Abstract
This experiment was conducted to test the predictions of two behavioral-economic approaches to quantifying relative reinforcer efficacy. The normalized demand analysis suggests that characteristics of averaged normalized demand curves may be used to predict progressive-ratio breakpoints and peak responding. By contrast, the demand analysis holds that traditional measures of relative reinforcer efficacy (breakpoint, peak response rate, and choice) correspond to specific characteristics of non-normalized demand curves. The accuracy of these predictions was evaluated in rats' responding for food or water: two reinforcers known to function as complements. Consistent with the first approach, predicted peak normalized response output values obtained under single-schedule conditions ordinally predicted progressive-ratio breakpoints and peak response rates obtained in a separate condition. Combining the minimum-needs hypothesis with the normalized demand analysis helped to interpret prior findings, but was less useful in predicting choice between food and water—two strongly complementary reinforcers. Predictions of the demand analysis had mixed success. Peak response outputs predicted from the non-normalized water demand curves were significantly correlated with obtained peak responding for water in a separate condition, but none of the remaining three predicted correlations was statistically significant. The demand analysis fared better in predicting choice—relative consumption of food and water under single schedules of reinforcement predicted preference under concurrent schedules significantly better than chance.
Keywords: behavioral economics, relative reinforcer efficacy, complement, minimum-needs, rat, lever press
Historically, three measures have been used to assess relative reinforcer efficacy: 1) progressive-ratio (PR) breakpoint; 2) peak response rate maintained by the reinforcer; and 3) choice. One reinforcer is deemed more effective than another if it maintains higher PR breakpoints, higher peak response rates, and is preferred over another reinforcer (e.g., Griffiths, Brady, & Bradford, 1979; Katz, 1990; Stafford, Lesage, & Glowa, 1998; Woolverton & Nader, 1990). However, as amply summarized by Bickel, Marsch, and Carroll (2000), these three measures do not always agree. Such inconsistencies have led some behavioral economists to question the utility of the concept of relative reinforcing efficacy (e.g., Bickel et al.; Johnson & Bickel, 2006).
This article aims to test the accuracy of predictions made by two behavioral-economic approaches to reinforcer efficacy. The first is the normalized demand analysis, which was proposed by Hursh and Winger (1995). Here the reinforcing efficacy of a good is quantified by examining normalized consumption of that good across a range of normalized prices (price is most often manipulated in behavioral-economic experiments by changing the number of responses required per unit of the reinforcer). The quantitative details of normalizing are presented below; for now it is important simply to note that normalizing expresses consumption and price in terms of comparable (normalized) units of reinforcer magnitude. Thereafter, measures derived from demand curves may be compared despite differences in reinforcer dose or potency, or differences in peak levels of consumption observed when access to the reinforcers is unconstrained.
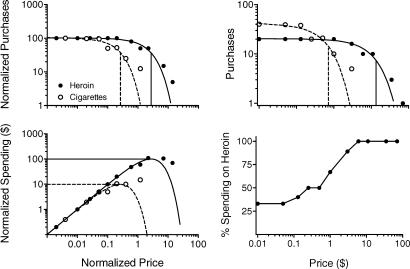
The upper-left panel of Figure 1 shows opioid-dependent outpatients' normalized median demand for hypothetical cigarettes and bags of heroin as reported by Jacobs and Bickel (1999). According to the normalized demand analysis, heroin is the relatively more effective reinforcer because increasing the normalized price of heroin produced smaller decrements in heroin purchases when compared with cigarettes. Hursh and Winger (1995) proposed two methods for quantifying sensitivity to normalized price increases. The first, Pmax, is shown as vertical lines in the upper-left panel of Figure 1. Pmax is the normalized price below which demand is inelastic (consumption decreases are proportionally smaller than price increases) and above which demand is elastic (consumption decreases are proportionally larger than price increases). Pmax is also the normalized price at which peak response output is predicted to be observed. At higher normalized prices, total responding per session should decline. The second measure, Omax, is the predicted peak response output that will be maintained by the reinforcer and is shown as the horizontal lines in the lower-left panel of Figure 1. Using both measures, the normalized demand analysis ranks heroin as the more reinforcing of the two reinforcers and this is consistent with the higher PR breakpoints and peak response rates reported by Jacobs and Bickel. More generally, the normalized demand analysis has successfully ranked the reinforcing efficacy of drugs within (Ko, Terner, Hursh, Woods, & Winger, 2002; Winger, Hursh, Casey, & Woods, 2002) and across (Hursh & Winger) drug classes. These rankings are consistent with epidemiological and receptor-efficacy studies (for a review see Hursh, Galuska, Winger, & Woods, 2005).
Fig 1.
Median hypothetical purchases and expenditures of opioid-dependent outpatients in Jacobs and Bickel (1999). See text for details.
Within the economic framework outlined by Hursh and Winger (1995), reinforcing efficacy is equated with effects of price on consumption, not relative consumption (choice). Accordingly, the normalized demand analysis holds that choice is not an appropriate measure of reinforcer efficacy. By contrast, the second behavioral-economic approach, the demand analysis, rejects the construct of relative reinforcing efficacy, suggesting instead that the three traditional measures of reinforcing efficacy, including choice, correspond to quantitative characteristics of non-normalized demand curves (Bickel et al., 2000). The upper-right panel of Figure 1 shows median non-normalized demand for hypothetical heroin and cigarettes as reported by Jacobs and Bickel (1999). Because Pmax provides a measure of the price at which responding for a reinforcer is expected to decline, the demand analysis holds that Pmax and PR breakpoint should be positively correlated. Consistent with this prediction, Jacobs and Bickel reported significant correlations between Pmax and PR breakpoint for both heroin and cigarettes (Spearman's rho = .98 and .99, respectively). Because Omax provides predicted maximum response output, the demand analysis holds that this measure should be positively correlated with peak response rates. Once again, Jacobs and Bickel found this to be the case with both heroin and cigarettes (rho = .83 and .99, respectively). Finally, the demand analysis holds that relative levels of consumption at a given price under single schedule conditions should be predictive of choice when both commodities are available at that price. As may be observed by comparing the upper- and lower-right panels of Figure 1, this prediction held 100% of the time in the Jacobs and Bickel study.
