Abstract
Formation of ER-derived protein transport vesicles requires three cytosolic components, a small GTPase, Sar1p, and two heterodimeric complexes, Sec23/24p and Sec13/31p, which comprise the COPII coat. We investigated the role of Lst1p, a Sec24p homologue, in cargo recruitment into COPII vesicles in Saccharomyces cerevisiae. A tagged version of Lst1p was purified and eluted as a heterodimer complexed with Sec23p comparable to the Sec23/24p heterodimer. We found that cytosol from an lst1-null strain supported the packaging of α-factor precursor into COPII vesicles but was deficient in the packaging of Pma1p, the essential plasma membrane ATPase. Supplementation of mutant cytosol with purified Sec23/Lst1p restored Pma1p packaging into the vesicles. When purified COPII components were used in the vesicle budding reaction, Pma1p packaging was optimal with a mixture of Sec23/24p and Sec23/Lst1p; Sec23/Lst1p did not replace Sec23/24p. Furthermore, Pma1p coimmunoprecipitated with Lst1p and Sec24p from vesicles. Vesicles formed with a mixture of Sec23/Lst1p and Sec23/24p were similar morphologically and in their buoyant density, but larger than normal COPII vesicles (87-nm vs. 75-nm diameter). Immunoelectronmicroscopic and biochemical studies revealed both Sec23/Lst1p and Sec23/24p on the membranes of the same vesicles. These results suggest that Lst1p and Sec24p cooperate in the packaging of Pma1p and support the view that biosynthetic precursors of plasma membrane proteins must be sorted into ER-derived transport vesicles. Sec24p homologues may comprise a more complex coat whose combinatorial subunit composition serves to expand the range of cargo to be packaged into COPII vesicles. By changing the geometry of COPII coat polymerization, Lst1p may allow the transport of bulky cargo molecules, polymers, or particles.
Keywords: ER, cargo packaging, transport vesicle, yeast, coat protein
Introduction
Secretory and plasma membrane proteins are sorted from intracellular proteins at several points along the secretory pathway. Proteins exit the ER en route to the Golgi in small vesicles whose budding is mediated by a proteinaceous coat termed COPII (Barlowe et al. 1994). Assays performed in vitro demonstrated that COPII vesicle formation from donor membranes in Saccharomyces cerevisiae requires guanine nucleotide and a minimum of three cytosolic components: the two heterodimers, Sec23/24p and Sec13/31p, and the small GTPase, Sar1p (Salama et al. 1993; Barlowe et al. 1994). This appears to be a universal mechanism as homologues of all COPII components have been reported in mammals (see Tang et al. 2000). Sar1p is recruited to the ER membrane by a small membrane glycoprotein, Sec12p, which catalyzes the exchange of Sar1p-bound GDP for GTP (Barlowe et al. 1993). Recruitment of Sec23/24p and Sec13/31p follows (Matsuoka et al. 1998), and the membrane curves to form a bud that pinches off as a COPII-coated vesicle.
In addition to the standard and essential COPII genes, several homologues have emerged in genetic screens and database searches. There are two Sec24p homologues that are not essential for viability in yeast and at least four mammalian forms of Sec24p (Pagano et al. 1999; Tani et al. 1999). A putative Sec23p homologue was also identified in both mammalian and yeast genomes (Paccaud et al. 1996). The yeast open reading frame (YHR035W) encodes a protein with 21% identity to Sec23p. The yeast Sec24p homologues, Lst1p (lethal with sec-thirteen) and Iss1p (interacting with sec-sixteen), are 23 and 55% identical, respectively, in their amino acid sequence to Sec24p (Roberg et al. 1999; Kurihara et al. 2000). These facts raise the possibility that multiple combinations of Sec23/Sec24p homologues are involved in vesicle budding from the ER in vivo.
During the process of vesicle formation, cargo proteins are specifically packaged, whereas ER residents are excluded. The mechanism of protein sorting remains controversial. According to one school of thought, cargo molecules contain no ER-export signals and are thus packaged by a default mechanism (known as the “bulk flow” hypothesis) (Wieland et al. 1987), which takes them from the ER towards later compartments where sorting of resident proteins is achieved by retention or recycling (Pelham 1991). Also, in support of a default process, two abundant secretory proteins appear not to be concentrated in COPII buds and vesicles leaving the ER, in pancreatic acinar cells (Martinez-Menarguez et al. 1999). A second view assumes the presence of sorting signals on cargo molecules and sorting receptors that drive their concentration into ER-derived vesicles (Kuehn and Schekman 1997). Although ER-exit signals are only partially defined (and cargo receptors remain poorly characterized), experimental evidence supports their existence. First, earlier studies document that several cargo molecules get concentrated into ER-derived vesicles (Mizuno and Singer 1993; Balch et al. 1994; Bednarek et al. 1995) implying the operation of active sorting at the level of the ER. Second, prebudding complexes between COPII components and both soluble proteins (e.g., glycosylated pro-α-factor [gpαF]) and membrane proteins (e.g., v-SNARES, amino acid permeases, and Emp24p in yeast and vesicular stomatitis virus glycoprotein (VSV-G) in mammalian cells) have been isolated (Aridor et al. 1998; Kuehn et al. 1998; Springer and Schekman 1998). Whereas membrane proteins may possess sorting signals recognized by components of the coat, the selection of soluble cargo would require membrane adaptor or receptor proteins, which would interact with the coat. Candidates for such cargo receptors include members of the p24 family of proteins and ERGIC53/58/EMP47 lectin-like proteins found in mammals and yeast (Hauri et al. 2000; Muniz et al. 2000). Notably, ER residents are excluded from all of these complexes, suggesting that at least some sorting of cargo occurs before the cargo exits the ER. A subset of the COPII components, Sar1p and Sec23/24p, is thought to be involved in cargo recognition and recruitment because prebudding complexes form in the absence of Sec13/31p, before the appearance of a coated membrane surface.
Several sorting signals by which cargo molecules interact with subunits of coats have been identified. Tyrosine-based signals on proteins packaged into clathrin-coated vesicles at the plasma membrane interact with the clathrin AP2 complex (Bonifacino et al. 1996). Likewise, cargo molecules to be selected by and concentrated into COPI vesicles (which mediate their retrograde transport from various Golgi and pre-Golgi compartments to the ER) bind either directly to components of the COPI coat through a di-lysine (K(X)KXX) motif (present on the COOH terminus of membrane cargo) (Cosson and Letourneur 1997) or indirectly via receptors such as the K/HDEL receptor, which binds lumenal cargo tagged with the K/HDEL COOH-terminal sequence. The identity, recognition, and decoding of packaging signals on cargo exiting the ER is less clear. However, two sorting motifs present on the cytoplasmic tails of membrane proteins have recently emerged from work on mammalian cells. A di-acidic motif (DXE, single amino acid code, where X represents any amino acid) was found to be required for the selective export and concentration of VSV-G and other cargo into vesicles budding from the ER (Nishimura and Balch 1997). A double phenylalanine (FF) motif found in the cytoplasmic domains of some transmembrane proteins that cycle between the ER and Golgi, such as p24 proteins and ERGIC-53/58, was shown to bind to the Sec23/24p complex in vitro and to be required for efficient transport of these proteins out of the ER in vivo (Fiedler et al. 1996; Kappeler et al. 1997; Dominguez et al. 1998). The di-phenylalanine motif tolerates other hydrophobic residues, but it is not sufficient by itself for the export of reporter cargo molecules, suggesting that it is part of a larger ER-exit signal (Hauri et al. 2000).
Previously, we have reported that soluble proteins, such as gpαF, and membrane cargo molecules like Gap1p (the general amino acid permease) are efficiently packaged into vesicles in a cell free reaction containing ER membranes and purified COPII components (consisting of Sar1p, Sec23/24p and Sec13/31p), whereas packaging of the plasma membrane ATPase, Pma1p, is inefficient (Kuehn et al. 1996). However, when cytosol is used instead of the purified COPII components, efficient packaging of Pma1p is observed, indicating that a cytosolic factor(s), in addition to the purified COPII components, is required for efficient packaging of Pma1p. The recent report that chromosomal deletion of LST1 inhibits Pma1p exit out of the ER (Roberg et al. 1999) suggests that Lst1p is the missing cytosolic component required for efficient packaging of Pma1p into COPII vesicles.
Here, we present biochemical and morphological data which show that Lst1p and Sec24p cooperate in COPII coat formation on vesicles budding from the ER membrane and in Pma1p packaging into these vesicles. The results imply that members of the Sec24p protein family join together to serve the dual function of coupling cargo recognition and packaging to the structural formation of a coat and a coated vesicle on ER membrane sites.
Materials and Methods
Reagents, Yeast Strains, and Plasmids
Reagents for yeast growth media were obtained from Difco Laboratories Inc. Rich medium (YPD) contained 1% Bacto-yeast extract, 2% Bacto-peptone, and 2% glucose. Minimal medium (SD) contained 0.67% yeast nitrogen base with ammonium sulfate, 2% glucose, and required nutrients (Sherman 1991). All other reagents were obtained from Sigma-Aldrich unless otherwise noted. Yeast transformations were performed by a modified (Schiestl and Gietz 1989) lithium acetate method (Ito et al. 1983). Standard recombinant DNA techniques were performed (Sambrook et al. 1989).
