Abstract
Background
The isoflavone genistein (GEN) is found in soy (Glycine max) and red clover (Trifolium pratense). The estrogenic activity of GEN is known, and it is widely advertised as a phytoestrogen useful in alleviating climacteric complaints and other postmenopausal disorders. Knowledge of effects of long-term administration of GEN in laboratory animals is scarce, and effects in the uterus and mammary gland after long-term administration have not been studied. The uterus and mammary gland are known to be negatively influenced by estrogens used in hormone therapy.
Objectives
We administered two doses of GEN [mean daily uptake 5.4 (low) or 54 mg/kg (high) body weight (bw)] orally over a period of 3 months to ovariectomized (ovx) rats and compared the effects with a treatment with two doses of 17β-estradiol [E2; 0.17 (low) or 0.7 mg/kg bw (high)]. Mammary glands, vaginae, and uteri were investigated morphologically and immunohistochemically. We quantified the expression of proliferating cell nuclear antigen (PCNA) and progesterone receptor (PR) in the mammary gland.
Results
In rats treated with either of the E2 doses or the high GEN dose, we found increased uterine weight, and histologic analysis showed estrogen-induced features in the uteri. In vaginae, either E2 dose or GEN high induced hyperplastic epithelium compared with the atrophic controls. In the mammary gland, E2 (either dose) or GEN increased proliferation and PR expression. Serum levels of luteinizing hormone were decreased by E2 (both doses) but not by GEN.
Conclusions
In summary, E2 and GEN share many effects in the studied organs, particularly in the vagina, uterus, and mammary gland but not in the hypothalamo/pituitary unit.
Keywords: estradiol, genistein, mammary gland, uterus, vagina
In climacteric and postmenopausal women, low serum levels of 17β-estradiol (E2) often result in symptoms such as hot flashes or degenerative processes such as osteoporosis. This has prompted women to receive hormone replacement therapy (HRT) to prevent these ageing-associated symptoms or diseases (Burger 2003). Because estrogens alone stimulate endometrial proliferation, which may result in cancer, they have to be given in combination with progestins (Albertazzi and Sharma 2005). This has resulted in massive consumption of estrogens in combination with progestins. The recent Women’s Health Initiative and the British Million Women Study have thrown doubts on the exclusively beneficial effect of HRT, particularly because a higher incidence of mammary cancers and arteriosclerotic complications such as heart attacks and strokes were reported (Beral 2003; Rossouw et al. 2002). This has resulted in a search for HRT alternatives and plant-derived, so-called phytoestrogens are vigorously promoted.
Genistein (GEN) is an isoflavone found mainly in soy (Glycine max) and red clover (Trifolium pratense), and its estrogenic activity has been reported (Boue et al. 2003; Cos et al. 2003). The estrogenicity of GEN can be explained by the molecular similarity between GEN and E2 and their transactivational properties via estrogen receptors (ERs) (Bovee et al. 2004; Mueller et al. 2004; Ricketts et al. 2005; Sirtori et al. 2005).
A second ER (ER-β) was cloned in 1996 from rat prostate tissue (Kuiper et al. 1996). This ER-β was also found to be expressed in humans (Mosselman et al. 1996). Its distribution pattern is different from the former known ER, now called ER-α (Pelletier et al. 2000; Saunders et al. 1997; Shughrue et al. 1998).
Companies that produce products containing soy or red clover as food additives claim exclusive beneficial effects in post-menopausal women; hence, they advertise their products as selective ER modulators.
E2 binds to both receptors with apparently no difference in affinity, and recent binding assays showed that GEN had a higher affinity to ER-β than to ER-α (Kuiper et al. 1998). In reporter cell systems, however, GEN transactivated both ERs with similar potency (Mueller et al. 2004). In short-term animal studies, GEN stimulated uterine growth (Diel et al. 2004; Kanno et al. 2003). In the uterus, E2 stimulates endometrial proliferation; this stimulation, without addition of progestins, will result in endometrial hyperplasia and eventually neoplasia (Albertazzi and Sharma 2005). In a placebo-controlled 5-year-long clinical study, Unfer et al. (2004) found that a soy product containing 150 mg isoflavones stimulated the endometrium of women such that 3.37% developed endometrial hyperplasia, which was not observed in any of the placebo-treated women.
Another target for E2 is the vagina, where proliferation and cornification of the epithelium are induced; these are desired estrogenic effects because the lactobacillae use these cells to produce lactic acid, which keeps the vaginal milieu acidic and thus prevents ascending infections (Heinemann and Reid 2005).
Another undesired effect of E2 is stimulation of proliferation of mammary gland tissue. Proliferation-promoting and -inhibiting effects of GEN on human mammary cancer cells and on mammary cancers in a variety of animal models have been described and remain controversial (Allred et al. 2004; Dave et al. 2005; Harris et al. 2005; Jeune et al. 2005; Kijkuokool et al. 2005; Kousidou et al. 2005; Liu et al. 2005; Schmidt et al. 2005; Vantyghem et al. 2005). Early exposure of pubertal rats or girls to soy or genistein has been shown to have a preventive effect on mammary cancer development during adulthood (Cabanes et al. 2004; Lamartiniere 2002). Negligible effects on climacteric complaints and bones have also been reported (Geller and Studee 2005; Krebs et al. 2004; Phipps et al. 2002) under the treatment with soy or red clover products. Hence, it is an open question whether GEN has exclusively beneficial effects, as claimed by industrial companies, or whether they are endocrine disruptors that endanger the mammary gland or the uterus. Furthermore, little is known about the biological effects of GEN after long-term oral administration, which is the common method of treatment in postmenopausal women.
