Abstract
Background
Animal and in vitro studies have indicated that human male reproductive disorders can arise as a result of disrupted androgen receptor (AR) signalling by persistent organic pollutants (POPs). Our aim in the present study was to compare serum xenoandrogenic activity between study groups with different POP exposures and to evaluate correlations to the POP proxy markers 2,2′,4,4′,5,5′-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis(p-chlorophenyl)-ethylene (p,p′-DDE).
Methods
We determined xenoandrogenic activity in the serum fraction containing the lipophilic POPs but free of endogenous hormones. Adult male serum (n = 261) from Greenland, Sweden, Warsaw (Poland), and Kharkiv (Ukraine) was analyzed. Xenoandrogenic activity was determined as the effect of serum extract alone (XAR) and in the presence of the synthetic AR agonist R1881 (XARcomp) on AR transactivated luciferase activity.
Results
The study groups differed significantly with respect to XARcomp activity, which was increased in the Inuits and decreased in the European study groups; we observed no difference for XAR activity. We found the highest level of the AR antagonist p,p′-DDE in Kharkiv, and accordingly, this study group showed the highest percent of serum samples with decreased XARcomp activities. Furthermore, the percentage of serum samples with decreased XARcomp activities followed the p,p′-DDE serum level for the European study groups. No correlations between serum XAR or XARcomp activities and the two POP markers were revealed.
Conclusions
The differences in XARcomp serum activity between the study groups suggest differences in chemical exposure profiles, genetics, and/or lifestyle factors.
Keywords: AR activity; CB-153; human serum; polychlorinated biphenyls; p,p′-DDE
The endocrine-disrupting potential of persistent organic pollutants (POPs) such as organochlorine pesticides, polychlorinated biphenyls (PCBs), and polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDDs/PCDFs) has received significant attention since the early 1990s. These endocrine-disrupting chemicals (EDCs) have the potential to mimic, enhance, or antagonize the biological activity of endogenous hormones and thereby interfere with the development and function of the immune, reproductive, and central nervous systems, and thus the cognitive development in animals and humans (Ahmed 2000; Bonefeld-Jørgensen 2004; Bonefeld-Jørgensen and Ayotte 2003; Hotchkiss et al. 2002; Jacobson and Jacobson 1996; McLachlan et al. 2001; Patandin et al. 1999). The use of many organochlorine pesticides and PCBs has been restricted or banned since the 1970s in most industrialized countries, but their lipophilic and persistent nature cause bioaccumulation and magnification in the marine food web and piscivorous birds (Dewailly et al. 1993), and thus continue to be a potential health threat to wildlife and humans. POPs are globally ubiquitous due to long-range transport by atmospheric and oceanic currents and are found in, for example, Arctic areas (Barrie et al. 1992; Macdonal et al. 2000). Many PCB congeners, PCDDs/PCDFs, and pesticides [e.g. 2,2-bis(p-chlorophenyl)-1,1,1-trichloroethane (DDT) and its main metabolite 1,1-dichloro-2,2-bis(p-chlorophenyl)-ethylene (p,p′-DDE)] have been found in human blood, adipose tissue, and breast milk (Archibeque-Engle et al. 1997; Bonefeld-Jørgensen and Ayotte 2003; Turusov et al. 2002).
The androgen receptor (AR) is the key regulatory element of androgen cell signaling and is essential for male reproductive functions and development, including spermatogenesis (Sharpe 2006). Antagonistic effects on the AR activity in vitro have been reported for several pesticides including p,p′-DDE (Kelce et al. 1995), dieldrin (Andersen et al. 2002; Roy et al. 2004), fenarimol (Andersen et al. 2002), methiocarb (Andersen et al. 2002; Birkhoj et al. 2004), prochloraz (Andersen et al. 2002; Birkhoj et al. 2004; Vinggaard et al. 2002), and vinclozolin (Kelce et al. 1994). Furthermore, several PCB congeners (CBs 49, 66, 74, 77, 105, 118, 126, 138, 153, and 156) have been reported to act as AR antagonists (Bonefeld-Jørgensen et al. 2001; Endo et al. 2003; Schrader and Cooke 2003).
During recent years there has been a concern regarding a possible time-related deterioration of human sperm production and a concomitant increase in the incidence of testicular germ cell cancer and congenital abnormalities of male reproductive organs, such as hypospadias and cryptorchidism (Huyghe et al. 2003; Manson and Carr 2003; Skakkebaek 2003; Toppari 1996; Vidaeff and Sever 2005). Virtanen et al. (2005) suggested that the so-called testicular dysgenesis syndrome (poor sperm counts, testicular cancer, hypospadias, and cryptorchidism) is the result of genetic predisposition combined with certain environmental- and lifestyle–related exposures, including EDCs. Many POPs have shown hormone-disrupting activities in experimental studies, and prenatal animal exposures to pesticides, PCBs, and p,p′-DDE clearly have shown reproductive disruptive effects, including decreased sperm quality and malformations (Gray et al. 1999, 2000; Kelce et al. 1997; Kitamura et al. 2003; Shono et al. 2004).
The combined effects of the large number of environmental contaminants present in human and wildlife are unexplored. Studies on the combined effects of some estrogenic and antiandrogenic chemicals performed in vitro and in rats (Birkhoj et al. 2004; Gray et al. 2001, 2004; Hotchkiss et al. 2004; Nellemann et al. 2003; Payne et al. 2001) have shown that the combined effects of single compounds of low potency cannot be ignored. In fact, Rajapakse et al. (2002) showed in vitro that xenoestrogen mixtures below their no observed effect concentration caused a dramatic additive enhancement of hormone actions.
The present study is part of a large collaborative research project, INUENDO, funded by the European Union Commission with the overall aim to estimate the impact of POP exposure on human fertility. Cohorts were established in three European countries—Sweden, Poland, and Ukraine—together with cohorts of Inuits from Greenland. 2,2′,4,4′,5,5′-Hexachlorobiphenyl (CB-153) and p,p′-DDE were used as proxy biomarkers of exposure to POPs. The specific aim of the present study was to determine the actual levels of xenoandrogenic activity in the serum fractions containing lipophilic POPs but free of endogenous hormones, and to evaluate whether xenoandrogenic activity was associated with the two POP proxy markers. We used the AR-mediated chemically activated luciferase expression (AR-CALUX) bioassay to assess the integrated biological activity of AR-active lipophilic POP compounds in their actual mixture in serum.
