remained intact in apoptotic MCF-7 cells, in which the nuclear permeability is increased, although it was processed in the cells that expressed caspase-3 or caspase-3 GFP (data not shown). Therefore, cleavage of lamins is not required for the increase in nuclear permeability during apoptosis.
Nuclear Pores Are Altered during Apoptosis
Because caspases disassemble a cell by processing their substrates, and because the increase in nuclear permeability required caspase activity, we reasoned that some components of the nuclear transport machinery, either soluble or insoluble, may be caspase targets. We found that none of the tested proteins were cleaved in MCF-7 cells (Table ), although two proteins, RanGap1 (a soluble component of the transport machinery) and p270/TPR (a component of the nuclear pore complex) were processed in MCF-7 cells that expressed caspase-3. Because nuclear transport is disrupted even without caspase-3, we concluded that processing of these two proteins is not required for the increase in nuclear permeability. We confirmed that nuclear pore protein NUP153 is cleaved in apoptotic HeLa cells (Buendia et al. 1999), although we failed to detect it in MCF-7 cells (data not shown), thus leaving open the question as to whether processing of this protein is required for the increase in nuclear permeability. It should be emphasized that our inspection of the transport machinery was limited by the available tools. We tested only 5 of the >50 proteins of the vertebrate nuclear pore, many of which have not been characterized (Doye and Hurt 1997; Talcott and Moore 1999). This leaves open the possibility that other nuclear pore complex components are caspase targets and their processing causes an increase in nuclear permeability. For example, deficiency in yeast nup188 and nup170 results in an increase of the nuclear permeability (Shulga et al. 2000), suggesting vertebrate homologues of these proteins as potential targets of caspases. Considering that caspases usually target the key elements of a system or structure, the putative caspase substrates in the nuclear pore are likely to be critical to the pore function in living cells.
Table 1.
Proteolytic Processing of Nuclear Transport Components during Apoptosis
| Protein | MCF-7 cells | MCF-7 cells expressing caspase-3 | |
|---|---|---|---|
| Soluble components | |||
| Importin β1 | NC | NC | |
| Transportin | NC | NC | |
| Ran | NC | NC | |
| RanBP1 | NC | NC | |
| RanGAP1 | NC | C | |
| RCC1 | NC | NC | |
| NTF2 | NC | NC | |
| CAS | NC | NC | |
| Nuclear pore components | |||
| p62 | NC | NC | |
| p153 | ND | NC | |
| p175 | NC | NC | |
| p214 | NC | NC | |
| p270 | NC | C | |
MCF-7 and MCF-7/C3 were either left untreated or were treated with 50 μM cisplatin for 12 h, which resulted in >75% apoptotic cells as scored by chromatin condensation. Cell lysates were analyzed by immunoblotting using monoclonal antibodies to nuclear transport proteins. NC, not cleaved; C, cleaved; ND, not detected.
Since the inspection of the nuclear pore components provided no clues for the increase in the permeability, we considered several speculative mechanisms. One speculation was that caspase activity leads to removal of the central plug of the nuclear pore, which would explain the increase in the nuclear permeability. To test this possibility, we used antibodies to p62, a component of the plug (Talcott and Moore 1999). As expected, the antibody detected nuclear pores in untreated MCF-7 cells, which appeared as a typical nuclear rim, whereas hardly any nuclear rim was detected in apoptotic cells (Fig. 5 A). We found that p62 is intact in apoptotic cells as detected by immunoblotting and reasoned that this protein may become soluble if the plug is removed. However, we found that p62 detectable by immunoblotting remained with the nuclear fraction of both living and apoptotic cells during cell fractionation (data not shown). Furthermore, a monoclonal antibody 114 (mAb114) (Davis and Blobel 1987), which detects several nuclear pore proteins in the plug and beyond, also failed to detect nuclear pores in apoptotic cells (Fig. 5 B), arguing against the plug hypothesis and suggesting that many pore proteins are affected. The failure to detect the proteins may be due to a modification of the epitopes in apoptotic cells or a failure of the antibodies to access them. Interestingly, a fusion of nuclear pore membrane protein POM121 with GFP was reported to be less detectable in apoptotic cells (Imreh et al. 1998), consistent with some changes in the nuclear pores other than a change in accessibility of the epitopes. Hence, it appears that some alterations in nuclear pores accompany the increase in their permeability during apoptosis, although what these changes are remains to be determined.