The ovariectomized (ovx) rat is a widely used model to study estrogen withdrawal and replacement because many phenomena in this rat model are similar to those occurring in postmenopausal women. Therefore, we analyzed a number of physiologic and morphologic effects induced in the mammary gland, uterus, and vagina after long-term (3 months) oral administration of two doses of GEN in ovx rats and compared them with those induced by E2 treatment. In the present study, tissue samples were analyzed histologically and immunocytochemically. E2-induced effects on body weight and in uterus and vagina are well known (Albertazzi and Sharma 2005; Asarian and Geary 2006; Heinemann and Reid 2005) and allow comparison with effects of test substances. E2-induced effects in the mammary gland have not been well described and therefore we defined own parameters; thus, this study provides not only information on effects of long-term oral administration of GEN but also quantitative data on the effects of E2 in this tissue. E2 stimulates mammary gland proliferation by a mechanism that involves ER-α (Tekmal et al. 2005); this has been associated with the increased risk of cancer development because DNA damage might occur during replication (Krebs et al. 2004). Therefore, we performed proliferation-specific analyses and determined expression of the progesterone receptor (PR), which is stimulated in the mammary gland after E2 exposure (Tekmal et al. 2005). Cells expressing both proliferating cell nuclear antigen (PCNA) protein and PR protein were quantified after immunohistochemical staining.
Materials and Methods
Animals and chemical exposures
Permission for this study was given by the Bezirksregierung (Az. 509.42502/01-36.03), which precludes inhumane treatment and painful experimentation. Sprague-Dawley rats were fed soy-free food during and after breeding, and body weights of each animal were recorded before and at termination of the experiment. Bilateral ovx was performed under isoflurane anesthesia at the age of 3 months, and animals were divided into five groups (n = 11–12/group). One group was left on soy-free food after ovx. Food was soy-free pelleted chow supplemented with potato proteins (D-59494; Ssniff Spezialdiäten GmbH, Soest, Germany). Immediately after ovx, animals in four of the groups received the same chow with added E2 [0.17 or 0.7 mg/kg body weight (bw)] or GEN (5.4 or 54 mg/kg bw].
E2 was given as estradiolbenzoate (catalog no. 8515, purity of 98.5%; Sigma, Munich, Germany). Genistein (catalog no. SG 20030618, purity of 98.5%) was a commercial product of Chemos (Regensburg, Germany). We checked the purity of the isoflavone by HPLC and ultraviolet (UV) detection at 260 nm. The HPLC conditions were gradient elution with 70% A (water containing 0.085% o-phosphoric acid), 30% B (acetonitril) for 0 min; 25% A, 75% B for 15 min; and a 250 × 4 mm C18 reverse-phase column with a flow rate of 1 mL/min.
Animals were fed these diets for 3 months and housed under standardized conditions (lights on 12 hr from 0600 hours to 1800 hours; room temperature, 23°C; 5–6 animals per cage) with free access to water and food. We estimated intake of the test substances from the mean food intake in each cage divided by the number of animals per cage. The daily food intake per animal was as follows: controls, 18.7 g; E2 low, 15.4 g; E2 high, 14.0 g; GEN low, 18.8 g; and GEN high, 15.5 g. On the basis of food intake, we estimated that the daily exposure to free E2 or GEN per animal (in milligrams per kilograms body weight) was as follows: E2 low, 0.17; E2 high, 0.7; GEN low, 5.4; GEN high, 53.
Tissue samples
Animals were sacrificed by decapitation under CO2 anesthesia between 0900 and 1200 hours; blood was collected from the trunk, and serum was stored at –20°C until analysis of E2 by radioimmunoassay (3rd Generation RIA Kit; DSL GmbH, Sinsheim, Germany). Sera of the GEN-treated animals were extracted using the SEP-PAK cartridges solid-phase method according to the standard protocol suggested by the manufacturer (Waters, Eschborn, Germany), and ethanol-redissolved aliquots were subjected to HPLC separation and UV-detection as described above.
The uteri were removed and weighed, and the fifth mammary glands and vaginae were collected. All samples were fixed in 10% buffered formalin for 48 hr. Uteri were cut such that three cross-sections per area could be prepared at proximal, medial, and distal areas. Mammary glands were cut to obtain sections from the nipple through the fat pad toward the abdominal muscles. Vaginae were prepared for longitudinal sections. All samples were embedded in paraffin, and 3-μm thick sections were cut, mounted, and stained with hematoxylin and eosin (H&E) for microscopic analysis.
Immunohistochemical analysis
We performed immunohistochemistry in mammary gland sections to identify PCNA and PR. After antigen retrieval using microwave radiation and citrate buffer (Ezaki 2000), we immunolabeled tissue sections with a monoclonal antibody for PCNA (PC-10, sc 56; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or a polyclonal antibody for PR (C19, sc 538; Santa Cruz Biotechnology). The tissue sections were then immunostained using peroxidase and diaminobenzidine (EnVision Detection System, Peroxidase/DAB, rabbit/mouse, K5007; Dako Cytomation, Hamburg, Germany). Sections were then stained with H&E. We quantified the number of PCNA-and PR-immunoreactive cells in alveolar and terminal buds of the mammary gland on one slide for each of the 11–12 animals per group; 400–600 cells were counted per slide. The number of PCNA- or PR-expressing cells was expressed as the percentage of the total number of mammary gland cells.
Morphologic analyses
For morphologic analyses of uterus, we recorded known E2-induced features including shape and polarity of the lamina propia cells, epithelial mitotic figures and necrosis, determination of hypertrophy and hyperplasia of glands and endometrial epithelium, and pathologic features such as squamous metaplasia and pyometra. We also measured the thickness of endometrium and myometrium. The parameters of estrogenic effects in the vaginae were number of epithelial cell layers and their cornification. In the mammary glands, we determined the degree of luminal formation and the presence or absence of secretion. Quantification of these parameters was performed on three slides per animal; at least 44 slides per group were evaluated.