Xenoestrogenic activity in human adipose tissue and the serum POP fraction free of endogenous hormones have previously been described (Bonefeld-Jørgensen et al. 2006; Fernandez et al. 2004; Ibarluzea et al. 2004; Rasmussen et al. 2003; Rivas et al. 2001), and total androgenic bioactivity has been measured in whole human serum, including endogenous hormones (Paris et al. 2002; Raivio et al. 2001; Roy et al. 2006). However, the present study is, to our knowledge, the first report of the actual xenoandrogenic activity of the lipophilic POP faction of human serum. Our hypothesis was that xenoandrogenic activity would differ between study groups because of different exposure profiles and that the selected POP proxy markers would correlate to the xenoandrogenic activities.
Methods
Study population and collection of blood samples
Subjects for the main INUENDO study were recruited among pregnant women and their male spouses from May 2002 through February 2004 in 19 cities and settlements in Greenland, in Warsaw (Poland), and in Kharkiv (Ukraine) (Toft et al. 2005). In addition to the pregnant couples, an already established cohort of fishermen from Sweden (Rignell-Hydbom et al. 2005) was included. Altogether, 798 men provided semen and blood samples. In the present study, we analyzed serum samples from a subset of the INUENDO study groups, consisting of 261 adult males (37 Inuits from Greenland, 83 men from Warsaw, 59 from Sweden, and 82 from Kharkiv). We had intended to analyze 400 serum samples of high/low POP exposure; however, in some cases too little blood was collected for the analysis. Because of time limitations, the serum samples were randomly selected before POP determination. The study was approved by the local ethical committees representing all participating populations, and all subjects gave written or oral informed consent. Venous blood samples were collected into 10-mL vacuum tubes; after centrifugation, serum samples were transferred to brown glass bottles (Termometerfabriken, Gothenburgh, Sweden).
Determination of CB-153 and p,p′-DDE
Serum concentrations of CB-153 and p,p′-DDE were determined using solid phase extraction (SPE) and on-column degradation of lipids, followed by analysis with gas chromatography–mass spectrometry (Rignell-Hydbom et al. 2004). The relative standard deviations, calculated from samples analyzed in duplicate on different days, was < 18% for CB-153 and < 11% for p,p′-DDE. The detection limits were 0.05 ng/mL for CB-153 and 0.1 ng/mL for p,p′-DDE. The analyses of CB-153 and p,p′-DDE were part of the Round Robin intercomparison program (H. Drexler, Institute and Out-Patient Clinic for Occupational, Social and Environmental Medicine, University of Erlangen-Nuremberg, Germany) with analysis results within the tolerance limits. The POP concentrations were adjusted for serum lipids analyzed with enzymatic methods, and the interassay coefficients of variation (CVs) were 1.5–2.0% (Jonsson et al. 2005).
SPE-HPLC fractionation of the serum samples
To obtain the serum fraction containing the actual mixture of bioaccumulated lipophilic POPs, we performed an SPE followed by high performance liquid chromatography (HPLC) fractionation on 3.6 mL serum (Bonefeld-Jørgensen et al. 2006; Hjelmborg et al. 2006). The first HPLC fraction (F1: 0.00–5.30 min, protected from light in brown tubes), which included most POPs while leaving out the endogenous hormones (Hjelmborg et al. 2006), was evaporated to near dryness and frozen for later AR-CALUX analysis. To verify that the endogenous hormones were separated from F1 but present in F2.1 (5.30–12.0 min), we performed AR-CALUX analyses of the F1 and F2.1 SPE-HPLC fractions of different male serum.
Batches of serum from the blood bank of Aarhus Sygehus (Denmark) were combined and distributed into 3.6-mL portions and stored at –80°C. This was done for male (KHM) and female (KHF) serum; on a weekly basis, one sample from each sex was processed by the SPE-HPLC method in parallel with the project samples; these samples served as serum controls for this cleanup procedure. The mean intra-CV (coefficient of variation within the same assay) of these serum controls was < 11%, and the inter-CV (day to day) was < 31% (n = 12).
On the day of analyses, the SPE-HPLC F1 fractions (project samples and serum controls) were dissolved as previously described (Bonefeld-Jørgensen et al. 2006; Hjelmborg et al. 2006). The serum samples were analyzed randomly, and we attempted to analyze serum from different study groups in each independent assay.
AR-CALUX assay
AR transactivity was determined in Chinese Hamster ovary cells (CHO-K1) by transient cotransfection with the MMTV-LUC reporter vector (kindly provided by R.M. Evans, The Salk Institute for Biological Studies, La Jolla, CA, USA) and the AR expression plasmid pSVAR0 (kindly provided by A.O. Brinkmann, Erasmus University, Rotterdam, the Netherlands).
The synthetic AR agonist methyltrienolone (R1881) and the antagonist hydroxyflutamide (HF; Mikromol GmbH, Luckenwalde, Germany), both protected from the light, were used as dose–response controls in each assay. We determined the EC50 (median effective concentration) of R1881 (0.025 nM) and the IC50 (median inhibitory concentration) of HF (58.5 nM) using the Chapman, 4 parameters equation in Sigma Plot (SPSS Inc., Chicago, IL, USA). The R1881 EC50 served as a positive control on each separate plate and was used to determine the competitive effect of HF and the F1 serum fraction. The solvent controls (± R1881 EC50) consisted of samples treated the same as the SPE-HPLC F1 fractions, but without the serum. The solvent control and the R1881 EC50 solvent control had inter-CVs of ≤ 11% (n = 13) and ≤ 13% (n = 13), respectively. The xeno-AR activity was determined in the F1 serum fraction alone (XAR), and the competitive xeno-AR activity (XARcomp) was determined upon co-treatment of cells with F1 and R1881 EC50.
We performed the reporter gene assay as described by Birkhoj et al. (2004) with minor modifications: The transfection was carried out with 100 ng DNA, and the transfection reagent Fugene was not removed before application of the samples; the dissolved SPE-HPLC F1 samples ± R1881 EC50, in triplicate, were added to the cells in parallel with the described controls (KHM, KHF, R1881 EC50, and solvent ± R1881 EC50). Cell lysis, followed by luciferase activity and protein measurements, were performed as described by Bonefeld-Jørgensen et al. (2006).