The yeast strains used in this study were as follows: RSY1795 (MATα leu2-3, 112 ura3-52 ade2-1 trp1-1 his3-11,15 PEP4::TRP1 SEC23 [pTKY9] LST1 [pTKY12]) was used for Sec23/Lst1p purification. pTKY9 is a pGAL426GAL1 2μ URA3 plasmid containing SEC23 (NcoI–HindIII) between the GAL1 promotor and CYC1 terminator. pTKY12 is a pGAL426GAL1 2μ LEU2 plasmid containing a His6-tagged version of LST1 between the GAL1 promotor and CYC1 terminator. RSY1801 (MATa ade2-101oc his3Δ200 leu2-Δ1 lys2-801am trp1-Δ63 ura3-52 lst1::HIS3, pma1::HA-PMA1::LEU2) was used for preparation of semiintact cells. The LST1 gene was deleted by gene disruption with HIS3 (Sikorski and Hieter 1989) and NH2-terminally HA-tagged PMA1 was introduced into the chromosome at the PMA1 locus by integrative recombination (Kuehn et al. 1996). YPH499 (Sikorski and Hieter 1989) and RSY1800 (YPH499/lst1::HIS3) were used for preparation of cytosol.
Purification of Sec23/24p, Sec23/Iss1p, and Sec23/Lst1p Complexes
Purification of Sec23/24p and Sec23/Iss1p was performed as previously described (Yeung et al. 1995; Kurihara et al. 2000). The Sec23/Lst1p complex was purified by a strategy similar to that described (Kurihara et al. 2000). In brief, an LST1-His6 construct (pTKY12) was generated, containing the LST1 gene modified at the COOH terminus to encode six histidine residues, under the control of the GAL1 promoter, as described above. RSY620 (Kurihara et al. 2000) was then transformed with pTKY12 and pTKY9, which carries SEC23 under the control of the GAL1 promoter, and cultivated with galactose for the cooverproduction of Lst1p and Sec23p as previously described (Kurihara et al. 2000). The Sec23/Lst1p complex was then purified on a Ni-NTA agarose column (QIAGEN) as previously described for Sec23/Iss1p purification (Kurihara et al. 2000), except that elution was obtained with buffer (50 mM potassium acetate, 50 mM Hepes [pH 7.0, at 4°C], 0.1 mM EGTA, 20% [wt/vol] glycerol) containing 250 mM imidazole (instead of 500 mM). Typically, >1 mg Sec23/Lst1p was obtained from 6 liters of a late log yeast culture (∼25 g wet wt). Aliquots containing purified Sec23/Lst1p were frozen in liquid nitrogen and stored at −80°C.
Production and Use of Antibodies
Anti-Iss1p was produced against purified Sec23/Iss1p complex, and anti-Lst1p was prepared against SDS-PAGE–isolated Lst1p bands. Antigen (50–100 μg protein) was injected into rabbits at 2-wk intervals, and the serum obtained was used at 1:1,000 dilution in immunoblot analysis. Preparation of anti-Sec24p (Hicke et al. 1992) and anti-Sec13p (Salama et al. 1993) antibodies has been described, and serum was used at a 1:10,000 and 1:1,000 dilution, respectively, in immunoblots. Purified monoclonal anti-HA (clone 16B12) was purchased from Berkeley Antibody Co. and used at 1:500 in immunoblots. Donkey anti–rabbit and sheep anti–mouse secondary antibodies coupled to HRP were obtained from Amersham Pharmacia Biotech. Anti-Lst1p and anti-Sec24p sera were further purified on concanavalin A (Con A) Sepharose for ultrastructural analysis.
Methods for SDS-PAGE (Laemmli 1970) and immunoblotting (Towbin et al. 1979) have been described, and immunodetection was performed by enhanced chemiluminescence using the ECL reagents (Amersham Pharmacia Biotech).
Preparation of Protein and Cellular Components
Semi-intact cells (SICs; also termed perforated yeast spheroplasts) and microsomes were prepared from RSY1801 (lst1-null cells expressing HA-Pma1p) as described (Wuestehube and Schekman 1992; Rexach et al. 1994). Part of an early log phase culture was employed in the preparation of metabolically pulse-labeled SICs (Kuehn et al. 1996), which were used for assessing HA-Pma1p packaging, whereas a mock-labeled portion of the cells was used for determining gpαF packaging (see below). For the preparation of labeled SICs, cells (25 ml, mid log) were washed in SD-Leu-Met and pulse-labeled for 3 min (to label all newly synthesized proteins) with 1 mCi 35S-promix protein labeling mix (1,200 Ci/mmol; Amersham Pharmacia Biotech) at 30°C. Metabolic activity was stopped by immediately placing the cells on ice in the presence of 20 mM ice-cold sodium azide for 15 min. The labeled cells were then washed, and perforated spheroplasts were prepared as reported (Rexach et al. 1994).
Cytosol was prepared from mid-log YPH499 and RSY1800 (YPH499/lst1Δ) yeast cells as described (Spang and Schekman 1998).
Sar1p, Sec23/24p and Sec13/31p were purified as previously described (Barlowe et al. 1994; Salama et al. 1997). Preparation of [35S]gpαF has also been previously described (Baker et al. 1988).
ER Vesicle Budding Assay
SICs (or urea washed microsomes in Fig. 6) from lst1-null yeast expressing HA-Pma1p (RSY1801) were used as the source of donor membranes in an assay based on a previously described procedure (Baker et al. 1988). Aliquots of unlabeled and pulse-labeled SICs (which were prepared in parallel from the same yeast culture) were thawed, washed, and used in two sets of reactions as follows. [35S]gpαF was posttranslationally translocated into the unlabeled SICs as previously described (Rexach et al. 1994), whereas the pulse-labeled SICs were mock-treated. The [35S]gpαF-loaded SICs and the pulse-labeled SICs were then distributed into two parallel sets of tubes for the budding step. For the experiments such as those presented in Fig. 2, Fig. 2 mg/ml cytosol prepared from either wild-type yeast or from lst1-null yeast and supplemented with the indicated amounts of purified Sec23/Lst1p, was used. In the experiments depicted in Fig. 3, purified COPII components were used to facilitate vesicle budding. The concentrations of purified COPII components were: 20 μg/ml Sar1p, 40 μg/ml Sec13/31p, plus Sec23/24p and Sec23/Lst1p as indicated. Reaction mixtures in B88 (20 mM Hepes, pH 6.8, 250 mM sorbitol, 150 mM KOAc, 5 mM Mg(OAc)2) included an ATP regeneration system and guanine nucleotides: 0.1 mM GTP or 0.1 mM GMP-PNP (in reactions where preservation of the coat was desired). After incubation at 25°C for 30 min, reactions were chilled on ice for 5 min and aliquots representing the total label were transferred to fresh tubes. The rest was sedimented in a microcentrifuge (12,000 g, 4 min), retaining the COPII vesicles in the medium speed supernatant (MSS). MSS and total fractions from [35S]gpαF-loaded SICs were treated with trypsin and then trypsin inhibitor. After denaturation with SDS, and Con A–Sepharose precipitation, bound [35S]gpαF was quantified in a scintillation counter, and packaging efficiency was calculated as the ratio of Con A-bound [35S]gpαF detected in the MSS to that present in the total fraction, as described (Rexach et al. 1994). MSS and total fractions from pulse-labeled SICs were solubilized in 1% SDS supplemented with 1 mM PMSF, incubated at 42°C for 3 min, and immunoprecipitated with protein G–Sepharose and anti-HA antibodies overnight at 4°C. Protein sample buffer was added to the washed immunoprecipitates and samples were separated on 7.5% SDS-PAGE. HA-Pma1p packaging efficiency was determined after exposure of the fixed dry gel to a PhosphorImager (STORM 860; Molecular Dynamics) and quantitation of the metabolically labeled HA-Pma1p protein bands in the MSS and total reactions.
Figure 6.
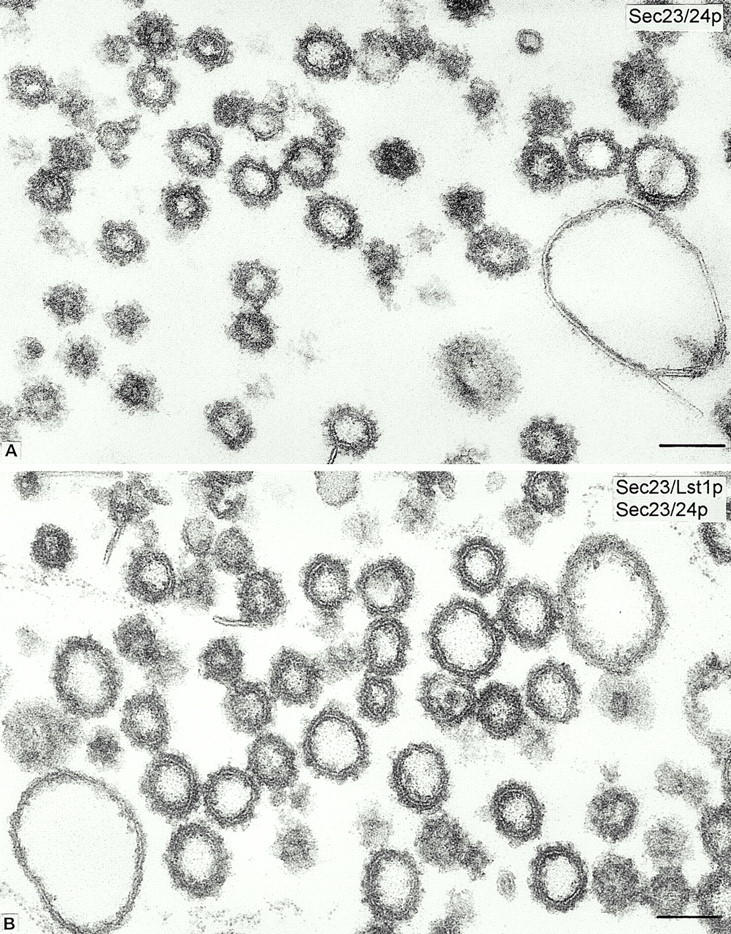
Conventional thin section samples from enriched fractions of COPII-coated vesicles produced from lst1-null microsomes in reactions containing Sec23/24p alone (20 μg/ml; A) or Sec23/Lst1p and Sec23/24p (10 μg/ml each; B). The overall vesicles morphology and the coat appearance are comparable in both incubations; the coated vesicles generated in mixture B are of slightly larger mean diameter (87 ± 14 nm SD) than those produced in mixture A (75 ± 10 nm SD). 800 vesicles were measured in each condition. The fractions also contain large vacuolar-type uncoated membrane contaminants. Bars, 100 nm.