Statistical analyses
We set the number of PCNA and PR positive cells per slide in relation to the total number of alveolar and terminal bud epithelial cells. The percentages of related values were used to calculate means. Similarly, we used individual serum hormone levels and uterine weights to calculate means and to perform statistical analysis of the data by analysis of variance followed by Newman-Keuls post hoc test. Morphologic features were statistically analyzed with contingency tables compared with controls. All analyses were performed with GraphPad PRISM 4 software (GraphPad Software, Inc., San Diego, CA, USA). Data are presented as mean ± SEM.
Results
Animal weights
Table 1 presents body weights of animals before ovx and on the day of termination of the experiments. The E2 low and GEN high animals gained significantly less weight and the E2 high gained no weight compared with the control animals. Serum E2 levels in controls and E2-treated animals are shown in Figure 1A. Serum E2 concentrations were barely detectable in control animals, whereas they were in physiologic range in the E2 low group and at supra-physiologic levels in the E2 high group. Serum luteinizing hormone (LH) levels were high in control animals, decreased dose-dependently in the E2 groups, and not reduced significantly in the GEN groups (Figure 1B).
Table 1.
Initial and final body weights (grams) of E2- and GEN-treated ovx rats (mean ± SEM).
| Treatment
|
|||||
|---|---|---|---|---|---|
| Time point | Control | E2 low | E2 high | GEN low | GEN high |
| Initial bw | 256 ± 5.5 | 268 ± 5.8 | 261 ± 3.1 | 264 ± 6.6 | 250 ± 3.8 |
| Final bw | 341 ± 8.1* | 286 ± 9.8*,** | 258 ± 5.2** | 354 ± 8.1* | 293 ± 4.3*,** |
p < 0.05 compared with initial control weight.
p < 0.5 compared with final control weight.
Figure 1.
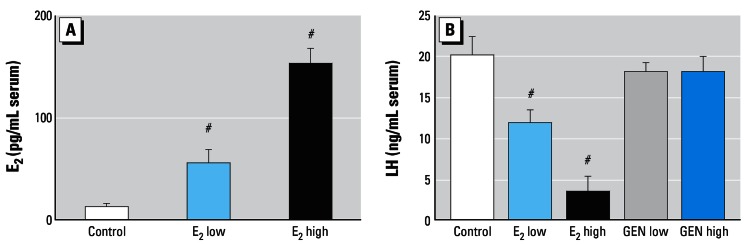
Serum levels (mean ± SEM) of E2 (A) and LH (B) in ovx rats after 3 months of treatment. Note the dose-dependent reduction of LH by E2 and the lacking effect of GEN.
#p > 0.01 compared with control.
Uterus
Both E2 doses and GEN high significantly increased uterine weight. With E2 (both doses), uterine weight was around 5-fold that of controls (Figure 2), and weights were in the range observed in intact adult female rats (441.1 ± 18.7 mg in previous experiments using the same rat strain). GEN high increased uterine weights only around 2-fold (Figure 2). Figure 3 shows microscopic preparations of representative uteri from one animal per treatment group. The morphologic findings in uteri of all animals were quantified and are presented in Table 2. In control uterus we observed atrophy affecting all structures (Figure 3A). The endometrium was composed of cuboidal inactive cells, and the connective tissue was an unorganized lax syncytium of round nuclei. No mitotic activity was detected in epithelial cells. E2 low (Figure 3B) induced estrogenic features such as large cytoplasms in endometrial cells, vacuolar degeneration, and necrosis; we also observed spindle lamina propria cells intermingled with areas mimicking proestrus of a normal cycle (smaller nucleus:cytoplasm ratio, less degeneration, and necrosis). Few pathologic findings, such as hypertrophic and hyperplastic glands, were detected. Mitotic activity was present in endometrial cells in most animals at various degrees.
Figure 2.
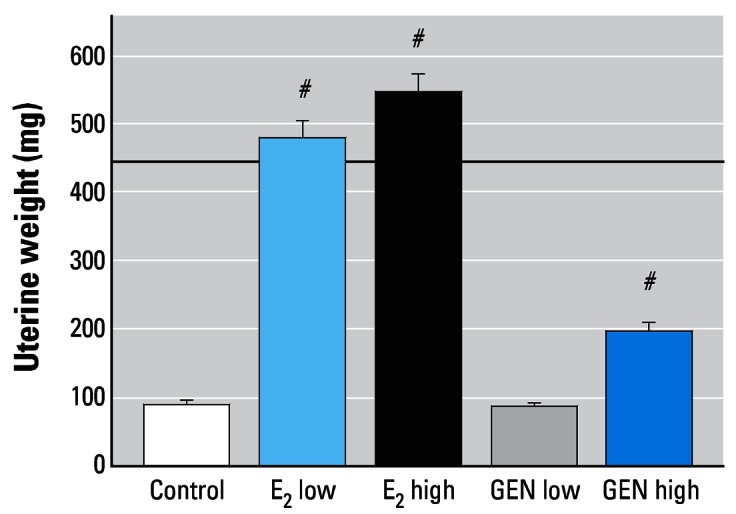
Uterine wet weights (mean ± SEM) of ovx rats after 3 months of treatment with E2 or GEN. Note the stimulating effects of both E2 doses and the GEN high. The dashed line indicates the average uterine wet weight for an intact female rat.
#p < 0.01 compared with control.
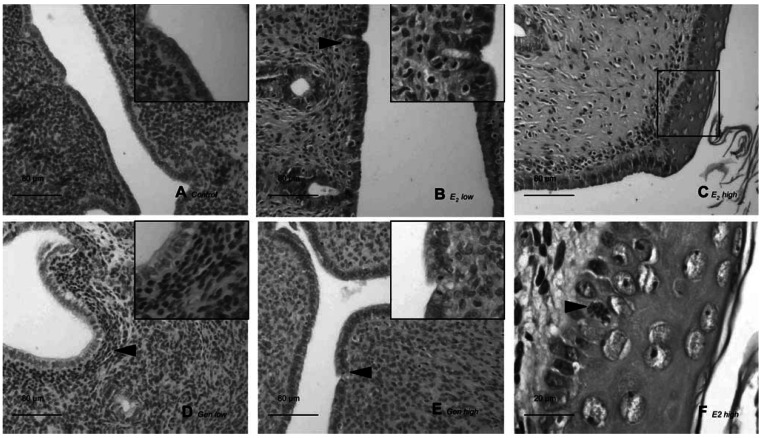
Figure 3.
