Conventional kinesin is a highly processive motor that can take >100 steps along a microtubule before dissociating. Various lines of evidence have led to a model of hand over hand processive motion, in which the trailing head detaches and rebinds to the next open tubulin dimer site on the same protofilament, leading to an 8-nm movement of the center of mass (Svoboda and Block 1994; Hancock and Howard 1998). Biochemical evidence for an alternating mechanism in which the ATPase cycles on the two heads are out of phase (Hackney 1994; Ma and Taylor 1997; Gilbert et al. 1998) supports the hand over hand mechanism. Processivity is a competition between the detachment and rebinding of one head in order to take a step, and the rate of dissociation of the complex while only one head is bound. Although processivity is thought to require two heads in the case of conventional kinesin, a monomeric kinesin construct of KIF1A is processive (Okada and Hirokawa 1999). This surprising result has been explained by a diffusive motion within the electrostatic field of the microtubule, biased by some conformational change coupled to the ATPase cycle (Okada and Hirokawa 2000).
It is not clear how dimeric kinesin takes the step to the next binding site on tubulin. Important progress in identifying the structural change necessary for a step is reported in two papers in this issue (Thorn et al. 2000; Tomishige and Vale 2000). In addition, the results point to an unexpected similarity in the mechanism of motion of conventional kinesin and KIF1A.
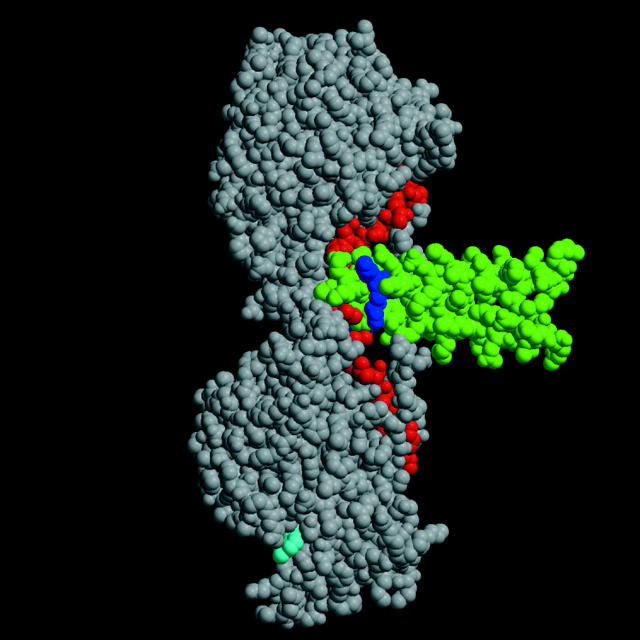
The determination of the structure of the rat kinesin dimer bound to ADP (Kozielski et al. 1997) raised the question of whether the two heads could ever be simultaneously bound to successive tubulin dimer units. The orientation of kinesin in Fig. 1 corresponds to the position with the trailing head bound and the plus end of the microtubule pointing up. Based on docking the crystal structure to the electron micrograph, the coiled-coil segment (Fig. 1, green) is along the surface of the microtubule, perpendicular to the direction of motion (Hoenger et al. 1998; Rice et al. 1999). An alternative docking mechanism has been proposed in which the coiled-coil is pointing away from the microtubule and the second head is detached (Hirose et al. 1999). If we tentatively accept the first alternative, the orientation of the dimer in the figure is roughly as it would appear while walking along the microtubule surface from top to bottom. The trailing head is bound to the microtubule and the leading head is free to rotate away from the previous microtubule binding site. However, the distance between heads is not sufficient to span the 8nm spacing between sites. Two solutions to the problem have been proposed. First, the coiled-coil may untwist sufficiently to allow the leading head to rotate and span the distance between sites (Tripet et al. 1997; Hoenger et al. 1998). A second possibility is that the neck linker (13 residues, colored red in Fig. 1) is disordered in the leading head, which could also allow the head to rotate and reach the next tubulin site without untwisting the coiled-coil (Rice et al. 1999). To try to decide between these alternatives, Tomishige and Vale 2000 introduced cross-links between appropriately placed cysteine residues either to attach the neck linker to the catalytic core, preventing neck linker motion, or to place a disulfide bridge at positions in the coiled-coil, preventing untwisting. The effects on processivity were strikingly different.