The luciferase activity was corrected to the cell protein content and expressed in relative luciferase units per microgram protein (RLU/protein). If one of the triplicate values deviated > 30% from the other two values, the mean was calculated from two wells only. To standardize the data for transfection efficiency, the XAR and XARcomp activity data was normalized to the respective solvent controls, which were set to 1. Finally, the data was given as activity per milliliters of serum, and the values of the solvent controls were 3.13 RLU/mL serum.
Statistical analysis
We checked data distribution using Q-Q plots. The XAR and XARcomp activities and lipid-adjusted CB-153 and p,p′-DDE were natural-log (ln) transformed to improve normality, and the statistical analyses were performed on the continuous ln-transformed data.
We performed the comparisons of means of XAR and XARcomp among the different study groups using one-way analysis of variance (ANOVA). If significant differences were observed between the groups, multiple comparison ad hoc tests were performed using the least-significant difference (LSD) pair-wise multiple comparison test for variables with equal variance (p > 0.05) and Dunnett’s T3 test for variables with an unequal variance (p ≤ 0.05). We tested for homogeneity of variance using Levene’s test.
We used Spearman’s rank correlation to evaluate associations of the xenoandrogenic activity both to POP markers and to endogenous serum estradiol and testosterone (total and free), which served as method verification that the sex hormones were excluded from the F1 fraction. The overall association between POP markers and xenoandrogenic activity across the study groups were evaluated by comparing the regression lines for each study group using multiple linear regression analysis. In the first step, we investigated whether the associations across the study groups are homogenous. If this hypothesis was accepted, we used a model with parallel regression lines to analyze common slopes and intercepts of the regression lines for each study group. If both the hypothesis of a common slope and the hypothesis of a common intercept were accepted, indicating that the regression lines are equal, the data for the study groups could be combined. However, if one or both of the hypotheses were rejected, the regression lines are not equal and the data cannot be combined.
Our hypothesis was that a potential determinant of POP bioaccumulation might also be a potential determinant for serum xeno-androgenic activity. From the literature and from an assessment of the total INUENDO study populations (Jonsson et al. 2005; Toft et al. 2005), age and seafood intake are known determinants affecting the POP serum level. We also evaluated lifestyle characteristics (Table 1) as potential determinants of XAR and XARcomp activities. Using multivariate linear regression to evaluate the impact of POP biomarkers and potential determinants on XAR/XARcomp, blocks of variables, together with either CB-153 or p,p′-DDE, were entered as follows: In block I, age and seafood intake were included in the model; and in block II, age, seafood, smoking status (smoked ever, yes/no), body mass index (BMI), and alcohol and coffee intake were included. Because many values of the potential determinants were missing, the number of available observations in the confounder analyses are much smaller than in the unadjusted analysis on the full data set (full data set, n = 250; block I of confounders, n = 192; block II, n = 104). Therefore, we performed the analysis only on the block II data set (n = 104) containing information about all the determinants. The statistical analysis was performed in SPSS 10.0 (SPSS Inc, Chicago, IL, USA). We considered a p-value ≤ 0.05 to be statistically significant.
Table 1.
Characteristics of the men in the study groups.
| Greenland | Warsaw | Sweden | Kharkiv | All | |
|---|---|---|---|---|---|
| Age (years) | |||||
| No. | 35 | 81 | 58 | 79 | 253 |
| Median (SD) | 30 (6.0) | 30 (4.1) | 44 (9.8) | 25 (5.4) | 30 (9.6) |
| Min–max | 18–46 | 24–46 | 24–68 | 16–45 | 16–68 |
| BMI (kg/m2) | |||||
| No. | 36 | 80 | 59 | 80 | 255 |
| Median (SD) | 26 (3.7) | 25 (3.4) | 26 (2.8) | 23 (3.1) | 25 (3.3) |
| Min–max | 19–35 | 19–38 | 22–36 | 19–36 | 19–38 |
| Alcohol (drinks/week) | |||||
| No. | 23 | 71 | 61 | 155 | |
| Median (SD) | 2.8 (4.6) | 4.0 (6.5) | NA | 3.0 (2.9) | 3.0 (5.2) |
| Min–max | 0–31 | 0–30 | 0.5–15 | 0–31 | |
| Smoking | |||||
| Percent (No.) | 86 (35) | 49 (81) | 64 (59) | 82 (81) | 68 (256) |
| Seafood (days/week) | |||||
| No. | 36 | 76 | 80 | 192 | |
| Median (SD) | 1.5 (1.5) | 1.0 (1.2) | NA | 4.0 (1.2) | 2.0 (1.6) |
| Min–max | 0–7 | 0–9 | 1–9 | 0–9 | |
| Coffee (cups/day) | |||||
| No. | 34 | 70 | 35 | 139 | |
| Median (SD) | 3.0 (4.0) | 1.3 (1.2) | NA | 2.0 (1.2) | 2.0 (2.5) |
| Min–max | 0–12 | 0–6 | 1–7 | 0–12 | |
| Total T (nmol/Ll) | |||||
| No. | 11 | 69 | 58 | 82 | 220 |
| Median (SD) | 15 (4.1) | 13 (4.3) | 11 (5.0) | 18 (4.5) | 15 (5.3) |
| Min–max | 10–23 | 6–23 | 4–28 | 8–31 | 4–31 |
| Free T (nmol/L) | |||||
| No. | 11 | 69 | 58 | 82 | 220 |
| Median (SD) | 0.32 (0.07) | 0.30 (0.08) | 0.24 (0.08) | 0.39 (0.10) | 0.30 (0.11) |
| Min-max | 0.11–0.49 | 0.15–0.51 | 0.09–0.50 | 0.20–0.64 | 0.09–0.64 |
| Estradiol (nmol/L) | |||||
| No. | 11 | 69 | 58 | 82 | 220 |
| Median (SD) | 59 (16) | 70 (32) | 64 (21) | 79 (24) | 71 (27) |
| Min–max | 31–85 | 45–297 | 25–154 | 33–144 | 25–297 |
Abbreviations: max, maximum; Min, minimum; NA, not available; T, testosterone. No. is the number of individuals with data for the specific demographic and lifestyle characteristics.
Results
Correlations between xenoandrogenic serum activity and endogenous hormones: method verification
AR-CALUX analysis of the SPE-HPLC serum fractions F1 and F2.1 showed that the F2.1 fraction (containing androsterone and testosterone,) elicited significantly higher XAR activity than the F1 fraction (free of endogenous hormones) (Figure 1A). This indicates that the endogenous androgens were excluded from the F1 fraction. The XAR activity of the KHM and KHF F1 serum controls did not differ significantly from the solvent control, whereas the XARcomp activity of KHM was significantly lower than the R1881 EC50 solvent control (Figure 1B).