Photomicrographs of representative uteri from one animal per treatment group. (A) Control: atrophic uterus with cuboidal endometrial epithelium and loose connective tissue composed of round nuclei in an unorganized pattern. (B) E2 low: the endometrium consists of tall single-layered epithelial cells with abundant mitotic figures and necrosis (arrowhead); stromal cells of endometrial lamina propria are well organized and spindle shaped. (C) E2 high: all structures are hypertrophic and hyperplastic; the endometrial epithelium is multilayered with squamous metaplasia and atypic mitotic figure (arrowhead) surrounded by anaplastic epithelial nuclei, and keratinized cells are present in the lumen. The square in (C) indicates the area shown at higher magnification in (F). (D) GEN low: lamina propria cells adopted an organized pattern (arrowhead), with more condensed nuclei of spindle shape. (E) GEN high: areas of high endometrial proliferation and unclear influences on lamina propria cells are present. Arrowheads indicate cells undergoing mitosis. Insets in (A), (B), (D), and (E) show higher magnifications of endometrium. Bar = 80 μm in (A–E) and 20 μm in (F).
Table 2.
Summary of physiologic and pathologic findings in uteri of ovx rats after treatment with E2 or GEN.
| Physiologic
|
Pharmacologic E2-induced findings by degree of severity
|
||||||
|---|---|---|---|---|---|---|---|
| Treatment | Uterine weight (mg) | Spindle-shaped lamina propria cells | Endometrial epithelium with mitosis | Hyperplastic/hypertrophic glands | Squamous metaplasia | Cystic glands | Pyometra |
| Control | 91.6 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 |
| E2 low | 478.4* | 11/11 | 11/11 | 6/11 | 0/12 | 0/12 | 0/12 |
| E2 high | 548.5* | 10/10 | 10/11 | 10/10 | 9/11 | 4/10 | 5/11 |
| GEN low | 87.92 | 6/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 |
| GEN high | 196.8* | 12/12 | 12/12 | 0/12 | 0/12 | 0/12 | 0/12 |
p < 0.05 vs. control.
With E2 high (Figure 3C) treatment, all uterine structures were hypertrophic and hyperplastic. Although differing in intensity, endometria were hypertrophic and hyperplastic in 10 of 11 animals (in 1 animal endometrium could not be analyzed due to severe pyometra) with squamous metaplasia and anaplastic signs such as hyperchromatic nuclei with dispersed chromatin and prominent nucleoli. We observed various mitotic figures in endometrial epithelium and, within these, bizarre mitotic figures were apparent. Pyometra also developed in 5 of 11 animals. Some of these effects in the E2 high group are shown in Figure 3F (a bizarre mitotic cell and keratinized cells). The treatment with GEN low (Figure 3D) induced negligible effects: Lamina propria cells were found in some areas in 6 of 12 animals but epithelial structures in endometrial epithelium remained unaltered, without hyperplastic/hypertrophic changes or mitotic activity. In GEN high animals (Figure 3E), endometrial cells were stimulated but no pathologic signs were detected. Endometrial mitotic activity was found abundantly in 1 of 12, rarely in 2 of 12, and absent in 9 of 12 animals.
Vagina
Figure 4 shows microscopic preparations of representative vaginae from one animal per treatment group; the morphologic findings in vaginae of all animals were quantified and are presented in Table 3. Atrophic vaginal epithelium was observed in control animals (Figure 4A); in 12 of 12 rats, only two to three cell layers were present, and these were composed of flattened cells with no cornification.
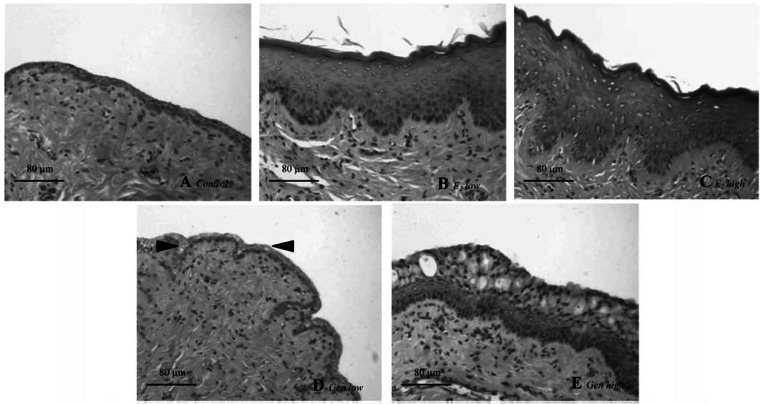
Figure 4.
Photomicrographs of representative vaginal sections from one animal per treatment group. (A) Control: atrophic vaginal epithelium is composed of a few layers of flattened cells. (B) E2 low: vaginal epithelium is hypertrophic and hyperplastic, with cornification in the upper layers. (C) E2 high: stronger hypertrophy and hyperplasia of epithelium are present; compared with E2 low, there are more cell layers and a higher degree of cornification. (D) GEN low: atrophic vaginal epithelium and the thickness and number of layers resemble that of control animals, but some areas have an incipient cytoplasmatic vacuolization (arrowheads). (E) GEN high: vaginal epithelium is increased (5–10 cells per layer on average), and large cytoplasmatic vacuoles are present in the upper layers. Bar = 80 μm.
Table 3.