Figure 5.
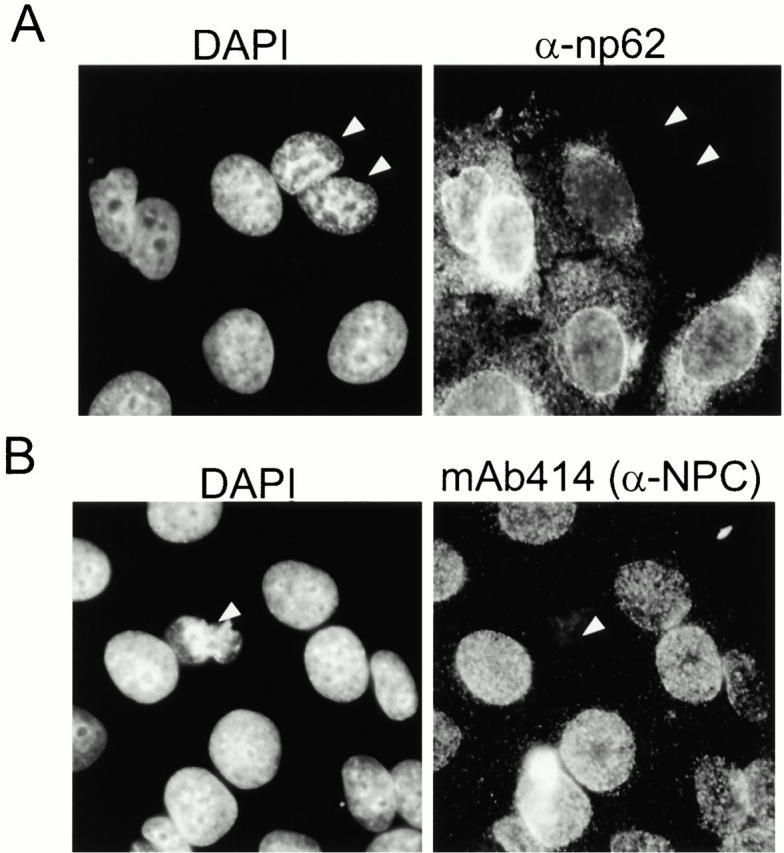
Nuclear pores in apoptotic cells are not detectable by antibodies to nuclear pore proteins. MCF-7 were treated with 50 μM cisplatin for 10 h, fixed, and stained with DAPI to visualize chromatin and with antibodies to nuclear pore protein np62 (A) or monoclonal antibody 414, which reacts with multiple nuclear pore proteins (B). White arrows indicate cells that have lost nuclear pore staining and have condensed chromatin.
A practical implication of the disappearance of nuclear pore staining during apoptosis may be in using this observation to identify apoptotic cells that, like MCF-7, do not have typical apoptotic morphology. We found that nuclear pores were hardly detectable by immunofluorescence during apoptosis not only in MCF-7 cells, but also all other cell lines that we tested (HeLa, Cos, U2OS, RKO, and U87) (data not shown), indicating that the apparent disappearance of the nuclear pore staining is a common phenomenon.