Figure 1.
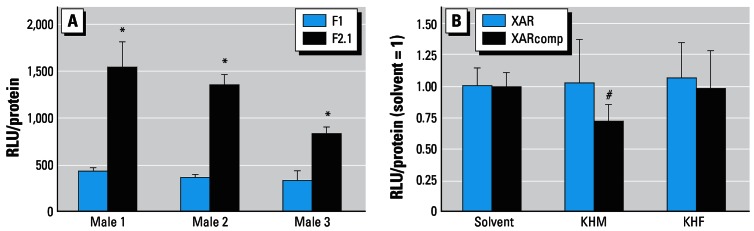
Verification of the HPLC procedure. (A) XAR activity of the HPLC fractions F1 (0.00–5.30 min; without endogenous hormones) and F2.1 (5.30–12.0 min; with endogenous hormones) from three different males were determined in the AR-CALUX assay. (B) Mean ± SE of the F1 fraction of KHM and KHF serum controls alone (XAR; n = 12) or in the presence of R1881 EC50 (XARcomp; n = 9).
*XAR activity of the F2.1 fraction significantly higher than for the F1 fraction. #Significantly lower than the R1881 EC50 solvent control.
We found no correlation between XAR and endogenous blood testosterone (free and total) and estradiol for the combined data of the study groups (data not shown).
Basic characteristics of the study groups
Demographic and lifestyle factors (Table 1) that may potentially influence the AR-mediated activities of the study groups were similar with those obtained for the total INUENDO study population (Toft et al. 2005). The levels of CB-153 and p,p′-DDE (Table 2) were in the same range as for the main study groups (Jonsson et al. 2005) as were the inter-correlations between serum concentration of CB-153 and p,p′-DDE (Greenland: rS = 0.93; Sweden: rS = 0.68; Kharkiv: rS = 0.52; Warsaw: rS = 0.35).
Table 2.
Xenoandrogenic serum activities and lipid-adjusted serum levels of CB-153 and p,p′-DDE.
| Greenland | Warsaw | Sweden | Kharkiv | All | |
|---|---|---|---|---|---|
| XAR RLU/mL serum | |||||
| No. | 37 | 83 | 59 | 82 | 261 |
| Mean ± SE | 3.85 ± 0.14 | 3.93 ± 0.21 | 3.83 ± 0.13 | 3.57 ± 0.09 | 3.78 ± 0.08 |
| Percent increased | 35 | 25 | 34 | 26 | 28 |
| Percent decreased | 3 | 5 | 5 | 2 | 4 |
| XARcomp RLU/mL serum | |||||
| No. | 37 | 83 | 59 | 82 | 261 |
| Mean ± SE | 4.05 ± 0.18 | 2.99 ± 0.07 | 3.01 ± 0.10 | 2.25 ± 0.06 | 2.91 ± 0.06 |
| Percent increased | 22 | 7 | 10 | 0 | 8 |
| Percent decreased | 3 | 21 | 8 | 50 | 26 |
| CB-153 (ng/g lipid) | |||||
| No. | 35 | 83 | 58 | 76 | 252 |
| Mean ± SE | 262 ± 34 | 20 ± 2 | 238 ± 27 | 58 ± 5 | 115 ± 10 |
| p,p′-DDE (ng/g lipid) | |||||
| No. | 35 | 83 | 58 | 76 | 252 |
| Mean ± SE | 678 ± 83 | 653 ± 37 | 299 ± 39 | 1,130 ± 77 | 718 ± 35 |
The percent increased and percent decreased indicate the percentage of serum samples (of the total number of samples) that elicited a significant increase or decrease in XAR/XARcomp activity, respectively. In each independent assay, the significant activity differences between the triple F1 fraction determinations and their respective solvent controls (percent increased and percent decreased) were determined by the Student’s f-test and t-test using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) (p ≤ 0.05).
The XAR activity of the study groups did not differ significantly (ANOVA, p = 0.51), reflecting that the percentage of serum extracts with increased XAR activity (Table 2) was similar for the four study groups (25–35%).
One-way ANOVA (p < 0.001) followed by multiple comparison of means showed that the XARcomp activity was significantly different between the study groups (LSD test, p < 0.001) except for Sweden and Warsaw (LSD test, p = 0.91). The four study groups also differed with respect to the percentage of serum samples with increased or decreased XARcomp activity (Table 2): in Greenland 22% and 3%, and in Kharkiv 0% and 50%, respectively. For the Warsaw and Swedish study groups, 21% and 8%, respectively, of the serum samples showed a decreased XARcomp activity. For the European study groups, the highest level of p,p′-DDE was found in Kharkiv, and this study group had the highest percentage of serum samples (50%) with decreased XARcomp activity. The lowest level of p,p′-DDE was observed in the Swedish study group, the group in which we found the lowest percentage of serum samples (8%) with decreased XARcomp activity.
Statistical analysis of xenoandrogenic activity and POP markers
We found no correlations between the POP markers and either XAR or XARcomp in the four study groups (data not shown). Adjusting for seafood and age (block I) and for seafood, age, smoking status, BMI, alcohol, and coffee (block II) in a multiple linear regression model did not change the results (data not shown).
Multiple regression analysis of both response variables (XAR and XARcomp) showed homogeneity of slopes between study groups and the POP markers (Table 3). Furthermore, in the model with parallel regression lines, we found common slopes and intercepts for XAR against CB-153 and p,p′-DDE (Table 3), which support the ANOVA analysis showing no difference between the study groups. However, no significant correlations were observed when data were combined (data not shown). For XARcomp against CB-153 and p,p′-DDE, we found common slopes but different intercepts (Table 3). Thus, the difference in XARcomp activity between the study groups (ANOVA, p < 0.001) still existed after adjustment for CB-153 and p,p′-DDE.
Table 3.
