Abstract
Surgeries involving transplantation of fetal dopamine (DA) neurons into the caudate-putamen of patients with Parkinson's disease (PD) have been performed in various clinical trials to examine a potential restoration of motor function. The absence of studies in nonhuman primates to define the best transplantation protocols have lead to the use of a broad variety of techniques that potentially could have a major impact on the clinical outcome. The effects of using different cell and tissue preparation, and surgical targets, remain unknown. For this purpose, 20 St. Kitts African Green Monkeys (AFG) rendered parkinsonian by i.m injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), were balanced into 4 groups and unilaterally grafted in the (a) caudate or (b) putamen with fetal ventral mesencephalic (VM) tissue as (c) solid pieces or as a (d) cell suspension. By 9 months post-transplantation all animals showed significant and similar behavioral improvement as determined by an UPDRS based PD scale. Postmortem analyses showed that VM transplants survived in all animals. They were located in both surgical target sites, producing a broad DA reinnervation of the targeted nuclei that could also extend to the non-grafted nucleus on the ipsilateral side. Although no differences between groups were found in survival of DA neurons or degree of DA reinnervation, there was a significant correlation between striatal reinnervation and behavioral recovery only in animals transplanted in the putamen surgical target. Additionally, there was in general, a stronger glial reaction to solid grafts than to cell suspensions. These studies provide data for the optimal time-course, cell preparation and surgical targets for systematic examinations of both potential benefits and side effects of dopamine neuron cell transplantation in primate models of PD.
INTRODUCTION
Transplantation of fetal mesencephalic cells to the striatum of patients with PD was explored in a series of open labeled clinical studies performed in the 90s. (Freed et al., 1992; Freed et al., 2001; Freeman et al., 1995; Hagell et al., 1999; Levivier et al., 1997; Lindvall et al., 1990; Lindvall et al., 1992; Lopez-Lozano et al., 1997b; Mendez et al., 2002; Mendez et al., 2005; Molina et al., 1994; Olanow et al., 2003; Peschanski et al., 1994; Piccini et al., 1999; Piccini et al., 2000; Spencer et al., 1992). In many cases patients showed significant clinical improvements and in several cases, they were able to eliminate their dopamine medication altogether (Lindvall et al., 1994). Follow up of some of these patients provided evidence of graft survival up to 11 years post transplantation (Piccini et al., 1999; Piccini et al., 2000). On the other hand, 2 placebo controlled studies performed in the last years failed to find statistical evidence of efficacy and noted several mild to severe side effects caused by the implantation of fetal DA cells (Freed et al., 2001; Olanow et al., 2003). The most troubling side effect was the development of “off medication” dyskinesias in some patients (Hagell et al., 2002; Ma et al., 2002; Olanow et al., 2003). Limited understanding of cell transplantation parameters and patient selection might explain why some transplants work, while others do not. It has been shown that in spite of good graft viability, there is sub-optimal reinnervation of the host striatum and sub-optimal cell composition of the surviving grafts. In addition, it has been never systematically tested, especially in nonhuman primate models, whether grafts placed in the putamen or caudate nucleus would be more effective or whether small pieces of tissue implanted are as effective as dissociated cells.
Both double-blinded clinical trials (Freed et al., 2001; Olanow et al., 2003) employed grafts composed of solid tissue pieces. In spite of excellent graft viability, post-mortem data from these clinical trials have indicated the presence of immune markers including T-cell and B-cell markers. Furthermore, graft efficacy was lost in one of the clinical trials following the cessation of cyclosporine (Olanow et al., 2003). This suggests that a slow immune process might be ongoing that deleteriously affects graft function and potentially be responsible for the lack of efficacy seen following fetal implants. Preclinical studies in rodents demonstrate that solid grafts augment the expression of immune markers relative to suspension grafts (Lindsay and Raisman, 1984). Thus in this study we have compared the expression of specific immune markers in parkinsonian monkeys receiving implants of solid tissue versus suspension grafts.
The optimal graft location within the striatum has also never been empirically determined in a controlled study in primates. There is a preponderance of thought that the post-commissural putamen is the best target for transplantation based upon the pattern of A9 nigrostriatal degeneration that preferentially effects this striatal region (Bernheimer et al., 1973; Kish et al., 1988; Leenders et al., 1990; Nyberg et al., 1983). The connectivity of the post-commissural putamen with the motor cortex, while the caudate nucleus connects with associative cortices has also supported the preference for this nucleus. However, multiple lines of evidence support the concept that dopaminergic reinnervation of the caudate nucleus may be important for the functional recovery. First, single cell recordings have shown neural activation prior to self initiating movements from both caudate and putamen of non human primates, implicating both nuclei in motor control (Schultz and Romo, 1992). Secondly, primate studies, as well as studies in PD patients have provided direct evidences that transplantation in caudate alone improve symptoms of PD (Lopez-Lozano et al., 1997a; Taylor et al., 1991). Lastly, all double blinded trials employing trophic factor delivery or fetal grafting have exclusively targeted the post-commissural putamen and all have failed to reach their primary endpoint, although alternative explanations for these failures are plentiful. While theoretical arguments exist for the choice of graft-type and or localization, they have not been compared empirically in the primate brain, which provides the best available animal model of PD. The present study was carried out to answer two important questions: (1) What are the effects of tissue composition (solid vs. suspension) upon graft efficacy and transplant survival; and (2) Whether implantation into the caudate or the putamen will produce better efficacy.
METHODS
Experimental design
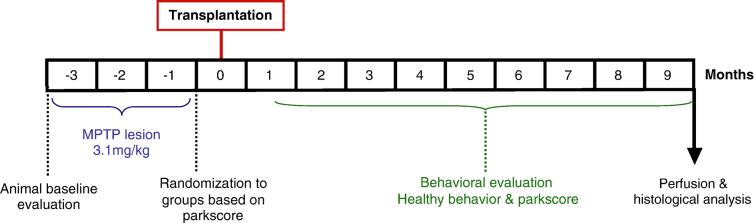
Twenty AFG monkeys, rendered parkinsonian by repetitive injections of MPTP (see below), were randomized into 4 groups by the severity of their parkinsonism and presence of normal activity of daily living behaviors. Employing a 2 × 2 design, they received fetal tissue as cell suspensions (VMsusp) or solid pieces (VMsolid), in the caudate (Cd) of putamen (Put) nucleus. Tissue pieces or cell suspensions from 1.5 embryos were grafted unilaterally to each host (Table 1A). The embryonic age of grafted fetal tissue was also randomized within the 4 treatment groups (Table 1A). After 9 months of behavioral observation, animals were sacrificed and the number of TH-ir neurons, striatal DA reinnervation, and host immune response to the graft, were analyzed by immunohistochemistry (Table 1B).
Table 1.
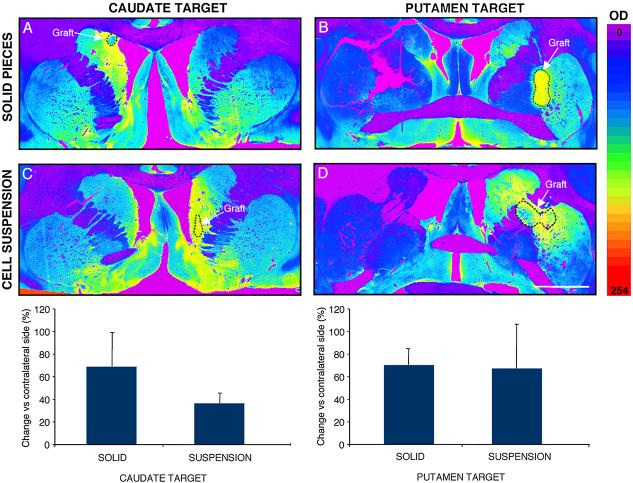
A. Characteristics of donors and host in the different transplantation groups. Host severity was determined by a parkinsonism rating scale (parkscore; see Methods. For this comparison, mild (parkscore 0-15)=1, moderate (parkscore 15-50)=2 or severe (parkscore >50)=3). Values of CRL correspond to the direct measure after hysterotomy. Estimation of embryonic day was calculated with conversion charts generated at SKBRF. There was no significant difference among the groups in host severity, donor age, or amount of grafted tissue (measured both in number of VMs grafted per host and in number of cells in the suspension groups. See methods). B. Measurements of the change in TH-OD in target location (Cd or Put), vs contralateral non-grafted side, showed no significant differences between the groups. Measurements of TH-OD in the ipsilateral and contralateral striatum (both Cd and Put) showed reinnervation of both striatal nuclei within the grafted side in all transplantation groups. Stereological estimations of TH-ir surviving cells within the grafts did not show significant differences between groups.