Caspase-8 Requires Caspase-9 Activity to Disrupt the Nuclear-Cytoplasmic Boundary
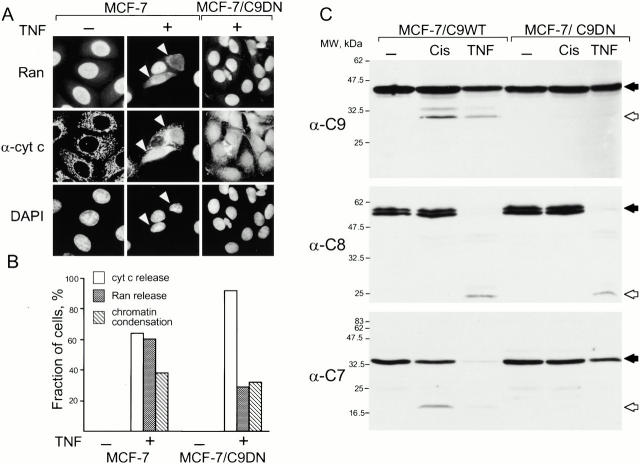
A current view is that common apoptotic changes can be induced by activating one of the several initiator caspases, such as caspase-8 or caspase-9. Therefore, we tested whether initiator caspases other than caspase-9 can inactivate nuclear transport. Because caspase-8 can indirectly activate caspase-9 (Li et al. 1998; Luo et al. 1998), we evaluated the effect of caspase-8 activity not only in MCF-7 cells, but also in the cells that express the dominant-negative mutant of caspase-9 (MCF-7/C9DN). Apoptosis in these cell lines was induced by TNF-α in the presence of cycloheximide, which, for unclear reasons, potentiates the effect of TNF-α. As expected, treatment with TNF-α released cytochrome c (Fig. 6 A) and processed caspase-8 in both cell lines (Fig. 6 C). However, Ran was released only from the nucleus of MCF-7 cells, but not of the cells that expressed C9DN (Fig. 6A and Fig. B), indicating that, in the absence of caspase-3, caspase-8 alone could not change the nuclear pore permeability, but required the activity of caspase-9 or its substrates. The finding that caspase-8 fails to process caspase-7, a substrate of caspase-9, in MCF-7/C9DN cells (Fig. 6 C) leaves both possibilities open.
Figure 6.
Activity of caspase-9 is required for the change in nuclear permeability even if apoptosis is induced by activating caspase-8. Caspase-9 activity is required for the release of Ran from the nucleus during apoptosis induced by TNF-α. MCF-7 and MCF-7/C9DN cells were treated with 35 ng/ml TNF-α and 10 μg/ml cycloheximide for 6 h, fixed, and stained with a monoclonal antibody to Ran and a polyclonal antibody to cytochrome c. White arrows indicate cells that have released cytochrome c. Cells were scored for the release of cytochrome c, loss of nuclear Ran staining, and change in chromatin structure (B). Note that expression of C9DN causes the decrease in the ratio between the cells that release cytochrome c and Ran. The processing of caspases-9, -8 and, -7 was confirmed by immunoblotting (C). Caspase precursors are indicated with black and the processed caspases with white arrows. Note that C9DN prevents processing of caspases-9 and -7, but not of caspase-8. Two experiments were performed, and >200 cells were scored for each experiment.
In summary, activation of caspase-9 during apoptosis increases permeability of the nuclear pore, which allows cytoplasmic caspases to reach their nuclear substrates and lets soluble proteins that are normally restricted to the nucleus or cytoplasm to distribute throughout the cell.
Acknowledgments
We are indebted to Ludwig Englmeier for generously sharing his expertise in nuclear transport, members of the Lazebnik lab and Mike Myers for suggestions, Ximena Opitz-Araya for excellent technical assistance, Regina Whitaker for expert help with tissue culture, June Blanchford for never refusing our requests, and Tamara Howard and David Spector for sharing their expertise in microscopy.
This work was supported by National Institutes of Health grant CA 13106-25 and by a Seraph Foundation grant to Y. Lazebnik.
Footnotes
Abbreviations used in this paper: C9DN, caspase-9 dominant-negative mutant; DAPI, 4′6-diamidino-2-phenylindole; GFP, green fluorescent protein; NLS, nuclear localization signal.