A number of previous studies have tested the predictions of the demand analysis and as summarized elsewhere (e.g., Johnson & Bickel, 2006; Madden, Smethells, Ewan, & Hursh, 2007) the results have largely been supportive. Where exceptions to this rule have been reported (Madden et al.; Shahan, Bickel, Madden, & Badger, 1999) the two reinforcers have functioned as economic substitutes. Economists classify the relation between reinforcers along a continuum ranging from substitutes to complements, with independent reinforcers falling in between (see Green & Freed, 1993, for a review). Substitutes are typically reinforcers that share some characteristics. For example, coffee and tea both contain caffeine. Tea would be classified as a substitute for coffee if when the price of coffee went up, tea consumption increased as coffee consumption declined.
Shahan et al. (1999) reported that the choice predictions of the demand analysis (the only predictions tested in that experiment) were incorrect when nicotine-containing and de-nicotinized cigarettes were arranged as reinforcers (Johnson, Bickel, & Kirshenbaum, 2004, reported that these two kinds of cigarettes function as partial substitutes). Similarly, Madden et al. (2007) reported that predicted correlations between Pmax and PR breakpoint and between Omax and peak response rate were not consistently observed when rats responded for food and fat reinforcers, which they demonstrated to be partial substitutes. Likewise, choice predictions of the demand analysis were not significantly better than chance in the latter study. By contrast, Pmax and Omax values derived from normalized demand curves ordinally predicted PR breakpoints and peak response rates, respectively.
Complements are at the end opposite to substitutes along the economic continuum. Complementary reinforcers tend to be consumed at a constant ratio (e.g., 1 part milk, 2 parts cereal). To be classified as a complement, consumption of Reinforcer A must decline with consumption of Reinforcer B when the price of Reinforcer B increases. For example, if the price of cereal doubles and per capita cereal consumption declines, then one might expect milk consumption also to decline even though the price of milk remains unchanged. Some commodities function as perfect complements in the sense that they are always consumed together in a constant ratio regardless of price. The classic example is left and right shoes. If we can imagine a world in which left and right shoes are sold separately, then if the price of left shoes were to double and purchasing of left shoes were to decline by 30%, then the perfect complementary relation would be reflected in a 30% decline in right shoe purchases, despite there being no change in the price of right shoes. The complementary relation between goods need not be perfect. For example, for many of us, coffee and cream are complementary goods and are consumed in an optimal ratio of about 20 1. If the price of cream were greatly to increase such that cream consumption declined by 50%, it would not surprise us to see less than a 50% reduction in coffee consumption.
This less-than-perfect complementary relation is illustrated with cigarettes and heroin in the lower-right panel of Figure 1. These two goods have been empirically found to function as economic complements in humans (Mello, Mendelson, Sellers, & Kuehnle, 1980). Jacobs and Bickel's (1999) opioid-dependent outpatients self-reported an optimal cigarette-to-heroin consumption ratio of approximately 3:1 when consumption of both was unconstrained ($0.01 per cigarette or bag of heroin). However, as the price of both commodities increased, cigarette consumption declined more rapidly than did heroin. In a perfect complementary relation (like left and right shoes), one would expect the consumption ratio to be unchanged across the price range.
The present experiment sought to compare the predictions of the demand and normalized demand analyses with food and water reinforcers—two commodities known to function as economic complements in a variety of species including humans (Adolph, 1947), monkeys (e.g., Hursh, 1978), dogs (e.g., Robinson & Adolph, 1943), and rats (e.g., Bolles, 1961; Kagel, Battalio, Green, & Rachlin, 1980; Verplanck & Hayes, 1953; although it should be noted that exceptions to this rule have been reported with schedule-induced polydipsia: Allison & Mack, 1982; Rachlin & Krasnoff, 1983).
Method
Subjects
Six experimentally naive male albino Sprague-Dawley rats (K2, R3, G2, R1, K4, and B1), about 3 months old at the start of the experiment, were individually housed in a continuously lit colony room.
Apparatus
Six identical two-lever operant chambers (Med Associates, St. Albans, VT) enclosed in sound-attenuation enclosures were used. Each chamber measured 210 mm high, 210 mm wide, and 280 mm long. Response levers were located 70 mm from the floor and 85 mm apart. A single 28-V stimulus lamp was located 50 mm above each response lever. A liquid dipper (Med Associates, St. Albans, VT) equipped with a 0.1 ml cup was positioned 40 mm from the floor, on the rear wall and directly across from the left lever. A 45-mg food-pellet dispenser (Coulbourn Instruments, Allentown, PA) was mounted on the rear wall directly across from the right lever, and 40 mm from the floor. A PC in a neighboring room used Med Associates® hardware and software to control experimental contingencies and to record responding.
Procedure
Preliminary Training
Rats' access to food was restricted for 23 hr before each training session in which food served as the reinforcer. During the first session, pressing the right lever was shaped by successive approximations using three 45-mg food pellets (Noyes Formula PJAI, Research Diets, Inc., New Brunswick, NJ) as the reinforcer (these pellets provide a complete diet). Once responding was established, the FR requirement was gradually increased to FR 20. In subsequent training sessions, responding on the left lever was shaped using water as the reinforcer (rats were deprived of water for 12 hr before these sessions). The dipper cup containing water was raised for 7 s, because a pilot study indicated that this was sufficient time to consume this amount. As with food, the FR requirement was gradually increased to FR 20.
General Procedures
For the remainder of the experiment, sessions were started at the same time (10:00 p.m.) 7 days a week, lasted 11 hr, and with the exception of the light(s) above the operative lever(s), were conducted in dark experimental chambers. When reinforcers were delivered all lights in the chamber were darkened for 7 s. During this time lever pressing had no programmed consequences.