| Table 1A. Group characteristics (n=5 per group) | ||||
|---|---|---|---|---|
| Surgical target | Graft type | Parkinsonism scale (1-4) | Donor age (days) | Grafted embryos/case |
| CAUDATE | SOLID | 1.8±0.37 | 42.6±0.17 | 1.5 |
| SUSPENSION | 1.4±0.24 | 44.58±0.51 | 1.51±0.1 | |
| PUTAMEN | SOLID | 2±0.44 | 45.3±1.5 | 1.5 |
| SUSPENSION | 2±0.36 | 44.77±0.46 | 1.48±0.1 | |
| Table 1B. Graft characteristics (n=5 per group) | ||||
| Surgical Target | Graft type | TH fiber density in target location (% above contralat) |
TH fiber density in Cd & Put (% above contralat) |
Surviving TH-ir cells (×103) |
| CAUDATE | SOLID | 51±19.2 | 34.4±16.9 | 42,37±13.78 |
| SUSPENSION | 26±5.6 | 12.7±13.2 | 37,53±12.69 | |
| PUTAMEN | SOLID | 59±14 | 43±16.1 | 33,21±10.94 |
| SUSPENSION | 49±25 | 47.7±0.8 | 63,10±19.81 | |
Subject assignment and MPTP Treatment
Mature adult male AFG (Chlorocebus sabaeus, St. Kitts) monkeys were used for these experiments. All monkeys were housed at the St. Kitts Biomedical Research Foundation facility. Monkeys were singly housed with natural daylight light/dark cycle at 17 degrees North Latitude. The animals received food and water ad lib or were supplemented with special feeding, if needed, during the course of the study. The study was performed in accordance with U.S. federal guidelines and with the approval of the Institutional Animal Care and Use Committees. The monkeys were injected intramuscularly with 5 doses of MPTP HCl (RBI, Natick, MA) given over a 5-day period (total dose 2.25 mg/kg), followed by additional doses over several weeks up to 3.1 mg/kg, until they demonstrated measurable and stable signs of parkinsonism as described previously (Elsworth et al., 2000; Taylor JR, 1994; Taylor et al., 1997). MPTP was handled using published guidelines (Przedborski et al., 2001).
Behavioral observations
A primate behavior and parkinsonism rating scale was used to assess the behavioral and functional status of the monkeys under normal, and MPTP treatment, and MPTP treatment + transplant conditions according to a validated quantitative scoring system developed by Redmond and coworkers (Taylor JR, 1994; Taylor et al., 1997). Occurrence of dyskinesias, psychological disturbances and vomiting were also recorded. Trained blinded observers ‘scored’ and ‘rated’ the behavior and motor movements of each monkey individually during 2 daily observation periods performed 5 days a week. From these quantitative time-sampled and rated assessments of 29 behaviors, a parkinsonian summary score was derived, based on a previous principal component factor analysis of 55,000 observations of 80 monkeys with varying signs of parkinsonism or normal daily behaviors. The parkinsonian summary score (parkscore) contains different behaviors scored from 0 to 5, with 0 being “normal’ and 5 being severely parkinsonian (Taylor JR, 1994; Taylor et al., 1997). Subjects were classified by severity, based on their average parkinsonian score after MPTP treatment, in mild (0-15) moderate (15 to 50) and severe (>50).
The parkinsonian summary score (parkscore) was used to classify subjects by severity, based on their average parkinsonian score after treatment. The parkscore is highly responsive to pharmacological changes in DA function and correlates highly with post-mortem striatal DA concentrations (Elsworth et al., 2000; Taylor JR, 1994; Taylor et al., 1991). Normal daily primate behaviors from these ratings were also analyzed as a sum factor “healthy behavior” categorized from prior factor analysis (Taylor JR, 1994). This sum score includes usual behaviors engaged in by normal healthy monkeys (scale 0-60) and is similar to the “activities of daily living scale” of the UPDRS used by clinical neurologists. A standardized video protocol was also used to assess and record the function of the monkeys in some experiments to provide a permanent record for later review. Observers did reliability training prior to testing to achieve a coefficient of concordance (Kendall's) greater than 0.95 in their ratings of all behaviors before beginning actual data collection. Inter-rater reliability was also evaluated at least once a week and any differences in rating were discussed regularly by the raters to eliminate observer “drift” or idiosyncrasy. Finally, rater differences were analyzed and controlled for during the statistical analysis of the data. For data analysis, pre-operative days were averaged into one-month treatment blocks designated MO1 and MO2. Similarly, months post-transplantation were averaged into blocks of 1 month of duration and were designated T1 to T10. In addition, all observational days were analyzed using a standard regression analysis during baseline, after MPTP, and after grafting until the end of the study. Animals were divided according to their parkscore in severe, moderate, mild or very mild, based on segregation into quintiles of a large group of animals treated with MPTP (Taylor et al., 1997).
Treatment Group Assignment
The monkeys were assigned to four transplantation groups after MPTP treatment based upon their parkscore. Ten monkeys were assigned to receive cell suspension grafts and 10 to receive small minced pieces of tissue. Half of each of these groups was assigned to receive grafts into the Put and the other half into the Cd, leading to a total of 10 monkeys grafted into each target. Previous data from rodent or primate experiments provided the basis for estimating the required number of monkeys in each experimental group. The Type 1 error (the false positive rate) was specified to be a 2-sided 0.05, and the power to detect differences was set at 80% with a ‘large’ standardized effect size as described by (Cohen, 1988). Because of multiple comparisons, the 2-group sample size was increased by 20%, which supported an estimate of 5 monkeys per group or a total of 20 monkeys to be transplanted in four groups. The monkeys were assigned to the treatment groups in a stratified random fashion to balance their parkscores in each group. The comparisons were designed to maximize the numbers in the treatment groups by comparing them also with prior long-term data on sham transplanted or un-transplanted MPTP-treated monkeys studied with identical conditions and methods (Taylor et al., 1995).
Breeding
Breeding-age females were exposed to males in a timed manner to produce fetuses of the desired fetal age for each transplant procedure. The age of the embryos was assessed by ultrasound, measuring the crown-rump length (CRL) and, in some cases, also the bi-parietal diameter, and confirmed by direct measurement after hysterotomy (Table 1A). The embryonic age was estimated based on unpublished data from almost 1000 pregnancies studied at the St. Kitts Biomedical Research Foundation.
VM dissection
The medial aspect of the VM was dissected using a dissecting microscope (Costantini et al., 1997; Haque et al., 1997). Briefly, the developing membranes and the duramater were carefully peeled off and removed entirely, so that the forebrain and midbrain were exposed. Both structures were then isolated by a horizontal section caudal to the mesencephalon. The remaining meninges, that at this embryonic stage already contain a rich capillary plexus, were then removed. To obtain all of the central midbrain dopaminergic neurons, the ventral mesencephalon was dissected by two horizontal cuts in the caudal and ventral limits of the mesencephalic flexure and two vertical cuts at both sides of the midline (resulting in a piece approximately 1/3 the size of the total mesencephalon; VM). This VM piece was then cut first through the midline, and then perpendicularly to the rostro-caudal axis to obtain 4 VM pieces, with approximately the same amount of dopaminergic neurons.
The mean CRL of the embryos used in the study was 16 mm. For the AFG, this corresponds to 44-45 days of pregnancy (Table 1A). Cells suspensions (See cell suspension graft method below) from 3 embryos were prepared each transplantation day (1.5 embryos grafted per host), and suspended in approximately 100μl (108 ± 9.8μl) in a final cell concentration of 24,296 ± 3,426 cells/μl. The mean cell viability pre-transplantation was 94.6±1.9% (post-transplantation 93.6 ± 3.05%). For these VMsusp grafts the total number of grafted cells was 1,01×106 ± 0.13×106 in the Cd location (corresponding to 1.51+/−0.1 embryos) and 1,02 106 ± 0.15 in the Put location (corresponding to 1.48+/−0.1 embryos). For the VMsolid grafts, VM tissue from 1.5 embryos was grafted per host (Table 1A).