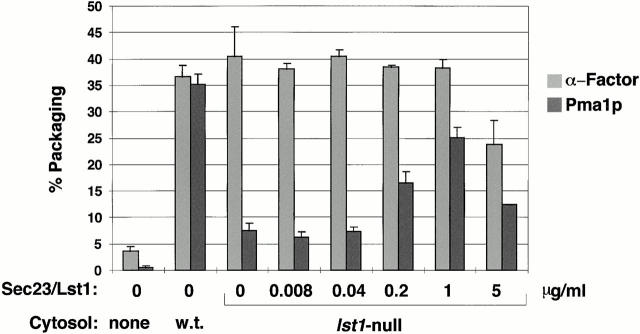
Figure 2.
α-Factor and Pma1p packaging into vesicles reconstituted with cytosol. Budding assays were performed in duplicates as described in Materials and Methods using donor membranes from lst1Δ, HA-Pma1p-containing cells primed with wild-type cytosol, or lst1Δ cytosol supplemented with Sec23/Lst1p as indicated. Percent packaging of α-factor and Pma1p was determined as described in Materials and Methods and is reported as the mean of at least two independent experiments with error bars indicating SD.
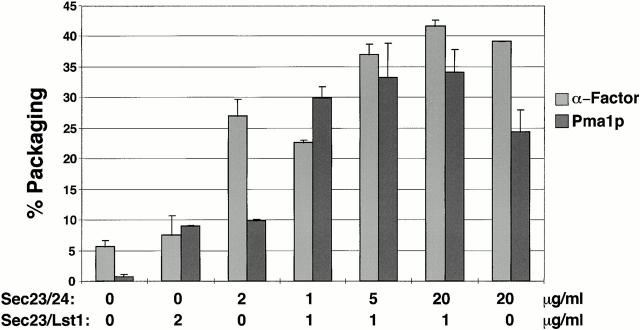
Figure 3.
α-Factor and Pma1p packaging into vesicles reconstituted with all purified COPII components. Budding was performed in duplicates using lst1Δ, HA-Pma1p containing SICs primed with GTP, 20 μg/ml Sar1p, and 40 μg/ml Sec13/31p, plus the indicated concentrations of Sec23/24p and Sec23/Lst1p. Percent packaging of α-factor and Pma1p was determined as described in Materials and Methods and is reported as the mean of at least two independent experiments with error bars indicating SD.
Coimmunoprecipitation
Budding reactions were performed with RSY1801 (lst1Δ cells that express HA-Pma1p) or wild type SICs as described above. Vesicle formation was confirmed by assaying [35S]gpαF packaging. For denaturing immunoprecipitations, vesicles in the MSS were cross-linked (20 min at 20°C) with 0.5 mM DSP (Pierce Chemical Co.) and quenched (5 min at 20°C) with 50 mM glycine. Membranes were sedimented in a TLA100.3 rotor (Beckman Instruments) at 50,000 rpm (100,000 g) for 20 min, solubilized with 1% SDS containing 1 mM PMSF, and an aliquot was kept aside as the input. The rest was precleared for 2 h at 4°C with protein A–Sepharose in IP buffer (150 mM NaCl, 1% TX100, 0.1% SDS, 15 mM Tris-HCl, pH 7.5, 2 mM NaN3). Supernatant fractions were transferred to fresh tubes and immunoprecipitated with the appropriate antibody plus protein A–Sepharose at 4°C overnight. Washed beads were heated in sample buffer for SDS-PAGE, followed by immunoblot analysis. The efficiency of coimmunoprecipitations usually ranged from 0.5–5%.
Vesicle Flotation
RSY1801 (lst1Δ) SICs were preincubated with [35S]gpαF and used in budding reactions which were scaled-up 5–10-fold, as described above. The MSS was then loaded on a 15/70% pad of sucrose made in B88 and ultracentrifuged in a SW55 rotor (Beckman Instruments) at 50,000 rpm (237,000 g) for 2 h. The 15/70% interface was collected, adjusted to 55% sucrose (final vol ∼0.5 ml), and placed at the bottom of a density gradient consisting of 250 μl each of 50, 45, 40, 35, and 25% sucrose/B88. After ultracentrifugation at 50,000 rpm for 16 h (SW55, Beckman Instruments), 10 fractions were collected from the top and either subjected to SDS-PAGE followed by immunoblot analysis or counted for [35S]gpαF distribution.
Electron Microscopy and Immunocytochemistry
Vesicles in the MSS were sedimented (40,000 rpm, 30 min) in an SW55 rotor (Beckman Instruments) and the pellets were fixed with 1.5–2% glutaraldehyde/B88 for 1 h on ice. For conventional EM, samples of the fixed pellets of vesicles were processed as previously described (Orci et al. 1991). For immunocytochemistry, fragments of the fixed pellets were infiltrated with 2.3 M sucrose and processed for cryoultramicrotomy (Tokuyasu 1986). Cryosections were incubated by the protein A-gold technique (Roth et al. 1978) with affinity-purified anti-Lst1p or anti-Sec24p antiserum diluted at 1:400 and 1:20, respectively, followed by protein A-gold (gold size 10 nm). The quantitative evaluation of the immunogold labeling on vesicles was performed as follows. Areas on suitable cryosections showing aggregates of closely packed, labeled and unlabeled vesicles were outlined with an electronic pen to delimit the total surface of the areas. Vesicle-free regions, if present, were outlined and subtracted from the total surface. The number of gold particles (ranging from 1–3) present on the vesicles were recorded and divided by the total vesicular surface. The data were expressed as number of gold particles per micrometer2 of vesicle surface, and as percent of labeled vesicles over the total number of vesicles (Table ). Ten pictures at final magnification of 96,000× were evaluated for each sample and for each antibody from two independent experiments (Table ). The diameter of vesicles was measured with a graduated reticle on calibrated (130,000×) photographs of Epon-embedded, tannic acid-stained samples. The diameter was defined as the distance between the outer leaflet of the vesicle's membrane, excluding the coat.
Table 1.
Statistical Analysis of the Immunogold Labeling
| Surface density (gold/μm2) | % Labeled vesicles | Vesicles counted | ||||
|---|---|---|---|---|---|---|
| Antibody | αLst1p | αSec24p | αLst1p | αSec24p | αLst1p | αSec24p |
| Experiment 1 | ||||||
| Reaction 1 | 359 ± 43 | 408 ± 28 | 63 ± 2 | 68 ± 3 | 830 | 1,038 |
| Reaction 2 | 36 ± 4 | 474 ± 31 | 12 ± 1 | 74 ± 3 | 873 | 1,167 |
| Experiment 2 | ||||||
| Reaction 1 | 240 ± 16 | 286 ± 28 | 58 ± 2 | 73 ± 4 | 815 | 650 |
| Reaction 2 | 24 ± 4 | 518 ± 73 | 10 ± 1 | 76 ± 4 | 724 | 662 |
Values are expressed as mean ± SEM. Reaction 1 contained Sec23/Lst1p and Sec23/24p and reaction 2 contained Sec23/24p alone.
Results
Lst1p and Sec23p Copurify as a Heterodimer
To study the mechanism by which Lst1p functions in cargo packaging, coat assembly, and vesicle budding, we overproduced the protein and purified it from yeast. We employed a strain harboring two 2μ vectors with a galactose inducible promoter; one carrying a His6-tagged version of the LST1 gene and the other carrying the SEC23 gene. A high speed supernatant from lysates of yeast overexpressing both Lst1p and Sec23p was loaded on a Ni-NTA agarose column and buffers containing increasing concentrations of imidazole were used for elution of bound proteins (see Materials and Methods and Kurihara et al., 2000). Sec23/Iss1p and Sec23/24p complexes were also purified as described (Yeung et al. 1995; Kurihara et al. 2000). The Sec23/Lst1p complex eluted at 250 mM imidazole, a concentration higher than that required for Sec23/24p elution (150 mM), but lower than needed for elution of Sec23/Iss1p (500 mM). All three Sec23p complexes were eluted as heterodimers as verified by native 0.3–1.3 M sucrose density gradients in the presence of molecular weight markers and by quantification of gel bands (data not shown). Over 1 mg purified Sec23/Lst1p heterodimer was obtained from ∼25 g yeast (wet wt), which was similar to the protein yield obtained for the Sec23/Iss1p complex (Kurihara et al. 2000). Interestingly, whereas the predicted molecular weight of Lst1p is slightly larger than that of Sec24p, its electrophoretic mobility was greater on reducing SDS-PAGE (Fig. 1 A). Iss1p, which has the smallest predicted molecular weight, migrated fastest among the three (Fig. 1 A).