Demand Curves
Following preliminary training, food and water demand curves were obtained separately by examining daily consumption of a single commodity at a range of FR values. Demand for food was assessed in the first part of this condition for 3 rats (K2, R3, and G2), followed by an assessment of the water demand curve. This order was reversed for the other 3 rats. When the food demand curve was being assessed, rats were given 24 hr free access to water via a standard lick-tube water bottle in both the chamber and the home cage but were given no supplemental feeding. In the water- demand-curve portion of this condition, rats were given 24 hr free access to Harlan Teklad (Madison, WI) standard rodent diet 2014 but were given no supplemental water. The food was held in a stainless steel cup that was transferred with the rat from home cage to chamber.
In assessing each demand curve, an FR 1 was programmed for at least five sessions and until the total number of reinforcers obtained per day showed no systematic trend over the last four sessions. The FR value was then increased daily according to the following progression: 2, 3, 4, 5, 6, 7, 8, 9, 10, 13, 20, 30, 42 and 60. After this sequence, the ratio value was increased by 10% daily until the rat either a) failed to earn a reinforcer for 2 consecutive days, b) showed signs of dehydration, or c) posted a presession weight of 70% of free-feeding or less. This rapid demand curve assay procedure yields demand curves that are replicable both within and between subjects (Raslear, Bauman, Hursh, Shurtleff, & Simmons, 1988). Once a complete demand curve was obtained, the process was repeated for the other reinforcer type.
Choice
During the next condition, sessions began by illuminating the stimulus lights above both levers. When one lever was pressed, the light above the other was darkened and that lever was deactivated until the FR requirement on the operative lever was completed. As before, three food pellets were delivered when the FR on the right lever was completed and 0.1 ml of water was presented when the FR on the left lever was completed. Choice was assessed at concurrent FR 1, 10, 30, 100, 250, and 1 (in that order), with the FR value always being the same on both levers. No supplemental food or water was given at any point during this condition. Sessions continued at each FR value until the following stability criteria were met: a) percent choice of food in the two most recent sessions deviated from the previous two by 5% or less; b) neither the highest nor lowest choice percentage appeared in the final four sessions; and c) no trend was visually apparent.
Pr Breakpoints
Following the choice condition, 3 rats (K2, R3, & G2) responded under a single PR schedule for food in the first phase, water in the second, and food in the final phase in this condition. This sequence was reversed for the other 3 rats. Supplemental food or water was presented according to the same protocol used when single-schedule demand curves were assessed. The PR values were generated using the following equation (Depoortere, Li, Lane, & Emmet-Oglesby, 1993):
| 1 |
With the exception of the 7-s reinforcer-delivery interval, the light above the operative lever remained on, and responses were counted toward completion of the ratio requirements throughout each session. Breakpoint was defined as the first ratio value not completed in each session. Each of the three phases completed in the PR condition was continued until breakpoints in the two most recent sessions deviated from the previous two by 5% or less, neither the highest nor lowest breakpoint appeared in the final four sessions, and no trend was visually apparent
Results
Demand Curves
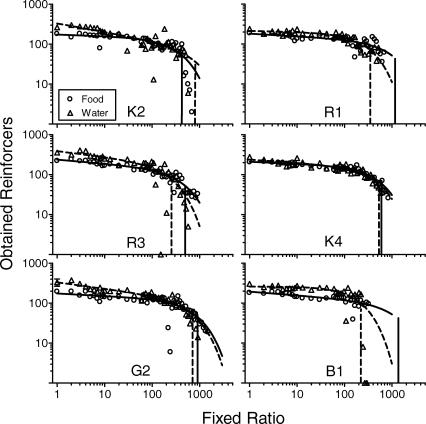
Figure 2 shows, for individual subjects, the average number of food and water reinforcers obtained in the final four sessions under FR 1, and the number obtained in each session at higher FR values (note the logarithmic axes). Error bars at FR 1 show one standard deviation in both directions, but are smaller than the data point in all cases except that of Rat G2's water reinforcers, showing that there was almost no variation in consumption over the final four sessions of FR 1. Where no food or water reinforcers were obtained in a session, consumption was set at 1 because zero is undefined in logarithmic coordinates. Recall that these values indicate the number of reinforcers obtained when the other reinforcer type was unavailable (i.e., only one lever was operative during each session). The best-fitting (by least squares) demand curves were fit by Graph Pad Prism® using the demand equation proposed by Hursh, Raslear, Shurtleff, Bauman, and Simmons (1988):
| 2 |
where L is predicted consumption at FR 1 and is commonly referred to as intensity of demand. In the present experiment, price (P) was the FR value. The parameters b and a are the initial slope and acceleration of the demand curve, respectively. The fitted parameter values and R2 values are shown in Table 1. Across subjects and reinforcer type, Equation 2 accounted for a median of 80.5% of the variance in consumption across the range of ratio values investigated.
Fig 2.
Individual rats' single-schedule demand curves (Equation 2) fit to the number of food and water reinforcers obtained per session. At FR 1, average values (and standard deviations, where visible) are shown from the last four stable sessions. Vertical solid and dashed lines show Pmax values obtained in the food and water conditions, respectively.
Table 1.
Parameters of individual rats' food and water single-schedule demand curves from least-squares fits of Equation 2. Pmax and Omax values were derived from these parameters using Equations 3 and 4.