Cell suspension grafts
The dissected VM pieces from 3 embryos were collected in ice cold Hanks' Balanced Salt Solution with 20mM d-glucose (HBSS-glucose). Pieces were incubated in HBSS-glucose containing 0.2% trypsin (Sigma, St Louis, MO), at 37°C, for 30 minutes. Trypsin enzymatic activity was stopped by washing 5 times in an HBSS-glucose solution containing 0.2% DNAse (Sigma, St Louis, MO). Tissue was then passed though a series of fire polished Pasteur pipettes of decreasing diameter until a single cell suspension was generated. Finally, cells were spun down at 900 rpm for 7 min, using an ultracentrifuge Marathon-21000R (Fisher, Needham, MA) re-suspended in DNAse solution, and kept on ice. Cell counts and viability assessments were made using trypan blue by exclusion method. After 2-3 hrs, the suspension was loaded in a 22-gauge Hamilton syringe that was placed into an automatic injector (Stoelting 310, Wood Dale, Illinois) mounted on a stereotactic frame (Kopf instruments®, Tujunga, California). In the Cd group, the suspension was divided into 2 tracts (18-24μl/tract) of 3mm depth (coordinates from earbar zero (mm); AP 18 and 19, Lat 4.0, DV 19.0). In the Put group the suspension was grafted in 3 tracts (12-16μl/tract) of 6mm depth (coordinates from earbar zero (mm); AP 18, 19 and 21.1, Lat 10.0, and DV 19.0). For each host animal, in either the Cd and Put groups, 1.5 VM equivalent cell suspension was grafted. Consequently, each Put tract contained suspension from 1/2 VM, whereas each Cd tract contained suspension from 3/4 of VM. The syringes were loaded right before grafting and flow was checked carefully. The suspensions were then injected using an automatic injector at a rate of 1μl/min, and the needle was kept 3 additional minutes before being retracted. After the completion of the surgery, cell viability was determined using trypan blue by exclusion method.
Solid grafts
The VM pieces obtained from the dissections (4 pieces per VM. See VM dissection method, above) were loaded in a 19-gauge Hamilton syringe with 20μl of 0.2% DNAse solution as vehicle. For each animal transplanted into the Cd, a total of 1.5 VMs were grafted (as for the VMsusp group), distributed in 2 tracts with 3 VM pieces per tract (3/4 of a VM). Similarly, for each Put location 1.5 VMs were grafted, distributed in this case in 3 tracts with 2 VM pieces per tract (1/2 VM). The placement and distribution of the grafts was done as described for the VMsusp. After each graft placement the needle tip was inspected under the dissecting microscope to make sure all VM pieces were successfully grafted.
Post-mortem analyses
Immunohistochemistry
Once the behavioral evaluation was completed, they were perfused through the left ventricle with cold heparinized saline followed by cold 4% paraformaldehyde. The brains were removed from the calvaria, post-fixed in 4% paraformaldehyde, and cryoprotected in increasing gradient of sucrose solutions. Brains were cut in 40 μm thick sections using a freezing microtome. Immunohistochemistry was performed on series of sections randomly selected that represented 1/16th of the total brain per primary antibody. Sections were treated for 10 minutes in 3% hydrogen peroxide (Humco, Texarkana, TX), washed 5 times in PBS, and incubated in 10% normal goat serum (NGS) and 0.1 % Triton X-100 for 1 hour prior to overnight incubation at room temperature with the primary antibody diluted in 10% NGS. The primary antibodies utilized were rabbit anti-tyrosine hydroxylase (TH) (Pel Freez, Rogers, AK; 1:300), mouse CD-68 (Dako A/S, Denmark; 1:250), rabbit anti-glial fibrillary acidic protein (GFAP) (Dako A/S, Denmark; 1:500). After a 3×10 minute wash in PBS, the sections were incubated in biotinilated goat anti-mouse/rabbit secondary antibody (Vector Laboratories, Burlingame, CA; 1:300) diluted in 5% NGS in PBS at room temperature for 1 hour. The sections were washed three times in PBS and incubated in streptavidin-biotin complex (Vectastain ABC Kit Elite, Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Following thorough wash with PBS, staining was visualized by incubation in 3, 3'-diaminobenzidine solution, sometimes with nickel enhancement (Vector Laboratories, Burlingame, CA). Controls with omission of the primary antibody were performed on selected sections that verified the specificity of staining. After immunostaining, floating tissue sections were mounted on glass slides, counterstained with cresyl violet (for CD68 staining), and finally coversliped with DPX (VWR, England). For immunofluorescence, sections were rinsed for 3×10 minutes in PBS, incubated in 10% normal donkey serum (Lindsay and Raisman, 1984) (Vector Laboratories, Burlingame, CA) with 0.1% Triton X-100 in PBS for 60 minutes and then incubated overnight at room temperature in primary antibody (sheep anti-TH Pel Freez, Rogers, AK, 1:300; rabbit anti-Girk2 Alomone Laboratories, Jerusalem, Israel, 1:80; mouse anti-Calb Swant, Bellinzona, Switzerland, 1:2000). After incubation sections were washed 3×10 minutes in PBS and incubated in fluorescent dye conjugated secondary antibodies (Alexa Fluor donkey anti-sheep/rabbit/mouse of 488/568/660 wavelengths respectively; Molecular Probes, Eugene, OR, 1:500) in PBS for 60 minutes at room temperature. After washing 3×10 minutes in PBS, sections were mounted onto superfrost plus slides and coverslipped in Gel/Mount (Biømeda Corporation, Foster City, CA). Sections were examined using a confocal microscope (LSM510 META, Carl Zeiss, Thornwood, NY).
Stereology
Stereological counts of grafted TH immunoreactive (TH-ir) nigral cells were be performed using an unbiased, stereological cell counting method as described in detail elsewhere (Cohen, 1988). The optical dissector system consisted of a computer assisted image analysis system including an Olympus BX-60 microscope hard-coupled to a Ludl computer-controlled x-y-z motorized stage, a high-sensitivity Hitachi 3CCD video camera system (Hitachi, Japan), and PC computer. Neuronal counts were performed using Microbrightfield stereological software. The instrumentation was calibrated before each series of measurements. The graft was outlined under a low magnification (1.25X), and 5% of the outlined region was measured with a systematic random design of dissector counting frames. The total number of surviving TH-ir and neurons was estimated employing a 60X planapo oil immersion objective with a 1.4 numerical aperture. Using the dissector principle, at least 200 TH-ir grafted neurons were sampled by optical scanning using uniform, systematic and random design procedures for all measurements. Four equally spaced sections were sampled from each subject. The absolute thickness of each section was determined empirically. The total number of TH-ir neurons (N) in the entire graft was calculated using the following formula N = NV × VSN. Here, NV is the numerical density and VSN is the volume of the graft. The variability within groups was assessed via the Coefficient of error (CE).
Analysis of DA innervation
The extent of dopaminergic innervation in the grafted striatum was quantified measuring the optical density of TH-ir fibers (TH-OD) in the Cd and in the Put using the non-grafted side as a control for each animal. Subtraction was made between TH-OD from grafted and non-grafted sides, and the difference was used in a statistical correlation with functional improvement (see above). TH-OD measurement has been shown to be a good method to measure striatal dopaminergic innervation (Burke et al., 1990). For the Put, the dorsal half of the nucleus was considered, as all the grafts were located in that area, and to exclude the ventromedial parts, less affected by the MPTP lesion. For each animal, photomicrographs from a whole coronal section were taken from 2 representative sections separated 480μm, using a Zeiss Axioscope 2 plus microscope, and the Microbrightfield Stereo Investigator and Virtual slice software (MicroBrightField, Williston, VT). All images were taken in the same session to ensure that the background levels and all the illumination parameters were identical for all the animals. OD measurements were performed using NIH image 1.61 software.
Analysis of glial response
The astroglial response around the grafts was measured in 3 representative sections per animal, each separated by 480 μm. Grafts were outlined with a 4X planapo objective (0.25 numerical aperture), using an integrated Axioskop 2 microscope (Carl Zeiss, Thornwood, NY) with an Optronics camera interfacing with a computer operating Stereo Investigator 6.02.2 software (MicroBrightField, Williston, VT). Graft borders were determined by using anatomical landmarks from an adjacent section stained with TH, as well as by the presence of a robust astroglial response with thick GFAP immunoreactive (GFAP-ir) processes, present within all the grafts. The size of the astrocyte halo was then measured at 3 different angles (0, 90 and 180°), using a 20X planapo objective (0.75 numerical aperture). The microglial reaction around the grafts was quantified using an unbiased, stereological cell counting method (West et al., 1991). Three representative sections in which the graft was on target were analyzed per case. Nissl stained cells containing CD68 immunoreactive (CD68-ir) inclusions were also counted using a 63X planapo oil immersion objective (1.25 numerical aperture), and a computer assisted image analysis system. Caudate or Putamen, were outlined at low magnification (2.5X), and the total number of Nissl stained-CD68-ir cells were estimated using the optical fractionator probe. The number of counting sites and other settings were experimentally determined in order to get a Gunderson coefficient of variance less than 0.1 for each case (West et al., 1991).