Effects of treatment with E2 or GEN on morphologic features of vagina in ovx rats.
| Cell layers
|
Vacuolization
|
||||||
|---|---|---|---|---|---|---|---|
| Group | 1–5 | > 5 to < 10 | 10 | > 10 | Keratinization | Incipient | Clear |
| Control | 12/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 |
| E2 low | 0/12 | 0/12 | 11/11 | 0/12 | 11/11* | 0/12 | 0/12 |
| E2 high | 0/12 | 0/12 | 0/12 | 11/11 | 11/11* | 0/12 | 0/12 |
| GEN low | 12/12 | 0/12 | 0/12 | 0/12 | 0/12 | 5/12 | 0/12 |
| GEN high | 0/12 | 0/12 | 5/12 | 0/12 | 1/12 | 0/12 | 12/12* |
p < 0.05 compared with control.
The E2 low animals (Figure 4B) displayed a typical squamous multilayered epithelium. Approximately 10 cell layers with cornification were observed in all 11 samples. Almost the same changes were seen with E2 high (Figure 4C) as with E2 low: All 11 animals had around 10–15 cell layers with cornification. We found no clear differences between controls and GEN animals (Figure 4D). In GEN animals, epithelium thickness seemed slightly augmented in some areas, the number of cell layers did not differ from controls (two to three on average), and no cornification was found. An incipient cytoplasmatic vacuolization of epithelial cells was observed in 5 of 12 rats. The treatment with GEN high (Figure 4E) increased epithelial thickness and also the number of cell layers (5–10 layers). Cornification was found only in 1 of 12 animals, and cytoplasmatic vacuolization was clearly present in all samples.
Mammary gland
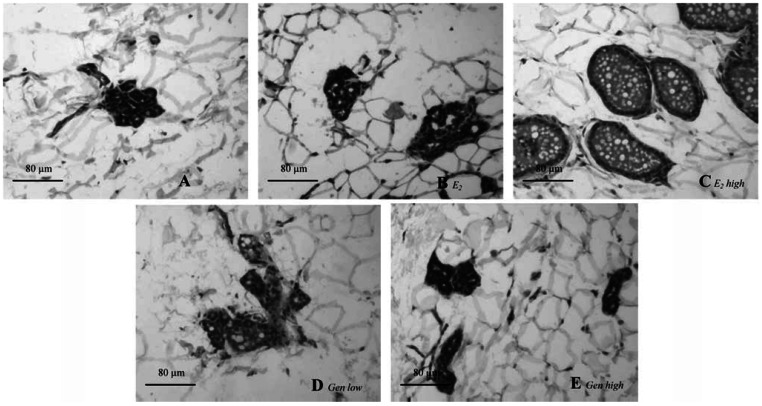
Figure 5 shows microscopic preparations of representative mammary glands from one animal per treatment group, and quantitative data obtained from glands of all animals are shown in Table 3. In controls (Figure 5A) all epithelial structures appeared atrophic. Deep in the fat pad, we observed scarce clusters of densely packed terminal structures, many of which did not show luminal formation. Treatment with E2 low (Figure 5B) induced formation of small lumina, but no secretory material was detected. Terminal epithelial structures were abundant after treatment with E2 high (Figure 5C). Luminal formation was present in all animals, and secretion was seen in 9 of 11 animals. Both GEN treatments (Figure 5D, E) were only slightly different from controls: No secretion was induced, and incipient luminal formation was present in one-half of the animals of both groups. An overview of all collected data, including their significance, is presented in Table 4.
Figure 5.
Photomicrographs of representative mammary gland sections from one animal per treatment group. (A) Control: a small cluster of densely packed epithelial cells without luminal formation are present in the deep subcutaneous fat pad. (B) E2 low: a few terminal structures with small lumina but without secretory material. (C) E2 high: well-formed acinar and luminal structures with secretory material in the lumina. In GEN low (D) and GEN high (E), there is almost no cellular or acinar difference from control. Bars = 80 μm.
Table 4.
Effects of treatment with E2 or GEN on morphologic features of mammary glands in ovx rats.
| Luminal formation
|
||||
|---|---|---|---|---|
| Group | Absent | Incipient | Clear | Secretion |
| Control | 6/12 | 6/12 | 0/12 | 0/12 |
| E2 low | 0/12 | 0/12 | 11/11* | 0/11 |
| E2 high | 0/12 | 0/12 | 11/11* | 9/11* |
| GEN low | 6/12 | 6/12 | 0/12 | 0/12 |
| GEN high | 6/12 | 6/12 | 0/12 | 0/12 |
p < 0.05 compared with control.
PCNA and PR immunostaining
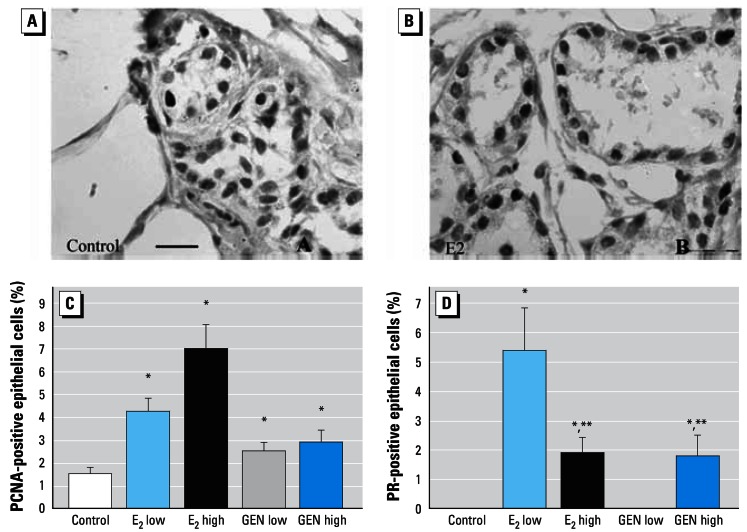
Figure 6A shows representative PCNA-immunostained section of mammary gland from a control animal, and Figure 6B shows a tissue section from an E2 high animal. In mammary gland samples immunostained for PCNA (Figure 6C) or PR (Figure 6D), the number of PCNA- or PR-expressing cells was counted and expressed as a percentage of the total number of cells. E2 stimulated expression of PCNA dose dependently, and GEN also stimulated expression of this proliferation marker (Figure 6C). PR-expressing nuclei were found only in mammary gland cells of animals treated with E2 and GEN (both doses of each; Figure 6D). E2 low was more effective in increasing the number of PR-expressing cells than was E2 high.