| Subject | Reinforcer | L | b | a | R2 | Pmax | Omax |
| K2 | Food | 173.8 | −0.0380 | −0.0023 | 0.78 | 423.8 | 22365 |
| Water | 329.4 | −0.2131 | −0.0010 | 0.76 | 817.3 | 29358 | |
| R3 | Food | 237.5 | −0.0998 | −0.0018 | 0.92 | 497.6 | 25852 |
| Water | 386.5 | −0.1414 | −0.0034 | 0.86 | 255.1 | 19083 | |
| G2 | Food | 177.9 | −0.0635 | −0.0010 | 0.71 | 907.4 | 41056 |
| Water | 332.0 | −0.1564 | −0.0012 | 0.90 | 713.7 | 36475 | |
| R1 | Food | 182.5 | −0.0668 | −0.0008 | 0.61 | 1159.0 | 51912 |
| Water | 213.5 | −0.0226 | −0.0028 | 0.83 | 348.0 | 24493 | |
| K4 | Food | 209.6 | −0.0472 | −0.0016 | 0.93 | 595.1 | 35583 |
| Water | 236.9 | −0.0786 | −0.0017 | 0.84 | 529.8 | 30513 | |
| B1 | Food | 193.7 | −0.0942 | −0.0007 | 0.69 | 1361.7 | 54025 |
| Water | 259.6 | 0.0048 | −0.0045 | 0.67 | 222.7 | 21730 |
Rats tended to consume more water than food at low ratio values. To quantify sensitivity to increasing ratio values, parameters of the food and water demand curves were used to calculate separate Pmax values using the equation proposed by Hursh, Raslear, Bauman, and Black (1989):
| 3 |
Obtained Pmax values are provided in Table 1 and are shown as vertical lines in Figure 2. Pmax for food exceeded that for water reinforcers in 5 of 6 rats, although nominally so for Rat K4. A Wilcoxon's matched-pairs signed-ranks test indicated that this difference did not achieve conventional levels of statistical significance (Z = −1.36, p = .17) although the lack of statistical power should be considered when interpreting this outcome. Table 1 also provides Omax values calculated using the following equation (a variant of Equation 2 with Pmax substituted for P):
| 4 |
As with Pmax, Omax values for food exceeded those for water in 5 of 6 rats, and this difference was not statistically significant (Z = −1.36, p = .17).
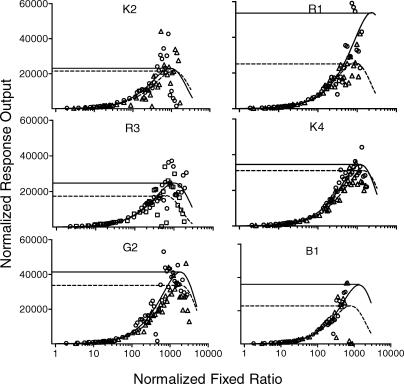
Figures 3 and 4 show normalized demand and response-output curves for food and water reinforcers (note the semi-logarithmic axes used in Figure 4). Parameters of the demand curves obtained using Equation 2 (with L = 100) are shown in Table 2. Across subjects and reinforcer type, Equation 2 accounted for a median of 80.5% of the variance across the range of normalized ratio values. For all rats, normalized demand for food and water were visually indistinguishable across most of the range of normalized ratio values. Pmax values (shown as vertical lines in Figure 3) were not systematically higher when food or water was the reinforcer (Z = −0.94, p = .34). By contrast, Omax values (horizontal lines in Figure 4) for food exceeded those for water in all rats and this difference was statistically significant (Z = −2.20, p = .03).
Fig 3.
Individual rats' normalized single-schedule demand curves. Vertical solid and dashed lines show normalized Pmax values obtained in the food and water conditions, respectively.
Fig 4.
Individual rats' normalized single-schedule response output curves. Horizontal solid and dashed lines show normalized Omax values obtained in the food and water conditions, respectively.
Table 2.
Normalized demand curve parameters, and the normalized FR value (Pmax) at which peak normalized response output (Omax) is predicted to occur.
| Subject | Reinforcer | b | a | R2 | Pmax | Omax |
| K2 | Food | −0.0694 | −0.0010 | 0.78 | 941.1 | 23078 |
| Water | −0.1116 | −0.0008 | 0.72 | 1149.6 | 21536 | |
| R3 | Food | −0.0661 | −0.0009 | 0.91 | 995.9 | 24792 |
| Water | −0.0404 | −0.0016 | 0.83 | 606.9 | 17941 | |
| G2 | Food | −0.0762 | −0.0005 | 0.71 | 1877.9 | 41351 |
| Water | −0.1144 | −0.0004 | 0.89 | 1942.1 | 33689 | |
| R1 | Food | −0.0687 | −0.0004 | 0.61 | 2261.4 | 52400 |
| Water | −0.0386 | −0.0011 | 0.84 | 850.8 | 25076 | |
| K4 | Food | −0.0408 | −0.0008 | 0.93 | 1230.3 | 35259 |
| Water | −0.0859 | −0.0006 | 0.84 | 1446.5 | 31030 | |
| B1 | Food | −0.0542 | −0.0007 | 0.66 | 1363.8 | 35817 |
| Water | −0.0432 | −0.0012 | 0.66 | 794.7 | 22876 |
Traditional Measures of Relative Reinforcer Efficacy
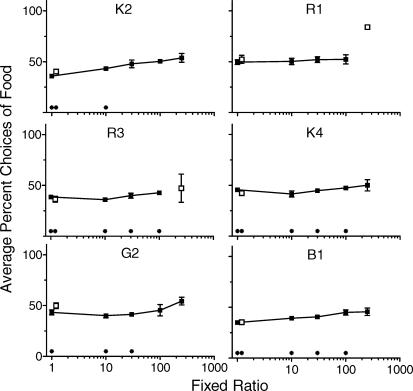
Figure 5 shows individual rats' average percent choices of food (and standard deviations) in the final four stable sessions, at each ratio value tested in the choice condition. The open data point at FR 1 shows choice percentages at this ratio value after the rats had completed all sessions arranged at FR 10–250. Stable choices could not be obtained at FR 250 in rats R1 and R3 because they were unable to maintain their weight in a healthy range at this ratio value. The open data points provided at FR 250 for these rats show the mean of the two sessions conducted at this ratio value; unless indicated, these data were not used in subsequent analyses. Wilcoxon's matched-pairs signed-ranks tests were used to determine if individual subjects' stable choices significantly deviated from indifference at each FR value. Asterisks along the x-axis in Figure 5 show those ratio values at which they did. A Friedman's test for correlated samples indicated a small but statistically significant tendency to choose food more frequently as ratio values increased; this trend was significant based on 4 (χ2F = 10.20, p < .05) or 6 rats (χ2F = 16.93, p < .01) depending on whether Rats R1 and R3 were included in the analysis. The modest change in the food-to-water choice ratio illustrates that our across-the-board price increases did little to disrupt the complementary relation between these commodities.
Fig 5.
Percent choices for the food reinforcer across the ratio values arranged. Choices are averaged over the final four sessions of the concurrent schedule phase and error bars correspond to one standard deviation in both directions. Open data points show the replication of FR 1 and the unstable choices at FR 250 (Rats R1 and R3).