Statistical analysis
The significance of the behavioral changes between treatment groups was analyzed by a multifactor analysis of variance, with post hoc tests using Student-Newman-Keuls. The null hypothesis was rejected when p<0.05. Analysis of linear regressions and the significance of slopes were determined for all monkeys together and each monkey individually, as well as correlated t tests of changes between the last month before to the last month after transplantation and before sacrifice. The analyses of change over time was repeated in the most severe animals (park-score >50, which corresponds to Hoehn-Yahr V) since these animals were previously shown to remain parkinsonian for long periods of time, and are most similar to severe end-stage parkinsonian patients. Unpaired t tests and the Mann-Whitney U-test were used in the analysis of the changes in striatal dopaminergic innervation, TH-ir cells in the graft, and glial reaction measurements. Correlations were done using both parametric and non-parametric methods (SAS analysis system, Glastonbury, CT).
RESULTS
All 20 monkeys analyzed as a group showed a stable parkinsonism after MPTP administration, with a mean parkscore for the group of 27.8+/−7.1. Based upon the parkscores, monkeys were matched into 4 transplantation groups (n=5 in each group) balanced for the severity of the parkinsonism (See Methods and Table 1A) that received either VMsusp or VMsolid in the Cd or Put locations. Groups were also balanced for the age of the donor tissue used and for the side of implantation of the host (both VMsusp vs. VMsolid, and Cd vs. Put) (See Methods and Table 1A).
Behavioral evaluations after transplantation showed an improvement in parkinsonism in the 20 animals that began at 6 months and became statistically significant by 9 months post transplantation (multifactor analysis of variance and post hoc Student-Newman-Keuls test at p<0.05; Fig. 2A). Post-hoc analysis by level of parkscore showed a marked improvement in the severe animals, which correspond to Hoehn-Yahr Stage 5 PD patients. Progressively smaller improvements were seen in moderate, mild and very mild animals (p<0.05 for moderate animals. Fig. 2B).
Figure 2.
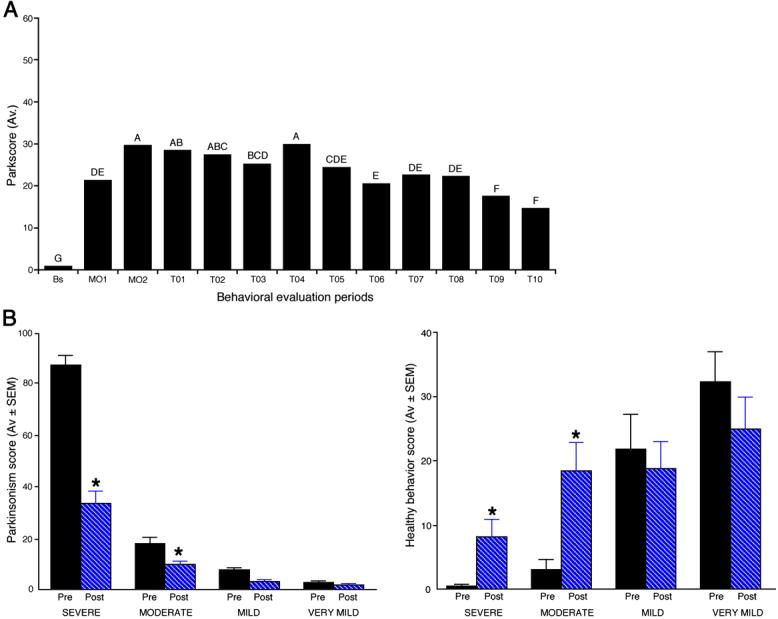
A. Parkinsonism was evaluated several times each week. The mean scores during each month from baseline, two months after MPTP treatment, and each of 10 months after transplantation until sacrifice are shown. An analysis of variance, including all 20 monkeys, showed that there were significant improvements in the final 2 months, compared with earlier periods after transplantation, although the parkscores did not return fully to baseline by that time (effect of treatment month [F=56.45, df=12, 2709, p=0.0001]). Significant differences (p<0.05) from a Newman Keuls post hoc analysis are indicated by letters: Months with the same letter are not different from each other. B. To illustrate the effect of parkinsonism severity, the mean parkscore and mean of normal daily activities included in “healthy behavior” during the last month before transplantation (M02) were compared with the mean scores during the last month of the study (T10). For this analysis animals with a parkscore <5 (“very mild”) were analyzed separately. Analysis by severity groups showed a dramatic improvement of parkscore in severe animals, and a quantitatively smaller improvement in moderate, mild and very mild animals, although the percentage improvements were very similar at 40 to 60% in all four groups. Similarly, “healthy behavior” improved significantly in severe and moderate animals, but not in the mild and very mild animals, which had more normal healthy behavior scores before transplantation.
Experimental group analysis to determine the best transplantation target, showed a significant interaction between “target” (Cd vs Put) and month after treatment (F=18.46, df=12,2626, p=0.0001). In the last month before transplantation and in the last month before sacrifice, tests of main effects showed no differences between the 2 transplantation locations (Cd and Put) (F=3.25, df=1,18, p=0.0882 before transplantation and F=0.18, df=1,18, p=0.6732 before sacrifice). Similarly, analysis of the effects of VMsolid vs VMsusp showed no overall difference in behavioral effects (F=0.13, df=1,18, p=0.7185), although there was a significant interaction between behavioral improvement in both groups (VMsolid and VMsusp) and time after transplantation (F=2.68, df=12,2709, p=0.0014).
The mean “activity of daily living” score (see Methods) for baseline monkeys before treatment in this study was, on average, 28.6, which was reduced to 12.4 one month after MPTP treatment. The number of normal daily behaviors improved, but did not return to normal by the end of the study, consistent with prior studies in the MPTP treated AFG (Taylor et al., 1991). However, when the most severe animals were evaluated separately, the behaviors recorded in such animals improved in an almost linear fashion from the mean low point of 0.091 in the first month after MPTP to a mean of 8.015 at 9 months post-transplantation (Fig. 3). The individual items included in this evaluation showed significant changes at high levels of statistical significance including “food response,” from 1.6 to 1.3 (mean value at last month before transplant vs last month of study), “delayed initiation of movement,” from 1.58 to 1.15, “poverty of movement,” from 1.73 to 1.27, and “resting tremor,” from 3.5 to 1.97. There was no left/right difference in any of these behaviors (p<0.05). The ratings for dyskinesia in these animals were consistently zero as expected for MPTP treated monkeys not exposed chronically to L-Dopa.
Figure 3.
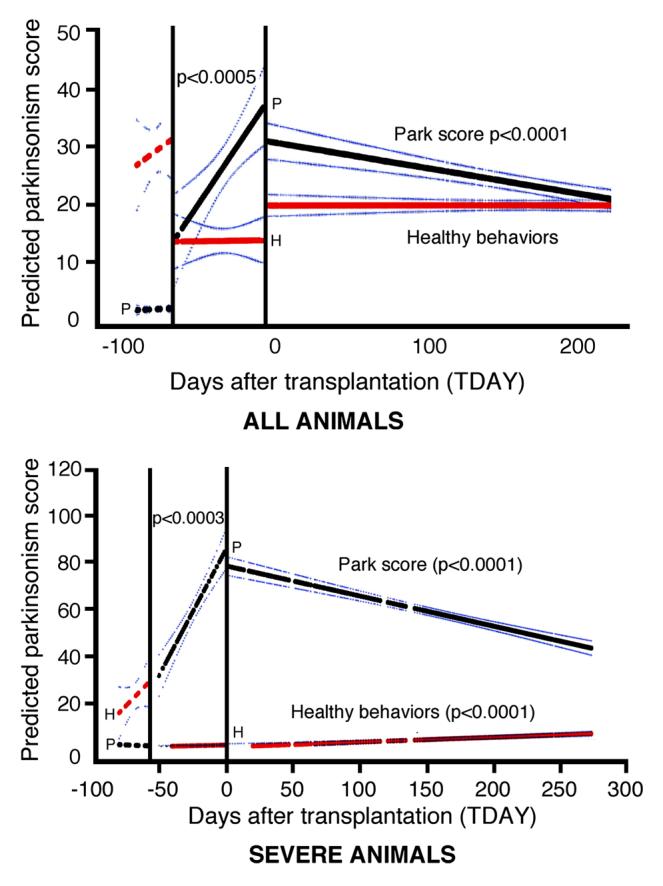
A regression analysis on the “park score” (black line) and the “healthy behavior” (red line) summary factors were calculated and the predicted slopes plotted for the baseline (Transplant Day ‘TDAY’) before −60, after MPTP (TDAY 0 to −60) and after grafting (0 to 270) were plotted for “all animals” and for the subset of the most “severe animals” with the 95% confidence interval of the predicted slope. Baselines did not show significant slopes All monkeys analyzed together, showed a positive slope in “park score” prior to grafting (slope +0.407, p<0.0005), and a significant improvement for the period after grafting (slope −0.046, p<0.0001). In the total group, the “healthy behaviors” showed no significant differences in the slope before grafting or after, whereas the severe animals (which had a lower pre-grafting level of “healthy behaviors”) showed a significant increase in these behaviors after grafting (slope +0.0235, p<0.0001). Slopes and significance levels were determined by linear regressions (GLM, Statistical Analysis System).