References
- Allen T.D. Ultrastructural aspect of cell death. In: Potten C.S., editor. Perspectives on Mammalian Cell Death. Oxford University Press; Oxford, UK: 1987. pp. 39–65. [Google Scholar]
- Ashkenazi A., Dixit V.M. Death receptorssignaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Budihardjo I., Oliver H., Lutter M., Luo X., Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell. Dev. Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Buendia B., Santa-Maria A., Courvalin J.C. Caspase-dependent proteolysis of integral and peripheral proteins of nuclear membranes and nuclear pore complex proteins during apoptosis. J. Cell Sci. 1999;112:1743–1753. doi: 10.1242/jcs.112.11.1743. [DOI] [PubMed] [Google Scholar]
- Chandler J.M., Cohen G.M., MacFarlane M. Different subcellular distribution of caspase-3 and caspase-7 following Fas-induced apoptosis in mouse liver. J. Biol. Chem. 1998;273:10815–10818. doi: 10.1074/jbc.273.18.10815. [DOI] [PubMed] [Google Scholar]
- Cryns V., Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- Davis L.I., Blobel G. Nuclear pore complex contains a family of glycoproteins that includes p62glycosylation through a previously unidentified cellular pathway. Proc. Natl. Acad. Sci. USA. 1987;84:7552–7556. doi: 10.1073/pnas.84.21.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doye V., Hurt E. From nucleoporins to nuclear pore complexes. Curr. Opin. Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- Enari M., Sakahira H., Yokoyama H., Okawa K., Iwamatsu A., Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Fearnhead H.O., Rodriguez J., Govek E.E., Guo W., Kobayashi R., Hannon G., Lazebnik Y.A. Oncogene-dependent apoptosis is mediated by caspase-9. Proc. Natl. Acad. Sci. USA. 1998;95:13664–13669. doi: 10.1073/pnas.95.23.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G.J., Sun P., Carnero A., Xie L.Y., Maestro R., Conklin D.S., Beach D. MaRXan approach to genetics in mammalian cells. Science. 1999;283:1129–1130. doi: 10.1126/science.283.5405.1129. [DOI] [PubMed] [Google Scholar]
- Imreh G., Beckman M., Iverfeldt K., Hallberg E. Noninvasive monitoring of apoptosis versus necrosis in a neuroblastoma cell line expressing a nuclear pore protein tagged with the green fluorescent protein. Exp. Cell Res. 1998;238:371–376. doi: 10.1006/excr.1997.3846. [DOI] [PubMed] [Google Scholar]
- Izaurralde E., Kutay U., von Kobbe C., Mattaj I.W., Gorlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke R.U., Sprengart M.L., Wati M.R., Porter A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Kohler C., Hakansson A., Svanborg C., Orrenius S., Zhivotovsky B. Protease activation in apoptosis induced by MAL. Exp. Cell Res. 1999;249:260–268. doi: 10.1006/excr.1999.4472. [DOI] [PubMed] [Google Scholar]
- Krajewska M., Wang H.G., Krajewski S., Zapata J.M., Shabaik A., Gascoyne R., Reed J.C. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res. 1997;57:1605–1613. [PubMed] [Google Scholar]
- Kuwana T., Smith J.J., Muzio M., Dixit V., Newmeyer D.D., Kornbluth S. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J. Biol. Chem. 1998;273:16589–16594. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y.A., Takahashi A., Moir R.D., Goldman R.D., Poirier G.G., Kaufmann S.H., Earnshaw W.C. Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc. Natl. Acad. Sci. USA. 1995;92:9042–9046. doi: 10.1073/pnas.92.20.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhu H., Xu C.J., Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Liu X., Li P., Widlak P., Zou H., Luo X., Garrard W.T., Wang X. The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc. Natl. Acad. Sci. USA. 1998;95:8461–8466. doi: 10.1073/pnas.95.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.S., Zou H., Slaughter C., Wang X.D. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Mancini M., Nicholson D.W., Roy S., Thornberry N.A., Peterson E.P., Casciola-Rosen L.A., Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distributionimplications for apoptotic signaling. J. Cell Biol. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I.W., Englmeier L. Nucleocytoplasmic transportthe soluble phase. Annu. Rev. Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nakamura Y., Ikeda H., Hiwasa T., Kuida K., Su M.S., Zhao H., Cnaan A., Sakiyama S. High levels of expression and nuclear localization of interleukin-1 beta converting enzyme (ICE) and CPP32 in favorable human neuroblastomas. Cancer Res. 1997;57:4578–4584. [PubMed] [Google Scholar]