Multiple regression of the combined study group data.
| Response variable | Exposure variable | Homogeneity of slopes (p-value) | Common slope estimate (SE); p-value | Common intercept | Adjusted R2 (p-value) |
|---|---|---|---|---|---|
| XAR (n = 252) | CB-153 | 0.63 | 0.003 (0.02); 0.91 | 0.45 | 0.00 |
| p,p′-DDE | 0.96 | −0.001 (0.03); 0.98 | 0.54 | 0.00 | |
| XARcomp (n = 252) | CB-153 | 0.92 | 0.01 (0.02); 0.80 | < 0.001* | 0.36 |
| p,p′-DDE | 0.56 | 0.05 (0.03); 0.10 | < 0.001* | 0.37 |
Response and exposure variables are ln-transformed. Test for homogeneity of association between exposure variable and outcome variable across study groups, p > 0.05; hypothesis of homogeneity accepted. Estimated common slope across study groups assuming homogeneity of slopes, p > 0.05; hypothesis of a common slope accepted. Test for a common intercept across study groups assuming a common slope, p > 0.05; hypothesis of a common intercept accepted. Adjusted R2 assuming a common slope, adjusted for degrees of freedom.
Statistically significant (p ≤ 0.05).
In the European study groups, the percentage of serum samples with decreased XARcomp activity followed the level of p,p′-DDE (Kharkiv > Warsaw > Sweden); therefore, we tried to analyze the combined European data. We observed a significant negative association (rS = –0.21; p = 0.001) between XARcomp and p,p′-DDE across the combined European study groups. In the multiple regression analysis of the combined European study groups, the hypothesis of a common intercept was rejected (data not shown).
Discussion
In the present study we found that a) the XARcomp activity in the Inuits was significantly different from that in the European study groups; b) the percentage of serum samples with decreased XARcomp activity in the European study groups [Kharkiv (50%) > Warsaw (21%) > Sweden (8%)] positively followed the p,p′-DDE serum level; c) the XAR activity was not significantly different between the study groups; and d) there were no significant correlations between xeno-AR activities and any of the two POP markers.
It is well documented, both in vitro and in vivo, that p,p′-DDE is an AR antagonist (Araki et al. 2005; Gray et al. 1999; Roy et al. 2004; Vinggaard et al. 1999), which is consistent with the observed decreased XARcomp activities in the European groups. Interestingly, in a parallel INUENDO study, the fecundability among couples in Kharkiv was lower than among couples from the other study groups (Toft et al. 2005). Furthermore, the lowest sperm motility in semen samples was found in the Kharkiv samples, compared with the three other study groups (Toft et al. 2005). However, in the present study, we found high p,p′-DDE levels in serum in the Inuits, who also had the highest percentage of serum samples (22%) with increased XARcomp activity. These data from the different ethnic groups could be caused by a different composition of serum POPs, despite the high amounts of p,p′-DDE in Inuits. In parallel INUENDO studies that investigated the xenoestrogenic (Bonefeld-Jørgensen et al. 2006) and dioxin-like (Long et al. 2006) activities in human serum samples, significant differences between Inuits and the European study groups were also observed. In addition to a different exposure pattern, dietary habits and/or lifestyle factors and the genetic difference between Inuits and Caucasians (de Maat et al. 1999) may also be taken into account when evaluating the observed differences.
We found no correlations between the xeno-AR activities and the two POP markers. This is in accordance with previous studies in human adipose tissue or serum that reported the lack of correlation between several suspected endocrine disruptors and estrogenic activities (Fernandez et al. 2004; Ibarluzea et al. 2004; Rasmussen et al. 2003; Rivas et al. 2001). Furthermore, CB-153 might not be optimal as a global exposure marker for POPs because the pattern of distribution of noncoplanar and coplanar CBs can differ geographically depending on occupational and/or nonoccupational exposures (Dewailly et al. 1994).
We rejected the hypothesis of a common intercept for XARcomp activities in the multiple linear regression model. However, when we assumed a common intercept, a significant negative correlation was found between the XARcomp activity and p,p′-DDE for the combined European study groups. This suggests that p,p′-DDE might be involved in the decreased XARcomp activity in a concentration-dependent manner in the European study groups.
The total androgenic bioactivity in whole human serum samples has been investigated in cell-based reporter gene assays (Paris et al. 2002; Raivio et al. 2001; Roy et al. 2006); these assays represented reliable methods for determining androgenic activities in biological samples. However, these studies determined the total androgenic bioactivity in whole human serum, which contained the endogenous hormones. In contrast, our assay determines the xenoandrogenic activity of the serum fraction of bioaccumulated POPs only.
The inter-CV was ≤ 13% for the solvent controls and < 31% for the serum controls (KHM and KHF). However, our CVs are within the range observed in other in vitro studies (Korner et al. 2004) using reporter gene assays (the CV for the androgen control was 22% in stably transfected human prostate adenocarcinoma PC-3 cells, 30% in transiently transfected CHO-K1 cells, and 57% in stably transfected human breast carcinoma MDA-kb2 cells).The higher CV of the serum controls compared with the solvent controls in our study can be explained by further variations introduced by the SPE-HPLC extraction.
The R1881 EC50 value of 0.025 nM in our assay was somewhat lower than that seen in other reporter gene assays using other cell lines (Blankvoort et al. 2001; Christiaens et al. 2005; Korner et al. 2004; Lemaire et al. 2004). In stable transfected PC3 cells (PALM), Lemaire et al. (2004) found an EC50 for R1881 of 0.066 nM in one study, whereas Korner et al. (2004), who also used PALM cells, reported an EC50 of 0.11 nM. Thus, even in the same cell line, the EC50 value can vary between laboratories.
Hjelmborg et al. (2006) previously showed that the F1 fraction, analyzed for xenoandrogenic effects, included PCB-180, PCB-126, PCB-81, endosulfan, PCB-153, PCB-138, bisphenol A dimethacrylate, o,p′-DDT, 4n-nonylphenol, 4-octylphenol, 4-OH-PCB-121, vinclozolin, bisphenol A, butyl hydroxy anisole, and methiocarb. The F2.1 fraction contained the endogenous hormones pregnenolone, androsterone, progesterone, estrone, and testosterone, as well as the pesticides fenarimol and prochloraz (Hjelmborg et al. 2006). To verify our SPE-HPLC AR-CALUX method, we analyzed the F2.1 serum fraction in parallel with the F1 serum fraction free of endogenous hormones. The significantly higher XAR activity of the F2.1 fraction compared with the F1 fraction indicates that the endogenous hormones, which are capable of activating the AR, are present in F2.1 but not in F1 fractions. Furthermore, the XAR activity of the KHM F1 fraction did not differ from the solvent control or KHF. Because total androgenic bioactivity is higher in men than in women (Paris et al. 2002), a significantly higher XAR activity for KHM, compared with KHF, would have been expected if the endogenous hormones were present in the F1 serum fraction. Vinggaard et al. (1999) previously showed that estradiol is able to compete with R1881 for transactivation of AR in an AR-CALUX assay similar to the one used in the present study. Therefore, we investigated correlations between XAR and the sex hormones testosterone (total and free) and estradiol. We found no correlations between XAR and the sex hormones, confirming optimal separation of the endogenous hormones from the SPE-HPLC F1 fraction. In investigations of total androgen bioactivity in whole human serum samples, including endogenous hormones, Raivio et al. (2001) and Roy et al. (2006) found significant correlations between serum testosterone levels and androgen bioactivity. In a parallel study using the same SPE-HPLC approach, Bonefeld-Jørgensen (et al. 2006) observed no correlations between the endogenous hormones in serum and the xeno-estrogenic activity of the F1 serum fraction; this further confirms the separation of the endogenous hormones.