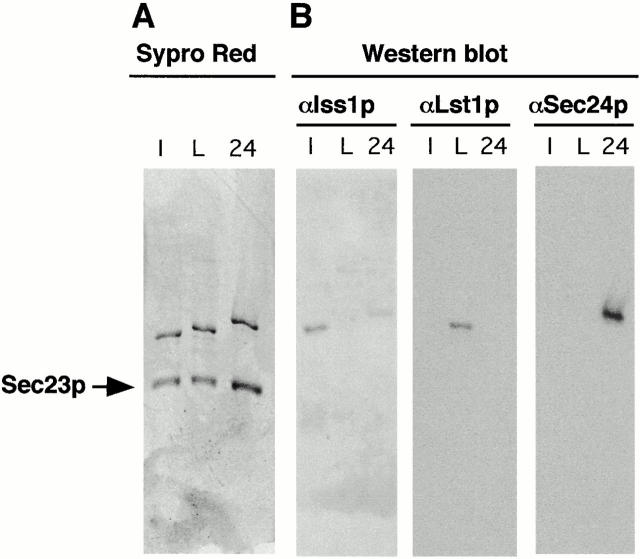
Figure 1.
Purification of Sec23p complexes. Heterodimers of Sec23/Iss1p (lane I), Sec23/Lst1p (lane L) and Sec23/24p (lane 24) were purified as described in Materials and Methods and separated on a 7.5% SDS-PAGE gel followed by Sypro Red staining (A) or immunoblot analysis (B) with either anti-Iss1p, anti-Lst1p, or anti-Sec24p antibodies as indicated.
Antibodies raised against each of the three purified yeast Sec24p homologues appeared highly specific to their respective antigen in immunoblot analysis (Fig. 1 B). Only slight cross-reaction was observed with anti-Iss1p serum and Sec24p (Fig. 1 B), but none of the antibodies reacted with Sec23p (the lower bands in Fig. 1 A). Using these antibodies and the purified Sec23p complexes as a standard ladder, we quantified by immunochemiluminescence the relative abundance of these complexes in yeast cells and found that both Sec24p and Lst1p were enriched in microsomal fractions relative to the cytosol and that Sec24p levels were two to seven times higher than those of Lst1p (data not shown and Peng et al. 2000). This agrees with the predicted mRNA level of Sec24p being about twice as high as those of either Lst1p or Iss1p. This transcript ratio is maintained and constant along the cell cycle (Spellman et al. 1998).
Lst1-null Cytosol Is Deficient in the Packaging of Pma1p into COPII Vesicles
To look for cargo whose packaging is Lst1p-dependent, we constructed an lst1-null strain and prepared membranes from this strain which were then used in budding assays. Cells were radiolabeled with [35S]-Met for three minutes to label secretory proteins newly assembled in the ER. SICs prepared from the labeled cells were used as the source of ER membranes and incubated with COPII proteins and either Sec23/Lst1p or Sec23/24p. The vesicles formed were purified by flotation on sucrose gradients and analyzed by SDS-PAGE, followed by exposure to film. The differences in the protein patterns were not prominent enough to allow the identification of Lst1p-dependent cargo (data not shown).
Given the discovery of Roberg et al. 1999, that lst1 mutant cells accumulate Pma1p in the ER, we examined whether Pma1p is an Lst1p-dependent cargo for packaging into COPII vesicles. To provide a source of membranes with a tagged form of Pma1p, we replaced the chromosomal PMA1 gene with an HA-tagged version of PMA1 in an lst1-null yeast strain. Cells were pulse-labeled for three minutes and converted to SICs, which served as a source of membranes for budding assays primed with cytosol. Packaging of Pma1p and α-factor was then assessed as described in Materials and Methods. When cytosol prepared from a wild-type strain was used, >35% of both α-factor and Pma1p was packaged into vesicles (Fig. 2). However, with cytosol prepared from the lst1-null, α-factor was efficiently packaged, whereas Pma1p was not. Supplementing the lst1-null cytosol with purified Sec23/Lst1p restored Pma1p packaging up to 25% of total (Fig. 2). This experiment demonstrated that Lst1p is required for efficient packaging of Pma1p into vesicles. It also showed that the purified tagged version of Sec23/Lst1p complex was functional in our budding assay. At the highest levels of Sec23/Lst1p, less efficient Pma1p packaging was observed (Fig. 2), raising the possibility that a proper balance between Lst1p and Sec24p concentrations may be important for optimal cargo packaging.
Lst1p and Sec24p Cooperate in Packaging of Pma1p
We next investigated whether Sec23/Lst1p is needed in addition to Sec23/24p or replaces the requirement for Sec23/24p in the capture of Pma1p into COPII vesicles. gpαF and Pma1p packaging assays were conducted with combinations of purified COPII components (Fig. 3). When 2 μg/ml Sec23/24p was employed in the absence of Sec23/Lst1p, gpαF was packaged, whereas Pma1p was not. With 2 μg/ml Sec23/Lst1p, but no Sec23/24p, neither cargo was efficiently packaged. In contrast, in the presence of a mixture of both, 1 μg/ml Sec23/Lst1p and 1 μg/ml Sec23/24p, efficient gpαF and Pma1p packaging was accomplished. Because the total concentration of Sec23p in these three reactions was kept constant (at 2 μg/ml of Sec23p complex), the observed differences in packaging efficiency must be attributed solely to the presence or absence of Lst1p or Sec24p (and not to the dosage of Sec23p). These results demonstrate that both Lst1p and Sec24p heterodimers are required for efficient Pma1p packaging. On the other hand, efficient packaging of α-factor requires only the Sec24p heterodimer.
A high concentration (20 μg/ml) of Sec23/Lst1p in the absence of Sec23/24p did not facilitate the packaging of α-factor and Pma1p (data not shown). In contrast, in budding experiments performed with a high concentration (20 μg/ml) of Sec23/24p in the absence Sec23/Lst1p, both α-factor and Pma1p were efficiently packaged (Fig. 3). Thus, although Lst1p can greatly facilitate Pma1p packaging, it is not an absolute requirement for Pma1p packaging into COPII vesicles. However, in the absence of Sec23/Lst1p, more than a tenfold higher concentration of Sec24p complex was needed for efficient Pma1p packaging to occur (Fig. 3).
Sec23/Lst1p and Sec23/24p Coassemble with Pma1p
We have shown previously that a subset of COPII components, Sar1p and Sec23/24p, form a complex with vesicle cargo (Kuehn et al. 1998). Because Lst1p and Sec24p were required concurrently for Pma1p packaging into COPII vesicles, Lst1p, Sec24p, and Pma1p could all be associated with each other in a complex on the vesicle membrane. To test whether Lst1p and Sec24p assemble on the same vesicles with Pma1p, we conducted budding reactions (from lst1-null SICs expressing HA-Pma1p or from wild-type SICs not expressing HA-tagged Pma1p) in the presence of GMP-PNP and a mixture of Sec23/Lst1p and Sec23/24p, or with only Sec23/24p, and the other COPII proteins Sar1p and Sec13/31p. COPII vesicles in the MSS fraction were treated with the cleavable cross-linking agent DSP and then subjected to denaturation in the absence of reducing agent. After immunoprecipitation with anti-Lst1p or anti-Sec24p antibodies, samples were incubated with reducing agent and subjected to immunoblot analysis using anti-HA antibodies to detect HA-Pma1p.
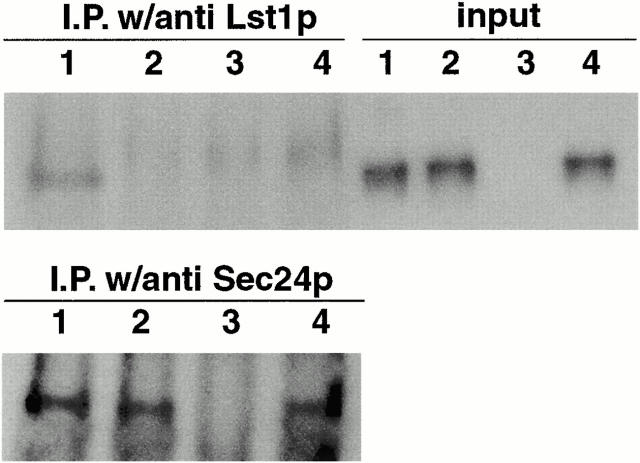
HA-Pma1p coimmunoprecipitated with either anti-Lst1p or anti-Sec24p antibodies from vesicles produced with a mixture of Sec23/Lst1p and Sec23/24p (depicted in Fig. 4, lanes 1). In vesicles produced with high levels of Sec23/24p, but without any Lst1p, an HA-Pma1p-Sec24p complex was immunoprecipitated with anti-Sec24p, but not with anti-Lst1p antibodies (Fig. 4, lanes 2). Membranes expressing untagged Pma1p generated no species that could be immunoprecipitated from vesicles (Fig. 4, lanes 3). A species migrating slightly slower than Pma1p, and found in all four samples, may correspond to antibody heavy chain dimers that failed to be fully reduced in sample buffer given the mild conditions necessary to prevent denaturing aggregation of Pma1p.