Table 3 shows the number of sessions completed by each rat in the PR conditions when food or water served as the sole reinforcer. Because there were no systematic differences between the initially determined and replicated PR breakpoints or peak response rates, these data are combined in Table 3. For all rats, average PR breakpoints and daily peak response outputs maintained by food were higher than those maintained by water. For 2 rats, K4 and B1, this difference was nominal as water maintained higher PR breakpoints and peak response outputs in at least one of the final four sessions of their respective conditions. Wilcoxon's matched-pairs signed ranks tests were conducted using breakpoints and peak response data collected in the final four stable sessions (the average of the initial exposure and replication data was used). These tests revealed that food reinforcers maintained significantly higher PR breakpoints (Z = −3.63, p < .05) and peak response outputs (Z = −3.77, p < .05) than did water.
Table 3.
Numbers of sessions to which individual subjects were exposed in the initial and replicated conditions, where applicable, in the PR condition. Average (and standard deviation) values for PR breakpoint and peak responses emitted per session in the final four stable sessions are provided.
| Subject | Reinforcer | Sessions | PR Breakpoint (SD) | Peak Resp. |
| Output (SD) | ||||
| K2 | Food | 11 (10) | 796.6 (64.0) | 19014 (1116) |
| Water | 13 | 487.5 (61.0) | 11475 (1486) | |
| R3 | Food | 16 (11) | 564.6 (76.7) | 13356 (1874) |
| Water | 12 | 321.2 (122.8) | 7506 (2956) | |
| G2 | Food | 12 (26) | 733.7 (72.6) | 17395 (1807) |
| Water | 16 | 494.5 (60.3) | 11650 (1468) | |
| R1 | Food | 23 | 579.2 (52.2) | 13708 (1269) |
| Water | 20 (12) | 369.4 (95.3) | 8626 (2270) | |
| K4 | Food | 11 | 698.7 (35.1) | 16630 (863) |
| Water | 12 (17) | 669.9 (170.1) | 15926 (4150) | |
| B1 | Food | 14 | 442.5 (51.8) | 10380 (1258) |
| Water | 21 (13) | 437.2 (62.3) | 10198 (1483) |
In sum, the three traditional measures of relative reinforcer efficacy did not present a consistent picture: two measures (PR breakpoint and peak response output) indicated that food was a more effective reinforcer, and one measure (choice) indicated that water was slightly more effective at the lower range of ratio values; otherwise, the two were equivalently reinforcing.
Normalized PMax and OMax as Predictors of Relative Reinforcer Efficacy
Normalizing was conducted using the procedures outlined by Hursh and Winger (1995). Normalized reinforcer magnitude units (q) were calculated separately for food and water: q = 100/B, where B was consumption at FR 1. Normalized consumption was obtained by multiplying consumption obtained at a given FR value by q. Normalized prices were obtained by dividing FR values by q.
Hursh and Winger (1995) suggested that grouped average normalized Pmax and Omax values could be used as measures of relative reinforcer efficacy. No significant difference was detected between normalized Pmax values for food and water. Therefore this measure made no predictions about other measures of reinforcer efficacy. By contrast, normalized Omax was significantly higher in the single-schedule food reinforcement condition. Therefore, this measure correctly predicted significantly higher PR breakpoints and peak response outputs for food over water.
Although Hursh and Winger (1995) had not proposed it, we also examined whether individual rats' normalized Pmax and Omax values ordinally predicted food and water PR breakpoints and peak response outputs. Of the 4 rats with consistently higher breakpoints and peak response outputs in the food condition (K2, R3, G2, and R1), only Rats R3 and R1 had higher normalized Pmax values in the single-schedule food condition. By contrast, all 6 rats had higher Omax values in the single-schedule food condition, and this was consistent with their mean breakpoints and peak responding although, as noted above, this was not observed in every stable session for Rats K4 and B1. Across rats, in the stable sessions in the PR condition, normalized Omax correctly predicted that food-maintained breakpoints would be higher in 21 of 24 cases, which was significantly better than chance (χ2 = 13.5, p < .05). Likewise, normalized Omax correctly ordinally predicted food- and water-maintained peak response outputs in 20 of 24 cases, which was also significantly better than chance (χ2 = 10.67, p < .05).
Demand Analysis
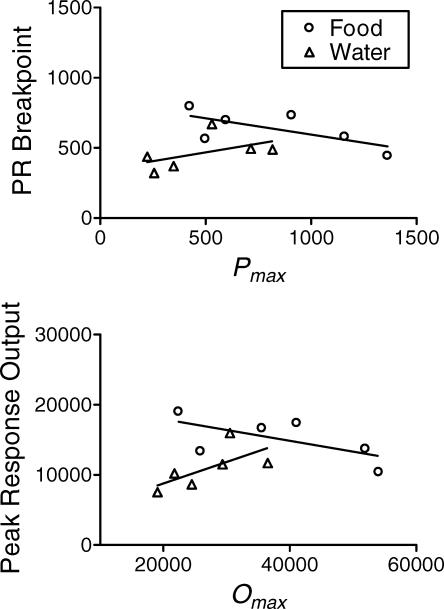
The upper panel of Figure 6 shows the correlations between PR breakpoints and non-normalized Pmax values obtained with food and water. Neither correlation was statistically significant (food: Spearman's rho = −0.60, p = .21; water: rho = 0.60, p = .21). Likewise, the correlation between non-normalized Omax for food was not significantly correlated with food-maintained peak response outputs (rho = −0.60, p = .21). However, the correlation between water-maintained peak responding was correlated with Omax (rho = 0.89, p < .05).
Fig 6.
Upper Panel: Scatter-plot illustrating the correlation between PR breakpoints and Pmax values derived from the non-normalized demand curves. Lower Panel: Scatter-plot illustrating the correlation between peak response output per session and Omax values derived from the non-normalized demand curves.