Figure 6.
Results of immunostaining for PCNA (A,C) and PR (B,D) in mammary gland. (A) Photomicrograph showing PCNA immunostaining in control tissue. (B) Photomicrograph showing PR immunostaining in E2 high mammary gland. Percentage of PCNA-positive (C) and PR-expressing (D) epithelial cells in mammary terminal structures. Immunostained PCNA cells were regularly seen in all animals (C). PR-expressing cells were seen in both in E2 groups and in the GEN high group (D); note the reduced number of PR-expressing cells in E2 high versus E2 low mammary glands. Bars = 20 μm.
*p < 0.05 compared with control; **p < 0.05 compared with E2 low.
Discussion
In the present study we compared the effects of two doses of genistein with those of two doses of E2 in ovx rats. We chose these doses on the basis of published data (Diel et al. 2004; Tinwell and Ashby 2004) and compared them with those used in recently published studies (Hertrampf et al. 2006; Michael McClain et al. 2006). The route and the duration of application were chosen because little information is available about the effects of subchronically and orally administered GEN in the uterus and mammary gland tissue of ovx rats (Hertrampf et al. 2006; Michael McClain et al. 2006). Michael McClain et al. (2006) reported that GEN at 500 mg/kg/day (higher than doses used in the present study) had no estrogenic effect in the uterus or the mammary gland; the animals in those experiments however were not ovx. Because we sought an animal model that might be comparable to postmenopausal women, we used ovx rats, which are in line with the Organisation for Economic Co-operation and Development recommendation to test for estrogenicity either in immature or ovx rats (Kanno et al. 2001). Hertrampf et al. (2006) used ovx rats and GEN doses similar to those used in the present study, but studied the effects after only 3 days of oral application. In agreement with our data, they observed estrogenic effects of GEN in the mammary gland (PR expression was increased) and a mild uterotrophic effect of GEN.
GEN is known to bind to both estrogen receptors, with a slight preference for ER-β. Data from ER-α–knock-out mice suggest that the ER-α is the receptor that mediates the typical effects of E2 in the uterus, vagina, and mammary gland (Couse and Korach 1999; Hewitt and Korach 2003). Most studies have addressed effects of E2 and GEN in these organs after a relatively short duration of application, and reports about longer-lasting effects particularly in the mammary gland are scarce. In the present study, administration of the two doses of E2 added to the daily food for 3 months resulted in high physiologic serum concentrations of E2 low and in supraphysiologic concentrations of E2 high. Serum LH concentrations were suppressed under both doses, which is typical for E2, whereas neither GEN dose affected LH serum values. Using LH serum levels as a marker of estrogenic effect in the hypothalamus—where the gonadotropin-releasing hormone pulse generator resides—GEN seems to be ineffective in reducing the activity of this brain structure. This may also explain the clinical finding that GEN did not substantially alleviate hot flashes (Geller and Studee 2005). Indeed, in women suffering from climacteric complaints, few placebo-controlled trials yielded significant effects of isoflavones. The placebo educed climacteric complaints by 30–50%, which was similar to the effectiveness of isoflavones (Geller and Studee 2005; Krebs et al. 2004; Phipps et al. 2002). Upon gross morphologic analyses, ovx animals had very low uterine weights; in animals treated with either E2 dose, uterine weight was increased to the range of that of intact animals. GEN high animals had increased uterine weight, whereas GEN low animals did not.
Histologic analysis of effects of both E2 doses revealed well-known morphologic changes in uterus (Cummings and Yochim 1984; Fazleabas and Strakova 2002). Under physiologic conditions of proestrus and estrus, the nuclei of lamina propria stromal cells depict a spindle shape and an organized cell pattern (Cummings and Yochim 1984; Fazleabas and Strakova 2002; Yuan 2002). We observed this pattern in one-half of the GEN low animals, in all GEN high animals, and in both E2 groups; in the controls, cells remained round and densely packed. Endometrial epithelial cells were also affected by E2 and GEN. During the course of an estrous cycle, E2 induces mitotic replication of cells and induces epithelial cells to adopt a tall cylindrical shape. Hence, in E2-treated animals, the epithelial cells depict such cylindrical shape with a high cytoplasmic:nuclear ratio. We observed changes like these mainly in the E2 low and GEN high animals. Under supraphysiologic doses of E2, we noted some pathologic features. Cells from epithelial glands were hypertrophic and hyperplastic, and the endometrial epithelium was multilayered and often developed squamous metaplasia. We did not observe such pathologic features in the GEN-treated animals. E2 also widens the lumen of the uterine cervix, which under chronic conditions allows bacterial ascension that may result in development of pyometras. Consequently, we found pyometras in some animals treated with E2 high. The morphologic changes induced by E2 or GEN in uterus are similar, differing only in degree. These results suggest that GEN has similar although weaker effects than E2 in the rat uterus.
Vaginal epithelium also subject to estrogenic action in that E2 causes proliferation and cornification. In the present study, we observed increased vaginal epithelial height, number of layers, and cornification in all samples from both E2 groups, with a stronger effect in the E2 high group. GEN low increased epithelial height only slightly; GEN high dose, however, increased numbers of layers comparable to E2 high. A different feature, namely, cytoplasmatic vacuolization, was detected in the GEN animals at incipient degrees in the low-dose animals and clearly in the high-dose animals. Cytoplasmatic vacuolization was not observed in any of the E2-treated animals. Hence, the morphologic changes induced by GEN in vaginal epithelium are not identical with those exerted by E2, suggesting that the mechanisms by which this phytoestrogen and its gut metabolites (e.g., equol) affect vaginal epithelium are different from those mediating the effects of E2.