- Nakielny S., Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- Orth K., Chinnaiyan A.M., Garg M., Froelich C.J., Dixit V.M. The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J. Biol. Chem. 1996;271:16443–16446. [PubMed] [Google Scholar]
- Porter A.G. Protein translocation in apoptosis. Trends Cell Biol. 1999;9:394–401. doi: 10.1016/s0962-8924(99)01624-4. [DOI] [PubMed] [Google Scholar]
- Posmantur R., McGinnis K., Nadimpalli R., Gilbertsen R.B., Wang K.K. Characterization of CPP32-like protease activity following apoptotic challenge in SH-SY5Y neuroblastoma cells. J. Neurochem. 1997;68:2328–2337. doi: 10.1046/j.1471-4159.1997.68062328.x. [DOI] [PubMed] [Google Scholar]
- Roberts P.M., Goldfarb D.S. In vivo nuclear transport kinetics in Saccharomyces cerevisiae . Methods Cell Biol. 1998;53:545–557. doi: 10.1016/s0091-679x(08)60894-8. [DOI] [PubMed] [Google Scholar]
- Sakahira H., Enari M., Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- Samejima K., Earnshaw W.C. ICAD/DFF regulator of apoptotic nuclease is nuclear. Exp. Cell Res. 1998;243:453–459. doi: 10.1006/excr.1998.4212. [DOI] [PubMed] [Google Scholar]
- Samejima K., Earnshaw W.C. Differential localization of ICAD-L and ICAD-S in cells due to removal of a C-terminal NLS from ICAD-L by alternative splicing. Exp. Cell Res. 2000;255:314–320. doi: 10.1006/excr.2000.4801. [DOI] [PubMed] [Google Scholar]
- Shulga N., Mosammaparast N., Wozniak R., Goldfarb D.S. Yeast nucleoporins involved in passive nuclear envelope permeability. J. Cell Biol. 2000;149:1027–1038. doi: 10.1083/jcb.149.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee E.A., Harte M.T., Kluck R.M., Wolf B.B., Casiano C.A., Newmeyer D.D., Wang H.G., Reed J.C., Nicholson D.W., Alnemri E.S. Ordering the cytochrome c–initiated caspase cascadehierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9–dependent manner. J. Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., Alnemri E.S., Lazebnik Y.A., Fernandes-Alnemri T., Litwack G., Moir R.D., Goldman R.D., Poirier G.G., Kaufmann S.H., Earnshaw W.C. Cleavage of lamin A by Mch2 alpha but not CPP32multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc. Natl. Acad. Sci. USA. 1996;93:8395–8400. doi: 10.1073/pnas.93.16.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott B., Moore M.S. Getting across the nuclear pore complex. Trends Cell Biol. 1999;9:312–318. doi: 10.1016/s0962-8924(99)01608-6. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A., Lazebnik Y. Caspasesenemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Woo M., Hakem R., Soengas M.S., Duncan G.S., Shahinian A., Kagi D., Hakem A., McCurrach M., Khoo W., Kaufman S.A. Essential contribution of caspase 3 CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–819. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A.H., Kerr J.F.R., Currie A.R. Cell deaththe significance of apoptosis. Int. Rev. Cytol. 1980;68:251–305. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yasuhara N., Eguchi Y., Tachibana T., Imamoto N., Yoneda Y., Tsujimoto Y. Essential role of active nuclear transport in apoptosis. Genes Cells. 1997;2:55–64. doi: 10.1046/j.1365-2443.1997.1010302.x. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B., Samali A., Gahm A., Orrenius S. Caspasestheir intracellular localization and translocation during apoptosis. Cell Death Differ. 1999;6:644–651. doi: 10.1038/sj.cdd.4400536. [DOI] [PubMed] [Google Scholar]