The third prediction of the demand analysis was that relative consumption under single schedules would predict choice under concurrent schedules. Thus, all rats should have chosen water reinforcers more often than food when both were available according to FR 1 schedules because under single schedules all 6 rats obtained more water than food reinforcers in each of the final four sessions of this condition (see Figure 2). Consistent with this prediction, in 9 of 12 cases (two for each rat) rats chose water reinforcers significantly more often than food (see Figure 5). The exceptions, Rat R1 and Rat G2 during the replication of FR 1, did not consistently choose one more often than the other.
At higher ratio values, during the single-schedule condition, a single session was completed at each ratio value when separately determining the food and water demand curves. The following criteria were used to conclude that more of one reinforcer was consumed than another in this condition: a) using a binomial distribution with a priori p = .5, the probability of obtaining such an unequal number of food and water reinforcers at the ratio value was < .05; and b) the relative consumption predicted by the fitted demand curves (see Figure 2) ordinally agreed with obtained relative consumption at that FR value. When these criteria were applied at FR 10, the demand analysis predicted preference for water reinforcers in all cases. With the exception of Rat R1, these predictions were correct. At FR 30, the demand analysis predicted either indifference (Rats K2, R1) or preference for water (Rats R3, G2, K4, & B1) and each of these predictions was correct. At FR 100, rats were predicted to prefer food (K2), water (R3, G2, & B1) or to be indifferent (R1, K4); these predictions were correct in three of six cases. Finally, at FR 250, for the 4 rats that completed choice sessions at this ratio value, the demand analysis predicted either preference for water (G2, B1) or indifference (K2, K4); these predictions were correct in two of four cases. Overall, predictions of choice by the demand analysis were qualitatively correct in 24 of 34 instances (70.6%) which was significantly better than chance (χ2 = 5.76, p < .05).
Discussion
As in previous experiments employing human and nonhuman subjects (see review by Bickel et al., 2000), in the present experiment the three traditional measures of relative reinforcer efficacy were not in agreement. Although food tended to maintain higher PR breakpoints and peak response rates, it was never chosen more often than water across the stable concurrent schedule sessions under any ratio requirement. Thus, the results of this experiment are similar to those that have supported the demand analysis in the past (e.g., Bickel & Madden, 1999; Johnson & Bickel, 2006). Despite this similarity, non-normalized Pmax values were not significantly correlated with PR breakpoints in the food or water conditions as the demand analysis predicted. The only correlation predicted by the demand analysis that attained statistical significance was that between Omax and peak responding when water was the reinforcer.
Although these findings are inconsistent with the majority of the experiments conducted to assess predictions of the demand analysis (see review by Johnson & Bickel, 2006), they are consistent with those reported by Madden et al. (2007), who examined these correlations with food and fat reinforcers (economic substitutes). As in Madden et al.'s experiment, the lack of the predicted correlations may be due to an inability to sample complete food and water demand curves. Demand for both commodities was inelastic over a majority of the demand curves shown in Figure 2 and very little, if any, of the demand curve to the right of Pmax was sampled. Examining consumption at higher prices was impossible without compromising the health of our subjects. Given this constraint, our estimates of Pmax and Omax may have been less representative of the values that would have been obtained had complete demand curves been assessed for both commodities. To assess adequately the correlations predicted by the demand analysis we will need two complete demand curves; perhaps curves obtained where the commodities are economic luxury goods and not required for the health of the organism.
Alternatively, the high correlations that have been reported in human subjects between Pmax and PR breakpoints and between Omax and peak response rate may be an artifact of the procedures used. Studies reporting these very strong correlations (Bickel & Madden, 1999; Jacobs & Bickel, 1999; Johnson & Bickel, 2006) assessed PR breakpoints and peak response rates from data collected in the same conditions in which the demand curves were derived. Because measures derived from the demand curves were not independent of the PR breakpoints and peak response rates with which they were found to be correlated, there is reason to suspect that correlations assessed from independent conditions (as in the present experiment) would be weaker.
The present study did not yield the correlations predicted by the demand analysis, but the relative positions of the single-schedule food and water demand curves proved useful in ordinally predicting choices under concurrent schedules. In sum, just as was reported by Madden et al. (2007), the data did not consistently support the predictions of the demand analysis, although further research is called for.
A clearer picture is provided about the predictions of the normalized demand analysis. Consistent with the findings of Madden et al. (2007), normalized Omax more accurately ordinally predicted PR breakpoints and peak response rates in the food and water conditions than did normalized Pmax. To our knowledge, the present experiment is the first test of the normalized demand analysis when the two reinforcers function as economic complements. When combined with Madden et al.'s experiment in which the reinforcers arranged were economic substitutes, these experiments provide broad (across the economic continuum) support for Hursh and Winger's (1995) contention that normalized Omax is a useful unitary measure of relative reinforcer efficacy. Converging evidence from the drug reinforcement literature considerably bolsters this position (for a review, see Hursh et al., 2005).
To some perspectives, a shortcoming of the normalized demand analysis is that it makes no predictions about choice, a measure widely viewed as an important metric of reinforcer efficacy. To address this shortcoming, Madden et al. (2007) suggested integrating the normalized demand analysis with the economic minimum-needs hypothesis (Kagel, Battalio, & Green, 1995; Kagel, Dwyer, & Battalio, 1985; Pollak & Wales, 1980; Shurtleff, Warren-Boulton, & Silberberg, 1987). According to this hypothesis, the sequence in which reinforcers are selected is determined by which reinforcer more effectively satisfies the organism's current state of deprivation. In Madden et al., food was hypothesized to fill the rats' food deprivation better than a fat solution because food maintained higher Omax values. Consistent with the minimum-needs hypothesis, the rats responded almost exclusively for food at the beginning of the choice sessions. At low ratio requirements, rats quickly filled their minimum food needs and then, when the marginal utility of additional food reinforcers was hypothesized to equal that of a fat reinforcer, roughly alternated between the food and fat reinforcers. Thus, at low prices most rats demonstrated moderate preferences for food. At higher prices (larger work requirements), minimum food needs were less likely to be met during the session and, therefore, less time was left to respond for fat reinforcers. Consistent with the minimum-needs hypothesis, preference for food became more extreme as the price of both commodities increased.