Figure 1.
Kinesin dimer structure. The structure of the kinesin dimer (Kozielski et al. 1997) with the catalytic core shown in gray, the neck linker in red, and the neck coiled-coil in green. The bound ADP is shown in cyan. The two terminal lysines of heptad one are shown in blue.
The human kinesin construct used in this study, K560, is highly processive. The run length for wild type is at least 1.5 μm. Disulfide cross-links were introduced from the neck linker to the catalytic core (C334/C222 and C330/C4, numbers refer to human kinesin). Processivity was essentially abolished as measured using the single molecule assay. ATPase activity of the construct was reduced by twofold, as though one head of the cross-linked kinesin could not interact with the microtubule. In a multiple motor microtubule gliding assay, the cross-linked kinesin produced a low velocity of motion that was comparable to that elicited by monomeric kinesin. These data are consistent with kinesin with a cross-linked neck linker acting as a nonprocessive monomer with only one head attaching and cycling during an encounter with a microtubule. In contrast, cross-linking of the coiled-coil by a disulfide bridge at C337, the beginning of the coil, or at C344, had only a small effect on run length or microtubule gliding velocity, indicating that they were still processive motors.
Do these results settle the question? They show that to attach the leading head, the neck linker has to be released from its interaction with the core. Any unwinding of the coiled-coil is not sufficient for attachment if the neck linker remains bound to the core. The results argue against the first model of Hoenger et al. 1998 which postulated that unwinding was sufficient for attachment with the neck linker remaining in the position seen in the crystal structure. A conservative interpretation is that release of the neck linker is a necessary condition. However, a cross-link at the beginning of the coiled-coil did have a small effect, a 40–50% reduction in run length. A small increase in the probability of dissociation (decrease in run length) could arise from a change in the distribution of states in the cycle, such as an increase in the relative amount of a weakly bound state. Thus, unwinding of the first heptad of the neck coiled-coil could play some role in aiding processivity.
A similar experiment in which the first residue of the coiled-coil was cross-linked using rat kinesin 379, yielded a different result (Hoenger et al. 2000). Single molecule motility was not measured directly, but the KM for microtubule activation of the ATPase was increased 13-fold with little change in the maximum rate (kcat). The decrease in the ratio of kcat/KM was interpreted as an indirect measure of the decrease in processivity (Hackney 1995). The authors adopted a revised model that now includes disorder of the neck linker, along with an unwinding of the first half of the coiled-coil to extend the span of the kinesin dimer by 1.2 nm. The results of Tomishige and Vale 2000 are consistent with an untwisting of the first heptad of the coiled-coil, but not with the extent of unwinding proposed in the Hoenger et al. 2000 model.
Does the coiled-coil structure have any additional effect on processivity? Thorn et al. 2000 report on the effect of altering the charge distribution of the coiled-coil. The first heptad, TAEQWKK, has charged groups in positions that reduce stability (lysines, colored blue in Fig. 1). The addition of three repeats of the first heptad increased the run length by more than fourfold. Increasing the positive charge of the five heptads of the coiled-coil also increased the run length twofold. Therefore, there is an electrostatic interaction of the coiled-coil with the microtubule, as might be expected if the coil projects close to the surface. Processivity also depends on the negative charge at the COOH-terminal of tubulin, the E-hook (Wang and Sheetz 2000; Thorn et al. 2000).