Postmortem analyses
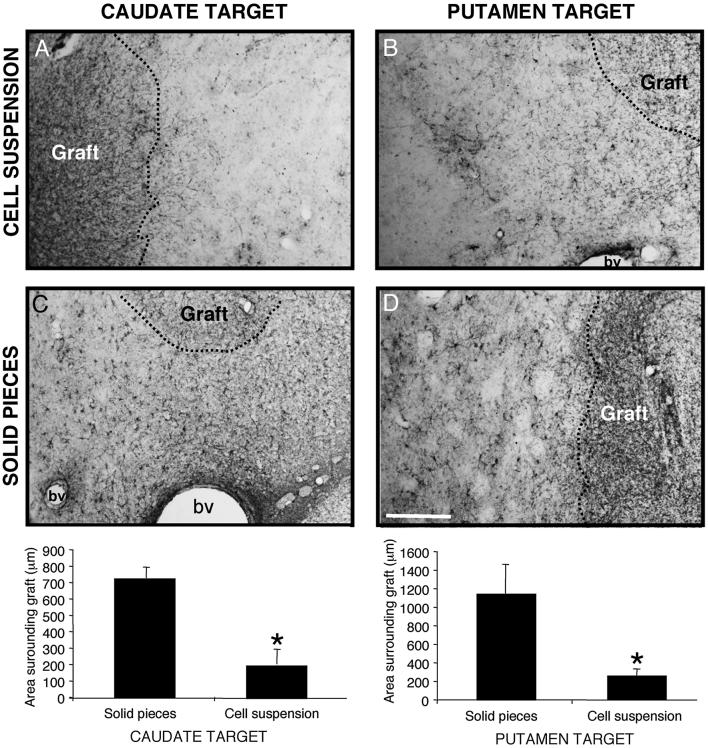
Histological evaluation showed that all 20 animals had surviving grafts 9 months after transplantation. All grafts were located in their target nuclei (Cd or Put), and, in some cases, close or partially in the neighboring internal capsule. Two solid grafts were partly located in the lateral ventricle. Individual grafts provided differing degrees of innervation to the targeted nuclei and, in many cases to both ipsilateral striatal nuclei (Fig. 5, and Table 1B). VMsusp grafts showed the typical organization, with aggregates of TH-ir cells in the periphery, and a reticular area with smaller TH-ir cells, in the centre of the graft. In VMsusp TH-ir cells almost completely aggregated in the periphery, leaving areas in the center of the graft without TH-ir cells (specially in VMsolid grafts located in the Cd) (Fig. 4). Analysis of the dopaminergic cell phenotypes showed both TH/Calb A10-like, and TH+/Girk-2+ A9-like cells in each graft type. In the suspension grafts, TH+/Girk-2+ cells were preferentially located in the periphery, whereas TH+/Calb+ cells were located in the center of the graft (data not shown), as it has been reported in human postmortem data (Mendez et al., 2005). Both dopaminergic cell types were found in the VMsolid, but without a well-defined cytoarchitechtonic organization (data not shown).
Figure 5.
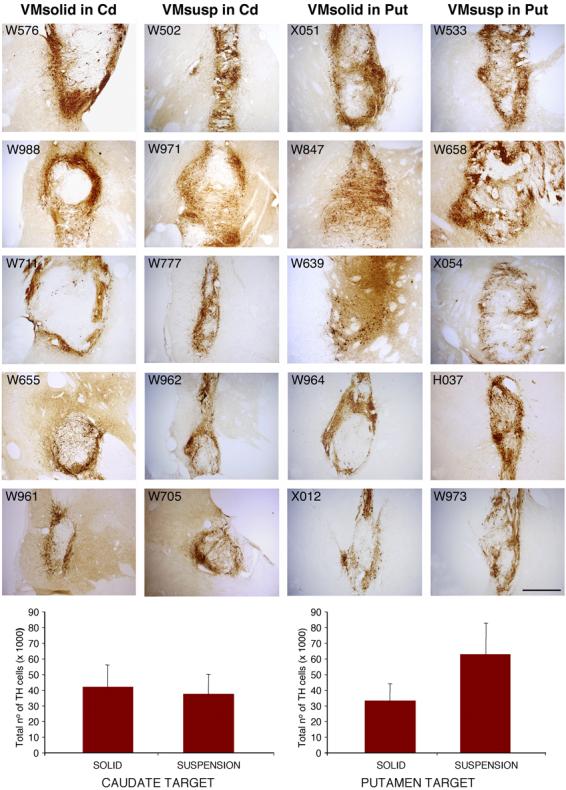
Representative low power photomicrographs of TH immunostained coronal sections from an animal per group. Sections were color-coded and optical density of TH innervation was quantified (scale 0-254), using NIH image 1.61 software. In all the transplantation groups, VM grafts provided a significant increase of dopaminergic innervation in the Cd or Put target, and also in the other striatal nucleus (Put or Cd respectively), compared with the non-grafted contralateral striatum (Table 1B). There were no significant differences between transplantation groups. Scale bar 5 mm.
Figure 4.
TH immunostaining showed surviving grafts in the 20 transplanted animals. Macroscopically, cell suspension grafts showed a typical organization, with aggregates of TH positive cells in the periphery, and a reticular area with small TH positive cells, in the centre of the graft. There was no significant difference in the number of surviving TH+ cells in the 4 transplantation groups (Two way ANOVA and Newman-Keuls post-hoc test). Scale bar 1mm.
Using stereological methods, the dopaminergic cell numbers were quantified (Table 1B). Statistical analyses of all grafts (VMsolid vs. VMsusp) showed that the number of surviving TH-ir neurons was not statistically different between the 2 fetal tissue preparations (p>0.05, post-hoc Newman-Keuls test). Further, there was no statistical difference between the numbers of surviving TH-ir neurons in different locations (Cd vs. Put; p>0.05. Fig. 4). The average increase of dopaminergic innervation using optical density comparisons of the grafted Cd and Put over the contralateral side was 46% (46.2±7.1%). VMsolid increased innervation by 51% and 59% when placed in the Cd or Put respectively. VMsusp achieved a 26% increase in Cd and a 49% in Put innervation. Overall, no significant differences were found between the innervation achieved by VMsolid vs VMsusp grafts, or between Cd vs Put locations (Table 1B). The differences in optical density of TH immunoreactivity between the grafted and contralateral Cd and Put and change in the behavioral measures from the last month before grafting (M02) to the last month of the study (T10) were significantly correlated in the Put location (r=0.5842, p=0.007, N=20) but not in the Cd location (r=0.14, p=NS).
In both the Cd and the Put locations, VMsolid elicited a greater astroglial response compared to the VMsusp (940±171μm VMsolid vs 240±51μm VMsusp. Mann-Whitney test, p<0.005. Fig. 6) both in the Cd (729±65μm VMsolid vs 204±89μm VMsusp; p<0.01) and in the Put location (1152±312μm VMsolid vs 268±67 μm VMsusp; p<0.05. Fig. 6). Within the area of astroglial reaction, the number of hypertrophic GFAP+ cells was higher in the VMsolid than in the VMsusp grafts (data not shown). No significant differences were found in the microglial reaction to the different cell preparations or target nuclei (Fig. 7).
Figure 6.
Representative photomicrographs from the 4 transplantation groups, of a GFAP immunostaining showing the astroglial reaction around the grafts. Solid grafts both at Cd (B) and Put (C) locations elicit a significantly larger astroglial reaction of fibrous hypertrophic astrocytes compared with cell suspension grafts at the same locations (A-B). * p<0.05, unpaired t test. Scale bar 250 μm.