This integration of the normalized demand analysis and minimum needs hypothesis also provides a potentially useful account of Jacobs and Bickel's (1999) findings shown in the lower-right panel of Figure 1. Recall that in their study, opioid-dependent individuals made choices between hypothetical cigarettes and bags of heroin, both available at the prices shown along the x-axis. Because normalized Omax was higher in the heroin demand condition (lower-left graph in Figure 1), the minimum need for heroin exceeded that of cigarettes. At low prices, sufficient heroin could be purchased to fulfill this minimum need and because subjects were instructed to purchase no more than they could consume in a day, they spent their remaining budget on cigarettes (presumably due to the risk of a hypothetical heroin overdose). Consequently, subjects purchased more cigarettes than bags of heroin in the lower range of prices. At higher prices preferences reversed, presumably because fulfilling minimum heroin needs left no funds for cigarettes.
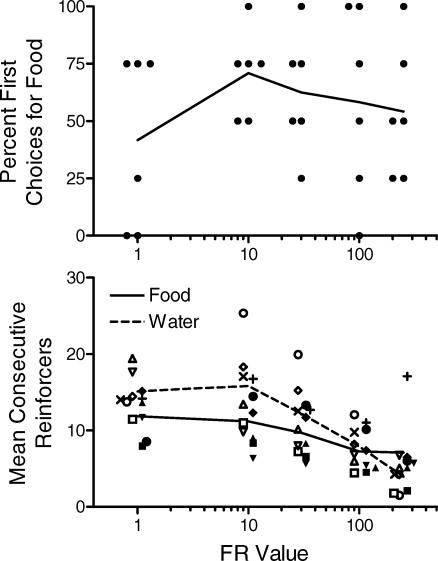
The present experiment is an interesting test of this integration of normalized demand with minimum needs because food and water are strong complements; therefore, the minimum daily intake of one reinforcer (e.g., dry food) cannot be met without also periodically consuming the other (water; Bolles, 1961; Collier & Knarr, 1966). Therefore, we would not expect our rats to fulfill their relatively stronger minimum need, in this case for food given higher Omax values, to the exclusion of water. Instead rats should start the session by responding for food but after consuming some relatively small number of food pellets, the marginal utility of more pellets should fall below that of water. Thus, responding for water should emerge before the minimum daily need for food is fulfilled.
The top panel of Figure 7 shows that the first of these predictions was not supported. When examined across the range of FR values, the rats did not reliably begin the four stable sessions by responding for either food or water (binomial χ2 = 2.79, p > .05). The lower panel of Figure 7 shows the average number of consecutive food (and water) reinforcers consumed by individual rats in a bout before switching to water (food). As expected, rats did not usually consume large quantities of a single reinforcer in a continuous bout. Instead they consumed between 5 and 20 of any one reinforcer at a time, and bout size was inversely related to FR value.
Fig 7.
Upper Panel: Percentage of the final four stable sessions that rats began by choosing a food reinforcer. Data points show individual subjects' percentages and lines connect the group averages. Lower Panel: Average number of consecutive food (open symbols to the left of each FR value) and water (closed symbols to the right of each FR) reinforcers obtained per consumption bout. The different symbols correspond to individual rats and the lines connect the group averages.
A final, and certainly the most important choice-related prediction of the integration of the normalized demand analysis and minimum needs hypothesis is that as FR values increase, rats should allocate their increasingly limited resources toward fulfilling their minimum need for food over their relatively weaker minimum need for water. As shown in Figure 5, the evidence for this prediction was marginal. Choosing food increased significantly as a function of FR value but the increase was modest and the majority of rats never selected food more than water even at FR 250.
Two comments appear warranted given the results of these predictions. First, as noted above, the minimum needs for strong complements like food and water are not independent. Thus, unlike in previous studies reviewed above and elsewhere (Madden et al., 2007), rats were not expected to fulfill their minimum food needs and then use their remaining session time to fulfill their need for water. Second, there are hints that somewhat stronger preferences for food may have emerged at higher FR values if they could have been investigated. Rat 1, for example, more often chose food over water in the two sessions completed at FR 250. Consistent with this, the lower panel of Figure 7 reveals that the difference in individual rats' food- and water-consumption bouts declined as ratio values increased. Whether water bouts would have dipped below bouts of consecutive food reinforcers at higher ratio values is unknown. As noted above, more definitive data could only come from an examination of demand for, and choice of, nonessential commodities that function as economic complements. With these commodities, steady-state demand and choice may be assessed at high ratio values without impinging on the nutritional and hydration requirements of the organism.
In sum, the results of the present study offer further support for the predictions of the normalized demand analysis and for Omax as a measure of relative reinforcer efficacy. The non-normalized demand analysis garnered mixed support, which may have been due to our inability to sample a sufficient portion of the complete food and water demand curves. Further research with nonessential commodities may help further to outline the relative merits of these two behavioral-economic approaches to the topic of relative reinforcer efficacy.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse (1 R15 DA016569-01). The authors thank Wayne Chen and Travis Smith for their assistance in data collection.