Mammary gland tissue was scarce in the ovx control rats, and the few detectable terminal structures were collapsed. E2 low induced luminar formation but no secretion, whereas in the mammary gland of E2 high animals, ample tissue and signs of secretion were observed. GEN did not have marked effects on mammary gland morphology; half of the animals in each GEN dose group had an incipient luminal formation. PCNA is a nuclear protein that is expressed in proliferating cells during the S phase of the cell cycle and is a useful tool in mammary cancer prognosis research (Kurki et al. 1986; Schimmelpenning et al. 1993). It can be visualized by immunohistochemistry; this allows the estimation of the percentage of proliferating cells in relation to the total number of cells. In deep terminal mammary structures, epithelial proliferation was increased by both E2 doses; PCNA analysis also indicates that GEN has a stimulatory effect on mammary gland epithelial cells. Stimulation of mammary cell proliferation is an increased risk factor for the development of cancer (Gadducci et al. 2005).
Stimulation of PR in mammary epithelial cells is another typical effect of E2, which we observed in the animals treated with both E2 doses and GEN high; however, we counted significantly fewer PR-expressing cells in the E2 high tissues compared with the E2 low tissues. The reason the E2 high did not stimulate PR receptor as profoundly as did E2 low remains obscure, particularly in view of the profound stimulation of mammary duct tissue with luminal formation and secretory activity by the E2 high.
In summary, we found that GEN had similar but milder effects in the uterus than E2, without showing any selective ER modulator properties. Also, mammary gland proliferation was increased under both E2 and GEN. In the hypothalamus and vagina GEN exerted different effects compared with E2. LH release was unaltered, suggesting that GEN did not exert the estrogenic negative feed back on GnRH in the rat hypothalamus. The clearest finding that GEN does not act exactly the same as E2 was found in the vaginal epithelium, where GEN high induced a different feature: cytoplasmatic vacuolization.
Footnotes
This article is part of the monograph “Endocrine Disruptors—Exposure Assessment, Novel End Points, and Low-Dose and Mixture Effects.”
This study was funded in part by the European Union (EURISKED contract EVK1-CT-2002-00128 and CASCADE contract Food-CT-2004-506319).
References
- Albertazzi P, Sharma S. Urogenital effects of selective estrogen receptor modulators: a systematic review. Climacteric. 2005;8(3):214–220. doi: 10.1080/13697130500117946. [DOI] [PubMed] [Google Scholar]
- Allred CD, Allred KF, Ju YH, Clausen LM, Doerge DR, Schantz SL, et al. Dietary genistein results in larger MNU-induced, estrogen-dependent mammary tumors following ovariectomy of Sprague-Dawley rats. Carcinogenesis. 2004;25(2):211–218. doi: 10.1093/carcin/bgg198. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Boue SM, Wiese TE, Nehls S, Burow ME, Elliott S, Carter-Wientjes CH, et al. Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J Agric Food Chem. 2003;51(8):2193–2199. doi: 10.1021/jf021114s. [DOI] [PubMed] [Google Scholar]
- Bovee TF, Helsdingen RJ, Rietjens IM, Keijer J, Hoogenboom RL. Rapid yeast estrogen bioassays stably expressing human estrogen receptors alpha and beta, and green fluorescent protein: a comparison of different compounds with both receptor types. J Steroid Biochem Mol Biol. 2004;91(3):99–109. doi: 10.1016/j.jsbmb.2004.03.118. [DOI] [PubMed] [Google Scholar]
- Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Report a a meeting held in Funchal, Madeira, February 24–25, 2003. Climacteric. 2003;6(suppl 1):11–36. [PubMed] [Google Scholar]
- Cabanes A, Wang M, Olivo S, DeAssis S, Gustafsson JA, Khan G, et al. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25(5):741–748. doi: 10.1093/carcin/bgh065. [DOI] [PubMed] [Google Scholar]
- Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck AJ. Phytoestrogens: recent developments. Planta Med. 2003;69(7):589–599. doi: 10.1055/s-2003-41122. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Yochim JM. Differentiation of the uterus in preparation for gestation: a model for the action of progesterone. J Theor Biol. 1984;106(3):353–374. doi: 10.1016/0022-5193(84)90035-3. [DOI] [PubMed] [Google Scholar]
- Dave B, Eason RR, Till SR, Geng Y, Velarde MC, Badger TM, et al. The soy isoflavone genistein promotes apoptosis in mammary epithelial cells by inducing the tumor suppressor PTEN. Carcinogenesis. 2005;26(10):1793–1803. doi: 10.1093/carcin/bgi131. [DOI] [PubMed] [Google Scholar]
- Diel P, Geis RB, Caldarelli A, Schmidt S, Leschowsky UL, Voss A, et al. The differential ability of the phytoestrogen genistein and of estradiol to induce uterine weight and proliferation in the rat is associated with a substance specific modulation of uterine gene expression. Mol Cell Endocrinol. 2004;221(1–2):21–32. doi: 10.1016/j.mce.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ezaki T. Antigen retrieval on formaldehyde-fixed paraffin sections: its potential drawbacks and optimization for double immunostaining. Micron. 2000;31(6):639–649. doi: 10.1016/s0968-4328(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Strakova Z. Endometrial function: cell specific changes in the uterine environment. Mol Cell Endocrinol. 2002;186(2):143–147. doi: 10.1016/s0303-7207(01)00655-4. [DOI] [PubMed] [Google Scholar]
- Gadducci A, Biglia N, Sismondi P, Genazzani AR. Breast cancer and sex steroids: critical review of epidemiological, experimental and clinical investigations on etiopathogenesis, chemoprevention and endocrine treatment of breast cancer. Gynecol Endocrinol. 2005;20(6):343–360. doi: 10.1080/09513590500128492. [DOI] [PubMed] [Google Scholar]