These results suggest that there are subtle interactions that have not yet been explored. High processivity may require an electrostatic interaction of the coiled-coil segment with the microtubule to reduce the rate of dissociation from weakly bound states. In the case of the KIF1A monomer, processivity is dependent on extra positive charges in loop L12, which may interact with the E-hook (Okada and Hirokawa 2000). The longer charged loop in this monomer may serve the same purpose as the charge distributed along the coiled-coil segment of a dimer. However, this is not a simple charge interaction because the construct in which the neck linker is cross-linked to the core showed a diffusive component of the motion, which is similar to the behavior of KIF1A. This component may be masked in the normal kinesin by the processive stepping. The results suggest that conventional kinesin and KIF1A can diffuse along the microtubule by interacting with the flexible E-hook that is present in both a and b tubulin (Kikkawa et al. 2000). A general treatment of the problem of the motion of a molecular motor in a periodic electrostatic potential was given by Atsumian and Bier 1996.
Independent of the structural mechanism by which processivity is supported, one may ask to what cellular purpose the mechanism is put and under what selection pressures it operates. One plausible suggestion raised by Thorn et al. 2000 is that ultra-high levels of kinesin processivity may be detrimental because of chance encounters with immovable obstacles. Were a kinesin motor to continue to grind away on the same microtubule track, the cargo might never reach its destination. A better strategy might be to dissociate from the microtubule stochastically. This would allow the possibility of finding another track that might circumvent the obstacle. From this perspective, an optimal degree of kinesin processivity would be dependent on the size of its cargo and the structure of the cytoplasm. Another possibility is that processivity is selected to achieve an optimal balance between vectorial transport and random motion. One example of this process is the fish melanophore, where functional coordination between kinesin transport on radially organized microtubules and myosin V transport on randomly arranged actin filaments is used to achieve a uniform distribution of pigment granules (Rodionov et al. 1998; Rogers and Gelfand 1998). The system requires frequent hand-offs between the microtubules and the actin filaments and, consequently, limitations on processivity. Similar considerations may underlie mechanisms of transport and interaction of vesicles in endocytosis and exocytosis, neuronal transport, assembly of the Golgi apparatus, and directional cell motility (Allan and Schroer 1999). In all of these cases, microtubule-based transport may deliver cargo to the neighborhood, but a trial-and-error mechanism of random exploration may be essential to find the proper address. Thus, handing off cargo is likely no less important than its delivery, and a balance between these two dimensions may represent the evolutionarily optimal degree of processivity. As in life, there is a time to hold on and a time to let go.
References
- Allan V., Schroer T. Membrane motors. Curr. Opin. Cell Biol. 1999;11:476–482. doi: 10.1016/s0955-0674(99)80068-4. [DOI] [PubMed] [Google Scholar]
- Atsumian R.D., Bier M. Mechanochemical coupling of the motion of molecular motors to ATP hydrolysis. Biophys. J. 1996;70:637–653. doi: 10.1016/S0006-3495(96)79605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S.P., Moyer M.L., Johnson K.A. Alternating site mechanism of the kinesin ATPase. Biochemistry. 1998;37:792–799. doi: 10.1021/bi971117b. [DOI] [PubMed] [Google Scholar]
- Hackney D.D. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc. Natl. Acad. Sci. USA. 1994;91:6865–6869. doi: 10.1073/pnas.91.15.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D.D. Highly processive microtubule-stimulated ATP hydrolysis by dimeric kinesin head domains. Nature. 1995;377:448–450. doi: 10.1038/377448a0. [DOI] [PubMed] [Google Scholar]