Figure 4.
Coimmunoprecipitation of HA-Pma1p with Lst1p and Sec24p. Vesicles were generated from lst1-null, HA-Pma1p containing SICs using either a mixture of 10 μg/ml each of Sec23/Lst1p and Sec23/24p (lanes 1) or 20 μg/ml Sec23/24p only (lanes 2). In lanes 3, a mixture of 10 μg/ml each of Sec23/Lst1p and Sec23/24p was used as in lanes 1, but wild-type SICs membranes, not expressing HA-tagged Pma1p, were used. Lanes 4 contained a mixture of the vesicles generated in reactions 2 and 3. After cross-linking, reactions were immunoprecipitated with anti-Lst1p or anti-Sec24p as indicated, and subjected to SDS-PAGE and immunoblot analysis using anti-HA antibodies. Input lanes represent 1% of the cross-linked reactions before immunoprecipitation.
To test whether HA-Pma1p and Sec23/Lst1p could be cross-linked from distinct vesicles, samples generated in the reactions examined in Fig. 4, lanes 2 (containing HA-Pma1p but no Lst1p) and 3 (containing Lst1p but no HA-Pma1p), were mixed and then cross-linked and immunoprecipitated. As shown in Fig. 4, lanes 4, HA-Pma1p was not immunoprecipitated with anti-Lst1p antibodies. However, HA-Pma1p was precipitated with Sec24p antibodies. The results of these experiments show that Pma1p can form a complex, directly or indirectly, with Sec23/Lst1p and Sec23/24p, and, together with the in vitro packaging results, suggest that the combination of complexes facilitates Pma1p packaging into a common vesicle species.
Lst1p and Sec24p Are Present on the Same COPII Vesicles
That Lst1p and Sec24p both interact with Pma1p and are required for efficient Pma1p packaging indicated that Lst1p and Sec24p cooperate in vesicle budding by forming a mixed coat containing both protein complexes. Alternatively, the two Sec23p-containing complexes could cooperate in the capture of Pma1p, but segregate into distinct COPII vesicles. To test this, we performed large-scale cell-free budding reactions using lst1-null membranes supplemented with Sec23/24p or Sec23/Lst1p, or both together with the other COPII proteins. Vesicle budding reactions were performed in the presence of the nonhydrolyzable GTP analogue, GMP-PNP, conditions that preserve the COPII coat. After being resolved from donor membranes, vesicles were separated from insoluble and soluble contaminants by sedimentation onto a sucrose cushion, followed by flotation in sucrose density gradients. Fractions were either subjected to immunoblot analysis using antibodies to various COPII subunits or counted to detect the radioactive cargo molecule, [35S]gpαF. When Sec23/24p was used for vesicle budding, the peak of [35S]gpαF coincided (at ∼40% sucrose) with the peaks of Sec24p and Sec13p, indicating that the coat remained associated with the vesicles under these conditions (Fig. 5 A). In budding reactions where no Sec24p homologues were included, vesicles did not form (Fig. 5 D). When Sec23/Lst1p was used instead of Sec23/24p, vesicle formation was inefficient, as seen by the lower levels of Sec13p detected in immunoblots (Fig. 5A and Fig. B) as well as the lower levels of the other coat components (all of which were added at identical concentrations in the two reactions, A and B) in stained gels (not shown). [35S]gpαF packaging was not detected (Fig. 5 B). Nevertheless, coinciding peaks of Lst1p and Sec13p were detected at ∼40% sucrose (Fig. 5 B). This indicates that Sec23/Lst1p can interact with the other COPII subunits, Sec13/31p and Sar1p, in the absence of Sec23/24p to form a coat and a vesicle, albeit inefficiently in comparison to Sec23/24p. Iss1p, the third yeast homologue of Sec24p, formed vesicles more readily in place of Sec23/24p in the budding assay (Kurihara et al. 2000). Iss1p vesicles, unlike Lst1p vesicles, did contain [35S]gpαF, but packaging efficiency was lower than in Sec24p vesicles (Kurihara et al. 2000).
Figure 5.
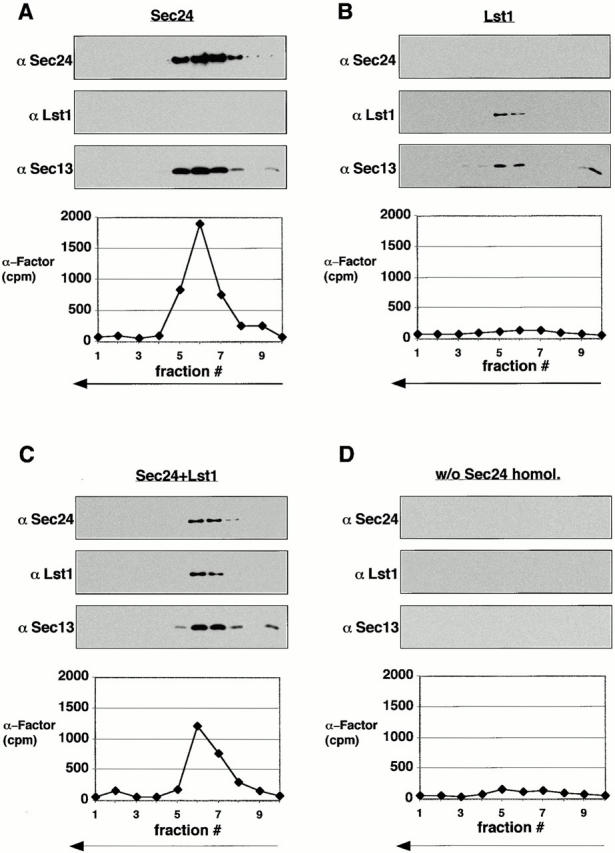
Density gradient analysis of COPII vesicles. Four large-scale budding reactions, using lst1-null membranes, Sar1p, Sec13/31p, and the indicated combinations of Sec23/24p (Sec24p) and Sec23/Lst1p (Lst1p) at a concentration (combined) of 5 μg/ml, were performed (A–D). Vesicles were floated in sucrose gradients as described in Materials and Methods. Fractions were either subjected to SDS-PAGE followed by immunoblot analysis with the indicated antibodies and exposed simultaneously to film, or counted in a scintillation counter for [35S]gpαF distribution.
In the reactions where Sec23/24p and Sec23/Lst1p were used together, coated vesicles of the same buoyant density as the Sec23/24p vesicles and Sec23/Lst1p vesicles were formed (Fig. 5 C). Although the total amount of Sec24p plus homologue and all other components was kept the same in both cases, only half the [35S]gpαF was packaged in a reaction containing the mixture compared with one containing Sec23/24p. The levels of Sec13p (as well as the other coat components, not shown) were also lower, indicating that not only less [35S]gpαF was packaged, but that fewer or less stable vesicles were formed. This is probably due to limiting levels of Sec23/24p (see also Fig. 3) again reflecting the packaging dependence of [35S]gpαF only on Sec23/24p. Notwithstanding, Lst1p vesicles, Sec24p vesicles, and vesicles produced with a mixture of both complexes, all cofractionated at a similar sucrose density. Thus, either a single vesicle species is formed in incubations containing mixed coat subunits or physically similar but molecularly distinct species are produced.
To distinguish these possibilities, we conducted conventional (Fig. 6) and immunoelectron microscopy (Fig. 7) on enriched fractions of vesicles produced in reactions containing Sec23/Lst1p and Sec23/24p or Sec23/24p alone. The coat morphology of vesicles in both reactions was similar, but the vesicles generated with both complexes were larger (Fig. 6). We observed a consistent difference in the range of vesicle size depending on the source of ER membrane used in a budding reaction. COPII vesicles generated from gently lysed SICs were smaller than those generated from microsomal membranes isolated by more vigorous means, including a urea washing step. However, with either membrane source, vesicles generated with a mixture of Sec23/Lst1p and Sec23/24p were on average 15% larger than those generated with Sec23/24p alone; 87 nm compared with 75 nm with microsomes (Fig. 6) or 74 nm vs. 64 nm with SICs (not shown). This is in agreement with our previous reported size of 60–65 nm for standard COPII vesicles (Barlowe et al. 1994). For immunoelectron microscopy, polyclonal, monospecific anti-Lst1p and anti-Sec24p antibodies (see Fig. 1 B) were used in single-label immunogold staining of cryosections of the vesicles. In budding reactions in which a mixture of Sec23 heterodimers was applied, ∼60–70% of the vesicles were labeled with either anti-Lst1p or anti-Sec24p antibodies (Fig. 7A and Fig. B, and Table ). In control reactions in which vesicles were produced with only Sec23/24p, only Sec24p immunolabeling was observed (∼75% of all vesicles), whereas anti-Lst1p antibodies labeled a background of ∼10% of all vesicles (Fig. 7C and Fig. D, and Table ). Lst1p and Sec24p labeling thus overlapped in at least 20–30% of all vesicles in the field, strongly suggesting that Lst1p and Sec24p can form a coat on the membrane of the same vesicle.
Figure 7.