Figure 7.
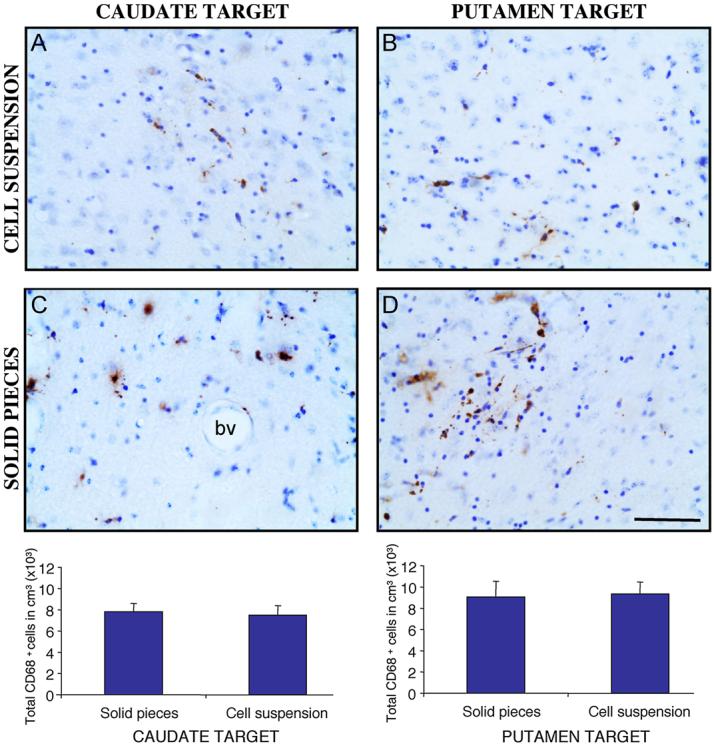
Representative photomicrographs from the 4 transplantation groups, of the graft-host border stained with CD68 to analyze activated microglia. Stereological quantification did not show differences in the microglial reaction between graft locations or between tissue preparations. p >0.05 unpaired t test. Scale bar 60 μm.
DISCUSSION
This relatively large primate transplant study was designed to examine parameters of tissue preparation and graft location relative to obtaining maximum functional benefit following striatal fetal cell transplantation. The study demonstrates that striatal fetal VM transplants both as cell suspensions and solid pieces, in the Cd and Put locations, are effective in improving parkinsonian signs and activities of normal daily living in a bilateral MPTP primate model of PD. We have also shown that, similarly to human tissue, long post-transplantation periods may be necessary to observe a meaningful functional recovery (at least 9 months post-transplantation in monkeys). This recovery is associated with the time fetal primate DA cells need for maturation, outgrowth and functional connectivity (Isacson et al., 2003; Isacson and Deacon, 1997) and is proportional to the reported outcomes of the clinical trials using human tissue, where substantial clinical benefits appear 12-24 months post-transplantation, but are not complete until about 3-4 years after surgery (Isacson et al., 2003; Lindvall et al., 1994; Piccini et al., 1999).
Functional benefits in our study were particularly convincing in the most severely parkinsonian monkeys (equivalent to Hoehn-Yahr V, PD patients). Previous studies from our group show that these severe MPTP treated monkeys usually do not survive for a extended period of time, despite medical treatment (D.E. Redmond, unpublished observations). In contrast, all these animals in the present study survived and showed functional improvements both in general health and in motor parameters. This highlights the fact that striatal cell transplantation can offer a substantial clinical benefit also in moderate to severe PD patients, as has been recently reported in an open label study (Mendez et al., 2005). It is also worth noting that these functional benefits were achieved with only unilateral striatal transplantation, in a bilateral PD primate model, similar to cases in clinical studies of fetal transplantation (Defer et al., 1996; Lindvall et al., 1994; Mendez et al., 2005; Piccini et al., 1999), unilateral striatal GDNF infusion (Slevin et al., 2007; Slevin et al., 2005), and DBS stimulation (Slowinski et al., 2007). In these previous reports, bilateral functional benefit included mild to moderate ipsilateral and axial improvements and a pronounced contralateral improvements (Bastian et al., 2003; Germano et al., 2004), although the magnitude of ipsilateral and axial changes was not studied in detail. In the current primate study, contralateral improvement may have been the first signs of graft function. To objectively determine ipsilateral and contralateral function in non-human primates would have required the use of tests specifically designed to analyze motor performance of both sides independently (Gash et al., 1999; Jenkins et al., 2004). The PRS and ADL behavioral scales used in the present study were not designed to measure side bias. However, although the scales used did not specifically address the amount of unilateral improvement achieved, we would have detected major asymetries in normal motor function. Such major asymmetries were not detected post-transplantation, indicating that while contralateral improvements may have dominated, there must also have been considerable ipsilateral improvements. Such bilateral changes after unilateral basal ganglia manipulations have been described, originally reported by Nieoullon et al (Nieoullon et al., 1977). The neural substrate responsible for these bilateral responses to unilateral basal ganglia manipulations appears to involve glutamatergic thalamocortical and corticostriatal circuits with extensive bilateral connections, as well as descending projections from the basal ganglia to the brainstem and spinal cord (Barbeito et al., 1989).
No differences in behavioral recovery were seen between the Cd and Put locations. Nevertheless, there was a significant correlation between reinnervation and functional recovery in the Put, but not in the Cd graft location. The functional improvement in these grafted animals clearly correlated with the net increase in DA innervation. This functional-histological correlation for animals grafted in the Put support the concept that these animals experienced graft-mediated functional benefits. It is also worth noting that, before transplantation, despite this similar recovery rate, monkeys grafted in the Put were somewhat more severe (although not significantly; see Table 1) than monkeys grafted in the Cd.
A factor that complicates the analysis, and could account for this lack of differences in behavioral improvement between target locations, is the presence of a considerable reinnervation of the non-grafted striatal nuclei ipsilateral to the transplant, regardless of the primary graft target. That is, when grafts were placed into the caudate nucleus they innervated they partially innervated the putamen as well as the caudate. Similarly, when the grafts were placed into the putamen, they innervated the caudate nucleus as well as the putamen. Extensive work both in animal models and in post-mortem evaluation studies of grafted PD patients have shown that immature DA cells preserve the capacity to grow to their natural target when placed in a denervated adult striatum (Haque et al., 1997; Isacson and Deacon, 1996; Isacson et al., 1995; Mendez et al., 2005; Thompson et al., 2005). The ventral mesencephalon, at this embryonic developmental stage, is normally composed of a mixture of SNc (A9) and VTA (A10) immature DA cells that normally project to motor (including Cd and post-commissural Put) or non-motor (precommissural putamen and Cd) striatal areas, respectively. Thus, this innervation of both striatal nuclei is, most likely, due to the presence of these 2 different DA cell types in the grafted fetal VM tissue.
Although there are anatomical and clinical reasons to favor the post-commissural putamen as the best target for cell transplantation in PD, there are also observations that support the possibility that Cd nucleus reinnervation could provide an additional improvement of motor function. The role of the Cd nucleus in motor planning (Schultz and Romo, 1992), as well as reports of clinical improvements after Cd transplantation in non-human primates and PD patients (Lopez-Lozano et al., 1997a; Taylor et al., 1991), supports this concept. This possibility is also supported by the results reported in an open trial where both Cd and Put nuclei received DA innervation from fetal cells (Kordower et al., 1996; Mendez et al., 2005).
No differences were found in the survival of grafted TH-ir neurons between the VMsolid and VMsusp transplantation groups. Among several factors, donor gestational age at the time of dissection plays an important role in this outcome. In order to have the optimal cell survival post-transplantation, fetal VM needs to be dissected at a very specific time of embryonic development (Sladek et al., 1993). This optimal period is limited by the beginning of DA neuron neurogenesis and the time these DA neurons differentiate and extend neurites. In the AFG, this period corresponds to embryonic days 36 to 44 (Sladek et al., 1993; Tarantal and Hendrickx, 1988a; Tarantal and Hendrickx, 1988b).
Postmortem analyses of PD patients grafted with cell suspensions, showed SNc-like (A9) DA neurons located along the graft-host border, and VTA-like (A10) DA neurons preferentially distributed towards central areas of the graft (Mendez et al., 2005). Here, analysis of graft structure and integration revealed that this typical distribution of DA cell types of VM suspension grafts, was not seen in VM solid grafts. Additionally, there were large areas in the center of solid grafts without any TH-ir neurons or fibers, especially in grafts located in the Cd, in close proximity to the lateral ventricle. Nevertheless overall, both graft types had similar distribution and cell composition, with TH-ir cells preferentially distributed along the periphery of the graft and good graft-host integration.