- Geller SE, Studee L. Botanical and dietary supplements for menopausal symptoms: what works, what does not. J Womens Health (Larchmt) 2005;14(7):634–649. doi: 10.1089/jwh.2005.14.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alpha- or beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood) 2005;230(8):558–568. doi: 10.1177/153537020523000807. [DOI] [PubMed] [Google Scholar]
- Heinemann C, Reid G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. Can J Microbiol. 2005;51(9):777–781. doi: 10.1139/w05-070. [DOI] [PubMed] [Google Scholar]
- Hertrampf T, Schmidt S, Seibel J, Laudenbach-Leschowsky U, Degen GH, Diel P. Effects of genistein on the mammary gland proliferation of adult ovariectomised Wistar rats. Planta Med. 2006;72(4):304–310. doi: 10.1055/s-2005-916229. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003;125(2):143–149. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- Jeune MA, Kumi-Diaka J, Brown J. Anticancer activities of pomegranate extracts and genistein in human breast cancer cells. J Med Food. 2005;8(4):469–475. doi: 10.1089/jmf.2005.8.469. [DOI] [PubMed] [Google Scholar]
- Kanno J, Onyon L, Haseman J, Fenner-Crisp P, Ashby J, Owens W. The OECD program to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: phase 1. Environ Health Perspect. 2001;109:785–794. doi: 10.1289/ehp.01109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno J, Onyon L, Peddada S, Ashby J, Jacob E, Owens W. The OECD program to validate the rat uterotrophic bioassay. Phase 2: coded single-dose studies. Environ Health Perspect. 2003;111:1550–1558. doi: 10.1289/ehp.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijkuokool P, Parhar IS, Malaivijitnond S. Genistein enhances N-nitrosomethylurea-induced rat mammary tumorigenesis. Cancer Lett. 2005;242(1):53–59. doi: 10.1016/j.canlet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Kousidou OC, Mitropoulou TN, Roussidis AE, Kletsas D, Theocharis AD, Karamanos NK. Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int J Oncol. 2005;26(4):1101–1109. doi: 10.3892/ijo.26.4.1101. [DOI] [PubMed] [Google Scholar]
- Krebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. 2004;104(4):824–836. doi: 10.1097/01.AOG.0000140688.71638.d3. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kurki P, Vanderlaan M, Dolbeare F, Gray J, Tan EM. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA. Timing of exposure and mammary cancer risk. J Mammary Gland Biol Neoplasia. 2002;7(1):67–76. doi: 10.1023/a:1015722507237. [DOI] [PubMed] [Google Scholar]
- Liu B, Edgerton S, Yang X, Kim A, Ordonez-Ercan D, Mason T, et al. Low-dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumor prevention. Cancer Res. 2005;65(3):879–886. [PubMed] [Google Scholar]
- Michael McClain R, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem Toxicol. 2006;44(1):56–80. doi: 10.1016/j.fct.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor α (ERα) and ERβ in human cells. Toxicol Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor alpha, oestrogen receptor beta and androgen receptors in the rat reproductive organs. J Endocrinol. 2000;165(2):359–370. doi: 10.1677/joe.0.1650359. [DOI] [PubMed] [Google Scholar]
- Phipps WR, Duncan AM, Kurzer MS. Isoflavones and post-menopausal women: a critical review. Treat Endocrinol. 2002;1(5):293–311. doi: 10.2165/00024677-200201050-00003. [DOI] [PubMed] [Google Scholar]
- Ricketts ML, Moore DD, Banz WJ, Mezei O, Shay NF. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J Nutr Biochem. 2005;16(6):321–330. doi: 10.1016/j.jnutbio.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Maguire SM, Gaughan J, Millar MR. Expression of oestrogen receptor beta (ER beta) in multiple rat tissues visualised by immunohistochemistry. J Endocrinol. 1997;154(3):R13–16. doi: 10.1677/joe.0.154r013. [DOI] [PubMed] [Google Scholar]
- Schimmelpenning H, Eriksson ET, Franzen B, Zetterberg A, Auer GU. Prognostic value of the combined assessment of proliferating cell nuclear antigen immunostaining and nuclear DNA content in invasive human mammary carcinomas. Virchows Arch A Pathol Anat Histopathol. 1993;423(4):273–279. doi: 10.1007/BF01606890. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Michna H, Diel P. Combinatory effects of phytoestrogens and 17beta-estradiol on proliferation and apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2005;94(5):445–449. doi: 10.1016/j.jsbmb.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I. Comparative distribution of estrogen receptor-alpha (ER-alpha) and beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids. 1998;63(10):498–504. doi: 10.1016/s0039-128x(98)00054-3. [DOI] [PubMed] [Google Scholar]
- Sirtori CR, Arnoldi A, Johnson SK. Phytoestrogens: end of a tale? Ann Med. 2005;37(6):423–438. doi: 10.1080/07853890510044586. [DOI] [PubMed] [Google Scholar]
- Tekmal RR, Liu YG, Nair HB, Jones J, Perla RP, Lubahn DB, et al. Estrogen receptor alpha is required for mammary development and the induction of mammary hyper-plasia and epigenetic alterations in the aromatase transgenic mice. J Steroid Biochem Mol Biol. 2005;95(1–5):9–15. doi: 10.1016/j.jsbmb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Tinwell H, Ashby J. Sensitivity of the immature rat uterotrophic assay to mixtures of estrogens. Environ Health Perspect. 2004;112:575–582. doi: 10.1289/ehp.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Di Renzo GC. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo-controlled study. Fertil Steril. 2004;82(1):145–148. doi: 10.1016/j.fertnstert.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Vantyghem SA, Wilson SM, Postenka CO, Al-Katib W, Tuck AB, Chambers AF. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 2005;65(8):3396–3403. doi: 10.1158/0008-5472.CAN-04-4109. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Foley JP. Female reproductive system. In: Haschok WM, Roussaux CG, Wallig MA, editors. Handbook of Toxicologic Pathology. 2. Vol. 2. San Diego, CA: Academic Press; 2002. pp. 847–894. [Google Scholar]