We used the serum (KHM, KHF) from Danish individuals as controls for the SPE-HPLC cleanup procedure. The XAR data of the serum controls from 12 independent assays were not significantly different from the solvent control, indicating that no compounds present in the serum sample were able to activate AR activity. However, compared with the XARcomp activity of the R1881 EC50 solvent control, the XARcomp activity of KHM was significantly lower but that of KHF was not. Thus the SPE-HPLC F1 fraction seems to contain a mixture of bioaccumulated lipophilic POPs that differ between samples and are able to interfere with the R1881-induced AR activity in the CALUX assay.
To our knowledge, this is the first study to determine the integrated biological activity of AR-active lipophilic compounds in their actual mixture in serum; therefore, potential determinants for the activity were unknown. We hypothesized seafood, age, smoking status, BMI, alcohol, and coffee to be potential determinants of AR activity. However, the data did not change upon adjustment for these potential determinants. A different possibility is that other exogenous chemicals might confound the obtained data and that the two selected POP markers do not adequately reflect and/or represent the chemicals primarily responsible for the determined xenoandrogenic effects.
Conclusions
We found a significantly higher percentage of serum samples with decreased XARcomp activity for the Kharkiv study group, which had the highest p,p′-DDE level of the four study groups. Moreover, in the European study groups, the percentage of serum samples with decreased XARcomp activity followed the p,p′-DDE serum levels. In the Inuit study group, we found a significantly higher percentage of serum samples with increased XARcomp activity compared with the European study groups. No consistent correlations were found between XAR/XARcomp and the two POP proxy markers for the individual study groups or across study groups, suggesting that neither CB-153 nor p,p′-DDE alone can predict the xenoandrogenic activity of the serum samples included in the present study.
Our hypothesis was that xenoandrogenic activity would differ between the study groups with different POP profiles, and that the two selected POP markers would correlate to the xenoandrogenic activities. The first hypothesis was accepted: The differences in XARcomp serum activity between the study groups seem to reflect differences in chemical exposure profiles, genetics, and/or lifestyle factors. However, the second hypothesis was rejected; therefore, the selected POP markers could not be used as biomarkers of exposure to determine the xenoandrogenic effects.
The present ex vivo biomarker AR-CALUX activity assay may contribute to the insight of the impact of the total bio-accumulated EDCs geographically, which is difficult to predict on the basis of chemical analysis alone. However, the determined XAR/XARcomp activities of the serum fraction containing lipophilic POPs may fail to detect effects accomplished by hydrophilic compounds or effects through other receptors or other mechanisms.
Footnotes
This article is part of the monograph “Endocrine Disruptors—Exposure Assessment, Novel End Points, and Low-Dose and Mixture Effects.”
We thank I. Sørensen, B.S. Andersen, H. Åkesson, B. Holmskov, and C. Held for technical assistance; M. Ghisari and M. Long for scientific support; and J.K. Ludwicki and V. Zvyezday for coordination of the blood sampling.
This study is part of the INUENDO Project supported by The European Commission (Contract no. QLK4-CT-2001-00202). The work was also funded by the Danish Environmental Protection Agency, the Swedish Research Council, and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning. The Ukrainian part of the study was made possible by a grant from INTAS (International Association for the Promotion of Cooperation with Scientists from the New Independent States of the Former Soviet Union; project 012 2205).
References
- Ahmed SA. The immune system as a potential target for environmental estrogens (endocrine disrupters): a new emerging field. Toxicology. 2000;150(1–3):191–206. doi: 10.1016/s0300-483x(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Andersen HR, Vinggaard AM, Rasmussen TH, Gjermandsen IM, Bonefeld-Jørgensen EC. Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol Appl Pharmacol. 2002;179(1):1–12. doi: 10.1006/taap.2001.9347. [DOI] [PubMed] [Google Scholar]
- Araki N, Ohno K, Takeyoshi M, Iida M. Evaluation of a rapid in vitro androgen receptor transcriptional activation assay using AR-EcoScreen cells. Toxicol In Vitro. 2005;19(3):335–352. doi: 10.1016/j.tiv.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Archibeque-Engle SL, Tessari JD, Winn DT, Keefe TJ, Nett TM, Zheng T. Comparison of organochlorine pesticide and polychlorinated biphenyl residues in human breast adipose tissue and serum. J Toxicol Environ Health. 1997;52(4):285–293. doi: 10.1080/00984109708984065. [DOI] [PubMed] [Google Scholar]
- Barrie LA, Gregor D, Hargrave B, Lake R, Muir D, Shearer R, et al. Arctic contaminants: sources, occurrence and pathways. Sci Total Environ. 1992;122(1–2):1–74. doi: 10.1016/0048-9697(92)90245-n. [DOI] [PubMed] [Google Scholar]
- Birkhoj M, Nellemann C, Jarfelt K, Jacobsen H, Andersen HR, Dalgaard M, et al. The combined antiandrogenic effects of five commonly used pesticides. Toxicol Appl Pharmacol. 2004;201(1):10–20. doi: 10.1016/j.taap.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Blankvoort BM, de Groene EM, van Meeteren-Kreikamp AP, Witkamp RF, Rodenburg RJ, Aarts JM. Development of an androgen reporter gene assay (AR-LUX) utilizing a human cell line with an endogenously regulated androgen receptor. Anal Biochem. 2001;298(1):93–102. doi: 10.1006/abio.2001.5352. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC. The Human Health Effect Programme in Greenland, a review. Sci Total Environ. 2004;331(1–3):215–231. doi: 10.1016/j.scitotenv.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated poly-chlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology. 2001;158(3):141–153. doi: 10.1016/s0300-483x(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Ayotte P. Toxicological Properties of Persistent Organic Pollutants and Related Health Effects of Concern for the Arctic Population. AMAP Assessment 2002—Human Health in the Arctic. 2003. [accessed 16 May 2007]. Available: http://amap.no/documents/