References
- Adolph E.F. Physiology of man in the desert. New York: Interscience; 1947. [Google Scholar]
- Allison J, Mack R. Polydipsia and autoshaping: Drinking and lever pressing as substitutes for eating. Animal Learning and Behavior. 1982;10:465–475. [Google Scholar]
- Bickel W.K, Madden G.J. A comparison of measures of relative reinforcing efficacy and behavioral economics: Cigarettes and money in smokers. Behavioural Pharmacology. 1999;10:627–637. doi: 10.1097/00008877-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Bickel W.K, Marsch L.A, Carroll M.E. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: A theoretical proposal. Psychopharmacology. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Bolles R.C. The interaction of hunger and thirst in the rat. Journal of Comparative and Physiological Psychology. 1961;54:580–584. doi: 10.1037/h0044595. [DOI] [PubMed] [Google Scholar]
- Collier G, Knarr F. Defense of water balance in the rat. Journal of Comparative and Physiological Psychology. 1966;61:5–10. doi: 10.1037/h0022867. [DOI] [PubMed] [Google Scholar]
- Depoortere R.Y, Li D.H, Lane J.D, Emmet-Oglesby M.W. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacology, Biochemistry & Behavior. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Green L, Freed D.E. The substitutability of reinforcers. Journal of the Experimental Analysis of Behavior. 1993;60:141–158. doi: 10.1901/jeab.1993.60-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R.R, Brady J.V, Bradford L.D. Predicting the abuse liability of drugs with animal drug self-administration procedures: Psychomotor stimulants and hallucinogens. In: Thompson T, Dews P.B, editors. Advances in Behavioral Pharmacology: Vol. 2. New York: Academic Press; 1979. pp. 163–208. [Google Scholar]
- Hursh S.R. The economics of daily consumption controlling food- and water-reinforced responding. Journal of the Experimental Analysis of Behavior. 1978;29:475–491. doi: 10.1901/jeab.1978.29-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh S.R, Galuska C.M, Winger G, Woods J.H. The economics of drug abuse: A quantitative assessment of drug demand. Molecular Interventions. 2005;5:20–28. doi: 10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- Hursh S.R, Raslear T.G, Bauman R, Black H. The quantitative analysis of economic behavior with laboratory animals. In: Grunert K.G, Ölander F, editors. Understanding economic behaviour. Boston: Kluwer Academic; 1989. pp. 393–407. [Google Scholar]
- Hursh S.R, Raslear T.G, Shurtleff D, Bauman R, Simons L. A cost-benefit analysis of demand for food. Journal of the Experimental Analysis of Behavior. 1988;50:419–440. doi: 10.1901/jeab.1988.50-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh S.R, Winger G. Normalized demand for drugs and other reinforcers. Journal of the Experimental Analysis of Behavior. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E.A, Bickel W.K. Modeling drug consumption in the clinic using simulation procedures: Demand for heroin and cigarettes in opioid-dependent outpatients. Experimental and Clinical Psychopharmacology. 1999;7:412–426. doi: 10.1037//1064-1297.7.4.412. [DOI] [PubMed] [Google Scholar]
- Johnson M.W, Bickel W.K. Replacing relative reinforcing efficacy with behavioral economic demand curves. Journal of the Experimental Analysis of Behavior. 2006;85:73–93. doi: 10.1901/jeab.2006.102-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.W, Bickel W.K, Kirshenbaum A.P. Substitutes for tobacco smoking: A behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes. Drug and Alcohol Dependence. 2004;74:253–264. doi: 10.1016/j.drugalcdep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Kagel J.H, Battalio R.C, Green L. Economic choice theory: An experimental analysis of animal behavior. New York: Cambridge University Press; 1995. [Google Scholar]
- Kagel J.H, Battalio R.C, Green L, Rachlin H. Consumer demand theory applied to choice behavior in rats. In: Staddon J.E.R, editor. Limits to action: The allocation of individual behavior. New York: Academic Press; 1980. pp. 237–267. [Google Scholar]
- Kagel J.H, Dwyer G.P, Jr, Battalio R.C. Bliss points vs. minimum-needs: Tests of competing motivational models. Behavioral Processes. 1985;11:61–77. doi: 10.1016/0376-6357(85)90103-2. [DOI] [PubMed] [Google Scholar]
- Katz J.L. Models of relative reinforcing efficacy of drugs and their predictive utility. Behavioural Pharmacology. 1990;1:283–301. doi: 10.1097/00008877-199000140-00003. [DOI] [PubMed] [Google Scholar]
- Ko M.C, Terner J, Hursh S, Woods J.H, Winger G. Relative reinforcing effects of three opioids with different durations of action. Journal of Pharmacology and Experimental Therapeutics. 2002;301:698–704. doi: 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- Madden G.J, Smethells J.R, Ewan E.E, Hursh S.R. Tests of behavioral-economic assessments of relative reinforcer efficacy: Economic substitutes. Journal of the Experimental Analysis of Behavior. 2007;87:219–240. doi: 10.1901/jeab.2007.80-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello N.K, Mendelson J.H, Sellers M.L, Kuehnle J.C. Effects of heroin self-administration on cigarette smoking. Psychopharmacology. 1980;67:45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- Pollak R.A, Wales T.J. Comparison of the quadratic expenditure system and translog demand systems with alternative specifications of demographic effects. Econometrica. 1980;48:595–612. [Google Scholar]
- Rachlin H, Krasnoff J. Eating and drinking: An economic analysis. Journal of the Experimental Analysis of Behavior. 1983;39:385–404. doi: 10.1901/jeab.1983.39-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raslear T.G, Bauman R.A, Hursh S.R, Shurtleff D, Simmons L. Rapid demand curves for behavioral economics. Animal Learning and Behavior. 1988;16:330–339. [Google Scholar]
- Robinson E.A, Adolph E.F. Patterns of normal water drinking in dogs. American Journal of Physiology. 1943;139:39–44. [Google Scholar]
- Shahan T.A, Bickel W.K, Madden G.J, Badger G.J. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: A behavioral economic analysis. Psychopharmacology. 1999;147:210–216. doi: 10.1007/s002130051162. [DOI] [PubMed] [Google Scholar]
- Shurtleff D, Warren-Boulton F.R, Silberberg A. Income and choice between different goods. Journal of the Experimental Analysis of Behavior. 1987;48:263–275. doi: 10.1901/jeab.1987.48-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, Lesage M.G, Glowa J.R. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: A review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Verplanck W.S, Hayes J.R. Eating and drinking as a function of maintenance schedule. Journal of Comparative Physiology and Psychology. 1953;45:96–102. doi: 10.1037/h0055380. [DOI] [PubMed] [Google Scholar]
- Winger G, Hursh S.R, Casey K.L, Woods J.H. Relative reinforcing strength of three n-methyl-d-aspartate antagonists with different onsets of action. Journal of Pharmacology and Experimental Therapeutics. 2002;301:690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- Woolverton W.L, Nader M.A. Experimental evaluation of the reinforcing effects of drugs. In: Adler M.W, Cowan A, editors. Testing and evaluation of drugs of abuse. Oxford, England: John Wiley & Sons; 1990. pp. 165–192. [Google Scholar]