Our data, to some extent, supports the general notion that fetal tissue grafted as solid pieces elicits a stronger host tissue reaction than tissue grafted as a cell suspension. We observed a stronger astroglial reaction by the host to solid pieces even though exactly the same dissection techniques were used. In contrast, we could not confirm previous observations of increased microglial reaction after transplantation of solid VM pieces versus cell suspensions in PD patients grafted with fetal VM tissue (Freed et al., 2001; Kordower et al., 1997; Mendez et al., 2005; Olanow et al., 2003). These and other studies in animal models of PD, show that the presence of donor blood vessels in grafted solid VM pieces caused a more pronounced inflammatory response than the response elicited by cell suspension grafts (Lindsay and Raisman, 1984). The pristine condition and easy access to fetal material in this study, in contrast with fetal tissue fragments obtained from elective abortions, allowed us to perform a more thorough dissection in which all the remaining meninges and other membranes were completely peeled from the VM during the preparation of both solid pieces and cell suspensions. This thorough dissection may have almost eliminated the presence of donor blood vessels in the grafted solid pieces, and thus the formation of the typical microglial response to this tissue preparation.
Inflammatory responses may interfere with the host-graft interaction and with the normal process of striatal DA reinnervation, thus affecting graft-mediated functional recovery. This role of inflammation is supported by the findings in one of the double labeled clinical trials, in which the presence of graft-induced dyskinesias (in particular off-state dyskinesias) occurred in 50% of the subjects following discontinuation of immunosuppression (Olanow et al., 2003). The interruption of cyclosporine treatment also correlated with loss of transplant-induced clinical benefit, despite continued presence of healthy-appearing grafted neurons (Olanow et al., 2003). This is consistent with findings from xenograft studies, where functional loss occurs slowly and prior to complete elimination of xenogenic transplanted cells, due to a rejection process (Galpern et al., 1996; Widner, 1999). Finally, there are other factors influencing survival and integration of the grafts, such as tissue storage method, number of grafted embryos, and distribution of grafted tissue (Isacson et al., 2003). In our study, all these factors were kept constant and even dissection procedures were performed in the exact same way for VMsolid and VMsusp.
The hope that stem cells may be the most practical donor material for use in cell replacement is largely based on potential advantages in the supply and quality control of the donor material, not based on the assumption that stem cell derived midbrain DA neurons would be functionally superior to DA neurons from dissected fetal VM. Stem cell-derived midbrain DA neurons will be at, or go through, the same developmental fetal stage as we have used in this study, prior to or after transplantation, as well as experience similar tissue and guidance cues for integration into the recipient brain(Isacson et al., 2003). It is therefore likely that stem cell derived DA neurons of VM phenotype will function in a manner similar to dissected fetal DA neurons, and studies would encounter similar problems to those observed using fetal DA neuron grafts(Isacson, 2003). Consequently, it is imperative to better understand the mechanisms behind functional improvements, and in particular the molecular and neuronal substrates underlying the side effects observed in some clinical cell replacement trials (Freed et al., 2001; Olanow et al., 2003).
In summary, we show that grafted VM tissue survives and provides a significant striatal dopaminergic reinnervation, resulting in improvements of motor and general health parameters, with a high success rate in a primate model of PD. Based on our data, there are no major differences between implantation sites in the Cd or the Put, although the putaminal location seems to have a better correlation with the functional outcome. The (1) lack of benefits of solid tissue over cell suspensions, (2) increased glial response induced by solid grafts, and (3) the control over cell numbers using dissociated cells and (4) easier translation of methods and results to stem cell derived neural transplantation, all support the use of cell suspensions in future studies of fetal cell transplantation in PD.
In the exploration of DA neuron transplantation for future rational clinical use, the PD primate model studies presented here provide a basis for additional studies of efficacy and safety. Work in progress involves the transplantation of fetal DA neurons in this primate model, in animals with prior L-dopa induced dyskinesia (Youngerman BE, 2005) to better model the clinical reality and therapeutic trials.
Figure 1.
Experimental design. Twenty animals were treated with MPTP (an average of 3.1 mg/kg for each monkey; described in text). According to baseline severity in the last month prior to transplantation, animals were randomly allocated into 4 groups to receive small solid pieces (less than 1 mm × 1 mm × 1 mm) or cell suspensions from monkey fetal VM, either in the caudate or the putamen targets. One week after transplantation parkinsonism and normal behavior were evaluated 5 days/week for 9 months. After these 9 months animals were perfused for histological evaluation.
ACKNOWLEDGEMENTS
This research is supported by a grant from the Michael J. Fox Foundation (MJFF) and NIH grant NS-39793.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barbeito L, et al. Activation of the bilateral corticostriatal glutamatergic projection by infusion of GABA into thalamic motor nuclei in the cat: an in vivo release study. Neuroscience. 1989;28:365–74. doi: 10.1016/0306-4522(89)90183-8. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, et al. Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson's disease. Mov Disord. 2003;18:1000–7. doi: 10.1002/mds.10493. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, et al. Brain dopamine and the syndromes of Parkinson and Huntington: Clinical, morphological, and neurochemical correlations. J. Neurol. Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Burke RE, et al. An assessment of the validity of densitometric measures of striatal tyrosine hydroxylase-positive fibers: relationship to apomorphine-induced rotations in 6-hydroxydopamine lesioned rats. J Neurosci Methods. 1990;35:63–73. doi: 10.1016/0165-0270(90)90095-w. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. [Google Scholar]
- Costantini LC, et al. Medial fetal ventral mesencephalon: a preferred source for dopamine neuron grafts. Neuroreport. 1997;8:2253–7. doi: 10.1097/00001756-199707070-00032. [DOI] [PubMed] [Google Scholar]
- Defer GL, et al. Long-term outcome of unilaterally transplanted parkinsonian patients. I. Clinical approach. Brain. 1996;119(Pt 1):41–50. doi: 10.1093/brain/119.1.41. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, et al. Striatal dopaminergic correlates of stable parkinsonism and degree of recovery in old-world primates one year after MPTP treatment. Neuroscience. 2000;95:399–408. doi: 10.1016/s0306-4522(99)00437-6. [DOI] [PubMed] [Google Scholar]
- Freed CR, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 and 46 months after transplantation for Parkinson's disease. New Engl. J. Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- Freed CR, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–9. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Freeman TB, et al. Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson's disease. Ann Neurol. 1995;38:379–88. doi: 10.1002/ana.410380307. [DOI] [PubMed] [Google Scholar]
- Galpern WR, et al. Xenotransplantation of porcine fetal ventral mesencephalon in a rat model of Parkinson's disease: functional recovery and graft morphology. Exp Neurol. 1996;140:1–13. doi: 10.1006/exnr.1996.0109. [DOI] [PubMed] [Google Scholar]
- Gash DM, et al. An automated movement assessment panel for upper limb motor functions in rhesus monkeys and humans. J Neurosci Methods. 1999;89:111–7. doi: 10.1016/s0165-0270(99)00051-5. [DOI] [PubMed] [Google Scholar]
- Germano IM, et al. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: a double-blind 12-month evaluation study. J Neurosurg. 2004;101:36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- Hagell P, et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- Hagell P, et al. Sequential bilateral transplantation in Parkinson's disease: effects of the second graft. Brain. 1999;122:1121–32. doi: 10.1093/brain/122.6.1121. [DOI] [PubMed] [Google Scholar]
- Haque NS, et al. Differential dissection of the rat E16 ventral mesencephalon and survival and reinnervation of the 6-OHDA-lesioned striatum by a subset of aldehyde dehydrogenase-positive TH neurons. Cell Transplant. 1997;6:239–48. doi: 10.1177/096368979700600307. [DOI] [PubMed] [Google Scholar]
- Isacson O. The production and use of cells as therapeutic agents in neurodegenerative diseases. Lancet Neurol. 2003;2:417–24. doi: 10.1016/s1474-4422(03)00437-x. [DOI] [PubMed] [Google Scholar]