- Hancock W.O., Howard J. Processivity of the motor protein kinesin requires two heads. J. Cell Biol. 1998;140:1395–1405. doi: 10.1083/jcb.140.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Lieue J., Alonso M., Cross R.A., Amos L.A. Congruent docking of dimeric kinesin and ncd into three dimensional electron cryomicroscopy images of microtubule motor ADP complexes. Mol. Biol. Cell. 1999;10:2063–2074. doi: 10.1091/mbc.10.6.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenger A., Sack S., Thormahlen M., Marx A., Muller J., Gross H., Mandelkow E. Image reconstructions of microtubules decorated with monomeric and dimeric kinesinscomparison with X-ray structure and implications for motility. J. Cell Biol. 1998;141:419–430. doi: 10.1083/jcb.141.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenger A., Thormahlen M., Diaz-Avalos R., Doerhoefer M., Goldie K.N., Muller J., Mandelkow E. A new look at the microtubule binding patterns of dimeric kinesins. J. Mol. Biol. 2000;297:1087–1103. doi: 10.1006/jmbi.2000.3627. [DOI] [PubMed] [Google Scholar]
- Kikkawa M., Okada Y., Hirokawa N. 15 Å resolution model of the monomeric kinesin motor, KIF1A. Cell. 2000;100:241–252. doi: 10.1016/s0092-8674(00)81562-7. [DOI] [PubMed] [Google Scholar]
- Kozielski F., Sack S., Marx A., Thormahlen M., Schonbrunn E., Biou V., Thompson A., Mandelkow E.-M., Mandelkow E. The crystal structure of dimeric kinesin and implications for microtubule-dependent motility. Cell. 1997;91:985–994. doi: 10.1016/s0092-8674(00)80489-4. [DOI] [PubMed] [Google Scholar]
- Ma Y.-Z., Taylor E.W. Interacting head mechanism of microtubule–kinesin ATPase. J. Biol. Chem. 1997;272:724–730. doi: 10.1074/jbc.272.2.724. [DOI] [PubMed] [Google Scholar]
- Okada Y., Hirokawa N. A processive single-headed motorkinesin superfamily protein KIF1A. Science. 1999;283:1152–1157. doi: 10.1126/science.283.5405.1152. [DOI] [PubMed] [Google Scholar]
- Okada Y., Hirokawa N. Mechanism of the single-headed processivitydiffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proc. Natl. Acad. Sci. USA. 2000;97:640–654. doi: 10.1073/pnas.97.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S., Lin A.W., Safer D., Hart C.L., Naber N., Carragher B.O., Cain S.M., Pechatnikova E., Wilson-Kubalek E.M., Whittaker M. A structural change in the kinesin motor protein that drives motility. Nature. 1999;402:778–784. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- Rodionov V.I., Hope A.J., Svitkina T.M, Borisy G.G. Functional coordination of microtubule-based and actin-based motors in melanocytes. Curr. Biol. 1998;8:165–168. doi: 10.1016/s0960-9822(98)70064-8. [DOI] [PubMed] [Google Scholar]
- Rogers S.L., Gelfand V.I. Myosin cooperation with microtubule based motors during organelle transport in melanocytes. Curr. Biol. 1998;1998. 8:161–164. doi: 10.1016/s0960-9822(98)70063-6. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Block S.M. Force and velocity measured for single kinesin molecules. Cell. 1994;77:773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Thorn K.S., Ubersax J.A., Vale R.D. Engineering the processive run length of the kinesin motor. J. Cell Biol. 2000;151:1093–1100. doi: 10.1083/jcb.151.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomishige M., Vale R.D. Controlling kinesin by reversible disulfide cross-linkingidentifying the motility-producing conformational change. J. Cell Biol. 2000;151:1081–1092. doi: 10.1083/jcb.151.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet B., Vale R.D., Hodges R.S. Demonstration of coiled-coil interactions within the kinesin neck region using synthetic peptidesimplications for motor activity. J. Biol. Chem. 1997;272:8946–8956. doi: 10.1074/jbc.272.14.8946. [DOI] [PubMed] [Google Scholar]
- Wang Z., Sheetz M.P. The C-terminus of tubulin increases cytoplasmic dynein and kinesin processivity. Biophys. J. 2000;78:1955–1964. doi: 10.1016/S0006-3495(00)76743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]