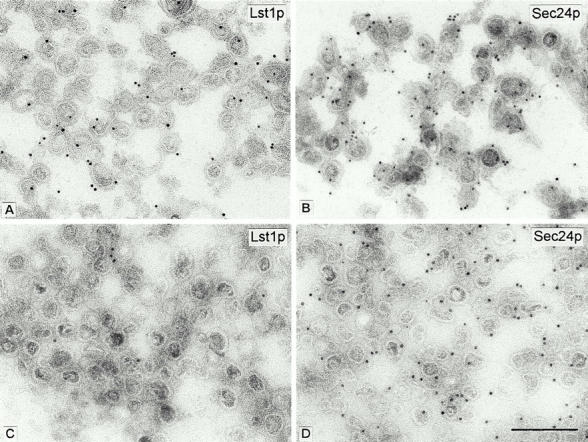
Ultrathin cryosections of vesicles from reaction 1 (A and B) performed with Sec23/Lst1p and Sec23/24p (10 μg/ml each), or from reaction 2 (C and D) performed with Sec23/24p only (20 μg/ml). In both conditions, lst1-null SICs were used as donor membranes. Sections were probed with either anti-Lst1p (A and C) or anti-Sec24p (B and D), followed by protein A-gold (10-nm gold). The quantitative evaluation of the respective immunolabelings is shown in Table . Bar, 0.2 μm.
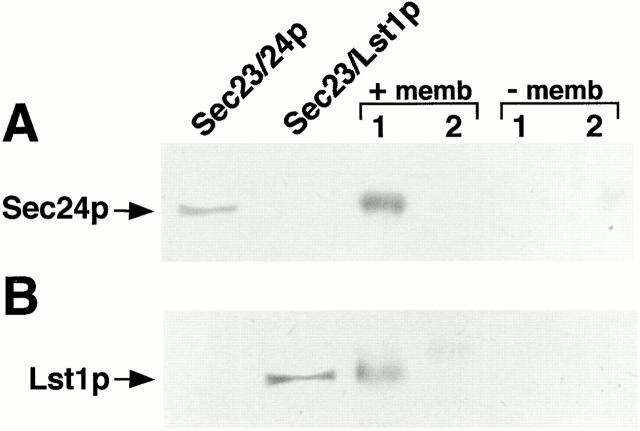
Further confirmation of the association of both complexes on the same vesicle was obtained by chemical cross-linking and coimmunoprecipitation of Lst1p and Sec24p. Vesicles were produced (assayed by [35S]gpαF packaging) from lst1-null SICs (Fig. 8, +memb) with either a mixture of Sec23 heterodimers (at 1 μg/ml each) or with Sec23/24p only (at 2 μg/ml) (Fig. 8, lanes 1 and 2 respectively) and mock reactions were incubated in the absence of the donor SICs membranes (−memb). Following cross-linking, reciprocal coimmunoprecipitation of Lst1p and Sec24p was observed only in the vesicle-containing fraction in which both heterodimers were present. No coprecipitation was detected in the reaction performed in the absence of donor membranes suggesting that soluble Sec23/24p and Sec23/Lst1p in the MSS were not associated. Thus, only membrane-tethered Sec23p complexes could be coimmunoprecipitated under these conditions.
Figure 8.
Coimmunoprecipitation of Lst1p and Sec24p is membrane-dependent. Reactions containing purified COPII components and a mixture of both Sec23/Lst1p and Sec23/24p (lanes 1) or Sec23/24p only (lanes 2) were set in the presence (+memb) or absence (−memb) of donor membranes and supernatants containing vesicles (+memb) or only soluble COPII proteins (−memb) were cross-linked and centrifuged as described under Materials and Methods. Solublized pellets were then either immunoprecipitated with anti-Lst1p serum and immunoblotted with anti-Sec24p serum (A) or vice versa (B). Purified Sec23/24p and Sec23/Lst1p were also separated on the gel for immunoblotting as indicated.
Finally, we considered the possibility that Sec23 heterodimers formed cross-links between vesicles in an aggregate. [35S]gpαF–loaded membranes were used in budding assays containing 2 μg/ml of Sec23/24p (but no Sec23/Lst1p), whereas mock-treated (unlabeled) SICs were used in budding assays containing 1 μg/ml of each heterodimer. The [35S]gpαF–labeled Sec23/24p vesicle pool was divided into three aliquots for cross-linking, followed by immunoprecipitation as described above. One aliquot was immunoprecipitated with anti-Sec24p antibodies, the second with anti-Lst1p antibodies, and the third was mixed with an MSS containing unlabeled vesicles produced with the mixture of heterodimers, and following cross-linking was immunoprecipitated with anti-Lst1p antibodies. All immunoprecipitates were washed and counted in a scintillation counter. In the Sec23/24p vesicles, ∼7% of the [35S]gpαF was coimmunoprecipitated by anti-Sec24p antibodies (data not shown). This is in agreement with previously reported experiments showing that ∼5% of translocated [35S]gpαF forms a complex with Sec24p (Kuehn et al. 1998). By contrast, only ∼1% of [35S]gpαF coimmunoprecipitated using anti-Lst1p antibodies, with either Sec23/24p vesicles (which were loaded with [35S]gpαF) or with similar vesicles mixed with the unlabeled preparation. Because anti-Lst1p antibodies coimmunoprecipitate Sec24p (Fig. 8 A, lanes 1), cross-linking between vesicles should have coprecipitated [35S]gpαF. However, only 1% of the [35S]gpαF was precipitated in the vesicle mixing reaction, the same percentage that precipitated in [35S]gpαF vesicles that do not contain Lst1p. Because neither vesicles nor soluble coat complexes in solution could be cross-linked to each other under these experimental conditions, the association of Sec23/24p with Sec23/Lst1p could have happened only if both were present on the same vesicle.
Discussion
LST1 encodes a nonessential SEC24 homologue that interacts in synthetic lethal combinations with other SEC genes known to be required for COPII-mediated vesicle budding from the ER (Roberg et al. 1999). Previous work showed that the deletion of LST1 causes the accumulation of the plasma membrane ATPase (Pma1p) in the ER membrane (Roberg et al. 1999). Thus, Roberg et al. 1999 suggested a role for Lst1p in COPII vesicle budding and Pma1p sorting. Evidence showing a direct role for Lst1p in packaging cargo proteins at the ER required a biochemical approach. To investigate the mechanism of Pma1p packaging and the involvement of Lst1p in this process, we employed a cell-free vesicle budding reaction (Rexach and Schekman 1991). This reaction allowed us to study the interplay between Lst1p and Sec24p in packaging cargo into vesicles and forming a coat. We found that Sec23/Lst1p is involved in Pma1p recruitment into COPII-coated vesicles.
In contrast to the prediction that Sec23/Lst1p may replace Sec23/Sec24p in the packaging of Pma1p, we found that Sec23/Lst1p functions poorly as a substitute for the normal heterodimer. Instead, we found that both heterodimers are required for efficient packaging of Pma1p into COPII vesicles. Vesicles formed in the presence of the Sec24p and Lst1p heterodimers were similar in buoyant density and morphological appearance, but were ∼15% larger in diameter than those formed in a standard incubation. Independent approaches using immunogold staining and chemical cross-linking, followed by denaturing immunoprecipitation with monospecific polyclonal antibodies showed that the two heterodimers copolymerize on a common vesicle species.
Because LST1 is not an essential gene, whereas PMA1 is essential, one would expect traffic of Pma1p not to depend solely on Lst1p. Indeed, we found that high levels of Sec23/Sec24p obviated the requirement for Sec23/Lst1p in our packaging reaction, whereas the converse was not true. At low pH, where Pma1p must function more vigorously, lst1-null cells die, whereas LST1 cells continue to grow. The normal level of Sec23/Sec24p may not suffice in mutant cells grown at low pH.
The Lst1p complex is necessary, but not sufficient for the reconstitution of efficient Pma1p packaging from Lst1-deprived donor membranes (see Fig. 2 and Fig. 3). However, when the cytosol was supplemented with high levels of purified Lst1p complex, a drop in the packaging efficiency of both Pma1p and gpαF was observed (Fig. 2). Because the Lst1p and Sec24p heterodimers cooperate in coat formation, an imbalance of the two may interfere with vesicle budding. This view is supported by the physiologic observation that overexpression of LST1 impairs the growth of wild-type yeast, but cooverexpression of SEC24 compensates for this defect (Roberg et al. 1999).
ISS1 is another nonessential SEC24 homologue of 55% identity (Kurihara et al. 2000). A Sec23/Iss1p heterodimer replaces Sec23/Sec24p to nearly 50% efficiency in a standard budding assay measuring the packaging of gpαF (Kurihara et al. 2000). No growth phenotype has yet been ascribed to the iss1 null and, as a result, no uniquely Iss1p dependent cargo has been identified. However, the existence of the homologues and the selective role of Lst1p suggests that distinct Sec24p species may cooperate to increase the range of cargo that can be accommodated in a mixed COPII vesicle. Because they are cytosolic proteins, the Sec24p homologues are easily recruited as needed to mediate the packaging of membrane cargo proteins by direct and most likely signal-mediated interaction.