- Isacson O, et al. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson's disease by stem cells. Ann Neurol. 2003;53(Suppl 3):S135–46. doi: 10.1002/ana.10482. discussion S146-8. [DOI] [PubMed] [Google Scholar]
- Isacson O, Deacon T. Neural transplantation studies reveal the brain's capacity for continuous reconstruction. Trends Neurosci. 1997;20:477–82. doi: 10.1016/s0166-2236(97)01081-3. [DOI] [PubMed] [Google Scholar]
- Isacson O, Deacon TW. Specific axon guidance factors persist in the adult brain as demonstrated by pig neuroblasts transplanted to the rat. Neuroscience. 1996;75:827–37. doi: 10.1016/0306-4522(96)00305-3. [DOI] [PubMed] [Google Scholar]
- Isacson O, et al. Transplanted xenogeneic neural cells in neurodegenerative disease models exhibit remarkable axonal target specificity and distinct growth patterns of glial and axonal fibres. Nat Med. 1995;1:1189–94. doi: 10.1038/nm1195-1189. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, et al. Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. J Neurosci. 2004;24:9553–60. doi: 10.1523/JNEUROSCI.1558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, et al. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. New Engl. J. Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Kordower J, et al. Functional fetal nigral grafts in a patient with Parkinson's disease: chemoanatomic, ultrastructural, and metabolic studies. J. Comp. Neurol. 1996;370:203–230. doi: 10.1002/(SICI)1096-9861(19960624)370:2<203::AID-CNE6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kordower JH, et al. Dopaminergic transplants in patients with Parkinson's disease: neuroanatomical correlates of clinical recovery. Experimental Neurology. 1997;144:41–46. doi: 10.1006/exnr.1996.6386. [DOI] [PubMed] [Google Scholar]
- Leenders KL, et al. The nigrostriatal dopaminergic system assessed in vivo by positron emission tomography in healthy volunteer subjects and patients with Parkinson's disease. Arch Neurol. 1990;47:1290–8. doi: 10.1001/archneur.1990.00530120034007. [DOI] [PubMed] [Google Scholar]
- Levivier M, et al. Intracerebral transplantation of fetal ventral mesencephalon for patients with advanced Parkinson's disease. Methodology and 6-month to 1-year follow-up in 3 patients. Stereotact Funct Neurosurg. 1997;69:99–111. doi: 10.1159/000099859. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Raisman G. An autoradiographic study of neuronal development, vascularization and glial cell migration from hippocampal transplants labelled in intermediate explant culture. Neuroscience. 1984;12:513–30. doi: 10.1016/0306-4522(84)90070-8. [DOI] [PubMed] [Google Scholar]
- Lindvall O, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science. 1990;247:574–7. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- Lindvall O, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson's disease. Ann Neurol. 1994;35:172–80. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- Lindvall O, et al. Transplantation of fetal dopamine neurons in Parkinson's disease: one-year clinical and neurophysiological observations in two patients with putaminal implants. Ann Neurol. 1992;31:155–65. doi: 10.1002/ana.410310206. [DOI] [PubMed] [Google Scholar]
- Lopez-Lozano J, et al. Long-term improvement in patients with severe Parkinson's disease after implantation of fetal ventral mesencephalic tissue in a cavity of the caudate nucleus: 5-year follow up in 10 patients. Journal of Neurosurgery. 1997a;86:931–942. doi: 10.3171/jns.1997.86.6.0931. [DOI] [PubMed] [Google Scholar]
- Lopez-Lozano JJ, et al. Regression of parkinsonian fetal ventral mesencephalon grafts upon withdrawal of cyclosporine A immunosuppression. The CPH Neural Transplantation Group. Transplant Proc. 1997b;29:977–80. doi: 10.1016/s0041-1345(96)00333-8. [DOI] [PubMed] [Google Scholar]
- Ma Y, et al. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol. 2002;52:628–34. doi: 10.1002/ana.10359. [DOI] [PubMed] [Google Scholar]
- Mendez I, et al. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: a pilot study. Report of three cases. J Neurosurg. 2002;96:589–96. doi: 10.3171/jns.2002.96.3.0589. [DOI] [PubMed] [Google Scholar]
- Mendez I, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain. 2005;128:1498–510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina H, et al. Neurotransplantation in Parkinson's disease: from open microsurgery to bilateral stereotactic approach: first clinical trial using microelectrode recording technique. Stereotact Funct Neurosurg. 1994;62:204–8. doi: 10.1159/000098620. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, et al. Interdependence of the nigrostriatal dopaminergic systems on the two sides of the brain in the cat. Science. 1977;198:416–8. doi: 10.1126/science.910137. [DOI] [PubMed] [Google Scholar]
- Nyberg P, et al. Dopaminergic deficiency is more pronounced in putamen than in nucleus caudatus in Parkinson's disease. Neurochem. Pathol. 1983;1:193–202. [Google Scholar]
- Olanow CW, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54:403–14. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Peschanski M, et al. Bilateral motor improvement and alteration of L-dopa effect in two patients with Parkinson's disease following intrastriatal transplantation of foetal ventral mesencephalon. Brain. 1994;117(Pt 3):487–99. doi: 10.1093/brain/117.3.487. [DOI] [PubMed] [Google Scholar]
- Piccini P, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci. 1999;2:1137–40. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- Piccini P, et al. Delayed recovery of movement-related cortical function in Parkinson's disease after striatal dopaminergic grafts. Ann Neurol. 2000;48:689–95. [PubMed] [Google Scholar]
- Przedborski S, et al. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–74. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R. Role of primate basal ganglia and frontal cortex in the internal generation of movements. I. Preparatory activity in the anterior striatum. Exp Brain Res. 1992;91:363–84. doi: 10.1007/BF00227834. [DOI] [PubMed] [Google Scholar]
- Sladek JR, Jr., et al. Fetal dopamine cell survival after transplantation is dramatically improved at a critical donor gestational age in nonhuman primates. Exp Neurol. 1993;122:16–27. doi: 10.1006/exnr.1993.1103. [DOI] [PubMed] [Google Scholar]
- Slevin JT, et al. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg. 2007;106:614–20. doi: 10.3171/jns.2007.106.4.614. [DOI] [PubMed] [Google Scholar]
- Slevin JT, et al. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–22. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- Slowinski JL, et al. Unilateral deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg. 2007;106:626–32. doi: 10.3171/jns.2007.106.4.626. [DOI] [PubMed] [Google Scholar]
- Spencer DD, et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson's disease. N Engl J Med. 1992;327:1541–8. doi: 10.1056/NEJM199211263272201. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Hendrickx AG. Characterization of prenatal growth and development in the crab-eating macaque (Macaca fascicularis) by ultrasound. Anat Rec. 1988a;222:177–84. doi: 10.1002/ar.1092220210. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Hendrickx AG. Use of ultrasound for early pregnancy detection in the rhesus and cynomolgus macaque (Macaca mulatta and Macaca fascicularis) J Med Primatol. 1988b;17:105–12. [PubMed] [Google Scholar]
- Taylor JR EJ, Roth RH, Sladek J, Jr., Redmond DE., Jr . Toxin-Induced Models of Neurological Disorders. Plenum Press; New York: 1994. Behavioral effects of MPTP administration in the St. Kitts monkey: a primate model of Parkinson's disease; pp. 139–174. [Google Scholar]
- Taylor JR, et al. Grafting of fetal substantia nigra to striatum reverses behavioral deficits induced by MPTP in primates: a comparison with other types of grafts as controls. Exp Brain Res. 1991;85:335–48. doi: 10.1007/BF00229411. [DOI] [PubMed] [Google Scholar]
- Taylor JR, et al. Severe long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the vervet monkey (Cercopithecus aethiops sabaeus) Neuroscience. 1997;81:745–55. doi: 10.1016/s0306-4522(97)00214-5. [DOI] [PubMed] [Google Scholar]
- Taylor JR, et al. Sham surgery does not ameliorate MPTP-induced behavioral deficits in monkeys. Cell Transplant. 1995;4:13–26. doi: 10.1177/096368979500400105. [DOI] [PubMed] [Google Scholar]
- Thompson L, et al. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J Neurosci. 2005;25:6467–77. doi: 10.1523/JNEUROSCI.1676-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, et al. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Widner H. Review of allo- and xenogeneic neural grafts in neurodegenerative disorders. Neural Tissue Transplantation Team(NETTLU) Transplant Proc. 1999;31:936–8. doi: 10.1016/s0041-1345(98)01845-4. [DOI] [PubMed] [Google Scholar]
- Youngerman BE RDJ, Kordower JH, Isacson O. Levodopa induced dyskinesia in the St. Kitts (African) green monkey. Parkinsonism and Related Disorders; Abstracts of the 16th International Congress on Parkinson's Disease and Related Disorders; 2005. p. 232. [Google Scholar]