- Bonefeld-Jørgensen EC, Hjelmborg PS, Reinert TS, Andersen BS, Lesovoy V, Lindh CH, et al. Xenoestrogenic activity in blood of European and Inuit populations. Environ Health. 2006;5(1):12. doi: 10.1186/1476-069X-5-12. Online 5 May 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens V, Berckmans P, Haelens A, Witters H, Claessens F. Comparison of different androgen bioassays in the screening for environmental (anti)androgenic activity. Environ Toxicol Chem. 2005;24(10):2646–2656. doi: 10.1897/05-126r.1. [DOI] [PubMed] [Google Scholar]
- de Maat MP, Bladbjerg EM, Johansen LG, de Knijff P, Gram J, Kluft C, et al. DNA-polymorphisms and plasma levels of vascular disease risk factors in Greenland Inuit—is there a relation with the low risk of cardiovascular disease in the Inuit? Thromb Haemost. 1999;81(4):547–552. [PubMed] [Google Scholar]
- Dewailly E, Ayotte P, Bruneau S, Laliberte C, Muir DC, Norstrom RJ. Inuit exposure to organochlorines through the aquatic food chain in Arctic Quebec. Environ Health Perspect. 1993;101:618–620. doi: 10.1289/ehp.93101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, Ryan JJ, Laliberte C, Bruneau S, Weber JP, Gingras S, et al. Exposure of remote maritime populations to coplanar PCBs. Environ Health Perspect. 1994;102(suppl 1):205–209. doi: 10.1289/ehp.94102s1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo F, Monsees TK, Akaza H, Schill WB, Pflieger-Bruss S. Effects of single non-ortho, mono-ortho, and di-ortho chlorinated biphenyls on cell functions and proliferation of the human prostatic carcinoma cell line, LNCaP. Reprod Toxicol. 2003;17(2):229–236. doi: 10.1016/s0890-6238(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Fernandez MF, Rivas A, Olea-Serrano F, Cerrillo I, Molina-Molina JM, Araque P, et al. Assessment of total effective xenoestrogen burden in adipose tissue and identification of chemicals responsible for the combined estrogenic effect. Anal Bioanal Chem. 2004;379(1):163–170. doi: 10.1007/s00216-004-2558-5. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58(2):350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7(3):248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Wolf C, Lambright C, Wilson V, et al. Toxicant-induced hypospadias in the male rat. Adv Exp Med Biol. 2004;545:217–241. doi: 10.1007/978-1-4419-8995-6_14. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Wolf C, Lambright C, Mann P, Price M, Cooper RL, et al. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p′-DDE, and ketoconazole) and toxic substances (dibutyl-and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health. 1999;15(1–2):94–118. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- Hjelmborg PS, Ghisari M, Bonefeld-Jørgensen EC. SPE-HPLC purification of endocrine-disrupting compounds from human serum for assessment of xenoestrogenic activity. Anal Bioanal Chem. 2006;385(5):875–887. doi: 10.1007/s00216-006-0463-9. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenburgh JG, Gray LE., Jr Androgens and environmental antiandrogens affect reproductive development and play behavior in the Sprague-Dawley rat. Environ Health Perspect. 2002;110(suppl 3):435–439. doi: 10.1289/ehp.02110s3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, et al. A mixture of the “anti-androgens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol Reprod. 2004;71(6):1852–1861. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol. 2003;170(1):5–11. doi: 10.1097/01.ju.0000053866.68623.da. [DOI] [PubMed] [Google Scholar]
- Ibarluzea JM, Fernandez MF, Santa-Marina L, Olea-Serrano MF, Rivas AM, Aurrekoetxea JJ, et al. Breast cancer risk and the combined effect of environmental estrogens. Cancer Causes Control. 2004;15(6):591–600. doi: 10.1023/B:CACO.0000036167.51236.86. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335(11):783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Jonsson BA, Rylander L, Lindh C, Rignell-Hydbom A, Giwercman A, Toft G, et al. Inter-population variations in concentrations, determinants of and correlations between 2,2′,4,4′,5,5′-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis (p-chlorophenyl)-ethylene (p,p′-DDE): a cross-sectional study of 3161 men and women from Inuit and European populations. Environ Health. 2005;4(1):27. doi: 10.1186/1476-069X-4-27. Online 11 November 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelce WR, Lambright CR, Gray LE, Jr, Roberts KP. Vinclozolin and p,p′-DDE alter androgen-dependent gene expression: in vivo confirmation of an androgen receptor-mediated mechanism. Toxicol Appl Pharmacol. 1997;142(1):192–200. doi: 10.1006/taap.1996.7966. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE., Jr Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994;126(2):276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375(6532):581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Ohta S, Fujimoto N. Antiandrogenic activity and metabolism of the organophosphorus pesticide fenthion and related compounds. Environ Health Perspect. 2003;111:503–508. doi: 10.1289/ehp.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner W, Vinggaard AM, Terouanne B, Ma R, Wieloch C, Schlumpf M, et al. Interlaboratory comparison of four in vitro assays for assessing androgenic and anti-androgenic activity of environmental chemicals. Environ Health Perspect. 2004;112:695–702. doi: 10.1289/ehp.112-1241964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire G, Terouanne B, Mauvais P, Michel S, Rahmani R. Effect of organochlorine pesticides on human androgen receptor activation in vitro. Toxicol Appl Pharmacol. 2004;196(2):235–246. doi: 10.1016/j.taap.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Long M, Andersen BS, Lindh CH, Hagmar L, Giwercman A, Manicardi GC, et al. Dioxin-like activities in blood across European and Inuit populations. Environ Health. 2006;5(1):14. doi: 10.1186/1476-069X-5-14. Online 25 May 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonal RW, Barrie LA, Bidleman TF, Diamond ML, Gregor DJ, Semkin RG, et al. Contaminants in the Canadian Arctic: 5 years of progress in understanding sources, occurrence and pathways. Sci Total Environ. 2000;254(2–3):93–234. doi: 10.1016/s0048-9697(00)00434-4. [DOI] [PubMed] [Google Scholar]