Coimmunoprecipitation experiments showed that Pma1p associated, directly or indirectly, with Sec23/Sec24p and Sec23/Lst1p. The interaction between Pma1p and Sec23/Sec24p was enhanced when Sec23/Lst1p was also present (Fig. 4). One possibility is that Lst1p makes a direct contact with a cytoplasmically exposed peptide signal on Pma1p. Sec24p may bind weakly to this signal. Further, we suggest that the Lst1p heterodimer is incapable of satisfying the role of the Sec24p heterodimer in COP II coat polymerization. Thus, Lst1p serves as an adaptor to engage Pma1p in the polymerization of a coat achieved by the normal COPII subunits.
Ideally, the Pma1p sorting signal resides within either one of two cytoplasmically oriented loops or termini providing direct access to Lst1p. It seems unlikely that either the NH2-terminal 27 residues or the COOH-terminal 18 residues is responsible for transport because deletions produce normal steady state levels of plasma membrane Pma1p (Portillo et al. 1989). Analysis of these mutants in our budding reaction revealed normal packaging efficiencies (not shown). Detailed mutagenesis analysis will be required to identify this signal. However, certain heterologous PMA1 gene expression experiments are instructive. Tobacco PMA1 complements a yeast pma1 mutation, whereas three Arabidopsis homologues (AHA1-3) produce active enzymes which largely remain in the ER (Palmgren and Christensen 1994; Dexaerde et al. 1995). The Arabidopsis homologues may contain a divergent sorting signal.
Do other cargo molecules depend on Lst1p for efficient transport out of the ER? Peng and colleagues observed a slight delay in the maturation of the glycolipid-anchored plasma membrane protein Gas1p in lst1-null cells (which they term Δsfb3) (Peng et al. 2000). However, we have previously shown that Gas1p is similarly packaged when either cytosol or normal purified COPII components are used in vesicle budding reactions (Doering and Schekman 1996). Pagano et al. 1999 found that several major proteins secreted into the growth medium are missing in culture supernatants from lst1-null cells (they call this gene SEC24C). These secretory proteins may derive from membrane-bound precursors that make direct contact with Lst1p in the ER, or they may be carried out of the ER in contact with Pma1p or with recycling membrane receptor proteins that are sorted by Lst1p. Alternatively, their production or secretion may depend on a pH gradient generated by Pma1p across the plasma membrane. The identity of these secreted proteins will help distinguish these possibilities.
Other membrane cargo proteins, such as the general amino acid permease (Gap1p), are recognized and packaged by the standard COPII subunits (Kuehn et al. 1996). We suggest that such standard passenger proteins are recognized directly or indirectly by Sec24p. Similar considerations apply to anterograde v-SNARE proteins (Springer and Schekman 1998) and to mammalian membrane proteins, such as the VSV-G protein (Aridor et al. 1998).
Sec24p and its homologues may exert a regulatory influence on the Sar1p-selective GAP (GTPase activating protein) activity of Sec23p (Yoshihisa et al. 1993). In the cycle of COPII vesicle formation, GTP hydrolysis serves to promote coat disassembly, which must be completed before the vesicle can dock and fuse with a target membrane. Although the Sec24p subunit of the heterodimer has no direct influence on the GAP activity of Sec23p, the existence of homologues, at least one of which confers some membrane cargo protein selectivity, suggests that cargo recognition by Sec24p could influence GTP hydrolysis linked to the protein sorting event.
A similar situation may apply in the sorting of cargo by the COPI coat. Coatomer, the assembly protomer of the COPI coat, assembles onto Golgi membranes in the presence of the GTPase, Arf, and GTP (Orci et al. 1993). Arf GAP and Arf nucleotide exchange activities influence the recruitment of Golgi membrane proteins, such as Erd2p and SNAREs (Aoe et al. 1998; Spang and Schekman 1998). Recently, Goldberg 1999, Goldberg 2000 has shown that coatomer stimulates the activity of Arf GAP and that certain cargo proteins retard the coatomer-stimulated rate of GTP hydrolysis. He suggests a kinetic proofreading model in which preferred cargo proteins delay GTP hydrolysis so as to favor the formation of a tight coatomer substrate complex. Likewise, Sec24p and its homologues may influence Sec23p GAP activity in response to preferred substrates: Gap1p for Sec24p and Pma1p for Lst1p.
Although our evidence is most consistent with a sorting signal on Pma1p decoded by the Lst1p subunit of the COP II coat, the issue of positive sorting vs. transport by default remains unresolved. Most recently, Martinez-Menarguez et al. 1999 resurrected the bulk flow hypothesis with the observation that two abundant secretory proteins in the pancreatic acinar cell, amylase and chymotrypsinogen, are not concentrated in COPII buds or vesicles leaving the ER, but are concentrated in the intermediate compartment by COPI-mediated retrieval of nonsecretory material. In interpreting the Martinez-Menarguez et al. 1999 data, Warren and Mellman 1999 suggested that abundant secretory or membrane cargo proteins may simply exceed the availability of any stoichiometric sorting receptors and therefore such molecules could only be packaged at their prevailing concentrations. They suggested that much less abundant proteins may be more selectively packaged to allow their rapid transport out of the ER. However, for membrane proteins that may be in direct contact with coat subunits, we suggest that even abundant cargo molecules could become concentrated within COPII buds and vesicles. Indeed, Pma1p comprises up to 50% of the total protein of the yeast plasma membrane (van der Rest et al. 1995).
It is possible to erect a model reconciling the bulk flow hypothesis and the role of Lst1p in sorting of Pma1p into COPII vesicles. Depending on the subunit structure of Pma1p as it assembles in the ER membrane, normal COPII vesicles may be too small or too sharply curved to admit an intact Pma1p oligomer. Pma1p has been crystallized as a hexameric ring with an outer diameter of 165 Å (Auer et al. 1998) and preliminary biosynthetic studies suggest an ER oligomer of Pma1p as large as a dodecamer (Lee, M., and R. Sheckman, unpublished observations). Furthermore, Bagnat et al. 2000, have demonstrated that Pma1p assembles in a glycolipid raft before its export from the ER. This structure may simply not fit in a standard COPII vesicle. Accordingly, we suggest as an alternative to the sorting signal model that the Sec23/Lst1p heterodimer may alter the geometry of COPII coat polymerization creating a vesicle that can accommodate the Pma1p oligomer. Conceivably, the ratio of Sec24p and Lst1p heterodimers in a COPII coat may be regulated to adapt the budding reaction to small or large cargo. In the extreme, large lipoprotein particles and collagen fibers must be transported out of the ER (Schekman and Mellman 1997), and this transport could require the mammalian equivalent of Lst1p.
Acknowledgments
We are grateful to all members of the Schekman lab for providing a pleasant and stimulating working environment. Special thanks to R. Lesch and P.-S. Huang for help in the preparation of reagents, and S. Hamamoto for help with sample fixation for EM.
Y. Shimoni was supported by a postdoctoral fellowship from the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation, DRG-1469, and T. Kurihara was supported by fellowships from the Human Frontier Science Program and the Ministry of Education, Science, Sports and Culture of Japan. Work in the Orci laboratory is supported by grants from the Swiss National Science Foundation and work in the Schekman laboratory is supported by the Howard Hughes Medical Institute.
Footnotes
Tatsuo Kurihara's present address is Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011, Japan.
Abbreviations used in this paper: DSP, Dithiobis (succinimidyl propionate); GMP-PNP, 5′-guanylylimidodiphosphate; gpαF, glycosylated pro-α-factor; HA, hemagglutinin; MSS, medium speed supernatant; Pma1p, plasma membrane ATPase; SICs, semi-intact cells; VSV-G, vesicular stomatitis virus glycoprotein.