- Manson JM, Carr MC. Molecular epidemiology of hypospadias: review of genetic and environmental risk factors. Birth Defects Res A Clin Mol Teratol. 2003;67(10):825–836. doi: 10.1002/bdra.10084. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Burow ME, Li SF. From malformations to molecular mechanisms in the male: three decades of research on endocrine disrupters. Apmis. 2001;109(4):263–272. doi: 10.1034/j.1600-0463.2001.d01-119.x. [DOI] [PubMed] [Google Scholar]
- Nellemann C, Dalgaard M, Lam HR, Vinggaard AM. The combined effects of vinclozolin and procymidone do not deviate from expected additivity in vitro and in vivo. Toxicol Sci. 2003;71(2):251–262. doi: 10.1093/toxsci/71.2.251. [DOI] [PubMed] [Google Scholar]
- Paris F, Servant N, Terouanne B, Sultan C. Evaluation of androgenic bioactivity in human serum by recombinant cell line: preliminary results. Mol Cell Endocrinol. 2002;198(1–2):123–129. doi: 10.1016/s0303-7207(02)00375-1. [DOI] [PubMed] [Google Scholar]
- Patandin S, Lanting CI, Mulder PG, Boersma ER, Sauer PJ, Weisglas-Kuperus N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J Pediatr. 1999;134(1):33–41. doi: 10.1016/s0022-3476(99)70369-0. [DOI] [PubMed] [Google Scholar]
- Payne J, Scholze M, Kortenkamp A. Mixtures of four organochlorines enhance human breast cancer cell proliferation. Environ Health Perspect. 2001;109:391–397. doi: 10.1289/ehp.01109391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio T, Palvimo JJ, Dunkel L, Wickman S, Janne OA. Novel assay for determination of androgen bioactivity in human serum. J Clin Endocrinol Metab. 2001;86(4):1539–1544. doi: 10.1210/jcem.86.4.7329. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. Combining xeno-estrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110:917–921. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen TH, Nielsen F, Andersen HR, Nielsen JB, Weihe P, Grandjean P. Assessment of xenoestrogenic exposure by a biomarker approach: application of the E-Screen bioassay to determine estrogenic response of serum extracts. Environ Health. 2003;2(1):12. doi: 10.1186/1476-069X-2-12. Online 15 October 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rignell-Hydbom A, Rylander L, Giwercman A, Jonsson BA, Lindh C, Eleuteri P, et al. Exposure to PCBs and p,p′-DDE and human sperm chromatin integrity. Environ Health Perspect. 2005;113:175–179. doi: 10.1289/ehp.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rignell-Hydbom A, Rylander L, Giwercman A, Jonsson BA, Nilsson-Ehle P, Hagmar L. Exposure to CB-153 and p,p′-DDE and male reproductive function. Hum Reprod. 2004;19(9):2066–2075. doi: 10.1093/humrep/deh362. [DOI] [PubMed] [Google Scholar]
- Rivas A, Fernandez MF, Cerrillo I, Ibarluzea J, Olea-Serrano MF, Pedraza V, et al. Human exposure to endocrine disrupters: standardisation of a marker of estrogenic exposure in adipose tissue. Apmis. 2001;109(3):185–197. doi: 10.1034/j.1600-0463.2001.090302.x. [DOI] [PubMed] [Google Scholar]
- Roy P, Franks S, Read M, Huhtaniemi IT. Determination of androgen bioactivity in human serum samples using a recombinant cell based in vitro bioassay. J Steroid Biochem Mol Biol. 2006;101(1):68–77. doi: 10.1016/j.jsbmb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Roy P, Salminen H, Koskimies P, Simola J, Smeds A, Saukko P, et al. Screening of some anti-androgenic endocrine disruptors using a recombinant cell-based in vitro bioassay. J Steroid Biochem Mol Biol. 2004;88(2):157–166. doi: 10.1016/j.jsbmb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Schrader TJ, Cooke GM. Effects of Aroclors and individual PCB congeners on activation of the human androgen receptor in vitro. Reprod Toxicol. 2003;17(1):15–23. doi: 10.1016/s0890-6238(02)00076-x. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab. 2006;20(1):91–110. doi: 10.1016/j.beem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Shono T, Suita S, Kai H, Yamaguchi Y. The effect of a pre-natal androgen disruptor, vinclozolin, on gubernacular migration and testicular descent in rats. J Pediatr Surg. 2004;39(2):213–216. doi: 10.1016/j.jpedsurg.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE. Testicular dysgenesis syndrome. Horm Res. 2003;60(suppl 3):49. doi: 10.1159/000074499. [DOI] [PubMed] [Google Scholar]
- Toft G, Axmon A, Giwercman A, Thulstrup A, Rignell-Hydbom A, Pedersen HS, et al. Fertility in four regions spanning large contrasts in serum levels of widespread persistent organochlorines: a cross-sectional study. Environ Health. 2005;4(1):26. doi: 10.1186/1476-069X-4-26. Online 9 November 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J. Is semen quality declining? Andrologia. 1996;28(6):307–308. doi: 10.1111/j.1439-0272.1996.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyl-trichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect. 2002;110:125–128. doi: 10.1289/ehp.02110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaeff AC, Sever LE. In utero exposure to environmental estrogens and male reproductive health: a systematic review of biological and epidemiologic evidence. Reprod Toxicol. 2005;20(1):5–20. doi: 10.1016/j.reprotox.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Joergensen EC, Larsen JC. Rapid and sensitive reporter gene assays for detection of antiandrogenic and estrogenic effects of environmental chemicals. Toxicol Appl Pharmacol. 1999;155(2):150–160. doi: 10.1006/taap.1998.8598. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Nellemann C, Dalgaard M, Jørgensen EB, Andersen HR. Antiandrogenic effects in vitro and in vivo of the fungicide prochloraz. Toxicol Sci. 2002;69(2):344–353. doi: 10.1093/toxsci/69.2.344. [DOI] [PubMed] [Google Scholar]
- Virtanen HE, Rajpert-De Meyts E, Main KM, Skakkebaek NE, Toppari J. Testicular dysgenesis syndrome and the development and occurrence of male reproductive disorders. Toxicol Appl Pharmacol. 2005;207(2 suppl):501–505. doi: 10.1016/j.taap.2005.01.058. [DOI] [PubMed] [Google Scholar]