References
- Aoe T., Lee A.J., van Donselaar E., Peters P.J., Hsu V.W. Modulation of intracellular transport by transported proteinsinsight from regulation of COPI-mediated transport. Proc. Natl. Acad. Sci. USA. 1998;95:1624–1629. doi: 10.1073/pnas.95.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M., Weissman J., Bannykh S., Nuoffer C., Balch W.E. Cargo selection by the COPII budding machinery during export from the endoplasmic reticulum. J. Cell Biol. 1998;141:61–70. doi: 10.1083/jcb.141.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer M., Scarborough G.A., Kühlbrandt W. Three-dimensional map of the plasma membrane H+-ATPase in the open conformation. Nature. 1998;392:840–843. doi: 10.1038/33967. [DOI] [PubMed] [Google Scholar]
- Bagnat M., Keranen S., Shevchenko A., Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Hicke L., Rexach M., Schleyer M., Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988;54:335–344. doi: 10.1016/0092-8674(88)90196-1. [DOI] [PubMed] [Google Scholar]
- Balch W.E., McCaffery J.M., Plutner H., Farquhar M.G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Barlowe C., d'Enfert C., Schekman R. Purification and characterization of SAR1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J. Biol. Chem. 1993;268:873–879. [PubMed] [Google Scholar]
- Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M.F., Ravazzola M., Amherdt M., Schekman R. COPIIa membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bednarek S.Y., Ravazzola M., Hosobuchi M., Amherdt M., Perrelet A., Schekman R., Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Bonifacino J.S., Marks M.S., Ohno H., Kirchhausen T. Mechanisms of signal-mediated protein sorting in the endocytic and secretory pathways. Proc. Assoc. Am. Phys. 1996;108:285–295. [PubMed] [Google Scholar]
- Cosson P., Letourneur F. Coatomer (COPI)-coated vesiclesrole in intracellular transport and protein sorting. Curr. Opin. Cell Biol. 1997;9:484–487. doi: 10.1016/s0955-0674(97)80023-3. [DOI] [PubMed] [Google Scholar]
- Dexaerde A.D., Supply P., Dufour J.P., Bogaerts P., Thines D., Goffeau A., Boutry M. Functional complementation of a null mutation of the yeast Saccharomyces cerevisiae plasma membrane H+-ATPase by a plant H+-ATPase gene. J. Biol.Chem. 1995;270:23828–23837. doi: 10.1074/jbc.270.40.23828. [DOI] [PubMed] [Google Scholar]
- Doering T.L., Schekman R. GPI anchor attachment is required for Gas1p transport from the endoplasmic reticulum in COP II vesicles. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:182–191. [PMC free article] [PubMed] [Google Scholar]
- Dominguez M., Dejgaard K., Fullekrug J., Dahan S., Fazel A., Paccaud J.P., Thomas D.Y., Bergeron J.J., Nilsson T. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J. Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Veit M., Stamnes M.A., Rothman J.E. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Goldberg J. Structural and functional analysis of the ARF1–ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell. 1999;96:893–902. doi: 10.1016/s0092-8674(00)80598-x. [DOI] [PubMed] [Google Scholar]
- Goldberg J. Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell. 2000;100:671–679. doi: 10.1016/s0092-8674(00)80703-5. [DOI] [PubMed] [Google Scholar]
- Hauri H.P., Kappeler F., Andersson H., Appenzeller C. ERGIC-53 and traffic in the secretory pathway. J. Cell Sci. 2000;113:587–596. doi: 10.1242/jcs.113.4.587. [DOI] [PubMed] [Google Scholar]
- Hicke L., Yoshihisa T., Schekman R. Sec23p and a novel 105-kDa protein function as a multimeric complex to promote vesicle budding and protein transport from the endoplasmic reticulum. Mol. Biol. Cell. 1992;3:667–676. doi: 10.1091/mbc.3.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler F., Klopfenstein D.R., Foguet M., Paccaud J.P., Hauri H.P. The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J. Biol. Chem. 1997;272:31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- Kuehn M.J., Schekman R. COPII and secretory cargo capture into transport vesicles. Curr. Opin. Cell Biol. 1997;9:477–483. doi: 10.1016/s0955-0674(97)80022-1. [DOI] [PubMed] [Google Scholar]
- Kuehn M.J., Schekman R., Ljungdahl P.O. Amino acid permeases require COPII components and the ER resident membrane protein Shr3p for packaging into transport vesicles in vitro. J. Cell Biol. 1996;135:585–595. doi: 10.1083/jcb.135.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn M.J., Herrmann J.M., Schekman R. COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature. 1998;391:187–190. doi: 10.1038/34438. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Hamamoto S., Gimeno R.E., Kaiser C.A., Schekman R., Yoshihisa T. Sec24p and Iss1p function interchangeably in transport vesicle formation from the endoplasmic reticulum in Saccharomyces cerevisiae . Mol. Biol. Cell. 2000;11:983–998. doi: 10.1091/mbc.11.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during assembly of the head of bacteiphage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martinez-Menarguez J.A., Geuze H.J., Slot J.W., Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Orci L., Amherdt M., Bednarek S.Y., Hamamoto S., Schekman R., Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- Mizuno M., Singer S.J. A soluble secretory protein is first concentrated in the endoplasmic reticulum before transfer to the Golgi apparatus. Proc. Natl. Acad. Sci. USA. 1993;90:5732–5736. doi: 10.1073/pnas.90.12.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz M., Nuoffer C., Hauri H.P., Riezman H. The Emp24 complex recruits a specific cargo molecule into endoplasmic reticulum-derived vesicles. J. Cell Biol. 2000;148:925–930. doi: 10.1083/jcb.148.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N., Balch W.E. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J.G., Lippincottschwartz J., Klausner R.D., Rothman J.E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991;64:1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Orci L., Palmer D.J., Amherdt M., Rothman J.E. Coated vesicle assembly in the Golgi requires only coatomer and Arf proteins from the cytosol. Nature. 1993;364:732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- Paccaud J.P., Reith W., Carpentier J.L., Ravazzola M., Amherdt M., Schekman R., Orci L. Cloning and functional characterization of mammalian homologues of the COPII component Sec23. Mol. Biol. Cell. 1996;7:1535–1546. doi: 10.1091/mbc.7.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano A., Letourneur F., Garcia-Estefania D., Carpentier J.L., Orci L., Paccaud J.P. Sec24 proteins and sorting at the endoplasmic reticulum. J. Biol. Chem. 1999;274:7833–7840. doi: 10.1074/jbc.274.12.7833. [DOI] [PubMed] [Google Scholar]
- Palmgren M.G., Christensen G. Functional comparisons between plant plasma membrane H(+)-ATPase isoforms expressed in yeast. J. Biol.Chem. 1994;269:3027–3033. [PubMed] [Google Scholar]
- Pelham H.R.B. Recycling of proteins between the endoplasmic reticulum and the Golgi complex. Curr. Biol. 1991;3:585–591. doi: 10.1016/0955-0674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Peng R.W., De Antoni A., Gallwitz D. Evidence for overlapping and distinct functions in protein transport of coat protein Sec24p family members. J. Biol. Chem. 2000;275:11521–11528. doi: 10.1074/jbc.275.15.11521. [DOI] [PubMed] [Google Scholar]
- Portillo F., de Larrinoa I.F., Serrano R. Deletion analysis of yeast plasma membrane H+-ATPase and identification of a regulatory domain at the carboxyl-terminus. FEBS Letters. 1989;247:381–385. doi: 10.1016/0014-5793(89)81375-4. [DOI] [PubMed] [Google Scholar]
- Rexach M.F., Schekman R.W. Distinct biochemical requirements for the budding, targeting, and fusion of ER-derived transport vesicles. J. Cell Biol. 1991;114:219–229. doi: 10.1083/jcb.114.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach M.F., Latterich M., Schekman R.W. Characteristics of endoplasmic reticulum-derived transport vesicles. J. Cell Biol. 1994;126:1133–1148. doi: 10.1083/jcb.126.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg K.J., Crotwell M., Espenshade P., Gimeno R., Kaiser C.A. LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. J. Cell Biol. 1999;145:659–672. doi: 10.1083/jcb.145.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J. Histochem. Cytochem. 1978;26:1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Salama N.R., Chuang J.S., Schekman R.W. Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol. Biol. Cell. 1997;8:205–217. doi: 10.1091/mbc.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama N.R., Yeung T., Schekman R.W. The Sec13p complex and reconstitution of vesicle budding from the ER with purified cytosolic proteins. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:4073–4082. doi: 10.1002/j.1460-2075.1993.tb06091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular Cloning. A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press, ; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Schekman R., Mellman I. Does COPI go both ways? Cell. 1997;90:197–200. doi: 10.1016/s0092-8674(00)80326-8. [DOI] [PubMed] [Google Scholar]
- Schiestl R.H., Gietz R.D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genetics. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sikorski R.S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae . Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A., Schekman R. Reconstitution of retrograde transport from the Golgi to the ER in vitro. J. Cell Biol. 1998;143:589–599. doi: 10.1083/jcb.143.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman P.T., Sherlock G., Zhang M.Q., Iyer V.R., Anders K., Eisen M.B., Brown P.O., Botstein D., Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S., Schekman R. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 1998;281:698–700. doi: 10.1126/science.281.5377.698. [DOI] [PubMed] [Google Scholar]
- Tang B.L., Zhang T., Low D.Y.H., Wong E.T., Horstmann H., Hong W.J. Mammalian homologues of yeast Sec31pan ubiquitously expressed form is localized to endoplasmic reticulum (ER) exit sites and is essential for ER-Golgi transport. J. Biol. Chem. 2000;275:13597–13604. doi: 10.1074/jbc.275.18.13597. [DOI] [PubMed] [Google Scholar]
- Tani K., Oyama Y., Hatsuzawa K., Tagaya M. Hypothetical protein KIAA0079 is a mammalian homologue of yeast Sec24p. FEBS Letts. 1999;447:247–250. doi: 10.1016/s0014-5793(99)00303-8. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K.T. Application of cryoultramicrotomy to immunocytochemistry. J. Microsc. 1986;143:139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheetsprocedure and some applications. Proc. Natl Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Rest M.E., Kamminga A.H., Nakano A., Anraku Y., Poolman B., Konings W.N. The plasma membrane of Saccharomyces cerevisiaestructure, function, and biogenesis. Microbiol. Rev. 1995;59:304–322. doi: 10.1128/mr.59.2.304-322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Mellman I. Bulk flow redux? Cell. 1999;98:125–127. doi: 10.1016/s0092-8674(00)81006-5. [DOI] [PubMed] [Google Scholar]
- Wieland F.T., Gleason M.L., Serafini T.A., Rothman J.E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987;50:289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Wuestehube L.J., Schekman R.W. Reconstitution of transport from endoplasmic reticulum to Golgi complex using endoplasmic reticulum-enriched membrane fraction from yeast. Methods Enzymol. 1992;219:124–136. doi: 10.1016/0076-6879(92)19015-x. [DOI] [PubMed] [Google Scholar]
- Yeung T., Yoshihisa T., Schekman R. Purification of Sec23p–Sec24p complex. Methods Enzymol. 1995;257:145–151. doi: 10.1016/s0076-6879(95)57020-9. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T., Barlowe C., Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]